Abstract
Normal functioning of the brain is dependent upon a complex web of communication between numerous cell types. Within neuronal networks, the faithful transmission of information between neurons relies on an equally complex organization of inter- and intra-cellular signaling systems that act to modulate protein activity. In particular, post-translational modifications (PTMs) are responsible for regulating protein activity in response to neurochemical signaling. The key second messenger, cyclic adenosine 3’,5’- monophosphate (cAMP), regulates one of the most ubiquitous and influential PTMs, phosphorylation. While cAMP is canonically viewed as regulating the addition of phosphate groups through its activation of cAMP-dependent protein kinases, it plays an equally critical role in regulating removal of phosphate through indirect control of protein phosphatase activity. This dichotomy of regulation by cAMP places it as one of the key regulators of protein activity in response to neuronal signal transduction throughout the brain. In this review we focus on the role of cAMP in regulation of the serine/threonine phosphatases protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) and the relevance of control of PP1 and PP2A to regulation of brain function and behavior.
Keywords: PP1, PP2A, cAMP, PKA, brain, striatum
I. Introduction
It is difficult to overstate the importance of protein phosphorylation and dephosphorylation in the control of neuronal function. Understanding the mechanisms governing phosphorylation status of proteins is critical to elucidating the details of diverse aspects of neuronal development, synaptic transmission and plasticity, and neurodegeneration, as well as how these processes are affected by both physiological and pathological stimuli. The importance of protein phosphorylation has been appreciated for some time [1,2]; however, even after decades of study, novel aspects of the complex regulation of phosphorylation networks continue to be discovered.
Protein phosphorylation is a fundamental principle guiding cellular function. This fact is especially poignant in the brain where cells must communicate and respond across a wide range of time scales. Phosphorylation provides a rapid and adaptable platform for extracellular signals to affect intracellular processes. Maintaining the proper balance of phosphorylation not only depends on the appropriate addition but also the appropriate removal of phosphates. Thus, signaling pathways must incorporate modification of both kinase and phosphatase activity in order to appropriately modify a target protein’s phosphorylation status. Proper functioning of these complex signaling pathways is critical for normal neuronal function as is evidenced by psychiatric and degenerative illnesses associated with deficits in these regulatory networks. Appropriate regulation is maintained by a variety of signaling molecules and pathways. One important source of regulation is cyclic adenosine 3’,5’-monophosphate (cAMP). In this review we will focus on the role cAMP plays in regulating two important phosphatases, protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) in neurons of the central nervous system.
II. Background
Cyclic AMP and protein kinase A
Since its initial discovery, cAMP has been recognized as one of the most important signaling molecules in cells throughout various tissues of the body, including the brain [3]. cAMP has served as a model second messenger presenting a blueprint for how a single type of molecule can coordinate a complex network of intracellular signaling in response to extracellular stimuli. A vast array of signals trigger cAMP rises through the transmembrane protein, adenylyl cyclase (AC). Signals that stimulate AC range from intracellular Ca2+ fluxes, such as those triggered by the opening of ER membrane channels [4], to Gαs protein stimulation from G-protein coupled receptors (GPCRs) such as type 1 dopamine receptors [5]. In addition to many possible stimuli of AC activity, there are also many isoforms of AC with a variety of properties of cAMP production [6]. Maintaining a proper balance of cAMP depends not only on its production but also on its degradation. Cyclic nucleotide phosphodiesterases (PDEs) are a family of molecules capable of hydrolyzing cAMP. PDEs come in a wide variety of forms, with 11 genes and more than 50 different isoforms of PDEs regulating cAMP in different regions and contexts [7]. It has been long understood that cells utilize the multitude of AC and PDE isoforms for both production and degradation to tightly regulate cAMP abundance [8]. The precision and depth of regulation made possible by this variety of isoforms, particularly in the central nervous system, continues to be expanded upon even after decades of study [9]. As a result of the array of distinct possibilities in protein expression and localization for both cAMP generation and degradation, there is a huge diversity in the patterns of cAMP that act to regulate downstream signaling. Numerous studies have demonstrated the versatility and importance of this regulation in the formation of gradients for developing neurons [10], cAMP nanodomains [11], and variable GPCR responses [12].
Down-stream effects of cAMP can be mediated by a variety of substrates, such as exchange protein directly activated by cAMP (EPAC) [13] and cyclic nucleotide-gated ion channels [14]. Nonetheless, cAMP-dependent protein kinase (or protein kinase A (PKA)) is a serine/threonine kinase that is generally considered to be the largest effector of cAMP signaling within cells. PKA activation requires a certain threshold of cAMP abundance and thus is tightly regulated by cAMP gradients generated by the interplay between ACs and PDEs. Through extensive and ongoing research it has been established that PKA is a heterotetromer composed of two regulatory subunits and two catalytic subunits; upon cAMP binding to the regulatory subunits the catalytic subunits are released from their autoinhibited state and are able to phosphorylate substrates [15–18]. PKA activity is dependent on both its activation and its proximity to an appropriate substrate. Kinases favor particular substrates for phosphorylation in a manner partially determined by the presence of a recognition sequence accessible on the given substrate. While the consensus motif has been long recognized as a derivative of the R-R-X-S/T-Φ (Φ being a hydrophobic residue) motif, it is clear that the presence of this motif is not the only determinant of kinase activity [19,20]. Appropriate docking and adaptor domain interactions are critical for kinase activity and specificity [20]. In the case of PKA, adaptor proteins that ensure the proximity of PKA to target substrates have long been recognized and appreciated for their role in regulating PKA activity [8,15,21–23]. For example, A-kinase anchoring proteins (AKAPs) create hubs of PKA regulation [21–25] by binding PKA itself as well as both the sources of cAMP production, ACs [26], and degradation, PDEs [27,28]. Thus, the regulation of PKA activity is a highly orchestrated process regulated by a variety of players in a spatiotemporally controlled manner.
Cyclic AMP and regulation of serine/threonine protein phosphatases
As mentioned above cAMP is capable of a broad range of actions that affect the behavior of a variety of downstream targets. In addition to increasing PKA activity, cAMP is able to modulate the actions of serine/threonine phosphatases, which are responsible for the removal of phosphate groups. While there are ~400 different serine/threonine kinases there are far fewer catalytic subunits of serine/threonine phosphatases, that are defined by four main sub-groups [29]. This review will focus on two phosphatases regulated by cAMP, protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A). Both PP1 and PP2A are part of the serine/threonine phosphoprotein phosphatase (PPP) family and account for the vast majority of eukaryotic phosphatase activity [29]. Thus, these two enzymes play critical roles in regulating phosphorylation status and proper signal transduction in many vital molecular pathways throughout the body.
Protein phosphatase 1
PP1 is ubiquitously expressed at high levels throughout the body. It is encoded by three separate genes which can encode 4 different catalytic isoforms [29]. Unlike the specificity afforded PKA by its consensus sequence, PP1 lacks inherent substrate specificity; rather, specificity is determined by interactions with some of its more than 200 known interacting proteins [30]. Interaction with different binding partners determines the localization and pattern of activity of PP1. Specific interactions with a particular binding protein can enhance PP1 activity toward selected substrates while limiting interactions with others [30]. Notably, one of PP1’s binding partners is AKAP79 which helps to localize PP1 near the cAMP regulatory machinery [31]. Concentrating multiple molecular regulators with a hub like AKAP79 ensures that the appropriate molecular targets are impacted. Altering the presence of various PP1 regulators and substrates can dramatically affect the behavior of the enzyme in different cell types or subcellular compartments, such as within the dendritic spines of neurons [32–34]. Thus, it is important to understand the regulatory proteins, signaling molecules, and substrates in the microenvironment surrounding PP1 in order to determine the protein’s behavior.
Protein phosphatase 2A
PP2A is a heterotrimer consisting of a scaffolding A subunit, a catalytic C subunit, and a variable regulatory B subunit [35]. There are four families of B subunits, B, B’, B”, an B”’ but only one is present in each holoenzyme [36]. Within the 4 families of B subunits there are also multiple isoforms. For example, the B’ family is encoded by 5 genes (B’α, β, γ, δ, or ε) with multiple splice variants (β1 and β2) [35]. In addition to the variation in B subunits, the A and C subunits also have α and β forms [35]. Thus, there is a huge variety in the composition of PP2A heterotrimers that affect its localization, activity, and as we will discuss below, its response to cAMP. While the mechanisms guiding PP2A specificity are still being discovered; it is nonetheless clear that specificity is in part dependent on protein location and subunit composition [37]. Both the abundant forms of PP2A and wide variety of its regulators are critical to appropriate enzymatic function in a complex network of signaling molecules ensuring proper protein phosphorylation.
III. Increasing phosphorylation through cAMP-regulated inhibition of protein phosphatases
Phosphatases are not typically thought of as drivers of phosphorylation; however, cAMP regulation of phosphatases can result in increased phosphorylation status of proteins. Specifically, cAMP is capable of orchestrating widespread rise in phosphorylation of a variety of substrates through specific inhibition of PP1.
DARPP-32: cAMP-dependent inhibition of PP1
DARPP-32 (dopamine and cyclic adenosine 3’,5’ monophosphate regulated phosphoprotein, 32 kDa) was originally discovered from an analysis of phosphoproteins regulated by the neurotransmitter, dopamine [38]. DARPP-32 is enriched specifically in dopaminoceptive medium spiny neurons in the striatum which express either D1 or D2 type dopamine receptors [38,39]. D1 dopamine receptors are coupled positively to AC through Gαs and can stimulate PKA activity, while D2-class receptors, couple negatively to AC via Gαi, thus decreasing cAMP levels and lowering PKA activity [40–42]. It was initially discovered that DARPP-32 phosphorylation is achieved by either dopamine stimulation or increasing cAMP levels [38]. Purification and characterization of DARPP-32 revealed biochemical similarities to inhibitor-1, a potent inhibitor of PP1, found in other regions of the body [43]. Ultimately it was discovered that DARPP-32 is a homolog of inhibitor-1 and behaves in much the same fashion. Both proteins are phosphorylated by PKA at a single threonine (Thr-34 in DARPP-32; Thr-35 in inhibitor-1) found within a highly conserved N-terminal region. DARPP-32 is effective at nanomolar concentrations, is specific to PP1, and is only active when phosphorylated by PKA just like inhibitor-1 [43–46].
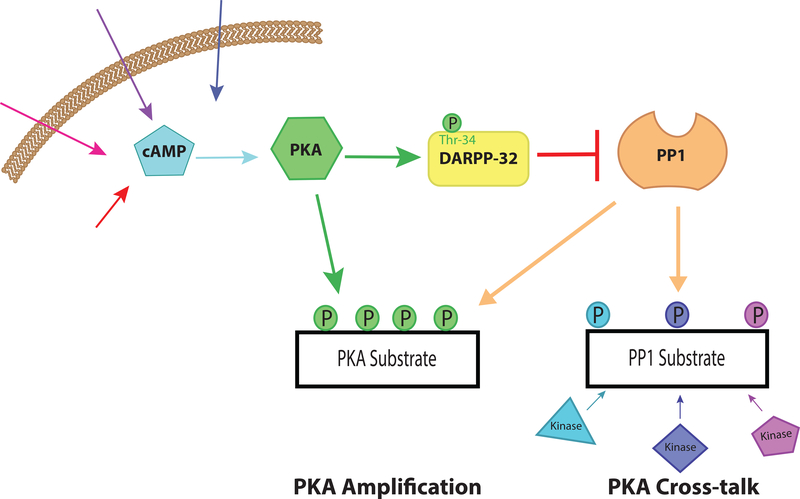
Both DARPP-32 and inhibitor-1 proteins interact with PP1 through two subdomains, a docking domain and an inhibitory domain [47–50]. The docking domain interacts with PP1 in a region removed from the active site of the enzyme, that also interacts with a variety of targeting proteins that utilize the so-called RVXF motif [51]. In DARPP-32, this motif is located in the N-terminal region, residues 6–11 (RKKIQF) [49]. Although the interaction of the RVXF motif is removed from the active site of PP1, it is nonetheless critical for the ability of PKA-phosphorylated DARPP-32 to inhibit PP1 activity [52]. A full-review of the structural basis of interaction and mechanism of regulation by DARPP-32 with PP1 is outside the scope of this review and has been well covered elsewhere [34,53,54]. Nonetheless, a large body of evidence indicates that cAMP plays a key physiological role in regulating DARPP-32-dependent inhibition of PP1 activity in striatal neurons. As illustrated in Figure 1, elevation of cAMP resulting from dopamine stimulation or various other neurochemical signals leads to an increase in PKA phosphorylation of DARPP-32 at Thr-34, which activates DARPP-32 to inhibit PP1. Other mechanisms regulating DARPP-32 activity will be discussed below; however, this is one pathway by which cAMP increases phosphorylation levels by decreasing PP1 phosphatase activity, in this case leading to amplification of D1 receptor signaling. This mechanism of PP1 regulation is regionally specific given that DARPP-32 is preferentially expressed in regions of the brain innervated by dopaminergic signaling [39]. Thus, providing a unique pathway for PP1 regulation in distinct brain regions as will be discussed further below.
Figure 1. cAMP-mediated inhibition of PP1.
A schematic of cAMP-mediated inhibition of PP1 via activation of DARPP-32. An elevation in cAMP, which can result from a variety of stimuli, leads to activation of PKA. Subsequently, PKA phosphorylates DARPP-32 at Thr-34 converting the protein into a potent inhibitor of PP1. As a result, suppression of PP1 activity contributes to increased phosphorylation of substrates for a variety of kinases, including PKA. This can lead to “cross-talk” between PKA signaling pathways and other kinase pathways or to “amplification” of PKA signaling by preventing dephosphorylation of substrates for PKA.
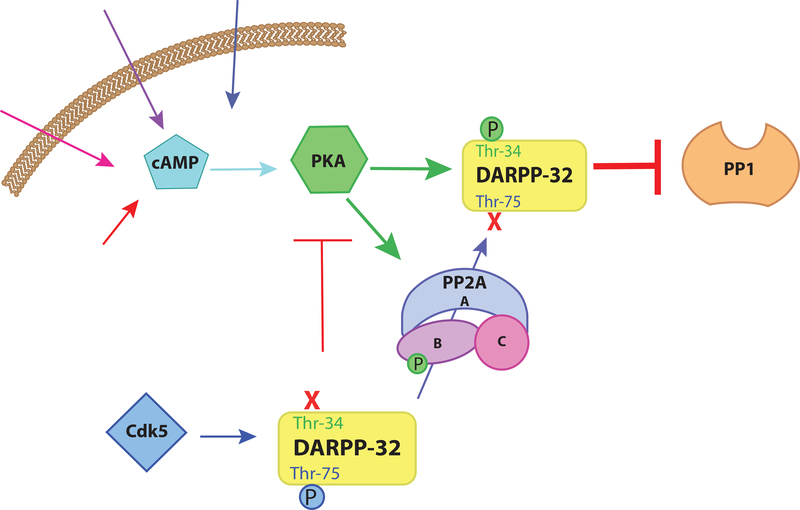
While phosphorylation of Thr-34 and inhibition of PP1 activity is the central feature of DARPP-32 function, its regulation involves a complex interplay between a wide variety of kinases and phosphatases, including both PP1 and PP2A acting at various phosphorylation sites within DARPP-32 (see 54–56 for detailed reviews). In brief, PP2A dephosphorylates two other phosphorylated residues on DARPP-32, Thr-75 and Ser-97. As will be discussed in greater detail below, PKA is able to phosphorylate and activate a specific heterotrimeric form of PP2A [57].Thus, elevation of cAMP levels can increase the dephosphorylation of a subset of PP2A substrates, one of which is DARPP-32. Specifically, upon PKA-dependent activation, PP2A dephosphorylates DARPP-32 at Thr-75 [58–60], a site phosphorylated by cyclin-dependent kinase 5 (Cdk5) [61]. When phosphorylated by Cdk5, DARPP-32 acts as an inhibitor of PKA [61]. Phosphorylation at Thr-75 antagonizes phosphorylation of Thr-34 [56]. Thus, decreasing phosphorylation of Thr-75 by activation of PP2A, cAMP not only reduces the inhibition of PKA but also enables PKA phosphorylation of DARPP-32 and subsequent inhibition of PP1 (Figure 2). The interaction between PP1 and PP2A mediated by differential phosphorylation and dephosphorylation of distinct sites in DARPP-32 is a powerful example of how cAMP can drive modifications that affect webs of interconnected molecular pathways.
Figure 2. PKA enhancement of DARPP-32-mediated PP1 inhibition.
cAMP activates PKA which phosphorylates DARPP-32 at Thr-34 resulting in DARPP-32-mediated inhibition of PP1. However, DARPP-32 is also phosphorylated by the kinase Cdk5 at Thr-75. When DARPP-32 is phosphorylated at Thr-75 it suppresses PKA activity. Additionally, the p-Thr-75 form of DARPP-32 antagonizes Thr-34 phosphorylation by PKA, diminishing the ability of DARPP-32 to inhibit PP1. PKA also phosphorylates and activates a specific heterotrimeric form of PP2A that selectively dephosphorylates DARPP-32 at Thr-75. Thus, PKA drives the phosphorylation of DARPP-32 as well as driving dephosphorylation of an antagonistic phosphorylation site thus enhancing PKA mediated inhibition of PP1 via DARPP-32.
IV. cAMP stimulation decreases phosphorylation
Increases in cAMP levels are typically associated with increases in phosphorylation due to the ubiquitous expression of its primary target, PKA. However, a variety of studies revealed a paradox of cAMP activation; despite activating kinases, increased cAMP led to decreases in phosphorylation of specific substrates in a number of cellular contexts [62–64]. This counter-intuitive response led to numerous studies of the role of cAMP and PKA in the regulation of protein phosphatase activity. Moreover, these studies revealed various examples of how phosphatases are involved in feedback control of kinases; reducing phosphorylation levels both at the level of kinase activation and substrate modification [65]. For example, PP2A dephosphorylates protein kinase B (Akt or PKB) at Thr-308, a phosphorylation site which normally activates the kinase [66,67]. Thus, phosphorylation is reduced both by the removal of phosphates and the prevention of phosphate addition. Through a network of signaling pathways cAMP is able to both increase and decrease phosphorylation depending upon the components, location, and basal phosphorylation state of the proteins involved. Here we discuss some examples related to the regulation of PP1 and PP2A by cAMP that alter phosphatase activity levels to decrease phosphorylation levels.
Regulation of PP1 targeting by cAMP-dependent phosphorylation
As mentioned above PP1 has a wide variety of protein binding partners. These protein interactions play a critical role in its regulation as discussed above for DARPP-32, and the localization of the catalytic subunit. Localization of PP1 by distinct targeting subunits typically enhances phosphatase activity against a preferred substrate, or in some cases enhances activity toward one substrate while suppressing activity toward another substrate [29,30,68]. Importantly, cAMP plays a role in regulating PP1 interactions as well as the interactions of PP1 targeting proteins. Thus cAMP can regulate PP1 activity, in either direction, toward specific substrates. Here we will address a few examples of this manner of PP1 regulation by cAMP in the context of neurons (see 32,68,69 for broader context).
Neurabin I is a neurally expressed filamentous-actin (F-actin) binding protein first identified in rat brain as critical for neurite formation [70]. Neurabin I shares significant structurally similarity with a PP1 binding protein highly enriched in dendritic spines, but ubiquitously expressed, called spinophilin or neurabin II [71,72]. In brain, neurabin I, spinophilin, and PP1 form actin-associated molecular networks [73,74]. Both proteins bind to PP1 via RVXF domains and contribute to targeting of the protein [75,76]. In vitro spinophilin and neurabin I inhibit PP1 activity [73,77,78] but in vivo they seem to direct PP1 activity rather that suppress it. In vivo both proteins target PP1 near AMPA-type glutamate receptors where PP1 is able to dephosphorylate the GluR1 subunit of the receptor [79–81]. Structural studies have provided a potential mechanism by which spinophilin can influence the substrate specificity of PP1 [77]. The binding of spinophilin to PP1 is able to block access to some substrates while leaving the active site able to dephosphorylate certain other substrates, like the subunits of the glutamate receptor [77]. The extent to which the features and roles of these two proteins align compared to how they diverge remains to be fully elucidated.
While neurabin and spinophilin are similar in terms of their structural interaction with PP1, their functions and regulation appear distinct. Knock-out (KO) of either protein in mice reveals dramatically different phenotypes; in corticostriatal synapses neurabin I KO animals exhibit deficits in long-term potentiation (LTP) rescued by D1 agonists while spinophilin KO animals exhibit deficits in long-term depression (LTD) rescued by D2 agonists [82]. In contrast, recent evidence in the hippocampus suggests that neurabin I/PP1 interactions, but not spinophilin/PP1 interactions, are critical for the induction of LTD [79]. It remains unclear exactly why the proteins have opposing effects in corticostriatal neurons and why this effect is different in hippocampal circuits. Although the detailed mechanism may be unclear it is evident that despite structural similarities the two proteins vary in their regulation of synaptic plasticity.
cAMP has the capacity to regulate neurabin and spinophilin in different ways. Neurabin I binding to PP1c is important for proper localization [73,78]. Proper neurabin I/PP1 interactions are important for maintaining cytoskeletal dynamics as well as dendritic maturation and synaptic plasticity [70,75,83,84]. The interaction between neurabin I and PP1 is inhibited by PKA phosphorylation at Ser-461, a site immediately C-terminal to the RVXF-motif [78]. This does relieve PP1 of inhibition from neurabin I as evidenced by increased PP1 activity in cell culture experiments; however, without the proper localization phosphatase activity within the synapse is diminished as evidenced by increased phosphorylation of certain synaptic targets [83]. Spinophilin is also phosphorylated by PKA; however, this phosphorylation is at Ser-97 and Ser-177, which decreases the affinity of spinophilin for F-actin but not PP1 [85]. Increased PKA activation thus dissociates the entire spinophilin-PP1 complex from its normal dendritic spine localization rather than PP1 from its targeting protein [85]. Thus, PKA disrupts this localization and may change the substrates that are accessible to PP1. cAMP activation of PKA will alter the localization of PP1 with modified substrate affinity. This will, for example, decrease the activity of PP1 in dendritic spines but may lead to enhanced PP1 activity in different cellular compartments by increasing the concentration of the enzyme outside of spines.
cAMP/PKA-dependent phosphorylation and activation of PP2A
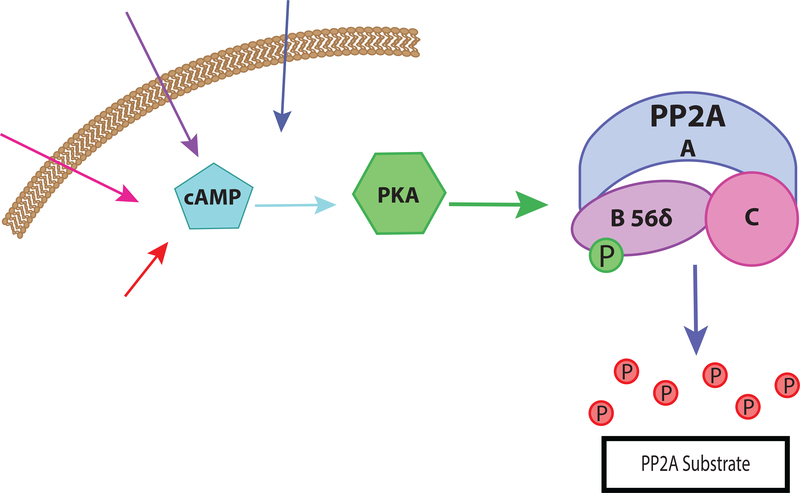
In addition to regulating protein modifiers of phosphatase activity, cAMP affects the activity of the phosphatase enzyme itself. As mentioned above, cAMP is capable of activating PP2A via phosphorylation by PKA. Initial studies in cell culture models concluded that increases in cAMP levels were associated with decreased phosphorylation of selected substrates leading to the hypothesis that a specific phosphatase may be responsible [64]. In addition, cAMP-mediated decreases in phosphorylation were sensitive to PP2A, but not PP1, inhibitors, and PP2A activity was increased [64]. PKA had also been shown in vitro to phosphorylate the regulatory B’ subunit (B56δ or PPP2R5D) of PP2A leading to enzyme activation [57].
The B56δ subunit can be phosphorylated at multiple sites by PKA, both in vitro and in intact cells [57,58] with phosphorylation of Ser-566 being responsible for enzyme activation [57,58]. The precise mechanism of activation of PP2A by PKA is not known, but both the Km and Vmax are affected by phosphorylation [58]. The structure of several assemblies of PP2A have been determined, including that of the B56γ holoenzyme [86], however, the crystal structure only shows the core alpha-helical region conserved in B56γ and B56δ and does not provide insight into the role of the C-terminal region of B56δ. The in vitro studies indicated a specific influence of PKA-dependent phosphorylation on some but not all substrates [57] suggesting some sort of effect of B56γ C-terminal phosphorylation on substrate recruitment.
In summary, an elevation of cAMP can lead to enhanced dephosphorylation of certain PP2A substrates as illustrated in Figure 3. This activation is not a universal property of PP2A. Activation by PKA was only observed in the B56δ-containing heterotrimers as opposed Bα-containing heterotrimers [58], and is important for creating a context-specific response of PP2A to cAMP elevation. Differential subunit expression is therefore able to tune PP2A response to appropriate signaling pathways and prevent off-target phosphatase activity.
Figure 3. cAMP mediated activation of PP2A.
cAMP activates PKA which phosphorylates the B56δ subunit of the PP2A heterotrimer. This phosphorylation increases the phosphatase activity of the enzyme resulting in greater dephosphorylation of selected PP2A substrates.
cAMP regulation of PP2A is dependent not only on the pattern of expression of the B56γ subunit, but also on the interaction with other regulatory proteins and their substrates. Anchoring proteins create nodes of activity to guide the network to respond in a specific fashion. As mentioned above, AKAPs anchor both PKA and PDEs to create a tight regulation of cAMP activity. PP2A can also be associated with these anchoring sites in specific locations where PKA mediated PP2A activity is required. In one such node, PP2A is anchored by mAKAP in coordination with PKA and PDE4D3 [87]. Normally, PKA phosphorylation of PDE4D3 serves as a negative feedback loop activating the PDE’s ability to hydrolyze cAMP, thus lowering local concentrations and diminishing PKA activation [88]. However, when B56δ containing PP2A is also present, an increase in PKA activity drives the phosphatase to dephosphorylate PDE4D3 switching the negative feedback loop into a positive feedback loop [87]. PP2A activity increases the hydrolysis of cAMP via PDE4D3 thus regulating the activation of PKA and the entire pathway. Given that certain forms of PP2A also inactivate PKA it is possible that these nodes operate on multiple levels to finely tune the pathways activated by cAMP.
ENSA and ARPP-16/19: disinhibition of PP2A by cAMP
As a continuation of the studies referenced above examining phosphoproteins regulated by cAMP in striatal neurons [38], two highly related proteins were identified and termed ARPP-16 and ARPP-19 (cAMP regulated phosphoprotein, 16 kDa or 19 kDa respectively) [89]. Subsequent studies identified a related gene product, termed endosulfine (ENSA) [90]. All of these proteins share a high degree of sequence similarity; ARPP-16 and ARPP-19 are alternatively spliced variants of the same gene with ARPP-19 containing 16 additional residues at its N-terminus [89]. ENSA (also termed ARPP-19e) is the product of a different gene and contains a unique N-terminus [91]. Two regions are highly conserved through evolution in all three proteins; one region containing a site (Ser-46 in ARPP-16; Ser-62 in ARPP-19) phosphorylated by Greatwall (Gwl) kinase [92] or MAST3 kinase [67] and the other region containing the PKA phosphorylation site (Ser-88 in ARPP-16; Ser-104 in ARPP-19) [93]. These regions are conserved not only between the three proteins but across species, indicating their importance to proper protein function [93]. The proteins have distinct expression profiles. ARPP-16 is expressed exclusively in the brain in various forebrain regions but is particularly enriched in medium spiny neurons of the striatum [94,95]. ARPP-19 is expressed throughout the body including within the brain but has a relatively low level of expression in the striatum [93]. ENSA represents the predominant 19 kDa ARPP-related protein in the striatum and is also found in other brain regions [93].
A full understanding of the function of these proteins both within the brain and other organ systems of the body is just beginning to be elucidated. Unlike DARPP-32, sequence analysis of the ARPP-16/19/ENSA proteins did not reveal an obvious function. However, there were indications that ARPP-16/19 served an important function in the brain, including an association between ARPP-19 and Alzheimer’s disease [96] as well as the observation that ARPP-16/19 knockout mice were embryonically lethal [56]. Evidence for an interaction between the ARPP-16/19 proteins and phosphatase activity first came from studies looking at the functional role of PP2A regulators in cell cycle progression [92,97]. Previous work had shown that in drosophila, ENSA played a key role in regulating meiotic maturation [98]. Furthermore, PP2A was known to be a critical regulator of the cell cycle that was somehow inactivated through the activity of the Gwl kinase, although not through direct inhibition [92,97]. Thus, these studies sought to discover what protein mediated PP2A inhibition via phosphorylation by Gwl. One group found that ARPP-19, while the other group found that ENSA, when phosphorylated, inhibited PP2A [92,97]. Subsequent studies indicated that in post-mitotic neurons, ARPP-16 was phosphorylated at Ser-46 by microtubule-associated serine/threonine kinase 3 (MAST3 kinase), converting the protein into a potent inhibitor of PP2A in an analogous fashion to that observed by Gwl in the cell cycle [67]. ARPP-16 interacts with the scaffolding A subunit and in certain heterotrimeric forms of PP2A, when phosphorylated at Ser-46, specifically inhibits B55α- and B56δ-containing forms [67]. Notably, ARPP-16 is highly phosphorylated by MAST3 kinase in vivo in the striatum suggesting that basally, certain PP2A heterotrimers are tonically inhibited. Consistent with this, knock-out of ARPP-16/19 in forebrain neurons leads to significant increases in phosphorylation of a subset of PP2A substrates [67].
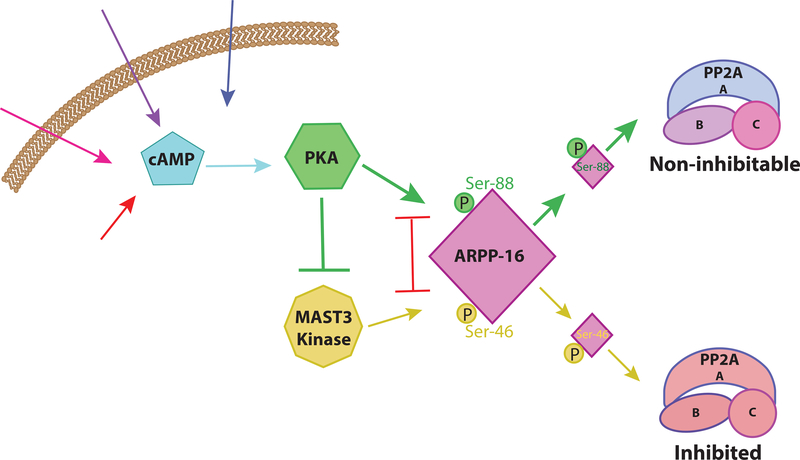
Given that ARPP-16 was originally identified as a substrate for PKA it was important to identify the effect of this regulation. Increased phosphorylation at the PKA site (Ser-88) was found to be associated with decreased phosphorylation at the MAST3 kinase site (Ser-46) [67]. In follow-up studies, it was found that PKA and MAST3 kinase phosphorylation of ARPP-16 are mutually antagonistic; phosphorylation at either site results in decreased phosphorylation of the opposing site [99]. PKA is also able to suppress MAST3 kinase phosphorylation of ARPP-16 by directly phosphorylating and inhibiting MAST3 kinase [99]. Therefore cAMP acts as a switch to regulate ARPP-16 inhibition of PP2A [99] (Figure 4). cAMP drives PKA activation which phosphorylates MAST3 kinase and ARPP-16. Once ARPP-16 is phosphorylated it pushes PP2A into a “non-inhibitable” form. Overall, an elevation in cAMP leads to a disinhibition of PP2A activity.
Figure 4. Disinhibition of PP2A by cAMP.
Schematic of cAMP-mediated disinhibition of PP2A via PKA and ARPP-16. Without PKA phosphorylation, MAST3 kinase phosphorylates ARPP-16 at Ser-46. This form of ARPP-16 selectively inhibits the activity of specific heterotrimeric forms of PP2A. cAMP activates PKA which phosphorylates ARPP-16 at Ser-88, which interacts with PP2A to make the enzyme non-inhibitable. Phosphorylation of the Ser-88 and Ser-46 sites is mutually antagonistic, thus phosphorylation at Ser-88 opposes phosphorylation at Ser-46 and vice versa. PKA is also able to phosphorylate and inhibit MAST3 kinase further reducing Ser-46 phosphorylation.
In summary, cAMP can act by two distinct, but possibly synergistic, pathways to increase PP2A activity. One pathway is through direct activation of PP2A heterotrimers containing the B56δ subunit; the second pathway is through disinhibition of B56δ or Bα containing PP2A. These pathways likely help explain the paradoxical observation of decreased phosphorylation in response to cAMP from earlier studies and suggest that such a mode of regulation may be widespread.
V. Physiological consequences of cAMP regulation of PP1 and PP2A in brain
Appropriate protein phosphorylation plays a critical role in normal neuronal function. Neurons rely on rapid adaptation to changes in stimuli in order to integrate information about the world around us and orchestrate complex behavioral responses. The building blocks of these computations are synapses where neurons connect and communicate with one another via chemical and electrical signals. Modulation of protein phosphorylation underlies the regulation of the electrical properties of a neuron [100], as well as the complex molecular changes related to learning and memory, including LTP and LTD [101]. Thus, a proper balance between kinase and phosphatase activity is critical for neuronal function, circuit function, and ultimately behavior. cAMP regulation of phosphatase activity plays an important role in mediating this balance and enabling effective neuronal communication.
cAMP-mediated phosphatase action and the regulation of neuronal ion channels
Ionic conductance is the basis of neuronal electrical communication. Manipulation of ion channels has dramatic effects on neuronal function. Essentially all ion channels are regulated by phosphorylation with the modulation of certain ion channels being critical for various aspects of brain development as well as learning and memory. The N-methyl-D-aspartate (NMDA) receptors are key calcium permeable glutamate receptors that serve as coincidence detectors important for synaptic potentiation. Post-translational modifications of NMDA receptors have significant effects on conductance thus altering their ability to potentiate synapses. Multiple studies have demonstrated a clear link between dopamine stimulation and the potentiation of NMDA receptors in a PP1-dependent manner [102–104]. The NR1 subunit of the NMDA receptor can be phosphorylated by PKA and dephosphorylated by PP1 [34]. PKA phosphorylation on the intracellular loop at Ser-684 increases the evoked response amplitude of the channel [105]. Since the discovery of this site it has been well understood that both kinases and phosphatases play similarly important roles in regulating its phosphorylation status [106]. Phosphorylation of the NMDA receptor is increased not only by the activity of PKA but also by reduction of phosphatase activity by DARPP-32 inhibition of PP1 [107]. When cAMP is activated, PKA signaling is stimulated and further potentiated by DARPP-32-mediated PP1 inhibition as discussed above. In neurons expressing PP2A B56δ, there is a greater enhancement of PKA activity via PP2A-mediated dephosphorylation of DARPP-32 at Thr-75 as discussed above [58,108]. Thus, elevated cAMP downstream of D1 receptor activation can potentiate NMDA conductance increases in medium spiny neurons [104,109].
Calcium channels also provide an excellent example of the importance of the microenvironment in cAMP regulation of phosphatases. In neurons, PKA is closely associated with L-type calcium channels via AKAP79/150 scaffolding protein mentioned earlier [110]. Furthermore, PP2A was found to bind the C-terminus of a specific subtype of these channels, CaV1.2, and dephosphorylate the PKA site in this channel resulting in decreased channel conductance [111,112]. The binding of PP2A and subsequent dephosphorylation of this channel was found to be specific to B’ and B” containing forms of the heterotrimer [112]. The ability of PP2A to regulate L-type calcium conductance is therefore dependent on subunit expression of both the channel and the phosphatase, which varies by brain region and cell type. cAMP regulation of PP2A is also subunit specific, activating only B56δ containing heterotrimers. Thus, the activity of PKA on even a single class of calcium channels is subject to regulation at a variety of levels. This example highlights the importance of context and local environment in understanding cAMP regulation of phosphatases.
Overall, phosphatases play an important role in regulating the activity of neurons. Numerous studies in brain have demonstrated roles for PP1 and PP2A in LTP [113–115] and LTD [116,117]. Ultimately, the mechanisms of regulation vary by brain region, cell type, and intracellular environment. Nonetheless it is clear that cAMP regulation of phosphatases plays an essential role in regulating neuronal potentiation and ionic conductance throughout the brain.
cAMP regulation of PP1/PP2A: disease relevance
Given the specificity of cAMP and subsequent phosphatase response in certain neuronal populations it can be instructive to take a larger scale view of behavior in order get a full picture of the actions of PP1 and PP2A. Both these phosphatases have been implicated in a wide variety of psychiatric illness such as Alzheimer’s disease [118,119], intellectual disability [120–122] and drug addiction [123,124]. Recently PP2A was linked to psychiatric illness and depression-like phenotypes through both animal behavior and genetic screens [125,126]. Furthermore, cAMP itself has long been associated with a wide variety of psychiatric illnesses [127–129]. The particular PP2A subunit regulated by PKA activity, PP2A B56δ, was implicated in intellectual disability [130]. Thus, all of the components of the above-described pathways appear likely to play a role in psychiatric and neurodegenerative illness; however, detailed mechanisms of how these components cause disease remain to be discovered. The interconnected regulation by cAMP/PKA pathways and a range of other kinases as well as signaling molecules makes identifying the contribution of single pathway extremely difficult at an organismal level. For example, stress is known to elevate the level of cAMP in the brain [131] and PP2A activity is increased in response to stressful behavioral paradigms [125]. While this is currently correlation it is not unreasonable to expect that PKA could play a role in regulating PP2A activity in response to stress. However, cAMP is far from the only signaling molecule affected by stress [132] and thus further studies are necessary to determine the specific contribution of cAMP regulation of PP2A in the neuronal stress response. In Down Syndrome and Alzheimer’s disease there are decreased levels of PKA and ARPP-19 suggesting aberrant cAMP-mediated phosphatase regulation [96]. The mechanism by which these altered pathways lead to degeneration remains to be determined but provides important insights into the necessity of appropriate cAMP regulation of phosphatase for neuronal health. Thus, while further research is required there is strong evidence that cAMP regulation of PP1 and PP2A plays an important role in appropriate neuronal function and aberrant regulation may contribute to psychiatric illness.
VI. Summary and concluding remarks
Protein phosphorylation is an important mechanism that cells utilize to modify behavior in response to external stimuli. A proper balance of kinase and phosphatase activity is important to neuronal function. cAMP is a critical second messenger that orchestrates a symphony of proteins to create appropriate cellular responses. Regulation of two proteins in particular, PP1 and PP2A, by cAMP has a dramatic impact on neuronal protein phosphorylation. cAMP is able to both increase and decrease phosphatase activity depending upon the local context. Through interactions with protein regulators like DARPP-32 and ARPP-16, cAMP is able to dynamically regulate the activity of both PP1 and PP2A in response to external stimuli. This degree of versatility enables neurons to use similar pathway components for vastly different cellular outcomes including synaptic potentiation versus depression. Understanding the diversity of ways these molecules interact is critical to knowing how neurons maintain proper protein phosphorylation balance. Furthermore, the connections between both PP1 and PP2A and a variety of psychiatric and neurological illnesses indicates that these molecules play an integral role in regulating proper neuronal communication. Further studies of the role of cAMP in regulation in PP1 and PP2A activities across and within neurons will be critical to discerning how neurons integrate and respond to the environment around them.
Acknowledgements:
We acknowledge the support from the NIH (AG047270, NS091336, Training Grant T32 NS41228). Support was also obtained from the State of Connecticut Department of Mental Health and Addiction Services and by a Gruber Fellowship.
The authors would like to thank Veronica Musante and Dan Li for their fruitful discussions
Abbreviations:
- cAMP
cyclic-adenosine 3’−5’-monophosphate
- PP1
protein phosphatase 1
- PP2A
protein phosphatase 2A
- PKA
cAMP-dependent protein kinase
- DARPP-32
dopamine and cyclic adenosine 3’,5’ monophosphate regulated phosphoprotein, 32 kDa
- AC
adenylyl cyclase
- PDE
phosphodiesterase
- MAST3 kinase
microtubule-associated serine/threonine kinase 3
- ARPP-16/ARPP-19
cAMP regulated phosphoprotein, 16 kDa or 19 kDa
- ENSA
endosulfine
- KO
knock-out
- AKAP
A-kinase anchoring protein
- GPCR
G-protein coupled receptor
- LTP
long-term potentiation
- LTD
long-term depression
- Gwl
greatwall
Citations:
- [1].Nestler EJ, Greengard P, Protein phosphorylation in the brain, Nature. 305 (1983) 583–588. doi: 10.1038/305583a0. [DOI] [PubMed] [Google Scholar]
- [2].Walaas SI, Greengard P, Protein phosphorylation and neuronal function., Pharmacol. Rev 43 (1991) 299–349. [PubMed] [Google Scholar]
- [3].Sutherland EW, Rall TW, Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles, J. Biol. Chem 232 (1958) 1077–1091. [PubMed] [Google Scholar]
- [4].Cooper DMF, Mons N, Karpen JW, Adenylyl cyclases and the interaction between calcium and cAMP signalling, Nature. 374 (1995) 421–424. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- [5].Beaulieu J-M, Espinoza S, Gainetdinov RR, Dopamine receptors - IUPHAR Review 13, Br. J. Pharmacol 172 (2015) 1–23. doi: 10.1111/bph.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sunahara RK, Dessauer CW, Gilman AG, Complexity and diversity of mammalian adenylyl cyclases, Annu. Rev. Pharmacol. Toxicol 36 (1996) 461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- [7].Lugnier C, Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents, Pharmacol. Ther 109 (2006) 366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- [8].Houslay MD, Milligan G, Tailoring cAMP-signalling responses through isoform multiplicity, Trends Biochem. Sci 22 (1997) 217–224. [DOI] [PubMed] [Google Scholar]
- [9].Neves-Zaph SR, Phosphodiesterase diversity and signal processing within cAMP signaling networks, Adv. Neurobiol 17 (2017) 3–14. doi: 10.1007/978-3-319-58811-7_1. [DOI] [PubMed] [Google Scholar]
- [10].Gorshkov K, Mehta S, Ramamurthy S, Ronnett GV, Zhou F-Q, Zhang J, AKAP-mediated feedback control of cAMP gradients in developing hippocampal neurons, Nat. Chem. Biol 13 (2017) 425–431. doi: 10.1038/nchembio.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lohse C, Bock A, Maiellaro I, Hannawacker A, Schad LR, Lohse MJ, Bauer WR, Experimental and mathematical analysis of cAMP nanodomains, PLoS One. 12 (2017) e0174856. doi: 10.1371/journal.pone.0174856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tsvetanova NG, von Zastrow M, Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis, Nat. Chem. Biol 10 (2014) 1061–1065. doi: 10.1038/nchembio.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bos JL, Epac: a new cAMP target and new avenues in cAMP research, Nat. Rev. Mol. Cell Biol 4 (2003) 733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- [14].Kaupp UB, Seifert R, Cyclic nucleotide-gated ion channels, Physiol. Rev 82 (2002) 769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- [15].Dell’Acqua ML, Scott JD, Protein kinase A anchoring, J. Biol. Chem 272 (1997) 12881–12884. doi: 10.1074/jbc.272.20.12881. [DOI] [PubMed] [Google Scholar]
- [16].Smith FD, Scott JD, Protein kinase A activation: Something new under the sun?, J Cell Biol. 217 (2018) 1895–1897. doi: 10.1083/jcb.201805011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Smith FD, Esseltine JL, Nygren PJ, Veesler D, Byrne DP, Vonderach M, Strashnov I, Eyers CE, Eyers PA, Langeberg LK, Scott JD, Local protein kinase A action proceeds through intact holoenzymes, Science. 356 (2017) 1288–1293. doi: 10.1126/science.aaj1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Taylor SS, Buechler JA, Yonemoto W, cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes, Annu. Rev. Biochem 59 (1990) 971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- [19].Kemp BE, Pearson RB, Protein kinase recognition sequence motifs, Trends Biochem. Sci 15 (1990) 342–346. doi: 10.1016/0968-0004(90)90073-K. [DOI] [PubMed] [Google Scholar]
- [20].Miller CJ, Turk BE, Homing in: mechanisms of substrate targeting by protein kinases, Trends Biochem. Sci 43 (2018) 380–394. doi: 10.1016/j.tibs.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Colledge M, Scott JD, AKAPs: from structure to function, Trends Cell Biol. 9 (1999) 216–221. doi: 10.1016/S0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- [22].Edwards AS, Scott JD, A-kinase anchoring proteins: protein kinase A and beyond, Curr. Opin. Cell Biol 12 (2000) 217–221. doi: 10.1016/S0955-0674(99)00085-X. [DOI] [PubMed] [Google Scholar]
- [23].Pidoux G, Taskén K, Specificity and spatial dynamics of protein kinase A signaling organized by A-kinase-anchoring proteins, J. Mol. Endocrinol 44 (2010) 271–284. doi: 10.1677/JME-10-0010. [DOI] [PubMed] [Google Scholar]
- [24].Schott M, Gonowolo F, Maliske B, Grove B, FRET biosensors reveal AKAP-mediated shaping of subcellular PKA activity and a novel mode of Ca2+/PKA crosstalk, Cell. Signal 28 (2016) 294–306. doi: 10.1016/j.cellsig.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Smith FD, Reichow SL, Esseltine JL, Shi D, Langeberg LK, Scott JD, Gonen T, Intrinsic disorder within an AKAP-protein kinase A complex guides local substrate phosphorylation, elife. 2 (2013) e01319. doi: 10.7554/eLife.01319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DMF, Dessauer CW, Scott JD, Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes, Mol. Cell 23 (2006) 925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carlisle Michel JJ, Dodge KL, Wong W, Mayer NC, Langeberg LK, Scott JD, PKA-phosphorylation of PDE4D3 facilitates recruitment of the mAKAP signalling complex, Biochem. J 381 (2004) 587–592. doi: 10.1042/BJ20040846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Koçer SS, Wang H, Malbon CC, “Shaping” of cell signaling via AKAP-tethered PDE4D: probing with AKAR2-AKAP5 biosensor, J. Mol. Signal 7 (2012) 4. doi: 10.1186/1750-2187-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bollen M, Peti W, Ragusa MJ, Beullens M, The extended PP1 toolkit: designed to create specificity, Trends Biochem. Sci 35 (2010) 450–458. doi: 10.1016/j.tibs.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Peti W, Nairn AC, Page R, Structural basis for protein phosphatase 1 regulation and specificity, FEBS J. 280 (2013) 596–611. doi: 10.1111/j.1742-4658.2012.08509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Le AV, Tavalin SJ, Dodge-Kafka KL, Identification of AKAP79 as a protein phosphatase 1 catalytic binding protein, Biochemistry. 50 (2011) 5279–5291. doi: 10.1021/bi200089z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cohen PTW, Protein phosphatase 1 – targeted in many directions, J. Cell Sci 115 (2002) 241–256. [DOI] [PubMed] [Google Scholar]
- [33].Virshup DM, Shenolikar S, From promiscuity to precision: protein phosphatases get a makeover, Mol. Cell 33 (2009) 537–545. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- [34].Greengard P, Allen PB, Nairn AC, Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 Cascade, Neuron. 23 (1999) 435–447. doi: 10.1016/S0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- [35].Janssens V, Goris J, Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling., Biochem. J 353 (2001) 417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mayer-Jaekel RE, Hemmings BA, Protein phosphatase 2A — a ‘ménage à trois,’ Trends Cell Biol. 4 (1994) 287–291. doi: 10.1016/0962-8924(94)90219-4. [DOI] [PubMed] [Google Scholar]
- [37].Slupe AM, Merrill RA, Strack S, Determinants for substrate specificity of protein phosphatase 2A, Enzyme Res. (2011). doi: 10.4061/2011/398751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ivar Walaas S, Aswad DW, Greengard P, A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions, Nature. 301 (1983) 69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- [39].Walaas SI, Greengard P, DARPP-32, a dopamine- and adenosine 3’:5’-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. I. Regional and cellular distribution in the rat brain, J. Neurosci 4 (1984) 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Beaulieu J-M, Gainetdinov RR, The physiology, signaling, and pharmacology of dopamine receptors, Pharmacol. Rev 63 (2011) 182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- [41].Enjalbert A, Bockaert J, Pharmacological characterization of the D2 dopamine receptor negatively coupled with adenylate cyclase in rat anterior pituitary., Mol. Pharmacol 23 (1983) 576–584. [PubMed] [Google Scholar]
- [42].Kebabian JW, Calne DB, Multiple receptors for dopamine, Nature. 277 (1979) 93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- [43].Hemmings HC, Nairn AC, Aswad DW, Greengard P, DARPP-32, a dopamine- and adenosine 3’:5’-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. II. Purification and characterization of the phosphoprotein from bovine caudate nucleus, J. Neurosci. Off. J. Neurosci 4 (1984) 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hemmings HC Jr, Greengard P, Tung HYL, Cohen P, DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1, Nature. 310 (1984) 503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- [45].Hemmings HC, Williams KR, Konigsberg WH, Greengard P, DARPP-32, a dopamine- and adenosine 3’:5’-monophosphate-regulated neuronal phosphoprotein. I. Amino acid sequence around the phosphorylated threonine, J. Biol. Chem 259 (1984) 14486–14490. [PubMed] [Google Scholar]
- [46].Williams KR, Hemmings HC, LoPresti MB, Konigsberg WH, Greengard P, DARPP-32, a dopamine- and cyclic AMP-regulated neuronal phosphoprotein. Primary structure and homology with protein phosphatase inhibitor-1., J. Biol. Chem 261 (1986) 1890–1903. [PubMed] [Google Scholar]
- [47].Desdouits F, Cheetham JJ, Huang HB, Kwon YG, da Cruz e Silva EF, Denefle P, Ehrlich ME, Nairn AC, Greengard P, Girault JA, Mechanism of inhibition of protein phosphatase 1 by DARPP-32: studies with recombinant DARPP-32 and synthetic peptides, Biochem. Biophys. Res. Commun 206 (1995) 652–658. [DOI] [PubMed] [Google Scholar]
- [48].Shenolikar S, Nairn AC, Protein phosphatases: recent progress, Adv. Second Messenger Phosphoprotein Res 23 (1991) 1–121. [PubMed] [Google Scholar]
- [49].Kwon Y-G, Huang H-B, Desdouits F, Girault J-A, Greengard P, Nairn AC, Characterization of the interaction between DARPP-32 and protein phosphatase 1 (PP-1): DARPP-32 peptides antagonize the interaction of PP-1 with binding proteins, Proc. Natl. Acad. Sci 94 (1997) 3536–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Endo S, Zhou X, Connor J, Wang B, Shenolikar S, Multiple structural elements define the specificity of recombinant human inhibitor-1 as a protein phosphatase-1 inhibitor, Biochemistry. 35 (1996) 5220–5228. doi: 10.1021/bi952940f. [DOI] [PubMed] [Google Scholar]
- [51].Egloff M-P, Johnson DF, Moorhead G, Cohen PTW, Cohen P, Barford D, Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1, EMBO J. 16 (1997) 1876–1887. doi: 10.1093/emboj/16.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Huang H, Horiuchi A, Watanabe T, Shih S-R, Tsay H-J, Li H-C, Greengard P, Nairn AC, Characterization of the inhibition of protein phosphatase-1 by DARPP-32 and Inhibitor-2, J. Biol. Chem 274 (1999) 7870–7878. doi: 10.1074/jbc.274.12.7870. [DOI] [PubMed] [Google Scholar]
- [53].Aggen JB, Nairn AC, Chamberlin R, Regulation of protein phosphatase-1, Chem. Biol 7 (2000) R13–R23. doi: 10.1016/S1074-5521(00)00069-7. [DOI] [PubMed] [Google Scholar]
- [54].Yger M, Girault J-A, DARPP-32, Jack of all trades… master of which?, Front. Behav. Neurosci 5 (2011). doi: 10.3389/fnbeh.2011.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Svenningsson P, Nishi A, Fisone G, Girault J-A, Nairn AC, Greengard P, DARPP-32: an integrator of neurotransmission, Annu. Rev. Pharmacol. Toxicol 44 (2004) 269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- [56].Walaas SI, Hemmings HCJ, Greengard P, Nairn AC, Beyond the dopamine receptor: regulation and roles of serine/threonine protein phosphatases, Front. Neuroanat 5 (2011). doi: 10.3389/fnana.2011.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hirofumi U, Inoue R, Tanabe O, Nishito Y, Shimizu M, Hideyuki H, Takeda M, Activation of protein phosphatase 2A by cAMP‐dependent protein kinase‐catalyzed phosphorylation of the 74‐kDa B″ (δ) regulatory subunit in vitro and identification of the phosphorylation sites, FEBS Lett. 430 (1998) 312–316. doi: 10.1016/S0014-5793(98)00684-X. [DOI] [PubMed] [Google Scholar]
- [58].Ahn J-H, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC, Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56δ subunit, Proc. Natl. Acad. Sci 104 (2007) 2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nishi A, Snyder GL, Nairn AC, Greengard P, Role of calcineurin and protein phosphatase-2A in the regulation of DARPP-32 dephosphorylation in neostriatal neurons, J. Neurochem 72 (1999) 2015–2021. [DOI] [PubMed] [Google Scholar]
- [60].Nishi A, Bibb JA, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P, Regulation of DARPP‐32 dephosphorylation at PKA‐ and Cdk5‐sites by NMDA and AMPA receptors: distinct roles of calcineurin and protein phosphatase‐2A, J. Neurochem 81 (2002) 832–841. doi: 10.1046/j.1471-4159.2002.00876.x. [DOI] [PubMed] [Google Scholar]
- [61].Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai L-H, Kwon YT, Girault J-A, Czernik AJ, Huganir RL, Hemmings HC Jr, Nairn AC, Greengard P, Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons, Nature. 402 (1999) 669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- [62].Christoffersen J, Smeland EB, Stokke T, Taskén K, Andersson KB, Blomhoff HK, Retinoblastoma protein is rapidly dephosphorylated by elevated cyclic adenosine monophosphate levels in human B-lymphoid cells, Cancer Res. 54 (1994) 2245–2250. [PubMed] [Google Scholar]
- [63].Begum N, Graham AL, Sussman KE, Draznin B, Role of cAMP in mediating effects of fasting on dephosphorylation of insulin receptor, Am. J. Physiol.-Endocrinol. Metab 262 (1992) E142–E149. doi: 10.1152/ajpendo.1992.262.2.E142. [DOI] [PubMed] [Google Scholar]
- [64].Feschenko MS, Stevenson E, Nairn AC, Sweadner KJ, A novel cAMP-stimulated pathway in protein phosphatase 2A activation, J. Pharmacol. Exp. Ther 302 (2002) 111–118. doi: 10.1124/jpet.302.1.111. [DOI] [PubMed] [Google Scholar]
- [65].Millward TA, Zolnierowicz S, Hemmings BA, Regulation of protein kinase cascades by protein phosphatase 2A, Trends Biochem. Sci 24 (1999) 186–191. doi: 10.1016/S0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- [66].Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA, Mechanism of activation of protein kinase B by insulin and IGF-1., EMBO J. 15 (1996) 6541–6551. [PMC free article] [PubMed] [Google Scholar]
- [67].Andrade EC, Musante V, Horiuchi A, Matsuzaki H, Brody AH, Wu T, Greengard P, Taylor JR, Nairn AC, ARPP-16 is a striatal-enriched inhibitor of protein phosphatase 2A regulated by microtubule-associated serine/threonine kinase 3 (Mast 3 Kinase), J. Neurosci 37 (2017) 2709–2722. doi: 10.1523/JNEUROSCI.4559-15.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Brautigan DL, Shenolikar S, Protein serine/threonine phosphatases: Keys to unlocking regulators and substrates, Annu. Rev. Biochem 87 (2018) 921–964. doi: 10.1146/annurev-biochem-062917-012332. [DOI] [PubMed] [Google Scholar]
- [69].Ceulemans H, Bollen M, Functional diversity of protein phosphatase-1, a cellular economizer and reset button, Physiol. Rev 84 (2004) 1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- [70].Nakanishi H, Obaishi H, Satoh A, Wada M, Mandai K, Satoh K, Nishioka H, Matsuura Y, Mizoguchi A, Takai Y, Neurabin: a novel neural tissue-specific actin filament-binding protein involved in neurite formation, J. Cell Biol 139 (1997) 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Allen PB, Ouimet CC, Greengard P, Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Mizoguchi A, Takai Y, Neurabin-II/spinophilin. An actin filament-binding protein with one pdz domain localized at cadherin-based cell-cell adhesion sites, J. Biol. Chem 273 (1998) 3470–3475. [DOI] [PubMed] [Google Scholar]
- [73].MacMillan LB, Bass MA, Cheng N, Howard EF, Tamura M, Strack S, Wadzinski BE, Colbran RJ, Brain actin-associated protein phosphatase 1 holoenzymes containing spinophilin, neurabin, and selected catalytic subunit isoforms, J. Biol. Chem 274 (1999) 35845–35854. doi: 10.1074/jbc.274.50.35845. [DOI] [PubMed] [Google Scholar]
- [74].Ryan XP, Alldritt J, Svenningsson P, Allen PB, Wu G-Y, Nairn AC, Greengard P, The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology, Neuron. 47 (2005) 85–100. doi: 10.1016/j.neuron.2005.05.013. [DOI] [PubMed] [Google Scholar]
- [75].Morishita W, Connor JH, Xia H, Quinlan EM, Shenolikar S, Malenka RC, Regulation of synaptic strength by protein phosphatase 1, Neuron. 32 (2001) 1133–1148. doi: 10.1016/S0896-6273(01)00554-2. [DOI] [PubMed] [Google Scholar]
- [76].Hsieh-Wilson LC, Allen PB, Watanabe T, Nairn AC, Greengard P, Characterization of the neuronal targeting protein spinophilin and its interactions with protein phosphatase-1, Biochemistry. 38 (1999) 4365–4373. doi: 10.1021/bi982900m. [DOI] [PubMed] [Google Scholar]
- [77].Ragusa MJ, Dancheck B, Critton DA, Nairn AC, Page R, Peti W, Spinophilin directs protein phosphatase 1 specificity by blocking substrate binding sites, Nat. Struct. Mol. Biol 17 (2010) 459–464. doi: 10.1038/nsmb.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].McAvoy T, Allen PB, Obaishi H, Nakanishi H, Takai Y, Greengard P, Nairn AC, Hemmings HC, Regulation of neurabin I interaction with protein phosphatase 1 by phosphorylation, Biochemistry. 38 (1999) 12943–12949. [DOI] [PubMed] [Google Scholar]
- [79].Gao J, Hu X-D, Yang H, Xia H, Distinct roles of protein phosphatase 1 bound on neurabin and spinophilin and its regulation in AMPA receptor trafficking and LTD Induction, Mol. Neurobiol (2018). doi: 10.1007/s12035-018-0886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Yan Z, Hsieh–Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P, Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP–32 and spinophilin, Nat. Neurosci 2 (1999) 13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- [81].Hu X, Huang Q, Yang X, Xia H, Differential Regulation of AMPA Receptor Trafficking by Neurabin-Targeted Synaptic Protein Phosphatase-1 in Synaptic Transmission and Long-Term Depression in Hippocampus, J. Neurosci 27 (2007) 4674–4686. doi: 10.1523/JNEUROSCI.5365-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Allen PB, Zachariou V, Svenningsson P, Lepore AC, Centonze D, Costa C, Rossi S, Bender G, Chen G, Feng J, Snyder GL, Bernardi G, Nestler EJ, Yan Z, Calabresi P, Greengard P, Distinct roles for spinophilin and neurabin in dopamine-mediated plasticity, Neuroscience. 140 (2006) 897–911. doi: 10.1016/j.neuroscience.2006.02.067. [DOI] [PubMed] [Google Scholar]
- [83].Oliver CJ, Terry-Lorenzo RT, Elliott E, Christensen Bloomer WA, Li S, Brautigan DL, Colbran RJ, Shenolikar S, Targeting protein phosphatase 1 (PP1) to the actin cytoskeleton: the neurabin I/PP1 complex regulates cell morphology, Mol. Cell. Biol 22 (2002) 4690–4701. doi: 10.1128/MCB.22.13.4690-4701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Terry-Lorenzo RT, Roadcap DW, Otsuka T, Blanpied TA, Zamorano PL, Garner CC, Shenolikar S, Ehlers MD, Neurabin/protein phosphatase-1 complex regulates dendritic spine morphogenesis and Maturation, Mol. Biol. Cell 16 (2005) 2349–2362. doi: 10.1091/mbc.e04-12-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hsieh-Wilson LC, Benfenati F, Snyder GL, Allen PB, Nairn AC, Greengard P, Phosphorylation of spinophilin modulates its interaction with actin filaments, J. Biol. Chem 278 (2003) 1186–1194. doi: 10.1074/jbc.M205754200. [DOI] [PubMed] [Google Scholar]
- [86].Cho US, Xu W, Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme, Nature. 445 (2007) 53–57. doi: 10.1038/nature05351. [DOI] [PubMed] [Google Scholar]
- [87].Dodge-Kafka KL, Bauman A, Mayer N, Henson E, Heredia L, Ahn J, McAvoy T, Nairn AC, Kapiloff MS, cAMP-stimulated protein phosphatase 2A activity associated with muscle A Kinase-Anchoring Protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3, J. Biol. Chem 285 (2010) 11078–11086. doi: 10.1074/jbc.M109.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sette C, Conti M, Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation, J. Biol. Chem 271 (1996) 16526–16534. [DOI] [PubMed] [Google Scholar]
- [89].Horiuchi A, Williams KR, Kurihara T, Nairn AC, Greengard P, Purification and cDNA cloning of ARPP-16, a cAMP-regulated phosphoprotein enriched in basal ganglia, and of a related phosphoprotein, ARPP-19, J. Biol. Chem 265 (1990) 9476–9484. [PubMed] [Google Scholar]
- [90].Peyrollier K, Héron L, Virsolvy-Vergine A, Le Cam A, Bataille D, Alpha endosulfine is a novel molecule, structurally related to a family of phosphoproteins, Biochem. Biophys. Res. Commun 223 (1996) 583–586. [DOI] [PubMed] [Google Scholar]
- [91].Heron L, Virsolvy A, Peyrollier K, Gribble FM, Le Cam A, Ashcroft FM, Bataille D, Human alpha-endosulfine, a possible regulator of sulfonylurea-sensitive KATP channel: molecular cloning, expression and biological properties, Proc. Natl. Acad. Sci. U. S. A 95 (1998) 8387–8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gharbi-Ayachi A, Labbé J-C, Burgess A, Vigneron S, Strub J-M, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T, The substrate of greatwall jinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A, Science. 330 (2010) 1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- [93].Dulubova I, Horiuchi A, Snyder GL, Girault JA, Czernik AJ, Shao L, Ramabhadran R, Greengard P, Nairn AC, ARPP-16/ARPP-19: a highly conserved family of cAMP-regulated phosphoproteins, J. Neurochem 77 (2001) 229–238. [DOI] [PubMed] [Google Scholar]
- [94].Girault JA, Horiuchi A, Gustafson EL, Rosen NL, Greengard P, Differential expression of ARPP-16 and ARPP-19, two highly related cAMP-regulated phosphoproteins, one of which is specifically associated with dopamine-innervated brain regions, J. Neurosci. Off. J. Neurosci 10 (1990) 1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Brené S, Lindefors N, Ehrlich M, Taubes T, Horiuchi A, Kopp J, Hall H, Sedvall G, Greengard P, Persson H, Expression of mRNAs encoding ARPP-16/19, ARPP-21, and DARPP-32 in human brain tissue, J. Neurosci. Off. J. Neurosci 14 (1994) 985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kim SH, Nairn AC, Cairns N, Lubec G, Decreased levels of ARPP-19 and PKA in brains of Down syndrome and Alzheimer’s disease, J. Neural Transm Suppl (2001) 263–272. [DOI] [PubMed] [Google Scholar]
- [97].Mochida S, Maslen SL, Skehel M, Hunt T, Greatwall phosphorylates an inhibitor of protein phosphatase 2Α that is essential for mitosis, Science. 330 (2010) 1670–1673. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- [98].Stetina JRV, Tranguch S, Dey SK, Lee LA, Cha B, Drummond-Barbosa D, α-Endosulfine is a conserved protein required for oocyte meiotic maturation in Drosophila, Development. 135 (2008) 3697–3706. doi: 10.1242/dev.025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Musante V, Li L, Kanyo J, Lam TT, Colangelo CM, Cheng SK, Brody AH, Greengard P, Novère NL, Nairn AC, Reciprocal regulation of ARPP-16 by PKA and MAST3 kinases provides a cAMP-regulated switch in protein phosphatase 2A inhibition, elife. 6 (2017) e24998. doi: 10.7554/eLife.24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Augustine CK, Bezanilla F, Phosphorylation modulates potassium conductance and gating current of perfused giant axons of squid, J. Gen. Physiol 95 (1990) 245–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Woolfrey KM, Dell’Acqua ML, Coordination of protein phosphorylation and dephosphorylation in synaptic plasticity, J. Biol. Chem 290 (2015) 28604–28612. doi: 10.1074/jbc.R115.657262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Cepeda C, Levine MS, Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum, Dev. Neurosci 20 (1998) 1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- [103].Chen G, Greengard P, Yan Z, Potentiation of NMDA receptor currents by dopamine D1 receptors in prefrontal cortex, Proc. Natl. Acad. Sci. U. S. A 101 (2004) 2596–2600. doi: 10.1073/pnas.0308618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Flores-Hernández J, Cepeda C, Hernández-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, Greengard P, Levine MS, Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: Role of D1 receptors and DARPP-32, J. Neurophysiol 88 (2002) 3010–3020. [DOI] [PubMed] [Google Scholar]
- [105].Raymond LA, Blackstone CD, Huganir RL, Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase, Nature. 361 (1993) 637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- [106].Wang L-Y, Orser BA, Brautigan DL, MacDonald JF, Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatases 1 and 2A, Nature. 369 (1994) 230–232. doi: 10.1038/369230a0. [DOI] [PubMed] [Google Scholar]
- [107].Blank T, Nijholt I, Teichert U, Kügler H, Behrsing H, Fienberg A, Greengard P, Spiess J, The phosphoprotein DARPP-32 mediates cAMP-dependent potentiation of striatal N-methyl-D-aspartate responses, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 14859–14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P, Amplification of dopaminergic signaling by a positive feedback loop, Proc. Natl. Acad. Sci 97 (2000) 12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Surmeier DJ, Bargas J, Hemmings HC, Nairn AC, Greengard P, Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons, Neuron. 14 (1995) 385–397. [DOI] [PubMed] [Google Scholar]
- [110].Davare MA, Dong F, Rubin CS, Hell JW, The A-kinase anchor protein MAP2B and cAMP-dependent protein kinase are associated with class C L-type calcium channels in Neurons, J. Biol. Chem 274 (1999) 30280–30287. doi: 10.1074/jbc.274.42.30280. [DOI] [PubMed] [Google Scholar]
- [111].Davare MA, Horne MC, Hell JW, Protein phosphatase 2A is associated with class C L-type calcium channels (Cav1.2) and antagonizes channel phosphorylation by cAMP-dependent protein kinase, J. Biol. Chem 275 (2000) 39710–39717. doi: 10.1074/jbc.M005462200. [DOI] [PubMed] [Google Scholar]
- [112].Hall DD, Feekes JA, Arachchige Don AS, Shi M, Hamid J, Chen L, Strack S, Zamponi GW, Horne MC, Hell JW, Binding of protein phosphatase 2A to the L-type calcium channel Cav1.2 next to Ser1928, its main PKA site, is critical for Ser1928 dephosphorylation, Biochemistry. 45 (2006) 3448–3459. doi: 10.1021/bi051593z. [DOI] [PubMed] [Google Scholar]
- [113].Allen PB, Hvalby O, Jensen V, Errington ML, Ramsay M, Chaudhry FA, Bliss TV, Storm-Mathisen J, Morris RG, Andersen P, Greengard P, Protein phosphatase-1 regulation in the induction of long-term potentiation: heterogeneous molecular mechanisms, J. Neurosci. Off. J. Neurosci 20 (2000) 3537–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Belmeguenai A, Hansel C, A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation, J. Neurosci. Off. J. Neurosci 25 (2005) 10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Fukunaga K, Muller D, Ohmitsu M, Bakó E, DePaoli-Roach AA, Miyamoto E, Decreased protein phosphatase 2A activity in hippocampal long-term potentiation, J. Neurochem 74 (2000) 807–817. [DOI] [PubMed] [Google Scholar]
- [116].Jouvenceau A, Billard J-M, Haditsch U, Mansuy IM, Dutar P, Different phosphatase-dependent mechanisms mediate long-term depression and depotentiation of long-term potentiation in mouse hippocampal CA1 area, Eur. J. Neurosci 18 (2003) 1279–1285. [DOI] [PubMed] [Google Scholar]
- [117].Mauna JC, Miyamae T, Pulli B, Thiels E, Protein phosphatases 1 and 2A are both required for long-term depression and associated dephosphorylation of cAMP response element binding protein in hippocampal area CA1 in vivo, Hippocampus. 21 (2011) 1093–1104. doi: 10.1002/hipo.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Braithwaite SP, Stock JB, Lombroso PJ, Nairn AC, Protein phosphatases and Alzheimer’s disease, Prog. Mol. Biol. Transl. Sci 106 (2012) 343–379. doi: 10.1016/B978-0-12-396456-4.00012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sontag J-M, Sontag E, Protein phosphatase 2A dysfunction in Alzheimer’s disease, Front. Mol. Neurosci 7 (2014). doi: 10.3389/fnmol.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ma L, Bayram Y, McLaughlin HM, Cho MT, Krokosky A, Turner CE, Lindstrom K, Bupp CP, Mayberry K, Mu W, Bodurtha J, Weinstein V, Zadeh N, Alcaraz W, Powis Z, Shao Y, Scott DA, Lewis AM, White JJ, Jhangiani SN, Gulec EY, Lalani SR, Lupski JR, Retterer K, Schnur RE, Wentzensen IM, Bale S, Chung WK, De novo missense variants in PPP1CB are associated with intellectual disability and congenital heart disease, Hum. Genet 135 (2016) 1399–1409. doi: 10.1007/s00439-016-1731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Shang L, Henderson LB, Cho MT, Petrey DS, Fong C-T, Haude KM, Shur N, Lundberg J, Hauser N, Carmichael J, Innis J, Schuette J, Wu YW, Asaikar S, Pearson M, Folk L, Retterer K, Monaghan KG, Chung WK, De novo missense variants in PPP2R5D are associated with intellectual disability, macrocephaly, hypotonia, and autism, Neurogenetics. 17 (2016) 43–49. doi: 10.1007/s10048-015-0466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Loveday C, Tatton-Brown K, Clarke M, Westwood I, Renwick A, Ramsay E, Nemeth A, Campbell J, Joss S, Gardner M, Zachariou A, Elliott A, Ruark E, van Montfort R, Childhood Overgrowth Collaboration, Rahman N , Mutations in the PP2A regulatory subunit B family genes PPP2R5B, PPP2R5C and PPP2R5D cause human overgrowth, Hum. Mol. Genet 24 (2015) 4775–4779. doi: 10.1093/hmg/ddv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault J-A, Greengard P, The role of DARPP-32 in the actions of drugs of abuse, Neuropharmacology. 47 Suppl 1 (2004) 14–23. doi: 10.1016/j.neuropharm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- [124].Philibin SD, Hernandez A, Self DW, Bibb JA, Striatal Signal Transduction and Drug Addiction, Front. Neuroanat 5 (2011). doi: 10.3389/fnana.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Lecca S, Pelosi A, Tchenio A, Moutkine I, Lujan R, Hervé D, Mameli M, Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice, Nat. Med 22 (2016) 254–261. doi: 10.1038/nm.4037. [DOI] [PubMed] [Google Scholar]
- [126].Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium, Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways, Nat. Neurosci 18 (2015) 199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Dwivedi Y, Pandey GN, Adenylyl cyclase-cyclicAMP signaling in mood disorders: role of the crucial phosphorylating enzyme protein kinase A, Neuropsychiatr. Dis. Treat 4 (2008) 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Garver DL, Johnson C, Kanter DR, Schizophrenia and reduced cyclic AMP production: evidence for the role of receptor-linked events, Life Sci. 31 (1982) 1987–1992. [DOI] [PubMed] [Google Scholar]
- [129].Nestler EJ, Cellular responses to chronic treatment with drugs of abuse, Crit. Rev. Neurobiol 7 (1993) 23–39. [PubMed] [Google Scholar]
- [130].Houge G, Haesen D, Vissers LELM, Mehta S, Parker MJ, Wright M, Vogt J, McKee S, Tolmie JL, Cordeiro N, Kleefstra T, Willemsen MH, Reijnders MRF, Berland S, Hayman E, Lahat E, Brilstra EH, van Gassen KLI, Zonneveld-Huijssoon E, de Bie CI, Hoischen A, Eichler EE, Holdhus R, Steen VM, Døskeland SO, Hurles ME, FitzPatrick DR, Janssens V, B56δ-related protein phosphatase 2A dysfunction identified in patients with intellectual disability, J. Clin. Invest 125 (2015) 3051–3062. doi: 10.1172/JCI79860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Arnsten AFT, Stress signalling pathways that impair prefrontal cortex structure and function, Nat. Rev. Neurosci 10 (2009) 410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lupien SJ, McEwen BS, Gunnar MR, Heim C, Effects of stress throughout the lifespan on the brain, behaviour and cognition, Nat. Rev. Neurosci 10 (2009) 434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]