Abstract
Methoxyamine (MX) is the first DNA base-excision-repair (BER) inhibitor evaluated in humans. This work described the development and validation of an LC-MS/MS method for quantitative determination of MX in human plasma. In this method, MX and its stable isotope methoxyl-d3-amine (MX-d3 as internal standard) were directly derivatized in human plasma with 4-(N,N-diethylamino)benzaldehyde. The derivatized MX and IS were extracted by methyl-tert-butyl ether, and separated isocratically on a Xterra C18 column (2.1 × 100 mm) using an aqueous mobile phase containing 45% acetonitrile and 0.4% formic acid at a flow rate of 0.200 ml/min. Quantitation of MX was carried out by multiple-reaction-monitoring (MRM) mode of positive turbo-ion-spray tandem mass spectrometry. This method has been validated according to FDA guidelines for bioanalytical method. The linear calibration range for MX was 1.25–500 ng/ml in human plasma with a correlation coefficient ≥ 0.9993. The intra- and inter-assay precision (%CV) at three concentration levels (3.50, 45.0 and 450 ng/ml) ranged 0.9–1% and 0.8–3%, respectively. The stability studies showed that MX met the acceptable criteria under all tested conditions. The method developed had been applied to the determination of plasma MX concentrations in the first-in-human phase I clinical trial, and PK data were presented.
Keywords: methoxyamine, temozolomide, LC-MS/MS, DNA base-excision-repair inhibitor, pharmacokinetic study
1. Introduction
Methoxyamine (MX) is a small organic amine (CH3ONH2, CAS registry # 67–62-9) with a molar mass of 47.06 g. MX is the first DNA base-excision-repair (BER) inhibitor evaluated in human clinical trials. This compound potentiates the cytotoxicity of alkylating and antimetabolic agents by condensing with DNA apurinic/apymidinic (AP) sites which are produced through the removal of alkylated or abnormal nucleobases from DNA backbone by DNA glycosylases [1–3]. The formation of AP-MX complex blocks the BER pathway, and hence reverses tumor resistance to the chemotherapeutic agents [4]. The further repair of MX-bound DNA by topoisomerase II via the formation of cleavable complex results in ds-DNA breaks, and leads to tumor cell death [5]. To study the pharmacokinetics (PK) of MX, a phase I clinical trial of MX in combination of temozolomide (TMZ) in patients with advanced solid tumors is currently ongoing [6].
To obtain MX concentration-time profiles in patients, a reliable and rugged analytical method for quantitative measurement of MX in human plasma samples was critically needed. Although CZE and LC-UV methods [7–9] had been reported for measurement of MX, these methods could not reach the detection limit required for human study and had never been applied to human plasma samples. We previously reported a flow injection analysis (FIA)-tandem mass spectrometry (MS/MS) method using on-line solid-phase extraction (SPE) for the measurement of MX in plasma samples [10]. The main concerns that prevented the use of the FIA-MS/MS method in clinical study of MX include (a) short lifespan of in-line filter due to clogging; (b) no chromatographic separation of the derivatized MX from the derivatizing agent and other endogenous compounds; (c) matrix effect of the co-eluted compounds; and (d) time-restraint of the analysis due to the use of on-line SPE. This led to the development and validation of a liquid chromatography tandem mass spectrometry (LC-MS/MS) method for determination of MX in human plasma in this work.
In the LC-MS/MS method, 4-(N,N-diethylamino)benzaldehyde was used as derivatizing agent for direct derivatization of MX and the internal standard (MX-d3 or IS) in human plasma. A liquid-liquid extraction (LLE) procedure was used to extract the derivatized MX and IS from human plasma using methyl tert-butyl ether as organic solvent. Both the derivatized MX and IS were then separated isocratically from the derivatizing agent on a reverse-phase C18 column using an aqueous mobile phase containing 45% acetonitrile and 0.4% formic acid. Quantitation of analytes was carried out by multiple-reaction-monitoring (MRM) mode of positive tandem mass spectrometry with mass transitions of m/z 207 > 178 for the derivatized MX and m/z 210 > 181 for the derivatized MX-d3, respectively. The LC-MS/MS method developed permitted batch sample preparation and baseline resolution for the analytes from the derivatizing agent and other endogenous compounds in plasma, which has been fully validated and successfully applied to the first-in-human PK study of MX.
2. Experimental
2.1. Chemicals and solutions
LC/MS-grade acetonitrile, HPLC-grade water, and HPLC-grade methyl tert-butyl ether were purchased from Fisher Scientific (Pittsburgh, PA, USA). Methoxyamine hydrochloride, formic acid, acetic acid, phosphoric acid, hydrochloric acid, and 4-(N,N-diethylamino)benzaldehyde were from Sigma-Aldrich (St. Louis, MO, USA). Methoxyl-d3-amine hydrochloride was from C/D/N Isotopes Inc. (Pointe-Claire, Quebec, Canada). Temozolomide (CAS registry # 85622–93-1) was obtained from the Developmental Therapeutics Program at the National Cancer Institute (Rockville, MD, USA). Pooled human plasma containing no detectable MX was obtained from Fisher Scientific (Pittsburgh, PA, USA) and used as blank plasma. Six randomly selected pre-dosed patients’ plasma samples were used as six sources of human plasma matrices for the studies of the selectivity and the lower limit of quantitation (LLOQ) of the method. Six individual human plasmas containing no detectable MX (i.e., 1M2070–01, 1M2070–02, 1M2070–03,1M2070–04, 1M2070–05, and 1M2070–06) were also obtained from Innovative Research (Novi, MI, USA) for the study of matrix effect.
The stock solution of MX (2.00 mg/ml) was prepared by dissolving appropriate amount of methoxyamine hydrochloride (CH3ONH2∙HCl) with a mass correction factor of 0.563 in a known volume of 0.100 N hydrochloric acid. The stock solution of MX-d3 (2.00 mg/ml) was prepared by dissolving appropriate amount of methoxyl-d3-amine hydrochloride (CD3ONH2∙HCl) with a mass correction factor of 0.579 in a known volume of 0.100 N hydrochloric acid. The stock solution of 4-(N,N-diethylamino)benzaldehyde (200 mg/ml) was prepared by dissolving appropriate amount of the compound in a known volume of 66.7 % acetic acid. The stock solution of TMZ (5.00 mg/ml) was prepared by dissolving appropriate amount of TMZ in 0.100 N hydrochloric acid. The working standard solutions of MX (5.00 μg/ml, 500 ng/ml, and 50.0 ng/ml) were prepared by a serial dilution of the stock solution of MX (2.00 mg/ml) with 0.100 N hydrochloric acid. The working internal standard solution of MX-d3 (100 ng/ml) was prepared from the serial dilution of the stock solution of MX-d3 (2.00 mg/ml) with 0.100 N hydrochloric acid. The working solution of 4-(N,N-diethylamino)benzaldehyde (500 μg/ml) was prepared from the serial dilution of stock solution of 4-(N,N-diethylamino)benzaldehyde (200 mg/ml) with 33.3% formic acid. The working solution of TMZ (500 μg/ml) was prepared by diluting the stock solution of TMZ (5.00 mg/ml) with 0.100 N hydrochloride acid. The mobile phase was prepared by mixing 450 ml of LC/MS-grade acetonitrile, 550 ml of HPLC-grade water, and 4 ml of formic acid.
2.2. Preparation of plasma calibrators and quality controls
Plasma MX calibrators (1.25, 2.50, 5.00, 12.5, 25.0, 50.0, 125, 250 and 500 ng/ml) were prepared by dilution of the working standard solutions of MX (50.0, 500 and 5.00 × 103 ng/ml) with blank plasma and 0.100 N hydrochloric acid to ensure each calibrator containing exactly 90% (in volume) of plasma and 10% of 0.100 N hydrochloric acid. Plasma matrix blank and plasma MX zero calibrator samples were prepared to contain 90% (in volume) of plasma and 10% of 0.100 N hydrochloric acid. The plasma MX QCs (3.50, 45.0 and 450 ng/ml) and plasma MX dilution QC (1.50 × 103 ng/ml) were prepared in the same manner as that of the plasma calibrators. Plasma MX calibrators (150 μl), QCs (150 μl) and dilution QCs (30 μl) were stored in small aliquots with capped borosilicate glass tubes (13 × 100 mm, Fisher Scientific) at −70 °C before use. The MX dilution QC samples (1.50 × 103 ng/ml, 30 μl) was diluted by a factor of 5 with 120-μl pooled human blank plasma prior to sample preparation.
Plasma TMZ control (20.0 μg/ml) was prepared by diluting the working solution of TMZ (500 μg/ml) with pooled human blank plasma. Plasma MX QCs (3.50, 45.0 and 450 ng/ml) containing TMZ (20.0 μg/ml) were prepared in the same manner as that of the plasma MX QCs except using plasma TMZ control instead of blank plasma as a diluent.
2.3. Sample preparation
Each aliquot of 150 μl plasma sample (i.e., plasma calibrators, plasma QCs, or patient plasma samples) was mixed with 75 μl of working internal standard solution of MX-d3 (100 ng/ml), 50 μl of 4-(N,N-diethylamino)benzaldehyde working solution (500 μg/ml), and 150 μl of 4% phosphoric acid (v/v) in a borosilicate glass tube with cap. The mixture was heated at 70 °C for 1 h in a dry-bath incubator (Denville Scientific, Roebling, NJ, USA) and then extracted with 2 ml of methyl tert-butyl ether. The organic phase was transferred into a fresh borosilicate glass tube (12 × 75 mm, Fisher Scientific) which was then dried in a TurboVap® LV evaporator (Caliper Life Sciences, Hopkinton, MA, USA) at 30 °C under nitrogen gas pressure of 15 psi for ca. 15 min. The residue was then reconstituted in 150 μl of aqueous solution containing 10% acetonitrile and 1% formic acid for LC-MS/MS analysis.
2.4. Instrumentation
The instrumentation system used consisted of a Shimadzu SIL-20AC autosampler (Shimadzu, Columbia, MD, USA), a Shimadzu LC-20AD HPLC unit with Waters XTerra® MS C18 precolumn (2.1 × 10 mm, 3.5 μm) and Waters XTerra® MS C18 column (2.1 × 100 mm, 3.5 μm) (Waters, Milford, MA, USA), and an AB Sciex API 3200 turbo-ion-spray® triple quadrupole tandem mass spectrometer (AB Sciex, Foster City, CA, USA). The system was controlled by AB Sciex Analyst® (version 1.5.1) software.
The API 3200 tandem mass spectrometer was operated under the positive turbo-ion-spray ionization mode. It was tuned by a reaction mixture of 500 ng/ml MX, 500 ng/ml MX-d3, and 500 μg/ml 4-(N,N-diethylamino)benzaldehyde in 45% acetonitrile and 1% formic acid aqueous solution for both the compound-dependent and the source-dependent parameters. MRM data were acquired with the following mass transitions and optimized instrument settings: m/z 207 > 178 for the derivatized MX, m/z 210 > 181 for the derivatized MX-d3, and m/z 178 > 134 for the derivatizing agent; curtain gas (CUR) at 25, collision assisted dissociation gas (CAD) at 5; ionization voltage (IS) at 5500 V; source temperature (TEM) at 550; sheath gas (GS1) at 40; desolvation gas (GS2) at 45; desolvation potential (DP) at 36; entrance potential (EP) at 3.5; collision energy (CE) at 21; collision cell exit potential (CXP) at 4; and resolution at unit.
Analytical separation of the derivatized analytes was accomplished on a XTerra® MS C18 column by isocratic elution with the mobile phase at a flow rate of 0.200 ml/min. During each run, 5 μl of reconstituted sample was injected into the system by the autosampler set at 15°C. The two-position switch valve on the API 3200 tandem mass spectrometer was programmed to direct the column eluate to the mass spectrometer for the first 4 min, then switch to the waste for the next 3 min, and return to the mass spectrometer for the last 2 min during each run. Quantitation of the derivatized analytes was carried out with MRM mode of the tandem mass spectrometer. The total instrument run time for each sample analysis was 9 min. Prior to initial sample analysis, the column was equilibrated with the mobile phase at the above flow rate for at least 30 min.
2.5. Data analysis
Data acquisition and peak integration were done using the Analyst® software with IntelliQuan-MQII algorithm. The peak area ratios of the derivatized MX to the derivatized MX-d3 (IS) were plotted against the MX concentrations in plasma calibrators for a linear regression calibration equation using a weighting factor of 1/x2. The MX concentration in a patient’s sample was calculated by the Analyst® software using the peak area ratio of the derivatized MX to that of the derivatized IS and the calibration equation.
2.6. Stability studies
The stability of MX stock solution (2.00 mg/ml) in refrigerator (4 °C) and at benchtop (23 °C), and the stability of plasma QCs (3.50, 45.0 and 450 ng/ml) at benchtop (23 °C), in autosampler (15 °C), by freeze-and-thaw cycles (−70 to 23 °C), and in deep freezer (−70 °C) were investigated over various time periods.
2.7. Pharmacokinetic study
In the phase I clinical trial of MX and TMZ in patients with advanced solid tumors, all patients enrolled have been histologically confirmed solid tumors that were considered incurable and were not amenable to conventional surgical, radiation therapy or chemotherapy treatment programs.
Two dosing regimens of MX have been examined in the phase I clinical trial. Initially, patients were given MX as an iv continuous infusion (15 mg/m2/day) for 5 days in combination with an oral dose of TMZ (100 mg/m2/day) for 5 days on a 28-day cycle. The dosing regimen of MX was amended from a 5-day iv continuous infusion to a single 1-h iv infusion (15 mg/m2) after first six patients. Dose-escalation of both MX and TMZ followed the schedule specified in the CASE 1Y05 protocol [6].
In the clinical trial, patients were given MX within 5 min of taking an oral dose of TMZ. Blood samples were drawn before and after MX administration according to the schedules specified in the CASE 1Y05 protocol. Plasma samples were collected for MX analysis by the procedure as follows: drew 3 ml of blood into a labeled lithium heparinized tube (green top) at each sampling time point, and kept all sample tubes in an ice bath before centrifugation; centrifuged the blood samples at 1500 × g at 4 ºC for 5 min; transferred the plasma into a labeled Nunc® CryoTube® vial; capped and mixed the sample tubes; and stored the sample tubes immediately at −70 ºC until LC-MS/MS analysis.
Pharmacokinetic modeling was done by Phoenix WinNonLin (Version 6.2) software from Pharsight Corporation (St. Louis, MO, USA). The 120-h iv infusion data fitted well with the PK model 2 (one-compartment iv-infusion, no lag time, first-order elimination), and the 1-h iv infusion data were modeled by non-compartmental analysis (NCA).
3. Results and discussion
3.1. LC-MS/MS method development
Although the measurement of MX in plasma samples had been reported by the FIA-MS/MS method [10]; however, there were several shortcomings of this method when applied to large-batch clinical samples, including: (a) frequent clogging of the in-line filter by plasma proteins, which required replacement of the filter to avoid pressure fluctuation of the system; (b) a large amount of unreacted derivatizing agent 4-(N,N-diethylamino)benzaldehyde presented in the plasma samples (ca. 16.7 μg of the derivatizing agent in each 10 μl sample injection), which could be co-extracted and co-eluted on/from the on-line SPE cartridge with the derivatized MX and IS, and harmful to a mass spectrometer if it entered on a routine basis; and (c) the matrix effect of the co-eluted of derivatizing agent and other endogenous compounds, which could suppress ionization of the derivatized MX and IS in mass spectrometer; and (d) the on-line SPE of the FIA-MS/MS method [10], which made sample re-run impossible in case of instrument failures. Therefore, an LC-MS/MS method has been developed, which uses methyl tert-butyl ether as organic solvent for the extraction of the derivatized MX and IS, and a Waters XTerra® MS C18 column (2.1 × 100 mm, 3.5 μm) for analytical separation of the derivatized analytes from the derivatizing agent 4-(N,N-diethylamino)benzaldehyde and other endogenous compounds.
3.1.1. Derivatizing agent 4-(N,N-diethylamino)benzaldehyde
The derivatization reaction between MX and 4-(N,N-diethylamino)benzaldehyde is a Schiff reaction [9]. In order to consume all Schiff reagent (MX), excess amount of 4-(N,N-diethylamino)benzaldehyde had been used [10]. As discussed in Section 3.1, the unreacted derivatizing agent in plasma samples could have an adverse effect on mass spectrometric detection. Therefore, the reaction between MX and 4-(N,N-diethylamino)benzaldehyde has been further optimized. In this work, the amount of the derivatizing agent was lowered by 20 times from a 1000-fold excess in the FIA-MS/MS method [10] to a 50-fold excess in the current method. The lower amount of the derivatizing agent was proven by the method validation data (see later sections) to be sufficient to drive the derivatization reaction to completion in plasma matrix under 70 °C for 1-h duration. Thus, 50 μl of 500 μg/ml 4-(N,N-diethylamino)benzaldehyde solution has been adopted for plasma MX derivatization in the LC-MS/MS method (see Section 2.3).
It was also found that the addition of 4% phosphoric acid to a plasma sample stabilized acidic medium required for the derivatization reaction, and helped to increase the clarity of the solution in the later LLE.
3.1.2. Liquid-liquid extraction and reconstitution of sample extract
The derivatized MX and IS were extracted from plasma by an LLE procedure. To find a better organic solvent, both ethyl acetate and methyl tert-butyl ether were tested. Methyl tert-butyl ether was found to be the one because it gave not only a higher extraction recovery for the derivatized MX and IS, but also a clearer solution of the reconstituted extract prepared in the later step in comparison to ethyl acetate. Therefore, methyl tert-butyl ether was chosen as the organic solvent for the LLE.
To reconstitute the dry extract after solvent evaporation, an aqueous solution of 10% acetonitrile and 1% formic acid was used. This solution had a lower organic content than that of the mobile phase. Therefore, it did not contribute to chromatographic peak broadening.
3.1.3. Mass spectrometric detection
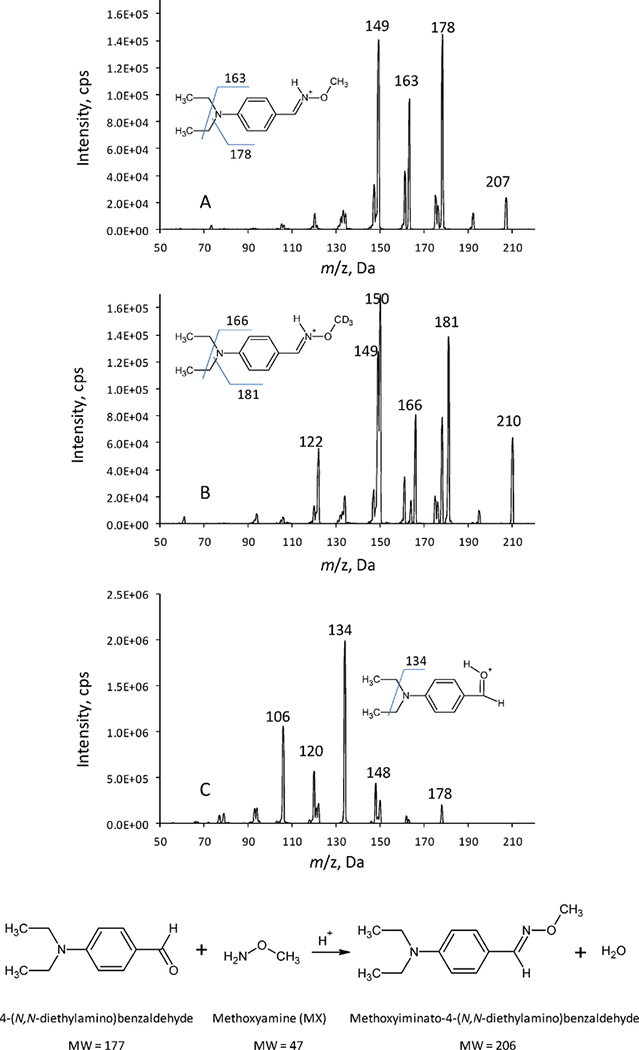
In this work, the derivatized MX, the derivatized IS, and the derivatizating agent 4-(N,N-diethylamino)benzaldehyde were readily protonated in the acidic conditions. Therefore, the positive turbo-ion-spray ionization mode was used for identification and quantitation of the analytes. As shown in the mass scans of the product ions (Figure 1), the major fragments were m/z 178 for the derivatized MX (Figure 1A), m/z 181 for the derivatized IS (Figure 1B), and m/z 134 for the derivatizing agent (1C) by selecting precursor ions at m/z 207 for the derivatized MX, m/z 210 for the derivatized IS, and m/z 178 for the derivatizing agent, respectively. Hence, mass transitions of m/z 207 > 178 for the derivatized MX, and m/z 210 > 181 for the derivatized IS were chosen for the determination of MX by MRM mode; and mass transition of m/z 178 > 134 for the derivatizing agent was chosen for the interference study.
Figure 1.
MX derivatization reaction, and mass spectra (product ions) of the derivatized MX (A), the derivatized MX-d3 (B), and the derivatizing agent 4-(N,N-diethylamino)benzaldehyde (C). The experimental conditions were the same as those described in Section 2.4.
3.1.4. Chromatographic separation
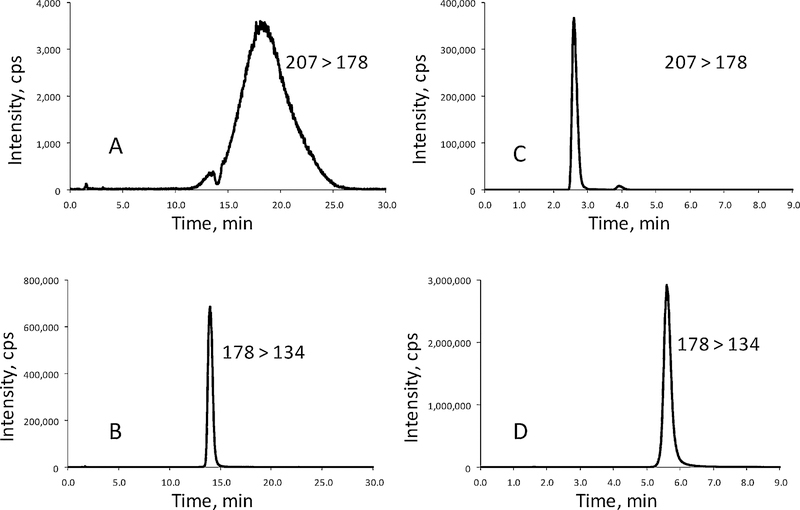
Separation of the derivatized MX and 4-(N,N-diethylamino)benzaldehyde was done on a Waters Xterra C18 column (2.1 × 100 mm, 3.5 um) using a mobile phase containing 45% acetonitrile at a flow rate of 0.2 ml/min. The addition of formic acid in the mobile phase could affect the elution order of the derivatized MX and 4-(N,N-diethylamino)benzaldehyde (Figure 2). When no formic acid was added to the mobile phase, the peak of the derivatized MX (Figure 2A) overlapped with that of 4-(N,N-diethylamino)benzaldehyde (Figure 2B). Moreover, the peak of the derivatized MX was severely suppressed and broadened (Figure 2A) due to the presence of a large amount of 4-(N,N-diethylamino)benzaldehyde in the sample. When formic acid was added in the mobile phase, the peak of the derivatized MX (Figure 2C) not only eluted out of the column earlier than that of 4-(N,N-diethylamino)benzaldehyde (Figure 2D), but also the peak of the derivatized MX was sharpened (Figure 2C). In this method, the formic acid concentration was optimized to be 0.4% in the mobile phase of 45% acetonitrile. This mobile phase gave retention times of 2.6 and 5.6 min on the Waters Xterra C18 column for the derivatized MX and the derivatizing agent 4-(N,N-diethylamino)benzaldehyde, respectively. Therefore, the matrix effect from 4-(N,N-diethylamino)benzaldehyde on the mass spectrometric detection of the derivatized MX could be avoided. Furthermore, to prevent contamination to the mass spectrometer, the eluate containing 4-(N,N-diethylamino)benzaldehyde was diverted to waste from the run times of 4.0 to 7.0 min by programming the two-position switch valve on the mass spectrometer.
Figure 2.
The effect of formic acid in the mobile phase on the chromatographic separation of the derivatized MX and the derivatizing agent 4-(N,N-diethylamino)benzaldehyde: (A) mass chromatogram of MX, no formic acid added; (B) mass chromatogram of the derivatizing agent, no formic acid added; (C) mass chromatogram of MX, 0.4% formic acid added; and (D) mass chromatogram of the derivatizing agent, 0.4% formic acid added. Mass transitions: m/z 207 > 178 for the derivatized MX, and m/z 178 > 134 for the derivatizing agent.
3.2. Method validation
The LC-MS/MS method was validated according to the guidelines for bioanalytical method validation set forth by the FDA and the industry [11–13].
3.2.1. Specificity and LLOQ
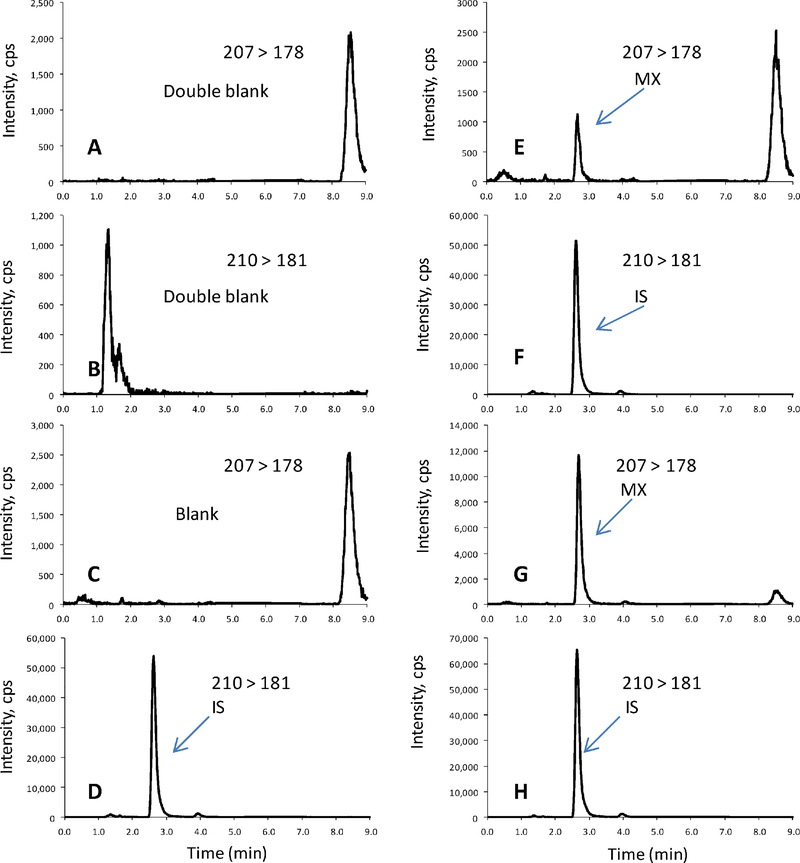
The specificity of the method was evaluated by the pre-dosed plasma samples from patient donors. In the CASE 1Y50, more than 50 patient pre-dosed plasma samples were collected and analyzed, there were no detectable interferences observed at the mass transitions and the retention times of the derivatized MX and IS. Figure 3 shows the representative mass chromatograms of human plasma matrix blank (Figures 3A and 3B), zero plasma MX calibrator with the IS (Figures 3C and 3D), and two non-zero plasma MX calibrators with the IS (Figures 3E–3H).
Figure 3.
The representative mass chromatograms of the derivatized MX and IS in human plasma samples: (A, B) plasma matrix blank (with neither MX nor IS); (C, D) plasma zero calibrator with IS (50.0 ng/ml); (E, F) plasma MX at LLOQ (1.25 ng/ml) with IS; and (G, H) patient sample with IS at 70-min sampling point by the dosing regimen of MX (single 1-h iv infusion), 15 mg/m2/day; and TMZ (oral once daily), 100 mg/m2/day for 5 days.
In this work, the LLOQ of the method was defined by the lowest concentration of plasma MX calibrator (1.25 ng/ml). Table 1 shows the accuracy and precision of the method at LLOQ, which were defined as percent relative error (%RE) and correlation coefficient (%CV). By five replicate measurements of each individual plasma matrix, the mean accuracy and precision from six individual pre-dosed patient plasma matrices were ≤ −0.8% and ≤ 0.8%, respectively. These values were much lower than those of the industry limits (± 20% and 20%), and implied that the actual LLOQ of the method could be lower than 1.25 ng/ml.
Table 1.
Accuracy and precision of MX at LLOQ in six patients’ pre-dosed plasmas (n = 5)
| Pre-dosed patient plasma | Nominal [MX] (ng/ml) | Measured [MX] (ng/ml) | SD (ng/ml) | %CV | %RE |
|---|---|---|---|---|---|
| #1 | 1.25 | 1.26 | 0.04 | 3 | 0.8 |
| #2 | 1.25 | 1.23 | 0.03 | 2 | −2 |
| #3 | 1.25 | 1.24 | 0.02 | 2 | −0.8 |
| #4 | 1.25 | 1.29 | 0.03 | 2 | 3 |
| #5 | 1.25 | 1.21 | 0.03 | 2 | −3 |
| #6 | 1.25 | 1.23 | 0.04 | 3 | −2 |
| Meana | 1.25 | 1.24 | 0.01 | 0.8 | −0.8 |
X = (x1 + x2 + …x6)/6; ; %CV =SD/X; %RE = [(measured value − nominal value)/(nominal value)] × 100%
3.2.2. Matrix factor and recovery
Matrix factor is a measure of matrix effect in a sample. Absolute matrix factor (MF) was determined by the mean-peak area of the derivatized MX in extracted plasma matrix over the mean-peak area of the derivatized MX in mobile phase; and IS normalized MF was determined by the MF of the derivatized MX over the MF of the derivatized IS. Whereas, absolute recovery was determined by the mean-peak area of the derivatized MX in plasma sample over the mean-peak area of the derivatized MX in extracted plasma matrix, and IS normalized recovery was determined by the recovery of the derivatized MX over the recovery of the derivatized IS.
Because MX-d3, a stable isotope of MX, was the IS in this work, the pooled human plasma instead of 6 individual sources was initially used as sample matrix for the studies of matrix effect and recovery. In these studies, MX samples at three concentrations (3.50, 45.0 and 450 ng/ml) were prepared in the pooled human plasma, the extracted pooled human plasma, and the mobile phase; then, subjected to the procedure described in the Section 2.3 before the LC-MS/MS analyses. As seen in Table 2, the absolute MFs were 0.42–0.54 for the derivatized MX and 0.42–0.56 for the derivatized IS; and the IS normalized MFs was 0.96–1.02. These data indicated that signal suppression did occur in plasma matrix by nearly 50%; however, the use of internal standard could correct the signal suppression by nearly 100%. In terms of recoveries (Table 3), high and consistent absolute recoveries (84–100% for the derivatized MX and 84–98% for the derivatized IS), and near 100% IS normalized recoveries (100–102%) were achievable by the method.
Table 2.
Matrix effect on MX QC samples in the pooled blank human plasma (n = 5)
| MX QC (ng/ml) | PAMX in extracted plasma ± SD (x 105)a | PAMX in mobile phase ± SD (x 105) | MFMX ± SDb | PAIS in extracted plasma ± SD (x 105) | PAIS in mobile phase ± SD (x 105) | MFIS ± SDb | IS Normalized MF ± SDb |
|---|---|---|---|---|---|---|---|
| 3.50 | 0.40 ± 0.02 | 0.960 ±0.005 | 0.42 ± 0.02 | 5.7 ± 0.2 | 13.4 ± 0.1 | 0.42 ± 0.02 | 1.00 ± 0.07 |
| 45.0 | 6.8 ± 0.3 | 12.5 ± 0.3 | 0.54 ± 0.03 | 7.5 ± 0.4 | 13.4 ± 0.2 | 0.56 ± 0.03 | 0.96 ± 0.07 |
| 450 | 52.0 ± 0.5 | 101 ± 2 | 0.51 ± 0.01 | 6.00 ± 0.09 | 11.9 ± 0.3 | 0.50 ± 0.01 | 1.02 ± 0.03 |
PA = mean peak area
Table 3.
Matrix effect on MX QC samples in the pooled blank human plasma (n = 5)
| MX QC (ng/ml) | PAMX in plasma ± SD (x 105) | PAMX in extracted plasma ± SD (x 105) | RecoveryMX ± SD (%)a | PAIS in plasma ± SD (x 105) | PAIS in extracted plasma ± SD (x 105) | RecoveryIS ± SD (%)b | IS Normalized Recovery ± SD (%)c |
|---|---|---|---|---|---|---|---|
| 3.50 | 0.40 ± 0.03 | 0.40 ± 0.02 | 100 ± 9 | 5.6 ± 0.4 | 5.7 ± 0.2 | 98 ± 8 | 102 ± 12 |
| 45.0 | 5.7 ± 0.3 | 6.8 ± 0.3 | 84 ± 6 | 6.3 ± 0.3 | 7.5 ± 0.4 | 84 ± 6 | 100 ± 10 |
| 450 | 49.8 ± 0.6 | 52.0 ± 0.5 | 96 ± 1 | 5.7 ± 0.1 | 6.00 ± 0.09 | 95 ± 2 | 101 ± 2 |
Recovery of MX = [(PA of MX in plasma matrix)/(PA of MX in extracted plasma matrix)] × 100%
Recovery ofIS = [(PA of IS in plasma matrix)/(PA of IS in extracted plasma matrix)] × 100%
IS normalized recovery = [(Recovery of MX)/(Recovery of IS)] × 100%.
Matrix effects in six individual lots of human blank plasma were also determined per reviewer’s request. As shown in Table 4, even though the absolute MFs of the derivatized MX and IS ranged from 0.66 to 1.7, the IS normalized MFs were 1.0. These results revealed that either signal suppression or enhancement could occur in each individual human plasma samples. The use of MX-d3 as the IS could normalize MFs in the samples to unity.
Table 4.
Matrix effect on MX QCs in six individual lots of blank human plasma matrices (n = 5)
| Plasma matrix | MX QC (ng/mL) | MFMX ± SDa | MFIS ± SDa | IS normalized MF ± SDa |
|---|---|---|---|---|
| Lot 1 | 3.50 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.0 ± 0.1 |
| 45.0 | 1.5 ± 0.1 | 1.44 ± 0.09 | 1.0 ± 0.1 | |
| 450 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.0 ± 0.1 | |
| Lot 2 | 3.50 | 1.27 ± 0.09 | 1.25 ± 0.08 | 1.0 ± 0.1 |
| 45.0 | 1.24 ± 0.09 | 1.23 ± 0.08 | 1.0 ± 0.1 | |
| 450 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0.1 | |
| Lot 3 | 3.50 | 0.82 ± 0.09 | 0.80 ± 0.08 | 1.0 ± 0.1 |
| 45.0 | 0.71 ±0.05 | 0.71 ± 0.05 | 1.0 ± 0.1 | |
| 450 | 0.74 ± 0.08 | 0.75 ± 0.08 | 1.0 ± 0.2 | |
| Lot 4 | 3.50 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.2 |
| 45.0 | 0.77 ± 0.09 | 0.77 ± 0.09 | 1.0 ± 0.2 | |
| 450 | 0.82 ± 0.07 | 0.82 ± 0.07 | 1.0 ± 0.1 | |
| Lot 5 | 3.50 | 0.67 ± 0.08 | 0.66 ± 0.08 | 1.0 ± 0.2 |
| 45.0 | 0.71 ± 0.08 | 0.71 ± 0.07 | 1.0 ± 0.2 | |
| 450 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.2 | |
| Lot 6 | 3.50 | 0.84 ± 0.09 | 0.82 ± 0.08 | 1.0 ± 0.1 |
| 45.0 | 0.70 ± 0.09 | 0.70 ± 0.08 | 1.0 ± 0.2 | |
| 450 | 0.75 ± 0.08 | 0.75 ± 0.08 | 1.0 ± 0.2 |
MFMX = (mean peak area of MX in the extracted plasma matrix)/(mean peak area of MX in the mobile phase)
MFIS = (mean peak area of IS in the extracted plasma matrix)/(mean peak area of IS in the mobile phase)
IS normalized MF = MFMX/MFIS.
3.2.3. Calibration curve
MX calibration curve in human plasma was constructed using a plasma matrix blank (with neither MX nor IS), a zero plasma calibrator (with only IS), and 9 non-zero plasma calibrators (with MX and IS). The IS concentration was 50.0 ng/ml, and the MX concentrations of the non-zero calibrators were 1.25, 2.50, 5.00, 12.5, 25.0, 50.0, 125, 250 and 500 ng/ml. The linear calibration range of 1.25–500 ml was established in human plasma by plotting the peak-area ratios of the derivatized MX to the derivatized IS versus the concentrations of MX. The calibration equation derived from five individual calibration curves from five validation batches with 1/x2 weighting was Y = 0.0174(±0.0002)X + 0.0006(±0.0001) with a correlation coefficient ≥ 0.993. The accuracy and precision of individual calibrators in human plasma as summarized in Table 5 were ≤ ±3% and ≤ 3%, respectively.
Table 5.
Accuracy and precision of MX plasma calibrators over five validation batches
| MX calibrator (ng/ml) | Measured [MX] (ng/ml) | SD (ng/ml) | %CV | %RE |
|---|---|---|---|---|
| 1.25 | 1.27 | 0.02 | 3 | 2 |
| 2.50 | 2.44 | 0.06 | 2 | −2 |
| 5.00 | 4.95 | 0.1 | 2 | −1 |
| 12.5 | 12.2 | 0.2 | 2 | −2 |
| 25.0 | 24.3 | 0.5 | 2 | −3 |
| 50.0 | 51.2 | 0.4 | 0.8 | 2 |
| 125 | 125 | 1 | 0.8 | 0 |
| 250 | 253 | 1 | 0.4 | 1 |
| 500 | 513 | 7 | 1 | 3 |
3.2.4. Accuracy and precision
The intra-run accuracy and precision were determined by 6 replicate measurements of a QC sample at each QC concentration (3.50, 45.0, 450, and 1.50 × 103 ng/ml) within a validation batch. The inter-run accuracy and precision were determined by 5 parallel measurements of 5 identical QC samples at each concentration (3.50, 45.0, 450, and 1.50 × 103 ng/ml) over five validation batches. As shown in Table 6, the intra-run accuracy and precision were ≤ 3% and ≤ 1%, and the inter-run accuracy and precision were ≤ 4% and ≤ 3%, respectively.
Table 6.
Intra- and inter-run accuracy and precision of plasma MX QC samples
| Intra-day (n = 6) | ||||
| MX QC (ng/ml) | Measured [MX] (ng/ml) | SD (ng/ml) | %CV | %RE |
| 3.50 | 3.50 | 0.04 | 1 | 0 |
| 45.0 | 46.4 | 0.4 | 0.9 | 3 |
| 450 | 458 | 5 | 1 | 2 |
| 1.50 × 103 | 1.50 ×103 | 2 × 101 | 1 | 0 |
| Intra-day (n = 5) | ||||
| MX QC (ng/ml) | Measured [MX] (ng/ml) | SD (ng/ml) | %CV | %RE |
| 3.50 | 3.55 | 0.03 | 0.8 | 1 |
| 45.0 | 46.0 | 1 | 3 | 2 |
| 450 | 466 | 4 | 0.9 | 4 |
| 1.50 × 103 | 1.54 × 103 | 2 × 101 | 1 | 3 |
3.2.5. TMZ interference study
For the CASE 1Y05 clinical trial [6], the maximum dose of TMZ was scheduled to be 150 mg/m2/day. According to the published reports [14–17], maximum concentrations (Cmax) of TMZ in human plasma from clinical trials were below 10 μg/ml by such dosing regimen. Hence, the TMZ interference study was conducted using MX QCs (low, mid, high) containing TMZ concentration of 20.0 μg/ml (> 2 times of Cmax). It was not only the mass chromatograms showed no interferences from TMZ at the mass transitions of MX and the IS (data not shown), but also there were no significant differences observed between the results of MX QCs with and without TMZ (Table 7). Therefore, the presence of TMZ in human plasma doesn’t affect the accurate determination of MX in plasma samples..
Table 7.
The interference study of TMZ on the measurement of MX in human plasma (n = 5)
| MX QC (ng/ml) | 3.50 | 45.0 | 450 | |||
| TMZ (20.0 μg/ml) | Yes | No | Yes | No | Yes | No |
| Measured [MX] (ng/ml) | 3.58 | 3.65 | 46.8 | 47.5 | 477 | 475 |
| SD (ng/ml) | 0.05 | 0.03 | 0.9 | 0.7 | 6 | 6 |
| %CV | 1 | 1 | 2 | 1 | 1 | 1 |
| %RE | 2 | 4 | 4 | 6 | 6 | 6 |
3.2.6. Stability
The stability of MX in human whole blood until separated into plasma and storage was evaluated in this work. At 70 min after infusion, 5 tubes of blood (2 ml each) from a patient underwent MX therapy were drawn (< 2 min) using lithium heparinized tubes (green top) and kept in an ice bath before centrifugation. Following the detail procedure specified in the Section 2.7, the blood samples were centrifuged at 0, 30, 60, 90, and 120 min after drawing, and the plasma samples were analyzed by the LC-MS/MS method at a later time. The measured MX concentrations from the patient were 10.5, 10.6, 10.4, 10.3 and 10.5 ng/ml, respectively, which gave a mean value of 10.5 ng/ml, a standard deviation of 0.1 ng/ml, and a %CV of 1%. This study revealed that there was no significant changes in MX concentration in human whole blood over a period of 2 hours when samples were kept in an ice bath. MX is stable in whole blood by following the sampling procedure specified in the CASE 1Y05 protocol [6].
The stability of a test sample (i.e., stock solution or plasma QC) was determined by the mean-peak-area ratio of the derivatized MX to the derivatized IS from six parallel measurements of six test solutions over that of the freshly prepared test solutions, which was expressed as percent recovery. As shown in Table 8, the recoveries of stock solutions were 96.4–98.4% for refrigerator (4 °C) and bench top (23 °C); and the recoveries of QC samples at three concentrations (3.50, 45.0 and 450 ng/ml) were 89.4–95.7%, 90.7–96.9%, 94.4–96.6%, and 95.7–103% for bench top, autosampler (15 °C), three freeze-and-thaw cycles (−70 to 23 °C), and long-term storage (5 months), respectively. The data indicated that MX was stable under all tested conditions.
Table 8.
Stability study of MX under various test conditions
| Condition | T (°C) | Samplea | Period | Recovery ± SD (%) (n = 6) |
|---|---|---|---|---|
| Refrigerator | 4 | Stock solution | 5 months | 96.4 ± 7 |
| Bench-top | 23 | Stock solution | 18 hours | 98.4 ± 6 |
| Bench-top | 23 | Low QC | 18 hours | 89.4 ± 4 |
| Mid QC | 18 hours | 94.5 ± 4 | ||
| High QC | 18 hours | 95.7 ± 2 | ||
| Autosampler | 15 | Low QC | 3 days | 90.7 ±6 |
| Mid QC | 3 days | 94.5 ± 5 | ||
| High QC | 3 days | 96.9 ±3 | ||
| Freeze & thaw | −70 to 23 | Low QC | 3 cycles | 96.6 ± 2 |
| Mid QC | 3 cycles | 94.4 ± 2 | ||
| High QC | 3 cycles | 96.2 ± 2 | ||
| Deep freezer (Long-term) | −70 | Low QC | 5 months | 95.7 ±3 |
| Mid QC | 5 months | 101 ± 2 | ||
| High QC | 5 months | 103 ± 2 |
The concentration of MX stock solution was 2.00 mg/ml which was measured by serial dilution to 45.0 ng/ml in 1% formic acid. The concentration of MX in LQC, MQC and HQC were 3.50, 45.0 and 450 ng/ml, respectively.
3.3. Application of the method to the first-in-human MX clinical trial
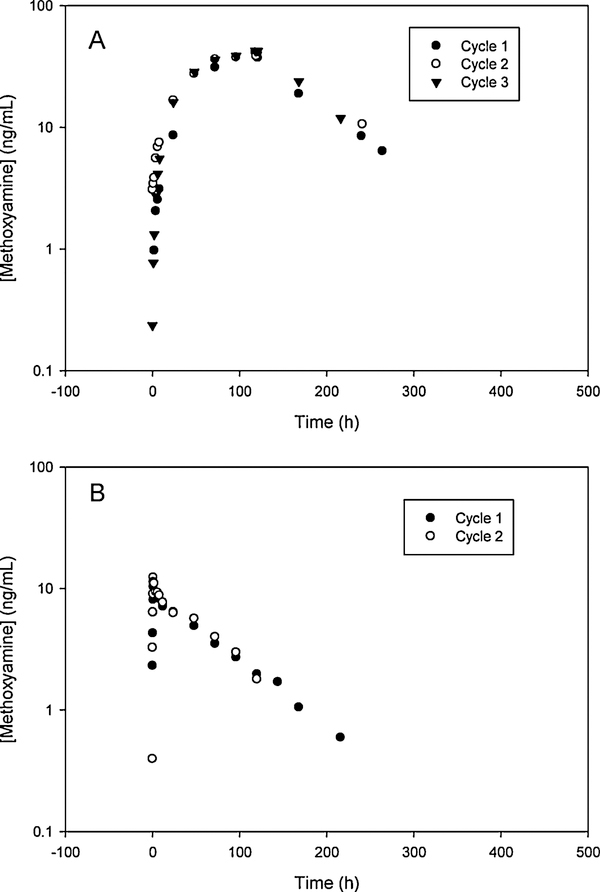
The LC-MS/MS method has been applied to the PK study of MX in patients with advanced solid tumors by two dosing regimens (120-h iv continuous infusion and 1-h iv infusion). The typical concentration-time profiles of MX in patients by 120-h and 1-h iv infusion of MX were shown in Figure 4. The PK parameters of first six patients (16 cycles) by 120-h iv continuous infusion had a mean elimination half-life (t1/2) of 45.07 h, and a mean maximum concentration (Cmax) of 40.62 ng/ml. The initial data demonstrated that MX has a distinct PK profile in humans compared to that in mice [10], which permitted an amendment to the initial regimen to a more convenient 1-h infusion regimen. The PK parameters of MX in 20 patients (46 cycles) with 1-h iv infusion had a mean t1/2 of 54.21 h, and a mean Cmax of 14.98 ng/ml. The t-test at 95% confidence level revealed that there was no significant difference in half-life estimates between the 120-h and 1-h infusion regimens.
Figure 4.
Representative plasma concentration-time profiles of MX in patients. (A) Dosing regimen: MX (iv continuous infusion), 15 mg/m2/day for 5 days; TMZ (oral once daily), 100 mg/m2/day for 5 days. (B) Dosing regimen: MX (single 1-h iv infusion), 15 mg/m2/day; and TMZ (oral once daily), 100 mg/m2/day for 5 days.
3.3.1. Incurred sample re-analysis
As part of the method validation, plasma samples from two patients’ treatment cycles were re-analyzed from their initial analyses after 9-month storage at −70 °C. The results indicated that the percent relative errors (%RE) between the two separate analyses over a period of 9 months were all less than 15%. Table 9 showed an example of such re-analysis. This study further demonstrated the stability of long-term sample storage and the robustness of the LC-MS/MS method.
Table 9.
Example of incurred patient sample re-analysis.
| Patient sampling schedule (h) | Measured [MX] (ng/ml) | E (ng/ml)a | %REb | |
|---|---|---|---|---|
| First analysis | Re-analysis after 9 months | |||
| Pre-dose | no peak | no peak | not applicable | not applicable |
| 0.250 | 5.36 | 5.69 | 0.33 | 6.2 |
| 0.500 | 8.95 | 10.2 | 1.25 | 14.0 |
| 0.750 | 16.2 | 18.2 | 2.0 | 12.3 |
| 0.983 | 20.5 | 22.1 | 1.6 | 7.8 |
| 1.17 | 25 | 27.1 | 2.1 | 8.4 |
| 2.00 | 24.2 | 24.3 | 0.1 | 0.4 |
| 4.00 | 18.3 | 20 | 1.7 | 9.3 |
| 6.00 | 19 | 19.5 | 0.5 | 2.6 |
| 8.00 | 18.9 | 17.5 | −1.4 | −7.4 |
| 12.0 | 15.6 | 16.2 | 0.6 | 3.8 |
| 24.0 | 13.3 | 13.2 | −0.1 | −0.8 |
| 48.0 | 9.46 | 10 | 0.54 | 5.7 |
| 72.0 | 6.56 | 6.46 | −0.10 | −1.5 |
| 96.0 | 4.46 | 4.76 | 0.3 | 6.7 |
| 120 | 3.55 | 3.69 | 0.14 | 3.9 |
| 168 | 2.11 | 2.29 | 0.18 | 8.5 |
| 192 | 1.55 | 1.76 | 0.21 | 13.5 |
| 216 | 1.22 | 1.39 | 0.17 | 13.9 |
E (error) = value of first analysis − value of re-analysis
%RE (percent relative error) = (error/value of first analysis) × 100%
4. Conclusion
An LC-MS/MS method for the quantitative determination of MX in human plasma has been developed and validated. In this work, MX and MX-d3 (IS) was derivatized directly in plasma with 4-(N,N-diethylamino)benzoaldehyde under acidic conditions. The derivatized MX could be extracted from human plasma by methyl tert-butyl ether and be readily separated from the derivatizing agent by Waters Xterra C18 column. Detection of the derivatized MX and IS was carried out by positive turbo-ion-spray MS/MS in MRM mode. Quantitation of MX was accomplished by internal calibration. This method has been successfully applied to the determination of MX in plasma samples from the first-in-human MX clinical trial.
Highlights.
An LC-MS/MS method for quantitative determination of methoxyamine in human plasma has been developed and fully validated.
This method has been applied to pharmacokinetic study of methoxyamine in a phase I clinical trial.
The concentration-time profiles and pharmacokinetic parameters of methoxyamine in patients were presented.
5. Acknowledgement
This research was supported by the Cancer Pharmacology Core Facility of the Case Comprehensive Cancer Center (P30 CA43703) and the National Cancer Institute of the US National Institutes of Health (5R21CA126149).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Liu L, Gerson SL, Current Opinion in Investigational Drugs, 5 (2004) 623–627. [PubMed] [Google Scholar]
- [2].Gerson SL, Liu L, PCT Int. Appl, WO 2001012199 A2 (2001). [Google Scholar]
- [3].Gerson SL, Liu L, US Pat. Appl. Publ, US2002198264 A1 (2002). [Google Scholar]
- [4].Liu L, Nakatsura Y, Gerson SL, Clinical Cancer Research, 8 (2002) 2985–2991. [PubMed] [Google Scholar]
- [5].Yan L, Bulgar A, Miao Y, Mahajan V, Donze JR, Gerson SL, Liu L, Clinical Cancer Research, 13 (2007) 1523–1539. [DOI] [PubMed] [Google Scholar]
- [6].Http://clinicaltrials.gov/show/NCT00892385 (accessed on February 15, 2012).
- [7].Liao Y, Syu M, Xu Y, Journal of Liquid Chromatography and Related Technologies 28 (2005), 2433–2443. [Google Scholar]
- [8].Wang E, Sreuble E, Liu P, Cheung AP, Journal of Pharmaceutical and Biomedical Analysis, 30 (2002) 415–427. [DOI] [PubMed] [Google Scholar]
- [9].Liao Y, Yang S, Syu M, Xu Y, Journal of Pharmaceutical and Biomedical Analysis, 39 (2005) 724–729. [DOI] [PubMed] [Google Scholar]
- [10].Yang S, Liu L, Gerson SL, Xu Y, Journal of Chromatography B, 795 (2003) 295–307. [DOI] [PubMed] [Google Scholar]
- [11].U.S. Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Guidance for Instrustry: Bioanalytical Method Validation, 2001, available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf (accessed on February 15, 2012).
- [12].Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, Shah VP, Skelly JP, Swann PG, Weiner R, The AAPS Journal, 9 (1) Article 4 (2007) E30–E42, available at http://www.springerlink.com/content/F3077L2771256145/fulltext.pdf (accessed on February 15, 2012). [Google Scholar]
- [13].Bansal S, DeStefano A, The AAPS Journal, 9 (1) Article 11 (2007) E109–E114, available at http://www.springerlink.com/content/N3865627384N57W1/fulltext.pdf (accessed on February 15, 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hammond LA, Eckardt JR, Baker SD, Eckhardt SG, Dugan M, Forral K, Reidenberg P, Statkevich P, Weiss GR, Rinaldi DA, Von Hoff DD, Rowinsky EK, Journal of Clinical Oncology, 17 (1999) 2604–2613. [DOI] [PubMed] [Google Scholar]
- [15].Britten CD, Rowinsky EK, Baker SD, Agarwala SS, Eckardt JR, Barrington R, Diab SG, Hammond LA, Johnson T, Villalona-Calero M, Fraass U, Statkevich P, Von Hoff DD, Eckhardt S. Gail, Clinical Cancer Research, 5 (1999) 1629–1637. [PubMed] [Google Scholar]
- [16].Brada M, Judson I, Beale P, Moore S, Reidenberg P, Statkevich P, Dugan M, Batra V, Cutler D, British Journal of Cancer, 81 (1999) 1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aoki T, Nishikawa R, Mizutani T, Nojima K, Mishima K, Adachi J, Matsutani M, International Journal of Clinical Oncology, 12 (2007) 341–349. [DOI] [PubMed] [Google Scholar]