Abstract
Multiple executive functions (EFs) are associated with trait anxiety and depression symptoms, but it is unclear how genetic and/or environmental factors account for these associations, and if they are explained by general variance underlying multiple EFs (i.e., Common EF). In this study, 1207 male twins completed seven EF tasks and measures of trait anxiety and depression symptoms at average age 62. The Common EF factor was associated with both anxiety (r= −.25) and depression symptoms (r= −.35). Anxiety and depression had near complete genetic overlap (rg=.96). Genetic influences shared with depression accounted for 83% of the phenotypic correlation between anxiety and Common EF. For depression, the phenotypic correlation with Common EF was explained by genetic influences shared with anxiety (69%), but also nonshared environmental influences distinct from anxiety (20%). These results suggest that genetic influences on Common EF play a role in the observed genetic overlap between anxiety and depression.
Keywords: executive control, trait anxiety, depression, heritability, working memory
A large body of research has examined the association between individual differences in anxiety and depression symptoms and executive functions (EFs), cognitive abilities that control and regulate behavior (Miyake & Friedman, 2012). According to attentional control theory, for example, subclinical levels of anxiety are associated with poor EFs in multiple contexts, including prepotent response inhibition, task-set shifting, and the updating of working memory representations (WM updating; Berggren & Derakshan, 2013; Eysenck & Derakshan, 2011; Eysenck, Derakshan, Santos, & Calvo, 2007). Associations between EFs and depression symptoms have also been observed in the general population (Franz et al., 2011; Holmes & Pizzagalli, 2007).
Despite the breadth of evidence for associations between EFs and tendencies to experience anxiety and depression symptoms, these findings have yet to be integrated into the unity and diversity framework of EF that emphasizes its genetic/environmental variance components and the general processes underlying many EF abilities (i.e., Common EF; Friedman et al., 2016; Miyake & Friedman, 2012). The strong correlation between trait anxiety and depression symptoms is explained largely by overlapping genetic influences (Eley & Stevenson, 1999; Jardine, Martin, Henderson, & Rao, 1984; Kendler, Heath, Martin, & Eaves, 1987), and the associations between EFs and other clinically-relevant constructs such as substance use, conduct disorder, and attention problems also appear to be due to genetic influences (Gustavson, Stallings, et al., 2017; Young et al., 2009). However, little research has examined whether genetic influences account for the associations between EFs and trait anxiety or depression symptoms (Franz et al., 2011; Friedman, du Pont, Corley, & Hewitt, 2018; Routledge et al., 2017), especially the overlapping genetic influences between anxiety and depression. Moreover, the associations between anxiety and separate EFs may reflect an underlying association with Common EF, rather than distinct associations with individual EF processes. The current study examined associations between EFs and anxiety and depression symptoms in a geneticallyinformative study of community-dwelling middle-aged male twins in the Vietnam Era Twin Study of Aging (VETSA).
Common Variance in Anxiety and Depression Symptoms
Anxiety and depression exhibit substantial overlap in the general population (Caci, Bayle, Dossios, Robert, & Boyer, 2003; Endler, Cox, Parker, & Bagby, 1992). Much of the phenotypic correlation between levels of anxiety and depression symptoms in the general population is explained by genetic influences regardless of whether symptoms are measured with diagnostic interviews (Hink et al., 2013; Krueger, McGue, & Iacono, 2001; Tackett et al., 2013) or with scales assessing trait tendencies (Eley & Stevenson, 1999; Jardine et al., 1984; Kendler et al., 1987). For example, a genetic correlation of rg = 1.0 between trait anxiety and depression symptoms was observed in children and adolescents (Eley & Stevenson, 1999), and smaller but substantial genetic correlations were observed in adults (rg = .79 to .88; Jardine et al., 1984).
Nonshared environmental influences were also correlated in both studies, but these associations were moderate (re = .43 to .63), suggesting that environmental factors also differentially influence anxiety and depression. These “nonshared” environmental influences refer to factors that make twins within a family less correlated, in contrast with shared environmental influences that makes twins more similar. Thus, both can be correlated across traits. These results are consistent with evidence from clinical studies suggesting that generalized anxiety disorder and major depressive disorder have nearly identical genetic influences, but only some overlapping nonshared environmental influences (Kendler & Myers, 2014; Kendler, Neale, Kessler, Heath, & Eaves, 1992; Lyons et al., 1998). Although many studies have examined how anxiety and depression symptoms are associated with cognition, few have considered the genetic and environmental overlap between cognition and anxiety/depression symptoms (Franz et al., 2011; Friedman et al., 2018; Routledge et al., 2017), especially EFs.
Unity and Diversity of Executive Functions
Existing research on EFs and anxiety or depression has focused on the three correlated EFs discussed by Miyake et al. (2000): inhibition, shifting, and WM updating. However, in the more recent unity and diversity model of EFs (Friedman & Miyake, 2017; Miyake & Friedman, 2012), variation in these well-known EF abilities can be re-conceptualized into different latent factors: a general factor explaining variation across all EF tasks (Common EF), and EF-specific factors that explain additional variance in certain processes not captured by Common EF (e.g., Shifting-Specific, WM-Specific). By re-conceptualizing variance in inhibition, shifting, and WM updating, researchers aim to better specify the cognitive/biological underpinnings and correlates of the variance unifying different aspects of EFs.
Our recent work on the present sample at an earlier wave of assessment identified evidence for the Common EF factor in middle age (Gustavson, Panizzon, Franz, et al., 2018). We also identified a WM-Specific factor accounting for additional variation in WM span tasks not captured by Common EF, but not a Shifting-Specific factor. As yet, there is no evidence for an Inhibition-Specific factor in adolescence, early adulthood, or middle age, suggesting that inhibition is entirely encapsulated by Common EF (Friedman et al., 2016; Gustavson, Stallings, et al., 2017; Ito et al., 2015).
Importantly, genetic influences account for a large portion of variance in Common EF and the specific factors. Heritability estimates of Common EF range from 46% to 81% in young and middle adulthood (Friedman et al., 2016; Gustavson, Panizzon, Franz, et al., 2018), and are essentially 100% in adolescence (Engelhardt, Briley, Mann, Harden, & Tucker-Drob, 2015; Friedman et al., 2008). Furthermore, genetic influences tend to explain most of the phenotypic correlations between Common EF and other cognitive and clinical constructs throughout the lifespan (Engelhardt et al., 2016; Gustavson, Panizzon, Franz, et al., 2018; Gustavson, Stallings, et al., 2017; Herd et al., 2014).
Executive Functions, Anxiety, Depression, and Aging
Theoretical reviews of attentional control theory have demonstrated that higher levels of subclinical anxiety symptoms are associated with poorer inhibition, shifting, and WM updating (Berggren & Derakshan, 2013; Eysenck & Derakshan, 2011; Eysenck et al., 2007). Associations between EFs and subclinical depression symptoms have received far less attention, and the findings are somewhat inconsistent. Studies of college students have yielded mixed results regarding associations between depression symptoms and EFs (Holmes & Pizzagalli, 2007; Wagner, Alloy, & Abramson, 2015). Several studies have also observed associations between EFs and trait anxiety above and beyond depression, and/or showed that depression symptoms were not associated with their candidate EF task in a model with trait anxiety (Edwards, Edwards, & Lyvers, 2015; Gustavson, Altamirano, Johnson, Whisman, & Miyake, 2017; Gustavson & Miyake, 2016).
Existing studies have focused on these associations in adolescence and young adulthood. When older adults are included, the samples have had generally wide age ranges (Bunce, Handley, & Gaines, 2008; Routledge et al., 2017). However, at an earlier wave of assessment of the present sample (age 51 to 60), levels of depression were weakly but significantly associated with performance on multiple EF tasks (rs = −.07 to −.12; Franz et al., 2011), and mostly explained by shared genetic influences. A more recent twin study of adults (aged 18 to 62) revealed that a combined measure of anxiety and depression symptoms (DASS-42), was associated with worse inhibition (β = −.14) and shifting (β = −.12; Routledge et al., 2017). Although nonsignificant, at least half of both associations were accounted for by genetic influences, with evidence for a significant environmental association for inhibition only.
Cognitive abilities begin to decline in late middle age, so this may be an important time to examine the associations among EFs, trait anxiety, and depression symptoms. For example, anxiety and depression are associated with increased risk for dementia (Byers & Yaffe, 2011; Gallacher et al., 2009; Petkus, Reynolds, et al., 2016). Additionally, EFs may be especially susceptible to age-related decline in middle age (de Frias, Dixon, & Strauss, 2006), and decline in EFs may underlie age-related decline in other cognitive abilities (Hasher & Zacks, 1988; Lustig, Hasher, & Zacks, 2007; Salthouse, Atkinson, & Berish, 2003). In fact, longitudinal analyses suggest that trait anxiety is associated with decline in multiple cognitive abilities including processing speed and attention, with evidence for some bi-directionality in these associations (Petkus, Reynolds, Wetherell, Kremen, & Gatz, 2017). However, the Petkus et al. study did not have EF or WM measures other than digit span. It is surprising that associations between anxiety, depression, and EFs have received so little attention in the aging literature, given their relevance to cognitive aging. Furthermore, anxiety and depression have been associated with dorsolateral prefrontal cortex structure and function (Engels et al., 2010; Herrington et al., 2010), an area also associated with EFs (Banich, 2009), suggesting that these constructs are linked through neurobiological factors that likely include genetic influences (Stollstorff et al., 2013).
The Current Study
We tested the hypothesis that anxiety and depression symptoms are associated with worse Common EF ability. We also hypothesized that the association between Common EF and anxiety/depression would be accounted for primarily by shared genetic influences. Specifically, we expected that most of the genetic influences on trait anxiety would be shared with depression symptoms, and that their genetic associations with Common EF would be primarily explained by genetic influences common to anxiety and depression rather than those unique to either one. This contrasts with an alternative (but potentially complementary) hypothesis that there are some unique genetic or environmental influences that also account for these associations. If so, these findings could shed light on the genetic/environmental factors that differentiate anxiety from depression. We also examined whether the WM-Specific factor would be associated with anxiety or depression, as these associations have yet to be explicitly studied. We tested these hypotheses in a twin study of community-dwelling middle-aged men who were not selected for their levels of anxiety or depression.
Method
Participants
Analyses were based on 1207 men from 563 full twin pairs (330 monozygotic [MZ] pairs, 233 dizygotic [DZ] pairs, 5 pairs with unknown zygosity, 71 twins unpaired) who participated in wave 2 of the VETSA (M = 61.72 years, SD = 2.44). VETSA participants were recruited from the Vietnam Era Twin Registry from a previous study (Tsuang, Bar, Harley, & Lyons, 2001). They are similar with respect to health and lifestyle factors to American men in their age group (e.g., M years of education = 13.81, SD = 2.11, Range = 5 to 20), and nearly 80% of the sample did not serve in combat or in Vietnam (Kremen, Franz, & Lyons, 2013; Kremen et al., 2006). Most of the sample identify as White, non-Hispanic (88.1%), with additional individuals identifying themselves as Black (5.1%), more than one race (2.7%), American Indian (< 1%), or Pacific Islander (< 1%). Hispanic individuals comprised 3.0% of the sample.
Measures
The neuropsychological tasks comprising the EF battery are widely used and have been described in previous work on this sample at an earlier wave of assessment (Gustavson, Panizzon, Franz, et al., 2018; Kremen, Moore, Franz, Panizzon, & Lyons, 2014; Kremen et al., 2006). After creating each of the dependent measures described below, we adjusted each measure for age by creating residualized scores. Moreover, because 193 individuals completed the EF tasks for the first time in this second wave of assessment, we also adjusted scores on each task to account for practice effects for those individuals who had completed the tests twice (Elman et al., 2018). These practice effect adjustments were based on procedures outlined by Rönnlund, Nyberg, Bäckman, and Nilsson (2005) in which returning participants are compared with matched replacement participants, and compared with dropouts to account for attrition effects.
Inhibition.
Inhibition was assessed with the Stroop task (Golden & Freshwater, 2002; Stroop, 1935) and the AX-Continuous Performance Test (AX-CPT; Braver et al., 2001; Kremen et al., 2011). For the Stroop, the dependent measure was a residualized score for the total number of words identified during the color-word condition after adjusting for performance on the two baseline conditions (word-only and color-only). For the AX-CPT, the dependent measure was an arcsine-transformed signal detection index (d’) based on the hit rate for AX trials minus the false alarm rate for BX trials. Additionally, d’ values less than 0 were set to 0 to reduce the tail of the distribution. On this test, the target stimulus is an X that is immediately preceded by an A. BX trials refer to trials on which an X is preceded by a non-A letter. Because 70% of trials are AX, BX trials require inhibition of a prepotent tendency to press the target button in response to an X.
Working memory span.
WM span was assessed with the letter-number sequencing subtest of the Wechsler Memory Scale-III (Wechsler, 1997), the reading span task (Daneman & Carpenter, 1980), and the combined forward and backward digit span subtests of the Wechsler Memory Scale-III. The dependent measures for letter-number sequencing and digit span were the total number of trials passed. The dependent measure for the reading span was the total number of correct words recalled across all 15 trials.
Shifting
Shifting was assessed using the Trail Making Test and the category switching trial of the verbal fluency test, both from the Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001). The dependent measure for the Trail Making Test was a residualized score for the time taken to complete the switching trial after adjusting for the time on the single-task trials (number sequencing and letter sequencing). The dependent measure of category switching was a residualized score for category switching accuracy (the number of times a participant correctly switched categories) after adjusting for the number of correct responses on the category fluency trials (animals and boys’ names).
Anxiety symptoms.
Levels of trait anxiety were measured with the trait form of the State-Trait Anxiety Inventory (STAI; Spielberger, 1983). The trait form of the STAI has 20 items that are responded to on a 1–4 rating scale and asks about “how you generally feel”. Reliability, computed using Cronbach’s Alpha, was high (α = .93). Additionally, this scale was log-transformed to improve the distributional characteristics. For use in additional analyses, we assessed state anxiety using the log-transformed state form of the STAI, which also had 20 items responded to on a 1–4 rating scale, and assesses anxiety symptoms based on “how you feel right now, that is, at this moment”. State anxiety was assessed on the day of testing; trait anxiety assessed through a mailed survey approximately four weeks prior to testing.
Depression symptoms.
Depressive symptoms were measured with the Center for Epidemiologic Studies–Depression scale (CESD; Radloff, 1977), which was also assessed through a mailed survey approximately four weeks prior to testing. The scale has 20 items, each responded to on a 0–3 rating scale, and asked about the experience of symptoms “during the past week”. Internal consistency was high (α =.89), and this scale was also log-transformed to improve distributional characteristics.
General cognitive ability.
For use in additional analyses, general cognitive ability was measured with the Armed Forces Qualification Test (AFQT; Bayroff & Anderson, 1963). The AFQT is a 100-question multiple choice paper-and-pencil test that is highly correlated with traditional measures of IQ and general cognitive ability (Lyons et al., 2009). Percentile scores were transformed based on military norms to normalize the distribution.
Data Analysis
Phenotypic analyses were conducted using Mplus Version 7.2 (Muthén & Muthén, 2010) and genetic/environmental analyses were conducted using the structural equation modeling package OpenMx in R (Boker et al., 2011). Both programs account for missing observations using full-information maximum likelihood. Mplus was preferred for phenotypic analyses to fit multiple regression analyses involving latent variables (i.e., controlling for general cognitive ability and state anxiety) and OpenMx was preferred for genetic analyses to obtain likelihoodbased 95% confidence intervals (95% CI).
Model fit was determined using χ2 tests, the Root Mean Square Error of Approximation (RMSEA), and the Comparative Fit Index (CFI). Good fitting models had χ2 values less than two times the degrees of freedom, RMSEA values < .06, and CFI values > .95 (Hu & Bentler, 1998). Significance of individual parameters was established with χ2 difference tests (by fixing those parameters to zero), or using likelihood-based 95% CIs. For phenotypic models, χ2 tests and standard errors were adjusted for the within-family clustering of the data using scaling factors provided in the output (i.e., by using the “type = complex” command in Mplus; Satorra & Bentler, 2001).
Genetic analyses.
Genetic analyses were based on the classical assumptions in twin designs. Additive genetic influences (A) are assumed to correlate at 1.0 for MZ twins and at 0.5 for DZ twins because MZ twins share 100% of their alleles identical-by-descent and DZ twins share, on average, 50% of their alleles identical-by-descent. Shared environmental influences (C) are assumed to correlate at 1.0 for both types of twins. Nonshared environmental influences (E), which also include measurement error, are not correlated in either MZ or DZ twins. We also assume that means and variances are identical across twin pair (twin 1 vs. twin 2) and across zygosity (MZ vs. DZ twins). These standard assumptions for univariate twin models extend to multivariate analyses and to analyses where phenotypic correlations are decomposed into their genetic (rg), shared environmental (rc), and nonshared environmental components (re).
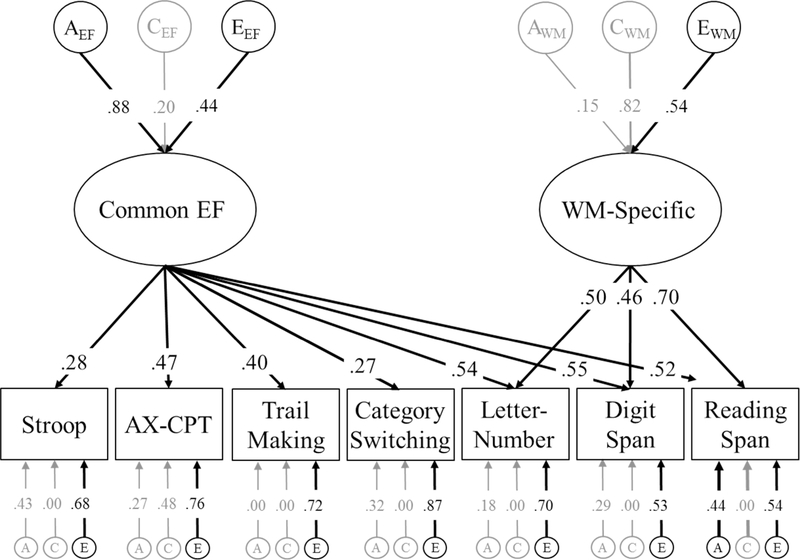
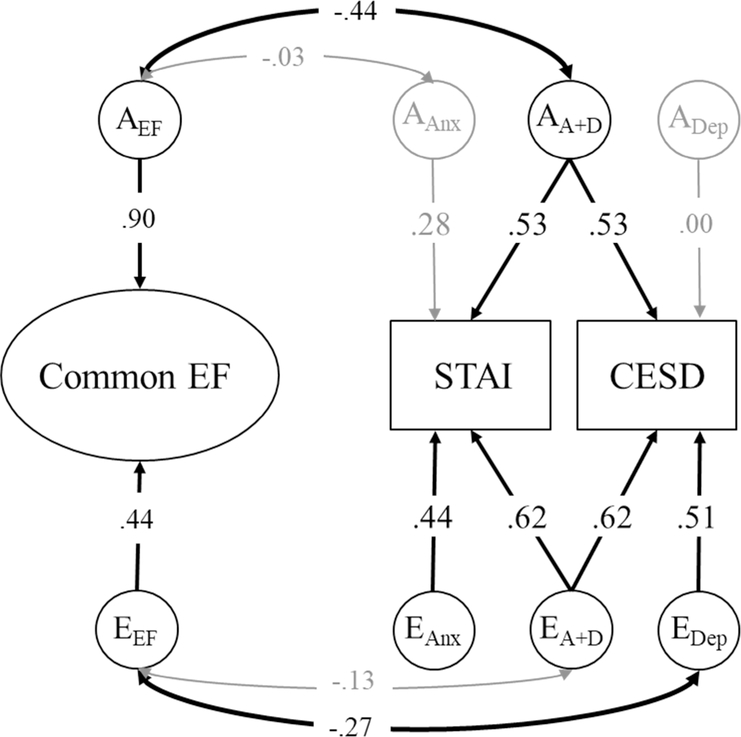
Using an approach developed in Gustavson et al., 2017b, we first fit the confirmatory common pathway model of EFs alone. This model, displayed in Figure 1, parses variance in the seven neuropsychological EF tasks into Common EF and WM-Specific factors, and each of these factors are decomposed into their genetic, shared environmental, and nonshared environmental components. Next, we combined this model of EFs with the measures of trait anxiety and depression symptoms using a Cholesky decomposition, which is a common first step in twin analyses. The Cholesky is a simple decomposition of the genetic and environmental variances and covariances among Common EF, WM-Specific, trait anxiety, and depression symptoms, and can be used to compute the genetic/environmental correlations between all constructs before examining more theoretically-driven models. Finally, we fit a confirmatory factor model in which we directly modeled the associations between Common EF and genetic/environmental influences capturing shared and unique variance in anxiety and depression. This model is displayed in Figure 2 and is described in further detail below.
Figure 1:
Model of the genetic (A), shared environmental (C), and nonshared environmental influences (E) on Common EF and WM-Specific. Circles and ellipses indicate latent variables and squares indicate measured variables. Variance explained by each latent factor can be computed by squaring the factor loading on that factor. Significant factor loadings are displayed with black text and lines (p < .05).
Figure 2:
Confirmatory model of the genetic and environmental associations between Common EF, trait anxiety, and depression symptoms. Variance in trait anxiety and depression symptoms are decomposed into genetic/environmental influences common to both anxiety and depression (AA+D, EA+D) and genetic/environmental influences unique to anxiety (AAnx, EAnx) or depression (ADep, EDep). Genetic/environmental influences on WM-Specific and shared environmental influences on all constructs are not pictured here, but all nonshared environmental influences were nonsignificant. Factor loadings and residual genetic/environmental influences on the EF tasks are also not shown here, but were similar to those displayed in Figure 1. Significant factor loadings and correlations are displayed in bold with black lines (p < .05).
Results
Preliminary Analyses
Descriptive statistics.
Descriptive statistics for all measures are summarized in Table 1, shown before adjusting for age on each of the dependent measures. The full phenotypic correlation matrix between all measures is displayed in the appendix. As would be expected in a middle-aged community-dwelling sample, the large majority were at normal levels of anxiety and depression. For example, only 172 individuals (14.3% of the sample) were at risk for clinical depression (i.e., CESD score > 15).
Table 1.
Descriptive Statistics for All Measures in the Study
| Measure | N | M | SD | Range | Skewness Kurtosis | rMZ | rDZ | |
|---|---|---|---|---|---|---|---|---|
| Executive Function Tasks | ||||||||
| Stroop | 1180 | 32.86 | 7.08 | −3.40, 5.42 | 0.00 | 2.39 | 0.31 | 0.14 |
| AX-CPT | 1059 | 1.09 | 0.26 | −4.54, 1.42 | −1.42 | 2.88 | 0.43 | 0.35 |
| Letter-Number | 1202 | 9.28 | 2.25 | −3.35, 3.23 | 0.10 | 0.47 | 0.36 | 0.21 |
| Reading Span | 1197 | 33.99 | 4.76 | −3.35, 2.04 | −0.38 | 0.06 | 0.56 | 0.36 |
| Digit Span | 1058 | 15.83 | 3.69 | −2.54, 3.25 | 0.36 | −0.06 | 0.57 | 0.39 |
| Trail Making Test | 1047 | 85.64 | 28.56 | −4.09, 2.33 | −1.71 | 4.97 | 0.22 | 0.12 |
| Category Switching | 1202 | 11.00 | 2.93 | −4.05, 2.78 | −0.53 | 0.98 | 0.18 | 0.11 |
| Anxiety and Depression Symptoms | ||||||||
| STAI - Trait | 1196 | 3.41 | 0.29 | 3.00, 4.33 | 0.50 | −0.52 | 0.42 | 0.19 |
| CESD | 1195 | 1.69 | 0.99 | 0, 3.99 | −0.17 | −0.72 | 0.32 | 0.22 |
| Additional Measures | ||||||||
| STAI - State | 1200 | 3.40 | 0.28 | 3.00, 4.22 | 0.31 | −0.75 | 0.30 | 0.17 |
| AFQT | 1203 | 0.34 | 0.67 | −2.05, 2.32 | 0.01 | −0.31 | 0.70 | 0.38 |
Note: Descriptive statistics for all dependent measures are shown before adjusting for age (for all measures), but after transformation (for measures of anxiety and depression). The final two columns display the cross-twin correlations for monozygotic (rMZ) and dizygotic twins (rDZ). Significant cross-twin correlations are displayed in bold (p < .05) AX-CPT = AX – Continuous Performance Test, STAI = State-Trait Anxiety Inventory, CESD = Center for Epidemiologic Studies – Depression scale, AFQT = Armed Forces Qualification Test percentile score (transformed).
Unity and diversity model of EF.
The genetic and environmental model of EFs alone, displayed in Figure 1, was largely consistent with the same genetic/environmental decomposition reported for these latent variables in the first wave of the VETSA (Gustavson, Panizzon, Franz, et al., 2018). Common EF accounted for variation and covariation in all seven neuropsychological EF tasks, and the WM-Specific factor accounted for residual variation and covariation across the three WM span tasks not already captured by Common EF. Common EF was explained primarily by genetic influences (heritability of a2 = .77, or the square of the factor loading of .88, 95% CI [.37, .90]), but also by some nonshared environmental influences, e2 = .19, 95% CI [.08, .29] and nonsignificant shared environmental influences c2 = .04, 95% CI [.00, .39]. The WM-Specific factor was also explained by nonshared environmental influences, e2 = .29, 95% CI [.08, .50]. Although shared environmental influences accounted for almost all the remaining variation in WM-Specific (c2 = .79), these influences were not significant due to the low power to detect the difference between genetic and shared environmental influences on this residual factor (Martin, Eaves, Kearsey, & Davies, 1978). Both the genetic and shared environmental influences on WM-Specific could not be removed from the model without a significant drop in fit, χ2diff(2) = 18.74, p < .001.
Phenotypic analyses
We examined the phenotypic associations between the two latent EF factors and the measures of anxiety and depression using a correlational measurement model displayed in Table 2a, which fit the data well χ2 (21) = 35.20, p = .027, RMSEA = .024, CFI = .992. Confirming our hypothesis, lower Common EF was significantly associated with greater trait anxiety (r = −.24) and depression symptoms (r = −.32), both χ2diff(1) > 14.05, ps < .001. In contrast, low WMSpecific was not significantly associated with trait anxiety (r = −.03) or depression symptoms (r = .03), both χ2diff(1) < .07, p > .785.
Table 2.
Latent Variable Correlations Between Executive Function and Anxiety/Depression Symptoms
| 1 | 2 | 3 | Variance Component |
|
|---|---|---|---|---|
| A. Phenotypic | ||||
| 1. Common EF | 1 | |||
| 2. WM-Specific | - | 1 | ||
| 3. Trait Anxiety | −.24 | −.03 | 1 | |
| 4. Depression Symptoms | −.32 | .03 | .73 | |
| B. Genetic | a2 | |||
| 1. Common EF | 1 | .72 | ||
| 2. WM-Specific | - | 1 | .15 | |
| 3. Trait Anxiety | −.40 | .39 | 1 | .36 |
| 4. Depression Symptoms | −.37 | .46 | .96 | .18 |
| C. Shared Environment | c2 | |||
| 1. Common EF | 1 | .08 | ||
| 2. WM-Specific | - | 1 | .55 | |
| 3. Trait Anxiety | −.64 | .08 | 1 | .05 |
| 4. Depression Symptoms | −.72 | −.21 | .98 | .12 |
| D. Nonshared Environment | e2 | |||
| 1. Common EF | 1 | .20 | ||
| 2. WM-Specific | - | 1 | .30 | |
| 3. Trait Anxiety | −.02 | −.17 | 1 | .59 |
| 4. Depression Symptoms | −.32 | .07 | .63 | .69 |
Note: The final column displays the proportion of variation in each trait that is explained by genetic influences (a2), shared environmental influences (c2), or nonshared environmental influences (e2) in the Cholesky decomposition. Genetic/environmental influences differ slightly from those reported in the main text because they were derived from the Cholesky decomposition rather than the confirmatory model (Figure 2). Factor loadings on the EF latent variables are not shown, but were similar to those displayed in Figure 1. Common EF and WMSpecific were fixed to be uncorrelated. Significant correlations and genetic/environmental variance components are displayed in bold (p < .05).
Genetic and Environmental Models of Anxiety, Depression, and Executive Functions
The genetic/environmental correlations among EF factors, trait anxiety, and depression symptoms derived from the Cholesky decomposition are displayed in Table 2b-2d. This basic model fit the data well, χ2 (313) = 400.14, p < .001, RMSEA = .021 CFI = .965.
To directly test the hypothesis that the associations between Common EF and anxiety/depression were due to those genetic influences underlying the commonality between anxiety and depression, we fit a confirmatory model based on one outlined by Loehlin (1996). In this model—displayed in Figure 2—there are genetic and environmental factors that capture the common variance between anxiety and depression symptoms (AA+D, EA+D; with equated factor loadings), and which correlate with the genetic/environmental influences on Common EF (AEF, EEF). Additionally, there are factors that capture the remaining genetic/environmental influences on anxiety (AAnx, EAnx) and depression symptoms (ADep, EDep), some of which are also correlated with Common EF.1 This model also fit the data well, χ2 (321) = 405.81, p < .001, RMSEA = .020, CFI = .966, and did not fit worse than the Cholesky decomposition, χ2diff(8) = 5.67, p = .684.
Although not displayed in Figure 2, WM-Specific was included in this model but restricted to not correlate with anxiety or depression, consistent with the lack of phenotypic correlations. Additionally, because shared environmental influences accounted for little variance in any construct (see below), these influences were estimated (CA+D, CAnx, CDep), but not allowed to correlate with those for Common EF (CEF), and are also not displayed in Figure 2. In both genetically-informative models, genetic/environmental correlations were not estimated directly, but derived algebraically from statistically-equivalent covariance matrices.
Genetic influences.
As shown in Figure 2, genetic influences explained a total of 36% of the variation in trait anxiety, a2 = .36, 95% CI [.12, .48] and 28% of the variation in depression symptoms, a2 = .28, 95% CI [.04, .40]. All of the genetic influences on depression were explained by the common factor, and there were only small and nonsignificant genetic influences unique to anxiety (AAnx), a2 = .08, 95% CI [.00, .12]. Thus, anxiety and depression have a nearcomplete genetic overlap.
Consistent with our hypothesis, there was a significant genetic correlation between Common EF (AEF) and the genetic influences shared between anxiety and depression (AA+D), rg = −.44, 95% CI [−1.0, −.25]. This correlation accounted for 83% of the phenotypic correlation between Common EF and trait anxiety and 69% of the phenotypic correlation between Common EF and depression symptoms. In contrast, the genetic correlation between Common EF and those genetic influences unique to anxiety (AAnx) was nonsignificant, rg = −.44, 95% CI [−1.0, 1.0], explaining only 3% of the phenotypic correlation.
Shared environmental influences.
Although not pictured in Figure 2, shared environmental influences accounted for a small and nonsignificant amount of variation in both anxiety, c2 = .04, 95% CI [.00, .25] and depression, c2 = .04, 95% CI [ .00, .25] in the confirmatory model. Although nonsignificant, these influences were explained entirely by the common factor for both anxiety and depression (CA+D), with no influences estimated on the specific shared environmental factors (CAnx, CDep).
As described above, we did not estimate shared environmental correlations between Common EF and anxiety/depression symptoms. If a shared environmental correlation between Common EF (CEF) and the influences shared between anxiety and depression (CA+D) were added to this model, it would be nonsignificant, rg = −1.0, 95% CI [−1.0, 1.0], and would explain less than 1% of the phenotypic correlation. In this alternate model including a shared environmental correlation, the significant genetic correlation between Common EF and genetic influences shared between anxiety and depression (AA+D) would remain similar in magnitude but would be nonsignificant, rg = −.38, 95% CI [−1.0, .06], most likely due to a loss of power (Martin et al., 1978).
Nonshared environmental influences.
As shown in Figure 2, nonshared environmental influences explained most of the variation in trait anxiety, e2 = .59, 95% CI [.52, .68] and depression symptoms e2 = .68, 95% CI [.60, .76]. Most of these influences were explained by the common factor (EA+D), explaining 38% of the total variance in either trait, but there were also influences unique to anxiety or depression (explaining the remaining 19% and 26% of variance, respectively).
The nonshared environmental correlation between Common EF (EEF) and the influences shared between anxiety and depression (EA+D) was nonsignificant, re = −.13, 95% CI [−.35, .09], explaining 14% and 12% of the phenotypic correlation between Common EF and anxiety or depression, respectively. Interestingly, there was a significant nonshared environmental correlation between Common EF and environmental influences specific to depression (EDep), re = −.27, 95% CI [−.52, −.04], explaining the remaining 20% of their phenotypic correlation.
Additional Analyses
We conducted two additional phenotypic multiple regression analyses to address potential confounds. First, there is a considerable phenotypic correlation between Common EF and measures of general cognitive ability or intelligence (Friedman et al., 2008; Gustavson, Panizzon, Franz, et al., 2018), as evidenced by the correlation of r = .71 between Common EF and general cognitive ability in these data. Therefore, it was possible that some of the associations between EF and anxiety or depression symptoms described here were explained by a broader association between general cognitive ability and anxiety and/or depression symptoms rather than Common EF. After controlling for general cognitive ability using multiple regression procedures, the phenotypic association between Common EF and trait anxiety remained significant (β = −.22, p = .016), as did the association between Common EF and depression symptoms (β = −.33, p = .001). Moreover, although general cognitive ability was negatively correlated with both trait anxiety (r = −.21, p < .001) and depression symptoms (r = −.25, p < .001), these associations were no longer significant after controlling for the EF latent factors (β = −.05, p = .499 for anxiety, β = −.01, p = .910 for depression). Thus, if anything, the association between general cognitive ability and anxiety/depression seems to be explained by variance in Common EF, rather than vice versa.
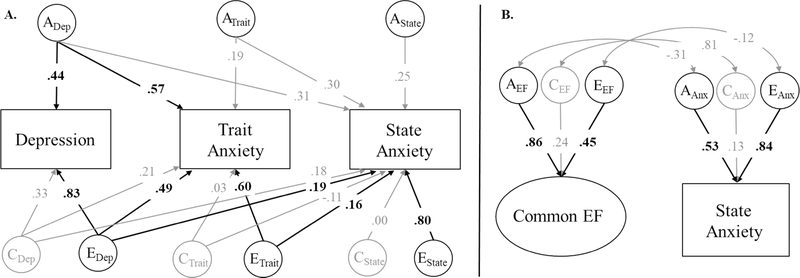
Second, although we focused here on the association between EFs and trait anxiety, it is possible that these associations are actually explained by state anxiety. Again using multiple regression, the phenotypic association between Common EF and trait anxiety remained significant after controlling for state anxiety (β = −.21, p = .001). State anxiety was correlated with both Common EF (r = −.16, p = .002) and WM-Specific (r = −.14, p = .022), but it was not associated with Common EF in the multiple regression model (β = −.05, p = .366). Additional genetic analyses involving state anxiety displayed in the appendix (Figure A1) revealed that state anxiety was not significantly explained by genetic influences common to trait anxiety and depression. Rather, it was primarily explained by nonshared environmental influences distinct from those on trait anxiety or depression (e2 = .64). There were no significant genetic or environmental correlations between Common EF and state anxiety, all χ2diff(1) < 3.51, p > .061. Therefore, the association between EFs and trait anxiety described above does not appear to be accounted for by people’s current level of anxiety.
Discussion
We examined the associations between EFs and anxiety and depression symptoms in a community dwelling sample of middle-aged male twins. First, we confirmed that the associations between EFs and anxiety/depression symptoms were due to common variance spanning multiple EFs (i.e., Common EF). Second, we showed that these associations were mostly explained by genetic influences. Specifically, the correlation between genetic influences on Common EF (AEF) and genetic influences shared between anxiety and depression (AA+D) accounted for most of the phenotypic correlation between Common EF and anxiety (83%) or depression (69%). Additionally, there was a significant correlation between the nonshared environmental influences on Common EF and those environmental influences unique to depression (EDep), accounting for 20% of their phenotypic correlation. Therefore, anxiety and depression were not associated with EFs entirely through the same mechanisms; depression was associated with Common EF through both genetic and environmental influences whereas anxiety was associated primarily through genetic influences.
These findings have some direct implications for theoretical models such as attentional control theory, which aim to explain the associations between EFs and subclinical anxiety (Eysenck & Derakshan, 2011; Eysenck et al., 2007). Three of attentional control theory’s hypotheses state that levels of anxiety are associated with inhibition, shifting, and WM updating using the framework inspired by Miyake et al. (2000), but these hypotheses have yet to be applied to the more recent unity and diversity model of EFs (Miyake & Friedman, 2012). Our results suggest that it may be more parsimonious to view this as an association between anxiety and the general processes underlying EFs across multiple subdomains (i.e., Common EF).
In contrast, the WM-Specific factor was uncorrelated with either anxiety or depression symptoms. Meta-analytic estimates have shown that there are significant associations between trait anxiety and WM span (Moran, 2016). Our results may appear to be in conflict with the meta-analytic results, but it should be noted that WM-Specific captures only the variance in WM span that is independent of Common EF. The remaining variance in WM is captured by Common EF, which is associated with trait anxiety. Models that directly quantify these sources of variance may be useful in further understanding how different aspects of WM processes are associated with other constructs. Although there was no Shifting-Specific factor in this sample, future work should investigate the extent to which anxiety is related to Common EF vs. Shifting-Specific processes as well, as there is some debate about whether associations between anxiety and shifting are specific to certain situations (Edwards et al., 2015; Gustavson, Altamirano, et al., 2017).
Previous work has suggested that almost all the genetic influences on trait anxiety are shared with those on depression symptoms, and that nonshared environmental influences are moderately correlated but are also unique to each construct (Eley & Stevenson, 1999; Jardine et al., 1984; Kendler et al., 1987). Similar results were observed here. All the genetic variance in depression was accounted for by the shared anxiety/depression genetic factor (AA+D) and only a small and nonsignificant portion of the variance in anxiety was accounted for by unique genetic influences (AAnx). Importantly, some of these shared genetic influences between anxiety and depression were the same as those genetic influences on Common EF, and there was no evidence that the unique influences on anxiety were associated with Common EF. Although genetic influences accounted for much of the covariance between Common EF and anxiety/depression, it should be noted that most of the genetic variance in Common EF is independent of anxiety or depression (i.e., only about 20% of the total genetic variance in Common EF was shared with genetic variance on anxiety and/or depression).
Interestingly, the environmental influences unique to depression symptoms (EDep) were negatively associated with Common EF (re = −.27), explaining 20% of their phenotypic correlation. It is unclear what environmental influences explain this association, only that they are distinct from the environmental influences on trait anxiety and are influences that differentiate twins (as opposed to shared environmental influences that make twins more similar). Moreover, we could have tested an alternate but statistically equivalent model where Common EF was negatively correlated with environmental influences common to anxiety/depression (ECom) but positively correlated with influences unique to anxiety (EAnx). We find it more likely that there are environmental influences unique to Common EF and depression and not anxiety, rather than environmental influences that act in opposite directions on Common EF and anxiety (completely cancelling each other out). Nevertheless, it will be important to examine these associations further as they may shed light on the situations that distinguish depression from anxiety. Given these results, it is also not surprising that despite their almost complete genetic overlap, anxiety and depression can have some independent associations with cognition and/or dementia (e.g., controlling for one another; Burton, Campbell, Jordan, Strauss, & Mallen, 2013; Petkus et al., 2017).
In addition to the novel genetic findings, our findings extend work on the association between EFs and anxiety and depressive disorders to show that these associations exist even in the normal range of anxiety and depression symptoms. These results are consistent with associations between EFs and generalized anxiety disorder or major depressive disorder (Caspi et al., 2014; Snyder, 2013; Snyder, Miyake, & Hankin, 2015), and evidence that much of these associations are explained by the shared variance between anxiety and depression (Caspi et al., 2014; Martel et al., 2017). Nevertheless, future work on clinical-level associations with EFs may benefit from similar multivariate models that emphasize Common EF (e.g., Martel et al., 2017).
These results are also relevant to recent efforts to use cognitive control training as an intervention for anxiety and/or depression. There is mounting evidence that interventions that target EF processes (e.g., attentional control, WM) are successful at reducing anxiety and depression symptoms in individuals with elevated symptoms (Bettis et al., 2017; Calkins, McMorran, Siegle, & Otto, 2015; Owens, Koster, & Derakshan, 2013; Sari, Koster, Pourtois, & Derakshan, 2016). Our findings that the associations between EFs and anxiety/depression symptoms are driven by Common EF suggests that interventions may be most successful if they incorporate EF tasks from multiple domains (inhibition, shifting, WM), rather than modular approaches that target only one process at a time (e.g., WM span only). Moreover, given the substantial overlap between anxiety and depression, EF training for anxious individuals may improve their depression symptoms (and vice-versa), but existing studies have typically considered only one construct or the other.
Strengths and Limitations
Examining associations at the level of latent variables was important for being able to draw conclusions. By isolating variance in Common EF and WM-Specific latent variables, we controlled for task-specific variance on each EF task, including measurement error. As displayed in the appendix, there were only weak correlations between individual EF tasks and trait anxiety or depressive measures (rs = −.01 to −.19), consistent with previous estimates (Franz et al., 2011; Routledge et al., 2017). Phenotypic correlations between Common EF and trait anxiety (r = −.26) or depression symptoms (r = −.33) were more substantial at the latent variable level, suggesting that the true associations may have been underestimated in prior studies.
These results also add to our growing understanding of the genetic influences on Common EF. Previous work using twin studies in adolescence and young adulthood has shown that the associations between Common EF and other clinically-relevant constructs tend to be explained by the genetic influences more so than environmental influences (Gustavson, Stallings, et al., 2017; Young et al., 2009). This was not as surprising in adolescence when nearly 100% of the individual differences are explained by genetic influences (Engelhardt et al., 2015; Friedman et al., 2016), but by midlife environmental influences also explain a considerable amount of the variance (Gustavson, Panizzon, Franz, et al., 2018). Nevertheless, genetic influences accounted for most of the phenotypic association between Common EF and trait anxiety, and much of the phenotypic correlation between Common EF and depression symptoms, suggesting that underlying genetic risk factors continue to account for its association with other constructs even in middle age.
Our results are cross-sectional and therefore cannot directly inform the causal associations between these constructs. Poor EFs may predispose individuals to experience uncontrollable negative thoughts (e.g., worry and rumination) and lead to anxiety or depression, but it is also possible that prolonged anxiety or depression episodes result in changes to the brain that affect cognitive control abilities involved in EFs. A longitudinal analysis has provided evidence for bi-directionality of associations between anxiety and cognition including processing speed, attention, and memory, suggesting that these associations may reinforce each other over time (Petkus et al., 2017), though EFs were not assessed in that study. Furthermore, the genetic variance in anxiety may increase in late life (past age 70), suggesting that these findings in middle age may differ with increasing age (Petkus, Gatz, Reynolds, Kremen, & Wetherell, 2016). To the extent that the associations among Common EF, anxiety, and depression are explained by genetic influences, shared genetic risk factors may be an underlying cause of both cognition and psychopathology.
Finally, although we examined individual differences in EFs at the level of latent variables, our assessment of anxiety and depression was more limited. The trait scale of the STAI does not assess anxiety symptoms as comprehensively as scales such as the DASS-42 or Index of Depression and Anxiety Symptoms (Lovibond & Lovibond, 1995; Watson et al., 2007), and has been criticized for assessing depression in addition to anxiety (Bieling, Antony, & Swinson, 1998; Caci et al., 2003). The CESD was designed to measure depression symptoms only in the past week. However, the CESD scores were correlated with CESD scores assessed in wave 1 of VETSA approximately 6 years earlier (r = .58, N = 1000; Franz et al., 2011), suggesting that they captured some relatively stable tendencies to experience depression symptoms. The genetic association between Common EF and depression symptoms also suggests that this association is explained in part by stable influences, especially considering the perfectly stable genetic influences on EFs over the past six years in this sample (rg = 1.0; Gustavson, Panizzon, Elman, et al., 2018). Nevertheless, it will be useful to further examine these associations with multiple measures of anxiety and depression symptoms, including those that assess tendencies for depression on longer time scales, to examine how strongly these associations are driven by current symptoms versus stable personality traits, and to model their shared and unique variance at the level of latent variables.
Conclusions
We found that EFs are associated with anxiety and depression symptoms through general sources of variance in Common EF and the genetic variance underlying anxiety and depression. These associations were observed in late middle age, a period during which many cognitive abilities begin to decline. Given that both anxiety and depression symptoms are relevant to agerelated cognitive decline and dementia (Byers & Yaffe, 2011; Gallacher et al., 2009; Petkus et al., 2017; Petkus, Reynolds, et al., 2016), and that the decline in EFs may underlie age-related decline in other cognitive abilities (Lustig et al., 2007; Persad, Abeles, Zacks, & Denburg, 2002), these results suggest that it is important to consider EFs when examining the dynamic association between anxiety, depression, and cognitive decline.
Acknowledgments
This research was supported by Grants AG050595, AG022381, and AG047903 from the National Institutes of Health.
The content of this manuscript is the responsibility of the authors and does not represent official views of NIA/NIH, or the Veterans’ Administration. Numerous organizations provided invaluable assistance in the conduct of the VET Registry, including: U.S. Department of Veterans Affairs, Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. The Cooperative Studies Program of the U.S. Department of Veterans Affairs provided financial support for development and maintenance of the Vietnam Era Twin Registry. The authors gratefully acknowledge the continued cooperation of the twins and the efforts of many staff members
Appendix
Table A1.
Phenotypic Correlations Between all Measures of the Study
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Stroop | ||||||||||
| 2. AX-CPT | 0.13 | |||||||||
| 3. Trail-Making Test | 0.16 | 0.22 | ||||||||
| 4. Category Switching | 0.13 | 0.12 | 0.15 | |||||||
| 5. Letter-Number | 0.18 | 0.25 | 0.26 | 0.12 | ||||||
| 6. Reading Span | 0.17 | 0.26 | 0.26 | 0.17 | 0.44 | |||||
| 7. Digit Span | 0.17 | 0.27 | 0.27 | 0.14 | 0.51 | 0.52 | ||||
| 8. STAI - Trait | −0.05 | −0.19 | −0.06 | −0.01 | −0.16 | −0.15 | −0.14 | |||
| 9. CESD | −0.09 | −0.21 | −0.09 | −0.02 | −0.17 | −0.21 | −0.15 | 0.73 | ||
| 10. STAI - State | −0.02 | −0.06 | −0.11 | −0.05 | −0.12 | −0.15 | −0.18 | 0.47 | 0.36 | |
| 11. AFQT | 0.23 | 0.38 | 0.26 | 0.12 | 0.37 | 0.46 | 0.38 | −0.21 | −0.25 | −0.14 |
Note: Significant correlations are displayed in bold (p < .05). AX-CPT = AX – Continuous Performance Test, STAI = State-Trait Anxiety Inventory, CESD = Center for Epidemiologic Studies – Depression scale, AFQT = Armed Forces Qualification Test.
Figure A1.
Additional twin analyses involving state anxiety. Panel A displays a Cholesky decomposition where variance in state anxiety is decomposed into genetic (A), shared environmental (C), and nonshared environmental (E) influences shared with depression and trait anxiety (ADep, CDep, EDep), shared with only trait anxiety (ATrait, CTrait, ETrait), and unique to state anxiety (AState, CState, EState). In summary, most of the variance in state anxiety was attributable to nonshard environmental influences unique to state anxiety (EState; e2 = .64). State anxiety was not significantly explained by the genetic influences shared between depression and trait anxiety (ADep), which explains why it was not significantly genetically correlated with Common EF in the modified Cholesky decomposition displayed in panel B. Significant factor loadings and correlations are displayed in bold with black lines (p < .05).
Footnotes
Due to model identification, only two of the three factors (AA+D, AAnx, ADep) can be correlated with the influences on Common EF (AEF) at a given time. For genetic influences, we chose to remove the correlation between Common EF and influences unique to depression (ADep) because these influences did not explain any variance in depression (a2 = .00) even if Common EF was not included in the model. For environmental influences, we chose to remove the correlation with influences unique to anxiety (EAnx). Based on the results of the Cholesky decomposition (Table 2d), there was little reason to suspect an environmental correlation with influences unique to anxiety (total re = −.02), but it did appear that there might be an environmental correlation between Common EF and depression (total re = −.32) beyond that observed for anxiety.
Contributor Information
Daniel E. Gustavson, Department of Psychiatry, Center for Behavior Genetics of Aging, University of California, San Diego
Carol E. Franz, Department of Psychiatry, Center for Behavior Genetics of Aging, University of California, San Diego
Matthew S. Panizzon, Department of Psychiatry, Center for Behavior Genetics of Aging, University of California, San Diego
Chandra A. Reynolds, Department of Psychology, University of California, Riverside
Hong Xian, Department of Biostatistics, St. Louis University and Clinical Epidemiology Center, Veterans Affairs St. Louis Healthcare System.
Kristen C. Jacobson, Department of Psychiatry and Behavioral Neuroscience, University of Chicago
Rosemary Toomey, Department of Psychological and Brain Sciences, Boston University.
Michael J. Lyons, Department of Psychological and Brain Sciences, Boston University
William S. Kremen, Department of Psychiatry, Center for Behavior Genetics of Aging, University of California, San Diego and Center of Excellence for Stress and Mental Health, Veterans Affairs San Diego Healthcare System.
References
- Banich MT (2009). Executive function: The search for an integrated account. Current Directions in Psychological Science, 18, 89–94. doi: 10.1111/j.1467-8721.2009.01615.x [DOI] [Google Scholar]
- Bayroff AG, & Anderson AA (1963). Development of Literacy Screening Scales for AFQT 7 and 8 Failures. Washington DC: [Google Scholar]
- Berggren N, & Derakshan N (2013). Attentional control deficits in trait anxiety: Why you see them and why you don’t. Biological Psychology, 92, 440–446. doi: 10.1016/j.biopsycho.2012.03.007 [DOI] [PubMed] [Google Scholar]
- Bettis AH, Coiro MJ, England J, Murphy LK, Zelkowitz RL, Dejardins L, . . . Compas BE (2017). Comparison of two approaches to prevention of mental health problems in college students: Enhancing coping and executive function skills. J Am Coll Health, 65, 313–322. doi: 10.1080/07448481.2017.1312411 [DOI] [PubMed] [Google Scholar]
- Bieling PJ, Antony MM, & Swinson RP (1998). The State-Trait Anxiety Inventory, Trait version: structure and content re-examined. Behavior Research and Therapy, 36, 777788. [DOI] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, . . . Fox J (2011). OpenMx: An open source extended structural equation modeling framework. Psychometrika, 76, 306–317. doi: 10.1007/s11336-010-9200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, . . . Reed (2001). Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology: General, 130, 746–763. doi: 10.1037/0096-3445.130.4.746 [DOI] [PubMed] [Google Scholar]
- Bunce D, Handley R, & Gaines SO Jr. (2008). Depression, anxiety, and within-person variability in adults aged 18 to 85 years. Psychology and Aging, 23, 848–858. doi: 10.1037/a0013678 [DOI] [PubMed] [Google Scholar]
- Burton C, Campbell P, Jordan K, Strauss V, & Mallen C (2013). The association of anxiety and depression with future dementia diagnosis: A case-control study in primary care. Family Practice, 30, 25–30. doi: 10.1093/fampra/cms044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL, & Yaffe K (2011). Depression and risk of developing dementia. Nat Rev Neurol, 7, 323–331. doi: 10.1038/nrneurol.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caci H, Bayle FH, Dossios C, Robert P, & Boyer P (2003). The Spielberger trait anxiety inventory measures more than anxiety. European Psychiatry, 18, 394–400. doi: 10.1016/j.eurpsy.2003.05.003 [DOI] [PubMed] [Google Scholar]
- Calkins AW, McMorran KE, Siegle GJ, & Otto MW (2015). The effects of computerized cognitive control training on community adults with depressed mood. Behavioural and cognitive psychotherapy, 43, 578–589. doi: 10.1017/S1352465814000046 [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, . . . Moffitt TE (2014). The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science, 2, 119–137. doi: 10.1177/2167702613497473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA, & Strauss E (2006). Structure of four executive functioning tests in healthy older adults. Neuropsychology, 20, 206–214. doi: 10.1037/0894-4105.20.2.206 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan executive function system (D-KEFS): Psychological Corporation. [Google Scholar]
- Edwards EJ, Edwards MS, & Lyvers M (2015). Cognitive trait anxiety, situational stress, and mental effort predict shifting efficiency: Implications for attentional control theory. Emotion, 15, 350–359. doi: 10.1037/emo0000051 [DOI] [PubMed] [Google Scholar]
- Eley TC, & Stevenson J (1999). Exploring the covariation between anxiety and depression symptoms: A genetic analysis of the effects of age and sex. Journal of Child Psychology and Psychiatry and Allied Disciplines, 40, 1273–1282. doi:Doi 10.1017/S0021963099004734 [DOI] [PubMed] [Google Scholar]
- Elman JA, Jak AJ, Panizzon MS, Tu X, Chen T, Reynolds CA, . . . Kremen WS (2018). Under-diagnosis of MCI in longitudinal studies: The problem of not accounting for practice effects. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, In press. doi: 10.1016/j.dadm.2018.04.003 [DOI] [Google Scholar]
- Endler NS, Cox BJ, Parker JD, & Bagby RM (1992). Self-reports of depression and state-trait anxiety: Evidence for differential assessment. Journal of Personality and Social Psychology, 63, 832–838. [DOI] [PubMed] [Google Scholar]
- Engelhardt LE, Briley DA, Mann FD, Harden KP, & Tucker-Drob EM (2015). Genes unite executive functions in childhood. Psychological Science, 26, 1151–1163. doi: 10.1177/0956797615577209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt LE, Mann FD, Briley DA, Church JA, Harden KP, & Tucker-Drob EM (2016). Strong genetic overlap between executive functions and intelligence. Journal of Experimental Psychology: General, 145, 1141–1159. doi: 10.1037/xge0000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels AS, Heller W, Spielberg JM, Warren SL, Sutton BP, Banich MT, & Miller GA (2010). Co-occurring anxiety influences patterns of brain activity in depression. Cognitive, Affective, & Behavioral Neuroscience, 10, 141–156. doi: 10.3758/CABN.10.1.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, & Derakshan N (2011). New perspectives in attentional control theory. Personality and Individual Differences, 50, 955–960. doi: 10.1016/j.paid.2010.08.019 [DOI] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, & Calvo MG (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7, 336–353. doi: 10.1037/1528-3542.7.2.336 [DOI] [PubMed] [Google Scholar]
- Franz CE, Lyons MJ, O’Brien R, Panizzon MS, Kim K, Bhat R, . . . Kremen WS (2011). A 35-year longitudinal assessment of cognition and midlife depression symptoms: The Vietnam Era Twin Study of Aging. The American Journal of Geriatric Psychiatry, 19, 559–570. doi: 10.1097/JGP.0b013e3181ef79f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, du Pont A, Corley RP, & Hewitt JK (2018). Longitudinal relations between depressive symptoms and executive functions from adolescence to early adulthood: A twin study. Clinical Psychological Science, 216770261876636. doi: 10.1177/2167702618766360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, & Miyake A (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. doi: 10.1016/j.cortex.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Altamirano LJ, Corley RP, Young SE, Rhea SA, & Hewitt JK (2016). Stability and change in executive function abilities from late adolescence to early adulthood: A longitudinal twin study. Developmental Psychology, 52, 326–340. doi: 10.1037/dev0000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, & Hewitt JK (2008). Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General, 137, 201–225. doi: 10.1037/0096-3445.137.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher J, Bayer A, Fish M, Pickering J, Pedro S, Dunstan F, . . . Ben-Shlomo Y (2009). Does anxiety affect risk of dementia? Findings from the caerphilly prospective study. Psychosomatic Medicine, 71, 659–666. doi: 10.1097/PSY.0b013e3181a6177c [DOI] [PubMed] [Google Scholar]
- Golden CJ, & Freshwater SM (2002). The Stroop color and word test: A manual for clinical and experimental uses[adult version]: Stoelting. [Google Scholar]
- Gustavson DE, Altamirano LJ, Johnson DP, Whisman MA, & Miyake A (2017). Is set shifting really impaired in trait anxiety? Only when switching away from an effortfully established task set. Emotion, 17, 88–101. doi: 10.1037/emo0000212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson DE, & Miyake A (2016). Trait worry is associated with difficulties in working memory updating. Cognition and Emotion, 30, 1289–1303. doi: 10.1080/02699931.2015.1060194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson DE, Panizzon MS, Elman JA, Franz CE, Reynolds CA, Jacobson KC, . . . Kremen WS (2018). Stability of genetic and environmental influences on executive functions in midlife. Psychology and Aging, 33, 219–231. doi: 10.1037/pag0000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson DE, Panizzon MS, Franz CE, Friedman NP, Reynolds CA, Jacobson KC, . . . Kremen WS (2018). Genetic and environmental architecture of executive functions in midlife. Neuropsychology, 32, 18–30. doi: 10.1037/neu0000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson DE, Stallings MC, Corley RP, Miyake A, Hewitt JK, & Friedman NP (2017). Executive functions and substance use: Relations in late adolescence and early adulthood. Journal of Abnormal Psychology, 126, 257–270. doi: 10.1037/abn0000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, & Zacks RT (1988). Working memory, comprehension, and aging: A review and a new view. Psychology of Learning and Motivation, 22, 193–225. [Google Scholar]
- Herd SA, O’Reilly RC, Hazy TE, Chatham CH, Brant AM, & Friedman NP (2014). A neural network model of individual differences in task switching abilities. Neuropsychologia, 62, 375–389. doi: 10.1016/j.neuropsychologia.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JD, Heller W, Mohanty A, Engels AS, Banich MT, Webb AG, & Miller GA (2010). Localization of asymmetric brain function in emotion and depression. Psychophysiology, 47, 442–454. doi: 10.1111/j.1469-8986.2009.00958.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hink LK, Rhee SH, Corley RP, Cosgrove VE, Hewitt JK, Schulz-Heik RJ, . . . Waldman ID (2013). Personality dimensions as common and broadband-specific features for internalizing and externalizing disorders. Journal of Abnormal Child Psychology, 41, 939–957. doi: 10.1007/s10802-013-9730-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, & Pizzagalli DA (2007). Task feedback effects on conflict monitoring and executive control: Relationship to subclinical measures of depression. Emotion, 7, 68–76. doi: 10.1037/1528-3542.7.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, & Bentler PM (1998). Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods, 3, 424–453. doi: 10.1037//1082-989x.3.4.424 [DOI] [Google Scholar]
- Ito TA, Friedman NP, Bartholow BD, Correll J, Loersch C, Altamirano LJ, & Miyake A (2015). Toward a comprehensive understanding of executive cognitive function in implicit racial bias. Journal of Personality and Social Psychology, 108, 187–218. doi: 10.1037/a0038557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine R, Martin N, Henderson A, & Rao D (1984). Genetic covariation between neuroticism and the symptoms of anxiety and depression. Genetic Epidemiology, 1, 89–107. doi: 10.1002/qepi.1370010202 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Martin NG, & Eaves LJ (1987). Symptoms of anxiety and symptoms of depression. Same genes, different environments? Archives of General Psychiatry, 44, 451–457. doi: 10.1001/archpsyc.1987.01800170073010 [DOI] [PubMed] [Google Scholar]
- Kendler KS, & Myers J (2014). The boundaries of the internalizing and externalizing genetic spectra in men and women. Psychological Medicine, 44, 647–655. doi: 10.1017/S0033291713000585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, & Eaves LJ (1992). Major depression and generalized anxiety disorder: Same genes, (partly) different environments? Archives of General Psychiatry, 49, 716–722. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Franz CE, & Lyons MJ (2013). VETSA: The Vietnam Era Twin Study of Aging. Twin Research and Human Genetics, 16, 399–402. doi: 10.1017/thg.2012.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Moore CS, Franz CE, Panizzon MS, & Lyons MJ (2014). Cognition in middle adulthood In Finkel D & Reynolds CA (Eds.), Behavior genetics of cognition across the lifespan (pp. 105–134): Springer; New York. [Google Scholar]
- Kremen WS, Panizzon MS, Xian H, Barch DM, Franz CE, Grant MD, . . . Lyons MJ (2011). Genetic architecture of context processing in late middle age: More than one underlying mechanism. Psychology and Aging, 26, 852–863. doi: 10.1037/a0025098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YM, Grant MD, Franz CE, Eisen SA, . . . Lyons MJ (2006). Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA). Twin Research and Human Genetics, 9, 1009–1022. doi: 10.1375/183242706779462750 [DOI] [PubMed] [Google Scholar]
- Krueger RF, McGue M, & Iacono WG (2001). The higher-order structure of common DSM mental disorders: Internalization, externalization, and their connections to personality. Personality and Individual Differences, 30, 1245–1259. doi:Doi 10.1016/S0191-8869(00)00106-9 [DOI] [Google Scholar]
- Loehlin JC (1996). The Cholesky approach: A cautionary note. Behavior Genetics, 26, 65–69. doi:Doi 10.1007/Bf02361160 [Google Scholar]
- Lovibond PF, & Lovibond SH (1995). The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behavior Research and Therapy, 33, 335–343. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L, & Zacks RT (2007). Inhibitory deficit theory: Recent developments in a “new view” In Gorfein DS (Ed.), Inhibition in cognition (pp. 145–162). Washington, DC: American Psychological Association. [Google Scholar]
- Lyons MJ, Xian H, Toomey R, Eisen S, Goldberg J, True W, . . . Tsuang MT (1998). The overlap of depression and generalized anxiety disorder: A study of male twins. American Journal of Medical Genetics, 81, 456–456. [Google Scholar]
- Lyons MJ, York TP, Franz CE, Grant MD, Eaves LJ, Jacobson KC, . . . Kremen WS (2009). Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychological Science, 20, 1146–1152. doi: 10.1111/j.1467-9280.2009.02425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Pan PM, Hoffmann MS, Gadelha A, do Rosário MC, Mari JJ, . . . Bressan RA (2017). A general psychopathology factor (P factor) in children: Structural model analysis and external validation through familial risk and child global executive function. Journal of Abnormal Psychology, 126, 137. doi: 10.1037/abn0000205 [DOI] [PubMed] [Google Scholar]
- Martin NG, Eaves LJ, Kearsey MJ, & Davies P (1978). The power of the classical twin study. Heredity (Edinb), 40, 97–116. doi: 10.1038/hdy.1978.10 [DOI] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21, 8–14. doi: 10.1177/0963721411429458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. doi: 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Moran TP (2016). Anxiety and working memory capacity: A meta-analysis and narrative review. Psychol Bull, 142, 831–864. doi: 10.1037/bul0000051 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2010). Mplus User’s Guide: Statistical Analysis with Latent Variables: User’s Guide: Muthén & Muthén. [Google Scholar]
- Owens M, Koster EHW, & Derakshan N (2013). Improving attention control in dysphoria through cognitive training: Transfer effects on working memory capacity and filtering efficiency. Psychophysiology, 50, 297–307. doi: 10.1111/psyp.12010 [DOI] [PubMed] [Google Scholar]
- Persad CC, Abeles N, Zacks RT, & Denburg NL (2002). Inhibitory changes after age 60 and their relationship to measures of attention and memory. Journals of Gerontology Series B-Psychological Sciences and Social Sciences, 57, 223–232. doi: 10.1093/geronb/57.3.P223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ, Gatz M, Reynolds CA, Kremen WS, & Wetherell JL (2016). Stability of genetic and environmental contributions to anxiety symptoms in older adulthood. Behavior Genetics, 46, 492–505. doi: 10.1007/s10519-015-9772-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ, Reynolds CA, Wetherell JL, Kremen WS, & Gatz M (2017). Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychology and Aging. doi: 10.1037/pag0000164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ, Reynolds CA, Wetherell JL, Kremen WS, Pedersen NL, & Gatz M (2016). Anxiety is associated with increased risk of dementia in older Swedish twins. Alzheimers & Dementia, 12, 399–406. doi: 10.1016/j.jalz.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement, 1, 385–401. [Google Scholar]
- Rönnlund M, Nyberg L, Bäckman L, & Nilsson LG (2005). Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and Aging, 20, 3–18. doi: 10.1037/0882-7974.20.1.3 [DOI] [PubMed] [Google Scholar]
- Routledge KM, Burton KLO, Williams LM, Harris A, Schofield PR, Clark CR, & Gatt JM (2017). The shared and unique genetic relationship between mental wellbeing, depression and anxiety symptoms and cognitive function in healthy twins. Cognition & Emotion, 31, 1465–1479. doi: 10.1080/02699931.2016.1232242 [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, & Berish DE (2003). Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General, 132, 566–594. doi: 10.1037/0096-3445.132.4.566 [DOI] [PubMed] [Google Scholar]
- Sari BA, Koster EHW, Pourtois G, & Derakshan N (2016). Training working memory to improve attentional control in anxiety: A proof-of-principle study using behavioral and electrophysiological measures. Biological Psychology, 121, 203–212. doi: 10.1016/j.biopsycho.2016.09.008 [DOI] [PubMed] [Google Scholar]
- Satorra A, & Bentler PM (2001). A scaled difference chi-square test statistic for moment structure analysis. Psychometrika, 66, 507–514. doi: 10.1007/Bf02296192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139, 81–132. doi: 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6, 328. doi: 10.3389/fpsyg.2015.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1983). Manual for the State-Trait Anxiety Inventory STAI (form Y)(“ self-evaluation questionnaire”).
- Stollstorff M, Munakata Y, Jensen AP, Guild RM, Smolker HR, Devaney JM, & Banich MT (2013). Individual differences in emotion-cognition interactions: emotional valence interacts with serotonin transporter genotype to influence brain systems involved in emotional reactivity and cognitive control. Frontiers in Human Neurocience, 7, 327. doi: 10.3389/fnhum.2013.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18, 643–662. doi: 10.1037/0096-3445.121.1.15 [DOI] [Google Scholar]
- Tackett JL, Lahey BB, van Hulle C, Waldman I, Krueger RF, & Rathouz PJ (2013). Common genetic influences on negative emotionality and a general psychopathology factor in childhood and adolescence. Journal of Abnormal Psychology, 122, 1142–1153. doi: 10.1037/a0034151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, & Lyons MJ (2001). The Harvard Twin Study of Substance Abuse: What we have learned. Harvard Review of Psychiatry, 9, 267–279. doi: 10.1093/hrp/9.6.267 [DOI] [PubMed] [Google Scholar]
- Wagner CA, Alloy LB, & Abramson LY (2015). Trait rumination, depression, and executive functions in early adolescence. Journal of Youth and Adolescence, 44, 18–36. doi: 10.1007/s10964-014-0133-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, . . Stuart S (2007). Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychological Assessment, 19, 253–268. doi: 10.1037/1040-3590.19.3.253 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997). WMS-III: Wechsler memory scale administration and scoring manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, & Hewitt JK (2009). Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology, 118, 117–130. doi: 10.1037/a0014657 [DOI] [PMC free article] [PubMed] [Google Scholar]