Abstract
Obesity compounds the negative health effects of human immunodeficiency virus (HIV) infection. We conducted the first randomized trial of behavioral weight loss for HIV-infected patients (n = 40). Participants randomized to an Internet behavioral weight loss program had greater 12-week weight loss (mean, 4.4 ± 5.4 kg vs 1.0 ± 3.3 kg; P = .02) and improvements in quality of life than controls.
Clinical Trials Registration
Keywords: weight loss, adherence, quality of life, HIV
Obesity is increasingly prevalent in human immunodeficiency virus (HIV)–infected patients, affecting approximately 40% of HIV-infected women and 20% of HIV-infected men in the United States [1], and adds to the risk for diabetes, hypertension, and cardiovascular disease and poor quality of life. Behavioral weight loss programs are recommended for overweight and obese individuals [2] but have not been systematically studied in people living with HIV. Given the many differences between the HIV-infected population and participants in typical weight loss studies, a randomized trial testing efficacy of an empirically validated behavioral weight loss program in HIV-infected patients is needed.
METHODS
Participants
HIV-infected patients on established antiretroviral therapy regimens, with an undetectable viral load, CD4 count >200 cells/µL, age 18–70 years, body mass index ≥27 kg/m2, and access to a computer and Internet were recruited between June 2015 and June 2016 from the outpatient clinic, Immunology Center at The Miriam Hospital, via self-referral, physician referral, and inquiries from research staff. Exclusion criteria included health problems that made participation unsafe, active substance use, pregnancy, plans to move outside area, current participation in a weight loss program, and non-English-speaking status. Participants completed informed consent (approved by The Miriam Hospital institutional review board) indicating their willingness to be randomly assigned to an Internet-delivered behavioral weight loss (WTLOSS) or Internet-delivered education (CONTROL) program.
Study Design
Subjects were randomly assigned with a 1:1 allocation, using a blocked randomization and variable block size prepared by the statistician. Both WTLOSS and CONTROL began with a face-to-face session where staff and participants learned the randomization assignment and participants were introduced to their Internet program. A sample size of 17 per group was needed to detect an effect size of 1.0 (mean difference in weight loss of 4.5 ± 4.5 kg; α = .05; power ≥.8). All participants received $75 for attending the 12-week visit.
Interventions
Behavioral Weight Loss Condition
The WTLOSS program is a 12-week behavioral program delivered via the Internet that has been used in prior studies [3–5]. The program includes 12 weekly interactive multimedia lessons that teach behavioral strategies for changing diet and exercise behaviors to promote weight loss. The program prescribes a low-calorie, low-fat diet (1200–1800 kcal per day, with <30% of kcal from fat) and gradual increases in physical activity using primarily brisk walking. Participants self-monitor their weight, intake, and physical activity, and submit this information to the study website; they receive a weekly automated message providing feedback.
Control Condition
A weekly educational lesson was posted on the study website to provide basic information about healthy eating, exercise, and weight loss. No behavioral strategies for changing diet and exercise were presented. Education alone has been associated with minimal weight loss [3, 6].
Measures
Baseline measures were obtained by direct measurement, questionnaires, and chart review. Height and weight were measured at baseline and 12 weeks in light clothes and no shoes, using a wall-mounted Harpenden stadiometer and calibrated standard digital scale (Tanita BWB 800). Adherence, measured automatically via the website, was defined as number of weeks with at least 1 website log-in for both groups and number of weeks with ≥5 days of self-monitoring data for WTLOSS group. Health-related quality of life was assessed using the Centers for Disease Control and Prevention (CDC) Healthy Days Core Module (CDC HRQOL-4) and the Short Form-36 Health Survey (SF-36) [7]. Although not pre-planned, clinic records were used to examine longer-term weight changes.
Statistical Analyses
T-tests and χ2 analyses were used to compare the 2 groups at baseline. One-way and repeated-measures analysis of variance tests were used to compare the 2 groups on adherence and changes in body weight and quality of life. Weight loss analyses followed the intent-to-treat principle, with missing data replaced by baseline values (to assume no weight loss). Pearson correlations were used to evaluate variables associated with percentage of weight loss. All analyses were conducted using PASW Statistics 19 (IBM SPSS, 2009, Chicago, Illinois).
RESULTS
Participants (N = 40) were randomized (20 WTLOSS, 20 CONTROL). Postprogram assessments were completed by 92.5% of participants. Two participants (1 WTLOSS, 1 CONTROL) dropped out in the first week and 1 WTLOSS participant was lost to follow-up. Table 1 shows the baseline characteristics. The groups were similar on most measures, except CONTROL participants were older (P = .01) and had higher baseline CD4 cell counts (P = .02) than WTLOSS; subsequent analyses controlled for these differences.
Table 1.
Baseline Demographics by Group
| Variable | Full Sample (n = 40) | WTLOSS (n = 20) | CONTROL (n = 20) | P Value |
|---|---|---|---|---|
| Age, y, mean ± SD | 49.9 ± 8.8 | 46.3 ± 9.8 | 53.6 ± 6.0 | .01 |
| Sex, male | 21 (52.5) | 12 (60) | 9 (45) | .34 |
| Race | .48 | |||
| White | 27 (67.5) | 15 (75) | 12 (60) | |
| African American | 5 (12.5) | 1 (5) | 4 (20) | |
| Native American | 3 (7.5) | 1 (5) | 2 (10) | |
| Other | 5 (12.5) | 3 (15) | 2 (10) | |
| Ethnicity | .21 | |||
| Non-Hispanic | 32 (80) | 18 (90) | 14 (70) | |
| Hispanic | 6 (15) | 2 (10) | 4 (20) | |
| Did not report | 2 (5) | 0 (0) | 2 (10) | |
| Education | .70 | |||
| High school or less | 18 (45) | 10 (50) | 8 (40) | |
| Some college | 14 (35) | 7 (35) | 7 (35) | |
| College degree | 8 (20) | 3 (15) | 5 (25) | |
| Annual household income | .34 | |||
| <$20 000 | 24 (60) | 14 (70) | 10 (50) | |
| $20 000–$60 000 | 8 (20) | 4 (20) | 4 (20) | |
| >$60 000 | 6 (15) | 2 (10) | 4 (20) | |
| Did not report | 2 (5) | 0 (0) | 2 (10) | |
| Smoker | 16 (40) | 8 (40) | 8 (40) | 1.00 |
| BMI, kg/m2, mean ± SD | 34.2 ± 6.7 | 33.0 ± 5.1 | 35.4 ± 7.9 | .25 |
| CD4 count, cells/µL, mean ± SD | 742.6 ± 339.2 | 619.1 ± 313.4 | 866.1 ± 325.2 | .02 |
| Years since HIV diagnosis, mean ± SD | 11.9 ± 6.4 | 11.9 ± 5.3 | 12.0 ± 7.4 | .98 |
| ART regimen | .21 | |||
| NNRTI | 17 (42.5) | 11 (55) | 6 (30) | |
| Protease inhibitor | 9 (22.5) | 5 (25) | 4 (20) | |
| Integrase inhibitor | 12 (30) | 3 (15) | 9 (45) | |
| Protease inhibitor + integrase inhibitor | 2 (5) | 1 (5) | 1 (5) | |
| Medications | ||||
| Hypertension | 10 (25) | 4 (20) | 6 (30) | .47 |
| Diabetes | 2 (5) | 0 (0) | 2 (10) | .15 |
| Dyslipidemia | 10 (25) | 5 (25) | 5 (25) | 1.00 |
| History of substance abuse | 13 (32.5) | 8 (40) | 5 (25) | .31 |
| History of alcohol abuse | 10 (25) | 6 (30) | 4 (20) | .47 |
| History of depression | 27 (67.5) | 11 (55) | 16 (80) | .09 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CONTROL, Internet-delivered education program; HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse transcriptase inhibitor; SD, standard deviation; WTLOSS, Internet-delivered behavioral weight loss program.
Primary Outcome: Weight Loss
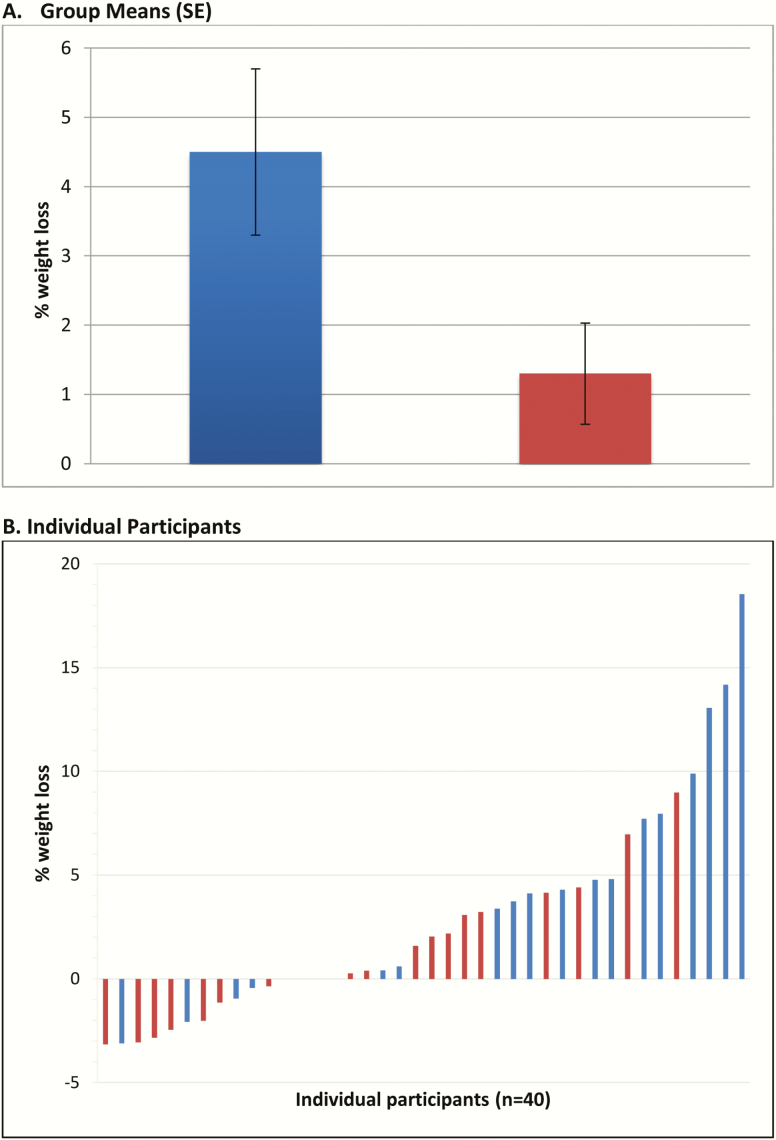
WTLOSS participants lost significantly more weight than CONTROL participants during the 12-week program (mean, –4.4 ± 5.4 kg vs –1.0 ± 3.3 kg; P = .021), which corresponded with greater percentage change in body weight (mean, –4.5% ± 5.8% vs –1.1% ± 3.3%, P = .028); controlling for age and CD4 count did not affect these results. Among completers, average weight losses were –4.9 kg vs –1.0 kg (–5.1% vs –1.2%) for WTLOSS (n = 18) and CONTROL (n = 19), respectively. The groups did not differ significantly in the percentage of participants who lost ≥5% of their body weight (30% in WTLOSS vs 10% in CONTROL; P = .11). Although there was marked variability in the weight losses (Figure 1), none of the baseline characteristics (eg, body mass index, CD4 cell count) was significantly related to weight losses. Based on clinic records, weight loss from baseline to 4–6 months after starting the program (mean, 6.3 months) was significant in the WTLOSS group (n = 17; mean, –3.8 ± 6.8 kg; P = .03), but not in controls (n = 16; mean, –1.1 ± 5.0 kg; P = .39).
Figure 1.
Percentage of body weight loss over the 12-week trial in the Internet-delivered behavioral weight loss (WTLOSS; blue) and Internet-delivered education (CONTROL; red) groups using intent-to-treat analysis. A, Group means (standard error [SE]). B, Individual participants.
Secondary Outcomes
Adherence
Both groups logged into the website frequently: mean, 8.0 ± 3.7 vs 8.5 ± 3.7 weeks in WTLOSS vs CONTROL, respectively (P = .645). Participants in WTLOSS submitted their data at least 5 days per week for (mean) 7.9 ± 4.1 of the 12 weeks. Adherence, measured by number of log-ins (r = .56, P = .01), lessons viewed (r = .66, P = .002), and submission of self-monitoring data (r = .61, P = .005) were each strongly related to weight loss in the WTLOSS arm.
Quality of Life
Participants in the WTLOSS arm were more likely to report an improvement in their overall health-related quality of life than those in the CONTROL group; in WTLOSS, 59%, 35%, and 6% reported improvements, no changes, and worsening in overall health, compared with 21%, 53%, and 26%, respectively, in CONTROL (χ2 = 6.146, P = .046). The 2 conditions did not differ on the other CDC questions. On the SF-36, WTLOSS had significant pre- to postintervention improvements in both physical function (mean, 11.5 ± 17.7; P < .02) and emotional function (mean, 9.7 ± 18.5; P < .05), whereas changes in CONTROL were not significant. Weight loss in WTLOSS was correlated with changes in reported overall health and emotional function (r = .35 and .40, P < .05).
DISCUSSION
We report the results of the first randomized trial testing the effects of an empirically validated behavioral weight loss program for overweight/obese HIV-infected patients. Participants adhered well to the Internet-delivered program, 92% completed the trial, and weight loss was significantly greater in WTLOSS than in CONTROL. Moreover, 59% of participants in the WTLOSS intervention reported improvements in overall health compared to 20% in CONTROL.
Weight losses achieved in this trial (mean, 4.4 kg) were similar to those reported in a previous trial (mean, 5.5 kg) [3] using this same Internet program with non-HIV-infected patients; adherence was also similar (data submitted on 6.7 weeks [3] vs 7.9 in this trial). In addition, we confirmed the strong relation between adherence and weight loss. The results achieved are particularly impressive given the many barriers to adherence in this patient population, including their low annual income, history of depression, alcohol and substance abuse, and complex medical regimen (Table 1).
Although the WTLOSS group had significant weight losses, there was a great deal of variability in outcome and only 30% of patients meeting the “clinically significant” 5% weight loss criterion. Baseline characteristics did not predict outcome, but adherence to the program did. These findings are consistent with the general literature on behavioral weight loss interventions [6, 8, 9]. Future studies of weight loss for HIV-infected patients should consider ways to improve adherence to the Internet program and evaluate the cost-effectiveness of other more intensive approaches to weight loss (eg, phone-based).
Strengths of this study include its randomized controlled design, intent-to-treat analysis, and high retention rate (92%). Inclusion criteria were broad, allowing us to recruit a diverse sample, with approximately equal numbers of men and women; needing Internet access did not lead to any exclusions. The program not only led to weight loss, but this weight loss was associated with improvements in health-related quality of life. Further, because the program is delivered via the Internet and is entirely automated, it could easily be disseminated to other HIV-infected patients seeking to lose weight. The study also has limitations. Although powered for the primary outcome of weight loss, the sample size was small and was drawn from 1 clinical site, and all participants volunteered to be in a clinical weight loss trial, thus limiting generalizability. Future studies should include larger sample and longer follow-up.
In conclusion, this study suggests that an Internet-delivered behavioral weight loss program may be an effective approach to promoting weight loss in people living with HIV. Given that weight loss provides an actionable approach to comorbidities associated with HIV infection and obesity, further research on the efficacy of behavioral weight loss interventions for changing both weight and improving health in this population is clearly needed.
Notes
Financial support. This project was supported by the Providence/Boston Center for AIDS Research (grant number P30 A1042853); the ACTG Clinical Research Site 2951 (The Miriam Hospital; grant number UM1AI069412); and the Cardiovascular Behavioral and Preventive Medicine Training Grant (grant number KB T32 HL076134).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Thompson-Paul AM, Wei SC, Mattson CL, et al. Obesity among HIV-infected adults receiving medical care in the United States: data from the cross-sectional medical monitoring project and national health and nutrition examination survey. Medicine (Baltimore) 2015; 94:e1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jensen MD, Ryan DH, Apovian CM, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the obesity society. J Am Coll Cardiol 2014; 63:2985–3023. [DOI] [PubMed] [Google Scholar]
- 3. Thomas JG, Leahey TM, Wing RR. An automated internet behavioral weight-loss program by physician referral: a randomized controlled trial. Diabetes Care 2015; 38:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leahey TM, Thomas G, Fava JL, et al. Adding evidence-based behavioral weight loss strategies to a statewide wellness campaign: a randomized clinical trial. Am J Public Health 2014; 104:1300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ross KM, Wing RR. Implementation of an internet weight loss program in a worksite setting. J Obes 2016; 2016:9372515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wadden TA, West DS, Neiberg RH, et al. ; Look AHEAD Research Group One-year weight losses in the look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009; 17:713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30:473–83. [PubMed] [Google Scholar]
- 8. MacLean PS, Wing RR, Davidson T, et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015; 23:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Unick JL, Leahey T, Kent K, Wing RR. Examination of whether early weight loss predicts 1-year weight loss among those enrolled in an Internet-based weight loss program. Int J Obes (Lond) 2015; 39:1558–60. [DOI] [PMC free article] [PubMed] [Google Scholar]