Abstract
Advances in understanding the biological bases of aging have intellectually revitalized the field of geriatric psychiatry and broadened its scope to include promoting successful aging and studying resilience factors in older adults. To describe the process by which this paradigm shift has occurred and illustrate its implications for treatment and research of late-life brain disorders, late-life depression is discussed as a prototype case. Prior phases of geriatric psychiatry research were focused on achieving depressive symptom relief, outlining pharmacokinetic and pharmacodynamic differences between older and younger adults, and identifying moderators of treatment response. Building on this work, current geriatric psychiatry researchers have begun to disentangle the etiologic complexity in late-life depression by focusing on the causative aging-related processes involved, identifying both neurobiological and behavioral intermediates, and finally delineating depression subtypes that are distinguishable by their underlying biology and the treatment approach required. In this review, we discuss several age-related processes that are critical to the development of late-life mood disorders, outline implications of these processes for the clinical evaluation and management of later-life psychiatric disorders, and finally put forth suggestions for better integrating aging and developmental processes into the National Institute of Mental Health’s Research Domain Criteria.
Keywords: Brain aging, Depression, Frailty, Successful aging
But an old age serene and bright,
And lovely as a Lapland night,
Shall lead thee to thy grave.
William Wordsworth, “To a Young Lady,” 1805
Psychiatry’s perspective on aging and late-life brain disorders is in the midst of a transformation that may soon render the term “geriatric psychiatry” obsolete. The subspecialty originated to address mental infirmities that were believed to result from the aging process, causing the term “geriatric” to be associated with senility and disease. Rather than endeavoring to ameliorate illnesses that affected older people, aging itself became the problem as physicians girded hospitals against the threat of “society [being] saddled with the care of…” “enfeebled,” “agitated,” and “helpless” aged patients (1). Tellingly, the inaugural issue of the first geriatric psychiatry journal (Journal of Geriatric Psychiatry, first published in 1967) featured a clinical case presentation titled “The Loneliness and Death of an Old Man” (2). Even as late as 1993, exhortations to undertake geriatric psychiatry training were not based on the inherent interest of aging-related processes, but rather were utilitarian calls to arms against an impending avalanche of needy elders (3).
Only recently has this impoverished conceptualization of aging been challenged, as neuroscientific advances permitted increased understanding of neurogenesis, synaptic plasticity, and other mechanisms of brain maturation. This evolution has intellectually revitalized research into late-life brain disorders and broadened geriatric psychiatry’s scope to include promoting brain health and maximizing mental functioning throughout the life span (4), a development that mirrors earlier shifts in geriatric medicine to promote maximal health span (eg, Fries’ compression of morbidity in 1980) (5). Late-life psychiatric disorders are increasingly recognized as the products of complex interactions between psychopathology and aging processes affecting brain structure and function. To study these interactions, geriatric mental health researchers have embraced an interdisciplinary approach spanning genetics, molecular biology, cellular physiology, and immunology in addition to maintaining traditional collaborations with geriatric medicine and neurology. Rather than focusing on a narrow age range (ie, older than 65), the field has adopted a developmental, life-span orientation in recognition of the fact that processes relevant to late-life disorders often begin in midlife and may be most therapeutically tractable in their early phases. As a result of these perspectival shifts, it is now the fascinating topic of geroscience, as opposed to pragmatic arguments about the health care needs of an aging population, that attracts practitioners and investigators to the “new” geriatric psychiatry.
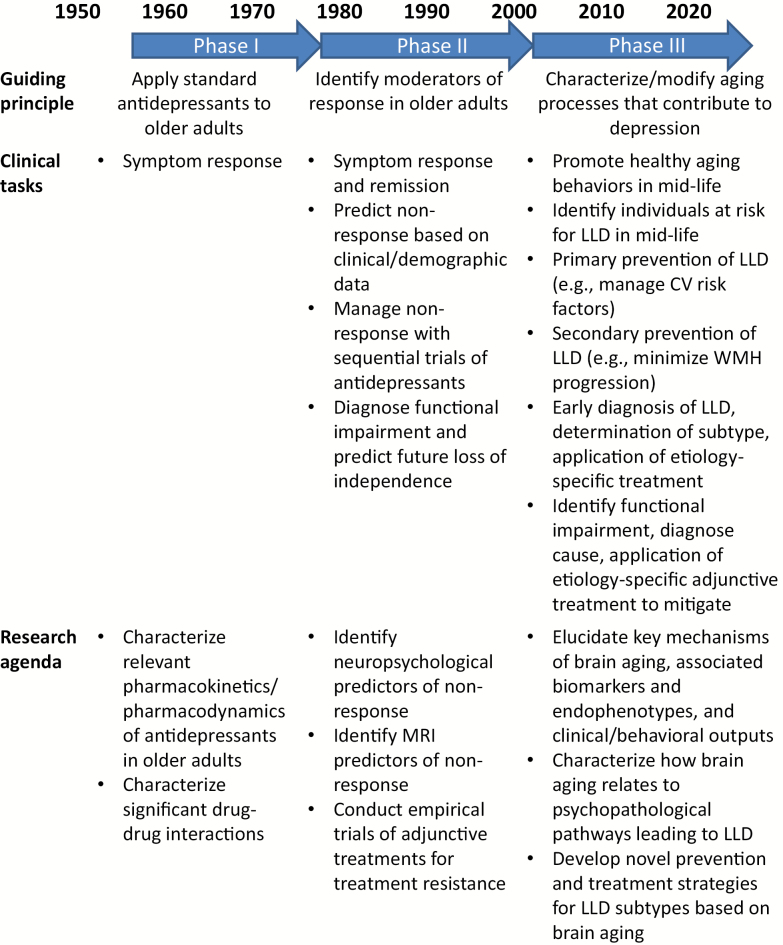
To describe this paradigm shift and illustrate its profound implications for the research and treatment of late-life brain disorders, we discuss depression in older adults as a prototype case and contrast the theoretical principles, clinical goals, and research agendas of successive models of late-life depression (LLD) emerging from the literature (see Figure 1). Rather than constituting a unitary phenomenon, depression in older adults increasingly is recognized to be a heterogenous later-life neuropsychiatric syndrome caused by pathophysiologically distinct brain disorders resulting in common behavioral manifestations. This is perhaps akin to how dementia may result from Alzheimer’s disease, vascular dementia, Lewy body dementia, or frontotemporal dementia, albeit with notable differences in neuropathology and initial clinical presentation. Focusing on the causative aging-related processes involved has allowed contemporary researchers to disentangle the etiologic complexity of LLD, identify neurobiological and behavioral endophenotypes, and finally delineate clinical depression subtypes that are distinguishable by virtue of their underlying biology and the treatment approach required.
Figure 1.
General schematic describing how late-life depression (LLD) has been conceptualized, treated, and studied over the past 40 years.
Phase I: “Late-Life Depression”
Concomitant with the development of geriatric psychiatry more generally, interest in older depressed patients grew as the Duke University Department of Psychiatry came to prominence in the 1950s and established the nation’s first fellowship program in geriatric psychiatry. The first antidepressant clinical trial for depression in an older patient population appeared in 1959 (6), and trials were published in increasing numbers throughout the early 1960s, generally involving tests of tricyclic antidepressants and lithium salts. Journal articles describing the phenomenology and clinical care of “depressive illnesses in late life” proliferated during this period and especially into the mid-1970s (7), a decade which also witnessed the founding of the National Institute on Aging, the establishment of Veterans Health Administration’s Geriatric Research, Education and Clinical Care Centers (GRECCs), and the publication of Paul Beeson’s seminal Institute of Medicine report on “Aging and Medical Education.”
The understanding of LLD that emerges from the geriatric psychiatry literature circa 1950–1980 can be summarized in this excerpt from a journal article of the period: “Treatment of depression in the aged is not generically different from treatment of depression in younger individuals. Because of certain age-related physical, physiological, and biochemical peculiarities, however, there are specific considerations in treatment of elderly depressed patients beyond those which routinely apply to younger adults” (8). That is, the clinical task with respect to depression was symptom improvement, whether the patient was a younger adult or an older patient. It was felt this could be accomplished with the standard psychopharmacopoeia so long as pharmacokinetic (eg, altered renal and hepatic clearance and increased volume of distribution for lipid-soluble drugs) and pharmacodynamic (eg, increased sensitivity to certain classes of drugs) considerations germane to aging were taken into account. Correspondingly, the relevant research to further these clinical goals involved developing better means of identifying cases of LLD, such as by describing the psychometric properties of new diagnostic interviews and rating scales or evaluating the diagnostic value of dexamethasone suppression tests. Clinical trials of antidepressant agents known to be effective in younger adults were undertaken in older patients, and laboratory research was performed aimed at clarifying the pharmacology of psychotropic drugs in older individuals (eg, how reduced gastric motility affects drug absorption and how declining hepatocyte number and function affects tricyclic demethylation and hydroxylation).
Phase II: “Vascular Depression”
Starting in the mid-1980s and increasingly in the 1990s, the relationship of so-called “early-onset” depression (ie, major depressive disorder in a younger individual, typically peaking in prevalence in the fourth decade of life) to “late-onset” depression (ie, a first depressive episode after the age of 55 years) began to be investigated. Initial studies reported phenomenological (ie, less depressed mood and more apathy, somatic concerns, anergia, and delusions in older patients (9)), cognitive (ie, more prevalent memory disturbance and executive dysfunction in older patients (10)), and treatment differences (ie, older patients being less responsive to antidepressant medication and experiencing a more chronic clinical course (11)) based on age of depression onset. However, it was quickly appreciated that categorization of depressive subtypes based on age of onset alone was problematic, owing to the facts that (i) many individuals’ recall of prior depressive episodes is poor and (ii) that nothing prevents a patient with a history of early-onset depression from subsequently experiencing age-related changes that shift their clinical presentation to being more consistent with what is observed in late-onset depression. Consequently, the field moved toward identifying more specific markers of depression in older adults that were more directly linked to etiology. For the purposes of this discussion, the term “late-life depression” is preferred and refers to depression in an older adult (aged 55–65 years or older) without specific reference to the age of initial depressive episode onset.
One such marker, first reported by Figiel and colleagues (12) and later elaborated by Krishnan and colleagues (13) and Alexopoulos and clooeagues (14), was the presence of small cerebrovascular infarcts appearing as white matter hyperintensities (WMH) on magnetic resonance imaging (MRI) scans. WMH accumulate with increasing age, perhaps due to aging-associated arterial thickening and stiffening due to calcium deposition, endothelial dysfunction, reduction in capillary number, and decreased elasticity of small vessels (15). These observations led to the vascular depression hypothesis, which proposes that vascular lesions in deep white matter tracts disconnect prefrontal cortical regions from striatal and limbic areas, disrupting reciprocal modulation between prefrontal cortical and subcortical structures, and causing depressive symptoms (13,14). Since the neural circuits supporting executive functions originate in the dorsolateral prefrontal cortex and course to subcortical components along the striato-pallido-thalamo-cortical pathway (16), strategically located deep white matter lesions also could compromise these structures, giving rise to a depression-executive dysfunction phenotype.
After the initial proposal, a large number of subsequent experiments confirmed the primary tenets of the vascular depression hypothesis. Greater baseline WMH burden is common in depressed elders and is associated with cardiovascular risk and neuropsychological dysfunction (17). Large prospective studies demonstrated that greater WMH burden and more severe baseline executive dysfunction are associated with poor therapeutic response to antidepressants (18,19). Progression of WMH and concomitant cognitive decline may reflect worsening of the underlying vascular disease, predict a poor course of depression, and confer treatment resistance (20). Recent functional neuroimaging studies associated WMH with patterns of dysregulated prefrontal cortical-subcortical activations typical of depression (eg, limbic hyperactivity in response to emotional face stimuli) (21). This work supports diagnostic criteria for a MRI-defined vascular depression diagnosis, a concept with demonstrated validity (22).
Despite this track record, the vascular depression hypothesis is limited in its ability to explain the mechanisms of depression pathogenesis and identify new therapeutic avenues. For example, the vascular depression model suggests that individuals with risk factors for cerebrovascular disease should be identified and treated vigorously, and those with existing disease must be managed closely to prevent WMH progression. There was already ample reasons to strive for these general clinical goals (ie, preventing myocardial infarction), and the model did not provide guidance about what to do when WMH were already present. Some studies focus on cognitive symptoms of vascular depression, finding that individuals with executive dysfunction may benefit preferentially from problem solving therapy (23), but this specialized intervention proved too complex for the average clinician to deliver with fidelity. In part, these limitations stem from the fact that the vascular depression hypothesis identifies baseline predictors, or moderators, of antidepressant outcome in older adults (eg, WMH and executive dysfunction) without explaining how or why these predictors are associated with diminished antidepressant response. Such mediators of treatment outcome would be more useful guides to improving treatment outcome (24), and their identification has been the focus of the current phase in geriatric psychiatry’s conceptualization of depression in older adults.
Phase III: “Depression in the Aging Patient”
By the 2000s, studies of the genetic, molecular, and homeodynamic mechanisms of aging began to identify physiologic processes that underlay and accompanied depressive symptoms in older adults. The current phase of research in geriatric psychiatry is distinguished by its increasing use of this information to unpack the heterogeneity of LLD, identify novel therapeutic targets, and personalize treatments to the specific underlying pathology present. As a consequence, the unitary construct of “late-life depression” has yielded to more complex, but potentially therapeutically useful, conceptualizations such as the “inflamed, slowed depressed patient,” in whom chronic inflammation leads to dopaminergic neurotoxicity and psychomotor slowing. Another example linking etiological contributors with clinical symptomatology is the “frail, fatigued depressed patient,” in whom the deleterious effects of oxidative stress lead to mitochondrial dysfunction, deficient energy metabolism, and physical decline. Along with the depression-executive dysfunction phenotype reviewed earlier, these syndromes form a triumvirate of prevalent and disabling LLD subtypes (see Table 1).
Table 1.
Biomarkers and Behaviors Associated with Late-Life Depression Subtypes
| Aging Processes | Biomarkers | Behaviors | Typical Phenotype |
|---|---|---|---|
| Cerebrovascular aging | Vascular | Neuropsychological | Depression-executive dysfunction patient |
| Systolic blood pressure | Response inhibition | ||
| Pulse wave velocity | Set shifting | ||
| Vessel calcification | Initiation/perseveration | ||
| T2 FLAIR MRI | Verbal fluency | ||
| Periventricular WMH | |||
| Deep WMH | |||
| Diffusion tensor imaging | |||
| FA in frontostriatal tracts | |||
| Inflammation and dopamine depletion | Inflammatory | Neuropsychological | Inflamed, slowed patient |
| IL-6 | Processing speed | ||
| TNF-alpha | Mobility | ||
| C-reactive protein | Gait speed | ||
| Dopaminergic function | Gait variability | ||
| D1/D2 receptor density | Motor speed (pegboard) | ||
| DAT activity | |||
| Response to stimulation | |||
| Oxidative stress and mitochondrial aging | Urinary | Mental fatigability | Frail, fatigued patient |
| F2 isoprostanes | Physical fatigability | ||
| Mitochondrial respiration | Short Physical Performance Battery | ||
| VO2 Max | Pittsburgh Fatigability Scale | ||
| Enzymatic activity | Physical testing | ||
| MRS | |||
| 1H MRS lactate | |||
| 1H MRS N-acetyl aspartate | |||
| 31P MRS phosphocreatine | |||
| Note: DAT = dopamine transporter; FA = fractional anisotropy; WMH = white matter hyperintensities. | |||
Inflammation, Dopamine, and Slowing
Inflammation is a fundamental aging process that also appears to initiate a deleterious physiologic cascade leading to LLD. An age-dependent increase in interleukin-6 (IL-6) levels is one of the most consistently found immunological abnormalities in older individuals, leading IL-6 to be referred to as the “cytokine for gerontologists” (25). Peripheral IL-6 levels are typically low or undetectable in young people, begin increasing as healthy individuals exceed 50 years of age, and are often found to be very high in extremely aged adults (25). This chronic, low-grade state of inflammation of older adults was termed “inflammaging” by Franceschi and colleagues (2000) (26), and it has been associated with adverse structural and functional changes in the aging central nervous system (CNS), including the development of depression (27). In addition to inflammaging, older adults are subjects to disorders, such as the metabolic syndrome (elevated abdominal obesity, triglycerides, blood pressure, fasting glucose, and low high-density lipoprotein), that may cause additional proinflammatory shifts and further increase depression risk (28). Depressed patients express increased levels of proinflammatory cytokines and their receptors in peripheral blood and cerebrospinal fluid (29,30), and administration of inflammatory cytokines (notably interferon-alpha [IFN-alpha]) or their inducers (eg, endotoxin) causes depressive symptoms in otherwise nondepressed individuals (31).
Mounting evidence suggests that reduced dopaminergic functioning may mediate the relationship between inflammation and depression and, more generally, that inflammaging may be an important cause of the linear decline in dopaminergic functioning that is observed with increasing age (32). Peripheral proinflammatory cytokines such as IL-6 and interferon-alpha (IFN-alpha) traverse the blood brain barrier via leaky regions such as circumventricular organs, undergo carrier-mediated transport across the blood brain barrier, and indirectly cause neuro-inflammation by recruiting activated immune cells to the brain (33). Once in the CNS, proinflammatory cytokines reduce dopaminergic transmission by limiting tetrahydrobiopterin (BH4) availability and decreasing dopamine synthesis, impairing vesicular release of dopamine in presynaptic neurons by decreasing expression of vesicular monoamine transporter 2 (VMAT2), increasing dopamine transporter reuptake of synaptic neurotransmitter, and decreasing glutamate-dependent dopamine signaling (34). Multimodal neuroimaging studies in humans have shown that exogenous inflammatory cytokine administration decreases the functionality of dopamine-dependent neural circuits, such as those subserving reward processing (35,36). Elegant studies in nonhuman primates by Felger and colleagues have related this inflammation-induced dysfunction in reward circuits to reduced striatal dopamine release, which can be reversed by levodopa (37,38).
Age-associated dopamine depletion (ie, reduced dopamine levels, decreased D1/D2 receptor density, and loss of dopamine transporter (32)) may lead to the onset of depressive symptoms directly (39) or via an intermediate step, namely the development of psychomotor slowing. Decreased dopaminergic tone has been associated with slowed processing speed on diverse cognitive tasks (40) as well as decreased gait speed and cadence (steps/min) (41). In addition to these effects of inflammation on psychomotor speed that are mediated by dopaminergic circuits, increased inflammation may also cause slowing via increased joint tissue destruction with resulting arthritic pain (42). Slowed cognition and motor functioning may lead to depression by increasing stress, impairing functioning, and ultimately decreasing activity levels. We have shown that this combination of increased inflammation, decreased gait speed, and depression in older adults merits urgent recognition and clinical intervention, as it is associated with extremely high mortality rates (85.2% over 10-year follow-up, which is more than double the mortality rate for individuals without these risk factors) (43).
Oxidative Stress, Mitochondria, and Fatigue
Oxidative stress, occurring when levels of oxidants and reactive oxygen species (ROS) exceed the body’s capacity to neutralize them, is a likely contributor to aging and is integrally related to other aging processes such as inflammation (44). Numerous studies have now reported decreased antioxidant levels and increased free radical and oxidative damage product levels in depressed patients relative to controls (45). The mitochondrial respiratory chain is simultaneously the primary source of ROS generation and an important target for ROS damage (46). High levels of oxidative stress negatively affect mitochondrial DNA, resulting in point mutations and deletions that compromise mitochondrial protein synthesis, oxidative capacity, and adenosine triphosphate synthesis (47). These deleterious effects are compounded by other aging-associated changes, such as reduced mitochondrial biogenesis, defects in mitochondrial fusion and fission proteins, and accumulation of morphologically abnormal mitochondria (48).
As mitochondria produce most of the body’s energy, their age-related decline constitutes part of the biological basis for the physical and mental fatigue that is a frequent target symptom in both depressed and nondepressed elders. Maximal energy expenditure (VO2 max) decreases by approximately 10% annually starting in the third decade of life, while simultaneously the energetic demands of maintaining basal metabolism increase in older adults (49). These opposing trends result in progressively diminishing amounts of energy available for voluntary activities (50), leading to increased fatigability with even basic tasks and eventually to a restriction in physical activities (51). Reduced activity levels exacerbate the cycle by causing sarcopenia and greater reductions in mitochondrial output, further impairing endurance, and eventually resulting in functional limitations and increased mortality (52).
Excessive fatigue is not only a cardinal feature of depression but also a core characteristic of frailty, a syndrome defined by weakness, decreased activity levels, slowed gait speed, and unintentional weight loss (53). This phenomenologic confluence suggests accelerated cellular aging may represent an overlapping causal pathway for these two syndromes. Indeed, epidemiologic data suggest depression and frailty are highly interrelated, with nearly all severely depressed older adults also meeting frailty criteria (54). Symptoms of frailty represent risk factors for the development of depression, while depression predicts incident frailty (55). Co-occurrence of depression and frailty is associated with greater levels of functional impairment and more dire clinical courses compared with either syndrome alone (56). The crucial role of senescence-related physiologic factors (eg, reduced mitochondrial respiration, sarcopenia, and undernutrition) in producing this subtype of LLD may explain why it responds poorly to antidepressant medication, a treatment which does not target any of these processes, and raises the possibility of alternative therapeutic approaches (eg, exercise).
Vulnerability and Resilience Factors
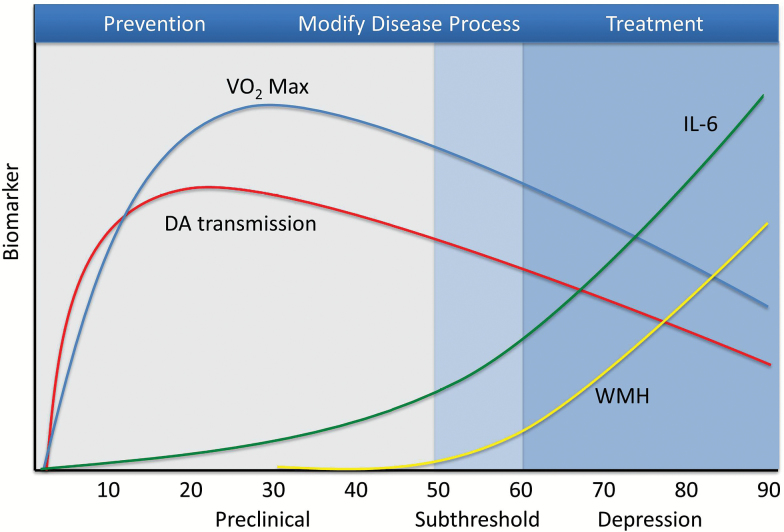
These examples suggest that aging-related processes such as vasculopathy, inflammation, and cellular senescence represent risk factors conferring increasing vulnerability for LLD across the life span (see Figure 2). Since these processes are normative to some degree and nearly universal, many individuals with milder vascular lesions or elevated levels of proinflammatory cytokines will not become depressed. Rather, a threshold may need to be crossed that results in a vulnerability to depression, and a higher threshold negatively affecting cortical circuits could be directly causal (57). Thus, individuals exhibiting “pathological” degrees of vasculopathy and inflammation (for example) are at high risk for illness, perhaps similar to how gradations of memory performance can be interpreted to gauge risk of future cognitive decline and differentiate pre-Alzheimer’s conditions from normal cognitive aging. Individuals might be classified on the basis of biomarker (eg, IL-6 levels) or behavioral (eg, gait speed) data into differing trajectories of aging, ranging from normative and healthy to unhealthy trajectories associated with premature functional decline and mortality.
Figure 2.
Aging processes and development of late-life depression over the life span. ATP = adenosine triphosphate; DA = dopamine; IL-6 = interleukin-6; VO2 max = maximal oxygen consumption; WMH = white matter hyperintensities.
It is also likely that vulnerability factors based on biological aging interact with one another as well as additional biological, psychological, and psychosocial vulnerabilities to determine whether an individual becomes depressed. According the inflammation-dopamine example above, gradual decrements in cellular function (eg, dopaminergic neurotransmission) may lead to dysfunction at higher biological scales (ventral striatal activations and functional connectivity of reward networks), eventually culminating in the emergence of behavioral alterations (psychomotor slowing) and depressive symptoms (anhedonia). Moderators may influence the trajectory of this dysregulation at each level of analysis, resulting in variability in the time course or severity of clinical symptoms related to the dopaminergic deficits. For example, a polymorphism in the catechol-o-methyl-transferase (COMT) gene (Val108/158Met) codes for different levels of enzymatic activity and results in higher (Met) or lower (Val) synaptic dopamine levels, thereby potentially exacerbating or ameliorating dopaminergic deficits (58). At a neural circuit level, dopamine-related depressive symptoms could be affected by damage to or alterations in prefrontal cortical inputs to reward circuitry or hippocampal-based memory inputs to reward learning (59).
From a psychosocial perspective, features of an individual’s environment could either exacerbate or alleviate the decline in activity levels typically observed with the development of psychomotor slowing and frailty. If no mechanisms are in place to address and reverse declining activity levels, the resultant social isolation and loneliness may accelerate development of syndromal LLD. For example, loneliness has been associated with diminished sleep duration, reduced sleep efficiency, and greater daytime fatigue, all of which could increase depression risk as well as further exacerbating subsequent loneliness (60). Loneliness also is a risk factor for cognitive decline and dementia and is associated with increased cardiovascular risk factors, heightened inflammatory responses, and increased morbidity and mortality (61). Not only could psychosocial deprivation reduce an individual’s resilience to increasing biological vulnerability over time, but also deleterious changes in an individual’s environment (eg, death of a spouse and retirement) could affect activity levels or hedonic potential and “tip” a previously stable biological vulnerability into full-blown LLD.
In this context, minimizing the impact of vulnerability factors and maximizing resilience to promote healthy aging trajectories are defining priorities for today’s geriatric psychiatrist (62). Clinical evidence now suggests that interventions to optimize social engagement (63) and increase physical activity (64) can enhance resilience to aging-related risk factors for depression. Neuroimaging studies suggest that older adults can learn new skills and employ compensatory strategies to offset cognitive declines (65), such as recruiting different brain regions to maintain task performance (66). Moreover, whereas depression is associated with reduced regional brain volumes (eg, hippocampus), animal models suggest that antidepressant treatment (67) and aerobic exercise (68) may help restore functioning in these areas via the stimulation of neurogenesis. Interventions to optimize physiologic resilience and build on positive psychological changes that occur with age (increased wisdom (69), knowledge (70), and happiness; decreased stress, worry, and anger (71)) to improve functioning and quality of life now seem to be obvious therapeutic strategies, but they were obscured in previous phases by geriatric psychiatry’s disproportionate focus on aging’s negative implications. Rather than focusing on the lower relative prevalence of depression in older age and seeking to understand the protective factors accounting for that fact (ie, what is adaptive about aging), for example, previous research focused on predictors of nonresponse to antidepressants (ie, what is “wrong” with older adults).
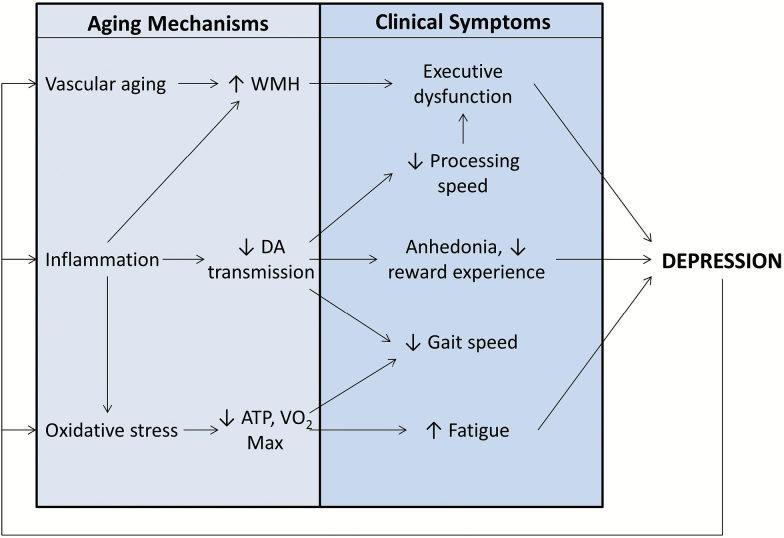
Finally, it should be noted that the relationship between aging and mental health is reciprocal, and disorders such as depression may leave biological “scarring” that accelerates aging (see Figure 3) (72). In the case of depression, chronic stress and hypothalamic–pituitary–adrenal axis activation may cause increased “wear and tear” on the body (73). For example, telomeres are repeating segments of deoxyribose nucleic acid found at the ends of chromosomes that help preserve genomic integrity by buffering against the small regions at the end of chromosomes that go uncopied during mitosis (74). Age-adjusted decreased telomere length is associated with increased incidence of age-related diseases, including coronary heart disease, heart failure, diabetes, increased cancer risk, and osteoporosis (75), and shortened telomeres confer a significantly increased risk of mortality (76). Initial studies suggest that lifetime depression exposure is associated with premature telomere shortening, even after accounting for related behaviors (eg, diet and smoking) that also influence telomere length (77).
Figure 3.
Reciprocal interactions between late-life depression and biological aging processes. ATP = adenosine triphosphate; DA = dopamine; VO2 max = maximal oxygen consumption; WMH = white matter hyperintensities.
Toward an Aging-Informed Management of Depression
From an aging perspective, the optimal clinical management of LLD must begin in midlife or earlier. The actual onset of depressive symptoms is likely to be accompanied by structural and functional CNS changes that confer treatment resistance, lead to functional impairment, and may be irreversible. Consequently, geriatric psychiatrists must work with medical and general psychiatric colleagues to pursue effective primary and secondary prevention strategies as patients enter their 30s and 40s rather than wait for patients to reach 65 years of age. The relevant biological data (hemoglobin A1c, serum cholesterol, C-reactive protein [CRP], and blood pressure) will already be available for many patients, such that individuals with incipient cardiovascular disease, high levels of proinflammatory cytokines, and diminished energy metabolism, could be identified and treated more vigorously in order to prevent worsening depression, gait and mobility deficits, and cognitive impairment (78,79). Such prevention strategies are already being studied in LLD, including vitamin D or omega-3 fatty acid supplementation, interventions that may reduce inflammation (80). Broader, lifestyle-focused strategies such as exercise, education, and maintaining active lifestyles should be advocated to help minimize inflammation, reduce oxidative stress, and increase physiologic and cognitive reserve (81). Such preventive strategies are mostly associated with small effect sizes and likely will never remove the need for effective treatment strategies, but even small reductions in depression risk could be highly significant from a public health perspective given the prevalence and potential lethality of LLD.
As ongoing research links biological aging to important mental health outcomes, metrics of aging and age-appropriate functional levels will need to be added to the clinical assessment of mid- and later-life individuals. Recent studies have begun to quantify biological aging in younger adults by constructing composite indices from a number of biomarkers and then proceeded to associate biological age with cognitive and physical decline (82). The establishment of norms may permit the clinical application of such indices, such as for providing prognostic information or targeting interventions to at risk populations. In addition to assessment of cardiovascular health, which is already routine, it is arguable that brain MRI should be performed in depressed adults with cardiovascular risk factors to document the presence, distribution, and severity of WMH. A relatively brief but thorough neuropsychological assessment can be performed with the National Institute of Health Toolbox Cognition Battery (83,84). Office-based assessment of physical function with tools such as the Short Physical Performance Battery allow the quantification of gait speed, determination of whether frailty criteria are met, and risk stratification for future disability and mortality (85). Finally, inflammatory status can be measured inexpensively with serum CRP, and noninvasive measures of oxidative stress (ie, urinary F2 isoprostanes) can be considered.
Antidepressant medication, extant adjunctive pharmacologic treatments, and evidence-based psychotherapeutic modalities remain important in the treatment of later-life depression due to the significant minority of individuals responding to these treatments. More efforts must be made to improve the availability of these treatments for older adults, who significantly underutilize mental health services and are rarely if ever seen by specialty mental health providers (86). However, the great promise of the new aging orientation in geriatric psychiatry lies in designing novel therapeutics tailored to the specific underlying pathology of individual patients. For example, dopamine receptor agonists (eg, piribedil, pramipexole, and ropinirole), stimulants (methylphenidate and amphetamine derivatives), and dopamine precursors such as levodopa may enhance dopaminergic function, ameliorate slowing, and treat depressive symptoms (87,88). For those who can tolerate and comply with it, exercise is a useful treatment for fatigability and mood symptoms in patients with co-occurring frailty and depression (89). Novel therapeutics to address mitochondrial dysfunction are actively being studied (eg, induction of peroxisome proliferator-activated receptor [PPAR]-γ-coactivator 1α [PGC-1α] (90) and NAD-dependent deacetylase sirtuin-1 [SIRT 1] activators (91)). Key to the success of these treatments will be targeting them to specific subgroups of older depressed patients having the relevant pathophysiology (eg, exercise for frail, fatigued patients and anti-inflammatory treatment for patients with high cytokine levels). Such personalized medicine may help avoid repeating previous failed efforts, which often tested the efficacy of new therapies in broadly defined and heterogenous samples of depressed patients.
Directions for Future Research With Application to RDoC
Efforts to develop improved prevention, early detection, and rationally designed treatment strategies for late-life disorders have traditionally been hampered by unsuccessful attempts to generalize research findings in younger populations to older adults. The current generation of research in geriatric psychiatry is remedying these mistakes by seeking to understand the complex interplay between aging-related processes and the pathophysiology underlying psychiatric disorders. This research agenda could be facilitated by ensuring aging and developmental processes are integrated into the National Institute of Mental Health’s (NIMH) new conceptual framework guiding research on psychiatric disorders, known as the Research Domain Criteria (RDoC). The initial deployment of RDoC construed developmental and environmental variables as orthogonal factors to the core domains and constructs articulated (92). However, as observers from the field of child and adolescent psychiatry have noted (93), the difficulties inherent to visually representing three- and four-dimensional RDoC matrices have resulted in a general neglect of developmental factors, and the influence of senescence and aging as specific types of developmental factors has been even less well appreciated. This circumstance is unfortunate given the critical importance of stimulating research into Aging × Psychopathology interactions, and a number of potential remedies are available.
First, it is important to expand RDoC to include physical domains that are clearly related to both cognitive processes and clinical outcomes. For example, defining a new domain termed “Mobility” could enhance RDoC’s relevance to mental disorders in older adults and promote research on interactions between central and peripheral processes. The general neglect of bodily processes in RDoC to date is understandable given its impetus was to identify behavioral processes that are fundamental to mental disorders and amenable to neuroscientific approaches. It is also true that RDoC’s scope must be limited, since RDoC qua psychiatric nosology cannot encompass all of medicine. However, a new domain of Mobility makes sense given that RDoC’s disembodiment greatly limits its applicability to older adults and that constructs such as gait exhibit significant, reciprocal relationships to mental disorders (eg, depression and mild cognitive impairment) and other RDoC domains (eg, cognitive systems) (94). Although many “nonpsychiatric” factors influence gait, walking requires cognitive processing (eg, multisensory integration, spatial awareness, and proprioception) that is evaluable at the levels of analysis required for RDoC constructs (eg, cells, circuits, and physiology) (95). The research-stimulating effect of a new “Mobility” construct is likely to be considerable, as has been the case for a similar concept proposed by Verghese and colleagues (2013) known as motoric cognitive risk syndrome (96,97). Nearly 20 publications over the past 3 years have investigated the epidemiologic characteristics, neural underpinnings, and health consequences of motoric cognitive risk syndrome, leading to a rapidly expanding body of knowledge.
Second, new constructs relevant to aging should be added to existing RDoC domains. For example, processing speed could be added as a subconstruct of the Cognitive Control construct within the Cognitive Systems domain. Processing speed influences nearly all cognitive tasks due to the limited time available to perform a given task and because degradation of information over time may cause the products of early processing to be lost by the time later processing is completed (98). Processing speed mediates performance on measures of verbal reasoning, fluency, and knowledge, and in many studies measures of working memory primarily depend upon speed of processing performance (99). Recently, functional MRI studies have begun to elucidate the neural circuitry underlying processing speed as a cognitive capacity (100), and, as reviewed earlier, processing speed has been repeatedly associated with clinically significant problems such as depression and executive dysfunction (101).
The concept of fatigability, which anchors an individual’s perceived fatigue to a specific activity having measurable intensity and duration (102), is central to the mental health and well-being of older adults, and yet it is notably missing from the current RDoC matrix. Performance-based measures of fatigability have been developed that demonstrate concurrent validity (103), and self-report rating scales are available whose results have been shown to be associated with performance as well as perceived exertion (104). Neuroimaging studies of subjects performing cognitively demanding tasks have begun to approach mental fatigue by investigating time-on-task effects, in which subject performance declines over the period of task engagement (105). Activation of frontoparietal attention networks is decreased by the performance of fatiguing tasks, and the magnitude of deactivation correlates with performance decrements (ie, reaction time). Similarly, the use of motor imagery paradigms in which subjects mentally simulate physical movement in the scanner has documented increased activation of compensatory brain regions supporting motor function in older adults as well as increased activation of hippocampal and medial temporal lobe regions related to topographical memory and mental navigation (106). A construct of fatigability would fit best within the Arousal and Regulatory Systems domain given its ultimate biological basis in energy dynamics, though it is also clearly related to the subconstruct of willingness to work within the Positive Valence Systems domain.
Conclusions
The purpose of this review has been to document geriatric psychiatry’s ongoing evolution from a static discipline concerned with the care of enfeebled elders to a more dynamic enterprise leveraging the science of aging to develop novel treatments for late-life brain disorders and lengthen the effective health span of older adults. Space considerations as well as the current incomplete state of knowledge about the different neurobiological pathways leading to LLD have unavoidably limited this discussion to a selective overview of this single disorder. Although the specific pathways (vascular, inflammation/slowing, and mitochondrial senescence) to LLD discussed are among the most prevalent, disabling, and best-supported by empirical evidence, additional mechanisms of aging other than those discussed (eg, neurodegenerative processes and aging effects on other monoamine systems) are undoubtedly relevant to depression pathogenesis and may eventually be integrated with the pathologic processes outlined earlier. The limited scope of this discussion also prohibited review of other late-life psychiatric disorders that are also are being reconceptualized in light of insights into the biology of aging (eg, post-traumatic stress disorder), leading to new research approaches and clinical possibilities. Nonetheless, we hope this review has served a useful purpose by highlighting the science of aging’s unfolding impact on psychiatric research and treatment. The manner in which late-life disorders are conceptualized, the priorities for clinical treatment, and the types of interesting research questions to be pursued will all change as “Geriatric Psychiatry” yields to the new field of “Brain Aging and Mental Health.” Clinicians, researchers, and patients all stand to benefit from the richer perspective afforded by this paradigm shift.
Funding
Work on this paper was supported by R01 MH102293 (B.R.R.), R01 MH102246 (W.D.T.), R21 MH099218 (W.D.T.), and T32 MH015144 (S.P.R.). B.R.R. reports receiving consulting fees from Pfizer.
Conflict of Interest
W.D.T., P.J.B., J.R.S., and S.P.R. have no disclosures to report. This paper has not been previously presented.
References
- 1. Group for the Advancement of Psychiatry. Report No. 14, The Problem of the Aged Patient in the Public Psychiatry Hospital. New York: Group for the Advancement of Psychiatry; 1950. [Google Scholar]
- 2. Da Silva G. The loneliness and death of an old man: three years’ psychotherapy of an eighty-one-year-old depressed patient. J Geriatr Psychiatry. 1967;1:5–27. [Google Scholar]
- 3. Small GW. Geriatric psychiatry fellowship recruitment: crisis or opportunity? Am J Geriatr Psychiatry. 1993;1:67–73. [DOI] [PubMed] [Google Scholar]
- 4. Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14:6–20. doi:10.1097/01.JGP.0000192501.03069.bc [DOI] [PubMed] [Google Scholar]
- 5. Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–135. doi:10.1056/NEJM198007173030304 [DOI] [PubMed] [Google Scholar]
- 6. Mouratoff GJ, Grossman AJ, Batterman RC. Effectiveness and tolerance of nialamide for geriatric patients. Dis Nerv Syst. 1959;20(suppl):38–40. [PubMed] [Google Scholar]
- 7. Post F. The management and nature of depressive illnesses in late life: a follow-through study. Br J Psychiatry. 1972;121:393–404. [DOI] [PubMed] [Google Scholar]
- 8. Fann WE. Pharmacotherapy in older depressed patients. J Gerontol. 1976;31:304–310. [DOI] [PubMed] [Google Scholar]
- 9. Krishnan KR, Hays JC, Tupler LA, George LK, Blazer DG. Clinical and phenomenological comparisons of late-onset and early-onset depression. Am J Psychiatry. 1995;152:785–788. doi:10.1176/ajp.152.5.785 [DOI] [PubMed] [Google Scholar]
- 10. Brodaty H, Luscombe G, Parker G, et al. Early and late onset depression in old age: different aetiologies, same phenomenology. J Affect Disord. 2001;66:225–236. [DOI] [PubMed] [Google Scholar]
- 11. Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56:713–718. [DOI] [PubMed] [Google Scholar]
- 12. Figiel GS, Krishnan KR, Doraiswamy PM, Rao VP, Nemeroff CB, Boyko OB. Subcortical hyperintensities on brain magnetic resonance imaging: a comparison between late age onset and early onset elderly depressed subjects. Neurobiol Aging. 1991;12:245–247. [DOI] [PubMed] [Google Scholar]
- 13. Krishnan KRR, McDonald WM: Arteriosclerotic depression. Med Hypotheses. 1995; 44:77–145. [DOI] [PubMed] [Google Scholar]
- 14. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562–565. [DOI] [PubMed] [Google Scholar]
- 15. North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Research. 2012;110:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alexopoulos GS. “The depression-executive dysfunction syndrome of late life”: a specific target for D3 agonists? Am J Geriatr Psychiatry. 2001;9:22–29. [PubMed] [Google Scholar]
- 17. Aizenstein HJ, Khalef A, Walker SE, Andreescu C. MRI predictors of treatment response in late-life depression. J Geriatr Psychiatry Neurol. 2014;27:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gunning-Dixon FM, Walton M, Cheng J, et al. MRI signal hyperintensities and treatment remission of geriatric depression. J Affect Disord. 2010;126:395–401. doi:10.1016/j.jad.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–285. doi:10.1001/archgenpsychiatry.2009.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor WD, Steffens DC, MacFall JR, et al. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003;60:1090–1096. doi:10.1001/archpsyc.60.11.1090 [DOI] [PubMed] [Google Scholar]
- 21. Aizenstein HJ, Andreescu C, Edelman KL, et al. fMRI correlates of white matter hyperintensities in late-life depression. Am J Psychiatry. 2011;168:1075–1082. doi:10.1176/appi.ajp.2011.10060853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sneed JR, Rindskopf D, Steffens DC, Krishnan KR, Roose SP. The vascular depression subtype: evidence of internal validity. Biol Psychiatry. 2008;64:491–497. doi:10.1016/j.biopsych.2008.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Areán PA, Raue P, Mackin RS, Kanellopoulos D, McCulloch C, Alexopoulos GS. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. Am J Psychiatry. 2010;167:1391–1398. doi:10.1176/appi.ajp.2010.09091327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kraemer HC. Messages for clinicians: moderators and mediators of treatment outcome in randomized controlled trials. Am J Psychiatry. Advance online publication. doi:10.1176/appi.ajp.2016.15101333 [DOI] [PubMed] [Google Scholar]
- 25. Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–181. [DOI] [PubMed] [Google Scholar]
- 26. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–254. [DOI] [PubMed] [Google Scholar]
- 27. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi:10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koponen H, Jokelainen J, Keinänen-Kiukaanniemi S, Kumpusalo E, Vanhala M. Metabolic syndrome predisposes to depressive symptoms: a population-based 7-year follow-up study. J Clin Psychiatry. 2008;69:178–182. [DOI] [PubMed] [Google Scholar]
- 29. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172:1075–1091. doi:10.1176/appi.ajp.2015.15020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. [DOI] [PubMed] [Google Scholar]
- 31. Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi:10.1016/S0893-133X(01)00407-9 [DOI] [PubMed] [Google Scholar]
- 32. Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006;30:791–807. doi:10.1016/j.neubiorev.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 33. D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi:10.1523/JNEUROSCI.3567-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33:315–327. doi:10.1016/j.yfrne.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi:10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Capuron L, Pagnoni G, Drake DF, et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 2012;69:1044–1053. doi:10.1001/archgenpsychiatry.2011.2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Felger JC, Hernandez CR, Miller AH. Levodopa reverses cytokine-induced reductions in striatal dopamine release. Int J Neuropsychopharmacol. 2015;18:pyu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Felger JC, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358–1365. doi:10.1038/mp.2015.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. JAMA Psychiatry. 2007;64:327–337. [DOI] [PubMed] [Google Scholar]
- 40. Eckart C, Bunzeck N. Dopamine modulates processing speed in the human mesolimbic system. Neuroimage. 2013;66:293–300. doi:10.1016/j.neuroimage.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 41. Cham R, Studenski SA, Perera S, Bohnen NI. Striatal dopaminergic denervation and gait in healthy adults. Exp Brain Res. 2008;185:391–398. doi:10.1007/s00221-007-1161-3 [DOI] [PubMed] [Google Scholar]
- 42. Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:412–420. doi:10.1038/nrrheum.2016.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brown PJ, Roose SP, Zhang J, et al. Inflammation, depression, and slow gait: a high mortality phenotype in later life. J Gerontol A Biol Sci Med Sci. 2016;71:221–227. doi:10.1093/gerona/glv156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi:10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- 45. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol. 2008;43:24–33. doi:10.1016/j.exger.2007.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gonzalez-Freire M, de Cabo R, Bernier M, et al. Reconsidering the role of mitochondria in aging. J Gerontol A Biol Sci Med Sci. 2015;70:1334–1342. doi:10.1093/gerona/glv070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Joseph AM, Adhihetty PJ, Buford TW, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell. 2012;11:801–809. doi:10.1111/j.1474-9726.2012.00844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tyrrell DJ, Bharadwaj MS, Van Horn CG, Kritchevsky SB, Nicklas BJ, Molina AJ. Respirometric profiling of muscle mitochondria and blood cells are associated with differences in gait speed among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2015;70:1394–1399. doi:10.1093/gerona/glu096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Santanasto AJ, Glynn NW, Jubrias SA, et al. Skeletal muscle mitochondrial function and fatigability in older adults. J Gerontol A Biol Sci Med Sci. 2015;70:1379–1385. doi:10.1093/gerona/glu134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:447–455. doi:10.1093/gerona/gls196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hardy SE, Studenski SA. Fatigue predicts mortality in older adults. J Am Geriatr Soc. 2008;56:1910–1914. doi:10.1111/j.1532-5415.2008.01957.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 54. Mezuk B, Lohman M, Dumenci L, Lapane KL. Are depression and frailty overlapping syndromes in mid- and late-life? A latent variable analysis. Am J Geriatr Psychiatry. 2013;21:560–569. doi:10.1016/j.jagp.2012.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Demakakos P, Cooper R, Hamer M, de Oliveira C, Hardy R, Breeze E. The bidirectional association between depressive symptoms and gait speed: evidence from the English Longitudinal Study of Ageing (ELSA). PLoS One. 2013;8:e68632. doi:10.1371/journal.pone.0068632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brown PJ, Roose SP, Fieo R, et al. Frailty and depression in older adults: a high-risk clinical population. Am J Geriatr Psychiatry. 2014;22:1083–1095. doi:10.1016/j.jagp.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–974. doi:10.1038/mp.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lotta T, Vidgren J, Tilgmann C, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. [DOI] [PubMed] [Google Scholar]
- 59. Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi:10.1038/nrn3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ong AD, Uchino BN, Wethington E. Loneliness and health in older adults: a mini-review and synthesis. Gerontology. 2016;62:443–449. doi:10.1159/000441651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cacioppo S, Capitanio JP, Cacioppo JT. Toward a neurology of loneliness. Psychol Bull. 2014;140:1464–1504. doi:10.1037/a0037618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237:143–149. [DOI] [PubMed] [Google Scholar]
- 63. Thomas PA. Trajectories of social engagement and limitations in late life. J Health Soc Behav. 2011;52:430–443. doi:10.1177/0022146511411922 [DOI] [PubMed] [Google Scholar]
- 64. Loprinzi PD, Loenneke JP, Blackburn EH. Movement-based behaviors and leukocyte telomere length among US adults. Med Sci Sports Exerc. 2015;47:2347–2352. doi:10.1249/MSS.0000000000000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi:10.1016/S0079-6123(06)57006-2 [DOI] [PubMed] [Google Scholar]
- 66. Gutchess A. Plasticity of the aging brain: new directions in cognitive neuroscience. Science. 2014;346:579–582. doi:10.1126/science.1254604 [DOI] [PubMed] [Google Scholar]
- 67. Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi:10.1038/nn1969 [DOI] [PubMed] [Google Scholar]
- 68. Nokia MS, Lensu S, Ahtiainen JP, et al. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol. 2016;594:1855–1873. doi:10.1113/JP271552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Baltes PB, Smith J. Toward a psychology of wisdom and its ontogenesis. In: Sternberg RJ, ed. Wisdom: Its Nature, Origins, and Development, . New York: Cambridge University Press;1990:87–120. [Google Scholar]
- 70. Grossmann I, Na J, Varnum ME, Park DC, Kitayama S, Nisbett RE. Reasoning about social conflicts improves into old age. Proc Natl Acad Sci USA. 2010;107:7246–7250. doi:10.1073/pnas.1001715107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stonea AA, Schwartz JE, Brodericka JE, Deaton A. A snapshot of the age distribution of psychological well-being in the United States. Proc Natl Acad Sci USA. 2010;22:9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Simon NM, Smoller JW, McNamara KL, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi:10.1016/j.biopsych.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 73. McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. [DOI] [PubMed] [Google Scholar]
- 74. Makarov VL, Hirose Y, Langmore JP. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell. 1997;88:657–666. [DOI] [PubMed] [Google Scholar]
- 75. Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr Opin Nutr Metab Care. 2011;14:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rode L, Nordestgaard BG, Bojeson SE. Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. J Natl Cancer Inst. 2015;107:djv074. [DOI] [PubMed] [Google Scholar]
- 77. Schutte NS, Malouff JM. The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety. 2015;32:229–238. doi:10.1002/da.22351 [DOI] [PubMed] [Google Scholar]
- 78. Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71:108–113. doi:10.1212/01.wnl.0000316799.86917.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Khalaf A, Edelman K, Tudorascu D, Andreescu C, Reynolds CF, Aizenstein H. White matter hyperintensity accumulation during treatment of late-life depression. Neuropsychopharmacology. 2015;40:3027–3035. doi:10.1038/npp.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Okereke OI, Lyness JM, Lotrich FE, Reynolds CF., 3rd Depression in late-life: a focus on prevention. Focus (Am Psychiatr Publ). 2013;11:22–31. doi:10.1176/appi.focus.11.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. doi:10.1016/S0140-6736(11)60749-6 [DOI] [PubMed] [Google Scholar]
- 82. Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015;112:E4104–E4110. doi:10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. National Institutes of Health Toolbox Cognition Battery (NIH Toolbox CB). Monographs Society Research Child Devel. 2013;78:1–172. [DOI] [PubMed] [Google Scholar]
- 84. Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11 suppl 3):S54–S64. doi:10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 86. Bartels SJ, Coakley EH, Zubritsky C, et al. ; PRISM-E Investigators. Improving access to geriatric mental health services: a randomized trial comparing treatment engagement with integrated versus enhanced referral care for depression, anxiety, and at-risk alcohol use. Am J Psychiatry. 2004;161:1455–1462. doi:10.1176/appi.ajp.161.8.1455 [DOI] [PubMed] [Google Scholar]
- 87. Castner SA, Goldman-Rakic PS. Enhancement of working memory in aged monkeys by a sensitizing regimen of dopamine D1 receptor stimulation. J Neurosci. 2004;24:1446–1450. doi:10.1523/JNEUROSCI.3987-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lavretsky H, Reinlieb M, St Cyr N, Siddarth P, Ercoli LM, Senturk D. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172:561–569. doi:10.1176/appi.ajp.2014.14070889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Richardson CA, Glynn NW, Ferrucci LG, Mackey DC. Walking energetics, fatigability, and fatigue in older adults: the study of energy and aging pilot. J Gerontol A Biol Sci Med Sci. 2015;70:487–494. doi:10.1093/gerona/glu146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi:10.1016/j.cell.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 91. Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi:10.1038/nature01960 [DOI] [PubMed] [Google Scholar]
- 92. Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biol Psychiatry. 2014;76:350–353. doi:10.1016/j.biopsych.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 93. Peterson BS. Research Domain Criteria (RDoC): a new psychiatric nosology whose time has not yet come. J Child Psychol Psychiatry. 2015;56:719–722. doi:10.1111/jcpp.12439 [DOI] [PubMed] [Google Scholar]
- 94. Chang M, Snaedal J, Einarsson B, et al. The association between midlife physical activity and depressive symptoms in late life: age gene/environment Susceptibility-Reykjavik Study. J Gerontol A Biol Sci Med Sci. 2016;71:502–507. doi:10.1093/gerona/glv196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi:10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68:412–418. doi:10.1093/gerona/gls191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Allali G, Ayers EI, Verghese J. Motoric cognitive risk syndrome subtypes and cognitive profiles. J Gerontol A Biol Sci Med Sci. 2016;71:378–384. doi:10.1093/gerona/glv092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. [DOI] [PubMed] [Google Scholar]
- 99. Bryan J, Luszcz MA. Speed of information processing as a mediator between age and free-recall performance. Psychol Aging. 1996;11:3–9. [DOI] [PubMed] [Google Scholar]
- 100. Habeck C, Steffener J, Barulli D, et al. Making cognitive latent variables manifest: distinct neural networks for fluid reasoning and processing speed. J Cogn Neurosci. 2015;27:1249–1258. doi:10.1162/jocn_a_00778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–595. doi:10.1001/archpsyc.61.6.587 [DOI] [PubMed] [Google Scholar]
- 102. Eldadah BA. Fatigue and fatigability in older adults. PM R. 2010;2:406–413. doi:10.1016/j.pmrj.2010.03.022 [DOI] [PubMed] [Google Scholar]
- 103. Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62:347–351. doi:10.1111/jgs.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Glynn NW, Santanasto AJ, Simonsick EM, et al. The Pittsburgh Fatigability scale for older adults: development and validation. J Am Geriatr Soc. 2015;63:130–135. doi:10.1111/jgs.13191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage. 2010;49:3426–3435. doi:10.1016/j.neuroimage.2009.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Allali G, van der Meulen M, Beauchet O, Rieger SW, Vuilleumier P, Assal F. The neural basis of age-related changes in motor imagery of gait: an fMRI study. J Gerontol A Biol Sci Med Sci. 2014;69:1389–1398. doi:10.1093/gerona/glt207 [DOI] [PubMed] [Google Scholar]