ABSTRACT
Background: Cystatin C may add explanatory power for associations with mortality in combination with other filtration markers, possibly indicating pathways other than glomerular filtration rate (GFR). However, this has not been firmly established since interpretation of associations independent of measured GFR (mGFR) is limited by potential multicollinearity between markers of GFR. The primary aim of this study was to assess associations between cystatin C and mortality, independent of mGFR. A secondary aim was to evaluate the utility of combining cystatin C and creatinine to predict mortality risk.
Methods: Cox regression was used to assess the associations of cystatin C and creatinine with mortality in 1157 individuals referred for assessment of plasma clearance of iohexol.
Results: Since cystatin C and creatinine are inversely related to mGFR, cystatin C−1 and creatinine−1 were used. After adjustment for mGFR, lower cystatin C−1 (higher cystatin C concentration) and higher creatinine−1 (lower creatinine concentration) were independently associated with increased mortality. When nested models were compared, avoiding the potential influence of multicollinearity, the independence of the associations was supported. Among models combining the markers of GFR, adjusted for demographic factors and comorbidity, cystatin C−1 and creatinine−1 combined explained the largest proportion of variance in associations with mortality risk (R2 = 0.61). Addition of mGFR did not improve the model.
Conclusions: Our results suggest that both creatinine and cystatin C have independent associations with mortality not explained entirely by mGFR and that mGFR does not offer a more precise mortality risk assessment than these endogenous filtration markers combined.
Keywords: creatinine, cystatin C, epidemiology, GFR, prognosis
INTRODUCTION
Chronic kidney disease (CKD) with reduced glomerular filtration rate (GFR) is an important risk factor for cardiovascular disease (CVD) and increased mortality [1]. Measurements of GFR by exogenous filtration markers (mGFR) and estimates of GFR (eGFR) using the endogenous filtration markers cystatin C (eGFRcys) and creatinine (eGFRcrea) display associations with mortality not fully explained by variation in GFR precision [2–9]. Non-GFR determinants of cystatin C and creatinine serum concentrations influencing production rate, sieving coefficient or extra-renal clearance may help explain differences and possibly contribute to mortality risk assessment.
Earlier studies suggest the existence of non-GFR factors associated with increased mortality that increase cystatin C concentration (lowering eGFRcys), but interpretation is complicated by the high degree of collinearity between cystatin C and mGFR [7, 10, 11]. Cystatin C has also been shown to have residual associations with cardiovascular risk factors, including markers of inflammation, after adjustment for mGFR [12–16]. Low creatinine production, mainly seen in individuals with low muscle mass, is a powerful predictor of mortality. This attenuates the association between higher creatinine concentration (lower eGFRcrea) and increased mortality when a single marker of GFR is used. Utilizing creatinine’s non-GFR associations with mortality has, apart from in dialysis patients with negligible GFR, required timed urinary samples to quantify creatinine excretion [17–21].
Here we present associations with all-cause mortality for cystatin C and creatinine after adjustment for mGFR in a clinical sample of 1157 individuals referred for assessment by plasma iohexol clearance. The primary aim was to assess whether cystatin C is associated with mortality independent of mGFR. A secondary aim was to evaluate the utility of combining cystatin C and creatinine to predict mortality risk. Filtration markers are highly collinear with mGFR and multicollinearity may influence the magnitude and the statistical significance of each measure’s association with mortality when included in the same models. However, combining them will not limit the evaluation of the independent information on mortality risk for each marker when nested models are compared [22]. Evaluation of possible collinearity influences was undertaken and the independent association with mortality risk for each marker of GFR is evaluated by comparing the performance of nested models. Since cystatin C and creatinine are inversely related to mGFR, cystatin C−1 and creatinine−1 are used to facilitate interpretation.
MATERIALS AND METHODS
Study sample
The study population comprises a consecutive patient series of 1286 Swedish residents ≥18 years of age referred to the Department of Laboratory Medicine at Örebro University Hospital for measurement of GFR by plasma iohexol clearance between 2004 and 2010 with sufficient serum to determine cystatin C and creatinine (>99%). Major indications for referral were a CKD diagnosis and follow-up, evaluation for treatment with drugs cleared by the kidneys (including chemotherapeutic drugs), evaluation of potential kidney donors and follow-up of patients treated with lithium. Individuals with an earlier diagnosis of end-stage renal disease (ESRD; n = 129) were excluded, resulting in a study population of 1157. All procedures involving patients and data were in accordance with the Helsinki Declaration of 1975 on ethical principles for medical research involving human subjects [23]. The study was approved by the ethical review board of Uppsala, Sweden (registration number 2013/065), which waived informed consent.
Baseline measurements
Details of the methods and instruments used for determination of plasma clearance of iohexol, serum cystatin C and serum creatinine are given in the Supplementary data. For plasma clearance of iohexol, the single-sample method was used [24–26]. All creatinine assays used calibrators traceable to isotope dilution mass spectrometry.
The Swedish National Patient Register (NPR) provided information on diagnoses before the date of iohexol clearance measurement. The NPR has information on inpatient diagnoses and procedures since 1964, with outpatient records since 2001 [27, 28]. Supplementary data, Table S1 contains the diagnostic codes used to define ESRD, diabetes, CVD and cancer.
Follow-up and outcome
Patients entered the study between 23 June 2004 and 21 December 2010. The end of follow-up was on 31 December 2012 and 706 (61.0%) patients were available for at least 5 years of follow-up. Vital status information was provided by the Swedish Cause of Death Register [29].
Statistical analysis
Associations of baseline markers of kidney function with mortality were examined using Cox regression. No exclusion of statistically suggested extreme outliers was performed, as they were considered biologically plausible. Time at risk was calculated from the date of the iohexol clearance examination to the date of death, emigration or end of follow-up, whichever occurred first. The adjusted models included age, sex, body mass index (BMI) and pre-existing diagnoses of CVD, diabetes and cancer. The variables were pre-specified and no data-dependent selection was performed. An interaction term between pre-existing diagnoses of CVD and cancer (evaluated on the multiplicative scale) was included. The adjusted Cox regression models selected for Table 3 and Supplementary data, Table S3 further allowed for different baseline functions by cancer status and tenths of the age distribution. Hazard ratios (HRs) were calculated comparing the median value of quintiles of cystatin C−1, creatinine−1 and mGFR with a reference value, which was chosen to approximate the median of the range of normal values for each marker (age < 50 years and averaging men and women): creatinine 75 µmol/L, cystatin C 0.925 mg/L and mGFR 100 ml/min/1.73 m2.
Table 3.
Filtration markers and all-cause mortality risk
| Adjusted modelsa |
||||||
|---|---|---|---|---|---|---|
| Quintiles of reciprocals of marker |
Unadjusted | Model 1b | Model 2c | Model 3d | ||
| Range | Median | Median−1 | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Cystatin C−1 | ||||||
| 0.16–0.39 | 0.32 | 3.10 | 6.66 (4.75–9.33) | 2.62 (1.96–3.51) | 9.37 (4.76–18.44) | 21.59 (11.92–39.10) |
| 0.39–0.56 | 0.47 | 2.14 | 4.57 (3.49–6.00) | 1.69 (1.44–1.98) | 4.61 (2.90–7.33) | 6.63 (4.60–9.56) |
| 0.56–0.79 | 0.67 | 1.49 | 2.77 (2.31–3.32) | 1.28 (1.19–1.38) | 2.40 (1.84–3.13) | 2.68 (2.22–3.24) |
| 0.80–1.04 | 0.91 | 1.10 | 1.52 (1.41–1.63) | 1.08 (1.05–1.10) | 1.36 (1.24–1.50) | 1.38 (1.30–1.47) |
| 1.05–2.78 | 1.22 | 0.82 | 0.70 (0.65–0.74) | 0.95 (0.94–0.97) | 0.79 (0.74–0.85) | 0.80 (0.76–0.83) |
| Creatinine−1 | ||||||
| 0.13–0.49 × 10−2 | 0.38 × 10−2 | 261.3 | 2.57 (1.99–3.32) | 1.27 (0.94–1.71) | 0.32 (0.21–0.47) | 0.13 (0.08–0.21) |
| 0.50–0.75 × 10−2 | 0.62 × 10−2 | 161.4 | 1.89 (1.57–2.27) | 1.06 (0.86–1.30) | 0.41 (0.30–0.56) | 0.26 (0.18–0.36) |
| 0.75–1.00 × 10−2 | 0.87 × 10−2 | 114.7 | 1.33 (1.20–1.46) | 0.95 (0.85–1.06) | 0.60 (0.50–0.72) | 0.52 (0.44–0.61) |
| 1.00–1.28 × 10−2 | 1.13 × 10−2 | 88.7 | 1.12 (1.07–1.17) | 0.95 (0.90–1.01) | 0.77 (0.71–0.85) | 0.75 (0.69–0.80) |
| 1.28–3.66 × 10−2 | 1.49 × 10−2 | 67.2 | 0.95 (0.90–0.99) | 1.10 (1.03–1.17) | 1.29 (1.18–1.42) | 1.29 (1.21–1.37) |
| mGFR | ||||||
| 12–25 | 19 | — | 5.95 (4.15–8.51) | 2.05 (1.28–3.29) | —c | — |
| 26–42 | 33 | — | 4.37 (3.25–5.88) | 1.81 (1.23–2.67) | —c | — |
| 43–64 | 55 | — | 2.69 (2.21–3.29) | 1.49 (1.15–1.94) | —c | — |
| 65–87 | 76 | — | 1.70 (1.52–1.89) | 1.24 (1.08–1.42) | —c | — |
| 88–141 | 102 | — | 0.96 (0.95–0.97) | 0.98 (0.97–0.99) | —c | — |
All adjusted Cox models included sex; BMI; pre-existing diagnoses of CVD, diabetes and cancer; an interaction term between baseline CVD and cancer and allowed for different baseline hazard functions by cancer status and tenths of age distribution.
Separate models for each marker.
Separate models for each marker adjusted for mGFR. HRs for mGFR are included in Supplementary data, Table S3.
Final model includes both cystatin C−1 and creatinine−1 but not mGFR.
HRs and 95% CIs are calculated at the median value of each quintile using 1.08 (cystatin C 0.925 mg/L) for cystatin C−1, 0.013 (creatinine 75 µmol/L) for creatinine−1 and 100 mL/min/1.73 m2 for mGFR as referents. Non-linearity of the associations for cystatin C−1, creatinine−1, mGFR and BMI were modeled as specified in the Supplementary data. An extended version of this table is provided as Supplementary data, Table S3.
Linearity of the relationship between the log hazard function and continuous variables (functional form) was assessed by applying the multivariable fractional polynomial (MFP) method [30, 31]. The MFP analysis detected non-linear associations and suggested transformations of creatinine−1, cystatin C−1, mGFR and BMI to achieve the best-fitting functional form. The transformations in each model are specified in the Supplementary data. The assumption of proportional hazards was assessed using a Grambsch–Therneau test of the scaled Schoenfeld residuals from a Cox model [32].
Pearson and Spearman correlations were used for the GFR markers to understand potential collinearity effects. Variance inflation factors (VIFs) and tolerance were calculated to examine possible multicollinearity in a model including the markers of GFR and the other variables. The independent contribution of each GFR marker to the performance of Cox regression models for all-cause mortality was assessed by comparing nested models where each kidney function marker was added to differently adjusted models (the base model included age, sex, BMI, pre-existing diagnoses of diabetes, CVD or cancer; base model and mGFR; base model and creatinine−1; base model, creatinine−1 and cystatin C−1). The likelihood ratio test was applied to evaluate statistically significant differences in model fit. The proportion of variance explained was evaluated by an adaption of the adjusted R2 proposed by Royston for censored survival data [33]. Discrimination was measured using Harrell’s concordance index (C-index) modified as proposed by Gönen and Heller [34]. The category free net reclassification improvement (NRI > 0) was calculated at 5 years (approximate mean follow-up time) as the sum of the event NRI > 0 (the net proportion of patients experiencing an event reclassified to a higher risk) and the non-event NRI > 0 (the net proportion of patients not experiencing an event reclassified to a lower risk). Bootstrapping with 1000 samplings was performed to attain 95% confidence intervals (CIs) for the NRI > 0 components [35, 36]. Sensitivity analyses included exclusion of extreme outliers for the markers of GFR and checking the adequacy of fractional polynomial functions in the final adjusted model using spline-based tests, detailed in the Supplementary data. P-values were two-sided and considered statistically significant at the 0.05 level. All statistical analyses were conducted using STATA 14 software (StataCorp, College Station, TX, USA).
RESULTS
Study population
Baseline characteristics of the study population and mortality rates are given in Table 1. By the end of follow-up on 31 December 2012, 312 patients had died, 1 patient had emigrated and no others were lost to follow-up. The mean follow-up time was 4.71 years and the overall mortality rate was 5.73 individuals/100 person-years. The main causes of death were CVD (n = 95), cancer (n = 78) and kidney failure (n = 24). The relatively high mortality rate reflects that the study population was referred for assessment of GFR by hospital departments rather than a general population sample.
Table 1.
Baseline characteristics and mortality
| n (%) | Deaths | Mortality rate | |
|---|---|---|---|
| mGFR (5ths; mL/min/1.73 m2) | |||
| 12–25 | 239 (20.7) | 115 | 11.46 (9.54–13.76) |
| 26–42 | 229 (19.8) | 86 | 8.41 (6.81–10.39) |
| 43–64 | 237 (20.5) | 53 | 4.66 (3.56–6.09) |
| 65–87 | 230 (19.9) | 36 | 3.26 (2.35–4.51) |
| 88–141 | 222 (19.2) | 22 | 1.87 (1.23–2.85) |
| Cystatin C−1 (5ths) | |||
| 0.16–0.39 | 233 (20.1) | 133 | 14.70 (12.40–17.43) |
| 0.39–0.56 | 234 (20.2) | 69 | 6.36 (5.02–8.05) |
| 0.56–0.79 | 229 (19.8) | 57 | 5.08 (3.92–6.59) |
| 0.80–1.04 | 235 (20.3) | 34 | 2.90 (2.07–4.05) |
| 1.05–2.78 | 226 (19.5) | 19 | 1.64 (1.05–2.57) |
| Creatinine−1 (5ths) | |||
| 0.13–0.49 × 10−2 | 232 (20.1) | 98 | 9.57 (7.85–11.66) |
| 0.50–0.75 × 10−2 | 231 (20.0) | 91 | 8.81 (7.17–10.81) |
| 0.75–1.00 × 10−2 | 232 (20.1) | 42 | 3.75 (2.77–5.07) |
| 1.00–1.28 × 10−2 | 231 (20.0) | 35 | 3.02 (2.17–4.20) |
| 1.28–3.66 × 10−2 | 231 (20.0) | 46 | 4.16 (3.11–5.51) |
| Age (years) | |||
| 18 ≤ 30 | 90 (7.8) | 4 | 0.89 (0.33–2.37) |
| 30 ≤ 50 | 247 (21.3) | 18 | 1.31 (0.82–2.07) |
| 50 ≤ 70 | 524 (45.3) | 132 | 5.40 (4.55–6.40) |
| 70 ≤ 80 | 229 (19.8) | 114 | 12.12 (10.08–14.56) |
| ≥80 | 67 (5.8) | 44 | 19.27 (14.34–25.90) |
| Sex | |||
| Male | 663 (57.3) | 191 | 6.13 (5.32–7.07) |
| Female | 494 (42.7) | 121 | 5.19 (4.35–6.21) |
| BMI (kg/m2) | |||
| Underweight, <18.5 | 35 (3.0) | 17 | 11.43 (7.11–18.40) |
| Normal weight, 18.5 < 25 | 396 (34.2) | 112 | 6.10 (5.07–7.34) |
| Overweight, 25 < 30 | 421 (36.4) | 101 | 4.87 (4.01–5.92) |
| Obese, ≥30 | 305 (26.4) | 82 | 5.91 (4.76–7.34) |
| CVD | |||
| No | 547 (47.3) | 91 | 3.31 (2.69–4.06) |
| Yes | 610 (52.7) | 221 | 8.21 (7.20–9.37) |
| Diabetes | |||
| No | 897 (77.5) | 196 | 4.51 (3.92–5.19) |
| Yes | 260 (22.5) | 116 | 10.57 (8.81–12.68) |
| Cancer | |||
| No | 968 (83.7) | 216 | 4.54 (3.97–5.18) |
| Yes | 189 (16.3) | 96 | 14.05 (11.50–17.16) |
Categorical variables are given as n (%) and mortality rate as deaths/100 person-years (95% CI).
Model evaluation
Correlation plots for the markers of GFR are presented in Supplementary data, Figure S1 and multicollinearity diagnostics in Supplementary data, Table S2. The VIFs indicate the degree to which the standard errors are inflated due to collinearity and tolerance (the reciprocal of VIF) values indicate the percentage of variance in one independent variable that is not accounted for by the other independent variables. When cystatin C−1, creatinine−1 and all covariates used in the adjusted models are included, the VIFs for the markers of GFR are 2.85–3.46 (tolerance 0.29–0.35) and with mGFR included 4.22–9.62 (tolerance 0.10–0.24).
The functional form for markers of GFR changed in the presence of a second filtration marker in the Cox model. Creatinine−1 had a J-shaped association with all-cause mortality in unadjusted analysis, where both the lowest and highest values were associated with increased mortality. After adjustment for cystatin C−1 or mGFR, the J-shape disappeared and higher creatinine−1 was associated with increased mortality risk. Lower cystatin C−1 was consistently associated with increased mortality before and after adjustment for creatinine−1 or mGFR. Adjustment for creatinine−1 increased the gradient of the linear association between lower mGFR and increased mortality, while adjustment for cystatin C−1 had the opposite effect, reversing the direction of the association.
The contribution of each filtration marker and mGFR to the performance of Cox regression models was evaluated by comparing nested models (Table 2). The single addition of cystatin C−1, creatinine−1 or mGFR significantly improved the models (likelihood ratio test) and increased the proportion of variance explained (adjusted R2 statistic) [33]. The addition of mGFR did not improve the adjusted model that included cystatin C−1 and creatinine−1. The largest differences and largest increase in explained variation were observed when cystatin C−1 was added to the models, indicating a highly statistically significant association with all-cause mortality independent of the potential confounding factors. Notably, both cystatin C−1 and creatinine−1 improved the model which included mGFR and the base model. Cystatin C−1 further improved the model including the base model and creatinine−1, reaching the highest R2 value.
Table 2.
Evaluation of Cox regression models for all-cause mortality
| Likelihood ratio test |
Adjusted R2 | C-index | NRI>0 |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline modela | Tested marker in the expanded modela | Deviance difference | P-value | Event (95% CI) | Non-event (95% CI) | Total (95% CI) | ||
| Base modelb | 0.492 | 0.755 | ||||||
| mGFR | 4.81 | 0.03 | 0.497 | 0.756 | 0.19 (0.07–0.31) | 0.09 (0.02–0.15) | 0.28 (0.15–0.42) | |
| Creatinine−1 | 14.00 | <0.01 | 0.508 | 0.757 | −0.12 (−0.24–0.00) | 0.41 (0.35–0.47) | 0.28 (0.14–0.42) | |
| Cystatin C−1 | 35.73 | <0.001 | 0.536 | 0.757 | 0.13 (0.01–0.25) | 0.26 (−0.20–0.33) | 0.40 (0.26–0.53) | |
| Base model + mGFR | Creatinine−1 | 26.13 | <0.001 | 0.532 | 0.763 | 0.00 (−0.13–0.12) | 0.28 (0.22–0.35) | 0.28 (0.14–0.42) |
| Cystatin C−1 | 48.70 | <0.001 | 0.556 | 0.760 | 0.13 (0.01–0.25) | 0.26 (0.19–0.32) | 0.39 (0.26–0.52) | |
| Base model + creatinine−1 | Cystatin C−1 | 104.16 | <0.001 | 0.613 | 0.771 | 0.15 (0.03–0.27) | 0.35 (0.29–0.41) | 0.50 (0.36–0.64) |
| Base model + creatinine−1 + cystatin C−1 | mGFR | 0.07 | 0.79 | 0.612 | 0.771 | −0.02 (−0.14–0.10) | 0.02 (−0.04–0.09) | 0.01 (−0.13–0.14) |
Non-linearity of the association for cystatin C−1, creatinine−1, mGFR and BMI was modeled as specified in the Supplementary data.
Base model includes age, sex, BMI and pre-existing diagnoses of CVD, diabetes and cancer.
The ability of the models to distinguish between high and low mortality risk were compared using Gönen and Hellers concordance index, which is not affected by censoring [34]. Since concordance indices are insensitive measures for quantifying the increment in discriminative power comparing nested models, we also included a reclassification measure [35]. The category free NRI allows for interpretation in terms of sensitivity and specificity and quantifies the increment in discrimination with less dependence on the distribution of other risk factors in the model, producing a measure that is more marker specific and less model specific [35, 36]. Both cystatin C−1 and creatinine−1 produced a positive NRI > 0 when added to the base model including mGFR (Table 2). For creatinine−1, the increase in predictive power was limited to the net proportion of patients without an event reclassified to a lower risk, which represents an increase in specificity. Cystatin C−1 consistently produced both positive event and non-event NRI > 0 values of the highest magnitude. When mGFR was added to the adjusted model combining cystatin C−1 and creatinine−1, no increase in discriminative power was observed.
Final model
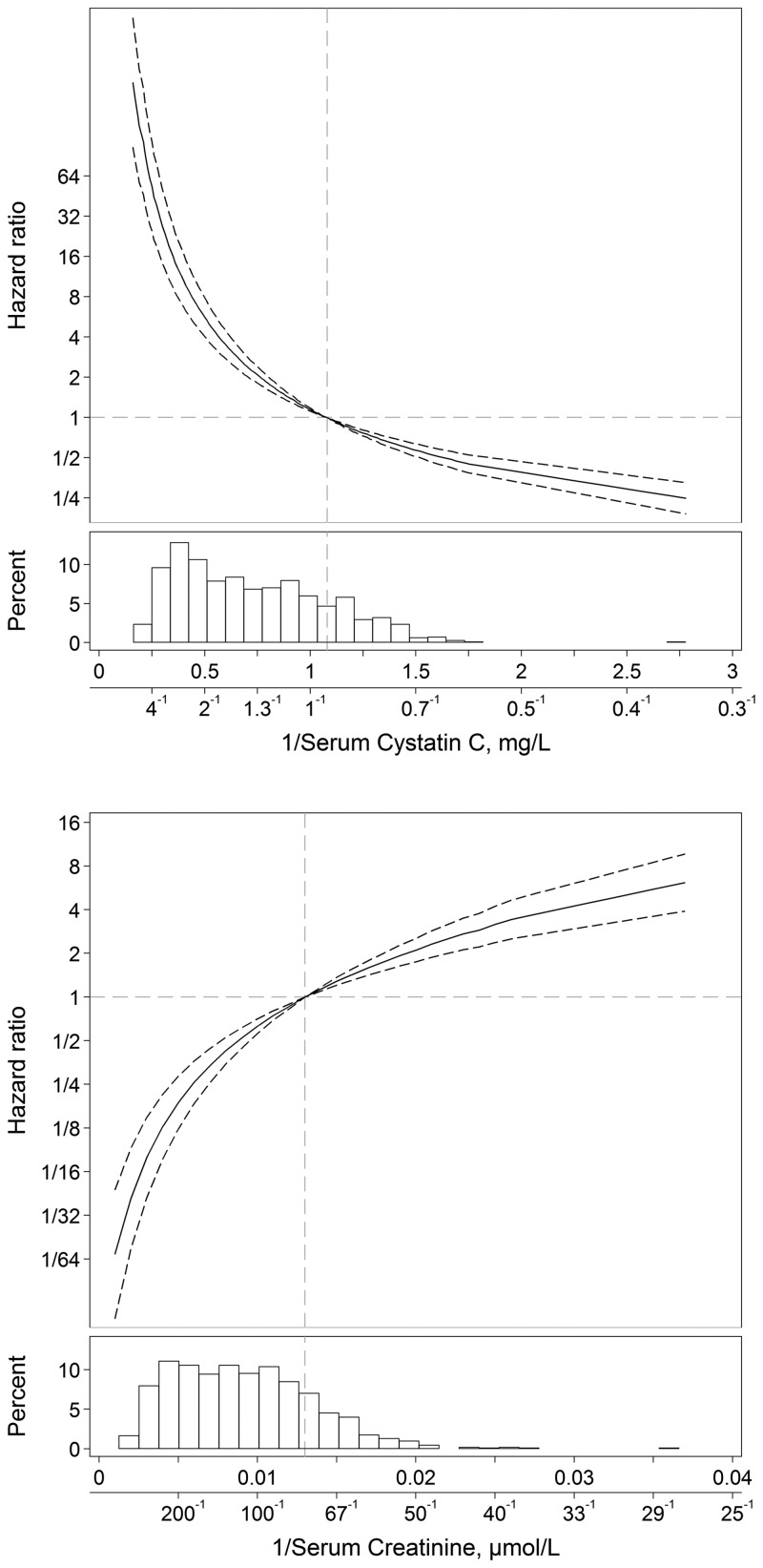
Table 3 displays the HRs for the associations of cystatin C−1, creatinine−1 and mGFR with all-cause mortality, comparing median values of fifths of the marker with reference values (cystatin C 0.925 mg/L, creatinine 75 μmol/L, mGFR 100 mL/min/1.73 m2) chosen from a range of normal values. The four models are unadjusted, adjusted for potential confounding factors (model 1), additionally adjusted for mGFR (model 2) and adjusted for potential confounders, cystatin C−1 and creatinine−1 (model 3). Plots of the HRs from model 3 and the distribution of each marker are presented in Figure 1. Lower cystatin C−1 (higher cystatin C and lower eGFRcys) was associated with increased mortality. Higher creatinine−1 (lower creatinine and a higher eGFRcrea) was associated with increased mortality. A full version of Table 3, with all models, is provided as Supplementary data, Table S3. In sensitivity analyses for influences of age and BMI or exclusion of patients with cancer the associations remained highly statistically significant. Age influenced the magnitude of the estimates for cystatin C, indicating age is a component of the association (Supplementary data, Table S4).
FIGURE 1.
Plots of HRs for all-cause mortality in the final fully adjusted Cox regression model. HRs are compared with reference points within the normal range using 1.08 (cystatin C 0.925 mg/L) for cystatin C−1 and 0.013 (creatinine 75 µmol/L) for creatinine−1. Dashed lines indicate 95% CIs. The histograms illustrate the distribution of each marker. The x-axes are labeled to illustrate both reciprocals and actual values.
DISCUSSION
Our aim was to assess the association of cystatin C with all-cause mortality in order to identify the extent to which it provides additional explanatory power. Using data from 1157 patients referred for determination of GFR by plasma clearance of iohexol, we found statistically significant associations with increased mortality risk for lower cystatin C−1 and higher creatinine−1 after adjustment for mGFR (which did not add additional explanatory power to the combination of cystatin C and creatinine). These three markers are highly correlated, so we assessed this issue.
Although multicollinearity diagnostics did not indicate problematic collinearity effects (VIF < 5 in models combining cystatin C−1 and creatinine−1 and < 10 when mGFR is also included), HR CIs are relatively wide in models combining markers of GFR, possibly influenced by collinearity [37]. Although collinearity may limit accurate determination of the magnitude of associations with all-cause mortality independent of mGFR for cystatin C−1 and creatinine−1, it does not limit evaluation of improvement in model performance for a predictor when comparing nested models [22]. To further evaluate the independent association of each marker of GFR with mortality, without the influence of collinearity and to address our aim to evaluate the utility of combining cystatin C and creatinine to predict mortality risk, we compared the proportion of variance explained and the discriminative power of nested models. This supported the assertion that both cystatin C−1 and creatinine−1 identify associations with mortality risk not accounted for entirely by mGFR. Once cystatin C−1 and creatinine−1 were combined in a fully adjusted model, the inclusion of mGFR did not add any additional explanatory or discriminatory power. These findings suggest that cystatin C and creatinine identify biological pathways related to mortality risk not measured by mGFR.
If mGFR had poor precision, our results might be explained by increased precision when additional markers of GFR are included [38]. In an extensive systematic review, plasma clearance of iohexol was assessed as an accurate method for measuring GFR comparable to inulin clearance, which is the reference method [26]. The analytical variation and the considerable within-subject biological variation of mGFR have been quantified as a total coefficient of variation in the range of 5–11% [26, 39]. Thus we cannot rule out that the associations with mortality for cystatin C−1 and creatinine−1 after adjustment for mGFR in our study are mediated by a stronger association with true GFR rather than mGFR. Cystatin C−1 and creatinine−1 are complementary in their associations with mortality and are influenced by non-GFR factors associated with increased mortality in opposite directions. Increased precision in determining GFR is unlikely to fully explain our findings.
Non-GFR mechanisms linking cystatin C with mortality have not been studied extensively. An association independent of mGFR for individuals with CKD is supported by data from the Modification of Diet in Renal Disease study [7]: the results suggest non-GFR associations of cystatin C with mortality, but also possibly reflecting residual associations with GFR itself or collinearity between cystatin C and mGFR. In African Americans with hypertensive kidney disease and reduced mGFR, Bhavsar et al. [11] found an association of cystatin C with mortality or ESRD, independent of mGFR. In a study from the CKD Biomarkers Consortium, eGFRcys was associated with mortality independent of mGFR in 250 southwestern Native Americans with type 2 diabetes [10]. Higher cystatin C has also been associated with fatal and non-fatal cardiovascular events in dialysis patients with a negligible GFR [40]. Indirect evidence can also be derived from several studies demonstrating associations with cardiovascular risk factors, including inflammatory markers, for lower eGFRcys or higher cystatin C after adjustment for mGFR [12–16]. It has also been shown that eGFRcys has a greater magnitude association with mortality than the more precise eGFR based on creatinine and cystatin C combined and that a decrease in mGFR is not more predictive of mortality than a decrease in eGFRcys or eGFRcrea [2, 6, 8, 9, 41–43]. This is consistent with our results, providing evidence for both a GFR and a non-GFR component in the association of cystatin C with mortality.
The physiological function of cystatin C is to inhibit the activity of cysteine proteases, which are central in both inflammation and degradation of damaged proteins [44]. Few non-GFR determinants directly influencing the plasma cystatin C level have been established conclusively, so possible biological pathways linking factors associated with cystatin C independent of mGFR and mortality risk are incompletely understood. There is evidence of an influence of thyroid disease and systemic high-dose treatment with corticosteroids, but conflicting results for the influence of age, sex, BMI and inflammatory states, which have been shown to not directly influence cystatin C level [16, 45–50]. It has been hypothesized that microvascular damage in the glomeruli reduces filtration of the larger cystatin C molecule (molecular weight 13.3 kDa) more than filtration of the smaller creatinine molecule (113 Da) and exogenous filtration markers like iohexol (821 Da) [51]. This pathophysiological state, called the ‘shrunken pore syndrome’, offers a potential mechanism linking vascular damage to serum levels of cystatin C and its association with CVD mortality [51]. Dardashti et al. [52] found that an eGFRcys <60% of eGFRcrea was associated with increased mortality in patients undergoing elective coronary artery bypass grafting. The study did not include assessment of creatinine production or adjustment for mGFR to distinguish low eGFRcys from high eGFRcrea. Purde et al. [53, 54] demonstrated an association between a higher cystatin C:creatinine ratio and increased mortality in ostensibly healthy Swiss volunteers ≥ 60 years of age. This increased ratio with the creatinine concentration was also observed for other molecules similar in size to cystatin C (3.5–66.5 kDa) but not in smaller or larger molecules, consistent with the shrunken pore syndrome concept.
Low urinary excretion of creatinine has been shown to be an important predictor of mortality in the general population and among those with CKD or CVD [19–21, 55]. In kidney failure, higher plasma creatinine is associated with longer survival [19]. In our study this is demonstrated when adjustment for cystatin C−1 transforms creatinine’s J-shaped association with mortality into a linear pattern with higher creatinine−1 associated with increased mortality. Thus, evaluating creatinine in conjunction with cystatin C identifies the increased mortality associated with low creatinine production without measuring creatinine excretion.
A potential limitation of our study is that participants were referred for measurement of GFR for clinical reasons that might introduce bias and limit generalizability. We examined the effect of excluding patients with cancer and that did not notably alter the results. Important risk factors for mortality, such as smoking, hypertension and hyperlipidemia, that may confound associations with mortality or represent explanatory factors were not measured in this study, which has implications for the precision of results. Many studies do not have access to such information, and if such influences are reflected in cystatin C levels, then inclusion of this measure in models will improve the precision of the estimates, albeit not to the extent offered by direct measures. The lack of an international reference for calibrating cystatin C measurements at the time of baseline examinations is another limitation. The larger number of patients assessed by mGFR compared with previous studies is a strength of our study, which together with the wider range of cystatin C, creatinine and mGFR values in our study population, contribute to attenuating potential multicollinearity effects [37].
Our results suggest that cystatin C and creatinine have associations with mortality not fully explained by mGFR. Collinearity or imprecision in mGFR do not seem to fully explain these associations. Mutual adjustment for cystatin C and creatinine seems to enhance mortality risk assessment to include both GFR and non-GFR factors. The combination of cystatin C and creatinine offers a more precise mortality risk assessment than GFR alone, and the addition of mGFR to cystatin C and creatinine is of no benefit. These non-GFR associations with mortality have implications for the choice of endogenous filtration markers and interpretation of results.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
FUNDING
This study was supported by grants from the Research Committee of the Örebro County Council (OLL-330601, OLL-408481 and OLL-506561). The study sponsor had no role in study design; collection, analysis and interpretation of data; writing the report or the decision to submit the report for publication.
CONFLICT OF INTEREST STATEMENT
None declared.
(See related article by Glassock and Rule. Optimally predicting mortality with kidney function markers is not the same as optimally determining how kidney function predicts mortality. Nephrol Dial Transplant 2017; 32: 585--587)
REFERENCES
- 1. Go AS, Chertow GM, Fan D. et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 2. Inker LA, Schmid CH, Tighiouart H. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levey AS, Coresh J, Greene T. et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007; 53: 766–772 [DOI] [PubMed] [Google Scholar]
- 4. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grubb A, Horio M, Hansson LO. et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem 2014; 60: 974–986 [DOI] [PubMed] [Google Scholar]
- 6. Shlipak MG, Matsushita K, Arnlov J. et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 2013; 369: 932–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tangri N, Inker LA, Tighiouart H. et al. Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol 2012; 23: 351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Helmersson-Karlqvist J, Arnlov J, Larsson A.. Cystatin C-based glomerular filtration rate associates more closely with mortality than creatinine-based or combined glomerular filtration rate equations in unselected patients. Eur J Prev Cardiol 2016; 23: 1649–1657 [DOI] [PubMed] [Google Scholar]
- 9. Salminen M, Laine K, Korhonen P. et al. Biomarkers of kidney function and prediction of death from cardiovascular and other causes in the elderly: a 9-year follow-up study. Eur J Intern Med 2016; 33: 98–101 [DOI] [PubMed] [Google Scholar]
- 10. Foster MC, Inker LA, Hsu CY. et al. Filtration markers as predictors of ESRD and mortality in southwestern American Indians with type 2 diabetes. Am J Kidney Dis 2015; 66: 75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhavsar NA, Appel LJ, Kusek JW. et al. Comparison of measured GFR, serum creatinine, cystatin C, and beta-trace protein to predict ESRD in African Americans with hypertensive CKD. Am J Kidney Dis 2011; 58: 886–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mathisen UD, Melsom T, Ingebretsen OC. et al. Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol 2011; 22: 927–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schei J, Stefansson VT, Mathisen UD. et al. Residual associations of inflammatory markers with eGFR after accounting for measured GFR in a community-based cohort without CKD. Clin J Am Soc Nephrol 2016; 11: 280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rule AD, Bailey KR, Lieske JC. et al. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int 2013; 83: 1169–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Melsom T, Fuskevag OM, Mathisen UD. et al. Estimated GFR is biased by non-traditional cardiovascular risk factors. Am J Nephrol 2015; 41: 7–15 [DOI] [PubMed] [Google Scholar]
- 16. Stevens LA, Schmid CH, Greene T. et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 2009; 75: 652–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heymsfield SB, Arteaga C, McManus C. et al. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr 1983; 37: 478–494 [DOI] [PubMed] [Google Scholar]
- 18. Rule AD, Bailey KR, Schwartz GL. et al. For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int 2009; 75: 1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Avram MM, Bonomini LV, Sreedhara R. et al. Predictive value of nutritional markers (albumin, creatinine, cholesterol, and hematocrit) for patients on dialysis for up to 30 years. Am J Kidney Dis 1996; 28: 910–917 [DOI] [PubMed] [Google Scholar]
- 20. Oterdoom LH, Gansevoort RT, Schouten JP. et al. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis 2009; 207: 534–540 [DOI] [PubMed] [Google Scholar]
- 21. Di Micco L, Quinn RR, Ronksley PE. et al. Urine creatinine excretion and clinical outcomes in CKD. Clin J Am Soc Nephrol 2013; 8: 1877–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer, 2001 [Google Scholar]
- 23. World Medical Association. World medical association declaration of helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194 [DOI] [PubMed] [Google Scholar]
- 24. Jacobsson L. A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol 1983; 3: 297–305 [DOI] [PubMed] [Google Scholar]
- 25. Krutzen E, Back SE, Nilsson-Ehle I. et al. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med 1984; 104: 955–961 [PubMed] [Google Scholar]
- 26. Soveri I, Berg UB, Bjork J. et al. Measuring GFR: a systematic review. Am J Kidney Dis 2014; 64: 411–424 [DOI] [PubMed] [Google Scholar]
- 27.National Patient Register. National Board of Health and Welfare http://www.socialstyrelsen.se/register/halsodataregister/patientregistret/inenglish (18 November 2016, date last accessed)
- 28. Ludvigsson JF, Andersson E, Ekbom A. et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cause of Death Register. National Board of Health and Welfare http://www.socialstyrelsen.se/register/dodsorsaksregistret (18 November 2016, date last accessed)
- 30. Royston P, Ambler G, Sauerbrei W.. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 1999; 28: 964–974 [DOI] [PubMed] [Google Scholar]
- 31. Sauerbrei W, Royston P, Binder H.. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med 2007; 26: 5512–5528 [DOI] [PubMed] [Google Scholar]
- 32. Grambsch PM, Therneau TM.. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81: 515–526 [Google Scholar]
- 33. Royston P. Explained variation for survival models. Stata J 2006; 6: 83–96 [Google Scholar]
- 34. Gönen M, Heller G.. Concordance probability and discriminatory power in proportional hazards regression. Biometrika 2005; 92: 965–970 [Google Scholar]
- 35. Pencina MJ, D’Agostino RB Sr, Steyerberg EW.. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011; 30: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leening MJ, Vedder MM, Witteman JC. et al. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Ann Intern Med 2014; 160: 122–131. [DOI] [PubMed] [Google Scholar]
- 37. Hair JF. Multivariate Data Analysis. Upper Saddle River, NJ: Prentice Hall, 2006 [Google Scholar]
- 38. Rule AD, Lieske JC.. Cystatin C is more than GFR, and this may be a good thing. J Am Soc Nephrol 2011; 22: 795–797 [DOI] [PubMed] [Google Scholar]
- 39. Stevens LA, Levey AS.. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 2009; 20: 2305–2313 [DOI] [PubMed] [Google Scholar]
- 40. Ho LC, Sung JM, Tsai YS. et al. Cystatin C as a predictor for outcomes in patients with negligible renal function. Blood Purif 2014; 38: 81–88 [DOI] [PubMed] [Google Scholar]
- 41. Nyman U, Grubb A, Sterner G. et al. Different equations to combine creatinine and cystatin C to predict GFR. Arithmetic mean of existing equations performs as well as complex combinations. Scand J Clin Lab Invest 2009; 69: 619–627 [DOI] [PubMed] [Google Scholar]
- 42. Tidman M, Sjostrom P, Jones I.. A comparison of GFR estimating formulae based upon s-cystatin C and s-creatinine and a combination of the two. Nephrol Dial Transplant 2008; 23: 154–160 [DOI] [PubMed] [Google Scholar]
- 43. Ku E, Xie D, Shlipak M. et al. Change in measured GFR versus eGFR and CKD outcomes. J Am Soc Nephrol 2016; 27: 2196–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grubb A. Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol 1992; 38(Suppl 1): S20–S27 [PubMed] [Google Scholar]
- 45. Knight EL, Verhave JC, Spiegelman D. et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 2004; 65: 1416–1421 [DOI] [PubMed] [Google Scholar]
- 46. Levey AS, Inker LA, Coresh J.. GFR estimation: from physiology to public health. Am J Kidney Dis 2014; 63: 820–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sjostrom PA, Jones IL, Tidman MA.. Cystatin C as a filtration marker–haemodialysis patients expose its strengths and limitations. Scand J Clin Lab Invest 2009; 69: 65–72 [DOI] [PubMed] [Google Scholar]
- 48. Sjostrom P, Tidman M, Jones I.. Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Invest 2005; 65: 111–124 [DOI] [PubMed] [Google Scholar]
- 49. Grubb A, Bjork J, Nyman U. et al. Cystatin C, a marker for successful aging and glomerular filtration rate, is not influenced by inflammation. Scand J Clin Lab Invest 2011; 71: 145–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martensson J, Martling CR, Oldner A. et al. Impact of sepsis on levels of plasma cystatin C in AKI and non-AKI patients. Nephrol Dial Transplant 2012; 27: 576–581 [DOI] [PubMed] [Google Scholar]
- 51. Grubb A, Lindstrom V, Jonsson M. et al. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: ‘shrunken pore syndrome’. Scand J Clin Lab Invest 2015; 75: 333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dardashti A, Nozohoor S, Grubb A. et al. Shrunken pore syndrome is associated with a sharp rise in mortality in patients undergoing elective coronary artery bypass grafting. Scand J Clin Lab Invest 2016; 76: 74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Purde M-T, Nock S, Risch L. et al. The cystatin C/creatinine ratio, a marker of glomerular filtration quality: associated factors, reference intervals, and prediction of morbidity and mortality in healthy seniors. Transl Res 2016; 169: 80–90.e2 [DOI] [PubMed] [Google Scholar]
- 54. Purde M-T, Nock S, Risch L. et al. Ratio of cystatin C and creatinine-based estimates of the glomerular filtration rate predicts mortality in healthy seniors independent of kidney function. Scand J Clin Lab Invest 2016; 76: 341–343 [DOI] [PubMed] [Google Scholar]
- 55. Ix JH, de Boer IH, Wassel CL. et al. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation 2010; 121: 1295–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.