Abstract
BACKGROUND: Using diffusion tensor imaging (DTI) in neurosurgical planning allows identification of white matter tracts and has been associated with a reduction in postoperative functional deficits.
OBJECTIVE: This study explores the relationship between the lesion-to-tract distance (LTD) and postoperative morbidity and mortality in patients with brain tumors in order to evaluate the role of DTI in predicting postoperative outcomes.
METHODS: Adult patients with brain tumors (n = 60) underwent preoperative DTI. Three major white matter pathways (superior longitudinal fasciculi [SLF], cingulum, and corticospinal tract) were identified using DTI images, and the shortest LTD was measured for each tract. Postoperative morbidity and mortality information was collected from electronic medical records.
RESULTS: The ipsilesional corticospinal tract LTD and left SLF LTD were significantly associated with the occurrence rate of total postoperative motor (P = .018) and language (P < .001) deficits, respectively. The left SLF LTD was also significantly associated with the occurrence rate of new postoperative language deficits (P = .003), and the LTD threshold that best predicted this occurrence was 1 cm (P < .001). Kaplan–Meier log-rank survival analyses in patients having high-grade tumors demonstrated a significantly higher mortality for patients with a left SLF LTD <1 cm (P = .01).
CONCLUSION: Measuring tumor proximity to major white matter tracts using DTI can inform clinicians of the likelihood of postoperative functional deficits. A distance of 1 cm or less from eloquent white matter structures most significantly predicts the occurrence of new deficits with current surgical and imaging techniques.
Keywords: Brain tumor, Diffusion tensor imaging, Morbidity, Mortality, Neurosurgery, White matter
A fundamental goal of neurosurgical management of intracranial tumors is to balance the extent of tumor resection with preservation of neurological function. Recent studies have shown that maximal tumor resection is associated with improved survival.1,2 To this end, various preoperative and intraoperative imaging techniques including fMRI (functional magnetic resonance imaging) and DTI (diffusion tensor imaging) have been implemented to improve delineation of normal vs pathological brain tissue and functional mapping of eloquent cortical and subcortical structures.3
DTI exploits Brownian motion of water molecules to measure microstructural characteristics of tissue, allowing visualization of subcortical white matter tracts. DTI has shown strong concordance with intraoperative subcortical stimulation, the gold standard for functional mapping of eloquent white matter.4 Given its reliability in delineation of white matter tracts, DTI has gained a wide range of applications in the field of neurosurgical oncology, including a role in preoperative diagnosis and tumor grading, predicting extent of resection, surgical planning, intraoperative neuronavigation, and characterization of postoperative changes in brain tissue.5 The addition of DTI to the preoperative workflow has clearly shown to have a positive impact on surgical outcomes after intracranial tumor resection.6-8
The commonplace use of DTI in preoperative imaging routines has allowed adjunctive exploration into the predictive value of DTI metrics for patients with brain tumors. DTI diffusion metrics such as fractional anisotropy (FA) or apparent diffusion coefficient of the tumors themselves can be prognostic.9 FA changes in white matter tracts can be predictive of postoperative outcomes.10,11 DTI tractography can be used to predict extent of resection.12
In this retrospective study, our goal is to further investigate the role of DTI in predicting postoperative outcomes in patients with brain tumors. Using DTI, we aim to characterize the relationship between the lesion-to-tract distance (LTD) and postoperative morbidity and mortality. Previous studies have used fMRI or intraoperative cortical stimulation to show the predictive value of the lesion-to-activation distance when evaluating how functional outcomes and mortality depend on tumor proximity to eloquent cortex.13-15 LTD could have similar predictive significance. Based on these analogous studies, we predict a higher incidence of new postoperative deficits with shorter LTDs and increased postoperative mortality with shorter LTDs.
METHODS
Subjects
Subjects were selected consecutively from a database of 531 patients who underwent advanced neuroimaging as part of preoperative neurosurgical planning at the University of Wisconsin-Madison between March 2004 and March 2012. Of 531 total patients, inclusion criteria selected 159 patients with intracranial tumors of variable pathology, of which 60 underwent preoperative DTI as well as motor and language mapping using fMRI. Patients gave informed consent according to the study protocol approved by the local institutional review board. Relevant clinical information was collected from electronic medical records. Any record of preoperative or postoperative weakness (lower extremity, upper extremity, or facial) or aphasia (Broca and Wernicke areas, conduction, global, etc.) was included in the analysis.
Mortality information was collected for all subjects by cross-referencing medical records with the Social Security Death Index. Original data from Social Security Administration were accessed via the http://stevemorse.org/ssdi/ssdi.html web site, which aggregates information from the following web sites: ancestry.com, familysearch.com, familytreelegends.com, geneaology.com, geneaologybank.com, newenglandancestors.org, rootsweb.com, and worldvitalrecords.com.
Surgical Technique
Between 2004 and 2012, multiple neurosurgeons operated on this set of patients with a mixture of extrinsic benign, and intrinsic low and high-grade tumors using stealth neuronavigational guidance, microsurgery dissection, and intraoperative ultrasound and/or intraoperative magnetic resonance imaging (MRI; activated April 2011) for residual tumor detection, when indicated. In addition, cortical mapping and/or awake craniotomy techniques were used when indicated. No subcortical or fiber tract stimulation was used. Preoperative DTI and fMRI were used in stealth neuronavigation and influenced decisions about use of cortical mapping and/or awake craniotomy. However, given the retrospective nature of this study, LTD measurements did not drive surgical decision-making.
Image Acquisition
MRI for this study was performed as part of the patients’ clinical exam at the University of Wisconsin Hospital and the Health and Emotions Research Institute (Madison, WI). Data were acquired at either GE 450W 1.5T or GE 750 3.0T MRI scanners (GE Healthcare, Milwaukee, WI) using standard clinical imaging protocols. DTI images were acquired in the same imaging session as contrast-enhanced anatomic T1 Bravo and T2 Cube Flair MRI scanning. Following acquisition, the axial DTI data were processed and reformatted for viewing in the Prism imaging suite (Prism Clinical Imaging Inc., Elmgrove, WI) and sent to McKesson PACS (McKesson Corp., San Francisco, CA). DTI data were acquired using 25 gradient directions with a b-value of 1000 s/mm2, 1 NEX, TE = 103 ms, TR = 12.5 s, parallel imaging acceleration factor 2, 128 × 128 matrix with 24 cm square FOV, 4-mm-thick axial slices covering the whole brain. Three-dimensional T1 Bravo images were acquired with a parallel imaging acceleration factor of 2, with a 26 × 19 cm FOV and a 320 × 320 imaging matrix, acquiring 100 axial locations with 1.5 mm thickness. The 3-D Cube Flair sequence was acquired with a parallel acceleration factor of 2, 25 × 23 cm FOV and a 256 × 256 image matrix size, acquiring 112 sagittal locations with 1.6 mm thickness.
Image Analysis and Interpretation
LTD measurements were performed for the cingulum, corticospinal tract (CST), and superior longitudinal fasciculus (SLF) in each hemisphere using the Prism software suite. The LTD is defined as the shortest distance in any orientation between the tract and the tumor margin. T1-weighted, T2-Flair-weighted, and contrast-enhanced T1 images were used to determine tumor margin. Tumor margin was defined as the enhancing margin for tumors that enhance with contrast or the peripheral margin of the solid portion of the tumor as noted in T2 FLAIR images. Directionally encoded colored FA maps were used to define tract borders. The Kenichi Oishi MRI Atlas of Human White Matter, 2nd Edition,16 was used as a standard for defining the cingulum, CST, and SLF. Translucent FA volumes were overlaid onto T2 volumes to allow visualization of tract location in reference to the tumor (Figure 1). Using Prism, markers with x, y, and z coordinates were assigned iteratively to tumor and tract boundaries until the shortest LTD had been determined. Distances were calculated using the distance formula. A medical student with 5 yr of experience in processing and interpreting DTI images determined all LTD measurements, and a neuroradiologist confirmed lesion and tract margins.
FIGURE 1.
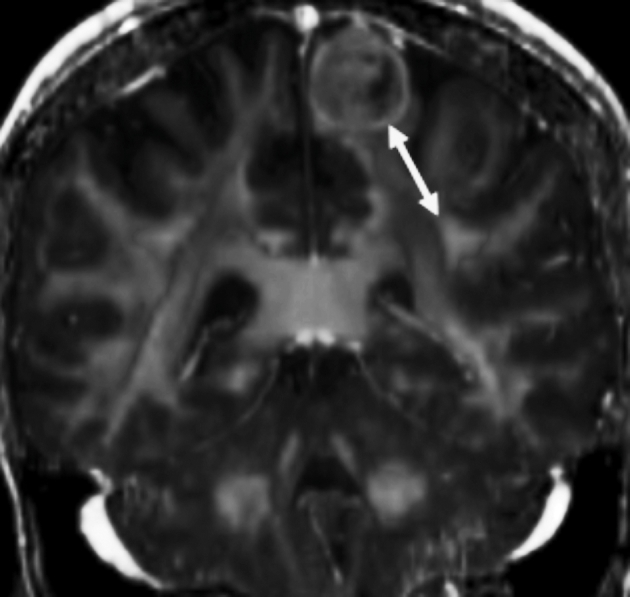
Example LTD measurement (white arrow) from tumor to left SLF shown in the coronal plane. A semitransparent color-coded map of FA (colors not demonstrable in gray scale) overlays the T2 MRI. The shortest distance between tumor and white matter structure, not always within one plane, was chosen for the LTD value.
Statistics
Statistical testing determined significant differences between subjects grouped by LTD (<1 cm, 1-2 cm, >2 cm) for the cingulum, CST, and SLF tracts. Our LTD distances were grouped according to an a priori decision that was based on data from past literature, which found these cutoff values to be of clinical relevance in functional imaging studies.3,14,17 Similar testing was done to evaluate significant differences between subjects with and without motor and language deficits. Pearson chi-square and Fisher's exact tests of independence were performed to compare categorical subject characteristics of interest (sex, handedness, and tumor grade, tumor lobe) between groups. For analyses involving tumor grade, subjects were grouped into low-grade (grade I, II, or benign) and high-grade (grade III, IV, or metastasis) groups. One-way ANOVA and independent-sample Kruskal–Wallis tests were performed to determine if age at the time of DTI significantly differed between groups. The associations between LTD groups and extent of resection and between tumor grade and extent of resection were investigated using Fisher's exact tests. Extent of resection was characterized, based on postoperative radiological notes, as total (no residual enhancement), subtotal (<10% residual tumor), or partial (>10% residual tumor). Fisher's exact tests were used to determine significant differences in postoperative functional deficits between LTD groups. Logistic regression analyses were used to investigate the dependency of new surgical language and motor deficits on tumor size, grade, and left and right-sided cingulum, and SLF LTD. Kaplan–Meier survival curves and Mantel–Cox log-rank tests were used to explore the relationship between LTD and survival. Analyses were performed by a researcher team member with a doctorate degree and completed statistical training using Statistical Package for the Social Science (SPSS, Chicago, Illinois) and SigmaPlot (SyStat Software Inc., San Jose, CA). All tests were considered significant at P < .05 (2-sided).
RESULTS
Demographics and Tumor Characteristics
Sixty subjects met selection criteria for this study. Intracranial tumors included 54 gliomas (35 astrocytoma, 12 oligodendroglioma, 3 mixed glioma or oligoastrocytoma, 2 glioblastoma, and 2 low-grade glioma), 3 meningiomas, 2 metastases, and 1 dysembryoplastic neuroepithelial tumor. Forty-six subjects had tumors in the left hemisphere, 14 had tumors in the right hemisphere. Fifty-four subjects demonstrated left hemisphere language dominance (as determined by radiologist reports) on fMRI language mapping, while 5 demonstrated mixed-hemisphere dominance and 1 did not have language lateralization data available. For the remaining analyses, only left SLF was considered given the predominance of left hemisphere language dominance.
Age, sex, handedness, and tumor grade information for subjects with and without postoperative motor and language deficits is presented in Table 1. Age, sex, handedness, and tumor grade information based on analysis of LTD to the 2 ipsilateral tracts (cingulum, CST) and left SLF tract is presented in Table 2. Of note, there was a significant relationship between tumor lobe location (frontal, parietal, or temporal) and ipsilateral CTS LTD, with 22 of 27 frontal tumors being within 1 cm of the ipsilateral CST (P = .003). There were no other significant relationships between tumor lobe location and LTD (see Analysis 1, Supplemental Digital Content, http://www.doi.org/10.1093/neuros/nyw040, for a more detailed discussion of the effect of tumor lobe location).
TABLE 1.
Demographics Information Based On Existence of Functional Deficits
| Patient characteristics | No deficits | Deficits | P-valuea |
|---|---|---|---|
| Motor deficits | |||
| No. of subjects | 40 | 20 | |
| Mean age ±SDb (years) | 48.8 ± 14.6 | 48.2 ± 15.7 | .89 |
| Gender (% male) | 55 | 85 | .022 |
| Handedness (% right) | 83 | 85 | .29 |
| Tumor grade (% high gradec) | 60 | 45 | .27 |
| Language deficits | |||
| No. of subjects | 34 | 26 | |
| Mean age ± SDb (years) | 45.5 ± 14.3 | 52.6 ± 14.9 | .066 |
| Gender (% male) | 68 | 62 | .62 |
| Handedness (% right) | 76 | 92 | .24 |
| Tumor grade (% high gradec) | 41 | 73 | .014 |
a P-value refers to Fisher's exact test or ANOVA.
bSD: standard deviation.
cHigh grade: grade III, grade IV, or metastasis.
TABLE 2.
Demographics Information Based on LTDa Group
| LTD group | ||||
|---|---|---|---|---|
| Patient characteristics | <1 cm | 1-2 cm | >2 cm | P-valueb |
| Ipsilateral cingulum | ||||
| No. of subjects (LTD range) | 14 | 29 | 17 | |
| LTD range (cm) | 0.00-0.72 | 1.20-1.99 | 2.01-6.07 | |
| Mean age ± SDb (years) | 47.9 ± 16.0 | 46.3 ± 14.5 | 52.9 ± 14.5 | .35 |
| Gender (% male) | 79 | 55 | 71 | .27 |
| Handedness (% right) | 64 | 90 | 88 | .12 |
| Tumor grade (% high gradec) | 50 | 48 | 71 | .31 |
| Ipsilateral CST | ||||
| No. of subjects (LTD range) | 39 | 9 | 12 | |
| LTD range (cm) | 0.00-0.87 | 1.10-1.99 | 2.05-5.13 | |
| Mean age ± SDc (years) | 47.3 ± 15.2 | 55.7 ± 7.5 | 47.5 ± 17.4 | .31 |
| Gender (% male) | 67 | 56 | 67 | .81 |
| Handedness (% right) | 82 | 100 | 75 | .54 |
| Tumor grade (% high gradec) | 46 | 67 | 75 | .16 |
| Left SLF | ||||
| No. of subjects | 27 | 13 | 20 | |
| LTD range (cm) | 0.00-0.98 | 1.03-1.97 | 2.73-7.31 | |
| Mean age ± SDb (years) | 56.2 ± 14.2 | 51.6 ± 11.8 | 44.5 ± 13.3 | .29 |
| Gender (% male) | 78 | 38 | 65 | .072 |
| Handedness (% right) | 89 | 85 | 75 | .66 |
| Tumor grade (% high graded) | 56 | 69 | 45 | .31 |
aLTD: lesion-to-tract distance.
b P-value refers to Fisher's exact test or ANOVA.
cSD: standard deviation.
dHigh grade: grade III, grade IV, or metastasis.
Total resection was performed for 24 subjects, subtotal resection for 24 subjects, and partial resection for 11 subjects. One patient did not have postoperative imaging available for evaluation of extent of resection. There were no significant differences in extent of resection between LTD groups for all 3 tracts and between subjects with low- and high-grade tumors.
Analyses of Functional Deficits
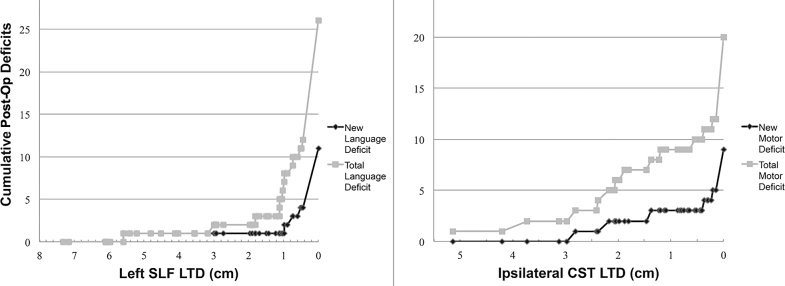
Cumulative distribution of new and total postoperative language and motor deficits as a function of LTD is shown in Figure 2. “Total deficits,” in this case, is defined as the sum of new postoperative deficits and old deficits that persisted despite surgery. A significant relationship was found between left SLF LTD (grouped as described in the Image Analysis and Interpretation section) and the existence of total postoperative language deficits (P < .001), with 74% of subjects with LTD <1 cm having language deficits compared to only 31% and 10% of subjects with LTD 1 to 2 cm and >2 cm, respectively. Furthermore, a significant relationship was found between left SLF LTD and occurrence of new postoperative language deficits (P = .003). A significant relationship was found between ipsilateral CST LTD and the existence of total postoperative motor deficits (P = .018), with 46% of subjects with LTD <1 cm having motor deficits compared to only 11% and 8% of subjects with LTD 1 to 2 cm and >2 cm, respectively. However, limiting this analysis to frontal tumors only did not yield significance (P = .730). There was no significant relationship between ipsilateral CST LTD and occurrence of new postoperative motor deficits (P = .255). No significant relationships were observed for ipsilateral cingulum LTD and postoperative functional outcomes.
FIGURE 2.
Left: cumulative language deficits are shown as a function of left SLF LTD, with comparison of new vs total (new deficits + old, persistent deficits) postoperative language deficits. Right: cumulative motor deficits as a function of ipsilateral CST LTD, with comparison of total vs new postop motor deficits.
The average left SLF LTD for new language deficits (0.7 ± 1.7 cm) compared to no new language deficits (2.3 ± 2.2 cm) was significantly different (Figure 3, P = .013). To investigate which LTD threshold predicted the new language deficit rate most accurately, LTD measurements were grouped into high- and low-LTD groups with threshold values ranging from 0.5 to 2 cm and compared to new language deficits. Agreements of observations with statistically significant differences from random occurrences (Fisher's exact test) were observed for LTD cutoff values between 0.5 and 1.5 cm, with the maximum agreement found at an LTD threshold of 1 cm (Figure 4, P < .001).
FIGURE 3.
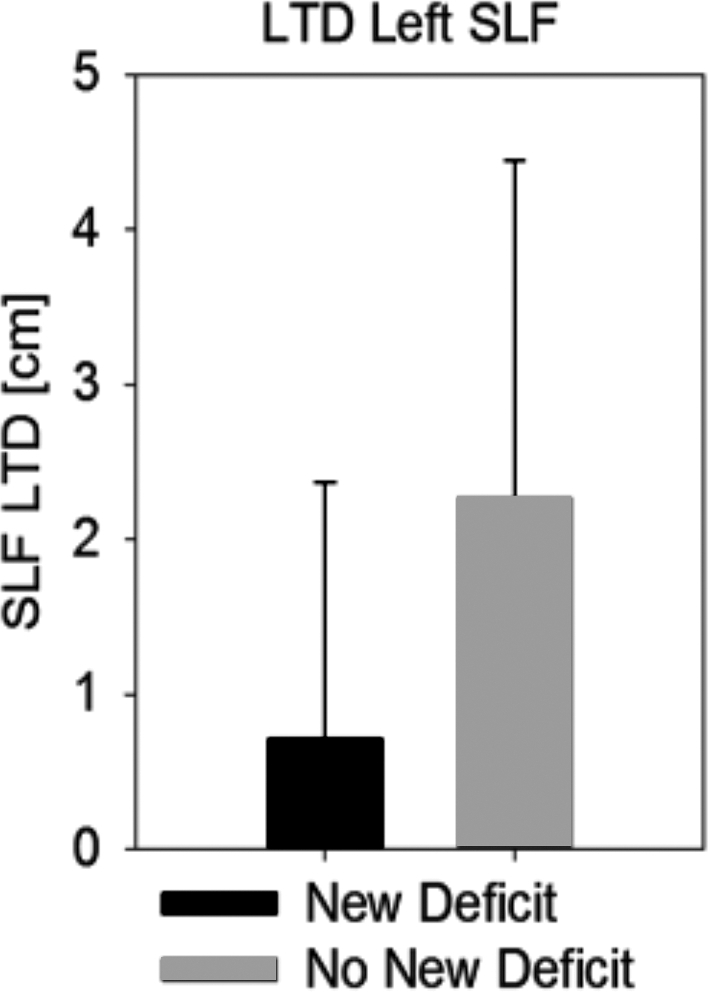
Average left SLF LTD (+/–SD) between subjects with new postoperative language deficits and subjects with no new language deficits (P = .013**).
FIGURE 4.
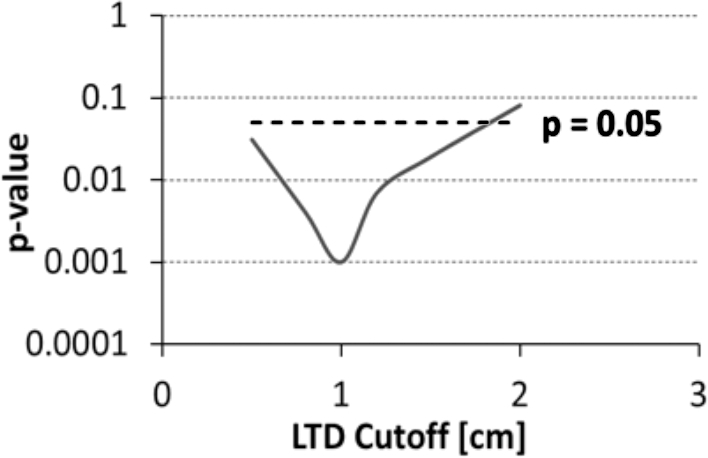
P-values (plotted logarithmically in the vertical axis of the figure) of left SLF LTD as a function of LTD cutoff value used as threshold for defining low vs high SLF LTD when comparing the rate of new surgical deficits.
Univariate logistic regression analyses investigating the dependency of new postoperative language deficits on tumor size, grade, and left and right-sided cingulum, and left SLF LTD yielded no significant findings. Narrowing the logistic regression to the highest ranking independent factors, left-sided SLF LTD and tumor grade, was nearly significant for left-sided SLF LTD (P = .055) but not for tumor grade. Selecting only subjects with left hemispheric dominance (n = 54) resulted in a significant dependency of new postoperative language deficits on left SLF LTD (P = .038), but not tumor grade. Logistic regression analysis investigating the dependence of new postoperative motor deficits on tumor size, grade, left and right (or minimum between left and right) cingulum, and CST LTD yielded no significance.
Survival Analyses
Of 60 subjects, 28 expired prior to this analysis. Cox regression analyses demonstrated that age (HR = 1.054, CI: 1.007-1.082, P = .002) and presence of high-grade tumor (HR = 12.66, CI: 3.731-43.48, P < .001) were significantly predictive of mortality. Gender, extent of resection, presence of postoperative language and motor deficits, and tumor hemisphere were not predictive of mortality. All further survival analyses were stratified by tumor grade, and 1 cm was used as the LTD threshold based on results from Figure 4.
There was no significant difference between LTD groups for subjects with low-grade tumors (n = 27). Figure 5 shows the Kaplan–Meier curves for high-grade tumors grouped into left SLF LTD <1 cm (n = 15) and >1 cm (n = 18). Pairwise, there is a significant difference between the survival curves for high-grade tumors and low-grade tumors (P < .001), and between the two high-grade curves (χ2 = 6.678, P = .01). There were no significant differences in survival rate due to LTD to ipsilateral cingulum (χ2 = 0.026, P = .873) or CST (χ2 = 0.013, P = .910).
FIGURE 5.
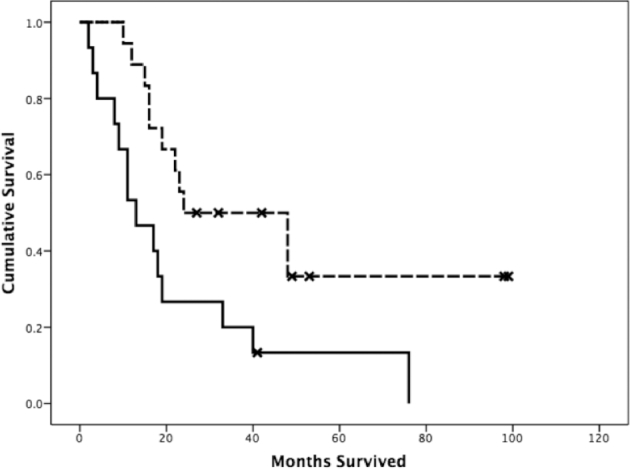
Kaplan–Meier log-rank survival functions (measured in months from DTI) grouped by left SLF LTD and tumor grade. The dashed line represents subjects with high-grade tumors and SLF LTD > 1 cm, while the bottom solid line represents subjects with high-grade tumors with SLF LTD < 1 cm.
DISCUSSION
DTI is an increasingly important part of preoperative planning and brain mapping for the purpose of maximizing tumor resection and minimizing the occurrence of postoperative deficits.7,18 However, the relationship between tumor proximity to major white matter tracts and surgical outcomes has not been completely characterized. In this retrospective study, we explored this relationship by measuring the LTD. This is analogous to the published lesion-to-activation distance for fMRI mapping of cortical function.13,14,19 We compared the LTD metric for 3 selected major white matter structures with postoperative functional deficits and survival time from date of DTI to investigate morbidity and mortality, respectively, taking into account critical factors such as tumor grade, tumor size, extent of resection, and hemispheric dominance (for language deficits).
This study demonstrates that left SLF LTD is significantly related to both new and total postoperative language deficits, and ipsilateral CST LTD is significantly related to total postoperative motor deficits. Left SLF LTD is significantly different for patients with and without postoperative deficits. The LTD threshold to create new deficits lies between 0.5 and 1.5 cm, as demonstrated by comparing the statistical significance for predicting new surgical language deficits from their thresholded left SLF LTD to actual new deficits over the same range. This significance was maximized at 1 cm with P < .001. A threshold of 1 cm has appeared several times in the literature, across different imaging modalities.14,20 The specific cutoff value is likely dependent on imaging and postprocessing technology as well as surgical techniques. New developments in high-resolution diffusion imaging, intraoperative MRI, and higher field strengths can improve the ability to accurately delineate tumor borders and critical white matter boundaries, allowing increasingly precise LTD measurement.21
The cumulative distributions for language and motor deficits (Figure 2) demonstrate that there are new deficits even with LTD >2 cm, suggesting that other mechanisms or dependencies on eloquent brain areas more distal to the white matter tracts influence the outcome. This includes the distance to cortical gray matter areas associated with the specific functional network.13 It is also possible that fewer deficits are seen in language systems, which are able to compensate due to their reliability on a more distributed network (multiple areas and tracts involved in language processing), in comparison to the motor system which is known to be “hard-wired” primarily to the primary motor cortex and CST. A combination of the LTD for white matter pathways with the lesion-to-activation distance for eloquent gray matter may provide a more complete morbidity prediction model.
Furthermore, our study demonstrates that, especially for high-grade tumors, the prediction of postoperative mortality can be improved by considering the LTD metric in addition to tumor grade. By grouping survival curves into low and high LTD in patients with high-grade tumors (using the cutoff of 1 cm as determined by our morbidity data), we demonstrated that higher left SLF LTDs are associated with longer survival time (P = .01). One reason for this finding may be that a large tumor is more likely to encroach on critical white matter tracts and surgeons need to be more aggressive removing larger tumors. Another reason may be that aggressive resections necessitated by invasive, encroaching tumors are inevitably subtotal. Another factor that may play a role is the patients’ quality of life postsurgery and their ability to interact with their environment.22 It must be emphasized that postoperative mortality is not equivalent to overall survival time. Overall survival is influenced by many variables and requires tracking patients longitudinally to confirm that advanced neuroimaging-aided preoperative planning leads to longer overall survival time.
Limitations
A significant limitation to our survival study is the lack of data on glioma histology and molecular genetics. This study was not designed to comprehensively address the question of survival time, and the relationship between LTD and survival time should be an area for further investigation.
While our study has shown a relationship between LTD and postoperative morbidity and mortality, these outcomes are more directly a function of extent of resection and the deft application of intraoperative subcortical stimulation to identify eloquent white matter and neuronavigation using registered DTI tractography. A limitation of this study may be that the precise extent of resection and exact surgical resection margins are not taken into account by our analysis. The interpretation of the results is limited to the analysis of LTD as measured from preoperative images and extent of resection categorized based on postoperative images. A detailed analysis of surgical resection margins requires postoperative DTI data, since nonlinear brain shifts impede accurate anatomic registration of preoperative DTI with postoperative computed tomography or anatomic MRI. This resection-to-tract distance would allow analyzing mortality and morbidity with respect to actual resection boundaries and comparing those to preoperative LTD data. The measurement of LTD using tumor borders from preoperative tumor MR images can have some variability for invasive tumors where the tumor borders are not clearly delineated. Adding to this variability is the limitation afforded by a 4-mm slice thickness.
The selection criteria for this study include patients that underwent functional imaging as part of presurgical planning. This introduces bias and may account for the high number of cases with lesions in the left hemisphere. This may also help explain the relatively low number of total resections, as our patient selection was biased toward those with lesions judged to be proximal to functional areas. It may also be that resections with any residual enhancement <10% were necessarily classified as subtotal, even if the enhancement was not definitively residual tumor. Furthermore, this study does not take into account the varying surgical approaches for different tumor types. The approach to glioma (n = 54) resection differs from brain metastases or meningiomas, and these differences need to be considered to completely understand the relationship between LTD and surgical outcomes. Finally, while we treat SLF as one tract, recent literature has subdivided the SLF into 3 to 4 distinct tracts, SLF I to III and the arcuate fasciculus (the arcuate fasciculus occasionally being considered entirely distinct from the SLF). We chose the SLF to be consistent with its use as a major tract involved in language.23 Our study is retrospective and spans over 10 yr; the methodology does not allow us to make a distinction of the subcomponents of the SLF.
CONCLUSIONS
This study takes steps toward establishing the value of measuring tumor proximity to major white matter tracts. LTD, as measured by DTI, can be used with relevant clinical information to inform clinicians of the likelihood of patient morbidity and mortality after resection of intracranial tumors, allowing clinicians and patients alike to make better informed decisions about the course of management. The evidence provided is especially valuable given the already proven utility of DTI in image-guided surgical resection of brain tumors.
Disclosures
This work was supported by UW Institute of Clinical and Translational Research CTSA program, through the NIH National Center for Advancing Translational Sciences NCATS, grant UL1TR000427 KL2 Scholar and Pilot Awards (VP); K23NS086852 NIH NINDS (VP); American Heart Association Midwest Postdoctoral Fellowship (VN), RC1MH090912-01 National Institute of Health, National Institute of Mental Health Challenge Grant (VN, VP, EM); Foundation of ASNR Award (VP); and by a training grant from the Computation and Informatics in Biology and Medicine Program at the UW-Madison and the National Library of Medicine NLM 5T15LM007359 (WG).
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Supplementary data are available at NEUROS online.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.neurosurgery-online.com).
Acknowledgments
We would like to thank C.H. Moritz and David Huss for their contribution to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.neurosurgery-online.com).
COMMENTS
The authors have taken a different tack to answer the question: “What are the risks associated with surgery near vital white matter tracts?” They have examined the preoperative distance from the tumor to the selected tracts (LTD) and found that distance matters. This finding is consistent with cited papers looking at subcortical mapping and DTI tractography. Indeed, they have even established that in high-grade tumors, lower LTD results in decreased survival. They add to the literature suggesting that worsened functional outcome results in worsened survival for high-grade tumors. Hopefully, future studies will strengthen our understanding of what risks neurosurgeons take in approaching these regions of the brain and how to lessen them and our ability to avoid these risks. These results appear to reinforce the tenet of maximal safe resection, providing further emphasis on the safety of the surgery.
Amit Goyal
Matthew A. Hunt
Minneapolis, Minnesota
This retrospective study investigates the relationship that the distance of intracranial neoplasms to vital white matter tracts plays in postoperative morbidity and mortality. It is of interest as DTI is increasingly used in preoperative planning for tumors near eloquent areas of the brain. The authors retrospectively examined the imaging and patient records for 60 patients to determine the role of DTI in predicting postoperative outcomes. This study confirms many things that were assumed to be true regarding lesion proximity to vital tracts. Namely, they found that lesion to distance (LTD) to SLF was significantly associated with preoperative and new postoperative language deficits. Interestingly, LTD to CST was not significantly associated with new motor deficits. The size and grade of tumors were not associated with deficits, suggesting that tumor location was key.
The authors commendably recognize the limitations of the retrospective nature of this study. Given that many of these vital tracts are often identified by direct stimulation intraoperatively, they may already be damaged by the point the surgeon is in position to test them. This study emphasizes that the thresholds of distance to these tracts need to be respected.
Michael Schulder
Manhasset, New York
REFERENCES
- 1. Sanai N, Berger MS. Operative techniques for gliomas and the value of extent of resection. Neurotherapeutics. 2009;6(3):478-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ius T, Isola M, Budai R et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg. 2012;117(6):1039-1052. [DOI] [PubMed] [Google Scholar]
- 3. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358(1):18-27. [DOI] [PubMed] [Google Scholar]
- 4. Zhu FP, Wu JS, Song YY et al. Clinical application of motor pathway mapping using diffusion tensor imaging tractography and intraoperative direct subcortical stimulation in cerebral glioma surgery: a prospective cohort study. Neurosurgery. 2012;71(6):1183-1174; discussion 1170-1183. [DOI] [PubMed] [Google Scholar]
- 5. Abdullah KG, Lubelski D, Nucifora PG, Brem S. Use of diffusion tensor imaging in glioma resection. Neurosurg Focus. 2013;34(4):E1. [DOI] [PubMed] [Google Scholar]
- 6. Wu JS, Zhou LF, Tang WJ et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61(5):948-939; discussion 935-948. [DOI] [PubMed] [Google Scholar]
- 7. Ulmer JL, Salvan CV, Mueller WM et al. The role of diffusion tensor imaging in establishing the proximity of tumor borders to functional brain systems: implications for preoperative risk assessments and postoperative outcomes. Technol Cancer Res Treat. 2004;3(6):567-576. [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez-Darder JM, Gonzalez-Lopez P, Talamantes F et al. Multimodal navigation in the functional microsurgical resection of intrinsic brain tumors located in eloquent motor areas: role of tractography. Neurosurg Focus. 2010;28(2):E5. [DOI] [PubMed] [Google Scholar]
- 9. Saksena S, Jain R, Narang J et al. Predicting survival in glioblastomas using diffusion tensor imaging metrics. J Magn Reson Imaging. 2010;32(4):788-795. [DOI] [PubMed] [Google Scholar]
- 10. Kinoshita M, Nakada M, Okita H, Hamada J, Hayashi Y. Predictive value of fractional anisotropy of the arcuate fasciculus for the functional recovery of language after brain tumor resection: a preliminary study. Clin Neurol Neurosurg. 2014;117:45-50. [DOI] [PubMed] [Google Scholar]
- 11. Laundre BJ, Jellison BJ, Badie B, Alexander AL, Field AS. Diffusion tensor imaging of the corticospinal tract before and after mass resection as correlated with clinical motor findings: preliminary data. AJNR Am J Neuroradiol. 2005;26(4):791-796. [PMC free article] [PubMed] [Google Scholar]
- 12. Castellano A, Bello L, Michelozzi C et al. Role of diffusion tensor magnetic resonance tractography in predicting the extent of resection in glioma surgery. Neuro-oncology. 2012;14(2):192-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Voss J, Meier TB, Freidel R et al. The role of secondary motor and language cortices in morbidity and mortality: a retrospective functional MRI study of surgical planning for patients with intracranial tumors. Neurosurg Focus. 2013;34(4):E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wood JM, Kundu B, Utter A et al. Impact of brain tumor location on morbidity and mortality: a retrospective functional MR imaging study. AJNR Am J Neuroradiol. 2011;32(8):1420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kundu B, Penwarden A, Wood JM et al. Association of functional magnetic resonance imaging indices with postoperative language outcomes in patients with primary brain tumors. Neurosurg Focus. 2013;34(4):E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oishi K. MRI Atlas of Human White Matter. 2nd ed Amsterdam: Elsevier Academic Press; 2011. [Google Scholar]
- 17. Sabbah P, Chassoux F, Leveque C et al. Functional MR imaging in assessment of language dominance in epileptic patients. NeuroImage. 2003;18(2):460-467. [DOI] [PubMed] [Google Scholar]
- 18. D’Andrea G, Angelini A, Romano A et al. Intraoperative DTI and brain mapping for surgery of neoplasm of the motor cortex and the corticospinal tract: our protocol and series in BrainSUITE. Neurosurg Rev. 2012;35(3):401-412; discussion 412. [DOI] [PubMed] [Google Scholar]
- 19. Krishnan R, Raabe A, Hattingen E et al. Functional magnetic resonance imaging-integrated neuronavigation: correlation between lesion-to-motor cortex distance and outcome. Neurosurgery. 2004;55(4):904-914; discussion 914-905. [DOI] [PubMed] [Google Scholar]
- 20. Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34(4):567-576; discussion 576. [DOI] [PubMed] [Google Scholar]
- 21. Prabhakaran V, Nair VA, Austin BP et al. Current status and future perspectives of magnetic resonance high-field imaging: a summary. Neuroimaging Clin N Am. 2012;22(2):373-397, xii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65(3):463-469; discussion 469-470. [DOI] [PubMed] [Google Scholar]
- 23. Friederici AD. The brain basis of language processing: from structure to function. Physiol Rev. 2011;91(4):1357-1392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at NEUROS online.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.neurosurgery-online.com).