Abstract
Background
Tinnitus is a symptom defined as the perception of sound in the absence of an external source. In England alone there are an estimated ¾ million general practice consultations every year where the primary complaint is tinnitus, equating to a major burden on healthcare services. Clinical management strategies include education and advice, relaxation therapy, tinnitus retraining therapy, cognitive behavioural therapy, sound enrichment using ear‐level sound generators or hearing aids, and drug therapies to manage co‐morbid symptoms such as sleep difficulties, anxiety or depression. As yet, no drug has been approved for tinnitus by a regulatory body. Nonetheless, over 100,000 prescriptions for betahistine are being filled every month in England, and nearly 10% of general practitioners prescribe betahistine for tinnitus.
Objectives
To assess the effects of betahistine in patients with subjective idiopathic tinnitus.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Register; Central Register of Controlled Trials (CENTRAL, via the Cochrane Register of Studies); Ovid MEDLINE; Ovid Embase; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 23 July 2018.
Selection criteria
Randomised controlled trials (RCTs) recruiting patients of any age with acute or chronic subjective idiopathic tinnitus were included. We included studies where the intervention involved betahistine and this was compared to placebo, no intervention or education and information. We included all courses of betahistine, regardless of dose regimens or formulations and for any duration of treatment.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcomes included tinnitus loudness and significant adverse effects (upper gastrointestinal discomfort). Our secondary outcomes included tinnitus symptom severity as measured by the global score on a multi‐item tinnitus questionnaire, depressive symptoms, symptoms of generalised anxiety, health‐related quality of life, other adverse effects (e.g. headache, drowsiness, allergic skin reactions (pruritis, rashes) and exacerbation of tinnitus) and tinnitus intrusiveness. We used GRADE to assess the quality of evidence for each outcome; this is indicated in italics.
Main results
This review included five studies (with a total of 303 to 305 participants) comparing the effects of betahistine with placebo in adults with subjective idiopathic tinnitus. Four studies were parallel‐group RCTs and one had a cross‐over design. The risk of bias was unclear in all of the included studies.
Due to heterogeneity in the outcomes measured and measurement methods used, very limited data pooling was possible. When we pooled the data from two studies for the primary outcome tinnitus loudness, the mean difference on a 0‐ to 10‐point visual analogue scale at one‐month follow‐up was not significant between betahistine and placebo (‐0.16, 95% confidence interval (CI) ‐1.01 to 0.70; 81 participants) (very low‐quality evidence). There were no reports of upper gastrointestinal discomfort (significant adverse effect) in any study.
As a secondary outcome, one study found no difference in the change in the Tinnitus Severity Index between betahistine and placebo (mean difference at 12 weeks 0.02, 95% CI ‐1.05 to 1.09; 50 participants) (moderate‐quality evidence). None of the studies reported the other secondary outcomes of changes in depressive symptoms or depression, anxiety symptoms or generalised anxiety, or health‐related quality of life as measured by a validated instrument, nor tinnitus intrusiveness.
Other adverse effects that were reported were not treatment‐related.
Authors' conclusions
There is an absence of evidence to suggest that betahistine has an effect on subjective idiopathic tinnitus when compared to placebo. The evidence suggests that betahistine is generally well tolerated with a similar risk of adverse effects to placebo treatments. The quality of evidence for the reported outcomes, using GRADE, ranged from moderate to very low.
If future research into the effectiveness of betahistine in patients with tinnitus is felt to be warranted, it should use rigorous methodology. Randomisation and blinding should be of the highest quality, given the subjective nature of tinnitus and the strong likelihood of a placebo response. The CONSORT statement should be used in the design and reporting of future studies. We also recommend the development of validated, patient‐centred outcome measures for research in the field of tinnitus.
Plain language summary
Betahistine for tinnitus
Background
Tinnitus describes 'ringing', 'whooshing' or 'hissing' sounds that are heard in the absence of any corresponding external sound. Between 5% and 43% of people experience this symptom and for some it has a significant negative impact on their quality of life. Tinnitus can be managed through education and advice, prescription devices that improve hearing, over‐the‐counter devices that generate background sounds, psychological therapy and relaxation therapy. Drug therapies are used to manage complaints associated with tinnitus such as sleep difficulties, anxiety or depression. No drug therapies exist that manage the tinnitus itself. Nonetheless, betahistine is often prescribed for tinnitus. The purpose of this review is to evaluate the evidence from high‐quality clinical trials to work out the effect of betahistine on people's tinnitus. We particularly wanted to look at the effect of betahistine on tinnitus loudness and the side effects of betahistine.
Study characteristics
Our review identified five randomised controlled trials with a total of 303 to 305 participants who suffered from tinnitus. These studies compared participants receiving betahistine to those receiving a placebo. Four study designs allocated participants into parallel groups. In one study, participants consented to take all study medications in a pre‐defined sequence. The outcomes that we evaluated included tinnitus loudness and intrusiveness, tinnitus symptoms and side effects.
Key results
The included studies did not show differences in tinnitus loudness, severity of tinnitus symptoms or side effects between participants receiving betahistine and participants receiving a placebo. No significant side effects were reported. We had planned to evaluate changes in tinnitus intrusiveness, depression and anxiety and quality of life, but these were not measured. The evidence suggests that betahistine is generally well tolerated with a similar risk of side effects to placebo.
Quality of the evidence
The quality of the evidence ranged from moderate to very low. The risk of bias in all of the included studies was unclear. The results were drawn from one or two studies only. In some studies, the participants that were included did not fully represent the entire population of people with tinnitus and so we cannot draw general conclusions.
Summary of findings
Summary of findings for the main comparison. Betahistine compared with placebo for tinnitus.
| Betahistine compared with placebo for tinnitus (without concurrent medication) | ||||||
|
Patient or population: patients with subjective idiopathic tinnitus Settings: departments of otorhinolaryngology in hospitals worldwide Intervention: betahistine Comparison: placebo | ||||||
| Outcomes | Treatment effects | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Betahistine | |||||
|
Tinnitus loudness measured by a visual analogue scale (range 0 to 10/more than 10) Follow‐up: 1 month |
The mean tinnitus loudness ranged across control groups from 4.8 to 8.5 | The mean tinnitus loudness in the intervention groups was 0.2 lower (0.3 lower to 1.0 higher) | ‐0.16 (‐1.01 to 0.70) | 81 (2) | ⊕⊝⊝⊝ very low1 | — |
|
Change in tinnitus loudness measured by a visual analogue scale (range 0 to more than 10) Follow‐up: 28 days |
The mean change in tinnitus loudness was 0.8 in the control group | The mean change in tinnitus loudness in the intervention group was 0.4 lower | ‐0.43 (‐1.20 to 0.34) | 11 (1) | ⊕⊝⊝⊝ very low2 | — |
|
Tinnitus loudness measured by a visual analogue scale (range 0 to 10) Follow‐up: 2 months |
The mean tinnitus loudness was 4.5 in the control group | The mean tinnitus loudness in the intervention group was 0.4 lower | ‐0.39 (‐1.37 to 0.60) | 70 (1) | ⊕⊝⊝⊝ very low3 | — |
|
Significant adverse effects (yes or no) Follow‐up: 28 days |
Study population | Not estimable | 11 (1) | ⊕⊝⊝⊝ very low2 | — | |
| 0 per 1000 | 0 per 1000 | |||||
|
Significant adverse effects (yes or no) Follow‐up: 3 months |
Study population | Not estimable | 41 (1) | ⊕⊕⊕⊝ moderate4 | — | |
| 0 per 1000 | 0 per 1000 | |||||
|
Change in Tinnitus Severity Index (range 0 to 56) Follow‐up: 12 weeks |
The mean change in Tinnitus Severity Index was 1.7 in the control group | The mean change in Tinnitus Severity Index in the intervention group was the same | 0.02 (‐1.05 to 1.09) | 50 (1) | ⊕⊕⊕⊝ moderate4 | — |
|
Tinnitus severity score (range 0 to 4) Follow‐up: 3 months |
The mean tinnitus severity score was 3.1 in the control group | The mean tinnitus severity score in the intervention group was 0.5 lower | ‐0.52 (‐1.34 to 0.30) | 36 (1) | ⊕⊕⊝⊝ low5 | |
| Depressive symptoms | Not measured | |||||
| Symptoms of generalised anxiety | Not measured | |||||
|
Other adverse effects (yes or no) Follow‐up: 28 days |
Study population | RR 3.50 (0.17 to 70.94) | 11 (1) | ⊕⊝⊝⊝ very low2 | — | |
| 0 per 1000 | 200 per 1000 | |||||
|
Other adverse effects (yes or no) Follow‐up: 3 months |
Study population | Not estimable | 41 (1) | ⊕⊕⊕⊝ moderate4 | — | |
| 0 per 1000 | 0 per 1000 | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to an unclear overall risk of bias in both studies; downgraded one level due to inconsistency (one study has a slight preference for betahistine and the other for placebo); downgraded one level due to indirectness (in one study a patient with Ménière's disease was included and in the other studies only male participants/military personnel with noise‐induced hearing loss were included); downgraded one level due to imprecision.
2Downgraded one level due to an unclear overall risk of bias, downgraded one level due to indirectness (a patient with Ménière's disease was included); downgraded one level due to imprecision.
3Downgraded one level due to an unclear overall risk of bias, downgraded one level due to indirectness (only male participants/military personnel with noise‐induced hearing loss were included); downgraded one level due to imprecision.
4Downgraded one level due to an unclear overall risk of bias.
5Downgraded one level due to an unclear overall risk of bias; downgraded one level due to imprecision.
Summary of findings 2. Betahistine versus placebo (with concurrent medication).
| Betahistine compared with placebo for tinnitus (with concurrent medication) | ||||||
|
Patient or population: patients with subjective idiopathic tinnitus Settings: departments of otorhinolaryngology in hospitals worldwide Intervention: betahistine Comparison: placebo (vitamin B6) | ||||||
| Outcomes | Treatment effects | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo (vitamin B6) | Betahistine | |||||
|
Change in tinnitus loudness match (range 0 to 5) Follow‐up: 1 week |
The mean tinnitus loudness match was 2.5 in the control group | The mean tinnitus loudness match in the intervention group was 0.1 lower | ‐0.10 (‐0.50 to 0.30) | 60 (1) | ⊕⊕⊕⊝ moderate1 | |
|
Significant adverse effects (yes or no) Follow‐up: 1 week |
Study population | RR 3.10 (0.13 to 73.14) | 59 (1) | ⊕⊕⊝⊝ low2 | ||
| 34 per 1000 | 0 per 1000 | |||||
| Tinnitus symptom severity | Not measured | |||||
| Depressive symptoms | Not measured | |||||
| Symptoms of generalised anxiety | Not measured | |||||
|
Other adverse effects (yes or no) Follow‐up: 1 week |
Study population | RR 0.44 (0.13 to 1.55) | 59 (1) | ⊕⊕⊕⊝ moderate1 | ||
| 103 per 1000 | 233 per 1000 | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to an unclear overall risk of bias.
2Downgraded one level due to an unclear overall risk of bias; downgraded one level due to imprecision.
Background
The 'Description of the condition' and 'Diagnosis and clinical management of tinnitus' sections below are based on the Cochrane Review 'Amplification with hearing aids for patients with tinnitus and co‐existing hearing loss' (Hoare 2014). Other sections are based on the Cochrane protocol 'Betahistine for Ménière's disease or syndrome' (van Esch 2018). They are both reproduced with permission.
Tinnitus is defined as the perception of sound in the absence of an external source (Jastreboff 2004). It is typically described by those who experience it as a ringing, hissing, buzzing or whooshing sound and is thought to result from abnormal neural activity at some point or points in the auditory pathway, which is erroneously interpreted by the brain as sound. Tinnitus can be either objective or subjective. Objective tinnitus refers to the perception of sound that can also be heard by the examiner and is usually due to turbulent blood flow or muscular contraction (Roberts 2010). Most commonly, however, tinnitus is subjective; the sound is only heard by the person experiencing it and no source of the sound is identified (Jastreboff 1988).
Tinnitus affects between 5% and 43% of the general population and prevalence increases with age (McCormack 2016). It can be experienced acutely, recovering spontaneously within minutes to weeks, but is considered chronic and unlikely to resolve spontaneously when experienced for more than three months (Gallus 2015; Hall 2011).
For many people, tinnitus is persistent and troublesome, and has disabling effects such as insomnia, difficulty concentrating, difficulties in communication and social interaction, and negative emotional responses such as anxiety and depression (Hall 2018a). In approximately 90% of cases, chronic subjective tinnitus is co‐morbid with some degree of hearing loss, which may confound these disabling effects (Fowler 1944; Sanchez 2002). Nevertheless, the association between hearing loss and tinnitus is not simple or straightforward; not all people with hearing loss experience tinnitus, and conversely some people with clinically normal hearing have tinnitus (Baguley 2013). It has been reported that 40% of patients are unable to identify what health condition is associated with their tinnitus onset, i.e. the tinnitus is idiopathic (Henry 2005). Hence for the purposes of this review, tinnitus that occurs in association with a hearing loss is considered to be idiopathic tinnitus, since the causal link is not known for certain.
Description of the condition
Diagnosis and clinical management of tinnitus
There is no standard procedure for the diagnosis or management of tinnitus. Practice guidelines and the approaches described in studies of usual clinical practice typically reflect differences between the clinical specialities of the authors or differences in the clinical specialities charged with meeting tinnitus patients' needs (medical, audiology/hearing therapy, clinical psychology, psychiatry), or the available resources of a particular country or region (access to clinicians or devices, for example) (Biesinger 2011; Cima 2012; Department of Health 2009; Hall 2011; Henry 2008; Hoare 2011). Common across current clinical guidelines for the assessment of subjective tinnitus (Fuller 2017) is a recommendation for the use of written questionnaires to assess tinnitus and its impact on patients by measuring tinnitus symptom severity (e.g. impact of tinnitus on quality of life, activities of daily living or sleep), and a judgement about patients who are experiencing a degree of psychological distress (including depression or anxiety).
Clinical management strategies include education and advice, relaxation therapy, tinnitus retraining therapy (TRT), cognitive behavioural therapy (CBT), sound enrichment using ear‐level sound generators or hearing aids, and drug therapies to manage co‐morbid symptoms such as sleep difficulties, anxiety or depression (for example, Department of Health 2009; Tunkel 2014). As yet, no drug has been approved for tinnitus by a regulatory body (e.g. the European Medicines Agency or US Food and Drug Administration).
Pathophysiology
Many people with chronic tinnitus have some degree of measurable hearing loss (Ratnayake 2009), and the prevalence of tinnitus increases with greater hearing loss (Han 2009; Martines 2010). The varying theories of tinnitus generation involve changes in either function or activity of the peripheral (cochlea and auditory nerve) or central auditory nervous systems (Henry 2005). Theories involving the peripheral systems include the discordant damage theory, which predicts that the loss of outer hair cell function, where inner hair cell function is left intact, leads to a release from inhibition of inner hair cells and aberrant activity (typically hyperactivity) in the auditory nerve (Jastreboff 1990). Such aberrant auditory nerve activity can also have a biochemical basis, resulting from excitotoxicity or stress‐induced enhancement of inner hair cell glutamate release with upregulation of N‐methyl‐D‐aspartate (NMDA) receptors (Guitton 2003; Sahley 2001).
In the central auditory system, structures implicated as possible sites of tinnitus generation include the dorsal cochlear nucleus (Middleton 2011; Pilati 2012), the inferior colliculus (Dong 2010; Mulders 2010), and the auditory and non‐auditory cortex (discussed further below). There is a strong rationale that tinnitus is a direct consequence of maladaptive neuroplastic responses to hearing loss (Moller 2000; Mühlnickel 1998). This process is triggered by sensory deafferentation and a release from lateral inhibition in the central auditory system allowing irregular spontaneous hyperactivity within the central neuronal networks involved in sound processing (Eggermont 2004; Rauschecker 1999; Seki 2003). As a consequence of this hyperactivity, a further physiological change noted in tinnitus patients is increased spontaneous synchronous activity occurring at the subcortical and cortical level, measurable using electroencephalography (EEG) or magnetoencephalography (MEG) (Dietrich 2001; Tass 2012; Weisz 2005). Another physiological change thought to be involved in tinnitus generation is a process of functional reorganisation, which amounts to a change in the response properties of neurons within the primary auditory cortex to external sounds. This effect is well demonstrated physiologically in animal models of hearing loss (Engineer 2011; Noreña 2005). Evidence in humans, however, is limited to behavioural evidence of cortical reorganisation after hearing loss, demonstrating improved frequency discrimination ability at the audiometric edge (Kluk 2006; McDermott 1998; Moore 2009; Thai‐Van 2002; Thai‐Van 2003), although Buss 1998 did not find this effect. For comprehensive reviews of these physiological models, see Adjamian 2009 and Noreña 2011.
It is also proposed that spontaneous hyperactivity could cause an increase in sensitivity or 'gain' at the level of the cortex, whereby neural sensitivity adapts to the reduced sensory inputs, in effect stabilising mean firing and neural coding efficiency (Noreña 2011; Schaette 2006; Schaette 2011). Such adaptive changes would be achieved at the cost of amplifying 'neural noise' due to the overall increase in sensitivity, ultimately resulting in the generation of tinnitus.
Increasingly, non‐auditory areas of the brain, particularly areas associated with emotional processing, are also implicated in bothersome tinnitus (Rauschecker 2010; Vanneste 2012). Vanneste 2012 describes tinnitus as "an emergent property of multiple parallel dynamically changing and partially overlapping sub‐networks", implicating the involvement of many structures of the brain more associated with memory and emotional processing in tinnitus generation. However, identification of the structural components of individual neural networks responsible for either tinnitus generation or tinnitus intrusiveness, which are independent of those for hearing loss, remains open to future research (Melcher 2013). One further complication in understanding the pathophysiology of tinnitus is that not all people with hearing loss have tinnitus and not all people with tinnitus have a clinically significant and measurable hearing loss. Other variables, such as the profile of a person's hearing loss, may account for differences in their tinnitus report. For example, König 2006 found that the maximum slope within audiograms was higher in people with tinnitus than in people with hearing loss who do not have tinnitus, despite the 'non‐tinnitus' group having the greater mean hearing loss. This suggests that a contrast in sensory inputs between regions of normal and elevated threshold may be more likely to result in tinnitus. However, this finding is not consistent across the literature (Sereda 2011; Sereda 2015).
Description of the intervention
First registered in 1968, betahistine is an oral drug that, by 2006, was estimated to have been prescribed to more than 130 million people worldwide (Jeck‐Thole 2006). The indication for taking betahistine is to treat patients with Ménière's disease (Electronic Medicines Compendium 2015). However, many patients are given betahistine off‐licence to treat idiopathic subjective tinnitus (Hall 2011; McFerran 2018) and vertigo (Murdin 2016), even when these symptoms are not associated with Ménière's disease. The recommended daily dose of betahistine is 24 mg to 48 mg per day divided into two or three single doses containing 8 mg, 16 mg or 24 mg (Jeck‐Thole 2006). Although gastrointestinal side effects are cited in many formularies, the rate of adverse effects in patients taking betahistine is not significantly different from those taking placebo in comparison studies (Murdin 2016).
Betahistine hydrochloride (or dihydrochloride) is a derivative of betahistine. Alternative formulations of betahistine include betahistine mesylate (or mesilate), dimesylate and maleate.
How the intervention might work
Betahistine is a weak histamine H1 receptor agonist and a potent histamine H3 receptor antagonist. Studies have shown that betahistine reaches a peak plasma concentration in about one hour and it has a plasma half‐life of approximately 3.5 hours (Electronic Medicines Compendium 2015). The maximal vestibular therapeutic effect will last approximately three to four hours (Electronic Medicines Compendium 2015).
The various effects of betahistine have been examined in controlled animal experiments showing effects on cochlear blood flow (Dziadziola 1999; Laurikainen 1993; Martinez 1972) and vestibular nerve firing rate (Unemoto 1982). Despite this body of work investigating the efficacy of betahistine, little is known about its mechanism of action. One postulated mechanism is a reduction in endolymphatic pressure through vasodilation of the vessels of the stria vascularis and spiral ligament of the lateral wall of the cochlea (Martinez 1972). For example, Dziadziola 1999 demonstrated that with histamine receptor activation, venules and arterioles larger than approximately 80 mm constrict, and vessels smaller than 80 mm dilate. An abnormality in endolymphatic pressure can lead to symptoms of hearing loss, tinnitus and vertigo.
Betahistine could also have effects on the symptoms of tinnitus via central nervous system activity. Betahistine can cross the blood‐brain barrier, the cell bodies of histamine‐containing neurons project throughout the brain, including the ventromedial hypothalamic nucleus, the thalamus and the cerebral cortex, and betahistine has measurable effects on regional cerebral blood flow (Barak 2008). Data from a single double‐blind, placebo‐controlled clinical study suggested significant effects of betahistine on some cognitive function tests (Pathy 1977). Inhibition of activity in the vestibular nuclei may contribute to rebalancing neural activity and expedite the recovery process (Lacour 2007; Timmerman 1994).
For Ménière's disease/syndrome the pharmacological characteristics of betahistine are thought to reduce the intensity and duration of vertigo symptoms in the short term (under three months) and additionally prevent attacks in the longer term (over three months). For tinnitus, betahistine is thought to work by reducing pressure and improving blood flow to certain areas within the inner ear. It is not fully understood how betahistine actually interacts within the inner ear to reduce tinnitus symptoms, but it is possible that the effect on tinnitus is secondary to an amelioration in hearing. Based on current knowledge, one might therefore speculate that betahistine is most likely to be effective in cases where a non‐specific tinnitus is associated with hearing difficulties (or vertigo).
In some countries, prescription behaviour differs according to whether tinnitus is acute (less than three months) or chronic (three months or more) (Hall 2011). This may reflect a belief that duration of tinnitus is a modifier of treatment success.
Why it is important to do this review
In England alone there are an estimated ¾ million general practice consultations every year where the primary complaint is tinnitus (El‐Shunnar 2011), equating to a major burden on healthcare services. A study reported over 100,000 prescriptions for betahistine being filled every month in England (Phillips 2008), and nearly 10% of general practitioners prescribe betahistine for tinnitus. There is a published Cochrane Review of betahistine for Ménière's disease (idiopathic) or Ménière's syndrome (secondary to established inner ear disorders) (James 2001), and a new protocol for an update (van Esch 2018). Both examine tinnitus as a secondary outcome. However, there is no existing Cochrane Review of betahistine for tinnitus as a primary outcome, without the co‐morbidities of vertigo and hearing loss. Assessment of the effect of betahistine in the treatment of subjective idiopathic tinnitus is therefore warranted.
Objectives
To assess the effects of betahistine in patients with subjective idiopathic tinnitus.
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
randomised controlled trials (RCTs), including cluster‐randomised (cross‐over trials were eligible if data from before the cross‐over could be extracted, to avoid the potential for a carry‐over phenomenon).
We excluded studies with the following design characteristics:
quasi‐randomised controlled studies.
We applied no restrictions on language, year of publication or publication status.
Types of participants
Patients of any age with acute or chronic subjective idiopathic tinnitus. Participants who had received betahistine previously were eligible for inclusion.
Patients with tinnitus as part of Ménière’s disease were not considered to have idiopathic tinnitus, because tinnitus is one of the defining features of the diagnosis. Therefore, we excluded studies that included a majority (more than 50%) of patients with Ménière’s disease. We considered patients with tinnitus and other co‐morbid conditions to have idiopathic tinnitus, because tinnitus is not a diagnostic feature in these disorders. Although tinnitus has been associated with a number of otologic conditions, such as noise‐induced hearing loss, presbyacusis and otosclerosis, there is no proven mechanistic link.
Types of interventions
We included all courses of betahistine, regardless of dose regimens or formulations and for any duration of treatment.
The comparators were placebo, no intervention or education and information only.
The main comparison was:
betahistine versus placebo.
Other possible comparison pairs included betahistine versus no intervention, or betahistine versus education and information only, but none of the included studies were of this design.
Concurrent use of other medication or other treatment was acceptable if used equally in each group. For example, betahistine with an additional intervention versus placebo with an identical additional intervention. Where an additional intervention was used equally in both groups, we planned to analyse this as a separate comparison.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Primary outcomes
Tinnitus loudness (a change in subjective perception) measured using either patient‐reported instruments (including visual analogue scales or numerical rating scales) or performance‐based procedures (including tinnitus loudness matching or minimum masking level).
Significant adverse effects: upper gastrointestinal discomfort.
Secondary outcomes
-
Tinnitus symptom severity (such as the impact of tinnitus on quality of life, activities of daily living and sleep), as measured by the global score on a multi‐item tinnitus questionnaire (Table 3). These included:
Tinnitus Functional Index (Meikle 2012);
Tinnitus Handicap Inventory (Newman 1996);
Tinnitus Handicap Questionnaire (Kuk 1990);
Tinnitus Reaction Questionnaire (Wilson 1991);
Tinnitus Questionnaire (Hallam 2009; Hiller 2006); and
Tinnitus Severity Scale (Sweetow 1990).
Depressive symptoms or depression as measured by a validated instrument including the Beck Depression Inventory (Beck 1988; Beck 1996), the depression scale of the Hospital Anxiety and Depression Scale (HADS; Zigmond 1983), or the Hamilton Rating Scale for Depression (Hamilton 1960).
Anxiety symptoms or generalised anxiety as measured by a validated instrument including the Beck Anxiety Inventory (Beck 1988a), the anxiety scale of the HADS (Zigmond 1983), or the Anxiety Sensitivity Index (Reiss 1986).
Health‐related quality of life as measured by any appropriate scale including the Short‐Form Health Survey (Hays 1993), WHOQOL‐BREF (Skevington 2004), other WHOQOL versions and the Health Utilities Index (Furlong 2001).
Other adverse effects: headache, drowsiness, allergic skin reactions (pruritis, rashes) and exacerbation of tinnitus.
1. Examples of questionnaires measuring tinnitus symptom severity.
| Measurement instrument (author, year) | Number of items and subscales | Internal consistency (Cronbach's alpha for the global score) |
| Tinnitus Functional Index (Meikle 2012) | 25 items, 8 subscales | 0.97 |
| Tinnitus Handicap Inventory (Newman 1996) | 25 items, 3 subscales | 0.93 |
| Tinnitus Handicap Questionnaire (Kuk 1990) | 27 items, 3 subscales | 0.94 |
| Tinnitus Reaction Questionnaire (Wilson 1991) | 26 items | 0.96 |
| Tinnitus Questionnaire, English version (Hallam 2009) | 52 items, 5 subscales | 0.94 |
| Tinnitus Questionnaire, German version (Hiller 2006) | 52 items, 6 subscales | 0.93 |
| Tinnitus Severity Scale (Sweetow 1990) | 15 items | Not reported |
In addition, we planned to report the new core outcome for trials of pharmacological interventions for tinnitus, this being tinnitus intrusiveness (Hall 2018b), as measured by a single‐item patient‐reported visual analogue scale or numerical rating scale. However, there were no reported outcomes of this type.
Based on the pharmacological properties of the drug described above, we planned to assess outcomes as short‐term (less than three months) and long‐term (three to six months). We also planned to consider whether these outcomes were sustained beyond six months, but the included studies did not conduct any long‐term follow‐up beyond six months.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 23 July 2018.
Electronic searches
The Information Specialist searched:
the Cochrane ENT Register (searched via the Cochrane Register of Studies 23 July 2018);
the Cochrane Central Register of Controlled Trials (CENTRAL) (searched via the Cochrane Register of Studies 23 July 2018);
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 23 July 2018);
Ovid EMBASE (1974 to 23 July 2018);
LILACS (Latin American and Caribbean Health Science Information database), lilacs.bvsalud.org (searched 23 July 2018);
Web of Knowledge, Web of Science (1945 to 23 July 2018);
ClinicalTrials.gov (searched via the Cochrane Register of Studies 23 July 2018);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), www.who.int/ictrp (searched 23 July 2018).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors if necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
We did not perform a separate search for adverse effects of betahistine for tinnitus. We considered adverse effects described in the included studies only.
Data collection and analysis
Selection of studies
Two authors (DAH and DM) independently scanned the initial search results to identify studies that appeared to meet the inclusion criteria. The authors then reviewed the full‐text articles of the retrieved studies and applied the inclusion and exclusion criteria independently. Any differences in opinion about which studies to include in the review were resolved by discussion.
Data extraction and management
Three authors (IW, DAH and ALS) independently extracted data from the studies using a purposefully designed data form (see Appendix 2). We extracted data so as to allow an intention‐to‐treat analysis. We piloted the data extraction form on a subset of articles and revised it as indicated before formal data extraction began.
Information extracted included: trial design, country of recruitment, setting, funding, conflict of interest (any author), methods of randomisation and blinding, power, number of participants, inclusion and exclusion criteria, type of intervention and control(s), total dose per day (mg), method of administration, concomitant treatment, treatment duration, treatment fidelity, type and duration of follow‐up, definition of outcomes and endpoints, and statistical tests.
Data extracted included: baseline characteristics of participants (age, sex, duration of tinnitus, tinnitus symptom severity, tinnitus loudness estimates, details of co‐morbid hearing loss, anxiety or depression), and details of any attrition or exclusion.
Outcome data extracted included: for continuous data the group mean, standard deviation and number of participants for each treatment group at pre‐ and post‐intervention and follow‐up, and results of any statistical tests of between‐group comparisons. For dichotomous data, the number of participants experiencing an event and the total number of participants assessed at the time point were extracted. For ordinal data, the number of participants in each category and the total number of participants assessed at the time point were extracted.
With regard to subgroup analysis, we planned to extract data to allow grading according to duration of tinnitus and treatment protocol (dose and duration of drug treatment). If betahistine doses differed among the intervention groups within a study, we planned to extract data on the highest dose and compare this to placebo. Extraction of data on co‐morbidity involved, for example, depressive symptoms, generalised anxiety and reduced sound level tolerance.
After independent data extraction, the authors (IW, DAH and ALS) reviewed the extracted data for disagreements, and revisited and discussed the relevant studies as required to reach a final consensus.
Assessment of risk of bias in included studies
Two authors (IW and IS) assessed the risk of bias of the included trials independently, with the following taken into consideration, as guided by theCochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other sources of bias (e.g. lack of an intervention control as a comparator, improper statistical analysis).
We used the Cochrane 'Risk of bias' tool in RevMan 5.3 (RevMan 2014), which involved describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias. We resolved differences of opinion by discussion. If no consensus was reached, we consulted a third author (ALS).
Measures of treatment effect
The primary outcome in this review was tinnitus loudness, which is likely to be a continuous variable. For intervention effect measures using continuous data, we planned to calculate the mean difference between groups with a 95% confidence interval (CI), provided that the selected studies used the same scale of measurement. If different scales were used, we planned to calculate the standardised mean difference (Cohen's d effect size (ES)).
We analysed dichotomous data as risk ratios with 95% CIs.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐RCTs with the cluster as the unit of analysis. If clusters had not been taken into account in the analyses, we planned to adjust for the clusters using the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). None of the studies that we included were cluster‐randomised trials.
Cross‐over trials
For subjective idiopathic tinnitus, it is unlikely that symptom severity returns to its baseline level after the first treatment period. Therefore, we only used data from cross‐over trials if data from before the cross‐over could be obtained.
Multi‐armed studies
In studies with more than two groups (e.g. two or more active treatments being tested against placebo), we established which of the comparisons were relevant to the systematic review. All included multi‐armed studies used independent groups of participants. Thus participants were not included in more than one group. We treated these studies as independent comparisons.
Repeated observations on participants
The unit of analysis was the participant. If studies evaluated the effect over a longer time period, we recorded the results at multiple time points. To avoid a unit of analysis error when combining study results in a single meta‐analysis (and therefore counting the same participants in more than one comparison), we defined different outcomes related to the periods of follow‐up and we performed separate analyses.
Dealing with missing data
Where necessary or where insufficient data were provided within the study publication, we contacted the authors of the study requesting further details about missing data and reasons for the incompleteness of the data, in all those cases where an email address was reported. If not reported or provided by the authors, we planned to estimate standard deviations in RevMan 5.3 (RevMan 2014) using the available data, such as standard errors, confidence intervals, P values and t values. We did not impute other missing data.
Assessment of heterogeneity
We determined whether the selected studies suffered from clinical, statistical and methodological heterogeneity. We planned to quantify statistical heterogeneity using the I2 statistic and the Chi2 test. With respect to the I2 statistic, an approximate guide to interpretation is provided in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). If the I2 value was 50% or higher, we considered the data to suffer from substantial or considerable heterogeneity. For the Chi2 test, we used the indicator that where the Chi2 is greater than the degrees of freedom (where the degrees of freedom are the number of studies K minus 1), then heterogeneity is likely to be present. We considered heterogeneity to be statistically significant if the P value was less than 0.10. Subsequently, we performed the meta‐analysis using fixed‐effect (in the absence of heterogeneity) and random‐effects modelling (in the presence of heterogeneity).
Assessment of reporting biases
We searched for and requested study protocols for the included studies and, where available, we evaluated whether there was evidence of selective reporting. We planned to assess publication bias using a funnel plot and Egger's test if a meta‐analysis contained at least 10 studies. Unfortunately, none of the meta‐analyses contained more than two studies.
Data synthesis
We planned to perform meta‐analyses using RevMan 5.3 if more than one study was identified for a given outcome (RevMan 2014). There was only one outcome for which two studies were identified.
Subgroup analysis and investigation of heterogeneity
There were insufficient data available for subgroup analyses. Although we planned to perform the following subgroup analyses, we were not able to do so:
age (children < 16 or 18 years and adults ≥ 16 or 18 years);
duration of tinnitus (acute ≤ 3 months and chronic > 3 months);
dose of betahistine administered (minimum daily dose of 8 mg to a maximum of 148 mg);
additional interventions (betahistine with and without an additional intervention).
Sensitivity analysis
We planned to conduct a sensitivity analysis by excluding those studies with a high risk of bias, thereby checking the robustness of the conclusion from the studies included in the meta‐analysis. In addition, we planned to use sensitivity analyses for studies in which data were imputed. However, all of the included studies carried a high or unclear risk of bias and in none of the studies were data imputed.
GRADE and 'Summary of findings' table
Two independent authors (IW and IS) used the GRADE approach to rate the overall quality of evidence using GRADEpro GDT (https://gradepro.org/). The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high‐quality of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low‐quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high‐quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision; and
publication bias.
We included 'Summary of findings' tables, constructed according to the recommendations described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), for the following comparisons:
betahistine versus placebo;
betahistine versus placebo, with concurrent medication.
We included the following outcomes in the 'Summary of findings' tables:
tinnitus loudness;
significant adverse effects (upper gastrointestinal discomfort);
tinnitus symptom severity;
other adverse effects (headache, drowsiness, allergic skin reactions (pruritis, rashes) and exacerbation of tinnitus);
depressive symptoms;
symptoms of generalised anxiety;
other adverse effects.
Results
Description of studies
Results of the search
Our electronic database search on 23 July 2018 identified 251 records. After removal of duplicates, we were left with 250 records. We discarded 235 records based on title and abstract screening, leaving 15 full‐text papers to be retrieved. From these we excluded 10 studies. Five studies were included. No further eligible records were identified from a handsearch of the reference lists for the five studies included in this review. We did not identify any ongoing studies and no studies are awaiting classification.
A flowchart of study retrieval and selection is provided in Figure 1.
1.

Study flow diagram.
Included studies
We included five published studies (Cekkayan 1996; Kay 1981; Ma 2006; Maqbool 2010; Mashali 2016). See Characteristics of included studies.
Three of the included studies had more than two treatment arms (Cekkayan 1996; Kay 1981; Mashali 2016). Cekkayan 1996 was a three‐armed study that compared betahistine hydrochloride, Gingko biloba and placebo. Kay 1981 was a four‐armed study comparing betahistine, mexiletine, diazepam and placebo. Mashali 2016 was a three‐armed study comparing betahistine, carbamazepine and placebo. For all three studies, we included the betahistine and placebo group in this review.
Study design
Four studies were parallel‐group RCTs (Cekkayan 1996; Ma 2006; Maqbool 2010; Mashali 2016). One study was a cross‐over trial (Kay 1981). This trial was stopped before enrolled participants had completed all cycles of medication because of an adverse event of mexiletine in an unrelated trial. For the first completed cycle, five test participants received betahistine and six control participants received a placebo.
Setting
Four studies were conducted in otorhinolaryngology departments within hospitals (Cekkayan 1996; Ma 2006; Maqbool 2010; Mashali 2016). Kay 1981 did not mention the study setting in his article, but the author was an otorhinolaryngologist. All studies were single‐centre (Cekkayan 1996; Kay 1981; Ma 2006; Maqbool 2010; Mashali 2016).
Participants
The total sample size for all included studies was between 303 and 305. In one study it was not clear how many participants were recruited in the treatment and control group (Mashali 2016). In the text of the results section the authors report 25 participants in the betahistine group and 24 participants in the placebo group. However, in table 1 they report 24 participants in the betahistine group and 25 participants in the placebo group. Three studies recruited adult participants (18 years or over) according to their eligibility criteria (Ma 2006; Maqbool 2010; Mashali 2016). The criteria for inclusion or exclusion varied between studies. Kay 1981 excluded patients with cardiovascular risk based on a clinical history and electrocardiographic examination, because one group of participants received mexiletine in their trial. Likewise, Mashali 2016 excluded patients with severe heart disease and medication that interferes with carbamazepine, because one group of participants received carbamazepine in their trial. Maqbool 2010 only included male military personnel with noise‐induced hearing loss. The participant groups in these three studies may not fully represent the tinnitus population (Kay 1981; Maqbool 2010; Mashali 2016).
Interventions and comparisons
All studies evaluated the effect of betahistine. Different salts of betahistine were used. Cekkayan 1996 and Maqbool 2010 evaluated the effect of betahistine hydrochloride, Ma 2006 evaluated betahistine mesilate and Kay 1981 and Mashali 2016 evaluated betahistine not otherwise specified.
The daily dosage varied from 16 mg daily to 48 mg daily. One study prescribed 8 mg twice daily initially followed by 8 mg three times daily for 28 days (Kay 1981). A second study prescribed 6 mg three times daily for one week (Ma 2006). A third study prescribed 16 mg three times daily for two months (Maqbool 2010) and a fourth study 8 mg twice daily for 12 weeks (Mashali 2016). Cekkayan 1996 did not report dosage or frequency.
Concurrent medication was prescribed in one study (Ma 2006). Both treatment arms received flunarizine hydrochloride, 5 mg daily for one week.
The comparator in three studies was a placebo not otherwise specified (Cekkayan 1996; Kay 1981; Maqbool 2010). In the other two studies vitamin B6 and a multivitamin were used as a placebo (Ma 2006 and Mashali 2016, respectively).
Outcomes
All included studies used one of the pre‐specified outcome measures (Types of outcome measures) (Cekkayan 1996; Kay 1981; Ma 2006; Maqbool 2010; Mashali 2016).
Primary outcomes
One study assessed clinical efficacy using an estimate of the change in tinnitus loudness with a matching procedure, but the details of the methods used were not reported (Ma 2006). Match estimates were subsequently graded on a five‐point scale according to the pre‐ versus post‐treatment difference in the tinnitus loudness match: 1 = loudness decreased to 0 dB; 2 = loudness reduced by 15 dB or more; 3 = loudness reduced by 5 dB or more and less than 15 dB; 4 = loudness reduced by less than 5 dB or increased by less than 5 dB; 5 = loudness increased by 5 dB or more. Two studies assessed post‐treatment tinnitus loudness using a visual analogue scale (Kay 1981; Maqbool 2010). One of these studies also assessed the change in tinnitus loudness using a visual analogue scale (Kay 1981). In Maqbool 2010 the scale ranged from 0 to 10, while in Kay 1981 the upper limit of the range was not reported. Outcomes were measured at one week (Ma 2006), 28 days (Kay 1981), one month (Maqbool 2010) and two months (Maqbool 2010).
Three studies assessed significant adverse effects (Cekkayan 1996; Kay 1981; Ma 2006). Outcomes were measured at one week (Ma 2006), 28 days (Kay 1981) and three months (Cekkayan 1996).
Secondary outcomes
Only one study reported changes in tinnitus symptom severity before and after treatment, as measured by the global score on a multi‐item questionnaire (Mashali 2016). The selected instrument was the Tinnitus Severity Index, a questionnaire comprising 12 questions rated on a five‐point Likert scale and a visual analogue scale of loudness between 0 and 10 (Folmer 2000). The Tinnitus Severity Index has not been published in a peer‐reviewed journal. The outcome was measured at 12 weeks.
Three studies assessed other adverse effects (Cekkayan 1996; Kay 1981; Ma 2006). Outcomes were measured at one week (Ma 2006), 28 days (Kay 1981) and three months (Cekkayan 1996).
None of the studies reported changes in depressive symptoms or depression, anxiety symptoms or generalised anxiety, or health‐related quality of life as measured by a validated instrument.
None of the studies included measures of tinnitus intrusiveness.
Other outcomes
Cekkayan 1996 reported change in tinnitus symptom severity using a single‐item five‐point Likert scale: 0 = the tinnitus disappeared completely; 1 = great relief, but the complaint was still ongoing; 2 = relieved by 50%; 3 = relief was very small; 4 = no changes were noticed.
Excluded studies
We excluded 10 studies after reviewing the full‐text paper. We discarded four records as they were review articles and so they did not address the research question (de Kernier 2000; Robson 1994; Sirimanna 1992; Werkman 1982). From the remainder, we discarded a further three records as they were the wrong study design (two were not randomised (Jakobs 1978; Sonmez 2013), and one did not include a control group and hence was also not randomised (Larikova 2005)), and three because they recruited patients on the basis of a diagnosis of Ménière’s disease or vertigo, not idiopathic subjective tinnitus (Kluyskens 1990; Oosterveld 1984; Singarelli 1979).
Risk of bias in included studies
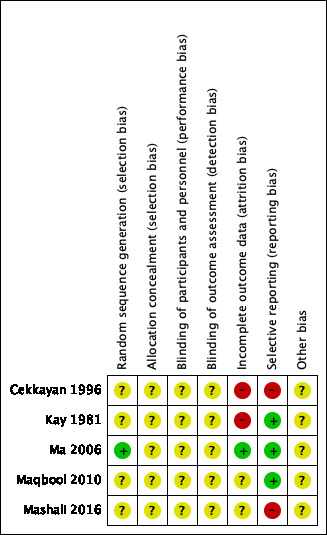
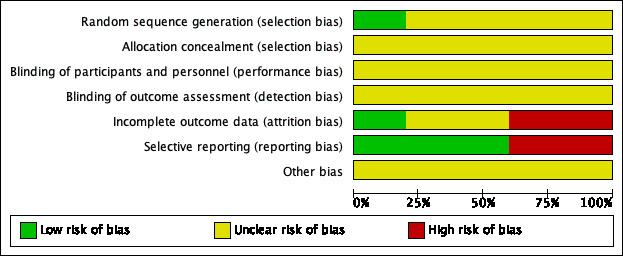
All of the included studies were randomised and controlled. Details on our judgements about the risk of bias items for each study can be found in Characteristics of included studies and Figure 2. Figure 3 shows a risk of bias graph that shows our judgements about each risk of bias item presented as percentages across all included studies. The risk of bias was unclear in all of the included studies (Cekkayan 1996; Kay 1981; Ma 2006; Maqbool 2010; Mashali 2016).
2.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
We considered the risk of selection bias due to inadequate sequence generation to be unclear in four studies (Cekkayan 1996; Kay 1981; Maqbool 2010; Mashali 2016) and low in one study (Ma 2006). Four studies reported that participants had been randomised to the treatment arms, but did not provide further information on the methods of sequence generation (Cekkayan 1996; Kay 1981; Maqbool 2010; Mashali 2016). In the study Ma 2006, randomisation was performed by workers from the Epidemiological Investigation Room, Peking University Third Hospital, using SAS software. In four studies, participants were randomised in a 1:1 ratio into the betahistine and placebo arms (Kay 1981; Ma 2006; Maqbool 2010; Mashali 2016). In one study, the results of 28 participants were analysed in the betahistine hydrochloride group and 13 in the placebo group (Cekkayan 1996). It was not reported whether participants were initially allocated in a 2:1 ratio or whether a large number of participants were lost to follow‐up in the placebo group.
Allocation concealment
We rated allocation concealment as unclear in all of the included studies (Cekkayan 1996; Kay 1981; Ma 2006; Maqbool 2010; Mashali 2016). None of the included studies provided any information about allocation concealment.
Baseline characteristics
In one study, baseline characteristics were not reported at all (Kay 1981). Two studies provided information about age and sex only. Cekkayan 1996 reported a difference of 10 years in mean age between the betahistine group and the placebo group. In the same study, 13 out of 28 participants (46%) in the betahistine hydrochloride group were male compared to 4 out of 13 (31%) in the placebo group. Age and sex were not significantly different when comparing the betahistine and the placebo group in the study performed by Mashali 2016, but no summary data were reported. Maqbool 2010 reported baseline tinnitus loudness, in addition to age and sex. There was no clinically relevant difference in mean age between the betahistine hydrochloride and multivitamin group (48 versus 49 years, respectively). All of the included participants were male. Baseline tinnitus loudness was 6 dB in both groups. Lastly, Ma 2006 evaluated age, sex, tinnitus duration, tinnitus loudness, tinnitus laterality, tinnitus pitch and presence of hearing loss at baseline. For example, the mean age in the betahistine and placebo groups was 47.1 and 46 years, respectively and 16 out of 30 participants (53%) in the betahistine group were male, while males accounted for 15 out of the 30 participants (50%) in the placebo group. In terms of hearing status, 17 (57%) in the betahistine group were reported to have hearing loss and 20 (67%) in the placebo group (Ma 2006). No clinically relevant or statistically significant differences were found between the betahistine mesilate and vitamin B6 group in these baseline characteristics.
Blinding
The risk of performance bias and detection bias as a result of inadequate blinding was unclear in all of the included studies (Cekkayan 1996; Kay 1981; Ma 2006; Maqbool 2010; Mashali 2016). In three articles, the authors stated that the studies were "double‐blinded", but no information was provided regarding who was blinded for what; nor did the authors describe which precautions were taken to prevent participants, personnel and/or outcome assessors from identifying the allocated treatment arm (Kay 1981; Ma 2006; Mashali 2016). The other two studies did not provide any information regarding blinding of participants, personnel and/or outcome assessors (Cekkayan 1996; Maqbool 2010).
Incomplete outcome data
We considered only one study to be at a low risk of attrition bias (Ma 2006). Three participants did not complete the study: one participant from the betahistine mesilate group and two participants from the vitamin B6 group. Intention‐to‐treat and per‐protocol analyses were both performed.
In one study, there was no mention of anyone dropping out of the trial or discontinuing for any reason (Maqbool 2010). However, the authors did not report how many participants were analysed for the reported outcomes and therefore we assessed the risk of attrition bias due to incomplete outcome data as unclear. In another study, the number of included participants was not mentioned in the materials and methods section and therefore it was unclear whether the number of participants evaluated was the same as the number of participants initially allocated to the treatment groups (Mashali 2016).
In the studies performed by Cekkayan 1996 and Kay 1981, we considered the risk of attrition bias due to incomplete outcome data to be high. Cekkayan 1996 provided no information regarding the lower number of participants in the placebo group (n = 13) compared to the betahistine hydrochloride group (n = 28). Furthermore, change in tinnitus severity categories were reported for eight out of 13 participants in the placebo group. Therefore there seemed to be missing data for five participants (38% of the placebo group). In the study performed by Kay 1981, 21 out of the included 42 participants (50%) were later excluded from the study on medical grounds or because they quickly became non‐compliant.
Selective reporting
We identified no study protocols for the included studies. In three studies, the outcomes that were mentioned in the abstract and/or methods section were also reported in the results section and therefore we considered the risk of selective reporting to be low in these studies (Kay 1981; Ma 2006; Maqbool 2010). We considered the risk of selective reporting to be high in two studies (Cekkayan 1996; Mashali 2016). In the study Cekkayan 1996, all participants were called to the clinic every 15 days for monitoring. During these visits, routine examinations and hearing tests were performed, and participants were asked to rate the severity of tinnitus subjectively on a scale of 0 to 4. The results of routine examinations and hearing tests were not reported in the results section and only the results at three months were reported. In the study performed by Mashali 2016, the abstract and the materials and methods section mentioned that audiometric tests were conducted as a pre‐ and post‐intervention measure, but these were not reported in the results section.
Other potential sources of bias
Conflicts of interest and funding were not reported in any of the included studies (Cekkayan 1996; Kay 1981; Ma 2006; Maqbool 2010; Mashali 2016). Cekkayan 1996 did not report which statistical tests were used and thus it was unclear whether these were proper.
Effects of interventions
Betahistine versus placebo
Primary outcomes
Tinnitus loudness
Both Kay 1981 and Maqbool 2010 reported no statistically significant differences in post‐treatment tinnitus loudness using a visual analogue scale ranging respectively from 0 to an undefined upper limit and from 0 to 10 at one‐month follow‐up. The mean difference observed in Kay 1981 was 1.00 (95% confidence interval (CI) ‐1.31 to 3.31) in favour of placebo, whereas the mean difference in Maqbool 2010 was ‐0.34 (95% CI ‐1.26 to 0.58) in favour of betahistine. The pooled mean difference was ‐0.16 (95% CI ‐1.01 to 0.70) (GRADE: very low‐quality) (Analysis 1.1). At two‐month follow‐up the mean difference in Maqbool 2010 was ‐0.39 (95% CI ‐1.37 to 0.60) (Analysis 1.2) (GRADE: very low‐quality). These differences were not statistically significant or clinically relevant.
1.1. Analysis.
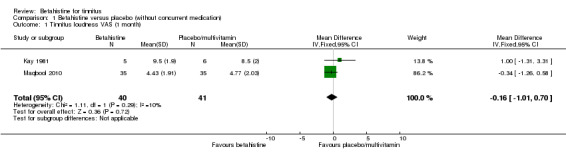
Comparison 1 Betahistine versus placebo (without concurrent medication), Outcome 1 Tinnitus loudness VAS (1 month).
1.2. Analysis.
Comparison 1 Betahistine versus placebo (without concurrent medication), Outcome 2 Tinnitus loudness VAS (2 months).
Kay 1981 also reported the change in tinnitus loudness using a visual analogue scale at 28‐day follow‐up. With a mean difference of ‐0.43 (95% CI ‐1.20 to 0.34) (Analysis 1.3), no statistically significant or clinically relevant difference was reported (GRADE: very low‐quality).
1.3. Analysis.
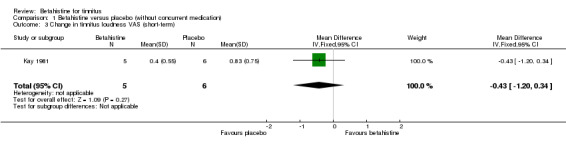
Comparison 1 Betahistine versus placebo (without concurrent medication), Outcome 3 Change in tinnitus loudness VAS (short‐term).
Significant adverse effects
Betahistine is frequently thought to cause upper gastrointestinal adverse effects. However, in the studies performed by Kay 1981 and Cekkayan 1996 none of the participants reported suffering from upper gastrointestinal discomfort at 28‐day and three‐month follow‐up (GRADE: very low‐quality and moderate‐quality, respectively).
Secondary outcomes
Tinnitus symptom severity measured by the global score on a multi‐item tinnitus questionnaire
Mashali 2016 found no difference in the change in Tinnitus Severity Index between betahistine and placebo (mean difference at 12 weeks 0.02, 95% CI ‐1.05 to 1.09) (Analysis 1.6) (GRADE: moderate‐quality).
1.6. Analysis.
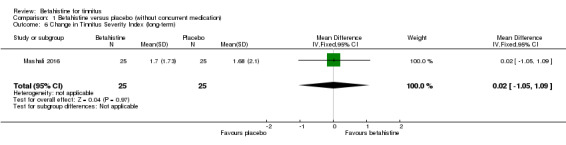
Comparison 1 Betahistine versus placebo (without concurrent medication), Outcome 6 Change in Tinnitus Severity Index (long‐term).
Depressive symptoms or depression, anxiety symptoms or generalised anxiety, and health‐related quality of life
None of the studies reported changes in depressive symptoms or depression, anxiety symptoms or generalised anxiety, or health‐related quality of life as measured by a validated instrument.
Other adverse effects
None of the participants suffered from other adverse effects at three‐month follow‐up in the study Cekkayan 1996 (GRADE: moderate‐quality). In the study Kay 1981, one participant in the betahistine group suffered from fatigue and none of the participants in the placebo group suffered from adverse effects (risk ratio 3.50, 95% CI 0.17 to 70.94, not statistically significant) (Analysis 1.8) (GRADE: very low‐quality).
1.8. Analysis.
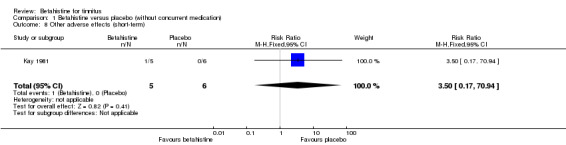
Comparison 1 Betahistine versus placebo (without concurrent medication), Outcome 8 Other adverse effects (short‐term).
Tinnitus intrusiveness
None of the studies included measures of tinnitus intrusiveness.
Betahistine versus placebo, with concurrent medication
Primary outcomes
Tinnitus loudness
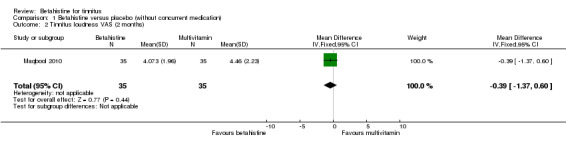
Ma 2006 assessed the change in tinnitus loudness using a psychoacoustic matching procedure, graded on a five‐point Likert scale, at one‐week follow‐up. The reported mean difference was not statistically significant or clinically relevant (‐0.10, 95% CI ‐0.50 to 0.30) (Analysis 2.1) (GRADE: moderate‐quality).
2.1. Analysis.
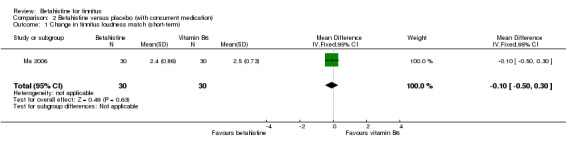
Comparison 2 Betahistine versus placebo (with concurrent medication), Outcome 1 Change in tinnitus loudness match (short‐term).
Significant adverse effects
In the study Ma 2006 one of the participants in the betahistine group suffered from mild nausea at one‐week follow‐up and none of the participants in the vitamin B6 group suffered from upper gastrointestinal discomfort (risk ratio 3.10, 95% CI 0.13 to 73.14) (Analysis 2.2) (GRADE: low‐quality). The difference was not statistically significant.
2.2. Analysis.
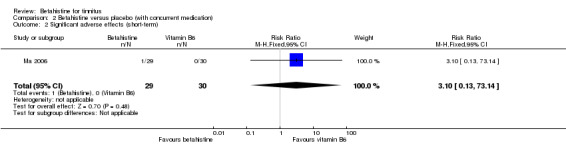
Comparison 2 Betahistine versus placebo (with concurrent medication), Outcome 2 Significant adverse effects (short‐term).
Secondary outcomes
Tinnitus symptom severity measured by the global score on a multi‐item tinnitus questionnaire
Ma 2006 did not report tinnitus symptom severity.
Depressive symptoms or depression, anxiety symptoms or generalised anxiety, and health‐related quality of life
Ma 2006 did not report changes in depressive symptoms or depression, anxiety symptoms or generalised anxiety, or health‐related quality of life as measured by a validated instrument.
Other adverse effects
In the study Ma 2006, with a follow‐up duration of one week, three participants in the betahistine group complained of mild drowsiness and six participants in the vitamin B6 group complained of mild drowsiness. One participant in the vitamin B6 group complained of a dry mouth. The risk ratio was 0.44 (95% CI 0.13 to 1.55) and was not statistically significant (Analysis 2.3) (GRADE: moderate‐quality).
2.3. Analysis.
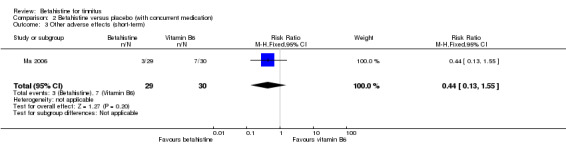
Comparison 2 Betahistine versus placebo (with concurrent medication), Outcome 3 Other adverse effects (short‐term).
Tinnitus intrusiveness
Ma 2006 did not report tinnitus intrusiveness.
Discussion
Summary of main results
The objective of this review was to assess the effects of betahistine in patients with subjective idiopathic tinnitus. This review includes five studies (with a total of 303 to 305 participants) comparing the effects of betahistine with placebo in adults with subjective idiopathic tinnitus (Table 1; Table 2). Due to heterogeneity in the outcomes measured and measurement methods used, very limited data pooling was possible. The included studies did not find a statistically significant or clinically relevant difference in our primary outcomes (tinnitus loudness and significant adverse events) or secondary outcomes (tinnitus symptom severity measured on a multi‐item tinnitus questionnaire and other adverse effects). One study did find a statistically significant and clinically relevant difference in tinnitus symptom severity measured using a single‐item scale with a response option range from 0 to 4 (Cekkayan 1996); however, this outcome measure was not usable according to our protocol. There is insufficient evidence to support the superiority or inferiority of betahistine over placebo.
None of the studies reported changes in depressive symptoms or depression, anxiety symptoms or generalised anxiety, health‐related quality of life as measured by a validated instrument or tinnitus intrusiveness.
Overall completeness and applicability of evidence
All of the included studies included patients with subjective idiopathic tinnitus. Only one study limited eligibility to patients with chronic tinnitus for more than six months (Mashali 2016), and none of the other studies described the duration of tinnitus experienced by their sample. Although one study reported numbers of participants with acute and chronic symptoms this was reported in a way that combined tinnitus and hearing loss (Ma 2006). Similarly, only one study reported baseline tinnitus severity (Mashali 2016), but the Tinnitus Severity Index used does not have a grading system to guide clinical interpretation of the numerical scores (Folmer 2000). Kay 1981 excluded patients with cardiovascular risk, because one group of participants received mexiletine in their trial. Likewise, Mashali 2016 excluded patients with severe heart disease and medication that interferes with carbamazepine, because one group of participants received carbamazepine in their trial. Maqbool 2010 only included male military personnel with noise‐induced hearing loss. The participant groups in these three studies may not fully represent the tinnitus population (Kay 1981; Maqbool 2010; Mashali 2016).
Different salts of betahistine were used in the included studies. Cekkayan 1996 and Maqbool 2010 evaluated the effect of betahistine hydrochloride, Ma 2006 evaluated betahistine mesilate and Kay 1981, and Mashali 2016 evaluated betahistine not otherwise specified. Furthermore, the daily dosage varied from 16 mg to 48 mg daily (Kay 1981; Ma 2006; Maqbool 2010; Mashali 2016). The treatment duration varied from one week to three months (Cekkayan 1996; Kay 1981; Ma 2006; Maqbool 2010; Mashali 2016). The comparator in three studies was a placebo not otherwise specified (Cekkayan 1996; Kay 1981; Maqbool 2010). In the other two studies vitamin B6 and a multivitamin were used as a placebo (Ma 2006; Mashali 2016, respectively) since these dietary supplements have no established benefit for tinnitus (Coelho 2016). In the analyses, we pooled the different salts of betahistine, dosages and types of placebos together.
The primary outcomes were often not reported and there was considerable inconsistency between studies with regard to outcomes and how they were measured and reported. Tinnitus loudness, for instance, was measured using a visual analogue scale ranging from 0 to 10 in one study (Maqbool 2010), a visual analogue scale whereof the upper limit is unknown in another study (Kay 1981) and a psychoacoustic loudness matching procedure in a third study where the methods were not described (Ma 2006).
Tinnitus loudness was assessed only over the short term (less than three months) (Kay 1981; Ma 2006; Maqbool 2010), while tinnitus symptom severity was assessed over the long term but only at the three‐month point (Mashali 2016). None of the studies followed up patients beyond the visit immediately following the end of treatment.
Quality of the evidence
The quality of the evidence ranged from moderate to very low. We are uncertain about the estimates for (change in) tinnitus loudness measured by a visual analogue scale and the loudness matching procedure and (significant) adverse effects at short‐term follow‐up. Further research is likely to have an important impact on our confidence in estimates of the change in tinnitus symptom severity and (significant) adverse effects at long‐term follow‐up. Further research is (very) likely to have an important impact on our confidence in the estimates of change in tinnitus loudness using a matching procedure and (significant) adverse effects at short‐term follow‐up, when concurrent medication is used.
The quality of the evidence was affected by a number of issues: risk of bias, indirectness of evidence and imprecision. The studies were characterised by poor reporting. Randomisation, allocation concealment and blinding of participants, personnel and outcomes assessors were particularly poorly reported.
None of the included studies had a pre‐published protocol available for inspection.
Potential biases in the review process
The review is based on a pre‐published protocol (Hall 2018); no significant changes have been made. Our searches of the electronic databases were comprehensive. Language was not a barrier for inclusion and, in addition to English, we reviewed full‐text articles in Chinese, French, German, Italian, Russian and Turkish for inclusion assessment, after appropriate translation. Author roles were pre‐defined in the review process. Two authors selected studies for inclusion and judged risk of bias independently, with recourse to a third author for resolution of any disagreement or uncertainty. Three authors independently extracted data to minimise personal bias. We considered both clinical and statistical heterogeneity before carrying out our analysis.
Agreements and disagreements with other studies or reviews
This is the first systematic review to examine exclusively the effects of betahistine on subjective idiopathic tinnitus. A broad‐ranging systematic review considered reports of all randomised clinical trials of any tinnitus intervention to map out known promising interventions that deserve further research and to identify aspects of research methodology that could be improved (Dobie 1999). One study evaluating betahistine was identified (Kay 1981), for which Dobie described the findings as not offering any advantage over placebo, just as we did.
Cochrane ENT has published two systematic reviews evaluating betahistine for other clinical indications in otorhinolaryngology. One review of betahistine for Ménière's disease identified seven trials comparing betahistine with placebo (243 participants) (James 2001). Only five trials assessed tinnitus as a secondary outcome using a single‐item numerical rating scale, and while some trial findings suggested a reduction in tinnitus the authors concluded that all these effects may have been caused by bias in the methods. Notably, one trial with good methods showed no effect of betahistine on tinnitus compared with placebo in 35 patients. Across the seven trials, adverse effects tended to be no different in the betahistine group from the placebo group. One study reported headache occurring more frequently in patients taking betahistine than placebo, but gastrointestinal symptoms were little reported. This review is currently being updated (van Esch 2018), with tinnitus again as a secondary outcome. The second review of betahistine for symptoms of vertigo identified 16 trials comparing betahistine with placebo (953 participants) (Murdin 2016). Tinnitus was not an outcome evaluated in this review, but adverse effects were. The authors reported that adverse effects (mostly gastrointestinal symptoms and headache) were common but medically serious effects in the study were rare and isolated, and there was no difference in the frequency of adverse effects between the betahistine and placebo groups.
In conclusion, previous reviews conclude that there is no evidence for any therapeutic benefit of betahistine for tinnitus, irrespective of whether the symptom reflects subjective idiopathic tinnitus or presents in the context of Ménière's disease. Existing work also concludes that betahistine is generally well tolerated with a low risk of treatment‐related adverse effects. Similar to the current review, the studies varied considerably in terms of types of participants, their diagnoses, the dose of betahistine and the length of time the drug was taken for, the study methods and the way improvements were measured.
Authors' conclusions
Implications for practice.
We did not find any evidence to support or refute the prescription of betahistine for subjective idiopathic tinnitus. The evidence suggests that betahistine is generally well tolerated with a similar risk of adverse effects to placebo treatments.
Given that the sample of participants was heterogeneous both within an individual trial and across trials, might betahistine have a positive effect on individual patients? For any pharmacological intervention to be personalised, then one would need to be able to make a differential clinical diagnosis based on the underlying tinnitus pathophysiology, and one would need to be confident that betahistine could influence this favourably. At present, it is not possible to do either of these things since the symptom of tinnitus has many possible causes, in the cochlea or brain, or the connections thereof, and the pharmacological effects of betahistine are not fully worked out. Nevertheless, the findings of this review do not negate the need for a proper clinical assessment of patients with the symptom of tinnitus with the goal of making an appropriate diagnosis. There are other evidence‐based treatments for tinnitus, which should be offered where appropriate.
Implications for research.
Future research into the effectiveness of betahistine in patients with tinnitus should use rigorous methodology. Randomisation and blinding should be of the highest quality, given the subjective nature of tinnitus and the strong likelihood of a placebo response. The CONSORT statement should be used in the design and reporting of future studies (CONSORT 2010).
We also recommend the development of validated, patient‐centred outcome measures for research in the field of tinnitus. Visual analogue scales have limited value in this regard because quantifying change using only a single item has inadequate measurement properties (e.g. internal consistency cannot be established and test‐retest scores are at greater risk of instability). Although most recent studies included in this review used multi‐item questionnaires of tinnitus symptom severity, other outcomes such as depression, anxiety and health‐related quality of life were not measured. None of the studies reported adverse effects. In future trials, in addition to multi‐item questionnaires of tinnitus symptom severity, validated instruments measuring depressive symptoms or depression, anxiety symptoms or generalised anxiety and health‐related quality of life should also be used.
At the time of the publication of this review, core outcome measures for adults with subjective tinnitus have only recently been identified (Hall 2018b). For pharmacology‐based interventions, these are tinnitus intrusiveness and loudness. None of the included studies assessed tinnitus intrusiveness, and while loudness was assessed in three out of the five included studies, the measurement methods varied. There is no standard test for tinnitus loudness, and psychoacoustic loudness matching and subjective rating methods are equally common. These measurement methods are generally applied, and findings interpreted, with the assumption that they measure the same underlying construct (i.e. that they have convergent validity). However, retrospective analysis of one randomised placebo‐controlled trial in 91 participants with subjective idiopathic tinnitus indicates otherwise (Hall 2017). Use of the core outcome set as a minimum standard for what should be assessed and reported in randomised controlled trials will facilitate comparison between studies and meta‐analyses (Tunis 2016).
Given the heterogeneity of tinnitus patients, future trials should assess and report baseline characteristics so that the risk of potential confounding factors can be better understood. Examples include tinnitus duration, tinnitus symptom severity, age, hearing loss and co‐morbidities since these might reasonably modify treatment success. Future trials might also consider, as a subgroup analysis, the differential effect of betahistine on acute (i.e. less that three months duration) versus chronic (more than three months duration) subjective idiopathic tinnitus. With the exception of one included trial (Ma 2006), trials either failed to describe this important participant baseline characteristic or recruited only chronic cases. None of the included studies performed a sample size estimation, and so future studies should seek to recruit an adequate sample size based on an appropriate power calculation for the primary outcome.
None of the included studies followed up patients beyond three months. Although betahistine would be expected to become effective over the short term, future studies might consider including long‐term follow‐up in order to explore whether any such changes are maintained.
Acknowledgements
We are grateful to Dr Louisa Murdin for peer reviewing the manuscript for this review and to Sandy Walsh for her input as the consumer referee.
Suyi Hu, PhD candidate, translated the article Ma 2006 from Chinese into English.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding for Cochrane ENT and a Senior Investigator award to DAH. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Samantha Cox, Cochrane Information Specialist, designed the search strategy for the review.
Appendices
Appendix 1. CENTRAL search strategy
1 MESH DESCRIPTOR Tinnitus EXPLODE ALL AND CENTRAL:TARGET
2 (tinnit*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET
3 #1 OR #2
4 MESH DESCRIPTOR betahistine EXPLODE ALL AND CENTRAL:TARGET
5 (betahistin* or serc or betaserc):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET
6 AEQUAMEN or BETASERK or BEATSERKA or EXTOVYL or FIDIUM or LECTIL or LOBIONE or MEGINALISK or MELOPAT or MENIACE or MERISLON or MICROSER or RIBRAIN or VASOMOTAL AND CENTRAL:TARGET
7 ((BY next vertin)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET
8 (Betavert or vertigon or pt9 or "pt 9"):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET
9 #4 OR #5 OR #6 OR #7 OR #8
10 #3 AND #9
Appendix 2. Data extraction form
| Study details and author contact details | |
| Ref‐ID | |
| Date of extraction | |
| Citation | |
| Corresponding author | |
| Address | |
| Additional identification data | |
| Characteristics of included study | |
| Methods | X‐armed, double/single/non‐blinded, parallel‐group/cross‐over/cluster randomised controlled trial, with x duration of treatment and x duration of follow‐up |
| Participants |
Location: place, country Setting: single‐/multicentre, department, hospital, study duration Sample size:
Participant baseline characteristics:
Inclusion criteria: Exclusion criteria: |
| Interventions |
Intervention group: drug name, method of administration, dosage per day, frequency of administration, duration of intervention Comparator group: drug name, method of administration, dosage per day, frequency of administration, duration of intervention Use of additional interventions: |
| Outcomes |
Outcome measure(s): Time point(s): Outcome type: continuous/dichotomous/adverse effects Reported as: mean with confidence intervals/standard deviation/standard error Range: Unit of measurement: Direction: lower is better/higher is better Statistical tests used: Treatment fidelity: |
| Funding sources | No information provided/none declared/state source of funding |
| Declarations of interest | No information provided/none declared/state conflict(s) |
| Notes | |
| Risk of bias assessment | ||
| Bias | Author's judgement (low/high/unclear) | Support for judgement |
| Random sequence generation | ||
| Allocation concealment | ||
| Blinding of participants and personnel | ||
| Blinding of outcome assessment | ||
| Incomplete outcome data | ||
| Selective reporting | ||
| Other sources of bias | ||
| Results (continuous data) | |||||||
| Outcome | Test group | Control group | Other summary statistics and notes | ||||
| Mean | SD | N | Mean | SD | N | Test, significance level, 95% confidence interval | |
| Pre‐intervention | |||||||
| Post‐intervention | |||||||
| Change/difference | |||||||
| Results (dichotomous data) | |||||
| Outcome | Test group | Control group | Other summary statistics and notes | ||
| Number of subjects with events | Number of subjects analysed | Number of subjects with events | Number of subjects analysed | Test, significance level, risk ratio, odds ratio, 95% confidence interval | |
Data and analyses
Comparison 1. Betahistine versus placebo (without concurrent medication).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tinnitus loudness VAS (1 month) | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐1.01, 0.70] |
| 2 Tinnitus loudness VAS (2 months) | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.37, 0.60] |
| 3 Change in tinnitus loudness VAS (short‐term) | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.20, 0.34] |
| 4 Significant adverse effects (short‐term) | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Significant adverse effects (long‐term) | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Change in Tinnitus Severity Index (long‐term) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐1.05, 1.09] |
| 7 Tinnitus severity score (long‐term) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.34, 0.30] |
| 8 Other adverse effects (short‐term) | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [0.17, 70.94] |
| 9 Other adverse effects (long‐term) | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Change in tinnitus severity score (long‐term) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.58, ‐0.14] |
1.4. Analysis.
Comparison 1 Betahistine versus placebo (without concurrent medication), Outcome 4 Significant adverse effects (short‐term).
1.5. Analysis.
Comparison 1 Betahistine versus placebo (without concurrent medication), Outcome 5 Significant adverse effects (long‐term).
1.7. Analysis.
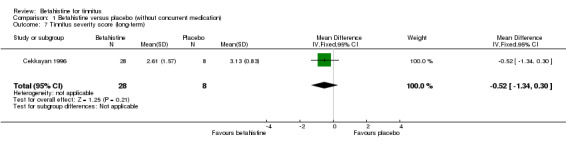
Comparison 1 Betahistine versus placebo (without concurrent medication), Outcome 7 Tinnitus severity score (long‐term).
1.9. Analysis.
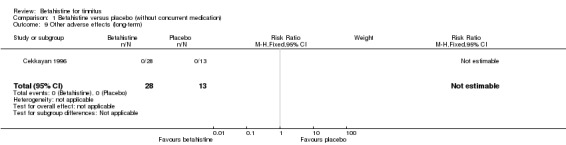
Comparison 1 Betahistine versus placebo (without concurrent medication), Outcome 9 Other adverse effects (long‐term).
1.10. Analysis.
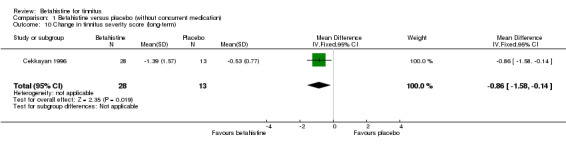
Comparison 1 Betahistine versus placebo (without concurrent medication), Outcome 10 Change in tinnitus severity score (long‐term).
Comparison 2. Betahistine versus placebo (with concurrent medication).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in tinnitus loudness match (short‐term) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.50, 0.30] |
| 2 Significant adverse effects (short‐term) | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.1 [0.13, 73.14] |
| 3 Other adverse effects (short‐term) | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.13, 1.55] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cekkayan 1996.
| Methods | A 3‐armed (betahistine, Gingko biloba and placebo), parallel‐group randomised controlled trial with 3 months duration of treatment and 3 months duration of follow‐up | |
| Participants |
Location: Malatya, Turkey Setting: single‐centre study, Department of Otorhinolaryngology, Inönü University, from September 1993 to April 1994 Sample size:
Participant baseline characteristics:
Inclusion criteria: patients with subjective tinnitus Exclusion criteria: none |
|
| Interventions |
Intervention group: betahistine hydrochloride tablets, 1 tablet 3 times a day, 3 months, dosage not reported Comparator group: placebo capsules, 1 capsule 3 times a day, 3 months Use of additional interventions: none reported |
|
| Outcomes |
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported whether participants and/or personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information is provided regarding the lower number of participants in the control group (n = 13) compared to the test group (n = 28). Change in tinnitus severity categories is reported only for 8 out of 13 participants in the placebo group. There seems to be missing data for 5 participants. |
| Selective reporting (reporting bias) | High risk | All participants were called to the clinic every 15 days for monitoring. During these visits, routine examinations and hearing tests were performed. Participants were asked to rate the severity of tinnitus subjectively on a scale of 0 to 4, compared to the first day of the treatment. The results of routine examinations and hearing tests were not reported in the results section. Only the results at 3 months were reported. |
| Other bias | Unclear risk | Unclear which statistical tests were used and thus whether these were proper. Conflicts of interest and funding were not reported. |
Kay 1981.
| Methods | A 4‐armed (betahistine, mexiletine, diazepam and placebo), double‐blinded, cross‐over randomised controlled trial with 28 days duration of treatment and 28 days duration of follow‐up | |
| Participants |
Location: England Setting: not reported Sample size:
Participant baseline characteristics:
Inclusion criteria: non‐treatable tinnitus Exclusion criteria: cardiovascular risk based on clinical history and electrocardiographic examination |
|
| Interventions |
Intervention group: betahistine capsules, 16 mg daily initially (8 mg 2 times a day) followed by 24 mg daily (8 mg 2 times a day), 28 days Comparator group: placebo capsules, 2 capsules daily initially, followed by 3 capsules daily, 28 days Use of additional interventions: none reported |
|
| Outcomes |
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | The trial was stopped after the first cycle of medication because of an adverse event of mexiletine in an unrelated trial. In this first cycle, 5 test participants received betahistine and 6 control participants received a placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | In the materials and methods section it was mentioned that the trial was double‐blind. However, the authors did not report who was blinded for what. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | In the materials and methods section it was mentioned that the trial was double‐blind. However, the authors did not report who was blinded for what. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21 out of 42 participants were excluded from the trial on medical grounds or because they quickly became non‐compliant. |
| Selective reporting (reporting bias) | Low risk | There is no protocol available. The outcomes listed in the materials and methods section of the article are all reported in the results section of the article. |
| Other bias | Unclear risk | Conflicts of interest and funding were not reported |
Ma 2006.
| Methods | A 2‐armed, double‐blinded, parallel‐group randomised controlled trial with 1 week duration of treatment and 1 week duration of follow‐up | |
| Participants |
Location: Beijing, China Setting: single‐centre study, Department of Otorhinolaryngology, Third Hospital Beijing, from January 2005 to April 2005 Sample size:
Participant baseline characteristics:
Inclusion criteria: patients aged 18 to 75 years with subjective tinnitus Exclusion criteria:
|
|
| Interventions |
Intervention group: betahistine mesilate tablets, 18 mg daily (6 mg 3 times a day), 1 week Comparator group: vitamin B6 tablets, 30 mg daily (10 mg 3 times a day), 1 week Use of additional interventions: flunarizine hydrochloride, 5 mg daily (5 mg once a day), 1 week, both treatment arms |
|
| Outcomes |
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by workers from the Epidemiological Investigation Room Peking University Third Hospital, using SAS software |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | In the abstract it was mentioned that this was a double‐blind trial. However, the authors did not report who was blinded for what. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | In the abstract it was mentioned that this was a double‐blind trial. However, the authors did not report who was blinded for what. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants did not complete the trial: 1 participant from the test group and 2 participants from the control group. An intention‐to‐treat and per‐protocol analysis was performed. |
| Selective reporting (reporting bias) | Low risk | There is no protocol available. The outcomes listed in the methods section of the article are all reported in the results section of the article. |
| Other bias | Unclear risk | Conflicts of interest and funding were not reported |
Maqbool 2010.
| Methods | A 2‐armed, parallel‐group randomised controlled trial with 2 months duration of treatment and 2 months duration of follow‐up | |
| Participants |
Location: Rawalpindi, Pakistan Setting: single‐centre study, Department of Otorhinolaryngology, Combined Military Hospital Rawalpindi, from July 2006 to December 2006 Sample size:
Participant baseline characteristics:
Inclusion criteria: patients aged over 18 years with tinnitus due to noise‐induced hearing loss with a confirmed history of exposure to noise and confirmed hearing loss at 3 or 4 kHz Exclusion criteria:
|
|
| Interventions |
Intervention group: betahistine hydrochloride tablets, 48 mg daily (16 mg 3 times a day), 2 months Comparator group: multivitamin tablets, 3 tablets daily (1 tablet 3 times a day), 2 months Use of additional interventions: none reported |
|
| Outcomes | Tinnitus loudness measured using a visual analogue scale (range 0 to 10) at 1 month and 2 months | |
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported whether participants and/or personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported whether participants dropped out or were lost to follow‐up. Not reported whether outcome data were missing. |
| Selective reporting (reporting bias) | Low risk | There is no protocol available. The outcomes listed in the patients and methods section of the article are all reported in the results section of the article. |
| Other bias | Unclear risk | Conflicts of interest and funding were not reported |
Mashali 2016.
| Methods | A 3‐armed (betahistine, carbamazepine and placebo), double‐blinded, parallel‐group randomised controlled trial with 12 weeks duration of treatment and 12 weeks duration of follow‐up | |
| Participants |
Location: Ahvaz, Iran Setting: single‐centre study, Department of Otorhinolaryngology, Ahvaz Jundishapur University of Medical Sciences, from May 2015 to May 2016 mentioned in the abstract and from August 2015 to August 2016 mentioned in the materials and methods section Sample size:
Participant baseline characteristics:
Inclusion criteria: patients aged over 21 to 65 years with non‐pulsatile tinnitus with a duration of at least 6 months Exclusion criteria:
|
|
| Interventions |
Intervention group: betahistine tablets, 16 mg daily (8 mg 2 times a day), 12 weeks Comparator group: placebo tablets, dosage and frequency not reported, 12 weeks Use of additional interventions: none reported |
|
| Outcomes | Tinnitus Severity Index (range 0 to 56 points) at 12 weeks | |
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | In the text of the results section the authors report 25 participants in the betahistine group and 24 participants in the placebo group. In table 1 the authors report 24 participants in the betahistine group and 25 participants in the placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly placed in 3 groups in terms of sex, age and tinnitus severity. Methods of randomisation were not reported. |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | In the introduction it was mentioned that this was a double‐blind trial. However, the authors did not report who was blinded for what. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | In the introduction it was mentioned that this was a double‐blind trial. However, the authors did not report who was blinded for what. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of included participants was not mentioned in the materials and methods section. It is unclear whether the number of participants evaluated was the same as the number of participants initially allocated to the treatment groups. |
| Selective reporting (reporting bias) | High risk | The abstract and the materials and methods section mentions that audiometric tests were conducted as a pre‐ and postintervention measure, but these were not reported in the results section |
| Other bias | Unclear risk | Conflicts of interest and funding were not reported |
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| de Kernier 2000 | Review article |
| Jakobs 1978 | ALLOCATION: no randomisation |
| Kluyskens 1990 | PARTICIPANTS: patients had a diagnosis of Ménière’s disease or vertigo |
| Larikova 2005 | ALLOCATION: no control group, no randomisation |
| Oosterveld 1984 | PARTICIPANTS: patients had a diagnosis of vertigo |
| Robson 1994 | Review article |
| Singarelli 1979 | PARTICIPANTS: patients had a diagnosis of vertigo |
| Sirimanna 1992 | Review article |
| Sonmez 2013 | ALLOCATION: no randomisation |
| Werkman 1982 | Review article |
Differences between protocol and review
We planned to report the new core outcome for trials of pharmacological interventions for tinnitus, this being tinnitus intrusiveness (Hall 2018b), as measured by a single‐item patient‐reported visual analogue scale or numerical rating scale. However, there were no reported outcomes of this type.
Other possible comparison pairs included betahistine versus no intervention, or betahistine versus education and information only, but none of the included studies were of this design.
We added drowsiness as an additional adverse effect for the secondary outcome 'Other adverse effects'.
Two (DAH and DM) instead of three authors (DAH, DM and IS) scanned the retrieved records for eligibility.
Our protocol did not explicitly cover mixed populations and how we would handle these. We decided to exclude studies that included a majority (more than 50%) of patients with Ménière’s disease.
Contributions of authors
The Cochrane ENT Information Specialist developed and ran the search strategy.
DAH obtained copies of studies with assistance from the NIHR Nottingham Biomedical Research Centre.
DAH and DM were responsible for selection of studies.
IW, DAH and ALS were responsible for data extraction.
IW and IS were responsible for assessing risk of bias.
IW entered data into RevMan.
IW and IS conducted and interpreted the analyses.
IW, DAH and IS drafted the final review.
DAH, ALS and IS will be responsible for updating the review.
All authors agreed on the final draft.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
NIHR Senior Investigator award, UK.
Declarations of interest
Deborah A Hall: is an NIHR Senior Investigator and Section Editor for the journal Hearing Research, Elsevier. She leads the Core Outcome Measures in Tinnitus (COMiT) initiative whose work is currently supported by the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie grant agreement No 764604 and the NIHR Nottingham Biomedical Research Centre.
Inge Wegner: none known.
Adriana L Smit: none known.
Don McFerran: receives royalties for writing books on tinnitus has received consultancy honoraria from GlaxoSmithKline, Autifony and Otonomy.
Inge Stegeman: none known.
New
References
References to studies included in this review
Cekkayan 1996 {published data only}
- Cekkayan S, Ozlüoğlu L, Yoloğlu S, Söylemezoğlu S, Erpek G. Comparison of the efficiency of betahistine hydrochloride and gingko biloba extract in tinnitus patients [Tinnituslu hastalarda betahistin ve gingko biloba ekstresinin etkinliginin karsilastirilmasi]. K.B.B. ve Baş Boyun Cerrahisi Dergisi 1996;4:19‐22. [Google Scholar]
Kay 1981 {published data only}
- Kay NJ. Oral chemotherapy for tinnitus. British Journal of Audiology 1981;15(2):123‐4. [PUBMED: 7225648] [DOI] [PubMed] [Google Scholar]
Ma 2006 {published data only}
- Ma F, Xin Y, Zhao YM, Lü JQ. Efficacy of betahistine mesilate combined with flunarizine hydrochloride for treating tinnitus [倍他司汀联合盐酸氟桂利嗪治疗耳鸣的随机双盲对照初步研究]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2006;41(4):269‐73. [PUBMED: 16848175] [PubMed] [Google Scholar]
Maqbool 2010 {published data only}
- Maqbool S, Ahmed B, Manzoor T. Eifficacy of betahistine hydrocholride in tinnitus due to noise‐induced hearing loss. Pakistan Armed Forces Medical Journal 2010; Vol. 60, issue 1.
Mashali 2016 {published data only}
- Mashali L, Rahimi S, Rekabi H, Rahimi P. The comparative study of two drugs of carbamazepine and betahistine on tinnitus improvement. International Journal of Pharmacy & Technology 2016;8(3):14774‐81. [Google Scholar]
References to studies excluded from this review
de Kernier 2000 {published data only}
- Kernier C. Vertigo and tinnitus [Vertiges et acouphenes]. Actualites Pharmaceutiques 2000;382:47‐50. [Google Scholar]
Jakobs 1978 {published data only}
- Jakobs P, Martin G. The therapy of tinnitus resulting from blast injury [Die Therapie des Tinnitus nach knalltraumatischer Schädigung]. HNO 1978;26(3):104‐6. [PUBMED: 346541] [PubMed] [Google Scholar]
Kluyskens 1990 {published data only}
- Kluyskens P, Lambert P, D'Hooge D. Trimetazidine versus betahistine in vestibular vertigo. A double blind study [Trimétazidine contre bétahistine dans les vertiges vestibulaires. Etude en double aveugle]. Annales d'Oto‐laryngologie et de Chirurgie Cervico Faciale 1990;107(1):11‐9. [PUBMED: 2240994] [PubMed] [Google Scholar]
Larikova 2005 {published data only}
- Larikova TI, Cherevikova GM. Betaserc and improvement of life quality in war veterans. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 2005;105(3):68‐70. [PUBMED: 15822746] [PubMed] [Google Scholar]
Oosterveld 1984 {published data only}
- Oosterveld WJ. Betahistine dihydrochloride in the treatment of vertigo of peripheral vestibular origin. A double‐blind placebo‐controlled study. Journal of Laryngology and Otology 1984;98(1):37‐41. [PUBMED: 6363584] [DOI] [PubMed] [Google Scholar]
Robson 1994 {published data only}
- Robson AK, Birchall JP. Management of tinnitus. Prescribers' Journal 1994;34(1):1‐7. [Google Scholar]
Singarelli 1979 {published data only}
- Singarelli S. Double‐blind trial on the efficacy of betahistine hydrochloride in a group of outpatients with positional vertigo and tinnitus [Studio in doppio cieco sull'efficacia del cloridato di betaistina nel trattamento ambulatoriale di un gruppo di pazienti affetti da vertigine di posizione e acufeni]. Nuovo Archivio Italiano di Otologia, Rinologia e Laringologia 1979;7:69‐72. [Google Scholar]
Sirimanna 1992 {published data only}
- Sirimanna T, Stephens D. Coping with tinnitus. Practitioner 1992;236(1518):821‐6. [PUBMED: 1461881] [PubMed] [Google Scholar]
Sonmez 2013 {published data only}
- Sönmez O, Külahlı I, Vural A, Sahin MI, Aydın M. The evaluation of ozone and betahistine in the treatment of tinnitus. European Archives of Oto‐rhino‐laryngology 2013;270(7):1999‐2006. [PUBMED: 23100082] [DOI] [PubMed] [Google Scholar]
Werkman 1982 {published data only}
- Werkman BK. The treatment of tinnitus. Clinical Otolaryngology and Allied Sciences 1982;7(5):354. [DOI] [PubMed] [Google Scholar]
Additional references
Adjamian 2009
- Adjamian P, Sereda M, Hall DA. The mechanisms of tinnitus: perspectives from human functional neuroimaging. Hearing Research 2009;253(1‐2):15‐31. [PUBMED: 19364527] [DOI] [PubMed] [Google Scholar]
Baguley 2013
- Baguley D, McFerran D, Hall D. Tinnitus. Lancet 2013;382(9904):1600‐7. [PUBMED: 23827090] [DOI] [PubMed] [Google Scholar]
Barak 2008
- Barak N. Betahistine: what’s new on the agenda?. Expert Opinion on Investigational Drugs 2008;17:795–804. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty‐five years of evaluation. Clinical Psychology Review 1988;8(1):77‐100. [DOI: ] [Google Scholar]
Beck 1988a
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56(6):893‐7. [PUBMED: 3204199] [DOI] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory‐II. San Antonio, Texas: Psychological Corporation, 1996. [Google Scholar]
Biesinger 2011
- Biesinger E, Bo L, Ridder D, Goodey R, Herraiz C, Kleinjung T, et al. Algorithm for the diagnostic & therapeutic management of tinnitus. http://www.tinnitusresearch.org/images/files/migrated/TRI_Tinnitus_Flowchart.pdf (accessed 21 May 2018) 2011.
Buss 1998
- Buss E, Hall JW 3rd, Grose JH, Hatch DR. Perceptual consequences of peripheral hearing loss: do edge effects exist for abrupt cochlear lesions?. Hearing Research 1998;125(1‐2):98‐108. [PUBMED: 9833964] [DOI] [PubMed] [Google Scholar]
Cima 2012
- Cima RF, Maes IH, Joore MA, Scheyen DJ, Refaie A, Baguley DM, et al. Specialised treatment based on cognitive behaviour therapy versus usual care for tinnitus: a randomised controlled trial. Lancet 2012;379(9830):1951‐9. [PUBMED: 22633033] [DOI] [PubMed] [Google Scholar]
Coelho 2016
- Coelho C, Tyler R, Ji H, Rojas‐Roncancio E, Witt S, Tao P, et al. Survey on the effectiveness of dietary supplements to treat tinnitus. American Journal of Audiology 2016;25(3):184‐205. [PUBMED: 27681261] [DOI] [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Department of Health 2009
- Department of Health. Provision of Services for Adults with Tinnitus. A Good Practice Guide. London: Central Office of Information, 2009. [Google Scholar]
Dietrich 2001
- Dietrich V, Nieschalk M, Stoll W, Rajan R, Pantev C. Cortical reorganization in patients with high frequency cochlear hearing loss. Hearing Research 2001;158(1‐2):95‐101. [PUBMED: 11506941] [DOI] [PubMed] [Google Scholar]
Dobie 1999
- Dobie RA. A review of randomized clinical trials in tinnitus. Laryngoscope 1999;109(8):1202‐11. [PUBMED: 10443820] [DOI] [PubMed] [Google Scholar]
Dong 2010
- Dong S, Rodger J, Mulders WH, Robertson D. Tonotopic changes in GABA receptor expression in guinea pig inferior colliculus after partial unilateral hearing loss. Brain Research 2010;1342:24‐32. [PUBMED: 20438718] [DOI] [PubMed] [Google Scholar]
Dziadziola 1999
- Dziadziola JK, Laurikainen EL, Rachel JD, Quirk WS. Betahistine increases vestibular blood flow. Otolaryngology ‐ Head and Neck Surgery 1999;120(3):400‐5. [PUBMED: 10064646] [DOI] [PubMed] [Google Scholar]
Eggermont 2004
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends in Neurosciences 2004;27(11):676‐82. [PUBMED: 15474168] [DOI] [PubMed] [Google Scholar]
El‐Shunnar 2011
- El‐Shunnar SK, Hoare DJ, Smith S, Gander PE, Kang S, Fackrell K, et al. Primary care for tinnitus: practice and opinion among GPs in England. Journal of Evaluation in Clinical Practice 2011;17(4):684‐92. [PUBMED: 21707872] [DOI] [PMC free article] [PubMed] [Google Scholar]
Electronic Medicines Compendium 2015
- Electronic Medicines Compendium. Betahistine dihydrochloride 16 mg tablets. https://www.medicines.org.uk/emc/medicine/26617 2015.
Engineer 2011
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature 2011;470(7332):101‐4. [PUBMED: 21228773] [DOI] [PMC free article] [PubMed] [Google Scholar]
Folmer 2000
- Folmer RL. Managing chronic tinnitus as phantom auditory pain. Audiology Online. Available at https://www.audiologyonline.com/articles/managing‐chronic‐tinnitus‐as‐phantom‐1268 2000 (accessed 29 September 2018).
Fowler 1944
- Fowler E. Head noises in normal and in disordered ears: significance, measurement, differentiation and treatment. Archives of Otolaryngology 1944;39(6):498‐503. [Google Scholar]
Fuller 2017
- Fuller TE, Haider HF, Kikidis D, Lapira A, Mazurek B, Norena A, et al. Different teams, same conclusions? a systematic review of existing clinical guidelines for the assessment and treatment of tinnitus in adults. Frontiers in Psychology 2017;8:206. [PUBMED: 28275357] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furlong 2001
- Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI) system for assessing health‐related quality of life in clinical studies. Annals of Medicine 2001;33(5):375‐84. [PUBMED: 11491197] [DOI] [PubMed] [Google Scholar]
Gallus 2015
- Gallus S, Lugo A, Garavello W, Bosetti C, Santoro E, Colombo P, et al. Prevalence and determinants of tinnitus in the Italian adult population. Neuroepidemiology 2015;45(1):12‐9. [PUBMED: 26182874] [DOI] [PubMed] [Google Scholar]
Guitton 2003
- Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL. Salicylate induces tinnitus through activation of cochlear NMDA receptors. Journal of Neuroscience 2003;23(9):3944‐52. [PUBMED: 12736364] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2011
- Hall DA, Lainez MJ, Newman CW, Sanchez TG, Egler M, Tennigkeit F, et al. Treatment options for subjective tinnitus: self reports from a sample of general practitioners and ENT physicians within Europe and the USA. BMC Health Services Research 2011;11:302. [PUBMED: 22053947] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2017
- Hall DA, Mehta RL, Fackrell K. How to choose between measures of tinnitus loudness for clinical research? A report on the reliability and validity of an investigator‐administered test and a patient‐reported measure using baseline data collected in a phase IIa drug trial. American Journal of Audiology 2017;26(3):338‐46. [PUBMED: 28841725] [DOI] [PubMed] [Google Scholar]
Hall 2018a
- Hall DA, Fackrell K, Li AB, Thavayogan R, Smith S, Kennedy V, et al. A narrative synthesis of research evidence for tinnitus‐related complaints as reported by patients and their significant others. Health and Quality of Life Outcomes 2018;16(1):61. [PUBMED: 29642913] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2018b
- Hall DA, Smith H, Hibbert A, Colley V, Haider HF, Horobin A, et al. The COMiT'ID study: developing core outcome domains sets for clinical trials of sound‐, psychology‐, and pharmacology‐based interventions for chronic subjective tinnitus in adults. Trends in Hearing 2018;22:2331216518814384. [PUBMED: 30488765] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallam 2009
- Hallam RS. TQ, Manual of the Tinnitus Questionnaire: Revised and Updated. 2nd Edition. London: Polpresa Press, 2009. [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960;23:56‐62. [PUBMED: 14399272] [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2009
- Han BI, Lee HW, Kim TY, Lim JS, Shin KS. Tinnitus: characteristics, causes, mechanisms, and treatments. Journal of Clinical Neurology (Seoul, Korea) 2009;5(1):11‐9. [PUBMED: 19513328] [DOI] [PMC free article] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hays 1993
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36‐Item Health Survey 1.0. Health Economics 1993;2(3):217‐27. [PUBMED: 8275167] [DOI] [PubMed] [Google Scholar]
Henry 2005
- Henry JA, Dennis KC, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. Journal of Speech, Language, and Hearing Research 2005;48(5):1204‐35. [PUBMED: 16411806] [DOI] [PubMed] [Google Scholar]
Henry 2008
- Henry JA, Zaugg TL, Myers PJ, Schechter MA. The role of audiologic evaluation in progressive audiologic tinnitus management. Trends in Amplification 2008;12(3):170‐87. [PUBMED: 18628281] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hiller 2006
- Hiller W, Goebel G. Factors influencing tinnitus loudness and annoyance. Archives of Otolaryngology‐‐Head & Neck Surgery 2006;132(12):1323‐30. [PUBMED: 17178943] [DOI] [PubMed] [Google Scholar]
Hoare 2011
- Hoare DJ, Hall DA. Clinical guidelines and practice: a commentary on the complexity of tinnitus management. Evaluation & the Health Professions 2011;34(4):413‐20. [PUBMED: 21177640] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoare 2014
- Hoare DJ, Edmondson‐Jones M, Sereda M, Akeroyd MA, Hall D. Amplification with hearing aids for patients with tinnitus and co‐existing hearing loss. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010151.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2001
- James AL, Burton MJ. Betahistine for Meniere's disease or syndrome. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001873] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jastreboff 1988
- Jastreboff PJ, Brennan JF, Coleman JK, Sasaki CT. Phantom auditory sensation in rats: an animal model for tinnitus. Behavioral Neuroscience 1988;102(6):811‐22. [PUBMED: 3214530] [DOI] [PubMed] [Google Scholar]
Jastreboff 1990
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neuroscience Research 1990;8(4):221‐54. [PUBMED: 2175858] [DOI] [PubMed] [Google Scholar]
Jastreboff 2004
- Jastreboff PJ, Hazell JWP. Tinnitus Retraining Therapy. Cambridge University Press, 2004. [DOI: ] [Google Scholar]
Jeck‐Thole 2006
- Jeck‐Thole S, Wagner W. Betahistine: a retrospective synopsis of safety data. Drug Safety 2006;29(11):1049‐59. [PUBMED: 17061910] [DOI] [PubMed] [Google Scholar]
Kluk 2006
- Kluk K, Moore BC. Dead regions in the cochlea and enhancement of frequency discrimination: effects of audiogram slope, unilateral versus bilateral loss, and hearing‐aid use. Hearing Research 2006;222(1‐2):1‐15. [PUBMED: 17071031] [DOI] [PubMed] [Google Scholar]
Kuk 1990
- Kuk FK, Tyler RS, Russell D, Jordan H. The psychometric properties of a tinnitus handicap questionnaire. Ear and Hearing 1990;11(6):434‐45. [PUBMED: 2073977] [DOI] [PubMed] [Google Scholar]
König 2006
- König O, Schaette R, Kempter R, Gross M. Course of hearing loss and occurrence of tinnitus. Hearing Research 2006;221(1‐2):59‐64. [PUBMED: 16962270] [DOI] [PubMed] [Google Scholar]
Lacour 2007
- Lacour M, Heyning PH, Novotny M, Tighilet B. Betahistine in the treatment of Meniere's disease. Neuropsychiatric Disease and Treatment 2007;3(4):429‐40. [PUBMED: 19300572] [PMC free article] [PubMed] [Google Scholar]
Laurikainen 1993
- Laurikainen EA, Miller JM, Quirk WS, Kallinen J, Ren T, Nuttall AL, et al. Betahistine induced vascular effects in the rat cochlea. American Journal of Otology 1993;14(1):24‐30. [PUBMED: 8424471] [PubMed] [Google Scholar]
Martines 2010
- Martines F, Bentivegna D, Piazza F, Martines E, Sciacca V, Martinciglio G. Investigation of tinnitus patients in Italy: clinical and audiological characteristics. International Journal of Otolaryngology 2010;2010:265861. [PUBMED: 20652075] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 1972
- Martinez DM. The effect of Serc (betahistine hydrochloride) on the circulation of the inner ear in experimental animals. Acta Oto‐laryngologica. Supplementum 1972;305:29‐47. [PUBMED: 4353749] [DOI] [PubMed] [Google Scholar]
McCormack 2016
- McCormack A, Edmondson‐Jones M, Somerset S, Hall DA. A systematic review of the reporting of tinnitus prevalence and severity. Hearing Research 2016;337:70‐9. [PUBMED: 27246985] [DOI] [PubMed] [Google Scholar]
McDermott 1998
- McDermott HJ, Lech M, Kornblum MS, Irvine DR. Loudness perception and frequency discrimination in subjects with steeply sloping hearing loss: possible correlates of neural plasticity. Journal of the Acoustical Society of America 1998;104(4):2314‐25. [PUBMED: 10491696] [DOI] [PubMed] [Google Scholar]
McFerran 2018
- McFerran D, Hoare DJ, Carr S, Ray J, Stockdale D. Tinnitus services in the United Kingdom: a survey of patient experiences. BMC Health Services Research 2018;18(1):110. [PUBMED: 29433479] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meikle 2012
- Meikle MB, Henry JA, Griest SE, Stewart BJ, Abrams HB, McArdle R, et al. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear and Hearing 2012;33(2):153‐76. [PUBMED: 22156949] [DOI] [PubMed] [Google Scholar]
Melcher 2013
- Melcher JR, Knudson IM, Levine RA. Subcallosal brain structure: correlation with hearing threshold at supra‐clinical frequencies (>8 kHz), but not with tinnitus. Hearing Research 2013;295:79‐86. [PUBMED: 22504034] [DOI] [PubMed] [Google Scholar]
Middleton 2011
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proceedings of the National Academy of Sciences of the United States of America 2011;108(18):7601‐6. [PUBMED: 21502491] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moller 2000
- Moller AR. Similarities between severe tinnitus and chronic pain. Journal of the American Academy of Audiology 2000;11(3):115‐24. [PUBMED: 10755808] [PubMed] [Google Scholar]
Moore 2009
- Moore BC, Vinay SN. Enhanced discrimination of low‐frequency sounds for subjects with high‐frequency dead regions. Brain 2009;132(Pt 2):524‐36. [PUBMED: 19036764] [DOI] [PubMed] [Google Scholar]
Mulders 2010
- Mulders WH, Seluakumaran K, Robertson D. Efferent pathways modulate hyperactivity in inferior colliculus. Journal of Neuroscience 2010;30(28):9578‐87. [PUBMED: 20631186] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murdin 2016
- Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD010696.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mühlnickel 1998
- Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proceedings of the National Academy of Sciences of the United States of America 1998;95(17):10340‐3. [PUBMED: 9707649] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newman 1996
- Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Archives of Otolaryngology‐‐Head & Neck Surgery 1996;122(2):143‐8. [PUBMED: 8630207] [DOI] [PubMed] [Google Scholar]
Noreña 2005
- Noreña AJ, Eggermont JJ. Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. Journal of Neuroscience 2005;25(3):699‐705. [PUBMED: 15659607] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noreña 2011
- Noreña AJ. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neuroscience and Biobehavioral Reviews 2011;35(5):1089‐109. [PUBMED: 21094182] [DOI] [PubMed] [Google Scholar]
Pathy 1977
- Pathy J, Menon G, Reynolds A, Strik R. Betahistine hydrochloride (Serc) in cerebrovascular disease a placebo‐controlled study. Age and Ageing 1977;6(3):179‐84. [DOI] [PubMed] [Google Scholar]
Phillips 2008
- Phillips JS, Prinsley PR. Prescribing practices for betahistine. British Journal of Clinical Pharmacology 2008;65(4):470‐1. [PUBMED: 18279470] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pilati 2012
- Pilati N, Large C, Forsythe ID, Hamann M. Acoustic over‐exposure triggers burst firing in dorsal cochlear nucleus fusiform cells. Hearing Research 2012;283(1‐2):98‐106. [PUBMED: 22085487] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratnayake 2009
- Ratnayake SA, Jayarajan V, Bartlett J. Could an underlying hearing loss be a significant factor in the handicap caused by tinnitus?. Noise & Health 2009;11(44):156‐60. [PUBMED: 19602769] [DOI] [PubMed] [Google Scholar]
Rauschecker 1999
- Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends in Neurosciences 1999;22(2):74‐80. [PUBMED: 10092047] [DOI] [PubMed] [Google Scholar]
Rauschecker 2010
- Rauschecker JP, Leaver AM, Muhlau M. Tuning out the noise: limbic‐auditory interactions in tinnitus. Neuron 2010;66(6):819‐26. [PUBMED: 20620868] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reiss 1986
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy 1986;24(1):1‐8. [PUBMED: 3947307] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2010
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. Journal of Neuroscience 2010;30(45):14972‐9. [PUBMED: 21068300] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sahley 2001
- Sahley TL, Nodar RH. A biochemical model of peripheral tinnitus. Hearing Research 2001;152(1‐2):43‐54. [PUBMED: 11223280] [DOI] [PubMed] [Google Scholar]
Sanchez 2002
- Sanchez TG, Ferrari GMS. The control of tinnitus through hearing aids: suggestions for optimal use. Pró‐Fono Revista de Atualização Científica 2002;14:111‐8. [Google Scholar]
Schaette 2006
- Schaette R, Kempter R. Development of tinnitus‐related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. European Journal of Neuroscience 2006;23(11):3124‐38. [PUBMED: 16820003] [DOI] [PubMed] [Google Scholar]
Schaette 2011
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. Journal of Neuroscience 2011;31(38):13452‐7. [PUBMED: 21940438] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seki 2003
- Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone‐induced hearing loss. Hearing Research 2003;180(1‐2):28‐38. [PUBMED: 12782350] [DOI] [PubMed] [Google Scholar]
Sereda 2011
- Sereda M, Hall DA, Bosnyak DJ, Edmondson‐Jones M, Roberts LE, Adjamian P, et al. Re‐examining the relationship between audiometric profile and tinnitus pitch. International Journal of Audiology 2011;50(5):303‐12. [PUBMED: 21388238] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sereda 2015
- Sereda M, Edmondson‐Jones M, Hall DA. Relationship between tinnitus pitch and edge of hearing loss in individuals with a narrow tinnitus bandwidth. International Journal of Audiology 2015;54(4):249‐56. [PUBMED: 25470623] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skevington 2004
- Skevington SM, Lotfy M, O'Connell KA. The World Health Organization's WHOQOL‐BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Quality of Life Research 2004;13(2):299‐310. [PUBMED: 15085902] [DOI] [PubMed] [Google Scholar]
Sweetow 1990
- Sweetow RW, Levy MC. Tinnitus severity scaling for diagnostic/therapeutic usage. Hearing Instruments 1990;41(20‐1):46. [Google Scholar]
Tass 2012
- Tass PA, Adamchic I, Freund HJ, Stackelberg T, Hauptmann C. Counteracting tinnitus by acoustic coordinated reset neuromodulation. Restorative Neurology and Neuroscience 2012;30(2):137‐59. [PUBMED: 22414611] [DOI] [PubMed] [Google Scholar]
Thai‐Van 2002
- Thai‐Van H, Micheyl C, Norena A, Collet L. Local improvement in auditory frequency discrimination is associated with hearing‐loss slope in subjects with cochlear damage. Brain 2002;125(Pt 3):524‐37. [PUBMED: 11872610] [DOI] [PubMed] [Google Scholar]
Thai‐Van 2003
- Thai‐Van H, Micheyl C, Moore BC, Collet L. Enhanced frequency discrimination near the hearing loss cut‐off: a consequence of central auditory plasticity induced by cochlear damage?. Brain 2003;126(Pt 10):2235‐45. [PUBMED: 12847078] [DOI] [PubMed] [Google Scholar]
Timmerman 1994
- Timmerman H. Pharmacotherapy of vertigo: any news to be expected?. Acta Oto‐laryngologica. Supplementum 1994;513:28‐32. [PUBMED: 7910713] [DOI] [PubMed] [Google Scholar]
Tunis 2016
- Tunis SR, Clarke M, Gorst SL, Gargon E, Blazeby JM, Altman DG, et al. Improving the relevance and consistency of outcomes in comparative effectiveness research. Journal of Comparative Effectiveness Research 2016;5(2):193‐205. [PUBMED: 26930385] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tunkel 2014
- Tunkel DE, Bauer CA, Sun GH, Rosenfeld RM, Chandrasekhar SS, Cunningham ER Jr, et al. Clinical practice guideline: tinnitus executive summary. Otolaryngology‐‐Head and Neck Surgery 2014;151(4):533‐41. [PUBMED: 25274374] [DOI] [PubMed] [Google Scholar]
Unemoto 1982
- Unemoto H, Sasa M, Takaori S, Ito J, Matsuoka I. Inhibitory effect of betahistine on polysynaptic neurons in the lateral vestibular nucleus. Archives of Oto‐rhino‐laryngology 1982;236(3):229‐36. [PUBMED: 7159275] [DOI] [PubMed] [Google Scholar]
van Esch 2018
- Esch B, Zaag‐Loonen HJ, Bruintjes T, Murdin L, James A, Benthem PP. Betahistine for Ménière's disease or syndrome. Cochrane Database of Systematic Reviews 2018, Issue 1. [DOI: 10.1002/14651858.CD012914] [DOI] [Google Scholar]
Vanneste 2012
- Vanneste S, Ridder D. The auditory and non‐auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Frontiers in Systems Neuroscience 2012;6:31. [PUBMED: 22586375] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisz 2005
- Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Medicine 2005;2(6):e153. [PUBMED: 15971936] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1991
- Wilson PH, Henry J, Bowen M, Haralambous G. Tinnitus reaction questionnaire: psychometric properties of a measure of distress associated with tinnitus. Journal of Speech and Hearing Research 1991;34(1):197‐201. [PUBMED: 2008074] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [PUBMED: 6880820] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hall 2018
- Hall DA, Wegner I, Smit AL, McFerran D, Stegeman I. Betahistine for tinnitus. Cochrane Database of Systematic Reviews 2018, Issue 8. [DOI: 10.1002/14651858.CD013093] [DOI] [PMC free article] [PubMed] [Google Scholar]


