Abstract
Background:
The fecal immunochemical test (FIT) is commonly used for colorectal cancer (CRC) screening. Despite demographic variations in stool hemoglobin concentrations, little data exist regarding optimal positivity thresholds by age and sex.
Objective:
Identify programmatic (multi-test) FIT performance characteristics and optimal FIT quantitative hemoglobin positivity thresholds in a large, population-based screening program.
Design:
Retrospective cohort study
Setting:
Kaiser Permanente Northern and Southern California
Participants:
Adults 50–75 years of age, screening eligible, with baseline quantitative FIT results (2013–2014), and 2 years follow-up. Nearly two-thirds (411,641) had a previous FIT within 2 years.
Measurements:
FIT programmatic sensitivity for CRC and number of positive tests per cancer detected, overall and by age and sex.
Results:
Among 640,859 persons completing a baseline FIT and followed for 2 years, 481,817 (75%) had ≥1 additional FIT and 1,245 (0.19%) were diagnosed with CRC. Cancer detection (programmatic sensitivity) increased at lower positivity thresholds, from 925/1,245 (74.3%) at 30 μg/g, to 950 (76.3%) at 20 μg/g, and 987 (79.3%) at 10 μg/g; the number of positive tests per cancer detected increased from 43 at 30 μg/g, to 52 at 20 μg/g, and 85 at 10 μg/g. Reducing the positivity threshold from 20 to 15 μg/g would detect 3% more cancers and require 23% more colonoscopies. At the conventional 20 μg/g FIT threshold, programmatic sensitivity decreased with increasing age (79.0%, 73.4%, and 68.9% for ages 50–59, 60–69 and 70–75 years, respectively, p=0.009) and was higher in men than women (77.0% vs. 70.6%, p=0.011).
Limitations:
Information on advanced adenoma were lacking.
Conclusion:
Increased cancer detection at lower positivity thresholds is counter-balanced by substantial increases in positive tests. Tailored thresholds may provide more comparable screening benefits for different demographic groups, dependent upon local resources.
Keywords: colorectal cancer, screening, fecal occult blood tests, sensitivity and specificity, number needed to scope
Introduction:
Colorectal cancer (CRC) is the second-leading cause of cancer mortality in the United States (US)(1). Screening with guaiac-based fecal occult blood tests reduced CRC mortality in randomized trials by 15 to 33% (2, 3). The fecal immunochemical test (FIT) has several advantages over guaiac-based tests, including greater specificity for human hemoglobin, the need for only a single fecal sample, and no requirement for dietary or medication restrictions (1, 4).
Quantitative FITs directly measure human hemoglobin concentrations in the stool. The quantitative threshold for a positive FIT result (positivity threshold) can be tailored to different settings and patient groups to optimize cancer detection (sensitivity), while limiting the number of colonoscopies triggered by positive FIT results. Population-based screening programs worldwide use a broad range of FIT positivity thresholds, ranging from 10 to 47 μg hemoglobin/g (5), although 20 μg/g is the convention in the US (6). Few comparisons between positivity thresholds exist from screening programs in large diverse populations (7, 8). Furthermore, there are differences in fecal hemoglobin concentrations between population groups, with reports of higher concentrations in men than women and increasing concentrations with age (9–11). Likewise, CRC incidence is higher in men than women, and increases with age (12). In contrast, women have a higher incidence of proximal cancers (right sided) that may be more difficult to detect by screening (13, 14). Consequently, utilizing the same FIT positivity threshold across subgroups may be suboptimal (15). Previous studies examining positivity thresholds have been limited by an insufficient number of cancer diagnoses needed to define the sensitivity of FIT, particularly in demographic subgroups (4, 7, 14, 16–18), were conducted with population samples that did not resemble the general US population (19), used outdated or qualitative tests (4), or lacked comprehensive follow-up of negative FIT results needed to identify missed CRCs (i.e., false negatives)(20, 21).
We examined FIT screening performance measures in two large, community-based, screening programs using mailed FIT outreach with linkage to comprehensive cancer registries. We assessed the balance of cancer detection and screening burden at varying FIT positivity thresholds. Our goal was to quantify the programmatic sensitivity of FIT (over multiple rounds of testing) and the potential impact of various positivity thresholds on a screening program, in a cohort representative of a community-based, diverse population.
Methods
Study Setting:
This retrospective cohort study included members of Kaiser Permanente Northern California (KPNC) and Southern California (KPSC) who completed FIT screening between January 1, 2013 and December 31, 2014. KPNC and KPSC are integrated healthcare systems with approximately 8 million members receiving care across 40 medical centers. These systems provide comprehensive care, an integrated electronic health record, and cancer registries reporting to the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) program. Their diverse membership closely approximates the adult census population in California, except at extremes of income (22–24). The study was approved by the institutional review boards of KPNC and KPSC, and was conducted within the National Cancer Institute-funded Population-based Research to Optimize the Screening Process (PROSPR II) consortium, which conducts multisite, coordinated, transdisciplinary research to evaluate and improve cancer screening processes.
Fecal immunochemical tests:
KPNC and KPSC have organized FIT-based CRC screening outreach programs that began between 2006 and 2008 (8, 25). Members 50–75 years of age without a colonoscopy within 10 years or sigmoidoscopy within 5 years were mailed a FIT kit annually. The kit included a one-sample FIT (OC FIT-CHEK; Polymedco), a standardized letter, directions for completing the test, and a preprinted laboratory requisition form. Patients mailed their completed FIT to a regional laboratory, where it was analyzed using an OC-Sensor Diana automated system (Polymedco) with the threshold for a positive test of >20 μg/g (corresponding to 100 nanograms of hemoglobin/ml of test buffer) (26). Patients with a result at the threshold were classified as negative. Patients with positive results were referred for colonoscopy. Patients and providers were informed if the result was “positive” or “negative”, but were not provided with the actual quantitative values. Tests with a value >200 μg/g were read as “over assay” and reported as positive.
Overall cohort eligibility criteria and follow-up:
Individuals were eligible if they had a quantitative FIT result available between January 1, 2013 and December 31, 2014, irrespective of whether it was their first-ever FIT; were 50–75 years of age; were at average risk for CRC (no personal history of CRC, total colectomy, or inflammatory bowel disease); and had been members of the health system for ≥2 years before the test result date (to capture prior endoscopy examinations or FIT).Patients were followed for 2 years after the test result date or until their CRC diagnosis date if it was within 2 years.
Data sources:
Quantitative FIT values were obtained directly from the automated OC-Sensor Diana machine. Patient characteristics (date of birth, sex, body mass index (BMI), race and Hispanic ethnicity) were obtained from the electronic health record. The number of primary care visits and Charlson comorbidity scores were computed with a standardized algorithm using International Classification of Diseases, Ninth Revision codes from care episodes in the calendar year before the baseline FIT (27). Colorectal adenocarcinoma diagnoses were obtained from the KPNC and KPSC cancer registries, which capture greater than 99% of cancers diagnosed among members, compared with manual review, and include the cancer site, morphology, and stage. Cancers were diagnosed in three main ways: by colonoscopy done for follow-up of positive FIT; by colonoscopy or radiologic imaging exams done after negative FIT because of symptoms or laboratory abnormalities (e.g. anemia); and by colonoscopy after negative FIT because subsequent to the FIT, the patient elected to continue future screening with an elective colonoscopy. We only considered adenocarcinomas, which represent approximately 90–95% of all CRCs, because these tumors are believed to follow the adenoma-carcinoma sequence and are potentially preventable through screening (28, 29). Cancer location was defined as the right colon (proximal to the splenic flexure), left colon, or rectum. Advanced-stage cancers were defined as stage III (regional lymph node involvement) or stage IV (distant metastasis) according to the American Joint Committee on Cancer staging system or as code 3 (disease in the regional lymph nodes), code 4 (regional disease with direct extension and spread to regional lymph nodes), or code 7 (distant metastasis) according to the 2013 SEER Program Coding and Staging Manual 2013 (30).
Data Analysis:
The distribution of baseline quantitative FIT values among those with and without CRC during follow-up, by age and sex, were summarized using percentiles. The primary analysis was the programmatic sensitivity, specificity, and corresponding confidence intervals at FIT positivity thresholds of 10, 15, 20, 25 and 30 μg/g. Programmatic sensitivity was the proportion of patients with a CRC diagnosis within 2 years of FIT screening who had a quantitative FIT result above a given positivity threshold at baseline or during follow-up testing; programmatic specificity was the proportion of patients without a CRC diagnosis with all quantitative FIT results at or below a given threshold. The number of cancers diagnosed and the number individuals with a positive test were calculated at each threshold. The number of participants with a positive test per cancer detected was calculated at each threshold. The same test characteristics were examined separately by age (50–59, 60–69 and 70–75 years) and sex (male and female), with comparisons between subgroups using the χ2 and ANOVA tests. Analyses were repeated using only the baseline FIT result and 1 year of follow-up in the cancer registry to evaluate the impact of a single test using the recommended 1-year screening interval in the United States, and to limit the effect of repeated screening on results (4). Analyses used SAS Version 9.4 (SAS Institute) and Stata Version 14 (StataCorp).
Role of Funding Sources:
The study was funded by the National Cancer Institute and the Swiss Cancer Research Foundation, neither of which had a role in the conception, design, analysis, or conduct of the study.
Results:
Overall cohort Characteristics:
Of 1,550,542 members who received a FIT, 1,212,461 (78%) completed ≥1 FIT in 2013–2014 (Figure 1). A total of 640,859 patients (53%), including 401,975 at KPNC and 238,884 at KPSC, had ≥1 quantitative FIT result and met the inclusion criteria; 411,241 (64%) completed a previous FIT within 2 years of the baseline FIT (Table 1). During 2 years of follow-up, 159,042 (25%) did not complete additional FIT screening after the baseline, 250,519 (39%) completed 1 additional FIT, and 231,298 (36%) completed ≥2 additional FITs (Figure 1). A total of 48,561 (7.6%) patients had a positive result at the conventional positivity threshold of 20 μg/g, of whom 36,906 (76%) had a colonoscopy within 2 years of the baseline FIT (median 33 days after the result). The distributions of positive tests, colonoscopy follow-up, and cancer diagnoses were similar between those with quantitative results and the overall population of KPNC and KPSC members tested during the study period (data not shown).
Figure 1:
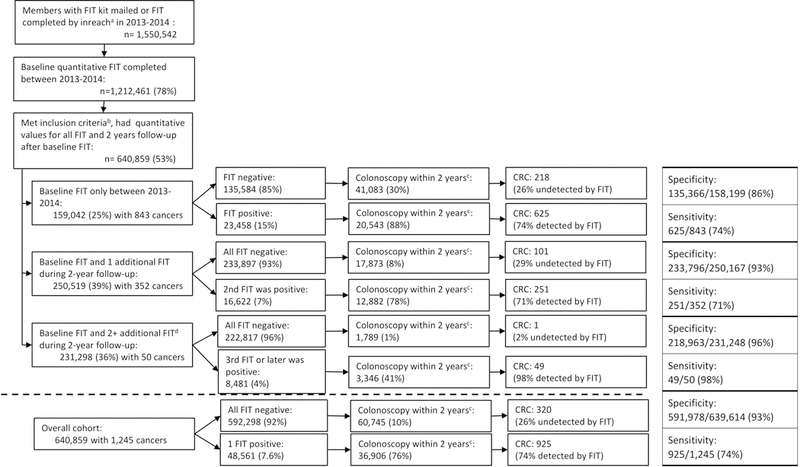
Number of fecal immunochemical tests (FIT) completed, FIT results, number of colonoscopies, and cancer diagnoses among patients with ≥1 FIT completed in 2013–2014 and followed for 2 years from their baseline FIT. aInreach is the local distribution of FIT by primary care staff or otherwise. bInclusion criteria: 50–75 years of age; ≥2 years Kaiser Permanente membership prior to baseline FIT; no prior diagnosis of inflammatory bowel disease or CRC; no prior colectomy; and quantitative values available for all FITs during 2-year study interval. Patients followed for 2 years after the baseline FIT. c2-year time interval started at time of the baseline FIT. Overall, 80% of patients received a colonoscopy within 180 days of a positive FIT. d226,984 completed 2 additional FIT, 4,161 completed 3 additional FIT, and 153 completed 4 additional FIT during the 2-year follow-up. FIT: fecal immunochemical test, CRC: colorectal cancer
Table 1:
Patient characteristics and colorectal cancer outcomes within 2 years of a baseline fecal immunochemical test (FIT) among participants who completed ≥1 FIT (n=640,859)
| Participant Characteristics | |
|---|---|
| Female sex | 337,588 (53%) |
| Age at time of FIT | |
| 50 to 59 years | 323,855 (51%) |
| 60 to 69 years | 234,665 (37%) |
| 70 to 75 years | 82,339 (13%) |
| Race/ethnicity | |
| Non-Hispanic white | 352,913 (55%) |
| Hispanic | 114,724 (18%) |
| Black | 46,925 (7%) |
| Asian/Pacific islander | 103,958 (16%) |
| Other or unknown | 22,339 (3%) |
| Obese (BMI ≥30 kg/m2)a | 215,312 (34%) |
| Primary care visits in year preceding test | |
| No primary care visit | 124,285 (19%) |
| 1 to 3 primary care visits | 367,406 (57%) |
| 4 or more primary care visits | 149,168 (23%) |
| Charlson comorbidity score year prior to baseline FIT | |
| Score = 0 | 443,577 (69%) |
| Score = 1 or 2 | 153,825 (24%) |
| Score ≥ 3 | 43,457 (7%) |
| Previous FIT within 2 years of baseline FIT | |
| Yes | 411,241 (64%) |
| No | 229,618 (36%) |
| Outcomes during follow-up | |
| Colonoscopy within 2 years | 97,651 (15%) |
| Baseline FIT > 20 μg/g | 26,654 (4.2%) |
| Colorectal cancer diagnosed within 2 years of baseline FIT | 1,245 (0.19%) |
| Right-sided colon cancer | 505 (41%) |
| Left-sided colon cancer | 358 (29%) |
| Rectal cancer | 362 (29%) |
| Location unknown | 20 (2%) |
| Colorectal cancer stage at diagnosis | |
| Early | 1,064 (85%) |
| Late | 181 (15%) |
Body mass index (BMI) was missing for 15,360 patients (2%). FIT: fecal immunochemical test
Of 1,245 cancers diagnosed at or within 2 years of the baseline FIT, 505 (41%) were in the right colon, 358 (29%) in the left colon, 362 (29%) in the rectum, and 20 (1.6%) of undefined primary location (Table 1). The proportion of right-sided cancers increased with age (29% between 50–59 years, 44% between 60–69 years, and 53% between 70–75 years, p<0.001), and was higher in women than men (46% vs. 36%, p=0.004) (supplemental Tables 1 and 2). Among those who did not develop cancer during follow-up, baseline stool hemoglobin values at the 95% percentile increased with age (13.4 μg/g for age 50–59, 16.2 μg/g for age 60–69, and 21.0 μg/g for age 70–75, p<0.001), and were higher in men than women (17.8 μg/g vs. 13.4 μg/g, p<0.001)(Supplemental table 3). For those who developed cancer, median stool hemoglobin values from the baseline FIT were higher for left-sided than right-sided cancers or rectal cancers (60.0 μg/g, 12.4 μg/g, and 24.4 μg/g respectively, p<0.001 for each 2-way comparison)(Supplemental table 3).
FIT performance at varying positivity thresholds in the overall cohort:
The proportion of cancers detected by FIT (programmatic sensitivity) decreased with higher positivity thresholds, from 987 of 1,245 cancers at 10 μg/g (79.3%), to 925 at 20 μg/g (74.3%), and 822 at 30 μg/g (66.0%) (Table 3). The number of participants with a positive baseline or subsequent FIT test also decreased, from 84,293 at 10 μg/g (13.2% with positive test, programmatic specificity 87.0%), to 48,561 at 20 μg/g (7.6% with positive test, specificity 92.6%), and 35,017 at 30 μg/g (5.5% with positive test, specificity 96.8%).
Table 3:
Colorectal cancer (CRC) detection within 2 years of a baseline fecal immunochemical test (FIT) at various positivity thresholds by an organized outreach program using annual FIT, stratified by age and sex.
| Threshold for FIT positivity: | |||||
|---|---|---|---|---|---|
| 30 μg/g | 25 μg/g | 20 μg/ga | 15 μg/g | 10 μg/g | |
| Age 50–59 years (n=323,855) | |||||
| Cancers detected | 298/428 | 315/428 | 338/428 | 342/428 | 354/428 |
| Programmatic sensitivityb (95% CI) | 69.6% (65.0–74.0) | 73.6% (69.2–77.7) | 79.0% (74.8–82.7) | 79.9% (72.6–81.0) | 82.7% (78.8–86.2) |
| Programmatic specificityc (95% CI) | 95.3% (95.2–95.3) | 94.6% (94.5–94.6) | 93.5% (93.4–93.6) | 92.0% (91.9–92.1) | 88.7% (88.6–88.8) |
| Positive FIT at baseline or within 2 yearsd | 15,605 (4.8%) | 17,878 (5.5%) | 21,321 (6.6%) | 26,230 (8.1%) | 36,892 (11%) |
| Positive results / 1 cancer (95% CI) | 52 (47 – 59) | 57 (51 – 64) | 63 (57 – 70) | 76 (69 – 85) | 104 (94 – 116) |
| Age 60–69 years (n=234,665) | |||||
| Cancers detected | 353/534 | 370/534 | 392/534 | 403/534 | 421/534 |
| Programmatic sensitivityb (95% CI) | 66.1% (61.9–70.1) | 69.3% (65.2–73.2) | 73.4% (69.4–77.1) | 75.5% (71.6–79.1) | 78.8% (75.1–82.2) |
| Programmatic specificityc (95% CI) | 94.3% (94.2–94.4) | 93.3% (93.2–93.4) | 91.9% (91.8–92.0) | 90.0% (89.8–90.1) | 85.7% (85.6–85.9) |
| Positive FIT at baseline or within 2 yearsd | 13,745 (5.9%) | 15,955 (6.8%) | 19,355 (8.3%) | 23,897 (10%) | 33,847 (14%) |
| Positive results / 1 cancer (95% CI) | 39 (35 – 43) | 43 (39 – 48) | 49 (45 – 55) | 59 (54 – 65) | 80 (73 – 89) |
| Age 70–75 years (n=82,056) | |||||
| Cancers detected | 171/283 | 182/283 | 195/283 | 205/283 | 212/283 |
| Programmatic sensitivityb (95% CI) | 60.4% (54.5–66.2) | 64.3% (58.4–69.9) | 68.9% (63.2–74.3) | 72.4% (66.8–77.6) | 74.9% (69.4–79.9) |
| Programmatic specificityc (95% CI) | 93.3% (93.1–93.5) | 92.2% (92.0–92.4) | 90.6% (90.4–90.8) | 88.4% (88.2–88.6) | 83.7% (83.5–84.0) |
| Positive FIT at baseline or within 2 yearsd | 5,667 (6.9%) | 6,566 (8.0%) | 7,885 (9.6%) | 9,742 (12%) | 13,554 (16%) |
| Positive results / 1 cancer (95% CI) | 33 (29 – 39) | 36 (31 – 42) | 40 (35 – 47) | 48 (42 – 55) | 64 (56 – 73) |
| Men (n=303,271) | |||||
| Cancers detected | 489/717 | 517/717 | 552/717 | 562/717 | 577/717 |
| Programmatic sensitivityb (95% CI) | 68.2% (64.7–71.6) | 72.1% (68.7–75.4) | 77.0% (73.7–80.0) | 78.4% (75.2–81.3) | 80.5% (77.4–83.3) |
| Programmatic specificityc (95% CI) | 93.9% (93.8–94.0) | 93.0% (92.9–93.1) | 91.6% (91.5–91.7) | 89.7% (89.6–89.8) | 85.6% (85.5–85.7) |
| Positive FIT at baseline or within 2 yearsd | 18,899 (3.8%) | 21,654 (7.1%) | 25,932 (8.6%) | 31,814 (11%) | 44,108 (15%) |
| Positive results / 1 cancer (95% CI) | 39 (35 – 42) | 42 (39 – 42) | 47 (43 – 51) | 57 (52 – 62) | 76 (70 – 83) |
| Women (n=429,590) | |||||
| Cancers detected | 333/528 | 350/528 | 373/528 | 388/528 | 410/528 |
| Programmatic sensitivityb (95% CI) | 63.1% (58.8–67.2) | 66.3% (62.1–70.3) | 70.6% (66.6–74.5) | 73.5% (69.5–77.2) | 77.7% (73.9–81.1) |
| Programmatic specificityc (95% CI) | 95.3% (95.2–95.4) | 94.5% (94.5–94.6) | 93.4% (93.3–93.5) | 91.8% (91.7–91.9) | 88.2% (88.1–88.3) |
| Positive FIT at baseline or within 2 yearsd | 16,118 (4.8%) | 18,745 (5.6%) | 22,629 (6.7%) | 28,055 (8.3%) | 40,185 (12%) |
| Positive results / 1 cancer (95% CI) | 48 (44 – 54) | 54 (48 – 60) | 60 (55 – 67) | 72 (66 – 80) | 98 (89 – 108) |
The current United States conventional FIT positivity threshold is 20 μg/g
Among those with colorectal cancer, the proportion with a positive FIT at baseline or within 2 years
Among those without cancer, the proportion without a positive FIT at baseline or within 2 years
Individuals with a positive FIT at baseline or within 2 years
The number of positive tests per cancer detected increased substantially at thresholds below 20 μg/g. Positive tests per cancer detected increased 11% from 25 to 20 μg/g (47 to 52), 22% from 20 to 15 μg/g (52 to 63), and 35% from 15 to 10 μg/g (63 to 85). Reducing the positivity threshold from 20 to 15 μg/g would have detected 25 additional cancers (3% increase) from 11,308 additional colonoscopies (23% increase), equating to 452 colonoscopies per additional cancer detected.
FIT performance at varying positivity thresholds stratified by age:
Programmatic sensitivity and specificity decreased significantly with increasing age at thresholds between 10 and 30 μg/g. At 20 μg/g, the programmatic sensitivity decreased from 79.0% (95%CI 74.8–82.7) among those 50–59 years, to 73.4% among those 60–69 years (95%CI 69.4–77.1), and 68.9% among those 70–75 years (95%CI 63.2–74.3, p=0.009)(Table 4). Programmatic specificity declined with age, from 93.5% (95%CI 93.4–93.6) among those 50–59 years, to 90.6% (95%CI 90.4–90.8) among those 70–75 years (p<0.001), as the number of positive tests per cancer detected decreased from 63 (57–70) to 40 (35–47).
FIT performance at varying positivity thresholds stratified by sex:
At all thresholds between 10 and 30 μg/g, FIT had lower programmatic sensitivity and higher programmatic specificity among women than men (Table 4). At 20 μg/g, the programmatic sensitivities were 77.0% (95%CI 73.7–80.0) and 70.6% (95%CI 66.6–74.5) for men and women respectively (p=0.011), and the programmatic specificities were 91.6% (95%CI 91.5–91.7) and 93.4% (95%CI 93.3–93.5) (p<0.001). Decreasing the threshold from 20 to 10 μg/g led to substantial increases in the number of positive tests per cancer detected for both sexes, increasing 62% from 47 (95%CI 43–51) to 76 (95%CI 70–83) in men, and 63% in women from 60 (95%CI 55–67) to 98 (95%CI 89–108). Decreasing the threshold for women from 20 to 10 μg/g yielded a programmatic sensitivity similar to that of men at the 20 μg/g threshold, but with a lower programmatic specificity (88.2% vs. 91.6%) and more than double the number of positive results per cancer detected (98 vs. 47).
Additional analyses with 1-year follow-up:
Similar patterns were seen for the trade-off of sensitivity and specificity, both overall and among subgroups, using only 1-year of follow-up from the baseline FIT result (Supplementary Materials). FIT sensitivity of the baseline test only with 1-year of follow-up was slightly higher (77.7% at 20 μg/g (95%CI 72.6–81.0)) compared to the programmatic sensitivity with 2 years of follow-up (74.3% (95%CI 71.8–76.7); FIT specificity was also higher (95.9% (95%CI 95.9–96.0) vs. a programmatic specificity of 92.6% (95%CI 92.5–92.6) (Supplemental Table 6).
Discussion:
In this large, diverse, community-based sample of adults participating in FIT screening, programmatic sensitivity for CRC increased modestly with decreasing positivity thresholds, from 74.3% at 20 μg/g, to 76.3% at 15 μg/g, and 79.3% at 10 μg/g. The number of positive tests per cancer detected over 2 years increased with decreasing positivity thresholds, especially below 20 μg/g. FIT performance varied by age and sex, with lower programmatic sensitivity and specificity with increasing age, and higher programmatic sensitivity and lower specificity in men than women.
The 5% point increase in CRC programmatic sensitivity observed when decreasing the positivity threshold from 20 to 10 μg/g is smaller than differences reported in some prior studies (31), although prior reports have been inconsistent and had small numbers of cancers, resulting in low precision (wide confidence intervals) (32–34). A systematic review of several FIT brands found that a threshold of <20 μg/g yielded an overall sensitivity of 86% (95%CI 75–92) vs. 63% (95%CI 43–79) for thresholds between 20–50 μg/g (4). Previous studies with colonoscopy follow-up of all screening participants have compared FIT sensitivity (using OC-Sensor Diana) for advanced adenomas at varying thresholds (32–35). They have shown 5–10% increases in advanced adenoma sensitivity when decreasing the positivity threshold from 20 to 10 μg/g. This increase is larger than we observed for cancers, and is clinically relevant given that approximately 25% of advanced adenomas will progress to CRC over 10 years (36).
A few prior studies with colonoscopy follow-up suggested that FIT sensitivity for CRC and advanced adenomas is higher and specificity lower in men than women (10, 34, 37), though reports are conflicting and did not allow precise estimates by quantitative FIT thresholds (19). Proposed explanations for this disparity include a higher proportion of harder-to-detect, right-sided cancers (34), lower serum hemoglobin concentrations (10), and longer colonic transit times in women than in men (38). Women in our cohort had a higher proportion of right-sided cancers than men, and women with cancer had lower mean stool hemoglobin concentrations than men with cancer (Supplemental table 3). Women also had smaller relative reductions in CRC mortality than men in previous randomized trials of guaiac tests (39). The apparent lower sensitivity of FIT in women is concerning given that sigmoidoscopy also appears less effective in women (40). There are few published reports of FIT performance trends by age, though FIT may perform better below age 50 than above (41), and worse above age 65 (42).
Our findings suggest that screening programs wishing to increase cancer detection by lowering their positivity threshold below the conventional 20 μg/g will require substantial additional colonoscopy and financial resources. The large increases in the number of positive tests per cancer detected are likely because CRC is rare in screening populations and more overlap in lower fecal hemoglobin concentrations between those with and without CRC. Some additional colonoscopies generated by positive tests without a cancer (false positives in this study) would detect and remove advanced adenomas, likely reducing future cancer incidence (8, 31). However, repeat annual testing can mean larger accumulated numbers of false positive tests; our 2-year programmatic specificity results were substantially lower than one-time test specificity with 1-year follow-up (supplementary materials). The optimal positivity threshold (acceptable number of positive tests per cancer detected) will depend on the availability of local colonoscopy resources. Lower resourced settings may raise their positivity threshold, although in our study sensitivity at 30 μg/g declined from 74.3% to 66.0%. Notably, a screening program in the Netherlands increased their threshold from 15 to 47 μg/g following unexpectedly high colonoscopy demand (43).
The implications for screening programs of the observed differences in programmatic sensitivity and specificity by age and sex are less clear, and likely depend on the relative importance given to colonoscopy burden or maximizing cancer detection across subgroups. As might be expected, given the lower baseline fecal hemoglobin concentrations in women, we observed a larger increase in programmatic sensitivity when decreasing the positivity threshold from 20 to 10 μg/g in women than in men (7.1% vs. 3.5% increase), although women also had a larger increase in the number of positive tests per cancer (38 vs. 29 more tests), likely due to their lower cancer incidence rates. Lowering the positivity threshold for women may achieve comparable sensitivity to men and fewer interval cancers (34, 44), although others have proposed more aggressive screening in men due to their higher risk (12, 45, 46). We are not aware of previous work advocating lower positivity thresholds in older age groups. While FIT programmatic sensitivity and the number of positive tests per cancer detected were lowest in those aged 70–75 years, both the risk of cancer and the risk of complications from colonoscopy increase with age (1).
Strengths of our study include a diverse, multi-center community-based screening cohort, with quantitative FIT results available, including from repeated testing; analyses from multiple laboratories; high overall screening rates, decreasing the potential for selection bias (47); high-quality cancer registries; more cancers than previous studies (7, 16–18); and a US screening population (19, 32).
Our study also has several potential limitations. First, we did not have quantitative FIT values available on all members who underwent FIT screening during the study interval. However, analyses by season and medical center did not reveal any pattern in missing quantitative values, and our positive predictive values were similar to previous publications from the entire population, including those without quantitative values (8, 48). Second, we assumed all cancers with quantitative values ≤20 μg/g would be detected if designated positive; although follow-up colonoscopy rates and cancer registry ascertainment are high in the KPNC and KPSC screening programs (49), imperfect follow-up would likely attenuate the benefit of lowering the positivity threshold. Third, despite cancer registry follow-up, estimates of programmatic sensitivity incorporate some differential verification bias: patients with a FIT result >20 μg/g were likely to receive immediate, invasive ascertainment sensitive for early-stage CRC (colonoscopy), rather than clinical follow-up with less sensitive ascertainment (cancer registry). Differential verification bias falsely increases sensitivity in studies of 1-time fecal-based cancer screening because of incomplete accrual of false negatives (50), and could make the current threshold of 20 μg/g appear more advantageous. However, our primary results focus on the performance of a screening program over 2 years rather than a single FIT. Fourth, we could not measure false negatives for advanced adenomas and thus our findings may reflect only part of the benefit of lower positivity thresholds. However, smaller studies have been able to estimate the effect of varying positivity thresholds on advanced adenoma detection using the same FIT brand (OC-Sensor Diana) (32–35). Fifth, because participants were followed for two calendar years and not precisely two rounds of screening, there may not have been sufficient time for colonoscopy follow-up of positive tests occurring towards the end of the 2-year follow-up. Finally, most participants had prior FIT screening, which, by removing some prevalent cancers, may have increased the number of positive tests per cancer detected as compared to screening naïve populations (8). However, as screening programs mature, it is important to measure test performance in heterogeneous populations of first-time and repeat screenees.
Conclusions
Among participants in a FIT-based screening program followed for 2 years from their baseline FIT, cancer detection and the number of positive tests per cancer detected varied significantly and substantially by age and sex, suggesting that modifications in positivity thresholds by subgroups could optimize screening program performance, although also with impacts on the number of false-positive tests. Modifying the positivity threshold for the overall study population from the current US conventional value of 20 to 15 μg/g would have resulted in 25 additional cancers detected (3% increase), and required the evaluation of 11,308 additional positive tests (23% increase). Further research is needed to assess the cost-effectiveness of such changes and practicality across settings with differing resources.
Supplementary Material
Table 2:
Colorectal cancer (CRC) detection within 2 years of a baseline fecal immunochemical test (FIT) at various positivity thresholds
| Overall cohort (n=640,859) | Threshold for test positivity | ||||
|---|---|---|---|---|---|
| 30 μg/g | 25 μg/g | 20 μg/ga | 15 μg/g | 10 μg/g | |
| Cancers detected | 822/1,245 | 867/1,245 | 925/1,245 | 950/1,245 | 987/1,245 |
| Programmatic sensitivityb (95% CI) | 66.0% (63.3–68.7) | 69.6% (67.0–72.2) | 74.3% (71.8–76.7) | 76.3% (73.8–78.6) | 79.3% (76.9–81.5) |
| Programmatic specificityc (95% CI) | 94.7% (94.6–94.7) | 93.8% (93.8–93.9) | 92.6% (92.5–92.6) | 90.8% (90.7–90.9) | 87.0% (86.9–87.1) |
| Positive FIT at baseline or within 2 yearsd | 35,017 (5.5%) | 40,399 (6.3%) | 48,561 (7.6%) | 59,869 (9.3%) | 84,293 (13.2%) |
| Positive results / 1 cancer detected (95% CI) | 43 (40–46) | 47 (44–50) | 52 (49–56) | 63 (59–67) | 85 (80–91) |
The current United States conventional FIT positivity threshold is 20 μg/g
Among those with colorectal cancer, the proportion with a positive FIT at baseline or within 2 years
Among those without cancer, the proportion without a positive FIT at baseline or within 2 years
Individuals with a positive result at baseline or within 2 years
Acknowledgements:
The authors would like to thank Peter Bacchetti and Robert Hiatt for their detailed feedback during manuscript preparation.
Funding Source: National Cancer Institute (U54 CA163262)
Funding/Support: This study was conducted within the National Cancer Institute-funded (grant U54 CA163262) Population-based Research to Optimize the Screening Process (PROSPR II) consortium, which conducts multisite, coordinated, transdisciplinary research to evaluate and improve cancer screening processes, and by grants K07 CA212057 from the National Cancer Institute and grant BIL KFS-3720–08-2015 of the Swiss Cancer Research Foundation.
Role of the Funder/Sponsor: The National Cancer Institute had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit for publication.
Footnotes
Reproducible research statement:
Protocol: not available
Statistical Code: Available to interested readers by contacting Dr. Kevin Selby at kevin.selby@hospvd.ch
Data: Data set can be available for collaborative efforts by contacting Dr. Douglas Corley at douglas.corley@kp.org.
Conflict of Interest Disclosures: No conflicts of interest to disclose.
References
- 1.United States Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr., et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564–75. [DOI] [PubMed] [Google Scholar]
- 2.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–9. [DOI] [PubMed] [Google Scholar]
- 3.Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O’Connor E, et al. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315(23):2576–94. [DOI] [PubMed] [Google Scholar]
- 4.Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: An update. World Journal of Gastroenterology. 2017;23(20):3632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;152(5):1217–37 e3. [DOI] [PubMed] [Google Scholar]
- 7.Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287–97. [DOI] [PubMed] [Google Scholar]
- 8.Jensen CD, Corley DA, Quinn VP, Doubeni CA, Zauber AG, Lee JK, et al. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann Intern Med. 2016;164(7):456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald Paula J, Strachan Judith A, Digby J, Steele Robert JC, Fraser Callum G. Faecal haemoglobin concentrations by gender and age: implications for population-based screening for colorectal cancer. Clinical Chemistry and Laboratory Medicine; 2012:935. [DOI] [PubMed] [Google Scholar]
- 10.Brenner H, Haug U, Hundt S. Sex differences in performance of fecal occult blood testing. The American journal of gastroenterology. 2010;105(11):2457–64. [DOI] [PubMed] [Google Scholar]
- 11.Fraser CG, Rubeca T, Rapi S, Chen LS, Chen HH. Faecal haemoglobin concentrations vary with sex and age, but data are not transferable across geography for colorectal cancer screening. Clin Chem Lab Med. 2014;52(8):1211–6. [DOI] [PubMed] [Google Scholar]
- 12.Ferlitsch M, Reinhart K, Pramhas S, et al. Sex-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. JAMA. 2011;306(12):1352–8. [DOI] [PubMed] [Google Scholar]
- 13.Kim S-E, Paik HY, Yoon H, Lee JE, Kim N, Sung M-K. Sex- and gender-specific disparities in colorectal cancer risk. World Journal of Gastroenterology : WJG. 2015;21(17):5167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner H, Niedermaier T, Chen H. Strong subsite-specific variation in detecting advanced adenomas by fecal immunochemical testing for hemoglobin. Int J Cancer. 2017;140(9):2015–22. [DOI] [PubMed] [Google Scholar]
- 15.van Turenhout ST, van Rossum LG, Oort FA, Laheij RJ, van Rijn AF, Terhaar sive Droste JS, et al. Similar fecal immunochemical test results in screening and referral colorectal cancer. World J Gastroenterol. 2012;18(38):5397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Wijkerslooth TR, Stoop EM, Bossuyt PM, Meijer GA, van Ballegooijen M, van Roon AH, et al. Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol. 2012;107(10):1570–8. [DOI] [PubMed] [Google Scholar]
- 17.Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E, et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med. 2007;146(4):244–55. [DOI] [PubMed] [Google Scholar]
- 18.Gies A, Cuk K, Schrotz-King P, Brenner H. Direct Comparison of Diagnostic Performance of 9 Quantitative Fecal Immunochemical Tests for Colorectal Cancer Screening. Gastroenterology. 2018;154(1):93–104. [DOI] [PubMed] [Google Scholar]
- 19.Chen CH, Wen CP, Tsai MK. Fecal immunochemical test for colorectal cancer from a prospective cohort with 513,283 individuals: Providing detailed number needed to scope (NNS) before colonoscopy. Medicine (Baltimore). 2016;95(36):e4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faivre J, Dancourt V, Manfredi S, Denis B, Durand G, Gendre I, et al. Positivity rates and performances of immunochemical faecal occult blood tests at different cut-off levels within a colorectal cancer screening programme. Dig Liver Dis. 2012;44(8):700–4. [DOI] [PubMed] [Google Scholar]
- 21.Chiang TH, Chuang SL, Chen SL, Chiu HM, Yen AM, Chiu SY, et al. Difference in performance of fecal immunochemical tests with the same hemoglobin cutoff concentration in a nationwide colorectal cancer screening program. Gastroenterology. 2014;147(6):1317–26. [DOI] [PubMed] [Google Scholar]
- 22.Gordon N 2015. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2011 California Health Interview Survey. Accessed at Kaiser Permanente Northern California Division of Research at https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/chis_non_kp_2011.pdf on June 6, 2018.
- 23.Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krieger N Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33:101–10. [DOI] [PubMed] [Google Scholar]
- 26.Fraser CG, Allison JE, Halloran SP, Young GP, Expert Working Group on Fecal Immunochemical Tests for Hemoglobin CCSCWEO. A proposal to standardize reporting units for fecal immunochemical tests for hemoglobin. J Natl Cancer Inst. 2012;104(11):810–4. [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 28.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319(9):525–32. [DOI] [PubMed] [Google Scholar]
- 29.Goodman M, Fletcher RH, Doria-Rose VP, Jensen CD, Zebrowski AM, Becerra TA, et al. Observational methods to assess the effectiveness of screening colonoscopy in reducing right colon cancer mortality risk: SCOLAR. J Comp Eff Res. 2015;4(6):541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SEER PROGRAM CODING AND STAGING MANUAL 2013. 2013. Accessed at National Cancer Institute at http://seer.cancer.gov/archive/manuals/2013/SPCSM_2013_maindoc.pdf on June 8, 2018.
- 31.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: Modeling study for the us preventive services task force. JAMA. 2016;315(23):2595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim NH, Yang HJ, Park SK, Park JH, Park DI, Sohn CI, et al. Does Low Threshold Value Use Improve Proximal Neoplasia Detection by Fecal Immunochemical Test? Dig Dis Sci. 2016;61(9):2685–93. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez V, Cubiella J, Gonzalez-Mao MC, Iglesias F, Rivera C, Iglesias MB, et al. Fecal immunochemical test accuracy in average-risk colorectal cancer screening. World J Gastroenterol. 2014;20(4):1038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Turenhout ST, Oort FA, van der Hulst RW, Visscher AP, Terhaar sive Droste JS, Scholten P, et al. Prospective cross-sectional study on faecal immunochemical tests: sex specific cut-off values to obtain equal sensitivity for colorectal cancer? BMC Gastroenterol. 2014;14:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aniwan S, Ratanachu Ek T, Pongprasobchai S, Limsrivilai J, Praisontarangkul OA, Pisespongsa P, et al. The Optimal Cut-Off Level of The Fecal Immunochemical Test For Colorectal Cancer Screening in a Country with Limited Colonoscopy Resources: A Multi-Center Study from Thailand. Asian Pac J Cancer Prev. 2017;18(2):405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840 149 screening colonoscopies. Gut. 2007;56(11):1585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner H, Qian J, Werner S. Variation of diagnostic performance of fecal immunochemical testing for hemoglobin by sex and age: results from a large screening cohort. Clin Epidemiol. 2018;10:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao SS, Kuo B, McCallum RW, Chey WD, DiBaise JK, Hasler WL, et al. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7(5):537–44. [DOI] [PubMed] [Google Scholar]
- 39.Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–14. [DOI] [PubMed] [Google Scholar]
- 40.Holme O, Loberg M, Kalager M, Bretthauer M, Hernan MA, Aas E, et al. Long-Term Effectiveness of Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality in Women and Men: A Randomized Trial. Ann Intern Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim NH, Park JH, Park DI, Sohn CI, Choi K, Jung YS. The fecal immunochemical test has high accuracy for detecting advanced colorectal neoplasia before age 50. Digestive and Liver Disease. 2017;49(5):557–61. [DOI] [PubMed] [Google Scholar]
- 42.Shin A, Choi KS, Jun JK, Noh DK, Suh M, Jung KW, et al. Validity of fecal occult blood test in the national cancer screening program, Korea. PLoS One. 2013;8(11):e79292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toes-Zoutendijk E, van Leerdam ME, Dekker E, van Hees F, Penning C, Nagtegaal I, et al. Real-Time Monitoring of Results During First Year of Dutch Colorectal Cancer Screening Program and Optimization by Altering Fecal Immunochemical Test Cut-Off Levels. Gastroenterology;152(4):767–75.e2. [DOI] [PubMed] [Google Scholar]
- 44.Steele RJ, McClements P, Watling C, Libby G, Weller D, Brewster DH, et al. Interval cancers in a FOBT-based colorectal cancer population screening programme: implications for stage, gender and tumour site. Gut. 2012;61(4):576–81. [DOI] [PubMed] [Google Scholar]
- 45.Arana-Arri E, Idigoras I, Uranga B, Perez R, Irurzun A, Gutierrez-Ibarluzea I, et al. Population-based colorectal cancer screening programmes using a faecal immunochemical test: should faecal haemoglobin cut-offs differ by age and sex? BMC Cancer. 2017;17(1):577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grobbee EJ, Wieten E, Hansen BE, Stoop EM, de Wijkerslooth TR, Lansdorp-Vogelaar I, et al. Fecal immunochemical test-based colorectal cancer screening: The gender dilemma. United European Gastroenterology Journal. 2017;5(3):448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singal AG, Corley DA, Kamineni A, Garcia M, Zheng Y, Doria-Rose PV, et al. Patterns and predictors or repeat fecal immunochemical and occult blood test screening in four large health care systems in the United States. Am J Gastroenterol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doubeni CA, Jensen CD, Fedewa SA, Quinn VP, Zauber AG, Schottinger JE, et al. Fecal Immunochemical Test (FIT) for Colon Cancer Screening: Variable Performance with Ambient Temperature. J Am Board Fam Med. 2016;29(6):672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chubak J, Garcia MP, Burnett-Hartman AN, Zheng Y, Corley DA, Halm EA, et al. Time to Colonoscopy after Positive Fecal Blood Test in Four U.S. Health Care Systems. Cancer Epidemiol Biomarkers Prev. 2016;25(2):344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosman AS, Korsten MA. Effect of verification bias on the sensitivity of fecal occult blood testing: a meta-analysis. J Gen Intern Med. 2010;25(11):1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


