Abstract
The lipid mediator lysophosphatidic acid (LPA) in biological fluids is primarily produced by cleavage of lysophospholipids by the lysophospholipase D enzyme Autotaxin (ATX). LPA has been identified and abundantly detected in the culture medium of various cancer cell types, tumor effusates, and ascites fluid of cancer patients. Our current understanding of the physiological role of LPA established its role in fundamental biological responses that include cell proliferation, metabolism, neuronal differentiation, angiogenesis, cell migration, hematopoiesis, inflammation, immunity, wound healing, regulation of cell excitability, and the promotion of cell survival by protecting against apoptotic death. These essential biological responses elicited by LPA are seemingly hijacked by cancer cells in many ways; transcriptional upregulation of ATX leading to increased LPA levels, enhanced expression of multiple LPA GPCR subtypes, and the downregulation of its metabolic breakdown. Recent studies have shown that overexpression of ATX and LPA GPCR can lead to malignant transformation, enhanced proliferation of cancer stem cells, increased invasion and metastasis, reprogramming of the tumor microenvironment and the metastatic niche, and development of resistance to chemo-, immuno-, and radiation-therapy of cancer. The fundamental role of LPA in cancer progression and the therapeutic inhibition of the ATX-LPA axis, although highly appealing, remains unexploited as drug development to these targets has not reached into the clinic yet. The purpose of this brief review is to highlight some unique signaling mechanisms engaged by the ATX-LPA axis and emphasize the therapeutic potential that lies in blocking the molecular targets of the LPA system.
Keywords: LPA, ENPP2, Invasion, Metastasis, Cancer stem cell, Therapy resistance
1. Introduction
The growth factor-like lipid mediator lysophosphatidic acid (LPA) activates many essential cellular responses that range from the regulation of cell proliferation, metabolism, motility, differentiation, and excitability to cell death (Benesch et al., 2018; Chun et al., 2018; Lee et al., 2018; Peyruchaud, 2009; Tigyi, 2010). In biological fluids the primary source of LPA is generated by the secreted lysophospholipase D designated autotaxin (ATX). Type I diacylglycerol kinases (DGKs), type II DGKs, and the type III DGK, which have substantial 2-monoacylglycerol kinase activity, can generate the sn-2 regioisomer of LPA contributing to intracellular pools of LPA (Sakane et al., 2018; Sato et al., 2016). LPA has multiple cellular targets but at the cell surface, the six G protein-coupled LPA receptors (LPAR) determine most of its cellular actions (Chun et al., 2018). LPA is inactivated by lipid phosphate phosphatase enzymes (LPP), which maintain low nanomolar levels of LPA in normal biological fluids (Leblanc et al., 2018; Leblanc and Peyruchaud, 2015). Research conducted on the ATX-LPAR-LPP axis has garnered a lot of attention in the field of oncology research because the molecular players of the ATX-LPAR axis have important effects on the growth, progression, metastasis and therapy resistance of cancers. The present review will cover three aspects of the ATX-LPA axis in malignancies by highlighting some of the reports we deemed are fundamental findings in the field. Thus, our objective is not to provide a comprehensive description of the many facets of LPA biology but instead to focus on the pivotal discoveries limited to oncology that have shaped our still rudimentary understanding of the ATX-LPAR axis in cancer.
2. The role of individual LPA receptors and ATX in tumor cells
After the original description of the mitogenic actions of LPA in non-transformed fibroblasts (van Corven et al., 1989, 1992), reports using cultured tumor cells have demonstrated that LPA can regulate the proliferation of transformed cells both as a mitogen (Xu et al., 1995a) or as an inhibitor of cell growth (i.e. anti-mitogen) (Tigyi et al., 1994). Whereas the mitogenic effect of LPA has been linked to pertussis toxin-sensitive Gi heterotrimeric G protein-mediated activation of the Ras – ERK1/2 signaling pathway (van Corven et al., 1993), inhibition of the growth of Sp2Age–O14 myeloma cells was accompanied by an elevation of cAMP level (Tigyi et al., 1994) in the cell, indicating a different mechanism of action and hinting that there might be multiple LPA receptors present in the different cell types that couple to distinct signaling pathways. Only after the molecular cloning and identification of six LPA GPCR (Kihara et al., 2014) has it become clear that LPA activation of LPA1, LPA2, LPA3, and LPA4 couple to the Gi—Ras-MAPK axis to promote cell proliferation, whereas LPA5, predominantly expressed in Sp2Age–O14 myeloma cells, via G activates a Ca2+-dependent adenylate cyclase that in turn inhibits cells growth. LPA GPCR identified and validated to date fall into two gene families. LPA1, LPA2 and LPA3 belong to the Endothelial Differentiation Gene (EDG) family, whereas LPA4, LPA5, and LPA6 represent a subcluster of the Purinergic GPCR family. The expression profile, signal transduction coupling and pharmacological ligands of these receptors can be accessed online in the IUPHAR/BPS Guide to Pharmacology (Chun et al., 2018). It is also important to note that LPA has additional molecular targets that include the nuclear hormone receptor PPARγ (Crowder et al., 2017; Tsukahara et al., 2006, 2010).
The LPA1, LPA2, and LPA3 receptors when overexpressed using the mammary tumor virus promoter in transgenic mice cause malignant transformation and lead to the development of metastatic breast carcinomas (Liu et al., 2009). Likewise, overexpression of these individual LPA receptors in SKOV-3 human ovarian cancer cells drove malignant transformation and promoted tumor growth when injected into nude mice (Yu et al., 2008). In addition to the EDG family of LPAR, LPA4 has also been shown to cause malignant transformation when overexpressed in mouse embryonic fibroblasts (MEF) overexpressing c-Myc and Tbx2 that are immortal but not transformed (Taghavi et al., 2008).
LPA1 has been shown to regulate tumor cell motility and invasion (Li et al., 2009) in several different neoplastic cell types (Shida et al., 2004). This property of LPA1 is likely related to its highly efficient coupling to the G12/13-Rho-Rac-ROCK pathway (Chen et al., 2007; Ishii et al., 2000; Shida et al., 2004; Takuwa et al., 2002). It is well documented that LPA1 mediates breast cancer metastasis particularly to the bone (Boucharaba et al., 2006, 2009; David et al., 2012, 2014b; Leblanc et al., 2014; Peyruchaud et al., 2003). However, this action of LPA is also influenced by stromal LPA1 receptors in osteoclasts (David et al., 2014b; Leblanc et al., 2014; Peyruchaud et al., 2013). Tumor cell invasion and the production of matrix metalloproteinases are also affected by LPA1 (Park et al., 2010; Shida et al., 2008).
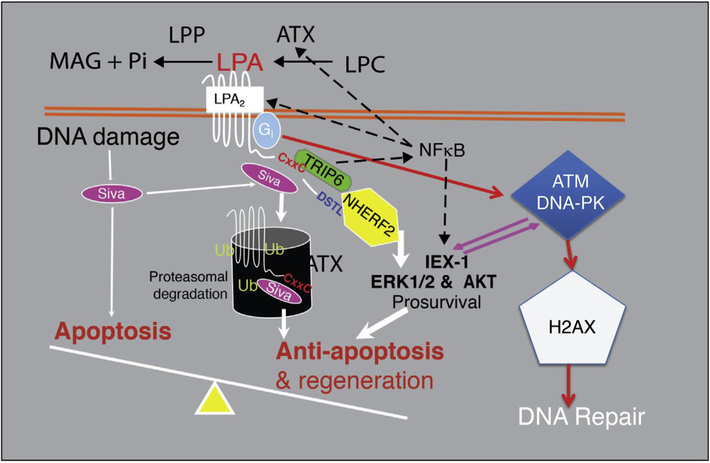
LPA2 has been linked to augmenting cell survival and rescuing apoptotically condemned cells from various noxious stimuli including radiation exposure (Deng et al., 2002, 2003, 2004, 2007, 2015). LPA2 differs from other EDG LPAR in its C terminus where it contains the LIM domain and a C-terminal PDZ protein binding motif that promotes ligand-dependent interaction with several signaling proteins (Fig. 1). The LIM domain, which is a half Zn-finger domain mediates its binding to the proapoptotic protein Siva-1 (Lin et al., 2007). Siva-1 is activated by DNA damage and binds to Bcl-xL, which destabilizes the mitochondrial outer membrane triggering the activation of the mitochondrial apoptosis pathway. LPA2 inhibits the proapoptotic activity of Siva-1 by binding to it upon ligand activation and the macromolecular complex becomes polyubiquitinated and targeted for proteosomal degradation. Another LIM domain interacting protein is the thyroid receptor interacting protein-6 (TRIP6). TRIP6 is also a PDZ-binding protein and makes a ternary complex with the Na–H exchange response factor-2 (NHERF2) and the C-terminal PDZ motif of LPA2 (E et al.,2009). Assembly of this ternary complex augments LPA2-mediated activation of the pro-survival Akt and ERK1/2 kinase pathways (E et al., 2009). The TRIP6-NHERF2 and Siva-1 macromolecular complexes play an important non-G protein-dependent signal coupled to LPA2 (Brindley et al., 2013). TRIP6 interaction with LPA2 is not only important for inhibition of FAS-induced apoptosis but also for the promotion of cell migration (Lai et al., 2010). Although not yet shown in tumor cells, such macromolecular complex-mediated signaling is important for the spatial recruitment and coupling to LPA2-interacting proteins that anchor the complex to the cytoskeleton. The interactome formed by LPA2-NHERF2-phospholipase Cβ governs gradient sensing and directional migration in response to LPA in fibroblasts (Ren et al., 2014). In addition, LPA2 has been reported to promote cell migration via activation of c-src kinase and downstream tyrosine phosphorylation of villin in Caco2 colon carcinoma cells. In these cells, LPA stimulated cell motility was insensitive to pertussis toxin, but was regulated by activation of PLC-gamma 1 (Khurana et al., 2008). LPA2 is also an integral player of the DNA damage response (DDR) pathway and is rapidly induced by γ-irradiation and the activation of NFkB (Balogh et al., 2015;Kuo et al., 2018). We have shown that the transcription factor NFkB mediates radiation-induced upregulation of LPA2, which in turn augment the resolution of DNA double strand breaks indicated by the accelerated resolution of γH2AX histone staining in wild type but not in LPA2 knockout (KO) mice. In the context of physical interaction of LPA2, it is noteworthy that a C-terminal-extended gain of function mutant has been described for this receptor in ovarian carcinoma cells (Huang et al., 2002). LPA2 has been found to accelerate tumorigenesis in the colon in an azoxymethane and dextran sulfate sodium induced experimental model of colitis-associated cancer of mice (Lin et al., 2009) and also in mice carrying the ApcMin mutation (Lin et al., 2010). Additionally, LPA2 KO mice showed reduced mucosal damage and fewer tumors than wild-type (WT) mice. Interestingly, in HCT116 and SW480 colon carcinoma cells, NHERF-2 promoted migration and invasion, whereas MAGI-3 inhibited these processes (Lee et al., 2011). MAGI-3 decreased the tumorigenic capacity of LPA2 by reducing the activities of c-Jun N-terminal kinase and NFkB.
Fig. 1.
Anti-apoptotic signaling pathways activated by LPA2.
LPA3 was first reported to be a receptor that prefers sn-2 fatty acyl substituted LPA species and is highly sensitive to the presence of albumin in the LPA delivery vehicle (Bandoh et al., 1999; Hama et al., 2002; Heise et al., 2001). To what extent LPA3 activation is affected by carrier proteins in vivo remains to be evaluated but LPA3 undoubtedly functions in different tissue environments (Hama et al., 2007; Ye et al., 2011). In the context of neoplasia, siRNA to LPA3 reduced proliferation, plasma membrane integrity and altered the morphology of A375 melanoma cells (Altman et al., 2010). Along with LPA2, the LPA3 GPCR has been shown to contribute to ovarian cancer aggressiveness (Yu et al., 2008). In particular, knockdown of the LPA2 or LPA3 receptors inhibited the production of IL-6, IL-8, and VEGF in SKOV-3 and OVCAR-3 cells (Yu et al., 2008). Although the crystal structure of LPA3 has not yet been solved, homology models of this receptor have been reported and characterized (Fujiwara et al., 2005). For example, the src homology 3 ligand binding motif, R/K-X-X-V/P-X-X-P or (216)-KTNVLSP-(222), within the third intracellular loop of LPA3 has been shown to be required for its prosurvival action (Jia et al., 2015). LPA3 has been implicated in promoting pancreatic cancer progression, invasiveness and migration (Kato et al., 2012). LPA3 has also been shown to regulate dendritic cell migration suggesting that the receptor may have a role in antitumor immunity (Chan et al., 2007).
LPA4 (P2Y9/GPR23) (Yanagida et al., 2009) has been linked to the regulation of cellular motility in nonmalignant (Lee et al., 2008) as well as malignantly transformed cells (Takahashi et al., 2017). Interestingly, LPA4 when expressed in the SQ-20B cell line derived from head and neck squamous cell carcinoma reduced cell proliferation, motility, invasiveness (Matayoshi et al., 2013). The most profound phenotypic abnormality of LPA4 KO mice in the C57/Bl6 background is the impaired development of lymphatic vessels (Sumida et al., 2010). However, this phenotype is not observed in LPA4 KO mice maintained on mixed 129/Sv and C57BL/6 background (Lee et al., 2008).
LPA5 (GPR92) (Kotarsky et al., 2006; Pasternack et al., 2008) similarly to several other LPAR has been found to augment the progression of liver carcinoma cells that is associated with its aberrant DNA methylation (Okabe et al., 2011; Tsujino et al., 2010). LPA5 acts as a chemorepellent in B16 mouse melanoma cells via receptor-mediated elevation of cAMP level (Jongsma et al., 2011). A unique action of LPA5 is that it inhibits immune response by attenuating the activation of the T cell (Oda et al., 2013) and the B cell receptor (Hu et al., 2014). LPA5-mediated inhibition of T cell receptor function attenuate tumor immunity and immune surveillance and might play a critical role in tumor progression in vivo (Oda et al., 2013).
The LPA6 (P2Y5) receptor (Yanagida et al., 2009) is essential for human hair growth. Mutation of the lpar6 gene causes woolly hair disorder (Kurban et al., 2013) and autosomal recessive hypotrichosis simplex (Nahum et al., 2011). In addition, LPA6 has a role in telencephalon development (Geach et al., 2014). This receptor prefers 2-acyl-LPA rather than 1-acyl-LPA and couples primarily to the G12/13-Rho signaling pathway, making the structure-activity studies with this receptor more complicated (Inoue et al., 2012). In terms of cancer, LPA6 exerts an inhibitory effect on pancreatic cancer invasiveness (Ishii et al., 2015), is uniquely upregulated in glioblastoma multiforme (Stangeland et al., 2015), and has been implicated in liver cancer, squamous cell carcinoma and metastatic prostate cancer (Younis and Rashid, 2017).
In biological fluids, LPA is generated by ATX, a lysophospholipase D that cleaves the choline/serine headgroup from lysophospholipids to generate LPA. ATX is a member of the ecto-nucleotide pyrophosphatase/phosphodiesterase (ENPP) enzyme family and is also known as ENPP2. ATX was originally identified as a secreted phosphatase in melanoma culture supernatant that promoted cancer cell motility (Stracke et al., 1992) but was later proven to function as a lysophospholipase D responsible for the production of LPA in blood (Tokumura et al., 2002; Umezu-Goto et al., 2002). ATX KO mice die in utero due to angiogenesis and neural tube defects (Tanaka et al., 2006; Fotopoulou et al., 2010 #154; van Meeteren et al., 2006).
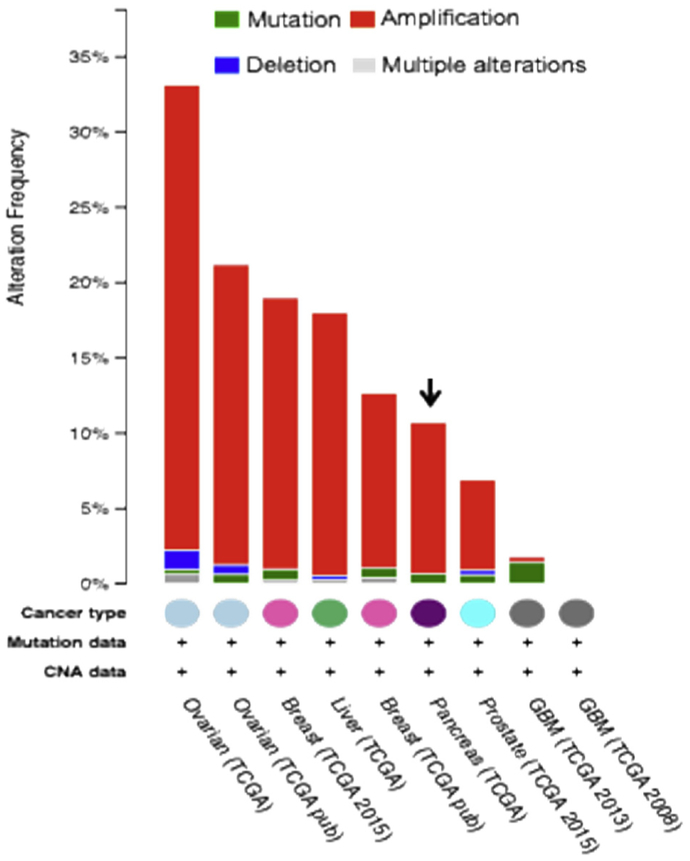
Many cancers secrete ATX, which contributes to their invasive properties. Ovarian cancer cells in particular produce high levels of LPA (Baker et al., 2002; Sutphen et al., 2004; Xu et al., 1995b). ATX is not only secreted by tumor cells but also from cells of the tumor microenvironment (TME) (Gotoh et al., 2012; Lee et al., 2015), where it prepares the metastatic niche, promotes invasion, and inhibits antitumor immunity (Hu et al., 2014; Oda et al., 2013). Gene copy numbers are elevated in ovarian cancers in chromosomal region 8q24 (Fig. 2), which contains the gene encoding ATX (Dimova et al., 2006). Ectopic expression of ATX in mice has been shown to lead to mammary intraepithelial neoplasia, which subsequently develop into invasive and metastatic tumors (Liu et al., 2009).
Fig. 2.
A cross-cancer query of enpp2, the gene encoding ATX using the cBio Cancer Genomics Portal. ATX (encoded by enpp2 gene) is amplified or upregulated predominantly in ovarian cancer. Datasets obtained from The Cancer Genome Atlas.
ATX becomes overexpressed in patients with recurrent disease after treatment cycles with chemotherapy (Jazaeri et al., 2005). It is also the second most upregulated gene in therapy-resistant cancer stem cells (CSC) (Gupta et al., 2009) and is one of the 40 most upregulated genes in aggressively metastatic carcinomas (Euer et al., 2002). Moreover, a genome-wide siRNA screen identified ATX as a candidate drug-resistance gene in ovarian cancer (Vidot et al., 2010). Indeed, studies have shown that the upregulated ATX-LPA2 axis makes ovarian cancer cells resistant to Cisplatin and Adriamycin (E et al., 2009), and breast carcinomas resistant to Paclitaxel-induced apoptosis (Samadi et al., 2009). In addition, treatment of ovarian cancer cells with an inhibitor of ATX reversed the resistance to paclitaxel (Vidot et al., 2010).
ATX is upregulated as part of DNA damage repair and diminishes the efficacy of apoptosis-inducing therapies (Balogh et al., 2015). Multiple types of integrins have been shown to mediate the responses of LPA (Valenick and Schwarzbauer, 2006), and β3 integrin has been reported to interact directly with ATX (Hausmann et al., 2011; Pamuklar et al., 2009). It has also been demonstrated that α6β4 integrin signaling activates transcription factors of the NFAT family, which in turn regulate ATX expression (Chen and O’Connor, 2005). Thus, feed-forward LPA-induced upregulation of NFAT activity can, at least theoretically, upregulate the expression of ATX and LPA production. ATX is also upregulated by TNFα-mediated activation of NFkB (Balogh et al., 2015). NFkB-dependent transcriptional regulation of ATX is particularly important in the context of the inflammatory milieu of the TME where several cytokines such as interleukin 6, interleukin 8, vascular endothelial growth factor, and urokinase plasminogen activator (Fang et al., 2004; Hu et al., 2001; Pustilnik et al., 1999; Schwartz et al., 2001) can potentially drive overexpression of ATX and the ensuing LPA production. Taken together, ATX provides all LPAR with their cognate ligand and is a target of gene duplication as well as transcriptional upregulation by a host of factors in the TME.
3. The role of the ATX-LPA receptor axis in cancer stem cells
The genetic instability of tumor cells leads to heterogeneity in a cancer cell population, be it of the primary or metastatic tumors. However, there is yet another type of heterogeneity that distinguishes cancer stem cells (CSC) from their daughter cells due to the unique properties of CSC in self-renewal, invasion, metastasis, and engraftment in distant tissues. Thus, even though cancer may start from a single cell that undergoes malignant transformation, the tumors that develop cannot be viewed as a single lineage. This property of malignancies represents significant challenges to cancer therapy (Prasetyanti and Medema, 2017). Along with recent advances in the successful eradication of primary tumors, more attention is being given to therapies that target CSC and cancer metastasis. An important mechanism in the development of metastatic lesions is the interconnected relationship between the invading CSC and the pre-metastatic niche. Recent studies suggest an important role for the ATX-LPAR signaling axis in CSC growth, invasion, metastasis and the reprogramming of the TME. There are many challenges in therapeutically targeting CSC, due to their rarity, dormancy, and resistance to therapy attributed in part by intrinsic properties of the CSC and the protective environment of the TME. There is increasing evidence that the ATX-LPA axis is involved in all of these mechanisms.
LPA has been recognized for its ability to promote epithelial-mesenchymal transition (EMT), which is a key step in the development of the invasive and metastatic ability of CSC (Burkhalter et al., 2015; Ha et al., 2016; Harma et al., 2012; Hashimoto et al., 2016; Jahn et al., 2012; Jiang et al., 2011; Ray et al., 2017; Xu et al., 2017). ATX upregulation has been recognized as an event accompanying EMT (Castellana et al., 2012; Dai et al., 2015; Ptaszynska et al., 2010). In particular, ATX-LPA has been reported to activate signaling pathways driving EMT such as the PI3K/AKT/mTOR/Skp2/p27 (Xu et al., 2017), p53 (Jiang et al., 2011), NFAT-1 (Dai et al., 2015), Transcriptional co-activator with PDZ-binding motif (TAZ) (Jeong et al., 2013), Kruppel-like factor 4 (KLF4) (Shin et al., 2014), and NFkB (Tobar et al., 2010).
In epithelial ovarian cancer cells, the ATX-LPA1 receptor axis has recently been recognized as a mechanism responsible for driving the expression of CSC-associated genes such as OCT4, SOX2, and aldehyde dehydrogenase 1 (Seo et al., 2016). Silencing LPA1, ATX or AKT1 prevented the upregulation of these markers in ovarian CSC (Seo et al., 2016). We showed that ATX inhibitors dose-dependently reduce the viability of 4T1 murine breast carcinoma-derived mammospheres (Banerjee et al., 2017). We have tested the effect of ATX inhibitors BMP-22 (Gupte et al., 2011) and BESA-3 (Banerjee et al., 2017) and the LPA2 antagonist compound 35 reported by Beck et al. from Amgen Inc., on the growth and viability of CSC mammosphere cultures established from 4T1 cells (Fig. 3). Both ATX inhibitors significantly reduced the growth and viability of 4T1 mammospheres. The LPA2 antagonist was most effective in reducing the growth and viability of the cultured mammospheres suggesting that this receptor subtype may have a profound role in the growth of cultured 4T1 CSC. Taken together, the ATX-LPA axis emerges as a major regulatory system of CSC growth and progression (Lee et al., 2018).
Fig. 3.
LPA2 inhibition disrupts spheroid formation in 4T1 mouse mammary carcinoma cells. 4T1 cells were plated in triplicate in ultra-low adherence 24-well plates at a density of 7.5 × 103 cells per well in spheroid formation medium (RPMI with 200 μM L-glutamine, 100 U penicillin, 100 μg/ml streptomycin, 1× N-2 supplement, 20 ng/ml mouse EGF and 20 ng/ml mouse FGF). Cells were incubated at 37 °C with 5% CO2 for 4 days to allow spheroid formation, then treated with a range of 0–3 μM Amgen 35 (LPA2 antagonist), BMP-22 (ATX inhibitor) or BESA-3 (ATX/LPA1 inhibitor) and incubated an additional 3 days. Cells were surveyed under 100× magnification (microscopy panels), and spheroids were manually counted, plotted versus Amgen 35 concentration and GraphPad Prism v. 5.0a was used to perform non-linear regression in a variable slope model to determine the IC50 of Amgen 35 for spheroid formation (dose-response panel). Finally, cell viability was determined using Promega CellTiter Blue reagent as per manufacturer’s instructions. Briefly, 50 μl CellTiter Blue were added to each well, and plates were incubated at 37 °C for 3 h prior to measuring fluorescence at excitation/emission wavelengths of 560/590 nm, respectively. Relative fluorescence was then background-corrected and normalized to percentage vehicle signal for each compound tested (bar graph panel). GraphPad Prism was used to perform one-factor ANOVA with Bonferroni’s post-test to determine whether cell viability differed significantly between vehicle and drug treatment (** = p < 0.01, *** = p < 0.001; n = 3).
4. The role of ATX and LPA in the tumor microenvironment
Apart from cancer cells, the TME is an equally important source of ATX and LPA. Compelling evidence for the role of stromal ATX and LPA1 in the bone metastasis of breast carcinoma has been provided by the Peyruchaud group (Boucharaba et al., 2004, 2006;David et al., 2014a, 2014b; Leblanc et al., 2014, 2018). Using MDA-B02, an ATX non-expressing variant of the breast carcinoma cell line MDA-MB-231, they have shown that treatment of mice either with the LPA1/3 inhibitor Ki16425 or the ATX inhibitor BMP-22 greatly reduced the number of bone metastases. The number of micrometastatic colonies from the bone marrow of BMP-22 treated mice showed a 93% reduction and the growth of already established osteolytic metastases were attenuated by 43% when compared to controls (Leblanc et al., 2014). Gotoh et al. demonstrated that the ATX inhibitor BMP-22 reduced the number of lung metastasis of B16eF10 melanoma (Gotoh et al., 2012). Lee et al. using the LPA1, LPA2 and LPA5 KO mice on the C57/BL6 genetic background syngeneic with the B16–F10 melanoma found that the number of metastatic nodules was reduced by approximately 50% in the LPA1 KO mice and almost completely absent in LPA5 KO host mice. They also found a similar decrease in the number of fluorescently-labeled B16–F10 cells attaching to the lungs of LPA1 and LPA5 KO mice, respectively 24 h after inoculation via the tail vein (Lee et al., 2015). Treatment of the mice with either the LPA1/3 antagonist Ki16425 or the ATX inhibitor BMP-22 alone partially reduced the number of lung metastases by B16eF10 whereas, combined treatment caused near 90% reduction in lung metastases. Because B16eF10 cells do not express LPA1 and LPA3 transcripts, this observation further emphasizes the importance of stromal LPAR in the seeding of metastasis (Lee et al., 2015). With regard to stromal ATX, adipocytes are a rich source (van Meeteren et al., 2006). The Brindley group has elegantly demonstrated that adipocyte-rich stroma of the mammary gland provide an abundant source of LPA for the growth and progression of breast carcinomas (Benesch et al., 2015a, 2015b; Gaetano et al., 2009; Samadi et al., 2011; Tang et al., 2015; Venkatraman et al., 2015). In addition, it is also important to recognize that the ATX-LPA axis is responsible for the production of a host of proinflammatory mediators from stromal elements in the TME that have a profound role in the progression and metastatic spread of carcinomas (Benesch et al., 2018).
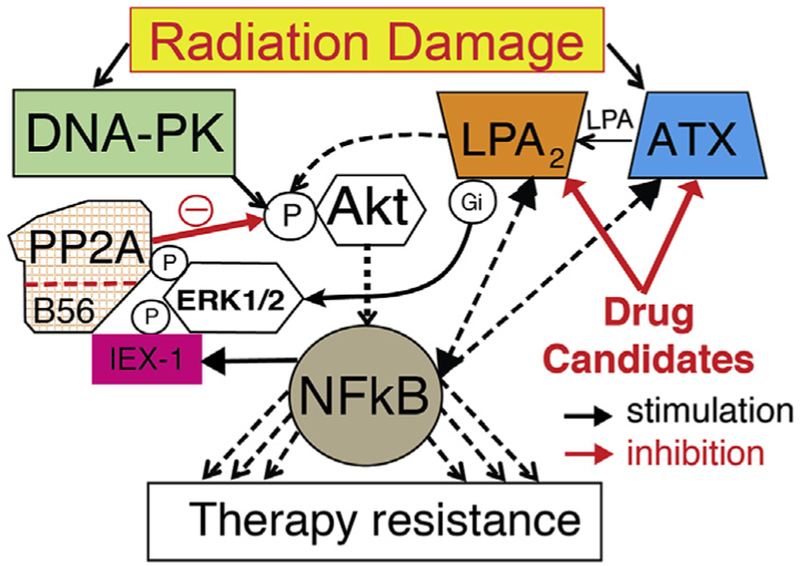
The ability of cancer to develop resistance to therapy is a major health problem which was identified recently by the NCI Cancer Moonshot initiative as a priority research topic for the scientific community. A critical barrier to progress in dealing with the problem of cancer resistance to therapy is the lack of drugs that reverse resistance in the tumor cell itself and also block the supportive actions of the TME. Recent research began addressing this need by identifying the ATX-LPA axis as a major system that regulates therapy resistance in various cancers. As we have described in an earlier section of this paper, ATX is the second most upregulated gene in therapy-resistant CSC, whereas the LPA inactivating enzyme lipid phosphate phosphatase (LPP) is the most downregulated gene (Gupta et al., 2009). As mentioned above, ATX becomes overexpressed in patients undergoing treatment with repeated cycles of chemotherapy (Jazaeri et al., 2005) and in a genome-wide siRNA screen ATX has been identified as candidate drug-resistance gene in ovarian cancer (Lee et al., 2018; Vidot et al., 2010). LPA has been reported to activate Arf6 and enhance invasion and drug resistance of renal carcinoma cells (Hashimoto et al., 2016; Yamauchi et al., 2017). ATX and LPA2 are upregulated as part of DNA damage repair and diminish the efficacy of apoptosis-inducing therapies. These observations lead to a novel hypothesis which predicts that when DNA damage repair goes awry it will transcriptionally and functionally upregulate the ATX-LPA2 signaling axis leading to therapy resistance (Fig. 4).
Fig. 4.
When DNA damage repair goes awry it can lead to therapy resistance. A new hypothesis.
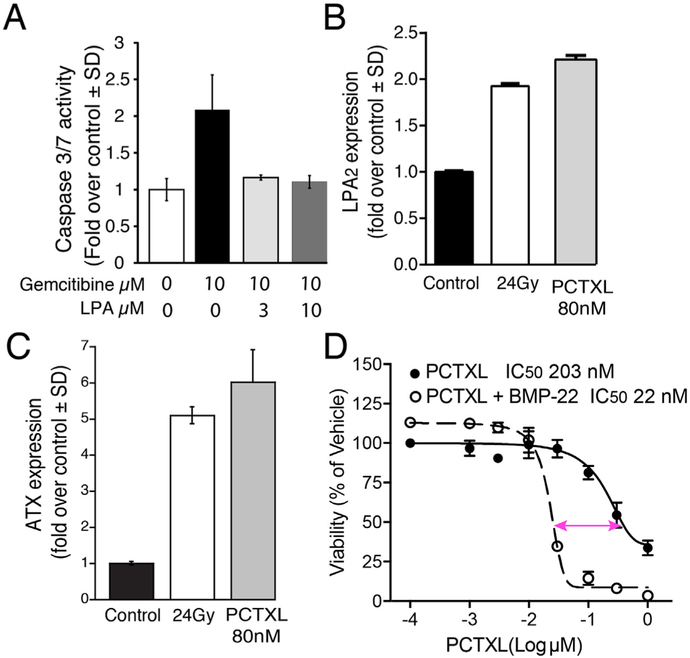
LPA2 has been demonstrated to protects cells from apoptosis elicited by exposure to ionizing radiation and chemotherapeutics (Fig. 5) (Deng et al., 2002; Deng et al., 2004; Deng et al., 2003; E et al., 2009; Kuo et al., 2018). We demonstrated that the ATX and LPA2 genes are induced by exposure to ionizing radiation (Balogh et al., 2015; Kuo et al., 2018). Specifically, the upregulation of LPA2 is downstream of the DDR as CGK-733, an ATM/ATR kinase inhibitor, blocked radiation-induced upregulation of lpa2 transcripts in IEC-6 cells (Balogh et al., 2015). We found that stimulation of the LPA2 receptor causes a sustained activation of the ERK1/2 and Akt kinases lasting more that 12 h (unpublished). These two kinases play key roles in the regulation of cell survival by inhibiting apoptosis. The central role of the Akt kinase–NFkB transcription factor is well documented in mediating resistance to radiation and chemotherapy (Oeck et al., 2017; Szymonowicz et al., 2018). We hypothesize that the upregulated ATX-LPA2 receptor axis via the IEX-1 scaffold protein (de Laval et al., 2014) and ERK1/2 are important amplifiers of the activity of Akt-NFkB.
Fig. 5.
ATX and LPA2 play key roles in resistance against radiation-induced programmed cell death. (A) LPA protects HPAF II human pancreatic cancer cells against gemcitabine-induced apoptosis. HPAF II cells were cultured in growth media (DMEM F12 supplemented with heat inactivated 10% FBS, 200 μM L-glutamine, 100 U penicillin and 100 μg/ml streptomycin). Next day, cells were serum starved for 24 h followed by exposure to 10 μM Gemcitabine with or without LPA (at 3 μM or 10 μM). Caspase 3/7 activity was measured after 24 h of treatment using the CaspaseGlo® Kit (Promega). (B) LPA2 and (C) ATX expression are upregulated in 4T1 CSC upon exposure to Paclitaxel or γ-irradiation. 4T1 CSC were generated as described in Fig. 3 and spheroids were allowed to form for 3 days. At day 4, CSCs were either treated with 80 nM of Paclitaxel (PCTXL) or fractionated doses of γ-irradiation (6Gy/day for 4 days). CSCs were harvested for QPCR analysis after 4 days of PCTXL treatment or 24 h after the last dose of fractionated radiation. GraphPad Prism was used to perform one-factor ANOVA with Bonferroni’s post-test to determine the significance in LPA2 or ATX expression in vehicle versus PCTXL or radiation treatment, respectively (*** = p < 0.001; n = 3). (D) The ATX inhibitor BMP-22 enhances 4T1 cell killing by PCTXL. 4T1 cells were plated in quadruplicate in 96-well plates at a density of 5 × 103 cells per well in RPMI with 1% (v/v) charcoal-stripped FBS and incubated overnight at 37 °C with 5% CO2. Cells were then treated for 24 h with 3 μM BMP-22 followed by 48 h treatment with a dose range of 0–1 μM PT in RPMI with 1% (v/v) charcoal-stripped FBS. After 48 h incubation, cells were allowed to recover in RPMI with 1% (v/v) charcoal-stripped FBS for an additional 72 h. Viability was then assessed via Promega CellTiter Blue (as per manufacturer’s instructions) and normalized to % vehicle control samples. Data were plotted using GraphPad Prism v. 5.0a, and non-linear regression analysis was performed in a variable slope model in order to determine LD50 concentrations for PCTXL in the presence and absence of BMP-22.
In particular, the uniquely long-lasting activation of LPA2-mediated antiapoptotic signal is mediated by an interactome, which inhibits protein phosphatase 2a (PP2A) – the key terminator of Akt and ERK1/2 phosphorylation (Garcia et al., 2002; Rocher et al., 2007). A key scaffold protein that brings together this interactome is the immediate early gene IEX-1. IEX-1 expression is driven by ATM kinase, a critical enzyme in the DDR pathway (Pawlikowska et al., 2010). We suggest that LPA2 activation of ERK1/2 via heterotrimeric Gi protein and MEK kinase, leads to the downstream phosphorylation of IEX-1 by ERK1/2, which stabilizes the complex and promotes IEX-1 association with the B56 subunit of PP2A (de Laval et al., 2014). This association allows for ERK1/2 to phosphorylate the B56 subunit of PP2A that triggers its dissociation from the PP2A holoenzyme (Letourneux et al., 2006). PP2A is constitutively active in cells and functions to inhibit ERK1/2 and AKT. We hypothesize that the reduced PP2A activity, as a result of ERK1/2-IEX-1-mediated dissociation of B56 subunit, will give rise to a prolonged activation state of ERK1/2 and Akt, which enhance cell survival and counteract therapeutically-induced apoptosis in neoplastic cells. We are currently testing this hypothesis (Fig. 5). Initial data reinforce the protective action of LPA against frontline chemotherapeutics like Gemcitibine (Fig. 5A) and that exposure of 4T1 breast CSC to either 24 Gy fractionated γ-irradiation or 80 nM Paclitaxel treatment causes transcriptional upregulation of LPA2 and ATX (Fig. 5 B & C). We are hopeful that drugs that inhibit LPA2 and ATX can be used as adjuvants to restore the sensitivity of CSC to radiation- and chemotherapy as we have reported for a group of ATX inhibitors (Banerjee et al., 2017). We further demonstrate in Fig. 5D that BMP-22 reduced the IC50 of Paclitaxel in 4T1 cancer cells by an order of magnitude.
As more proof of principle studies will demonstrate the utility of drug candidates that block the ATX-LPAR targets in different malignancies, it is conceivable that companies with compounds already at hand will launch clinical trials for the experimental treatment of cancer patients.
Acknowledgements
This research was supported by grants from the National Cancer Institute CA 092160 (GT), the National Institute of Allergy and Infectious Diseases Radiation and Nuclear Countermeasure Program U01AI080405 (GT), the Biomedical Laboratory Research and Development Service of the VA Office of Research and Development I01BX007080 (GT), the Harriet Van Vleet Endowment in Basic Oncology Research of the University of Tennessee (G.T.) and Hungarian Scientific Research Fund K-112964 & K-125174 (AB & GT).
Abbreviations:
- ATX
Autotaxin
- BESA-3
utotaxin inhibitor benzene sulfonamide compound 3
- BMP-22
Autotaxin inhibitor benzyl methyl phosphonic acid compound 22
- CSC
Cancer stem-like cell
- EDG
Endothelial Differentiation Gene
- ENPP
ecto-nucleotide pyrophosphatase/phosphodiesterase
- LPA
lysophosphatidic acid
- LPAR
LPA G protein-coupled receptor
- NHERF-2
NaH exchange response factor-2
- TRIP6
thyroid receptor interacting protein-6
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbior.2018.09.008.
References
- Altman MK, Gopal V, Jia W, Yu S, Hall H, Mills GB, McGinnis AC, Bartlett MG, Jiang G, Madan D, Prestwich GD, Xu Y, Davies MA, Murph MM, 2010. Targeting melanoma growth and viability reveals dualistic functionality of the phosphonothionate analogue of carba cyclic phosphatidic acid. Mol. Canc 9, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DL, Morrison P, Miller B, Riely CA, Tolley B, Westermann AM, Bonfrer JM, Bais E, Moolenaar WH, Tigyi G, 2002. Plasma lysophosphatidic acid concentration and ovarian cancer. Jama 287 (23), 3081–3082. [DOI] [PubMed] [Google Scholar]
- Balogh A, Shimizu Y, Lee SC, Norman DD, Gangwar R, Bavaria M, Moon C, Shukla P, Rao R, Ray R, Naren AP, Banerje S, Miller DD, Balazs L, Pelus L, Tigyi G, 2015. The autotaxin-LPA2 GPCR axis is modulated by gamma-irradiation and facilitates DNA damage repair. Cell. Signal 27 (9), 1751–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K, Tsujimoto M, Arai H, Inoue K, 1999. Molecular cloning and characterization of a novel human G-protein- coupled receptor, EDG7, for lysophosphatidic acid. J. Biol. Chem 274 (39), 27776–27785. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Norman DD, Lee SC, Parrill AL, Pham TC, Baker DL, Tigyi GJ, Miller DD, 2017. Highly potent non-carboxylic acid autotaxin inhibitors reduce melanoma metastasis and chemotherapeutic resistance of breast cancer stem cells. J. Med. Chem 60 (4), 1309–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch MG, Ko YM, Tang X, Dewald J, Lopez-Campistrous A, Zhao YY, Lai R, Curtis JM, Brindley DN, McMullen TP, 2015a. Autotaxin is an inflammatory mediator and therapeutic target in thyroid cancer. Endocr. Relat. Canc 22 (4), 593–607. [DOI] [PubMed] [Google Scholar]
- Benesch MG, Tang X, Dewald J, Dong WF, Mackey JR, Hemmings DG, McMullen TP, Brindley DN, 2015b. Tumor-induced inflammation in mammary adipose tissue stimulates a vicious cycle of autotaxin expression and breast cancer progression. Faseb. J 29 (9), 3990–4000. [DOI] [PubMed] [Google Scholar]
- Benesch MGK, MacIntyre ITK, McMullen TPW, Brindley DN, 2018. Coming of age for autotaxin and lysophosphatidate signaling: clinical applications for preventing, detecting and targeting tumor-promoting inflammation. Cancers 10 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucharaba A, Guillet B, Menaa F, Hneino M, van Wijnen AJ, Clezardin P, Peyruchaud O, 2009. Bioactive lipids lysophosphatidic acid and sphingosine 1-phosphate mediate breast cancer cell biological functions through distinct mechanisms. Oncol. Res 18 (4), 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O, 2004. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J. Clin. Invest 114 (12), 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clezardin P, Peyruchaud O, 2006. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc. Natl. Acad. Sci. U. S. A 103 (25), 9643–9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley DN, Lin FT, Tigyi GJ, 2013. Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim. Biophys. Acta 1831 (1), 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter RJ, Westfall SD, Liu Y, Stack MS, 2015. Lysophosphatidic acid initiates epithelial to mesenchymal transition and induces beta-Catenin-mediated transcription in epithelial ovarian carcinoma. J. Biol. Chem 290 (36), 22143–22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellana B, Escuin D, Peiro G, Garcia-Valdecasas B, Vazquez T, Pons C, Perez-Olabarria M, Barnadas A, Lerma E, 2012. ASPN and GJB2 are implicated in the mechanisms of invasion of ductal breast carcinomas. J. Canc 3, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LC, Peters W, Xu Y, Chun J, Farese RV Jr., Cases S, 2007. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA). J. Leukoc. Biol 82 (5), 1193–1200. [DOI] [PubMed] [Google Scholar]
- Chen M, O’Connor KL, 2005. Integrin alpha6beta4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells. Oncogene 24 (32),5125–5130. [DOI] [PubMed] [Google Scholar]
- Chen M, Towers LN, O’Connor KL, 2007. LPA2 (EDG4) mediates Rho-dependent chemotaxis with lower efficacy than LPA1 (EDG2) in breast carcinoma cells. Am. J. Physiol. Cell Physiol 292 (5), C1927–C1933. [DOI] [PubMed] [Google Scholar]
- Chun J, Hla T, Moolenaar W, Spiegel S, Yung YC, Mpamhanga C, 2018. Lysophospholipid (LPA) Receptors, vol. 2018. [Google Scholar]
- Crowder MK, Seacrist CD, Blind RD, 2017. Phospholipid regulation of the nuclear receptor superfamily. Adv Biol Regul 63, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Wang F, He L, Lin C, Wu S, Chen P, Zhang Y, Shen M, Wu D, Wang C, Lu J, Zhou Y, Xu X, Xu L, Guo C, 2015. Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial-mesenchymal transition: partial mediation by the transcription factor NFAT1. Mol. Carcinog 54 (4), 301–311. [DOI] [PubMed] [Google Scholar]
- David M, Machuca-Gayet I, Kikuta J, Ottewell P, Mima F, Leblanc R, Bonnelye E, Ribeiro J, Holen I, Lopez Vales R, Jurdic P, Chun J, Clezardin P, Ishii M, Peyruchaud O, 2014a. Lysophosphatidic acid receptor type 1 (LPA1) plays a functional role in osteoclast differentiation and bone resorption activity. J. Biol. Chem 289 (10), 6551–6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M, Machuca-Gayet I, Kikuta J, Ottewell P, Mima F, Leblanc R, Bonnelye E, Ribeiro J, Holen I, Vales RL, Jurdic P, Chun J, Clezardin P, Ishii M, Peyruchaud O, 2014b. Lysophosphatidic acid receptor type 1 (LPA1) plays a functional role in osteoclast differentiation and bone resorption activity. J. Biol. Chem 289 (10), 6551–6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M, Ribeiro J, Descotes F, Serre CM, Barbier M, Murone M, Clezardin P, Peyruchaud O, 2012. Targeting lysophosphatidic acid receptor type 1 with Debio 0719 inhibits spontaneous metastasis dissemination of breast cancer cells independently of cell proliferation and angiogenesis. Int. J. Oncol 40 (4), 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laval B, Pawlikowska P, Barbieri D, Besnard-Guerin C, Cico A, Kumar R, Gaudry M, Baud V, Porteu F, 2014. Thrombopoietin promotes NHEJ DNA repair in hematopoietic stem cells through specific activation of Erk and NF-kappaB pathways and their target, IEX-1. Blood 123 (4), 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Balazs L, Wang DA, Van Middlesworth L, Tigyi G, Johnson LR, 2002. Lysophosphatidic acid protects and rescues intestinal epithelial cells from radiation- and chemotherapy-induced apoptosis. Gastroenterology 123 (1), 206–216. [DOI] [PubMed] [Google Scholar]
- Deng W, Kimura Y, Gududuru V, Wu W, Balogh A, Szabo E, Thompson KE, Yates CR, Balazs L, Johnson LR, Miller DD, Strobos J, McCool WS, Tigyi GJ, 2015. Mitigation of the hematopoietic and gastrointestinal acute radiation syndrome by octadecenyl thiophosphate, a small molecule mimic of lysophosphatidic acid. Radiat. Res 183 (4), 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Poppleton H, Yasuda S, Makarova N, Shinozuka Y, Wang DA, Johnson LR, Patel TB, Tigyi G, 2004. Optimal lysophosphatidic acid-induced DNA synthesis and cell migration but not survival require intact autophosphorylation sites of the epidermal growth factor receptor. J. Biol. Chem 279 (46), 47871–47880. [DOI] [PubMed] [Google Scholar]
- Deng W, Shuyu E, Tsukahara R, Valentine WJ, Durgam G, Gududuru V, Balazs L, Manickam V, Arsura M, VanMiddlesworth L, Johnson LR, Parrill AL, Miller DD, Tigyi G, 2007. The lysophosphatidic acid type 2 receptor is required for protection against radiation-induced intestinal injury. Gastroenterology 132 (5), 1834–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Wang DA, Gosmanova E, Johnson LR, Tigyi G, 2003. LPA protects intestinal epithelial cells from apoptosis by inhibiting the mitochondrial pathway.Am. J. Physiol. Gastrointest. Liver Physiol 284 (5), G821–G829. [DOI] [PubMed] [Google Scholar]
- Dimova I, Raitcheva S, Dimitrov R, Doganov N, Toncheva D, 2006. Correlations between c-myc gene copy-number and clinicopathological parameters of ovarian tumours. Eur. J. Canc 42 (5), 674–679. [DOI] [PubMed] [Google Scholar]
- E S, Lai YJ, Tsukahara R, Chen CS, Fujiwara Y, Yue J, Yu JH, Guo H, Kihara A, Tigyi G, Lin FT, 2009. Lysophosphatidic acid 2 receptor-mediated supramolecular complex formation regulates its antiapoptotic effect. J. Biol. Chem 284 (21), 14558–14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euer N, Schwirzke M, Evtimova V, Burtscher H, Jarsch M, Tarin D, Weidle UH, 2002. Identification of genes associated with metastasis of mammary carcinoma in metastatic versus non-metastatic cell lines. Anticancer Res 22 (2A), 733–740. [PubMed] [Google Scholar]
- Fang X, Yu S, Bast RC, Liu S, Xu HJ, Hu SX, LaPushin R, Claret FX, Aggarwal BB, Lu Y, Mills GB, 2004. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J. Biol. Chem 279 (10), 9653–9661. [DOI] [PubMed] [Google Scholar]
- Fotopoulou S, Oikonomou N, Grigorieva E, Nikitopoulou I, Paparountas T, Thanassopoulou A, Zhao Z, Xu Y, Kontoyiannis DL, Remboutsika E, Aidinis V,2010. ATX expression and LPA signalling are vital for the development of the nervous system. Dev. Biol 339 (2), 451–464. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Sardar V, Tokumura A, Baker D, Murakami-Murofushi K, Parrill A, Tigyi G, 2005. Identification of residues responsible for ligand recognition and regioisomeric selectivity of lysophosphatidic acid receptors expressed in mammalian cells. J. Biol. Chem 280 (41), 35038–35050. [DOI] [PubMed] [Google Scholar]
- Gaetano CG, Samadi N, Tomsig JL, Macdonald TL, Lynch KR, Brindley DN, 2009. Inhibition of autotaxin production or activity blocks lysophosphatidylcholine-induced migration of human breast cancer and melanoma cells. Mol. Carcinog 48 (9), 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Ye Y, Arranz V, Letourneux C, Pezeron G, Porteu F, 2002. IEX-1: a new ERK substrate involved in both ERK survival activity and ERK activation. EMBO J 21 (19), 5151–5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geach TJ, Faas L, Devader C, Gonzalez-Cordero A, Tabler JM, Brunsdon H, Isaacs HV, Dale L, 2014. An essential role for LPA signalling in telencephalon development. Development 141 (4), 940–949. [DOI] [PubMed] [Google Scholar]
- Gotoh M, Fujiwara Y, Yue J, Liu J, Lee S, Fells J, Uchiyama A, Murakami-Murofushi K, Kennel S, Wall J, Patil R, Gupte R, Balazs L, Miller DD, Tigyi GJ, 2012. Controlling cancer through the autotaxin-lysophosphatidic acid receptor axis. Biochem. Soc. Trans 40 (1), 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES, 2009. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138 (4), 645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte R, Patil R, Liu J, Wang Y, Lee SC, Fujiwara Y, Fells J, Bolen AL, Emmons-Thompson K, Yates CR, Siddam A, Panupinthu N, Pham TC, Baker DL, Parrill AL, Mills GB, Tigyi G, Miller DD, 2011. Benzyl and naphthalene methylphosphonic acid inhibitors of autotaxin with anti-invasive and anti-metastatic activity. ChemMedChem 6 (5), 922–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha JH, Ward JD, Radhakrishnan R, Jayaraman M, Song YS, Dhanasekaran DN, 2016. Lysophosphatidic acid stimulates epithelial to mesenchymal transition marker Slug/Snail2 in ovarian cancer cells via Galphai2, Src, and HIF1alpha signaling nexus. Oncotarget 7 (25), 37664–37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama K, Aoki J, Inoue A, Endo T, Amano T, Motoki R, Kanai M, Ye X, Chun J, Matsuki N, Suzuki H, Shibasaki M, Arai H, 2007. Embryo spacing and implantation timing are differentially regulated by LPA3-mediated lysophosphatidic acid signaling in mice. Biol. Reprod 77 (6), 954–959. [DOI] [PubMed] [Google Scholar]
- Hama K, Bandoh K, Kakehi Y, Aoki J, Arai H, 2002. Lysophosphatidic acid (LPA) receptors are activated differentially by biological fluids: possible role of LPA-binding proteins in activation of LPA receptors. FEBS (Fed. Eur. Biochem. Soc.) Lett 523, 187–192. [DOI] [PubMed] [Google Scholar]
- Harma V, Knuuttila M, Virtanen J, Mirtti T, Kohonen P, Kovanen P, Happonen A, Kaewphan S, Ahonen I, Kallioniemi O, Grafstrom R, Lotjonen J, Nees M, 2012. Lysophosphatidic acid and sphingosine-1-phosphate promote morphogenesis and block invasion of prostate cancer cells in three-dimensional organotypic models. Oncogene 31 (16), 2075–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Mikami S, Sugino H, Yoshikawa A, Hashimoto A, Onodera Y, Furukawa S, Handa H, Oikawa T, Okada Y, Oya M, Sabe H, 2016. Lysophosphatidic acid activates Arf6 to promote the mesenchymal malignancy of renal cancer. Nat. Commun 7, 10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann J, Kamtekar S, Christodoulou E, Day JE, Wu T, Fulkerson Z, Albers HM, van Meeteren LA, Houben AJ, van Zeijl L, Jansen S, Andries M, Hall T, Pegg LE, Benson TE, Kasiem M, Harlos K, Kooi CW, Smyth SS, Ovaa H, Bollen M, Morris AJ, Moolenaar WH, Perrakis A, 2011. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat. Struct. Mol. Biol 18 (2), 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise CL, Santos WL, Schreihofer AM, Heasley BM, Mukhin YV, Macdonald TL, Lynch KR, 2001. Activity of 2-substituted LPA analogs at LPA receptors: discovery of a LPA1/LPA3 receptor antagonist. Mol. Pharm XX, XX–YY. [DOI] [PubMed] [Google Scholar]
- Hu J, Oda SK, Shotts K, Donovan EE, Strauch P, Pujanauski LM, Victorino F, Al-Shami A, Fujiwara Y, Tigyi G, Oravecz T, Pelanda R, Torres RM,2014. Lysophosphatidic acid receptor 5 inhibits B cell antigen receptor signaling and antibody response. J. Immunol 193 (1), 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YL, Tee MK, Goetzl EJ, Auersperg N, Mills GB, Ferrara N, Jaffe RB, 2001. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J. Natl. Canc. Inst 93 (10), 762–768. [DOI] [PubMed] [Google Scholar]
- Huang MC, Graeler M, Shankar G, Spencer J, Goetzl EJ, 2002. Lysophospholipid mediators of immunity and neoplasia. Biochim. Biophys. Acta 1582 (1–3),161–167. [DOI] [PubMed] [Google Scholar]
- Inoue A, Ishiguro J, Kitamura H, Arima N, Okutani M, Shuto A, Higashiyama S, Ohwada T, Arai H, Makide K, Aoki J, 2012. TGFalpha shedding assay: an accurate and versatile method for detecting GPCR activation. Nat. Methods 9 (10), 1021–1029. [DOI] [PubMed] [Google Scholar]
- Ishii I, Contos JJA, Fukushima N, Chun J, 2000. Functional comparisons of the lysophosphatidic acid receptors, LPA1/VZG-1/EDG-2, LPA2/EDG-4, and LPA3/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol. Pharmacol 58 (5), 895–902. [DOI] [PubMed] [Google Scholar]
- Ishii S, Hirane M, Fukushima K, Tomimatsu A, Fukushima N, Tsujiuchi T, 2015. Diverse effects of LPA4, LPA5 and LPA6 on the activation of tumor progression in pancreatic cancer cells. Biochem. Biophys. Res. Commun 461 (1), 59–64. [DOI] [PubMed] [Google Scholar]
- Jahn SC, Law ME, Corsino PE, Parker NN, Pham K, Davis BJ, Lu J, Law BK, 2012. An in vivo model of epithelial to mesenchymal transition reveals a mitogenic switch. Canc. Lett 326 (2), 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazaeri AA, Awtrey CS, Chandramouli GV, Chuang YE, Khan J, Sotiriou C, Aprelikova O, Yee CJ, Zorn KK, Birrer MJ, Barrett JC, Boyd J, 2005. Gene expression profiles associated with response to chemotherapy in epithelial ovarian cancers. Clin. Canc. Res 11 (17), 6300–6310. [DOI] [PubMed] [Google Scholar]
- Jeong GO, Shin SH, Seo EJ, Kwon YW, Heo SC, Kim KH, Yoon MS, Suh DS, Kim JH, 2013. TAZ mediates lysophosphatidic acid-induced migration and proliferation of epithelial ovarian cancer cells. Cell. Physiol. Biochem. : international journal of experimental cellular physiology, biochemistry, and pharmacology 32 (2), 253–263. [DOI] [PubMed] [Google Scholar]
- Jia W, Tran SK, Ruddick CA, Murph MM, 2015. The Src homology 3 binding domain is required for lysophosphatidic acid 3 receptor-mediated cellular viability in melanoma cells. Canc. Lett 356 (2 Pt B), 589–596. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Xie X, Li Z, Wang Z, Zhang Y, Ling ZQ, Pan Y, Wang Z, Chen Y, 2011. Functional cooperation of RKTG with p53 in tumorigenesis and epithelialmesenchymal transition. Canc. Res 71 (8), 2959–2968. [DOI] [PubMed] [Google Scholar]
- Jongsma M, Matas-Rico E, Rzadkowski A, Jalink K, Moolenaar WH, 2011. LPA is a chemorepellent for B16 melanoma cells: action through the cAMP-elevating LPA5 receptor. PLoS One 6 (12), e29260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Yoshikawa K, Tanabe E, Kitayoshi M, Fukui R, Fukushima N, Tsujiuchi T, 2012. Opposite roles of LPA1 and LPA3 on cell motile and invasive activities of pancreatic cancer cells. Tumour Biol 33 (5), 1739–1744. [DOI] [PubMed] [Google Scholar]
- Khurana S, Tomar A, George SP, Wang Y, Siddiqui MR, Guo H, Tigyi G, Mathew S, 2008. Autotaxin and lysophosphatidic acid stimulate intestinal cell motility by redistribution of the actin modifying protein villin to the developing lamellipodia. Exp. Cell Res 314 (3), 530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara Y, Maceyka M, Spiegel S, Chun J, 2014. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol 171 (15), 3575–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B, 2006. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J. Pharmacol. Exp. Therapeut 318 (2), 619–628. [DOI] [PubMed] [Google Scholar]
- Kuo B, Szabó E, Lee SL, Balogh A, Norman DD, Inoue A, Ono Y, Aoki J, Tigyi GJ, 2018. The LPA2 receptor agonist Radioprotectin-1 spares LGR-5 positive intestinal stem cells from radiation injury in murine enteroids. Cell. Signal 51, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurban M, Wajid M, Shimomura Y, Christiano AM, 2013. Mutations in LPAR6/P2RY5 and LIPH are associated with woolly hair and/or hypotrichosis. J. Eur. Acad. Dermatol. Venereol 27 (5), 545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YJ, Lin VT, Zheng Y, Benveniste EN, Lin FT, 2010. The adaptor protein TRIP6 antagonizes Fas-induced apoptosis but promotes its effect on cell migration. Mol. Cell Biol 30 (23), 5582–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc R, Houssin A, Peyruchaud O, 2018. Platelets, autotaxin and lysophosphatidic acid signalling: win-win factors for cancer metastasis. Br. J. Pharmacol 175(15), 3100–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc R, Lee SC, David M, Bordet JC, Norman DD, Patil R, Miller D, Sahay D, Ribeiro J, Clezardin P, Tigyi GJ, Peyruchaud O, 2014. Interaction of platelet-derived autotaxin with tumor integrin alphaVbeta3 controls metastasis of breast cancer cells to bone. Blood 124 (20), 3141–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc R, Peyruchaud O, 2015. New insights into the autotaxin/LPA axis in cancer development and metastasis. Exp. Cell Res 333 (2), 183–189. [DOI] [PubMed] [Google Scholar]
- Lee D, Suh DS, Lee SC, Tigyi GJ, Kim JH, 2018. Role of autotaxin in cancer stem cells. Canc. Metastasis Rev 10.1007/s10555-018-9745-x.[Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Lee SC, Fujiwara Y, Liu J, Yue J, Shimizu Y, Norman DD, Wang Y, Tsukahara R, Szabo E, Patil R, Banerjee S, Miller DD, Balazs L, Ghosh MC, Waters CM, Oravecz T, Tigyi GJ, 2015. Autotaxin and LPA1 and LPA5 receptors exert disparate functions in tumor cells versus the host tissue micro-environment in melanoma invasion and metastasis. Mol. Canc. Res 13 (1), 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Ritter SL, Zhang H, Shim H, Hall RA, Yun CC, 2011. MAGI-3 competes with NHERF-2 to negatively regulate LPA2 receptor signaling in colon cancer cells. Gastroenterology 140 (3), 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Z, Cheng CT, Zhang H, Subler MA, Wu J, Mukherjee A, Windle JJ, Chen CK, Fang X, 2008. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol. Biol. Cell 19 (12), 5435–5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneux C, Rocher G, Porteu F, 2006. B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK. EMBO J 25 (4), 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TT, Alemayehu M, Aziziyeh AI, Pape C, Pampillo M, Postovit LM, Mills GB, Babwah AV, Bhattacharya M, 2009. Beta-arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells. Mol. Canc. Res 7 (7), 1064–1077. [DOI] [PubMed] [Google Scholar]
- Lin FT, Lai YJ, Makarova N, Tigyi G, Lin WC, 2007. The lysophosphatidic acid 2 receptor mediates down-regulation of Siva-1 to promote cell survival. J. Biol. Chem 282 (52), 37759–37769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Lee SJ, Shim H, Chun J, Yun CC, 2010. The absence of LPA receptor 2 reduces the tumorigenesis by ApcMin mutation in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol 299 (5), G1128–G1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Wang D, Iyer S, Ghaleb AM, Shim H, Yang VW, Chun J, Yun CC, 2009. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology 136 (5), 1711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, Yu S, Stephens LC, Cui X, Murrow G, Coombes K, Muller W, Hung MC, Perou CM, Lee AV, Fang X, Mills GB, 2009. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Canc. Cell 15 (6), 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matayoshi S, Chiba S, Lin Y, Arakaki K, Matsumoto H, Nakanishi T, Suzuki M, Kato S, 2013. Lysophosphatidic acid receptor 4 signaling potentially modulates malignant behavior in human head and neck squamous cell carcinoma cells. Int. J. Oncol 42 (5), 1560–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum S, Morice-Picard F, Taieb A, Sprecher E, 2011. A novel mutation in LPAR6 causes autosomal recessive hypotrichosis of the scalp. Clin. Exp. Dermatol 36 (2), 188–194. [DOI] [PubMed] [Google Scholar]
- Oda SK, Strauch P, Fujiwara Y, Al-Shami A, Oravecz T, Tigyi G, Pelanda R, Torres RM, 2013. Lysophosphatidic acid inhibits CD8 T cell activation and control of tumor progression. Cancer Immunol Res 1 (4), 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeck S, Al-Refae K, Riffkin H, Wiel G, Handrick R, Klein D, Iliakis G, Jendrossek V, 2017. Activating Akt1 mutations alter DNA double strand break repair and radiosensitivity. Sci. Rep 7, 42700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K, Hayashi M, Yamawaki Y, Teranishi M, Honoki K, Mori T, Fukushima N, Tsujiuchi T, 2011. Possible involvement of lysophosphatidic acid receptor-5 gene in the acquisition of growth advantage of rat tumor cells. Mol. Carcinog 50 (8), 635–642. [DOI] [PubMed] [Google Scholar]
- Pamuklar Z, Federico L, Liu S, Umezu-Goto M, Dong A, Panchatcharam M, Fulerson Z, Berdyshev E, Natarajan V, Fang X, van Meeteren LA, Moolenaar WH, Mills GB, Morris AJ, Smyth SS, 2009. Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J. Biol. Chem 284 (11), 7385–7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Jeong KJ, Panupinthu N, Yu S, Lee J, Han JW, Kim JM, Lee JS, Kang J, Park CG, Mills GB, Lee HY, 2010. Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression. Oncogene 30 (11), 1351–1359. [DOI] [PubMed] [Google Scholar]
- Pasternack SM, von Kugelgen I, Al Aboud K, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A, Nurnberg P, Nothen MM, Betz RC, 2008. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat. Genet 40 (3), 329–334. [DOI] [PubMed] [Google Scholar]
- Pawlikowska P, Leray I, de Laval B, Guihard S, Kumar R, Rosselli F, Porteu F, 2010. ATM-dependent expression of IEX-1 controls nuclear accumulation of Mcl-1 and the DNA damage response. Cell Death Differ 17 (11), 1739–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyruchaud O, 2009. Novel implications for lysophospholipids, lysophosphatidic acid and sphingosine 1-phosphate, as drug targets in cancer. Anticancer Agents Med Chem 9 (4), 381–391. [DOI] [PubMed] [Google Scholar]
- Peyruchaud O, Leblanc R, David M, 2013. Pleiotropic activity of lysophosphatidic acid in bone metastasis. Biochim. Biophys. Acta 1831 (1), 99–104. [DOI] [PubMed] [Google Scholar]
- Peyruchaud O, Serre CM, NicAmhlaoibh R, Fournier P, Clezardin P, 2003. Angiostatin inhibits bone metastasis formation in nude mice through a direct anti-osteoclastic activity. J. Biol. Chem 278 (46), 45826–45832. [DOI] [PubMed] [Google Scholar]
- Prasetyanti PR, Medema JP, 2017. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Canc 16 (1), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptaszynska MM, Pendrak ML, Stracke ML, Roberts DD, 2010. Autotaxin signaling via lysophosphatidic acid receptors contributes to vascular endothelial growth factor-induced endothelial cell migration. Mol. Canc. Res 8 (3), 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustilnik TB, Estrella V, Wiener JR, Mao M, Eder A, Watt MA, Bast RC Jr., Mills GB, 1999. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin. Canc. Res 5 (11), 3704–3710. [PubMed] [Google Scholar]
- Ray U, Roy SS, Chowdhury SR, 2017. Lysophosphatidic acid promotes epithelial to mesenchymal transition in ovarian cancer cells by repressing SIRT1. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol 41 (2), 795–805. [DOI] [PubMed] [Google Scholar]
- Ren A, Moon C, Zhang W, Sinha C, Yarlagadda S, Arora K, Wang X, Yue J, Parthasarathi K, Heil-Chapdelaine R, Tigyi G, Naren AP, 2014. Asymmetrical macromolecular complex formation of lysophosphatidic acid receptor 2 (LPA2) mediates gradient sensing in fibroblasts. J. Biol. Chem 289 (52), 35757–35769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher G, Letourneux C, Lenormand P, Porteu F, 2007. Inhibition of B56-containing protein phosphatase 2As by the early response gene IEX-1 leads to control of Akt activity. J. Biol. Chem 282 (8), 5468–5477. [DOI] [PubMed] [Google Scholar]
- Sakane F, Mizuno S, Takahashi D, Sakai H, 2018. Where do substrates of diacylglycerol kinases come from? Diacylglycerol kinases utilize diacylglycerol species supplied from phosphatidylinositol turnover-independent pathways. Adv Biol Regul 67, 101–108. [DOI] [PubMed] [Google Scholar]
- Samadi N, Bekele RT, Goping IS, Schang LM, Brindley DN, 2011. Lysophosphatidate induces chemo-resistance by releasing breast cancer cells from taxol-induced mitotic arrest. PLoS One 6 (5), e20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi N, Gaetano C, Goping IS, Brindley DN, 2009. Autotaxin protects MCF-7 breast cancer and MDA-MB-435 melanoma cells against Taxol-induced apoptosis. Oncogene 28 (7), 1028–1039. [DOI] [PubMed] [Google Scholar]
- Sato Y, Murakami C, Yamaki A, Mizuno S, Sakai H, Sakane F, 2016. Distinct 1-monoacylglycerol and 2-monoacylglycerol kinase activities of diacylglycerol kinase isozymes. Biochim. Biophys. Acta 1864 (9), 1170–1176. [DOI] [PubMed] [Google Scholar]
- Schwartz BM, Hong G, Morrison BH, Wu W, Baudhuin LM, Xiao YJ, Mok SC, Xu Y, 2001. Lysophospholipids increase interleukin-8 expression in ovarian cancer cells. Gynecol. Oncol 81 (2), 291–300. [DOI] [PubMed] [Google Scholar]
- Seo EJ, Kwon YW, Jang IH, Kim DK, Lee SI, Choi EJ, Kim KH, Suh DS, Lee JH, Choi KU, Lee JW, Mok HJ, Kim KP, Matsumoto H, Aoki J, Kim JH, 2016. Autotaxin regulates maintenance of ovarian cancer stem cells through lysophosphatidic acid-mediated autocrine mechanism. Stem Cell 34 (3), 551–564. [DOI] [PubMed] [Google Scholar]
- Shida D, Fang X, Kordula T, Takabe K, Lepine S, Alvarez SE, Milstien S, Spiegel S, 2008. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Canc. Res 68 (16), 6569–6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida D, Kitayama J, Yamaguchi H, Hama K, Aoki J, Arai H, Yamashita H, Mori K, Sako A, Konishi T, Watanabe T, Sakai T, Suzuki R, Ohta H, Takuwa Y, Nagawa H, 2004. Dual mode regulation of migration by lysophosphatidic acid in human gastric cancer cells. Exp. Cell Res 301 (2), 168–178. [DOI] [PubMed] [Google Scholar]
- Shin SH, Kwon YW, Heo SC, Jeong GO, Kim BR, Seo EJ, Kim JH, 2014. Kruppel-like factor 4 mediates lysophosphatidic acid-stimulated migration and proliferation of PC3M prostate cancer cells. Exp. Mol. Med 46, e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangeland B, Mughal AA, Grieg Z, Sandberg CJ, Joel M, Nygard S, Meling T, Murrell W, Vik Mo EO, Langmoen IA, 2015. Combined expressional analysis, bioinformatics and targeted proteomics identify new potential therapeutic targets in glioblastoma stem cells. Oncotarget 6 (28), 26192–26215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, Liotta LA, 1992. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J. Biol. Chem 267 (4), 2524–2529. [PubMed] [Google Scholar]
- Sumida H, Noguchi K, Kihara Y, Abe M, Yanagida K, Hamano F, Sato S, Tamaki K, Morishita Y, Kano MR, Iwata C, Miyazono K, Sakimura K, Shimizu T, Ishii S, 2010. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood 116 (23), 5060–5070. [DOI] [PubMed] [Google Scholar]
- Sutphen R, Xu Y, Wilbanks GD, Fiorica J, Grendys EC Jr., LaPolla JP, Arango H, Hoffman MS, Martino M, Wakeley K, Griffin D, Blanco RW, Cantor AB, Xiao YJ, Krischer JP, 2004. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol. Biomark. Prev 13 (7), 1185–1191. [PubMed] [Google Scholar]
- Szymonowicz K, Oeck S, Krysztofiak A, van der Linden J, Iliakis G, Jendrossek V, 2018. Restraining Akt1 phosphorylation attenuates the repair of radiation-induced DNA double-strand breaks and reduces the survival of irradiated cancer cells. Int. J. Mol. Sci 19 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi P, Verhoeven E, Jacobs JJ, Lambooij JP, Stortelers C, Tanger E, Moolenaar WH, van Lohuizen M, 2008. In vitro genetic screen identifies a cooperative role for LPA signaling and c-Myc in cell transformation. Oncogene 27 (54), 6806–6816. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Fukushima K, Onishi Y, Inui K, Node Y, Fukushima N, Honoki K, Tsujiuchi T, 2017. Lysophosphatidic acid (LPA) signaling via LPA4 and LPA6 negatively regulates cell motile activities of colon cancer cells. Biochem. Biophys. Res. Commun 483 (1), 652–657. [DOI] [PubMed] [Google Scholar]
- Takuwa Y, Takuwa N, Sugimoto N, 2002. The edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. J. Biochem 131 (6), 767–771. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H, 2006. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem 281 (35), 25822–25830. [DOI] [PubMed] [Google Scholar]
- Tang X, Benesch MG, Brindley DN, 2015. Lipid phosphate phosphatases and their roles in mammalian physiology and pathology. J. Lipid Res 56 (11), 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigyi G, 2010. Aiming drug discovery at lysophosphatidic acid targets. Br. J. Pharmacol 161 (2), 241–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigyi G, Dyer D, Miledi R, 1994. Lysophosphatidic acid possesses dual action in cell proliferation. Proc. Natl. Acad. Sci. U.S.A 91, 1908–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobar N, Villar V, Santibanez JF, 2010. ROS-NFkappaB mediates TGF-beta1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol. Cell. Biochem 340 (1–2), 195–202. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K, 2002. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem 277 (42), 39436–39442. [DOI] [PubMed] [Google Scholar]
- Tsujino M, Fujii M, Okabe K, Mori T, Fukushima N, Tsujiuchi T, 2010. Differential expressions and DNA methylation patterns of lysophosphatidic acid receptor genes in human colon cancer cells. Virchows Arch 457 (6), 669–676. [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Tsukahara R, Fujiwara Y, Yue J, Cheng Y, Guo H, Bolen A, Zhang C, Balazs L, Re F, Du G, Frohman MA, Baker DL, Parrill AL, Uchiyama A, Kobayashi T, Murakami-Murofushi K, Tigyi G, 2010. Phospholipase D2-dependent inhibition of the nuclear hormone receptor PPARgamma by cyclic phosphatidic acid. Mol. Cell 39 (3), 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Tsukahara R, Yasuda S, Makarova N, Valentine WJ, Allison P, Yuan H, Baker DL, Li Z, Bittman R, Parrill A, Tigyi G, 2006. Different residues mediate recognition of 1-O-oleyllysophosphatidic acid and rosiglitazone in the ligand binding domain of peroxisome proliferator-activated receptor gamma. J. Biol. Chem 281 (6), 3398–3407. [DOI] [PubMed] [Google Scholar]
- Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H, 2002. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol 158 (2), 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenick LV, Schwarzbauer JE, 2006. Ligand density and integrin repertoire regulate cellular response to LPA. Matrix Biol 25 (4), 223–231. [DOI] [PubMed] [Google Scholar]
- van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenar WH, 1989. Lysophosphatidic-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell 59, 45–54. [DOI] [PubMed] [Google Scholar]
- van Corven EJ, Hordijk PL, Medema RH, Bos JL, Moolenaar WH, 1993. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc. Natl. Acad. Sci. USA 90, 1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Corven EJ, Rijswijk AV, Jalink K, Bend R.V.d., Blitterswijk WV, Moolenaar WH, 1992. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Biochem. J 281, 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J, 2006. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell Biol 26 (13), 5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman G, Benesch MG, Tang X, Dewald J, McMullen TP, Brindley DN, 2015. Lysophosphatidate signaling stabilizes Nrf2 and increases the expression of genes involved in drug resistance and oxidative stress responses: implications for cancer treatment. Faseb. J. 29 (3), 772–785. [DOI] [PubMed] [Google Scholar]
- Vidot S, Witham J, Agarwal R, Greenhough S, Bamrah HS, Tigyi GJ, Kaye SB, Richardson A, 2010. Autotaxin delays apoptosis induced by carboplatin in ovarian cancer cells. Cell. Signal 22 (6), 926–935. [DOI] [PubMed] [Google Scholar]
- Xu M, Liu Z, Wang C, Yao B, Zheng X, 2017. EDG2 enhanced the progression of hepatocellular carcinoma by LPA/PI3K/AKT/mTOR signaling. Oncotarget 8 (39), 66154–66168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Casey G, Mills GB, 1995a. Effect of lysophospholipids on signaling in the human Jurkat T cell line. J. Cell. Physiol 163 (3), 441–450. [DOI] [PubMed] [Google Scholar]
- Xu Y, Gaudette DC, Boynton JD, Frankel A, Fang X-J, Sharma A, Hurteau J, Casey G, Goodbody A, Mellors A, Holub BJ, Mills GB, 1995b. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin. Canc. Res 1, 1223–1232. [PubMed] [Google Scholar]
- Yamauchi Y, Miura Y, Kanaho Y, 2017. Machineries regulating the activity of the small GTPase Arf6 in cancer cells are potential targets for developing innovative anti-cancer drugs. Adv Biol Regul 63, 115–121. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y, Taguchi R, Shimizu T, Ishii S, 2009. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J. Biol. Chem 284 (26), 17731–17741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Herr DR, Diao H, Rivera R, Chun J, 2011. Unique uterine localization and regulation may differentiate LPA3 from other lysophospholipid receptors for its role in embryo implantation. Fertil. Steril 95 (6), 2107–2113 2113 e2101–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis S, Rashid S, 2017. Alpha conotoxin-BuIA globular isomer is a competitive antagonist for oleoyl-L-alpha-lysophosphatidic acid binding to LPAR6; A molecular dynamics study. PLoS One 12 (12), e0189154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J, Stephens C, Fang X, Mills GB, 2008. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J. Natl. Canc. Inst 100 (22), 1630–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]