Abstract
Background:
Viscoelastic tests (VETs), specifically thromboelastography (TEG) and rotational thromboelastometry (ROTEM), are gaining popularity in the management of critically ill surgical patients with hemorrhage or thrombosis due to their comprehensive characterization of the coagulation process and point-of-care availability in comparison to conventional coagulation tests (CCTs). We review current evidence for VET use in patients in the surgical intensive care unit (SICU).
Methods:
We searched PUBMED, EMBASE and the Cochrane Library through May 30, 2018 for articles that evaluated the use of VETs in patient populations and clinical scenarios germane to the surgical intensivist. Individual articles were critically evaluated for relevance and appropriate methodology using a structured technique. Information on patient characteristics, timing and methods of CCTs/VETs, and outcomes was collected and summarized in narrative form.
Results:
Of 2,589 identified articles, 36 were included. Five (14%) were interventional studies and 31 (86%) were observational. Twenty-five (69%) evaluated TEG, 11 (31%) ROTEM and 18 (50%) CCTs. Investigated outcomes included quantitative blood loss (13 (36%)), blood product transfusion (9 (25%)), thromboembolic events (9 (25%)) and mortality (6 (17%)). We identified 12 clinical scenarios with sufficient available evidence, much of which was of limited quantity and poor methodological quality. Nonetheless, research supports the use of VETs for guiding early blood product administration in severe traumatic hemorrhage and for the prediction of excess bleeding following routine cardiac surgery. In contrast, evidence suggests VET-based heparin dosing strategies for venous thromboembolism prophylaxis are not superior to standard dosing in SICU patients.
Conclusions:
While VETs have the potential to impact the care of critically ill surgical patients in many ways, current evidence for their use is limited, mainly because of poor methodological quality of most available studies. Further high-quality research, including several ongoing randomized controlled trials, is needed to elucidate the role of TEG/ROTEM in the SICU population.
Keywords: Thromboelastography, Rotational Thromboelastometry, Intensive Care, Hemorrhage, Blood Transfusion
INTRODUCTION
The interpretation and application of blood coagulation test results and the diagnosis and treatment of associated pathologic hemorrhage or thrombosis is fundamental to the practice of surgical critical care. Combined with clinical findings, the diagnosis of abnormal blood clotting is most often made through analysis of conventional coagulation tests (CCTs) such as prothrombin time (PT), activated partial thromboplastin time (PTT), platelet count and fibrinogen level. Despite their ubiquity, these measurements have several important fundamental limitations; they fail to account for the interdependence of the multiple aspects (cellular and enzymatic) of the coagulation cascade, they focus on quantity rather than functionality of clotting components, and they provide information on clot formation but do not address clot stability/dissolution(1).
Viscoelastic testing (VET), in the form of thromboelastography (TEG) or rotational thromboelastometry (ROTEM), is an alternative method of measuring blood coagulation status that has existed for nearly as long as CCTs but until recently had not been adopted into bedside practice outside the operating room (OR)(2, 3). These technologies measure multiple aspects of the clot formation-dissolution spectrum that reflect the combined function rather than individual quantity of coagulation elements(1). Despite these theoretical advantages, VET technology has only recently been effectively operationalized and its usage in clinical practice is just now undergoing evidence-based analysis(4, 5). Current literature has focused on VET use in the OR, particularly in cardiac surgery (CS) and liver transplantation (LT), but there are numerous potential applications relevant to the surgical intensivist caring for patients with disordered coagulation(4–6). We sought to systematically review the available evidence for the use of TEG or ROTEM in critically ill surgical patients applicable specifically to their condition within the intensive care unit (ICU).
Research Question (PICOS)
Our goal was to answer the following two questions in critically ill surgical patients residing in an ICU: 1) In the setting of hemorrhage or pathologic thromboembolism, are VET-guided treatment strategies superior to CCT-guided treatment strategies with regard to blood product transfusion, thrombotic complications, or mortality 2) Are VETs predictive of various subsequent clinical outcomes (excess bleeding, blood transfusion, thrombosis, mortality) and what are the strength of these associations compared to CCTs. All study designs were eligible.
METHODS
The study was conducted according to standard systematic review methodology, except where noted(7). A formal protocol was not published prior to this manuscript.
Eligibility Criteria
We included clinical studies of VETs applied to any patient population that could be encountered in a surgical ICU (SICU). All study designs were eligible, but articles were limited to those including human participants, age ≥13, published in English, in full manuscript form.
Studies had to obtain VETs in the ICU or immediately prior to ICU admission in the OR/emergency department (ED) and measure outcomes on patients while they were admitted to the SICU or immediately thereafter on the hospital ward. Studies measuring VETs and outcomes on patients solely while in the OR, prior to ICU admission, were excluded. Specifically, randomized controlled trials evaluating VET vs. CCT-based intraoperative transfusion strategies in patients undergoing CS or LT were excluded, as these results have been previously thoroughly reviewed(4, 5). Additionally, studies measuring VETs in the ED and their association with subsequent outcomes in trauma patients were also excluded, as these have also been previously reviewed(6). Studies measuring VETs and outcomes in patients solely outside of the ICU (hospital ward, outpatient setting, healthy volunteers) were excluded. Finally, studies involving retrospective ex-vivo modification of blood samples with a therapeutic agent (‘spiking’) were excluded.
Data Sources and Search Strategy
The search was performed on PUBMED, EMBASE and the Cochrane Library databases as of May 30, 2018. For PUBMED, the following terms were included: (“Thrombelastography”[Mesh] OR thromboelastograph*[tiab] OR ROTEM[tiab] OR TEG[tiab] OR ROTEG[tiab] OR thromboelastometry[tiab] OR thromboelastrometr*[tiab]). For EMBASE, the search strategy was: (‘thromboelastography’/exp OR thromboelastograph*:ab,ti OR rotem:ab,ti OR teg:ab,ti OR roteg:ab,ti OR thromboelastometry:ab,ti OR thromboelastometr*:ab,ti’). Both database results were filtered with the limits: humans, English language, age ≥13. For the Cochrane library, the search terms were: (thrombelastography OR thromboelastograph* OR ROTEM OR TEG OR ROTEG OR thromboelastometry OR thromboelastrometr*). The reference lists of applicable systematic reviews and all included articles were also examined for further potentially relevant studies.
Study Selection and Data Collection
Given the specifically broad inclusion criteria, we anticipated that our search strategy would identify research with a wide variety of study designs and patient groups. Studies were initially screened based on titles and abstracts by one investigator (B.C.D.) and if potentially relevant, were grouped by patient population and research question. Initial selection of articles for full-text review was based on the overall relevance of the study population to the SICU setting and the number and type of studies involving similar patient groups. Selection was performed by committee involving all five authors, requiring at least four of five in favor of inclusion. Upon subsequent review of initially selected full-text articles by one investigator (B.C.D.), studies were excluded for the following reasons: incorrect population, singular case reports, inadequate presentation of data, invalid/no longer available VET methods, inappropriate study design/conduct, or inappropriate statistical methods. Final selection was confirmed using the same committee method.
Data extracted from each study included: patient inclusion/exclusion criteria, study size, method, timing and reference range of VET/CCT measurement, location of patients at the time of blood sampling, and outcome measures, including but not limited to quantitative blood loss, occurrence of hemostasis, blood product transfusions, thromboembolic events and mortality. Data collection was performed independently by one author for each study, unblinded and with the use of a pre-specified data collection form.
Assessment of Bias and Synthesis of Results
Scoping searches confirmed that nearly all eligible articles would not be in the form of randomized, controlled trials given the design of the review and would be of low overall quality and high risk of bias. Additionally, the heterogenous nature of the study designs and outcome measures would preclude quantitative data synthesis. Thus, we did not perform standardized assessment of evidence quality or risk of bias for included articles. Study results are summarized in narrative/tabular form and relevant methodological aspects are highlighted. Consensus recommendations for use of VETs in the SICU in specific clinical scenarios are presented.
RESULTS
Search Results and Study Characteristics
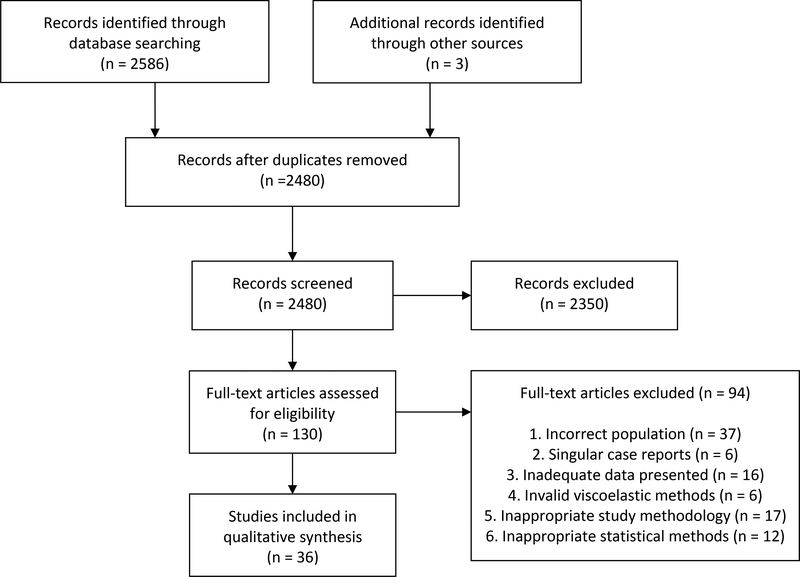
Our systematic search revealed a total of 2,586 eligible articles (PUBMED – 1703, EMBASE not MEDLINE – 879, Cochrane Library - 4) and 3 additional studies were identified by review of reference lists (Figure 1). Of the 2,480 non-duplicate articles, 130 were initially selected for full-text review. Ninety-four were excluded and 36 were selected for final inclusion (Table 1). We identified 12 clinical scenarios relevant to the surgical intensivist with available evidence.
Figure 1.
PRISMA diagram
Table 1 –
Methodological characteristics of included studies (n=36)
| Characteristic | n, (%) |
|---|---|
| Study design | |
| Randomized controlled trial | 3 (8) |
| Quasi-experimental, before-after | 2 (6) |
| Prospective cohort | 18 (50) |
| Retrospective cohort | 11 (31) |
| Case control | 2 (6) |
| Coagulation tests examined* | |
| Conventional coagulation tests | 18 (50) |
| Thromboelastography | 25 (69) |
| Rotational thromboelastometry | 11 (31) |
| Study setting* | |
| Intensive care unit | 36 (100) |
| Operating room | 20 (56) |
| Non-critical care bed | 4 (11) |
| Emergency department | 7 (19) |
| Outcome Measures* | |
| Quantitative blood loss | 13 (36) |
| Incidence of hemostasis/bleeding | 4 (11) |
| Blood product transfusion | 9 (25) |
| Thromboembolic events | 9 (25) |
| Mortality | 6 (17) |
| Re-operation | 3 (8) |
| Year of Publication | |
| 2000–2005 | 2 (6) |
| 2006–2010 | 3 (8) |
| 2011–2015 | 19 (53) |
| 2016–2018 | 12 (33) |
Multiple items possible within each study
Early blood product resuscitation in patients with traumatic hemorrhage using VET versus CCT or ratio-based treatment algorithms
We identified three studies that compared VET-guided transfusion algorithms to CCT-based or ratio-based protocols in the setting of traumatic hemorrhage. Gonzalez et al. conducted a single center, pragmatic, randomized controlled trial of CCT-guided versus rapid TEG (rTEG)-guided massive transfusion protocol (MTP) administration in patients with severe traumatic hemorrhage (systolic blood pressure (SBP)<70mmHg or 70–90mmHg and heart rate ≥108 plus 1 of: penetrating torso injury, unstable pelvic fracture, positive focused abdominal sonography for trauma (FAST))(8). All patients received initial transfusion of 4 units of packed red blood cells (PRBC) and 2 units of fresh frozen plasma (FFP) upon MTP activation; patients in the CCT-guided group had FFP, platelets, cryoprecipitate and transexamic acid (TXA) administered based on international normalized ratio (INR) (≥1.5), platelet count (<100 cells/mm3), fibrinogen level (<150 mg/dl) and d-dimer (>500 mcg/l) respectively, while patients in the rTEG group had transfusion based upon ACT (>110 sec), angle (<63̊), maximum amplitude (MA) (<55) and LY30 (≥7.5, changed to ≥3 after 61% enrollment). Patients with ACT>140 sec on initial rTEG received 2 units FFP, 1 unit of platelets, and 10 units of cryoprecipitate, which was unique to only the initial rTEG measurement. Randomization was performed on a weekly basis and the primary outcome, 28-day mortality, was compared with a modified intention-to-treat basis (excluding ineligible patients or those that died prior to any blood transfusion/blood draw). All patients had both VETs and CCTs measured when triggered by the clinicians, but only the assigned study group test results were available to the treating providers. In the 111 patients analyzed (56 rTEG, 55 CCT, 68% blunt mechanism, mean injury severity score (ISS)=30, mean admission base deficit (BD)=12 mEq/l, mean initial SBP 92mmHg), 28-d survival was greater in the rTEG group compared with the CCT group (log-rank test, p=0.032, crude 28-d mortality 19.6% vs 36.4%). PRBC administration was similar between groups, while the CCT group received more FFP at 2 and 4 hours, more platelets at 2 hours and more cryoprecipitate at 24 hours compared with the rTEG group. CCT/VET measurements were similar between groups in the first 24 hours.
Two quasi-experimental studies evaluated VET vs ratio-based MTP administration. Kashuk et al. evaluated 68 patients (34 ratio-before, 34 rTEG-after) requiring 6 or more PRBCs in the first 6 hours after introduction of an rTEG-based transfusion strategy and found no difference in 6 hour blood product requirements(9). Additional conclusions were limited by the small sample size. Tapia et al. evaluated 163 patients (98 kaolin TEG (kTEG)-before, 65 ratio-after) requiring 10 or more PRBCs in 24 hours and found no difference in overall 30-d mortality(10). Post-hoc analysis revealed 30-d mortality was lower with kTEG-guided resuscitation in penetrating trauma patients (33% vs. 54%, p=0.04). Ratio-guided patients received significantly more FFP but less crystalloid resuscitation, questioning whether changes in overall resuscitation practice over time occurred during the study period.
Prediction of post-operative bleeding in cardiac surgery
We identified 14 studies that evaluated the ability of perioperative VETs to identify patients that subsequently developed excess post-operative bleeding. Given the heterogeneity in the study populations, we grouped them according to the overall pre-operative expected bleeding risk; elective, first-time, simple cardiopulmonary bypass (CPB) cases (8 studies) and urgent/emergent, complex, or redo CPB cases (6 studies)(11–24). Tables 2a and 2b display the relevant study details.
Table 2a.
Prediction of post-operative bleeding after cardiac surgery – low bleeding risk studies
| Author | Type | Size | Population | VET | CCT | Timing | Outcome | Exposure | Post-op Transfusion Strategy | Results | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Sensitivity | Specificity | ||||||||||
| Ghavidel | PC | 400 | Elective 1st CABG | ROTEM (IN, EX) | -- | 20 min post-protamine | Excess bleeding: >200cc/hr*1 or >1000cc/24 hrs (binary) | Any abnl ROTEM (binary) | PRBC: not reported Hemostatic products: not reported Blinding to VETs: not reported | -- | VET - 87.5 | VET - 100 |
| Gozdzik | RC | 114 | Elective CPB | ROTEM (IN, EX) | -- | Pre-operative | 48 hr CTD (contin) | Any abnl ROTEM (binary) | PRBC: Hct<25% Hemostatic products: not reported Blinding to VETs: not reported | Median CTD abnl ROTEM: 700 (330–1060) v nl ROTEM: 530 (390–770), p=0.03 | ||
| Ostrowsky | PC | 35 | Elective CPB | TEG | -- | 1 hr post-op | CTD ?duration (contin) | Individual TEG values (contin) | PRBC: not reported Hemostatic products: not reported Blinding to VETs: not reported | No significant correlation between TEG values (continuous) and CTD | ||
| Rafiq | PC | 170 | Elective or urgent CABG | kTEG | PT, PTT, plt, fib | Pre-op | 18 hr CTD (contin) | Individual TEG, CCT values (contin) | PRBC: not reported Hemostatic products: not reported Blinded to VETs | Modeled CTD using multivariable linear regression, no TEG or CCTs significantly associated with CTD | ||
| Ti | PC | 40 | Elective CABG | TEG (heparinase) | -- | 10/60 min post-protamine | Excess bleeding: >250cc/hr*2 or >1000cc/24 hrs (binary) | Any TEG value >20% outside nl range (K>4, A<40, MA<40, LY60>15) (binary) | PRBC: Hgb<8 g/dl Hemostatic products: CCTs only Blinded to VETs | -- | VET 10 min - 70 VET 60 min - 100 | VET 10 min - 83 VET 60 min - 73 |
| Welsby | PC | 30 | Elective CPB | kTEG | PT, PTT, plt, fib | ICU admission | Excess bleeding: >400cc/4 hrs (binary) | Abnl individual VET/CCTs (binary) | PRBC: not reported Hemostatic products: CCTs only Blinded to VETs | MA- 0.78 Fib – 0.73 | -- | -- |
| Sharma | PC | 50 | Elective CPB | kTEG (heparinase) | PT, PTT, plt, fib | ICU admission | Excess bleeding: >100cc/hr*3 or >300cc/hr*1 or >1000cc/24 hr (binary) | Abnl individual VET/CCTs (binary) | PRBC: not reported Hemostatic products: not reported Blinding to VETs: not reported | *R- ~0.75 MA- ~0.75 CCTs - ns | -- | -- |
| Agarwal | PC | 54 | Elective CABG ± valve | kTEG-platelet mapping | PT, PTT, plt, fib | 10 min post-protamine | 4 hr CTD (contin) | Individual TEG, CCT values (contin) | PRBC: not reported Hemostatic products: VETs only | Modeled CTD using multivariable linear regression, no TEG or CCTs significantly associated with CTD | ||
Exact AUC values not stated, only ranges given (0.7–0.8)
VET=viscoelastic test, CCT=conventional coagulation test, AUC=area under receiver operating characteristics curve, PC=prospective cohort, CABG=coronary artery bypass graft, IN=INTEM, EX=EXTEM, RC=retrospective cohort, abnl=abnormal according to manufacturer’s standards, CPB=cardiopulmonary bypass, CTD=chest tube drainage, PT=prothrombin time, PTT=activated partial thromboplastin time, plt=platelet count, fib=fibrinogen, R=R-time, K=K-time, A=alpha angle, MA=maximum amplitude, LY60=percent lysis at 60 minutes, PRBC=packed red blood cell
Table 2b.
Prediction of postoperative bleeding in cardiac surgery – high bleeding risk articles
| Author | Type | Size | Population | VET | CCT | Timing | Outcome | Exposure | Post-op Transfusion Strategy | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Lee | PC | 321 | Any CPB, 1/3 high bleeding risk | ROTEM (IN, EX, FIB) | PT, PTT, plt, fib | 5 min post-protamine; ICU admission | 8 hr CTD (contin) | Individual ROTEM, CCT values (contin) | PRBC: clinician discretion Hemostatic products: clinician discretion Blinded to VETs | Modeled CTD using multivariable linear regression. Addition of post-protamine INTEM CT and FIBTEM angle significantly improved model fit statistics (F-test, p<0.001). |
| Tarzia | PC | 100 | ACS, urgent/emergent CABG | ROTEM (IN, EX) w/ calculated “AUC” EXTEM MCF | PT, PTT, plt, fib | Pre-operative | Excess bleeding: >3 U PRBCs/8 hrs or surgical re-exploration (binary) | Individual ROTEM, CCT values (contin) | PRBC: not reported Hemostatic products: CCTs and VETs | Modeled excess bleeding using multivariable logistic regression. Only “AUC” EXTEM MCF significantly associated with excess bleeding (Wald x2, p=0.008) |
| Azarfarin | PC | 60 | ACS, failed PCI, clopidogrel loaded -> urgent CABG | ROTEM (EX) | -- | Pre-operative | 24 hr CTD (contin); need for surgical re-exploration (binary) | EXTEM MCF<50 (binary) | PRBC: not reported Hemostatic products: not reported Blinded to VETs | Mean CTD: 1259±555cc v 447±165cc, p<0.0001 Re-exploration: 31.3% (5/16) v 6.8% (3/44), p=0.026 |
| Sharma | RC | 400 | Mixed CPB (25% complex) | kTEG (heparinase) | PT, PTT, plt, fib | Before decannulation or post-protamine | 8 hr CTD (contin) | Individual TEG, CCT values (contin) | PRBC: clinician discretion Hemostatic products: clinician discretion Aware of VETs | Modeled CTD using multivariable linear regression, no CCTs sign associated. Addition of TEG values did not improved model fit statistics (F-test, p=0.3743) |
| Welsh | RC | 67 | Complex CPB (heart transplant, VAD, etc) | kTEG | PT, PTT, plt, fib | 10 min post-protamine | Excess bleeding: >150/hr “sustained” or >2000cc/24 hr (binary) | Individual TEG, CCT values (contin) | PRBC: not reported Hemostatic products: CCTs and VETs | Modeled excess bleeding using multivariable logistic regression. Only PTT significantly associated with excess bleeding (Wald x2, p=0.0094) |
| Wasowicz | RC | 434 | Complex CPB (redo, double valve, etc) | kTEG (heparinase) | -- | Pre-CPB and on-CPB before decannulation | Excess bleeding: ≥5 U PRBCs/24 hrs (binary) | Individual TEG values (contin) | PRBC: Hct<24% Hemostatic products: CCTs only Aware of VETs | Modeled excess bleeding using multivariable logistic regression. Addition of TEG values did not improve model fit statistics (N.P.) |
VET=viscoelastic test, CCT=conventional coagulation test, AUC=area under receiver operating characteristics curve, PC=prospective cohort, CPB=cardiopulmonary bypass, IN=INTEM, EX=EXTEM, FIB=FIBTEM, PT=prothrombin time, PTT=activated partial thromboplastin time, plt=platelet count, fib=fibrinogen, ICU=intensive care unit, CTD=chest tube drainage, CT=clotting time, ACS=acute coronary syndrome, CABG=coronary artery bypass graft, MCF=maximum clot firmness, U=units, PRBC=packed red blood cells, PCI=percutaneous coronary intervention, RC=retrospective cohort, VAD=ventricular assist device, Hct=hematocrit, N.P.=not performed
Prediction of bleeding in patients on extracorporeal membrane oxygenation (ECMO)
We identified two studies that evaluated the association between VETs and bleeding complications in patients on ECMO. Nair et al. prospectively studied 10 patients on ECMO (7 venoarterial, 3 venovenous) with daily morning ROTEM testing (110 patient-days in total) and evaluated the ability of each morning’s ROTEM values to predict subsequent bleeding episodes in the next 24 hours that required transfusion of FFP, platelets or cryoprecipitate guided by CCTs and clinical findings, but blinded to ROTEM results(25). They found EXTEM MCF<50mm to have the strongest association, but only with a sensitivity of 64% and specificity of 83%. No CCTs were evaluated in a similar fashion.
Panigada et al. retrospectively studied 32 patients on venovenous ECMO (316 patient-days in total) and similarly evaluated the ability of daily CCTs and heparinase, kTEG R-time to predict bleeding requiring reduction in heparin dose or blood transfusion, triggered by CCTs or clinical parameters(26). They evaluated the association of same day, 1 day prior, and 2 days prior values with the first bleeding episode on ECMO using random effects logistic regression and found no significant association between any laboratory values and subsequent bleeding.
Prediction of bleeding in women with post-partum hemorrhage
We identified two studies that evaluated the ability of VETs to predict excess bleeding in women with post-partum hemorrhage (PPH). Karlsson et al. performed a case-control study comparing kTEG and CCTs in 45 women undergoing cesarean section with a greater than 2 liter estimated blood loss (EBL) to 49 women with routine vaginal deliveries with less than 600 ml blood loss(27). They found the women with PPH had significantly lower R-time, alpha angle, MA, and LY30 values, lower platelet counts and fibrinogen levels, and higher PT and PTT values, however the mean values were all within the normal range and no data was presented on the incidence of abnormal values.
Collins et al. performed a prospective cohort study of women experiencing moderate-severe PPH (all at least >1 liter EBL) and evaluated the association between FIBTEM A5 and fibrinogen levels drawn at the time of PPH diagnosis and subsequent blood loss and transfusion requirements(28). PRBCs were transfused for hemodynamic instability or a point-of-care hemoglobin <8.0 g/dl and hemostatic blood products were guided by CCTs; clinicians were blinded to ROTEM results. EBL was measured prospectively using a validated gravimetric method plus drain outputs. Of the 347 women enrolled (50% spontaneous delivery, median initial EBL 1.2 L (1.0–1.5)), 11% progressed to an EBL>2.5L and 30% received 1 or more PRBC transfusions, which were the co-primary outcomes. AUCs for FIBTEM A5 were 0.61 (0.54–0.68) for any PRBC transfusion and 0.75 (0.66–0.85) for EBL>2.5 liters, while for fibrinogen, AUCs were 0.67 (0.6–0.74) and 0.71 (0.61–0.81) respectively. The authors performed a large number of secondary analyses using multiple outcome measures. Overall, the results suggested that both FIBTEM A5 and fibrinogen had improved prognostic ability for larger measures of hemorrhage, such as AUCs of 0.78 (0.69–0.88) and 0.78 (0.67–0.88) respectively for prediction of ≥ 4 units of PRBC transfusion. While FIBTEM A5 results were theoretically available an average of 50 minutes faster than fibrinogen levels, the median time to initial blood transfusion was 225 minutes (80–1327) from blood sampling.
Prediction of post-operative bleeding in liver transplantation
We found one study that addressed the association of VETs with post-operative bleeding after LT. Dotsch et al. retrospectively analyzed data on 243 patients undergoing LT to evaluate whether ROTEM or CCTs obtained at ICU admission could predict the occurrence of bleeding requiring relaparotomy or transfusion of 3 or more PRBCs within 48 hours, which occurred in 12% of subjects(29). Patients were treated with a comprehensive, evidence-based VET-guided transfusion algorithm intra-operatively and in the ICU. PTT (AUC 0.69 (0.63–0.75)), INR (0.62 (0.56–0.69)), EXTEM clotting time (0.68 (0.62–0.74)), and FIBTEM A10 (0.64 (0.57–0.70)) were significant associated with major postoperative bleeding, but all demonstrated inadequate predictive capability.
Prediction of worsening intracranial bleeding in patients with traumatic brain injury (TBI)
We identified one study that evaluated whether VETs are predictive of worsening intracranial bleeding in patients with severe TBI. Folkerson et al. retrospectively evaluated the association between admission rTEG and CCT levels and worsening head computed tomography (CT) findings at 6-hour follow up in a cohort of 279 patients with isolated, severe TBI(30). While they found no significant differences in median rTEG or CCT values between patients with and without progressive injury, presence of at least 1 abnormal rTEG or CCT measurement indicating coagulopathy was independently associated with an increased odds of injury progression in multivariable logistic regression (OR 1.81 (1.09–3.01), p=0.021). Unfortunately, the investigators did not individually evaluate the predictive ability of rTEG or CCTs.
Prediction of free flap thrombosis after microvascular reconstructive surgery
We found one study that addressed whether VETs are associated with flap thrombosis and flap loss after free tissue transfer surgery. Kolbenschlag et al. performed an elegant retrospective cohort study in which they evaluated whether preoperative ROTEM values were associated with post-operative pedicle thrombosis or flap loss among 181 patients (60% trauma, 25% malignancy) undergoing free tissue transfer surgery without routine postoperative anticoagulation (31). Patients with hypercoagulable ROTEM (66/181 (36.5%), defined as INTEM or EXTEM MCF>72 mm or FIBTEM MCF>25mm) did not have a different incidence of flap thrombosis as patients with normal ROTEM values (16.6% v 15%), but were more likely to suffer overall flap loss (13.6% v 4.3%, p=0.024), which was driven by the inability to successfully salvage almost all thrombosed flaps in patients with hypercoagulable ROTEM values. Nonetheless, while the presence of hypercoagulable ROTEM was significantly associated with flap loss after controlling for age, sex and comorbidities in multivariable regression analysis, the AUC was only 0.63. Unfortunately, the prognostic ability of CCTs was not evaluated and it was not reported whether ROTEM results were incorporated into clinical decision making.
Prediction of post-operative thrombotic complications in liver transplantation
We identified one study that examined the association between VETs and post-operative thrombotic complications in LT. Zahr Eldeen et al. retrospectively examined the association between preoperative kTEG and CCTs and early hepatic artery thrombosis (HAT) (≤21 days) among a cohort of 828 patients undergoing LT at a high-volume center treated with routine postoperative thromboprophylaxis(32). Early HAT occurred in 23 (2.7%) patients; AUC for preoperative MA>65mm was 0.75 and for preoperative platelet count>145 cells/cubic mm was 0.71. However, these cutoffs were determined post-hoc based upon the 75th percentile values in patients that did not develop HAT and do not reflect the upper limit of normal for kTEG MA(33, 34).
Prediction of venous thromboembolism (VTE) in critically ill/injured surgical patients
We identified five studies that investigated the predictive ability of VETs for VTE in critically ill surgical patients (Table 3)(35–39). Only the studies by Brill et al. and Van et al. provided data on prophylactic anticoagulation use, with both papers reporting an overall high (>90%) adherence rate. In all studies, VETs were not used to guide prophylactic anticoagulation or VTE diagnosis.
Table 3.
Studies evaluating viscoelastic tests for the prediction of pathological thromboembolism
| Author | Type | Size | Population | VET | CCT | Outcome | Outcome Diagnostic Modality | Exposure | Results |
|---|---|---|---|---|---|---|---|---|---|
| Brill | PC | 684 | Trauma ICU + ward | Admission kTEG | -- | LE DVT | ≥2 screening US | Hypercoagulable TEG (R<5, A>72, or MA>74) | Hypercoagulable TEG: AUC 0.54 |
| Cotton | RC | 2080 | Trauma ICU + ward | Admission rTEG | -- | PE | CTPA – symptom triggered | MA>72 | MA>72: Sensitivity: 49%, Specificity: 87% |
| McCully | Case-control | 558 | Trauma, MTP | Admission rTEG | -- | DVT or PE | LE US – screening or symptom triggered CTPA – symptom triggered | MA>65 | MA>65: VTE+ 43% vs. VTE- 26%, p<0.001 |
| Hincker | PC | 313 | Major non-CV surgery | Preoperative ROTEM | PT, PTT, Plt, Fib | Post-op arterial, venous or catheter thrombosis | Not described, retrospectively abstracted from chart | Abnormal individual ROTEM/CCT values | INTEM MCF>72: AUC 0.74 EXTEM MCF>71: AUC 0.73 INTEM CFT<71: AUC 0.75 EXTEM CFT<46: AUC 0.74 All CCTs: non-significant |
| Van | PC | 61 | SICU | Daily kTEG for 4 days | -- | LE DVT | ≥1 screening US | Mean R over 4 days | Mean R: DVT+ 6.1 min vs. DVT- 7.6 min, p<0.01 |
VET=viscoelastic test, CCT=conventional coagulation test, PC=prospective cohort, ICU=intensive care unit, LE=lower extremity, DVT=deep vein thrombosis, US=ultrasound, R=R-time, A=alpha angle, MA=maximum amplitude, TEG=thromboelastography, AUC=area under receiver operating characteristics curve, RC=retrospective cohort, PE=pulmonary embolism, CTPA=computed tomography pulmonary arteriography, MTP=massive transfusion protocol, VTE=venous thromboembolism, CV=cardiovascular, ROTEM=rotational thromboelastometry, PT=prothrombin time, PTT=activated partial thromboplastin time, plt=platelet count, fib=fibrinogen, MCF=maximum clot firmness, CFT=clot formation time, SICU=surgical intensive care unit
Titration of DVT prophylaxis in critically ill/injured surgical patients using VETs compared to standard care
We identified two studies that evaluated VET-based dosing strategies for administration of DVT chemoprophylaxis. Connelly et al. conducted a multicenter, randomized controlled trial evaluating the efficacy of low molecular weight heparin (LMWH) dosing titrated using TEG measurements compared to standard fixed dosing in 185 trauma and general surgery patients with an expected hospital stay >3 days(40). In the intervention group, enoxaparin dosing started at standard levels (30 mg twice daily) and was uptitrated every 48 hours to reach a goal value of ΔR (R-time without heparinase – R-time with heparinase) of 1–2 minutes, up to a maximum of 1 mg/kg twice daily. Primary outcomes were the incidence of VTE (mixed screening strategy, all patients received at least one surveillance LE US) and major bleeding. They found no difference in the proportion of patients suffering VTE in the intervention group vs standard dosing (6.3% v 6.7%, p>0.99) and a suggestion of increased bleeding (13.5% v 5.6%, p=0.08) when the trial was halted at the mid-point interim analysis. Only 10.4% of patients in the intervention group achieved the goal ΔR (vs. 13.5% in control, p=0.68) after receiving median enoxaparin doses of 37.5 mg (35–42.2) twice daily.
Harr et al. performed a very similar single-center randomized controlled trial of TEG-based LMWH dosing compared to standard fixed dosing in 50 ICU trauma patients(41). They targeted a kTEG ΔR > 1.4 min and uptitrated dosing more rapidly (every 24 hours) compared with Connelly et al. Additionally, if the maximum dalteparin dose (10,000 units twice daily) was reached without achieving goal ΔR, aspirin was added (required in 20% of patients in intervention arm). Despite this aggressive protocol, only 12% of the treatment group (vs 8% in control) achieved the goal ΔR. Only one VTE event occurred in the entire study population and major bleeding was not reported.
Prediction of mortality in ICU patients with sepsis
The association of VETs with mortality in ICU patients with sepsis was evaluated in 3 studies. Ostrowski et al. prospectively evaluated whether abnormal admission kTEG MA, either hypo (<51mm) or hypercoagulable (>69mm), was associated with 28-d mortality in 60 ICU patients with sepsis(42). They found patients with low MA on ICU admission had significantly worse survival compared to those who had normal or high MA, whose survival curves were similar (p=0.003, log-rank test), and that a low MA was independently associated with mortality after controlling for disease severity (hazard ratio (HR) 4.3 (1.4–13.7), p=0.014). No CCTs were evaluated.
Haase et al. used data from a randomized, controlled trial of hetastarch resuscitation in sepsis to evaluate whether early hypocoagulability, measured by abnormally low kTEG values (except R-time), was associated with 90-d mortality or 90-d major bleeding, as compared to normo or hypercoagulability(43). They used TEG results obtained from 260 patients on ICU admission and daily for 5 days and modeled time to death/bleeding using cox proportional hazards, with the most proximate TEG results to the event/censoring incorporated as time-dependent variables. Hypocoagulable K-time, MA, alpha angle and functional fibrinogen-MA (FF-MA) were all significantly associated with mortality, compared to normo-coagulability. Additionally, hypercoagulable MA (>69mm) was associated with a decreased risk of death compared with normo-coagulability (HR 0.52 (0.33–0.81), p=0.004). Only low FF-MA (<14mm) was significantly associated with major bleeding. No CCTs were evaluated.
Finally, Massion et al. prospectively studied 39 patients with septic shock obtaining ROTEM and CCT measurements at 5 time points from ICU admission to day 7 to determine whether VETs/CCTs were associated with in-hospital mortality(44). They analyzed ROTEM and CCT values continuously using two-way repeated measures analysis of variance. Serial median PT, PTT and INTEM clotting time values showed significant differences between survivors and non-survivors but there was no information presented on the occurrence of abnormal values.
Prediction of mortality in patients with traumatic brain injury at ICU admission
We found one study that evaluated the association between VETs obtained on ICU admission and mortality in patients with severe TBI. Daley et al. retrospectively studied a group of 90 patients with severe TBI, the majority of whom had isolated neurological injury, to determine whether kTEG-platelet mapping (kTEG-PM) values obtained on ICU admission were associated with in-hospital mortality(45). In an older population (mean age 65), 30% of whom were taking pre-injury antiplatelet therapy, they found patients with a degree of adenosine diphosphate (ADP) inhibition >60% (normal 0–30%) had significantly greater mortality than those with less platelet inhibition (32% v. 8%. p<0.01) and that high platelet inhibition was independently associated with mortality even after controlling for other known prognostic factors. The specific prognostic ability of TEG-PM or CCTs, however, was not presented and no information was provided on the prescription of platelet transfusions. Furthermore, patients classified as not having severe ADP inhibition (≤60%) still showed values far from normal (mean 44±19%).
DISCUSSION
Although we identified a large number and wide variety of studies evaluating the use of VETs in the SICU setting, the poor quality of most of the research limits related evidence-based conclusions. Nonetheless, current literature suggests that VETs have a role in guiding early blood product administration in severe traumatic hemorrhage and in predicting excess bleeding following routine CS, whereas VET-based dosing strategies of prophylactic LMWH are not superior to standard dosing in critically ill trauma or surgical patients.
Interest in the use of VETs has increased significantly in recent years (see Table 1), but proper evidence-based evaluation of such strategies is somewhat lagging. Our review identified primarily observational studies (120/130 (92%) of full-text reviewed articles), many of which were not conducted in accordance with current basic recommendations (46–48). This led to 45 of the 94 (48%) excluded articles being removed because of research methodology limitations (Figure 1). Furthermore, interpretation of a significant number of the included studies mentioned above was hampered by additional methodological shortcomings, such as a categorization of VET results as continuous variables incorporating both normal and abnormal results, incomplete reporting of predictive measures (AUC, sensitivity, specificity), and lack of comparison with CCTs. Future research should incorporate scientifically robust methodology to more appropriately evaluate the accuracy of VETs and CCTs to predict abnormal bleeding or thromboembolism in SICU patients.
We identified five clinical scenarios with sufficient evidence upon which to make recommendations (Table 4). In the past two decades, blood component resuscitation for severe traumatic hemorrhage has undergone significant advancement with the increased understanding of trauma-induced coagulopathy and adverse effects of crystalloid resuscitation leading to institution of MTPs with balanced blood component administration(49). Debate exists over the prescription of the hemostatic elements of MTPs (FFP, platelets, cryoprecipitate, TXA), with strategies incorporating CCT-guided algorithms, fixed ratio algorithms, and VET-guided algorithms all being employed(50, 51). We found that in patients with severe traumatic hemorrhage, evidence suggests early VET-based transfusion strategies result in decreased blood product requirements and decreased mortality compared to CCT-based or ratio-based strategies. This is supported primarily by the well-designed, albeit single center study by Gonzalez et al. that compared an rTEG guided transfusion algorithm to one guided by CCTs(8). Interestingly, the trial found that patients in the rTEG group received less FFP and platelets during very early resuscitation yet had increased short and long-term survival. Results from several recently completed/ongoing randomized clinical trials comparing VET vs. CCT-based transfusion strategies for initial trauma resuscitation should soon provide further high-quality evidence on this topic (Table 5). As such, we conditionally recommend the use of VET-based transfusion algorithms in the early resuscitation of patients with severe traumatic hemorrhage.
Table 4 –
Recommendations for Clinical Use of Viscoelastic Tests in the SICU
| Scenario | Recommendation |
|---|---|
| Early blood product resuscitation in patients with traumatic hemorrhage using VET versus CCT or ratio-based treatment algorithms | We conditionally recommend TEG-guided blood product administration compared to CCT or ratio-based treatment. |
| Prediction of post-operative bleeding in elective, simple, cardiac surgery | We recommend risk stratification for excessive post-operative bleeding using VETs for elective cardiac surgery patients. The presence of any abnormal VETs obtained soon after surgery is predictive of the occurrence of excessive postoperative bleeding, more so than abnormal CCT results. |
| Titration of DVT prophylaxis in critically ill/injured surgical patients using VET compared to standard care | We do not recommend TEG-based titration of LMWH dosing targeting ΔR critically ill trauma and surgical patients. |
| Prediction of VTE in critically ill/injured surgical patients | We do not recommend routine risk stratification of SICU patients using VETs in the prediction of subsequent VTE. |
| Prediction of mortality in SICU patients with sepsis | We do not recommend routine risk stratification for mortality based upon VETs in SICU patients with sepsis. |
VET=viscoelastic test, CCT=conventional coagulation test, DVT=deep vein thrombosis, TEG=thromboelastography, LMWH=low molecular weight heparin, VTE=venous thromboembolism, SICU=surgical intensive care unit
Table 5 –
Ongoing Randomized Clinical Trials Comparing Viscoelastic Test vs Conventional Coagulation Test-based Transfusion Strategies
| Name | Design | Location | Population | Setting | Timing of Results* |
|---|---|---|---|---|---|
| STATA (NCT02416817) | Single-center | South America | Trauma | ED, OR, ICU | Completed 7/2016 |
| iTACTIC (NCT02593877) | Multi-center | Europe | Trauma | ED, OR, ICU | Completed 7/30/2018 |
| VISCOTRAUMA (NCT03380767) | Single-center | Europe | Trauma | ED, OR, ICU | Expected 9/2021 |
| ROTEM-PPH (NCT02461251) | Multi-center | Europe | Post-partum hemorrhage | OR, ICU | Expected 12/2019 |
| NCT03064152 | Single-center | United States | Post-partum hemorrhage | OR, ICU | Expected 9/2018 |
Based on information provided at www.clinicaltrials.gov, accessed October 1, 2018
ED=emergency department, OR=operating room, ICU=intensive care unit
Available research also supports the use of VETs to predict excess early post-operative hemorrhage in patients undergoing on-pump CS with a low predicted risk of bleeding (Table 2a). Reduction of postoperative bleeding in CS requiring CPB is of major importance due to its association with worse outcomes and increased resource utilization(52). Multiple studies, using either TEG or ROTEM values obtained post-protamine administration in the OR or upon ICU admission, showed that the presence of any abnormal VET had good ability to predict early excess bleeding in this patient population, with AUCs > 0.7 or sensitivity/specificity > 80%(11, 15–17). Although only analyzed in two of four studies, the prognostic capacity of VETs appeared to be at least equal, or superior to, CCTs, particularly fibrinogen(16, 17). Thus, we recommend routine risk stratification of patients undergoing elective CS with VETs to help identify patients at risk for excess post-operative hemorrhage. Of note, intra and post-operative transfusion strategies in most of the included studies were not guided by VETs, so these results may not be generalizable to scenarios in which VET-based intraoperative transfusion is performed. While we identified several studies that evaluated the association between VETs and bleeding in CS patients with a higher baseline bleeding risk, in whom an effective risk stratification tool would be even more valuable, the use of non-prognostic statistical methods to present the results impeded our ability to draw conclusions from these findings.
Prior research has demonstrated a high rate of VTE in severely injured trauma patients despite prescription of standard dose thromboprophylaxis(53). This is hypothesized to be due, in part, to inadequate LMWH dosing and/or relative antithrombin III deficiency(54). We identified two similarly designed randomized controlled trials that evaluated whether VET-guided dosing of LMWH thromboprophylaxis was more effective than fixed dosing in SICU patients(40, 41). Based upon prior observational data showing patients with a TEG ΔR > ~1 min had a decreased incidence of VTE, both studies used a ΔR guided LWMH dosing strategy(39). Unfortunately, in both trials the VET-guided strategy was rarely able to achieve the desired ΔR and the results did not show a decrease in VTE rates. Thus, we cannot recommend LWMH dosing guided by a TEG ΔR-based protocol. Future studies using other VET-based parameters could have differing results.
On two additional topics, prediction of VTE in SICU patients and prediction of mortality in septic ICU patients, preliminary data suggests an association between VET results and subsequent outcomes, but the magnitude and significance of this association are unclear. Prior studies have evaluated the association of VETs with the development of VTE after routine surgery but the results were severely limited by methodological flaws(55). Similarly, of the 5 studies we found that evaluated the association between VETs and VTE in SICU patients only the study by Hincker et al. reported sufficient predictive ability of VETs, but the authors outcome measure included arterial thromboembolic events and asymptomatic catheter-related thrombosis, limiting the generalizability of their findings(38). While the studies by Ostrowski et al. and Haase et al. both showed that early TEG-based coagulopathy was independently associated with mortality in sepsis patients, neither group evaluated the concomitant significance of CCTs(42, 43). Further, what to do about such early coagulopathy detected by VETs is unclear, as the association with bleeding events or need for blood product transfusion was only evaluated in one study(43). Further research is needed to elucidate the value of VETs in these settings and we cannot recommend their routine use at this time.
Finally, we identified a number of important clinical scenarios where VETs may have the potential to address gaps in current treatment strategies, but available evidence is inadequate to guide recommendations (Table 6). Post-partum hemorrhage is the most frequent cause of perinatal maternal morbidity and mortality, but predicting which patients will develop bleeding requiring aggressive resuscitation is difficult(56). Only one study evaluated VETs in the prediction of excess bleeding in PPH and found FIBTEM A5 to be equivalent to fibrinogen for predicting major bleeding(28). Results from two ongoing randomized clinical trials evaluating VET vs CCT-based transfusion strategies in women with PPH will hopefully provide additional high-quality evidence on this topic (Table 5). There was also very limited data on the prediction of post-operative bleeding/thrombosis in liver transplant patients and the prediction of hemorrhage progression/mortality in severe TBI. Current familiarity with VET use in the OR in these settings makes this area ripe for further study. Finally, VETs may prove useful in managing uncommon, but clinically challenging scenarios, such as predicting flap thrombosis in microvascular reconstructive surgery and identifying ECMO patients that will suffer bleeding complications, but current evidence is prohibitively scarce to recommend their use.
Table 6 –
Clinical Scenarios with Inadequate Evidence to Recommend Clinical Use of Viscoelastic Tests in the Surgical Intensive Care Unit
| Scenario |
|---|
| Prediction of post-operative bleeding in complex/emergent cardiac surgery |
| Prediction of bleeding in patients on extracorporeal membrane oxygenation |
| Prediction of bleeding in women with post-partum hemorrhage |
| Prediction of post-operative bleeding in liver transplantation |
| Prediction of worsening intracranial bleeding in patients with traumatic brain injury |
| Prediction of free flap thrombosis after microvascular reconstructive surgery |
| Prediction of post-operative thrombotic complications in liver transplantation |
| Prediction of mortality in patients with traumatic brain injury at intensive care admission |
Our review methodology has certain limitations, including the inability to present quantitative summaries of results given the lack of consistent data reporting in the included studies and the use of unstructured analysis of study quality, compared with formal validated assessment tools(57). On the other hand, our search strategy was quite broad and allowed for identification of a wide spectrum of topics relevant to the surgical intensivist, while critically evaluating the methodology of eligible studies and not incorporating results from studies with clear bias or inappropriate design. We were also unable to address every possible clinical scenario relevant to the surgical intensivist, but rather chose to include those of maximum applicability with available robust evidence.
In conclusion, while VETs have the potential to impact the care of critically ill surgical patients in many ways, current evidence for their use is limited. Further scientifically sound research is urgently needed to elucidate the role of TEG and ROTEM in the SICU population with particular attention to the anticipation and management of severe hemorrhage and the prediction and treatment of pathologic thromboembolism.
ACKNOWLEDGEMENTS
We would like to thank Emilie Ludeman, MSLIS, research librarian at the Health Sciences and Human Services Library of the University of Maryland School of Medicine for assistance with the electronic database searches.
Footnotes
This manuscript was not presented at any regional or national meeting. The authors are disclosing no conflict of interests. No extramural funding was involved in the creation of this work.
References
- 1.Ganter MT, Hofer CK. Coagulation monitoring: current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth Analg. 2008;106(5):1366–75. [DOI] [PubMed] [Google Scholar]
- 2.Hartert H Blutgerinnungsstudien mit der Thrombelastographie; einem neuen Untersuchungs verfahren. Klin Wochenschr. 1948;26(37–38):577–83. [DOI] [PubMed] [Google Scholar]
- 3.Haas T, Gorlinger K, Grassetto A, Agostini V, Simioni P, Nardi G, Ranucci M. Thromboelastometry for guiding bleeding management of the critically ill patient: a systematic review of the literature. Minerva Anestesiol. 2014;80(12):1320–35. [PubMed] [Google Scholar]
- 4.Wikkelso A, Wetterslev J, Moller AM, Afshari A . Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016(8):CD007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, Misso K, Ross J, Severens J, Kleijnen J. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2015;19(58):1–228, v-vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veigas PV, Callum J, Rizoli S, Nascimento B, da Luz LT. A systematic review on the rotational thrombelastometry (ROTEM(R)) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med. 2016;24(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. 2016;263(6):1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashuk JL, Moore EE, Wohlauer M, Johnson JL, Pezold M, Lawrence J, Biffl WL, Burlew CC, Barnett C, Sawyer M, et al. Initial experiences with point-of-care rapid thrombelastography for management of life-threatening postinjury coagulopathy. Transfusion. 2012;52(1):23–33. [DOI] [PubMed] [Google Scholar]
- 10.Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ Jr., Mattox KL, Suliburk J. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378–85; discussion 85–6. [DOI] [PubMed] [Google Scholar]
- 11.Ghavidel AA, Toutounchi Z, Shahandashti FJ, Mirmesdagh Y. Rotational thromboelastometry in prediction of bleeding after cardiac surgery. Asian Cardiovasc Thorac Ann. 2015;23(5):525–9. [DOI] [PubMed] [Google Scholar]
- 12.Gozdzik W, Adamik B, Wysoczanski G, Gozdzik A, Rachwalik M, Skalec T, Kubler A. Preoperative thromboelastometry for the prediction of increased chest tube output in cardiac surgery: A retrospective study. Medicine (Baltimore). 2017;96(30):e7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostrowsky J, Foes J, Warchol M, Tsarovsky G, Blay J. Plateletworks platelet function test compared to the thromboelastograph for prediction of postoperative outcomes. J Extra Corpor Technol. 2004;36(2):149–52. [PubMed] [Google Scholar]
- 14.Rafiq S, Johansson PI, Kofoed KF, Olsen PS, Steinbruchel DA. Preoperative hemostatic testing and the risk of postoperative bleeding in coronary artery bypass surgery patients. J Card Surg. 2016;31(9):565–71. [DOI] [PubMed] [Google Scholar]
- 15.Ti LK, Cheong K-F, Chen F-G. Prediction of excessive bleeding after coronary artery bypass graft surgery: The influence of timing and heparinase on thromboelastography. J Cardiothorac Vasc Anesth. 2002;16(5):545–50. [DOI] [PubMed] [Google Scholar]
- 16.Welsby IJ, Jiao K, Ortel TL, Brudney CS, Roche AM, Bennett-Guerrero E, Gan TJ. The kaolin-activated Thrombelastograph predicts bleeding after cardiac surgery. J Cardiothorac Vasc Anesth. 2006;20(4):531–5. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Kumar S, Tewari P, Pande S, Murari M. Utility of thromboelastography versus routine coagulation tests for assessment of hypocoagulable state in patients undergoing cardiac bypass surgery. Ann Card Anaesth. 2018;21(2):151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee GC, Kicza AM, Liu KY, Nyman CB, Kaufman RM, Body SC. Does rotational thromboelastometry (ROTEM) improve prediction of bleeding after cardiac surgery? Anesth Analg. 2012;115(3):499–506. [DOI] [PubMed] [Google Scholar]
- 19.Tarzia V, Bortolussi G, Buratto E, Paolini C, Dal Lin C, Rizzoli G, Bottio T, Gerosa G. Single vs double antiplatelet therapy in acute coronary syndrome: Predictors of bleeding after coronary artery bypass grafting. World J Cardiol. 2015;7(9):571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azarfarin R, Noohi F, Kiavar M, Totonchi Z, Heidarpour A, Hendiani A, Koleini ZS, Rahimi S. Relationship between maximum clot firmness in ROTEM((R)) and postoperative bleeding after coronary artery bypass graft surgery in patients using clopidogrel. Ann Card Anaesth. 2018;21(2):175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma AD, Al-Achi A, Seccombe JF, Hummel R, Preston M, Behrend D. Does incorporation of thromboelastography improve bleeding prediction following adult cardiac surgery? Blood Coagul Fibrinolysis. 2014;25(6):561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh KJ, Padilla A, Dasgupta A, Nguyen AN, Wahed A. Thromboelastography is a suboptimal test for determination of the underlying cause of bleeding associated with cardiopulmonary bypass and may not predict a hypercoagulable state. Am J Clin Pathol. 2014;142(4):492–7. [DOI] [PubMed] [Google Scholar]
- 23.Wasowicz M, McCluskey SA, Wijeysundera DN, Yau TM, Meinri M, Beattie WS, Karkouti K. The incremental value of thrombelastography for prediction of excessive blood loss after cardiac surgery: an observational study. Anesth Analg. 2010;111(2):331–8. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal S, Johnson RI, Kirmani BH. Pre- and Post-Bypass Platelet Function Testing With Multiple Electrode Aggregometry and TEG Platelet Mapping in Cardiac Surgery. J Cardiothorac Vasc Anesth. 2015;29(5):1272–6. [DOI] [PubMed] [Google Scholar]
- 25.Nair P, Hoechter DJ, Buscher H, Venkatesh K, Whittam S, Joseph J, Jansz P. Prospective observational study of hemostatic alterations during adult extracorporeal membrane oxygenation (ECMO) using point-of-care thromboelastometry and platelet aggregometry. J Cardiothorac Vasc Anesth. 2015;29(2):288–96. [DOI] [PubMed] [Google Scholar]
- 26.Panigada M, Iapichino G, L’Acqua C, Protti A, Cressoni M, Consonni D, Mietto C, Gattinoni L. Prevalence of “Flat-Line” Thromboelastography During Extracorporeal Membrane Oxygenation for Respiratory Failure in Adults. Asaio J. 2016;62(3):302–9. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson O, Jeppsson A, Hellgren M. Major obstetric haemorrhage: monitoring with thromboelastography, laboratory analyses or both? Int J Obstet Anesth. 2014;23(1):10–7. [DOI] [PubMed] [Google Scholar]
- 28.Collins PW, Lilley G, Bruynseels D, Laurent DB, Cannings-John R, Precious E, Hamlyn V, Sanders J, Alikhan R, Rayment R, et al. Fibrin-based clot formation as an early and rapid biomarker for progression of postpartum hemorrhage: a prospective study. Blood. 2014;124(11):1727–36. [DOI] [PubMed] [Google Scholar]
- 29.Dotsch TM, Dirkmann D, Bezinover D, Hartmann M, Treckmann JW, Paul A, Saner FH. Assessment of standard laboratory tests and rotational thromboelastometry for the prediction of postoperative bleeding in liver transplantation. Br J Anaesth. 2017;119(3):402–10. [DOI] [PubMed] [Google Scholar]
- 30.Folkerson LE, Sloan D, Cotton BA, Holcomb JB, Tomasek JS, Wade CE. Predicting progressive hemorrhagic injury from isolated traumatic brain injury and coagulation. Surgery. 2015;158(3):655–61. [DOI] [PubMed] [Google Scholar]
- 31.Kolbenschlag J, Daigeler A, Lauer S, Wittenberg G, Fischer S, Kapalschinski N, Lehnhardt M, Goertz O. Can rotational thromboelastometry predict thrombotic complications in reconstructive microsurgery? Microsurgery. 2014;34(4):253–60. [DOI] [PubMed] [Google Scholar]
- 32.Zahr Eldeen F, Roll GR, Derosas C, Rao R, Khan MS, Gunson BK, Hodson J, Mergental H, Ferraz-Neto BH, Isaac J, et al. Preoperative Thromboelastography as a Sensitive Tool Predicting Those at Risk of Developing Early Hepatic Artery Thrombosis After Adult Liver Transplantation. Transplantation. 2016;100(11):2382–90. [DOI] [PubMed] [Google Scholar]
- 33.Sun JB, Bian MH, Zhong T, Lu YY, Zhu BQ, Wen HQ, Hu HL. Reference values for kaolin-activated thromboelastography in volunteers of Anhui Province in China. J Clin Lab Anal. 2017;31(6):e22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scarpelini S, Rhind SG, Nascimento B, Tien H, Shek PN, Peng HT, Huang H, Pinto R, Speers V, Reis M, et al. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res. 2009;42(12):1210–7. [DOI] [PubMed] [Google Scholar]
- 35.Brill JB, Badiee J, Zander AL, Wallace JD, Lewis PR, Sise MJ, Bansal V, Shackford SR. The rate of deep vein thrombosis doubles in trauma patients with hypercoagulable thromboelastography. J Trauma Acute Care Surg. 2017;83(3):413–9. [DOI] [PubMed] [Google Scholar]
- 36.Cotton BA, Minei KM, Radwan ZA, Matijevic N, Pivalizza E, Podbielski J, Wade CE, Kozar RA, Holcomb JB. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg. 2012;72(6):1470–5; discussion 5–7. [DOI] [PubMed] [Google Scholar]
- 37.McCully BH, Connelly CR, Fair KA, Holcomb JB, Fox EE, Wade CE, Bulger EM, Schreiber MA. Onset of Coagulation Function Recovery Is Delayed in Severely Injured Trauma Patients with Venous Thromboembolism. J Am Coll Surg. 2017;225(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hincker A, Feit J, Sladen RN, Wagener G. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit Care. 2014;18(5):549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van PY, Cho SD, Underwood SJ, Morris MS, Watters JM, Schreiber MA. Thrombelastography versus AntiFactor Xa levels in the assessment of prophylactic-dose enoxaparin in critically ill patients. J Trauma. 2009;66(6):1509–15; discussion 15–7. [DOI] [PubMed] [Google Scholar]
- 40.Connelly CR, Van PY, Hart KD, Louis SG, Fair KA, Erickson AS, Rick EA, Simeon EC, Bulger EM, Arbabi S, et al. Thrombelastography-Based Dosing of Enoxaparin for Thromboprophylaxis in Trauma and Surgical Patients: A Randomized Clinical Trial. JAMA Surg. 2016;151(10):e162069. [DOI] [PubMed] [Google Scholar]
- 41.Harr JN, Moore EE, Chin TL, Ghasabyan A, Gonzalez E, Wohlauer MV, Banerjee A, Silliman CC, Sauaia A. Platelets are dominant contributors to hypercoagulability after injury. J Trauma Acute Care Surg. 2013;74(3):756–62; discussion 62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ostrowski SR, Windelov NA, Ibsen M, Haase N, Perner A, Johansson PI. Consecutive thrombelastography clot strength profiles in patients with severe sepsis and their association with 28-day mortality: a prospective study. J Crit Care. 2013;28(3):317 e1–11. [DOI] [PubMed] [Google Scholar]
- 43.Haase N, Ostrowski SR, Wetterslev J, Lange T, Moller MH, Tousi H, Steensen M, Pott F, Soe-Jensen P, Nielsen J, et al. Thromboelastography in patients with severe sepsis: a prospective cohort study. Intensive Care Med. 2015;41(1):77–85. [DOI] [PubMed] [Google Scholar]
- 44.Massion PB, Peters P, Ledoux D, Zimermann V, Canivet JL, Massion PP, Damas P, Gothot A. Persistent hypocoagulability in patients with septic shock predicts greater hospital mortality: impact of impaired thrombin generation. Intensive Care Med. 2012;38(8):1326–35. [DOI] [PubMed] [Google Scholar]
- 45.Daley MJ, Enright Z, Nguyen J, Ali S, Clark A, Aydelotte JD, Teixeira PG, Coopwood TB, Brown CV. Adenosine diphosphate platelet dysfunction on thromboelastogram is independently associated with increased morality in traumatic brain injury. Eur J Trauma Emerg Surg. 2017;43(1):105–11. [DOI] [PubMed] [Google Scholar]
- 46.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18(6):805–35. [DOI] [PubMed] [Google Scholar]
- 47.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, Lijmer JG, Moher D, Rennie D, de Vet HC, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Bmj. 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55–63. [DOI] [PubMed] [Google Scholar]
- 49.Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, Dubose JJ, Fox EE, Inaba K, Rodriguez CJ, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82(3):605–17. [DOI] [PubMed] [Google Scholar]
- 50.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. Jama. 2015;313(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore EE, Moore HB, Chapman MP, Gonzalez E, Sauaia A. Goal-directed hemostatic resuscitation for trauma induced coagulopathy: Maintaining homeostasis. J Trauma Acute Care Surg. 2018;84(6S Suppl 1):S35–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levy JH, Tanaka KA, Steiner ME. Evaluation and management of bleeding during cardiac surgery. Curr Hematol Rep. 2005;4(5):368–72. [PubMed] [Google Scholar]
- 53.Velmahos GC, Nigro J, Tatevossian R, Murray JA, Cornwell EE 3rd, Belzberg H, Asensio JA, Berne TV, Demetriades D. Inability of an aggressive policy of thromboprophylaxis to prevent deep venous thrombosis (DVT) in critically injured patients: are current methods of DVT prophylaxis insufficient? J Am Coll Surg. 1998;187(5):529–33. [DOI] [PubMed] [Google Scholar]
- 54.Malinoski D, Jafari F, Ewing T, Ardary C, Conniff H, Baje M, Kong A, Lekawa ME, Dolich MO, Cinat ME, et al. Standard prophylactic enoxaparin dosing leads to inadequate anti-Xa levels and increased deep venous thrombosis rates in critically ill trauma and surgical patients. J Trauma. 2010;68(4):874–80. [DOI] [PubMed] [Google Scholar]
- 55.Dai Y, Lee A, Critchley LA, White PF. Does thromboelastography predict postoperative thromboembolic events? A systematic review of the literature. Anesth Analg. 2009;108(3):734–42. [DOI] [PubMed] [Google Scholar]
- 56.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–73. [DOI] [PubMed] [Google Scholar]
- 57.Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–7. [DOI] [PubMed] [Google Scholar]