Abstract
Low T-cell counts and acute rejection are associated with increased cardiovascular events (CVE), and T cell depleting agents decrease both T cell counts and acute rejection rates. Thus, we aimed to characterize risk of CVE by induction agent used in kidney transplant recipients. We conducted a secondary data analysis of those with Medicare as their primary insurance from 1999–2010. Outcomes of interest were incident CVE, all-cause mortality, CVE-related mortality, and a composite outcome of mortality and CVE. Of 47,258 recipients, 29.3% received IL-2 receptor antagonist (IL-2RA), 33.3% received anti-thymocyte globulin (ATG), 7.3% received alemtuzumab, and 30.0% received no induction. Compared with IL-2RA, there was no difference in the risk of CVE in the ATG [aHR=0.98, 95% CI: 0.92–1.05] and alemtuzumab group [aHR=1.01, 95% CI: 0.89–1.16], but slightly higher in the no induction group [aHR=1.06, 95% CI: 1.00–1.14]. Acute rejection did not modify this association in the latter group but did increase CVE by 46% in the alemtuzumab group. There was no difference in the hazard of all-cause or CVE-related mortality. Only in the ATG group, 7% lower hazard of the composite outcome of mortality and CVE was noted. Induction agents are not associated with incident CVE; although prospective trials are needed to determine a personalized approach to prevention.
Introduction
Cardiovascular events (CVE) are among the leading causes of mortality in kidney transplant (KT) recipients.1–9 The annual rate of fatal or nonfatal CVE is 3.5–5.0%, and this rate is 50-fold higher than that in the general population.7 Despite this high disease burden, little is known regarding prevention of CVE in KT recipients. Clinical recommendations are often extrapolated from studies conducted in patients with chronic kidney disease or from the general population. However, there is growing evidence that conventional cardio-protective therapies might be ineffective in KT recipients.10–12 Hence, there is a need to better understand the risk factors associated with CVE in this specific population and to develop therapeutics tailored to prevent and treat these events.
Induction immunosuppressive agents are commonly used during KT. Induction agents can be lymphocyte depleting, such as anti-thymocyte globulin (ATG) and alemtuzumab (AZM), or can prevent lymphocyte activation and replication, such as IL-2 receptor antagonist (IL-2RA). T cell depleting agents are associated with lower incidence and severity of acute rejection.13–19. Acute rejection post-KT is a risk factor for CVE.20–22 Thus, intuitively one would assume that use of T cell depleting agents as an induction therapy would be associated with lower rates of CVE. On the other hand, B and T-cells and their subsets are important determinants of cardiovascular health.23–28 T lymphocytes play critical roles in the development of angiotensin II, deoxycorticosterone salt-sensitive and Dahl salt-sensitive hypertension, and in the progression of vascular remodeling and atherosclerosis.26,27 In patients with HIV, low CD4 counts are reported to be an independent risk factor for incident CVE.25 Thus, it would seem depleting T cells would be a risk factor for CVE. Indeed, a single center study and an older registry analysis in KT recipients reported that use of ATG, when compared with no induction or IL-2RA, is a risk factor for CVE and mortality.27,29,30 The authors suggested that the evaluation of cardiovascular risk should contribute to the decision on which induction agent to use.27
Given this conflicting evidence and the high burden of CVE among KT recipients, we sought to address this knowledge gap using a national registry and Medicare claims data. The objectives of this study were to characterize the risk of CVE and mortality in the KT recipient population according to induction agent used during transplantation, and whether induction agents are indeed a modifiable risk factor for CVE in KT recipients.
Methods
Study population
Using the United States Renal Data System, we studied first-time adult KT recipients between January 1, 1999 and December 31, 2010 who had no previous CVE events and used Medicare as their primary insurance. This was a secondary data analysis of a national, mandated prospective registry of KT recipients. Among 162,998 KT recipients in the study period, we only included 86,649 who had Medicare as primary insurance for at least 365 days prior to KT and had no CVE-related claim in this time period. We excluded KT recipients aged <18 years (n=3914), those without complete immunosuppression records (n=1,374), those who received more than one type of induction agent prior to discharge (n=3,862), those with previous CVE events as captured from Medicare claims during the 365 days prior to KT (n=29,351), and those with any previous solid organ transplant (n=890). Our final study population included 47,258 KT recipients. Recipients were followed until one of the following events, whichever occurred first: death, CVE, end of Medicare Primary coverage, or end of follow-up (12/31/2011).
Cardiovascular events
The main outcome of interest was incident CVE and based on outcomes reported in previous literature was defined as one of the following:27,31–41 myocardial infarction, cardiac catheterization, coronary artery bypass grafting, congestive heart failure, atrial fibrillation, stroke, transient ischemic attack, venous thromboembolism, amputation, and peripheral arterial disease and revascularization (angioplasty, atherectomy, endarterectomy, or arterial bypass). These events were ascertained through Medicare inpatient claims as recorded in the United States Renal Data System using a list of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and the Current Procedural Terminology (CPT) codes, and as performed in prior studies of CVE epidemiology in this population.42–46 We employed externally validated ICD-9 and CPT codes that have been reported to be a sensitive measure of CVE after KT.42 We also studied the following outcomes: (1) a composite outcome of CVE and all-cause mortality, (2) all-cause mortality, and (3) mortality due to CVE. Date and cause of death were collected from multiple sources, including Center for Medicare and Medicaid Service Death Notification, Organ Procurement and Transplant Network follow-up form, and Social Security Death Master File.
Statistical analysis
Cumulative incidence of CVE was estimated using the Kaplan-Meier method. The hazards of CVE were compared by induction agents using a Cox proportional hazard model. To account for the variations between transplant centers, we used the shared frailty approach, which is analogous to including random-intercept terms in mixed-effects models.47 We adjusted for recipient factors: age, race, gender, panel reaction antibody, body mass index, cause of end stage renal disease, time on dialysis, hepatitis C virus status, education level, panel reactive antibody level, maintenance regimen at discharge; donor factors: age, race, gender, living vs. deceased, donation after cardiac death, expanded criteria donor, creatinine, hepatitis C virus status; and transplant factors: calendar year of transplant, cold ischemic time, HLA mismatch, regionally shared organ, ABO compatibility. IL-2RA was used as the reference group as the 2009 Kidney Disease: Improving Global Outcomes clinical practice guidelines recommends that it be the first-line induction agent.48 However, we did perform a pairwise comparison between each induction agent to ensure our results were not drastically different. The hazards of the mortality outcomes were compared using shared-frailty Cox models, adjusting for the same set of variables used in the CVE model. Missing values were handled by missingness indicators. All analyses were performed using Stata 14.0/MP for Linux (College Station, Texas).
Effect heterogeneity and sensitivity analysis
We conducted interaction term analyses to examine whether the association of induction agent and CVE was modified by the following variables: recipient gender, race, age (<65 years versus ≥ 65), donor type (living versus deceased donor), use of steroid maintenance at discharge, and acute rejection. In other words, we examined if the association between induction agent and CVE was heterogeneous in any subset of the population. Younger (age<65) KT recipients lose Medicare primary coverage at 3 years after KT. To examine if our estimates were biased by the difference in the length of follow up between age groups, we conducted a sensitivity analysis for primary outcome in which all recipients were censored at 3 years after KT.
Results
Study population
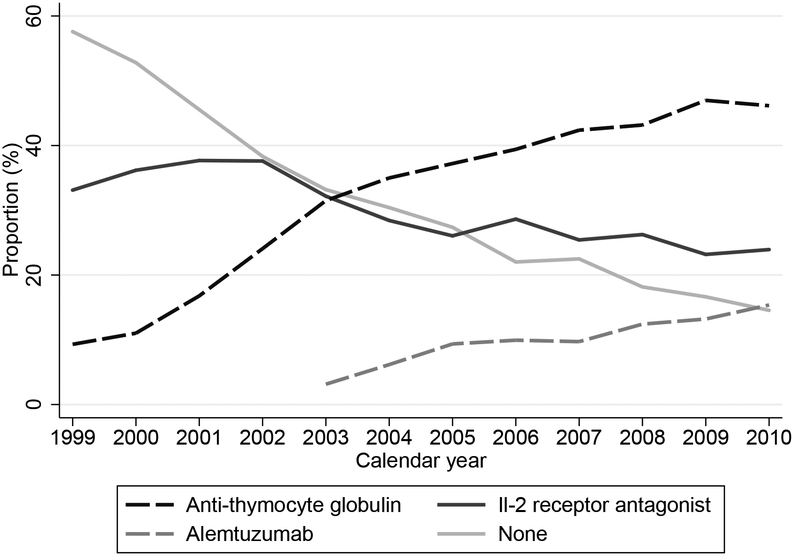
Among 47,258 KT recipients included in our study, 29.3% received IL-2RA, 33.3% received ATG, 7.3% received AZM, and 30.0% received no induction. Use of ATG and AZM has consistently increased, while use of IL-2 RA and no induction has steadily declined in the last decade (Figure 1). Recipient age, gender, race, body mass index, cause of end stage renal disease, and cold ischemia time were similar in all four groups. Donor age, gender, race and creatinine were also similar. However, recipients with traditional risk factors for rejection were more likely to receive lymphocyte-depleting agents. For instance, the ATG and AZM groups had a higher percentage of recipients who received kidneys from expanded criteria donors, higher peak panel reactive antibody level, longer time on dialysis, and fewer zero HLA mismatches. In terms of maintenance immunosuppression, tacrolimus was used more frequently in the ATG and AZM groups (82.2% and 92.0%) compared to the IL-2RA and no induction groups (64.3% and 64.6%). Only 22.7% of those who received AZM induction were on steroid maintenance when compared with 68.5% who received ATG, 89.6% who received IL-2RA, and 87.8% who received no induction. The median follow-up for the composite outcome analysis was 3.05 years. (Table 1)
Figure 1:
Temporal pattern and use of induction agents in first time kidney transplantation recipients from 1999 −2010 with Medicare as their primary insurance
Table 1:
Baseline Characteristics
| IL-2RA (n=13,864) | ATG (n=15,756) | AZM (n=3,438) | No induction (n=14,200) | |
|---|---|---|---|---|
| Recipient factors | ||||
| Age (Years) | 49.0 (38.0, 58.0) | 49.0 (39.0, 58.0) | 50.0 (40.0, 59.0) | 48.0 (37.0, 57.0) |
| Female sex | 37.4% | 43.2% | 40.1% | 38.6% |
| BMI (kg/m2) | 26.3 (23.1, 30.2) | 27.0 (23.6, 31.1) | 27.4 (23.8, 31.6) | 26.2 (23.0, 30.2) |
| Race/Ethnicity | ||||
| White | 47.1% | 43.7% | 48.7% | 47.3% |
| African American | 24.5% | 32.8% | 31.0% | 28.0% |
| Hispanic/Latino | 18.5% | 15.2% | 14.6% | 17.0% |
| Other/multi-racial | 9.9% | 8.3% | 5.7% | 7.7% |
| Attended college | 37.4% | 37.4% | 43.0% | 34.4% |
| Preemptive transplant | 14.3% | 13.8% | 19.7% | 17.7% |
| Years on dialysis | 2.5 (1.0, 4.6) | 3.0 (1.2, 5.0) | 2.6 (0.5, 4.9) | 2.3 (0.5, 4.3) |
| Cause of ESRD | ||||
| GN | 26.3% | 24.5% | 22.8% | 24.6% |
| DM | 20.9% | 21.5% | 23.3% | 19.3% |
| HTN | 18.8% | 22.1% | 23.5% | 21.0% |
| Others | 34.0% | 31.8% | 30.4% | 35.1% |
| HCV(+) | 4.8% | 5.0% | 3.3% | 5.3% |
| Peak PRA | 0.0 (0.0, 8.0) | 2.0 (0.0, 22.0) | 3.0 (0.0, 19.0) | 0.0 (0.0, 10.0) |
| Zero HLA mismatch | 11.6% | 8.3% | 8.3% | 12.6% |
| ABO incompatible | 0.3% | 0.6% | 0.6% | 0.4% |
| Cold ischemia time (Hr) | 13.0 (3.0, 20.9) | 14.0 (6.0, 21.0) | 15.0 (2.0, 22.1) | 14.0 (2.6, 21.0) |
| Maintenance Immunosuppression | ||||
| Steroid maintenance | 89.6% | 68.5% | 22.7% | 87.8% |
| Cyclosporine | 29.7% | 12.9% | 3.3% | 30.1% |
| Tacrolimus | 64.3% | 82.2% | 92.0% | 64.6% |
| MMF | 88.5% | 88.9% | 83.4% | 80.8% |
| mTOR | 9.1% | 8.7% | 1.5% | 9.5% |
| Azathioprine | 1.8% | 1.6% | 0.2% | 2.2% |
| Donor factors | ||||
| Age (Years) | 39.0 (26.0, 50.0) | 41.0 (26.0, 51.0) | 42.0 (29.0, 52.0) | 39.0 (26.0, 49.0) |
| Female sex | 46.7% | 46.0% | 47.8% | 46.0% |
| Race/Ethnicity | ||||
| White | 65.5% | 67.8% | 68.3% | 66.6% |
| African American | 12.3% | 14.6% | 15.5% | 13.5% |
| Hispanic/Latino | 17.1% | 13.7% | 13.0% | 15.9% |
| Other/multi-racial | 5.2% | 3.9% | 3.2% | 4.0% |
| Deceased donor | 64.6% | 72.8% | 63.2% | 63.9% |
| Among deceased donors: | ||||
| Serum Creatinine (mg/dL) | 0.9 (0.7, 1.2) | 1.0 (0.7, 1.3) | 1.0 (0.7, 1.3) | 1.0 (0.7, 1.2) |
| DCD | 3.8% | 7.8% | 9.0% | 3.5% |
| ECD | 9.9% | 13.2% | 14.6% | 9.9% |
AZM: alemtuzumab, ATG: anti-thymocyte globulin, DM: Diabetes mellitus, DCD: Donation after cardiac death, ECD: expanded criteria donor, ESRD: end stage renal disease, GN: glomerulonephritis, HCV: hepatitis C virus, HTN: Hypertension, IL-2RA: IL-2 receptor antagonist, mTOR: mammalian target of rapamycin, MMF: mycophenolate mofetil, PRA: panel reactive assay
Incidence rates
Overall, there were 7,659 incident CVE, and 9,148 deaths, of which 1,346 were attributed to cardiovascular causes. The crude incidence rate for CVE was 41 per 1,000 person-years, for all-cause mortality was 34 per 1,000 person-years, and for CVE-related mortality was 5 per 1,000 person-years. Congestive heart failure, deep venous thrombosis, and atrial fibrillation were the three most common CVEs (congestive heart failure: 4181, venous thromboembolism: 2252, atrial fibrillation: 1758, myocardial infarction: 1377, cardiac catheterization: 1261, stroke: 1208, transient ischemic attack: 623, peripheral vascular disease: 512, amputation: 502, coronary artery bypass grafting: 270 incident events). When categorized by induction therapy, those who received AZM had the lowest rate of CVE (36 per 1,000 person-years), all-cause mortality (28 per 1,000 person-years) and CVE-related mortality (4 per 1,000 person-years). In those who received IL-2RA, ATG and no induction, the incidence rates for CVE were 42, 43 and 39 per 1,000 person-years, all-cause mortality was 36, 31 and 35 per 1,000 person-years, and CVE-related mortality was 5, 4 and 5 per 1,000 person-years, respectively. When analyzing each induction agent, congestive heart failure, deep venous thrombosis, and atrial fibrillation were the most common CVEs in all four groups. However, the crude incidence ratio of myocardial infarction, cardiac catheterization and coronary artery bypass grafting was lower in the AZM group compared with other groups. (Table 2)
Table 2:
Crude incidence rates of cardiovascular events by induction agent
| IL-2RA | ATG | AZM | None | Overall | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of events | Person-years^ | IR | Number of events | Person-years^ | IR | Number of events | Person-years^ | IR | Number of events | Person-years^ | IR | Number of events | Person-years^ | IR | |
| MI | 440 | 62.8 | 7.01 | 385 | 57.7 | 6.67 | 60 | 10.5 | 5.70 | 492 | 72.4 | 6.79 | 1377 | 203.4 | 6.77 |
| Cath | 411 | 62.6 | 6.57 | 351 | 57.5 | 6.11 | 42 | 10.5 | 3.99 | 457 | 72.2 | 6.33 | 1261 | 202.8 | 6.22 |
| CABG | 98 | 63.6 | 1.54 | 67 | 58.3 | 1.15 | 5 | 10.6 | 0.47 | 100 | 73.3 | 1.36 | 270 | 205.8 | 1.31 |
| CHF | 1364 | 60.5 | 22.53 | 1187 | 55.9 | 21.23 | 188 | 10.3 | 18.18 | 1442 | 70.1 | 20.58 | 4181 | 196.8 | 21.24 |
| AFib | 583 | 62.6 | 9.31 | 475 | 57.5 | 8.25 | 81 | 10.5 | 7.70 | 619 | 72.2 | 8.57 | 1758 | 202.9 | 8.66 |
| TIA | 197 | 63.3 | 3.11 | 189 | 58.0 | 3.26 | 28 | 10.6 | 2.65 | 209 | 73.1 | 2.86 | 623 | 205.0 | 3.04 |
| Stroke | 367 | 63.2 | 5.81 | 365 | 57.8 | 6.32 | 58 | 10.5 | 5.51 | 418 | 72.8 | 5.74 | 1208 | 204.3 | 5.91 |
| VTE | 717 | 62.0 | 11.56 | 687 | 56.9 | 12.08 | 111 | 10.4 | 10.62 | 737 | 71.6 | 10.29 | 2252 | 201.0 | 11.20 |
| Amputation | 173 | 63.4 | 2.73 | 119 | 58.2 | 2.05 | 11 | 10.6 | 1.04 | 199 | 73.1 | 2.72 | 502 | 205.2 | 2.45 |
| PVD | 172 | 63.3 | 2.72 | 133 | 58.1 | 2.29 | 22 | 10.6 | 2.08 | 185 | 73.0 | 2.53 | 512 | 204.9 | 2.50 |
| Any CVE | 2426 | 57.2 | 42.42 | 2287 | 53.0 | 43.14 | 359 | 10.0 | 35.89 | 2587 | 66.1 | 39.11 | 7659 | 186.3 | 41.10 |
| All-cause mortality | 3034 | 84.3 | 35.97 | 2325 | 74.5 | 31.19 | 351 | 12.6 | 27.73 | 3438 | 97.3 | 35.34 | 9148 | 268.8 | 34.03 |
| CVE-related mortality | 447 | 84.3 | 5.30 | 333 | 74.5 | 4.47 | 50 | 12.6 | 3.95 | 516 | 97.3 | 5.30 | 1346 | 268.8 | 5.01 |
Unit for IR: (Cases/Person-years)*1,000
expressed in 1,000 person-years
AZM: alemtuzumab, ATG: anti-thymocyte globulin, AFib: atrial fibrillation, Cath: cardiac catheterization, CVE: cardiovascular event, CHF: congestive heart failure, CABG: coronary artery bypass graft, VTE: venous thromboembolism, IR: incidence rate, IL-2RA: IL-2 receptor antagonist, MI: myocardial ischemia, PVD: peripheral vascular disease, TIA: transient ischemic attack
Cardiovascular events
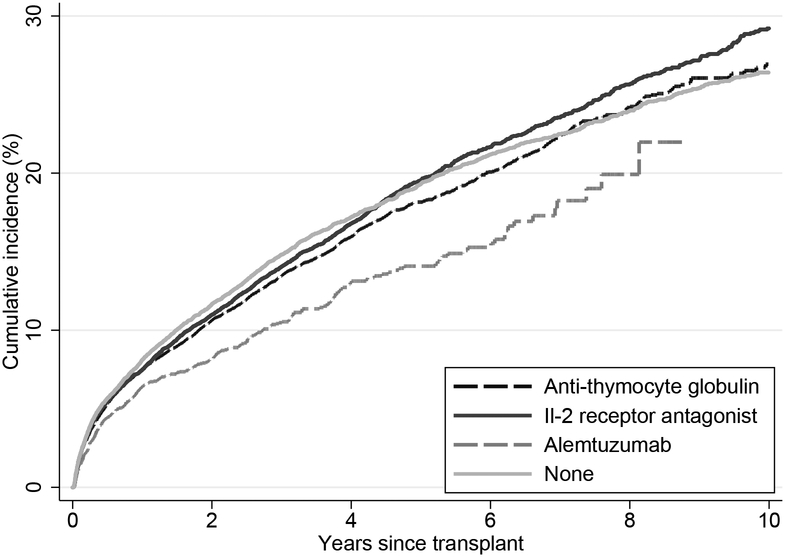
The incidence of CVE was similar across the induction groups: 10-year Kaplan-Meier cumulative incidences were 29.2%, 26.9%, 14.1%, 26.4% in the IL-2RA, ATG, AZM and no induction groups, respectively (Figure 2). The incidence of CVE for the median time observed in our cohort, i.e. 3-year, was also similar; Kaplan-Meier cumulative incidences were 14.0%, 13.5%, 10.5%, 14.8% in the IL-2RA, ATG, AZM and no induction groups respectively. Compared to those who received IL-2RA, there was no difference in the adjusted hazard ratio (aHR) of CVE in the ATG [aHR=0.98, 95% confidence interval (CI): 0.92–1.05, p=0.5] and AZM group [aHR=1.01, 95% CI: 0.89–1.16, p=0.8]. The risk of CVE was higher in the no induction groups, although this did not reach statistical significance [aHR=1.06, 95% CI: 1.00–1.14, p=0.054]. (Table 3) In our pairwise comparisons, the aHR ratios for CVE are as follows: ATG vs. no induction aHR = 0.92 (0.86 – 0.98), AZM vs no induction aHR = 0.95 (0.83 – 1.09), and AZM vs ATG aHR = 1.04 (0.91 – 1.18).
Figure 2:
Kaplan-Meier cumulative incidence of cardiovascular events by induction agent following kidney transplantation.
Table 3:
Adjusted hazard of cardiovascular events and mortality by induction agent
| IL-2RA | ATG | AZM | None | |
|---|---|---|---|---|
| CVE | Ref | 0.98 (0.92–1.05) | 1.01 (0.89–1.16) | 1.06 (1.00–1.14) |
| Composite of mortality and CVE | Ref | 0.93 (0.88–0.99) | 0.91 (0.81–1.01) | 1.02 (0.97–1.08) |
| All-cause mortality | Ref | 0.94 (0.88–1.00) | 0.96 (0.85–1.10) | 1.01 (0.95–1.06) |
| CVE-related mortality | Ref | 0.95 (0.81–1.12) | 0.99 (0.70–1.38) | 1.03 (0.89–1.18) |
AZM: alemtuzumab, ATG: anti-thymocyte globulin, CVE: cardiovascular events, IL-2RA: IL-2 receptor antagonist
Mortality outcomes
Compared to those who received IL-2RA, no difference in the hazard of all-cause or CVE-related mortality was noted in the ATG, AZM or no-induction groups. However, the hazard of the composite outcomes of mortality and CVE was lower among those who received ATG [aHR=0.93, 95% CI: 0.88–0.99, p=0.02]. There was no significant difference in the hazard of the composite outcome in the AZM group [aHR=0.91, 95% CI: 0.81–1.01, p=0.1] or no induction group [aHR=1.02, 95% CI: 0.97–1.08, p=0.5]. (Table 3)
Effect heterogeneity
The association between ATG and CVE was modified by gender and recipient diabetes status. ATG was associated with a lower hazard of CVE among males [aHR=0.92; 95% CI: 0.85–1.00] but not among females [aHR=1.08, 95% CI: 0.98–1.19, p for interaction=0.01]. Similarly, ATG was associated with a lower hazard of CVE among recipients without diabetes [aHR=0.89, 95% CI: 0.81–0.98] but not among those with diabetes [aHR=1.04, 95% CI: 0.96–1.13, p-for interaction=0.01]. This effect modification was not seen in the AZM or no induction groups. Recipient age and race, and donor type, and steroid maintenance at discharge did not significantly modify the association between induction agents and CVE. Interestingly, acute rejection did not modify the association between no induction and CVE [aHR=0.97, 95% CI: 0.83–1.14, p-for interaction=0.2], and ATG and CVE [aHR=1.04, 95% CI: 0.88–1.22 p-for interaction=0.6]. However, the association between AZM and CVE was significantly modified by recipients experiencing acute rejection. [aHR=1.46, 95% CI: 1.12–1.92 p-for interaction=0.005] (Table 4)
Table 4:
Adjusted hazard of cardiovascular events by induction agent, stratified by gender, race, donor source, age, diabetes mellitus, steroid maintenance and acute rejection
| ATG | AZM | No induction | ||||
|---|---|---|---|---|---|---|
| aHR (95% CI) | p value for interaction | aHR (95% CI) | p value for interaction | aHR(95% CI) | P value for interaction | |
| Gender | ||||||
| Male | 0.92 (0.85–1.00) | 0.01 | 0.99 (0.84–1.16) | 0.5 | 1.03 (0.96–1.12) | 0.2 |
| Female | 1.08 (0.98–1.19) | 1.06 (0.88–1.29) | 1.12 (1.02–1.24) | |||
| Race | ||||||
| Non-AA | 0.99 (0.91–1.07) | 0.7 | 0.99 (0.85–1.17) | 0.6 | 1.04 (0.97–1.12) | 0.3 |
| AA | 0.97 (0.87–1.08) | 1.06 (0.87–1.29) | 1.12 (1.00–1.24) | |||
| Donor type | ||||||
| LD | 0.94 (0.80–1.10) | 0.5 | 0.85 (0.63–1.14) | 0.2 | 1.00 (0.88–1.14) | 0.3 |
| DD | 0.99 (0.92–1.06) | 1.05 (0.91–1.21) | 1.08 (1.01–1.16) | |||
| Age | ||||||
| Age<65 | 0.97 (0.90–1.05) | 0.8 | 1.03 (0.89–1.19) | 0.7 | 1.06 (0.99–1.14) | 0.8 |
| Age ≥ 65 | 0.99 (0.88–1.12) | 0.98 (0.77–1.23) | 1.08 (0.96–1.21) | |||
| Diabetes | ||||||
| Yes | 1.04 (0.96–1.13) | 0.01 | 1.04 (0.87–1.23) | 0.8 | 1.07 (0.99–1.15) | 0.8 |
| No | 0.89 (0.81–0.98) | 1.00 (0.84–1.20) | 1.08 (0.98–1.19) | |||
| Steroid maintenance | ||||||
| Yes | 1.00 (0.93–1.07) | 0.1 | 1.07 (0.87–1.32) | 0.3 | 1.07 (1.00–1.14) | 0.7 |
| No | 0.86 (0.72–1.03) | 0.92 (0.75–1.13) | 1.03 (0.84–1.26) | |||
| One-year acute Rejection | ||||||
| Yes | 1.04 (0.88–1.22) | 0.6 | 1.46 (1.12–1.92) | 0.005 | 0.97 (0.83–1.14) | 0.2 |
| No | 0.99 (0.92–1.06) | 0.97 (0.84–1.12) | 1.08 (1.01–1.16) | |||
AA: African American, AZM: alemtuzumab, ATG: anti-thymocyte globulin, CI: confidence interval, CVE: cardiovascular events, DD: deceased donor, IL-2RA: IL-2 receptor antagonist, LD: living donor
Sensitivity analysis
We found similar results from our sensitivity analysis in which all recipients were censored at 3 years post-transplant. Compared to those who received IL-2RA, there was no difference in the hazard of CVE in the ATG [aHR=0.96, 95% CI: 0.90–1.03, p=0.3] and AZM group [aHR=1.03, 95% CI: 0.90–1.17, p=0.7]. However, in the no induction group a higher hazard of CVE was noted [aHR=1.08, 95% CI: 1.01–1.16, p=0.02].
Discussion
In this national study of 47,258 KT recipients in the U.S., lymphocyte-depleting induction agents, when compared with IL-2RA, were not associated with higher risk of CVE, all-cause mortality or CVE-related mortality. ATG was associated with 7% lower risk of the composite outcomes of mortality and CVE. In fact, administering no induction was associated with a higher risk of CVE. This association was not modified in recipients who had acute rejection in the first year post-KT. In our subgroup analyses, ATG was associated with 8% lower hazard of CVE among male recipients and 11% lower hazard among recipients without diabetes. However, AZM use was associated with a 46% higher hazard of CVE amongst those recipients who experienced acute rejection. Overall, our findings suggest that lymphocyte-depleting induction immunosuppression agents are not associated with high risk of CVE in KT recipients. However, this association may vary by recipient gender, diabetes status, and acute rejection.
Our results are in contrast to an older registry study conducted by Meier-Kriesche and colleagues that examined KT recipients between 1988 and 1997.30 In primary KT recipients, those who received lymphocyte-depleting agents had higher early deaths due to cardiovascular causes, when compared with those who did not receive antibody induction therapy. However these results are not applicable to the current era, as lymphocyte-depleting agents in use are different.49 Also, the mean age of donors (34) and recipients (43) was much younger than the current KT donor and recipient populations. The demographics of recipient and donor populations have changed substantially over the past two decades with efforts to maximize the use of kidneys from older donors and to expand the candidacy for KT among older end stage renal disease patients.50 Lastly, it is unclear how the authors defined “cardiovascular death”. Another retrospective single-center study of 302 KT recipients reported that ATG use was an independent risk factor for CVE.27 Compared to this single-center study, we believe our national study of over 47,000 KT recipients has greater external validity. Also, this center had several changes in clinical practices over different eras (ATG was used in all patients until 1998, from 1998 to 2005 only given to those under the age of 59, and from 2005 onwards only to those with second transplants and those with higher panel reactive antibody) potentially introducing a selection bias.
Some of our other findings merit further discussion, in particular the increased risk of CVE in those who receive no induction. We initially thought that the no induction group may have experienced more rejection rates and since acute rejection is a risk factor for CVE, this could explain our findings.20–22 Alas, we report acute rejection did not modify this association. Thus, we speculate increased CVE in the no induction group could be due to other factors such as higher long-term doses of maintenance immunosuppression, or residual confounding factors such as center level practices or patient level characteristics such as frailty. Instead we report that acute rejection was associated with more CVE in the AZM group. In a recently published 1:1 matched-cohort study, when compared with ATG, patients who received AZM had higher odds of acute rejection by 1 year.18 Acute rejection leads to augmented immunosuppression and acute kidney injury, both of which can increase the risk of CVE; but why it would modify the association of CVE only in the AZM group is not known. We hypothesize that this could be related to other factors such as cytomegalovirus (CMV). Increased incidence of CMV disease has been reported in AZM treated recipients by a Cochrane systematic review.19 Both CMV exposure and post-KT CMV replication contribute to the increased risk of cardiovascular disease in transplant recipients.51 Even the above mentioned study by Ducloux and colleagues did report that the effect of ATG was restricted to the CMV-exposed patients.27 However, more granular data and center level studies are needed to test for our finding; in particular if the characteristics and severity of acute rejection is different in those patients who receive AZM. We also report that in non-diabetic patients and in men, lower incidence of CVE was noted with ATG. Gender variation in risk factor association with CVE and mortality has been previously reported.2,52 Sex and gender consideration in health outcomes of KT recipients is an active area of research and immunologic factors alone likely do not explain these variations.53 Lastly, diabetes is a well-known risk factor of CVE2,6,8 but why it would modify the effect on CVE only in the ATG group is not known.
Our analysis continues to report the significant and sobering higher incidence of CVE and mortality in the KT recipient population. The crude incidence rate for CVE was 41 per 1,000 person-years, all-cause mortality was 34 per 1,000 person-years. This is much higher than the current reported incidence rates in several different populations.54–57 A Canadian study of about 5,000 KT recipients reported a composite outcome of death and major cardiovascular events as 3.2 events per 100 person-years.56 This was much higher than a comparator group that included the general population and those with chronic kidney disease; 0.89 events per 100 person-years. Using data from the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study, between 1982–1992, the cardiovascular disease incidence rates was 225 per 10,000 person-years.57 Several primary and secondary prevention measures in the general population have led to a temporal decline in cardiovascular disease and mortality.57,58 This is unlikely to occur in the KT recipient population, as mean recipient age at transplantation is rising, there is a high prevalence of cardiovascular disease with age in this population, and conventional therapeutic strategies are often ineffective.10–12,59 We also note that congestive heart failure, and not ischemic heart disease, is the most common cause of CVE after KT, with a crude incidence of 21.23 events per 1000 person-years. A previous study has reported a higher incidence of de-novo heart failure but a similar incidence of de-novo ischemic heart disease among KT recipients when compared to the population-based cohorts from Framingham and Minnesota.60 Using United States Renal Data System records for 67,591 KT candidates, another study reported an increased early risk of heart failure.61 KT is a thought to be a state of “accelerated heart failure” more than a state of “accelerated atherosclerosis”.60 Our results support this statement.
Important strengths of our study include that we ascertained inpatient diagnoses for ten different CVE, as well as mortality, and that our analysis included over 47,000 patients. CVE is often an umbrella-term that encompasses several different cardiovascular outcomes. A previous review to evaluate the heterogeneity and validity of composite end points, major adverse cardiac events noted a substantial heterogeneity in the study-specific individual outcomes used to define this term.62 Most studies have used fatal or nonfatal acute myocardial infarction, coronary revascularization, and mortality to define CVE; however, some have added cerebrovascular events, congestive heart failure and severe peripheral vascular disease.27,31–41 We provide a composite of these outcomes and report incidence rates for each. Also, the validity of our analysis rests on reliable capturing of the CVE using ICD-9 and CPT codes. We employed externally validated ICD-9 and CPT codes that are sensitive measures of CVE after KT.42
Our study also has limitations. The registry data source does not include all characteristics related to cardiovascular health, leaving the possibility of unmeasured confounding. This includes prolonged lymphopenia. However, in most patients lymphocyte count reconstitution of 500 cells/mm3 occurs by day 90,63,64 and recipient age is one of the most important predictors of T cell profile and lymphocyte reconstitution.65,66 We aimed to minimize the impact of the unmeasured confounding by building extensive multi-variable models that include all potential confounders available in this national registry. Also, we restricted our analysis to recipients who used Medicare as their primary insurance from at least 365 days before the date of transplant; although this might affect generalizability, Medicare-primary recipients comprise half of the entire KT population in the United States and have historically been considered representative.67–71
In conclusion, our findings suggest that lymphocyte-depleting induction agents are not associated with a higher risk of CVE in KT recipients. However, in a certain subset of patients ATG use is associated with lower CVE and in those who develop acute rejection AZM use is associated with higher CVE. Prospective trials are needed to assess these in recipients with different cardiac and immunologic risk profiles and to determine an optimal and personalized approach to preventing CVE post-transplantation.
Acknowledgments
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government. This study was supported by NIH K24DK101828 (Segev). Shaifali Sandal was supported by an educational grant from Amgen to the Nephrology Division, at the McGill University Health Centre and a bursary from the Société Québécoise De Transplantation. Mara McAdams-DeMarco was supported by K01AG043501 and R01AG055781 from the National Institute on Aging.
Abbreviations
- aHR
adjusted hazard ratio
- AZM
Alemtuzumab
- ATG
Anti-thymocyte globulin
- CVE
cardiovascular events
- CI
confidence interval
- CPT
Current Procedural Terminology
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IL-2RA
IL-2 receptor antagonist
- KT
kidney transplant
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Segev receives speaking honoraria from Sanofi and Novartis. The other authors have no conflicts of interest to disclose.
References
- 1.Aakhus S, Dahl K, Wideroe TE. Cardiovascular morbidity and risk factors in renal transplant patients. Nephrol Dial Transplant. 1999;14(3):648–654. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol. 2000;11(9):1735–1743. [DOI] [PubMed] [Google Scholar]
- 3.Ducloux D, Kazory A, Chalopin JM. Predicting coronary heart disease in renal transplant recipients: a prospective study. Kidney Int. 2004;66(1):441–447. [DOI] [PubMed] [Google Scholar]
- 4.Aakhus S, Dahl K, Wideroe TE. Cardiovascular disease in stable renal transplant patients in Norway: morbidity and mortality during a 5-yr follow-up. Clin Transplant. 2004;18(5):596–604. [DOI] [PubMed] [Google Scholar]
- 5.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 Annual Data Report: Kidney. American Journal of Transplantation. 2017;17:21–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghanta M, Kozicky M, Jim B. Pathophysiologic and treatment strategies for cardiovascular disease in end-stage renal disease and kidney transplantations. Cardiol Rev. 2015;23(3):109–118. [DOI] [PubMed] [Google Scholar]
- 7.Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation. 2006;82(5):603–611. [DOI] [PubMed] [Google Scholar]
- 8.Shirali AC, Bia MJ. Management of cardiovascular disease in renal transplant recipients. Clin J Am Soc Nephrol. 2008;3(2):491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lentine KL, Costa SP, Weir MR, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation: endorsed by the American Society of Transplant Surgeons, American Society of Transplantation, and National Kidney Foundation. Circulation. 2012;126(5):617–663. [DOI] [PubMed] [Google Scholar]
- 10.Dad T, Tighiouart H, Joseph A, et al. Aspirin Use and Incident Cardiovascular Disease, Kidney Failure, and Death in Stable Kidney Transplant Recipients: A Post Hoc Analysis of the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. Am J Kidney Dis. 2016;68(2):277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leberkuhne LJ, Ebtehaj S, Dimova LG, et al. The predictive value of the antioxidative function of HDL for cardiovascular disease and graft failure in renal transplant recipients. Atherosclerosis. 2016;249:181–185. [DOI] [PubMed] [Google Scholar]
- 12.Hiremath S, Fergusson DA, Fergusson N, Bennett A, Knoll GA. Renin-Angiotensin System Blockade and Long-term Clinical Outcomes in Kidney Transplant Recipients: A Meta-analysis of Randomized Controlled Trials. Am J Kidney Dis. 2017;69(1):78–86. [DOI] [PubMed] [Google Scholar]
- 13.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355(19):1967–1977. [DOI] [PubMed] [Google Scholar]
- 14.Noel C, Abramowicz D, Durand D, et al. Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J Am Soc Nephrol. 2009;20(6):1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellemans R, Hazzan M, Durand D, et al. Daclizumab Versus Rabbit Antithymocyte Globulin in High-Risk Renal Transplants: Five-Year Follow-up of a Randomized Study. Am J Transplant. 2015;15(7):1923–1932. [DOI] [PubMed] [Google Scholar]
- 16.Brennan DC, Schnitzler MA. Long-term results of rabbit antithymocyte globulin and basiliximab induction. N Engl J Med. 2008;359(16):1736–1738. [DOI] [PubMed] [Google Scholar]
- 17.Dharnidharka VR, Naik AS, Axelrod DA, et al. Center practice drives variation in choice of US kidney transplant induction therapy: a retrospective analysis of contemporary practice. Transpl Int. 2018;31(2):198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyawala N, Silber JH, Rosenbaum PR, et al. Comparing Outcomes between Antibody Induction Therapies in Kidney Transplantation. J Am Soc Nephrol. 2017;28(7):2188–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill P, Cross NB, Barnett AN, Palmer SC, Webster AC. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev. 2017;1:Cd004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ. Cardiovascular disease after renal transplantation. J Am Soc Nephrol. 1996;7(1):158–165. [DOI] [PubMed] [Google Scholar]
- 21.Kuo HT, Sampaio MS, Vincenti F, Bunnapradist S. Associations of pretransplant diabetes mellitus, new-onset diabetes after transplant, and acute rejection with transplant outcomes: an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database. Am J Kidney Dis. 2010;56(6):1127–1139. [DOI] [PubMed] [Google Scholar]
- 22.Stoumpos S, Jardine AG, Mark PB. Cardiovascular morbidity and mortality after kidney transplantation. Transpl Int. 2015;28(1):10–21. [DOI] [PubMed] [Google Scholar]
- 23.Matusik P, Guzik B, Weber C, Guzik TJ. Do we know enough about the immune pathogenesis of acute coronary syndromes to improve clinical practice? Thromb Haemost. 2012;108(3):443–456. [DOI] [PubMed] [Google Scholar]
- 24.Hilgendorf I, Theurl I, Gerhardt LM, et al. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation. 2014;129(16):1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51(4):435–447. [DOI] [PubMed] [Google Scholar]
- 26.Schiffrin EL. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens. 2010;19(2):181–186. [DOI] [PubMed] [Google Scholar]
- 27.Ducloux D, Courivaud C, Bamoulid J, et al. Polyclonal antithymocyte globulin and cardiovascular disease in kidney transplant recipients. J Am Soc Nephrol. 2014;25(6):1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma F, Feng J, Zhang C, et al. The requirement of CD8+ T cells to initiate and augment acute cardiac inflammatory response to high blood pressure. J Immunol. 2014;192(7):3365–3373. [DOI] [PubMed] [Google Scholar]
- 29.Ducloux D, Courivaud C, Bamoulid J, et al. Prolonged CD4 T cell lymphopenia increases morbidity and mortality after renal transplantation. J Am Soc Nephrol. 2010;21(5):868–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meier-Kriesche HU, Arndorfer JA, Kaplan B. Association of antibody induction with short- and long-term cause-specific mortality in renal transplant recipients. J Am Soc Nephrol. 2002;13(3):769–772. [DOI] [PubMed] [Google Scholar]
- 31.Jeon HJ, Kim CT, An JN, et al. Time-varying maximal proteinuria correlates with adverse cardiovascular events and graft failure in kidney transplant recipients. Nephrology (Carlton). 2015;20(12):945–951. [DOI] [PubMed] [Google Scholar]
- 32.Arnol M, de Mattos AM, Chung JS, Prather JC, Mittalhenkle A, Norman DJ. Late steroid withdrawal and cardiovascular events in kidney transplant recipients. Transplantation. 2008;86(12):1844–1848. [DOI] [PubMed] [Google Scholar]
- 33.Silver SA, Huang M, Nash MM, Prasad GV. Framingham risk score and novel cardiovascular risk factors underpredict major adverse cardiac events in kidney transplant recipients. Transplantation. 2011;92(2):183–189. [DOI] [PubMed] [Google Scholar]
- 34.Kiberd B, Panek R. Cardiovascular outcomes in the outpatient kidney transplant clinic: the Framingham risk score revisited. Clin J Am Soc Nephrol. 2008;3(3):822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Israni AK, Snyder JJ, Skeans MA, et al. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am J Transplant. 2010;10(2):338–353. [DOI] [PubMed] [Google Scholar]
- 36.Jardine AG, Fellstrom B, Logan JO, et al. Cardiovascular risk and renal transplantation: post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) Study. Am J Kidney Dis. 2005;46(3):529–536. [DOI] [PubMed] [Google Scholar]
- 37.Soveri I, Holdaas H, Jardine A, Gimpelewicz C, Staffler B, Fellstrom B. Renal transplant dysfunction--importance quantified in comparison with traditional risk factors for cardiovascular disease and mortality. Nephrol Dial Transplant. 2006;21(8):2282–2289. [DOI] [PubMed] [Google Scholar]
- 38.Vanrenterghem YF, Claes K, Montagnino G, et al. Risk factors for cardiovascular events after successful renal transplantation. Transplantation. 2008;85(2):209–216. [DOI] [PubMed] [Google Scholar]
- 39.Weiner DE, Carpenter MA, Levey AS, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. Am J Transplant. 2012;12(9):2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ducloux D, Challier B, Saas P, Tiberghien P, Chalopin JM. CD4 cell lymphopenia and atherosclerosis in renal transplant recipients. J Am Soc Nephrol. 2003;14(3):767–772. [DOI] [PubMed] [Google Scholar]
- 41.Neale J, Smith AC. Cardiovascular risk factors following renal transplant. World J Transplant. 2015;5(4):183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lentine KL, Schnitzler MA, Abbott KC, Bramesfeld K, Buchanan PM, Brennan DC. Sensitivity of billing claims for cardiovascular disease events among kidney transplant recipients. Clin J Am Soc Nephrol. 2009;4(7):1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16(2):496–506. [DOI] [PubMed] [Google Scholar]
- 44.Lentine KL, Schnitzler MA, Abbott KC, et al. De novo congestive heart failure after kidney transplantation: a common condition with poor prognostic implications. Am J Kidney Dis. 2005;46(4):720–733. [DOI] [PubMed] [Google Scholar]
- 45.Lentine KL, Schnitzler MA, Abbott KC, et al. Incidence, predictors, and associated outcomes of atrial fibrillation after kidney transplantation. Clin J Am Soc Nephrol. 2006;1(2):288–296. [DOI] [PubMed] [Google Scholar]
- 46.Lentine KL, Rocca Rey LA, Kolli S, et al. Variations in the risk for cerebrovascular events after kidney transplant compared with experience on the waiting list and after graft failure. Clin J Am Soc Nephrol. 2008;3(4):1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersen PK, Klein JP, Knudsen KM, Tabanera y Palacios R. Estimation of variance in Cox’s regression model with shared gamma frailties. Biometrics. 1997;53(4):1475–1484. [PubMed] [Google Scholar]
- 48.Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney International.77(4):299–311. [DOI] [PubMed] [Google Scholar]
- 49.Cai J, Terasaki PI. Induction immunosuppression improves long-term graft and patient outcome in organ transplantation: an analysis of United Network for Organ Sharing registry data. Transplantation. 2010;90(12):1511–1515. [DOI] [PubMed] [Google Scholar]
- 50.Wynn JJ, Alexander CE. Increasing organ donation and transplantation: the U.S. experience over the past decade. Transplant International. 2011;24(4):324–332. [DOI] [PubMed] [Google Scholar]
- 51.Courivaud C, Bamoulid J, Chalopin JM, et al. Cytomegalovirus exposure and cardiovascular disease in kidney transplant recipients. J Infect Dis. 2013;207(10):1569–1575. [DOI] [PubMed] [Google Scholar]
- 52.Cheng S, Claggett B, Correia AW, et al. Temporal trends in the population attributable risk for cardiovascular disease: the Atherosclerosis Risk in Communities Study. Circulation. 2014;130(10):820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laprise C, Sridhar VS, West L, Foster B, Pilote L, Sapir-Pichhadze R. Sex and gender considerations in transplantation research: protocol for a scoping review. Syst Rev. 2017;6(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104(23):2855–2864. [DOI] [PubMed] [Google Scholar]
- 55.Engelen SE, van der Graaf Y, Stam-Slob MC, et al. Incidence of cardiovascular events and vascular interventions in patients with type 2 diabetes. Int J Cardiol. 2017;248:301–307. [DOI] [PubMed] [Google Scholar]
- 56.Lam NN, Kim SJ, Knoll GA, et al. The Risk of Cardiovascular Disease Is Not Increasing Over Time Despite Aging and Higher Comorbidity Burden of Kidney Transplant Recipients. Transplantation. 2017;101(3):588–596. [DOI] [PubMed] [Google Scholar]
- 57.Ergin A, Muntner P, Sherwin R, He J. Secular trends in cardiovascular disease mortality, incidence, and case fatality rates in adults in the United States. Am J Med. 2004;117(4):219–227. [DOI] [PubMed] [Google Scholar]
- 58.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stevens LA, Viswanathan G, Weiner DE. Chronic kidney disease and end-stage renal disease in the elderly population: current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis. 2010;17(4):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rigatto C, Parfrey P, Foley R, Negrijn C, Tribula C, Jeffery J. Congestive heart failure in renal transplant recipients: risk factors, outcomes, and relationship with ischemic heart disease. J Am Soc Nephrol. 2002;13(4):1084–1090. [DOI] [PubMed] [Google Scholar]
- 61.Lentine KL, Xiao H, Brennan DC, et al. The impact of kidney transplantation on heart failure risk varies with candidate body mass index. American heart journal. 2009;158(6):972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kip KE, Hollabaugh K, Marroquin OC, Williams DO. The problem with composite end points in cardiovascular studies: the story of major adverse cardiac events and percutaneous coronary intervention. J Am Coll Cardiol. 2008;51(7):701–707. [DOI] [PubMed] [Google Scholar]
- 63.Hanaway MJ, Woodle ES, Mulgaonkar S, et al. Alemtuzumab induction in renal transplantation. N Engl J Med. 2011;364(20):1909–1919. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Tao Y, Chopra M, et al. Differential reconstitution of T cell subsets following immunodepleting treatment with alemtuzumab (anti-CD52 monoclonal antibody) in patients with relapsing-remitting multiple sclerosis. J Immunol. 2013;191(12):5867–5874. [DOI] [PubMed] [Google Scholar]
- 65.Moro-Garcia MA, Alonso-Arias R, Lopez-Larrea C. When Aging Reaches CD4+ T-Cells: Phenotypic and Functional Changes. Front Immunol. 2013;4:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Longuet H, Sautenet B, Gatault P, et al. Risk factors for impaired CD4+ T-cell reconstitution following rabbit antithymocyte globulin treatment in kidney transplantation. Transpl Int. 2014;27(3):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Massie AB, Kucirka L, Segev DL. Big Data in Organ Transplantation: Registries and Administrative Claims. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(8):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stirnemann PM, Takemoto SK, Schnitzler MA, et al. Agreement of immunosuppression regimens described in Medicare pharmacy claims with the Organ Procurement and Transplantation Network survey. J Am Soc Nephrol. 2006;17(8):2299–2306. [DOI] [PubMed] [Google Scholar]
- 69.McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL. Early hospital readmission after kidney transplantation: patient and center-level associations. Am J Transplant. 2012;12(12):3283–3288. [DOI] [PubMed] [Google Scholar]
- 70.Axelrod D, Segev DL, Xiao H, et al. Economic Impacts of ABO-Incompatible Live Donor Kidney Transplantation: A National Study of Medicare-Insured Recipients. Am J Transplant. 2016;16(5):1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rose C, Gill J, Zalunardo N, Johnston O, Mehrotra A, Gill JS. Timing of Pregnancy After Kidney Transplantation and Risk of Allograft Failure. American Journal of Transplantation. 2016;16(8):2360–2367. [DOI] [PubMed] [Google Scholar]