Abstract
Atherosclerosis is the devastating underlying cause of cardiovascular disease and it preferentially develops at arterial regions exposed to disturbed flow (DF), while much less at regions of unidirectional laminar flow (UF). Recent studies have demonstrated that DF and UF differentially regulate important aspects of endothelial function, such as vascular inflammation, oxidative stress, vascular tone, cell proliferation, senescence, mitochondrial function, and glucose metabolism. DF and UF regulate vascular pathophysiology via differential regulation of mechanosensitive transcription factors (MSTFs) (KLF2, KLF4, NRF2, YAP/TAZ/TEAD, HIF-1α, NF-κB, AP-1 and others). Emerging studies show that MSTFs represent promising therapeutic targets for the prevention and treatment of atherosclerosis. Here, we present a comprehensive overview of the role of MSTFs in atherosclerosis and highlight future directions for developing novel therapeutic agents by targeting MSTFs.
Keywords: endothelial cells, shear stress, unidirectional laminar flow, disturbed flow, transcription factors, atherosclerosis
Atherosclerosis and shear stress
Atherosclerosis (see Glossary) is the major underlying pathology of cardiovascular disease and the leading cause of global morbidity and mortality [1,2]. Atherosclerosis often occurs in medium- and large-sized arteries that consist of endothelial cells, vascular smooth muscle cells (VSMCs), and other vascular cells (Figure 1). The development of atherosclerosis commences with endothelial dysfunction, followed by the retention and modification of low-density lipoproteins (LDL), concomitant with adhesion, rolling, and subsequent transmigration of leukocytes into the sub-endothelial space. Transmigrated monocytes can differentiate into macrophages in the vessel wall and avidly uptake various forms of modified LDL (oxidized LDL in particular), to form “foam cells”, which is considered as the early pathological hallmark of fatty streaks and atherosclerotic plaques [3]. Lineage tracing studies have shown that VSMCs can also form “macrophage-like” cells and foam cells during atherogenesis [4, 5]. The formation and eventual rupture of vulnerable plaques (characterized by plaques with a large necrotic core covered by a thin fibrous cap), eventually leads to arterial thrombosis, vessel occlusion, tissue ischemia and the acute cardiovascular events (such as heart attacks and ischemic strokes) [3].
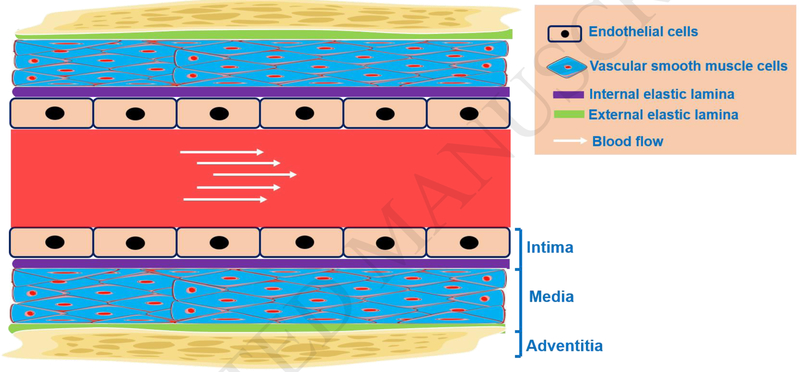
Figure 1. Vascular components of the artery.
Blood vessels are usually composed of three layers: the tunica intima, tunica media, and tunica adventitia. The intima consists of an endothelial cells monolayer lining the vessel lumen. Often, the internal elastic lamina separates the tunica intima from the tunica media. The tunica media is mainly composed of vascular smooth muscle cells. The external elastic lamina separates the tunica media from the tunica adventitia. The tunica adventitia is primarily composed of connective tissues made up of fibroblasts, macrophages, and associated collagen-rich matrix proteins.
It has been well established that the distribution of atherosclerotic plaques is not uniform in medium and large-sized arteries, with regions having disturbed flow (DF, such as arterial bends and branching points) being more prone to atherosclerosis. The regions with unidirectional laminar flow (UF, such as straight parts of aorta) are protected from atherosclerosis [6-14]. The cause of the uneven and focal nature of atherosclerosis lies in the different cellular responses elicited by different pattern of blood flow. Among all the vascular cells in the arterial wall, endothelial cells (the innermost monolayer in the blood vessel) are the major cell type that are constantly exposed to hemodynamic forces from blood flow, including shear stress, the frictional dragging force acting on the surface of vascular lumen [12]. Shear stress modulates endothelial structure and function, and hence shear stress is critically involved in cardiovascular development, physiology, and pathology [12].
Shear stress on the vascular endothelium
Endothelial cells are the main cell type that sense (by membrane-localized mechanosensors and mechanosensing complexes) and transduce (by mechanotransducers) mechanosignals from shear stress to regulate various signaling pathways and biological functions [12]. In mechanobiology, UF and DF are two important types of shear stress occurring in vivo. UF occurs in straight parts of arteries; where blood flow is generally uniform and steady. UF is associated with high laminar shear stress and provides antiinflammatory and anti-atherosclerotic effects by maintaining endothelial cell quiescence, alignment to the direction of flow, and thus preserving endothelial homeostasis [6, 12-14]. DF (also known as turbulent/oscillatory flow) is a complicated flow pattern that has low magnitude of shear stress, and is irregular and non-uniform. Other features of DF include spatiotemporal changes in gradients of shear stress and flow direction [14]. DF normally occurs in regions of arteries that curve sharply or arteries with branching points. DF, in conjunction with other cardiovascular risk factors (such as hyperlipidemia and hyperglycemia), leads to endothelial dysfunction and focal distribution of atherosclerotic plaques [6, 12-14]. DF-dependent mechanisms promote the initiation of atherosclerosis as well as contribute to plaque rupture. Therefore, DF represents one major biomechanical mechanism for endothelial dysfunction and the progression of coronary artery disease [18, 19].
UF and DF exert differential effects on the functions of endothelial cells (such as inflammation, oxidative stress, proliferation, cell alignment, mitochondrial function, and glucose metabolism), mainly through mechanosensitive transcription factors (MSTFs)-dependent gene expression (Figure 2, Key Figure) [16]. Considering the important role of MSTFs in regulating endothelial cell gene expression, we present an overview of the recent studies examining the role of MSTFs in regulating endothelial function and atherosclerosis. We also highlight possible therapeutic pathways to new cardiovascular drug discovery arising from the targeting MSTFs.
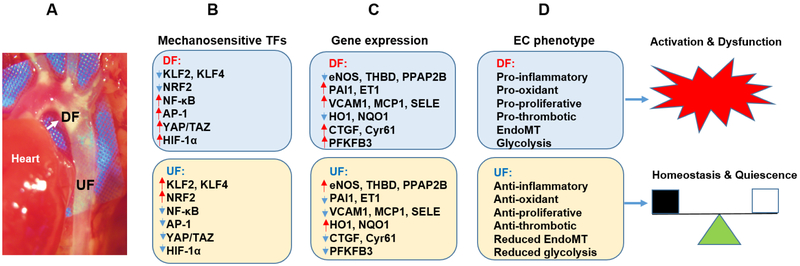
Figure 2, Key figure. Role of MSTFs in regulating endothelial phenotype.
A. Image showing the focal distribution of lesions in a mouse model of atherosclerosis. The lesions preferentially develop at regions with disturbed flow (DF), such as branches and bends of the blood vessel (indicated by white arrow), but much less in regions of unidirectional laminar flow (UF), such as the straight part of the blood vessel (unpublished data). Note: color saturation of the image is enhanced for presentation purposes.
B. Major MSTFs that are regulated by UF and DF in ECs. The downregulation in shown by blue arrows and upregulation is shown by red arrows.
C. The genes that are regulated by each of the MSTFs in DF and UF treated endothelial cells, are shown.
D. Flow-dependent endothelial gene expression regulates EC phenotype. DF treated endothelial cells show a pro-inflammatory, pro-oxidant, pro-proliferative response, and enhanced endothelial to mesenchymal transition (EndoMT) phenotype. By doing so, DF causes endothelial cell activation and ensuing dysfunction. In contrast, UF treated ECs show anti-inflammatory, anti-oxidant, and anti-proliferative phenotype along with reduced EndoMT and glycolysis, leading to endothelial cell quiescence and vascular homeostasis.
Role of MSTFs in regulating endothelial function and atherosclerosis
As the master regulators of shear stress-induced gene expression in endothelial cells, MSTFs control endothelial function and the development of atherosclerosis. For example, Kruppel like factor 2 (KLF2), Kruppel like factor 2 (KLF4), and nuclear factor (erythroid-derived 2)-like 2 (NRF2) are important MSTFs which are upregulated after exposure to UF [21, 22]. In contrast, transcription factors such as activator protein 1 (AP-1), nuclear factor kappa B (NF-κB) [23-25], yes-associated protein (YAP)/WW domain-containing transcription regulator 1 (TAZ, also known as WWTR1)/TEA domain transcription factor (TEAD) [26-28], and hypoxia inducible factor 1 alpha (HIF-1α) [29, 30], are suppressed by UF (Figure 2). However, DF has opposite effects on these MSTFs [6, 12-14, 31]. In the sections below, we review the specific role of these MSTFs in endothelial function and atherosclerosis.
KLF2 and KLF4
Among all the MSTFs, endothelial KLF2 and KLF4 are the best characterized. KLF2 and KLF4 are vascular homeostasis-associated molecular patterns, which regulate the expression of a wide range of antiinflammatory, anti-oxidant, and anti-thrombotic genes in endothelial cells [32]. KLF2 is upregulated by UF, but decreased by DF [33, 34]. Important downstream targets of KLF2 include endothelial nitric oxide synthase (eNOS) and thrombomodulin (THBD) and many others (Figure 2) [10]. Mechanistically, KLF2 increases the expression of target genes via binding to the KLF2 binding sites (consensus binding motif: 5’-C(A/T)CCC-3’) in target gene promoters/enhancers [32]. KLF2 also negatively regulates multiple pro-inflammatory, pro-thrombotic, and vasoconstrictive genes, such as vascular cell adhesion molecule (VCAM1), monocyte chemoattractant protein (MCP-1), E-selectin (SELE), endothelin 1 (ET1), and plasminogen activator inhibitor-1 (PAI1) (Figure 2) [10]. KLF2 inhibits the expression of pro-inflammatory genes via recruiting transcriptional co-activator p300 [32] to inhibit the hyperactivation of NF-κB induced by pro-inflammatory cytokines. KLF4, another important MSTF, also regulates the expression of an appreciable portion of overlapping genes with KLF2 (such as eNOS, THBD, PAI1, and VC AMI) [35].
Further in vivo studies have shown that KLF2 and KLF4 protect aortic vessels from atherosclerosis [35, 36]. The protective roles of KLF2 and KLF4 is evidenced by the fact that KLF2 deficiency and KLF4 deficiency accelerate atherosclerosis in apolipoprotein E deficient (ApoE−/−) mice and LDL receptor deficient (LDLR−/−) mice, two frequently used animal models to assess the effect of genetic manipulations on the development of atherosclerosis [37]. Specifically, KLF2+/−; ApoE−/− mice display an increase of lipid uptake, foam cell formation, and atherosclerotic lesions [36], providing the first direct link between KLF2 and atheroprotection. Similarly, myeloid cell specific knockout of KLF2 also increases the development of atherosclerosis in LDLR−/− mice by increasing the content of neutrophils and macrophages as well as the level of nitroxidative stress (Table 1) [38]. KLF4 has similar atheroprotective functions. Endothelial cell specific deletion of KLF4 increases, while endothelial cell specific KLF4 transgene reduces atherosclerosis in ApoE−/− mice [35], suggesting that activating endothelial KLF2 and KLF4 could confer atheroprotective effects (Table 1).
Table 1.
Role of MSTFs in atherosclerosis in vivo
| Animal model | Effect on atherosclerosis |
Observed atherosclerotic characteristics | References |
|---|---|---|---|
| KLF2+/−; ApoE−/− mice | ↑ | ↑ Foam cell formation | [36] |
| KLF2 ΔMye; LDLR−/− mice | ↑ | ↑ Lesional neutrophils and macrophages | [38] |
| KLF4 ΔEC; ApoE−/− | ↑ | ↑ Endothelial inflammation and thrombosis | [35] |
| KLF4Tg(EC); ApoE−/− | ↓ | ↓ Endothelial inflammation and thrombosis | [35] |
| NRF2−/−; ApoE−/− | ↓ | ↓ Foam cell formation | [95, 96] |
| NRF2−/−; LDLR−/− ApoB 100/100 | Mixed |
↓ Atherosclerotic lesion size ↑ Atherosclerotic plaque vulnerability |
[97] |
| NF-kBΔEC; ApoE−/− | ↓ | ↓ Adhesion molecules and inflammation | [63] |
| I-κBmtOE(EC); ApoE−/− | ↓ | ↓ Chronic intermittent hypoxia induced atherosclerosis; NF-kB activity | [64] |
| HIF-1αΔEC; ApoE−/− | ↓ | ↓ Monocyte adhesion | [61] |
| HIF-1αΔMye; ApoE−/− | ↓ | ↓ Inflammatory gene expression | [98] |
| YAP/TAZKD; ApoE−/− | ↓ | ↓ Promotes EC proliferation, inflammation | [27] |
| YAPOE (EO); ApoE−/−; YAPTg (EC); ApoE−/−; YAP/TAZCA; ApoE−/− | ↑ | ↑ Monocyte adhesion, macrophage accumulation | [26] |
| YApOE (EC); ApoE−/− | ↑ | ↑ VCAM1 dependent monocyte adhesion | [28] |
Abbreviations:
KD, knockdown; OE (EC), endothelial cell-specific overexpression; Tg (EC), endothelial cell specific transgene; ΔMye, myeloid cell-specific knockout; ΔEC, endothelial cell-specific knockout
A recent study has shown that deficiency of KFL2 and KLF4 alters the endothelial cell transcriptome [39], and additional shear stress-responsive KLF2- dependent genes have recently been revealed. Fang group reported that KLF2 regulates the expression of a novel flow sensitive gene, phosphatidic acid phosphatase type 2B (PPAP2B ), a gene product associated with coronary artery disease and ischemic stroke (CAD/IS). Specifically, PPAP2B is essential for UF-induced endothelial cell alignment and antiinflammatory effects [40]. Moreover, Fang group showed that a genetic variant of PPAP2B, rs17114036, generates a binding site for KLF2 and has CAD/IS-protective effect via regulating endothelial cell responses to hemodynamic changes [41]. In addition, KLF2 is implicated in the regulation of endothelial cell metabolism. A recent study [42] has shown that UF induces KLF2 upregulation, thereby repressing glycolysis and mitochondrial respiration. This study further showed that 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase-3 (PFKFB3), an important gene regulating glycolysis, is a direct target of KLF2, thus extending the functions of KLF2 from maintaining vascular homeostasis to the regulation of endothelial cellular metabolism [42].
Collectively, emerging evidence indicate that endothelial KLF2 and KLF4 represent two major MSTFs that regulate vascular homeostasis and represent promising therapeutic targets to prevent atherosclerosis by pharmacological intervention. Since recent studies have identified that other members of KLF family, such as KLF3 [43] and KLF14 [44] also exhibit similar anti-inflammatory functions as KLF2 and KLF4, it would be interesting to assess whether KLF3 and KLF14 are also mechanosensitive and if so, their potential interaction with KLF2/KLF4 in the regulation of endothelial homeostasis and atherosclerosis.
NRF2
NRF2 is a crucial transcript factor that regulates the expression of a plethora of genes responsible for cytoprotection, phase II detoxification (a process that conjugates drug metabolites or endobiotics via glucuronidation, glutathionylation, and sulphation etc), inflammation, and metabolism in response to oxidative stress. NRF2 functions by binding to a conserved NRF2 binding motif (5’-A/GTGAC/GTCAGCA-3’) in the target gene promoters [45, 46]. Well-characterized downstream target genes of NRF2 include: heme oxygenase-1 (HO1) and NAD(P)H quinone oxidoreductase 1 (NQO1) [45, 46]. NRF2 expression/activity is controlled transcriptionally, epigenetically, post-translationally, and pharmacologically [45, 46]. NRF2 activation in response to UF is important for endothelial cell adaptation to oxidative and nitrosative stress as well as being anti-inflammatory [10]. In endothelial cells, UF increases eNOS-dependent nitric oxide (NO) production and represses oxidative stress, while DF promotes oxidative stress via NADPH oxidase, xanthine oxidase, eNOS uncoupling, and mitochondrial-derived reactive oxygen species (ROS) [10]. In contrast, NRF2 depletion or knockout reverses UF induced upregulation of cytoprotective enzymes [47].
In addition to the known role of NRF2 in maintaining cellular redox homeostasis via phase 2 detoxification and antioxidant responses, NRF2 has been implicated in metabolism and inflammation related to endothelial cell functions and atherosclerosis. For example, a recent study [48] has revealed that NRF2 binds to the proximal promoter region of IL-6 and IL-1β genes and suppresses the expression of IL-6 and IL-1β independent of redox control. In addition, NRF2 activation protects against TNFα-induced activation of VCAM1 gene expression in endothelial cells [49]. NRF2 also regulates the metabolism of carbohydrate, lipid, heme, iron, amino acid, and nucleotides, as well as the generation of NADPH through regulating key enzymes in these metabolic processes, indicating the essential role of NRF2 in dictating metabolic reprogramming under stress conditions [45, 46].
Since both KLF2 [50] and NRF2 [51] function downstream of ERK5 and some NRF2 downstream genes (NQO1 and HO1) are upregulated in KLF2-overexpressed endothelial cells [52], it is plausible that both MSTFs function synergistically to regulate UF-induced endothelial cell gene expression. KLF2 served as a priming factor for UF-induced NRF2 activation since knockdown of KLF2 by small interfering RNA (siRNA) blocks NRF2-dependent antioxidant gene expression in endothelial cells [52, 53]. By analyzing the transcriptomes of endothelial cells exposed to UF, KLF2 overexpression, and NRF2 overexpression, a considerable pool of overlapping genes (76%) were observed between UF treatmen and KLF2/NRF2 overexpression in endothelial cells. These results indicate that KLF2 and NRF2 contribute to the vast majority of UF-induced transcriptional networks [52].
YAP/TAZ/TEAD
YAP/TAZ/TEAD in the Hippo pathway emerged as a master regulator of multiple cellular processes, such as cell proliferation, migration, and inflammation [54]. A recent study in YAP/TAZ knockout cells indicates that YAP and TAZ have functionally redundant roles and YAP has an overall stronger influence than TAZ [55]. Connective tissue growth factor (CTGF, also known as CCN2) and cysteine rich angiogenic inducer 61 (Cyr61, also known as CCN1), two well-studied YAP/TAZ target genes, are highly expressed in advanced atherosclerotic plaques [56], indicating the potential pro-atherogenic role of YAP/TAZ activation. In 2016, three independent groups reported that UF suppressed the transactivation of YAP/TAZ/TEAD [26, 27, 57]. Specifically, UF promotes YAP phosphorylation (at serine 127), reduces YAP nuclear localization, and inhibits the expression of YAP-dependent pro-atherogenic genes (CTGF and Cyr61) in cultured human endothelial cells and mouse aortic endothelium. In contrast, DF promotes the activation of YAP/TAZ in endothelial cells, providing an alternative explanation for the uneven development of atherosclerosis. In addition, DF also promotes YAP activation and endothelial VCAM1-dependent inflammatory responses by increasing YAP phosphorylation (at tyrosine 357) dependent on tyrosine protein kinase activity [28]. In addition to DF pro-inflammatory cytokine TNFα also activates YAP/TAZ/TEAD in endothelial cells [58].
Recently, the precise role of YAP/TAZ in driving vascular inflammation and atherosclerosis has been addressed in vivo. In particular, systemic deficiency of YAP/TAZ (by morpholino oligos) [27], or endothelial cell specific deficiency of YAP (by CRISPR/Cas9 technology) reduces atherosclerosis in ApoE−/− mice [26]. In contrast, overexpression of endothelial YAP, or systemic TAZ, or a constitutively active mutant of YAP/TAZ promotes atherosclerosis in ApoE−/− mice [26, 28]. These series of gain- and loss-of-function studies in mice provide proof-of-concept that YAP/TAZ/TEAD represents a promising therapeutic target to prevent atherosclerosis [26]. Studies in cultured human endothelial cells indicate that activation of YAP/TAZ/TEAD leads to endothelial cell proliferation, cell cycle progression, and inflammation (increased expression of VCAM1 and leukocyte-endothelium interaction) [26-28]. These observations suggest that local and systemic risk factors could drive atherosclerosis by inducing sustained activation of YAP/TAZ/TEAD.
HIF-1α
Activation of the HIF-1α pathway drives vascular permeability, leukocyte recruitment, and intraplaque angiogenesis in atherosclerotic plaques from mouse models and human subjects [59, 60]. Specifically, endothelial HIF-1α aggravates DF-induced atherosclerosis development in ApoE−/− mice after partial carotid ligation, by triggering the miR-19a/CXCL1 (C–X–C motif ligand 1) axis and stimulating leukocyte adhesion to activated endothelial cells [61]. HIF-1α is activated by DF in vitro in both human umbilical vein endothelial cells and human aortic endothelial cells under normoxic conditions [29, 30]. More importantly, HIF-1α is also activated by DF in vivo in areas prone to atherosclerosis of the mouse and porcine aorta (near branches or bends of arteries) [29, 30].
Upon exposure to DF, HIF-1α expression is increased through a dual mechanism which involves transcriptional activation of NF-κB as well as HIF-1α protein stabilization by the deubiquitinating enzyme Cezanne [29]. NADPH oxidase 4 (NOX4)-dependent generation of ROS is also involved in DF-induced HIF-1α stabilization [30]. Activation of HIF-1α not only drives the expression of multiple pro-inflammatory and pro-adhesive genes (MCP1, SELE, and VCAM1), represses mitochondria respiration, but also enhances the expression of multiple glycolytic enzymes (such as enolase 2 (ENO2); hexokinase 2 (HK2); PFKFB3, and pyruvate dehydrogenase kinase-1 (PDK-1)) [29, 30]. This evidence suggests that HIF-1α is critical not only in the initiation of atherosclerosis (by regulating inflammatory responses) [62], but also in advanced stages of atherosclerosis (by regulating intraplaque angiogenesis).
NF-κB and AP-1
NF-κB promotes the expression of multiple pro-inflammatory and pro-adhesive genes (VCAM1, MCP1, and SELE) in endothelial cells [24]. Activity of NF-κB is tightly controlled by its phosphorylation, and subcellular localization. AP-1 is a heterodimer composed of c-Fos, c-Jun, activating transcription factor (ATF) families. Both NF-κB and AP1 are redox sensitive MSTFs that mediate inflammation of the endothelium [24]. NF-κB and AP1 are activated by prolonged DF in cultured endothelial cells and endothelium from DF area of mouse aorta [23-25].
Endothelial cell-specific inhibition of NF-κB reduces and stabilizes atherosclerotic plaques from ApoE−/− mice fed an atherogenic diet or treated with chronic intermittent hypoxia [63, 64]. The mechanism is related to reduced macrophage recruitment via decreased expression of adhesion molecules, and pro-inflammatory cytokines/chemokines [63, 64]. Conversely, UF protects endothelial cells via inhibition of NF-κB and AP-1 activation induced by TNFα [65]. Overall, these results indicate that NF-κB and AP-1 as two established pro-inflammatory MSTFs, are differentially regulated by different patterns of blood flow, with important implications in the development of atherosclerosis.
Therapeutic targeting MSTFs in atherosclerosis
As described above, MSTFs regulate the development of atherosclerosis by influencing the expression of genes responsible for endothelial function. In this section, we will overview the effect and mechanism of known MSTFs-targeting pharmacological agents in atherosclerosis (Table 2).
Table 2.
Examples of atheroprotective drugs that target MSTFs
| Name of drug | Effect on atherosclerosis |
Targeted MSTFs | References |
|---|---|---|---|
| Statins | ↓ | (+) KLF2; (+) KLF4; (+) NRF2; (−) NF-κB; (−) AP1; (−) YAP/TEAD; (−) HIF-1α | [26, 27, 66, 67, 99-102] |
| Resveratrol | ↓ | (+) KLF2; (+) KLF4; (+) NRF2; (−) NF-κB | [22, 68, 69, 103, 104] |
| Tannic acid | ↓ | (+) KLF2 | [71, 72] |
| SAHA | ↓ | (+) KLF2 | [70] |
| Sulforaphane | ↓ | (+) NRF2; (−) NF-κB | [75, 105] |
| Verteporfin | ↓ | (−) YAP/TEAD | [77, 78] |
| Evodiamine | ↓ | (−) HIF-1α | [79, 106] |
| Digoxin | ↓ | (−) HIF-1α | [80, 81] |
| 2-Methoxyestradiol | ↓ | (−) HIF-1α | [82, 83] |
(+) activate; (−) inhibit.
Activators of KLF2 and KLF4
KLF2 and KLF4 elicit potent anti-inflammatory effects in both endothelial cells and macrophages [32]. Therefore, pharmacological activators of KLF2 and KLF4 are promising agents to treat endothelial dysfunction and atherosclerosis. Many pharmaceutical agents have been identified/repurposed as activators of KLF2 and/or KLF4, such as statins, resveratrol, suberanilohydroxamic acid (SAHA), tannic acid (TA), and many others.
HMG-CoA reductase inhibitors (statins) are well characterized activators of KLF2 and KLF4 both in vitro and in vivo. Statins activate KLF2 via the mevalonate- [66] and MEF2 (myocyte enhancer factor-2)-dependent pathways [67] since exogenous addition of mevalonate or mutation of the MEF2 binding site in the KLF2 promoter reverses the effects of statin-mediated KLF2 upregulation.
Resveratrol is a natural and pharmacological activator of both KLF2 and KLF4 in endothelial cells. Resveratrol activates the KLF2 via SIRT1 (Sirtuin 1)/MEK5 (MAP2K5 mitogen-activated protein kinase kinase 5)/MEF2 [68] and AMPKα (AMP-activated protein kinase alpha)/ERK5/MEF2 pathways [69]. Resveratrol also activates KLF4 via the MEK5/MEF2-dependent signaling pathway in endothelial cells [22]. SAHA (also known as vorinostat), a pan-inhibitor of histone deacetylases, has been identified as a pharmacological activator of KLF2 by luciferase reporter assay-based high throughput drug screening [70]. SAHA exhibits potent anti-inflammatory effects and reduces monocyte adhesion to activated endothelial cells. The protective effects against inflammation of the endothelium are reversed in KLF2 deficiency or depletion, suggesting that SAHA attenuates inflammation in a KLF2-dependent manner. Furthermore, luciferase assays indicate that SAHA activates KLF2 via the MEF2-dependent pathway [70].
In addition, TA has recently been identified as a new pharmacological activator of KLF2 [71]. TA activates KLF2 via the ERK5/MEF2 pathway, thereby reducing monocyte adhesion to activated endothelium in a KLF2-dependent manner [71]. Most importantly, both SAHA [70] and TA [71, 72] have protective effects against atherosclerosis in ApoE−/− mice fed a high fat diet.
A recent study from the García-Cardeña laboratory [73] reported another KLF2 reporter system using green fluorescence protein (GFP). This screening system is also compatible with high throughput drug screening by assessing GFP fluorescence using a microplate reader. This reporter system has been used successfully to validate current known KLF2 activators (simvastatin) or treatments (UF or infection with constitutively active mutant of MEK5) [73]. The use of KLF2-luciferase reporter assays and GFP-KLF2 reporter assays, is potentially useful for identifying new KLF2 activators. It remains to be determined whether or not compounds identified by these methods also activate KLF4. In addition, the potential side effects of systemic KLF2 and KLF4 activators needs to be carefully assessed.
NRF2 activators
Despite the controversial role of NRF2 in atherosclerosis in pre-clinical animal models of atherosclerosis, pharmacological activators of NRF2 have shown overall atheroprotective effects [74]. One such NRF2 activator is sulforaphane, which is a bioactive natural product from broccoli. An elegant study [75] from Evans’ laboratory has shown that NRF2 is activated in endothelial cells at sites of great curvature (atheroprotective sites) of C57BL/6J mouse aorta by en face immunostaining. Pharmacological activation of NRF2 by sulforaphane reduces endothelial cell activation at atheroprone sites (inner curvature) by suppressing p38/VCAM1 signaling pathway in a KLF2-dependent manner [75]. Additionally, statins also activate the NRF2 pathway, and NRF2-dependent expression of various cytoprotective genes, such as HO-1. Simultaneous treatment with UF and atorvastatin has synergistic effects in protecting against H2O2-induced oxidant injury in endothelial cells [76].
YAP/TAZ/TEAD inhibitors
Verteporfin, also known as Visudyne, is a benzoporphyrin derivative photosensitizer widely used in photodynamic therapy in patients with macular degeneration. Verteporfin is the first identified pharmacological inhibitor of YAP by disrupting the YAP/TEAD interaction [77]. Intra-arterial administration of verteporfin combined with photoactivation reduces atherosclerosis and macrophage apoptosis in ApoE−/− mice [78]. In addition, many existing FDA-approved drugs, such as statins, have anti-atherosclerotic functions partially by inhibiting YAP/TAZ transactivation [26, 27]. These lines of evidence indicate the therapeutic potential of the use of YAP/TAZ/TEAD inhibitors to treat atherosclerosis.
HIF-1α inhibitors
HIF-1α is mainly induced by hypoxic stress which is very common during the development of atherosclerosis. The activation of HIF-1α promotes the development and enhances the vulnerability of atherosclerotic plaques [59, 60]. Therefore, down-regulation of HIF-1α expression and signaling holds promise for treating atherosclerosis. For example, evodiamine, a bioactive anti-inflammatory alkaloid isolated from the medicinal herb-Evodia rutaecarpa, can repress hypoxia-induced cyclooxygenase 2 (COX-2) expression by inhibiting HIF-1α [79]. Digoxin, a drug traditionally used in treating cardiac arrhythmia and heart failure, also inhibits HIF-1α activation. Specifically, digoxin treatment decreases HIF-1α protein expression in endothelial cells, and consequently digoxin inhibits endothelial cell growth and survival both in vitro and in vivo [80]. Importantly, digoxin has a dose-dependent inhibitory effect on atherosclerotic lesion formation and plasma lipid levels [81]. 2-Methoxyestradiol (2ME2) is an orally bioavailable small molecule inhibitor of HIF-1α with anti-tumor and anti-angiogenic activities. 2ME2 inhibits HIF-1α by depolymerizing microtubules and blocking the nuclear accumulation and transcriptional activity of HIF-1α [82]. In ApoE−/− mice, 2ME2 (66.6 μg/day) reduces atherosclerotic lesions [83], suggesting 2ME2 could be a potential anti-atherosclerotic drug.
NF-κB inhibitors
The NF-κB signaling pathway plays a crucial role in driving endothelial inflammation and dysfunction by orchestrating the expression of inflammatory cytokines/chemokines involved in atherosclerosis. In this scenario, endothelium-restricted inhibition of NF-κB, reduces atherosclerotic plaque formation in ApoE−/− mice [63, 64], indicating that blocking NF-κB-dependent pro-inflammatory gene expression in the arterial wall could retard the progression of the atherosclerotic process. In this regard, many NF-κB inhibitors, such as BMS-06 and vinpocetine, have been shown to reduce atherosclerosis in mice [84].
Concluding remarks and future perspectives
Studies in the past several decades have elucidated multiple mechanisms of atherosclerosis, such as endothelial dysfunction, oxidative stress, chronic inflammation, lipid deposition, and epigenetic dysregulation [2, 3, 15]. Despite significant advances in the understanding of pathogenesis of atherosclerosis and emerging pharmacotherapies, atherosclerosis and its clinical sequalae remains the major cause of morbidity and mortality worldwide. A deeper understanding of molecular and cellular mechanisms of atherogenesis and its progression will lead to the identification of novel therapeutic targets for atherosclerosis and accelerate the discovery process of target-based new cardiovascular drugs.
MSTFs are important master regulator of endothelial gene expression. Researches in the past decade have identified multiple MSTFs that are involved in regulating endothelial function and atherosclerosis. In this review, we mainly focused on the role of main MSTFs in regulating endothelial function, atherosclerosis and targeted pharmacotherapies. In addition to the MSTFs described above, there are several other MSTFs in endothelial cells, for example, early growth response-1 gene (Egr-1) [87], Hand2 [88], and Snail (which mediates endothelial mesenchymal transition (EndoMT)) [89]. In-depth elucidation of the regulation and roles of MSTFs in atherosclerosis is critical for designing effective therapeutic strategies against atherosclerosis.
However, there are several key questions (see Outstanding Questions) and challenges in translating findings from mechanobiological studies into new MSTF-targeted therapeutics. For example, the potential interaction network of multiple MSTFs regulates the expression of important genes in oxidative stress, inflammation, and metabolism in endothelial cells. Important interactions, such as interaction of KLF2 with NRF2 [51, 52, 90],NF-κB with HIF-1α [29, 30] have been described recently. Firstly, KLF2 potently inhibits the expression and activity of HIF-1α, while HIF-1α is also involved in DF-induced KLF2 downregulation [62]. Secondly, redox-independent regulation of inflammatory and metabolic gene expression by NRF2 [45, 91, 92] further highlights the potential interaction between KLF2 and NRF2. Thirdly, glucose metabolism also regulates inflammation in endothelial cells evidenced by the fact that PFKFB3-mediated increased glycolysis promotes endothelial inflammation [93]. Therefore, the interplay of oxidative stress, inflammation, and glucose metabolism (glycolysis) in endothelial cells is complex and may represent a new direction to study the effect of hemodynamic forces on endothelial function [29, 30, 51, 52, 90, 93] (Figure 3).
Outstanding questions.
How to identify novel mechanosensitive transcription factors (MSTFs)?
How do MSTFs crosstalk with each other to regulate vascular pathophysiology?
How are MSTFs regulated epigenetically (by DNA methylation, histone modification, and non-coding RNAs)?
How to identify novel atheroprotective drugs by targeting MSTFs?
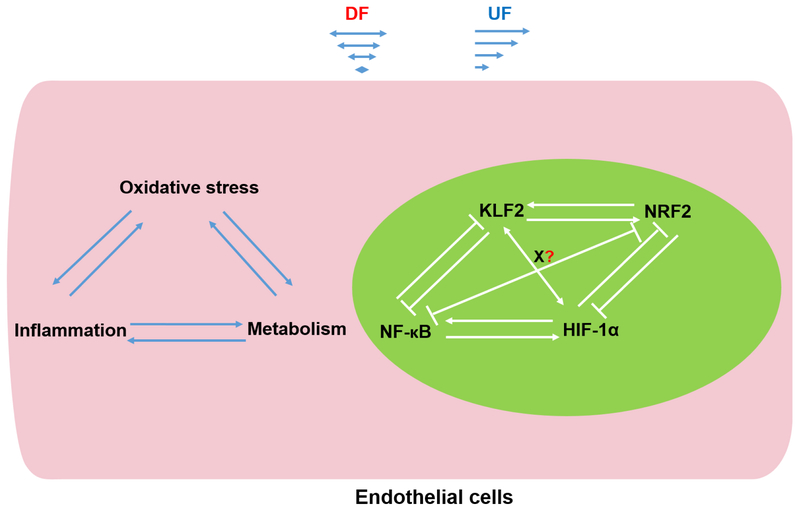
Figure 3. Interaction network of MSTFs.
The interaction of multiple MSTFs represent a complex interaction network that regulates the expression of genes involved in oxidative stress, inflammation, and metabolic programing (such as glycolysis) in ECs. Three biological processes-oxidative stress, inflammation and metabolism cross talk with each other. The KLF2/NRF2 crosstalk, and NF-κB/HIF-1α crosstalk are two examples. Glycolysis promotes endothelial inflammation, while KLF2 suppresses vascular inflammation, glycolysis, and HIF-1α expression/activity. It is plausible that KLF2 may negatively regulate DF induced upregulation of HIF-1α. On the other hand, HIF-1α could also possibly be involved in DF induced KLF2 downregulation. Additionally, redox-independent regulation of inflammatory and metabolic gene expression by NRF2 further pinponint the potential interaction network between KLF2 and NRF2. The elucidation of new MSTFs (transcription factor X) and their cross regulation with other MSTFs are critical for further understanding flow-induced transcriptional program and functional consequences.
In light of the important role of MSTFs in regulating endothelial function and atherosclerosis, the identification and elucidation of new endothelial MSTFs seem to be a pressing issue. Fortunately, recent biotechnological advances, such as genome-wide transcriptional profiling [94] could possibly assist us in identifying new MSTFs and accelerate MSTFs-based drug discovery (Figure 4). The increasing ease of accessibility to transcriptional profiling datasets in public repositories (such as NCBI Gene Expression Ominbus and Arrayexpress) will also assist in defining MSTF-regulated gene signatures. Further validation of the role of new MSTFs in atherosclerosis using genetically engineered mice will be needed for potential development of MSTFs targeting therapeutics (Figure 4). Last but not least, the pharmacokinetic/pharmacodynamic profile and potential side effects of MSTFs-tragetted therapeutics is another important consideration in translating preclinical studies into effective therapeutics of atherosclerosis.
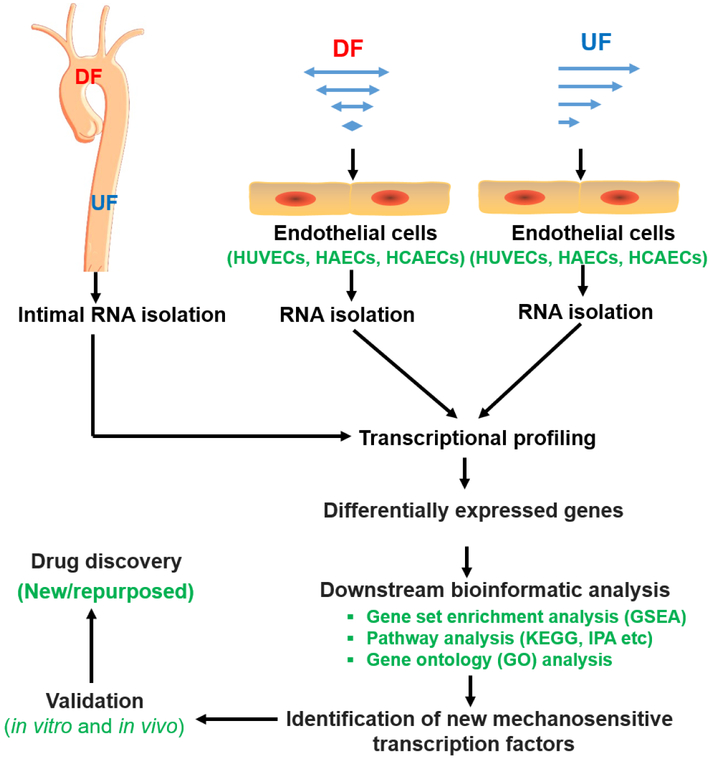
Figure 4. Discovery pipeline of novel MSTFs and targeted therapeutics.
Transcriptional profiling (such as RNA-sequencing or microarray) is commonly used to identify novel MSTFs. In vivo, intimal RNA samples from regions of DF and regions of unidirectional UF are collected for RNA-sequencing or microarray. In vitro, human ECs are exposed to UF, or DF before RNA is collected for transcriptional profiling. New MSTFs or gene regulation pathways can be identified by downstream bioinformatics analysis of differentially expressed genes. The newly identified MSTFs need to be validated both in cultured cells and in pre-clinical animal models. After validation, drug-screening system can be established to identify novel/repurposed pharmacological modulators of MSTFs, which can be further tested pre-clinically and clinically.
Abbreviations: HAEC, human aortic endothelial cells; HCAECs, human coronary artery endothelial cells; HUVECs, human umbilical vein endothelial cells; IPA, ingenuity® pathway analysis; KEGG, Kyoto encyclopedia of genes and genomes
Highlights.
Unidirectional laminar flow and disturbed flow differentially modulate several mechanosensitive transcription factors (MSTFs) in endothelial cells.
MSTFs regulate multiple aspects of endothelial function and vascular homeostasis, such as inflammation, proliferation, thrombosis, oxidative stress, and endothelial cell metabolism.
MSTFs crosstalk with each other and functionally regulates endothelial gene expression and function.
Targeting MSTFs could be a promising therapeutic strategy for atherosclerosis.
Acknowledgments
This work was supported by grants from NIH (HL09502, HL114570, HL128363 and HL130167 to Z.G.J) and the American Heart Association (17GRNT33660671 to Z.G.J) and Career Development Award from the American Heart Association (18CDA34110359 to S. X.). Figures in this review were prepared using elements from Science Slides and Servier Medical Arts (https://smart.servier.com/).
Glossary
- Atherosclerosis:
Atherosclerosis is a common lipid-driven disease in which lipids are retained and accumulated in the vessel wall. As a result, the vessel lumen become narrowed and hardened. At initial stage, atherosclerosis is asymptotic. When atherosclerosis progresses to more advanced stage, it causes severe symptoms, such as: heart attacks, stroke, and so on. Many risk factors (such as hyperlipidemia, hyperglycemia, physical inactivity, smoking, aging, and obesity) can lead to this disease.
- Disturbed flow (DF):
DF (also known as turbulent/oscillatory flow) is a complicated flow pattern that is irregular and non-uniform, including spatiotemporal changes in gradients of shear stress and flow direction. DF typically includes periods of reciprocating flow reversal that generates oscillatory wall shear stress. DF is associated with endothelial dysfunction (such as vascular inflammation), and focal distribution of atherosclerotic plaques.
- Unidirectional laminar flow (UF):
UF is also called as steady laminar flow. It is a well-ordered normal flow pattern in the blood vessel. UF is associated with high shear stress and is antiinflammatory as well as atheroprotective.
- Mechanotransducer:
In biomedical engineering, mechanotransducer indicates the specific molecules through which endothelial cells and other mechanotransducing cells sense and respond to mechanical cues (such as shear stress or cyclic strain) by converting them to biochemical/ electrochemical signals that can elicit specific cellular responses.
- Shear stress:
Shear stress is the mechanical force generated by flowing blood fluid. The magnitude of shear stress is defined as force/wall area (e.g., dyn/cm2) and the direction of shear stress is parallel to the direction of blood flow.
- Mechanosensitive transcription factors (MSTFs):
Specialized DNA binding proteins that respond to biomechanical treatments and control the rate of transcription of genetic information from DNA to mRNA. MSTFs regulate downstream gene expression by binding to specific sequences of DNA.
- Transcriptional profiling:
Transcriptional profiling, also known as expression profiling, refers to high-throughput sequencing techniques that are used to detect the expression amount of many transcripts (such as genes) in cells or tissues at the same time. The most common methods of transcriptional profiling include DNA microarray and RNA-sequencing.
Footnotes
Disclaimer Statement
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ et al. (2018) Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 137 (12), e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Xu S et al. (2018) Targeting epigenetics and non-coding RNAs in atherosclerosis: from mechanisms to therapeutics. Pharmacol Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasikara C et al. (2018) The role of non-resolving inflammation in atherosclerosis. J Clin Invest 128 (7), 2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankman LS et al. (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21 (6), 628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherepanova OA et al. (2016) Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective. Nat Med 22 (6), 657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu JJ and Chien S (2011) Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91 (1), 327–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rezvan A et al. (2011) Animal, in vitro, and ex vivo models of flow-dependent atherosclerosis: role of oxidative stress. Antioxid Redox Signal 15 (5), 1433–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies PF (2009) Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6 (1), 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe J and Berk BC (2014) Novel mechanisms of endothelial mechanotransduction. Arterioscler Thromb Vasc Biol 34 (11), 2378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmons RD et al. (2016) The role of endothelial mechanosensitive genes in atherosclerosis and omics approaches. Arch Biochem Biophys 591, 111–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak BR et al. (2014) Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J 35 (43), 3013–20, 3020a-3020d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeyens N et al. (2016) Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest 126 (3), 821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimbrone MA Jr. and Garcia-Cardena G (2016) Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res 118 (4), 620–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies PF et al. (2013) The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res 99 (2), 315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu S et al. (2018) Atherosclerosis Is an Epigenetic Disease. Trends Endocrinol Metab 29 (11), 739–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theodorou K and Boon RA (2018) Endothelial Cell Metabolism in Atherosclerosis. Front Cell Dev Biol 6, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eelen G et al. (2018) Endothelial Cell Metabolism. Physiol Rev 98 (1), 3–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatzizisis YS et al. (2007) Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 49 (25), 2379–93. [DOI] [PubMed] [Google Scholar]
- 19.Stone PH et al. (2012) Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation 126 (2), 172–81. [DOI] [PubMed] [Google Scholar]
- 20.Xu J et al. (2018) GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 173 (3), 762–775.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boon RA and Horrevoets AJ (2009) Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie 29 (1), 39–40, 41-3. [PubMed] [Google Scholar]
- 22.Villarreal G Jr. et al. (2010) Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun 391 (1), 984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajra L et al. (2000) The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A 97 (16), 9052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noguchi N and Jo H (2011) Redox going with vascular shear stress. Antioxid Redox Signal 15 (5), 1367–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohan S et al. (1997) Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am J Physiol 273 (2 Pt 1), C572–8. [DOI] [PubMed] [Google Scholar]
- 26.Wang L et al. (2016) Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. [DOI] [PubMed] [Google Scholar]
- 27.Wang KC et al. (2016) Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci U S A 113 (41), 11525–11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B et al. (2019) c-Abl regulates YAPY357 phosphorylation to activate endothelial atherogenic responses to disturbed flow. J Clin Invest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng S et al. (2017) Mechanical Activation of Hypoxia-Inducible Factor 1alpha Drives Endothelial Dysfunction at Atheroprone Sites. Arterioscler Thromb Vasc Biol 37 (11), 2087–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu D et al. (2017) HIF-1alpha is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Min E and Schwartz M (2019) Translocating transcription factors in fluid shear stress-mediated vascular remodeling and disease. Exp Cell Res doi: 10.1016/j.yexcr.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweet DR et al. (2018) Kruppel-Like Factors in Vascular Inflammation: Mechanistic Insights and Therapeutic Potential. Front Cardiovasc Med 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu W et al. (2011) Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation 124 (5), 633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang Y and Davies PF (2012) Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol 32 (4), 979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou G et al. (2012) Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest 122 (12), 4727–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkins GB et al. (2008) Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circ Res 103 (7), 690–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emini Veseli B et al. (2017) Animal models of atherosclerosis. Eur J Pharmacol 816, 3–13. [DOI] [PubMed] [Google Scholar]
- 38.Lingrel JB et al. (2012) Myeloid-specific Kruppel-like factor 2 inactivation increases macrophage and neutrophil adhesion and promotes atherosclerosis. Circ Res 110 (10), 1294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sangwung P et al. (2017) KLF2 and KLF4 control endothelial identity and vascular integrity. 2 (4), e91700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu C et al. (2015) Mechanosensitive PPAP2B Regulates Endothelial Responses to Atherorelevant Hemodynamic Forces. Circ Res 117 (4), e41–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krause MD et al. (2018) Genetic variant at coronary artery disease and ischemic stroke locus 1p32.2 regulates endothelial responses to hemodynamics. Proc Natl Acad Sci U S A 115 (48), E11349–e11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doddaballapur A et al. (2015) Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler Thromb Vasc Biol 35 (1), 137–45. [DOI] [PubMed] [Google Scholar]
- 43.Dunn J et al. (2014) Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest 124 (7), 3187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu W et al. (2018) Kruppel-like factor 14, a coronary artery disease associated transcription factor, inhibits endothelial inflammation via NF-kappaB signaling pathway. Atherosclerosis 278, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayes JD and Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39 (4), 199–218. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki T et al. (2013) Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci 34 (6), 340–6. [DOI] [PubMed] [Google Scholar]
- 47.Takabe W et al. (2011) Anti-atherogenic effect of laminar shear stress via Nrf2 activation. Antioxid Redox Signal 15 (5), 1415–26. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi EH et al. (2016) Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 7, 11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen XL et al. (2003) Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem 278 (2), 703–11. [DOI] [PubMed] [Google Scholar]
- 50.Parmar KM et al. (2006) Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest 116 (1), 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim M et al. (2012) Laminar flow activation of ERK5 protein in vascular endothelium leads to atheroprotective effect via NF-E2-related factor 2 (Nrf2) activation. J Biol Chem 287 (48), 40722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fledderus JO et al. (2008) KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol 28 (7), 1339–46. [DOI] [PubMed] [Google Scholar]
- 53.Dai G et al. (2007) Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res 101 (7), 723–33. [DOI] [PubMed] [Google Scholar]
- 54.Panciera T et al. (2017) Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol 18 (12), 758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plouffe SW et al. (2018) The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J Biol Chem 293 (28), 11230–11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schober JM et al. (2002) Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood 99 (12), 4457–65. [DOI] [PubMed] [Google Scholar]
- 57.Xu S et al. (2016) Atheroprotective laminar flow inhibits Hippo pathway effector YAP in endothelial cells. Transl Res 176, 18–28 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi HJ et al. (2018) TNF-alpha-Induced YAP/TAZ Activity Mediates Leukocyte-Endothelial Adhesion by Regulating VCAM1 Expression in Endothelial Cells. Int J Mol Sci 19 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo L et al. (2018) CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J Clin Invest 128 (3), 1106–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sluimer JC et al. (2008) Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol 51 (13), 1258–65. [DOI] [PubMed] [Google Scholar]
- 61.Akhtar S et al. (2015) Endothelial Hypoxia-Inducible Factor-1alpha Promotes Atherosclerosis and Monocyte Recruitment by Upregulating MicroRNA-19a. Hypertension 66 (6), 1220–6. [DOI] [PubMed] [Google Scholar]
- 62.Li X et al. (2017) Beyond Impressions: How Altered Shear Stress Connects Hypoxic Signaling to Endothelial Inflammation. Arterioscler Thromb Vasc Biol 37 (11), 1987–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gareus R et al. (2008) Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab 8 (5), 372–83. [DOI] [PubMed] [Google Scholar]
- 64.Song D et al. (2018) Selective inhibition of endothelial NF-kappaB signaling attenuates chronic intermittent hypoxia-induced atherosclerosis in mice. Atherosclerosis 270, 68–75. [DOI] [PubMed] [Google Scholar]
- 65.Partridge J et al. (2007) Laminar shear stress acts as a switch to regulate divergent functions of NF-kappaB in endothelial cells. Faseb j 21 (13), 3553–61. [DOI] [PubMed] [Google Scholar]
- 66.Parmar KM et al. (2005) Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem 280 (29), 26714–9. [DOI] [PubMed] [Google Scholar]
- 67.Sen-Banerjee S et al. (2005) Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 112 (5), 720–6. [DOI] [PubMed] [Google Scholar]
- 68.Gracia-Sancho J et al. (2010) Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res 85 (3), 514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gan W et al. (2016) ERK5/HDAC5-mediated, resveratrol-, and pterostilbene-induced expression of MnSOD in human endothelial cells. Mol Nutr Food Res 60 (2), 266–77. [DOI] [PubMed] [Google Scholar]
- 70.Xu Y et al. (2017) Suberanilohydroxamic Acid as a Pharmacological Kruppel-Like Factor 2 Activator That Represses Vascular Inflammation and Atherosclerosis. J Am Heart Assoc 6 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Y et al. (2017) Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells. Sci Rep 7 (1), 6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Do GM et al. (2011) Tannic acid is more effective than clofibrate for the elevation of hepatic beta-oxidation and the inhibition of 3-hydroxy-3-methyl-glutaryl-CoA reductase and aortic lesion formation in apo E-deficient mice. Br J Nutr 106 (12), 1855–63. [DOI] [PubMed] [Google Scholar]
- 73.Slegtenhorst BR et al. (2018) A Mechano-Activated Cell Reporter System as a Proxy for Flow-Dependent Endothelial Atheroprotection. SLAS Discov 23 (8), 869–876. [DOI] [PubMed] [Google Scholar]
- 74.Mimura J and Itoh K (2015) Role of Nrf2 in the pathogenesis of atherosclerosis. Free Radic Biol Med 88 (Pt B), 221–232. [DOI] [PubMed] [Google Scholar]
- 75.Zakkar M et al. (2009) Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol 29 (11), 1851–7. [DOI] [PubMed] [Google Scholar]
- 76.Ali F et al. (2009) Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J Biol Chem 284 (28), 18882–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu-Chittenden Y et al. (2012) Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev 26 (12), 1300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jain M et al. (2016) Intra-Arterial Drug and Light Delivery for Photodynamic Therapy Using Visudyne(R): Implication for Atherosclerotic Plaque Treatment. Front Physiol 7, 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu YN et al. (2009) Evodiamine represses hypoxia-induced inflammatory proteins expression and hypoxia-inducible factor 1alpha accumulation in RAW264.7. Shock 32 (3), 263–9. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H et al. (2008) Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci U S A 105 (50), 19579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi H et al. (2016) Digoxin reduces atherosclerosis in apolipoprotein E-deficient mice. Br J Pharmacol 173 (9), 1517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mabjeesh NJ et al. (2003) 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3 (4), 363–75. [DOI] [PubMed] [Google Scholar]
- 83.Bourghardt J et al. (2007) The endogenous estradiol metabolite 2-methoxyestradiol reduces atherosclerotic lesion formation in female apolipoprotein E-deficient mice. Endocrinology 148 (9), 4128–32. [DOI] [PubMed] [Google Scholar]
- 84.Pamukcu B et al. (2011) The nuclear factor--kappa B pathway in atherosclerosis: a potential therapeutic target for atherothrombotic vascular disease. Thromb Res 128 (2), 117–23. [DOI] [PubMed] [Google Scholar]
- 85.Nicholls SJ et al. (2016) Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. Jama 316 (22), 2373–2384. [DOI] [PubMed] [Google Scholar]
- 86.Ridker PM et al. (2017) Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377 (12), 1119–1131. [DOI] [PubMed] [Google Scholar]
- 87.Khachigian LM et al. (1997) Egr-1 is activated in endothelial cells exposed to fluid shear stress and interacts with a novel shear-stress-response element in the PDGF A-chain promoter. Arterioscler Thromb Vasc Biol 17 (10), 2280–6. [DOI] [PubMed] [Google Scholar]
- 88.Bjorck HM et al. (2012) Characterization of shear-sensitive genes in the normal rat aorta identifies Hand2 as a major flow-responsive transcription factor. PLoS One 7 (12), e52227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mahmoud MM et al. (2017) Shear stress induces endothelial-to-mesenchymal transition via the transcription factor Snail. Sci Rep 7 (1), 3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuosmanen SM et al. (2018) NRF2 regulates endothelial glycolysis and proliferation with miR-93 and mediates the effects of oxidized phospholipids on endothelial activation. Nucleic Acids Res 46 (3), 1124–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki T and Yamamoto M (2017) Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J Biol Chem 292 (41), 16817–16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamamoto M et al. (2018) The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol Rev 98 (3), 1169–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loscalzo J and Xiao W (2018) PFKFB3-Mediated Enhancement of Gycolysis Supports Inflammation in Human Systemic Arterial Endothelial Cells. Circulation 138, A13027. [Google Scholar]
- 94.Xu S (2017) Transcriptome Profiling in Systems Vascular Medicine. Front Pharmacol 8, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sussan TE et al. (2008) Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS One 3 (11), e3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barajas B et al. (2011) NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler Thromb Vasc Biol 31 (1), 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruotsalainen AK et al. (2018) Nrf2 deficiency impairs atherosclerotic lesion development but promotes features of plaque instability in hypercholesterolemic mice. Cardiovasc Res. [DOI] [PubMed] [Google Scholar]
- 98.Aarup A et al. (2016) Hypoxia-Inducible Factor-1alpha Expression in Macrophages Promotes Development of Atherosclerosis. Arterioscler Thromb Vasc Biol 36 (9), 1782–90. [DOI] [PubMed] [Google Scholar]
- 99.Maejima T et al. (2014) Direct evidence for pitavastatin induced chromatin structure change in the KLF4 gene in endothelial cells. PLoS One 9 (5), e96005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hwang AR et al. (2017) Fluvastatin inhibits AGE-induced cell proliferation and migration via an ERK5-dependent Nrf2 pathway in vascular smooth muscle cells. PLoS One 12 (5), e0178278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sorrentino G et al. (2014) Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol 16 (4), 357–66. [DOI] [PubMed] [Google Scholar]
- 102.Dichtl W et al. (2003) HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 23 (1), 58–63. [DOI] [PubMed] [Google Scholar]
- 103.Deng YH et al. (2011) Inhibition of TNF-alpha-mediated endothelial cell-monocyte cell adhesion and adhesion molecules expression by the resveratrol derivative, trans-3,5,4"-trimethoxystilbene. Phytother Res 25 (3), 451–7. [DOI] [PubMed] [Google Scholar]
- 104.Ungvari Z et al. (2010) Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299 (1), H18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shehatou GS and Suddek GM (2016) Sulforaphane attenuates the development of atherosclerosis and improves endothelial dysfunction in hypercholesterolemic rabbits. Exp Biol Med (Maywood) 241 (4), 426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wei J et al. (2013) Essential role of transient receptor potential vanilloid type 1 in evodiamine-mediated protection against atherosclerosis. Acta Physiol (Oxf) 207 (2), 299–307. [DOI] [PubMed] [Google Scholar]
- 107.Mazurek R et al. (2017) Vascular Cells in Blood Vessel Wall Development and Disease. Adv Pharmacol 78, 323–350. [DOI] [PMC free article] [PubMed] [Google Scholar]