Abstract
Generalized spike-wave discharges (SWD) are the hallmark of generalized epilepsy on the electroencephalogram (EEG). In clinically obvious cases, generalized SWD produce myoclonic, atonic/tonic or absence seizures with brief episodes of staring and behavioral unresponsiveness. However, some generalized SWD have no obvious behavioral effects. A serious challenge arises when patients with no clinical seizures request driving privileges and licensure, yet their EEG shows generalized SWD. Specialized behavioral testing has demonstrated prolonged reaction times or missed responses during SWD, which may present a driving hazard even when patients or family members do not notice any deficits. On the other hand, some SWD are truly asymptomatic in which case driving privileges should not be restricted. Clinicians often decide on driving privileges based on SWD duration or other EEG features. However, there are currently no empirically-validated guidelines for distinguishing generalized SWD that are “safe” versus “unsafe” for driving. Here we review the clinical presentation of generalized SWD and recent work investigating mechanisms of behavioral impairment during SWD with implications for driving safety. As a future approach, computational analysis of large sets of EEG data during simulated driving utilizing machine learning could lead to powerful methods to classify generalized SWD as safe vs. unsafe. This may ultimately provide more objective EEG criteria to guide decisions on driving safety in people with epilepsy.
Keywords: subclinical epileptiform discharges, driving simulation, driving safety, consciousness, absence seizures, epilepsy
1. Introduction
On the electroencephalogram (EEG), generalized seizures and epilepsies are characterized by generalized spike-wave discharges (SWDs). These epileptiform discharges are most common in childhood absence epilepsy (CAE) and juvenile myoclonic epilepsy (JME). However, varieties of generalized SWDs may be present in other disorders (such as metabolic encephalopathies) or even as benign EEG variants (as in 6Hz “phantom” spike-wave) [1, 2]. Mechanistically, spike-wave discharges are thought to share a similar involvement of thalamocortical networks, but the EEG features (i.e., amplitude, frequency, rhythmicity, etc.) of the discharges and the accompanying clinical features differ among seizure and epilepsy types. Emerging evidence from hemodynamic, electrophysiology, and behavior studies suggest that EEG features of the discharges may predict the extent of behavioral impairment, indicating a possibility of using EEG characteristics to predict driving safety of patients with epilepsy, particularly those with subclinical spike-wave discharges.
2. Natural history and clinical course of generalized spike-wave epilepsy
The four classic generalized epilepsy syndromes consist of Childhood Absence Epilepsy (CAE), Juvenile Absence Epilepsy (JAE), Juvenile Myoclonic Epilepsy (JME), and Generalized Tonic-Clonic Alone (GTCA) [3, 4]. These epilepsies are similar in their suggested genetic etiologies, non-focal onsets, involvement of thalamocortical networks, and characteristic generalized SWDs on EEG that can be provoked by sleep deprivation, hyperventilation, and photic stimulation. With frontally predominant 2.5–4Hz generalized SWDs over normal EEG background, absence seizures are observed in CAE and JAE and are often associated with brief (3–10 seconds) lapses in consciousness with cessation of ongoing activity followed by a rather immediate return to activity [5, 6]. JME typically presents in adolescence with myoclonic, tonic-clonic and absence seizures exhibiting frontally predominant 3.5–5Hz polyspike-wave discharges on EEG. 10–37% of patients with JME have absence seizures, and subclinical epileptiform discharges may persist and confer cognitive impairments [7–9]. GTCA typically presents in late adolescence with generalized tonic-clonic seizures (GTCs); absence seizures are uncommon manifestations. Cessation of activity in absence seizures and GTCs pose an obvious risk to complex behaviors such as driving. The generalized epilepsies are more thoroughly reviewed elsewhere [10, 11].
Generalized SWDs, and for that matter generalized seizures and epilepsies, confer variable behavioral impairments, suggesting that generalized seizures do not always involve the brain the same way [12–14]. Absence seizures commonly seen in CAE, JAE, and JME have been best studied for their varied behavioral effects among patients and among seizure episodes in the same patient. These studies, which have employed behavior testing paradigms ranging from simple repetitive tapping to responding to verbal stimuli, have identified interesting behavioral features of absence seizures. Tasks that place higher demands on decision-making and attention are more likely to identify behavioral and cognitive impairments during seizures [13, 15–17]. In investigations using tasks requiring verbal responses during seizures, subjects demonstrate varied levels of impairments: while some do not respond at all, others turn their gaze to the examiner without verbally responding, still others respond appropriately [18–20]. Absence seizures are less frequent in mentally demanding tasks and they can be ended by external stimulation [19, 21, 22]. Studies have reached differing conclusions on whether behavioral impairments differ between absence seizures in CAE, JAE and JME [23–26]. The possible role of physiological properties of SWD in determining behavioral severity is discussed further in the sections that follow.
It is important to clearly define the difference between absence seizures and subclinical generalized SWD. Absence seizures are defined based on the presence of both generalized 3–4 Hz SWD and impaired behavioral responsiveness. In contrast, truly subclinical generalized SWD produce no behavioral deficits. However, behavioral deficits in absence seizures are not always easy to detect by family members or even by basic clinical observation, making this distinction challenging in some patients. Apparently “subclinical” generalized SWD, i.e. those that are not accompanied by obvious clinical impairments may nevertheless demonstrate subtle behavioral deficits that are not appreciated by the patient, relatives or clinicians but can be demonstrated on specialized behavior testing [13, 17, 27–30]. Specialized testing may be needed to detect deficits either because the deficits are subtle (e.g. slowed reaction times) or because they are severe (e.g. completely missed responses) but very brief and therefore will be missed without critically timed and brief stimuli. Because such behavioral testing is not readily available to many clinicians, some have proposed defining absence seizures purely based on EEG duration greater than 3 or 4 seconds. Although it is true that longer generalized SWD on EEG are more likely to impair behavior than shorter ones [23, 31] there are many exceptions to this rule. For example, several studies have demonstrated transient impairments in cognition in generalized SWD as brief as 0.5 seconds long, and conversely no impairment with generalized SWD lasting much longer than 4 seconds [28, 30, 32–35]. Therefore, absence seizures should not be defined based on EEG duration alone. Careful behavioral testing is needed to determine whether generalized SWD cause deficits or not. Importantly, deficits in apparently subclinical SWD can include subtle changes in reaction times, but can also include brief episodes of complete behavioral arrest [34, 36, 37]. Research on driving has demonstrated that glancing away from the road for as brief as 1 second significantly increases the risk of a crash [38, 39]. Therefore, transient behavioral arrest even during a brief SWD could potentially be catastrophic if it coincides with appearance of an obstacle while driving. Conversely, some SWD may be truly asymptomatic and should not lead to restriction of driving privileges. More research is needed to understand how to best distinguish these situations and determine driving safety in patients with generalized SWD.
Generalized seizures and epilepsies demonstrate different courses over the lifetime of patients. In one meta-analysis of 23 study cohorts representing 2,303 patients with CAE, seizure remission rates ranged from 21% to 89%, reflecting heterogeneous inclusion criteria and methodologies of the different studies [40]. An estimated two-thirds of patients with CAE achieve remission of seizures later in adolescence, ceasing to need treatment indefinitely [40–43]. Yet patients with CAE who develop generalized tonic-clonic seizures in the course of their disease are less likely to achieve remission of absence seizures [40]. Unlike patients with CAE whose seizures often remit with age, patients with JAE, JME, GTCA, and other epilepsy syndromes characterized by generalized SWDs are also less likely to achieve remission of seizures without medications and typically need treatment for life [44–46]. Even in clinical remission, patients may still have apparently subclinical generalized SWDs on EEG, and as already noted these epileptiform discharges may be associated with neurocognitive and/or behavioral impairments detectable only with specialized testing [17, 27–30]. Because the true behavioral significance of apparently asymptomatic generalized SWD is often uncertain in standard clinical practice, clinicians are presented with a challenging dilemma when asked about driving privileges.
3. Driving safety and epilepsy
Given its obvious relation to employment, access to social, educational, and economic opportunities, it is no surprise that in one survey of 81 patients with epilepsy driving was the most frequently cited area of concern[47]. Although patients highly value their driving privileges, most nations (and states, in the U.S.) have regulations restricting these privileges. For lawmakers, the challenge is in establishing restrictions that suitably balance patients’ driving privileges and safety as well as the public’s safety. This challenge is reflected in the varied and often-changing driving regulations. For example, in the United States, individual states maintain the right to enact laws restricting driving among people with epilepsy. While most states require seizure-free intervals of 3 months or longer (median of 6 months), some states adopt evaluations by physicians and/or medical advisory boards, giving room for consideration of factors such as changes in antiepileptic drugs, presence of auras, or nocturnal seizures [48, 49]. In the United States, 6 states – California, Oregon, Nevada, Delaware, Pennsylvania, and New Jersey – mandate healthcare providers to report seizures to driver licensing authorities. In general, countries in the European Union require 1 year of seizure-freedom before granting driving privileges, but there are allowances for patients with nocturnal seizures only, seizures that never impair consciousness, etc [50, 51]. The Epilepsy Foundation of America maintains a database for up-to-date driving laws in the various US states. Similarly, the UK Epilepsy Society maintains an online interactive guide on driving privileges of people with epilepsy [52].
Variations in regulations on driving privileges of people with epilepsy is very much a reflection of our limited knowledge of risk of motor vehicle crashes (MVCs) among people with epilepsy. Most studies attempting to quantify MVC rates among people with epilepsy have relied on surveys and retrospective reviews of medical and government databases. These studies have largely suggested a moderate increase in MVC rates among people with epilepsy, but there are a few studies that have found no increase in MVC rate among people with epilepsy [53]. One study reviewing Wisconsin transportation records and medical records of people with epilepsy from one medical center (241 people with epilepsy and 30,420 controls) estimated the relative risk of a MVC at 1.33 (p-value = 0.04), suggesting a slightly increased risk of MVCs among people with epilepsy [54]. In a more recent study that surveyed 16,958 people with epilepsy and 8,888 controls, the investigators found no increase in the risk of MVCs among people with epilepsy (odd ratio, OR = 0.77) after adjusting for driving experience, mileage, age, and sex [55]. However, that same study, found a 40% increase in risk for serious injuries from MVCs among people with epilepsy. In the US, an estimated 0.2% of annual fatalities from MVCs are attributed to seizures, but there is some evidence that seizure-related MVCs are more likely to result in severe injuries and/or property damage when compared to MVCs not related to seizures [55–57].
More complex than defining the MVC rate among people with epilepsy is determining the appropriate length of seizure freedom before granting driving privileges. In an earlier study, seizure freedom for more than 3 years significantly reduced the risks of having any MVC (OR: 0.74, 95% CI: 0.62, 0.87) and MVCs that result in injuries (OR: 0.66, 95% CI: 0.46, 0.93) [55]. Subsequent studies have shown an association between longer seizure-free intervals and significantly reduced MVC rates [58, 59]. In the 2005 European Union’s regulations, 20% seizure recurrence rate was adopted as the tolerable Chance of an Occurrence of Seizure in the next Year (COSY) among non-commercial drivers. Using data from more than 630 subjects in the Multicentre study of early Epilepsy and Single Seizures (MESS), Bonnett et al. showed that at six months after an unprovoked index seizure the risk of seizure recurrence in the subsequent 12 months was less than 20% for those who started antiepileptic drugs[59]. The risk for those who did not start AEDs was 18%. Similar findings have been reported using data from the UK-based multicenter Standard versus New Antiepileptic Drugs (SANAD) study [60].
The current evidence on auras in reducing risk of MVCs among people with epilepsy is inconclusive. Some previous studies had suggested that people with seizures that are preceded by auras were less likely to have MVCs, but findings in more recent studies have differed [55, 61–63]. In a recent study of 215 patients reporting seizures that occurred while driving, 40.4% of patients with history of seizure-related MVCs reported having reliable auras; this was similar (p-value = 0.56) to the 44.6% of those without histories of seizure-related MVCs who reported having reliable auras.
Seizure semiology such as hallucination, visual or motor impairment, and loss of consciousness may be related to risk of MVCs [64]. In a limited study recording 22 seizures in 13 patients playing a computerized driving game, rFactor, driving impairment during seizures was highly variable, showing severe impairment in some seizures and no impairment in others [65]. Differences in the reported rates of MVCs among people with epilepsy clearly demonstrate the limitations of retrospective methods employed in previous studies. Well-designed prospective studies are needed to establish the risk of MVCs and to conclusively identify associations between seizure-related features and MVC rates [66].
In addition to deficits during seizures, other relevant factors for driving safety in epilepsy patients may include patient age, effect of anti-epileptic medications, and other epilepsy comorbidities including structural and connectivity abnormalities and cognitive impairments associated with the underlying disorder [67–70]. In JME, for instance, there is evidence of impaired working memory and increased risk-taking behavior, and ethosuximide, the most commonly used medication in treating absence epilepsy, may result in psychomotor slowing and attentional dysfunction.
The possible role of apparently subclinical or interictal epileptiform discharges in epilepsy driving safety is also important to consider. Effects of focal interictal spikes on cognition are discussed extensively elsewhere, and here we instead focus on apparently subclinical generalized SWD. Given the previously discussed influence of generalized spike-wave discharges on simple behavioral tests like repetitive tapping, it is necessary to investigate the influence of generalized SWDs on driving, a more complex behavior. Generalized SWD even as brief as 1 second or less can interfere with behavior, including episodes of complete behavioral arrest [34, 36, 37]. Given that distractions or glancing away from the road for as brief as 1 second can significantly increase crash risk while driving this is an important clinical problem. Aldenkamp has pointed out that the acute behavioral effects of subclinical epileptiform discharges may be overestimated and that deficits may instead be due to chronic interictal attentional impairment, or subtle seizures misidentified as interictal discharges [71]. Nevertheless, although most clinicians will allow patients with brief focal interictal epileptiform discharges to drive, in the case of frequent brief generalized SWD many clinicians are reluctant to let patients drive without direct evidence that their ability to respond is intact. Such evidence is challenging to obtain in a clinical setting and no current widely accepted standards exist for evaluating driving safety in patients with subclinical generalized SWD. Limited studies of driving during epileptiform activity, including generalized SWD, have provided some initial evidence of impaired driving in some cases including virtual crashes [28, 65, 74–76]. Therefore, more prospective research is needed to fully investigate behavioral impairment specifically during generalized SWD and to determine the mechanisms and electrographic characteristics of SWD that do or do not present a safety hazard. In the sections that follow we first discuss recent work elucidating possible mechanisms of impaired consciousness in generalized SWD, and next return to applications to driving safety.
4. Mechanisms of behavior impairment in generalized spike-wave discharges
In earlier works employing EEG and video recordings while subjects completed cognitive and IQ tests, Aldenkamp and colleagues demonstrated that occurrence of generalized epileptiform discharges - even when subclinical - resulted in transient impairments in cognitive function that potentially accumulated over time and partly accounted for long-term cognitive and intelligence deficits in some epilepsy syndromes [77–79]. Presumably, transient cognitive impairments such as prolongation of reaction time during subclinical generalized epileptiform discharges affect more than performance on neuropsychological testing and may even affect driving as more recent studies have demonstrated [74, 75]. Recent advancements in EEG analysis and functional neuroimaging have provided opportunities to investigate associations between features of generalized SWDs and behavioral impairments. While there remains much work to be done in this area, findings from the few available studies have advanced our understanding of the mechanisms underlying behavioral deficits observed in generalized SWDs, and we are gaining grounds in predicting the influence of SWDs on behavior.
It has long been recognized that alterations in consciousness vary at different times during a given episode of spike-wave discharge. Earlier studies using motor and verbal tests reported only subtle behavioral impairments in the first 3 seconds and the last 3–5 seconds of absence seizures; more severe impairments occurred in the intervening seconds of the discharges [15, 80, 81]. Later studies employing reaction time studies demonstrated that impairments are detectable at onset of SWDs or even in the preceding seconds [15, 82]. Further, there is evidence from tapping tasks that behavioral deficits in generalized SWDs may persist for a few seconds after cessation of the epileptiform discharge [15, 20]. The differing results of these studies is reflective of the different behavioral tests employed, suggesting that the severity of behavioral and cognitive impairments during generalized SWDs may be associated with specific tasks and time courses during the seizures.
More recent studies using Blood Oxygen Level Dependent functional Magnetic Resonance Imaging (BOLD-fMRI) have replicated and extended some of the findings of these earlier studies. Bai et al. recorded EEG and fMRI during 88 typical absence seizures in 9 children while completing continuous performance tasks (CPT) and repetitive tapping tests (RTT) [83]. Significant impairments in performance on both behavior tests were noticed during SWDs, but was more severe on the more attentionally demanding CPT task compared to the easier RTT task. In addition, behavior tended to gradually improve towards the end of SWD for both tasks. Concurrent BOLD-fMRI showed signal increases in orbital/medial frontal and medial/lateral parietal cortex more than 5 seconds before onset of electrographic seizures; signal decreases were noticed to last more than 20 seconds after seizure end. Another recent study of children with CAE showed variable behavioral impairment associated with variable fMRI amplitude from one SWD to the next even in the same patient [34]. Of note, behavioral impairment in some cases included complete behavioral arrest in response to external stimuli during SWD lasting as briefly as 2 seconds, whereas other SWD lasting as long as 8 seconds caused no behavioral impairment [34]. In an interesting single case report, Moeller et al. reported the absence of any cognitive impairment in a 14 year old girl who had generalized SWDs lasting up to 10 seconds during EEG-fMRI recording with CPT [84]. Again, generalized SWDs demonstrate dynamic behavioral influences based on the difficulty of the task, the involved cognitive domains, and the specific time during the course of the discharge. This dynamism of SWDs suggests involvement of different subcortical-cortical networks, or involvement of networks to varying degree, in generation, maintenance, propagation, and termination of these seizures.
Given the variable impact of generalized SWDs on behavior and cognition, some investigators have explored the associations between clinical features (like age and epilepsy syndrome) and EEG features of the SWDs. Moreover, others have explored associations between EEG features of SWDs and behavioral impairments. Reviewing 509 seizures in 70 children with CAE, JAE, or JME, Sadleir et al. observed that the specific epilepsy syndrome was associated with the number of spikes per wave in a given seizure, with JME and JAE having similar number of spikes per wave but more than CAE[85]. In the same study, older subjects were more likely to have organized SWDs, and neither seizure duration (p-value = 0.66) nor presence of clinical features (p-value = 0.14) was associated with the number of spikes per wave. Seizures provoked by photic stimulation were at increased likelihood to have polyspikes on EEG than did seizures provoked by hyperventilation. These results demonstrate the intricate relationship between clinical features and EEG features of generalized SWDs.
Earlier behavioral tests suggested that longer seizures, larger amplitude or other EEG features lead to more severe impairments in behavior, although differing results have been reported by some studies [15, 17, 23, 35, 86–91]. In one study reviewing video EEG recordings of 509 absence seizures in 70 treatment-naive children with diagnosis of CAE, JAE or JME, patient’s age and seizure duration significantly influenced the level of awareness during seizures [23]. Older children were more likely to be aware during seizures, and children were more likely to be aware during briefer seizures.
Recent work combining EEG and fMRI in absence seizures demonstrates that both the duration and the physiological amplitude of generalized SWDs are associated with extent of behavioral impairment during the discharges. Guo and colleagues recorded EEG-fMRI of seizures in 39 children who also completed behavioral testing with CPT and RTT [31]. As had been previously shown, performance on both CPT and RTT markedly declined during SWDs. Of more interest, SWDs during which subjects performed more poorly on behavior tests lasted longer (7.9 seconds vs. 3.8 seconds; p-value < 0.0001) and were associated with larger amplitudes of EEG and fMRI signals in widespread regions of the brain (Figures 1, 2, and 3). The larger EEG and fMRI signal amplitudes in the behavior-impairing SWDs were observable in the seconds preceding seizure onset and in three established neural networks (i.e., default mode, task-positive, and primary sensorimotor-thalamic networks) (Figure 3).
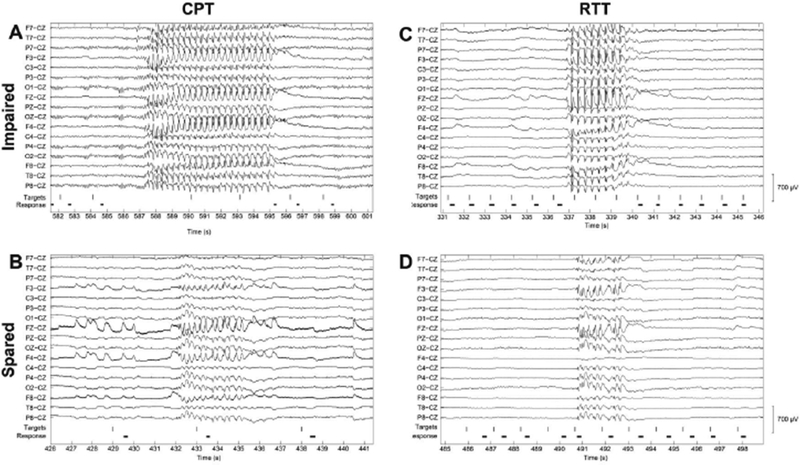
Figure 1. Examples of EEG recordings showing generalized spike-wave discharges with impaired versus spared performance.
On both the continuous performance task (CPT, A and B) and repetitive tapping task (RTT, C and D), generalized SWDs that resulted in impaired performance (A and C) lasted longer than those that spared performance (B and D). Shown here are out-of-scanner high-density EEG recordings with a limited number of channels shown for ease of viewing. Vertical lines indicate presentation of target letters during behavioral tasks. For CPT (A and B), the targets consisted of the letter “X” presented in a stream of other letters appearing once each second. For RTT (C and D), the targets consisted of any letter presented once each second. Durations of button presses are indicated by the heavy black horizontal lines. Reproduced with permission from Guo et al., Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet Neurology, 2016; 15(13) 1336–1345.
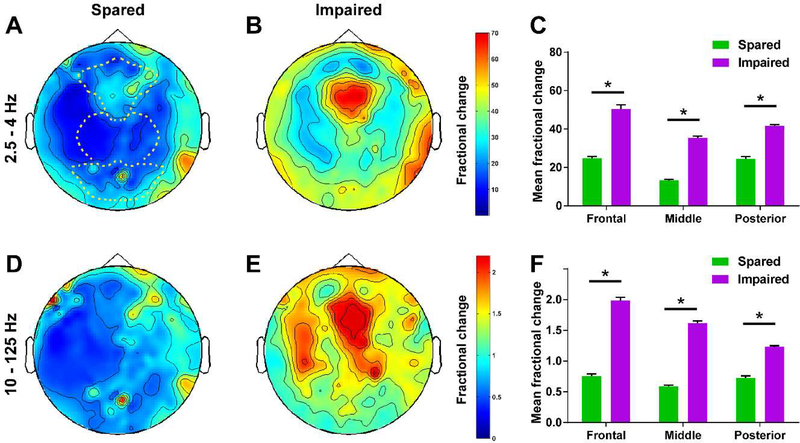
Figure 2. Generalized SWDs with impaired performance on CPT and RTT are associated with greater EEG amplitude in widespread brain regions.
(A and B) Wave component of SWDs represented by head maps of 256 channel high-density EEG power in the 2.5–4Hz frequency range for both performance sparing (A) and performance-impairing (B) SWDs. (C) Mean fractional EEG power in the 2.5–4Hz frequency range for seizures with spared versus impaired performance. (D and E) Spike component of SWDs represented by head maps of EEG power in the 10–125Hz frequency range for both spared (D) or impaired (E) performance. (F) Mean fractional EEG power in the 10–125Hz frequency range for seizures with spared versus impaired performance. Color scale bars are EEG power during seizures divided by baseline power before seizures (fractional power). The top color bar is for panels (A) and (B), and the bottom bar is for (D) and (E). Dashed lines in in (A) show regions used for analysis in (C) and (F) (frontal, middle, and posterior EEG contacts). *p<0.0001. Error bars represent standard error. Analysis is based on data from 30 performance-sparing seizures in 5 patients and 26 performance-impairing seizures in 8 patients. Reproduced with permission from Guo et al., Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet Neurology, 2016; 15(13) 1336–1345.
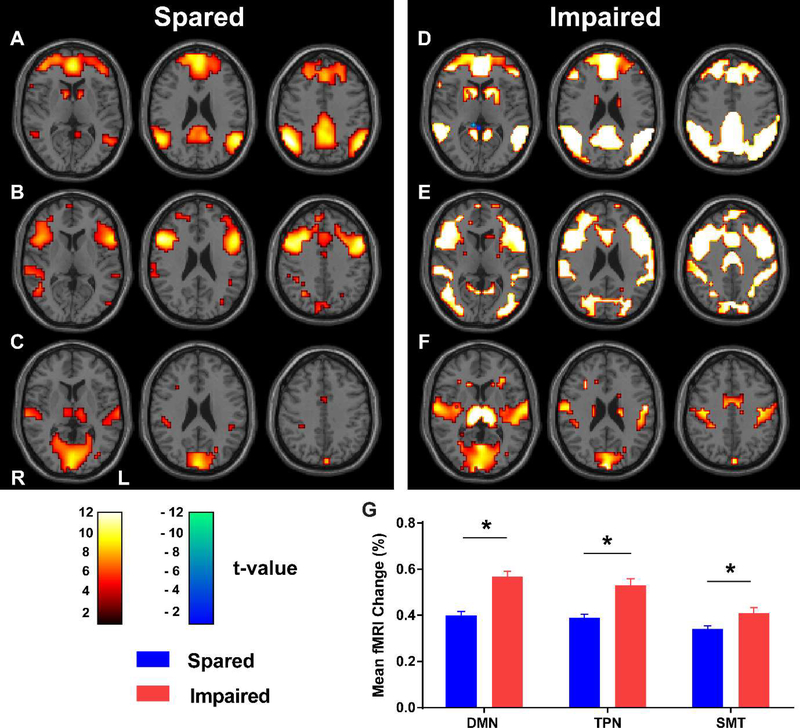
Figure 3. fMRI signals in three established brain networks during generalized spike-wave seizures that spared or impaired performance on behavior tests.
(A, B and C) Axial brain t-maps with fMRI signals for generalized SWDs with spared performance in the default-mode network (DMN) (A), task-positive network (TPN) (B), and primary sensorimotor-thalamic network (SMT) (C). (D, E and F) Corresponding axial brain t-maps with fMRI signals for generalized SWDs with impaired performance. Color scale bars show t values. Hot colors (white-orange) indicate significant fMRI changes in the same direction as the network-specific hemodynamic response functions. Cool colors (green-blue) indicate changes in the opposite direction. (G) Mean percentage change in BOLD-fMRI signal across seizures in each network. Analysis is based on data from 93 performance-sparing seizures in 17 patients and 112 performance-impairing seizures in 22 patients. Reproduced with permission from Guo et al., Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet Neurology, 2016; 15(13) 1336–1345.
The involvement of generalized SWDs with established networks necessary for attention and information processing has been demonstrated in other EEG-fMRI studies, shedding light on the subcortical-cortical networks involved in these epileptiform discharges [34, 92–105]. Together, these findings suggest that generalized SWDs that impair consciousness and behavior take advantage of preceding vulnerable states, recruit more neurons, and induce intense physiologic changes in established neural networks resulting in widespread cortical and subcortical involvement. While fMRI offers valuable insights into SWD pathophysiology, EEG remains a more clinically accessible tool for widespread use. Therefore, further investigation of EEG features which may predict altered responsiveness and impaired driving safety with SWD will be crucial in order to provide clinically useful guidance to patients and their health care providers regarding driving safety.
5. Driving evaluation in generalized spike-wave discharges
In some jurisdictions like Australia and Britain, an individual who continues to have epileptiform discharges on EEG may be denied driving privileges even after satisfying the required seizure-freedom period [106, 107]. This raises questions on the utility of the EEG and its predictive value in evaluating driving safety among people with epilepsy. Studies attempting to answer these questions are few, but their results have been informative.
Prolonged EEG evaluation is preferable to routine ambulatory EEG in defining seizure freedom and predicting seizure relapse among people with epilepsy. One study retrospectively analyzed reports from routine EEG and 6-hour video-EEG monitoring of 34 patients, 26 (76%) of whom had genetic generalized epilepsy [108]. 27 of the 34 patients were assessed as fit to drive based on interpretation of video-EEG by treating neurologists. Within 2 years of follow-up, 5 (19%) of the 27 deemed fit to drive had seizure relapses, albeit with identifiable precipitating factors. All 7 patients who had been assessed unfit to drive had seizure relapses (unprovoked in 4 patients) in the follow-up period. Thus, the relative risk of seizure relapse following an assessment of being unfit to drive based on 6-hour VEM was 5.4 (p-value = 0.00015). The relative risk among patients with genetic generalized epilepsy was 4.0 (p-value = 0.002). Interestingly, using the routine 30 minutes EEG reports alone, the relative risk of seizure relapse after being assessed fit to drive was 3.4 (p-value = 0.037) [108].
Prolonged EEG monitoring may be invaluable in identifying epileptiform activities that would otherwise be missed on routine EEG or are not reported by the patient and/or relatives during evaluation for granting driving privileges to people with epilepsy. A retrospective study analyzing 24-hour ambulatory EEGs of 1100 patients identified 57 patients, 15 of whom had genetic generalized epilepsy, with ictal events on EEG that were not reported by the patients and their relatives[109]. Of the 57, 21 patients had been assessed as seizure-free and at least 18 of them were driving regularly. Patients and their relatives may fail to recognize and report some manifestations of seizures due to limited knowledge of their diseases [110], post-ictal amnesia, or even in attempt to avoid driving restrictions. In such cases, prolonged EEG monitoring can help direct decision-making by physicians, medical advisory boards, and driver licensing authorities during assessments for granting or renewing driving privileges.
Despite the utility of prolonged EEG, without concurrent behavioral testing the true risk of generalized SWD without obvious behavioral manifestations remains unknown. Conceivably, the gold standard for evaluating driving in people with epilepsy would be a realistic, EEG-monitored driving experience on a road for a sufficient length of time. Such test poses several concerns with regards to safety and defining the appropriate length of recording time and parameters for assessing driving safety, but this effort has been attempted. Trenité and colleagues recruited six patients with spontaneous subclinical epileptiform discharges to drive in a car equipped to record EEG and monitor driving parameters like positioning, speed, and steering [29]. The subjects drove for 420km (i.e., 3 runs of 140km each) on a motorway. All 4 subjects who had been seizure-free for 4 or more years had subclinical generalized (poly)spike-wave discharges on EEG during the test sessions. In 3 subjects (2 with generalized SWDs and 1 with sharp waves), the epileptiform discharges were associated with significant deviations in car positioning, demonstrating impaired driving during the discharges.
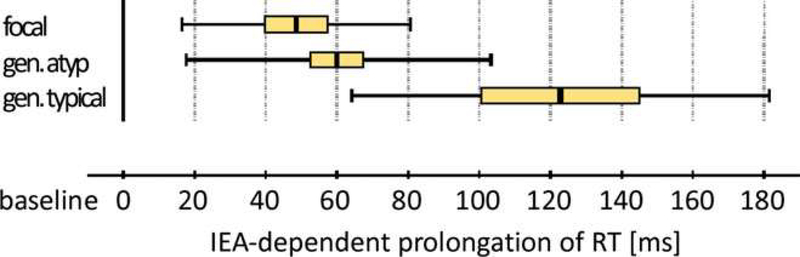
Simulated driving experiences have similarly demonstrated impaired driving behavior in the interictal period and during epileptiform discharges, particularly generalized SWDs. As aforementioned, using the rFactor computerized game, our group has shown a likely association between seizure type and collisions [65]. Researchers in Switzerland have developed another computerized driving game, Steer Clear, with which they have studied the effect of interictal epileptiform activity on driving [74, 75]. In this game, an obstacle is manually introduced in the driving course during the discharges. In one study involving 46 adults (34 of whom had generalized interictal epileptiform activity), both focal and generalized EEG discharges resulted in significant prolongation of reaction time (Figure 4) [75]. On average, focal and atypical generalized interictal epileptiform activity resulted in 49 ± 32 milliseconds (ms) and 60 ± 42ms prolongation in reaction time, respectively. Typical generalized interictal epileptiform activity showed the most profound prolongation in reaction time (123 ± 59ms) on this test in which ≥ 100ms prolongation in reaction time corresponds to ≥ 2.8 meters increase in braking distance in a car traveling at 100km/hr (approx. 62mph). Further, the probability of virtual accidents per obstacle presentation was markedly increased during typical generalized interictal epileptiform activity (32.0%) compared to during focal (3.5%) and atypical generalized interictal epileptiform activity (4.1%). Like prolonged EEG monitoring, simulated driving carries great promise of utility in evaluating driving safety among people with epilepsy, whether for seizure relapse or impaired reaction to road hazards. Ideally, with additional investigation of EEG features of SWD associated with impaired driving ability, it may be possible to predict driving safety based on EEG alone, with no need for driving simulation in each individual patient.
Figure 4. Typical generalized spike-wave discharges prolong reaction time more than focal and atypical generalized spike-wave discharges on a computerized driving game.
Reaction times during interictal epileptiform activity (IEA) including focal, atypical and typical generalized spike-wave discharges are shown. IEA resulting in lapses or crashes during testing are excluded in calculating the depicted reaction times. Center value = mean, colored box = standard error of the mean, range bar = standard deviation. Reproduced with permission from Nirkko et al., Virtual car accidents of epilepsy patients, interictal epileptic activity, and medication. Epilepsia, 2016, 57(5) 832–840.
5. Conclusion and future directions
Although previous studies have elucidated the clinical course, EEG and fMRI pathophysiology of seizures and epilepsies characterized by generalized SWDs, driving safety remains an open question for patients with SWD on EEG but no clinically obvious seizures. Many clinicians currently will use basic EEG characteristics such as SWD duration, frequency of SWD occurrence in prolonged recordings, and whether the SWD only occur during sleep, as common-sense criteria to decide on driving privileges for people with epilepsy. However, clinical practice varies widely in this regard and there are no accepted evidence-based criteria to decide on driving privileges in patients with generalized SWD on EEG. Initial evidence suggests that EEG features such as SWD duration and amplitude may, distinguish between “safe” and “unsafe” SWD, at least on a population level [31]. However, further work is needed with realistic driving testing during SWD to better characterize the EEG features of SWD that do or do not present a safety hazard. With sufficient EEG and behavioral data it may ultimately be possible to develop a machine-learning-based classifier which could predict driving safety based on EEG recordings in individual patients. Combining simulated driving, EEG and advanced computational analysis represent the next phase of our quest to understand the influences of epileptiform discharges on complex behaviors like driving. We hope that future work will enable more objective guidelines to be developed which will better inform clinicians and improve safe decision-making for people with epilepsy and generalized SWD who wish to drive.
Highlights.
Generalized SWD are associated with varied levels of behavioral impairments.
Discernible EEG features of generalized SWD may predict behavior impairment.
Simulated driving can reveal behavioral impairment in subclinical SWD.
Acknowledgments
Funding
This work was supported by the National Institutes of Health [R01 NS055829, R37NS100901, UL1TR000142, and CTSA TL1TR000141]; the Betsy and Jonathan Blattmachr family, and the Loughridge Williams Foundation.
Footnotes
Conflicts of Interest
None of the authors has any conflict of interest to disclose regarding this article.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Blumenfeld H. Cellular and Network Mechanisms of Spike-Wave Seizures. Epilepsia 2005;46: 21–33. [DOI] [PubMed] [Google Scholar]
- [2].Ebersole JS, Pedley TA. Current Practice of Clinical Electroencephalography, 3rd Edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- [3].Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, Hirsch E, Jain S, Mathern GW, Moshe SL, Nordli DR, Perucca E, Tomson T, Wiebe S, Zhang YH, Zuberi SM. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58: 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Scheffer IE, Berg AT. Classification and clinical features of absence epilepsies: how evidence leads to changing concepts. Epilepsia 2008;49: 2140–1. [DOI] [PubMed] [Google Scholar]
- [5].Penry JK, Porter RJ, Dreifuss RE. Simultaneous recording of absence seizures with video tape and electroencephalography. A study of 374 seizures in 48 patients. Brain 1975;98: 427–40. [DOI] [PubMed] [Google Scholar]
- [6].Sadleir LG, Scheffer IE, Smith S, Connolly MB, Farrell K. Automatisms in absence seizures in children with idiopathic generalized epilepsy. Arch Neurol 2009;66: 729–34. [DOI] [PubMed] [Google Scholar]
- [7].Panayiotopoulos CP, Obied T, Waheed G. Differentiation of typical absence seizures in epileptic syndromes. Brain 1989;112: 1039–1056. [DOI] [PubMed] [Google Scholar]
- [8].Iqbal N, Caswell HL, Duncan S. The Effects of Sub-Clinical EEG on Cognition; A Case of Two Patients with JME. Journal of Neurology and Neurophysiology 2014;5. [Google Scholar]
- [9].Matsouka H, Nakamura M, Ohno T, Shimabukuro J, Suzuki T, Numachi Y, Awata S. The Role of Cognitive–Motor Function in Precipitationand Inhibition of Epileptic Seizures. Epilepsia 2005;46: 17–20. [DOI] [PubMed] [Google Scholar]
- [10].Mullen SA, Berkovic SF, Commission IG. Genetic generalized epilepsies. Epilepsia 2018;59: 1148–1153. [DOI] [PubMed] [Google Scholar]
- [11].Gallentine WB, Mikati MA. Genetic generalized epilepsies. Journal of Clinical Neurophysiology 2012;2012: 408–419. [DOI] [PubMed] [Google Scholar]
- [12].Nersesyan H, Herman P, Erdogan E, Hyder F, Blumenfeld H. Relative changes in cerebral blood flow and neuronal activity in local microdomains during generalized seizures. J Cereb Blood Flow Metab 2004;24: 1057–68. [DOI] [PubMed] [Google Scholar]
- [13].Blumenfeld H. Consciousness and epilepsy: why are patients with absence seizures absent? Prog Brain Res 2005;150: 271–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McNally KA, Blumenfeld H. Focal network involvement in generalized seizures: new insights from electroconvulsive therapy. Epilepsy & Behavior 2004;5: 3–12. [DOI] [PubMed] [Google Scholar]
- [15].Mirsky AF, Vanburen JM. On the Nature of the “Absence” in Centrencephalic Epilepsy: A Study of Some Behavioral, Electroencephalographic and Autonomic Factors. Electroencephalogr Clin Neurophysiol 1965;18: 334–48. [DOI] [PubMed] [Google Scholar]
- [16].Geller MR, Geller A. Brief amnestic effects of spike wave discharges. Neurology 1970;20: 380–1. [PubMed] [Google Scholar]
- [17].Guey J, Tassinari CA, Charles C, Coquery C. [Variations in the efficiency level in relation to paroxysmal epileptic discharges]. Rev Neurol (Paris) 1965;112: 311–7. [PubMed] [Google Scholar]
- [18].Kooi KA, Hovey HB. Alterations in mental function and paroxysmal cerebral activity. AMA Arch Neurol Psychiatry 1957;78: 264–71. [PubMed] [Google Scholar]
- [19].Boudin G, Barbizet J, Masson S. Etude de la dissolution de la conscience dans 3 cas de petit mal avec crises prolongees. Rev Neurol (Paris) 1958;99: 483–487. [Google Scholar]
- [20].Goldie L, Green JM. Spike and wave discharges and alterations of conscious awareness. Nature 1961;191: 200–1. [DOI] [PubMed] [Google Scholar]
- [21].Davidoff RA, Johnson LC. PAROXYSMAL EEG ACTIVITY AND COGNITIVE-MOTOR PERFORMANCE. Electroencephalogr Clin Neurophysiol 1964;16: 343–54. [DOI] [PubMed] [Google Scholar]
- [22].Bureau M, Guey J, Dravet C, Roger J. A study of the distribution of petit mal absences in the child in relation to his activities. Electroencephalogr Clin Neurophysiol 1968;25: 513. [PubMed] [Google Scholar]
- [23].Sadleir LG, Scheffer IE, Smith S, Carstensen B, Carlin J, Connolly MB, Farrell K. Factors influencing clinical features of absence seizures. Epilepsia 2008;49: 2100–7. [DOI] [PubMed] [Google Scholar]
- [24].Giannakodimos S, Panayiotopoulos CP. Eyelid myoclonia with absences in adults: a clinical and video-EEG study. Epilepsia 1996;37: 36–44. [DOI] [PubMed] [Google Scholar]
- [25].Appleton RE, Panayiotopoulos CP, Acomb BA, Beirne M. Eyelid myoclonia with typical absences: an epilepsy syndrome. J Neurol Neurosurg Psychiatry 1993;56: 1312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Janz D, Beck-Mannagetta G, Sproder B, Sproder J, Waltz S. Childhood absence epilepsy (pyknolepsy) and juvenile: absence epilepsy: one or two syndromes? In: Wolf P, editor. Epileptic seizures and syndromes. London: John Libbey and Company Ltd; 1994, p. 115–126. [Google Scholar]
- [27].Grisell JL, Levin SM, Cohen BD, Rodin EA. EFFECTS OF SUBCLINICAL SEIZURE ACTIVITY ON OVERT BEHAVIOR. Neurology 1964;14: 133–5. [DOI] [PubMed] [Google Scholar]
- [28].Kasteleijn-Nolst Trenite DG, Vermeiren R. The impact of subclinical epileptiform discharges on complex tasks and cognition: relevance for aircrew and air traffic controllers. Epilepsy Behav 2005;6: 31–4. [DOI] [PubMed] [Google Scholar]
- [29].Kasteleijn-Nolst Trenite DG, Riemersma JBJ, Binnie CD, Smit AM, Meinardi H. The influence of subclinical epileptiform EEG discharges on driving and behavior. Electroencephalography and clinical Neurophysiology 1987;67: 167–170. [DOI] [PubMed] [Google Scholar]
- [30].Tuvo F Contribution a l’etude des niveaux de conscience au cours des paroxysmes epileptiques infraclinique. Electroenceph and Clin Neurophysiol 1958;10: 715–718. [DOI] [PubMed] [Google Scholar]
- [31].Guo JN, Kim R, Chen Y, Negishi M, Jhun S, Weiss S, Ryu JH, Bai X, Xiao W, Feeney E, Rodriguez-Fernandez J, Mistry H, Crunelli V, Crowley MJ, Mayes LC, Constable RT, Blumenfeld H. Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet Neurol 2016;15: 1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rugland AL. Neuropsychological Assessment of Cognitive Functioning in Children with Epilepsy. Epilepsia 1990;31: s41–s44. [DOI] [PubMed] [Google Scholar]
- [33].Aldenkamp AP, Arends J. Effects of epileptiform EEG discharges on cognitive function: Is the concept of “transient cognitive impairment” still valid? Epilepsy & Behavior 2004;5: 25–34. [DOI] [PubMed] [Google Scholar]
- [34].Berman R, Negishi M, Vestal M, Spann M, Chung M, Bai X, Purcaro M, Motelow JE, Dix-Cooper L, Enev M, Novotny EJ, Constable RT, Blumenfeld H. Simultaneous EEG, fMRI, and behavioral testing in typical childhood absence seizures. Epilepsia 2010;51(10): 2011–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Browne TR, Penry JK, Porter RJ, Dreifuss FE. Responsiveness before, during and after spike-wave paroxysms. Neurology 1974;24: 659–665. [DOI] [PubMed] [Google Scholar]
- [36].Mirsky AF, Van Buren JM. On the Nature of the “Absence” in Centrencephalic Epilepsy: A Study of some Behavioral, Electroencephalographic, and Autonomic Factors. Electroencephalogr Clin Neurophysiol 1965;18: 334–348. [DOI] [PubMed] [Google Scholar]
- [37].Tizard B, Margerison JH. Psychological functions during wave-spike discharges. Brit J Soc Clin Psychol 1963;3: 6–15. [Google Scholar]
- [38].Simons-Morton BG, Guo F, Klauer SG, Ehsani JP, Pradhan AK. Keep your eyes on the road: young driver crash risk increases according to duration of distraction. J Adolesc Health 2014;54: S61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Klauer SG, Guo F, Simons-Morton BG, Ouimet MC, Lee SE, Dingus TA. Distracted driving and risk of road crashes among novice and experienced drivers. N Engl J Med 2014;370: 54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bouma PA, Westendorp RG, van Dijk JG, Peters AC, Brouwer OF. The outcome of absence epilepsy: a meta-analysis. Neurology 1996;47: 802–8. [DOI] [PubMed] [Google Scholar]
- [41].Loiseau P, Pestre M, Dartigues JF, Commenges D, Barberger-Gateau C, Cohadon S. Long-term prognosis in two forms of childhood epilepsy: typical absence seizures and epilepsy with rolandic (centrotemporal) EEG foci. Ann Neurol 1983;13: 642–8. [DOI] [PubMed] [Google Scholar]
- [42].Berg AT, Levy SR, Testa FM, Blumenfeld H. Long-term seizure remission in childhood absence epilepsy: might initial treatment matter? Epilepsia 2014;55: 551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Grosso S, Galimberti D, Vezzosi P, Farnetani M, Di Bartolo RM, Bazzotti S, Morgese G, Balestri P. Childhood absence epilepsy: evolution and prognostic factors. Epilepsia 2005;46: 1796–801. [DOI] [PubMed] [Google Scholar]
- [44].Seneviratne U, Cook M, D’Souza W. The prognosis of idiopathic generalized epilepsy. Epilepsia 2012;53: 2079–90. [DOI] [PubMed] [Google Scholar]
- [45].Camfield C, Camfield P. Management guidelines for children with idiopathic generalized epilepsy. Epilepsia 2005;46 Suppl 9: 112–6. [DOI] [PubMed] [Google Scholar]
- [46].Camfield PR, Camfield CS. What happens to children with epilepsy when they become adults? Some facts and opinions. Pediatr Neurol 2014;51: 17–23. [DOI] [PubMed] [Google Scholar]
- [47].Gilliam F, Kuzniecky R, Faught E, Black L, Carpenter G, Schrodt R. Patient-validated content of epilepsy-specific quality-of-life measurement. Epilepsia 1997;38: 233–6. [DOI] [PubMed] [Google Scholar]
- [48].Kang JY, Mintzer S. Driving and Epilepsy: a Review of Important Issues. Curr Neurol Neurosci Rep 2016;16: 80. [DOI] [PubMed] [Google Scholar]
- [49].Ma BB, Bloch J, Krumholz A, Hopp JL, Foreman PJ, Soderstrom CA, Scottino MA, Matsumoto M, Krauss GL. Regulating drivers with epilepsy in Maryland: Results of the application of a United States consensus guideline. Epilepsia 2017;58: 1389–1397. [DOI] [PubMed] [Google Scholar]
- [50].Beghi E, Sander JW. Epilepsy and driving: regulations in the European Union need harmonisation as well as greater flexibility. BMJ 2005;331: 60–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Epilepsy and driving in Europe: A report of the second European Working Group on Epilepsy and Driving, an advisory board to the Driving Licence Committee of the European Union. 2005.
- [52].Society E Driving. In: Living with Epilepsy.
- [53].Chen WC, Chen EY, Gebre RZ, Johnson MR, Li N, Vitkovskiy P, Blumenfeld H. Epilepsy and driving: Potential impact of transient impaired consciousness. Epilepsy & Behavior 2014;30: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hansotia P, Broste SK. The effect of epilepsy or diabetes mellitus on the risk of automobile accidents. N Engl J Med 1991;324: 22–6. [DOI] [PubMed] [Google Scholar]
- [55].Taylor J, Chadwick D, Johnson T. Risk of accidents in drivers with epilepsy. J Neurol Neurosurg Psychiatry 1996;60: 621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sheth SG, Krauss G, Krumholz A, Li G. Mortality in epilepsy: driving fatalities vs other causes of death in patients with epilepsy. Neurology 2004;63: 1002–7. [DOI] [PubMed] [Google Scholar]
- [57].Bener A, Murdoch JC, Achan NV, Karama AH, Sztriha L. The effect of epilepsy on road traffic accidents and casualties. Seizure 1996;5: 215–9. [DOI] [PubMed] [Google Scholar]
- [58].Krumholz A, Wiebe S, Gronseth GS, Gloss DS, Sanchez AM, Kabir AA, Liferidge AT, Martello JP, Kanner AM, Shinnar S, Hopp JL, French JA. Evidence-based guideline: Management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2015;84: 1705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bonnett LJ, Tudur-Smith C, Williamson PR, Marson AG. Risk of recurrence after a first seizure and implications for driving: further analysis of the Multicentre study of early Epilepsy and Single Seizures. BMJ 2010;341: c6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bonnett LJ, Powell GA, Tudur Smith C, Marson AG. Risk of a seizure recurrence after a breakthrough seizure and the implications for driving: further analysis of the standard versus new antiepileptic drugs (SANAD) randomised controlled trial. BMJ Open 2017;7: e015868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Krauss GL, Krumholz A, Carter RC, Li G, Kaplan P. Risk factors for seizure-related motor vehicle crashes in patients with epilepsy. Neurology 1999;52: 1324–9. [DOI] [PubMed] [Google Scholar]
- [62].Gastaut H, Zifkin BG. The risk of automobile accidents with seizures occurring while driving: relation to seizure type. Neurology 1987;37: 1613–6. [DOI] [PubMed] [Google Scholar]
- [63].Punia V, Farooque P, Chen W, Hirsch LJ, Berg AT, Multicenter Study of Epilepsy S, Blumenfeld H. Epileptic auras and their role in driving safety in people with epilepsy. Epilepsia 2015;56: e182–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chen WC, Chen EY, Gebre RZ, Johnson MR, Li N, Vitkovskiy P, Blumenfeld H. Epilepsy and driving: potential impact of transient impaired consciousness. Epilepsy & Behavior 2014;30: 50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yang L, Morland TB, Schmits K, Rawson E, Narasimhan P, Motelow JE, Purcaro MJ, Peng K, Raouf S, Desalvo MN, Oh T, Wilkerson J, Bod J, Srinivasan A, Kurashvili P, Anaya J, Manza P, Danielson N, Ransom CB, Huh L, Elrich S, Padin-Rosado J, Naidu Y, Detyniecki K, Hamid H, Farooque P, Astur R, Xiao B, Duckrow RB, Blumenfeld H. A prospective study of loss of consciousness in epilepsy using virtual reality driving simulation and other video games. Epilepsy Behav 2010;18: 238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Naik PA, Fleming ME, Bhatia P, Harden CL. Do drivers with epilepsy have higher rates of motor vehicle accidents than those without epilepsy? Epilepsy Behav 2015;47: 111–4. [DOI] [PubMed] [Google Scholar]
- [67].Wolf P, Yacubian EM, Avanzini G, Sander T, Schmitz B, Wandschneider B, Koepp M. Juvenile myoclonic epilepsy: A system disorder of the brain. Epilepsy Res 2015;114: 2–12. [DOI] [PubMed] [Google Scholar]
- [68].Shinnar RC, Shinnar S, Cnaan A, Clark P, Dlugos D, Hirtz DG, Hu F, Liu C, Masur D, Weiss EF, Glauser TA, Childhood Absence Epilepsy Study G. Pretreatment behavior and subsequent medication effects in childhood absence epilepsy. Neurology 2017;89: 1698–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bourgeois BF. Determining the effects of antiepileptic drugs on cognitive function in pediatric patients with epilepsy. J Child Neurol 2004;19 Suppl 1: S15–24. [DOI] [PubMed] [Google Scholar]
- [70].Selassie AW, Wilson DA, Martz GU, Smith GG, Wagner JL, Wannamaker BB. Epilepsy beyond seizure: a population-based study of comorbidities. Epilepsy Research 2014;108: 305–15. [DOI] [PubMed] [Google Scholar]
- [71].Aldenkamp AP, Arends J. Effects of epileptiform EEG discharges on cognitive function: is the concept of “transient cognitive impairment” still valid? Epilepsy Behav 2004;5 Suppl 1: S25–34. [DOI] [PubMed] [Google Scholar]
- [72].Kleen JK, Scott RC, Holmes GL, Roberts DW, Rundle MM, Testorf M, Lenck-Santini PP, Jobst BC. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology 2013;81: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kleen JK, Scott RC, Holmes GL, Lenck-Santini PP. Hippocampal interictal spikes disrupt cognition in rats. Annals of Neurology 2010;67: 250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Krestel HE, Nirkko A, von Allmen A, Liechti C, Wettstein J, Mosbacher A, Mathis J. Spike-triggered reaction-time EEG as a possible assessment tool for driving ability. Epilepsia 2011;52: e126–9. [DOI] [PubMed] [Google Scholar]
- [75].Nirkko AC, Bernasconi C, von Allmen A, Liechti C, Mathis J, Krestel H. Virtual car accidents of epilepsy patients, interictal epileptic activity, and medication. Epilepsia 2016;57: 832–40. [DOI] [PubMed] [Google Scholar]
- [76].Kasteleijn-Nolst Trenite DG, Riemersma JB, Binnie CD, Smit AM, Meinardi H. The influence of subclinical epileptiform EEG discharges on driving behaviour. Electroencephalography & Clinical Neurophysiology 1987;67: 167–70. [DOI] [PubMed] [Google Scholar]
- [77].Aldenkamp AP, Beitler J, Arends J, van der Linden I, Diepman L. Acute effects of subclinical epileptiform EEG discharges on cognitive activation. Funct Neurol 2005;20: 23–28. [PubMed] [Google Scholar]
- [78].Nicolai J, Ebus S, Biemans DP, Arends J, Hendriksen J, Vles JS, Aldenkamp AP. The cognitive effects of interictal epileptiform EEG discharges and short nonconvulsive epileptic seizures. Epilepsia 2012;53: 1051–9. [DOI] [PubMed] [Google Scholar]
- [79].Tromp SC, Weber JW, Aldenkamp AP, Arends J, van der Linden I. Relative influence of epileptic seizures and of epilepsy syndrome on cognitive function. J Child Neurol 2003;18: 407–412. [DOI] [PubMed] [Google Scholar]
- [80].Shimazono Y, Hirai T, Okuma T, Fukuda T, Yamamasu E. Disturbance of consciousness in petit mal epilepsy. Epilepsia 1953;2: 49–55. [Google Scholar]
- [81].Goode DJ, Penry JK, Dreifuss FE. Effects of paroxysmal spike-wave on continuous visual-motor performance. Epilepsia 1970;11: 241–54. [DOI] [PubMed] [Google Scholar]
- [82].Browne TR, Penry JK, Proter RJ, Dreifuss FE. Responsiveness before, during, and after spike-wave paroxysms. Neurology 1974;24: 659–65. [DOI] [PubMed] [Google Scholar]
- [83].Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, Desalvo M, Novotny EJ, Constable RT, Blumenfeld H. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci 2010;30: 5884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Moeller F, Muhle H, Wiegand G, Wolff S, Stephani U, Siniatchkin M. EEG-fMRI study of generalized spike and wave discharges without transitory cognitive impairment. Epilepsy Behav 2010;18: 313–6. [DOI] [PubMed] [Google Scholar]
- [85].Sadleir LG, Scheffer IE, Smith S, Carstensen B, Farrell K, Connolly MB. EEG features of absence seizures in idiopathic generalized epilepsy: impact of syndrome, age, and state. Epilepsia 2009;50: 1572–8. [DOI] [PubMed] [Google Scholar]
- [86].Schwab RS. The influence of visual and auditory stimuli on the electroencephalographic tracing of petit mal. The American Journal of Psychiatry 1941;97: 1301–1312. [Google Scholar]
- [87].Schwab RS. Reaction time in petit mal epilepsy. Research Publications- Association for Research in Nervous and Mental Disease 1947: 339–341. [Google Scholar]
- [88].Blumenfeld H. Consciousness and epilepsy: why are patients with absence seizures absent? In: The Boundaries of Consciousness: Neurobiology and Neuropathology; 2005, p. 271–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Courtois GA, Ingvar DH, Jasper HH. Nervous and mental defects during petit mal attacks. Electroenceph and Clin Neurophysiol 1953;Suppl 3: 87. [Google Scholar]
- [90].Jus A, Jus C. Etude electro-clinique des alterations de conscience dans le petit mal. Studii si cercetari de Neurol 1960;5: 243–254. [Google Scholar]
- [91].Porter RJ, Penry JK. Responsiveness at the Onset of Spike-Wave Bursts. Electroencephalogr Clin Neurophysiol 1973;34: 239–245. [DOI] [PubMed] [Google Scholar]
- [92].Dong L, Luo C, Zhu Y, Hou C, Jiang S, Wang P, Biswal BB, Yao D. Complex discharge-affecting networks in juvenile myoclonic epilepsy: A simultaneous EEG-fMRI study. Hum Brain Mapp 2016;37: 3515–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Li Q, Luo C, Yang T, Yao Z, He L, Liu L, Xu H, Gong Q, Yao D, Zhou D. EEG-fMRI study on the interictal and ictal generalized spike-wave discharges in patients with childhood absence epilepsy. Epilepsy Res 2009;87: 160–8. [DOI] [PubMed] [Google Scholar]
- [94].Tenney JR, Kadis DS, Agler W, Rozhkov L, Altaye M, Xiang J, Vannest J, Glauser TA. Ictal connectivity in childhood absence epilepsy: Associations with outcome. Epilepsia 2018. [DOI] [PubMed] [Google Scholar]
- [95].Moeller F, LeVan P, Muhle H, Stephani U, Dubeau F, Siniatchkin M, Gotman J. Absence seizures: individual patterns revealed by EEG-fMRI. Epilepsia 2010;51: 2000–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Tyvaert L, Chassagnon S, Sadikot A, LeVan P, Dubeau F, Gotman J. Thalamic nuclei activity in idiopathic generalized epilepsy: an EEG-fMRI study. Neurology 2009;73: 2018–22. [DOI] [PubMed] [Google Scholar]
- [97].Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proceedings of the National Academy of Sciences of the United States of America 2005;102: 15236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Li Q, Luo C, Yang T, Yao Z, He L, Liu L, Xu H, Gong Q, Yao D, Zhou D. EEG-fMRI study on the interictal and ictal generalized spike-wave discharges in patients with childhood absence epilepsy. Epilepsy Research 2009;87: 160–8. [DOI] [PubMed] [Google Scholar]
- [99].Carney PW, Masterton RA, Flanagan D, Berkovic SF, Jackson GD. The frontal lobe in absence epilepsy: EEG-fMRI findings. Neurology 2012;78: 1157–65. [DOI] [PubMed] [Google Scholar]
- [100].Carney PW, Masterton RA, Harvey AS, Scheffer IE, Berkovic SF, Jackson GD. The core network in absence epilepsy. Differences in cortical and thalamic BOLD response. Neurology 2010;75: 904–11. [DOI] [PubMed] [Google Scholar]
- [101].Labate A, Briellmann RS, Abbott DF, Waites AB, Jackson GD. Typical childhood absence seizures are associated with thalamic activation. Epileptic Disorders 2005;7: 373–7. [PubMed] [Google Scholar]
- [102].Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI “deactivation” of the posterior cingulate during generalized spike and wave. Neuroimage 2003;20: 1915–22. [DOI] [PubMed] [Google Scholar]
- [103].Hamandi K, Laufs H, Nöth U, Carmichael DW, Duncan JS, Lemieux L. BOLD and perfusion changes during epileptic generalised spike wave activity. NeuroImage 2008;39: 608–618. [DOI] [PubMed] [Google Scholar]
- [104].Salek-Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR. Functional magnetic resonance imaging of human absence seizures. Ann Neurol 2003;53: 663–7. [DOI] [PubMed] [Google Scholar]
- [105].Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, Desalvo M, Novotny EJ, Constable RT, Blumenfeld H. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. Journal of Neuroscience 2010;30: 5884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Assessing fitness to drive for commercial and private vehicle drivers In. Reprinted 2017 ed. https://www.onlinepublications.austroads.com.au/items/AP-G56-13: Australia National Transport Commission; 2016. [Google Scholar]
- [107].For medical practitioners. At a glance guide to the current medical standards of firness to drive Issued by Drivers Medical Group. In: Driver & Vehicle Licensing Agency; 2013. [Google Scholar]
- [108].Kamel JT, Christensen B, Odell MS, D’Souza WJ, Cook MJ. Evaluating the use of prolonged video-EEG monitoring to assess future seizure risk and fitness to drive. Epilepsy Behav 2010;19: 608–11. [DOI] [PubMed] [Google Scholar]
- [109].Fattouch J, Di Bonaventura C, Lapenta L, Casciato S, Fanella M, Morano A, Manfredi M, Giallonardo AT. Epilepsy, unawareness of seizures and driving license: the potential role of 24-hour ambulatory EEG in defining seizure freedom. Epilepsy Behav 2012;25: 32–5. [DOI] [PubMed] [Google Scholar]
- [110].Detyniecki K, Blumenfeld H. Consciousness of seizures and consciousness during seizures: are they related? Epilepsy & Behavior 2014;30: 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]