Abstract
Problem
Chronic endometritis, inflammation of the uterine lining caused by common gram-negative bacterial strains or mycoplasma, has been associated with unexplained implantation failure and infertility. However, limited models of bacteria-induced implantation loss exist to study the molecular changes that occur in vivo. The goal of this study was to provide a new resource to study the process of bacteria-induced inflammation and implantation loss utilizing common experimental models: C57Bl/6 mice and primary human endometrial stromal cells.
Method of Study
Prior to implantation, mated C57Bl/6 females were administered vehicle (saline) or gram-negative bacterial lipopolysaccharide (LPS) at a range of concentrations by intraperitoneal injection. Implantation sites were counted, and uteri were harvested to evaluate the molecular changes that accompany LPS-mediated implantation loss. Primary human endometrial stromal cells were decidualized in vitro in the presence and absence of LPS. Total RNA and conditioned media were harvested to evaluate the expression of known decidualization-associated genes and various cytokines and chemokines.
Results
LPS treatment resulted in fewer implantation sites in mice, decreased expression of decidualization-associated genes, and altered expression and release of cytokines and chemokines. Immunohistological analysis of the uterus from LPS-exposed mice demonstrated increased apoptosis and decreased proliferation during decidualization.
Conclusions
LPS exposure disrupted implantation and decidualization in mice and human endometrial stromal cells. This model could be used to study the pathophysiology of implantation failure in patients with chronic endometritis or to test potential therapeutic interventions.
1. Introduction
The establishment of pregnancy in placental species relies on a number of factors, including synchronous development of the fertilized blastocyst and the transformation of the uterus into a receptive state. Uterine receptivity refers to the window of time that the endometrium has obtained the capacity to accept a competent blastocyst. The ovarian hormones drive uterine receptivity by inducing endometrial decidualization, a process of remodeling required for implantation, invasion, and to support embryonic growth 1. The maternal immune system also contributes to uterine remodeling and receptivity by altering the expression of epithelial attachment molecules, regulating the degree of trophoblast invasion, enhancing breakdown of the extracellular matrix, and supporting vascular remodeling and angiogenesis 2, 3. In fact, the importance of some pro-inflammatory activity during implantation has been demonstrated by improved IVF success rates in patients with biopsy-induced local inflammation4. This local injury induces the expression of several pro-inflammatory cytokines and chemokines, such as tumor necrosis factor alpha (TNF-α) and growth-regulated oncogene alpha (GRO-α)5. However, cytokine and chemokine expression must be precisely regulated, as inappropriately elevated concentrations of certain cytokines and chemokines are associated with implantation failure 6, 7.
It is critical that the local immune environment be tightly regulated for implantation to occur, and one potential contributing factor to an altered immune environment in humans is chronic endometritis, a condition estimated to affect approximately 10% of women 8, 9. Chronic endometritis refers to inflammation of the endometrium and is often caused by gram-negative bacterial strains such as Escherichia coli and Gardnerella vaginalis or by Ureaplasma urealyticum and Mycoplasma species 10. Notably, the prevalence of endometritis is much higher in women with infertility, and specifically those with recurrent implantation failure (RIF). A recent study of infertile women with RIF identified chronic endometritis in almost 34% of their study population, although others have reported the prevalence as high as 57% in women with RIF 11, 12. Interestingly, endometritis is also common in the dairy industry, and bovine uterine infections are estimated to cost the United States 650 million dollars annually 13.
Genital tract infections by ascending microbial invasion with gram-negative bacteria have well documented negative effects on gestation, including pregnancy loss and preterm labor. Exposure of pregnant mice to the gram-negative bacterial lipopolysaccharide (LPS) during mid-pregnancy results in spontaneous preterm labor and fetal loss 14–16. Infections present at the time of conception may also cause reproductive dysfunction by disrupting uterine receptivity. However, there is limited literature documenting the immunologic changes occurring in response to bacterial infection prior to implantation which may affect gestational success. Studies of LPS-mediated implantation loss have been undertaken in the Park strain mouse, although the minimum dose required to induce implantation loss likely varies by experimental strain. Moreover, studies with the Park strain mouse provided limited information on the molecular and histologic changes that accompany implantation failure 17.
Our study aims to provide a new model for the investigation into the effects of gram negative bacteria on implantation and uterine receptivity. Establishing this model with the C57Bl/6 mouse line will provide a basis for comparison in future studies utilizing transgenic mice, often developed on the C57Bl/6 background. Furthermore, studying the effects of LPS on decidualization in primary human endometrial stromal cells will provide additional insight into the direct effects of gram-negative bacteria on human endometrial stromal cell signaling. Both models provide an opportunity to understand the pathophysiology of infection-mediated pregnancy loss in eutherian mammals.
2. Materials and Methods
2.1. Reagents
Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from Life Technologies (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Sigma-Aldrich (St. Louis, MO). Charcoal dextran-treated (stripped) FBS was purchased from Gemini Bio Products (West Sacramento, CA). Estradiol (E2; 1,3,5[10] estratrien-3,17β-diol; ≥99%) was purchased from Steraloids (Newport, RI). Progesterone (P4; 4-pregnene-3,20-dione; ≥99%) and dibutyryl cyclic AMP (cAMP; N6, 2’-O-dibutyryladenosine 3’−5’-cyclic monophosphate sodium salt; ≥96% HPLC) were purchased from Sigma Aldrich. Ki-67 antibody (D3B5) was purchased from Cell Signaling Technology (Danvers, MA). Primer-probe sets for TaqMan assays were purchased from Applied Biosystems (Thermo Fisher Scientific, Waltham, MA). The 384-well mouse cytokine and chemokine panel assay (cat. #10034635) and human cytokine and chemokine panel assay (cat. #10034472) were purchased from BioRad Laboratories (Hercules, CA). The ApopTag® Peroxidase In Situ Apoptosis Detection Kit (cat. #S7100) was purchased from Millipore Sigma (Temecula, CA). Harris Hematoxylin (cat. #S212) and Eosin Phloxine Alcoholic Working Solution (cat. #S176) were purchased from Poly Scientific R&D Corp. (Bay Shore, NY).
2.2. Animals
All experiments were conducted with the approval of the Institutional Animal Care and Use Committee at Yale University and followed the National Institute of Health Guide for the Care and Use of Laboratory Animals. Food and water were provided ad libitum, and mice were maintained on a 12:12 hour light:dark schedule. Adult (6–10-week-old) C57Bl/6 mice were utilized for all experiments. Female mice were placed with proven-fertile male mice overnight at a 1:1 ratio and evaluated for the presence of a copulatory plug the following morning. The morning that the copulatory plug was observed was designated 0.5 days post coitum (dpc). Male mice were removed once the copulatory plug was observed. On the afternoon of 0.5 dpc, plug-positive female mice were administered vehicle (saline), 1 μg LPS, 2.5 μg LPS, 5 μg LPS, or 10 μg LPS diluted in saline in a total volume of 100 μl by intraperitoneal injection. Mice were weighed at the time of injection, 1 day post-injection, and 5 days post-injection. Implantation sites were visualized and counted on 5.5 dpc. Uteri were harvested on 1.5 and 5.5 dpc for histology or RNA extraction.
2.3. Cell Culture
Primary human endometrial stromal cells (hESCs) were obtained from endometrial biopsies as previously described and maintained at 37ᵒC with 95% humidity and 5% carbon dioxide 18. hESCs were used before the sixth passage for all experiments. hESCs were grown in DMEM media supplemented with heat-inactivated 10% FBS. Prior to treatment, cells were cultured in 6-well plates and media were changed to phenol red-free DMEM supplemented with 5% heat-inactivated, charcoal dextran-treated (stripped) FBS. Decidualization was induced by supplementing media with 10 nM E2, 100 nM P4, and 0.5 mM dibutyryl cAMP (decidualization cocktail). Cells were additionally treated with .1 μg/mL LPS or vehicle (PBS). The dose of LPS was chosen based on previous reports of induced inflammatory responses in cultured mammalian endometrial cells 19. Every 48 hours, half of the culture medium was removed and replaced by fresh media containing the decidualization cocktail. Control wells underwent the same media replacement protocol were half of the phenol red-free DMEM supplemented with 5% heat-inactivated, charcoal dextran-treated (stripped) FBS was replaced every 48 hours. Cells treated with LPS also had the LPS treatment replaced every 48 hours. For gene array and quantitative RT-PCR analysis, RNA was harvested 3 and 7 days after the addition of the decidualization cocktail. Transcript levels of amphiregulin (Areg) and wingless-related integration site 4 (Wnt4) were used as markers for implantation and decidualization in mice. Transcript levels of insulin-like growth factor binding protein-1 (IGFBP1) and prolactin (PRL) were used as markers for the decidual response in hESCs 20.
2.4. RNA Extraction
Total RNA was harvested from the mouse uterus using the Qiagen QIAshredder and the Qiagen RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Total RNA was harvested from the hESCs using the Qiagen RNeasy mini kit. A deoxyribonuclease (DNase) treatment was performed on-column for all samples using the ribonuclease-free DNase Kit (Qiagen) according to the manufacturer’s instructions. Total RNA quantity and purity was assessed using the NanoDrop One Spectrophotometer (ThermoFisher Scientific) based on the absorbance ratios at 260 and 280 nM and at 260 and 230 nM.
2.5. Quantitative RT-PCR
Individual mRNA abundance was determined using a TaqMan One-Step procedure on the CFX Connect Real-Time System (Bio-Rad Laboratories) with predesigned TaqMan assays (ThermoFisher Scientific) and 100 ng of total RNA as input. Quantitative RT-PCR was performed in a 10 μl reaction volume with the following thermocycling parameters: 48ᵒC for 30 minutes, 95ᵒC for 10 minutes, followed by 40 cycles of 95ᵒC for 15 seconds and 60ᵒC for 60 seconds. Expression values for each gene were calculated using a standard curve. In both the mouse and human samples, the signal from each probe was normalized to the reference gene peptidylprolyl-isomerase B (Ppib and PPIB respectively). Each gene primer-probe was evaluated with technical duplicates for each sample with a minimum of four biological replicates per treatment group. A list of TaqMan assays used in this study is shown in Supplemental Table S1.
2.6. Cytokine Array
Transcript levels of various cytokines and chemokines in the mouse uterus and hESCs were measured from a total of 50 ng RNA using predeveloped mouse and human cytokine and chemokine 384-well panel assays from BioRad Laboratories. Array data was analyzed using the Bio-Rad CFX Manager 3.1 software. The transcript levels were normalized using the delta-delta Ct method and the reference gene TATA-binding protein (Tbp). Relative expression was determined for the mouse uterus by graphing data relative to transcript levels in the uterus of non-pregnant, diestrous-staged mice. Relative expression for decidualized hESCs was determined by graphing data relative to transcript levels in non-decidualized (Day 0) hESCs.
2.7. Immunohistochemistry
Uterine mouse tissue samples isolated at 1.5 and 5.5 dpc were fixed overnight in 10% buffered formalin and stored in 70% ethanol until processing and embedding. Tissues were paraffin-embedded and sectioned at 5μm by the Yale School of Medicine Pathology Core. Tissue slides were stored at room temperature in dust-free slide boxes. Histology was visualized by hematoxylin and eosin staining. For immunostaining, slides were deparaffinized, rehydrated, and endogenous peroxide blocked with 3% hydrogen peroxide. Heat-induced antigen retrieval was performed with a 0.01 M citrate buffer at pH 6 (diluted from a stock solution of 0.1 M sodium citrate and 0.1 M citric acid). Slides were then blocked with 10% normal goat serum (Vector Laboratories Inc., Burlington, CA) at room temperature for 1 hour, and incubated at 4ᵒC overnight with primary antibody (Ki-67 1:400). The next day, slides were incubated with anti-rabbit secondary antibody (cat. #BA-1000, Vector Laboratories) for 1 hour at room temperature. After washing, the antigen-antibody complex was visualized using the Vectastain ABC Kit (cat. #PK-6100, Vector Laboratories) and the chromogenic substrate 3,3’ diaminobenzidine tetrahydrochloride (DAB, cat #SK-4100, Vector Laboratories) solution. Apoptotic cells were detected through the procedures outlined by the manufacturer in the ApopTag® Peroxidase In Situ Apoptosis Detection Kit. Sections were counterstained with Hematoxylin, dehydrated, cleared with Xylenes, and cover slipped. Sections were visualized, and images were captured using the Revolve microscope (Echo Laboratories Inc., San Diego, CA).
2.8. Cytokine Analysis in Conditioned Media
Cytokines were measured in conditioned media from hESCs treated for decidualization cocktail in the presence and absence of 0.1 μg LPS utilizing the cytokine multiplex assay from BioRad Laboratories (cat. #12009709 and 171B6007M). The 17-plex assay measured GRO-α, Il-1β, IL-6, IL-8, IL-10, IL-12, IL-17A, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES, TNF-α, and VEGF. Analytes were quantified using the LUMINEX 200 (LUMINEX, Austin, TX).
2.9. Statistical Analysis
The data represent the average of at least 4 biological replicates and are presented as means ± SEM. Statistical significance was determined by ANOVA with Tukey’s post-hoc analysis. Statistical significance was defined as *p < 0.05 or **p < 0.01 using GraphPad Prism Software version 7.0.
3. Results and Discussion
3.1. Determining the optimal dose for LPS-induced implantation failure in C57Bl/6 mice.
In order to determine the minimum dose of LPS required to induce implantation loss in C57Bl/6 mice, successfully mated females were administered increasing doses of LPS on the day the copulatory plug was visualized (0.5 dpc). The experimental regimen is depicted in Figure 1A. Because LPS exposure can induce hypoglycemia and hypertriglyceridemia leading to weight loss in mice, body weight was measured prior to LPS injection, one day following LPS injection, and five days post injection (Figure 1B) 21. Mice receiving 5 μg of LPS on 0.5 dpc demonstrated a significant loss in body weight on 1.5 dpc. However, body weights for all treatment groups were equivalent to controls by 5.5 dpc, suggesting that acute LPS administration did not result in sustained weight loss.
Figure 1. LPS-induced implantation failure.
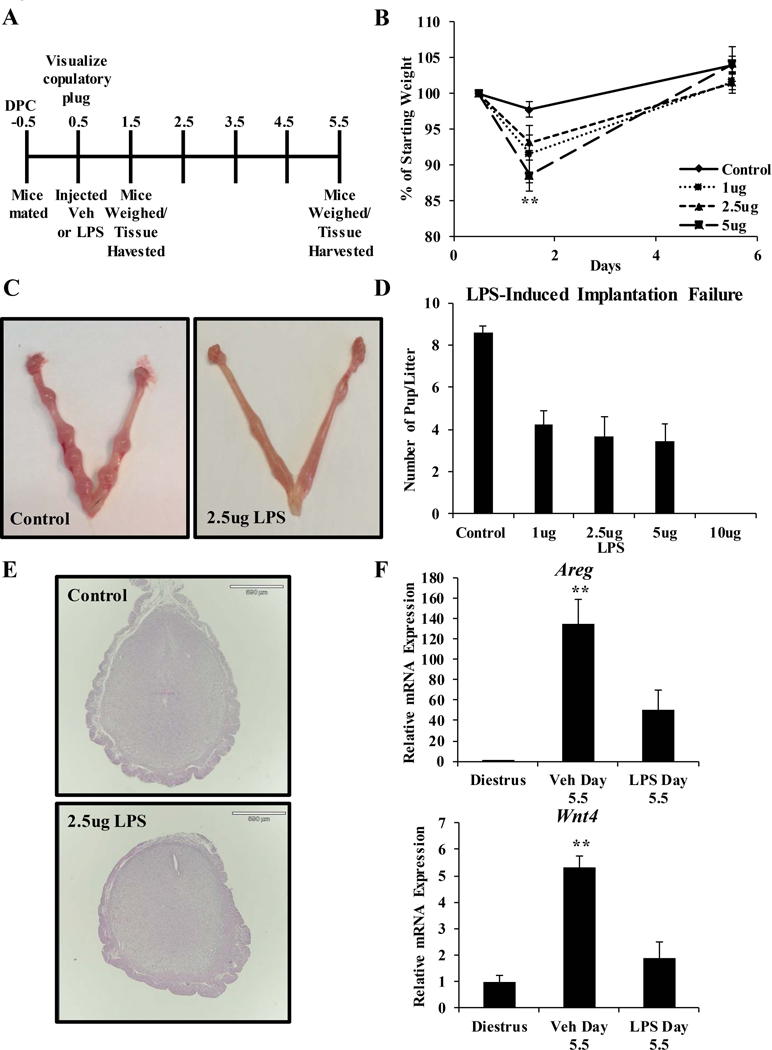
(A) Schematic of the LPS exposure regimen is shown. Mice were injected with saline or various concentrations of LPS diluted in saline on the day the copulatory plug was visualized (0.5 days post coitum; dpc). Mice received a total injection volume of 100 μl by intraperitoneal injection. Mice were weighed on 0.5, 1.5, and 5.5 dpc. Uterine tissue was harvested on 1.5 and 5.5 dpc. (B) Mice were weighed immediately prior to injection, 1 day post-injection, and 5 days post-injection. Body weight relative to starting body weight is graphed. (C) Representative images of uterine horns from vehicle and LPS (2.5 μg) treated mice on 5.5 dpc. (D) Implantation sites were counted on 5.5 dpc in all treatment groups and the average per group was graphed. (E) Gross examination of uterine histology in mice treated with vehicle or 2.5 μg LPS. Cross-sections were stained with hematoxylin and eosin. (F) Transcript levels of decidual biomarkers Areg and Wnt4 were measured by qRT-PCR in mouse uterine RNA samples harvested from diestrus staged mice and 5.5 dpc mice treated with vehicle (saline) or 2.5 μg LPS. Transcript levels were normalized to the reference gene Ppib and set relative to vehicle samples. Results are representative of at least 4 independent experiments. Data represent the mean ± SEM, **p < 0.01 as determined by ANOVA.
All concentrations of LPS tested reduced the number of implantation sites visualized at 5.5 dpc (Figures 1C and 1D). Although the Park strain mouse tolerated LPS to 50 μg per animal, treatment with 10 μg of LPS per mouse in the C57Bl/6 strain resulted in total implantation failure, along with indications of morbidity and mortality 17. Therefore, experiments at the 10 μg dose were suspended. Compared to Park strain mice, which demonstrated 100% implantation failure at 5 μg, C57Bl/6 mice were more resistant to LPS-induced implantation failure and demonstrated approximately 61% inhibition of implantation at 5 μg LPS 17. This suggests that C57Bl/6 mice have a reduced range of tolerated LPS exposure leading to implantation loss, between 1 and 10 ug per mouse. Mice exposed to 2.5 μg LPS demonstrated significant implantation failure with the least initial weight loss ( Figures 1B and 1D). Therefore, we further characterized the implantation sites in mice exposed to 2.5 μg of LPS. Gross examination of uterine histology at 5.5 dpc in LPS-treated mice revealed slightly smaller implantation sites and incomplete closure of the luminal epithelium compared to control mice (Figure 1E). In addition to the observed morphological changes, the transcript levels of implantation and decidualization marker genes were blunted by preimplantation exposure to LPS (Figure 1F). Expression of Areg, a member of the epidermal growth factor family expressed in the luminal epithelial cells at the time of implantation, was induced in the uterus of control mice but not in the uterus of mice exposed to LPS 22, 23. Transcript levels of Wnt4, which is critical to the process of embryo implantation and uterine decidualization, were attenuated at 5.5 dpc in the uterus of mice exposed to LPS compared to controls 24, 25. The reduced expression of uterine marker genes is similar to what is reported in genetic models of implantation failure 26–28.
The process of murine decidualization is characterized by pronounced stromal cell proliferation 29. Therefore, a Ki-67 stain was performed to visualize cell proliferation in the uterus of control and LPS-treated mice (Figure 2A). Fewer Ki-67 positive decidual cells were observed in the uterus of mice treated with LPS compared to control mice. This is similar to reports of fewer mitotic bodies found in the uterus of the Park strain mouse treated with LPS 17. The authors report this difference on day 14 of pregnancy, which suggests that bacterial endotoxin exposure early in pregnancy can produce long-term effects that persist throughout pregnancy. LPS exposure has also been shown to inhibit proliferation in several other cell types, including lung and skin fibroblasts and sub-types of epithelial cells 30–32. In macrophages, LPS inhibits proliferation by decreasing the expression of cyclin D1, a key mediator of protein synthesis and cell growth 33, 34. LPS treatment has also been shown to suppress cell proliferation through the nuclear factor-kB pathway 31. The mechanism by which pre-implantation exposure to LPS inhibits proliferation during the process of decidualization in the mouse is not clear. However, we found that the expression of several proliferation-associated cytokines and growth factors was dysregulated at 5.5 dpc following LPS treatment, which likely contributes to altered levels of stromal cell proliferation 35–37. Interestingly, others have reported that in non-pregnant mice, LPS treatment resulted in greater numbers of Ki-67-positive cells, indicating that the uterine response to LPS is context specific 38. However, the identity of the proliferating cells was not determined in that study and may include infiltrating immune cell types.
Figure 2. Immunohistological analysis of cell proliferation and cell death markers in response to LPS.
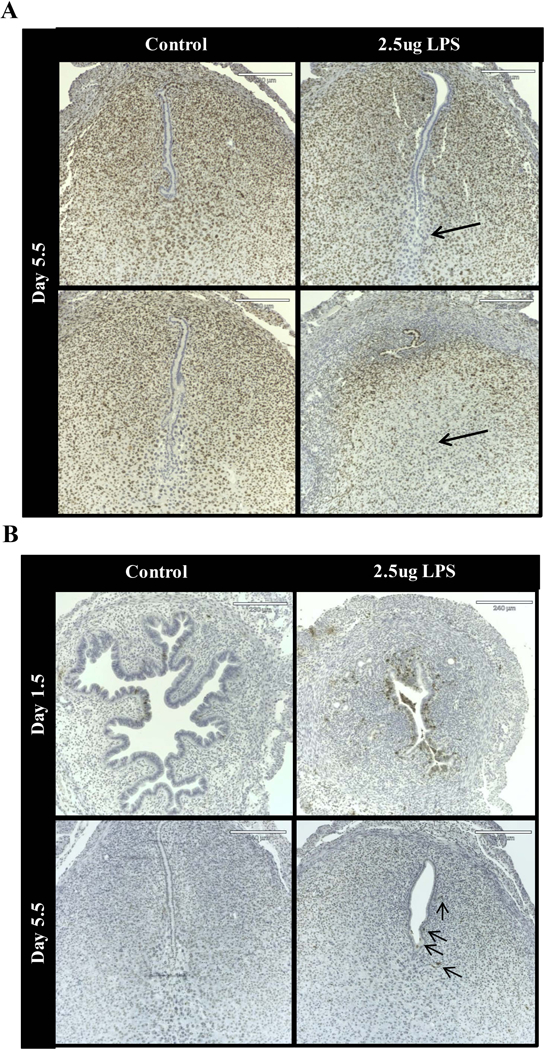
(A) Immunoreactive Ki-67 in cross-sections of uteri from vehicle and 2.5 μg LPS-treated mice on 1.5 and 5.5 dpc. Arrows point to regions of reduced Ki-67 reactivity. (B) ApopTag staining in cross-sections of uteri from vehicle and 2.5 μg LPS-treated mice on 1.5 and 5.5 dpc. Arrows point to ApopTag positive cells. n = 3 mice per group per time point.
To further evaluate the effect of preimplantation LPS exposure on the process of cell survival, apoptotic cells were visualized by TUNEL immunostaining (Figure 2B). LPS treatment resulted in an acute rise in apoptosis, evident on 1.5 dpc primarily in the luminal epithelial cells and the adjacent stroma. Apoptotic cells were also apparent in the stroma of 5.5 dpc uterine sections from mice treated with LPS. Rare TUNEL positive cells were observed in uterine sections from control mice at 1.5 dpc and none were evident at 5.5 dpc in control mice. Cell death in response to LPS treatment has also been observed in Human Hepatoma (HepG2), Human Microvascular Endothelial (HMVEC), and CD4 T cells and has been associated with increased reactive oxygen species production and oxidative stress in these cell types 39–41. Altered levels of proliferation and cell death likely contribute to implantation failure in this model, as these cellular processed are critical to the establishment of pregnancy.
3.2. Identifying the molecular changes that accompany LPS-induced implantation failure
To detect the molecular changes that accompany implantation failure in LPS-treated mice, we evaluated the transcript levels of various cytokines and chemokines in the uterus one day following LPS treatment (1.5 dpc) and at decidualization (5.5 dpc) (Figure 3A). Differentially expressed genes were identified by comparing uterine transcript levels in LPS-exposed mice to those in the control mice at the same time-point. Transcript levels in the uterus of non-pregnant, diestrus-staged mice served as a relative baseline for expression analysis. Plotting relative gene expression revealed several patterns of differentially expressed genes (Figures 3B, 3C, 3D, and 3E).
Figure 3. LPS treatment alters the expression of uterine cytokines and chemokines.
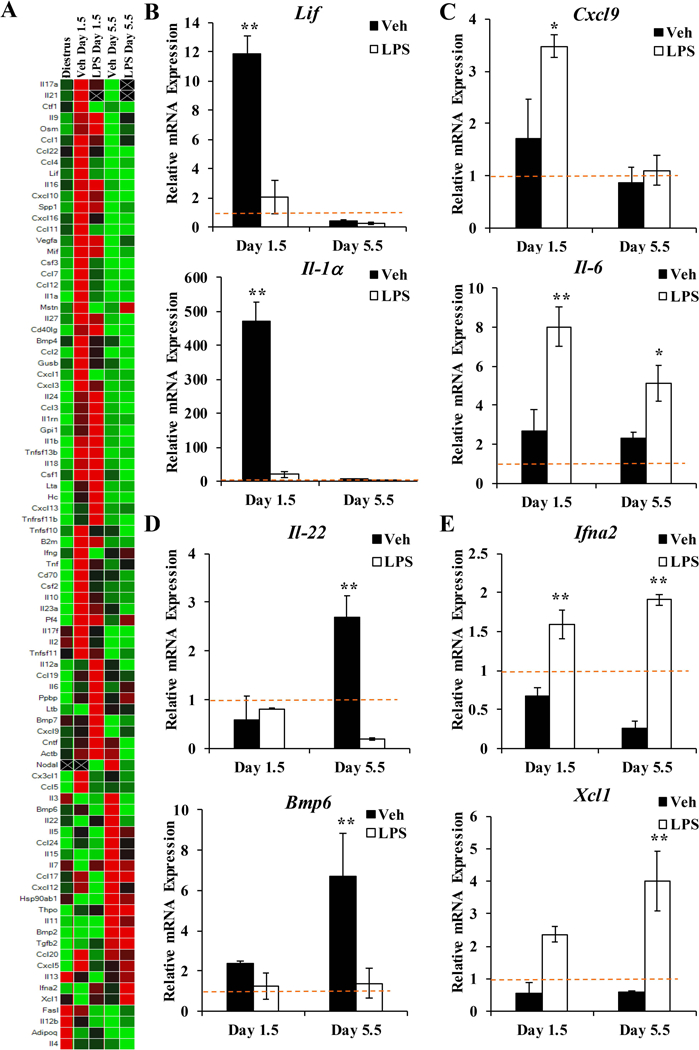
(A) Expression profile of cytokines and chemokines in the uteri of vehicle and LPS-treated mice at 1.5 and 5.5 dpc was evaluated by qRT-PCR and compared to levels in diestrus-staged non-pregnant uteri. Several patterns of differential gene expression were identified: (B) Expression of Lif and Il1α mRNA was induced in the uterus of control mice at 1.5 dpc not in mice exposed to LPS. (C) Expression of Cxcl9 and Il6 mRNA was acutely induced in the uterus in response to LPS exposure. (D) Expression of Il22 and Bmp6 mRNA was induced in the uterus of control mice on 5.5 dpc but not mice exposed to LPS. (E) Expression of Ifna2 and Xcl1 mRNA was repressed in the uterus of control mice at both timepoints but induced in the uterus of mice exposed to LPS. Expression data represent the mean of at least 4 independent experiments ± SEM. *p < 0.05; as determined by ANOVA.
In the control mice, transcript levels of leukemia inhibitory factor (Lif) were induced on 1.5 dpc compared to non-pregnant mice (Figure 3B). Lif is a pleiotropic cytokine that plays a critical role in embryo implantation by regulating growth and development of the embryo, enhancing embryo-uterine interactions, and stimulating receptivity of the endometrial stoma 42, 43. The early induction of Lif in the uterus prior to implantation was not evident in LPS-exposed mice. Likewise, interleukin-1 alpha (II-1α), which is also essential for implantation, was induced in the uterus of control mice on 1.5 dpc but not the uterus of mice exposed to LPS 44. The pattern representing genes induced immediately following mating in control mice but not LPS-exposed mice was evident for 34 of the 87 cytokine and chemokine genes analyzed. Conversely, transcript levels of cysteine-X-cysteine motif chemokine ligand 9 (Cxcl9), a chemokine that mediates T-cell trafficking, were acutely elevated in response to LPS (Figure 3C). Studies evaluating T-cell numbers in women with unexplained, recurrent spontaneous abortion (RSA) have correlated elevated levels of T helper 17 cells with RSA 45. Apart from the overall T cell number, the balance of T-cell subsets (e.g. Th1 and Th2) further influences implantation success, indicating that altered levels of Cxcl9 may disrupt the immune cell balance required for the establishment of pregnancy 46, 47. The pro-inflammatory cytokine interleukin-6 (Il-6) was also acutely induced in the uterus of LPS-exposed mice compared to controls (Figure 3C). Interestingly, levels of Il-6 remained significantly higher at 5.5 dpc in LPS treated mice, demonstrating persistent effects of inflammatory stimuli occurring prior to implantation. In addition, 19 genes were induced by LPS on 1.5 dpc compared to control samples at the same time-point.
Post-implantation, certain genes were uniquely induced during decidualization in the control mice compared to LPS-exposed mice. For example, transcript levels of interleukin-22 (Il-22) and bone morphogenic protein 6 (Bmp6) were increased in control mice on 5.5 dpc but not LPS-exposed mice (Figure 3D). mRNA expression of ciliary neurotrophic factor (Cntf), actin beta (Actb), nodal growth differentiation factor (Nodal), and interleukin 3 (Il-3) also followed this pattern. Previous reports have characterized the expression profiles of some of these genes in the uterus, demonstrating up-regulation during implantation and decidualization 48–50. However, their function during early pregnancy has yet to be determined. For example, Cntf, a member of the gp130 family that includes the critical cytokines Il-6 and Lif, binds the LIF receptor but does not have a demonstrated role in the uterus 51.
Interestingly, some genes found to be uniquely upregulated by LPS exposure were persistently upregulated at 5.5 dpc (Figure 3E). Transcript levels of interferon alpha 2 (Ifna2) and X-C motif chemokine ligand 1 (Xcl1) were elevated on 1.5 dpc, then significantly higher on 5.5 dpc. In control mice, the expression of these genes was reduced at both timepoints compared to levels in non-pregnant mice. The immunomodulatory functions of these chemokines are established 52, but it is unclear whether elevated levels of Ifna2 and Xcl1 in the uterus contribute to LPS-mediated implantation loss. Interferons are important for establishing uterine receptivity in domestic mammals 53, however the role of Ifna2 during pregnancy in humans has not been defined. The immune regulating effects of Xcl1 include tissue-specific recruitment of T lymphocytes, specifically dendritic cells 54, 55. Dendritic cells are potent antigen presenting cells found localized to the endometrium during pregnancy 56. Both elevated and low levels of uterine dendritic cells have been associated with RSA, suggesting that precise levels of dendritic cells are required for pregnancy success 57, 58. These results demonstrate that early exposure to bacterial endotoxins can result in acute and persistent effects on the expression of cytokines and chemokines in the mouse uterus during pregnancy. Moreover, the functions associated with these signaling molecules may disrupt the critical immune balance required for the establishment of pregnancy.
3.3. Characterizing Cytokine and Chemokine Release in Primary Human Endometrial Stromal Cell in Response to Decidualization and LPS
As in the mouse, a complex network of signaling molecules, originating from the endometrial stroma, are necessary for establishing the microenvironment in human endometrium that can support and regulate embryo attachment, trophoblast invasion, stromal cell decidualization, and vascular remodeling 59, 60. The signaling molecules required to generate this local milieu include secreted cytokines and chemokines, and disturbances in their expression related to maternal infection and inflammation can lead to implantation failure 61, 62. To determine the direct effect of bacterial endotoxin exposure on cytokine and chemokine secretion by endometrial stromal cells, hESCs were decidualized in vitro in the presence and absence of 0.1 μg/mL LPS. The response of hESCs to decidualization was first characterized in the absence of LPS. The addition of decidualization cocktail results in the robust upregulation of decidual biomarkers IGFBP1 and PRL compared to the undecidualized hESCs on Day 0 (Figure 4A) 63, 64.
Figure 4. hESC response to decidualization.
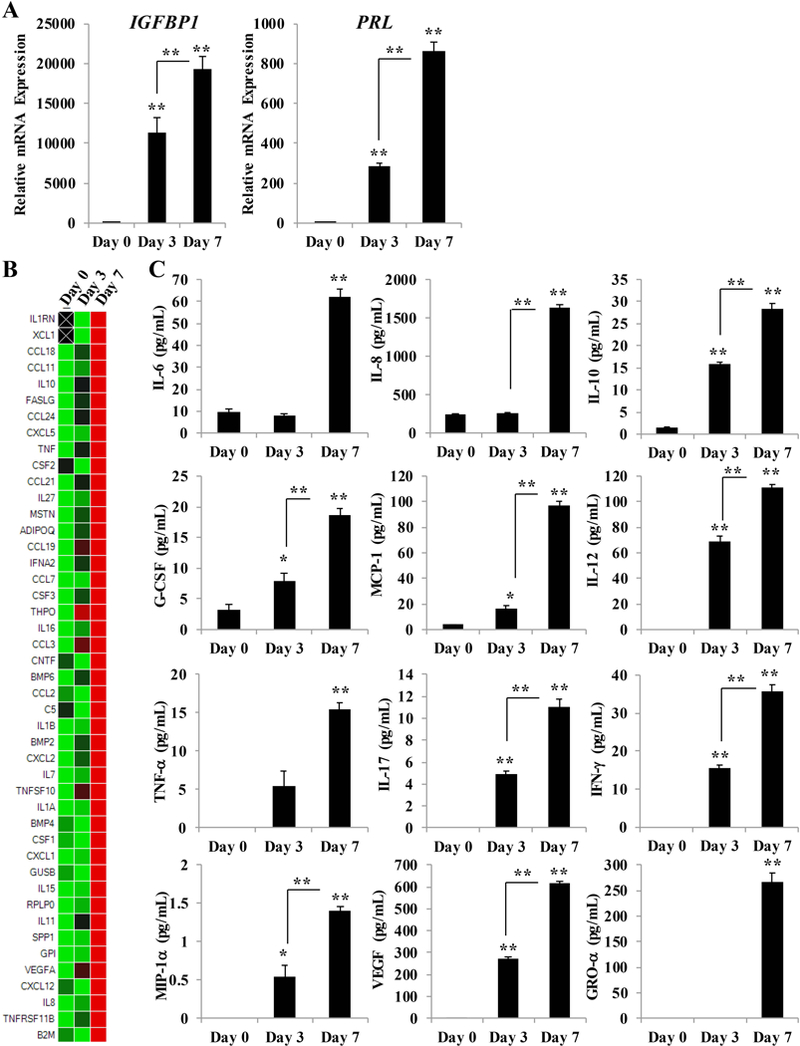
(A) qRT-PCR analysis of IGFBP1 and PRL mRNA in hESCs treated with decidualization cocktail on day 0, 3, and 7. mRNA values were normalized to the reference gene PPIB and set relative to Day 0 samples. (B) Transcript levels of cytokines and chemokines were measured by qRT-PCR using a predesigned array panel, and expression of genes regulated in response to decidualization was compared to levels in non-decidualized (Day 0) hESCs. (C) Measurement of cytokines and chemokines released into the media from hESCs on Day 0, 3, and 7 of decidualization by Luminex-based cytoplex. Bar graphs represent the mean of at least 4 independent experiments ± SEM. *p< 0.05 and **p < 0.01, as determined by ANOVA with Tukey’s post-hoc analysis.
To identify the decidualization-associated cytokines and chemokines, focused gene expression profiling was performed using a predesigned human cytokine and chemokine array panel (Figure 4B). mRNA expression in hESCs exposed to decidualization cocktail for 3 or 7 days was compared to expression levels in non-decidualized cells (Day 0). Following 7 days of hormone stimulus, 45 cytokine and chemokine genes were upregulated compared to non-decidualized cells. The genes evaluated by the human array panel included some that were also evaluated by the mouse cytokine and chemokine array panel in Figure 3A. We found that the following genes were upregulated in response to decidualization in both hESCs and the mouse uterus: CCL24, CXCL5, THPO, BMP6, BMP2, IL7, IL15, IL11, and CXCL12. We performed cytoplex analysis to identify the cytokines and chemokines secreted from hESCs in response to decidualization and found that IL-6, IL-8, IL-10, G-CSF, MCP-1, IL-12, TNF-α, IL-17, IFN-γ, MIP-1α, VEGF, and GRO-α were progressively released in a time-dependent manner following hormone stimulus (Figure 4C). Levels of GM-CSF, MIP-1β, and IL-1β were below the limit of detection at all timepoints evaluated. RANTES levels did not appreciably change with addition of the decidualization cocktail and IP-10 levels were significantly lower on Day 3 and Day 7 (data not shown). Elevated levels of proinflammatory/immunostimulatory cytokines characterize early implantation, and the expression of many of these cytokines and chemokines has been clinically associated with implantation rates 65. For example, intrauterine concentrations of TNF-α are positively associated with clinical pregnancy 66. Likewise, the TNF-α 308 promoter polymorphism, which is associated with high TNF-α production, has been linked to increased implantation rates and a reduced rate of miscarriage 67, 68. Conversely, a polymorphism at position 405 in the VEGF gene, which is associated with reduced gene expression, is associated with reduced embryo implantation rates 69, 70. Moreover, recent studies have demonstrated that G-CSF treatment in women with recurrent IVF failure improved implantation and pregnancy rates 71. It is important to note that it is necessary for the concentration and ratio of certain cytokines to remain within a narrow range to be associated with positive pregnancy outcomes, where levels above this range are associated with implantation failure and pregnancy loss 72, 73.
hESCs decidualized in the presence of LPS expressed significantly lower transcript levels of decidual biomarkers IGFBP1 and PRL compared to those decidualized without LPS (Figure 5A). Importantly, others have shown that decidualized stromal cells from patients with chronic endometritis secrete lower levels of IGFBP1 and PRL compared to patients without chronic endometritis 74. While the expression of decidual biomarkers was attenuated, the secretion of many cytokines and chemokines was enhanced when decidualized hESCs were exposed to LPS (Figure 5B). Secreted levels of IL-6, IL-8, IL-10, G-CSF, MCP-1, VEGF, GRO-α, RANTES, and IP-10 were significantly higher in hESCs exposed to the combination of decidualization cocktail and LPS compared to those exposed to only the decidualization cocktail on Day 7. These results confirm previous findings that demonstrated decidualized hESCs secrete higher levels of MCP-1 following exposure to LPS 75. A prospective cohort study found that women with successful clinical pregnancies had lower endometrial levels of MCP-1 than those with failed pregnancies, indicating a sensitivity to MCP-1 levels 76. Therefore, elevated cytokine levels in response to endotoxin exposure may be detrimental to the establishment of pregnancy. Induced cytokine expression in response to LPS has also been demonstrated in non-decidualized human, mouse, and bovine endometrial cells 77–80. However, this study is one of the first to evaluate the direct response of hESCs to bacterial endotoxin exposure during decidualization. Interestingly, secreted levels of IL-12 and IFN-γ were significantly decreased in decidualized hESCs exposed to LPS compared to those that were not exposed to LPS. In most cell types studied, LPS induced the expression of IL-12 and IFN-γ, suggesting a unique response to LPS in the context of decidualization 81, 82.
Figure 5. hESC combination LPS and decidualization effects on day 7.
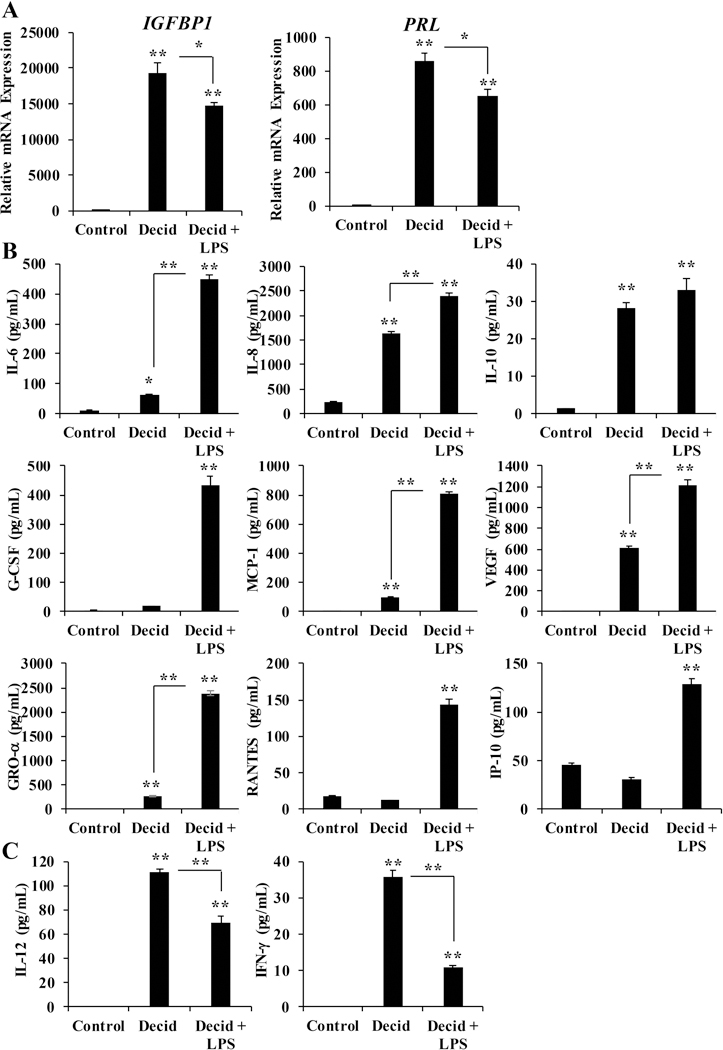
(A) mRNA expression of IGFBP1 and PRL in response to a combination of 0.1 μg/mL LPS and decidualization. mRNA values were normalized to the reference gene PPIB and set relative to control samples. (B) Secreted levels of cytokines and chemokines were measured by Luminex-based cytoplex in conditioned media from hESCs treated with decidualization cocktail +/− 0.1 μg/mL LPS. (C) Secreted levels of IL-12 and IFN-γ from hESCs exposed to decidualization cocktail +/− 0.1 μg LPS. Bar graphs represent the mean of at least 4 independent experiments ± SEM. **p< 0.01 as determined by ANOVA.
This study provides two relevant models to study the impact of bacteria endotoxin exposure on the establishment of pregnancy. We found that LPS exposure occurring prior to implantation and decidualization resulted in stark changes to the mouse endometrium and in hESCs that would be expected to blunt the initial stages of pregnancy. As chronic endometritis is associated with poor reproductive outcomes, these new models are directly relevant to enhancing our understanding of how bacterial endotoxins alter the uterine environment 83. Furthermore, use of these in vivo and in vitro models provide an avenue to identify and test targets for therapeutic intervention, ultimately improving fertility 84.
Supplementary Material
Acknowledgments
Funding Information:
This work was supported in part by a National Institutes of Health grant from the National Institute of Environmental Health Sciences R00 ES022983 (to S.W.) and an Albert McKern Scholar Award for Perinatal Research (to S.W.).
References
- 1.Park DW, Yang KM: Hormonal regulation of uterine chemokines and immune cells. Clin Exp Reprod Med 2011;38:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura H, Jasper MJ, Hull ML, Aplin JD, Robertson SA: Macrophages regulate expression of alpha1,2-fucosyltransferase genes in human endometrial epithelial cells. Mol Hum Reprod 2012;18:204–215. [DOI] [PubMed] [Google Scholar]
- 3.Mor G, Aldo P, Alvero AB: The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 2017;17:469–482. [DOI] [PubMed] [Google Scholar]
- 4.Barash A, Dekel N, Fieldust S, Segal I, Schechtman E, Granot I: Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil Steril 2003;79:1317–1322. [DOI] [PubMed] [Google Scholar]
- 5.Gnainsky Y, Granot I, Aldo PB, Barash A, Or Y, Schechtman E, Mor G, Dekel N: Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertil Steril 2010;94:2030–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zollner U, Bischofs S, Lalic I, Zollner K-P: LIF and TNF alpha concentrations in embryo culture media are predictive for embryo implantation in IVF, 2012.
- 7.Buyuk E, Asemota OA, Merhi Z, Charron MJ, Berger DS, Zapantis A, Jindal SK: Serum and follicular fluid monocyte chemotactic protein-1 levels are elevated in obese women and are associated with poorer clinical pregnancy rate after in vitro fertilization: a pilot study. Fertil Steril 2017;107:632–640 e633. [DOI] [PubMed] [Google Scholar]
- 8.Kitaya K, Yasuo T: Immunohistochemistrical and clinicopathological characterization of chronic endometritis. Am J Reprod Immunol 2011;66:410–415. [DOI] [PubMed] [Google Scholar]
- 9.Polisseni F, Bambirra EA, Camargos AF: Detection of chronic endometritis by diagnostic hysteroscopy in asymptomatic infertile patients. Gynecol Obstet Invest 2003;55:205–210. [DOI] [PubMed] [Google Scholar]
- 10.Haggerty CL, Hillier SL, Bass DC, Ness RB, Evaluation PID, Clinical Health Study I: Bacterial Vaginosis and Anaerobic Bacteria Are Associated with Endometritis. Clinical Infectious Diseases 2004;39:990–995. [DOI] [PubMed] [Google Scholar]
- 11.Cicinelli E, Matteo M, Tinelli R, Lepera A, Alfonso R, Indraccolo U, Marrocchella S, Greco P, Resta L: Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod 2015;30:323–330. [DOI] [PubMed] [Google Scholar]
- 12.Kitaya K, Matsubayashi H, Takaya Y, Nishiyama R, Yamaguchi K, Takeuchi T, Ishikawa T: Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am J Reprod Immunol 2017;78. [DOI] [PubMed]
- 13.Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ: Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol Reprod 2009;81:1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardenas I, Mor G, Aldo P, Lang SM, Stabach P, Sharp A, Romero R, Mazaki-Tovi S, Gervasi M, Means RE: Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol 2011;65:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidel PL Jr., Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB: Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 1994;170:1467–1475. [DOI] [PubMed] [Google Scholar]
- 16.Robertson SA, Skinner RJ, Care AS: Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol 2006;177:4888–4896. [DOI] [PubMed] [Google Scholar]
- 17.Deb K, Chaturvedi MM, Jaiswal YK: A ‘minimum dose’ of lipopolysaccharide required for implantation failure: assessment of its effect on the maternal reproductive organs and interleukin-1 alpha expression in the mouse. Reproduction 2004;128:87–97. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Wu J, Bagchi IC, Bagchi MK, Sidell N, Taylor RN: Disruption of gap junctions reduces biomarkers of decidualization and angiogenesis and increases inflammatory mediators in human endometrial stromal cell cultures. Mol Cell Endocrinol 2011;344:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healy LL, Cronin JG, Sheldon IM: Endometrial cells sense and react to tissue damage during infection of the bovine endometrium via interleukin 1. Sci Rep 2014;4:7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kommagani R, Szwarc MM, Vasquez YM, Peavey MC, Mazur EC, Gibbons WE, Lanz RB, DeMayo FJ, Lydon JP: The Promyelocytic Leukemia Zinc Finger Transcription Factor Is Critical for Human Endometrial Stromal Cell Decidualization. PLoS Genet 2016;12:e1005937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strassmann G, Fong M, Windsor S, Neta R: The role of interleukin-6 in lipopolysaccharide-induced weight loss, hypoglycemia and fibrinogen production, in vivo. Cytokine 1993;5:285–290. [DOI] [PubMed] [Google Scholar]
- 22.Plowman GD, Green JM, McDonald VL, Neubauer MG, Disteche CM, Todaro GJ, Shoyab M: The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Molecular and Cellular Biology 1990;10:1969–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK: Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol 1995;9:691–705. [DOI] [PubMed] [Google Scholar]
- 24.Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong J-W, Lydon JP, Bagchi IC, Bagchi MK, DeMayo FJ: WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. The FASEB Journal 2011;25:1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC: Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 2007;282:31725–31732. [DOI] [PubMed] [Google Scholar]
- 26.Kim TH, Yoo JY, Wang Z, Lydon JP, Khatri S, Hawkins SM, Leach RE, Fazleabas AT, Young SL, Lessey BA, Ku BJ, Jeong JW: ARID1A Is Essential for Endometrial Function during Early Pregnancy. PLoS Genet 2015;11:e1005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubel CA, Wu SP, Lin L, Wang T, Lanz RB, Li X, Kommagani R, Franco HL, Camper SA, Tong Q, Jeong JW, Lydon JP, DeMayo FJ: A Gata2-Dependent Transcription Network Regulates Uterine Progesterone Responsiveness and Endometrial Function. Cell Rep 2016;17:1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whirledge SD, Oakley RH, Myers PH, Lydon JP, DeMayo F, Cidlowski JA: Uterine glucocorticoid receptors are critical for fertility in mice through control of embryo implantation and decidualization. Proc Natl Acad Sci U S A 2015;112:15166–15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant DR: Physiological and molecular determinants of embryo implantation. Molecular aspects of medicine 2013;34:939–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H, Hu C, Li F, Liang L, Liu L: Effect of lipopolysaccharide on the biological characteristics of human skin fibroblasts and hypertrophic scar tissue formation. IUBMB Life 2013;65:526–532. [DOI] [PubMed] [Google Scholar]
- 31.Muller-Decker K, Manegold G, Butz H, Hinz DE, Huttner D, Richter KH, Tremmel M, Weissflog R, Marks F: Inhibition of cell proliferation by bacterial lipopolysaccharides in TLR4-positive epithelial cells: independence of nitric oxide and cytokine release. J Invest Dermatol 2005;124:553–561. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Wu L, Qu JM: Inhibited proliferation of human lung fibroblasts by LPS is through IL-6 and IL-8 release. Cytokine 2011;54:289–295. [DOI] [PubMed] [Google Scholar]
- 33.Vadiveloo PK, Keramidaris E, Morrison WA, Stewart AG: Lipopolysaccharide-induced cell cycle arrest in macrophages occurs independently of nitric oxide synthase II induction. Biochim Biophys Acta 2001;1539:140–146. [DOI] [PubMed] [Google Scholar]
- 34.Nelsen CJ, Rickheim DG, Tucker MM, Hansen LK, Albrecht JH: Evidence that cyclin D1 mediates both growth and proliferation downstream of TOR in hepatocytes. J Biol Chem 2003;278:3656–3663. [DOI] [PubMed] [Google Scholar]
- 35.Brizzi MF, Garbarino G, Rossi PR, Pagliardi GL, Arduino C, Avanzi GC, Pegoraro L: Interleukin 3 stimulates proliferation and triggers endothelial-leukocyte adhesion molecule 1 gene activation of human endothelial cells. J Clin Invest 1993;91:2887–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He Z, Jiang J, Kokkinaki M, Dym M: Nodal signaling via an autocrine pathway promotes proliferation of mouse spermatogonial stem/progenitor cells through Smad2/3 and Oct-4 activation. Stem Cells 2009;27:2580–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitra A, Raychaudhuri SK, Raychaudhuri SP: IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine 2012;60:38–42. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y, Zhang J, Qin Y: S100A4 promotes the development of lipopolysaccharide-induced mouse endometritis. Biol Reprod 2018. [DOI] [PubMed]
- 39.Raza H, John A, Shafarin J: Potentiation of LPS-Induced Apoptotic Cell Death in Human Hepatoma HepG2 Cells by Aspirin via ROS and Mitochondrial Dysfunction: Protection by N-Acetyl Cysteine. PLoS ONE 2016;11:e0159750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HL, Akinci IO, Baker CM, Urich D, Bellmeyer A, Jain M, Chandel NS, Mutlu GM, Budinger GR: The intrinsic apoptotic pathway is required for lipopolysaccharide-induced lung endothelial cell death. J Immunol 2007;179:1834–1841. [DOI] [PubMed] [Google Scholar]
- 41.McAleer JP, Vella AT: Understanding how lipopolysaccharide impacts CD4 T cell immunity. Critical reviews in immunology 2008;28:281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ: Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992;359:76–79. [DOI] [PubMed] [Google Scholar]
- 43.Salleh N, Giribabu N: Leukemia inhibitory factor: roles in embryo implantation and in nonhormonal contraception. TheScientificWorldJournal 2014;2014:201514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon C, Frances A, Piquette GN, el Danasouri I, Zurawski G, Dang W, Polan ML: Embryonic implantation in mice is blocked by interleukin-1 receptor antagonist. Endocrinology 1994;134:521–528. [DOI] [PubMed] [Google Scholar]
- 45.Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH, Lin QD: Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol 2010;84:164–170. [DOI] [PubMed] [Google Scholar]
- 46.Lee SK, Kim JY, Hur SE, Kim CJ, Na BJ, Lee M, Gilman-Sachs A, Kwak-Kim J: An imbalance in interleukin-17-producing T and Foxp3(+) regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod 2011;26:2964–2971. [DOI] [PubMed] [Google Scholar]
- 47.Ledee N, Petitbarat M, Chevrier L, Vitoux D, Vezmar K, Rahmati M, Dubanchet S, Gahery H, Bensussan A, Chaouat G: The Uterine Immune Profile May Help Women With Repeated Unexplained Embryo Implantation Failure After In Vitro Fertilization. Am J Reprod Immunol 2016;75:388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park CB, Dufort D: Nodal expression in the uterus of the mouse is regulated by the embryo and correlates with implantation. Biol Reprod 2011;84:1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garrido-Gomez T, Dominguez F, Lopez JA, Camafeita E, Quinonero A, Martinez-Conejero JA, Pellicer A, Conesa A, Simon C: Modeling human endometrial decidualization from the interaction between proteome and secretome. J Clin Endocrinol Metab 2011;96:706–716. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Xu B, Li MQ, Li DJ, Jin LP: IL-22 secreted by decidual stromal cells and NK cells promotes the survival of human trophoblasts. International journal of clinical and experimental pathology 2013;6:1781–1790. [PMC free article] [PubMed] [Google Scholar]
- 51.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L: Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. The Biochemical journal 1998;334 (Pt 2):297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul F, Pellegrini S, Uze G: IFNA2: The prototypic human alpha interferon. Gene 2015;567:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bazer FW, Spencer TE, Johnson GA: Interferons and uterine receptivity. Seminars in reproductive medicine 2009;27:90–102. [DOI] [PubMed] [Google Scholar]
- 54.Kelner GS, Kennedy J, Bacon KB, Kleyensteuber S, Largaespada DA, Jenkins NA, Copeland NG, Bazan JF, Moore KW, Schall TJ, et al. : Lymphotactin: a cytokine that represents a new class of chemokine. Science 1994;266:1395–1399. [DOI] [PubMed] [Google Scholar]
- 55.Kroczek RA, Henn V: The Role of XCR1 and its Ligand XCL1 in Antigen Cross-Presentation by Murine and Human Dendritic Cells. Frontiers in immunology 2012;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sutton L, Mason DY, Redman CW: HLA-DR positive cells in the human placenta. Immunology 1983;49:103–112. [PMC free article] [PubMed] [Google Scholar]
- 57.Liu S, Wei H, Li Y, Huang C, Lian R, Xu J, Chen L, Zeng Y: Downregulation of ILT4(+) dendritic cells in recurrent miscarriage and recurrent implantation failure. Am J Reprod Immunol 2018:e12998. [DOI] [PubMed]
- 58.Quenby S, Bates M, Doig T, Brewster J, Lewis-Jones DI, Johnson PM, Vince G: Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum Reprod 1999;14:2386–2391. [DOI] [PubMed] [Google Scholar]
- 59.PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, McCune JM, Mor G, Schulz L, Soares M, Spencer T, Strominger J, Way SS, Yoshinaga K: Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nature immunology 2015;16:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holmberg JC, Haddad S, Wunsche V, Yang Y, Aldo PB, Gnainsky Y, Granot I, Dekel N, Mor G: An in vitro model for the study of human implantation. Am J Reprod Immunol 2012;67:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guzeloglu-Kayisli O, Kayisli UA, Taylor HS: The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Seminars in reproductive medicine 2009;27:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dimitriadis E, White CA, Jones RL, Salamonsen LA: Cytokines, chemokines and growth factors in endometrium related to implantation. Human reproduction update 2005;11:613–630. [DOI] [PubMed] [Google Scholar]
- 63.Daly DC, Maslar IA, Riddick DH: Prolactin production during in vitro decidualization of proliferative endometrium. Am J Obstet Gynecol 1983;145:672–678. [DOI] [PubMed] [Google Scholar]
- 64.Giudice LC, Dsupin BA, Irwin JC: Steroid and peptide regulation of insulin-like growth factor-binding proteins secreted by human endometrial stromal cells is dependent on stromal differentiation. J Clin Endocrinol Metab 1992;75:1235–1241. [DOI] [PubMed] [Google Scholar]
- 65.van Mourik MS, Macklon NS, Heijnen CJ: Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. Journal of leukocyte biology 2009;85:4–19. [DOI] [PubMed] [Google Scholar]
- 66.Boomsma CM, Kavelaars A, Eijkemans MJ, Lentjes EG, Fauser BC, Heijnen CJ, Macklon NS: Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum Reprod 2009;24:1427–1435. [DOI] [PubMed] [Google Scholar]
- 67.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW: Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci U S A 1997;94:3195–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vialard F, El Sirkasi M, Tronchon V, Boudjenah R, Molina-Gomes D, Bergere M, Mauduit C, Wainer R, Selva J, Benahmed M: Tumor necrosis factor-308 polymorphism increases the embryo implantation rate in women undergoing in vitro fertilization. Hum Reprod 2013;28:2774–2783. [DOI] [PubMed] [Google Scholar]
- 69.Boudjenah R, Molina-Gomes D, Torre A, Boitrelle F, Taieb S, Dos Santos E, Wainer R, de Mazancourt P, Selva J, Vialard F: Associations between Individual and Combined Polymorphisms of the TNF and VEGF Genes and the Embryo Implantation Rate in Patients Undergoing In Vitro Fertilization (IVF) Programs. PLoS One 2014;9:e108287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watson CJ, Webb NJ, Bottomley MJ, Brenchley PE: Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine 2000;12:1232–1235. [DOI] [PubMed] [Google Scholar]
- 71.Aleyasin A, Abediasl Z, Nazari A, Sheikh M: Granulocyte colony-stimulating factor in repeated IVF failure, a randomized trial. Reproduction 2016;151:637–642. [DOI] [PubMed] [Google Scholar]
- 72.Gazvani MR, Bates M, Vince G, Christmas S, Lewis-Jones DI, Kingsland C: Follicular fluid concentrations of interleukin-12 and interleukin-8 in IVF cycles. Fertil Steril 2000;74:953–958. [DOI] [PubMed] [Google Scholar]
- 73.Kwak-Kim JY, Chung-Bang HS, Ng SC, Ntrivalas EI, Mangubat CP, Beaman KD, Beer AE, Gilman-Sachs A: Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum Reprod 2003;18:767–773. [DOI] [PubMed] [Google Scholar]
- 74.Wu D, Kimura F, Zheng L, Ishida M, Niwa Y, Hirata K, Takebayashi A, Takashima A, Takahashi K, Kushima R, Zhang G, Murakami T: Chronic endometritis modifies decidualization in human endometrial stromal cells. Reproductive biology and endocrinology : RB&E 2017;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu X, Wang Q, Deng B, Wang H, Dong Z, Qu X, Kong B: Monocyte chemoattractant protein-1 secreted by decidual stromal cells inhibits NK cells cytotoxicity by up-regulating expression of SOCS3. PLoS One 2012;7:e41869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rahiminejad ME, Moaddab A, Ebrahimi M, Rabiee S, Zamani A, Ezzati M, Abdollah Shamshirsaz A: The relationship between some endometrial secretion cytokines and in vitro fertilization. Iran J Reprod Med 2015;13:557–562. [PMC free article] [PubMed] [Google Scholar]
- 77.Arima K, Nasu K, Narahara H, Fujisawa K, Matsui N, Miyakawa I: Effects of lipopolysaccharide and cytokines on production of RANTES by cultured human endometrial stromal cells. Mol Hum Reprod 2000;6:246–251. [DOI] [PubMed] [Google Scholar]
- 78.Cronin JG, Turner ML, Goetze L, Bryant CE, Sheldon IM: Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol Reprod 2012;86:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koh YQ, Mitchell MD, Almughlliq FB, Vaswani K, Peiris HN: Regulation of inflammatory mediator expression in bovine endometrial cells: effects of lipopolysaccharide, interleukin 1 beta, and tumor necrosis factor alpha. Physiological reports 2018;6:e13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheldon IM, Roberts MH: Toll-like receptor 4 mediates the response of epithelial and stromal cells to lipopolysaccharide in the endometrium. PLoS One 2010;5:e12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kubin M, Chow JM, Trinchieri G: Differential regulation of interleukin-12 (IL-12), tumor necrosis factor alpha, and IL-1 beta production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood 1994;83:1847–1855. [PubMed] [Google Scholar]
- 82.Wysocka M, Kubin M, Vieira LQ, Ozmen L, Garotta G, Scott P, Trinchieri G: Interleukin-12 is required for interferon-gamma production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol 1995;25:672–676. [DOI] [PubMed] [Google Scholar]
- 83.Kitaya K, Takeuchi T, Mizuta S, Matsubayashi H, Ishikawa T: Endometritis: new time, new concepts. Fertil Steril 2018;110:344–350. [DOI] [PubMed] [Google Scholar]
- 84.Vitagliano A, Saccardi C, Noventa M, Di Spiezio Sardo A, Saccone G, Cicinelli E, Pizzi S, Andrisani A, Litta PS: Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: a systematic review and meta-analysis. Fertil Steril 2018;110:103–112 e101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


