Abstract
Face vascularized composite allografts (FVCA) have helped severe patient disfigurement, with acute rejection now largely controlled through iatrogenic immunosuppression. However, little is known regarding the incidence and mechanism(s) of more long-term pathologic alterations in FVCAs that may affect function and graft durability. Protocol surveillance biopsies for up to an 8 year interval in seven patients who received FVCAs at our institution revealed histopathologic evidence of chronic rejection. Clinical manifestations included features of premature aging, mottled leukoderma accentuating suture lines, telangiectasia, and dryness of nasal mucosa. Pathologic changes consisted of epidermal thinning accompanied by discrete foci of lymphocyte-mediated cytotoxicity, hyperkeratosis, follicular plugging, vascular ectasia, and sclerosis beneath the epidermal layer associated with collagen type I deposition. Genomic interrogation and immunohistochemistry of sclerotic zones revealed upregulation of the AP-1 pathway components, JunB and c-Fos, previously implicated in overproduction of type I dermal collagen in the setting of systemic sclerosis. We conclude that some patients develop chronic rejection in FVCAs with striking similarities to alterations seen in certain autoimmune cutaneous disorders (lupus erythematosus and scleroderma/chronic sclerodermoid GVHD). Identification of relevant pathways and genes, such as JunB and c-Fos, may provide new targets into preventative therapies for chronic immune-mediated changes in vascularized composite allografts.
Introduction
Successful allotransplantion of complex composite tissues such as a face has dramatically transformed restorative options for severely disfigured patients.(1) Ongoing development and adaptation of iatrogenic immunosuppressive regimen have significantly reduced the risks of acute rejection episodes. Chronic changes in allografts, known as chronic rejection (CR), are responsible for long term allograft deterioration and the potential for graft loss in solid organ transplantation (SOT). In both solid organ transplantation and VCA, CR has been associated with graft vasculopathy involving vascular narrowing due to intimal hyperplasia and fibrosis.(2–4) Initially, antibody-mediated graft injury was implicated in CR. More recently, cell mediated rejection, total number of rejection episodes and innate immune response mechanisms have also been linked to CR in solid organ transplantation. Further studies have revealed a potential role for other factors in the production of chronic graft changes, such as genetic predisposition, ischemia reperfusion injury, infections, smoking and arterial hypertension.(5)
Recent reports indicate that graft loss through chronic rejection in long term FVCA recipients has become part of clinical reality.(3, 6) However, little is known regarding the incidence and mechanism(s) of more long-term pathologic alterations in FVCAs that may affect function and graft durability.(7) Herein we report the occurrence of CR in our patient cohort of seven FVCA recipients, including aspects of their clinical, histologic and molecular presentations. Our data suggests that chronic graft alterations in FVCA share similarities to alterations seen in certain autoimmune cutaneous disorders.
Material and Methods
Study subjects
Between 2009 and 2016, seven patients received a facial transplantation after extensive evaluation of risks and benefits and providing written informed consent under the Partners Human Research Committee protocol 2008P000550.
Evaluation of over 250 skin biopsies of face and sentinel flaps of our recipients during routine surveillance at pre-determined set time points and during episodes of rejection, we identified three patients with histological changes in facial biopsies that are suggestive of chronic pathological alterations. For every patient, we evaluated samples that were histologically graded Grade 0 for non-rejection (NR), Grade III for acute rejection (AR) and those showing signs of chronic rejection (CR). Only samples with signs of acute grade III were selected to allow clear differentiation between AR, non-rejection (NR), and CR.
Histomorphology
Punch biopsy specimens (3–4 mm) were formalin-fixed and paraffin-embedded for further analysis by the dermatopathology core facility of Brigham and Women’s Hospital. Sections were stained with hematoxylin and eosin and evaluated by two different experienced dermatopathologists (GFM and CGL). All samples were assessed according to the most recent Banff 2007 Working Classification of Skin-Containing Composite Tissue Allograft Pathology. Additionally, we specifically surveyed for signs of dermal fibrosis and abnormal collagen deposition, basement membrane thickening, vasculopathy, keratinocyte apoptosis, and any other signs of chronic low-grade injury to the epidermis. Herovici staining was performed to detect the presence and distribution of collagen I.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on 4-μm-thick formalin-fixed paraffin-embedded tissue sections following pressure cooker heat-induced epitope retrieval (Target Retrieval Solution, pH 6.1; DAKO, Carpinteria, CA) with the rabbit polyclonal anti-CD31 (1:100 dilution, clone polyclonal; Abcam, Cambridge, MA, USA), rabbit polyclonal anti-c-Fos (1:50 dilution, clone polyclonal; Abcam), and rabbit monoclonal anti-JunB (1:40 dilution, clone C37F9; Cell Signaling Technology, Danvers, MA) antibodies. The Envision Plus Detection System (DAKO) was used for all antibodies for the NR, AR, and CR slides.
Gene expression profiling
We assessed samples of all three face transplant recipients with changes suspicious of CR (n=4) by gene expression profiling. We also included NR (n=5) and AR (n=5) samples of other face transplant recipient within our cohort. mRNA extraction was performed using 4–10 10mm scrolls from paraffin embedded biopsy blocks and the High Pure FFPET RNA Isolation Kit (#06650775001, Roche Pharmaceuticals). Gene expression profiling was performed using the NanoString® platform with a Human PanCancerImmuno Panel consisting of 840 genes (XT_PGX_HuV1_CancerImmune_KIT XT-GXA-HIP1–12, Nanostring Technologies, Seattle, WA). After preparation as per protocol, samples were analyzed using codesets CodesetNS_CancerImmune_C2929 and HuV1_CancerImmu_v1_1.
Gene expression levels were quantified on the NanoString nCounter™ console. Raw counts were normalized using the nSolver™ software (nSolver v3.0; NanoString Technologies, Seattle, WA) against nine genes, that were selected to have the least variance with the geNorm algorithm. (8)
Bioinformatic analysis of the gene expression data was provided as a service by the Harvard Chan Bioinformatics Core, Harvard T.H. Chan School of Public Health, Boston, MA. Differential gene expression was examined with DESeq2 using the Wald Test for differential expression and the Benjamini-Hochberg multiple test correction.(9)
Results
Clinical and Histopathological Signs of CR
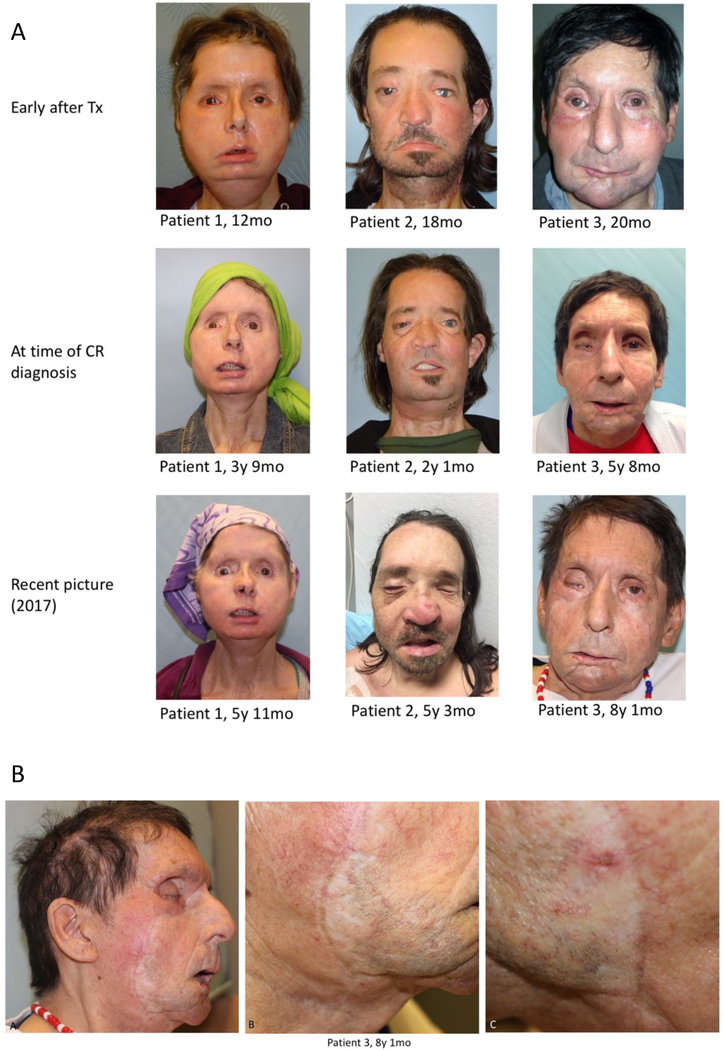
Routine assessment of biopsy specimen revealed histological changes that were indicative of chronic pathological alterations in three patients. Demographic characteristics as well as indications and extent of the transplant are summarized in Table 1. Although the clinical presentation of these three patients at the time of first histopathological diagnosis of suspected CR did not indicate clear macroscopic correlates of these histological changes (Fig. 1a), over the course of time, clinical alterations were noted. Patient 1 developed minute dry rhagades at the tip of the nose, one of which evolved into a visible indentation (Fig. 1b). Patient 2 showed signs consistent with accelerated chronologic aging, with facial laxity and wrinkling. Patient 3 showed increasingly prominent white demarcation of the suture lines over time, as well as telangiectasias that blanched under pressure. In both Patients 1 and 3, the facial allografts seemed to tighten over time. In all three patients, but particularly Patients 1 and 3, the skin of the allograft appeared thin and atrophic. Only these three patients reported repeated episodes of epistaxis over time, which required interventional treatment in one patient with coagulation (Fig. S1).
Table 1:
Characteristics of patients suspected for chronic rejection after face vascularized composite tissue transplantation compared to all patients of the Brigham and Women’s Hospital face transplant cohort. Tx – transplantation; CR – chronic rejection; AR – acute rejection; Tac – Tacrolimus; MMF – Mycophenolate mofetil; Pred – Prednisone; Myf – Myfortic; Sir - Sirolimus; Bela - Belatacept
| Mean | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|---|
| Type of Transplant | Full Face | Full Face | Partial Face | Full Face | Full Face | Partial Face | Partial Face | |
| Initial injury | Chimpanzee attack | Electrical Burn | Electrical Burn | Electrical Burn | Acid Burn | Ballistic Trauma | Ballistic Trauma | |
| Age at Tx | 47 (±19.1) | 57 | 25 | 59 | 30 | 44 | 39 | 33 |
| Donor age | 50 (±9.2) | 42 | 48 | 60 | 31 | 56 | 23 | 51 |
| Sex | female | male | male | male | female | male | male | |
| Signs of chronic rejection | Yes | Yes | Yes | No | No | No | No | |
| Time to CR in months | 46 (±21.5) | 45 | 25 | 68 | - | - | - | - |
| Total time post-transplantion | 76,4 (±23.5) | 86 | 88 | 112 | 87 | 65 | 45 | 52 |
| Total # AR episodes | 4,4 (±1.3) | 4 | 5 | 4 | 5 | 6 | 2 | 5 |
| # AR episodes before CR | 2(±1) | 2 | 1 | 3 | - | - | - | - |
| Donor Specific Antibodies | No | No | No | No | Yes | No | No | |
| Immunosuppression | Tac, Sir, Myf | Tac, MMF, Pred | Tac, MMF, Pred | Tac, MMF, Pred | Tac, MMF, Pred | Tac, MMF | Tac, MMF, Pred, Bela |
Fig. 1:
Clinical evolution of FVCAs for up to an 8-year interval showing changes associated with chronic rejection (A). Whitey patches on allograft with permanent telangiectasias in one of the FVCA recipients, suggestive of chronic changes (B).
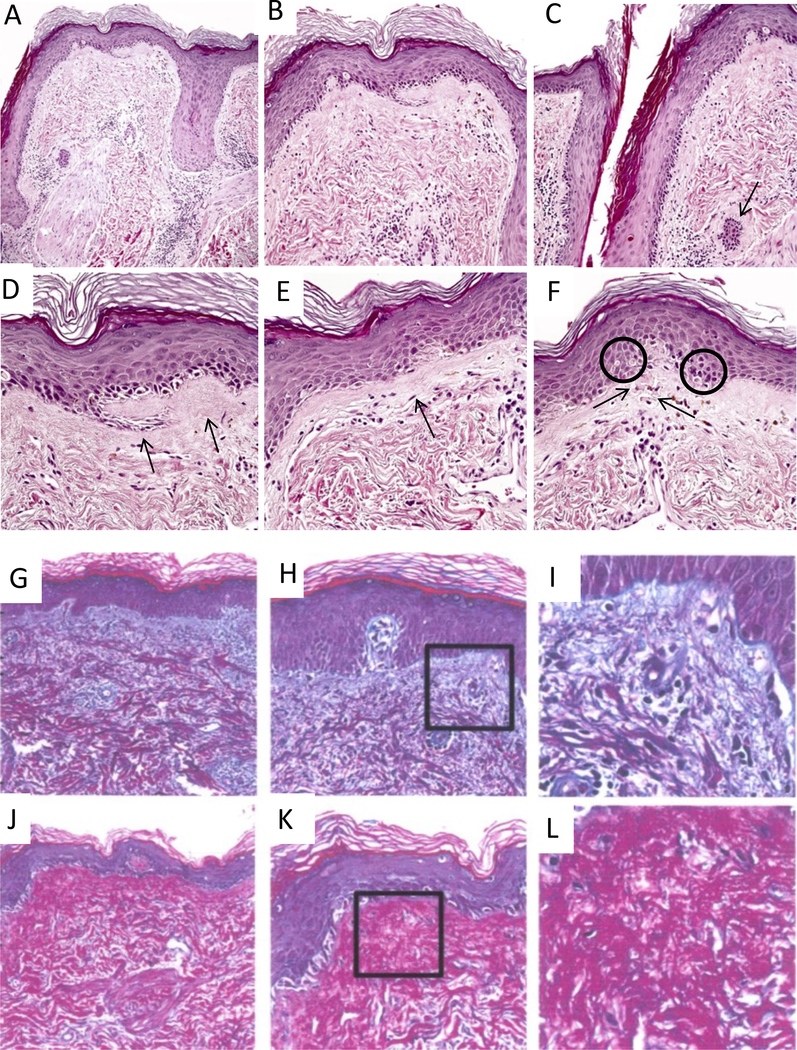
Histologically, there was epidermal thinning with loss of rete ridges, hyperkeratosis, and increased numbers of apoptotic cells (Fig. 2). Hair follicles demonstrated patchy lymphoid infiltration and infundibular plugging (Fig 2A and C), and the epidermal basement membrane and papillary dermis appeared thickened and fibrotic, containing scattered colloid bodies consistent with remote injury to basal keratinocytes (Fig. 2D-F). Herovici stains showed changes in distribution of different collagen types (Fig. 2G-I) when compaired to NR and AR specimens. CR biopsies demonstrated a shift of type I collagen, which is normally restricted to the reticular dermis, to the subepidermal papillary dermis (Fig. 2J-L). In some samples, this was accompanied by complete confluence of the layers of papillary and reticular dermis (Fig. 2J-L). The dermal microvasculature, as assessed morphologically and via CD31 immunohistochemistry, did not appear to be qualitatively or quantitatively altered in the CR specimens.
Fig. 2:
Evidence of chronicity in face transplant rejection in this biopsy includes destruction and deformation of hair follicles (f in panel A), patchy perifollicular lymphocytic infiltrates and arrector pili muscles (a, panel A) dissociated from follicles; epidermal thinning with loss of rete ridges (A-F); follicular plugging due to infundibular hyperkeratosis (C); fibrous thickening of subepidermal basement membrane zone (D&E, arrows); and intraepidermal and intradermal colloid body formation (F, encircled and arrows, respectively). Specimen A shows the typical distribution of collagen in null rejection tissue, where type III collagen, shown in blue, resides in the papillary dermis and type I collagen, shown in red, is in the reticular dermis (G-I). Specimen B, however, illustrate the shift of type I collagen into the papillary dermis in the case of chronic rejection (J-L).
Gene Expression Analysis of CR Biopsies
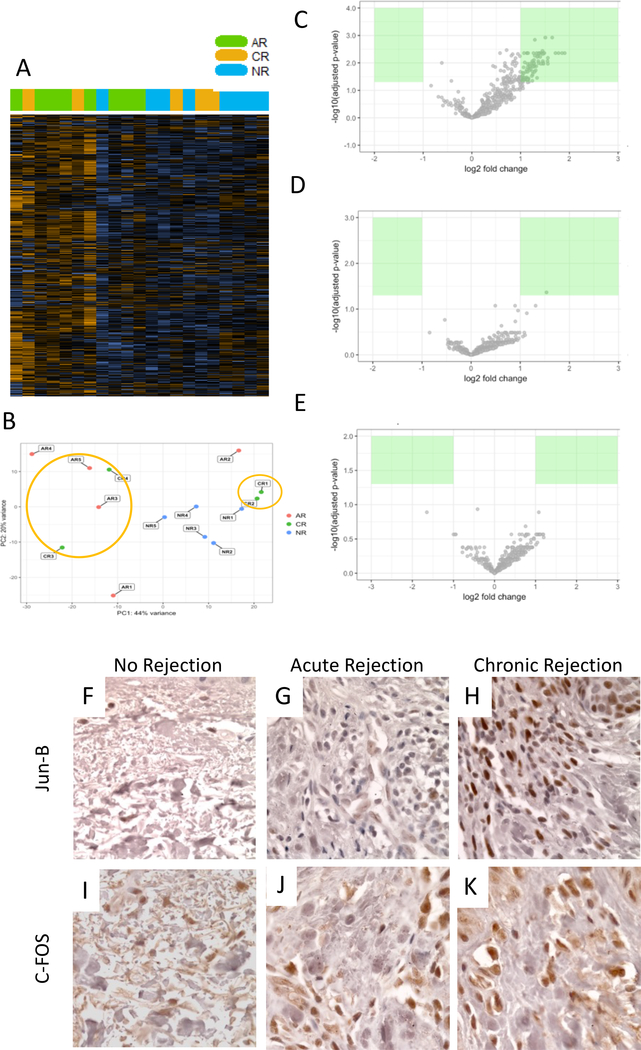
A total of four biopsy specimens suggestive of CR were compared to grade III acute rejection (AR) and samples with no signs of rejection (NR). An unsupervised heat map revealed distinct expression patterns (Fig. 3A). A principal component analysis of all samples disclosed a clustering of two CR samples with AR samples and two CR samples with NR samples (Fig. 3B). Re-assessment of histopathological slides confirmed co-existence of alterations of both acute on chronic rejection – also termed active chronic rejection - in two of the patients. Volcano plots confirmed distinct, yet not always significantly differentially-expressed, profiles (Fig. 3C-E).
Fig. 3:
Unsupervised clustering shows separation of AR and NR, and mixed distribution of CR (A). Principal component analysis indicates clustering of some CR specimen with AR and some with NR (B) suggesting partial acute on chronic presentation. Gene expression pointed distinct expression patterns in subsequent analysis; c-Fos (FOS) suggestive as marker for CR (C-E). IHC for c-Fos and JunB confirmed increased expression in CR samples (F-K).
Given the small sample size and variability of the samples, we were unable to reach statistical significance for differential expression in our comparison of CR and NR samples at an adjusted p-value < 0.1. However, the gene expression results suggest that innate immune responses may be activated in CR compared to NR. Expression of 16 genes was upregulated more than twofold (Table 2). These pertained mainly to immunomodulatory gene families of the innate immune system, particularly those pertaining to dendritic cells and macrophages.
Table 2:
Gene expression of non-rejection, acute and chronic rejection samples. Table S2 shows the top 20 genes with fold changes greater than twofold for chronic rejection (CR) vs non rejection (NR), acute rejection (AR) vs NR, and CR vs AR. Expression profiles were assessed using 840 gene containing panCancer immune Nanostring panel. FOS, aka c-Fos as part of AP-1 pathway, appears to associate with CR.
| CR vs NR | AR vs NR | CR vs AR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Fold Change | Adj. p | Gene | Fold Change | Adj. p | Gene | Fold Change | Adj. p | ||||
| FOS | 2.62 | NA | ↑ | CXCL11 | 3.74 | 0.0044 | ↑ | FOS | 2.19 | NA | ||
| CXCL11 | 2.29 | 0.3000 | ISG15 | 3.61 | 0.0044 | |||||||
| MX1 | 2.28 | 0.2714 | MX1 | 3.43 | 0.0044 | |||||||
| CXCL1 | 2.26 | 0.2714 | CCL19 | 3.28 | 0.0044 | |||||||
| C1QB | 2.17 | 0.2714 | ITGAX | 3.13 | 0.0012 | |||||||
| IFITM1 | 2.16 | 0.2714 | IFIT1 | 2.94 | 0.0066 | |||||||
| IFI27 | 2.14 | 0.2714 | CD83 | 2.94 | 0.0108 | |||||||
| FCGR2A | 2.12 | 0.2714 | CTLA4 | 2.93 | 0.0087 | |||||||
| SERPINB2 | 2.11 | 0.1289 | LY9 | 2.84 | 0.0012 | |||||||
| C3 | 2.11 | NA | PTPRC | 2.80 | 0.0044 | |||||||
| CD209 | 2.10 | 0.2714 | KLRK1 | 2.78 | 0.0044 | |||||||
| CCL8 | 2.06 | 0.3565 | CCL8 | 2.76 | 0.0108 | |||||||
| MSR1 | 2.06 | 0.2714 | PRF1 | 2.76 | 0.0102 | |||||||
| OAS3 | 2.05 | 0.2714 | SELPLG | 2.74 | 0.0048 | |||||||
| STAT1 | 2.02 | 0.2714 | IRF1 | 2.74 | 0.0087 | |||||||
| CD3E | 2.02 | 0.3822 | MSR1 | 2.73 | 0.0036 | |||||||
| BST2 | 2.70 | 0.0044 | ||||||||||
| IL2RG | 2.67 | 0.0108 | ||||||||||
| STAT1 | 2.64 | 0.0061 | ||||||||||
| IRF4 | 2.63 | 0.0044 | ||||||||||
Assessing specifically for genes expressed in chronic rejection samples compared to non-rejection as well as acute rejection, c-Fos (FOS) appeared to be upregulated in CR in both settings. Of interest was also an increased fold change of CXCL-1 in AR vs NR. (Table 2). Using immunohistochemistry, c-Fos upregulation was confirmed in the CR rejection samples, as well as the apparent upregulation of JunB, suggesting a role for AP-1 pathway activation in CR Fig 3F-K).
Discussion
Changes in clinical morphology and histology of facial allografts distinct from those of non-rejection and acute rejection occurred in three of seven facial transplant recipients. While macroscopic changes were more subtle and variable among patients, histology of these chronic changes shared alterations in epidermal thickness, collagen distribution and hair follicles. Profiling of gene expression showed nuanced differences in terms of innate and adaptive immune responses pathways between chronic, acute and non-rejection specimens, with findings implicating activation of AP-1 pathway in chronic rejection.
Although chronic rejection is a well-established complication in SOT, in FVCA there are only two previously-reported pathologically-proven cases.(6, 10, 11) Indeed, prompt diagnosis and treatment of AR episodes are considered as a potential explanation for the low incidence of CR episodes across all VCA centers (3), although the time-course required for eventual development of CR remains unknown. The first patient previously described in the literature developed T cell-mediated CR of the allograft after a programmed reduction of immunosuppressive (IS) therapy due to complications.(11) The clinical presentation entailed progressive fibrosis and retraction of the face with hypopigmented and hyperpigmented areas on the skin along with telangiectasias.(11) The second patient had developed chronic vascular rejection (described by the authors as chronic antibody-mediated rejection) with partial loss of the face allograft, presenting clinically as zones of tissue necrosis.(3)
The alterations in our patients with chronic FVCA alterations were more subtle and less dramatic, clinically consisting of early fibrotic changes of the skin (rhagades on the lips and nostrils), telangiectasias, and skin thinning, tightening, and/or accelerated wrinkling. Moreover, the development of epistaxis could herald nasal mucosal involvement in the spectrum of CR alterations. Because histopathology preceded the development of clinical changes, biopsies may provide an early indicator of CR at its earlier developmental stages.
Compared to the typical levels of collagen type I in the reticular dermis and collagen type III in the papillary dermis, our skin samples demonstrating CR showed collagen type I deposition in the papillary dermis, as well as occasional confluence of the reticular and papillary dermis. Bakker et al. noted increased levels of collagen I in the interstitial extracellular matrix of the kidney allografts to be unique to chronic rejection.(12) Significant deposits of dense collagen bundles were also noted by Petruzzo et al. in chronic rejection in a face allograft.(11, 13) In terms of basement membrane thickening, similar changes have also been described in discoid form of lupus erythematosus.(14)
Skin biopsies of a French face transplant recipient revealed thickening of the epidermis with dyskeratotic keratinocytes (apoptosis) and edema of the upper dermis in the absence of inflammatory infiltration.(11) Both epidermal thickening and thinning were similarly observed in our study, with epidermal thinning associated with the loss of rete ridges.
Unlike previous reports of chronic rejection in VCA(2–4), we could not identify consistent vascular changes in terms of intimal hyperplasia in our cohort of recipients with chronic rejection. Only one patient in our cohort had donor-specific antibodies and an episode of antibody mediated rejection – yet this patient never showed signs of chronic rejection in protocol biopsies.(15) We did though document endothelial changes in acute cellular rejection (lymphocytic vasculopathy). Small post capillary venues and arterioles in skin (the microvessels) generally don’t show intimal hyperplasia, although small arteries and venules in deeper dermis may. The microvessels, however, tend to show basement membrane reduplication and thickening (i.e. in diabetes, aging, and connective tissue diseases), and these features were not seen in our CR samples.(16) We suspect that CR in skin presents differently from other organs.
The histological changes of epidermal thickness, collagen type I and III distribution, and follicular and endothelium structure may present a more defined and consistent basis of determining the criteria for chronic rejection in VCA. Skin changes after organ transplantation have mainly been described in the context of tumor development, particularly squamous cell carcinoma. Skin related ailments such as pruritus and xerosis together with dialysis-induced microangiopathic changes in patients with chronic renal failure have been described to even improve after renal transplantation.(17, 18)
It has been argued that fibrotic graft changes could be the result of wound repair mechanisms due to ongoing tissue injury such as viral infections, T-cell mediated rejection, and calcineurin inhibitor toxicity.(19, 20) These changes are closely related to extracellular matrix remodelling and inflammatory pathways on a RNA and microRNA level.(21, 22) Mengel et al. and Einecke et al. described changes in gene expression for mast cell associated transcripts as well as plasma cell associated transcript to correlate with fibrosis in human kidney transplants.(23, 24) Transcripts of fibrillary collagen have been correlated to acute kidney injury as well as fibrosis.(20) Venner et al. demonstrated that progression of fibrosis in kidney transplants is independent of time, but more related to continuing graft injury.(25) It can be speculated, that fibrosis is skin is likely due to ongoing, potentially subclinical, inflammatory rejection like events - smouldering rejection – that over time is capable of inducing fibrotic changes. This could potentially explain the simultaneous histological presentation of acute and chronic changes in two of our samples – a phenomenon also described as active chronic rejection in SOT.
Molecular analysis identified c-Fos and CXCL1 with the largest fold change in CR vs NR; comparing CR vs AR, only c-Fos indicated an increased fold change. Activation of the AP-1 pathway via connective tissue growth factor induces collagen type 1 deposition, leading to fibrosis and sclerosis.(26) c-Fos is part of the AP-1 family of transcription factors, which form homo- and heterodimers together with other gene products of the JunB family. Growth-regulated-protein-1 (Gro-1/CXCL1) has been identified as a target gene of JunB via the RELA and SP1 pathways, and is an important mediator for neutrophil chemotaxis and leukocyte infiltration and persistent inflammation.(27)
It has been shown that an accumulation of JunB leads to an overexpression of type 1 collagen, which causes tissue fibrosis in patients with SSc.(28)
The increase in gene expression of c-Fos and JunB activity in samples with CR suggests that the AP-1 family may play a role in the collagen type 1 deposition in CR.
By its nature, our study is mainly limited by the small sample size, as facial allotransplantation has been performed in less than 40 patients worldwide.(1) Furthermore it is known from kidney biopsies, that biopsy location bias does exist – rejection is a focal process, thusly sampling error can occur.
In accordance with previous reports our investigation of chronic changes in composite vascularized facial allografts shows hallmarks of dermal fibrosis on a macroscopic, microscopic and likely also molecular level. Future research may be directed towards AP-1 related targets, which may yield the possibility of disrupting disturbed collagen deposition and inflammatory chemokines alike.
Supplementary Material
Acknowledgments
The project described was conducted with the support of Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH award #UL1 RR 025758 and financial contributions from participating institutions). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The authors (N.K., and B.P.) receive partial support from U.S. Department of Defense through a Biomedical Translational Initiative research contract (#W911QY-09-C-0216).
Abbreviation
- AMR
antibody mediated rejection
- AR
acute rejection
- CR
chronic rejection
- ACR
active chronic rejection i.e. with signs of acute rejection
- FVCA
face vascularized composite allograft
- IFTA
interstitial fibrosis and tubular atrophy
- NR
non-rejection
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found online in the supporting info tab for this article.
References
- 1.Sosin M, Rodriguez ED. The Face Transplantation Update: 2016. Plast Reconstr Surg. 2016;137(6):1841–50. [DOI] [PubMed] [Google Scholar]
- 2.Cendales LC, Kanitakis J, Schneeberger S, Burns C, Ruiz P, Landin L, et al. The Banff 2007 Working Classification of Skin-Containing Composite Tissue Allograft Pathology. American Journal of Transplantation. 2008;8(7):1396–400. [DOI] [PubMed] [Google Scholar]
- 3.Kanitakis J, Petruzzo P, Badet L, Gazarian A, Thaunat O, Testelin S, et al. Chronic Rejection in Human Vascularized Composite Allotransplantation (Hand and Face Recipients): An Update. Transplantation. 2016;100(10):2053–61. [DOI] [PubMed] [Google Scholar]
- 4.Kanitakis J, Petruzzo P, Gazarian A, Karayannopoulou G, Buron F, Dubois V, et al. Capillary Thrombosis in the Skin: A Pathologic Hallmark of Severe/Chronic Rejection of Human Vascularized Composite Tissue Allografts? Transplantation. 2016;100(4):954–7. [DOI] [PubMed] [Google Scholar]
- 5.Heemann U, Lutz J. Pathophysiology and treatment options of chronic renal allograft damage. Nephrol Dial Transplant. 2013;28(10):2438–46. [DOI] [PubMed] [Google Scholar]
- 6.Morelon E, Petruzzo P, Kanitakis J, Dakpe S, Thaunat O, Dubois V, et al. Face Transplantation: Partial Graft Loss of the First Case 10 Years Later. Am J Transplant. 2017;17(7):1935–40. [DOI] [PubMed] [Google Scholar]
- 7.Thaunat O, Badet L, Dubois V, Kanitakis J, Petruzzo P, Morelon E. Immunopathology of rejection: do the rules of solid organ apply to vascularized composite allotransplantation? Curr Opin Organ Transplant. 2015;20(6):596–601. [DOI] [PubMed] [Google Scholar]
- 8.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris P, Bradley A, Doyal L, Earley M, Hagen P, Milling M, et al. Face transplantation: a review of the technical, immunological, psychological and clinical issues with recommendations for good practice. Transplantation. 2007;83(2):109–28. [DOI] [PubMed] [Google Scholar]
- 11.Petruzzo P, Kanitakis J, Testelin S, Pialat JB, Buron F, Badet L, et al. Clinicopathological Findings of Chronic Rejection in a Face Grafted Patient. Transplantation. 2015;99(12):2644–50. [DOI] [PubMed] [Google Scholar]
- 12.Bakker RC, Koop K, Sijpkens YW, Eikmans M, Bajema IM, De Heer E, et al. Early interstitial accumulation of collagen type I discriminates chronic rejection from chronic cyclosporine nephrotoxicity. J Am Soc Nephrol. 2003;14(8):2142–9. [DOI] [PubMed] [Google Scholar]
- 13.Kanitakis J, Jullien D, Petruzzo P, Hakim N, Claudy A, Revillard J-P, et al. Clinicopathologic features of graft rejection of the first human hand allograft. Transplantation. 2003;76(4):688–93. [DOI] [PubMed] [Google Scholar]
- 14.Tachaudomdach C, Kantachuvesiri S, Wongpraphairot S, Worawichawong S, Tankee P, Riengrojpitak S, et al. High collagen I gene expression as an independent predictor of adverse renal outcomes in lupus nephritis patients with preserved renal function. Arch Pathol Lab Med. 2015;139(3):378–87. [DOI] [PubMed] [Google Scholar]
- 15.Chandraker A, Arscott R, Murphy GF, Lian CG, Bueno EM, Marty FM, et al. The management of antibody-mediated rejection in the first presensitized recipient of a full-face allotransplant. Am J Transplant. 2014;14(6):1446–52. [DOI] [PubMed] [Google Scholar]
- 16.Giannini C, Dyck PJ. Basement membrane reduplication and pericyte degeneration precede development of diabetic polyneuropathy and are associated with its severity. Ann Neurol. 1995;37(4):498–504. [DOI] [PubMed] [Google Scholar]
- 17.Ulrich C, Arnold R, Frei U, Hetzer R, Neuhaus P, Stockfleth E. Skin changes following organ transplantation: an interdisciplinary challenge. Dtsch Arztebl Int. 2014;111(11):188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilchrest BA, Rowe JW, Mihm MC Jr., Clinical and histological skin changes in chronic renal failure: evidence for a dialysis-resistant, transplant-responsive microangiopathy. Lancet. 1980;2(8207):1271–5. [DOI] [PubMed] [Google Scholar]
- 19.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Chapman JR, Allen RD. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation. 2004;78(4):557–65. [DOI] [PubMed] [Google Scholar]
- 20.Famulski KS, Reeve J, de Freitas DG, Kreepala C, Chang J, Halloran PF. Kidney transplants with progressing chronic diseases express high levels of acute kidney injury transcripts. Am J Transplant. 2013;13(3):634–44. [DOI] [PubMed] [Google Scholar]
- 21.Park WD, Griffin MD, Cornell LD, Cosio FG, Stegall MD. Fibrosis with inflammation at one year predicts transplant functional decline. J Am Soc Nephrol. 2010;21(11):1987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scian MJ, Maluf DG, David KG, Archer KJ, Suh JL, Wolen AR, et al. MicroRNA profiles in allograft tissues and paired urines associate with chronic allograft dysfunction with IF/TA. Am J Transplant. 2011;11(10):2110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mengel M, Reeve J, Bunnag S, Einecke G, Sis B, Mueller T, et al. Molecular correlates of scarring in kidney transplants: the emergence of mast cell transcripts. Am J Transplant. 2009;9(1):169–78. [DOI] [PubMed] [Google Scholar]
- 24.Einecke G, Reeve J, Mengel M, Sis B, Bunnag S, Mueller TF, et al. Expression of B cell and immunoglobulin transcripts is a feature of inflammation in late allografts. Am J Transplant. 2008;8(7):1434–43. [DOI] [PubMed] [Google Scholar]
- 25.Venner JM, Famulski KS, Reeve J, Chang J, Halloran PF. Relationships among injury, fibrosis, and time in human kidney transplants. JCI Insight. 2016;1(1):e85323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CH, Yu MC, Tung WH, Chen TT, Yu CC, Weng CM, et al. Connective tissue growth factor induces collagen I expression in human lung fibroblasts through the Rac1/MLK3/JNK/AP-1 pathway. Biochim Biophys Acta. 2013;1833(12):2823–33. [DOI] [PubMed] [Google Scholar]
- 27.Florin L, Hummerich L, Dittrich BT, Kokocinski F, Wrobel G, Gack S, et al. Identification of novel AP-1 target genes in fibroblasts regulated during cutaneous wound healing. Oncogene. 2004;23(42):7005–17. [DOI] [PubMed] [Google Scholar]
- 28.Ponticos M, Papaioannou I, Xu S, Holmes AM, Khan K, Denton CP, et al. Failed degradation of JunB contributes to overproduction of type I collagen and development of dermal fibrosis in patients with systemic sclerosis. Arthritis Rheumatol. 2015;67(1):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.