Abstract
Post-Traumatic Stress Disorder (PTSD) is an extremely debilitating disease with a broad array of associated symptoms, making the disorder difficult to diagnose and treat. In humans, patients seem to benefit from group therapy or other means of promoting social behavior. To test these effects on our rodent model of PTSD, adult, male rats were housed in either single or pair conditions prior to and during an acute stressor to induce PTSD-like behaviors in these rats. Subsequently, rats were assessed for PTSD-like symptoms in order to determine the effect of social housing on stress-induced phenotypes. Post-trauma phenotypes, including enhanced fear conditioning and anxiety-related behavior, persisted regardless of the animal’s housing condition. It is possible that any housing driven improvements to stress-induced phenotypes would require longer periods of pair housing than were used in these experiments. Although PTSD patients show improved health outcomes following social interaction or group therapy, the fear and anxiety phenotypes seen following an acute stressor in an animal model of the disease endured despite an animal’s housing condition.
Keywords: Post-Traumatic Stress Disorder, housing, fear, anxiety, stress
Fear is a set of highly conserved neural and behavioral mechanisms that are critical for a living organism’s survival. When kept at adaptive levels, fear allows an organism to respond appropriately to threats in their environment. However, in psychiatric diseases including Post-Traumatic Stress Disorder (PTSD), exposure to a traumatic event produces symptoms and behaviors in which the fear circuit sensitizes to generate maladaptive responses. These responses include exaggerated fear to mild threats, increased anxiety and depression, and other changes in affective mood (Acheson et al., 2015; Fani et al., 2012; Powers, Cross, Fani, & Bradley, 2015; Spinhoven, Penninx, van Hemert, de Rooij, & Elzinga, 2014).
Interestingly, PTSD and social anxiety disorder (SAD) can exhibit a high level of comorbidity, leading to social isolation among PTSD patients (McMillan & Asmundson, 2016; McMillan, Sareen, & Asmundson, 2014). It has also been suggested that co-morbidity of PTSD and SAD can heighten other PTSD symptoms, including exaggerated fear responses, depression, and anxiety (McMillan et al., 2014). In fact, social isolation ranks highly among problems that PTSD patients hope to overcome when beginning treatment (Rosen, Adler, & Tiet, 2013). Including techniques to reduce social isolation or adding group therapy components could be particularly important for the efficacy of PTSD treatment (Echeburúa, Sarasua, & Zubizarreta, 2014; Ellis, Peterson, Bufford, & Benson, 2014; Resick et al., 2015).
In animal models, isolation housing can be used as a model of chronic stress. Social isolation in animals has been shown to produce PTSD-like phenotypes including enhanced contextual fear conditioning and impaired fear extinction (Pibiri, Nelson, Guidotti, Costa, & Pinna, 2008; Zelikowsky et al., 2018). Many factors including the sex of the animal (male vs. female), the age at which the animal is housed in isolation (adolescent vs. adulthood), and the measure being analyzed (fear vs. depression vs. anxiety) all seem to play a critical role in determining the effect of isolation housing, often yielding results in disagreement (Arndt et al., 2009; Garrido et al., 2013; McCormick, Mongillo, & Simone, 2013; Pibiri et al., 2008; Skelly, Chappell, Carter, & Weiner, 2015). Conversely, group housing animals after a stressful event has been shown to mitigate anxiety and depression phenotypes (Liu et al., 2013).
Our laboratory has developed a rodent model of PTSD called Stress Enhanced Fear Learning (SEFL), in which animals undergo an acute traumatic experience which leads to a variety of maladaptive behavioral consequences (Rau, DeCola, & Fanselow, 2005). These behaviors include an exaggerated fear response to a mild stressor and increased baseline anxiety (Perusini et al., 2016; Poulos, Zhuravka, Long, Gannam, & Fanselow, 2015). To date, all studies conducted to understand the behavioral consequences of SEFL have involved animals that are housed in isolation. As isolation housing can serve as a powerful stress inducer, it is critical to understand if isolated housing conditions are necessary for SEFL or in some way interacts with the SEFL phenotype.
Here, we describe the behavioral portfolio of post-trauma SEFL rats in either single or pair housed conditions. We have previously shown the drastic effects of an acute traumatic experience in singly housed rats without demonstrating necessity of isolation housing in producing these maladaptive behaviors. Therefore, we compared our standard isolated housed SEFL protocol with a similar SEFL protocol that maintained rats in a paired housing condition. The effect of housing condition on SEFL behaviors was tested through analyzing subsequent enhanced fear learning, changes in anxiety (open field), and extinction to the context in which animals received a mild stressor. We subsequently analyze the contribution of housing condition to generalization in a novel context following the acute stressor.
Method and Materials
Animals
A total of 142 adult (PND 60–90), male Long Evans rats (Experiment 1: n = 30, Experiment 2: n = 32, Experiment 3: n = 32, Experiment 4: n = 48) were used. All rats were housed in a vivarium at UCLA with food and water given ad libitum except during experimental periods. Animals were housed on a 12-h on/off light cycle. These rats were assigned to their specific housing condition for at least one week prior to experiments and were handled for approximately 1 minute per day. Within the pair-housed condition, one rat received stress while its cage mate served as an unstressed control. The rats generally did not fight when housed in pairs, however one cage of rats in Experiment 1 was separated due to immediate fighting. Both of those animals were excluded from data analyses. All procedures were approved by the Chancellor’s Animal Research Committee at UCLA.
Behavioral Testing
Apparatus.
All fear conditioning took place in Med Associates conditioning chambers (28 × 21 × 21 cm; Lafayette Instrument Co.; Lafayette, IN) that were controlled through Med Associates Video Freeze software, as described previously (Zelikowsky et al., 2013). The conditioning chambers were configured into 3 distinct contexts: Context A, Context B, and Context C. Footshocks were delivered to the animals through Med Associates shock scramblers in each conditioning chamber (ENV 414-S). Conditioning sessions were recorded by near infrared cameras. Freezing behavior was analyzed via Med Associates Video Freeze software (Zelikowsky et al., 2013).
Context A consisted of a flat grid floor, windex odor, and white light illumination. Animals were transported to Context A in their homecages, which were mounted on a hanging rack. Context B consisted of an alternating thick and thin grid floor, acetic acid odor, red light illumination, and an A-frame insert. Animals were transported to Context B in a novel, large plastic box. Context C consisted of a white plastic floor, simple green odor, red light illumination, and a white plastic insert. Animals were transported to Context C in a plastic cage with fresh bedding.
Trauma (Experiments 1, 2, 3 and 4).
On Day 1, animals were subjected to an acute traumatic experience consisting of 15 pseudorandom, unsignaled footshocks (1-sec, 1.0-mA) across a 90-minute session in Context A. In all experiments, “Stressed” designates exposure to the acute traumatic experience while “Unstressed” designates control animals exposed to the context for 90 minutes without any footshock presentations.
Trauma Context Test (Experiment 1).
One day later, animals were placed back in Context A, the trauma context, for 8 minutes to assess contextual fear. Transport and all contextual configurations were identical to the trauma session.
One Shock Conditioning (Experiments 1, 2 and 4).
Following the trauma, animals were placed in a novel context, Context B, which was distinct from Context A in contextual configuration (distinct grid flooring, odor, illumination, and contextual cues) and animal transportation method. Animals could explore the context for 3 minutes (pre-shock baseline) before receiving a single 1-sec, 1.0-mA footshock. Thirty seconds following the footshock, animals were removed from the conditioning chamber.
One Shock Context Test (Experiments 1 and 4).
One day later, animals were placed back in Context B, the one shock context, for 8 minutes to assess contextual fear. Transport and all contextual configurations were identical to the one shock context. Greater freezing in the stressed rats compared to the unstressed rats during this test indicates the presence of stress-enhanced fear learning.
Generalization Test (Experiment 1).
Animals were placed in a novel context, Context C, that was distinct from both Contexts A and B in contextual configuration (distinct grid flooring, odor, illumination, and contextual cues) and animal transportation method. Animals could explore the context for 8 minutes, and freezing was measured to assess generalization of contextual fear to the novel chamber.
One Shock Extinction (Experiment 2).
Animals underwent 20-minute extinction sessions to the one shock context (Context B) across 4 days following the one shock conditioning. Freezing was assessed during the first 8 minutes of the session to analyze contextual fear. Transport and all contextual configurations were identical to the one shock context.
Open Field (Experiment 3).
One day after trauma, animals were placed in an open field arena for 8 minutes. The open field arena measured 91.4 cm x 91.4 cm with 30.5 cm high walls. All behavior was digitally recorded and subsequently blindly hand scored to determine mobility of the animals, as measured by number of times an animal crossed a gridline on the open field floor. Animal transportation method and the contextual configuration (odor and illumination) were distinct from those used during the trauma.
Data Analyses
All data were exported from Med Associates software and analyzed using R (RStudio, v1.0.136). Data were analyzed by between-subjects ANOVAs or repeated-measures ANOVAs where appropriate. Data were considered statistically significant with a p-value < 0.05. If there were significant effects found with the ANOVA, independent 2-group t-tests were performed as post hoc analyses. Data are plotted as group means ± SEM.
Results
Experiment 1: Social housing does not alter the context memory of trauma, stress-enhanced fear learning, or generalization to a novel context.
Trauma Context.
To test whether housing condition affected memory to the trauma context, animals were placed back in the trauma context for 8 minutes, one day following exposure to the acute traumatic event (15 or 0 unsignaled footshocks). Animals that experienced the 15 footshocks showed increased levels of fear compared to animals that were not shocked in the context (Stress: F(1, 26) = 209.16, p < 0.001; Fig 1b), but there was no effect of housing condition on contextual fear (Housing: F(1, 26) = 0.24, p = 0.63; Stress x Housing: F(1, 26) = 1.92, p = 0.18; Fig 1b). Housing condition does not affect contextual fear of the trauma-associated context.
Figure 1.
Social housing animals does not alter the context memory of trauma, stress-enhanced fear learning, or generalization to a novel context. (a) Experimental design. (b) Freezing across an 8-minute context test in the 15 shock context. (c) Freezing across an 8-minute context test in the one shock context. (d) Freezing across an 8-minute context test in a novel context.
Stress-Enhanced Fear Learning.
Previous findings indicate that stressed animals show enhanced fear to a mild stressor in a novel context (Rau et al., 2005). However, it was previously unknown whether housing condition interacted with this enhanced fear learning as all prior studies were conducted in single housed animals. To understand whether housing condition interacted with the post-trauma sensitization phenotype, single and pair housed animals underwent a mild stressor in a novel environment (1 shock in Context B). One day later, animals were placed back in the one shock context for 8 minutes to assess contextual fear. Stressed animals showed enhanced fear in this context (Stress: F(1, 26) = 98.70, p < 0.001; Fig 1c), but there was no effect of housing condition (Housing: F(1, 26) = 2.89, p = 0.10; Stress x Housing: F(1, 26) = 0.023, p = 0.88; Fig 1c). Stressed animals display the stress-enhanced fear learning phenotype regardless of housing condition.
Generalization.
Animals were placed in a third novel context (Context C) for 8 minutes the day after the one shock context test to test for housing condition’s effect on fear generalization. Stressed animals showed increased generalization compared to unstressed animals (Stress: F(1, 26) = 137.72, p < 0.001; Fig 1d), but housing condition did not alter generalization (Housing: F(1, 26) = 1.10, p = 0.30; Stress x Housing: F(1, 26) = 0.31, p = 0.58; Fig 1d). These findings indicate that housing condition does not affect generalization in stressed animals.
Experiment 2: Social housing does not alter generalization to a novel context or extinction of the one shock context.
Pre-One Shock Baseline.
Prior to the mild stressor (1 shock in Context B), animals can explore the context for 3 minutes, referred to as the pre-shock period or baseline. To understand whether housing condition altered animals’ behavior during the pre-shock period, we analyzed freezing during this period across stress and housing conditions. Stressed animals exhibited more freezing compared to unstressed animals (Stress: F(1, 28) = 22.23, p < 0.001; Fig 2b), but housing condition did not alter freezing behavior during this period (Housing: F(1, 28) = 1.11, p = 0.30; Stress x Housing: F(1, 28) = 1.07, p = 0.31; Fig 2b). These findings indicate that group housing does not change freezing behavior during the pre-shock period in Context B.
Figure 2.
Social housing animals does not significantly impact extinction to the one shock context. (a) Experimental design. (b) Freezing during the 3-minute baseline period prior to the one shock. (c) Freezing across the first 8 minutes of each extinction day for a four day extinction period.
Extinction of One Shock Context.
To test whether extinction behavior to the one shock context differs due to housing condition, animals underwent 20-minute extinction sessions for 4 consecutive days. Freezing behavior during the first 8 minutes of the extinction session was analyzed to determine between-session extinction. The first 8 minutes of the extinction session was analyzed to match the 8-minute context tests given to assess fear in Experiments 1 and 4. While there was a large effect of both stress (Stress: F(1, 120) = 155.03, p < 0.001; Fig 2c) and extinction day (Day: F(1, 120) = 162.62, p < 0.001; Stress x Day: F(1, 120) = 78.17, p < 0.001; Fig 2c), there was no effect of housing across extinction days (Housing: F(1, 120) = 3.57, p = 0.061; Fig 2c). Additionally, there were no significant interactions of housing with stress or extinction day (Housing x Stress: F(1, 120) = 1.32, p = 0.25; Housing x Day: F(1, 120) = 0.62, p = 0.43; Housing x Stress x Day: F(1, 120) = 1.59, p = 0.21; Fig 2c). Overall, there was no effect of housing on extinction rate to the one shock context.
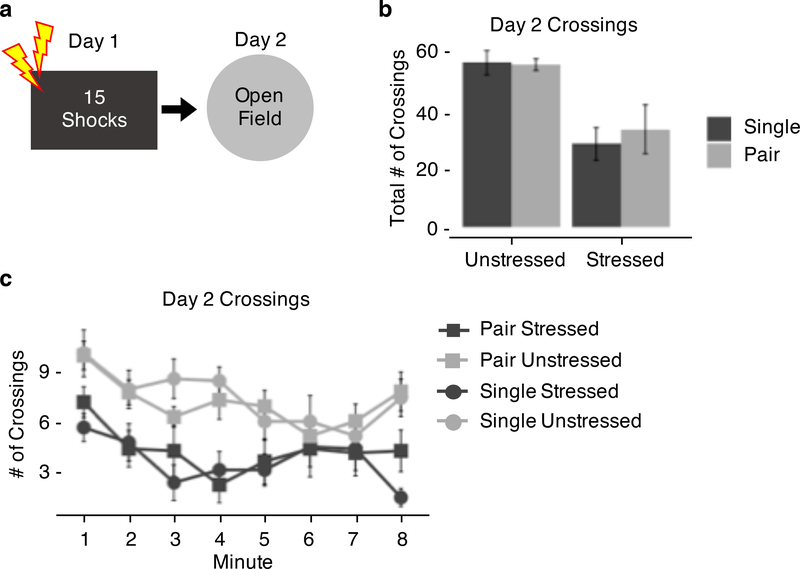
Experiment 3: Social housing does not change behavior in the open field following an acute traumatic experience.
Total Open Field Crossings.
Previous findings indicate that stressed animals show decreased mobility in an open field task (Perusini et al., 2016). To test whether housing condition interacts with behavior in an open field, animals were placed in an open field for 8 minutes on the day following trauma (15 or 0 unsignaled footshocks). Mobility was measured by the number of times animals crossed gridlines on the open field. Across the full 8-minute session, stressed animals were significantly less mobile than unstressed animals (Stress: F(1, 28) = 20.29, p < 0.001; Fig 3b), but there was no effect of housing on mobility in the open field (Housing: F(1, 28) = 0.16, p = 0.70; Stress x Housing: F(1, 28) = 0.26, p = 0.62; Fig 3b). Animals show decreased mobility in the open field following an acute traumatic experience, but housing condition does not interact with this anxious phenotype.
Figure 3.
Social housing animals does not change behavior in the open field test following an acute traumatic experience. (a) Experimental design. (b) Total number of crossings across an 8-minute test in the open field. (c) Minute by minute number of crossings across an 8-minute test in the open field.
Open Field Crossings by Minute.
To test whether mobility was differentially expressed across the 8-minute session, data were analyzed across 1-minute bins (Fig 3c). Across the 1-minute bins, stressed animals were significantly less mobile (Stress: F(1, 244) = 66.92, p < 0.001; Fig 3c), and animals were significantly less mobile across the session (Minute: F(1, 244) = 14.48, p < 0.001; Fig 3c), but there were no effects of housing on mobility (Housing: F(1, 244) = 0.14, p = 0.71; Stress x Housing: F(1, 244) = 1.47, p = 0.23; Stress x Housing x Minute: F(1, 244) = 0.092, p = 0.76; Fig 3c). These findings indicate that housing condition does not interact with the anxious phenotype shown by stressed animals in the open field task.
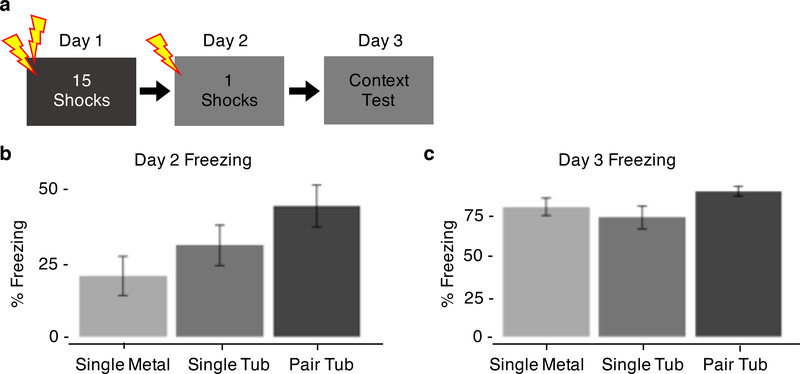
Experiment 4: Housing condition alters generalization to a novel context
Pre-One Shock Baseline.
In past SEFL studies, rats do not generalize fear to the novel context as measured by low pre-shock baseline freezing (Rau et al., 2005). However, animals in Experiments 1 – 3 froze significantly higher than typically observed in SEFL studies, indicating a generalization of fear (Fig 2b). To understand why rats were generalizing fear, we systematically compared the housing conditions used in Experiments 1 – 3 (single and pair housed in large, plastic tubs) with the housing conditions typically used in our laboratory (single housed in metal cages). There was a significant effect of housing condition, indicating that housing condition modulates fear generalization to a novel context (Housing: F(2, 42) = 3.65, p = 0.035; Fig 4b). Post-hoc analyses indicate that there is a significant decrease in freezing between single metal and pair tub housed rats (t(29) = 2.44, p = 0.010; Fig 4b). The single tub housed rats displayed an intermediate level of generalization between the single metal and pair tub housed rats, although these differences were not statistically significant (Single Tub vs. Single Metal: t(29) = 1.09, p = 0.14; Single Tub vs. Pair Tub: t(29) = 1.35, p = 0.094; Fig 4b). These data indicate that housing condition affects generalization to a novel context.
Figure 4.
Housing condition alters generalization to a novel context. (a) Experimental design. (b) Freezing during the 3-minute baseline period prior to the one shock. (c) Freezing during the 8-minute context test of the one shock context.
One Shock Context.
To verify that housing condition does not affect subsequent expression of fear to the one shock context, animals were given an 8-minute context test in the one shock context the day after conditioning. There was no significant effect of housing condition on this fear expression (Housing: F(2, 42) = 2.73, p = 0.077; Fig 4c). Housing condition does not affect expression of fear to the one shock context as measured during a subsequent context test.
Discussion
The above studies indicate that PTSD-like phenotypes observed following an acute stressor in our animal model of the disease persist regardless of the housing conditions imposed one week prior to the acute stressor and maintained throughout testing. Stressed animals continue to show post-trauma increases in fear and anxiety independent of housing condition. Given the lack of evidence of fear and anxiety differences between single and pair housed animals following stress, it seems unlikely that housing condition contributes substantially to developing these PTSD-like phenotypes after a 15-shock stressor. In our model, this indicates that the potential mild, chronic stress of single housing does not impact the behaviors associated with the acute, severe stressor (15 shocks) which produces enhanced fear conditioning and heightened anxiety behaviors.
Given the evidence in both animal models and humans for the role of isolation housing in exacerbating effects of stress, it is somewhat surprising that housing does not contribute to enhanced fear and anxiety changes following an acute stressor. Data from humans indicates that there can be marked benefits to individuals who suffer from PTSD when given social support through activities including group therapy (Ellis et al., 2014; Resick et al., 2015). Likewise, some animal models of stress indicate a relationship between housing condition and the effects of stress on subsequent phenotypes (Liu et al., 2013; Murínová, Hlaváčová, Chmelová, & Riečanský, 2017; Pibiri et al., 2008; Ravenelle, Santolucito, Byrnes, Byrnes, & Donaldson, 2014). However, in our model of PTSD we fail to find an effect of housing condition on an array of subsequent fear and anxiety related phenotypes, including enhanced fear conditioning and decreased activity in the open field task.
There are a few reasons as to why we might have failed to find an effect of housing condition on the PTSD-like symptoms seen in stressed animals. First, animals undergoing the 15-shock stressor show an extreme phenotype, particularly in subsequent fear conditioning where the animals are freezing at a near maximum rate (typically, 90–100%) across the context test. While we might have expected to see a reduction in this freezing for animals that were group-housed, there is not much room for a more severe phenotype in isolated animals. Additionally, this ceiling effect could have prevented the ability to disentangle a mitigating effect from pair housing animals. Future studies could address this ceiling effect by reducing the severity of the stress in order to tease apart a potential mitigation in fear and anxiety symptoms following an acute stressor.
Animals were housed in their respective condition (isolated or pair) for approximately one week prior to stress, and immediately following stress through the remainder of the experimental timeline. Some animal studies indicate a necessity for chronic isolation or pair housing to yield effects of this housing condition on behavioral measures, so it is possible that the present studies failed to produce effects of housing condition on PTSD-like measures because of the timeframe of our housing condition (Baker & Bielajew, 2007; Garrido et al., 2013). For example, one study indicating a stress-reducing effect of social housing in rats involved housing rats either in isolation or in pairs for a period of three weeks, across which time the rats were subjected to a chronic stress procedure (Westenbroek et al., 2005). Another critical difference between the positive results of this study and the experiments presented here is that our PTSD model involves an acute stressor to initiate PTSD-like symptoms while studies indicating an effect of social housing on mitigating fear, anxiety, and depression phenotypes often involve a chronic stressor (Babygirija, Zheng, Bülbül, Ludwig, & Takahashi, 2010; Westenbroek, Ter Horst, et al., 2003).
Finally, all of the experiments presented here were done in male Long-Evans rats. Our rationale for using male rats was to match most of the previous work done with SEFL, as we were primarily interested in understanding whether isolation housing was necessary to produce PTSD-like symptoms seen after an acute stressor. A variety of studies have indicated a sex-dependent nature of housing effect on susceptibility to stress with female rats showing a heightened response to pair housing compared to male rats (Baker & Bielajew, 2007; Leasure & Decker, 2009; Westenbroek, Den Boer, & Ter Horst, 2003; Westenbroek, Den Boer, Veenhuis, & Ter Horst, 2004; Westenbroek et al., 2005). Therefore, it is possible that female rats undergoing the same acute stress protocol presented here would show benefits of social housing following the acute stressor while few to no advantages were seen in male rats. Critically, the neuropeptide oxytocin has been shown to reduce CORT following a stressor and administration of oxytocin following stress can protect hippocampal function, which is normally impaired following a stressor (Lee et al., 2015; Park, Kim, Park, Han, & Choi, 2017; Windle, Shanks, Lightman, & Ingram, 1997). Additionally, oxytocin-deficient female mice exhibit increased anxiety behavior on the Elevated Plus Maze, and elevated levels of CORT alongside increased fos expression in the medial amygdala following stress (Amico, Mantella, Vollmer, & Li, 2004; Mantella, Vollmer, Rinaman, Li, & Amico, 2004). Thus, if oxytocin is mediating subsequent development of PTSD-like symptoms after an acute stressor, it is possible these effects would be seen selectively in a female population of rats compared to the male population used in these experiments. Future studies to disentangle the contribution of housing condition to the development of PTSD-like symptoms in our animal model should probe for these effects in female rats to get a more holistic understanding of the potential sex differences.
Prior work with SEFL indicates that the enhanced fear learning is due to nonassociative sensitization. Therefore, animals tend to exhibit minimal generalization when placed in a novel context that is very different from the stress context on the single shock day as indicated by a low level of freezing during the pre-shock baseline. In these experiments, the rats demonstrated higher than expected levels of fear during this pre-shock baseline, which indicates an increase in generalization. This increase in generalization could be due to one, or both, of the major differences in housing compared to prior work with SEFL: that animals were housed in large, plastic tubs with bedding rather than hanging metal cages with wire floors, or that animals were pair housed. To investigate the contribution of these housing manipulations to post-trauma generalization, these factors were manipulated and animals were tested for their fear generalization to a novel context. Single-housed animals in the metal cages froze significantly less than pair-housed animals in the plastic tubs, with single-housed animals in the plastic tubs exhibiting an intermediate level of freezing. Thus, these data indicate that both the type of cage and the presence of a cage-mate contribute to fear generalization.
Given that the fear conditioning boxes may more closely resemble the metal cages that animals are housed in, there is a larger contextual overlap for the single-housed animals in the metal cages than those living in the plastic tubs, which do not resemble the fear conditioning boxes. Thus, it could be the degree of similarity between housing condition and the fear conditioning boxes that drives the magnitude of generalization due to either higher (single-housed metal cages) or lower (pair-housed plastic tubs) contextual overlap. Animals with high contextual overlap might generalize less because their home cage, a safe environment, more closely resembles the fear conditioning boxes, an unsafe environment. Understanding how housing condition contributes to measures of fear, including generalization, has important implications in designing experiments where these effects might unintentionally influence data.
In the present set of studies, we sought to understand the role of pair housing animals on PTSD-like symptoms following an acute stressor. As housing rats in isolation can be a stressor independently, it was important for us to understand whether isolation housing rats prior to, during, and following an acute stressor was necessary for developing these PTSD-like symptoms including exaggerated subsequent fear conditioning and increases in anxiety-like behavior. These studies indicate that PTSD-like symptoms arising from an acute stressor persist regardless of the animal’s housing condition. Future studies to understand the contribution of housing condition to development of PTSD-like symptoms could include longer periods of respective housing condition, a chronic stress protocol rather than the acute stressor used here, or inclusion of female rats as animal studies indicate a sex-specific effect of housing condition on the development of stress-dependent changes in fear, anxiety and depression.
Acknowledgments
This work was supported by NIH R01-MH115678 and the Staglin Center for Brain & Behavioral Health.
References
- Acheson DT, Geyer MA, Baker DG, Nievergelt CM, Yurgil K, Risbrough VB, & Team, MRS-II. (2015). Conditioned fear and extinction learning performance and its association with psychiatric symptoms in active duty Marines. Psychoneuroendocrinology, 51, 495–505. doi: 10.1016/j.psyneuen.2014.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico JA, Mantella RC, Vollmer RR, & Li X (2004). Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol, 16(4), 319–324. doi: 10.1111/j.0953-8194.2004.01161.x [DOI] [PubMed] [Google Scholar]
- Arndt SS, Laarakker MC, van Lith HA, van der Staay FJ, Gieling E, Salomons AR, … Ohl F (2009). Individual housing of mice--impact on behaviour and stress responses. Physiol Behav, 97(3–4), 385–393. doi: 10.1016/j.physbeh.2009.03.008 [DOI] [PubMed] [Google Scholar]
- Babygirija R, Zheng J, Bülbül M, Ludwig K, & Takahashi T (2010). Beneficial effects of social attachment to overcome daily stress. Brain Res, 1352, 43–49. doi: 10.1016/j.brainres.2010.07.028 [DOI] [PubMed] [Google Scholar]
- Baker S, & Bielajew C (2007). Influence of housing on the consequences of chronic mild stress in female rats. Stress, 10(3), 283–293. doi: 10.1080/10253890701265362 [DOI] [PubMed] [Google Scholar]
- Echeburúa E, Sarasua B, & Zubizarreta I (2014). Individual Versus Individual and Group Therapy Regarding a Cognitive-Behavioral Treatment for Battered Women in a Community Setting. J Interpers Violence, 29(10), 1783–1801. doi: 10.1177/0886260513511703 [DOI] [PubMed] [Google Scholar]
- Ellis CC, Peterson M, Bufford R, & Benson J (2014). The importance of group cohesion in inpatient treatment of combat-related PTSD. Int J Group Psychother, 64(2), 208–226. doi: 10.1521/ijgp.2014.64.2.208 [DOI] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, … Jovanovic T (2012). Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med, 42(3), 533–543. doi: 10.1017/S0033291711001565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido P, De Blas M, Ronzoni G, Cordero I, Antón M, Giné E, … Mora F (2013). Differential effects of environmental enrichment and isolation housing on the hormonal and neurochemical responses to stress in the prefrontal cortex of the adult rat: relationship to working and emotional memories. J Neural Transm (Vienna), 120(5), 829–843. doi: 10.1007/s00702-012-0935-3 [DOI] [PubMed] [Google Scholar]
- Leasure JL, & Decker L (2009). Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus, 19(10), 907–912. doi: 10.1002/hipo.20563 [DOI] [PubMed] [Google Scholar]
- Lee SY, Park SH, Chung C, Kim JJ, Choi SY, & Han JS (2015). Oxytocin Protects Hippocampal Memory and Plasticity from Uncontrollable Stress. Sci Rep, 5, 18540. doi: 10.1038/srep18540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wu R, Tai F, Ma L, Wei B, Yang X, … Jia R (2013). Effects of group housing on stress induced emotional and neuroendocrine alterations. Brain Res, 1502, 71–80. doi: 10.1016/j.brainres.2013.01.044 [DOI] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Rinaman L, Li X, & Amico JA (2004). Enhanced corticosterone concentrations and attenuated Fos expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. Am J Physiol Regul Integr Comp Physiol, 287(6), R1494–1504. doi: 10.1152/ajpregu.00387.2004 [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mongillo DL, & Simone JJ (2013). Age and adolescent social stress effects on fear extinction in female rats. Stress, 16(6), 678–688. doi: 10.3109/10253890.2013.840283 [DOI] [PubMed] [Google Scholar]
- McMillan KA, & Asmundson GJG (2016). PTSD, social anxiety disorder, and trauma: An examination of the influence of trauma type on comorbidity using a nationally representative sample. Psychiatry Res, 246, 561–567. doi: 10.1016/j.psychres.2016.10.036 [DOI] [PubMed] [Google Scholar]
- McMillan KA, Sareen J, & Asmundson GJ (2014). Social anxiety disorder is associated with PTSD symptom presentation: an exploratory study within a nationally representative sample. J Trauma Stress, 27(5), 602–609. doi: 10.1002/jts.21952 [DOI] [PubMed] [Google Scholar]
- Murínová J, Hlaváčová N, Chmelová M, & Riečanský I (2017). The Evidence for Altered BDNF Expression in the Brain of Rats Reared or Housed in Social Isolation: A Systematic Review. Front Behav Neurosci, 11, 101. doi: 10.3389/fnbeh.2017.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Kim YJ, Park JC, Han JS, & Choi SY (2017). Intranasal Oxytocin following Uncontrollable Stress Blocks Impairments in Hippocampal Plasticity and Recognition Memory in Stressed Rats. Int J Neuropsychopharmacol, 20(10), 861–866. doi: 10.1093/ijnp/pyx061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusini JN, Meyer EM, Long VA, Rau V, Nocera N, Avershal J, … Fanselow MS (2016). Induction and Expression of Fear Sensitization Caused by Acute Traumatic Stress. Neuropsychopharmacology, 41(1), 45–57. doi: 10.1038/npp.2015.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, & Pinna G (2008). Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc Natl Acad Sci U S A, 105(14), 5567–5572. doi: 10.1073/pnas.0801853105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Zhuravka I, Long V, Gannam C, & Fanselow M (2015). Sensitization of fear learning to mild unconditional stimuli in male and female rats. Behav Neurosci, 129(1), 62–67. doi: 10.1037/bne0000033 [DOI] [PubMed] [Google Scholar]
- Powers A, Cross D, Fani N, & Bradley B (2015). PTSD, emotion dysregulation, and dissociative symptoms in a highly traumatized sample. J Psychiatr Res, 61, 174–179. doi: 10.1016/j.jpsychires.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP, & Fanselow MS (2005). Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev, 29(8), 1207–1223. doi: 10.1016/j.neubiorev.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Ravenelle R, Santolucito HB, Byrnes EM, Byrnes JJ, & Donaldson ST (2014). Housing environment modulates physiological and behavioral responses to anxiogenic stimuli in trait anxiety male rats. Neuroscience, 270, 76–87. doi: 10.1016/j.neuroscience.2014.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resick PA, Wachen JS, Mintz J, Young-McCaughan S, Roache JD, Borah AM, … Peterson AL (2015). A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol, 83(6), 1058–1068. doi: 10.1037/ccp0000016 [DOI] [PubMed] [Google Scholar]
- Rosen C, Adler E, & Tiet Q (2013). Presenting concerns of veterans entering treatment for posttraumatic stress disorder. J Trauma Stress, 26(5), 640–643. doi: 10.1002/jts.21841 [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Chappell AE, Carter E, & Weiner JL (2015). Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: Possible role of disrupted noradrenergic signaling. Neuropharmacology, 97, 149–159. doi: 10.1016/j.neuropharm.2015.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinhoven P, Penninx BW, van Hemert AM, de Rooij M, & Elzinga BM (2014). Comorbidity of PTSD in anxiety and depressive disorders: prevalence and shared risk factors. Child Abuse Negl, 38(8), 1320–1330. doi: 10.1016/j.chiabu.2014.01.017 [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, & Ter Horst GJ (2003). Gender-specific effects of social housing on chronic stress-induced limbic Fos expression. Neuroscience, 121(1), 189–199. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, & Ter Horst GJ (2004). Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull, 64(4), 303–308. doi: 10.1016/j.brainresbull.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Snijders TA, den Boer JA, Gerrits M, Fokkema DS, & Ter Horst GJ (2005). Pair-housing of male and female rats during chronic stress exposure results in gender-specific behavioral responses. Horm Behav, 47(5), 620–628. doi: 10.1016/j.yhbeh.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Ter Horst GJ, Roos MH, Kuipers SD, Trentani A, & den Boer JA (2003). Gender-specific effects of social housing in rats after chronic mild stress exposure. Prog Neuropsychopharmacol Biol Psychiatry, 27(1), 21–30. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, & Ingram CD (1997). Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology, 138(7), 2829–2834. doi: 10.1210/endo.138.7.5255 [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Hast TA, Bennett RZ, Merjanian M, Nocera NA, Ponnusamy R, & Fanselow MS (2013). Cholinergic blockade frees fear extinction from its contextual dependency. Biol Psychiatry, 73(4), 345–352. doi: 10.1016/j.biopsych.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Hui M, Karigo T, Choe A, Yang B, Blanco MR, … Anderson DJ (2018). The Neuropeptide Tac2 Controls a Distributed Brain State Induced by Chronic Social Isolation Stress. Cell, 173(5), 1265–1279.e1219. doi: 10.1016/j.cell.2018.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]