Abstract
Background and Purpose:
We investigated cortical superficial siderosis (cSS) progression and its clinical relevance for incident lobar intracerebral hemorrhage (ICH) risk, in probable cerebral amyloid angiopathy (CAA) presenting with neurological symptoms and without ICH.
Methods:
Consecutive patients meeting modified Boston criteria for probable CAA from a single-center cohort who underwent MRI at baseline and during follow-up were analyzed. cSS progression was assessed by comparison of the baseline and follow-up images. Patients were followed prospectively for incident symptomatic ICH. cSS progression and first-ever ICH risk were investigated in Cox proportional hazard models adjusting for confounders.
Results:
The cohort included 118 probable CAA patients: 72 (61%) presented with transient focal neurological episodes and 46 (39%) with cognitive complaints prompting the baseline MRI investigation. Fifth-two patients (44.1%) had cSS at baseline. During a median scan-interval of 2.2 years (IQR: 1.2–4.4 years) between the baseline (i.e. first) MRI and the latest MRI, cSS progression was detected in 33 (28%) patients. In multivariable logistic regression, baseline cSS presence (OR: 4.04; 95%CI: 1.53–10.70, p=0.005), especially disseminated cSS (OR: 9.12; 95%CI: 2.85–29.18, p<0.0001) and appearance of new lobar microbleeds (OR: 4.24; 95%CI: 1.29–13.9, p=0.017) were independent predictors of cSS progression. For patients without an ICH during the inter-scan interval (n=105) and subsequent follow-up (median post-final MRI: 1.34, IQR: 0.3–3 years), cSS progression independenly predicted increased symptomatic ICH risk (HR: 3.76; 95%CI: 1.37–10.35, p=0.010).
Conclusions:
Our results suggest that cSS evolution may be a useful marker for assessing disease progression and ICH risk in CAA patients and a candidate biomarker for clinical studies and trials.
Keywords: cerebral amyloid angioapathy, intracerebral hemorrhage, cortical superficial siderosis
Introduction
Cerebrovascular deposition of amyloid affecting superficial cortical microvessels, defined as cerebral amyloid angiopathy (CAA), in an important cause of spontaneous lobar intracerebral hemorrhage (ICH) in older individuals.1 Growing evidence suggests that CAA can present without major ICH,2 but instead with transient focal neurological episodes (TFNEs) or cognitive impairment in stroke or memory clinics. In these settings, the disease is diagnosed by the presence of characteristic hemorrhagic lesions on blood-sensitive MRI sequences, including isolated lobar cerebral microbleeds (CMBs) and cortical superficial siderosis (cSS), by the Boston criteria.3 cSS in particular, is found in about 30%−50% of symptomatic CAA cohorts,4 depending on the clinical setting.
Patients presenting with isolated strictly lobar CMBs have a neuroimaging profile suggestive of advanced CAA and might be at risk of incident ICH.5 Little is known about cSS in these patients, which has been identified as not only a useful CAA diagnostic marker but an independent, dose-dependent predictor of future ICH.4, 6 It remains unknown whether cSS progresses over follow-up time, and if yes, whether progression rate carries added prognostic information beyond the absolute cSS severity.
We analysed cSS progression using a new visual scale (designed in our cohort), and its role as an independent predictor of subsequent ICH. We designed a cohort study of CAA patients presenting with neurological symptoms without lobar ICH at baseline, who had blood-sensitive MRI sequences at two time points and clinical follow-up. We sought to investigate: (a) the rate and risk factors for cSS progression on MRI; and (b) whether the appearance of progressing cSS is associated with an increased risk of future first-ever symptomatic lobar ICH, in patients who were stroke free through the MRI follow-up period.
Methods
The authors declare that all supporting data are available within the article (and its online supplementary files).
Study population and patient selection
We analyzed prospectively collected data from an established consecutive patient cohort meeting modified Boston criteria for probable CAA3 in the absence of ICH (symptomatic or asymptomatic) seen at Massachusetts General Hospital Stroke service (including stroke unit and outpatient clinics) or Memory Clinic (March 2000-November 2015).7 For this study, inclusion criteria were: 1) suspected CAA based on initial non-stroke clinical presentations and diagnosis of probable CAA by modified Boston criteria (i.e. ≥2 hemorrhagic lesions, strictly lobar CMBs or cSS in any combination)3 and 2) at least two interpretable brain MRIs (at baseline and follow-up) with sequences including T2*-weighted/SWI. We excluded patients with ICH history at baseline or inflammatory CAA.7
Baseline clinical information were obtained as described,7 using standardised data collection forms. Two investigators independently reviewed and classified baseline clinical presentations: TFNEs, cognitive complaints or non-focal neurological symptoms. All patients with TFNEs (i.e. episodes of positive or negative neurological symptoms lasting for minutes with subsequent complete resolution) underwent a thorough diagnostic evaluation,8 including intracranial CT or MR angiography or carotid ultrasound, transthoracic echocardiogram, and cardiac monitoring for paroxysmal atrial fibrillation. TFNE were attributed to CAA in the absence of a causative diagnostic finding and a brain MRI supportive of probable CAA.
Neuroimaging data acquisition and analysis
Key baseline CAA MRI markers were assessed blinded to clinical and follow-up data by two trained observers (AC and GB, 7 and 5 years experience respectively) for the purpose of the study, according to STandards for ReportIng Vascular changes on nEuroimaging (STRIVE).9 Images were obtained at 1.5 Tesla MR scanner and included whole brain T2-weighted, T2*-weighted gradient-recalled echo (T2*-GRE; echo time [TE] 750/50ms, 5mm slice thickness, 1mm inter-slice gap) and fluid attenuated inversion recovery (FLAIR; TR/TE 10000/140ms, 5mm slice thickness, 1mm inter-slice gap). For a subset of patients (n=21) the paramagnetic MRI sequences included susceptibility weighted imaging (SWI, TR/TE 27/20ms, 1.5mm slice thickness).
CMBs presence and number were evaluated on axial T2*-GRE/SWI images using current consensus criteria.9 cSS was jointly assessed by two raters in line with consensus recommendations.4 cSS was defined as curvilinear hypointensities following the cortical surface, distinct from vessels, and was assessed on axial blood-sensitive sequences according to a validated scale: absent, focal (restricted to ≤3 sulci) or disseminated (affecting ≥4 sulci).3, 10 cSS ratings were performed independently of other imaging markers ratings, blinded to baseline and follow-up data.
Enlarged perivascular spaces (EPVS) were assessed on axial T2-weighted MRI, in the centrum semiovale (CSO), using a validated 4-point visual rating scale (0=no PVS, 1=<10 PVS, 2=11–20 PVS, 3=21–40 PVS and 4=>40 PVS).7 WMH volumes were calculated on axial FLAIR sequences with a previously described semi-automated planimetric method using MRICron software.7 Periventricular and deep WMH were also classified using the Fazekas scale.7
Follow-up MRI analysis
The indication for follow-up MRI was regular clinical follow-up or new symptoms/suspicion of stroke. The follow-up MRI protocol had overall identical acquisition parameters as the baseline MRI, at 1.5 Tesla. A composite hierarchical variable was created to incorporate potential differences in blood sensitive MRI sequences between baseline and follow-up MRI (1-scan type same at follow-up/baseline: SWI/SWI, T2*-GRE/ T2*-GRE, 2-less sensitive at follow-up: SWI/T2*-GRE, or 3-more sensitive at follow-up SWI/T2*-GRE, respectively).
We analyzed the evolution of CMBs and cSS using the following visual examination methods. The baseline MRI at index presentation and latest available follow-up MRI scans were aligned manually using the linking tool in IMPAX 6.2 Agfa ®. cSS evolution was considered any change in patient cSS status from baseline to follow-up MRI, including patients who progressed from no cSS at baseline to developing cSS during follow-up. For cSS, we defined progression on a new ordinal scale as follows: grade 0, No cSS progression; 1, cSS extension within an already present focus of cSS at the baseline MRI, without a new cSS focus; 2, appearance of one new cSS focus; 3, appearance of two new cSS foci and; 4, appearance of three or more cSS foci. According to this scale, categories 1–2 represent mild cSS progression, while categories 3–4 severe progression, easily identified and appreciated clinically on follow-up MRI. This new rating tool was developed based on cSS progression in a separate subset of CAA patients and after structured discussions with reference to the advised standards for evaluating cSS in CAA research studies.4 All scoring rules were formulated prior to the current analysis. Using a subset of 40 representative cases from the current paper cohort, inter-rater reliability for cSS progression assessment between two trained raters was excellent (kappa=0.81, 92.5% agreement). Evolution of cSS severity from none to focal, focal to disseminated or none to disseminated was also obtained from the initial and follow-up ratings.
For the current analysis, evolution on the follow-up MRI by comparison to the baseline was rated in consensus by two trained raters. In five cases in which ICH occurred during the inter-scan period, cSS progression was assessed where not connected to the hematoma. As a sensitivity analysis and to account for different blood-sensitive sequences at baseline and follow-up scans (SWI vs. T2*-GRE or vice versa), all cases were also rated blinded to MRIs timing and in random order. The results on cSS progression were consistent with the main rating method.
For CMBs evolution, we used the latest available follow-up MRI and comparatively analyzed the baseline MRI by visual inspection to assess whether each CMB was present at baseline. We categorized CMBs evolution using the following scale: 0, No new CMB; 1, One or 2 new CMB(s); 2, three to 5 new CMBs; 3, six to 10 new CMBs, 4, ten or more additional CMBs. MRI analyses were performed and recorded masked to all clinical information.
Clinical follow-up data
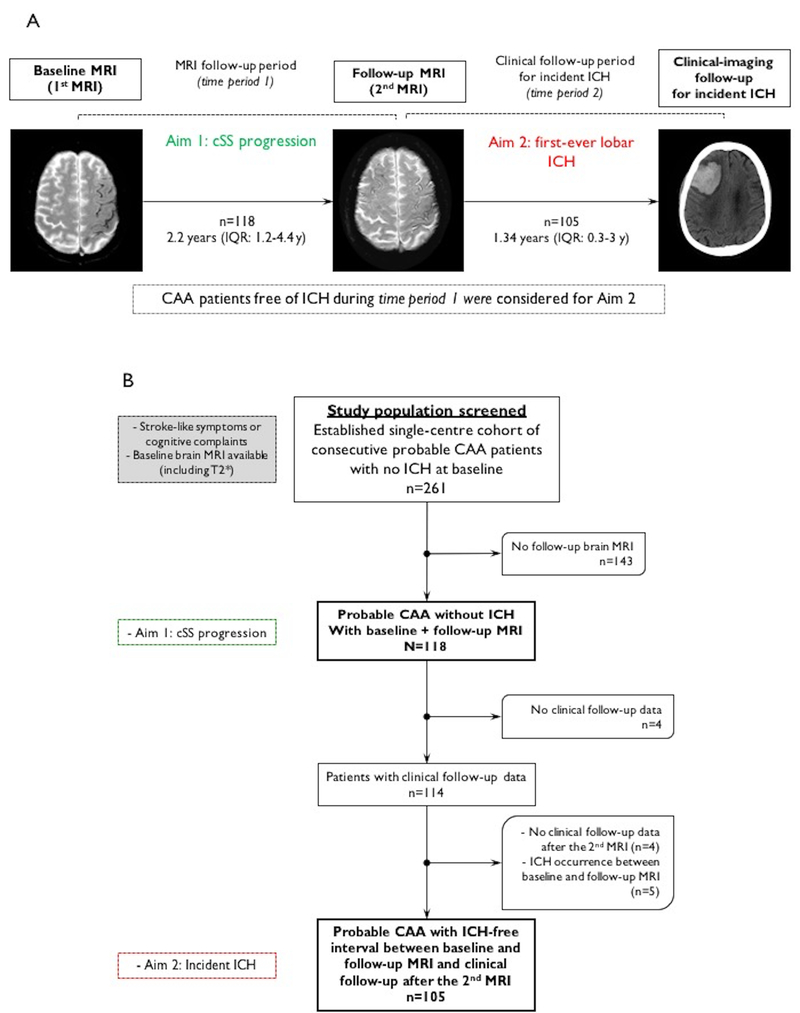
Follow-up clinical data were obtained by phone calls at 3 months after enrollment and every 6 months thereafter, supplemented by a systematic chart review of all available information, using standardized data collection forms. We collected information on clinically symptomatic lobar ICH. All outcome events were assessed using available clinical and radiologic information, masked to cSS presence at baseline. To study the relationship between cSS progression and incident ICH, we used clinical follow-up data starting from the end of the MRI follow-up period (i.e. 2nd MRI), in CAA patients who remained stroke-free during this period (Figure 1).
Figure 1.
(A) Study methods schematic according to the two basic aims: (a) cSS progression prevalence and predictors between baseline and follow-up MRI (i.e. MRI follow-up period); (b) incident first-ever lobar ICH risk during clinical follow-up (i.e. clinical follow-up period) in relation to cSS progression, in stroke-free CAA patients during the MRI follow-up period. (B) Flow chart of patient selection.
Standard protocol approvals, registrations, and patient consents
This study was approved by institutional review boards and with participants written informed consent.
Statistics
To determine whether patients with follow-up MRI differed from those without, we compared the main baseline and imaging characteristics between those 2 groups, using using 2-sample t-test, Wilcoxon rank sum, Pearson’s χ-square and Fisher exact tests, as appropriate. The same tests were used for comparison of baseline characteristics of probable CAA patients with versus without cSS progression.
For all analyses, cSS progression was specified pre-hoc (before looking at the results) as: (a) presence of any new cSS (Yes/No, i.e. 0 vs. 1–4 in the progression scale); (b) extent of cSS progression on an ordinal scale: no new cSS (grade 0), cSS progression in the same focus or a single new independent focus (grades 1–2), and cSS progression involving ≥2 new foci (grades 3–4), remote from previously seen cSS foci. These progression categories were specified to create balanced clinical relevant groups reflecting cSS progression severity.
We investigated characteristics associated with cSS progression during MRI follow-up using logistic regression analysis, adjusted for scan interval. Multivariable logistic regression analysis of presence or absence of new cSS was performed, adjusting for variables that demonstrated p<0.1 for an association in the univariate analyses, in addition to scan time interval, age and appearance of lobar CMBs (pre-specified). Using a similar approach, we examined associations with cSS progression categories in ordinal logistic regression. In sensitivity analyses, we additionally adjusted for differences in baseline and follow-up blood-sensitive MRI sequences using the 3-level variable defined in Methods.
We analyzed cSS progression as a univariable predictor of subsequent ICH among patients without ICH during the inter-scan interval (see Fig. 1), using Kaplan-Meier plot with significance testing by the log-rank test. Survival time was calculated from follow-up MRI scan date until the date of ICH at follow-up or the last known date without the outcome event. Pre-specified adjusted Cox regression analyses were performed to calculate multivariable hazard ratios (HR) for any cSS progression (grade >0) and progression category (as defined above) during the inter-scan interval in relation to subsequent first-ever ICH. We adjusted for candidate covariates demonstrating a univariable trend for an association with the outcome in Cox regression analysis (p<0.1) and biologically plausible predictors identified in previous studies, including age and appearance of new lobar CMBs.11 In sensitivity analyses, multivariable Cox regression models were further adjusted for potential differences in baseline and follow-up blood-sensitive MRI sequences, sex, cSS presence at baseline, and interscan interval. The proportional hazard assumption was tested using graphical checks and Schoenfeld residuals-based tests.
Significance level was set at 0.05. Stata software (Version 13, StataCorp.) was used for all analyses.
Results
The overall design according to the basic aims of our study is schematically summarised in Figure 1A. A flow chart of patient selection is provided in Figure 1B.
Baseline characteristics
From our original cohort, a total of 261 potentially eligible probable CAA patients with baseline MRI and without lobar ICH were screened for this analysis (130 presenting to the stroke service and 131 to the memory clinic). After excluding 143 patients without adequate MRI follow-up, 118 patients were included in the final analysis (Fig. 1B). Patients without follow-up MRI were not different from patients included in the study in baseline characteristics, apart from being older and trending towards lower prevalence of cSS at baseline (Supplementary Table I). Excluded patients more commonly presented to the memory clinic rather than the stroke service (61% vs. 39% respectively, p<0.0001).
Among 118 analyzed CAA patients (mean age: 73, SD: 8.3 years), 72 (61%) presented with TFNEs (often related to cSS or convexity SAH) and 46 (39%) with cognitive complaints (both subjective and clinical diagnosis of cognitive impairment, with mean CDR=0.5) prompting the baseline MRI investigation for their symptoms. Baseline clinical and MRI characteristics of the whole cohort are summarised in Table 1. Fifty-two patients (44.1%) had cSS at baseline. There was not significant difference in the prevalence of cSS in CAA patients presenting with cognitive symptoms vs. stroke-like presentations (46% vs. 44%). The indication for the follow-up MRI was regular clinical follow-up in 86 (73%) cases, investigation of cognitive complaints in 15 (13%) and suspicion of new focal neurological symptoms/stroke in 17 (14%) patients. There was no diffence in cSS prevalence or progression according to indication.
Table 1.
Comparison of baseline characteristics of probable CAA patients according to cSS progression during follow-up. (*p<0.05, **p<0.001)
| Characteristic | Whole CAA cohort (N=118) | With cSS progression (N=33) |
Without cSS progression (N=85) |
p-value | Adj. OR (95%CI) MRI interval |
|---|---|---|---|---|---|
| Age at MRI, mean (SD), years | 73 (8.3) | 71.2 (7.1) | 73.7 (8.7) | 0.146 | 0.96 (0.92–1.01) |
| Sex, male n (%) | 75 (64) | 26 (79) | 49 (58) | 0.032* | 2.63 (1.01–6.83) |
| Hypertension, n (%) | 78 (66.1) | 23 (69.7) | 55 (64.7) | 0.607 | 1.2 (0.49–2.90) |
| Hypercholesterolemia, n (%) | 70 (57.9) | 21 (60) | 49 (57) | 0.994 | 1.16 (0.5–2.7) |
| Warfarin use at baseline* | 14 (11.9) | 2 (5.7) | 12 (14.5) | 0.226 | 0.36 (0.08–1.69) |
| Severe (Fazekas 5–6) WMH, n (%) | 32 (27.1) | 8 (24.2) | 24 (28.2) | 0.661 | 0.73 (0.28–1.91) |
| WMH volume, median (IQR), cc | 19.1 (10–35.6) | 20.5 (12.8–30.7) | 18.4 (7.7–38.8) | 0.636 | 1 (1–1.01) |
| LogWMH volume, mean (95% CI) | 3 (2.4–3.6) | 3.1 (2.6–3.5) | 3 (2.2–3.7) | 0.636 | 1.20 (0.80–1.79) |
| High grade CSO-EPVS (>20), n (%) | 58 (49.2) | 18 (54.6) | 40 (47.1) | 0.465 | 1.60 (0.69–3.72) |
| Lobar CMBs count, median (IQR) | 4 (2–28) | 5 (2–17) | 4 (2–28) | 0.976 | 1 (1–1.01) |
| ≥5 CMBs presence, n (%) | 58 (49.2) | 19 (57.6) | 39 (45.9) | 0.254 | 1.82 (0.78–4.22) |
| Presence of cSS, n (%) | 52 (44.1) | 23 (69.7) | 29 (34.1) | <0.0001** | 4.99 (2.1–12.35)* |
| Focal cSS, n (%) | 29 (24.6) | 10 (30.3) | 19 (22.4) | <0.0001** | 2.98 (1.03–8.64)* |
| Disseminated cSS, n (%) | 23 (19.5) | 13 (39.4) | 10 (11.8) | 9.72 (3.11–30.3) | |
| New CMBs at follow-up, n (%) | 21 (18.3) | 11 (35.5) | 10 (11.9) | 0.011* | 3.70 (1.36–10.1) |
|
Follow-up vs. baseline scan types 1-Same (GRE-GRE/SWI-SWI) 2-Less sensitive at follow-up (SWI-GRE) 3-More sensitive at follow-up (GRE-SWI) |
46 (39) 41 (34.8) 31 (26.3) |
13 (39.4) 12 (36.4) 8 (24.2) |
33 (38.8) 29 (34.1) 23 (27.1) |
0.947 | 0.88 (0.52–1.49) |
|
MRI follow-up indication Regular clinical follow-up Investigation of cognitive complaints Suspicion of new focal neurological symptoms |
86 (73%) 15 (13%) 17 (14%) |
24 (73%) 4 (12%) 6 (18%) |
62 (73%) 11 (13%) 11 (13%) |
All p>0.05 |
- |
No patients were on direct oral anticoagulants or dual antiplatelets. 15/72 patients with available data were taking aspirin.
Aim 1: cSS progression and prognostic factors for new cSS
The median MRI scan interval between the baseline (i.e. first) MRI performed after the index presentation and the latest follow-up MRI available was 2.2 years (IQR: 1.2–4.4 years).
Any degree of cSS progression during MRI follow-up was detected in 33 (28%) patients, an incidence rate of 8.94 per 100 person-years of follow-up. In the whole cohort, 2 patients had cSS progression within a cSS focus already present at baseline (1.7%), 11 patients had a single new cSS focus (9.3%), 6 (5.1%) showed two new cSS foci, and 14 patients had three or more new cSS areas (11.9%). Figure 2 demonstrates some representative examples. Among patients with no cSS at baseline (n=66), 7 developed focal and 3 disseminated cSS at follow-up scans. In patients with focal cSS at baseline (n=29), 10 progressed to disseminated cSS. In patients with disseminated cSS at baseline (n=23), 10 progressed to more disseminated due to the appearance of new cSS areas. During the same time period, new lobar CMBs appeared in 21 (18.3%) patients (Table 1).
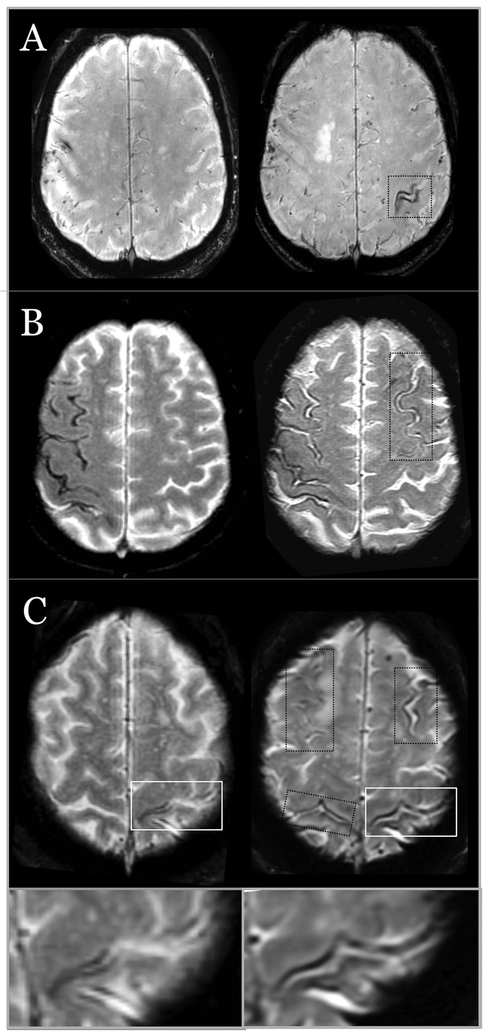
Figure 2.
Representative examples of probable CAA patients without ICH at baseline and cSS progression in follow-up MRI. (A) One new focus of cSS (right image) after a follow-up of 2.5 years. (B) A new focus of cSS (after 6 months) in a patient with disseminated cSS at baseline. (C) Three new cSS areas in a patient with a single focus of cSS at baseline (black squares) and progression of cSS in the same area as baseline (white square and lower inset), after 2 years. All images are T2*-GRE MRI at 1.5T.
The results of the bivariate analyses comparing patients with and without new cSS are summarized in Table 1. Patients who showed cSS progression during MRI follow-up, were more likely to be male, have cSS at baseline imaging (especially disseminated) and show new lobar CMBs. These results were consistent after adjusting for MRI time interval (Table 1). There was no difference in the prevalence of cSS progression according to follow-up/baseline scan types sensitivities (same at follow-up/baseline, less sensitive at follow-up, more sensitive at follow-up) (Table 1).
In multivariable logistic regression, cSS presence at baseline (especially disseminated rather than focal cSS) and appearance of new lobar CMBs were the only independent predictors of cSS progression after adjusting for potential confounders (Table 2). Baseline cSS presence (in particular disseminated, but not focal cSS) and new lobar CMBs were also independently associated with cSS progression category (see Methods) during follow-up in ordinal logistic regression analysis (Table 2).
Table 2.
Multivariable logistic regression of predictors of any cSS progression (model 1) and cSS progression category (model 2).
| Variables | OR (95%CI) | p-value |
|---|---|---|
| Model 1: any cSS progression* | ||
| Age, per year increase | 0.97 (0.91–1.02) | 0.237 |
| Sex, male | 2.98 (0.95–9.37) | 0.062 |
|
cSS presence at baseline - Focal cSS† - Disseminated cSS† |
4.04 (1.53–10.70) 2.34 (0.77–7.14) 9.12 (2.85–29.18) |
0.005 0.136 <0.0001 |
| CMBs progression | 4.24 (1.29–13.9) | 0.017 |
| Model 2: ordinal logistic regression of cSS severity progression* | ||
| Age, per year increase | 0.96 (0.91–1.02) | 0.159 |
| Sex, male | 2.47 (0.81–7.51) | 0.112 |
|
cSS presence at baseline - Focal cSS† - Disseminated cSS† |
4.12 (1.60–10.64) 2.54 (0.88–7.37) 7.57 (2.54–22.60) |
0.003 0.086 <0.0001 |
| CMBs progression | 4.56 (1.46–14.22) | 0.009 |
Both models additionally adjusted for potential differences in blood-sensitive sequences and MRI interval (months) between baseline/follow-up.
For analyses including cSS severity at baseline, i.e. focal cSS vs. disseminated cSS, all models remained consistenf and of similar effect size and statistical significance with regards to all other co-variated included (e.g. Age, Sex and CMBs progression).
Aim 2: Risk of symptomatic lobar ICH
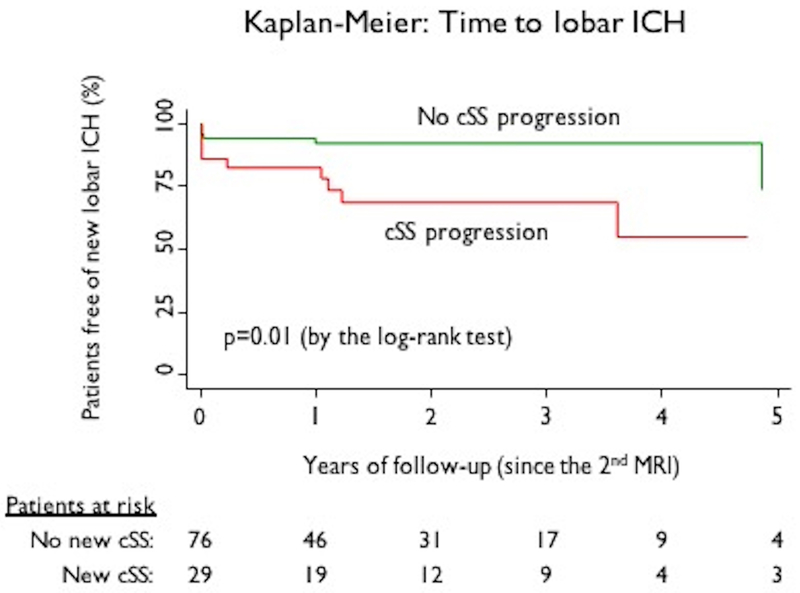
After excluding 5 patients with a first ICH during the inter-scan interval and 9 for absence of sufficient follow-up data, reliable clinical follow-up information (after the second follow-up MRI) were available in 105 CAA patients (Fig. 1). Symptomatic lobar ICH occurred in 17 (16.2%), after a median of 1.34 years (IQR: 0.3–3 years; 194.8 patient-years of follow-up) after the second MRI. The incidence rate was 8.7 (95%CI: 5.2–13.6) per 100 patient-years of follow-up. In Kaplan-Meier analysis of these 105 subjects, the appearance of new cSS was a predictor of time until subsequent ICH (p=0.01, by the log-rank test) (Figure 3). In univariable Cox regression analysis, cSS progression was a predictor of incident symptomatic lobar ICH (HR: 3.24; 95%CI: 1.23–8.59; p=0.018). Hazard for subsequent ICH increased with greater extent of cSS progression, with HRs of 1.30 (95%CI: 0.25–6.7) for category 1 (<2 new foci) and 4.86; (95%CI: 1.74–13.6; p=0.003) for 2 or more new foci. The appearance of new lobar CMBs were not associated with ICH risk (HR: 1.69; 95%CI: 0.53–5.43; p=0.375). Increasing age showed a trend towards association with the occurrence of ICH (HR: 1.06; 95%CI: 0.99–1.13; p=0.079, per year increase). None of the other demographic, clinical or imaging variables (including lobar CMBs burden at baseline, WMH, CSO-PVS) showed an association with future ICH in univariable analysis (all p>0.1).
Figure 3.
Kaplan-Meier estimates of time to symptomatic lobar ICH based on cortical superficial siderosis (cSS) progression.
In multivariable Cox regression models adjusting for biologically important risk factors (including age and CMBs progression), cSS progression was an independent predictor of increased symptomatic ICH risk during follow-up (Table 3). In a similar model using the pre-defined ordinal cSS progression severity categories, cSS progression in ≥2 new foci, but not cSS extension within an already present cSS focus at baseline MRI/progression in a single new focus, was strongly associated with future ICH risk. These results remained consistent and of similar effect size in sensitivity multivariable models further adjusted for sex, differences in baseline and follow-up blood-sensitive MRI sequences, MRI interval (months) between baseline and follow-up MRI and cSS presence/severity at baseline MRI (data not shown).
Table 3.
Multivariable cox regression of cSS presence and other potential predictors of first-ever symptomatic lobar intracerebral hemorrhage.
| Variables | HR (95%CI) | p-value |
|---|---|---|
| a. Main model (any cSS progression) | ||
| Age, per year increase | 1.08 (1–1.16) | 0.045 |
| Any cSS progression (vs. no progression) | 3.76 (1.37–10.35) | 0.010 |
| New lobar CMBs (Yes vs. No) | 0.95 (0.30–3) | 0.926 |
| b. Sensitivity analysis model (severity of cSS progression) | ||
| Age (for each year increase) | 1.11 (1.02–1.20) | 0.016 |
| cSS progression in the same focus/1 new focus | 1.21 (0.22–6.57) | 0.823 |
| cSS progression ≥2 new foci | 7.90 (2.47–25.21) | <0.0001 |
| New lobar CMBs (Yes vs. No) | 0.76 (0.23–2.46) | 0.644 |
Both models remain consistent/similar effect sizes when additionally adjusted for differences in baseline and follow-up blood-sensitive MRI sequences, sex, cSS presence at baseline and MRI interval (months).
In a sensitivity multivariable Cox regression analysis, cSS progression (≥2 new foci) was associated with an increased risk of ICH (HR: 7.21; 95%CI: 1.22–42.8; p=0.030) independent of cSS severity level on the final MRI. In a similar model exploring the interaction between cSS progression severity and cSS severity on final MRI, the combination of cSS progression (≥2 new foci) in patients with disseminated cSS on final MRI (but not other combinations) increased ICH risk (HR: 9.5; 95%CI: 2.62–34.4; p=0.001).
Discussion
A major finding of this cohort of probable CAA patients presenting with neurological symptoms but without ICH, is that cSS progression assessed on MRI over time by a new rating tool, is common and increases the risk of subsequent symptomatic lobar ICH. Hence, cSS progression may help stratify future bleeding risk in symptomatic CAA patients without major ICH, with implications for prognosis and treatment decisions.
cSS is a common (prevalence 40–60%) and reasonably specific hemorrhagic marker of CAA,4, 10 which affects the cerebral convexities, and reflects blood-breakdown residues in the outermost surface of the cortex or subarachnoid space.3 Studies systematically evaluating cSS progression are lacking. The only available data come from the population-based Rotterdam Study.4 Among 1425 individuals >60 years old who had two brain scans ~3 years apart, two developed new cSS (incidence 0.14%). Among participants who had cSS at baseline, 4/7 showed cSS progression.4
The pathophysiological mechanisms underlying cSS evolution and the currently observed independent association with future ICH risk in CAA remain to be determined.4, 12 A plausible interpretation is that MRI-detectable cSS progression might be a marker of more severe and aggressive underlying vascular disease and, thereby, predict subsequent ICH risk.6 In explorative sensitivity analyses, it seems that the future ICH risk driver is cSS progression independently of cSS severity on final MRI, indicating a more active trajectory. We note, however, that cSS progression correlates to some extent with cSS severity on final MRI, hence requiring external validation. Presumably, cSS progression reflects silent repeated episodes of superficial bleeding from fragile CAA-laden vessels.4, 12 A CAA cohort presenting with acute cSAH (n=29), recently suggested that leakage from CAA-affected leptomeningeal vessels may be an important mechanism for recurrent episodes of intrasulcal bleeding, which may indicate increased vulnerability sites for future ICH.12 Recent evidence suggests that APOE ε2 allele (thought to promote vasculopathic changes, i.e. vessel cracking and rupture) is more common in CAA patients with vs. without cSS.13, 14 CMBs burden at baseline and progression were not associated with future ICH risk. This finding, although counterintuitive at first glance, is in line with evolving thinking about CMBs in CAA, in that they are good markers for the disease’s presence, but not necessarily its hemorrhagic-prone burden. New evidence from multiple cohorts suggested that when cSS is taken into account, lobar CMBs are no longer independently associated with future ICH risk,4, 6 even in CAA-related lobar ICH at baseline. Finally, cSS progression was not related to vascular risk factors (e.g. hypertension, typically well controlled in our cohort), in line with CAA not being primarily dependent on vascular risk factors. However, data on blood pressure and cholesterol control were not availlable in our cohort and their interaction with MRI markers evolution in CAA should be an area for future research.
Strengths of our study are the systematic evaluation of a large sample of CAA patients without ICH from the two main settings through which they come to medical attention: stroke services, where CAA patients are commonly seen for TFNEs and memory clinics.11 The absence of lobar ICH in our CAA population, means that cSS foci detected, truly represent markers of primary hemorrhagic events, as opposed to secondary extension from ICH – a common challenge in interpreting cSS in CAA-ICH cohorts.4 Also, the exploration of MRI biomarkers in this ICH-free CAA population might help inform early clinical trials and guide future prevention in possibly less advanced CAA cases (compared to CAA-ICH). Another important limitation is that although the study cohort represents consecutive patients meeting the study’s inclusion/exclusion criteria, there is likely substantial selection bias due to the requirement for MRI follow-up performed as part of clinical care. Indeed, excluded patients were somewhat older and had a trend towards lower prevalence of cSS at baseline compared to those included in the study. It is possible that ICHs during the inter-scan interval were decreased by the study design, e.g. anyone with a devastating ICH wouldn’t get a follow-up MRI and hence not be eligible for the study. There is a possibility for referral bias to our tertiary centre. Also, given the observational clinical design of this study, the time point and indications of follow-up MRI were not standardized. These biases might have affected our results in that patients perceived to have high future CAA-ICH risk or being more symptomatic (i.e. presenting with TFNEs) were more likely to get a follow-up MRI. In this case, we may have overestimated the true incidence of cSS progression. Also the range of follow-up MRI timepoints could affect the observed cSS progression (less progression in patients with shorter follow-up).
For the same reason, the blood-sensitive MRI sequences were often different between the baseline and follow-up MRIs. While we have made every effort to incorporate this difference in our models by creating a compound variable (SWI-T2*-GRE pair combinations for baseline and follow-up MRI), this might not be adequate. SWI and T2*-GRE sequences have different sensitivities for blood-product detection. Thus, the lack of association between CMB accumulation with ICH, might be partly related to variability in MRI detection of CMB progression. There are no studies quantifying the difference in sensitivity of the two techniques for cSS classification. Given that cSS represents a much higher volume of blood-breakdown products compared to CMBs, the differences in sensitivity might be less pronounced. Of note, the combination raising this concern in our study is in patients with T2*-GRE at baseline and SWI at follow-up. Quite reassuringly, when MRIs were rated blinded to whether the scans were baseline or follow-up, the cSS progression results were consistent (i.e. no patients demonstrated cSS seemingly disappearing on follow-up T2*-GRE when the baseline MRIs were SWI). Any misclassification of cSS progression due to the properties of the visual method and imaging technique would be expected to be unrelated to the risk for subsequent symptomatic ICH and would therefore be predicted to bias towards a null result rather than a positive association. Finally, the number of ICHs during follow-up was low, leading to wide estimates around cSS progression effect size. Detailed data on treatments during follow-up, including antihypertensives, antithrombotics etc. were not available. Hence, our findings need to be interpreted with caution and could be only generalised to similar CAA populations.
Despite the limitations, our findings offer support for using cSS progression on MRI, presumably representing new CAA-bleeding events, as a new biomarker in CAA studies. They raise the possibility that cSS progression might be sufficiently predictive of future ICH to affect clinical decision making and be potential usefull as an outcome marker for candidate disease-modifying interventions trials. Our newly developed cSS progresson scale requires external validation and our findings await replication in further prospective studies in the field.
Supplementary Material
Sources of Funding
NIH grants R01-AG026484 (S.M. Greenberg). Bodossaki Foundation postdoctoral fellowship (A. Charidimou). NIH grant (J. Rosand). Consultant/advisory relationship with New Beta Innovations, Pfizer and Boehringer Ingelheim (J. Rosand).
Footnotes
Disclosures
No disclosures relevant to this work.
References
- 1.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: Recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83:124–137 [DOI] [PubMed] [Google Scholar]
- 2.Greenberg SM, Vonsattel JP, Stakes JW, Gruber M, Finklestein SP. The clinical spectrum of cerebral amyloid angiopathy: Presentations without lobar hemorrhage. Neurology. 1993;43:2073–2079 [DOI] [PubMed] [Google Scholar]
- 3.Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charidimou A, Linn J, Vernooij MW, Opherk C, Akoudad S, Baron JC, et al. Cortical superficial siderosis: Detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain. 2015;138:2126–2139 [DOI] [PubMed] [Google Scholar]
- 5.van Etten ES, Auriel E, Haley KE, Ayres AM, Vashkevich A, Schwab KM, et al. Incidence of symptomatic hemorrhage in patients with lobar microbleeds. Stroke. 2014;45:2280–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charidimou A, Peeters AP, Jager R, Fox Z, Vandermeeren Y, Laloux P, et al. Cortical superficial siderosis and intracerebral hemorrhage risk in cerebral amyloid angiopathy. Neurology. 2013;81:1666–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulouis G, Charidimou A, Jessel MJ, Xiong L, Roongpiboonsopit D, Fotiadis P, et al. Small vessel disease burden in cerebral amyloid angiopathy without symptomatic hemorrhage. Neurology. 2017;88:878–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charidimou A, Peeters A, Fox Z, Gregoire SM, Vandermeeren Y, Laloux P, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: Multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke. 2012;43:2324–2330 [DOI] [PubMed] [Google Scholar]
- 9.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charidimou A, Jager RH, Fox Z, Peeters A, Vandermeeren Y, Laloux P, et al. Prevalence and mechanisms of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology. 2013;81:626–632 [DOI] [PubMed] [Google Scholar]
- 11.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415–1420 [DOI] [PubMed] [Google Scholar]
- 12.Beitzke M, Enzinger C, Wunsch G, Asslaber M, Gattringer T, Fazekas F. Contribution of convexal subarachnoid hemorrhage to disease progression in cerebral amyloid angiopathy. Stroke. 2015;46:1533–1540 [DOI] [PubMed] [Google Scholar]
- 13.Shoamanesh A, Martinez-Ramirez S, Oliveira-Filho J, Reijmer Y, Falcone GJ, Ayres A, et al. Interrelationship of superficial siderosis and microbleeds in cerebral amyloid angiopathy. Neurology. 2014;83:1838–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Na HK, Park JH, Kim JH, Kim HJ, Kim ST, Werring DJ, et al. Cortical superficial siderosis: A marker of vascular amyloid in patients with cognitive impairment. Neurology. 2015;84:849–855 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.