Abstract
Background and Purpose—
Mitoquinone (MitoQ) has been reported as a mitochondria-targeting antioxidant to promote mitophagy in various chronic diseases. Here, our aim was to study the role of MitoQ in mitophagy activation and oxidative stress-induced neuronal death reduction after subarachnoid hemorrhage (SAH) in rats.
Methods—
Endovascular perforation was used for SAH model of male Sprague–Dawley rats. Exogenous MitoQ was injected intraperitoneally (i.p.) 1 hour after SAH. ML385, an inhibitor of NF-E2-related factor 2 (Nrf2) was given intracerebroventricularly (i.c.v.) 24 hours before SAH. Small interfering ribonucleic acid (siRNA) for Prohibitin 2 (PHB2) was injected i.c.v 48 hours before SAH. Nuclear, mitochondrial and cytoplasmic fractions were gathered using nucleus and mitochondria isolation kits. SAH grade evaluation, short- and long- term neurological function tests, oxidative stress and apoptosis measurements were performed. Pathway related proteins were investigated with Western blot and immunofluorescence staining.
Results—
Expression of Kelch-like ECH-associated protein (Keap1, 2.84 times at 24h), Nrf2 (2.78 times at 3h) and LC3II (1.94 times at 24h) increased, while PHB2 (0.46 times at 24h) decreased after SAH compared with Sham group. MitoQ treatment attenuated oxidative stress and neuronal death, both short-term and long-term. Administration of MitoQ resulted in a decrease in expression of Keap1 (0.33 times), Romo1 (0.24 times), Bax (0.31 times), Cleaved Caspase-3 (0.29 times) and an increase in Nrf2 (2.13 times), Bcl-xl (1.67 times), PINK1 (1.67 times), Parkin (1.49 times), PHB2 (1.60 times) and LC3II (1.67 times) proteins compared with SAH+vehicle group. ML385 abolished the treatment effects of MitoQ on behavior and protein levels. PHB2 siRNA reversed the outcomes of MitoQ administration through reduction in protein expressions downstream of PHB2.
Conclusions—
MitoQ inhibited oxidative stress related neuronal death by activating mitophagy via Keap1/Nrf2/PHB2 pathway after SAH. MitoQ may serve as a potential treatment to relieve brain injury after SAH.
Keywords: Mitoquinone, NF-E2-related factor 2, Prohibitin 2, Mitophagy, Subarachnoid hemorrhage
Introduction
Early brain injury (EBI) is the primary reason behind high rates of mortality and delayed neurological deficits in patients after subarachnoid hemorrhage (SAH).1 Recent studies report that oxidative stress-induced neuronal death is an underlying cause of poor outcomes in EBI,2 and mitochondrial dysfunction is the source of reactive oxygen species (ROS) generation and apoptosis.3 An effective treatment for attenuation of mitochondrial injury would play an important role in therapeutic strategy for patients with SAH. Selective elimination of damaged mitochondria by autophagy, which is also known as mitophagy, was reported to safeguard against neurological deficits after SAH.4 However, the role of mitophagy in reducing oxidative stress-induced neuronal death is still unclear.
Mitoquinone (MitoQ), a potent mitochondria-targeting antioxidant, which contains several hundred-fold antioxidant properties when compared to untargeted antioxidants, is very effective in prevention of mitochondrial dysfunction.5 Studies show that it effectively attenuates oxidative stress and apoptosis by repairing damaged mitochondria,6 and is associated with induction of mitophagy.7 However, mechanisms of MitoQ treatment have never been investigated in the context of SAH. Furthermore, oxidative stress regulator complex Keap1/Nrf2 has been identified to be the main target of antioxidants in various diseases, as well as in the SAH model.8 Although MitoQ has been reported to dissociate and active Nrf2 from aforementioned complex in kidney injury,9 it still needs to be investigated after induction of SAH. We postulate that the Keap1/Nrf2 complex participates in the MitoQ induced mitophagy after induction of SAH.
Prohibitin 2 (PHB2), a mitophagy receptor that binds to the autophagosomal membrane-associated protein LC3II and is a viable explanation underlying induction of mitophagy and establishment of protective effects against dysneuria.10 Moreover, compared to traditional mitophagy markers PINK1 and Parkin,11 the role of PHB2 in mitophagy would be a worthwhile and a novel target to pursue as a therapeutic strategy for SAH.
Thus, we hypothesize that MitoQ will inhibit oxidative stress and neuronal death via Nrf2/Keap1/PHB2 pathway in mitophagy after subarachnoid hemorrhage in rats.
Material and Methods
The authors declare that all supporting data are available within the article and the Online Supplemental Data.
SAH Animal Model
Adult male Sprague–Dawley rats (weight 280–330 g) were housed in a 12/ 12 hours light/dark cycle in 25°C room temperature, humidity control, free access to food and water before surgery. The endovascular perforation SAH model was performed as reported previously.12 All experiments were approved by the Institutional Animal Care and Use Committee of Loma Linda University, which in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental Protocols (Supplemental Figure I)
Experiment 1
36 (n=6 per group) rats were randomly divided into 6 groups: Sham and SAH after 3, 6, 12, 24, 72 hours after SAH. Additionally, 6 rats (n=3 per group) were equally distributed to Sham and SAH after 24 h groups for double immunohistochemistry staining (n=3 per group). Expression of Keap1, Nrf2, PHB2 and LC3I/LC3II was analyzed by Western blot. Immunofluorescence staining was performed for colocalization with neurons.
Experiment 2
In a short-term outcome evaluation, 30 (n=6 per group) rats were randomly assigned into 5 groups: Sham, SAH+vehicle (1 mL of sterile 0.9 % of NaCl), SAH+MitoQ 1 mg/kg, SAH+MitoQ 3 mg/kg, SAH+MitoQ 9 mg/kg groups for neurological scoring. Additionally, 9 (n=3 per group) rats were equally used in Sham, SAH+vehicle and SAH+MitoQ-Best dosage (3 mg/kg) groups for immunofluorescence, DHE and Fluoro-Jade C (FJC) staining. SAH+MitoQ-Best dosage (3 mg/kg) was administered in experiments 3, 4 and 5.
Experiment 3
In a long-term outcome evaluation, 24 (n=8 per group) rats were equally divided into Sham, SAH+vehicle, and SAH+MitoQ groups. Long-term neurobehavioral experiments, Nissl’s and FJC staining were performed.
Experiment 4
To investigate the proposed molecular mechanism, 24 (n=6 per group) rats were randomly divided into SAH+ML385+MitoQ, SAH+dimethylsulfoxide (DMSO)+MitoQ, SAH+PHB2 siRNA+MitoQ and SAH+Scramble siRNA (Scr siRNA)+MitoQ groups for Western blot. Another 12 (n=3 per group) rats were used for DHE and FJC double staining. Samples of Sham (n=6 per group), SAH+vehicle and SAH+MitoQ groups (n=9 per group) were shared with Experiment 2. Samples for MDA measurement were using 10mg from frozen brain samples prepared for Western blot in each group.
Experiment 5
Nuclear and mitochondrial proteins were gathered from 18 rats (6 rats per group) of Sham, SAH+vehicle, and SAH+MitoQ groups. Pathway related proteins were detected in their individual organelles with Western blot.
Drug Administration
MitoQ (MedKoo Biosciences, Morrisville, NC) was administered i.p. after SAH. ML385 (AOBIOUS, Gloucester, MA) diluted in DMSO i.c.v. injected before SAH. Both 500 pmol of PHB2 siRNA and Scr RNA (Origene Technologies, Inc., Rockville, MD) in 5μl was injected i.c.v. 48 hours pre-surgery as previously reported.12
Severity of SAH
The severity of SAH was blindly measured using the SAH grading scale after euthanasia as previously described.12 Rats with SAH grading scores ≤7 at 24 hours were excluded from this study.
Assessment of Short-term Neurobehavioral Outcomes
18-point scoring system with a modified Garcia scale and another 4- point scoring system with beam balance test were utilized to blindly evaluate as previous described.12
Assessment of Long-term Neurobehavioral Outcomes
Foot-fault test was performed in the first three weeks after SAH to evaluate sensorimotor coordination and balance as previously described.13 At 21–25 days after SAH rotarod and water maze tests were utilized as previous reported.14
Western Blot
Left hemispheres of brain samples were collected without surface hematoma 24 h after SAH. Western blot was performed as previous described.12
Histomorphology Measurement
Double immunofluorescence, DHE, FJC were performed as previously described.12, 14, 15 A series of 8 μm slices were prepared. Positive cells were counted in six sections per brain for the mean at ×200 magnification by an independent observer.
Nissl’s staining and evaluation of hippocampus injury was performed as previous description.14 The prepared slides (16 μm) were viewed for neurons in the different regions. The mean number of neurons at ×200 magnification (six sections per brain) was counted manually by an observer blinded to the treatment conditions. The neuronal density loss was estimated according to established method.16
Assessment of Intracellular MDA Levels
The lipid peroxidation assay kit purchased from Sigma-Aldrich (St. Louis, Mo) was utilized to measure the MDA level as previously reported.17
Assessment of Nuclear and Mitochondrial Proteins
Nuclear and cytoplasmic extraction reagents and mitochondria isolation kit for tissue were both purchased from Thermo Fisher Scientific (Grand Island, NY). The method for two types of extractions was described previously.18
Statistical Analysis
All experiments were performed with full blinding, allocation concealment, and randomization. Before analysis, Shapiro-Wilk test was used to test the normality and variables were log transformed when necessary. Based on the distribution of normality, Student t-test test was used to compare the continuous variables after intervention between two groups, one-way analysis of variance (ANOVA) followed by the Tukey’s post-hoc test were used for when multiple group comparisons were involved (>2 groups) with continuous independent variables, means ± SD were used for expression. If data were not normally distributed even with log transformation, Kruskal-Wallis test followed by the Dunn’s post-hoc test was used for statistics, the medians (interquartile range) were used for expression. P value of <0.05 was defined as statistically significant. All statistical analyses were performed using SPSS 24.0 (IBM Corp., USA).
Results
Mortality and SAH Severity Scores
32 rats were part of Sham group, 170 rats underwent SAH induction from which 7 rats were excluded. There was no mortality in Sham group and the total mortality in SAH groups was 22.09% (36 of 163) (Figure 1A). Subarachnoid blood clots were observed around the circle of Willis and ventral brain stem 24 hours after SAH (Supplemental Figure II). There was no significant difference in average SAH grading scores among all of the SAH induced groups (Supplemental Figure II).
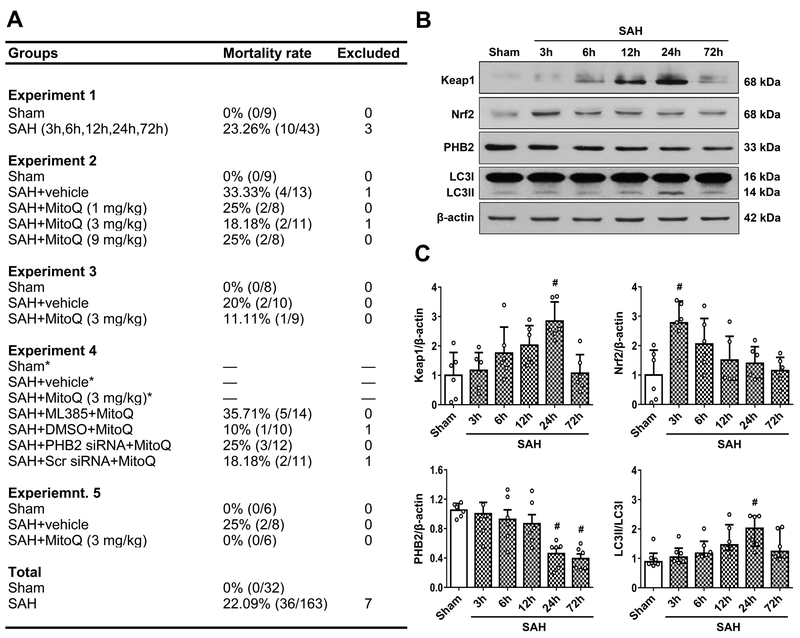
Figure 1. Mortality rate and expression changes of Kelch-like ECH-associated protein (Keap1), NF-E2-related factor 2 (Nrf2), Prohibitin 2 (PHB2) and LC3 after subarachnoid hemorrhage (SAH).
(A) The number of mortality and excluded rats of each group. * means shared groups with Experiment 2. (B) Representative Western blot images and (C) quantitative analysis of Keap1, Nrf2, PHB2 and LC3 time course expression. n=6 per group. Data of LC3 time course expression was expressed as the medians with interquartile range using Kruskal–Wallis test followed by the Dunn’s post hoc test. Other data were expressed as the means ± SD using one-way analysis of variance (ANOVA) followed by the Tukey post hoc test. #P < 0.05 vs. Sham group.
Time Course Expression of Keap1, Nrf2, PHB2, LC3II in EBI after SAH
Western blot expressions at 3, 6, 12, 24 and 72 h after SAH indicated that elevation of Keap1 started at 3 h and peaked at 24 h (2.84 ± 0.65 vs. 1 ± 0.78) (P<0.05; Figure 1B and 1C left upper panel). However, expression of Nrf2 rapidly increased to its highest level at 3 h (2.78 ± 0.74 vs. 1 ± 0.85), and gradually decreased until 72 h (P<0.05; Figure 1B and 1C right upper panel). Additionally, PHB2 levels declined and were notably lower than Sham (1 ± 0.09) at both 24 h (0.46 ± 0.18) and 72 h (0.39 ± 0.15) (P<0.05; Figure 1B and C left lower panel). Expression of LC3II was evaluated by its ratio with LCI and the peak was significantly higher compared with Sham rats at 24 h [2.02 (1.02) vs. 0.88 (0.38)] (P<0.05; Figure 1B and 1C, right lower panel). Double immunofluorescence staining revealed Keap1, Nrf2, PHB2 and LC3 colocalizing with neurons. The expression of Keap1 was increased while PHB2 was decreased in the left hemispheres of SAH rats compared with Sham group (P<0.05; Supplemental Figure III). Meanwhile, Nrf2 and LC3 did not changed much between the two groups (Supplemental Figure III).
MitoQ Treatment Improved Short-term Neurobehavior, Increased Expression of Nrf2 and PHB2 in Neurons, and Attenuated Oxidative Stress and Neuronal Death
Modified Garcia and beam balance data indicated impairments in SAH+vehicle group when compared to Sham group (P<0.01; Figure 2A). Administration of 3 mg/kg MitoQ significantly improved neurological scores most in all MitoQ treated groups compared to SAH+vehicle group in both Modified Garcia [16 (1.5) vs. 11.5 (1.5)] and beam balance tests [3.5 (0.75) vs. 1.33 (1.33)] (P<0.05; Figure 2A). Therefore, we chose 3mg/kg as the best dosage of MitoQ.
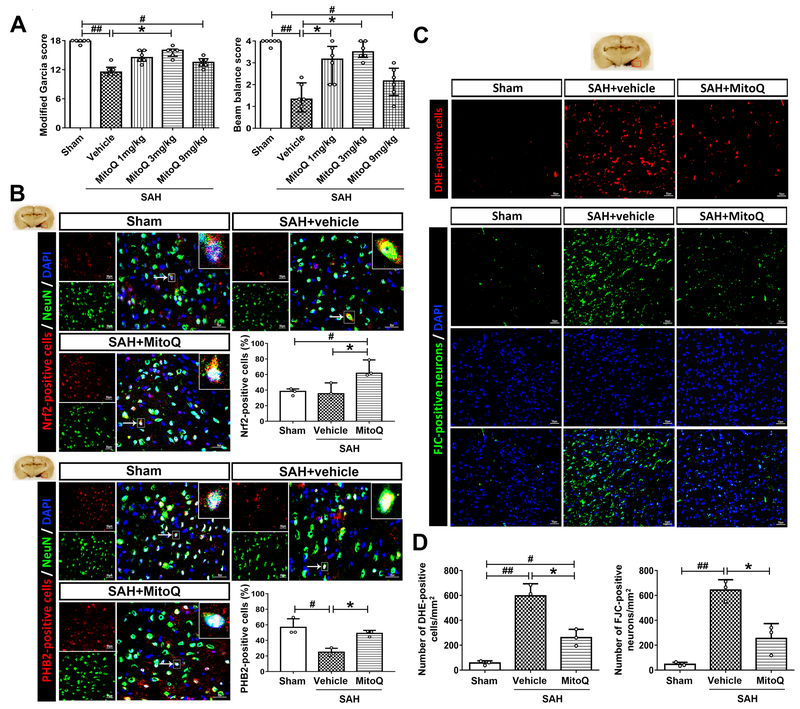
Figure 2. Mitoquinone (MitoQ) attenuated short-term neurological deficits, oxidative stress and neuronal death and increased the expression of Nrf2 and PHB2 after subarachnoid hemorrhage (SAH).
(A) Modified Garcia and beam balance scores. n=6 per group. (B) Double immunofluorescence staining and quantifications for Nrf2 (red) and PHB2 (red) in neuron (NeuN, green) of Sham, SAH+vehicle and SAH+MitoQ groups. Nuclei are stained with DAPI (blue). Top panel indicates the location of staining (small red box). Arrows indicate a cell chosen for 10 times magnification in right upper corner of merged panels. n=3 per group. (C) DHE and FJC staining in three groups above. (D) The quantifications of DHE and FJC staining. n=3 per group. Scale bar = 50μm. Data of Modified Garcia and beam balance scores were expressed as the medians with interquartile range using Kruskal–Wallis test followed by the Dunn’s post hoc test. Other data were expressed as the means ± SD using one-way analysis of variance (ANOVA) followed by the Tukey post hoc test. #P < 0.05 vs. Sham group; ##P < 0.01 vs. Sham group; *P < 0.05 vs. SAH+vehicle group.
Double immunofluorescence staining revealed an increase in expression Nrf2 (66.6 ± 10.6 vs. 37.57 ± 10.83) and PHB2 (49.01 ± 3.85 vs. 56.82 ± 10.99) proteins in MitoQ group compared to SAH+vehicle group at 24 h after SAH (P<0.05; Figure 2B). Meanwhile, DHE and FJC staining results concluded that the number of both DHE-positive cells and FJC-positive neurons was significantly increased in SAH+vehicle group when compared to Sham group (P<0.01; Figure 2C and 2D). Conversely, MitoQ administration could reverse the alteration (261.1 ± 66.67 vs. 596.1 ± 97.6; 254.4 ± 119.4 vs. 642.8 ± 83.37) (P<0.05; Figure 2C and 2D).
MitoQ Treatment Improved Long-term Neurobehavior and Reduced Hippocampal Neuronopathy
Rotarod test showed a remarkably shorter falling latency in SAH+vehicle group compared to Sham group during both the 5 RPM [39.05 (17.5) vs. 59.05 (6.6)] and 10 RPM [28.25 (9.78) vs. 49 (7.76)] accelerating velocity tests (P<0.05; Figure 3A). On the contrary, MitoQ treatment reversed the poor performance seen in the SAH+vehicle group, and in 10 RPM [39 (5.11) vs. 28.25 (9.78)] test the difference was significant (P<0.05; Figure 3A).
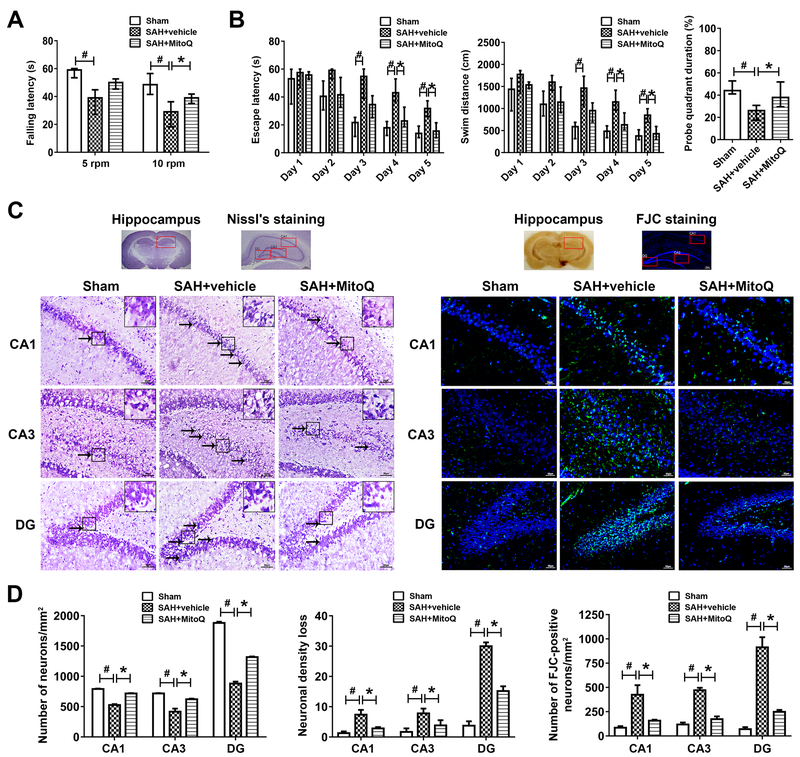
Figure 3. Mitoquinone (MitoQ) attenuated long-term neurological deficits and hippocampus injury after subarachnoid hemorrhage (SAH).
(A) Rotarod test of 5 RPM and 10 RPM on the 21th day. n=8 per group. (B) Water maze test related escape latency and swim distance and probe quadrant duration for 21–25 days. n=8 per group. (C) Representative images and neuronal quantifications of Nissl’s and FJC staining for hippocampus in CA1, CA3 and DG regions. (D) Quantitative analysis of Nissl’s and FJC staining. n=8 per group. Arrows indicate shrunken pyramidal or died neurons. Scale bar = 200μm (general) and 50 μm (regions). Data of long-term neurological behavior tests were expressed as the medians with interquartile range using Kruskal–Wallis test followed by the Dunn’s post hoc test. Other data were expressed as the means ± SD using one-way analysis of variance (ANOVA) followed by the Tukey post hoc test. #P < 0.05 vs. Sham group; *P < 0.05 vs. SAH+vehicle group.
Water maze test results supported spatial memory loss in SAH+vehicle group compared with Sham group in terms of longer escape latency and more distance to find the platform, but MitoQ treatment significantly ameliorated the performance of day 4–5 [Day 4 escape latency, 24.4 (8.7) vs. 44.45 (9.18); Day 5 escape latency, 16.51 (7.1) vs. 32.56 (7.66); Day 4 swim distance, 672.3 (239.8) vs. 1219 (313.1); Day 5 swim distance, 467.5 (193.3) vs. 869.8 (204.6)] (P<0.05; Figure 3B). In probe quadrant trail, compared to Sham group, SAH+vehicle group spent more time in wrong quadrants (P<0.05; Figure 3B). MitoQ treatment notably enhanced the duration of time spent in the target quadrant compared with SAH+vehicle group [38.15 (14.26) vs. 24.37 (9.07)] (P<0.05; Figure 3B).
Nissl’s staining and FJC staining revealed more shrinkage in cell morphology and more neuronal loss with cell death in CA1, CA3 and dentate gyrus (DG) of SAH+vehicle group compared with Sham group (P<0.05; Figure 3C and 3D). When given MitoQ treatment, the hippocampal neuronopathies were safeguarded against. Less neuronal loss was happened in SAH+MitoQ group compared with SAH+vehicle group among CA1 (2.41 ± 1.13 vs. 7.83 ± 0.99), CA3 (3.03 ± 0.7 vs. 8.24 ± 0.99) and DG (15.44 ± 1.22 vs. 29.12 ± 2.66) (P<0.05; Figure 3C and 3D). Besides, less cell death was occurred in SAH+MitoQ group compared with SAH+vehicle group among CA1 (158.3 ± 8.33 vs. 445 ± 70.49), CA3 (182.2 ± 16.86 vs. 465 ± 35.47) and DG (253.9 ± 12.62 vs. 926.7 ± 84.13) (P<0.05; Figure 3C and 3D).
Foot fault test revealed deficits in all limbs especially amelioration of forelimbs was noted in the SAH+vehicle group compared with Sham group for 3 weeks (P<0.05; Supplemental Figure IV). In contrast, MitoQ treatment improved performance compared to SAH+vehicle group (P<0.05; Supplemental Figure IV).
MitoQ Improved Neurobehavior and Activated Mitophagy through Nrf2 and PHB2 Dependent Pathway
Compared with SAH+DMSO+MitoQ group, ML385 abolished the protective effects of MitoQ administration both in modified Garcia [11.5 (3.75) vs. 15 (2)] and beam balance [1.33 (0.42) vs. 3 (0.65)] results (P<0.05; Figure 4A and 4B). Compared with SAH+Scr siRNA+MitoQ group, PHB2 siRNA also blocked the treatment effect in modified Garcia [12 (1.25) vs. 14.5 (2.75)] and beam balance [1.83 (1.67) vs. 3.16 (0.83)] scores (P<0.05; Figure 4A and 4B). Western blot revealed a significant increase in expression of Keap1, PINK1, Parkin, LC3 and no change in Nrf2, while decrease in PHB2 of SAH+vehicle group when compared with Sham group (P<0.05; Figure 4C and 4D). Administration of MitoQ resulted in a significant decrease in expression of Keap1 (1 ± 0.61 vs. 3.08 ± 1.29), while Parkin (3.33 ± 0.37 vs. 2.23 ± 0.6), PHB2 [0.98 (0.27) vs. 0.56 (0.09)] and LC3II (4.01 ± 0.91 vs. 2.4 ± 0.81) significantly increased along with an increase trend of Nrf2 [2.73 (1.2) vs. 1.38 (0.65)] when compared with SAH+vehicle group (P<0.05; Figure 4C and 4D).
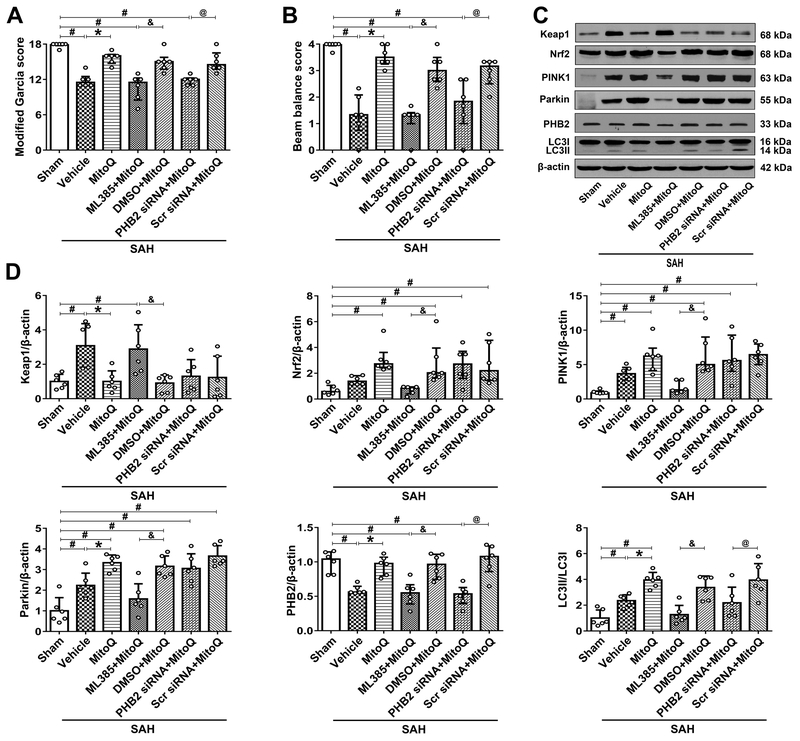
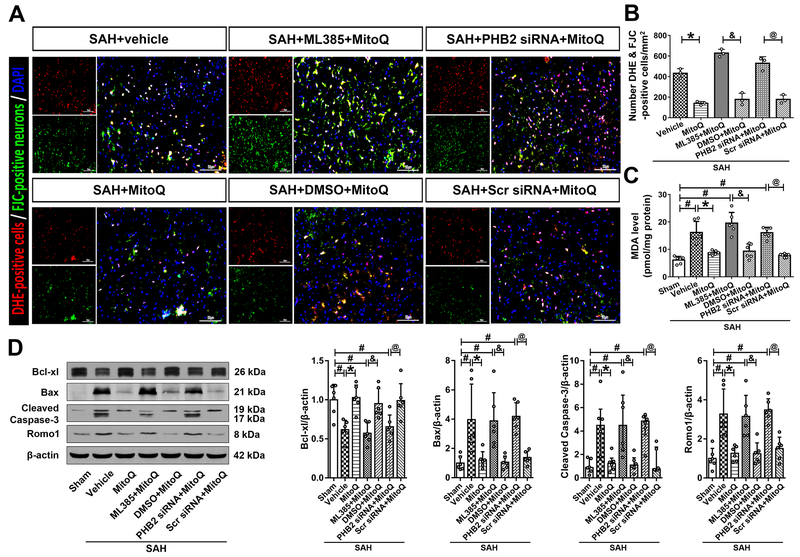
Figure 4. Blokade of Nrf2 and PHB2 abolished mitoquinone (MitoQ) treatment effects on the neurological function and mitophagy 24 h after subarachnoid hemorrhage (SAH).
(A) Modified Garcia score. (B) Beam balance score. (C) Representative Western bolt images of Keap1, Nrf2, PINK1, Parkin, PHB2 and LC3. (D) Quantitative analyses of those proteins above. n=6 per group. Data of Modified Garcia scores, beam balance scores and Nrf2, PINK1, PHB2 protein expressions were expressed as the medians with interquartile range using Kruskal–Wallis test followed by the Dunn’s post hoc test. Other data were expressed as the means ± SD using one-way analysis of variance (ANOVA) followed by the Tukey post hoc test. #P < 0.05 vs. Sham group; *P < 0.05 vs. SAH+vehicle group; &P< 0.05 vs. SAH+ML385+MitoQ group; @P< 0.05 vs. SAH+PHB2 siRNA+MitoQ group.
Inhibition of Nrf2 with ML385 abolished effects of exogenous MitoQ, which led to an increase in Keap1 (2.88 ± 1.42 vs. 0.9 ± 0.47), downregulation of Nrf2 [0.84 (0.54) vs. 2.03 (2.31)] and other downstream proteins (P<0.05; Figure 4A–D). Downregulation of PHB2 [0.53 (0.23) vs. 1.08 (0.37)] and LC3II (1.85 ± 1.95 vs. 3.75 ± 2.17) occurred upon administration of PHB2 siRNA, when compared to SAH+Scramble siRNA+MitoQ group (P<0.05; Figure 4C and 4D).
Blockade of Nrf2 and PHB2 Abolished the Protective Effect of MitoQ in Oxidative Stress-induced Neuronal Death
DHE and FJC double straining showed that SAH+vehicle group, SAH+ML385+MitoQ group and SAH+PHB2 siRNA+MitoQ group respectively contained significantly more positive and merge cells compared with treatment control groups (SAH+MitoQ group, SAH+DMSO+MitoQ group and SAH+Scr siRNA+MitoQ group) (P<0.05; Figure 5A and 5B). Similar results were also obtained in MDA measurements. Intracellular MDA increased significantly in SAH+vehicle when compared with Sham group (P<0.05; Figure 5C). Upon administration of MitoQ, MDA levels decreased when compared to SAH+vehicle group (8.77 ± 0.76 vs. 16.17 ± 4) (P<0.05; Figure 5C). In addition, MDA levels in SAH+ML385+MitoQ (19.57 ± 3.9 vs. 9.34 ± 2.62), SAH+PHB2 siRNA+MitoQ (16.08 ± 1.95 vs. 7.78 ± 0.65) groups significantly increased when compared with their treatment control groups (P<0.05; Figure 5C).
Figure 5. Blokade of Nrf2 and PHB2 reversed the effects of mitoquinone (MitoQ) on the oxidative stress-induced neuronal death 24 h after subarachnoid hemorrhage (SAH).
(A) Representative images and (B) quantitative analyses of Double staining for DHE and FJC. (C) MDA levels. (D) Representative Western blot images and quantitative analyses of Bcl-xl, Bax, Cleaved Caspase-3 and Romo1. Data of Cleaved Caspase-3 protein expression was expressed as the medians with interquartile range using Kruskal–Wallis test followed by the Dunn’s post hoc test. Other data were expressed as the means ± SD using one-way analysis of variance (ANOVA) followed by the Tukey post hoc test. #P <0.05 vs. Sham group; *P < 0.05 vs. SAH+vehicle group. &P< 0.05 vs. SAH+ML385+MitoQ group; @P< 0.05 vs. SAH+PHB2 siRNA+MitoQ group.
Expression of apoptosis related proteins showed that Bcl-xl decreased, while Bax, Cleaved Caspase-3 increased significantly in SAH+vehicle group compared with Sham group (P<0.05; Figure 5D). Meanwhile, ROS related protein Romo1 also increased notably after SAH (P<0.05; Figure 5D). Treatment with MitoQ reversed the aforementioned changes, while administration of ML385 and PHB2 siRNA terminated the protective effects of MitoQ leading to decreased expression of Bcl-xl (0.57 ± 0.13 vs. 0.95 ± 0.19; 0.65 ± 0.15 vs. 0.99 ± 0.22) and an increase in Bax (3.88 ± 1.92 vs. 1.06 ± 0.42; 4.19 ± 0.93 vs. 1.37 ± 0.44), Cleaved Caspase-3 [4.48 (4.77) vs. 1.09 (0.95); 4.85 (1.59) vs. 0.77 (1.96)] and Romo1 (3.15 ± 1.08 vs. 1.24 ± 0.56; 3.48 ± 0.57 vs. 1.51 ± 0.58) (P<0.05; Figure 5D).
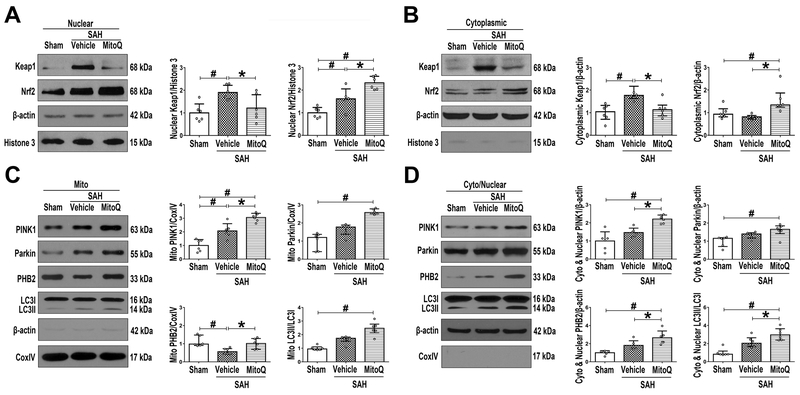
MitoQ Activates Mitophagy through Nuclear Nrf2/Keap1 and Mitochondrial PINK1/Parkin/PHB2/LC3II Pathways
Upon analysis of nuclear extraction samples, both Keap1 and Nrf2 increased significantly in SAH+vehicle group compared to Sham group (P<0.05; Figure 6A). Meanwhile, after MitoQ treatment, Keap1 (1.21 ± 0.6 vs. 1.9 ± 0.32) decreased, while Nrf2 (2.32 ± 0.29 vs. 1.61 ± 0.44) increased when compared with SAH+vehicle group (P<0.05; Figure 6A). Cytoplasmic extraction samples revealed that expression of Keap1/Nrf2 complex remained as in the whole cell extraction (P<0.05; Figure 6B).
Figure 6. The effects of Mitoquinone (MitoQ) on nuclear Keap1/Nrf2 and mitochondrial mitophagy-associated proteins after subarachnoid hemorrhage (SAH).
Representative Western blot images and quantitative analyses of Nrf2 and Keap1 in (A) nuclear and in (B) cytoplasmic fractions. Representative Western blot images and quantitative analyses of PINK1, Parkin, PHB2 and LC3 in (C) mitochondrial and in (D) cytoplasmic (except mitochondria) plus nuclear fractions. Data of Keap1 and Nrf2 cytoplasmic expressions; Parkin, PHB2 and LC3 mitochondrial expressions; Parkin and LC3 cytoplasmic (except mitochondria) plus nuclear expressions were expressed as the medians with interquartile range using Kruskal–Wallis test followed by the Dunn’s post hoc test. Other data were expressed as the means ± SD using one-way analysis of variance (ANOVA) followed by the Tukey post hoc test. #P < 0.05 vs. Sham group; *P< 0.05 vs. SAH+vehicle group.
In mitochondrial extraction, expression of PINK1 were significantly increased while PHB2 was decreased in SAH+vehicle group compared to Sham group (P<0.05; Figure 6C). However, MitoQ treatment group exhibited a further increase in expression of PINK1 (3.06 ± 0.31 vs. 2.07 ± 0.53) while PHB2 [1.01 (0.6) vs. 0.56 (0.29)] expression was reversed when compared with SAH+vehicle group (P<0.05; Figure 6C). In cytoplasm (except mitochondria) and nucleus portions, similar trends continued to be present in the SAH+vehicle group as were seen in the mitochondrial extraction (P<0.05; Figure 6D). Although not significant, Parkin [1.65 (0.43) vs. 1.33 (0.29)] expression also followed the trend of increase, however, the other three proteins PINK1 (2.2 ± 0.23 vs. 1.46 ± 0.24), PHB2 (2.65 ± 0.75 vs. 1.81 ± 0.51) and LC3II [2.96 (0.66) vs. 2.03 (0.61)] were significantly increased in SAH+MitoQ group compared to SAH+vehicle group (P<0.05; Figure 6D).
Discussion
Present study revealed that 24 hours after SAH, there is an increase in neuronal expression of Keap1, LC3II, no changes in Nrf2, and a decrease in PHB2. MitoQ treatment improved both short- and long- term neurological impairment, attenuated neuronal death, and is associated with upregulation of Nrf2 and PHB2. Inhibition of Nrf2 abolished neurological improvement associated with MitoQ, which was related to the overexpression of Keap1 and downregulation of Nrf2, PINK1, Parkin, PHB2 and LC3II. Moreover, knockdown of PHB2 using PHB2 siRNA abolished MitoQ treatment effect in a similar manner as inhibition of Nrf2, which was associated with a decrease of LC3II, but did not affect Keap1, Nrf2, PINK1 or Parkin. In addition, we discovered changes in expression of Keap1/Nrf2 complex in the nuclear extraction samples, as well as PINK1, Parkin, PHB2, LC3II component in the mitochondrial extraction. Taken together, our findings suggest that MitoQ promotes mitophagy, attenuation of oxidative stress-related neuronal death and associated improvement in neurological impairment through Keap1/Nrf2/PHB2 pathway after SAH.
MitoQ was initially discovered as a mitochondria-targeting antioxidant, which when compared with cytoplasm-targeted antioxidant, such as Coenzyme Q10, mitochondria-targeted therapies have a more specific and effective outcome for inhibition of oxidative stress and apoptosis.7 Recently, MitoQ was utilized as an effective treatment in kidney injury9 and received extensive attention due to its neuroprotective effects.7 However, no research has been published to evaluate MitoQ as a treatment modality for stroke. In this study, we have discovered that treatment with MitoQ has the capacity to attenuate oxidative stress and neuronal death, both short-term and long-term after SAH. At same time, we found a reasonable mechanism for how MitoQ and mitophagy improve neurological function. In addition, liposoluble MitoQ can easily bypass the blood brain barrier,7 thereby allowing utilization of intraperitoneal injection, which is more suitable for clinical application. In order to address a previously reported concern regarding a pro-oxidant effect being associated with higher concentrations of MitoQ,19 we used three different dosages in our investigation of treatment for SAH. Our results suggest that 3 mg/kg of MitoQ is most effective for neurological recovery. Although higher concentrations of MitoQ may not result in an increase in its therapeutic effects, it is also important to note that three times our recommended dosage (9mg/kg) did not make the outcome worse than that seen in the SAH+vehicle group. Thus, appropriate dosage of MitoQ is beneficial for treatment of SAH in rats.
Keap1/Nrf2 complex is a well-documented target of the antioxidant signaling pathway.20 During homeostasis Nrf2 remains in the cytoplasm and is degraded by Keap1. After acute stress, the junction between the two complexes is disrupted, allowing Nrf2 to increase rapidly and translocate to the nucleus for transcriptional activities.21 Our present investigation concurred that Keap1 increased gradually, which mediated degradation of Nrf2 in both the cytoplasm and the nucleus. However, upon administration of MitoQ, Keap1 decreased dramatically, and more Nrf2 transmitted into the nucleus for antioxidant-response. Keap1 contains several cysteine residues for specific electrophiles or unique sets to target. It is our hypothesis that MitoQ may bind to Keap1 for degradation and improve the function of Nrf2.
Neuronal autophagy occurs with stroke as well as SAH, therefore mitophagy, a mitochondria-targeted autophagy, is assumed to be involved.22 The PINK1/Parkin-mediated pathway is the most well-known mitophagy pathway, which is associated with the Keap1/Nrf2 complex.9 In physiological conditions, PINK1 is continuously imported into mitochondria for degradation, however, it will accumulate on the outer membrane of mitochondria during oxidative stress.11 At the same time, Parkin is recruited from the cytoplasm to mitochondria for activation, which promotes mitophagy.11 It has been recently discovered that a novel mitochondrial protein called PHB2 serves as a receptor of mitophagy, it binds to LC3II with its LC3-interaction domain, which then results in Parkin induced outer membrane rupture.10 Thus, we hypothesized that PHB2 may serve as a downstream protein of the PINK1/Parkin pathway. In the present investigation, we found that PINK1, Parkin and LC3II were enhanced after SAH, which proved the theory of activation of mitophagy in a SAH model. However, when separating the mitochondrial fraction, activation was not that significant, and mitophagy receptor PHB2 was found to decrease after SAH. It means that mitophagy is insufficient in SAH induction, and that the relationship between PINK1/Parkin pathway and PHB2 is not one of simple positive regulation. Our study revealed an increase in oxidative stress-stimulated deaths of cells after SAH and poor outcomes associated with administration of PHB2 siRNA. It was our aim to prove our hypothesis that stable PHB2, which is normally located in the inner membrane of mitochondria, is exposed during mitophagy to promote clearance of damaged mitochondria in order to encourage survival of other mitochondria. However, if the cells suffer severe damage such as oxidative stress, mitophagy would not be effective and mitochondria would inevitably undergo apoptosis. An increase in mitochondrial death would lead to a dramatic decrease in PHB2.
Oxidative stress and apoptosis are essential factors that play a significant role in early brain injury.2 Studies reveal that mitochondrial dysfunction is strongly associated with ROS generation, the accumulation of which may directly deplete the mitochondrial membrane potential and lead to neuronal death.23 Our investigation demonstrates that MitoQ promotes mitophagy, improves mitochondrial function and thereby attenuates oxidative stress-related apoptosis. It has been previously reported that Romo1 is a marker of mitochondrial oxidative stress.24 The results of our investigation support the association between oxidative stress and Romo1, but also underscore the importance of Bcl-xl, other than Bcl-2, as critical factor in this dynamic relationship.25
A recent study reported the presence of hippocampal injury 24 h after SAH.26 Our study also supports widely distributed neuronal death in the hippocampus, especially in CA1, CA3 and DG regions in long-term SAH rats, while MitoQ has the capacity to reverse this harmful effect. As we only administered one dose of treatment, the long-term improvement seen with MitoQ use is likely due to its effect on EBI.
The results of our investigation support MitoQ as treatment to promote mitophagy in order to attenuate oxidative stress-related neuronal death. However, there are limitations. First, while PHB2 locates in the mitochondria, it has also been reported that it can modulate the mitochondrial fusion protein OPA1 for mitochondrial dynamic regulation.27 Second, because of the limitation of LC3B antibody, we only tested LC3II in Western blot but not with immunofluorescence. In addition, there may be other factors that influence the protective effects of MitoQ on mitophagy, which must be further investigated.
In conclusion, we demonstrated that MitoQ is able to promote mitophagy and attenuate mitochondrial oxidative stress related neuronal apoptosis through Keap1/Nrf2/PHB2 pathway in EBI after SAH, which is associated with improvement in short-term and long-term neurological impairment. MitoQ may therefore be useful as a therapeutic antioxidant against EBI, as well as treatment for delayed neurological deficits after SAH.
Supplementary Material
Sources of Funding
This study was supported by grants from NIH (NS081740 and NS082184) to John H. Zhang, and a grant from the Harbin Medical University scientific research innovation fund (2017LCZX30).
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab 2006;26:1341–1353 [DOI] [PubMed] [Google Scholar]
- 2.Han Y, Zhang T, Su J, Zhao Y, Chenchen W, Li X. Apigenin attenuates oxidative stress and neuronal apoptosis in early brain injury following subarachnoid hemorrhage. J. Clin. Neurosci 2017;40:157–162 [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Wu H, Tang J, Zhang J, Zhang JH. Neurovascular events after subarachnoid hemorrhage: Focusing on subcellular organelles. Acta Neurochir. Suppl 2015;120:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jing CH, Wang L, Liu PP, Wu C, Ruan D, Chen G. Autophagy activation is associated with neuroprotection against apoptosis via a mitochondrial pathway in a rat model of subarachnoid hemorrhage. Neuroscience. 2012;213:144–153 [DOI] [PubMed] [Google Scholar]
- 5.McManus MJ, Murphy MP, Franklin JL. The mitochondria-targeted antioxidant mitoq prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of alzheimer’s disease. J. Neurosci 2011;31:15703–15715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maiti AK, Saha NC, More SS, Panigrahi AK, Paul G. Neuroprotective efficacy of mitochondrial antioxidant mitoq in suppressing peroxynitrite-mediated mitochondrial dysfunction inflicted by lead toxicity in the rat brain. Neurotox. Res 2017;31:358–372 [DOI] [PubMed] [Google Scholar]
- 7.Ham PB, 3rd, Raju R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog. Neurobiol 2017;157:92–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Qiu J, Wang Z, You W, Wu L, Ji C, et al. Dimethylfumarate alleviates early brain injury and secondary cognitive deficits after experimental subarachnoid hemorrhage via activation of keap1-nrf2-are system. J. Neurosurg 2015;123:915–923 [DOI] [PubMed] [Google Scholar]
- 9.Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, et al. The mitochondria-targeted antioxidant mitoq ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via nrf2/pink1. Redox biology. 2017;11:297–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Y, Chiang WC, Sumpter R, Jr., Mishra P, Levine B. Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell. 2017;168:224–238.e210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummins N, Gotz J. Shedding light on mitophagy in neurons: What is the evidence for pink1/parkin mitophagy in vivo? Cell. Mol. Life Sci. 2018;75:1151–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Z, Enkhjargal B, Wu L, Zhou K, Sun C, Hu X, et al. Exendin-4 attenuates neuronal death via glp-1r/pi3k/akt pathway in early brain injury after subarachnoid hemorrhage in rats. Neuropharmacology. 2017;128:142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Q, Vakhmjanin A, Li B, Tang J, Zhang JH. Hyperbaric oxygen therapy fails to reduce hydrocephalus formation following subarachnoid hemorrhage in rats. Med. Gas Res 2014;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherchan P, Lekic T, Suzuki H, Hasegawa Y, Rolland W, Duris K, et al. Minocycline improves functional outcomes, memory deficits, and histopathology after endovascular perforation-induced subarachnoid hemorrhage in rats. J. Neurotrauma 2011;28:2503–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto E, Tamamaki N, Nakamura T, Kataoka K, Tokutomi Y, Dong YF, et al. Excess salt causes cerebral neuronal apoptosis and inflammation in stroke-prone hypertensive rats through angiotensin ii-induced nadph oxidase activation. Stroke. 2008;39:3049–3056 [DOI] [PubMed] [Google Scholar]
- 16.Ullah N, Lee HY, Naseer MI, Ullah I, Suh JW, Kim MO. Nicotinamide inhibits alkylating agent-induced apoptotic neurodegeneration in the developing rat brain. PLoS One. 2011;6:e27093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Yuksel Y, Guven M, Kaymaz B, Sehitoglu MH, Aras AB, Akman T, et al. Effects of aloe vera on spinal cord ischemia-reperfusion injury of rats. J. Invest. Surg 2016;29:389–398 [DOI] [PubMed] [Google Scholar]
- 18.Qiu H, Lizano P, Laure L, Sui X, Rashed E, Park JY, et al. H11 kinase/heat shock protein 22 deletion impairs both nuclear and mitochondrial functions of stat3 and accelerates the transition into heart failure on cardiac overload. Circulation. 2011;124:406–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid. Redox Signal 2007;9:1825–1836 [DOI] [PubMed] [Google Scholar]
- 20.Dodson M, Redmann M, Rajasekaran NS, Darley-Usmar V, Zhang J. Keap1-nrf2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem. J 2015;469:347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bresciani A, Missineo A, Gallo M, Cerretani M, Fezzardi P, Tomei L, et al. Nuclear factor (erythroid-derived 2)-like 2 (nrf2) drug discovery: Biochemical toolbox to develop nrf2 activators by reversible binding of kelch-like ech-associated protein 1 (keap1). Arch. Biochem. Biophys 2017;631:31–41 [DOI] [PubMed] [Google Scholar]
- 22.Cao S, Shrestha S, Li J, Yu X, Chen J, Yan F, et al. Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of nlrp3 inflammasome activation. Sci. Rep 2017;7:2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Shahrani M, Heales S, Hargreaves I, Orford M. Oxidative stress: Mechanistic insights into inherited mitochondrial disorders and parkinson’s disease. J. Clin Med 2017;6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swarnabala S, Gattu M, Perry B, Cho Y, Lockey RF, Kolliputi N. Romo1 links oxidative stress to mitochondrial integrity. J. Cell Commun Signal 2015;9:73–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JJ, Lee SB, Park JK, Yoo YD. Tnf-alpha-induced ros production triggering apoptosis is directly linked to romo1 and bcl-x(l). Cell Death Differ. 2010;17:1420–1434 [DOI] [PubMed] [Google Scholar]
- 26.Veldeman M, Coburn M, Rossaint R, Clusmann H, Nolte K, Kremer B, et al. Xenon reduces neuronal hippocampal damage and alters the pattern of microglial activation after experimental subarachnoid hemorrhage: A randomized controlled animal trial. Front. Neurol 2017;8:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowno M, Watanabe-Susaki K, Ishimine H, Komazaki S, Enomoto K, Seki Y, et al. Prohibitin 2 regulates the proliferation and lineage-specific differentiation of mouse embryonic stem cells in mitochondria. PLoS One. 2014;9:e81552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.