Abstract
Objectives:
To determine the risk and risk factors for mental illness among colorectal cancer (CRC) survivors across short- and long-term follow-up periods.
Methods:
We used the Utah Cancer Registry to identify CRC survivors diagnosed between 1997 and 2013. Mental health diagnoses were available in electronic medical records and statewide facilities data that were linked by the Utah Population Database. CRC survivors were matched to individuals from a general population cohort. The risk of developing a mental illness was compared between cohorts. The association between mental illness and mortality was also analyzed.
Results:
8,961 CRC survivors and 35,897 individuals in a general population cohort were identified. CRC survivors were at increased risk for any mental health diagnosis at 0-2 years (HR 3.70, 95% CI 3.47-3.95), >2-5 years (HR 1.23, 95% CI 1.09-1.38), and >5 years (HR 1.20, 95% CI 1.07-1.36) after cancer diagnosis. CRC survivors were also at increased risk of depressive disorders specifically during the same time periods. At >5 years, CRC survivors still had an increased risk of developing many mental health diagnoses. Factors associated with increased risk of any mental health disorder among CRC survivors included colostomy and Charlson comorbidity index of 1+. There was an increased risk of death for CRC survivors diagnosed with any mental health disorder (HR 2.18, 95% CI 2.02-2.35) and depression (HR 2.10, 95% CI 1.92-2.28).
Conclusions:
Colorectal cancer survivors are at increased risk for mental health disorders in the short- and long-term. Survivors who develop mental health disorders also experience decreased survival.
Keywords: population health, depression, anxiety, survivorship, colostomy
Introduction
Cancer survivorship is associated with significant emotional distress. The knowledge that one might die of the disease, the physical effects of the cancer and its treatments, and the strain that this brings to personal relationships are all documented cancer survivor stressors and can affect quality of life [1, 2]. Colorectal cancer (CRC) presents distinct stressors including the use of colostomy and multimodality treatments with additive side effects in many patients. It is unknown whether these stressors increase the risk of mental illness in CRC survivors.
Five-year survival rates for non-metastatic CRC range from 92% for Stage I to 53% for stage IIIC patients, while 5-year survival for metastatic CRC patients is 11% [3]. Because CRC survivors often live for many years, it is important to understand the relative risks of various mental health problems in both the short- and long-term periods after diagnosis.
While studies have demonstrated a detrimental effect in many quality of life domains [4–8], no study with long-term follow up has studied the specific risks of mental health disorders in CRC survivors. The purpose of this study was to determine whether CRC survivors experience an increased risk of mental health diagnoses compared to a general population cohort, to determine the demographic and clinical risk factors for mental health diagnoses among CRC survivors, and to assess the relationship between having a mental health diagnosis and overall survival among CRC survivors.
Methods
The Utah Population Database (UPDB) links patient data from the Utah Cancer Registry (one of the original NCI Surveillance Epidemiology, and End-Results [SEER] databases); ambulatory surgery and inpatient data for the entire state; electronic medical records from the two largest healthcare providers (The University of Utah Healthcare and Intermountain Healthcare) which serve the majority of residents in the state; voter registration records; residential histories; family lineage records; birth, death, and marriage certificates; Utah driver’s licenses; vital records; and the social security death index (nationwide). Utah is considered to have minimal percent of residents who seek healthcare out of the state based on a report by the National Association of Health Data Organizations that reviewed inter-state exchange of non-resident data for health research and public health purposes [9]. Additionally, according to the US Census Bureau’s state to state migration flow data for 2016, approximately 2.9% of Utahans left the state, thus the out-migration rate is fairly low [10]. Studies using UPDB data have been approved by the University of Utah’s Resource for Genetic and Epidemiologic Research and its Institutional Review Board.
We queried the UPDB to identify individuals diagnosed with CRC (cancers of all segments of the colon and rectum) between 1997 and 2013 who were ≥18 years of age and a resident of the state of Utah at the time of diagnosis. Each CRC patient was matched to up to 5 individuals from a general population cohort also queried from the UPDB by birth year, birth state, follow-up time, and gender. We excluded CRC survivors with an “unknown” (n=412) or “in-situ” (n=630) cancer stage, an “appendix” (n=184) cancer site, and without any matched individuals from the general population cohort (n=14). We excluded individuals with any prior history of mental illness in both cohorts.
The diagnosis of mental illness among CRC survivors and the general population cohort was identified by ICD-9 diagnostic codes and categorized into medically similar diagnostic groupings utilizing Clinical Classifications Software (CSS) for ICD-9-CM. A comprehensive variety of mental illnesses was considered as listed in Table 3. CSS is a categorization scheme that collapses ICD-9 codes into clinically meaningful groups that are more amenable to analyzing and presenting clinical data. Mental illnesses diagnosed before the diagnosis of CRC were not included. Descriptive statistics were performed for both groups. These included birth year, gender, race, socioeconomic factors, baseline body mass index (BMI), baseline Charlson comorbidity index (CCI), follow-up period, age at follow up, family history of any cancer, and family history of colorectal cancer.
Table 3.
Adjusted Hazard Ratios (95% CI) for Mental Illness in Colorectal Cancer Survivors (11-11-18 Update)
| 0-2 Years after Cancer Diagnosis | 2-5 Years after Cancer Diagnosis | 5+ Years after Cancer Diagnosis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Cases | Gen. | Cancer Cases | Gen. | Cancer Cases | Gen. | ||||||||||
| Diagnosis (CCS Code) | n | % | n | % | Adj. HR (95% CI) | n | % | n | % | Adj. HR (95% CI) | n | % | n | % | Adj. HR (95% CI) |
| Mental Illness (5) | 1,517 | 24.8 | 2,340 | 8.7 | 3.70 (3.47, 3.95) | 437 | 12.8 | 1,961 | 11.4 | 1.23 (1.09, 1.38) | 541 | 26.9 | 2,031 | 21.7 | 1.2 (1.07, 1.36) |
| Adjustment disorders (5.1) | 54 | 0.61 | 73 | 0.2 | 3.56 (2.49, 5.09) | 23 | 0.37 | 52 | 0.21 | 1.76 (1.04, 3.00) | 29 | 0.75 | 56 | 0.4 | 1.99 (1.26, 3.12) |
| Anxiety disorders (5.2) | 522 | 6.4 | 875 | 2.7 | 2.84 (2.55, 3.17) | 214 | 3.98 | 743 | 3.39 | 1.24 (1.06, 1.47) | 321 | 9.68 | 991 | 8 | 1.30 (1.13, 1.50) |
| Delirium dementia and cognitive disorders (5.4) | 319 | 3.7 | 871 | 2.5 | 1.99 (1.72, 2.29) | 159 | 2.67 | 722 | 3.06 | 0.81 (0.67, 0.98) | 346 | 9.28 | 955 | 7.07 | 1.20 (1.03, 1.38) |
| Developmental disorders (5.5) | 46 | 0.5 | 62 | 0.17 | 3.78 (2.41, 5.94) | 27 | 0.43 | 76 | 0.31 | 1.37 (0.85, 2.20) | 33 | 0.84 | 90 | 0.64 | 1.22 (0.78, 1.91) |
| Communication disorders (5.5.1) | 36 | 0.4 | 48 | 0.13 | 4.14 (2.49, 6.90) | 24 | 0.38 | 62 | 0.25 | 1.39 (0.82, 2.34) | 30 | 0.76 | 83 | 0.59 | 1.19 (0.75, 1.90) |
| Mood disorders (5.8) | 643 | 8.2 | 1,106 | 3.5 | 2.98 (2.68, 3.32) | 267 | 5.22 | 939 | 4.48 | 1.18 (1.02, 1.37) | 315 | 10.1 | 1,016 | 8.6 | 1.24 (1.07, 1.43) |
| Bipolar disorders (5.8.1) | 47 | 0.53 | 94 | 0.26 | 1.97 (1.50, 2.59) | 30 | 0.49 | 85 | 0.35 | 1.45 (0.95, 2.21) | 33 | 0.85 | 134 | 0.97 | 1.14 (0.82, 1.58) |
| Depressive disorders (5.8.2) | 629 | 7.94 | 1,087 | 3.42 | 2.62 (2.35, 2.93) | 266 | 5.17 | 927 | 4.41 | 1.17 (1.06, 1.29) | 289 | 9.04 | 683 | 4.74 | 1.21 (1.10, 1.34) |
| Personality disorders (5.9) | 14 | 0.16 | 40 | 0.11 | 1.04 (0.48, 2.23) | 3 | 0.05 | 29 | 0.12 | 0.53 (0.15, 1.89) | 6 | 0.15 | 28 | 0.2 | 0.84 (0.32, 2.17) |
| Schizophrenia and other psychotic disorders | 167 | 1.89 | 339 | .96 | 2.8 (2.21, 3.53) | 81 | 1.33 | 244 | 1.01 | 1.28 (0.97, 1.68) | 161 | 4.2 | 392 | 2.85 | 1.28 (1.03, 1.58) |
| Alcohol-related disorders (5.11) | 109 | 1.25 | 169 | 0.48 | 3.55 (2.69, 4.67) | 42 | 0.69 | 141 | 0.59 | 1.2 (0.83, 1.73) | 51 | 1.34 | 182 | 1.32 | 1.23 (0.88, 1.73) |
| Substance-related disorders (5.12) | 141 | 1.6 | 179 | 0.51 | 5.41 (4.04, 7.25) | 81 | 1.33 | 190 | 0.79 | 1.93 (1.45, 2.56) | 81 | 2.12 | 204 | 1.48 | 1.41 (1.06, 1.87) |
| Suicide and intentional self-inflicted injury | 20 | 0.22 | 72 | 0.2 | 1.28 (0.76, 2.16) | 12 | 0.19 | 64 | 0.26 | 0.74 (0.39, 1.43) | 28 | 0.72 | 89 | 0.64 | 1.19 (0.75, 1.89) |
| Screening and history of MH and SA codes (5.14) | 850 | 11.4 | 905 | 2.8 | 5.70 (5.14, 6.32) | 213 | 4.5 | 813 | 3.76 | 1.28 (1.09, 1.51) | 231 | 7.84 | 893 | 7.32 | 1.18 (1.02, 1.36) |
| Codes related to mental health disorders (5.14.1) | 770 | 9.52 | 654 | 1.92 | 5.85 (5.26, 6.50) | 234 | 4.52 | 634 | 2.77 | 1.75 (1.49, 2.06) | 258 | 8.05 | 803 | 6.17 | 1.37 (1.19, 1.58) |
| Codes related to substance-related disorders | 370 | 4.54 | 457 | 1.35 | 4.45 (3.85, 5.15) | 88 | 1.61 | 393 | 1.73 | 1.00 (0.79, 1.28) | 98 | 2.83 | 373 | 2.88 | 0.97 (0.75, 1.26) |
| Miscellaneous mental disorders (5.15) | 106 | 1.22 | 265 | 0.76 | 2.81 (2.11, 3.74) | 71 | 1.18 | 250 | 1.06 | 1.18 (0.89, 1.57) | 79 | 2.1 | 262 | 1.95 | 1.09 (0.83, 1.44) |
| Eating disorders (5.12.2) | 6 | 0.07 | 16 | 0.04 | 1.81 (0.72, 4.56) | 1 | 0.02 | 7 | 0.03 | 1.33 (0.31, 5.81) | 1 | 0.03 | 12 | 0.09 | 0.27 (0.03, 2.28) |
| Psychogenic disorders (5.15.4) | 8 | 0.09 | 15 | 0.04 | 3.28 (1.23, 8.78) | 5 | 0.08 | 24 | 0.1 | 0.38 (0.09, 1.52) | 8 | 0.2 | 27 | 0.19 | 0.83 (0.31, 2.21) |
| Sleep disorders (5.15.6) | 11 | 0.12 | 37 | 0.1 | 1.49 (0.71, 3.12) | 14 | 0.22 | 42 | 0.17 | 1.32 (0.67, 2.58) | 16 | 0.4 | 52 | 0.37 | 1.02 (0.55, 1.88) |
| Somatoform disorders (5.15.7) | 27 | 0.3 | 72 | 0.2 | 1.79 (1.11, 2.89) | 15 | 0.24 | 84 | 0.35 | 0.69 (0.38, 1.25) | 22 | 0.57 | 78 | 0.56 | 1.10 (0.67, 1.80) |
| Mental disorders due to GMCs(5.15.8) | 35 | 0.39 | 43 | 0.12 | 3.72 (2.28, 6.07) | 14 | 0.22 | 44 | 0.18 | 1.08 (0.56, 2.12) | 22 | 0.56 | 60 | 0.43 | 1.36 (0.80, 2.31) |
| Other miscellaneous mental conditions | 6 | 0.07 | 19 | 0.05 | 1.96 (0.69, 5.54) | 4 | 0.06 | 5 | 0.02 | 1.41 (0.08, 23.6) | 3 | 0.08 | 15 | 0.11 | 0.63 (0.13, 2.99) |
Adjusted for baseline BMI, baseline CCI, and race.
Socioeconomic status for CRC survivors and the general population was measured at the county level. County data was taken from the closest year of cancer diagnosis using NCI SEER*Stat software which calculates county data from the US Census available from 1997-2012. For the general population, county data was taken from the year of cancer diagnosis of their matched cancer case. Data included education (% at least Bachelor degree 2000), income (median family income 2000), and poverty (% families below poverty 2000). [11]. We compared the percent insured at the county level of CRC survivors and the general population cohort using a student’s t-test.
BMI values at least one year prior to cancer diagnosis were calculated from the self-reported height and weight from the driver’s license records. For the cancer-free individuals, the most recent BMI value recorded at least one year prior to the cancer diagnosis date of the matched cancer patient was included. Approximately 27% of the cancer survivors cohort and 23% of the general population cohort were missing baseline BMI. For those individuals, BMI values were imputed using FCS discriminant function methods with cancer diagnosis, baseline CCI, and age at CRC cancer diagnosis as covariates. We compared estimates with and without the BMI imputation to assure that inferences did not change due to the imputation.
Chi-square tests were used to compare baseline characteristics between the cancer survivor and general population cohorts. The risk of developing any mental health diagnosis and depressive disorders at 0-2 years, >2-5 years, and >5 years after cancer diagnosis was compared between CRC survivors and the general population cohort using Cox proportional hazards models adjusting for SEER summary stage, age at diagnosis, sex, baseline BMI, and baseline CCI. Crude and adjusted hazard ratios (HR) were obtained using Cox regression analysis. Follow-up time for incident cases of each outcome was calculated separately from the CRC cancer survivor’s initial cancer diagnosis to the date of diagnosis for each outcome, last date of follow-up, or date of death. Individuals who did not have that outcome were censored at the date of last follow-up (last residence date in Utah or death) if that date fell within the analysis time period (0-2 years, >2-5 years or >5 years) or at the end of each analysis time period if their date of last-follow-up exceeded the end of the analysis time period. The proportional hazards assumption was checked for each model. Models that were in violation of the proportional hazards assumption were then tested with flexible parametric survival models with restricted cubic splines. Hazard ratios from the Cox proportional hazard models were reported where there were no changes in inference. As a sensitivity analysis, the risk of developing any mental health diagnosis and depressive disorders over the same time periods was analyzed controlling for county level socioeconomic factors: the percent with at least a Bachelor degree, and the percent living below the poverty level.
Among CRC survivors, risk factors for the development of mental health disorders were examined. The association between mental health diagnoses and survival was analyzed with Cox proportional hazards models. Because the diagnosis of a mental disorder affects survival in a time-dependent manner, the Mantel-Byar test was used for comparison of survival curves. All statistical analyses were done with SAS version 9.4.
Results
A total of 8,961 colorectal cancer survivors were identified along with 35,897 individuals in the general population cohort. Baseline characteristics of each group are listed in Table 1. CRC survivors were slightly older (p<0.001), less likely to be white (p=0.029), more likely to be obese (p<0.001), had a higher incidence of Charlson Comorbidity Index 1+ (p<0.001), and had a higher likelihood of a family history of colorectal cancer (p<0.001). There was no difference in the percent insured at the county level between CRC survivors (77.9%) and the general population cohort (78.0%) (p=0.56). Mean follow up was 5.57 (SD 4.87) years among CRC survivors and 8.16 (SD 4.62) years among the general population cohort. Clinical characteristics of CRC survivors are listed in Table 2.
Table 1.
Demographic Characteristics of Colorectal Cancer Survivors and General Population (11-11-18 Update)
| Colorectal Cancer | General Pop. | ||||
|---|---|---|---|---|---|
| n = 8,961 | % | n = 35,897 | % | p-value | |
| Birth Year | <0.001 | ||||
| Before 1920 | 869 | 9.70 | 3,445 | 9.60 | |
| 1920 - 1929 | 1,741 | 17.5 | 6,286 | 17.5 | |
| 1930 - 1939 | 2,064 | 21.6 | 7,766 | 21.6 | |
| 1940 - 1949 | 1,883 | 21.3 | 7,643 | 21.3 | |
| 1950 - 1959 | 1,459 | 16.3 | 6,385 | 17.8 | |
| 1960 or Later | 945 | 10.6 | 4,368 | 12.2 | |
| Sex | 0.4075 | ||||
| Female | 4,313 | 48.1 | 17,453 | 48.6 | |
| Male | 4,648 | 51.9 | 18,444 | 51.4 | |
| Vital Status | <0.0001 | ||||
| Dead | 4,766 | 53.2 | 8,829 | 24.6 | |
| Alive | 4,195 | 46.8 | 27,068 | 75.4 | |
| Race | 0.0287 | ||||
| White | 8,611 | 96.1 | 33,364 | 96.2 | |
| Black | 62 | 0.70 | 158 | 0.46 | |
| Nat. American | 94 | 1.05 | 372 | 1.07 | |
| Asian | 142 | 1.59 | 634 | 1.83 | |
| Pacific Islander | 49 | 0.55 | 169 | 0.49 | |
| Baseline BMI | <0.0001 | ||||
| < 18.5 kg/m2 | 108 | 1.2 | 465 | 1.30 | |
| 18.5 - 24.9 kg/m2 | 3,099 | 34.6 | 13,836 | 38.5 | |
| 25 - 29.9 kg/m2 | 3,618 | 40.4 | 14,577 | 40.6 | |
| 30+ kg/m2 | 2,136 | 23.8 | 7,019 | 19.6 | |
| Baseline CCI | <0.0001 | ||||
| 0 | 4,783 | 53.4 | 22,839 | 63.6 | |
| ≥1 | 4,178 | 46.6 | 13,058 | 36.4 | |
| Follow-up Period (years) | <0.0001 | ||||
| 0-1 | 1,833 | 20.5 | 287 | 0.80 | |
| 1 - 5 | 3,140 | 35.0 | 10,946 | 30.5 | |
| 5 - 10 | 2,172 | 24.2 | 12,851 | 35.8 | |
| 10 - 15 | 1,310 | 14.6 | 8,119 | 22.6 | |
| 15+ | 506 | 5.68 | 3,694 | 10.3 | |
| Age at Follow-up (years) | <0.0001 | ||||
| <35 | 99 | 1.10 | 293 | 0.80 | |
| 35 - 44 | 299 | 3.30 | 1,016 | 2.80 | |
| 45 - 54 | 761 | 8.50 | 2,756 | 7.70 | |
| 55 - 64 | 1,717 | 19.2 | 6,402 | 17.8 | |
| 65 - 74 | 2,137 | 23.9 | 8,117 | 22.6 | |
| 75 - 84 | 2,347 | 26.2 | 9,242 | 25.8 | |
| 85+ | 1,601 | 17.9 | 8,067 | 22.5 | |
| Missing | 0 | 0 | 4 | 0.01 | |
| Family History Any Cancer | 0.975 | ||||
| Yes | 5,168 | 57.7 | 20,696 | 57.7 | |
| No | 3,793 | 42.3 | 15,201 | 42.4 | |
| Family History Colorectal Cancer | <0.0001 | ||||
| Yes | 2,830 | 31.5 | 9,575 | 26.7 | |
| No | 6,138 | 68.5 | 26,322 | 73.3 | |
| % Bachelors Degree | 0.0041 | ||||
| < 15% | 1,019 | 11.4 | 4,228 | 11.8 | |
| 15-24.9% | 2,283 | 25.5 | 8,549 | 23.8 | |
| ≥ 25% | 5,659 | 31.2 | 23,120 | 64.4 | |
| % Families below Poverty | 0.0387 | ||||
| < 7% | 5,314 | 59.3 | 21,800 | 60.7 | |
| 7-9% | 2,566 | 28.6 | 9,992 | 27.8 | |
| > 9% | 1,081 | 12.1 | 4,105 | 11.4 | |
| Median Family Income | 0.6478 | ||||
| < 50,000 | 1,936 | 21.6 | 7,676 | 21.4 | |
| ≥ 50,000 | 7,052 | 78.4 | 28,221 | 78.6 | |
Table 2.
Clinical Characteristics of Colorectal Cancer Survivors (11-11-18 Update)
| Colorectal Cancer |
||
|---|---|---|
| n = 8,961 | % | |
| Diagnosis Year | ||
| 1997 - 2000 | 1,986 | 22.1 |
| 2001 - 2005 | 2,643 | 29.5 |
| 2006 - 2010 | 2,721 | 30.4 |
| 2011 - 2013 | 1,611 | 18.0 |
| Age at Diagnosis | ||
| <40 | 454 | 5.10 |
| 40-49 | 763 | 8.50 |
| 50-59 | 1,873 | 20.9 |
| 60-69 | 2,152 | 24.0 |
| 70-79 | 2,120 | 23.7 |
| 80+ | 1,599 | 17.8 |
| Cancer Stage | ||
| I | 2,879 | 32.3 |
| II | 1,988 | 22.3 |
| III | 2,318 | 26.0 |
| IV | 1,716 | 19.3 |
| Missing | 60 | 0.70 |
| Procedure (-ostomy) | ||
| None | 8,430 | 94.1 |
| Colostomy only | 455 | 5.10 |
| Ileostomy only | 45 | 0.50 |
| Colostomy and Ileostomy | 31 | 0.40 |
| Cancer Site | ||
| Cecum | 1,583 | 17.7 |
| Ascending Colon | 1,009 | 11.3 |
| Hepatic Flexure | 275 | 3.10 |
| Transverse Colon | 489 | 5.50 |
| Splenic Flexure | 185 | 2.10 |
| Descending Colon | 359 | 4.00 |
| Sigmoid Colon | 2,009 | 22.4 |
| Large Intestine | 208 | 2.30 |
| Rectosigmoid Junction | 614 | 6.90 |
| Rectum | 2,260 | 24.9 |
CRC survivors were at increased risk for any mental health diagnosis at 0-2 years (HR 3.70, 95% CI 3.47-3.95), >2-5 years (HR 1.23, 95% CI 1.09-1.38), and >5 years (HR 1.20, 95% CI 1.07-1.36) from cancer diagnosis (Table 3). The proportion of CRC patients diagnosed with a new mental health diagnosis was 24.8% during 0-2 years, 12.8% during >2-5 years, and 26.9% at >5 years from diagnosis. CRC survivors were also at increased risk of depressive disorders specifically at 0-2 years (HR 2.62, 95% CI 2.35-2.93), >2-5 years (HR 1.17, 95% CI 1.06-1.29), and >5 years (HR 1.21, 95% CI 1.10-1.34). The proportion of CRC patients with depressive disorders was 7.9% during 0-2 years, 5.2% during >2-5 years, and 9.0% during >5 years from cancer diagnosis.
At >5 years after cancer diagnosis, CRC survivors continued to have an increased risk of developing many mental health diagnoses including adjustment disorders (HR 1.99, 95% CI 1.26-3.12); anxiety disorders (HR 1.30, 95% CI 1.13-1.50); delirium, dementia, and other cognitive disorders (HR 1.20, 95% CI 1.03-1.38); and substance related disorders (HR 1.41, 95% CI 1.06-1.87). The sensitivity analysis adjustment for poverty and education level at the county level did not change the risks of developing mental health disorders.
Colostomy was associated with increased risk of depression at 0-2 years, and 2-5 years, while this was true of ileostomy only at 0-2 years (Table 4). Charlson comorbidity index of 1+ was also associated with increased risk of any mental health disorder and depression at >5 years from cancer diagnosis. Other factors associated with increased risk of any mental health disorder in early time periods but not in the >5 year time period included male gender, advanced stage, radiation therapy, and chemotherapy. Female gender was a risk factor for depression in all time periods. Cancer site (colon vs rectum) was not associated with risk of mental health disorders.
Table 4.
Risk factors for Mental Illness and Depression among Colorectal Cancer Survivors (11-11-18 Update)
| 0-2 Years after Cancer Diagnosis | >2-5 Years after Cancer Diagnosis | >5 Years after Cancer Diagnosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mental Illness | Depression | Mental Illness | Depression | Mental Illness | Depression | |||||||
| Factor | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Procedure (-ostomy)1 | ||||||||||||
| None | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Colostomy only | 1.8 | (1.48, 2.18) | 1.99 | (1.52, 2.61) | 1.54 | (0.99, 2.39) | 2.23 | (1.46, 3.4) | 1.55 | (0.98, 2.46) | 1.19 | (0.65, 2.17) |
| Ileostomy only | 1.13 | (0.57, 2.28) | 3.13 | (1.55, 6.31) | 1.17 | (0.29, 4.7) | 1.01 | (0.14, 7.21) | 1.13 | (0.28, 4.57) | 3.52 | (1.3, 9.54) |
| Colostomy or Ileostomy | 1.61 | (0.89, 2.91) | 1.39 | (0.45, 4.33) | 1.4 | (0.45, 4.36) | - | - | 1.89 | (0.84, 4.24) | 1.1 | (0.27, 4.41) |
| Treatment1 | ||||||||||||
| Surgery | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Radiation (RT) | 2.48 | (1.03, 5.98) | 2.74 | (0.68, 11.02) | - | - | - | - | - | - | - | - |
| Chemotherapy | 2.28 | (1.7, 3.06) | 2.04 | (1.27, 3.29) | 4.36 | (1.08, 17.63) | 6.58 | (2.41, 17.94) | - | - | - | - |
| Surgery + RT | 0.93 | (0.53, 1.65) | 1.45 | (0.72, 2.92) | 1.17 | (0.48, 2.84) | 1.7 | (0.63, 4.6) | 0.8 | (0.33, 1.94) | 0.38 | (0.05, 2.69) |
| Surgery + Chemo | 1.34 | (1.17, 1.54) | 1.23 | (0.99, 1.52) | 1.23 | (1.05, 1.59) | 1.2 | (0.87, 1.66) | 0.85 | (0.66, 1.1) | 0.89 | (0.64, 1.25) |
| RT + Chemo | 1.82 | (1.31, 2.53) | 1.4 | (0.76, 2.55) | 3.02 | (1.34, 6.78) | 2.92 | (1.08, 7.89) | 2.06 | (0.51, 8.27) | 1.18 | (0.17, 8.48) |
| Surgery + RT + Chemo | 1.5 | (1.3, 1.74) | 1.73 | (1.39, 2.16) | 1.45 | (1.09, 1.91) | 1.25 | (0.87, 1.8) | 0.71 | (0.52, 0.97) | 1.2 | (0.86, 1.66) |
| No Treatment | 1.38 | (1.04, 1.84) | 1.33 | (0.82, 2.14) | 0.8 | (0.26, 2.5) | - | - | 0.65 | (0.16, 2.63) | 1.83 | (0.68, 4.96) |
| AJCC Stage1 | ||||||||||||
| I | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| II | 1.31 | (1.13, 1.51) | 1.38 | (1.11, 1.72) | 1.15 | (0.9, 1.48) | 1.02 | (0.74, 1.41) | 0.99 | (0.81, 1.21) | 1.03 | (0.79, 1.35) |
| III | 1.34 | (1.17, 1.53) | 1.35 | (1.1, 1.67) | 1.38 | (1.09, 1.74) | 1.39 | (1.03, 1.87) | 0.86 | (0.69, 1.07) | 1.08 | (0.81, 1.43) |
| IV | 1.71 | (1.48, 1.99) | 1.68 | (1.32, 2.13) | 2.63 | (1.84, 3.74) | 2.59 | (1.68, 3.98) | 1.03 | (0.58, 1.85) | 0.92 | (0.43, 1.98) |
| Baseline CCI4 | ||||||||||||
| 0 | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| 1+ | 1.1 | (0.99, 1.22) | 1.53 | (1.31, 1.79) | 1.23 | (1.01, 1.49) | 1.12 | (0.88, 1.44) | 1.67 | (1.39, 2.00) | 1.51 | (1.19, 1.91) |
| Baseline BMI5 | ||||||||||||
| < 18 kg/m^2 | 0.87 | (0.53, 1.44) | 1.12 | (0.58, 2.19) | 1.37 | (0.64, 2.93) | 0.87 | (0.28, 2.75) | 1.08 | (0.51, 2.3) | 0.57 | (0.14, 2.33) |
| 18 - 24.9 kg/m^2 | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| 25 - 29.9 kg/m^2 | 0.93 | (0.83, 1.05) | 0.99 | (0.82, 1.2) | 0.94 | (0.75, 1.17) | 0.92 | (0.69, 1.23) | 1.08 | (0.89, 1.33) | 1 | (0.77, 1.31) |
| > 30 kg/m^2 | 0.98 | (0.85, 1.12) | 1.37 | (1.12, 1.68) | 1.09 | (0.85, 1.4) | 1.26 | (0.92, 1.72) | 1.11 | (0.88, 1.41) | 1.24 | (0.92, 1.67) |
| Cancer Site1 | ||||||||||||
| Cecum | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Ascending Colon | 0.86 | (0.7, 1.05) | 1 | (0.74, 1.36) | 1.29 | (0.88, 1.9) | 1.75 | (1.07, 2.86) | 0.82 | (0.58, 1.15) | 0.86 | (0.55, 1.35) |
| Hepatitic Flexure | 0.9 | (0.64, 1.28) | 0.77 | (0.43, 1.37) | 1.65 | (0.92, 2.97) | 0.8 | (0.28, 2.27) | 0.81 | (0.44, 1.52) | 1.7 | (0.96, 3.02) |
| Transverse Colon | 1.1 | (0.86, 1.41) | 1.45 | (1.03, 2.06) | 1.76 | (1.12, 2.76) | 2.18 | (1.24, 3.84) | 0.79 | (0.48, 1.3) | 0.83 | (0.44, 1.56) |
| Spleneic Flexure | 0.65 | (0.43, 0.99) | 1.07 | (0.61, 1.88) | 1.68 | (0.93, 3.02) | 1.75 | (0.77, 4) | 0.93 | (0.54, 1.61) | 1.03 | (0.49, 2.18) |
| Descending Colon | 0.81 | (0.6, 1.08) | 1.03 | (0.67, 1.59) | 1.28 | (0.77, 2.13) | 1.41 | (0.71, 2.81) | 0.64 | (0.39, 1.04) | 0.49 | (0.24, 1.04) |
| Sigmoid Colon | 0.85 | (0.72, 1) | 0.82 | (0.63, 1.07) | 1.05 | (0.76, 1.47) | 1.33 | (0.86, 2.06) | 0.92 | (0.71, 1.19) | 0.87 | (0.61, 1.23) |
| Large Intestine | 1.24 | (0.84, 1.82) | 1.17 | (0.63, 2.19) | 1.2 | (0.43, 3.3) | 2.13 | (0.75, 6.04) | 0.23 | (0.03, 1.62) | - | - |
| Rectosigmoid Junction | 1.17 | (0.94, 1.45) | 1.27 | (0.91, 1.76) | 1.52 | (0.99, 2.35) | 1.47 | (0.82, 2.64) | 1.02 | (0.7, 1.5) | 0.79 | (0.46, 1.36) |
| Rectum | 1.08 | (0.93, 1.27) | 1.09 | (0.85, 1.39) | 1.43 | (1.04, 1.96) | 1.52 | (0.99, 2.34) | 0.91 | (0.7, 1.18) | 0.91 | (0.64, 1.29) |
| Sex2 | ||||||||||||
| Male | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Female | 0.79 | (0.88, 0.71) | 1.38 | (1.18, 1.62) | 0.99 | (0.82, 1.2) | 1.48 | (1.16, 1.88) | 0.95 | (0.8, 1.13) | 1.15 | (0.92, 1.44) |
| Age at Diagnosis3 | ||||||||||||
| < 65 Years | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| ≥ 65 Years | 1.05 | (0.95, 1.17) | 0.94 | (0.80, 1.11) | 1.42 | (1.17, 1.72) | 0.98 | (0.77, 1.25) | 2.23 | (1.87, 2.67) | 2.16 | (1.81, 2.57) |
| Race7 | ||||||||||||
| Non-White | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| White | 1.04 | (0.79, 1.38) | 0.96 | (0.64, 1.44) | 1.39 | (0.76, 2.54) | 1.18 | (0.56, 2.51) | 0.9 | (0.57, 1.42) | 1.27 | (0.60, 2.69) |
| Rural6 | ||||||||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Yes | 1.05 | (0.91, 1.21) | 0.93 | (0.74, 1.17) | 1.23 | (0.96, 1.60) | 1.1 | (0.79, 1.53) | 1.05 | (0.82, 1.34) | 1.2 | (0.89, 1.63) |
| % Bachelors Degree | ||||||||||||
| < 15% | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| 15-24.9% | 0.9 | (0.75, 1.09) | 1.01 | (0.76, 1.35) | 1.18 | (0.8, 1.74) | 0.97 | (0.6, 1.58) | 1.35 | (0.93, 1.95) | 0.8 | (0.51, 1.27) |
| ≥ 25% | 0.81 | (0.68, 0.96) | 1.02 | (0.79, 1.33) | 0.94 | (0.65, 1.36) | 0.84 | (0.53, 1.33) | 1.02 | (0.72, 1.46) | 0.71 | (0.46, 1.1) |
| % Families below poverty | ||||||||||||
| < 7% | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| 7-9% | 1.1 | (0.98, 1.24) | 1.14 | (0.96, 1.36) | 0.85 | (0.68, 1.06) | 0.77 | (0.57, 1.03) | 1.13 | (0.91, 1.41) | 1.04 | (0.78, 1.39) |
| > 9% | 1.03 | (0.87, 1.21) | 1.05 | (0.82, 1.34) | 0.97 | (0.73, 1.31) | 1.2 | (0.85, 1.69) | 1.04 | (0.8, 1.36) | 0.85 | (0.58, 1.23) |
| Median Family Income | ||||||||||||
| < 50,000 | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| ≥ 50,000 | 1.01 | (0.89, 1.14) | 1.28 | (1.05, 1.58) | 0.74 | (0.60, 0.93) | 0.62 | (0.47, 0.81) | 0.85 | (0.70, 1.03) | 0.69 | (0.54, 0.89) |
Adjusted for Race, Sex, BMI, and Age at Diagnosis
Crude Hazard Ratio
Adjusted for Sex and Cancer Site
Adjusted for Stage at Diagnosis
Adjusted for Sex and Stage at Diagnosis
Adjusted for Baseline BMI and Treatment
Adjusted for Baseline BMI and Baseline CCI
Overall survival was compared between CRC survivors who developed mental health disorders throughout follow up and those who did not. After adjusting for SEER summary stage, diagnosis age, gender, baseline BMI, baseline CCI, and time from cancer diagnosis to the mental health diagnosis, there was an increased risk of death for CRC survivors diagnosed with any mental health disorder (HR 2.18, 95% CI 2.02-2.35) and depression (HR 2.10, 95% CI 1.92-2.28) compared to CRC survivors without (Figure 1).
Figure 1:
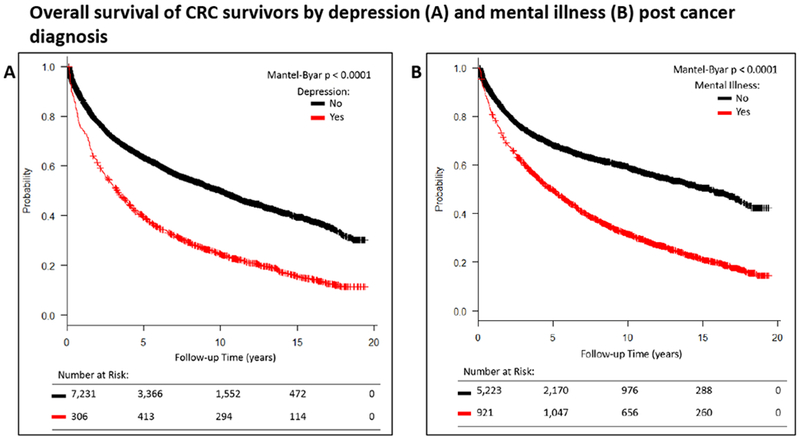
The effect of mental health disorders with any mental illness (A) or depression alone (B) adjusting for SEER summary stage, age at diagnosis, gender, baseline BMI, and baseline CCI and the time lag to diagnosis of a mental health disorder. Because the diagnosis of a mental disorder affects survival in a time-dependent manner, the Mantel-Byar test was used for comparison of survival curves.
Discussion
Quality of life and depression are known to be issues for survivors of CRC. Our analysis reveals a more granular level of detail about mental health disorders in this population, demonstrating an increased risk of any mental health disorder, depressive disorders, and many individual mental health disorders including anxiety, cognitive disorders, and substance abuse among CRC survivors compared to the general population cohort. In addition, we show that these increased risks persist over the short- and long-terms of CRC survivorship. Our results also demonstrate risk factors for mental health disorders including colostomy and medical comorbidities across all time periods, while male gender, advanced stage, radiation therapy, and chemotherapy increased the risk in the early time periods only. Finally, we show that survival for CRC survivors who are diagnosed with depression or any mental illness is lower than for those who are not.
The UPDB has the capability to analyze long-term issues in cancer survivorship due to its long follow-up and the level of detail contained in the linkages which include the EMRs of the majority of the patients in the registry boundaries. Our results are consistent with registry data from Seattle that showed increased rates of depression among surveyed CRC survivors compared to population controls [7]. Interestingly, our results would seem to be in contrast to survey data collected in a retrospective quality of life analysis of patients randomized to cooperative group trials which showed better overall mental health, as well as increased positive body image, and less fatigue when compared to non-CRC controls [6]. Our findings, which are conducted on the population-health level, suggest that patients treated in the community outside of a clinical trial setting may be at higher risk of significant mental health challenges.
The mental illnesses of CRC survivors are concomitant with several documented challenges including fatigue, fear of recurrence, negative body image, sensory neuropathy, and sexual dysfunction [1, 2], as well as low scores in measures of emotional and social functioning, and financial difficulties [5]. Managing these issues without resources is difficult and it is estimated that 40% of CRC survivors lack the confidence to manage illness-related problems [12] greatly increasing the stress on individual mental health.
Many of the general and specific mental health issues associated with CRC survivorship in the present study are novel findings. We found the proportion CRC patients diagnosed with mental health diseases and conditions to be higher than previously reported [13] and new diagnoses persisted through all time periods. Schizophrenia and other psychotic disorders were increased in CRC survivors. This finding runs counter to a long-held suspicion, as well as a recent analysis, that cancer is less likely in patients with schizophrenia [14]. It is possible that in our patient set, a cancer diagnosis brought schizophrenic individuals to medical care that would otherwise have gone undiagnosed. Finally, the finding of increased substance abuse is also novel and points to the need for survivorship caregivers to reinforce the importance of healthy life habits in the face of a long life expectancy for many patients. Our hope is that these findings will add texture to the psychosocial distress and mental health screening during survivorship care as recommended by major medical societies [15, 16].
Some of the diagnoses detected were expected. Adjustment disorders and “mental disorders due to general medical conditions” are understandably common in cancer patients and our findings do not likely represent the full scope of this phenomenon had all patients been formally screened. Adjustment disorders were found in 12% of inpatients who were screened extensively for DSM diagnoses in one study [17]. Delirium, dementia, and cognitive disorders are increased across all time periods, likely relating to CRC survivors’ increased incidence of polypharmacy, inpatient admissions, and metabolic abnormalities compared to population controls.
Colorectal cancer survivors who developed mental illness or depression specifically had increased mortality. There are likely two issues that help explain this association. Firstly, mental illness may lead to higher mortality because of poorer coping and/or compliance, and even suicide. In general, all cancer survivors face an increased risk of death when they score low on a wide variety of health-related quality of life measures [18]. Additionally, depressive disorders are believed to affect the outcomes of chronic diseases [19] and mental disorders are believed to be an underappreciated cause of mortality among the non-cancer population worldwide. Results from a meta-analysis of studies from 29 countries in 6 continents showed a pooled relative risk of mortality among people with mental health disorders was 2.22 (95% CI 2.12-2.33) [20]. Our results add to previous findings by Adams et al [4] that among CRC survivors lower quality of life scores portend increased mortality. Interestingly, in their report it was the physical component scores, and not mental component scores of quality of life that were significantly associated with increased mortality. Our data suggests that the external diagnosis of mental illness is associated with higher mortality rather than self-reported quality of life scores. A second possible explanation is that mental illness is triggered by the stress of cancer recurrence, increased pain, or declining health [21] and thus more prevalent in those nearing the end of life.
Among the risk factors we identified for mental illness among CRC survivors, female gender has been shown to be associated with depression in this patient population in previous studies [22]. Among female CRC survivors, those with enhanced social networks are at decreased risk of mental illness [23]. Other studies have shown lower health-related quality of life [8, 24], negative psychosocial effects [25], and higher rates of depression [26] among patients living with a stoma after CRC treatment. These patients have body image problems, sexual function problems, and poorer social functioning. Bowel dysfunction is also a persistent problem for many survivors that may lead to mental health issues [27]. Chemotherapy and radiation therapy may increase the incidence of mental illness through the increased range of toxicities that patients face, or these treatment types may stand as a proxy for more aggressive or advanced disease. Chemotherapy can affect cognition in colorectal cancer which may herald problems with mental illness [28]. Radiation therapy was a predictor of mental illness in the early time periods and patients undergoing radiation therapy have been shown to have lower quality of life scores when anxiety and depression are at play [29].
This study has some important limitations. Mental health diagnoses were determined by physicians and extracted from EMRs and do not represent patient recorded outcomes (PROs). PROs have been shown to provide distinct prognostic information beyond traditional clinical and demographic variables in cancer survivors [30–32]. It is possible that the increased incidence of mental illness was due to the increased interface with medical care expected among cancer patients compared to the general population. This may be true for mental illnesses that are often diagnosed early in life such as schizophrenia. Our results for 5+ years after cancer diagnosis would be less likely to be subject to surveillance bias, and increased risks were observed in this time period.
Conclusions
This study demonstrates an increase in any mental illness or depression in colorectal cancer survivors. This study is unique in that we surveyed a wide range of mental illnesses. Risk factors for mental illness among CRC survivors include colostomy, female gender (depression), radiation therapy, chemotherapy, advanced age, more advanced disease, and comorbid conditions. Survivors with one or more of these factors may benefit most from regular screening for mental illness. Diagnosis with mental illness is concomitant with increased mortality in this patient population. Further research should be done to determine whether the incidence of mental illness, as studied here, or the duration of time one suffers from a mental illness is more significantly associated with mortality. The need for supportive care among CRC survivors persists throughout all periods of follow up. Mental health screening should be incorporated into surveillance and survivorship visits.
Acknowledgments
Funding:
This work was supported by a grant from the National Cancer Institute at the National Institutes of Health (R21 CA185811), by the Huntsman Cancer Institute, Cancer Control and Population Sciences Program (HCI Cancer Center Support Grant P30CA042014), and the NCRR grant, “Sharing Statewide Health Data for Genetic Research” (R01 RR021746, G. Mineau, PI) with additional support from the Utah State Department of Health and the University of Utah. We thank the Pedigree and Population Resource of the Huntsman Cancer Institute, University of Utah (funded in part by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance and support of the Utah Population Database (UPDB). We thank the University of Utah Center for Clinical and Translational Science (CCTS) (funded by NIH Clinical and Translational Science Awards), the Pedigree and Population Resource, University of Utah Information Technology Services and Biomedical Informatics Core for establishing the Master Subject Index between the Utah Population Database, the University of Utah Health Sciences Center and Intermountain Healthcare.
Footnotes
Conflict of Interest: Shane Lloyd has received fees from Sirtex. The remaining authors declare no conflict of interest.
References
- 1.Den Oudsten BL, et al. , Higher prevalence of sexual dysfunction in colon and rectal cancer survivors compared with the normative population: a population-based study. Eur J Cancer, 2012. 48(17): p. 3161–70. [DOI] [PubMed] [Google Scholar]
- 2.Denlinger CS and Barsevick AM, The challenges of colorectal cancer survivorship. J Natl Compr Canc Netw, 2009. 7(8): p. 883–93; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. Early Detection, Diagnosis, and Staging - Colorectal Cancer 2017. [cited 2017 12/15/17]; Available from: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html.
- 4.Adams SV, Ceballos R, and Newcomb PA, Quality of Life and Mortality of Long-Term Colorectal Cancer Survivors in the Seattle Colorectal Cancer Family Registry. PLoS One, 2016. 11(6): p. e0156534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arndt V, et al. , Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: a population-based study. J Clin Oncol, 2004. 22(23): p. 4829–36. [DOI] [PubMed] [Google Scholar]
- 6.Kunitake H, et al. , Quality of life and symptoms in long-term survivors of colorectal cancer: results from NSABP protocol LTS-01. J Cancer Surviv, 2017. 11(1): p. 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey SD, et al. , Quality of life in long term survivors of colorectal cancer. Am J Gastroenterol, 2002. 97(5): p. 1228–34. [DOI] [PubMed] [Google Scholar]
- 8.Downing A, et al. , Health-related quality of life after colorectal cancer in England: a patient-reported outcomes study of individuals 12 to 36 months after diagnosis. J Clin Oncol, 2015. 33(6): p. 616–24. [DOI] [PubMed] [Google Scholar]
- 9.Next Steps for the Inter-State Exchange of Nonresident Data Between State Health Data Organizations, NAHDO Summary of Findings and Proposed Next Steps. September 2009.
- 10.US Census Bureau (2017). State-to-State Migration Flows. 2017; Available from: https://www.census.gov/data/tables/time-series/demo/geographic-mobility/state-to-state-migration.html.
- 11.U.S. Census Bureau, American Community Survey, 1999, 2005-2009, 2006-2010, 2007-2011, and 2008-2012 Estimates, Tables B15002, B19113, C17002, P57, P117, and P107A.
- 12.Grimmett C, et al. , Colorectal cancer patient’s self-efficacy for managing illness-related problems in the first 2 years after diagnosis, results from the ColoREctal Well-being (CREW) study. J Cancer Surviv, 2017. 11(5): p. 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang AY and Cooper GS, Recognition of Depression and Anxiety among Elderly Colorectal Cancer Patients. Nurs Res Pract, 2010. 2010: p. 693961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen M, Dembling B, and Schorling J, The association between schizophrenia and cancer: a population-based mortality study. Schizophr Res, 2002. 57(2-3): p. 139–46. [DOI] [PubMed] [Google Scholar]
- 15.Pirl WF, et al. , Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer, 2014. 120(19): p. 2946–54. [DOI] [PubMed] [Google Scholar]
- 16.Andersen BL, et al. , Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol, 2014. 32(15): p. 1605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derogatis LR, et al. , The prevalence of psychiatric disorders among cancer patients. JAMA, 1983. 249(6): p. 751–7. [DOI] [PubMed] [Google Scholar]
- 18.Montazeri A, Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes, 2009. 7: p. 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman DP, Perry GS, and Strine TW, The vital link between chronic disease and depressive disorders. Prev Chronic Dis, 2005. 2(1): p. A14. [PMC free article] [PubMed] [Google Scholar]
- 20.Walker ER, McGee RE, and Druss BG, Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry, 2015. 72(4): p. 334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiegel D, Sands S, and Koopman C, Pain and depression in patients with cancer. Cancer, 1994. 74(9): p. 2570–8. [DOI] [PubMed] [Google Scholar]
- 22.Kurtz ME, et al. , Predictors of depressive symptomatology of geriatric patients with colorectal cancer: a longitudinal view. Support Care Cancer, 2002. 10(6): p. 494–501. [DOI] [PubMed] [Google Scholar]
- 23.Sapp AL, et al. , Social networks and quality of life among female long-term colorectal cancer survivors. Cancer, 2003. 98(8): p. 1749–58. [DOI] [PubMed] [Google Scholar]
- 24.Hart TL, et al. , Symptom Severity and Quality of Life Among Long-term Colorectal Cancer Survivors Compared With Matched Control Subjects: A Population-Based Study. Dis Colon Rectum, 2018. 61(3): p. 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMullen CK, et al. , Greatest Challenges of Rectal Cancer Survivors: Results of a Population-Based Survey. Dis Colon Rectum, 2016. 59(11): p. 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross L, et al. , Quality of life of Danish colorectal cancer patients with and without a stoma. Support Care Cancer, 2007. 15(5): p. 505–13. [DOI] [PubMed] [Google Scholar]
- 27.Alavi M, et al. , Predictors of Bowel Function in Long-term Rectal Cancer Survivors with Anastomosis. Ann Surg Oncol, 2017. 24(12): p. 3596–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodgson KD, et al. , A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev, 2013. 39(3): p. 297–304. [DOI] [PubMed] [Google Scholar]
- 29.Frick E, Tyroller M, and Panzer M, Anxiety, depression and quality of life of cancer patients undergoing radiation therapy: a cross-sectional study in a community hospital outpatient centre. Eur J Cancer Care (Engl), 2007. 16(2): p. 130–6. [DOI] [PubMed] [Google Scholar]
- 30.Gotay CC, et al. , The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol, 2008. 26(8): p. 1355–63. [DOI] [PubMed] [Google Scholar]
- 31.Efficace F, et al. , Does a patient’s self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer? Eur J Cancer, 2006. 42(1): p. 42–9. [DOI] [PubMed] [Google Scholar]
- 32.Fournier E, et al. , Health-related quality of life is a prognostic factor for survival in older patients after colorectal cancer diagnosis: A population-based study. Dig Liver Dis, 2016. 48(1): p. 87–93. [DOI] [PubMed] [Google Scholar]


