Abstract
Individuals with posttraumatic stress disorder (PTSD) show deficits in recruiting neural regions associated with cognitive control. In contrast, trauma exposed individuals (TEIs) show increased recruitment of these regions. While many individuals who experience a trauma exhibit some PTSD symptoms, relatively few develop PTSD. Despite this, no work has examined the relationship between changes in PTSD symptoms and changes in neural functioning in TEIs longitudinally. This study examined the neural correlates of changing PTSD symptom levels in TEIs. Twenty-one military service members completed the affective stroop task while undergoing fMRI within 2 months of returning from deployment and a second scan 6–12 months later. Participants with PTSD or depression at baseline were excluded. PTSD symptom improvement was associated with greater increase in response to incongruent relative to congruent negative stimuli in dorsal anterior cingulate cortex and inferior frontal gyrus/anterior insula and increased BOLD response over time to emotional relative to neutral stimuli in inferior parietal cortex. Improvement in PTSD symptoms were not associated with changes in amygdala responsiveness to emotional stimuli. In short, the current data indicate that TEIs who become more able to recruit regions implicated in cognitive control show greater reductions in PTSD symptom levels.
Keywords: Posttraumatic stress disorder, Affective stroop, Emotion regulation, Longitudinal, Attentional control
1. Introduction
Posttraumatic stress disorder (PTSD) impacts approximately 15 million individuals each year in the United States alone (Kilpatrick et al., 2013). Neuroimaging work has begun to describe at least some of the pathophysiology underpinning PTSD. Individuals with PTSD show an increased amygdala response to emotional stimuli (Blair et al., 2013; El Khoury-Malhame et al., 2011; Hayes et al., 2012; Rauch et al., 2006; Shin and Liberzon, 2010), as well as disrupted recruitment of regions implicated in cognitive control, including emotion regulation (Blair et al., 2013; New et al., 2009; Pannu Hayes et al., 2009; Rabinak et al., 2014; Xiong et al., 2013) and response control (Offringa et al., 2013; Shin et al., 2001). PTSD treatment studies using emotionally evocative tasks suggest that successful intervention is associated with i) reduced amygdala response to emotional stimuli (Aupperle et al., 2013; Felmingham et al., 2007; Peres et al., 2011; Roy et al., 2010) and ii) increased recruitment of regions implicated in cognitive control including medial prefrontal (Peres et al., 2011; Roy et al., 2010), dorsal anterior cingulate cortex (dACC; Roy et al., 2010) and lateral prefrontal cortex (Lansing et al., 2005; Roy et al., 2010). It has been suggested that PTSD, at least with respect to some symptoms, is the result of hyperresponsivity to emotional information that interferes with cognitive control processes, including emotional regulation (Blair et al., 2013; New et al., 2009) and response control (Shin et al., 2001).
Critically, however, most individuals exposed to traumatic events do not go on to develop PTSD (Kilpatrick et al., 2013). Indeed, while reporting some symptoms immediately following a trauma, only about 20% of individuals go on to develop PTSD (McFarlane et al., 1997; Osofsky et al., 2015; Shalev and Yehuda, 1998; Yehuda et al., 1998). Despite this, relatively little is known about the neural correlates of PTSD symptoms in trauma-exposed individuals who do not go on to develop PTSD (TEIs). In some respects, TEIs appear to simply show a less severe response to trauma than patients with PTSD. When passively viewing emotional stimuli, TEIs show reduced amygdala response to emotional stimuli relative to patients with PTSD (Felmingham et al., 2010). Moreover, within TEIs, BOLD response in the amygdala to emotional stimuli has been reported to increase with PTSD symptom severity during passive viewing (White et al., 2015). TEIs do show greater amygdala response to emotional stimuli relative to healthy controls (van Wingen et al., 2011).
However, during paradigms with cognitive control demands, TEIs show increased response in some regions relative to PTSD patients. TEIs, relative to patients with PTSD, show increased recruitment of dorsal (New et al., 2009; Rabinak et al., 2014) and lateral prefrontal cortex (Morey et al., 2009; New et al., 2009) during cognitive reappraisal and increased recruitment of lateral frontal and parietal cortices during an automatic emotion regulation task (Blair et al., 2013; White et al., 2015). TEIs, compared to PTSD patients, also show increased activation in dACC during incongruent relative to congruent trials (Bremner et al., 2004; Offringa et al., 2013; Shin et al., 2001) and when inhibiting a primed response (Stevens et al., 2016). Indeed, TEI’s have been reported to show heightened recruitment of these regions not only relative to patients with PTSD but also comparison healthy adults (Blair et al., 2013; New et al., 2009). Notably, successful treatment of PTSD is associated with an increased in activity within medial frontal, lateral prefrontal and parietal cortices (as well as decreased amygdala responsiveness; Peres et al., 2007). Thus, while TEIs show less amygdala response to emotional stimuli than PTSD patients, TEIs show greater recruitment of regions implicated in cognitive control completing paradigms with task demands.
The relationship between change in neural functioning within cognitive control regions and change in PTSD symptom severity has not, however, been examined in the literature. In a series of studies, van Wingen and colleagues (2011) reported increased post-relative to pre-combat deployment amygdala and insula response, as well as disrupted functional connectivity between amygdala and dACC and lateral prefrontal cortex, to emotional stimuli in military personnel relative to military controls. These effects, however, were not directly related to PTSD symptomology and, as they were largely absent at long-term follow-up, may have been related to combat as opposed to PTSD symptoms.
The current study examined TEIs, recently returned from military deployment to a war zone, during fMRI. Participants were scanned at baseline and then again 6–12 months later. Given that the severity of PTSD symptoms fluctuates over time (e.g. Hussain et al., 2013), it was expected that some individuals would show improving and others worsening levels of PTSD symptoms. The goal of the current study was to leverage this naturally occurring variability in order to determine the neural correlates of changing PTSD symptom levels using the affect stroop task. The affective stroop paradigm includes “task trials,” where participants see a positive, negative or neutrally valenced distractor image, followed by a numerical display, followed by another distractor image (Blair et al., 2007). During task trials, participants must indicate how many digits are presented. During view trials, the distractor images bracket a blank screen and no participant response is required. During task trials, healthy participants show increased activation of cognitive control regions and do not show increased amygdala response to the emotional distractors, which is presumed to be a function of implicit emotion regulation (Blair et al., 2007). However, during emotional view trials, when no emotion regulation is required to complete the task, healthy participants show greater amygdala response to emotional stimuli (Blair et al., 2007). The affective stroop task has been used several times to identify neural dysfunction within regions implicated in cognitive control in both patients with PTSD and TEIs (Blair et al., 2013; White et al., 2015). We made two predictions: First, given previous research, we predicted reductions in PTSD symptom levels would be associated with reductions in amygdala responsiveness to emotional stimuli. Second, we predicted reductions in PTSD symptom levels would be associated with increased activation in regions involved in cognitive control (lateral prefrontal cortex, parietal cortex, dACC, dlPFC, aIC/iFG).
2. Methods
2.1. Participants
Study participants were 21 military service members (4 female) who were initially scanned within 8 weeks of returning from at least a 90-day deployment to either Iraq or Afghanistan and for a second time between 6 and 12 months later. The data from the first scan for these participants has been reported previously (White et al., 2015). Participants were 17 European-Americans (80.95%), 1 African-American (4.76%), 2 Asian-American/Pacific Islander (9.52%) and 1 Hispanic American 4.76%) with an average age of 30.64 (21.0–44.8 years, standard deviation = 7.72). All participants were exposed to relatively severe traumatic experiences that met Criterion A of PTSD (according to DSM-IV or DSM-5). The most commonly reported type index trauma was being on a base that was being attacked (e.g. mortar or rocket fire, n = 10), followed by being in combat (e.g. firefights, hit by improvised explosive device [IED], n = 6), witnessing combat related violence (e.g. watching truck in convoy be hit by an IED, n = 3) and dealing with comrades being either killed-in-action or missing-in-action (n = 2). Participants endorsed a moderate number of PTSD symptoms at the time of the initial scan [mean PTSD checklist-military version = 25.76, standard deviation = 6.89, range= 17–48] and at the follow-up scan [mean PTSD checklist-military version = 23.24, standard deviation = 6.90, range = 17–44]. Participants mostly showed modest change over time [mean PTSD checklist-military version change = 2.52, standard deviation=4.87, range = −7 to 11]. Exclusion criteria included Glasgow Coma Scale scores of less than 14, any loss of consciousness greater than 60 min, or post-concussive syndrome. Participants were also excluded if they met criteria for PTSD, major depression or active/past psychosis at the time of enrollment as determined by a clinician. No participants reported receiving either psychotherapeutic or pharmacological treatment for mental health problems between the baseline and follow-up scans. No participants met criteria for either PTSD or major depressive disorder at follow-up. Participants were also excluded if they were taking calcium channel or alpha-blockers. The Uniformed Services University of the Health Sciences and Walter Reed National Military Medical Center Institutional Review Boards approved this study.
2.2. Study measures
2.2.1. The affective stroop task
The affective stroop task used here was an adapted version of a paradigm described previously (Blair et al., 2007). The baseline scan data for the current participants were included in the report of a larger sample (White et al., 2015). Each trial began with a fixation cross centrally presented for 400 ms (see Fig. 1). This was followed by a 400 ms image presentation. In view trials, participants were then presented with a blank screen for 400 ms. During task trials, a numerical display was presented for 400 ms. For both view and task trials, there was then a second 400 ms period when the first image was presented again. This was followed by a blank screen for 1300 ms. The subjects had to determine the quantity of digits in the numerical display. That is, how many of the numbers were displayed, not the actual value of the numbers. For congruent trials, the quantity of numbers displayed was the same as the number value (e.g., three 3 s and four 4 s). For incongruent trials, the quantity of numbers displayed did not equal the number values (e.g., three 2 s and four 3 s). Participants could respond at any time from the presentation of the numerical display until the end of the blank screen. View trials required no response.
Fig. 1.
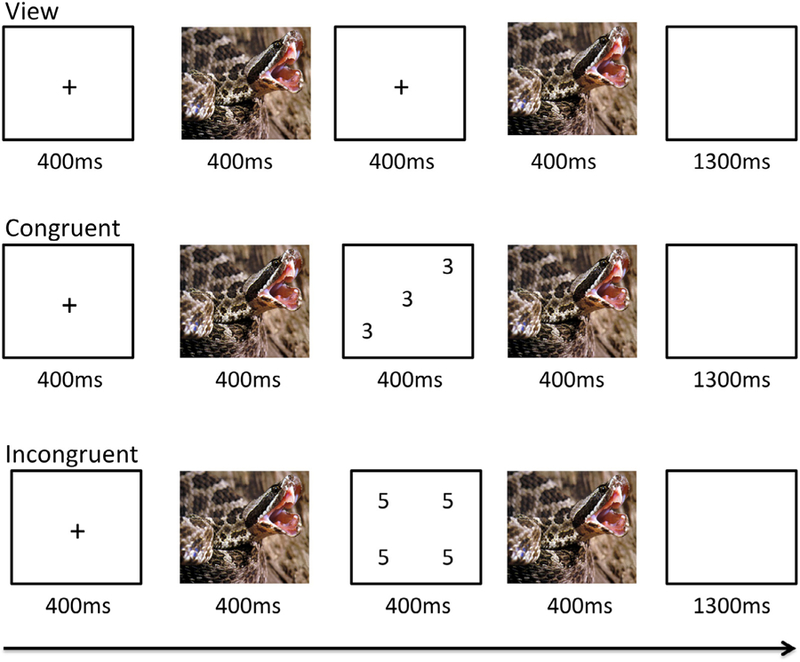
The affective stroop task Participants were exposed to neutral, positive or negative images followed either by a fixation cross (view trials), a screen showing digits congruent in their value and quantity (e.g. three 3 s; congruent trials) or a screening digits incongruent in their value and quantity (e.g. four 5 s; incongruent trials). The fixation/numeric images were followed by a second exposure to the neutral positive or negative image. A negative image is depicted here.
The individual numerical stimuli consisted of three, four, five, or six 3 s, 4 s, 5 s, or 6 s randomly presented within a 9-point grid (see Fig. 1). The emotional stimuli consisted of 32 positive, 32 negative, and 32 neutral pictures selected from the International Affective Picture System (Lang et al., 2005). The normative mean valence and arousal values on a 9-point scale were respectively 3.35 ± 0.77 and 5.97 ± 1.07 for negative pictures, 7.43 ± 0.52 and 4.99 ± 1.10 for positive pictures, and 4.87 ± 0.28 and 2.66 ± 0.54 for neutral pictures. There were nine trial types: view, congruent, and incongruent trials involving negative, positive, and neutral emotional stimuli. Subjects completed 2 runs each consisting of 16 trials of each of the nine trial classes and 48 fixation-point trials to generate a baseline. Each image was presented once in a congruent trial, once in an incongruent trial and once in a view trial. Each image appeared only in one run. There were 32 trials of each of the nine conditions presented and 96 fixation-point trials. Trials were randomized within each run for each participant and counterbalanced between participants.
2.2.2. Post-traumatic stress disorder checklist-military version (PCL; Weathers, et al. 1993)
The PCL is a self-administered screen for PTSD. The PCL has demonstrated good internal consistency (Cronbach’s alpha = 0.96) and convergent validity with both self-and clinician-report measures (Forbes et al., 2001; Weathers et al., 1993). The military version has minor differences in wording to focus more specifically on military experiences, compared to the civilian version. For the current study, a change score (PCL scores at baseline minus PCL scores at follow-up) was used to operationalize change in PTSD symptomology over time. Mean PCL scores at baseline were 25.76 (range = 17 to 48; standard deviation = 6.89). Mean PCL scores at follow-up were 23.24 (range = 17 to 44; standard deviation = 6.90). Average change in PCL scores was 2.52 (range = −8 to 11; standard deviation = 4.87). Change scores were entered into the model as a covariate-of-interest in order to continuously examine the relationship between change in neural responses and changes in PTSD symptomology over time.
2.3. MRI parameters
Participants were scanned using a 3T Siemens Magnetom scanner. A total of 166 functional images per run were taken with a gradient echo planar imaging (EPI) sequence (repetition time = 2900 ms; echo time = 27 ms; 64*64 matrix; 90° flip angle; 22 cm field of view). Whole-brain coverage was obtained with 44 axial slices (thickness, 2.5 mm; .5 mm spacing; in-plane resolution, 3.44*3.44 mm). A high-resolution anatomical scan (3-dimensional spoiled gradient recalled acquisition in a steady state; repetition time=2530 ms; echo time=3.03 ms; 25.6 cm field of view; 7° flip angle; 176 axial slices; thickness, 1.0 mm; 256*256 matrix) in register with the EPI data set was obtained covering the whole brain.
2.4. Imaging data preprocessing
Data were analyzed within the general linear model framework using analysis of functional neuroimages (AFNI; Cox, 1996). Each scan series began after equilibrium magnetization was reached through a Siemens automated process. Functional images from the time series were despiked, slice-time corrected, motion corrected and spatially smoothed with a 6mm full-width half-maximum gaussian filter. The time series were normalized by dividing the signal intensity of a voxel at each point by the mean signal intensity of that voxel for each run and multiplying the result by 100. Resultant regression coefficients represented a percentage of signal change from the mean. The participants’ anatomical scans were then individually registered to the Talairach and Tournoux atlas (Talairach and Tournoux, 1988). The individuals’ functional EPI data were then registered to their Talairach anatomical scan.
Following this, nine regressors were generated and included in the model: (i) negative congruent, (ii) negative incongruent, (iii) negative view, (iv) neutral congruent, (v) neutral incongruent, (vi) neutral view, (vii) positive congruent, (viii) positive incongruent and (ix) positive view. There was also a regressor for incorrect trials. All regressors were created by convolving the train of stimulus events with a gamma variate hemodynamic response function to account for the slow hemodynamic response. Linear regression modeling was performed using the 9 regressors described above plus regressors to model a first-order baseline drift function. This produced a β coefficient and associated t statistic for each voxel and regressor.
2.5. fMRI data analysis
The group analysis of the BOLD data was then performed on the change regression coefficients from individual subject analyses. These were created by, at the voxel level, subtracting BOLD at follow-up baseline scanning from BOLD at baseline scanning. These change scores were examined with a 3 (emotion; negative, positive, neutral) × 3 (condition; congruent, incongruent, view) repeated measures analysis of covariance (ANCOVA) with PCL change score [baseline minus follow-up] as a covariate-of-interest within specific regions of interest (ROI). PCL change scores were mean centered before being entered into the ANCOVA. ROI included the amygdala, lateral frontal cortex, inferior parietal cortex, dACC and aIC/iFG. The amygdala was interrogated using an anatomically defined mask (Eickhoff-Zilles Architectonic Atlas: 50% probability mask; Amunts et al., 2005). Bilateral ROIs were defined by 15 mm spheres centered on coordinates from Blair et al (2007) in lateral frontal cortex (x,y,z = +/−40, 38, 33), inferior parietal cortex (x,y,z= ± 61, −26, 19), dACC (x,y,z = ± 8, 16, 28), dlPFC (x,y,z = ± 36, 36, −6) and aIC/iFG (x,y,z = ± 37, 21, 0). Blair et al (2007) utilized the same paradigm as the current study, but in a sample of healthy adults.
The AFNI 3dClustSim autocorrelation function (-acf) was used to generate small-volume corrected (SVC) extant thresholds for the ROIs for a corrected p value of 0.05. Due to its small size, the amygdala SVC was established at an initial p value of 0.02 for the amygdala which yielded an extent threshold of 7 voxels. The SVCs for the remaining ROIs were established at an initial p value of 0.005 which yielded an extent threshold of 8 voxels (range k = 7.9 to 8.1). Furthermore, in order to facilitate future meta-analytic work, effect sizes for all clusters/follow-up t-tests are reported. Post hoc analyses of significant main effects and interactions were assessed with planned t-tests within SPSS 22.0 (IBM, 2012). Key interactions and main effects are presented below. Remaining effects and whole-brain results are reported in the Supplemental Results.
3. Results
3.1. Behavioral results
Initially, we examined the impact of emotion and task condition on changes in accuracy and response latencies across the sample over time, through two 3 (emotion; negative, positive, neutral) × 2 (condition; congruent, incongruent) repeated measures ANOVAs. With respect to accuracy, there was a significant PCL change score-by-task condition interaction effect [F(1,19)=5.73, p= 0.027]. Improvement in PTSD symptoms was associated with greater increases in accuracy in congruent relative to incongruent trials [Steiger’s Z= 2.32, p= 0.02]. With respect to change in response latencies over time for correct responses, no significant main effects or interactions were observed [F (1,19) = 0.16 to 1.52, p > 0.23].
3.2. fMRI results
3.2.1. PCL change score-by-emotion-by-task condition interaction
Significant PCL Change Score-by-Emotion-by-Task Condition interactions were observed in dACC and left iFG/aIC (Table 1, Fig. 2). In order to break down the interaction, the group was divided into those showing above average change (average change = 2.5, n = 12) and those showing below average change (n = 9). In both regions, individuals showing above average change showed a greater increase in response to incongruent relative to congruent negative stimuli [dACC: t (20) = 2.99, p = 0.008; iFG/aIC: t(20) = 3.51, p = 0.002]. However, there was not a significant change in response to neutral [dACC: t (20) = 0.68, p = 0.504; iFG/aIC: t(20) = 1.56, p= 0.129] or positive stimuli [dACC: t(20) = 0.82, p = 0.422; iFG/aIC: t(20) = 0.17, p = 0.864]. A significant interaction was not observed in dlPFC.
Table 1.
Brain regions showing significant activations within regions of interest in hypothesized contrasts.
| Coordinates of peak activationb | ||||||||
|---|---|---|---|---|---|---|---|---|
| Regiona | Left/Right | BA | x | y | z | F | p | Voxels |
| PCL change score-by-emotion-by-task condition dorsal anterior cingulate cortex | Right | 32 | 1.5 | 25.5 | 26.5 | 5.825 | 0.0004 | 14 |
| anterior insula cortex/inferior frontal gyrus | Left | 13 | −34.5 | 10.5 | −6.5 | 7.332 | <0.0001 | 18 |
| PCL change score-by-emotion inferior parietal cortex | Left | 40 | −61.5 | −31.5 | 26.5 | 14.56 | <0.0001 | 38 |
According to the Talairach Daemon Atlas (http://www.nitrc.org/projects/tal-daemon/).
Based on the Tournoux & Talairach standard brain template, BA = Brodmann’s area
PCL = posttraumatic stress disorder checklist-military version
Fig. 2.
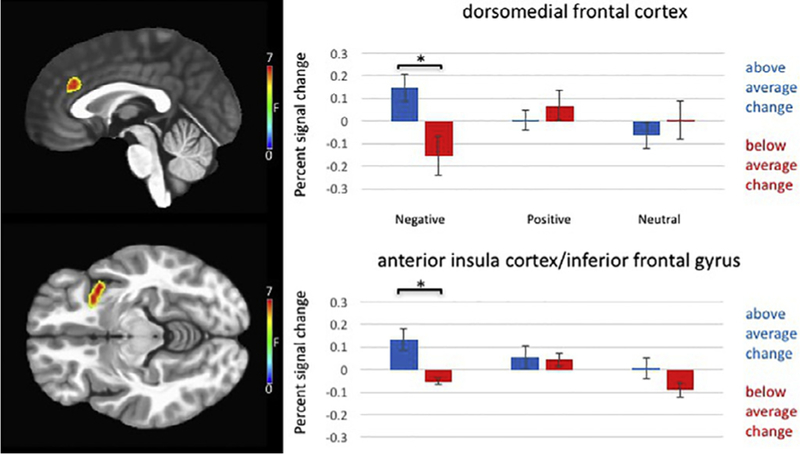
PTSD checklist change score-by-emotion-by-task condition interaction in dorsomedial frontal cortex and anterior insula cortex/inferior frontal Gyrus in 21 trauma-exposed combat veterans.
Participants showing above average improvement in PTSD symptoms also showed greater difference in activation to incongruent relative to congruent negative trials over time in dorsomedial frontal cortex and anterior insula cortex/inferior frontal gyrus.
3.2.2. PCL change score-by-emotion interaction
A significant PCL change score-by-emotion interaction was observed in left inferior parietal cortex (Table 1). Improvement in PTSD symptoms was significantly associated with increased BOLD response over time to negative relative to neutral [Steiger’s Z= 2.89, p= 0.004] and positive relative to neutral stimuli [Steiger’s Z= 2.62, p= 0.008]. Improvement in PTSD symptoms was not significantly associated with increased BOLD response over time to negative relative to positive stimuli [Steiger’s Z = 0.05, p = 0.958]. A significant interaction was not observed in lateral frontal cortex or amygdala ROIs.
3.2.3. PCL change score-by-task condition interaction
No significant interactions were observed in this contrast.
4. Discussion
The goal of the current study was to determine the neural correlates of changing PTSD symptom levels using the affect stroop task in those with recent exposure to combat stress. Consistent with predictions, greater improvement in PTSD symptoms was associated with greater activation in regions involved in cognitive control. TEIs experiencing above average PTSD symptom improvement showed a greater increase in response to incongruent relative to congruent negative stimuli in dACC and iFG/aIC. Furthermore, improvement in PTSD symptoms was significantly associated with increased BOLD response over time to emotional relative to neutral stimuli in inferior parietal cortex. Despite finding evidence of increased activation in regions involved in cognitive control, improvement in PTSD symptoms was not associated with changes in amygdala responsiveness to emotional stimuli.
Consistent with predictions, reductions in PTSD symptom levels were associated with increased recruitment of elements of the frontoparietal cognitive control network during emotional relative to neutral trials (inferior parietal cortex) and during incongruent relative to congruent negative trials (dACC, iFG/aIC). Reductions in PTSD symptoms were also associated with improved response accuracy in congruent trials. Cognitive control is thought to be generated via a fronto-parietal network (including inferior parietal cortex, dACC and iFG/aIC) involved in top-down modulatory processes (Cole and Schneider, 2007; Cromheeke and Mueller, 2014). Selective attention of visual stimuli is achieved when task relevant stimuli (here, numerical displays) are primed by top-down control regions and are represented more strongly in temporal cortex than task-irrelevant stimuli (here, images; Pessoa and Ungerleider, 2004). Top-down priming by this fronto-parietal network is also implicated in priming working memory representations of task relevant stimuli during response selection/response control (Gazzaley and Nobre, 2012). It has been argued that one way in which emotion regulation occurs is when non-emotional stimuli/stimulus features are primed at the expense of emotional features (Ochsner and Gross, 2005). This process inhibits the representation of emotional informational and is associated with reduced representation of emotion in amygdala (Blair et al., 2007; Mitchell et al., 2007). Increased activation of top-down control regions has been associated with improvement in PTSD symptoms in previous work (Offringa et al., 2013; Peres et al., 2011; Roy et al., 2010; Shin et al., 2001). It is possible that the improved behavioral performance and improvement in PTSD symptoms associated with increased recruitment of frontoparietal cognitive control network regions reflects increased effort during cognitive control in TEIs. However, it is possible that these regions are involved in some other form of processing during this task and this is reflected in the increased BOLD response observed over time. Future work will need to examine this possibility directly.
Notably, no relationship between change in PTSD symptoms and change in amygdala response was observed. This is inconsistent with some previous work, (Felmingham et al., 2010; White et al., 2015). However, Felmingham and colleagues (2010) used a passive, backward-masked face viewing task with no task demands. This task, therefore, required no emotion regulation. Furthermore, White et al (2015) found amygdala response only at a very lenient threshold (p = 0.05 uncorrected). The absence of an amygdala finding in the current study may be a result of type II error, though it should be noted that a main effect of emotion was observed within amygdala (see Supplemental Results). Alternatively, it is possible that change in amygdala response over time is not related to changes in PTSD symptom reports. Future work will need to examine these possibilities.
These data need to be interpreted in light of five caveats. First, the sample size was modest, though larger than most studies involving pre/post-scanning of TEIs (Aupperle et al., 2013; Laugharne et al., 2016; Lindauer et al., 2008; MacNamara et al., 2016; Peres et al., 2007; N ≤ 20). Second, while a cut-off score of 50 on the PCL has been shown to distinguish those likely to have a diagnosis of PTSD from those who likely do not (Forbes et al., 2001; Weathers et al., 1993), the psychometric validity of the PCL as a continuous measure of PSTD symptom severity has not been established. Third, while initial scans all occurred within 8 weeks of returning from deployment, deployment length varied between participants and the exact timing of traumatic events is unknown. Therefore, issues of timing of PTSD symptom onset relative to the initial trauma cannot be addressed in the current study. Fourth, the range for the timing of follow-up scans included in this analysis was rather large (6–12 months from the baseline scan) due to the military obligations of the active-duty military personnel participating in the study. Future work should examine a narrower follow-up window. Fifth, the same task was used at baseline and follow-up. While this allowed for the direct comparison of task-performance at both time points, the possibility that practice effects influenced the current results cannot be ruled out (anonymous reviewer suggestion). The current study focuses on the brain regions that change with symptom change; it is unlikely that practice effects would be selectively associated with brain regions that change with symptom change.
The current study revealed a significant relationship between the degree of improvement in PTSD symptoms over a 6–12 month period and the degree of increased activation within cognitive control regions in a population of TEIs. Previous work (e.g. Blair et al., 2013; New et al., 2009) suggests that TEIs who have not developed PTSD (trauma controls) more strongly recruit regions involved in cognitive control relative to both PTSD patients and healthy controls. The current data indicate that TEIs who become more able to recruit regions implicated in cognitive control show greater reductions in PTSD symptom levels. Of course, these data cannot address whether an increased recruitment of cognitive control regions causes a reduction in PTSD symptoms or whether a reduction in PTSD symptoms allows for increased recruitment of cognitive control regions. However, they suggest at least that it would be useful to determine whether interventions designed to increase cognitive control capacities might reduce symptom levels in patients with PTSD.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health under grant number 1-ZIA-MH002860-08 to Dr. Blair. Further support was provided by the National Institute of Mental Health, National Institutes of Health in grants to Dr. Blair (1-K22-MH109558) and Dr. White (1-K01-MH110643) and by the Center for Neuroscience and Regenerative Medicine under grant number 300601 8.01 60855510005 to Michael Roy.
This work was approved by the Uniformed Services University of the Health Sciences and Walter Reed National Military Medical Center Institutional Review Boards. All procedures performed in this study were conducted in accordance with the ethical standards of these Institutional Review Boards. NCT number: NCT01296126.
Footnotes
The authors report no conflicts of interest. Any opinions or assertions expressed are solely those of the authors and do not necessarily represent those of Uniformed Services University, the U.S. Army, U.S. Navy, Department of Defense, or the U.S. Government.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.pscychresns.2018.06.006.
References
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. , 2005. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. Berl 210, 343–352. 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Simmons AN, Flagan T, Thorp SR, Norman SB, et al. , 2013. Neural responses during emotional processing before and after cognitive trauma therapy for battered women. Psychiatry Res 214, 48–55. 10.1016/j.pscychresns.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, et al. , 2007. Modulation of emotion by cognition and cognition by emotion. Neuroimage 35, 430–440. 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Vythilingam M, Crowe SL, McCaffrey DE, Ng P, Wu CC, et al. , 2013. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol. Med 43, 85–95. 10.1017/s0033291712000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, et al. , 2004. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol. Psychiatry 55, 612–620. 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W, 2007. The cognitive control network: integrated cortical regions with dissociable functions. NeuroImage 37, 343–360. 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29, 162–173. https://doi.org/S0010480996900142 [pii]. [DOI] [PubMed] [Google Scholar]
- Cromheeke S, Mueller SC, 2014. Probing emotional influences on cognitive control: an ALE meta-analysis of cognition emotion interactions. Brain Struct. Funct 219, 995–1008. 10.1007/s00429-013-0549-z. [DOI] [PubMed] [Google Scholar]
- El Khoury-Malhame M, Reynaud E, Soriano A, Michael K, Salgado-Pineda P, Zendjidjian X, et al. , 2011. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia 49, 1969–1973. 10.1016/j.neuropsychologia.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A, et al. , 2007. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol. Sci 18, 127–129. 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, et al. , 2010. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. J. Abnorm. Psychol 119, 241–247. 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- Forbes D, Creamer M, Biddle D, 2001. The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD. Behav. Res. Ther 39, 977–986. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC, 2012. Top-down modulation: bridging selective attention and working memory. Trends Cogn. Sci 16, 129–135. 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Vanelzakker MB, Shin LM, 2012. Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front Integr. Neurosci 6, 89 10.3389/fnint.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Weisæth L, Heir T, 2013. Posttraumatic stress and symptom improvement in Norwegian tourists exposed to the 2004 tsunami–a longitudinal study. BMC Psychiatry 13, 232 10.1186/1471-244X-13-232. IBM, 2012. IBM SPSS Statistics For MacOSX. IBM Corp., Armonk, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ, 2013. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J. Trauma Stress 26, 537–547. 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, 2005. International Affective Picture System (IAPS): Affetive Ratings of Pictures and Instruction Manual University of Florida, Gainesville, FL. [Google Scholar]
- Lansing K, Amen DG, Hanks C, Rudy L, 2005. High-resolution brain SPECT imaging and eye movement desensitization and reprocessing in police officers with PTSD. J. Neuropsychiatry Clin. Neurosci 17, 526–532. 10.1176/appi.neuropsych.17.4.526. [DOI] [PubMed] [Google Scholar]
- Laugharne J, Kullack C, Lee CW, McGuire T, Brockman S, Drummond PD, et al. , 2016. Amygdala volumetric change following psychotherapy for posttraumatic stress disorder. J. Neuropsychiatry Clin. Neurosci appineuropsych16010006. 10.1176/appi.neuropsych.16010006. [DOI] [PubMed]
- Lindauer RJL, Booij J, Habraken JBA, van Meijel EPM, Uylings HBM, Olff M, et al. , 2008. Effects of psychotherapy on regional cerebral blood flow during trauma imagery in patients with post-traumatic stress disorder: a randomized clinical trial. Psychol. Med 38, 543–554. 10.1017/S0033291707001432. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Rabinak CA, Kennedy AE, Fitzgerald DA, Liberzon I, Stein MB,et al. , 2016. Emotion regulatory brain function and SSRI treatment in PTSD: neural correlates and predictors of change. Neuropsychopharmacology 41, 611–618. Off. Publ. Am. Coll. Neuropsychopharmacol. 10.1038/npp.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane AC, Atchison M, Yehuda R, 1997. The acute stress response following motor vehicle accidents and its relation to PTSD. Ann. N. Y. Acad. Sci 821, 437–441. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJ, 2007. The impact of processing load on emotion. Neuroimage 34, 1299–1309. 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Dolcos F, Petty CM, Cooper DA, Hayes JP, LaBar KS, et al. , 2009. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. J. Psychiatr. Res 43, 809–817. 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, et al. , 2009. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol. Psychiatry 66, 656–664. 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ, 2005. The cognitive control of emotion. Trends Cogn. Sci 9, 242–249. 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Offringa R, Handwerger Brohawn K, Staples LK, Dubois SJ, Hughes KC, Pfaff DL, et al. , 2013. Diminished rostral anterior cingulate cortex activation during trauma-unrelated emotional interference in PTSD. Biol. Mood Anxiety Disord 3, 10 10.1186/2045-5380-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osofsky JD, Osofsky HJ, Weems CF, King LS, Hansel TC, 2015. Trajectories of post-traumatic stress disorder symptoms among youth exposed to both natural and technological disasters. J. Child Psychol. Psychiatry 56, 1347–1355. 10.1111/jcpp.12420. [DOI] [PubMed] [Google Scholar]
- Pannu Hayes J, Labar KS, Petty CM, McCarthy G, Morey RA, 2009. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Res 172, 7–15. 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres JF, Foerster B, Santana LG, Fereira MD, Nasello AG, Savoia M, et al. , 2011. Police officers under attack: resilience implications of an fMRI study. J. Psychiatr. Res 45, 727–734. 10.1016/j.jpsychires.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Peres JF, Newberg AB, Mercante JP, Simao M, Albuquerque VE, Peres MJ, et al. , 2007. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol. Med 37, 1481–1491. 10.1017/s003329170700997x. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG, 2004. Neuroimaging studies of attention and the processing of emotion-laden stimuli. Prog. Brain Res 144, 171–182. 10.1016/S0079-6123(03)14412-3. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, MacNamara A, Kennedy AE, Angstadt M, Stein MB, Liberzon I,et al. , 2014. Focal and aberrant prefrontal engagement during emotion regulation in veterans with posttraumatic stress disorder. Depression Anxiety 31, 851–861. 10.1002/da.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA, 2006. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biol. Psychiatry 60, 376–382. 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Roy MJ, Francis J, Friedlander J, Banks-Williams L, Lande RG, Taylor P, et al. , 2010. Improvement in cerebral function with treatment of posttraumatic stress disorder. Ann. N. Y. Acad. Sci 1208, 142–149. 10.1111/j.1749-6632.2010.05689.x. [DOI] [PubMed] [Google Scholar]
- Shalev A, Yehuda R, 1998. Longitudinal development of traumatic stress disorders. Psychological Trauma American Psychiatric Press, Washington, DC, pp. 31–66. [Google Scholar]
- Shin LM, Liberzon I, 2010. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169–191. 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. , 2001. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol. Psychiatry 50, 932–942. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Ely TD, Sawamura T, Guzman D, Bradley B, Ressler KJ, et al. , 2016. Childhood maltreatment predicts reduced inhibition-related activity in the rostral anterior cingulate in PTSD, but not trauma-exposed controls. Depression Anxiety 33, 614–622. 10.1002/da.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, 1988. Co-Planar Stereotaxic Atlas of the Human Brain Thieme, Stuttgart. [Google Scholar]
- van Wingen GA, Geuze E, Vermetten E, Fernández G, 2011. Perceived threat predicts the neural sequelae of combat stress. Mol. Psychiatry 16, 664–671. 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Litz B, Herman D, Huska J, Keane T, 1993. The PTSD checklist (PCL): reliability, validity, and diagnostic utility. In: Presented at the Annual Convention of the International Society for Traumatic Stress Studies. [Google Scholar]
- White SF, Costanzo ME, Blair JR, Roy MJ, 2015. PTSD symptom severity is associated with increased recruitment of top-down attentional control in a traumaexposed sample. Neuroimage Clin 7, 19–27. 10.1016/j.nicl.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Zhang Y, Qiu M, Zhang J, Sang L, Wang L, et al. , 2013. Negative emotion regulation in patients with posttraumatic stress disorder. PloS One 8, e81957 10.1371/journal.pone.0081957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, McFarlane A, Shalev A, 1998. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol. Psychiatry 44, 1305–1313. 10.1016/S0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


