Abstract
Purpose:
This review compiles what is known about extracellular vesicles, their bioactive cargo, and how they might be used to treat radiation-induced brain injury. Radiotherapy (RT) is effective in cancer treatment, but can cause substantial damage to normal central nervous system tissue. Stem cell therapy has been shown to be effective in treating cognitive dysfunction arising from RT, but there remain safety concerns when grafting foreign stem cells into the brain (i.e. immunogenicity, teratoma). These limitations prompted the search for cell-free alternatives, and pointed to extracellular vesicles (EV) that have been shown to have similar ameliorating effects in other tissues and injury models.
Conclusions:
EV are nano-scale and lipid-bound vesicles that readily pass the blood-brain barrier. Arguably the most important bioactive cargo within EV are RNAs, in particular microRNAs (miRNA). A single miRNA can modulate entire gene networks and signalling within the recipient cell. Determining functionally relevant miRNA could lead to therapeutic treatments where synthetically-derived EV are used as delivery vectors for miRNA. Stem cell-derived EV can be effective in treating brain injury including radiation-induced cognitive deficits. Of particular interest are systemic modes of administration which obviate the need for invasive procedures.
Keywords: extracellular vesicles, stem cells, cognitive function, miRNA, radiotherapy
Introduction
While survival is rightfully considered the primary criteria for successful cancer treatment, increased success in oncology translates into an increasing population of survivors suffering from unintended side effects of treatment. A significant number of patients surviving more than six months post radiotherapy (RT) suffer cognitive impairments that impact quality of life. These decrements are debilitating, persistent and progressive, and are especially problematic in pediatric patients (Roman & Sperduto 1995; Abayomi 1996; Anderson et al. 2000). These studies suggest that neurocognitive endpoints should be considered a major criteria for successful therapeutic outcome. So what is the etiology and pathology of these problems associated with RT? While effective in solid tumor treatment and prevention of metastasis to the CNS, RT also causes brain injury - ranging from acute (within days to weeks), to early delayed (1–6 months), to late delayed (6+ months) post-RT. Acute and early side-effects such as nausea, vomiting, and headaches can be managed, but the late delayed side-effects such as intellectual impairment, memory loss, and dementia are usually irreversible. The pathogenesis of radiation-induced cognitive impairment is very complex and not fully understood, but the current model suggests a combination of persistent oxidative stress (Robbins et al. 2002), chronic inflammation (Hong et al. 1995; Rola et al. 2005; Ramanan et al. 2008), demyelination (Nagesh et al. 2008), morphometric degradation of neurons (Burger et al. 1979; Parihar et al. 2015), inhibition of neurogenesis (Madsen et al. 2003; Rola et al. 2004; Manda et al. 2009), disruption of neurogenic niche signaling (Monje et al. 2002) and angiogenesis (Warrington et al. 2013) that hinders hippocampal and non-hippocampal-dependent learning, memory, and spatial information processing (reviewed in (L. Zhang et al. 2015)). These phenomena combine and interact to create conditions that are remarkably similar to aging, and in addition, to those seen in progressive degenerative disorders such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, highlighting the extensive overlapping pathology between neurodegenerative diseases and cranial irradiation, where resultant cognitive deficits impact the memory of 40–50% of surviving patients (Meyers 2000). Despite improvements in technology and technique, standard of care treatments continue to induce cognitive impairments in survivors. Promising innovations in immune therapy and gene editing aside, development of clinical resources to remediate the radiation-injured brain remains a critical priority for the growing numbers of affected patients suffering from treatment-associated toxicities. This review explores the possiblity that stem cell derived extracellular vesicles might just represent that therapeutic candidate.
Therapeutic strategies to ameliorate radiation-induced brain injury
Ever since their discovery, stem cells have captured the imagination, holding great potential for regenerative medicine. The term stem cell is broad and includes pluripotent embryonic stem cells (ESCs), and multipotent derivatives including mesenchymal stem cells (MSCs), neural stem cells (NSCs), hematopoietic stem cells (HSCs) and various progenitor cells from all over the body. ESCs are fully pluripotent and can differentiate into any type of tissue. MSCs, NSCs, and HSCs are multipotent and therefore partially lineage-limited, able to differentiate into bone/cartilage/muscle/fat, neural cells, and blood cells, respectively. As a renewable source of undifferentiated cells, able to continuously grow and divide, it was shown that they could be transplanted to a new host or location and the new environmental cues would stimulate the cells to differentiate (Shihabuddin et al. 2000) to replace and repopulate the damaged tissue (reviewed in (Benderitter et al. 2014)). Unfortunately this simple and idealistic view has generally not borne out experimentally, with the notable exception of bone marrow transplantation (i.e. HSC transplantation), used to replenish the hematopoietic system after ablative radio/chemotherapy. However, there is promising research in a number of areas using stem cell therapy including: stroke (Bang et al. 2005; Savitz et al. 2011), severe burns (Lataillade et al. 2007), rheumatoid arthritis (Gonzalez-Rey et al. 2009; González et al. 2009), myocardial infarction (Meyer 2006; Schächinger et al. 2006; Lunde et al. 2006; Zhang et al. 2009), hearing loss (Li et al. 2004; Zhou et al. 2011), retinal disease (Meyer et al. 2011; Ng 2014), and neurodegenerative diseases (Lindvall et al. 2004; Joyce et al. 2010). Given the nature and scope of RT-induced side effects (e.g. normal tissue damage surrounding tumors) in the brain, cognitive deficits represent an adverse condition primed for stem cell therapy.
Indeed, it has been demonstrated that stem cell-based therapies can be effective in treating physical brain or spinal cord injury in rodents (Chopp et al. 2000; Tsuji et al. 2010). Radiation exposure has been well established to deplete neural stem cell populations, and previous efforts led by Dr. Limoli’s group employed NSC therapy to treat radiation-induced cognitive deficits. Specifically, athymic nude (ATN) rats received intra-hippocampal transplantation of human neural stem cells (hNSCs, H9 derived) or induced pluripotent stem cells (iPSC) after cranial irradiation (Acharya et al. 2011; Acharya et al. 2014; Acharya et al. 2015). The stem cell treated rats performed consistently better over a battery of behavioral tests compared to irradiated rats receiving vehicle. In addition, the neuronal structures were preserved, and the host hippocampus had less neuroinflammation as measured by microglial activation. A small percentage of the grafted stem cells were also shown to integrate into the host hippocampal circuitry. While these data are very promising, there are issues associated with stem cells therapies. Beyond the ethical concerns, other risks include teratoma formation and immunorejection (Bradley et al. 2002; Chopp & Zhang 2015). To avoid immunorejection, rodent studies relied on the use of immunocompromised hosts (Acharya et al. 2011). In human patients receiving non-self stem cells, immunosuppression would be necessary and can be problematic since the long-term use of immunosuppressants can result in toxicity, particularly in aged individuals (Mollison et al. 1998). To address these critical issues associated with stem cell therapies, researchers have been looking for safer and more efficacious alternatives.
Extracellular vesicles
Extracellular vesicles (EV) were originally thought to be a “disposal mechanism” for cellular garbage (Pan & Johnstone 1983), but have now been shown to be important both for cell-to-cell communication and microenvironment maintenance (Théry 2011). This autocrine/paracrine signaling mechanism is now considered a short or long distance mode of communication common to most all cells and tissues of the body. The classification of various EV is of some debate, however it is generally accepted that these membrane-bound vesicles are divided into two groups based on size and mode of formation. Microvesicles (MV) tend to be larger - ranging from 100 nm to 1 μm - and are created by direct assembly and outward budding from the cell membrane (Bucki et al. 1998). External stimuli such as hypoxia or the influx of Ca2+ can trigger the release of MV from the cell (Bucki et al. 1998). The biogenesis of exosomes which tend to be smaller - 30 to 100 nm - involves the release of intraluminal vesicles contained inside the endosome-derived multivesicular body (MVB) by fusion with the plasma membrane (György et al. 2011). During this process, the bioactive cargo from the cytosol is sorted into the tiny vesicles. MVB formed by this mechanism release exosomes into the extracellular space when they fuse with and bud off from the plasma membrane (Cocucci & Meldolesi 2015). The release of exosomes is known to involve Rab GTPases (Abels & Breakefield 2016).
EV are secreted by cells throughout the body both in normal physiological conditions and diseased states including cancer. Because they are found and readily characterized in a wide range of bodily fluids including blood, urine and cerebrospinal fluid (Raposo & Stoorvogel 2013) EV are of tremendous interest as circulating biomarkers of disease or exposure (Luga et al. 2012; Frühbeis et al. 2013). Importantly, EV have low immunogenicity, a long half-life in circulation, and are able to cross the blood-brain barrier (Alvarez-Erviti et al. 2011; Kalani et al. 2013; Zhu et al. 2017). While further study is needed to confirm a lack of immunogenicity or off target effects, these features further bolster enthusiasm for the use of EV, not just as biomarkers, but also as promising therapies for regenerative medicine. While extensive long-term studies have yet to be completed, evidence suggests that EV therapy will be well tolerated. Macrophage derived EV, loaded with catalase were administered to mice every other day for a total of 10 treatments and were well tolerated and effective in reducing Parkinson’s Disease related neuroinflammation in mice (Haney et al. 2015). Similarly, daily curcumin-loaded EV therapy for 31 consecutive days caused no adverse side effects and was effective in three mouse models, reducing LPS-mediated neuroinflammation, auto-immune encephalomyelitis, and also in delaying brain tumor growth (Zhuang et al. 2011).
EV Uptake
EV travel through the extracellular space to nearby cells or even through circulation to distant cells. It is thought that EV are able to identify target cells using interactions between transmembrane proteins on the EV and specific receptors on the surface of the target cell. Recipient cells internalize EV via either fusion with the plasma membrane or by endocytosis (Mulcahy et al. 2014). One factor that seems to be important for EV uptake is surface heparin sulfate proteoglycans (HSPGs). It has been shown that blocking HSPGs decreases EV uptake by target cells (Atai et al. 2013). While direct entry can be achieved by membrane fusion, the most common method of EV uptake is through endocytosis. Despite the fact that this pathway generally leads to degradation or re-export, functional transfer of nucleic acids has been demonstrated both in vitro and in vivo. Intact transfer of functional nucleic acids may be directed by a cell-specific ligand/receptor interaction in specific target cell types.
EV Cargo
EV cargo can include proteins, lipids, mitochondria, and nucleic acids. It has been found that bioactive cargo is responsible for cell-to-cell signaling and environmental responses (Raposo & Stoorvogel 2013). The membrane protects EV contents from extracellular proteases and nucleases (Théry et al. 2002). EV have been readily isolated from serum-free conditioned cell culture media using a number of different techniques including differential centrifugation, sucrose gradient centrifugation, microfiltration, immune-affinity capture, microfluidics devices, polymer based products such as ExoQuick™, and size-exclusion liquid chromatography (Momen-Heravi et al. 2013; Nordin et al. 2015). Quantification of EV can be achieved using nanoparticle tracking instruments (e.g. Nanosight and Zetaview) that utilize Brownian motion to determine concentration and size distribution of EV samples. Once isolated, EV have been shown to be stable and biologically active for 20 months at 4°C (Kumeda et al. 2017) and likely even longer at −80°C. Due to variations in EV isolation protocols and analysis, characterization of EV subtypes and the bioactive cargo within has been difficult. However, given the promise of EV-based therapies a significant a concerted effort is being made to refine isolation protocols, and to identify and catalog the various types of cargo found within EV from a variety of cell types. These data are conveniently searchable in online databases including Exocarta (Keerthikumar et al. 2016), Vesiclepedia (Kalra et al. 2012), and EVpedia (Kim et al. 2015) and include purification procedures for reproducibility and consistency. Further refinement and standardization of isolation methods of EV remains, for now, a techological challenge to the advancement of EV therapies, as does the characterization of EV cargo and understanding the function of those cargo in targeting specific recipient cells and effecting phenotypic changes in those cells following uptake.
Proteins
In general, the most common proteins found in EV are involved in the packing, biogenesis, and release of the EV themselves. EV generally have tetraspanins - namely CD63, CD81, and CD9 - and other transmembrane proteins such as LAMP1. Other common proteins include those involved in signal transduction and antigen presentation (Abels & Breakefield 2016).
Lipids
Lipid content of EV varies, but usually mimics the lipid content of the cell type from which the EV are derived. However, some lipids are specifically associated with EV types. Sphingomyelin, cholesterol, ganglioside GM3, disaturated lipids, phosphatidylserine, and ceramide are enriched in EV (Llorente et al. 2013), while phosphatidylcholine and diacyl-glycerol are depleted (Laulagnier et al. 2004).
Mitochondria
Mitochondrial components (including DNA) have been described in EV, though little is known about how the phenomenon occurs. Based on the size and capacity of the EV, either mitochondrial fragments/proteins and DNA in smaller EV (Guescini et al. 2009) or full functional mitochondria in larger EV (Hayakawa et al. 2016) have been observed. As such, EV are known to participate in mitochondrial transfer whereby mitochondrial components or full mitochondria are horizontally transferred between cells (Torralba et al. 2016). Whether the mitochondrial content of certain EV contribute to the regenerative properties of stem cells has yet to be determined.
Nucleic acids
In a minority of cases, some DNA has been found in EV, namely genomic and mitochondrial DNA. To date, there is little evidence for EV-mediated horizontal gene transfer in normal cells though, but rather that transfer of EV-DNA might play a role in intercellular communication by cancer cells in the cancer microenvironment or in metastasis. It has been shown that normal cells have cellular defense mechanisms that prevent the delivery and integration of EV-DNA into the genome of those normal cells (Kawamura et al. 2017). However, an overwhelming majority of the nucleic acid material in the EV is RNA. Many different types of RNA have been found in EV, including: mRNAs, microRNAs (miRNAs), rRNAs, long and short non-coding RNAs (ncRNAs), tRNA fragments, piwi-interacting RNA, vault RNA, and Y RNA (Abels & Breakefield 2016). For the most part the fragments of RNA are limited to 200 bp with a small portion extending to up to 4 kB indicating that most mRNAs and long ncRNAs are fragmented (Batagov & Kurochkin 2013). In fact, the mRNAs are thought to play more of a regulatory role, attracting specific miRNAs to the EV, than a functional one. However, it has also been shown that full-length mRNAs transferred by EV can be readily transcribed in the recipient cell (Valadi et al. 2007).
MicroRNAs
MiRNAs are a family of non-coding RNAs ranging between 20 and 25 bases in length that are able to affect protein levels by post-transcriptional regulation of messenger RNAs (mRNAs). miRNAs are small single-stranded RNAs that compliment the 3’ UTR of mRNA and are able to shut down the translation of the mRNA transcript as a part of the RNA-induced silencing complex (RISC) (Rana 2007). In addition, RISC-mediated silencing promotes deadenylation which hastens the degradation of mRNA transcripts. miRNAs have been discovered to be important in the brain and CNS in both healthy and disease states. One such miRNA, miR-124, is the most highly enriched in the brain and has been shown to be important for adult neurogenesis (Makeyev et al. 2007; Cheng et al. 2009). Another miRNA, miR-125a, has been shown to downregulate synthesis of postsynaptic density protein 95 (PSD-95) (Muddashetty et al. 2011), which is elevated in the irradiated hippocampus (Chmielewski et al. 2016). Replenishing or restoring normal miRNA levels has been suggested as a method for treating various types of brain injury.
miRNA as Active EV Cargo
Based on the evidence available to date, miRNAs are considered to be critical functional elements of EV (J. Zhang et al. 2015). In general, the miRNA contents of the EV match the contents of the cytoplasm of the cells from which they are derived, with some exceptions. It has been shown that miRNA overexpression in the EV-producing cell led to an increased level of the miRNA in EV, while miRNA depletion causes a decreased level in EV (Squadrito et al. 2014). However, there is evidence of selection in terms of which miRNAs are loaded into the EV. There are a four known mechanisms for this that suggest that the selection process is very complex and tightly regulated.
GGAG motif - a binding site for specific ribonucleoprotein thought to be involved in EV loading (Villarroya-Beltri et al. 2013)
3’ end uridylation - post-transcriptional modification that appears to contribute to sorting miRNAs into EV (Koppers-Lalic et al. 2014)
nSMase2 route - overexpression leads to more exported miRNAs (Kosaka et al. 2010)
RISC/AGO2 association - RISC components are also involved in targeted loading of EV (Guduric-Fuchs et al. 2012)
miRNAs are stable, transferred and delivered intact, and are able to change the target cell phenotype and/or physiology, influencing not just one gene, but entire cellular pathways or signaling cascades (Baumann & Winkler 2014). This can be done through two modes, the more canonical silencing RNA (siRNA) pathway by which the miRNA can bind to sites on mRNA and silence translation, and a more recently discovered ability to act as a Toll-Like Receptor ligand and activate immune cells (Fabbri et al. 2012). It is even possible for overexpressed exogenous miRNAs to get loaded into EV (Pegtel et al. 2010), leading to exciting possibilities for EV-mediated delivery of therapeutic miRNAs to a targeted cell population. In fact, targeted mesenchymal stem cell-derived EV loaded with exogenous miR-124 have been used to promote neurogenesis following stroke in mice (Yang et al. 2017). Further, mir-199a-laden EV were able to increase proliferation and decrease apoptosis in cardiomyocytes in vitro (Ferguson et al. 2018). That same group also loaded EV with miR-130a-3p and demonstrated an increase in all angiogenic endpoints in HUVECs. Examples of EV-associated miRNAs that have been isolated from specific cell types and have known biological effects in target cells can be found in Table 1. It will be essential that these engineered EV be carefully tested for off-target effects since it is possible, depending on the mode of delivery, that the these EV are introduced to cells that were not the intended target. However, the Yang et al. (2017) study used a specific targeting peptide to ensure fusion with target cells. Despite the caveats to this approach, these data strongly support the hypothesis that miRNAs are functional important cargo of EV able to exert profound effects in recipient cells.
Table 1.
miRNAs associated with EV from specific cell types and known functions based on peer-reviewed literature.
| MicroRNA ID | Cells Derived From | Biological Outcome in Target Cells | Reference |
|---|---|---|---|
| let-7 Family | Multiple Types | Suppresses oncogenes and cell-cycle regulators | (Kumar et al. 2008) |
| miR-19a | Astrocyte | Suppresses tumor suppressor PTEN | (L. Zhang et al. 2015) |
| miR-21 | CPC | Protects myocardial cells against oxidative stress-related apoptosis | (Xiao et al. 2016) |
| miR-124 | CNS cells | Promotes neural differentiation | (Yang et al. 2017) |
| miR-130a-3p | MSC | Increases angiogenesis | (Ferguson et al. 2018) |
| miR-132 | CPC | Enhances tube formation in epithelial cells | (Barile et al. 2014) |
| miR-133b | MSC | Promotes neurite outgrowth |
(Xin et al. 2012; Xin et al. 2013) |
| Recovers brain function after stroke | |||
| miR-199a | MSC | Cardiomyocyte proliferation | (Ferguson et al. 2018) |
| miR-210 | CPC |
Inhibits apoptosis |
(Barile et al. 2014) |
| Breast Cancer | Increases angiogenesis | (Kosaka et al. 2013) | |
| miR-294 | mESC | Increases survival, proliferation, and commitment of CPCs |
(Khan et al. 2015) |
| Increases formation, persistence, and proliferation of cardiomyocytes | |||
EV as Therapeutic Agents in Regenerative Medicine
EV Therapy for Radiation Induced Cognitive Deficit
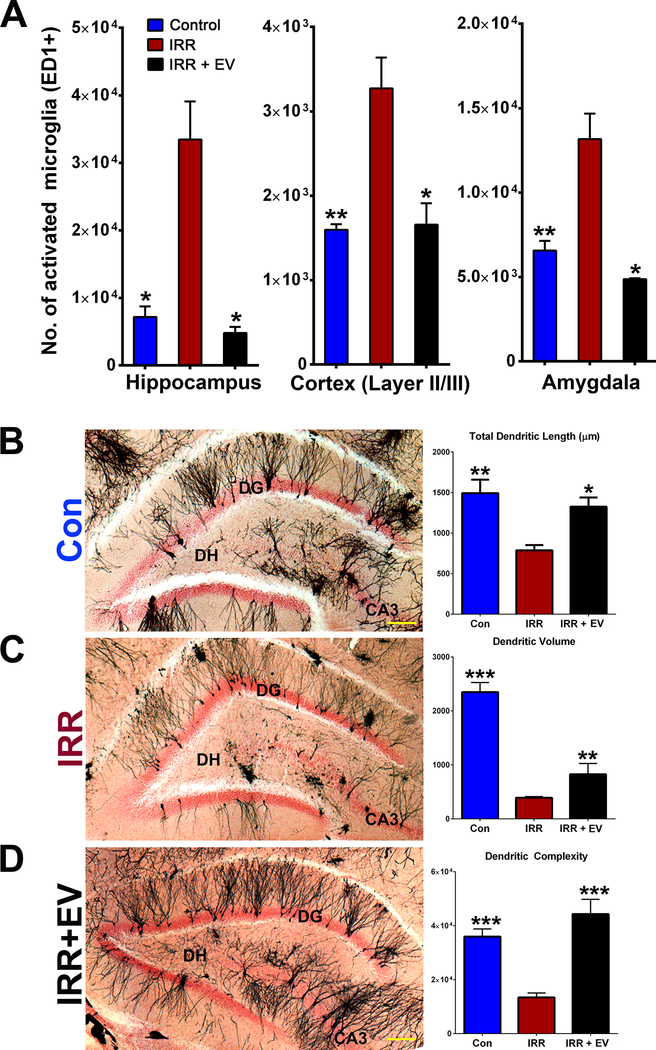
As alternatives to stem cell therapy, studies have found that stem cell-derived EV can provide equivalent beneficial effects compared to stem cells themselves in damaged tissues without the risk of teratoma formation (Doeppner et al. 2015). In inflammation-induced and physical injury-induced cases of cognitive dysfunction in rodents, mesenchymal stem cell-derived EV have reduced inflammation and increased performance in behavioral testing (Y. Zhang et al. 2015; Drommelschmidt et al. 2017; Zhang et al. 2017). Indeed, the Limoli lab has engrafted EV as opposed to the hNSCs from which they were derived (Baulch et al. 2016). The results were striking. Similar to engrafted hNSC, administration of EV ameliorated the adverse effects of cranial irradiation, as indicated by improved cognition, reduced inflammation, and preserved neuronal architecture (Figure 1). Further, the reduced inflammation in not only the hippocampus, but also in the cortex and the amygdala suggested that EV grafting could exert significant effects in regions of the brain both proximal and distal to the engraftment site. Combined with the previous study (Acharya et al. 2011) that showed that only 12–15% of grafted hNSCs remained four months after transplantation surgeries and that out of those remaining cells, less than a quarter of them had differentiated into neuronal lineages, these data suggest a trophic support role of engrafted stem cells is more likely than one of cell replacement. However, the EV study was performed in immunocompromised rats as opposed to wild-type animals. Clearly, proof-of-principle studies need to be carried out in wild-type, immunocompetent animals to define any adverse immune response of human NSC-derived EV therapy.
Figure 1.
EV treatment in cranially-irradiated athymic nude rats ameliorated radiation-induced neuroinflammation and damage to neuronal structure. (A) Immunohistochemical identification and stereology quantification of activated microglia showed that, compared with controls, irradiation significantly increased the number of activated microglia in all regions of the brain evaluated. Compared with the irradiated (IRR) cohort, IRR+EV animals had significantly lower numbers of activated microglia in the hippocampus, cortex (layer II/III), and amygdala. (B-D) Representative images of Golgi–Cox-impregnated hippocampal tissue sections from Control (Con), IRR, and IRR+EV illustrate the gross disruption of neuronal structure (black) in the dentate gyrus, dentate hilus and CA3 regions of the hippocampus (DG; nuclear fast red counterstained) after cranial irradiation that is resolved in animals receiving EV. Structural parameters of dendritic morphology (length, volume, complexity) quantified in each cohort demonstrate that radiation-induced reductions in dendritic morphology were ameliorated by EV grafting. Data are presented as mean ± SEM (n = 3–4 rats per group). *P ≥ 0.05, **P ≥ 0.01, ***P ≥ 0.001 (ANOVA and Bonferroni’s multiple comparisons test). [Scale bars, 50 μm (B–D).] [adapted from (Baulch et al. 2016)].
Systemic Administration of EV
A newer area of research is systemic administration of therapeutic EV. The capability of EV to readily cross the blood-brain barrier (Kalani et al. 2013), opens a number of opportunities for systemic delivery of EV, thereby avoiding invasive surgical procedures. Methods of systemic administration include intranasal, intraperitoneal, retro-orbital sinus, and tail vein injections (e.g. intraveneous or IV). These relatively new strategies have been used successfully for a number of different conditions including stroke (Xin et al. 2013), traumatic brain injury (Y. Zhang et al. 2015), and myocardial infarction (Timmers et al. 2011; Barile et al. 2014; Zhao et al. 2015; Khan et al. 2015). More specifically, intranasal administration of EV has been successful in treating Parkinson’s Disease (Haney et al. 2015), nasal allergies (Prado et al. 2010), stroke (Kalani et al. 2016), epilepsy-related brain damage (Long et al. 2017), and lung injury (Rice et al. 2017; Tan et al. 2018). In addition, the previously mentioned EV-mediated delivery of miR-124 post-stroke utilized tail vein injections (Yang et al. 2017). The potential benefits of these methods should be readily apparent - bypassing the need for invasive heart, brain, or lung surgical procedures to treat patients. In the context of treating the radiation injured brain, intranasal treatment represents a more direct delivery to the brain, bypassing peripheral organ filtration or dilution that occurs via IV administration, promoting a more rapid and direct delivery of EV to the brain (Haney et al. 2015). While gaps in knowledge remain to be closed regarding EV, the minimal immunogenicity, lack of teratoma concerns, and potential for systemic administration, therapeutic stem-cell derived EV represent a very promising direction for the treatment of radiation-induced cognitive dysfunction, in addition to many other side effects of radiation exposure.
Conclusion and Future Perspectives
The use of EV represent an emerging and innovative cell-free approach for the treatment of a variety of adverse conditions without the need for immunosuppression. Such approaches will likely include modified biological EV or synthetically manufactured EV loaded with specific therapeutic miRNAs. Ultimately, these therapies will help an underserved population of cancer survivors, desperately in need of novel interventions designed to minimize normal tissue complications and improve quality of life following RT.
It is possible and perhaps inevitable that in the future, EV-based therapies will be used to treat more than just radiation-induced cognitive deficits. Other potential targets could include traumatic brain injury, age-related memory loss, and neurodegenerative diseases. Eventually, it may even be possible to specifically tailor EV therapy to protect first responders to a radiologic accident such as Fukushima, victims of a terrorist mediated nuclear attack, or even a space traveler over the course of a deep space mission to Mars. The potential of this technology to resolve the adverse effects of radiation exposure, as well as other adverse indications, is extremely exciting and the future of the field looks bright.
Table 2.
Pros and cons of stem cell therapy as compared to stem cell-derived EV therapy
| Stem Cell Therapy | EV Therapy | ||
|---|---|---|---|
| Pros | Cons | Pros | Cons |
| Multipotent | Immunogenic (if not self) | Non-immunogenic | No cell replacement |
| Cell replacement | Possible Teratoma | Cannot form teratomas | Need to produce large quantities in vitro |
| Includes EV | Cannot cross BBB | Reduce inflammation | Consistency is needed in isolation protocol |
| Reduce inflammation | Brain surgery necessary for grafting | Readily cross BBB | Can be difficult to culture |
| Repair of neurogenic niche microenvironment | Can be difficult to culture | Can be administered intravenously or intra-nasally | |
| Possibly restore neurogenic niche microenvironment | |||
| Easily isolated from conditioned media | |||
Acknowledgments
This work was supported by the Defense Threat Reduction Agency (HDTRA 1–13-1–0022, CLL), NIH NINDS R01 NS074388 (CLL), California Institute for Regenerative Medicine (DISC1–10079, JEB), UCI Research Seed Funding Program (JEB).
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abayomi OK. 1996. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 35:659–663. [DOI] [PubMed] [Google Scholar]
- Abels ER, Breakefield XO. 2016. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol. 36:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya MM, Christie L-A, Lan ML, Giedzinski E, Fike JR, Rosi S, Limoli CL. 2011. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 71:4834–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya MM, Martirosian V, Christie L-A, Limoli CL. 2014. Long-term cognitive effects of human stem cell transplantation in the irradiated brain. Int J Radiat Biol. 90:816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya MM, Rosi S, Jopson T, Limoli CL. 2015. Human neural stem cell transplantation provides long-term restoration of neuronal plasticity in the irradiated hippocampus. Cell Transplant. 24:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 29:341–345. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Godber T, Smibert E, Weiskop S, Ekert H. 2000. Cognitive and academic outcome following cranial irradiation and chemotherapy in children: a longitudinal study. Br J Cancer. 82:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atai NA, Balaj L, van Veen H, Breakefield XO, Jarzyna PA, Van Noorden CJF, Skog J, Maguire CA. 2013. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J Neurooncol. 115:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. 2005. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 57:874–882. [DOI] [PubMed] [Google Scholar]
- Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. 2014. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 103:530–541. [DOI] [PubMed] [Google Scholar]
- Batagov AO, Kurochkin IV. 2013. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3′-untranslated regions. Biol Direct. 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulch JE, Acharya MM, Allen BD, Ru N, Chmielewski NN, Martirosian V, Giedzinski E, Syage A, Park AL, Benke SN, et al. 2016. Cranial grafting of stem cell-derived microvesicles improves cognition and reduces neuropathology in the irradiated brain. Proc Natl Acad Sci U S A. 113:4836–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann V, Winkler J. 2014. miRNA-based therapies: strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Future Med Chem. 6:1967–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benderitter M, Caviggioli F, Chapel A, Coppes RP, Guha C, Klinger M, Malard O, Stewart F, Tamarat R, van Luijk P, Limoli CL. 2014. Stem Cell Therapies for the Treatment of Radiation-Induced Normal Tissue Side Effects. Antioxid Redox Signal. 21:338–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JA, Bolton EM, Pedersen RA. 2002. Stem cell medicine encounters the immune system. Nat Rev Immunol. 2:859–871. [DOI] [PubMed] [Google Scholar]
- Bucki R, Bachelot-Loza C, Zachowski A, Giraud F, Sulpice J-C. 1998. Calcium Induces Phospholipid Redistribution and Microvesicle Release in Human Erythrocyte Membranes by Independent Pathways†. Biochemistry. 37:15383–15391. [DOI] [PubMed] [Google Scholar]
- Burger PC, Mahley MS Jr, Dudka L, Vogel FS. 1979. The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer. 44:1256–1272. [DOI] [PubMed] [Google Scholar]
- Cheng L-C, Pastrana E, Tavazoie M, Doetsch F. 2009. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 12:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski NN, Caressi C, Giedzinski E, Parihar VK, Limoli CL. 2016. Contrasting the effects of proton irradiation on dendritic complexity of subiculum neurons in wild type and MCAT mice. Environ Mol Mutagen. 57:364–371. [DOI] [PubMed] [Google Scholar]
- Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, Lu M, Rosenblum M. 2000. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 11:3001–3005. [DOI] [PubMed] [Google Scholar]
- Chopp M, Zhang ZG. 2015. Emerging potential of exosomes and noncoding microRNAs for the treatment of neurological injury/diseases. Expert Opin Emerg Drugs. 20:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E, Meldolesi J. 2015. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 25:364–372. [DOI] [PubMed] [Google Scholar]
- Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig A-K, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. 2015. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med. 4:1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drommelschmidt K, Serdar M, Bendix I, Herz J, Bertling F, Prager S, Keller M, Ludwig A-K, Duhan V, Radtke S, et al. 2017. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun. 60:220–232. [DOI] [PubMed] [Google Scholar]
- Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, et al. 2012. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proceedings of the National Academy of Sciences. 109:E2110–E2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, Nguyen J. 2018. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep. 8:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Möbius W, Goebbels S, Nave K-A, et al. 2013. Neurotransmitter-Triggered Transfer of Exosomes Mediates Oligodendrocyte–Neuron Communication. PLoS Biol. 11:e1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. 2009. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 60:1006–1019. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Gonzalez MA, Varela N, O’Valle F, Hernandez-Cortes P, Rico L, Buscher D, Delgado M. 2009. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 69:241–248. [DOI] [PubMed] [Google Scholar]
- Guduric-Fuchs J, O’Connor A, Camp B, O’Neill CL, Medina RJ, Simpson DA. 2012. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 13:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guescini M, Genedani S, Stocchi V, Agnati LF. 2009. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. 117:1–4. [DOI] [PubMed] [Google Scholar]
- György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger É, Pap E, Kittel Á, et al. 2011. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 68:2667–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. 2015. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 207:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH. 2016. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 535:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Chiang CS, Campbell IL, Sun JR, Withers HR, McBride WH. 1995. Induction of acute phase gene expression by brain irradiation. Int J Radiat Oncol Biol Phys. 33:619–626. [DOI] [PubMed] [Google Scholar]
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. 2010. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 5:933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, Tyagi SC, Tyagi N. 2016. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol. 79:360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Tyagi A, Tyagi N. 2013. Exosomes: Mediators of Neurodegeneration, Neuroprotection and Therapeutics. Mol Neurobiol. 49:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik V, et al. 2012. Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 10:e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Yamamoto Y, Sato T-A, Ochiya T. 2017. Extracellular vesicles as trans-genomic agents: Emerging roles in disease and evolution. Cancer Sci. 108:824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, et al. 2016. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol. 428:688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VNS, Benedict C, et al. 2015. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 117:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-K, Lee J, Simpson RJ, Lötvall J, Gho YS. 2015. EVpedia: A community web resource for prokaryotic and eukaryotic extracellular vesicles research. Semin Cell Dev Biol. 40:4–7. [DOI] [PubMed] [Google Scholar]
- Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MAJ, Sadek P, Sie D, Zini N, Middeldorp JM, Ylstra B, de Menezes RX, et al. 2014. Nontemplated Nucleotide Additions Distinguish the Small RNA Composition in Cells from Exosomes. Cell Rep. 8:1649–1658. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. 2010. Secretory Mechanisms and Intercellular Transfer of MicroRNAs in Living Cells. J Biol Chem. 285:17442–17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumeda N, Ogawa Y, Akimoto Y, Kawakami H, Tsujimoto M, Yanoshita R. 2017. Characterization of Membrane Integrity and Morphological Stability of Human Salivary Exosomes. Biol Pharm Bull. 40:1183–1191. [DOI] [PubMed] [Google Scholar]
- Lataillade JJ, Doucet C, Bey E, Carsin H, Huet C, Clairand I, Bottollier-Depois JF, Chapel A, Ernou I, Gourven M, et al. 2007. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med. 2:785–794. [DOI] [PubMed] [Google Scholar]
- Laulagnier K, Grand D, Dujardin A, Hamdi S, Vincent-Schneider H, Lankar D, Salles J-P, Bonnerot C, Perret B, Record M. 2004. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 572:11–14. [DOI] [PubMed] [Google Scholar]
- Li H, Corrales CE, Edge A, Heller S. 2004. Stem cells as therapy for hearing loss. Trends Mol Med. 10:309–315. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Martinez-Serrano A. 2004. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 10 Suppl:S42–50. [DOI] [PubMed] [Google Scholar]
- Llorente A, Skotland T, Sylvänne T, Kauhanen D, Róg T, Orłowski A, Vattulainen I, Ekroos K, Sandvig K. 2013. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1831:1302–1309. [DOI] [PubMed] [Google Scholar]
- Long Q, Upadhya D, Hattiangady B, Kim D-K, An SY, Shuai B, Prockop DJ, Shetty AK. 2017. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U S A. 114:E3536–E3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. 2012. Exosomes Mediate Stromal Mobilization of Autocrine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell. 151:1542–1556. [DOI] [PubMed] [Google Scholar]
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, et al. 2006. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 355:1199–1209. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PEG, Bolwig TG, Wörtwein G. 2003. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 119:635–642. [DOI] [PubMed] [Google Scholar]
- Makeyev EV, Zhang J, Carrasco MA, Maniatis T. 2007. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 27:435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda K, Ueno M, Anzai K. 2009. Cranial irradiation-induced inhibition of neurogenesis in hippocampal dentate gyrus of adult mice: attenuation by melatonin pretreatment. J Pineal Res. 46:71–78. [DOI] [PubMed] [Google Scholar]
- Meyer GP. 2006. Intracoronary Bone Marrow Cell Transfer After Myocardial Infarction: Eighteen Months’ Follow-Up Data From the Randomized, Controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) Trial. Circulation. 113:1287–1294. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, Pinilla I, Martin JM, Tian S, Stewart R, et al. 2011. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 29:1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers CA. 2000. Neurocognitive dysfunction in cancer patients. Oncology. 14:75–9; discussion 79, 81–2, 85. [PubMed] [Google Scholar]
- Mollison KW, Fey TA, Krause RA, Andrews JM, Bretheim PT, Cusick PK, Hsieh GC, Luly JR. 1998. Nephrotoxicity studies of the immunosuppressants tacrolimus (FK506) and ascomycin in rat models. Toxicology. 125:169–181. [DOI] [PubMed] [Google Scholar]
- Momen-Heravi F, Balaj L, Alian S, Mantel P-Y, Halleck AE, Trachtenberg AJ, Soria CE, Oquin S, Bonebreak CM, Saracoglu E, et al. 2013. Current methods for the isolation of extracellular vesicles. Biol Chem. 394:1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. 2002. Irradiation induces neural precursor-cell dysfunction. Nat Med. 8:955–962. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, Nalavadi VC, Gross C, Yao X, Xing L, Laur O, Warren ST, Bassell GJ. 2011. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol Cell. 42:673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy LA, Pink RC, Carter DRF. 2014. Routes and mechanisms of extracellular vesicle uptake. Journal of Extracellular Vesicles. 3:24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesh V, Tsien CI, Chenevert TL, Ross BD, Lawrence TS, Junick L, Cao Y. 2008. Radiation-induced changes in normal-appearing white matter in patients with cerebral tumors: a diffusion tensor imaging study. Int J Radiat Oncol Biol Phys. 70:1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TK. 2014. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J Stem Cells. 6:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin JZ, Lee Y, Vader P, Mäger I, Johansson HJ, Heusermann W, Wiklander OPB, Hällbrink M, Seow Y, Bultema JJ, et al. 2015. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. 11:879–883. [DOI] [PubMed] [Google Scholar]
- Pan B-T, Johnstone RM. 1983. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell. 33:967–978. [DOI] [PubMed] [Google Scholar]
- Parihar VK, Pasha J, Tran KK, Craver BM, Acharya MM, Limoli CL. 2015. Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Struct Funct. 220:1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MAJ, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. 2010. Functional delivery of viral miRNAs via exosomes. Proceedings of the National Academy of Sciences. 107:6328–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado N, Cañamero M, Villalba M, Rodríguez R, Batanero E. 2010. Bystander suppression to unrelated allergen sensitization through intranasal administration of tolerogenic exosomes in mouse. Mol Immunol. 47:2148–2151. [DOI] [PubMed] [Google Scholar]
- Ramanan S, Kooshki M, Zhao W, Hsu F-C, Robbins ME. 2008. PPARalpha ligands inhibit radiation-induced microglial inflammatory responses by negatively regulating NF-kappaB and AP-1 pathways. Free Radic Biol Med. 45:1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana TM. 2007. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol. 8:23–36. [DOI] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 200:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice TC, Pugh AM, Xia BT, Seitz AP, Whitacre BE, Gulbins E, Caldwell CC. 2017. Bronchoalveolar Lavage Microvesicles Protect Burn-Injured Mice from Pulmonary Infection. J Am Coll Surg. 225:538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins ME, Zhao W, Davis CS, Toyokuni S, Bonsib SM. 2002. Radiation-induced kidney injury: a role for chronic oxidative stress? Micron. 33:133–141. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. 2004. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 188:316–330. [DOI] [PubMed] [Google Scholar]
- Rola R, Sarkissian V, Obenaus A, Nelson GA, Otsuka S, Limoli CL, Fike JR. 2005. High-LET radiation induces inflammation and persistent changes in markers of hippocampal neurogenesis. Radiat Res. 164:556–560. [DOI] [PubMed] [Google Scholar]
- Roman DD, Sperduto PW. 1995. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 31:983–998. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Chopp M, Deans R, Carmichael T, Phinney D, Wechsler L, STEPS Participants. 2011. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke. 42:825–829. [DOI] [PubMed] [Google Scholar]
- Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, et al. 2006. Intracoronary Bone Marrow–Derived Progenitor Cells in Acute Myocardial Infarction. N Engl J Med. 355:1210–1221. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. 2000. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 20:8727–8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, Lyle R, Ibberson M, De Palma M. 2014. Endogenous RNAs Modulate MicroRNA Sorting to Exosomes and Transfer to Acceptor Cells. Cell Rep. 8:1432–1446. [DOI] [PubMed] [Google Scholar]
- Tan JL, Lau SN, Leaw B, Nguyen HPT, Salamonsen LA, Saad MI, Chan ST, Zhu D, Krause M, Kim C, et al. 2018. Amnion Epithelial Cell-Derived Exosomes Restrict Lung Injury and Enhance Endogenous Lung Repair. Stem Cells Transl Med. 7:180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C 2011. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Zitvogel L, Amigorena S. 2002. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2:569–579. [DOI] [PubMed] [Google Scholar]
- Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AAM, Goumans MJ, Strijder C, Sze SK, Choo A, et al. 2011. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 6:206–214. [DOI] [PubMed] [Google Scholar]
- Torralba D, Baixauli F, Sánchez-Madrid F. 2016. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front Cell Dev Biol. 4:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, Tomisato S, et al. 2010. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 107:12704–12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 9:654–659. [DOI] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sánchez-Madrid F. 2013. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 4:2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington JP, Ashpole N, Csiszar A, Lee YW, Ungvari Z, Sonntag WE. 2013. Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications. J Vasc Res. 50:445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. 2013. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 33:1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang X, Chen X, Wang L, Yang G. 2017. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol Ther Nucleic Acids. 7:278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. 2015. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genomics Proteomics Bioinformatics. 13:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang H, Tian Y. Radiation-induced cognitive impairment. 2015. Ther Targets Neurol Dis. 2:e837. [Google Scholar]
- Zhang S, Sun A, Xu D, Yao K, Huang Z, Jin H, Wang K, Zou Y, Ge J. 2009. Impact of timing on efficacy and safety of intracoronary autologous bone marrow stem cells transplantation in acute myocardial infarction: a pooled subgroup analysis of randomized controlled trials. Clin Cardiol. 32:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. 2015. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 122:856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, Ali M, Mahmood A, Xiong Y. 2017. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 111:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, Zhu W, Xu W. 2015. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Acute Myocardial Ischemic Injury. Stem Cells Int. 2015:761643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yuan J, Zhou B, Lee AJ, Lee AJ, Ghawji M Jr, Yoo TJ. 2011. The therapeutic efficacy of human adipose tissue-derived mesenchymal stem cells on experimental autoimmune hearing loss in mice. Immunology. 133:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, et al. 2011. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 19:1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, Jiang J, Elgamal OA, Mo X, Perle KL, et al. 2017. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 6:1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]