Abstract
Objective:
The burden of PD extends beyond physical limitations and includes significant psychosocial adjustments as individuals undergo changes to their self-perception and how others perceive them. There is limited quantitative evidence of the factors that contribute to selfperceived stigma, which we addressed in the present study.
Methods:
In 362 individuals with PD (157 women, 205 men), self-perceived stigma was measured by the four-item stigma subscale of the Parkinson’s Disease Questionnaire (PDQ-39). Hierarchical linear modeling was used to assess predictors of stigma including demographics (age, gender) and disease characteristics: duration, stage (Hoehn & Yahr Scale), motor severity (Unified Parkinson’s Disease Rating Scale, UPDRS, Part 3), activities of daily living (UPDRS Part 2), and depression (Geriatric Depression Scale). Predictor variables were chosen based on their significant correlations with the stigma subscale. Further analyses were conducted for men and women separately.
Results:
For the total sample, the full model accounted for 14% of the variance in stigma perception (p < .001). Younger age and higher depression scores were the only significant predictors (both p<.001). This pattern was also seen for the men in the sample. For the women, only depression was a significant predictor. Depression mediated the relation between stigma and activities of daily living.
Conclusions:
Younger age (men) and depression (men and women) were the primary predictors of self-perceived stigma in PD. Disease characteristics (motor and ADL) did not contribute to stigma perception. Depression is a potential treatment target for self-perceived stigma in PD.
Keywords: Parkinson’s disease, stigma, depression
1. Introduction
Though PD has traditionally been characterized as a motor disorder, the disease burden extends far beyond physical limitations. Diagnosis of PD is a major life transition, and requires adjusting to its multifaceted demands [1]. The impact of disease-related stigma on quality of life is notable [2] and stigmatizing beliefs and experiences are common in PD [3]. Self-perceived stigma may result from exposure to negative attitudes toward the disease (i.e., enacted stigma), which may ultimately foster subjective acceptance of negative stereotypes, discrimination, and prejudice (i.e., internalized stigma) [4]. Motor symptoms such as facial masking can fuel negative social perceptions even among trained healthcare providers [5]. Neurosurgical interventions targeted toward relief of motor dysfunction have demonstrated a reduction in selfperceived stigma, suggesting a relation between motor symptoms and stigma [6, 7]. PD-specific challenges such as medication fluctuations (i.e., off periods) and motor complications (e.g., dyskinesias) have important psychosocial implications [4] and are related to self-perceived stigma [7].
Although research has offered qualitative descriptions of stigma in PD [5], there is limited quantitative evidence of its contributing factors.. Considering the complex motor and non-motor symptomatology of PD, stigma may manifest differently according to different demographic and disease characteristics. For example, younger age of PD onset is associated with greater disease burden, motor complications, unemployment due to disability, poorer quality of life, depression, and greater stigmatization and marital dissatisfaction[8]. Men and women experience PD differently. Women experience later disease onset, greater severity of dyskinesias, and higher likelihood of depression; men more commonly experience sleep disorders and behavioral problems. [9]. The conceptualization and treatment of stigma in PD is limited by an incomplete understanding of these contributory factors.
The purpose of this study was to assess the contributions of clinical and demographic characteristics to self-perceived stigma in a PD sample that was in the early to middle stages of the disease, when potential self-perceived stigma may first be appearing. The hypotheses were as follows:
-
(1)
Demographic characteristics: Age and gender. Younger age would relate to increased self-perceived stigma, as diagnosis of PD typically results in more drastic life adjustments for younger individuals[8]. Women would endorse more stigma than men, reflecting greater societal emphasis on physical appearance in women [10].
-
(2)
Disease characteristics and functional impairment. Visibility of symptoms and severity of dysfunction would predict self-perceived stigma; hence, those with tremor-dominant symptoms (TD), which may be apparent during most social situations, would endorse more stigma than those with postural instability and gait difficulty (PIGD), whose problems may not be as obvious to the observer in certain situations (as when sitting). Limits to independence as indicated by self-reported challenges in activities of daily living (ADLs) would predict stigma.
-
(3)
Depression. Depression is associated with negative cognitive biases [11, 12]; hence, depression would predict self-perceived stigma.
2. Methods
2.1. Participants
Participants included 362 individuals with idiopathic PD (157 women, 205 men) who were assessed as part of two larger PD studies (Table 1). Data from 262 participants were collected as described in Dibble et al. (2010) [13] and the remaining 100 participants were assessed in Boston University’s Vision and Cognition Laboratory. All procedures were approved by the Institutional Review Board of each data collection site, and consent was obtained according to the Declaration of Helsinki.
Table 1.
Participant Characteristics, Mean (SD)
| Age (years) | PD Duration (years) | MDSUPDRS Part 2 (ADL) | MDSUPDRS Part 3 (motor) | GDS | PDQ-39Stigma | LED (mg/day) | |
|---|---|---|---|---|---|---|---|
| Entire sample (N=362) | 67.0 (8.8) | 6.1 (4.7) | 12.7 (7.5) | 32.2 (14.6) | 7.3 (5.4) | 14.9 (17.6) | 652.0 (556.8) |
| Men (n=205) | 67.4 (8.9) | 6.3 (4.7) | 13.1 (7.8) | 34.2 (15.0) | 7.4 (5.3) | 14.5 (18.5) | 680.4 (531.5) |
| Women (n=157) | 66.5 (8.5) | 5.8 (4.6) | 12.3 (7.0) | 29.6 (13.8) | 7.2 (5.6) | 15.3 (16.4) | 614.9 (588.0) |
PD = Parkinson’s disease; MDS-UPDRS = Movement Disorders Society Unified Parkinson’s Disease Rating Scale; ADL = Activities of Daily Living; GDS = Geriatric Depression Scale; PDQ-39 = 39-item Parkinson’s Disease Questionnaire; LED = Levodopa equivalent dosage; SD = standard deviation; The sample size for all variables was 362 with the exception of the GDS (n=384; 198 men, 150 women) and LED (n=360; 204 men, 156 women). The numbers of participants categorized as tremor dominant, postural instability and gait difficulty, and indeterminate were 110, 223, and 29, respectively. MDS-UPDRS values reflect the onmedication state.
Participants from the study by Dibble et al (2010) were recruited from the Movement Disorders Clinic at Boston Medical Center (N=76), University of Utah (N=38), Washington University in St. Louis (N=78) and University of Alabama (N=70). The 100 participants assessed in the Vision and Cognition Laboratory were recruited from the Movement Disorders Clinic at Boston Medical Center and other community resources including Fox Trial Finder and PD support groups. Inclusion criteria relevant to the current study consisted of a diagnosis of idiopathic PD according to the UK Brain Bank Criteria [14] and a modified Hoehn and Yahr stage less than or equal to 4 [15]. Exclusion criteria included a diagnosis of atypical parkinsonism, early onset PD (<40 years of age), or previous surgical management of PD.
The total sample had an average age of 67.0 years (SD = 8.7) and disease duration of 6.1 years (SD = 4.7). Median modified Hoehn & Yahr (H&Y) stage was 2 (range 1–4) [15]. Average motor severity as measured by the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS; Goetz et al., 2008)[16] was 32.2 (SD = 14.6). Motor symptom subtypes [17, 18] consisted of 110 with tremor-dominant profile (TD; 30.4%), 223 characterized by postural instability and gait difficulty (PIGD; 61.6%), and 29 indeterminate (IND; 8.0%), all as per time of assessment. Each participant had a Mini-Mental State Examination score of 24 or higher. All were tested while on medication.
2.2. Procedures
Data from demographics and from disease characteristics of duration, stage (Hoehn and Yahr), motor severity, ADLs, and depression were analyzed with respect to self-perceived stigma. The measures of stigma perception, motor severity, ADLs, and depression are as follows.
Stigma perception: Parkinson’s Disease Questionnaire-39 (PDQ-39).
The PDQ-39 is a standard 39-item measure of subjective quality of life in PD. There were four items on stigma (items 2326). These were: (#23) Felt you had to hide your Parkinson’s from people; (#24) Avoided situations which involve eating or drinking in public; (#25) Felt embarrassed in public; (#26) Felt worried about other people’s reaction to you. Each item was scored on a 5-point ordinal scale of 0–4 with 0 = never and 4 = always. Participants responded to each item with respect to their experiences within the last month. The scores ranged from 0–16. PDQ-39stigma scores were converted to a 100 point scale by multiplying the mean of the stigma items by 100, following the methods of Peto, Jenkinson, & Fitzpatrick, 1998 [19].
Motor severity and activities of daily living: Unified Parkinson’s Disease Rating Scale (UPDRS).
The MDS-UPDRS [16] was administered to all participants in Dibble et al. (2010), and the original UPDRS [20] was administered to the 100 participants recruited by the Vision and Cognition Laboratory of Boston University. In order to pool data across the samples, a standard formula was used to convert sections (B) Activities of Daily Living (ADL) and (C) Motor Examination (observer rated) of the UPDRS scores to the analogous MDS-UPDRS sections, which are, respectively, (2) Motor Experiences of Daily Living and (3) Motor Examination [21]. Such conversions are not available for sections A and D, and therefore analyses were limited to data from sections B and C.
Motor symptom subtype was calculated by the ratio of mean tremor to mean PIGD symptoms. For the original UPDRS, a ratio greater than or equal to 1.5 was classified as tremor dominant (TD), a ratio less than or equal to 1.0 was classified as postural instability and gait difficulty (PIGD), and a ratio between 1.0–1.5 was classified as indeterminate (IND) [17]. For the MDS-UPDRS, a ratio greater than or equal to 1.15 was classified as tremor dominant, a ratio less than or equal to 0.90 was classified as PIGD, and a ratio between 0.90–1.15 was classified as indeterminate [18]. Seventeen participants had a tremor score of 0 and were classified as PIGD; 12 participants had a PIGD score of 0 and were classified as TD [18]. No participant had scores of 0 for both tremor and PIGD symptoms. There were a total of 110 TD, 223 PIGD, and 29 IND.
Depression: Geriatric Depression Scale.
This is a standard 30-item depression measure [22]. Participants responded either yes or no to the experience of depression symptoms within the last week. The sum of all items was calculated, with a possible range of 0–30. Higher scores were indicative of greater depression severity.
2.3. Statistical analyses
For each variable, the mean and standard deviation were calculated. Two-tailed Pearson correlations were conducted with an alpha of p<.01 as significant in order to account for multiple correlations. Correlations between stigma and age, disease duration, disease stage (H&Y), motor severity (MDS-UPDRS – Part 3), activities of daily living (ADLs; MDS-UPDRS – Part 2), and depression (GDS) were examined. Significant correlates were entered as predictors in a hierarchical regression analysis with self-perceived stigma (PDQ-39stigma) as the dependent variable. T-tests were used to assess if self-reported stigma was different between men and women, and between TD and PIGD. Because gender differences are well documented in PD[9], separate regressions were conducted for men and women in addition to the whole-group analyses.
Regression models were built by entering significant correlates of stigma as the predictors in a three-block linear regression. The order of block entry was determined according to the following rationale. Block 1: Age and disease duration were entered first as general, nonmodifiable demographic and disease characteristics; Block 2: Disease stage (H&Y), motor severity (MDS-UPDRS – Part 3), and activities of daily living (MDS-UPDRS – Part 2) were entered as PD-specific indices of disease severity; Block 3: depression was entered last as a nonmotor feature of PD. Each successive block of predictors was entered to assess the predictive value of those variables above and beyond the preceding factors. Using an F test (α = 0.05), the significance of the r2 and r2 change values was examined to identify predictors of variance in stigma score.
Mediation analyses were performed to assess whether significant predictors mediated the relation between PD motor dysfunction (MDS-UPDRS sections 2 and 3) and PDQ-39stigma. The total, direct, and indirect effect was assessed with the software PROCESS, which allows for modeling mediation in SPSS [23]. A bootstrap estimation approach with 1,000 samples was used to measure the indirect effect [24], with 95% confidence intervals. The indirect effect was considered significant when the 95% confidence intervals did not contain zero [25]. A predictor was considered a significant mediator when the total effect was significant; the indirect effect was significant; and the confidence intervals of the direct effect contained zero [26].
Analyses were performed with SPSS 20.0 (SPSS, Inc., Chicago, IL).
3. Results
For the whole group, the significant correlates of PDQ-39stigma were age (p = .009), ADLs (p < .0001) and depression (p < .0001). The three-block linear regression model included (1) age, (2) ADLs, (3) depression. This full model accounted for 14.2% of the variance in PDQ-39stigma (r2 = .142, F = 18.99, p < .0001). Younger age (B = −.380, p < .0003) and higher depression scores (B = .966, p < .0001) were the only significant predictors of the PDQ-39 stigma score.
For women, only depression correlated with PDQ-39stigma (r = .22, p < .007), and this was a significant predictor of stigma, accounting for 4.8% of the variance in PDQ-39stigma (r2 = .048, F = 7.52, B = .65, p < .007). For men, the significant correlates were age (p < .002), depression (p < .0001), and ADLs (p < .00005). Age (B = −.49, p < .001) and depression (B = 1.18, p <.001) were significant predictors of stigma and together accounted for 22% of the variance in PDQ-39stigma (r2 = .22, F = 18.26, p < .001).
Self-perceived stigma was not significantly different for men and women (t(360) = −.44, p = .66) or for the TD and PIGD subgroups (t(325) = −1.51, p = .88).
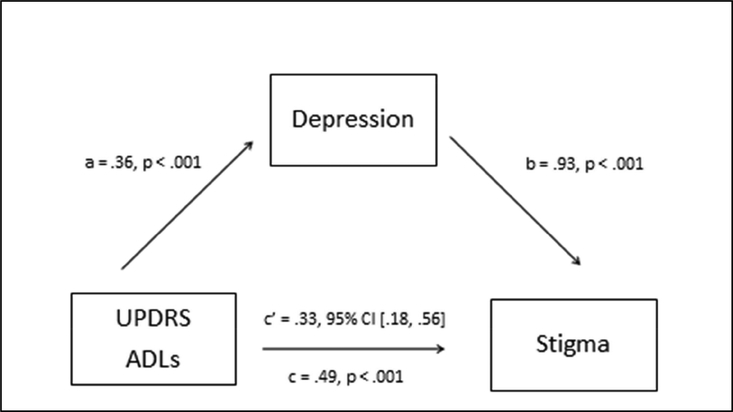
The results of the mediation analyses are presented in Table 2. When depression was entered in the mediation model for MDS-UPDRS-ADLs and PDQ-39stigma (Model 1), the total effect was significant [B = .49, p = .0008], the indirect effect was significant [B = .33, 95% CI: .18-.56], and the direct effect of MDS-UPDRS-ADLs on PDQ-39stigma approached zero [B = .16, 95% CI: −.12, .44], representing a decline in the strength of the direct effect (Figure 1). This supports depression as a full mediator of the relation between MDS-UPDRS-ADLs and PDQ39stigma. In this model, the indirect effect through depression accounted for a significant portion of the total effect, whereas the contribution of the direct effect between ADLs and stigma was not significant. In other words, the significant relation between challenges with ADLs and stigma perception was driven entirely by the relation to depression.
Table 2.
Mediation Analyses
| Model 1 | Predictor: UPDRS-A | DL DV: Stigma | Mediator: GDS | |||
|---|---|---|---|---|---|---|
| Path | B | SE | T | P | 95% CI | |
| a | 0.36 | 0.04 | 9.13 | <.0001 | 0.28, 0.43 | |
| b | 0.93 | 0.23 | 4.14 | <.0001 | 0.49, 1.38 | |
| total effect | c | 0.49 | 0.15 | 3.37 | 0.0008 | 0.20, 0.77 |
| direct effect | c - (a*b) | 0.16 | 0.14 | 1.11 | 0.27 | −0.12, 0.44 |
| indirect effect | c’ | 0.33 | 0.10 | 0.18, 0.56 | ||
| Model 2 | Predictor: UPDRSMotor | DV: Stigma | Mediator: GDS | |||
|---|---|---|---|---|---|---|
| Path | B | SE | T | P | 95% CI | |
| a | 0.14 | 0.02 | 7.73 | <.0001 | 0.11, 0.18 | |
| b | 1.05 | 0.27 | 4.86 | <.0001 | 0.63, 1.48 | |
| total effect | c | 0.14 | 0.08 | 1.79 | 0.07 | −0.01, 0.30 |
| direct effect | c - (a*b) | −0.01 | 0.08 | −0.11 | 0.92 | −0.16, 0.14 |
| indirect effect | c’ | 0.15 | 0.04 | 0.09, 0.25 | ||
| Model 3 | Predictor: UPDRS-A | DL DV: Stigma | Mediator: Age | |||
|---|---|---|---|---|---|---|
| Path | B | SE | T | P | 95% CI | |
| a | 0.21 | 0.06 | 3.46 | 0.0006 | 0.09, 0.33 | |
| b | −0.37 | 0.12 | −2.98 | 0.0031 | −0.61, −0.13 | |
| total effect | c | 0.51 | 0.14 | 3.58 | 0.0003 | 0.23, 0.80 |
| direct effect | c - (a*b) | 0.59 | 0.15 | 4.04 | <.0001 | 0.30, 0.88 |
| indirect effect | c’ | −0.08 | −0.03 | −0.16, −0.03 | ||
| Model 4 | Predictor: UPDRSMotor | DV: Stigma | Mediator: Age | |||
|---|---|---|---|---|---|---|
| Path | B | SE | T | P | 95% CI | |
| a | 0.15 | 0.04 | 4.15 | <.0001 | 0.08, 0.23 | |
| b | −0.37 | 0.14 | −2.71 | 0.007 | −0.64, −0.10 | |
| total effect | c | 0.16 | 0.08 | 2.00 | 0.046 | 0.003, 0.31 |
| direct effect | c - (a*b) | 0.21 | 0.08 | 2.63 | 0.009 | 0.05, 0.37 |
| indirect effect | c’ | −0.06 | 0.02 | −0.11, −0.03 | ||
UPDRS-ADL = Unified Parkinson’s Disease Rating Scale Activities of Daily Living Section; UPDRS-Motor = Unified Parkinson’s Disease Rating Scale Motor Section; DV = dependent variable; B = unstandardized regression coefficient; SE = Standard Error; t = t-value; 95% CI = 95% confidence interval
Figure 1. Depression mediates MDS-UPDRS ADLs and PDQ-39stigma.
Direct effect: B = .16, 95% CI: −.12, .44
UPDRS ADLs = Unified Parkinson’s Disease Rating Scale Activities of Daily Living Section
When depression was assessed as a mediator of MDS-UPDRS-motor and PDQ-39stigma (Model 2), the total effect was not significant [B = .14, p = .07], the indirect effect was significant [B = .15, 95% CI: .09, .25], and the direct effect was zero [B = −0.01, 95% CI: −.16, .14]. The mediation models with age (Models 3 & 4) as the mediator did not support age as a significant mediator. Inclusion of age in the model enhanced the strength of the direct effect, though the indirect effect was not significant. In other words, the relation between PD motor dysfunction and stigma was primarily driven by the direct effect; the contribution of age was not significant.
4. Discussion
Self-perceived stigma was predicted by younger age and greater depression severity. The prediction by depression held for both men and women, whereas the prediction by age was specific to men. The relation between PD ADLs and stigma was mediated by depression: as depression increased, the relation between PD ADLs and stigma strengthened. Contrary to expectations, disease-specific motor impairments were not significant predictors, nor did the severity of stigma differ according to gender or motor symptom subtypes.
Depression was most strongly related to stigma and impacted the relation between ADLs and stigma. This finding is in accord with work that has recognized depression as a prevalent, significant contributor to quality of life in PD [2]. The relation between younger age and stigma is consistent with research that has demonstrated greater disease burden for younger individuals with PD [8]. Younger age was related to increased perception of stigma in men with PD. The experience of diagnosis of a neurodegenerative disease relatively early in life may be qualitatively different than diagnosis in later life, having a greater impact on self-perception and self-expectations in family, social, and occupational roles [27]. Although age did not predict stigma perception in women in our sample, further research should consider whether gender differences arise for individuals who experience an even younger age of PD onset, as our sample was restricted to individuals who were age 40 and above at time of diagnosis.
The significant impact of depression on stigma perception has important implications for conceptualizing stigma in PD and its potential treatment targets. In this study, depression fully mediated the relation between ADLs and stigma. A similar pattern was demonstrated for the relation between the MDS-UPDRS motor score and stigma. The indirect effect of depression was significant, but the total effect was not significant (p = .07). Age, however, was not a significant mediator: the relation of stigma to motor symptoms and ADLs was not significantly influenced by age. Further, there was no difference in stigma perception for those categorized as having TD vs PIGD motor symptoms. Together, these results suggest that PD motor symptoms and their impact on daily life have relatively little contribution to the experience of stigma outside of the mediating role of depression. This suggests that treatments focused entirely on alleviating motor symptoms that do not target the emotional response to PD will not alleviate stigma perception. Future research can distinguish between whether this emotional response is a perceived reaction to PD symptoms (e.g., a sense of uncertainty or helplessness toward PD, feelings of failure toward attaining role expectations) or whether this emotional response is a reaction to actual barriers (physical, occupational, social) imposed by PD.
Our results provide direction for further investigation of mechanisms of perceived stigma and treatment targets. Awareness of the burden of (young) age and depression may prove particularly useful for providers, caregivers, and persons with PD themselves. Mood disorders in PD are commonly underdiagnosed [28], and untreated depression is associated with poorer quality of life [29]. Although the motor symptoms and limitations of PD are often a major focus of diagnosis and treatment, targeting motor dysfunction without also targeting mood disturbance could be a major disservice to individuals with PD who experience stigma. Whether mood interventions can independently improve motor function and activities of daily living is an important question for future research. Improvements in self-perceived stigma have been reported to follow neurosurgical treatments targeting motor function [6, 7], but the degree to which these improvements are mediated by improved mood has not been established. Without recognizing the impact of mood on functional outcomes in PD, the conceptualization and treatment of PD motor limitations may be incomplete.
This study was subject to limitations. First, we found that only 14.5% of the variance in stigma was accounted for by the regression model. Though this was a strong effect statistically, it suggests that other variables also account for stigma perception in PD. Second, other scales designed specifically for assessing stigma may offer a more thorough description of this phenomenon in PD than the stigma measure used in the present study (PDQ-39), which was limited to four items. Using a stigma scale with more items may yield greater variability in responses and allow for a more robust assessment of variance in PD stigma perception. It must be noted, however, that to date no other stigma scales have been validated specifically for PD; one study of stigma associated with chronic illness included individuals with PD along with individuals with Alzheimer’s disease, amyotrophic lateral sclerosis, epilepsy, stroke, and multiple sclerosis [30]. Finally, our study sample comprised persons with early to moderate PD without dementia, aged 40 and higher, with no history of surgical intervention, which precluded the consideration of potential interactions of serious cognitive impairment, younger age of disease onset, and neurosurgical treatment on self-perceived stigma in PD, as well as precluding consideration of potentially stigma-inducing assistive devices such as canes and walkers. The significant results from this study warrant further investigation into stigma in other PD subgroups, which may provide additional insight into underlying mechanisms and correlates, and in particular may be important to evaluating our finding that stigma perception in especially heightened in earlier-onset PD.
Our quantitative, model-based approach to address direct hypotheses about contributors to self-perceived stigma gives us a data/evidence-based perspective on this aspect of PD. It further allows us to comment on potential mechanisms, highlighting the role of depression, and indicating that the outward physical limitations of PD may drive stigma less than the subjective and emotional burden of managing the disease. These results support a patient-centered approach to treatment, which considers how demographic characteristics such as age and gender may interact with PD burden and promote stigma perception and depression. Interventions that target mood may have greater efficacy in improving stigma than motor treatments alone, and may be critical to improving quality of life.
Highlights.
This study assessed predictors of self-perceived stigma in Parkinson’s disease (PD).
Younger age and greater depression predicted self-perceived stigma for men with PD.
Depression predicted self-perceived stigma for women with PD.
Depression mediated the relation between Activities of Daily Living and stigma.
Acknowledgments
This research was supported by National Institute of Neurological Disorders and Stroke grants, R01 NS067128 and R21 NS043730 and a grant from the American Parkinson’s Disease Association to ACG, as well as NIH K12 HD043444 Building Interdisciplinary Research in Women’s Health, the Davis Phinney Foundation, and the Parkinson Disease Foundation to TE. Our recruitment efforts were supported, with our gratitude, by Marie Saint-Hilaire, MD and Cathi Thomas, RN, MSN, of Boston Medical Center Neurology Associates, by the Fox Foundation Trial Finder, and by the support staff of the Vision and Cognition Laboratory. We thank Karina Stavitsky Gilbert, Ph.D., and Abhishek Jaywant, Ph.D., for assistance with data collection. We are especially grateful to the individuals who participated in this study.
Footnotes
Conflict of interest
The authors do not have a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Phillips LJ, Dropping the bomb: the experience of being diagnosed with Parkinson’s disease. Geriatric Nursing, 2006. 27(6): p. 362–369. [DOI] [PubMed] [Google Scholar]
- 2.Ma H-I, et al. , Stigma as a key determinant of health-related quality of life in Parkinson’s disease. Quality of Life Research, 2016. 25(12): p. 3037–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermanns M, The invisible and visible stigmatization of Parkinson’s disease. Journal of the American Association Nurse Practitioners, 2013. 25(10): p. 563–6. [DOI] [PubMed] [Google Scholar]
- 4.Molina Y, et al. , (2013). The stigma scale for chronic illnesses 8-item version (SSCI-8): development, validation and use across neurological conditions. International Journal of Behavioral Medicine, 20(3): p. 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tickle-Degnen L, Zebrowitz LA, and Ma HI, Culture, gender and health care stigma: Practitioners’ response to facial masking experienced by people with Parkinson’s disease. Social Sicence & Medicine, 2011. 73(1): p. 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis TM, et al. , Reoperation for suboptimal outcomes after deep brain stimulation surgery. Neurosurgery, 2008. 63(4): p. 754–60; 760–761. [DOI] [PubMed] [Google Scholar]
- 7.Lyons KE and Pahwa R, Long-term benefits in quality of life provided by bilateral subthalamic stimulation in patients with Parkinson disease. Journal of Neurosurgery, 2005. 103(2): p. 252–5. [DOI] [PubMed] [Google Scholar]
- 8.Schrag A, et al. , Young-versus older-onset Parkinson’s disease: impact of disease and psychosocial consequences. Movement Disorders, 2003. 18(11): p. 1250–6. [DOI] [PubMed] [Google Scholar]
- 9.Miller IN and Cronin-Golomb A, Gender differences in Parkinson’s disease: clinical characteristics and cognition. Movement Disorders, 2010. 25(16): p. 2695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posen J, et al. , Young women with PD: a group work experience. Social Work in Health Care, 2000. 32(1): p. 77–91. [DOI] [PubMed] [Google Scholar]
- 11.Barlow DH, Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist, 2000. 55(11): p. 1247. [DOI] [PubMed] [Google Scholar]
- 12.Beck AT, The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry, 2008. 165(8): p. 969–977. [DOI] [PubMed] [Google Scholar]
- 13.Dibble LE, et al. , Charting the progression of disability in Parkinson disease: study protocol for a prospective longitudinal cohort study. BMC Neurology, 2010. 10(1): p. 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes AJ, et al. , Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery & Psychiatry, 1992. 55(3): p. 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz CG, et al. , Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Movement Disorders, 2004. 19(9): p. 10201028. [DOI] [PubMed] [Google Scholar]
- 16.Goetz CG, et al. , Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement Disorders, 2008. 23(15): p. 2129–2170. [DOI] [PubMed] [Google Scholar]
- 17.Jankovic J, et al. , Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology, 1990. 40(10): p. 1529–1534. [DOI] [PubMed] [Google Scholar]
- 18.Stebbins GT, et al. , How to identify tremor dominant and postural instability/gait difficulty groups with the Movement Disorder Society Unified Parkinson’s Disease Rating Scale: Comparison with the Unified Parkinson’s Disease Rating Scale. Movement Disorders, 2013. 28(5): p. 668–670. [DOI] [PubMed] [Google Scholar]
- 19.Peto V, Jenkinson C, and Fitzpatrick R, PDQ-39: A review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. Journal of Neurology, 1998. 245(1): p. S10–S14. [DOI] [PubMed] [Google Scholar]
- 20.Fahn S and Elton R, Unified Rating Scale for Parkinson’s Disease Recent developments in Parkinson’s disease. Florham Park. New York: Macmillan, 1987: p. 153–163. [Google Scholar]
- 21.Goetz CG, Stebbins GT, and Tilley BC, Calibration of Unified Parkinson’s Disease Rating Scale scores to Movement Disorder Society-Unified Parkinson’s Disease Rating Scale scores. Movement Disorders, 2012. 27(10): p. 1239–1242. [DOI] [PubMed] [Google Scholar]
- 22.Yesavage JA, et al. , Development and validation of a geriatric depression screening scale: a preliminary report. Journal Psychiatric Research, 1982. 17(1): p. 37–49. [DOI] [PubMed] [Google Scholar]
- 23.Hayes AF, PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. 2012.
- 24.Shrout PE and Bolger N, Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods, 2002. 7(4): p. 422–45. [PubMed] [Google Scholar]
- 25.Preacher KJ and Hayes AF, SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers, 2004. 36(4): p. 717–731. [DOI] [PubMed] [Google Scholar]
- 26.Hayes AF, Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. 2013: Guilford Press. [Google Scholar]
- 27.Calne SM, Lidstone SC, and Kumar A, Psychosocial issues in young-onset Parkinson’s disease: current research and challenges. Parkinsonism & related disorders, 2008. 14(2): p. 143–150. [DOI] [PubMed] [Google Scholar]
- 28.Prediger RD, et al. , Anxiety in Parkinson’s disease: a critical review of experimental and clinical studies. Neuropharmacology, 2012. 62(1): p. 115–24. [DOI] [PubMed] [Google Scholar]
- 29.Shulman L, et al. , Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism & related disorders, 2002. 8(3): p. 193–197. [DOI] [PubMed] [Google Scholar]
- 30.Rao D, et al. , Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Quality of Life Research, 2009. 18(5): p. 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]