Abstract
Intrahepatic cholangiocarcinoma (iCCA) has over the last 10–20 years become the focus of increasing concern largely due to its rising incidence and high mortality rates worldwide. The significant increases in mortality rates from this primary hepatobiliary cancer, particularly over the past decade or so, has coincided with a rapidly growing interest among clinicians, investigators, and patient advocates seeking greater mechanistic insights and more effective biomarker-driven targeted approaches for managing and/or preventing this challenging liver cancer. In addition to discussing challenges posed by this aggressive cancer, this review will emphasize recent epidemiological, basic and translational research findings for iCCA. In particular, we highlight emerging demographic changes and evolving risk factors, the critical role of the tumor microenvironment, extracellular vesicle biomarkers and therapeutics, inter- and intra-tumoral heterogeneity, and current and emerging targeted therapies regarding iCCA. Specifically, recent evidence linking non-bile duct medical conditions, such as non-alcoholic fatty liver disease and nonspecific cirrhosis to intrahepatic cholangiocarcinogenesis, together with geographic and ethnic variation are assessed. Recent developments concerning the roles played by transforming growth factor-β and platelet derived growth factor-D in driving the recruitment and expansion of cancer-associated myofibroblasts within cholangiocarcinoma stroma, as well as their therapeutic implications are also discussed. In addition, the potential significance of extracellular vesicles as novel bile and serum biomarkers and therapeutic delivery systems for iCCA are described. An integrated systems approach to classifying heterogeneous iCCA sub-types is further highlighted, and recent clinical trials and emerging targeted therapies are reviewed, along with recommendations for future translational research opportunities. Established international cholangiocarcinoma networks are now acting to facilitate collaborations aimed at advancing iCCA translational and clinical research.
Keywords: risk factors, desmoplastic microenvironment, extracellular vesicles, molecular profiling, targeted therapy
Intrahepatic cholangiocarcinoma (iCCA) is a rare, highly aggressive and often times fatal primary epithelial cancer arising within liver. iCCAs are largely diagnosed at an advanced non-curable stage (e.g., multicentric disease within the liver, lymph node and/or peritoneal metastasis) and are usually occurring sporadically in patients without recognizable risk factors. We now recognize that many previous cancers reported as adenocarcinomas of unknown primary (CUP) are in fact iCCA.
More than 90% of iCCAs are adenocarcinomas exhibiting biliary differentiation biomarkers, and can be subdivided by genomic and epigenomic profiling into etiologically distinct subtypes (1). Unlike hepatocellular carcinoma (HCC), most iCCAs develop in non-cirrhotic liver, although it has been reported that about 8–10% of all iCCAs occur in patients with liver cirrhosis (2). Unlike conventional HCCs, mass-forming iCCAs are typically characterized by a prominent desmoplastic and hypovascularized tumor stroma, which often represents the dominant histological feature of the tumor (3).
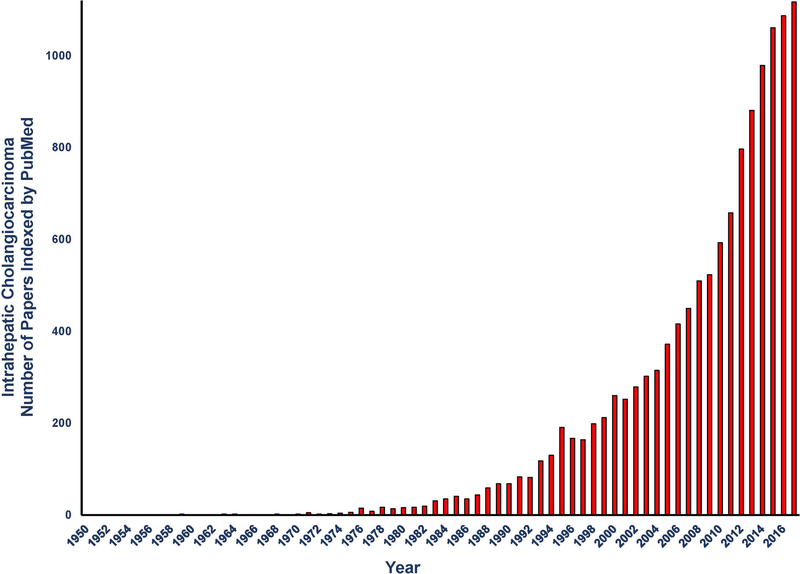
The increasing incidence and accompanying rising mortality rates for iCCA particularly during the last 10 to 20 years has coincided with a rapidly growing interest in iCCA as an important and highly challenging liver disease. This rising interest is reflected in Figure 1 showing a dramatic exponential increase in the numbers of iCCA papers indexed yearly by PubMed from between 1950 and 2017.
Figure 1.
Graphic representation of the number of intrahepatic cholangiocarcinoma (iCCA) articles published yearly in PubMed from 1950 to 2017. Between 1950 and 1970, only 14 publications on iCCA were indexed compared with 10,852 being indexed from the years 2000 to 2017. From Alexandru Dan Corlan. Medline trend: automated yearly statistics of PubMed results for any query. Web resource at URL:http://dancorlan.net/medline-trend.html.
Despite recent progress in the standard of care and management options for iCCA, the prognosis for this devastating cancer remains dismal (4). Curative intent surgical resection is the best option for achieving long-term survival outcomes. However, only a fraction (e.g., 30–40%) of iCCA patients are candidates for curative intent surgery. Moreover, the recurrence rates for iCCA after surgery are very high, reported to range from 40 to 80% (5), with less than one-third of the patients who undergo curative-intent surgery found to survive beyond 5 years after resection (4). The combination of gemcitabine and cisplatin is the first line systemic therapy for patients with locally advanced iCCA, but this treatment is not curative and at best provides only a modest increase in overall survival times. To date, limited clinical trials with targeted therapies to treat iCCA have also been disappointing (6).
Although the clinical challenges currently posed by iCCA are daunting, rising awareness leading to increased iCCA research over the past several years are providing new insights into the evolving risk factors, cellular and molecular pathogenesis, whole genomic and epigenomic profiling, and emerging targeting and prevention opportunities for individualizing iCCA therapy and/or prevention. This review will highlight a number of such recent advances, particularly as they relate to current developments in the areas of changing demographics and risk factors, tumor microenvironment and molecular pathway alterations, iCCA heterogeneity, identification of novel diagnostic and/or prognostic biomarkers, as well as emerging targeted agent and immunotherapies.
Epidemiology, Demographics, and Risk Factors
There is considerable geographic variability in the world-wide incidence of iCCA, with the highest rates seen in Eastern Asia when compared with Western countries (7). Geographic variation in iCCA incidence in the United States has also been reported, with the highest rates seen in the Northeast, and upper Midwest, and in the southwest from Texas to California and coastal counties of the Pacific, particularly in Hawaii and Alaska (8). iCCA risk factors together with environmental, genetic, ethnic, and age population differences in particular regions might partially explain these demographic variations, but do not appear to fully explain the dramatic apparent rise in iCCA incidence and mortality rates observed in the United States over the past four decades. It is also important to note that most cases of cholangiocarcinoma (e.g., 40–70%) appear to occur sporadically without obvious risk factors.
The Surveillance, Epidemiology, and End Results (SEER) data have been studied to assess long-term trends in the age-standardized incidence of iCCA and extrahepatic cholangiocarcinoma. In a recently reported analysis of SEER data to assess the trends in age-adjusted incidence of iCCA versus that of extrahepatic cholangiocarcinoma over the past 40-years between 1973 and 2012, it was found that the incidence of iCCA increased from 0.44 to 1.18 cases per 100,000; an annual percentage change (APC) of 2.3%. Disturbingly, this rise was also seen to have progressed during the past 10-years with an APC of 4.4% (9). In comparison, the incidence of extrahepatic cholangiocarcinoma increased slightly during this same 40-year time frame to 1.02 per 100,000. Thus, from this analysis, which corrected for systematic coding errors that avoided misclassifying perihilar CCA (described as “Klatskin tumors”) as iCCA, as well as took into account trends in CUP incidence over the same time period, it was concluded that the number of cases of iCCA in the U.S. continues to increase, while the level of extrahepatic cholangiocarcinoma appears to have stabilized (9).
Considering the current lack of an accurate and consistent international classification practice for CCA, it has been suggested that bile duct cancers be sub-classified as iCCA, perihilar CCA, and distal CCA (with the term Klatskin being omitted altogether) when assessing CCA incidence trends (7). Such a system would, if adopted, represent a major advance towards validating the results described above, as well as provide over the coming decades more accurate assessments of CCA incidence trends.
iCCA is also overrepresented as a health disparity in minority communities, including African-Americans, Hispanics, Asian or Pacific Islanders, and American Indian/Alaskan Native populations (10). Moreover, iCCA incidence and mortality rates were significantly higher among men of all races at age 45 years compared with those younger than 45 years and with women, respectively.
Various other risk factors prevalent in the advanced economically developed world and commonly associated with HCC are now also being recognized as being linked to an increased risk of iCCA. Prominent among these non-bile duct specific diseases are chronic hepatitis C virus (HCV) and hepatitis B virus (HBV) infection, non-alcoholic fatty liver disease, non-alcoholic steatohepatitis (NASH), alcoholic liver disease, and autoimmune hepatitis (11). Non-specific cirrhosis has been identified as a particularly strong risk factor for iCCA (11). The mechanisms by which these other risk factors contribute to iCCA development are not yet clear.
The Desmoplastic Stromal Reaction in iCCA: Key Molecular Drivers and Therapeutic Implications
iCCAs typically exhibit a prominent desmoplastic reaction largely characterized by the formation of a dense collagen type 1 fiber-enriched tumor stroma, and containing α-smooth muscle actin-positive cancer-associated myofibroblasts (α-SMA+CAFs), whose increasing prominence in the tumor stroma was found to correlate with poorer survival outcomes in iCCA patients following surgical resection of their primary liver tumors (3). Varying degrees of inflammatory cells, most notably tissue associated macrophages (TAMs) and tumor associated neutrophils, as well as endothelial cells, are also seen (albeit, typically accumulated to lesser extents than α-SMA+CAFs) in desmoplastic stroma of iCCAs, with an increased density of the M2-TAM subtype in iCCA having been shown to relate to metastasis potency (12). Increased deposition of extracellular matrix (ECM) proteins [e.g., periostin (Postn), tenascin-C (Tnc)] whose hypersecretion has also been associated with poorer prognosis in iCCA patients following surgical resection (13, 14) is another characteristic feature of the iCCA desmoplastic stroma.
There is now a growing body of data to convincingly implicate the desmoplastic reaction in iCCA as playing an active and crucial role in promoting progressive iCCA invasive growth and metastasis, cholangiocarcinoma cell survival, and resistance to chemo- and targeted agent therapies, and immunosuppression (13, 14). Underlying this relationship between the desmoplastic reaction and increased malignant behavior is the deleterious interplay between accumulating numbers of α-SMA+CAFs (and other stromal cell types), ECM proteins, and cholangiocarcinoma cells interacting to fuel malignant aggressiveness and therapeutic resistance (15).
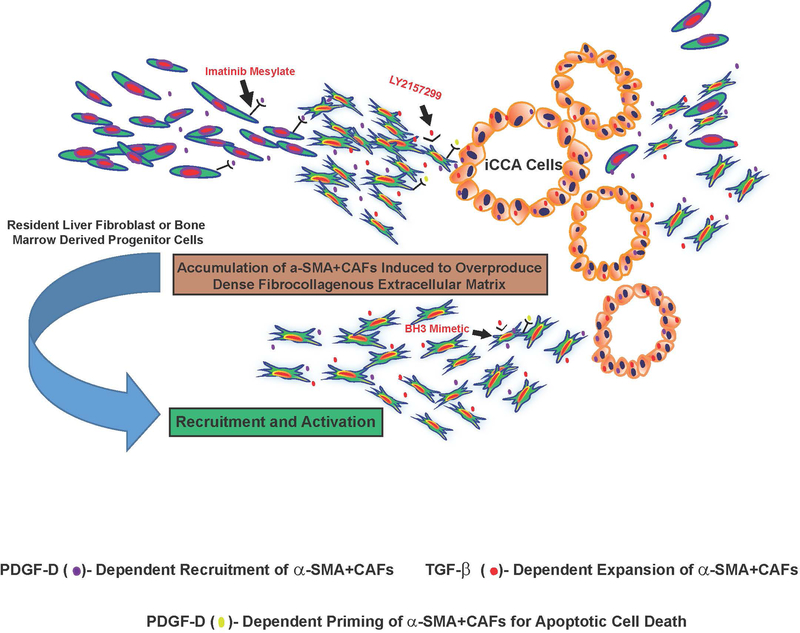
It is beyond the scope of this review to provide a comprehensive discussion of the complex cellular and molecular events that provoke, sustain, and remodel the desmoplastic stroma in iCCA. Rather, our focus will be on recent developments concerning the key roles played by TGF-β and PDGF-D in driving the recruitment and expansion of α-SMA+CAFs within iCCA stroma and their therapeutic implications (Figure 2).
Figure 2.
Schematic representation illustrating PDGF-D and TGF-β as key drivers of the desmoplastic reaction in iCCA. Resident liver fibroblasts that include portal fibroblasts, hepatic stellate cells, and periductular fibroblasts, as well as possibly fibroblastic cells derived from bone marrow progenitor cells have been suggested as potential sources of CAFs in iCCA stroma. By interacting with its cognate receptor PDGFRβ expressed by fibroblastic cells, PDGF-D secreted by cholangiocarcinoma cells has been shown to stimulate fibroblast migration mediated by Rho GTPases, notably Rac1, Cdc42 and JNK, thereby providing a novel mechanism for CAF recruitment in cholangiocarcinoma. Selective inhibition of PDGFRβ with imatinib mesylate, a tyrosine kinase inhibitor currently in clinical use for other cancer inhibitory indications, significantly blocked fibroblast migration by cholangiocarcinoma cells in vitro. In addition, PDGF-D was demonstrated to prime CAFs for apoptosis via Puma-mediated Bak activation, which could then be converted to full-blown apoptosis by BH3 mimetics. TGF-β, which is overexpressed in iCCA, being produced by both cholangiocarcinoma cells and by stromal cells, has further been shown to be an essential mediator of the desmoplastic cholangiocarcinoma phenotype in a 3-dimensional cholangiocarcinoma cell-α-SMA+CAF co-culture model, by provoking significant increases in proliferative α-SMA+CAFs together with the formation of a dense fibrocollagenous extracellular matrix characteristic of the in situ tumor. Galunisertib (LY2157299), a clinically relevant TGF-β signaling pathway inhibitor was further determined to produce a prominent dose-dependent attenuation of the dense fibrocollagenous matrix, which was accompanied by a significant decrease in α-SMA+CAFs accumulated within the supporting matrix. These findings collectively support PDGF-D and TGF-β as being important regulators of the desmoplastic reaction in iCCA and suggest novel strategies for abrogating CAF recruitment and accumulation within iCCA that may be of potential therapeutic benefit.
Transforming growth factor (TGF)-β, expressed in both cholangiocarcinoma cells and in α-SMA+CAFs was recently demonstrated to be essential for provoking a prominent desmoplastic-like reaction in vitro (dense collagen type 1 fibrogenesis, together with prominent accumulation and proliferation of α-SMA+CAFs) within a novel organotypic culture model established by co-culturing of rat cholangiocarcinoma cells (TDECC cells) with rat SMA+CAFs (TDFSM cells) within a dilute collagen type 1 hydrogel (16). Co-culturing of the TDFSM CAFs with the TDECC cholangiocarcinoma cells was further found to markedly increase mature TGF-β production within the gel cultures over that elaborated by either cell type alone, as well as to promote significantly increased cholangiocarcinoma cell growth and progression, which became increasingly more anaplastic and hyperproliferative when TGF-α was added to the culture medium (16). It is also relevant that human liver myofibroblasts isolated from colon cancer patients were shown to express heparin-binding epidermal growth factor, which not only activated epidermal growth factor receptor to promote cholangiocarcinoma cell invasive properties, but also significantly enhanced TGF-β1 expression in human cholangiocarcinoma cells, thereby providing an interactive paracrine signaling loop that favored keeping hepatic myofibroblasts in an activated state (17).
Increased cholangiocarcinoma progression manifested in vivo by enhanced cholangiocarcinoma tumor growth and increased numbers of gross metastasis was also observed in a rat cholangiocarcinoma model in which syngeneic rats were inoculated intrahepatically with tumorigenic BDEneu rat cholangiocytes transfected to overexpress TGF-β1 (18), although the specific effects of overexpressing this cytokine on the desmoplastic reaction in the tumor was not investigated in this study. However, in addition to its effects on promoting α-SMA+CAF expansion, TGF-β1 is also a known inducer of ECM proteins, notably collagen type 1, Postn and Tnc, which are highly germane to supporting and sustaining iCCA’s desmoplastic microenvironment. Of further note, a novel TGF-β-induced circular isoform of long noncoding RNA was just recently demonstrated to be highly up-regulated in human iCCA and shown to promote an inflammatory microenvironment in human iCCA, associated with an overexpression of proinflammatory cytokines (19).
Like TGF-β, PDGF-D, an isoform belonging to the platelet-derived growth factor (PDGF) family, is also considered to be a key driver of the desmoplastic reaction, particularly in relation to its effects on α-SMA+CAF recruitment. Based on results from a compelling study by Cadamuro et al. (20), these authors have proposed a novel model in which PDGF-D, selectively overexpressed in cholangiocarcinoma cells via a hypoxia mediated mechanism, is released into the tumor cell environment where it binds to and activates its cognate receptor PDGFRβ present on fibroblastic cells. This interaction, in turn, then provides a proliferative stimulus, as well as elicits a strong migratory response that involves Rho GTPases (Rac-1 and Cdc42) and JNK activation that leads to fibroblast recruitment by cholangiocarcinoma cells. This study further extended the role of PDGFRβ molecular targeting in iCCA, as fibroblast migration was shown to be significantly inhibited with imatinib mesylate, a clinically relevant PDGFR inhibitor, as well as by silencing PDGF-D expression in cholangiocarcinoma cells.
The therapeutic relevance of specifically targeting molecular pathways that drive and support the desmoplastic reaction in iCCA and other solid cancers has in recent years become an area of growing interest. Dosing of thioacetamide-treated rats with 1D11 monoclonal antibody against TGF-β was found to significantly reduce TGF-β1 transcription, significantly decrease collagen deposition, ameliorate pre-existing fibrosis, and strikingly reduce cholangiocarcinoma area within the livers of these animals (21). In vitro screening of several clinically relevant targeted agents in the TDECC-TDFSM co-culture model described above also recently revealed the TGF-β pathway inhibitors (galunisertib and halofuginone) to be the most potent among the various other agents tested for their ability to produce a significant concentration-dependent attenuation of the desmoplastic-like stroma induced by 3-D co-culturing of the cholangiocarcinoma cells with α-SMA+CAFs (16).
Interestingly, PDGF-D and PDGF-B, both of which are abundantly produced by cholangiocarcinoma cells, were shown to prime hepatic myofibroblasts for apoptosis triggered by the BH3 mimetics navitoclax and ABT-199, respectively (22). In the rat BDEneu cholangiocarcinoma model, navitoclax treatment induced α-SMA+CAF apoptosis, diminished the expression of desmoplastic stroma Tnc, suppressed BDEneu liver tumor size and metastasis, and improved survival (23). Similarly, ABT-199 was demonstrated to reduce tumor formation and tumor burden in a murine model of cholangiocarcinoma (22).
The preclinical results with galunisertib and with BH3 mimetics offer a proof of concept that treatments aimed at ablating the desmoplastic stroma in iCCA may provide a therapeutic benefit, although major challenges still remain and must be overcome before translating these findings into a suitable and well tolerated clinical treatment strategy for iCCA therapy. For example, targeting the TGF-β pathway in chronic liver diseases might also possibly increase the risk for cholangiocarcinoma development by diminishing the restrictive function of TGF-β on cholangiocyte proliferation in risk conditions, as was shown by Mu et al. (24) in a liver specific TGF-β receptor 2/PTEN double knockout mouse model. Clearly, a better understanding of the functions of the desmoplastic reaction in iCCA is needed in order to exploit its potential as a therapeutic target while avoiding any possibility of accelerating malignant aggressiveness.
Extracellular Vesicles
Extracellular vesicles (EVs) are derived from multivesicular bodies or plasma membrane and carry RNA species, as well as proteins, amplified gene sequences, and lipids. Although their physiologic role is currently unknown, EVs are released into the peri-cellular environment, as well as into body fluids and circulating blood, and as such, may transmit signals from donor to recipient cells.
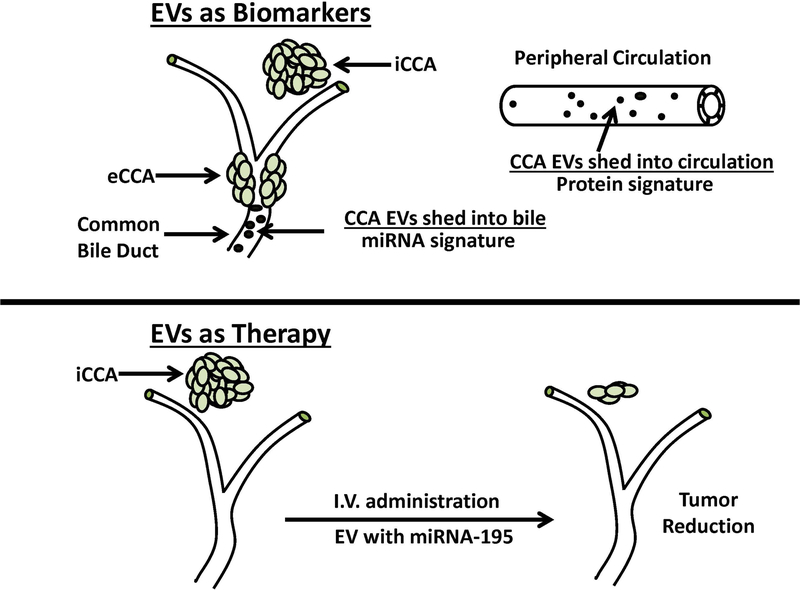
Recent translational studies have focused on utilizing EVs for 2 main utilizations: (1) EVs as markers of disease and (2) therapeutic interference by EVs with built in signaling pathways. Figure 3 highlights the potential utility of EVs as novel biological markers and miRNA delivery systems for CCA diagnosis and therapy.
Figure 3.
Extracellular vesicles (EVs) as bile and serum biomarkers for cholangiocarcinoma diagnosis and as carriers of tumor growth suppressing miRNA for cholangiocarcinoma therapy. As illustrated in the upper panel of the figure, human bile was shown to contain abundant EVs with a distinctive 5-miRNA signature panel for cholangiocarcinoma diagnosis that demonstrated a sensitivity of 67% and a specificity of 96%, and which was further reported to be better than CA19–9 in identifying patients with early cancers. As also illustrated here, human serum EVs were determined to contain a distinctive proteomic signature for cholangiocarcinoma that had differentiated diagnostic capacity in distinguishing cholangiocarcinoma patients from those with primary sclerosing cholangitis (PSC). Interestingly, some EV containing protein biomarkers, such as fibrinogen gamma chain, alpha-1-acid glycoprotein 1, and S100A8 showed higher diagnostic values in early stage cholangiocarcinoma versus PSC patients than CA19–9. The lower panel depicts the results of a preclinical study demonstrating that LX2 hepatic stellate cell-derived EVs loaded with miRNA-195 administered by intravenous (I.V) injection in a rat model of aggressive orthotopic cholangiocarcinoma significantly suppressed intrahepatic tumor growth and improved the survival of the treated animals over the control group. iCCA, intrahepatic cholangiocarcinoma. eCCA, extrahepatic cholangiocarcinoma. See text for additional details.
iCCA EV biomarkers.
EVs have the ability to protect their cargo (including RNA species) from enzyme degradation in plasma and bile. Therefore, EVs were investigated as microRNA-based biomarkers of inflammatory liver diseases in plasma (25). A more recent study further demonstrated that bile EVs contain an abundance of microRNA species (26). Interestingly, this study outlined a panel of microRNA species isolated from bile EVs that were able to diagnose CCA with a sensitivity of 67% and specificity of 96%.
Proteins within serum EVs were also found to be excellent biomarkers, able to differentiate between patients with primary sclerosing cholangitis (PSC) versus those with CCA or HCC, as well as normal liver controls (27). Since PSC is the most important risk factor for perihilar CCA in the USA and since clinically, it is oftentimes difficult to differentiate between a non-malignant stricture in PSC and frank CCA, the differentiation between PSC and CCA was of utmost importance. The study found that fibrinogen gamma chain, alpha 1-acid glycoprotein 1, and S100A8 were reliably overexpressed in EVs from CCA versus PSC. Here, also, SpyGlass single operator choledochoscopy can provide a valuable complementary approach for localizing and facilitating the diagnosis of intraductal malignant lesions occurring as complications of high risk conditions for cholangiocarcinoma, such as PSC.
iCCA therapeutic interference by EVs.
The involvement of EVs in physiologic and pathologic inter-cellular crosstalk prompted their utilization as carriers of active molecules. Notably, miR-195 within EVs was successfully delivered to iCCA cells in a rat cholangiocarcinoma model, resulting in decreased tumor size and increased survival (28). This study further demonstrated that EVs concentrate in the tumor mass, as opposed to being distributed throughout the liver in a non-discriminating fashion. The implication, although not yet demonstrated, is that the production of EVs by activated stellate cells in this study, could have conferred specificity for CCA cells. The topic of specificity, as well as efficient production of EVs for clinical utilization remain areas of intense investigation, as well as controversy. Further studies are necessary to precisely outline the role of EVs as therapeutics in liver diseases and to contrast them to existing micro-delivery platforms, such as liposomal and polymeric nanoparticles.
Molecular Profiling and iCCA Heterogeneity
Molecular profiling of iCCA has revealed a complex mutational landscape with vast inter-tumor heterogeneity (sample by sample) and intra-tumor (within each tumor) heterogeneity that are attributable in part to diverse multifactorial etiologies among different ethnic groups, histological tumor differences, and evolving malignant progression. Such inter- and intra-tumor molecular heterogeneity represents a major obstacle for early diagnosis and effective treatment and poses a major challenge to defining drivers responsible for early stage cholangiocarcinogenesis, as well as in developing new and more specific diagnostic tools and effective treatment modalities for iCCA.
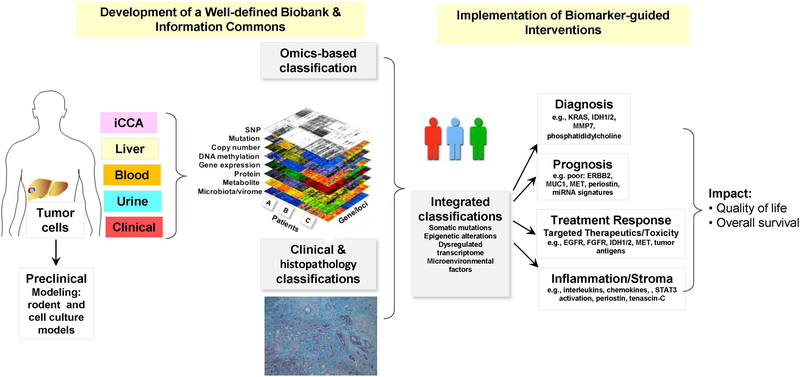
An integrated systems biology approach to classifying heterogenous iCCA subtypes, including combinations of genomic, transcriptome, metabolome, protein, epigenetic, and chromosomal analyses (29), has important implications for clinical practice, as schematically illustrated in Figure 4. Integrative molecular analysis was used to identify two distinct classes of iCCA, a proliferation subclass based on activation of oncogene signaling pathways [e.g., KRAS/RAF/ERK, IGF1R, EGFR, ERBB2, MET, NOTCH, PI3K/AKT/mTOR] and an inflammation subclass characterized by the activation of inflammatory pathways [e.g., interleukins/chemokines/STAT3 activation] (30). In this study, the proliferation class was demonstrated to exhibit a more aggressive clinical behavior, whereas the inflammation subclass defined a class of iCCA with a more favorable prognosis. In a separate study, iCCA with cholangiocellular differentiation (cd) was found to resemble an inflammation-related state, while iCCA without cd resembled a proliferation subtype (31). A subset of human cholangiocarcinomas exhibiting a strong-upregulation of ERBB2 signaling in the epithelial cell compartment of the tumor together with a concomitant overexpression in the tumor stroma of inflammatory cytokines, including IL-6, was further reported to be predictive of a highly malignant phenotype (32). Other relevant genomic alterations that include fibroblast growth factor receptor 2 (FGFR2) fusions and isocitrate dehydrogenase 1/2 (IDH1/2) mutations have also been reported to have prognostic significance for iCCA patients, and are emerging as novel targeted therapy options (see section below). Personalized genomics has also been used to identify immunogenic epitopes in iCCA, leading to novel clinical approaches based on adoptive immunity with mutation-specific CD4+ T cells (33) or personalized mutation-specific vaccination (34) to obtain positive clinical responses in a select few patients with metastatic iCCA.
Figure 4.
Schematic depiction of an integrated systems biology-based approach to sub-classifying iCCAs into distinct subgroups having important clinical implications for their diagnosis, prognosis, and targeted treatment designs. Adapted from Wang XW and Thorgeirsson SS (29). iCCA, intrahepatic cholangiocarcinoma; SNP, Single nucleotide polymorphism.
Considering the presence of complex, multifactorial etiologies in addition to gender and ethnic disparities linked to both HCC and iCCA, the Thailand Initiative in Genomics and Expression Research for Liver Cancer (TIGER-LC) Consortium was established to create a comprehensive biorepository to better classify molecular subtypes and related drivers in liver cancer (35). Utilizing an integrated systems approach involving genomics, transcriptomics and metabolomics, a Phase I study of the TIGER-LC consortium has revealed some surprising molecular similarities between HCC and iCCA, which traditionally had been treated as clinically-distinct diseases with different treatment guidelines and options. Of note, one of the common molecular subtypes of HCC and iCCA, designated C2, which was linked to obesity, inflammation (T cell infiltration) and altered bile acid metabolism was only present in the Asian, but not in the Caucasian populations analyzed in this study.Transcriptomic and metabolomic similarities between HCC and iCCA have also been detected by others (36, 37) suggesting the possibility, at least in some subtypes, of a common cellular origin.
Linking a distinct molecular footprint of iCCA to a specific causative etiology also has important implications with respect to potentially establishing whether the tumor developed as a result of past exposures to an extrinsic carcinogen or to an intrinsic genetic alteration independent of an environmental risk factor. Here, the recently reported results of an international team using an integrated genomic, epigenetic, and transcriptomic analysis of over 400 iCCA cases from 10 different countries were able to identify a liver-fluke-associated iCCA subtype uniquely enriched in ERBB2 amplifications and TP53 mutations (1). This finding is noteworthy, since it exemplifies how assessment of whole genome and epigenomic landscapes of distinct subtypes of iCCA may be of potential value in distinguishing extrinsic from intrinsic carcinogenic processes. However, as a cautionary note, the link of liver fluke to a particular iCCA subtype requires further validation, since this subtype also coincides with a particular geographic location and certain ethnicity.
Little is known about intratumor heterogeneity of iCCA. Understanding molecular features of tumor cells at a single cell level may provide a better understanding of tumor cell communities including an interplay between tumor cells and stroma cells, and help define key drivers responsible for tumor metastasis. A recent single cell analysis study has revealed cancer stem cell heterogeneity and tumor aggressiveness in HCC (38). Based on these findings, extension of single cell molecular profiling to iCCA is clearly warranted, since this approach offers new insights into intratumor heterogeneity, tumor progression and their implications for clinical management.
Molecular Targeting and Immunotherapy: IDH, FGFR, and Immune Checkpoint Inhibitors
Pharmacological Inhibitors.
IDH mutations occur frequently in iCCA (39, 40). IDH1 is more common than IDH2 and “hotspot” IDH1/2 mutations are point mutations located in the arginine 132 (R132) residue in IDH1 or the arginine 172 (R172) residue in IDH2. These mutations are ubiquitously higher in iCCA than extrahepatic cholangiocarcinoma. Mutant IDH loses its normal enzymatic activity and gains a new ability to produce the oncometabolite 2-hydroxyglutarate (2-HG), which can be detected in the tumor and blood (39, 41). Pharmacologic inhibitors highly specific to the individual IDH-mutant alleles (e.g., IDH1-R132 and IDH2-R172) have been developed. These block the function of mutant IDH1 or IDH2 at nanomolar concentrations, leading to reduced 2-HG levels.
AG-120 is a first-in-class, potent, oral inhibitor of mutant IDH1 and was examined in a phase I study in mutant IDH1 solid tumors including iCCA (42). AG-120 was well tolerated and of the 73 patients with IDH1 mutant advanced CCA enrolled, 72 patients were evaluable for efficacy. Six percent (n = 4) had a confirmed partial response and 56% (n = 40) experienced stable disease. The progression-free survival (PFS) rate at 6 months was 40%, and 8 patients were on treatment with AG-120 for ≥1 year. All of the patients responding to AG-120 exhibited a reduction in circulating 2-HG levels ranging from 73% to 99%, and a reduction in Ki67 nuclear staining ranging from 22% to 96% from baseline. A global, phase III, randomized, placebo-controlled study of AG-120 in mutant IDH1 cholangiocarcinoma is ongoing (ClarIDHy) (NCT02073994). Other IDH1 and IDH2 inhibitors are also now in clinical trials (NCT02273739, NCT02381886, and NCT02481154) and are enrolling patients with iCCA.
The recent discovery of recurrent FGFR2 fusions in 11–45% of patients with iCCA has rapidly translated this into a promising therapeutic target. Several selective reversible FGFR2 inhibitors have entered clinical trials in iCCA. The most mature data of selective FGFR inhibition in iCCA is with the oral agent BGJ-398 (Infigratinib, Novartis/QED). In the phase II study of BGJ-398 in advanced iCCA with FGFR aberrations after first-line chemotherapy (43), 61 patients with FGFR2 fusion (n = 48), mutation (n = 8), or amplification (n = 3) were enrolled. The overall response rate was 14.8% (18.8% in patients with FGFR2 fusions), disease control rate was 75.4%, and estimated median PFS was 5.8 months (95% CI, 4.3 to 7.6 months). As observed with other tumors treated with tyrosine kinase inhibitors, acquired resistance limited the durability of response in some patients.
Goyal, et al. (44) reported the first evidence of clinically acquired resistance to FGFR inhibition in an analysis of three patients with FGFR2-fusion positive iCCA who were treated with BGJ-398. Sequencing of cell-free DNA and biopsy samples collected at baseline and post-progression revealed polyclonal secondary mutations in the FGFR2 kinase domain, including the gatekeeper mutation FGFR2 V564F in all three patients. However, in vitro studies have identified structurally distinct FGFR inhibitors, which may overcome the resistance, offering promise for overcoming resistance to FGFR inhibition.
Other selective FGFR inhibitors, including INCB54828 (Incyte, NCT02924376), BAY1163877 (Bayer, NCT01976741), ARQ-087 (Arqule, NCT01752920), and Debio1347 (Debiopharm International, NCT01948297), have also been tested in phase I trial in solid tumors, including iCCA, with early evidence of antitumor activity. TAS-120, an oral, highly selective, irreversible FGFR1–4 tyrosine kinase inhibitor, has demonstrated inhibition of cancer cell growth in human xenografts of tumors bearing FGFR aberrations and has exhibited potent inhibition against both mutant and wild-type FGFR2. In a Phase I study of TAS-120 in patients with advanced solid tumors, including iCCA, antitumor activity was observed with a response rate of approximately 25% in iCCA patients carrying the FGFR2 fusions (45).
Immunotherapy for cholangiocarcinoma.
Cytotoxic T lymphocytes can recognize and destroy cancer cells, but are often inhibited by a variety of mechanisms inherent to cancer biology. For example, malignant cells and/or other cell types in the tumor immune microenvironment (TIME) express so called checkpoint proteins, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and others (46). Immune-checkpoint inhibitors, antibodies that block these check point proteins have been applied to a variety of human cancers with durable and impressive response rates in a subset of patients, and are now approved for use in a variety of cancers. Immune-checkpoint genes and high-mutational load with abundant tumors-specific neoantigens have been reported in iCCA, justifying the use of these checkpoint inhibitors in iCCA (47). Interim data from the KEYNOTE-028 basket trial (NCT02054806) of the anti-PD-1 antibody pembrolizumab have been reported in abstract form for patients with biliary tract cancer (48). A signal for efficacy was apparent in a subset of patients, leading to a larger trial of pembrolizumab in biliary tract cancers, the KEYNOTE-158 basket trial (NCT02628067). The results of these trials are awaited with anticipation.
Adjuvant Therapy
As pointed out in the Introduction, the recurrence rate for iCCA after curative-intent surgery remains very high, prompting a much greater need to develop and support novel strategies for adjuvant chemo- and targeted agent therapeutic trials. The recently completed BILCAP phase III randomized trial (ISRCTN72785446; NCT00363584)) has now led to adjuvant capecitabine being recommended as the standard of care following curative surgery for biliary tract cancers of all types, including iCCA, based on an overall survival outcome of 53 months for the capecitabine group compared with 36 months for the observational group not receiving capecitabine. The randomized PRODIGE12-ACCORD 18 phase III trial (NCT01313377) aimed at evaluating adjuvant gemcitabine and oxaliplatin in resected biliary tract cancers of all types was also recently completed, but without any demonstrable benefit in any of the analyzed subgroups.
Translational Opportunities
Herein, we highlighted key areas of current and evolving translational research focused on addressing critical challenges that continue to limit progress towards achieving significant improvements in iCCA clinical management and patient survival outcomes. Towards this end, we need more information on how HCV and HBV-related cirrhosis, metabolic syndrome, and NASH contribute to increased iCCA risk. The discovery that iCCA can originate from transdifferentiation of hepatocytes is an important clue that links parenchymal diseases to iCCA development. Moreover, there needs to be a universally accepted standardized classification system for CCA so as to provide more accurate estimates of global iCCA incidence relative to that of perihilar CCA, distal CCA and CUP. It is also now becoming increasingly evident that the desmoplastic microenvironment of iCCA plays an active and crucial role in cholangiocarcinoma cell development, malignant progression, immunosuppression, and therapeutic resistance. Clearly there is a compelling need to expand funding for basic and translational research aimed at defining mechanisms of tumor stromal-cholangiocarcinoma cell interactions and on identifying and testing novel strategies that target the tumor stroma in combination with those against the cholangiocarcinoma cells. Research aimed at developing non-invasive serological tests based on multiplexing of discreet cholangiocarcinoma and associated CAF biomarkers could be highly useful as early diagnostic and/or prognostic platforms for iCCA. Furthermore, both CCA cell and associated CAF-derived extracellular vesicles containing protein and micro-RNA markers should be more fully investigated for their diagnostic/prognostic potential, and perhaps even more so for their ability to serve as unique carriers of therapeutic molecules. Efforts to advance and validate an integrated systems biology approach to classifying iCCA subtypes and for identifying the genetic and molecular drivers of iCCA progression should continue to be actively pursued as an important part of a global effort to stratify iCCA patients for personalized systemic therapies. Although enrolling sufficient numbers of iCCA patients into stratified, randomized clinical trials remains challenging due to the relative rarity of this “orphan” cancer, efforts on the part of well organized international CCA research networks, such as TIGER-LC consortium, the European Network for the Study of Cholangiocarcinoma (ENS-CCA), and the International Cholangiocarcinoma Research Network (ICRN), together with patient advocate groups like the Cholangiocarcinoma Foundation are helping to facilitate establishing international translational and clinical research collaborations that hopefully will evolve into multicenter clinical trials with sufficient power to assess personalized iCCA therapies.
Acknowledgments
Financial Support: Supported by National Institutes of Health grants R01 CA 083650 and R13 CA 216895 to A.E.S, R01 CA 190610, R35 CA 197222, and P30 CA 006973 to J.D.G., R01 EB 017742 and R01 CA 190040 to F.M.S., R01 DK 079005, R01 DK 096096, and P30 DK 034989: Silvio O. Conte Digestive Diseases Research Core Centers to M.S., and by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute grants Z01 BC 010877, Z01 BC 010876 and Z01 BC 010313 to X.W.W.
Abbreviations:
- iCCA
intrahepatic cholangiocarcinoma
- CUP
adenocarcinomas of unknown primary
- HCC
hepatocellular carcinoma
- SEER
Surveillance, Epidemiology, and End Results
- APC
annual percentage change
- HCV
hepatitis C virus
- HBV
hepatitis B virus
- NASH
non-alcoholic steatohepatitis
- α-SMA
alpha-smooth muscle actin
- CAF
cancer-associated fibroblast
- TAMs
tissue associated macrophages
- ECM
extracellular matrix
- Postn
periostin
- Tnc
tenascin-C
- TGF
transforming growth factor
- PDGF
platelet-derived growth factor
- EVs
extracellular vesicles
- PSC
primary sclerosing cholangitis
- FGFR2
fibroblast growth factor receptor 2
- IDH1/2
isocitrate dehydrogenase 1/2
- 2-HG
2-hydroxyglutarate
- PFS
progression-free survival
- cd
cholangiocellular differentiation
- CTLA-4
cytotoxic T-lymphocyte-associate protein 4
- PD-1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
Footnotes
G.J.G, J.D.G., F.M.S., M.S., X.W.W., and A.X.Z. are each equal contributors to the writing of this review article, which is partially derived from presentations and discussions held at the conference “Hepatobiliary Cancers: Pathobiology and Translational Advances” co-sponsored in part by the American Association for the Study of Liver Diseases and The Cholangiocarcinoma Foundation, and held in Glen Allen, VA on December 7–10, 2017.
References
- 1.Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov 2017; 7: 1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jesper D, Heyn SG, Schellhaas B, Pfeifer L, Goertz RS, Zopf S, et al. Effects of liver cirrhosis and patient condition on clinical outcomes in intrahepatic cholangiocarcinoma: a retrospective analysis of 156 cases in a single center. Eur J Gastroenterol Hepatol 2018; 30: 552–556. [DOI] [PubMed] [Google Scholar]
- 3.Sirica AE, Gores GJ. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology 2014; 59: 2397–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatmen and prognosis for patients with intrahepatic cholangiocarcinoma Systematic review and meta-analysis. JAMA Surg. 2014; 149: 565–574. [DOI] [PubMed] [Google Scholar]
- 5.Wirth TC and Vogel A. Surveillance in cholangiocellular carcinoma. Best Pract Res Clin Gastroenterol 2016; 30: 987–999. [DOI] [PubMed] [Google Scholar]
- 6.Moeini A, Sia D, Bardeesy N, Mazzaferro V, Llovet JP. Molecular pathogenesis and targeted therapies for intrahepatic cholangiocarcinoma. Clin Cancer Res; 22: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridgewater J, Galle PR, Khan SA, Llovet JM, Park J-W, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014; 60: 1268–1289. [DOI] [PubMed] [Google Scholar]
- 8.Altekruse SF, Petrick JL, Rolin AI, Cuccinelli JE, Zou Z, Tatalovich Z et al. Geographic variation of intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and hepatocellular carcinoma in the United States. PLos One 2015; 10: e0120574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist 2016; 21: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antwi SO, Mousa OY, Patel T. Racial, ethnic, and age disparities in incidence and survival of intrahepatic cholangiocarcinoma in the United States; 1995–2014. Ann Hepatol 2018; 17: 274–285. [DOI] [PubMed] [Google Scholar]
- 11.Massarweh NN, El-Serag HB. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control 2017; 24: 1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thanee M, Loilome W, Techasen A, Namwat N, Boonmars T, Pairojkul P et al. Quantitative changes in tumor-associated M2 macrophages characterize cholangiocarcinoma and their association with metastasis. Asian Pac J Cancer Prev 2015; 16: 3043–3050. [DOI] [PubMed] [Google Scholar]
- 13.Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2012; 9: 44–54. [DOI] [PubMed] [Google Scholar]
- 14.Affo S, Yu L-X, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol Mech Dis 2017; 12: 153–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cadamuro M, Stecca T, Brivio S, Mariotti V, Fiorotto R, Spirli C et al. The deleterious interplay between tumor epithelia and stroma in cholangiocarcinoma. Biochim Biophys Acta 2018: 1864: 1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manzanares MÁ, Usui A, Campbell DJ, Dumur CI, Maldonado GT, Fausther M et al. Transforming growth factors α and β are essential for modeling cholangiocarcinoma desmoplasia and progression in a three-dimensional organotypic culture model. Am J Pathol 2017: 187: 1068–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clapéron A, Mergey M, Aoudjehane L, Ho-Bouldoires THN, Wendum D, Prignon A, et al. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology 2013; 58: 2001–2011. [DOI] [PubMed] [Google Scholar]
- 18.Huang C-K, Aihara A, Iwagami Y, Yu T, Carlson R, Koga H et al. Expression of transforming growth factor β1 promotes cholangiocarcinoma development and progression. Cancer Lett 2016; 380: 153–162, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merdrignac A, Angenard G, Allain C, Petitjean K, Bergeat D, Bellaud P et al. A novel transforming growth factor beta-induced long noncoding RNA promotes an inflammatory microenvironment in human intrahepatic cholangiocarcinoma. Hepatol Commun 2018; 2: 254–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cadamuro M, Nardo G, Indraccolo S, Dall’Olmo L, Sambado L, Moserle L et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 2013; 58: 1042–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling H, Roux E, Hempel D, Tao J, Smith M, Lonning S et al. Transforming growth factor β neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLoS One 2013; 8: e54499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizvi S, Mertens JC, Bronk SF, Hirsova P, Dai H, Roberts LR, et al. Platelet-derived growth factor primes cancer-associated fibroblasts for apoptosis. J Biol Chem 2014; 289: 22835–22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res 2012; 73: 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mu X, Pradere J-P, Affò S, Dapito DH, Friedman R, Lefkovitch JH et al. Epithelial transforming growth factor-β signaling does not contribute to liver fibrosis but protects mice from cholangiocarcinoma. Gastroenterology 2016; 150: 720–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 2012; 56: 1946–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Masica D, Ishida M, Tomuleasa C, Umegaki S, Kalloo AN, et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology 2014; 60: 896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbelaiz A, Azkargorta M, Krawczyk M, Santos-Laso A, Lapitz A, Perugorria MJ, et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2017; 66: 1125–1143. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Piontek K, Ishida M, Fausther M, Dranoff JA, Fu R, et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology 2017; 65: 501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang XW, Thorgeirsson SS. The biological and clinical challenge of liver cancer heterogeneity. Hepat Oncol 2014; 1: 349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 2013; 144: 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee H, Ko JE, Chung T, Jee BA, Kwon SM, Nahm JH et al. Transcriptomic and histopathological analysis of cholangiolocellular differentiation trait in intrahepatic cholangiocarcinoma. Liver Int 2018; 38: 113–124. [DOI] [PubMed] [Google Scholar]
- 32.Andersen J, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 2012; 142: 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran E, Turcotte S, Gros A, Robbins PF, Lu Y-C, Dudley ME et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014; 344: 641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Löffler MW, Chandran PA, Laske K, Schroeder C, Bonzheim I, Walzer M et al. Personalized peptide vaccine-induced immune response associated with long-term survival of a metastatic cholangiocarcinoma patient. J Hepatol. 2016; 65: 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaisaingmongkol J, Budhu A, Dang H, Rabibhadana S, Pupacdi B, Kwan SM et al. Common molecular subtypes among asian hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell 2017; 32: 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo HG, Lee JH, Yoon JH, Kim CY, Lee HS, Jang JJ et al. Identification of a cholangiocarcinoma-like gene expression trait in hepatocellular carcinoma. Cancer Res 2010; 70: 3034–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami Y, Kubo S, Tamori A, Itami S, Kawamura E, Iwaisako K et al. Comprehensive analysis of transcriptome and metabolome analysis in intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Sci Rep 2015; 5: 16294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng H, Pomyen Y, Hernandez MO, Li C, Livak F, Tang W et al. Single cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology 2018; Epub ahead of print. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 2012; 17: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kipp BR, Voss JS, Kerr SE, Barr Fritcher EG, Graham RP, Zhang L, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol 2012; 43: 1552–1558. [DOI] [PubMed] [Google Scholar]
- 41.Borger DR, Goyal L, Yau T, Poon RT, Ancukiewicz M, Desphande V, et al. Circulating oncometabolite 2-hydroxyglutarate is a potential surrogate biomarker in patients with isocitrate dehydrogenase-mutant intrahepatic cholangiocarcinoma. Clin Cancer Res 2014; 20: 1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowery MA, Abou-Alfa GK, Burris HA, Janku F, Shroff RT, Cleary JM, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol 2017; 3515_suppl.4015 [Google Scholar]
- 43.Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol 2018; 36: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goyal L, Sasha SK, Liu LY, Siravegna G, Leshchiner I, Ahronian LG, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov 2017; 7: 252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goyal L, Hendrik-Tobias A, Ben T, Soria J-C, Bahleda R, Mak G, et al. Early clinical efficacy of TAS-120, a covalently bound FGFR inhibitor, in patients with cholangiocarcinoma. Ann Oncol 2016; 28: 145. [Google Scholar]
- 46.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018; 24: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015; 47: 1003–1010. [DOI] [PubMed] [Google Scholar]
- 48.Rizvi S, Khan SA, Hallemeier CL, Kelley RK,Gores GJ. Cholangiocarcinoma-evolving concepts and therapeutic strategies. Nature Rev Clin Oncol 2018; 15: 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]