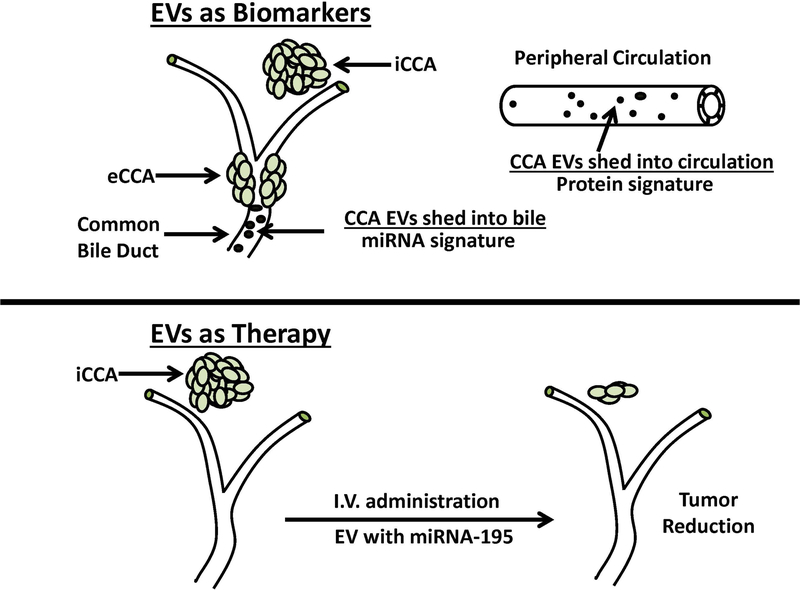
Figure 3.
Extracellular vesicles (EVs) as bile and serum biomarkers for cholangiocarcinoma diagnosis and as carriers of tumor growth suppressing miRNA for cholangiocarcinoma therapy. As illustrated in the upper panel of the figure, human bile was shown to contain abundant EVs with a distinctive 5-miRNA signature panel for cholangiocarcinoma diagnosis that demonstrated a sensitivity of 67% and a specificity of 96%, and which was further reported to be better than CA19–9 in identifying patients with early cancers. As also illustrated here, human serum EVs were determined to contain a distinctive proteomic signature for cholangiocarcinoma that had differentiated diagnostic capacity in distinguishing cholangiocarcinoma patients from those with primary sclerosing cholangitis (PSC). Interestingly, some EV containing protein biomarkers, such as fibrinogen gamma chain, alpha-1-acid glycoprotein 1, and S100A8 showed higher diagnostic values in early stage cholangiocarcinoma versus PSC patients than CA19–9. The lower panel depicts the results of a preclinical study demonstrating that LX2 hepatic stellate cell-derived EVs loaded with miRNA-195 administered by intravenous (I.V) injection in a rat model of aggressive orthotopic cholangiocarcinoma significantly suppressed intrahepatic tumor growth and improved the survival of the treated animals over the control group. iCCA, intrahepatic cholangiocarcinoma. eCCA, extrahepatic cholangiocarcinoma. See text for additional details.