TO THE EDITOR:
Aspirin-exacerbated respiratory disease (AERD) is a condition of the upper and lower respiratory tract characterized by chronic rhinosinusitis with nasal polyps (NP), asthma, tissue eosinophilia, and respiratory reactions to nonsteroidal anti-inflammatory drugs (NSAIDs) that inhibit cyclooxygenase-1.1 Daily high-dose aspirin therapy has been shown to reduce the rate of NP regrowth and improve asthma symptoms in 67%−78% of patients with AERD and is a recommended therapeutic.2, 3 In addition to the acute respiratory symptoms following NSAID exposure, a subset of patients with AERD also experience gastrointestinal symptoms including abdominal pain, cramping and dyspepsia upon acute and long-term exposure to NSAIDs.2, 4, 5
While most patients with AERD tolerate aspirin therapy without incident, it has been linked to eosinophilic esophagitis, coronary artery vasospasm, and gastric irritation which may lead to the discontinuation of aspirin.4, 6–8 In one retrospective analysis of patients with AERD desensitized to aspirin, 24 (13%) of 172 patients discontinued aspirin in the first year of treatment because of side effects, with the vast majority (53%) discontinuing due to epigastric pain.2 While most cases of epigastric pain on high-dose aspirin are attributed clinically to gastric irritation from NSAIDs, other etiologies exist. In our clinical experience, some patients with AERD who reported gastrointestinal symptoms demonstrated biopsy-proven esophageal eosinophilia (EE). Here we describe the presence of EE before and during aspirin therapy in a large cohort of patients with AERD.
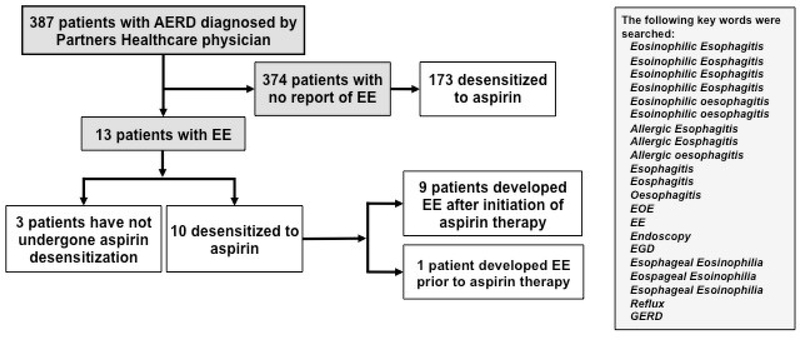
We performed a retrospective chart review of 387 patients enrolled in the Brigham and Women’s Hospital (BWH) AERD registry between September 2013 and April 2018. All subjects were diagnosed with AERD by a Partners Healthcare (BWH, Massachusetts General Hospital and Massachusetts Eye and Ear Infirmary) physician. Charts were reviewed for the presence of agreed-upon terms and then audited in detail (Figure 1). A patient-reported history of biopsy-proven EE was required for inclusion as a case of EE. This study was approved by the Partners Healthcare IRB and all subjects provided written consent. Data were extracted from the electronic medical record (Epic Systems, Verona, Wis) using a query tool.9 All analyses were performed using R™ software (version 3.3.3; R Foundation for Statistical Computing, Vienna, Austria) with means and standard deviations reported.
Figure 1. Flow diagram of study population.
387 charts were reviewed for the presence of agreed-upon terms and then audited in detail for history and biopsy-proven EE. Of the 387 charts reviewed, 13 patients had a history of EE and 10 underwent aspirin desensitization. 9 of the 10 patients with EE were diagnosed with EE after initiation of daily aspirin therapy.
Of the 387 charts reviewed, 13 (3.4%) patients had a history of EE documented. Of these 13 patients, 10 (77%) underwent aspirin desensitization with 9 (90%) of the 10 diagnosed with EE after initiation of daily aspirin therapy (Figure 1). Among those placed on aspirin, 8 had information available on the treatments used to achieve symptom improvement: 6 modified the aspirin dose (5 discontinued including 1 patient with a prior history of EE who reported recurrence of symptoms 6 months on aspirin and 1 dose reduced before being lost to follow-up), 4 initiated a proton pump inhibitor (PPI), 2 employed dietary modification, and 2 began steroids. Those never on aspirin initiated PPIs with 2 adding dietary modification. Pathology reports from an esophagogastroduodenoscopy (EGD) were available for 4 patients after initiation of aspirin therapy. These patients underwent EGD due to reflux symptoms and/or dysphagia. The 9 remaining patients underwent EGD outside of the Partners Healthcare system.
Of the 4 patients with available pathology reports, patients began aspirin 3 months, 1 year, 3 years and 6 years prior to biopsy confirmed EE. Three reports were notable for eosinophils in the esophageal mucosa, while 1 showed eosinophils in the gastroesophageal junction. All biopsies showed > 15 eosinophils/high power field (hpf), ranging from 21 to 90 eosinophils/hpf.
The 13 patients with EE showed no difference from the non-EE population in sex, self-reported age at diagnosis of asthma, NP and first NSAID-induced reaction, or baseline blood eosinophil counts. Prior to a trial of aspirin therapy, the EE population reported a history of faster NP regrowth, defined as the time from previous sinus surgery to visualization of NPs by physician exam or imaging, as compared to the non-EE population (4.75±3.35 months versus 14.9±20.8 months, P=<.0001). The EE and non-EE population had similar follow up periods after aspirin desensitization (Table 1).
Table 1.
Analysis of AERD populations with and without Esophageal Eosinophilia
| Total BWH AERD Cohort | Completed Aspirin Desensitization | |||
|---|---|---|---|---|
| Descriptive Property | AERD without EE n=374 | AERD with EE n=13 | AERD without EE n=173 | AERD with EE n=10 |
| Sex (Female), % | 57.3 | 53.8 | 53.1 | 60 |
| Age at 1st NSAID Reaction* | 37.0 ± 13.0 | 38.6 ± 11.3 | 38.2 ± 13.9 | 36.7 ± 8.1 |
| Age at NP Diagnosis | 37.0 ± 12.3 | 37.5 ± 11.1 | 37.6 ± 12.5 | 35.5 ± 8.8 |
| Time to polyp regrowth (months) | 14.9 ± 20.8 | 4.75 ± 3.35# | 16.1 ± 23.8 | 4.6 ± 3.3 |
| Prior history of reflux, n | 180 (48%) | 8 (62%) | 79 (43%) | 5 (50%) |
| FEV1% Predicted | 84.2 ± 19.5 | 88.3 ± 15.5 | 86.0 ± 19.8 | 85.9 ± 16.0 |
| Absolute eosinophil count (/μL) | 670 ± 390 | 530 ± 370 | ||
| Patients with gastric irritation during desensitization | 30 (16%) | 2 (20%) | ||
| Patients with gastric irrigation at follow-up visits** | 7 (4%) | 7 (70%)^ | ||
| Patients that discontinued aspirin due to gastric irritation** | 6 (3%) | 2 (20%)@ | ||
| Duration of follow-up periods (months) | 27.5 ± 27.2 | 17.5 ± 15.4 | ||
Means and standard deviations are reported.
Test of significance was performed using an independent two-sample t-test assuming an un-pooled variance (Welch’s t-test). A two-tailed p-value was reported.
Test of independence was performed with a Pearson’s Chi-Squared test with 1 degree of freedom, demonstrating significance at an alpha level of 0.05 for rejection of independence.
p<0.0001
p<.001
p<0.05
Among those in the BWH registry who underwent aspirin desensitization, the non-EE (n=173) and EE (n=10) groups reported similar rates of acid reflux symptoms prior to aspirin exposure and gastrointestinal symptoms during the acute aspirin-induced reaction. AERD subjects with EE demonstrated greater rates of gastric irritation at follow-up visits (P<0.001) and of aspirin discontinuation due to gastric irritation (P<0.05) than the non-EE group (Table 1).
Our study is the first to explore the relationship between aspirin therapy in AERD patients and the development of EE. We observed that EE can develop in subjects with AERD both before and after initiation of aspirin therapy. We found that AERD patients with EE had significantly faster regrowth of NPs than the general AERD registry population suggesting more severe sinonasal mucosal pathology. There were no other baseline clinical differences noted between the two subgroups to help in identifying which patients are most likely to experience EE. This observation of increased tissue eosinophils outside the respiratory tract highlights the disease spectrum of AERD can include systemic involvement.
Respiratory tract eosinophilia is well described in AERD. Tissue mast cell hyperplasia and activation with subsequent release of prostaglandin (PG)D2 is understood as one driving factor of tissue eosinophilia in AERD3, 4. High levels of PGD2 were previously associated with abdominal symptoms during aspirin-induced reactions in patients with AERD. PGD2 is also implicated as a potent chemotactic factor for eosinophils in eosinophilic esophagitis10. Although our retrospective data is insufficient to confirm or exclude the diagnosis of eosinophilic esophagitis in those subjects with EE or explain the mechanism by which eosinophils accumulate in the esophagus in AERD, it is plausible that similar biochemical pathways are implicated in the trafficking of eosinophils to the esophagus in AERD.
Larger prospective studies are needed to further characterize the pathophysiology and relationship between patients with AERD and the development of EE at baseline and after starting aspirin therapy. It is important for clinicians to recognize EE as potential cause of epigastric pain in patients with AERD on and off aspirin therapy.
Clinical implication:
This study provides clinical evidence for the presence of esophageal eosinophilia in a subgroup of patients with severe aspirin-exacerbated respiratory disease before and during treatment with high-dose aspirin therapy. Patients with AERD and upper gastrointestinal symptoms should be evaluated for the presence of esophageal eosinophilia.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (NIH grant nos U19AI095219, K23HL111113, K23AI118804, R01HL128241, T32AI00730), and by generous contributions from the Vinik and Kaye Families.
Footnotes
Conflict of Interest: T Laidlaw has served on scientific advisory boards for GlaxoSmithKline and Allakos and has received consultancy fees from Knopp Biosciences and Sanofi-Genzyme. K Cahill has served on scientific advisory boards for Teva, Regeneron, and Optinose. R Eid, M Palumbo, and K Buchheit have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J 2000; 16:432–6. [DOI] [PubMed] [Google Scholar]
- 2.Berges-Gimeno MP, Simon RA, Stevenson DD. Long-term treatment with aspirin desensitization in asthmatic patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2003; 111:180–6. [DOI] [PubMed] [Google Scholar]
- 3.Buchheit KM, Laidlaw TM. Update on the Management of Aspirin-Exacerbated Respiratory Disease. Allergy Asthma Immunol Res 2016; 8:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2015; 135:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee RU, White AA, Ding D, Dursun AB, Woessner KM, Simon RA, et al. Use of intranasal ketorolac and modified oral aspirin challenge for desensitization of aspirinexacerbated respiratory disease. Ann Allergy Asthma Immunol 2010; 105:130–5. [DOI] [PubMed] [Google Scholar]
- 6.Hempel SL, Elliott DE. Chest pain in an aspirin-sensitive asthmatic patient. Eosinophilic esophagitis causing esophageal dysmotility. Chest 1996; 110:1117–20. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier T, Tamayev R, Iammatteo M, Nautsch D, Hudes G, Lukin D, et al. Eosinophilic esophagitis as possible complication of aspirin treatment in patient with aspirinexacerbated respiratory disease. Ann Allergy Asthma Immunol 2017; 118:120–2. [DOI] [PubMed] [Google Scholar]
- 8.Shah NH, Schneider TR, DeFaria Yeh D, Cahill KN, Laidlaw TM. Eosinophilia-Associated Coronary Artery Vasospasm in Patients with Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract 2016; 4:1215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill KN, Johns CB, Cui J, Wickner P, Bates DW, Laidlaw TM, et al. Automated identification of an aspirin-exacerbated respiratory disease cohort. J Allergy Clin Immunol 2017; 139:819–25 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuta GT. Eosinophils in the esophagus: acid is not the only cause. J Pediatr Gastroenterol Nutr 1998; 26:468–71. [DOI] [PubMed] [Google Scholar]