Abstract
Animals must ingest water via drinking to maintain fluid homeostasis, yet the neurons that specifically promote drinking behavior are incompletely characterized. The lateral hypothalamic area (LHA) as a whole is essential for drinking behavior but most LHA neurons indiscriminately promote drinking and feeding. By contrast, activating neurotensin (Nts)-expressing LHA neurons (termed LHA Nts neurons) causes mice to immediately drink water with a delayed suppression of feeding. We therefore hypothesized that LHA Nts neurons are sufficient to induce drinking behavior and that these neurons specifically bias for fluid intake over food intake. To test this hypothesis we used designer receptors exclusively activated by designer drugs (DREADDs) to selectively activate LHA Nts neurons and studied the impact on fluid intake, fluid preference and feeding. Activation of LHA Nts neurons stimulated drinking in water-replete and dehydrated mice, indicating that these neurons are sufficient to promote water intake regardless of homeostatic need. Interestingly, mice with activated LHA Nts neurons drank any fluid that was provided regardless of its palatability, but if given a choice they preferred water or palatable solutions over unpalatable (quinine) or dehydrating (hypertonic saline) solutions. Notably, acute activation of LHA Nts neurons robustly promoted fluid but not food intake. Overall, our study confirms that activation of LHA Nts neurons is sufficient to induce drinking behavior and biases for fluid intake. Hence, LHA Nts neurons may be important targets for orchestrating the appropriate ingestive behavior necessary to maintain fluid homeostasis.
Keywords: Fluid Homeostasis, Osmolality, Water intake, Feeding, Ingestive Behavior, DREADDs
1. Introduction
Water intake is necessary to maintain cellular osmolality and homeostasis, therefore drinking is an essential behavior for survival. Dysregulation of drinking behavior, either too much or too little water, endangers health. For example, excessive water intake after extreme exercise, use of the drug Ecstasy or psychogenic polydipsia can lead to hyponatremia and severe neurological consequences that can cause death (Bourque, 2008; Dundas et al., 2007; Hawken et al., 2009; McKinley et al., 2004). Inadequate water intake promotes life-threatening cardiovascular impairment (Baron et al., 2015; Maughan, 2012) and may be due to voluntary or involuntary restriction of drinking. While individuals with anorexia purposely limit fluid intake to control body weight (Steffen et al., 2007), elderly persons have impaired ability to sense thirst that leads to unintentionally reduced drinking (Koch and Fulop, 2017; Phillips et al., 1984). Yet, despite the exigency of maintaining appropriate hydration for health there is still much to understand about how drinking behavior is regulated.
The brain contributes to fluid homeostasis by monitoring serum osmolality and, in turn, coordinates appropriate peripheral actions and drinking behavior needed to restore any imbalance (Zimmerman et al., 2017). Neurons in the lateral terminalis (LT) are the first-line osmolality sensors and detect whether there is a “need” for water (Abbott et al., 2016; Matsuda et al., 2016; Nation et al., 2016; Oka et al., 2015; Zimmerman et al., 2016) but they do not themselves modulate water handling. Instead, LT neurons send afferents to numerous brain regions that, in sum, control water intake and fluid management. For example, LT projections to the supraoptic nucleus and paraventricular hypothalamus (PVH) coordinate release of the hormone arginine vasopressin to regulate peripheral fluid handling (Kinsman et al., 2017; Larsen and Mikkelsen, 1995; Oldfield et al., 1994) and these circuits are vital for whole-body fluid balance. However, the brain regions that orchestrate the behavioral drinking response remain incompletely defined.
The lateral hypothalamic area (LHA) is a neuronally heterogeneous brain region that is positioned to orchestrate drinking because it receives inputs from LT neurons (Goto et al., 2005; Hahn and Swanson, 2012, 2010; Saper et al., 1979) and is known for controlling ingestive behavior. Indeed, electrical stimulation of the whole LHA induces drinking (Mogenson and Stevenson, 1967) whereas lesion of the LHA causes adipsia, aphagia and death from self-inflicted dehydration and starvation (Morrison et al., 1958; Stricker, 1976). Hence, the LHA contributes to both ingestive behaviors, but most research has focused on how LHA neurons regulate feeding (Stuber and Wise, 2016). By comparison, the role of the LHA in mediating water intake behavior has been less studied. The best characterized LHA populations are those expressing the neuropeptides melanin-concentrating hormone (MCH) (Bittencourt et al., 1992; Zamir et al., 1986) or orexin/hypocretin (OX) (de Lecea et al., 1998; Sakurai et al., 1998) and the large population of LHA neurons expressing the classical neurotransmitter GABA (Jennings et al., 2015; Karnani et al., 2013). Multiple independent reports show that the MCH, OX and LHA GABA neurons comprise separate, non-overlapping populations (Kurt et al., 2017; Brown et al., 2017; Jennings et al., 2015; Karnani et al., 2013; Stuber and Wise, 2016). Although some MCH and OX neurons express GABA synthesis enzymes, they lack the proteins necessary for vesicular packaging and release of GABA, and hence are functionally distinct from LHA GABA signaling neurons (Mickelsen et al., 2017). These MCH, OX and GABA populations are activated by specific peripheral cues and pathways, but they all generally promote ingestive behavior and do not appear to specifically coordinate feeding vs. drinking. For example, MCH neurons in the LHA promote intake of both food and liquids (Clegg et al., 2003; Domingos et al., 2013; Sakamaki et al., 2005). Since MCH neurons lack inputs from osmosensory centers (Kumar et al., 2008), and preferentially increase intake of palatable solutions but not water (Domingos et al., 2013), it is likely that MCH neurons primarily direct reward-based drinking rather than drinking to resolve osmolality imbalance. Activation of the adjacent OX neurons broadly induces intake of food, water and any rewarding liquids (Inutsuka et al., 2014; McGregor et al., 2011; Yamanaka et al., 1999), hence these neurons do not bias for food or liquid intake. Experimentally activating the entire population of LHA GABA neurons also generally increases ingestive behaviors, promoting intake of solid food, caloric-liquids and gnawing at non-caloric objects (e.g. wood, the home cage) (Jennings et al., 2015; Navarro et al., 2015; Nieh et al., 2015). It is now recognized, however, that LHA GABA neurons are heterogeneous (Jennings et al., 2015) and it is unlikely that they are all physiologically activated by the same cues or at the same time. Thus, while the LHA neurons studied to date generally promote food and liquid intake, it remained possible that specific subsets of LHA neurons might contribute uniquely to feeding and drinking.
Here we investigated the role of a specific subset of LHA GABA neurons that co-express the neuropeptide Neurotensin (Nts) in ingestive behavior, referred to as LHA Nts neurons (Brown et al., 2017; Goforth et al., 2014; Leinninger et al., 2011; Patterson et al., 2015). Nts is considered an anorectic neuropeptide and has been primarily studied in the context of feeding (Boules et al., 2000; Cooke et al., 2009; Luttinger et al., 1982; Sahu et al., 2001). Since dehydration increases Nts expression in the LHA (Watts and Sanchez-Watts, 2007) and pharmacologic Nts promotes drinking (Hawkins et al., 1989), we reasoned that LHA Nts neurons might contribute to drinking behavior. Indeed, we previously showed that activating LHA Nts neurons causes an immediate, voracious increase in water intake with a delayed suppression of feeding (Woodworth et al., 2017). This divergent effect on ingestive behavior is strikingly different from the general increase in drinking and feeding induced by activating MCH, OX or all LHA GABA neurons. It remained unclear, however, if LHA Nts neurons generally promote fluid intake or if they selectively promote intake of water. We, therefore, investigated the nature of the fluid intake triggered by activation of LHA Nts neurons and whether activation of LHA Nts neurons is sufficient to bias intake of any fluid, regardless of its palatability, over food intake.
2. Materials and Methods
2.1. Animals
Mice were bred and housed on a 12-hr light and 12-hr dark cycle. Mice had ad libitum access to rodent chow (Teklad 7913) and water unless noted otherwise. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Michigan State University in accordance with Association for Assessment and Accreditation of Laboratory Animal Care and National Institute of Health guidelines. Male adult mice (14–90 wk old) were studied, and despite this substantial age range we found no alteration of LHA Nts-induced drinking behavior with aging (data not shown). Most tests were performed in a single cohort of mice between 50–90 weeks of age. Sucrose preference was performed in a separate cohort of mice between 14–35 weeks of age.
2.2. Surgery
NtsCre mice (Leinninger et al., 2011) (n=8, 2–3 months old) were stereotaxically injected with 300 nL of AAV-hSyn-DIO-hM3D(Gq)-mCherry, serotype 2 (University of North Carolina Vector Core) into each side of the LHA according to coordinates from the Paxinos and Franklin mouse brain atlas (Paxinos and Franklin, 2001) (anteroposterior: −1.34, mediolateral: +1.05, dorsoventral: 5.20). These mice were tested via a crossover design, such that each mouse received vehicle or clozapine N-oxide (CNO) injections. Due to equipment malfunction during the final sucrose preference assay the data for this cohort of mice could not be recovered. Since the mice had been euthanized the assay could not be repeated, so a new cohort of NtsCre mice with bilateral LHA injections of AAV-hSyn-DIO-hM3D(Gq)-mCherry was generated for the sucrose preference experiment (n=7).
2.3. Acute Drinking, Osmolality and Body Temperature Measurements
Mice were studied during the light cycle in their home cages, where they received intraperitoneal injections of vehicle (VEH) [1X 0.2M pH 7.4 sterile PBS] or clozapine N-oxide (CNO) [0.3 mg/kg]. Before and 4 hours after each injection the body mass, food and liquid-bottles (containing either water, 0.014% Quinine solution or 2% NaCl solution) were weighed using an electronic balance. Body temperature was measured using a digital rectal thermometer. Urine was collected prior to and 4 hours after injections, and urine osmolalities were measured using a Wescor 5520 Vapro Vapor Pressure Osmometer. For most measures n=8, but there is reduced sample size for the urine osmolality analysis due to difficulty in obtaining fresh urine samples (Figure 1E, VEH=6, CNO=7). The concentration of NaCl (2%) was chosen based on prior literature that this solution is acutely palatable to mice (such as during the 4-hr window assessed here) but prolonged access induces osmotic dehydration (Johnson et al., 2015; Mizuno et al., 2003). Several concentrations of quinine solution were screened to identify the minimum quinine concentration that caused all mice to avoid the quinine solution and to prefer water (Supp. Figure 2). Based on this assessment 0.014% quinine was selected for testing because it produced quinine avoidance (< 50% preference for quinine) in all mice.
Figure 1. Acute activation of the LHA Nts neurons promotes water intake, but not food intake.
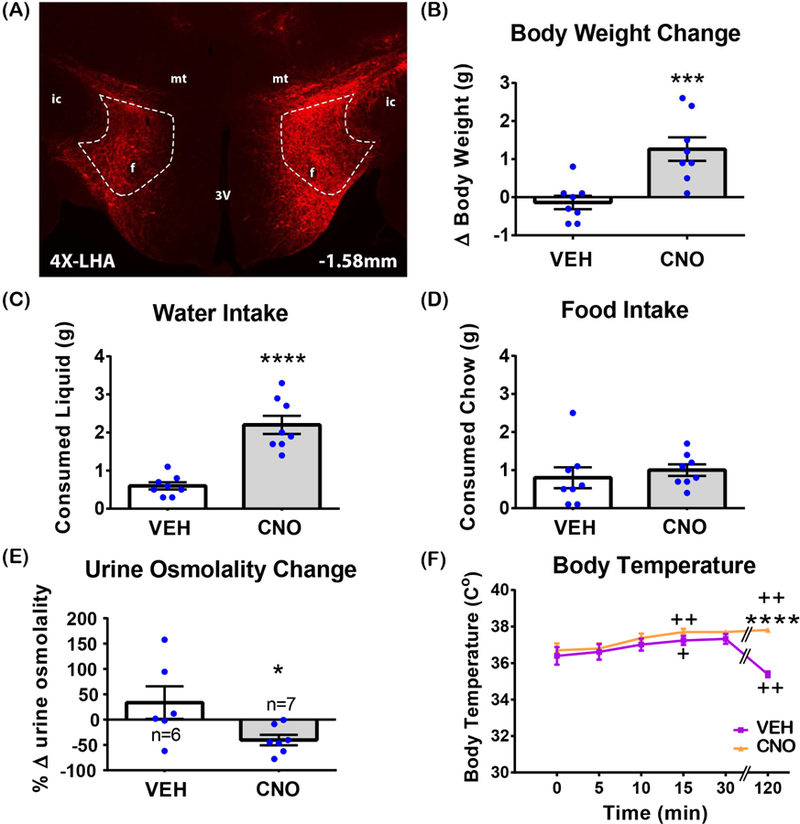
(A) Representative image of m-Cherry immunofluorescence in the LHA of NtsCre mice, confirming bilateral expression of hM3Dq-mCherry within LHA Nts neurons. Area within the dashed lines indicates the LHA. 3V - 3rd ventricle, mt-mammillothalamic tract, f- fornix, ic - internal capsule. 4X magnification. (B-F) Unless otherwise indicated on the graph, n=8. Significant differences were determined by unpaired t-tests. (B) Body weight change 4 hours fter i.p. VEH and CNO injections. p=0.0003. (C) Consumed water 4 hours after i.p. VEH or CNO injections. p<0.0001. (D) Consumed food 4 hours after i.p. VEH or CNO injections. p=0.5908. (E) Urine osmolality change 4 hours after i.p. VEH or CNO injections. p=0.0395. (F) Rectal temperature after i.p. injection of VEH or CNO. “+” indicates a time at which temperature was significantly different from t=0 and “*” indicates significant difference between VEH and CNO at the same time point. 2-way RM-ANOVA for change within a treatment group, t=0 vs t=i: F (5, 70) = 6.967, p<0.0001; (time) F (5, 70) = 6.572, p<0.0001; (treatment) F (1, 14) = 5.917, p=0.0290. Sidak`s multiple comparison: (VEH) t=0 vs t=5min p=0.9680, t=0 vs t=10min p=0.4706, t=0 vs t=15min p=0.0354, t=0 vs t=30min p=0.1552, t=0 vs t=120min p=0.0092; (CNO) t=0 vs t=5min p= 0.9680, t=0 vs t=10min p= 0.1162, t=0 vs t=15min p=0.0041, t=0 vs t=30min p= 0.0692, t=0 vs t=120min p=0.0036. 2-way RM-ANOVA for CNO versus VEH: F (5,35) = 16.38, p<0.0001; (time) F (5, 35) = 4.173, p=0.0044; (treatment) F (1, 7) = 38.71, p=0.0004. Sidak`s multiple comparison: VEH vs CNO at t=5min p= 0.6008, VEH vs CNO at t=10min p= 0.0763, VEH vs CNO at t=15min p= 0.0763, VEH vs CNO at t=30min p= 0.2674, VEH vs CNO at t=120min p<0.0001. *p<0.05; ***p<0.001; ****p<0.0001.
2.4. Euhydration vs. Dehydration Experiment
Liquid, food and body weight measurements were taken manually as indicated in section 2.3, just prior to the dark cycle, then mice were either euhydrated (EU) or dehydrated (DE) overnight(~18 hr). Mice received VEH or CNO injections in the morning and then were either given water(EU) or remained dehydrated (DE) for 5 hours, after which liquid-bottles, food and body weight were measured. Thus, this experiment had three test groups (n=8 for each): EU-EU control group (overnight euhydrated, 5-hour euhydrated), DE-EU (overnight dehydrated, 5-hour eu/rehydrated) dehydration motivated group, DE-DE (overnight dehydrated, 5-hour dehydrated) prolonged dehydration control group.
2.5. 2-Bottle Choice Tests
2 bottles, each containing water, were provided for 48 hours to acclimatize mice to the two-bottle arrangement. The position of the bottles in the cage was swapped every 24-hours to control for any place preference effects; observance of place preference during the water pre-testing would be cause for exclusion, but this was not noted in any of the mice. Next, one of the water bottles was replaced with a bottle containing the test solution so that mice had a choice of either water or 0.014% Quinine hydrochloride dihydrate (Sigma) solution, or water or 2% NaCl (Sigma), or water or 1% sucrose (Sigma). During 2-bottle preference testing the mice received twice daily injections of either VEH or CNO in the morning (9:30–10:00 am) and just before the dark cycle (5:30–6:00 pm). Since this was a crossover design, all mice served as their own controls, receiving both treatments. Mice were given at least 24-hours before repeating the experiment with the alternate treatment (e.g. 2 bottles of water for 48-hours, then water vs. other solution for 48-hours while being treated). Body mass, food and liquid bottles were weighed every 12 hr during the experiment using an electronic balance.
2.6. Verification of hM3D(Gq)-mCherry expression in LHA
After conclusion of experiments, mice received a lethal i.p. dose of pentobarbital (Fatal Plus, Vortech) followed by transcardial perfusion with 0.2M PBS (pH 7.4) and then 10% formalin (Fisher Scientific, Pittsburgh, PA). Brains were extracted, post-fixed in 10% formalin, then dehydrated in 30% sucrose solution prior to coronal sectioning (30 µm) using a sliding microtome (Leica, Buffalo Grove, IL). Sections from each brain were collected into four separate series. One series of sections from each mouse was assessed via immunofluorescence microscopy as previously described (Woodworth et al., 2017) to verify mice in which hM3D(Gq)-mCherry expression was targeted to, and confined within the LHA. The mCherry was detected using a rabbit dsRed primary antibody (1:1000, cat #:AB_10013483, Clontech), followed by anti-rabbit secondary antibody conjugated to Alexa-568(1:200, cat #: AB_2534017, Life Technologies). Images were collected with an Olympus BX53fluorescence microscope outfitted with transmitted light as well as FITC and Texas Red filters, by use of Cell Sens software and a Qi-Click 12 Bit cooled camera. Images were analyzed using Photoshop software (Adobe). Mice with bilateral mCherry expression in the LHA, without spread to neighboring brain regions, were included in the final dataset. Additionally, one mouse with unilateral mCherry expression confined to the LHA was included in the final dataset, because it demonstrated a comparable induction of drinking following CNO activation (Supp. Figure 1, mouse DQ60 identified by magenta arrow). This is consistent with our previous observations of comparable CNO-induced drinking in mice with bilateral or very well-target edunilateral hM3D(Gq)-mCherry expression in the LHA (data not shown). Importantly, CNO does not induce drinking in control mice that do not express hM3D(Gq)-mCherry in the LHA (Woodworth et al., 2017), thus the effects observed in this study are not due to off-target actions of CNO.
2.7. Statistics
2-tailed student’s t tests and 2-way ANOVA with 95% confidence intervals and alpha=0.05 were applied for the analyses by use of GraphPad Prism 7.0. All t-tests are paired unless indicated otherwise. For all data analyzed by ANOVA, either Sidak`s or Dunnett’s multiple comparisons post-hoc test was applied depending on whether every group was compared to each other or to a control group, respectively. For body temperature analyses, repeated measures 2-way ANOVA (RM-ANOVA) was applied. For the effects of hydration status/motivation tests, 2-way ANOVA was used to compare each hydration condition to the control (EU-EU) condition and within each condition. Treatment effects between two groups were analyzed by t-tests, including acute water, 2% NaCl and quinine solution intake data as well as 2-bottle preference tests.
3. Results
3.1. Activation of LHA Nts neurons induces water consumption but not feeding
NtsCre mice were injected in the LHA with AAV-hSyn-DIO-hM3D(Gq)-mCherry so that hM3D(Gq)-mCherry expressing LHA Nts neurons could be selectively activated via treatment with CNO (Figure 1A). First, the acute effects of VEH (control) or CNO-induced activation of LHA Nts neurons were assessed 4 hours after treatment. VEH treatment did not cause any alteration in body weight, but mice with CNO-mediated activation of LHA Nts neurons had significantly higher body weights (Figure 1B, p < 0.001). At first glance these data seemed contradictory with prior work showing that chemogenetic activation of LHA Nts neurons decreased body weight after 24 hours (Woodworth et al., 2017). We hypothesized that the short-term (4 hour) LHA Nts-induced weight gain could be due to the weight of immediately ingested water and/or food, which may have been missed in the prior study that only assessed long-term effects on body weight (24 hours or longer). Indeed, CNO-induced activation of LHA Nts neurons promoted voracious drinking over 4 hours (Figure 1C), but did not induce food intake during this time (Figure 1D). CNO treatment also led to a significant reduction in urine osmolality, indicating that LHA Nts-activated mice actually consumed water (Figure 1E). Thus, the acutely increased weight of LHA Nts-activated mice may be explained by the weight of the water they ingested. Over a longer time course, however, LHA Nts-induced drinking, locomoto activity and energy expenditure concomitant with a delayed restraint of feeding can produce modest weight loss (Woodworth et al., 2017). We reasoned that LHA Nts-induced energy expenditure and locomotor behavior might increase body temperature, which could drive mice to ingest water in an effort to cool the body (Sladek and Johnson, 2013). Injection of VEH and CNO both led to increased body temperature 15 minutes after injection as compared to pre-injection temperature (Figure 1F). VEH-treated control mice decreased body temperature 120min after treatment, as might be expected of mice that are no longer being handled (e.g. injected) and have resumed the low arousal and activity state typical of mice during the light cycle (Alföldi et al., 1990; Mochizuki et al., 2006 ). By contrast, body temperature of CNO-treated mice remained elevated two hours later, consistent with the prior finding that activating LHA Nts neurons increases physical activity and energy expenditure. Overall, these data reveal that acute activation of LHA Nts neurons specifically increases body temperature and promotes water, but not food, consumption.
3.2. Activation of LHA Nts neurons augments dehydration-induced drinking
We next investigated if activation of LHA Nts neurons can promote drinking behavior in the face of physiologic water need, e.g. dehydration. Water bottles were removed from the cages of mice expressing hM3D(Gq)-mCherry in LHA Nts neurons just prior to the dark cycle (when mice drink most of their daily water), thereby causing dehydration. The following morning the dehydrated mice were treated with VEH or CNO then water bottles were returned to assess drinking. As expected, dehydrated VEH-treated control mice drank water when it was restored, but CNO-mediated activation of LHA Nts neurons promoted significantly more water intake (Figure 2A). We next evaluated whether physiologic hydration status impacted LHA Nts neuronal control of feeding and body weight. CNO-mediated activation of LHA Nts neurons produced comparable food intake and body weight in consistently euhydrated (EU-EU) or dehydrated (DE-DE) mice (Figures 2B, C). By contrast, activation of LHA Nts neurons in dehydrated mice that are restored water (DE-EU mice) caused them to drink (Figure 2A) and to eat more food compared to CNO-treated euhydrated (EU-EU) controls (Figure 2C). In general, dehydration suppresses feeding in rodents (dehydration anorexia), but once water is restored they will drink and then eat (Watts, 1999). Accordingly, the CNO-induced drinking in formerly dehydrated mice (DE-EU) resolves their dehydration-induced anorexia to reinstate feeding. Although re-hydrated LHA Nts activated mice consumed more water and food they only exhibited a trend for higher body weight compared to the euhydrated (EU-EU) and dehydrated (DE-DE) controls. These data suggest that activation of LHA Nts neurons can induce water intake even beyond physiologic need.
Figure 2. Acute activation of the LHA Nts neurons increases drinking beyond physiologic need.
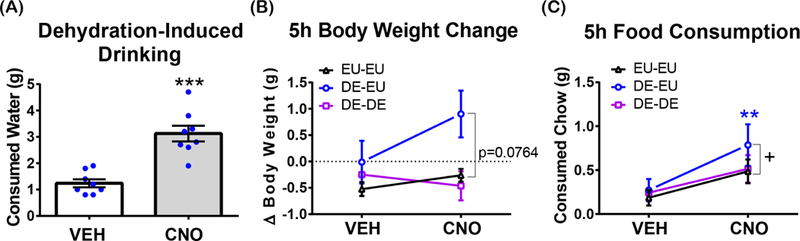
n=8 for all panels. EU-EU: mice with access to water for the entire study. DE-EU: mice that were dehydrated overnight then restored water just after treatment with VEH or CNO. DE-DE: mice that were dehydrated overnight and after treatment with VEH or CNO. (A) Dehydration-induced drinking 5 hours after i.p. injection of VEH or CNO in overnight dehydrated (DE) mice upon restoration of water (DE-EU mice). ***p=0.0005. (B) Body weight change 5 hours after i.p. injection of VEH or CNO. 2-way RM-ANOVA (2×3) for interaction of hydration status and njection condition, F (2, 14) = 1.171, p=0.3387. Hydration status: F (2, 14) = 15.58, p=0.0003. Dunnett’s multiple comparisons test for the difference between EU-EU (CNO) and DE-EU (CNO), p=0.0764. Injection condition: F (1, 7) = 2.112, p=0.1894 (C) Total food intake 5 hours after i.p. injection of VEH or CNO in overnight dehydrated. Dunnett’s multiple comparisons test for the difference between EU-EU (CNO) and DE-EU (CNO), p=0.0359. Sidak`s multiple comparisons test for the difference between DE-EU (VEH) and DE-EU (CNO), p=0.0018. 2-way RM-ANOVA (2×3) for interaction of hydration status and injection condition, F (2, 12) = 1.414, p=0.2809. Injection condition: F (1, 6) = 4.13, p=0.0884. Hydration status: F (2, 12) = 1.989, p=0.1795. “+” indicates significant difference between groups within a treatment and “*” indicates significant difference within the group between different treatments.
3.3. Activation of LHA Nts neurons promotes liquid intake regardless of palatability
LHA Nts neurons robustly induce water intake but we sought to determine if they also induce intake of other consumable liquids, and if the palatability of the liquid influences intake. To test this, mice expressing hM3D(Gq)-mCherry in LHA Nts neurons were treated with VEH or CNO, then their water bottle was replaced with one containing either hypertonic saline (2% NaCl, Figures 3A-C) or 0.014% quinine solution (Figure 3D-F). Liquid intake, food intake and body weight were measured 4 hours later. Acute activation of LHA Nts neurons significantly increased intake of 2% NaCl that increased body weight, but did not induce food intake (Figure 3A-C). Since mice find 2% NaCl palatable in the short term (Bachmanov et al., 2002), these data show that activation of LHA Nts neurons can promote intake of palatable liquids, not just water. Similarly, CNO-mediated activation of LHA Nts neurons also promoted consumption of non-palatable quinine solution compared to VEH-treated controls (Figure 3D) and acutely increased body weight (Figure 3E). Intriguingly, only the CNO-stimulated quinine intake was accompanied by an increase in feeding (Figure 3F), while consumption of an innocuous liquid (water) or a palatable liquid (2% NaCl) did not. It is possible that mice eat chow to counteract the bitter taste of the quinine. Overall, these data suggest that activation of LHA Nts neurons promotes intake of any available water-based solution regardless of its taste.
Figure 3. Acute activation of LHA Nts neurons promotes intake of available liquids.
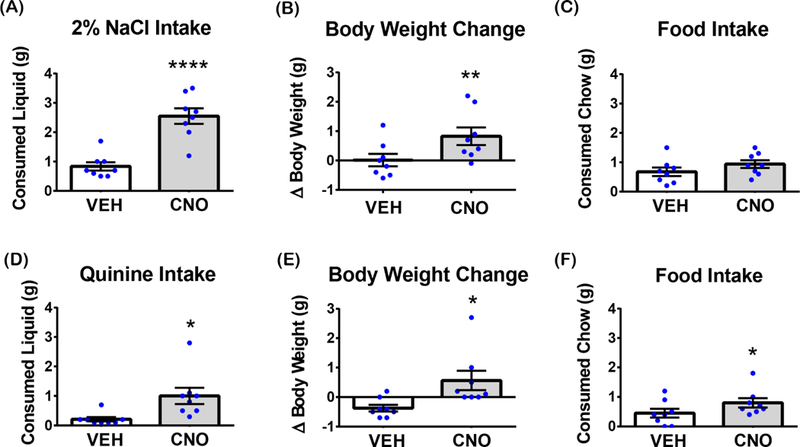
Graphed data shows the mean ± SEM, n=8. Significant differences were determined by unpaired t-tests. (A) 2% NaCl solution intake 4 hours after i.p. VEH or CNO injections, p<0.0001. (B) Body weight change 4 hours after i.p. VEH or CNO injections during acute 2% NaCl consumption, p=0.0051. (C) Food consumption 4 hours after i.p. VEH or CNO injections during acute 2% NaCl consumption, p=0.1031. (D) 0.014% quinine solution intake 4 hours after i.p. VEH or CNO injections, p=0.0272. (E) Body weight change 4 hours after i.p. VEH or CNO injections during acute 0.014% quinine consumption, p=0.0358 (F) Food consumption 4 hours after i.p. VEH or CNO injections during acute 0.014% quinine consumption, p=0.0495. *p<0.05, **p<0.01, ****p<0.0001.
3.4. Mice with activated LHA Nts neurons prefer water or palatable solutions
LHA Nts neurons are sufficient to drive intake of innocuous (water), palatable and unpalatable liquids, but this could occur via distinct mechanisms. LHA Nts neurons might promote liquid intake by increasing the physiological drive to obtain any liquid, possibly in an effort to cool the body. Alternately, activation of LHA Nts neurons might enhance preference for a liquid, which could also spur intake. To explore the latter possibility, mice expressing hM3D(Gq)-mCherry in LHA Nts neurons were treated with VEH or CNO during 2-bottle preference tests over 48 hours. First mice were given 48-hours of ad libitum access to water and 2% NaCl. Although 2% NaCl is acutely palatable, prolonged consumption causes osmotic dehydration, an unpleasant state that rodents are eager to resolve by drinking water (Watts, 1999). Hence, in this extended access experiment, 2% NaCl is a physiologically dehydrating liquid that mice should avoid drinking. VEH and CNO-treated mice exhibited a similar < 50% preference for prolonged hypertonic 2% NaCl, indicating that LHA Nts-activated mice avoided 2% NaCl in favor of water (Figure 4A). Yet, activation of LHA Nts neurons resulted in a significant increase in water intake compared to 2% NaCl over 48-hours, showing that induction still spurred drinking behavior (Figure 4B). No significant changes in body weight (Figure 4C) or feeding were observed 48 hours later (Figure 4D). Thus, these data show that LHA Nts neurons (unlike other orexigenic LHA populations) selectively promote intake of water that does not promote weight gain.
Figure 4. Activation of LHA Nts neurons does not alter NaCl solution preference.
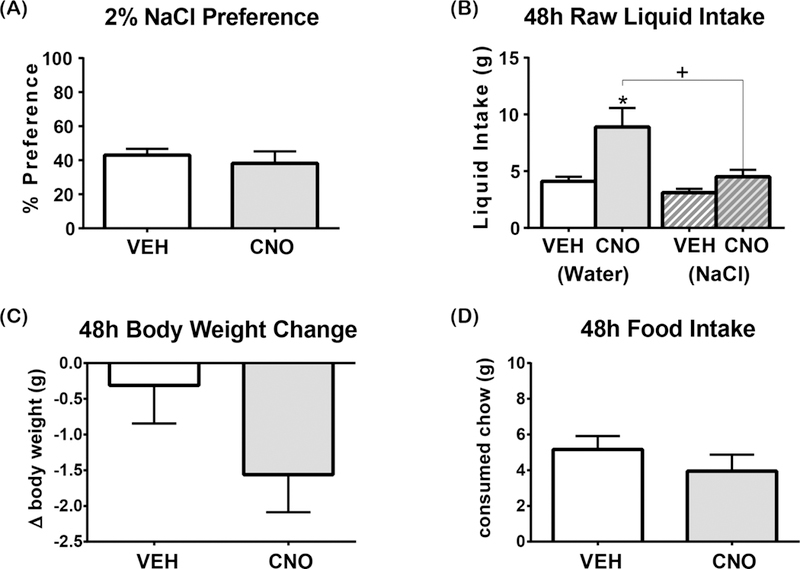
Graphed data shows the mean ± SEM, n=8. 48-hour 2-bottle preference test in which one bottle contains water and the other contains 2% NaCl. All statistical tests are two-tailed paired t-test with alpha: 0.05. (A) 2% NaCl solution preference [t(7)=0.7271, p=0.4908]. (B) Water intake [t(7)=3.334, p= 0.0125] and 2% NaCl solution intake [t(7)=2.287, p=0.0560]. (C) Body weight change over 48 hours [ t(7)=1.657, p=0.1414] (D) Food intake [t(7)=1.189, p=0.2732]. “+” indicates significant difference between the consumption of different liquids with the same treatment and “*” indicates significant difference in consumption of the same liquid between different treatments. *p<0.05, +p<0.05
We then tested if activation of LHA Nts neurons modified preference for a liquid that is normally not preferred due to its bitter taste, 0.014% quinine. Preference for quinine was similarly low (<30%) in VEH and CNO-treated mice, indicating that mice avoided drinking quinine solution and preferred drinking water (Figure 5A). CNO-mediated activation of LHA Nts neurons robustly stimulated water intake, showing that this system remains sufficient to induce drinking, and even modestly increased quinine intake (Figure 5B). Despite the increased liquid intake, mice with activated LHA Nts neurons did not exhibit weight gain over 48 hours (Figure 5C) and, in fact, showed a nearly significant reduction in feeding (Figure 5D, p = 0.0528). Thus, although activation of LHA Nts neurons will promote intake of quinine if it is the only liquid available, water is still preferred over this unpalatable substance. Together, the quinine and 2% NaCl data confirm that activation of LHA Nts neurons does not indiscriminately promote liquid intake, but does increase drinking drive that demands to be satisfied, as possible.
Figure 5. Activation of LHA Nts neurons does not alter quinine solution preference.
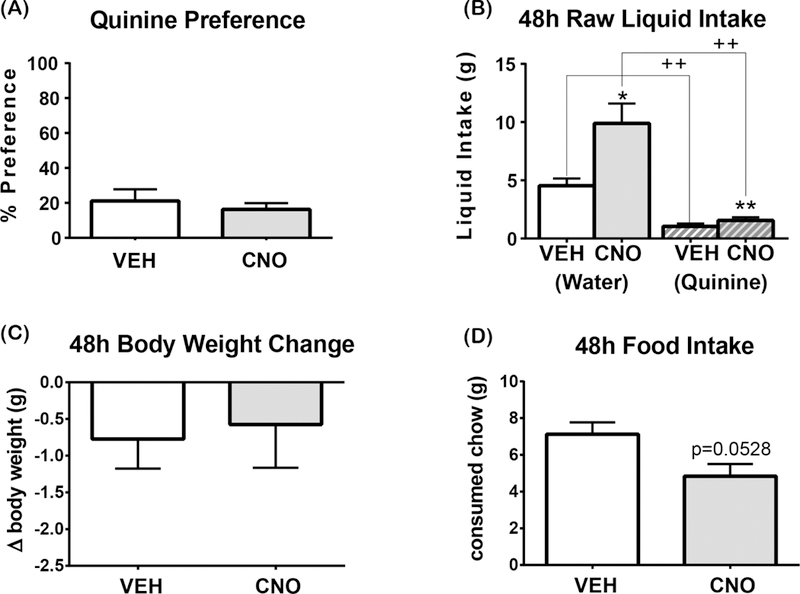
Graphed data shows the mean ± SEM, n=8. 48-hour 2-bottle preference test in which one bottle contains water and the other contains 0.014% quinine. All statistical tests are two-tailed paired t-test with alpha: 0.05. (A) 0.014% quinine preference, [t(7)=0.8685, p=0.4139]. (B) Water intake [t(7)=2.833, p=0.0253] and 0.014% quinine solution intake, [t(7)=5.274, p=0.0012]. VEH induced water vs quinine consumption: t(7)=4.41, p=0.0031. CNO-induced water vs quinine consumption: t(7)=4.549, p=0.0026. (C) Body weight change in 48 hours [t(7)=0.2587 p=0.8033]. (D) Food intake, [ t(7)=2.328, p=0.0528]. “+” indicates significant difference between the consumption of different liquids with the same treatment and “*” indicates significant difference in consumption of the same liquid between different treatments. *p<0.05, **p<0.01, ++ p<0.01.
Lastly, we investigated whether the increased drinking drive that occurs due to activating LHA Nts neurons could modify preference for palatable solutions, in this case a 1 %sucrose solution. VEH and CNO-treated mice prefer sucrose to water (>50% preference) but CNO-mediated activation of LHA Nts neurons significantly increased sucrose preference compared to the VEH-treated controls (Figure 6A). While LHA Nts activation promoted increased intake of sucrose and water, the mice consumed significantly more sucrose solution than water (Figure 6B). Importantly, the mice did not gain weight over this time despite increased caloric intake from the sucrose (Figure 6C). LHA Nts activated mice also consumed less food (Figure 6D), perhaps because they were ingesting extra calories via the sucrose solution and no longer needed to derive as many calories from food. In sum, these data indicate that activation of LHA Nts neurons is sufficient to induce drinking behavior, but that water or palatable solutions are preferred over physiologically dehydrating or unpalatable liquids.
Figure 6. Sucrose preference is increased when LHA Nts neurons are activated.
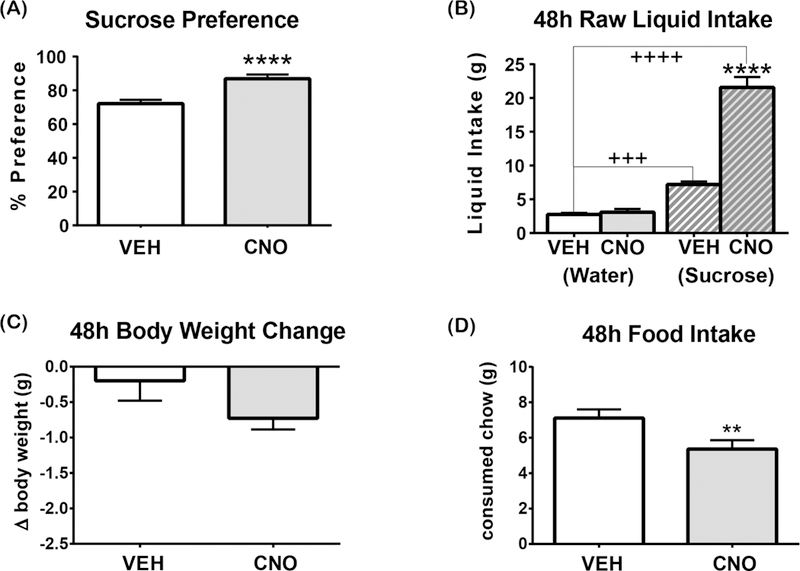
Graphed data shows the mean ± SEM, n=7. 48-hour 2-bottle preference test in which one bottle contains water and the other contains 1% sucrose. All statistical tests are two-tailed paired t-test with alpha: 0.05. (A) Sucrose preference, ****p<0.0001. [ t(6)=16.48, p<0.0001] (B) 1% sucrose intake [t(6)=11.74 , p<0.0001] and water intake [t(6)=1.202, p=0.2746]. VEH-induced water vs1% sucrose solution consumption: t(6)=8.955, p=0.0001. CNO-induced water vs 1% sucrose solution consumption: t(6)=9.896, p<0.0001. (C) Body weight change in 48 hours, [t(6)=1.584, p=0.1642]. (D) Food intake [ t(6)=4.822, p=0.0029]. “+” indicates significant difference between the consumption of different liquids with the same treatment and “*” indicates significant difference in consumption of the same liquid between different treatments. **p<0.01, ****p<0.0001, +++p<0.001, ++++p<0.0001.
4. Discussion
The LHA has long been recognized as essential for feeding and drinking behaviors, yet there remains much to understand about how the molecularly diverse LHA populations mediate these behaviors. Most of the characterized LHA populations promote intake of both food and water (Clegg et al., 2003; Domingos et al., 2013; Inutsuka et al., 2014; Jennings et al., 2015;McGregor et al., 2011; Navarro et al., 2015; Nieh et al., 2015; Sakamaki et al., 2005; Watts and Sanchez-Watts, 2007; Yamanaka et al., 1999), but other recently described populations restrain feeding without modulating water intake (Laque et al., 2015; Leinninger et al., 2009; Qualls-Creekmore et al., 2017). Thus, ingestive behaviors can be separately regulated by some LHA populations, but a population that biased for drinking behavior was yet to be described. Here we found that LHA Nts neurons are such a population because they strongly induce drinking but not feeding. Moreover, activation of LHA Nts neurons is sufficient to induce drinking behavior regardless of fluid balance status or the palatability of a single provided liquid. However, LHA Nts-induced drinking is informed by the physiologic valence and palatability of whatever liquids are available to drink, such that palatable or non-dehydrating liquids are preferred. Hence, LHA Nts neurons are a novel LHA population to bias for drinking over feeding. Going forward, it will be important to define the mechanisms by which LHA Nts neurons control drinking behavior to understand normal physiology and if disruption of LHA Nts neurons contributes to aberrant fluid balance states that threaten health, such as dehydration or excessive water ingestion.
Given that LHA Nts neurons can promote drinking behavior it will be useful to determine which precise signals mediate this physiology. Although we refer to them as “LHA Nts neurons”, the neurons express Nts and some also express the neuropeptides galanin (Laque et al., 2013; Qualls-Creekmore et al., 2017), CRH (Watts and Sanchez-Watts, 2007) and the neurotransmitter GABA (Patterson et al., 2015). It remains to be determined how each of these releasable transmitters contributes to LHA Nts-induced physiology and if there is a certain signal that mediates drinking behavior. CRH is a logical candidate via which LHA Nts neurons might regulate drinking behavior, based on its regulation in dehydration anorexia (Watts, 1992). The role of LHA Nts-induced Nts itself will be particularly important to investigate, given that Nts has been documented both as a dipsogenic (Hawkins et al., 1989) and anorectic (Boules et al.,2000; Cooke et al., 2009; Luttinger et al., 1982; Sahu et al., 2001) neuropeptide. LHA Nts-induced promotion of drinking may be partially mediated through the release of Nts and actions via the neurotensin receptor 1 (NtsR1) (Woodworth et al., 2017). Alternatively, Nts released from LHA Nts neurons may act via neurotensin receptor 2 (NtsR2). In the future, reagents to modulate the expression and function of NtsR1 or NtsR2 in a site-specific manner will be useful to determine if Nts modulates drinking behavior via either of these receptor isoforms. Furthermore, it is possible that the immediate effects of activating LHA Nts neurons (promoting drinking) and the delayed suppression of feeding (Woodworth et al., 2017) are mediated by distinct signals or pathways. Indeed, since signaling via NtsR1 is necessary for the LHA Nts-mediated suppression of feeding, but exerts a modest effect on drinking (Woodworth et al., 2017), Nts signaling may primarily contribute to regulating feeding. Alternately, other LHA Nts neuron-released neurotransmitters and/or neuropeptides may account for the immediate changes in drinking behavior, whereas long-term alteration of transcription or intracellular signaling (due in part to NtsR1) may be required to restrain feeding. Going forward, it will be important to determine how each of the releasable signals within LHA Nts neurons contribute to the control of drinking and/or feeding, and if there are dissociable mechanisms to control specific ingestive behaviors.
Our data show that LHA Nts neurons are sufficient for inducing drinking but further studies are required to determine if they are also necessary for drinking behavior and water homeostasis. The brain must coordinate osmosensory status with motivated behavior (e.g. the act of seeking and consuming water) to appropriately regulate body water balance. Given that lesioning the LHA causes adipsia (Morrison et al., 1958; Stricker, 1976) that persists even in the face of severe dehydration, this area likely contributes to coordinating physiologic water need and intake behavior. Indeed, the LHA receives input from osmosensory regions and projects to numerous brain areas that could modulate goal directed behaviors, including the ventral tegmental area (VTA) (Brown et al., 2017; Opland et al., 2013; Woodworth et al., 2017), the locus coeruleus (Laque et al., 2015), the preoptic area (Chou et al., 2002; Zuure et al., 2016) and the paraventricular hypothalamic nucleus (PVH) (Wu et al., 2015). Thus, it is not unreasonable that Nts neurons within the LHA might contribute to coordinating physiologic drinking, and their role in this process will be important to determine. Since osmoregulatory-linked drinking behavior is crucial for survival, however, it is highly likely that there are redundant mechanisms to modulate drinking behavior, including within the LHA. For example, LHA OX neurons are modulated by changes in fluid balance, and experimental activation of OX neurons induces water as well as food intake (Inutsuka et al., 2014; Watts and Sanchez-Watts, 2007).
LHA Nts neurons might conceivably be downstream targets of the neural circuitry that mediates need-based physiologic fluid intake (Bourque, 2008; Sladek and Johnson, 2013). LT neurons of the subfornical organ (SFO), median preoptic area (MnPO) and organum vasculosum of the latera terminalis (OVLT), monitor osmolality and precipitate changes in fluid intake, although the behavioral actuators are thought to be outside of the LT (Augustine et al.,2018; Matsuda et al., 2016). Since LT neurons project to the LHA (Goto et al., 2005; Hahn and Swanson, 2012, 2010; Saper et al., 1979) they could directly engage LHA Nts neurons to induce drinking behavior, though this has yet to be explored. Our current experiments using DREADD-mediated activation of LHA Nts neurons, however, may have masked their contributions to physiologic drinking behavior. For example, DREADD-mediated activation of LHA Nts neurons increased water consumption in dehydrated mice beyond physiologic need (Figure 2A), but such experimentally sustained activation is not likely to reflect the dynamics of normal physiologic regulation. Thirst-induced activation of LHA Nts neurons might be expected to attenuate once water balance has been restored. For example, thirst-activated SFO neurons are inhibited by GABAergic MnPO neurons immediately after fluid ingestion, and act as a brake to prevent drinking beyond homeostatic need (Augustine et al., 2018; Zimmerman et al., 2016). Thus, the sustained experimental activation of LHA Nts neurons in our studies may have prevented observance of an inhibitory brake in dehydrated mice, and could account for their enhanced drinking beyond homeostatic need. Future circuit based work is needed to determine if osmosensory neurons specifically project to LHA Nts neurons, and if their activity is temporally modulated with water need.
It is also possible that LHA Nts neurons mediate purely motivational aspects of drinking behavior, hence they may be sufficient to induce drinking but not required for maintenance of water homeostasis. Indeed, our finding that LHA Nts activation increased sucrose preference (Figure 6A) indicates that these neurons can enhance non-need based intake, possibly via altering motivation. Since LHA Nts neurons project to midbrain dopamine neurons (Brown et al., 2017; Opland et al., 2013; Woodworth et al., 2017) that modulate goal-directed behaviors, they are anatomically positioned to regulate motivated behaviors. Yet, motivational systems are also important for resolving homeostatic disruption. For example, recent work shows that dopamine neurons track physiologic hydration status, and only liquid intake that satisfied physiologic need caused increases in dopamine signaling (Fortin and Roitman, 2018). Thus, one possibility is that LHA Nts neurons engage dopamine systems to modulate motivated fluid intake to resolve physiologic need, but are also sufficient to induce motivated drinking beyond physiologic need (accounting for the enhanced intake of sucrose solution). Intriguingly, however, activation of LHA Nts neurons suppresses the motivation to obtain sucrose pellets in fasted mice (Woodworth et al., 2017). Therefore, it is possible that LHA Nts neurons differentially modify intake of caloric foods vs. liquids, and that promoted drinking can be additionally enhanced by palatability. By this rationale, it would be intriguing to determine if LHA Nts activation similarly increases intake of a palatable but calorically irrelevant solution such as saccharin. If palatability is sufficient to enhance intake, we would expect that saccharin and sucrose solutions would be similarly preferred. However, if the caloric-content of the solution and post-ingestive effects impact intake, then saccharin might be less preferred compared to sucrose. These questions will be important to address in future work.
Our current study focused on understanding how LHA Nts neurons modify drinking behavior, but going forward it will be important to determine if these neurons also engage endocrine mechanisms to modulate fluid balance. The two most well studied hormones in water balance are angiotensin II and vasopressin. Systemic and central angiotensin injection promotes drinking (Epstein et al., 1970; Fitzsimons et al., 1978; Tanaka et al., 2001), and it is possible that this system might intersect with LHA Nts neurons to induce water intake. Given that circulating angiotensin signaling via osmosensory regions (Almeida et al., 1999; Camargo et al., 2000; Tanaka et al., 2003) precedes changes in adrenergic signaling in the LHA, the physiologic effects of angiotensin II may occur prior to any induction of LHA Nts neurons. On the other hand, vasopressin neurons are important contributors to peripheral water balance, and dehydration-induced increases in vasopressin decrease excretion of water through the kidneys to conserve water (Bourque, 2008). By contrast, LHA Nts-induced drinking leads to water-excretion, as shown by decreased urine osmolality (Figure 1E). Therefore, it is possible that activation of LHA Nts neurons might suppress vasopressin neurons so as to dissipate the increased water load via excretion. In the future, circuit tracing and temporal assessments of angiotensin II and vasopressin will be useful to determine if LHA Nts neurons engage and modulate endocrine drinking systems.
We cannot yet determine whether LHA Nts-induced drinking is a response to elevated body temperature in an effort to cool the body. Our current data indicate that activation of LHA Nts neurons may sustain increased body temperature in mice. This possibility is consistent with prior work showing that activation of LHA Nts neurons increases locomotor activity and energy expenditure (Woodworth et al., 2017), both of which might maintain elevated body temperature as we saw in this study. Notably, however, our data suggest that LHA Nts neurons do not contribute to the Nts-mediated hypothermia that is observed with systemic or central Nts treatment (Bissette et al., 1976; Feifel et al., 2010; Martin et al., 1980). This is important because it suggests that LHA Nts neurons do not mediate all of the disparate physiology that has been ascribed to Nts (Schroeder and Leinninger, 2018), and that they may exert discrete facets of Nts-mediated signaling. Given that hypothermia and hypotension are induced by systemic and central Nts, the absence of LHA Nts-induced hypothermia raises hope that LHA Nts neurons may avoid such adverse physiologic effects. In sum, the unique role of LHA Nts neurons in specifically promoting drinking behavior suggests that these neurons serve a unique role amongst the LHA populations and within Nts signaling. Future studies to elaborate the signals and circuits whereby LHA Nts neurons modulate behavior may be useful to inform development of treatments for maladaptive drinking behaviors.
Supplementary Material
Highlights.
Activation of LHA Nts neurons is sufficient to promote voracious drinking.
Activation of the LHA Nts neurons biases for fluid consumption over food intake
LHA Nts neuronal activation induces drinking of any provided liquid
LHA Nts neuron-activated mice prefer water over dehydrating or unpalatable liquids.
LHA Nts neuron-activated mice prefer palatable liquids over water.
Acknowledgements
We thank Dr. Michelle Mazei-Robison for guidance on the quinine drinking experiments. We are also grateful to the Michigan State University Lyman Briggs College for providing an undergraduate research award that enabled Sabrina Fowler’s work on this project. This work was supported by a grant from the National Institutes of Health to GML (R01-DK103808).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Abbott SBG, Machado NLS, Geerling JC, Saper CB, 2016. Reciprocal Control of Drinking Behavior by Median Preoptic Neurons in Mice. J. Neurosci 36, 8228–8237. 10.1523/JNEUROSCI.1244-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alföldi P, Rubicsek G, Cserni G, Obál F, 1990. Brain and core temperatures and peripheral vasomotion during sleep and wakefulness at various ambient temperatures in the rat. Pfl??gers Arch. Eur. J. Physiol 417, 336–341. 10.1007/BF00371001 [DOI] [PubMed] [Google Scholar]
- Almeida NAA, Antunes VR, Saad WA, De Arruda Camargo LA, 1999. Effects of the alpha antagonists and agonists injected into the lateral hypothalamus on the water and sodium intake induced by angiotensin II injection into the subfornical organ. Brain Res. Bull 48, 521–525. 10.1016/S0361-9230(99)00032-5 [DOI] [PubMed] [Google Scholar]
- Augustine V, Gokce SK, Lee S, Wang B, Davidson TJ, Reimann F, Gribble F, Deisseroth K, Lois C, Oka Y, 2018. Hierarchical neural architecture underlying thirst regulation. Nature 10.1038/nature25488 [DOI] [PMC free article] [PubMed]
- Bachmanov AA, Beauchamp GK, Tordoff MG, 2002. Voluntary Consumption of NaCl , KCl , CaCl 2 , and NH 4 Cl Solutions by 28 Mouse Strains 32 [DOI] [PMC free article] [PubMed]
- Baron S, Courbebaisse M, Lepicard EM, Friedlander G, 2015. Assessment of hydration status in a large population. Br. J. Nutr 113, 147–158. 10.1017/S0007114514003213 [DOI] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM, 2015Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521,180–185. 10.1038/nature14416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissette G, Nemeroff CB, Loosen PT, Prange AJ, Lipton MA, 1976. Hypothermia and intolerance to cold induced by intracisternal administration of the hypothalamic peptide neurotensin. Nature 262, 607–609. 10.1038/262607a0 [DOI] [PubMed] [Google Scholar]
- Bittencourt J, Presse F, Arias C, Peto C, Vaughan J, Nahon J, Vale W, Sawchenko P,1992. The melanin concentrating hormone system of the rat brain An immuno and hybridisation histochemical characterisation.pdf. J. Comp. Neurol 319, 218–245. 10.1002/cne.903190204 [DOI] [PubMed] [Google Scholar]
- Boules M, Cusack B, Zhao L, Fauq A, McCormick DJ, Richelson E, 2000. A novel neurotensin peptide analog given extracranially decreases food intake and weight in rodents. Brain Res 865, 35–44. [DOI] [PubMed] [Google Scholar]
- Bourque CW, 2008. Central mechanisms of osmosensation and systemic osmoregulation. Nat. Rev. Neurosci 9, 519–531. 10.1038/nrn2400 [DOI] [PubMed] [Google Scholar]
- Brown JA, Bugescu R, Mayer TA, Gata-Garcia A, Kurt G, Woodworth HL, Leinninger GM, 2017. Loss of action via neurotensin-leptin receptor neurons disrupts leptin and ghrelin-mediated control of energy balance. Endocrinology 158, 1271–1288. 10.1210/en.2017-00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo LADA, Saad WA, Camargo GPDA, 2000. Effects of subtypes alpha- and beta-adrenoceptors of the lateral hypothalamus on the water and sodium intake induced by angiotensin II injected into the subfornical organ. Brain Res 881, 176–181. 10.1016/S0006-8993(00)02840-7 [DOI] [PubMed] [Google Scholar]
- Chou TC, Bjorkum A.a Gaus SE, Lu J, Scammell TE, Saper CB, 2002. Afferents to the ventrolateral preoptic nucleus. J. Neurosci 22, 977–990. https://doi.org/22/3/977 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Benoit SC, Sakai RS, Seeley RJ, Woods SC, 2003. Intraventricular melanin-concentrating hormone stimulates water intake independent of food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol 284, R494–9 [DOI] [PubMed] [Google Scholar]
- Cooke JH, Patterson M, Patel SR, Smith KL, Ghatei MA, Bloom SR, Murphy KG,2009. Peripheral and central administration of xenin and neurotensin suppress food intake in rodents. Obesity 17, 1135–1143. 10.1038/oby.2008.652 [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X-B, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG, 1998. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci 95, 322–327. 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos AI, Sordillo A, Dietrich MO, Liu ZW, Tellez LA, Vaynshteyn J, Ferreira JG, Ekstrand MI, Horvath TL, de Araujo IE, Friedman JM, 2013. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. Elife 2013, 1–15. 10.7554/eLife.01462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundas B, Harris M, Narasimhan M, 2007. Psychogenic polydipsia review: Etiology, differential, and treatment. Curr. Psychiatry Rep 9, 236–241. 10.1007/s11920-007-0025-7 [DOI] [PubMed] [Google Scholar]
- Epstein AN, Fitzsimons JT, Rolls BJ, 1970. Drinking induced by injection of angiotensin into the brain of the rat. J. Physiol 210, 457–474 10.1113/jphysiol.1970.sp009220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Goldenberg J, Melendez G, Shilling PD, 2010. The acute and subchronic effects of a brain-penetrating, neurotensin-1 receptor agonist on feeding, body weight and temperature. Neuropharmacology 58, 195–198. 10.1016/j.neuropharm.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons JT, Kucharczyk J, Richards G, 1978. Systemic angiotensin-induced drinking in the dog: a physiological phenomenon. J. Physiol 276, 435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin SM, Roitman MF, 2018. Challenges to body fluid homeostasis differentially recruit phasic dopamine signaling in a taste-selective manner. J. Neurosci 0399–18. 10.1523/JNEUROSCI.0399-18.2018 [DOI] [PMC free article] [PubMed]
- Furutani N, Hondo M, Kageyama H, Tsujino N, Mieda M, Yanagisawa M, Shioda S,Sakurai T, 2013. Neurotensin Co-Expressed in Orexin-Producing Neurons in the Lateral Hypothalamus Plays an Important Role in Regulation of Sleep/Wakefulness States. PLoS One 8 10.1371/journal.pone.0062391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goforth PB, Leinninger GM, Patterson CM, Satin LS, Myers MG, 2014. Leptin Acts via Lateral Hypothalamic Area Neurotensin Neurons to Inhibit Orexin Neurons by Multiple GABA-Independent Mechanisms. J. Neurosci 34, 11405–11415. 10.1523/JNEUROSCI.5167-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Canteras NS, Burns G, Swanson LW, 2005. Projections from the subfornical region of the lateral hypothalamic area. J. Comp. Neurol 493, 412–438. 10.1002/cne.20764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JD, Swanson LW, 2012. Connections of the lateral hypothalamic area juxtadorsomedial region in the male rat. J. Comp. Neurol 520, 1831–1890. 10.1002/cne.23064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JD, Swanson LW, 2010. Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and supra fornical regions of the lateral hypothalamic area in the malerat. Brain Res. Rev 64, 14–103. 10.1016/j.brainresrev.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawken ER, Crookall JM, Reddick D, Millson RC, Milev R, Delva N, 2009. Mortality over a 20-year period in patients with primary polydipsia associated with schizophrenia: A retrospective study. Schizophr. Res 107, 128–133. 10.1016/j.schres.2008.09.029 [DOI] [PubMed] [Google Scholar]
- Hawkins MF, Baker JD, Baumeister AA, 1989. Neurotensin-induced polydipsia: a structure-activity study. Brain Res 487, 188–91. [DOI] [PubMed] [Google Scholar]
- Inutsuka A, Inui A, Tabuchi S, Tsunematsu T, Lazarus M, Yamanaka A, 2014Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology 85, 451–460. 10.1016/j.neuropharm.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, Veleta K, Kantak PA, Aita M, Shilling-Scrivo K, Ramakrishnan C, Deisseroth K, Otte S,Stuber GD, 2015. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527. 10.1016/j.cell.2014.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Hindmarch CCT, Salinas YD, Shi Y, Greenwood M, Hoe SZ, Murphy D, Gainer H, 2015. A RNA-Seq Analysis of the Rat Supraoptic Nucleus Transcriptome: Effects of Salt Loading on Gene Expression. PLoS One 10, e0124523 10.1371/journal.pone.0124523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani MM, Szabó G, Erdélyi F, Burdakov D, 2013. Lateral hypothalamic GAD65 neurons are spontaneously firing and distinct from orexin- and melanin-concentrating hormone neurons. J. Physiol 591, 933–53. 10.1113/jphysiol.2012.243493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsman BJ, Nation HN, Stocker SD, 2017. Hypothalamic Signaling in Body Fluid Homeostasis and Hypertension. Curr. Hypertens. Rep 19, 50. 10.1007/s11906-017-0749-7 [DOI] [PubMed] [Google Scholar]
- Koch CA, Fulop T, 2017. Clinical aspects of changes in water and sodium homeostasis in the elderly. Rev. Endocr. Metab. Disord 18, 49–66. 10.1007/s11154-017-9420-5 [DOI] [PubMed] [Google Scholar]
- Kumar S, Szymusiak R, Bashir T, Suntsova N, Rai S, McGinty D, Alam MN, 2008. Inactivation of median preoptic nucleus causes c-Fos expression in hypocretin- and serotonin-containing neurons in anesthetized rat. Brain Res 1234, 66–77. 10.1016/j.brainres.2008.07.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt G, Woodworth HL, Leinninger GM, 2017. Lateral Hypothalamic Control of Energy Balance. Colloq. Ser. Integr. Syst. Physiol. From Mol. to Funct 9, i–106. 10.4199/C00159ED1V01Y201711ISP079 [DOI] [Google Scholar]
- Laque A, Yu S, Qualls-Creekmore E, Gettys S, Schwartzenburg C, Bui K, Rhodes C, Berthoud HR, Morrison CD, Richards BK, Mün zberg H, 2015. Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons. Mol. Metab 4, 706–717. 10.1016/j.molmet.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laque A, Zhang Y, Gettys S, Nguyen T-A, Bui K, Morrison CD, Münzberg H, 2013. Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. Am. J. Physiol. Endocrinol. Metab 304, E999–1011. 10.1152/ajpendo.00643.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Mikkelsen JD, 1995. Functional identification of central afferent projections conveying information of acute “stress” to the hypothalamic paraventricular nucleus. J. Neurosci 15, 2609–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DE, Zimmerman CA, Poormoghaddam A, Huey EL, Ahn JS, Lin Y-C, Tan CL, Chen Y, Knight ZA, 2017. The Forebrain Thirst Circuit Drives Drinking through Negative Reinforcement. Neuron 96, 1272–1281.e4. 10.1016/j.neuron.2017.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S, Diano S, Horvath TL, Seeley RJ, Becker JB, Münzberg H, Myers MG, 2009. Leptin Acts via Leptin Receptor-Expressing Lateral Hypothalamic Neurons to Modulate the Mesolimbic Dopamine System and Suppress Feeding. Cell Metab 10, 89–98. 10.1016/j.cmet.2009.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Opland DM, Jo Y, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF, Thompson RC, Myers MG, 2011. Leptin Action via Neurotensin Neurons Controls Orexin, the Mesolimbic Dopamine System and Energy Balance. Cell Metab 14, 313–323. 10.1016/j.cmet.2011.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttinger D, King RA, Sheppard D, Strupp J, Nemeroff CB, Prange AJ, 1982. The effect of neurotensin on food consumption in the rat. Eur. J. Pharmacol 81, 499–503. 10.1016/0014-2999(82)90116-9 [DOI] [PubMed] [Google Scholar]
- Martin GE, Bacino CB, Papp NL, 1980. Hypothermia elicited by the intracerebral microinjection of neurotensin. Peptides 1, 333–339. 10.1016/01969781(80)90011-X [DOI] [PubMed] [Google Scholar]
- Matsuda T, Hiyama TY, Niimura F, Matsusaka T, Fukamizu A, Kobayashi K, Kobayashi K, Noda M, 2016. Distinct neural mechanisms for the control of thirst and salt appetite in the subfornical organ. Nat. Neurosci 10.1038/nn.4463 [DOI] [PubMed]
- Maughan RJ, 2012. Hydration, morbidity, and mortality in vulnerable populations. Nutr. Rev 70 Suppl 2, S152–5. 10.1111/j.1753-4887.2012.00531.x [DOI] [PubMed] [Google Scholar]
- McGregor R, Wu M-F, Barber G, Ramanathan L, Siegel JM, 2011. Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. J. Neurosci 31, 15455–67. 10.1523/JNEUROSCI.4017-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, Cairns MJ, Denton DA, Egan G, Mathai ML, Uschakov A, Wade JD,Weisinger RS, Oldfield BJ, 2004. Physiological and pathophysiological influences on thirst. Physiol. Behav 81, 795–803. 10.1016/j.physbeh.2004.04.055 [DOI] [PubMed] [Google Scholar]
- Mickelsen LE, Kolling FW, Chimileski BR, Fujita A, Norris C, Chen K, Nelson CE Jackson AC, 2017. Neurochemical Heterogeneity among Lateral Hypothalamic Hypocretin/Orexin and Melanin-Concentrating Hormone Neurons Identified through Single Cell Gene Expression Analysis. Eneuro 4, ENEURO.0013–17.2017 10.1523/ENEURO.0013-17.2017 [DOI] [PMC free article] [PubMed]
- Mizuno A, Matsumoto N, Imai M, Suzuki M, 2003. Impaired osmotic sensation in mice lacking TRPV4. Am. J. Physiol. Cell Physiol 285, C96–C101. 10.1152/ajpcell.00559.2002 [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Klerman EB, Sakurai T, Scammell TE, 2006. Elevated body temperature during sleep in orexin knockout mice. Am. J. Physiol. Integr. Comp. Physiol 291, R533–R540. 10.1152/ajpregu.00887.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Stevenson JA, 1967. Drinking induced by electrical stimulation of the lateral hypothalamus. Exp. Neurol 17, 119–27 [DOI] [PubMed] [Google Scholar]
- Morrison SD, Barrnett RJ, Mayer J, 1958. Localization of Lesions in the Lateral Hypothalamus of Rats With Induced Adipsia and Aphagia. Am J Physiol -- Leg. Content 193, 230–234 [DOI] [PubMed] [Google Scholar]
- Nation HL, Nicoleau M, Kinsman BJ, Browning KN, Stocker SD, 2016. DREADD-induced activation of subfornical organ neurons stimulates thirst and salt appetite. J. Neurophysiol 115, 3123–9. 10.1152/jn.00149.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Olney JJ, Burnham NW, Mazzone CM, Lowery-Gionta EG, Pleil KE, Kash TL, Thiele TE, 2015. Lateral Hypothalamus GABAergic Neurons Modulate Consummatory Behaviors Regardless of the Caloric Content or Biological Relevance of the Consumed Stimuli. Neuropsychopharmacology 41, 1–27 10.1038/npp.2015.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, Neve R, Wildes CP, Tye KM, 2015. Decoding neural circuits that control compulsive sucrose seeking. Cell 160, 528–541. 10.1016/j.cell.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Ye M, Zuker CS, 2015. Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature 520, 349–52. 10.1038/nature14108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield BJ, Badoer E, Hards DK, McKinley MJ, 1994. Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neuroscience 60, 255–62. [DOI] [PubMed] [Google Scholar]
- Opland D, Sutton A, Woodworth H, Brown J, Bugescu R, Garcia A, Christensen L, Rhodes C, Myers M, Leinninger G, 2013. Loss of neurotensin receptor-1 disrupts the control of the mesolimbic dopamine system by leptin and promotes hedonic feeding and obesity. Mol. Metab 2, 423–434. 10.1016/j.molmet.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CM, Wong JMT, Leinninger GM, Allison MB, Mabrouk OS, Kasper CL, Gonzalez IE, Mackenzie A, Jones JC, Kennedy RT, Myers MG, 2015. Ventral tegmental area neurotensin signaling links the lateral hypothalamus to locomotor activity and striatal dopamine efflux in male mice. Endocrinology 156, 1692–1700. 10.1210/en.2014-1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin B, 2001. The Mouse Brain in Stereotaxic Coordinates, 2nd ed. Academic Press [Google Scholar]
- Phillips PA, Rolls BJ, Ledingham JG, Forsling ML, Morton JJ, Crowe MJ, Wollner L, 1984. Reduced thirst after water deprivation in healthy elderly men. N. Engl. J. Med 311, 753–759 [DOI] [PubMed] [Google Scholar]
- Qualls-Creekmore E, Yu S, Francois M, Hoang J, Huesing C, Bruce-Keller A, Burk D, Berthoud H-R, Morrison CD, Münzberg H, 2017. Galanin-Expressing GABA Neurons in the Lateral Hypothalamus Modulate Food Reward and Noncompulsive Locomotion. J. Neurosci 37, 6053–6065. 10.1523/JNEUROSCI.0155-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A, Carraway RE, Wang YP, 2001. Evidence that neurotensin mediates the central effect of leptin on food intake in rat. Brain Res 888, 343–347. 10.1016/S0006-8993(00)03107-3 [DOI] [PubMed] [Google Scholar]
- Sakamaki R, Uemoto M, Inui A, Asakawa A, Ueno N, Ishibashi C, Hirono S, Yukioka H, Kato A, Shinfuku N, Kasuga M, Katsuura G, 2005. Melanin-concentrating hormone enhances sucrose intake. Int. J. Mol. Med 15, 1033–9. [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, 1998. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- Saper CB, Swanson LW, Cowan WM, 1979. An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J. Comp. Neurol 183, 689–706. 10.1002/cne.901830402 [DOI] [PubMed] [Google Scholar]
- Schroeder LE, Leinninger GM, 2018. Role of central neurotensin in regulating feeding: Implications for the development and treatment of body weight disorders. Biochim. Biophys. Acta - Mol. Basis Dis 1864, 900–916. 10.1016/j.bbadis.2017.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek CD, Johnson AK, 2013. Integration of thermal and osmotic regulation of water homeostasis: the role of TRPV channels. AJP Regul. Integr. Comp. Physiol 305, R669–78. 10.1152/ajpregu.00270.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen KJ, Mitchell JE, Roerig JL, Lancaster KL, 2007. The eating disorders medicine cabinet revisited: A clinician’s guide to ipecac and laxatives. Int. J. Eat. Disord 40, 360– 368. 10.1002/eat.20365 [DOI] [PubMed] [Google Scholar]
- Stricker EM, 1976. Drinking by rats after lateral hypothalamic lesions: A new look at the lateral hypothalamic syndrome. J. Comp. Physiol. Psychol 90, 127–143 10.1037/h0021469 [DOI] [PubMed] [Google Scholar]
- Stuber GD, Wise RA, 2016. Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci 19, 198–205. 10.1038/nn.4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Hori K, Nomura M, 2001. Dipsogenic response induced by angiotensinergic pathways from the lateral hypothalamic area to the subfornical organ in rats. Behav. Brain Res 118, 111–116. 10.1016/S0166-4328(00)00321-1 [DOI] [PubMed] [Google Scholar]
- Tanaka J, Kariya K, Nomura M, 2003. Drinking attenuates the noradrenaline release in the lateral hypothalamic area induced by angiotensin II activation of the subfornical organ in rats. Behav. Brain Res 140, 49–55. 10.1016/S0166-4328(02)00277-2 [DOI] [PubMed] [Google Scholar]
- Watts AG, 1999. Dehydration-associated anorexia: Development and rapid reversal. Physiol. Behav 65, 871–878. 10.1016/S0031-9384(98)00244-3 [DOI] [PubMed] [Google Scholar]
- Watts AG, 1992. Osmotic stimulation differentially affects cellular levels of corticotropin-releasing hormone and neurotensin/neuromedin N mRNAs in the lateral hypothalamic area and central nucleus of the amygdala. Brain Res 581, 208–16. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G, 2007. Rapid and preferential activation of fos protein in hypocretin/orexin neurons following the reversal of dehydration-anorexia. J. Comp. Neurol 502, 768–782. 10.1002/cne.21316 [DOI] [PubMed] [Google Scholar]
- Woodworth HL, Beekly BG, Batchelor HM, Bugescu RPP-B, Perez-Bonilla P, Schroeder LE, Leinninger GM, Perez-Bonilla P, Schroeder LE, Leinninger GM 2017. Lateral Hypothalamic Neurotensin Neurons Orchestrate Dual Weight Loss Behaviors via Distinct Mechanisms. Cell Rep 21, 3116–3128. 10.1016/j.celrep.2017.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Kim ER, Sun H, Xu Y, Mangieri LR, Li D-P, Pan H-L, Xu Y, Arenkiel BR, Tong Q, 2015. GABAergic Projections from Lateral Hypothalamus to Paraventricular Hypothalamic Nucleus Promote Feeding. J. Neurosci 35, 3312–3318. 10.1523/JNEUROSCI.3720-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Sakurai T, Katsumoto T, Masashi Yanagisawa, Goto K, 1999. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res 849, 248–252. 10.1016/S0006-8993(99)01905-8 [DOI] [PubMed] [Google Scholar]
- Zamir N, Skofitsch G, Bannon MJ, Jacobowitz DM, 1986. Melanin-concentrating hormone: unique peptide neuronal system in the rat brain and pituitary gland. Proc Natl Acad Sci U S A 83, 1528–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CA, Leib DE, Knight ZA, 2017. Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci 18, 459–469. 10.1038/nrn.2017.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CA, Lin Y-C, Leib DE, Guo L, Huey EL, Daly GE, Chen Y, Knight ZA, 2016. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 537, 680–684. 10.1038/nature18950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuure WA, Quennell JH, Anderson GM, 2016. Leptin Responsive and GABAergic Projections to the Rostral Preoptic Area in Mice. J. Neuroendocrinol 28 10.1111/jne.12357 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


