Abstract
Background
Receiving a diagnosis of cancer and the subsequent related treatments can have a significant impact on an individual's physical and psychosocial well‐being. To ensure that cancer care addresses all aspects of well‐being, systematic screening for distress and supportive care needs is recommended. Appropriate screening could help support the integration of psychosocial approaches in daily routines in order to achieve holistic cancer care and ensure that the specific care needs of people with cancer are met and that the organisation of such care is optimised.
Objectives
To examine the effectiveness and safety of screening of psychosocial well‐being and care needs of people with cancer. To explore the intervention characteristics that contribute to the effectiveness of these screening interventions.
Search methods
We searched five electronic databases in January 2018: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycINFO, and CINAHL. We also searched five trial registers and screened the contents of relevant journals, citations, and references to find published and unpublished trials.
Selection criteria
We included randomised controlled trials (RCTs) and non‐randomised controlled trials (NRCTs) that studied the effect of screening interventions addressing the psychosocial well‐being and care needs of people with cancer compared to usual care. These screening interventions could involve self‐reporting of people with a patient‐reported outcome measures (PROMs) or a semi‐structured interview with a screening interventionist, and comprise a solitary screening intervention or screening with guided actions. We excluded studies that evaluated screening integrated as an element in more complex interventions (e.g. therapy, coaching, full care pathways, or care programmes).
Data collection and analysis
Two review authors independently extracted the data and assessed methodological quality for each included study using the Cochrane tool for RCTs and the Risk Of Bias In Non‐randomised Studies ‐ of Interventions (ROBINS‐I) tool for NRCTs. Due to the high level of heterogeneity in the included studies, only three were included in meta‐analysis. Results of the remaining 23 studies were analysed narratively.
Main results
We included 26 studies (18 RCTs and 8 NRCTs) with sample sizes of 41 to 1012 participants, involving a total of 7654 adults with cancer. Two studies included only men or women; all other studies included both sexes. For most studies people with breast, lung, head and neck, colorectal, prostate cancer, or several of these diagnoses were included; some studies included people with a broader range of cancer diagnosis. Ten studies focused on a solitary screening intervention, while the remaining 16 studies evaluated a screening intervention combined with guided actions. A broad range of intervention instruments was used, and were described by study authors as a screening of health‐related quality of life (HRQoL), distress screening, needs assessment, or assessment of biopsychosocial symptoms or overall well‐being. In 13 studies, the screening was a self‐reported questionnaire, while in the remaining 13 studies an interventionist conducted the screening by interview or paper‐pencil assessment. The interventional screenings in the studies were applied 1 to 12 times, without follow‐up or from 4 weeks to 18 months after the first interventional screening. We assessed risk of bias as high for eight RCTs, low for five RCTs, and unclear for the five remaining RCTs. There were further concerns about the NRCTs (1 = critical risk study; 6 = serious risk studies; 1 = risk unclear).
Due to considerable heterogeneity in several intervention and study characteristics, we have reported the results narratively for the majority of the evidence.
In the narrative synthesis of all included studies, we found very low‐certainty evidence for the effect of screening on HRQoL (20 studies). Of these studies, eight found beneficial effects of screening for several subdomains of HRQoL, and 10 found no effects of screening. One study found adverse effects, and the last study did not report quantitative results. We found very low‐certainty evidence for the effect of screening on distress (16 studies). Of these studies, two found beneficial effects of screening, and 14 found no effects of screening. We judged the overall certainty of the evidence for the effect of screening on HRQoL to be very low. We found very low‐certainty evidence for the effect of screening on care needs (seven studies). Of these studies, three found beneficial effects of screening for several subdomains of care needs, and two found no effects of screening. One study found adverse effects, and the last study did not report quantitative results. We judged the overall level of evidence for the effect of screening on HRQoL to be very low. None of the studies specifically evaluated or reported adverse effects of screening. However, three studies reported unfavourable effects of screening, including lower QoL, more unmet needs, and lower satisfaction.
Three studies could be included in a meta‐analysis. The meta‐analysis revealed no beneficial effect of the screening intervention on people with cancer HRQoL (mean difference (MD) 1.65, 95% confidence interval (CI) −4.83 to 8.12, 2 RCTs, 6 months follow‐up); distress (MD 0.0, 95% CI −0.36 to 0.36, 1 RCT, 3 months follow‐up); or care needs (MD 2.32, 95% CI −7.49 to 12.14, 2 RCTs, 3 months follow‐up). However, these studies all evaluated one specific screening intervention (CONNECT) in people with colorectal cancer.
In the studies where some effects could be identified, no recurring relationships were found between intervention characteristics and the effectiveness of screening interventions.
Authors' conclusions
We found low‐certainty evidence that does not support the effectiveness of screening of psychosocial well‐being and care needs in people with cancer. Studies were heterogeneous in population, intervention, and outcome assessment.
The results of this review suggest a need for more uniformity in outcomes and reporting; for the use of intervention description guidelines; for further improvement of methodological certainty in studies and for combining subjective patient‐reported outcomes with objective outcomes.
Plain language summary
Systematic screening and assessment of psychosocial well‐being and care needs of people with cancer
Background People with cancer may experience physical, psychological, and social problems due to the disease and its treatment. It is therefore important to take into account all of these aspects during the diagnosis and treatment of people with cancer. Nowadays, screening for psychosocial well‐being and care needs is often recommended. This means that patients are systematically queried about their well‐being and needs related to several psychosocial aspects (e.g. cognitive functioning, emotions, relationships and communication with loved ones, sexuality, social participation, employment). This is applied with self‐report questionnaires, or interviews in which the content of these questionnaires or checklists is used as interview guide. The current review had two objectives: to examine the effects and possible harms of screening of psychosocial well‐being and care needs of people with cancer, and to examine which characteristics of screening are more or less effective.
Study characteristics We found 26 studies including a total of 7654 adults with cancer. Most studies included both males and females. With regard to cancer type, most studies included people with a specific type of cancer, but some included a variety of cancer types. Furthermore, the type of screening differed: half of the studies asked participants to self‐complete a screening questionnaire about their psychosocial health, while in the remaining studies screening interviews were conducted in which a healthcare professional questioned participants about their well‐being face‐to‐face.
Key results Several studies showed benefits of screening on psychosocial well‐being of cancer patients, such as their health‐related quality of life, distress, care needs, and patient satisfaction. However, some studies also found negative effects. There were important differences between the studies: they assessed different psychosocial aspects (e.g. health‐related quality of life, distress, care needs, and patient satisfaction) and differed in their modes of screening (i.e. self‐report screening questionnaire versus screening interview), timing and frequency of the screening (1 to 12 times), outcome measures, and outcome time points. Due to these differences, only three studies studying the same intervention could be included in the analysis.
Certainty of the evidence Our results do not support the screening of psychosocial well‐being and care needs in people with cancer. The certainty of the evidence was low, which means that we are uncertain about the results of the review due to variations in characteristics, and results of the studies and study designs.
Summary of findings
Summary of findings for the main comparison. Screening of psychosocial well‐being and care needs compared to usual care in people with cancer.
| Screening of psychosocial well‐being and care needs compared to usual care in people with cancer | ||||
|
Patient or population: People with cancer Settings: Inpatient and outpatient cancer care Intervention: Screening of psychosocial well‐being and care needs Comparison: Usual care | ||||
| Outcomes | Impacts | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
HRQoL assessed with: EORTC QLQ‐C30 and EORTC QLQ‐H&N36; SF‐36; PCQoL; EQ‐5D‐3L; FACT‐G; FACT‐C; HRQoL‐LASA. Follow‐up: range 1 months to 14 months |
? : 1 study presented no data on this outcome. 0 : 10 studies found no effect of the intervention. ‐ : 1 study found a negative effect of the intervention for "role functioning". + : 8 studies found beneficial effects of the intervention for several subdomains or HRQoL in total, at 1 or several time points. |
5752 (14 RCTs, 6 NRCTs) |
⊕⊝⊝⊝ VERY LOW 1 2 |
Based on the narrative analysis of all 26 studies included in the current review.8 Meta‐analysis of 3 studies on the CONNECT intervention revealed no effect: MD 1.65, 95% CI (−4.83, 8.12), 2 RCTs, 6‐month follow‐up.9 |
|
Distress assessed with: POMS; HADS; GHQ‐12; CES‐D; SO; DT; PSYCH‐6; a modified version of an existing distress tool for breast cancer patients. Follow‐up: range 1 months to 14 months |
0 : 14 studies found no effect of the intervention. + : 2 studies found beneficial effects of the intervention for a subdomain or distress in total. |
5577 (13 RCTs, 3 NRCTs) |
⊕⊝⊝⊝ VERY LOW 3 4 |
Based on the narrative analysis of all 26 studies included in the current review.8 Meta‐analysis of 3 studies on the CONNECT intervention revealed no effect: MD 0.0, 95% CI (−0.42, 0.42), 2 RCTs and 1 NRCT, 6‐month follow‐up.9 |
|
Care needs assessed with: SCNS; NA‐ACP; NA‐ALCP. Follow‐up: range 1 months to 6 months |
? : 1 study presented no data on this outcome. 0 : 2 studies found no effect of the intervention on supportive care needs. ‐ : 1 study found a negative effect of the intervention for subdomains and care needs in total. + : 3 studies found a positive effect of the intervention for subdomains at certain time points. |
2331 (4 RCTs, 3 NRCTs) |
⊕⊝⊝⊝ VERY LOW 1 5 6 7 |
Based on the narrative analysis of all 26 studies included in the current review.8 Meta‐analysis of 2 studies on the CONNECT intervention revealed no effect: MD 2.32, 95% CI (−7.49, 12.14), 2 RCTs, 3‐month follow‐up.9 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HRQoL: health‐related quality of life; MD: mean difference; NRCT: non‐randomised controlled trial; RCT: randomised controlled trial | ||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||
1Downgraded for inconsistency: variability in study findings; some studies found positive effects, other studies found negative effects (and other studies found no effect). 2Downgraded for risk of bias: for overall risk of bias, 3 studies were labelled as 'high risk' study, 4 as 'low risk' study, 5 as 'risk of bias unclear', 5 as 'serious risk' study, and 1 as 'critical risk' study. 3Downgraded for imprecision: low sample sizes. 4Downgraded for risk of bias: for overall risk of bias, 4 studies were labelled as 'high risk' study, 3 as 'low risk' study, 3 as 'risk of bias unclear', 2 as 'serious risk' study, and 1 as 'critical risk' study. 5Downgraded for publication bias: one study presented no data on this outcome. 6Downgraded for imprecision: low sample sizes and lack of data; no raw data from Thewes, not possible to judge 95% CI. 7Downgraded for risk of bias: for overall risk of bias, 1 study was labelled as 'high risk' study, 1 as 'risk of bias unclear', 2 as 'serious risk' study, and 1 as 'critical risk' study. 8Due to the large amount of heterogeneity in outcome reporting (some studies reported on MD and 95% CI from all subscales separately, others of the instruments' total score, and others of both), it is not possible to provide a concise overview of these effects measures in this table, however these can be found for all outcomes in the Evidence Summary table (Table 5, Table 6, Table 7). 9The meta‐analyses included three studies of the same research group on exactly the same intervention, resulting in findings for that specific intervention, which were not representative of all psychosocial screening interventions studied in the current review.
Background
Description of the condition
Cancer is one of the leading causes of mortality and morbidity worldwide. According to the latest global statistics, there were 14.1 million new cancer cases in 2012, and this number is expected to increase to 24 million by 2035 (Ferlay 2015). Cancer accounted for 8.2 million deaths in 2012. With the increase of more successful therapeutic approaches, the life expectancy of people with cancer is increasing, resulting in a growing population of people with cancer and survivors. In 2012, there were 32.6 million people living with cancer (within five years of diagnosis) worldwide (Ferlay 2015).
Cancer and related treatments have a bio‐psychosocial impact on patients' health and well‐being. People with cancer may experience physical consequences such as pain, hair loss, nausea, weight gain/loss, fatigue, and sleeping difficulties varying from short to long term in nature (Carlson 2013; Feyer 2008; Heins 2013). Their psychosocial health is put to the test by emotional distress, fear of recurrence, memory changes, worries about the well‐being of relatives, sexual problems, social issues, and employment and financial difficulties, often resulting in supportive care needs (Boyes 2012; Browne 2011; Knobf 2012; Mikkelsen 2008; Parry 2012).
The term ‘psychosocial well‐being’ is used in this review as an umbrella term comprising the experience of psychological, emotional, cognitive, spiritual, existential, relational, familial, and social functioning of a person. In clinical practice and research, the psychosocial well‐being of people with cancer, or its disruption, is measured on the basis of the above components, and with the degree to which supportive care needs are experienced. Psychosocial well‐being is also frequently conceptualised and measured as a whole in terms of 'quality of life' (QoL) (Moons 2006), 'health‐related quality of life' (HRQoL) (Aaronson 1993; Ganz 1992), or 'distress' (NCCN 1999). The resulting 'care needs' can be defined as 'the requirement of some action or resource in care that is necessary, desirable, or useful to attain optimal well‐being' for the person (Sanson‐Fisher 2000).
Depending on the studies and participating populations, the prevalence of distress in people with cancer varies from 35% to 55% (Carlson 2013). The experienced distress can result in supportive care needs with a high individual variability for all life domains, resulting in a wide range of people with cancer who desire extra support, from 1% to 93% (Harrison 2009). People with cancer who experience high levels of distress or psychosocial burden do not necessarily desire extra supportive care. We believe that this wide difference in the desire for extra support indicates the need for quality cancer care that is organised and driven by patient‐centred initiatives in order to spend limited healthcare budgets as efficiently as possible.
In order to address the bio‐psychosocial impact on the well‐being of people with cancer, cancer care should be comprehensive, and should integrate psychosocial concerns in follow‐up (IOM 2008; Wolff 2015). The Institute of Medicine stated that care should be patient‐centred, respectful of, and responsive to, people with cancer experiences, needs, preferences, and values, and that patients’ input on these aspects should guide all clinical decisions (IOM 2001). National cancer plans were launched to integrate the psychosocial approach in cancer care (Grassi 2012), and routine screening of distress and needs is recommended as good practice across international cancer systems and in guidelines (Accreditation Canada 2008; Breitbart 2015; Holland 2011; IOM 2008; Meyer 2015; NBCC 2003; NCCN 2007).
Description of the intervention
In this review, the intervention of interest is the screening of psychosocial well‐being and care needs in people with cancer. A literature search showed wide variation in screening terms and definitions, the types of measure instruments used, the timing of screening, and the included participants (Carlson 2003; Carlson 2012; Meijer 2013). We defined screening of psychosocial well‐being as a concise measurement of psychosocial well‐being in all people with cancer, and not only in those with certain symptoms or complaints. For this screening, a patient‐reported outcome measure (PROM) or a structured interview is used. An assessment was seen as a more extended or profound form of screening.
How the intervention might work
Screening for distress and supportive care needs in cancer care is primarily recommended to integrate the psychosocial topic in daily routine to achieve ‘cancer care for the whole patient’ (IOM 2008). This screening of psychosocial well‐being and care needs can stimulate (1) detection of, (2) communication on, and (3) tailored referral for psychosocial concerns (Bauwens 2014; Heyn 2013; Ristevski 2015), all of which may increase the chance that patients with psychosocial difficulties receive the appropriate support. If the application of interventions for screening of people with cancers' psychosocial well‐being and care needs contributes to a more efficient and effective healthcare delivery, it is expected that it can improve the well‐being of people with cancer (Whitney 2014; Zabora 2012). Likewise, actively querying patients’ experiences and needs could stimulate patients to fulfil a more active role in their own care trajectory (Cox 2006). These approaches further promote the patient‐centeredness that is needed to create a good match between the care needs of people with cancer and the delivered care. Comprehensiveness, efficiency, and patient‐centeredness are essential components in achieving high‐quality cancer care (Hess 2013; Zucca 2014).
Why it is important to do this review
Several Cochrane Reviews focus on the effect of psychological and psychosocial interventions for people with cancer (Galway 2012; Goedendorp 2009; Parahoo 2013; Semple 2013). However, the results of these reviews were inconclusive. A significant variation in participants, mode of intervention delivery, discipline of the involved care professionals, and intervention content was observed (Galway 2012; Semple 2013). To respond to these findings, we chose to focus on a specific type of psychosocial intervention, which is the screening of patients' psychosocial well‐being and care needs. It is expected that these interventions bring an added value to the organisation of health care, and have a positive impact on the well‐being of patients. This type of screening in cancer care is widely recommended. However, the implementation of such screening is often based on the consensus of professionals and policymakers. The existence of evidence‐based data, collected in earlier reviews, seemed to be scarce and quite often contradictory (Bidstrup 2011; Carlson 2012; Meijer 2013). Consequently, the question as to whether systematic screening of psychosocial well‐being and care needs has a positive effect on cancer patients’ well‐being remains unanswered.
We are aware that many factors contribute to the psychosocial well‐being and care needs of cancer patients. Both patients’ sociodemographic, as well as medical characteristics such as age, gender, socioeconomic and other social factors, health status, tumour, and treatment type are important (Armes 2009; Boyes 2012;Choi 2012;Hack 2010; McIllmurray 2001). We assume that the characteristics of care interventions can also have an important role. We therefore also explored the characteristics of psychosocial screening interventions, and the extent to which these contribute to the effectiveness of such interventions. Such analysis might aid the development of effective screening interventions by combining promising characteristics of the intervention.
Consequently, we addressed the following two research questions in this systematic review.
What is the effect of screening of psychosocial well‐being and care needs on the well‐being of people with cancer?
Which intervention characteristics are important for effective screening interventions of cancer for the psychosocial well‐being of people with cancer and their care needs?
We expected that this systematic review would add value compared to earlier reviews on this topic and related topics (Bidstrup 2011; Carlson 2012; Howell 2012; Luckett 2009; Meijer 2013). Firstly, we used a more extensive collection of sources for the search of studies. Secondly, we included randomised clinical trials (RCTs) as well as non‐randomised controlled trials (NRCTs). Randomised controlled trial designs are seen as the most reliable and bias‐resistant research designs, and several of the previous reviews have focused only on this type of study design. However, the nature of the clinical field and interventions make it unrealistic to only evaluate the evidence of RCTs (Sidani 2015). Thirdly, our search strategy focused on a wider range of outcomes than those used in previous studies. Fourthly, we did not limit our focus to the final effect of the specific psychosocial screening interventions. Like Ranchor and colleagues (Ranchor 2012), we intended to describe the specific characteristics and components of these interventions (e.g. the instruments used, the procedures undertaken, the conditions set, as well as the care professionals that are involved in the intervention). Finally, considering the recent calls for screening intervention research and study protocol papers (Carlson 2012; Singer 2017), the availability of evidence‐based data on the topic has likely grown since the publication of the previous reviews.
The present systematic review provides a complete summary of international studies on the topic and is relevant for research, policy, and practice. We identified shortcomings in research, which provide information for future research into the composition of, or conditions for, effective screening of psychosocial well‐being and care needs. This review also provides policymakers with comprehensive evidence‐based data to support future decisions. Likewise, the findings of studies of this review clarify the effects or value of psychosocial screening for clinical practice.
Objectives
To examine the effectiveness and safety of screening of psychosocial well‐being and care needs of people with cancer. To explore the intervention characteristics that contribute to the effectiveness of these screening interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs on psychosocial screening interventions. Randomised controlled trials are the gold standard to evaluate intervention effects. However, due to the nature of the field, RCTs are often not available to address questions about the effects of health system interventions and implementation strategies (Sidani 2015). Consequently, we also included NRCTs, such as controlled before‐and‐after trials (CBAs), interrupted time series studies (ITS), repeated measures studies (RMS), and historically controlled studies (HCTs).
Types of participants
We included adults over 18 years of age with cancer, at any time point of their care trajectory (at diagnosis, in active treatment, at completion of treatment, in follow‐up, or in survivorship). We excluded research literature specifically on children, teenagers, and adolescents. We excluded references when the study authors had appointed their study population specifically with the term 'children', 'teenagers', 'adolescents', or related terms.
Types of interventions
The intervention of interest in this review was the screening of psychosocial well‐being and care needs in people with cancer. The term ‘psychosocial well‐being’ should be interpreted in terms of psychosocial, psychological, emotional, or social well‐being, quality of life, distress, anxiety or depression, or supportive care needs.
We included studies that focused on the evaluation of:
solitary or simple screening interventions (e.g. PROM or face‐to‐face screening, followed by the availability of screening results for healthcare professionals with no further instructions);
screening interventions followed by interventions based on the screening results, or ‘guided actions’ (e.g. PROM or face‐to‐face screening, followed by the use of screening results according to previously described guidelines on results discussion, interdisciplinary referral, computer‐generated care algorithms).
We excluded studies that evaluated screening followed by more complex interventions (e.g. therapy, coaching, full care pathways, or care programmes), as such studies would mostly evaluate the effects of the complex interventions.
The studies of interest compared the screening intervention with a usual care condition. We considered 'usual care' as the control condition that is described by the study authors as standard care or usual care, and does not contain any form of interventional screening or assessment of psychosocial well‐being and care needs.
We excluded studies that used the same PROM as screening tool in the intervention condition (e.g. combined with sharing the screening results with the medical team), and as outcome tool in the intervention and control condition, as there would be a lack of contrast between the two groups in such studies.
Types of outcome measures
Outcomes had to be collected with validated self‐report questionnaires or through interviews with the use of validated PROMs. Requirements for timing and frequency of outcome measurement were not specified.
Primary outcomes
Psychosocial well‐being and care needs of people with cancer had to be measured in terms of the following.
HRQoL, e.g. measured with the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ‐C30) or the Short Form Health Survey (SF‐36) (Aaronson 1993; Aaronson 1998).
Distress, e.g. measured with the Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983), the Beck Depression Inventory (BDI) (Beck 1996), or the Distress Thermometer (DT) (Tuinman 2008).
Supportive care needs, e.g. measured with the Supportive Care Needs Survey (SCNS), Sanson‐Fisher 2000, or the Cancer Survivors' Unmet Needs measure (CaSUN) (Hodgkinson 2007).
Adverse events: overburdening of patients by screening procedures, or induced fear or stress by discussing potential concerns and care needs with people with cancer who normally might prefer to use an avoidance‐coping strategy.
Secondary outcomes
Psychosocial well‐being measured by contributing components, defined by the study authors as follows: cognitive, emotional, psychological, social, or spiritual well‐being; mental health; and symptoms of anxiety or depression.
Patients’ satisfaction, e.g. measured with the EORTC cancer in‐patient satisfaction with care measure (EORTC IN‐PATSAT32), Bredart 2004; Bredart 2005, or the Patient Satisfaction and Quality in Oncological Care (PASQOC) questionnaire (Kleeberg 2005; Kleeberg 2008).
Search methods for identification of studies
We used several sources to identify records for inclusion in the review. We included studies written in English, French, and Dutch (the language capabilities of the authors). Publication status was not an exclusion criterion.
Electronic searches
We searched the following databases up to 26 January 2018:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1) in the Cochrane Library;
MEDLINE Ovid (from 1946 to January week 3 2018);
Embase Ovid (from 1980 to 2018 week 2);
PsycINFO to 29 January 2018;
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) to 29 January 2018.
The search strategies consisted of a combination of controlled vocabulary and free‐text terms for 'cancer', 'care model', 'psychosocial', 'screening', and 'assessment'. The initial search strategy was developed for MEDLINE and subsequently adjusted for the other databases (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5).
Searching other resources
Reference lists
We screened the reference lists of all included records as well as the reference lists of relevant reviews or clinical guidelines for relevant records.
Focused literature search
We searched the tables of contents of the last seven years (January 2010 to January 2018) in the journals Psycho‐Oncology and Supportive Care in Cancer.
Trial registers
We also searched the following trial registers to identify unpublished screening studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov);
UK National Research Register (webarchive.nationalarchives.gov.uk);
ISRCTN registry (www.isrctn.com/);
Dutch trial register (NRT) (www.trialregister.nl/trialreg/index);
RePORT Expenditures and Results (RePORTER) query tool (report.nih.gov).
These registries were consulted with a search combining 'cancer' with 'care model', 'psychosocial' and 'screening' or 'assessment' (Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10).
Conference abstracts
We searched relevant research initiatives presented on the World Congress of Psycho‐Oncology, organised by the International Psycho‐Oncology Society (IPOS). We screened the abstract proceedings of the IPOS conferences organised from 2010 to 2017.
We introduced the trial registers search and the conference abstracts search in order to minimise the risk of publication bias.
Data collection and analysis
We carried out data collection and analysis in accordance with the guidelines published in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Details are provided below.
Selection of studies
All records retrieved from the electronic search in the databases were imported into Covidence, systematic review software developed in collaboration with Cochrane (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org), and duplicates were removed. Two review authors independently screened titles and abstracts for relevancy (BS paired with AVH, BA, GB, JM, PV). Two review authors independently inspected the full texts of the relevant records (BS paired with AVH or BA) to assess their eligibility according to the inclusion and exclusion criteria. Where necessary, BS contacted study authors (two attempts) to obtain additional information or results to support the screening decision. We documented reasons for exclusion. Any cases of disagreement between the two review authors were resolved by discussion or by involving a third review author (AVH or BA). We included a PRISMA flow diagram to display the screening process (Liberati 2009).
Data extraction and management
Two review authors (BS paired with AVH or BA) independently extracted data from the included studies. Hereto, a data extraction file was constructed in accordance with the checklist proposed by Cochrane and the CReDECI 2‐guideline (Higgins 2011; Mohler 2015). Where possible, we obtained the following data from every study (Appendix 11).
Study information: authors, publication year, source of publication, funding of studies, and any conflicts of interest reported by the authors.
Methods: study design, study duration.
Participants: country of recruitment, description of people with cancer population, setting of recruitment, inclusion criteria, exclusion criteria.
Intervention: type of randomisation, aim of the study, content of screening or assessment, interventionist or executor of the concrete screening intervention, description of the screening or assessment intervention procedure (defined as 'solitary screening intervention' or 'screening intervention with co‐intervention to use screening results' added with a description of the intervention procedure), conditions for intervention implementation (e.g. necessary equipment for the screening, training for involved professionals, developed guidelines or handbooks, care or referral protocols, scheduled inter‐ or multidisciplinary meetings), theoretical basis of the studied screening or assessment intervention, description of the procedure for the comparative condition(s), protocol adherence, length of follow‐up.
Outcomes: primary and secondary outcome(s) defined by the study authors, outcome time points.
Study results: sample size, number of participants on which the analysis was based, mean age of sample, ratio of gender in sample, results of primary outcomes relevant to the review focus, results of secondary outcomes relevant to the review focus. We extracted continuous data as means with standard deviation per intervention group, if available. We extracted dichotomous data as number of events and totals per intervention group. If these data were not reported as such in the original papers, we converted them from the data available.
Review authors’ conclusion: conclusion on the results of the primary and secondary outcomes belonging to the scope of this review.
Evaluation of potential bias: sample size calculation, sequence generation, allocation concealment, blinding of personnel and people with cancer, blinding of outcome assessors, completeness of outcome data, reporting on outcome data, other sources of bias.
Any disagreements were resolved by discussion or by involving an additional review author when necessary (AVH or BA). When any of the record information was missing or unclear, BS made multiple attempts to contact the study authors to obtain further details.
Assessment of risk of bias in included studies
Randomised controlled trials
Two review authors (BS paired with AVH or BA) independently assessed the risk of bias of the included RCTs using Cochrane's tool for assessing the risk of bias (Higgins 2011). Each of the domains of potential bias was labelled as 'high risk', 'low risk', or 'unclear risk'. Any disagreements between the two review authors were resolved by discussion or by involvement of a third review author (AVH or BA).
We based the overall bias judgement of included RCTs on the following three domains of Cochrane's tool for assessing the risk of bias (Higgins 2011): adequate sequence generation, blinding of outcome assessors, and selective outcome reporting. An RCT at low risk on all of these domains was labelled as a low‐risk study. An RCT at high risk on one of these domains was labelled as a high‐risk study. If there was no clear information on the risk of bias for one or more key domains, but the RCT was not at high risk for any domain, we indicated that the risk of bias in the study was unclear.
Selection bias
Sequence generation
We assessed the method used to allocate participants to the conditions in the intervention and the control groups to determine whether it could produce comparable groups. We assessed the method as 'low risk' if random components were used (coin‐tossing, throwing dice, random computer assignment); 'high risk' if allocation was predictable (alternation; assignment based on date of birth, case record number, and date of presentation); or 'unclear risk' if there was insufficient information to judge sequence generation.
Allocation concealment
We evaluated the method used to conceal the allocation sequence to determine whether condition allocation could be foreseen. We labelled the method as 'low risk' if allocation could not have been foreseen (central or telephone randomisation; consecutively numbered, sealed envelopes); 'high risk' if allocation could have been foreseen (printed lists of computer‐randomised allocation, unsealed envelopes, date of birth ); or 'unclear risk' if there was insufficient information to judge allocation concealment.
Performance bias
We assessed the method used, if any, to blind study participants and personnel to the received intervention. Due to the nature of the studied screening intervention, blinding participants is difficult. We assessed the method as 'low risk' (participants and personnel blinded, or if we judged that a lack of blinding would not have affected the results); 'high risk' (incomplete or no blinding); or 'unclear risk' if there was insufficient information on blinding.
Detection bias
All outcomes in the scope of this review were subjective outcomes queried using self‐report measures or through interviews. All outcomes were thus ‐ strictly speaking ‐ sensitive to potential bias (influence of social desirability in answering). However, there were differences between studies in the efforts made to blind interviewers or other outcome assessors, or to prevent an extra person from inducing potential bias by knowledge of condition allocation. Consequently, we used the domain of detection bias to evaluate the blinding of outcome assessors to knowledge of condition allocation. We assessed studies as 'high risk' (outcome assessor was familiar with the intervention the participant received); 'low risk' (outcome assessor was not aware of the intervention the participant received, or outcomes were retrieved by self‐report of people with cancer); or 'unclear risk' if there was insufficient information to assess potential detection bias.
Attrition bias
We assessed the amount, nature, or handling of incomplete data to evaluate the potential for attrition bias. We assessed methods as 'low risk' (e.g. no missing outcome data; missing outcome data balanced across groups); 'high risk' (e.g. missing data for one or more of the primary outcome measures; numbers or reasons for missing data unbalanced across groups); or 'unclear risk' if there was insufficient information to assess potential attrition bias. We felt the need to assign a cut‐off for judging dropout rates as high or low. In reference to the literature, we chose to consider rates above 15% as a high dropout rate (Dettori 2011; Kristman 2004; Sacket 1997), resulting in a determination of high risk of attrition bias.
Reporting bias
We evaluated the data supporting the assessment of selective outcome reporting. For this domain, we coded studies as 'low risk' (study protocol was available, and all of the study’s prespecified outcomes were reported in the prespecified way; or the study protocol is not available, but it was clear that all the published reports included all expected outcomes, including those that were prespecified); 'high risk' (not all the prespecified primary outcomes were reported); or 'unclear risk' (insufficient information to judge reporting bias).
Non‐randomised controlled trials
Two review authors (BS paired with AVH or BA) independently assessed the risk of bias of the included NRCTs using the Cochrane tool for bias assessment in NRCTs, the Risk Of Bias In Non‐randomized Studies ‐ of Interventions (ROBINS‐I) tool (Sterne 2016). Using the ROBINS‐I tool, we assessed the risk of bias of studies based on the following seven domains.
Bias due to confounding
Bias in selection of participants into the study
Bias in classification of interventions
Bias due to deviations from the intended intervention
Bias due to missing data
Bias in measurement of outcomes
Bias in selection of the reported result
Our 'Risk of bias' judgements led to labelling the studies on these domains as 'critical risk', 'serious risk', 'moderate risk', 'low risk', or 'no information'. How we reached our 'Risk of bias' judgements for the pre‐intervention and at‐intervention domains is shown in Table 3, and how we reached these judgements for post‐intervention domains is provided in Table 4. Any disagreements between the two review authors were resolved by discussion or with the involvement of a third review author (AVH or BA).
1. Reaching risk of bias judgements in ROBINS‐I: pre‐intervention and at‐intervention domains.
| Judgement | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions |
| Low risk of bias (the study is comparable to a well‐performed RCT with regard to this domain) | No confounding expected. | All participants who would have been eligible for the target trial were included in the study and start of follow‐up and start of intervention coincide for all participants. | Intervention status is well‐defined and based solely on information collected at the time of intervention. |
| Moderate risk of bias (the study is sound for an NRCT with regard to this domain but cannot be considered comparable to a well‐performed RCT) | Confounding expected, all known important confounding domains appropriately measured and controlled for; and reliability and validity of measurement of important domains were sufficient, such that we do not expect serious residual confounding. |
Selection into the study may have been related to intervention and outcome, but the authors used appropriate methods to adjust for the selection bias; or start of follow‐up and start of intervention do not coincide for all participants, but (a) the proportion of participants for which this was the case was too low to induce important bias; (b) the authors used appropriate methods to adjust for the selection bias; or (c) the review authors are confident that the rate (hazard) ratio for the effect of intervention remains constant over time. |
Intervention status is well‐defined, but some aspects of the assignments of intervention status were determined retrospectively. |
| Serious risk of bias (the study has some important problems) | Switches in treatment, co‐interventions, or problems with implementation fidelity are apparent and are not adjusted for in the analyses. | Proportions of missing participants differ substantially across interventions; or reasons for missingness differ substantially across interventions; and missing data were addressed inappropriately in the analysis; or the nature of the missing data means that the risk of bias cannot be removed through appropriate analysis. |
The methods of outcome assessment were not comparable across intervention groups; or the outcome measure was subjective (i.e. likely to be influenced by knowledge of the intervention received by study participants) and was assessed by outcome assessors aware of the intervention received by study participants; or error in measuring the outcome was related to intervention status. |
| Critical risk of bias (the study is too problematic to provide any useful evidence on the effects of the intervention) | Substantial deviations from the intended intervention are present and are not adjusted for in the analysis. | (Unusual) There were critical differences between interventions in participants with missing data that were not, or could not, be addressed through appropriate analysis. | The methods of outcome assessment were so different that they cannot reasonably be compared across intervention groups. |
| No information on which to base a judgement about risk of bias for this domain | No information is reported on whether there is deviation from the intended intervention. | No information is reported about missing data or the potential for data to be missing. | No information is reported about the methods of outcome assessment. |
| Source:Sterne 2016. | |||
Abbreviations:
NRCT: non‐randomised controlled trial RCT: randomised controlled trial
2. Reaching risk of bias judgements in ROBINS‐I: postintervention domains.
| Judgement | Bias due to deviation from intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result |
| Low risk of bias (the study is comparable to a well‐performed RCT with regard to this domain) | No bias due to deviation from the intended intervention is expected, e.g. if both the intervention and comparator are implemented over a short time period, and subsequent interventions are part of routine medical care, or if the specified comparison relates to initiation of intervention regardless of whether it is continued. | Data were reasonably complete; or proportions of and reasons for missing participants were similar across intervention groups; or analyses that addressed missing data are likely to have removed any risk of bias. |
The methods of outcome assessment were comparable across intervention groups; and the outcome measure was unlikely to be influenced by knowledge of the intervention received by study participants (i.e. is objective) or the outcome assessors were unaware of the intervention received by study participants; and any error in measuring the outcome is unrelated to intervention status. |
There is clear evidence (usually through examination of a pre‐registered protocol or statistical analysis plan) that all reported results correspond to all intended outcomes, analyses, and subcohorts. |
| Moderate risk of bias (the study is sound for an NRCT with regard to this domain but cannot be considered comparable to a well‐performed RCT) | Bias due to deviation from the intended intervention is expected, and switches, co‐interventions, and some problems with intervention fidelity are appropriately measured and adjusted for in the analyses. Alternatively, most (but not all) deviations from intended intervention reflect the natural course of events after initiation of intervention. | Proportions of missing participants differ across interventions; or reasons for missingness differ minimally across interventions; and missing data were not addressed in the analysis. |
The methods of outcome assessment were comparable across intervention groups; and the outcome measure is only minimally influenced by knowledge of the intervention received by study participants; and any error in measuring the outcome is only minimally related to intervention status. |
The outcome measurements and analyses are consistent with an a priori plan; or are clearly defined and both internally and externally consistent; and there is no indication of selection of the reported analysis from among multiple analyses; and there is no indication of selection of the cohort or subgroups for analysis and reporting on the basis of the results. |
| Serious risk of bias (the study has some important problems) | Switches in treatment, co‐interventions, or problems with implementation fidelity are apparent and are not adjusted for in the analyses. | Proportions of missing participants differ substantially across interventions; or reasons for missingness differ substantially across interventions; and missing data were addressed inappropriately in the analysis; or the nature of the missing data means that the risk of bias cannot be removed through appropriate analysis. |
The methods of outcome assessment were not comparable across intervention groups; or the outcome measure was subjective (i.e. likely to be influenced by knowledge of the intervention received by study participants) and was assessed by outcome assessors aware of the intervention received by study participants; or error in measuring the outcome was related to intervention status. |
Outcome measurements or analyses are internally or externally inconsistent; or there is a high risk of selective reporting from among multiple analyses; or the cohort or subgroup is selected from a larger study for analysis and appears to be reported on the basis of the results. |
| Critical risk of bias (the study is too problematic to provide any useful evidence on the effects of the intervention) | Substantial deviations from the intended intervention are present and are not adjusted for in the analysis. | (Unusual) There were critical differences between interventions in participants with missing data that were not, or could not, be addressed through appropriate analysis. | The methods of outcome assessment were so different that they cannot reasonably be compared across intervention groups. | There is evidence or strong suspicion of selective reporting of results, and the unreported results are likely to be substantially different from the reported results. |
| No information on which to base a judgement about risk of bias for this domain | No information is reported on whether there is deviation from the intended intervention. | No information is reported about missing data or the potential for data to be missing. | No information is reported about the methods of outcome assessment. | There is too little information to make a judgement (e.g. if only an abstract is available for the study). |
| Source:Sterne 2016. | ||||
Abbreviations:
NRCT: non‐randomised controlled trial RCT: randomised controlled trial
As specified in the ROBINS‐I tool manual, we labelled an NRCT as a low‐risk study if we judged the study to be at low risk of bias for all domains; a moderate‐risk study if we judged the study to be at low or moderate risk of bias for all domains; a serious‐risk study if we judged the study to be at serious risk of bias in at least one domain, but not at critical risk of bias in any domain; a critical‐risk study if we judged the study to be at critical risk of bias in at least one domain; and we indicated that there was ‘no information on an NRCT‘ if there was no clear indication that the study was at serious or critical risk of bias, and there was a lack of information on one or more key domains.
For the NRCTs, we checked if covariance analyses were performed. Doing so, there was a correction of the results in function of potential influences from other variables than the intervention of interest, and the risk of bias in results was reduced.
Measures of treatment effect
We analysed continuous data of similar measures with the mean difference (MD), and used standardised mean difference (SMD) when measures were different. For dichotomous data, we used risk ratio (RR) for presentation of results. The method for handling ordinary scales depended on the length of the scale. We used the RR for scales that could be dichotomised, and calculated the MD for five‐point Likert scales or longer. We determined the 95% confidence intervals (CIs) for all measures of treatment effect.
According to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we have corrected the direction of the scales in case similar outcomes were reported with different scales, but with a different direction of magnitude. This allowed for correct meta‐analyses, and facilitated interpretation of data presented in an evidence summary in case meta‐analyses were not possible. The symptom subscales of the quality of life tools FLIC (Functional Living Index‐Cancer), SF‐36, FACT‐C (Functional Assessment of Cancer Therapy‐Colorectal), and PCQoL (Prostate Cancer‐Related Quality of Life Scales) all represent fewer symptoms with higher scores, while the EORTC symptom subscales represent more symptoms with higher scores. The reported symptom scales of the EORTC were therefore adjusted by subtracting the reported score from the maximal score possible (100). We made similar adjustments to two scales used for measuring psychosocial well‐being: the Locke‐Wallace Marital Adjustment Scale (LWMAS) (reported data subtracted from the maximal score of 158) and the Dyadic Adjustment Scale (DAS) (reported data multiplied by −1, as data were presented as a change from baseline).
Unit of analysis issues
If possible, cluster RCT was used to examine the effect of screening and assessment on the psychosocial well‐being and care needs of people with cancer. In the meta‐analysis we conducted, the results were analysed together with the results from the individually randomised trial after adjustment of the sample sizes as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For this purpose, an estimate of the intracluster correlation coefficient (ICC) was used, preferably from a similar trial. If no such estimate was available, a conservative ICC of 0.05 was used. We assessed the impact of cluster RCT on the results in a sensitivity analysis if applicable.
Dealing with missing data
When possible, we evaluated dropout rates of all included studies. In case of ambiguity or incompleteness of data, one review author (BA) undertook multiple attempts to contact the study authors for additional information. In the absence of response, the lack of data for the Evidence Summary was indicated with N/A (not available) and N/E (not estimable).
Assessment of heterogeneity
In concordance with Cochrane guidelines, we decided to only perform a meta‐analysis when a group of studies was sufficiently homogeneous to provide a meaningful summary; in case of heterogeneity, we decided to perform a narrative data synthesis. In our meta‐analysis, we used the Chi2 test included in the forest plots to examine heterogeneity in intervention effects. We calculated the I2 statistic to quantify inconsistency of the observed effects. With these results, we calculated the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). We adopted the guide for interpretation suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
The significance of the observed value was interpreted in the context of the magnitude and direction of effects, and on the strength of evidence for heterogeneity (i.e. P value from the Chi2 test).
Assessment of reporting biases
To assess publication bias, we planned to produce funnel plots (estimated treatment effects against their standard error) if more than 10 studies were included in the meta‐analyses.
Data synthesis
If two or more eligible studies were identified and found to be sufficiently similar, we performed a meta‐analysis using Review Manager 5 (RevMan 2014). We used a random‐effects model, as we expected at least some heterogeneity between the studies. For continuous outcomes (HRQoL, distress, anxiety), we used the inverse‐variance method. If studies reported similar outcomes, but with distinct scales, we would have used the generic inverse‐variance method and reported the pooled SMD. For dichotomous outcomes, we planned to use the Mantel‐Haenszel method.
For all of the studies that could not be included in the meta‐analysis due to heterogeneity, we described the results narratively.
For each outcome, we presented all available data from the studies in the Evidence Summary (Table 5; Table 6; Table 7) and summarised the results in a 'Summary of findings' table. We graded the certainty of the evidence based on: study design, inconsistency of results, indirectness of evidence, imprecision, and publication bias. Our overall judgement on the certainty of evidence for each outcome is displayed in Table 1.
3. Evidence summary ‐ continuous outcomes.
| Raw data: continuous outcomes | |||||||||||
| Main outcome | Suboutcome | Time postintervention | Scale used | Intervention (screening) | Control (usual care) | MD (95% CI) | Study ID | ||||
| Mean | SD | N | Mean | SD | N | ||||||
| HRQoL (continuous) | Global health status | 1 month | EORTC QLQ‐C30 | 65.18 | 17.43 | 28 | 51.49 | 26.16 | 28 | 13.69 [2.05, 25.33] | Bramsen 2008 |
| 61.8 | 20.9 | 109 | 61.2 | 18.2 | 103 | 0.60 [−4.67, 5.87] | Hollingworth 2013 | ||||
| EQ‐5D | 0.739 | 0.223 | 109 | 0.74 | 0.249 | 103 | −0.00 [−0.06, 0.06] | ||||
| FACT‐C | 107.8 | 11.9 | 20 | 101.2 | 22.3 | 21 | 6.60 [−4.27, 17.47] | Young 2010 | |||
| 96.1 | 18.5 | 35 | 98.3 | 19.7 | 31 | 0.21 [−2.47, 2.89] | Harrison 2011 | ||||
| 100.61 | 17.78 | 346 | 100.4 | 18.6 | 363 | 0.01 [−0.14, 0.16] | Young 2013 | ||||
| 6 weeks | EORTC QLQ‐C30 | 63.1 | 25 | 42 | 65.5 | 25 | 44 | −0.10 [−0.52, 0.33] | Nimako 2015 | ||
| 65.4 | 20.825 | 45 | 65.5 | 25 | 44 | −0.00 [−0.42, 0.41] | |||||
| 2 months | 57.5 | 4.74 | 103 | 58 | 4.75 | 192 | −0.11 [−0.34, 0.13] | Waller 2012 | |||
| 3 months | EORTC QLQ‐C30 | 72.61 | 20.08 | 268 | 71.48 | 19.62 | 300 | 0.06 [−0.11, 0.22] | Braeken 2013 | ||
| FLIC | 116.5 | 21.1 | 60 | 114.1 | 24.7 | 60 | 0.10 [−0.23, 0.44] | Rosenbloom 2007 | |||
| 112.1 | 20.6 | 60 | 114.1 | 24.7 | 60 | −0.09 [−0.41, 0.24] | |||||
| FACT‐C | 114.2 | 13.5 | 20 | 101.5 | 19.1 | 21 | 0.75 [0.11, 1.39] | Young 2010 | |||
| 97.5 | 21.4 | 34 | 96.2 | 21.9 | 29 | 0.06 [−0.44, 0.55] | Harrison 2011 | ||||
| 103.48 | 18.17 | 336 | 103.26 | 18.58 | 351 | 0.01 [−0.14, 0.16] | Young 2013 | ||||
| 4 months | EORTC QLQ‐C30 | 56.5 | 5.09 | 85 | 58 | 4.75 | 192 | −0.31 [−0.56, −0.05] | Waller 2012 | ||
| 6 months | 69.3 | 22.14 | 281 | 68 | 21.89 | 297 | 0.06 [−0.10, 0.22] | Bergholdt 2013 | |||
| 77 | 16 | 80 | 80 | 18 | 80 | −0.18 [−0.49, 0.14] | de Leeuw 2013 | ||||
| 68.6 | 17.7 | 108 | 68.3 | 18.2 | 101 | 0.02 [−0.25, 0.29] | Hollingworth 2013 | ||||
| 57.5 | 5.39 | 67 | 58 | 4.75 | 192 | −0.10 [−0.38, 0.18] | Waller 2012 | ||||
| EQ‐5D | 0.783 | 0.217 | 108 | 0.79 | 0.246 | 103 | −0.03 [−0.30, 0.24] | Hollingworth 2013 | |||
| FLIC | 115.8 | 22.9 | 51 | 112.2 | 21.4 | 52 | 0.16 [−0.17, 0.49] | Rosenbloom 2007 | |||
| 113.3 | 24.5 | 51 | 112.2 | 21.4 | 52 | 0.05 [−0.28, 0.37] | |||||
| FACT‐C | 106 | 19.3 | 28 | 98.6 | 23.4 | 30 | 0.34 [−0.18, 0.86] | Harrison 2011 | |||
| 105.1 | 17.88 | 322 | 105.35 | 19.5 | 350 | −0.01 [−0.16, 0.14] | Young 2013 | ||||
| 12 months | EORTC QLQ‐C30 | 75.95 | 18.7 | 268 | 76.09 | 17.53 | 300 | −0.01 [−0.17, 0.16] | Braeken 2013 | ||
| 81 | 18 | 80 | 80 | 17 | 80 | 0.06 [−0.25, 0.37] | de Leeuw 2013 | ||||
| 68.5 | 20.2 | 106 | 69.6 | 20.4 | 103 | −0.05 [−0.33, 0.22] | Hollingworth 2013 | ||||
| EQ‐5D | 0.742 | 0.268 | 106 | 0.788 | 0.257 | 103 | −0.17 [−0.45, 0.10] | ||||
| 14 months | EORTC QLQ‐C30 | 72.1 | 19.66 | 240 | 72.8 | 19.71 | 246 | −0.04 [−0.21, 0.14] | Bergholdt 2013 | ||
| 4th follow‐up visit | SF‐36 | 46 | N/A | 108 | 47 | N/A | 110 | N/E | Hilarius 2008 | ||
| Physical functioning | 1 month | EORTC QLQ‐C30 | 80.48 | 20.84 | 28 | 63.63 | 23.42 | 28 | 16.85 [5.24, 28.46] | Bramsen 2008 | |
| 81.9 | 20.5 | 109 | 80.7 | 20.5 | 103 | 1.20 [−4.32, 6.72] | Hollingworth 2013 | ||||
| FACT‐C | 22.8 | 3.3 | 20 | 21.8 | 5.1 | 21 | 1.00 [−1.62, 3.62] | Young 2010 | |||
| 6 weeks | EORTC QLQ‐C30 | 74.6 | 21.675 | 42 | 72.2 | 21.675 | 43 | 2.40 [−6.82, 11.62] | Nimako 2015 | ||
| 73.8 | 25 | 43 | 72.2 | 21.675 | 43 | 1.60 [−8.29, 11.49] | |||||
| 2 months | 57.21 | 30.33 | 55 | 60.2 | 29.99 | 53 | −2.99 [−14.37, 8.39] | Schofield 2013 | |||
| 3 months | 79.63 | 21.02 | 268 | 81.78 | 17.83 | 300 | −2.15 [−5.38, 1.08] | Braeken 2013 | |||
| 63.49 | 27.66 | 55 | 59.09 | 26.57 | 53 | 4.40 [−5.83, 14.63] | Schofield 2013 | ||||
| FLIC | 45.9 | 12 | 69 | 45.7 | 11.9 | 71 | 0.20 [−3.76, 4.16] | Rosenbloom 2007 | |||
| 44.5 | 10.4 | 60 | 45.7 | 11.9 | 60 | −1.20 [−5.20, 2.80] | |||||
| FACT‐C | 24.4 | 3.2 | 20 | 22.1 | 5.2 | 21 | 2.30 [−0.33, 4.93] | Young 2010 | |||
| 6 months | EORTC QLQ‐C30 | 79.7 | 22.1 | 280 | 79 | 22.65 | 294 | 0.70 [−2.96, 4.36] | Bergholdt 2013 | ||
| 83 | 17 | 80 | 86 | 16 | 80 | −3.00 [−8.12, 2.12] | de Leeuw 2013 | ||||
| 84.2 | 19 | 108 | 83.8 | 18.6 | 101 | 0.40 [−4.70, 5.50] | Hollingworth 2013 | ||||
| FLIC | 46.7 | 11.6 | 51 | 45.2 | 9.8 | 52 | 1.50 [−2.65, 5.65] | Rosenbloom 2007 | |||
| 45 | 20.6 | 51 | 45.2 | 9.8 | 52 | −0.20 [−6.45, 6.05] | |||||
| 12 months | EORTC QLQ‐C30 | 81.99 | 18.06 | 268 | 85 | 17.76 | 300 | −3.01 [−5.96, −0.06] | Braeken 2013 | ||
| 86 | 17 | 80 | 87 | 16 | 80 | −1.00 [−6.12, 4.12] | de Leeuw 2013 | ||||
| 83.8 | 19.3 | 106 | 85.5 | 17.8 | 103 | −1.70 [−6.73, 3.33] | Hollingworth 2013 | ||||
| 14 months | 82 | 20.19 | 234 | 81.9 | 20.45 | 240 | 0.10 [−3.56, 3.76] | Bergholdt 2013 | |||
| 4th follow‐up visit | SF‐36 | 53 | 28 | 104 | 52 | 26 | 95 | 1.00 [−6.50, 8.50] | Detmar 2002 | ||
| 69 | N/A | 108 | 62 | N/A | 110 | N/E | Hilarius 2008 | ||||
| Role functioning | 1 month | EORTC QLQ‐C30 | 57.14 | 27.75 | 28 | 39.88 | 35.35 | 28 | 17.26 [0.61, 33.91] | Bramsen 2008 | |
| 69.4 | 31.3 | 109 | 68 | 28.8 | 103 | 1.40 [−6.69, 9.49] | Hollingworth 2013 | ||||
| FACT‐C | 13.8 | 6.1 | 20 | 12.1 | 5.8 | 21 | 1.70 [−1.95, 5.35] | Young 2010 | |||
| 6 weeks | EORTC QLQ‐C30 | 70.63 | 25 | 42 | 69.7 | 25 | 44 | 0.93 [−9.64, 11.50] | Nimako 2015 | ||
| 62.5 | 25 | 44 | 69.7 | 25 | 44 | −7.20 [−17.65, 3.25] | |||||
| 2 months | 57.03 | 36.71 | 55 | 56.82 | 35.82 | 53 | 0.21 [−13.47, 13.89] | Schofield 2013 | |||
| 3 months | 72.77 | 29.77 | 268 | 72.87 | 27.52 | 300 | −0.10 [−4.83, 4.63] | Braeken 2013 | |||
| 58.48 | 36.86 | 55 | 65.01 | 35.16 | 53 | −6.53 [−20.11, 7.05] | Schofield 2013 | ||||
| FACT‐C | 21.4 | 4.8 | 20 | 17.4 | 7.3 | 21 | 4.00 [0.24, 7.76] | Young 2010 | |||
| 6 months | EORTC QLQ‐C30 | 72.5 | 31.28 | 277 | 71.3 | 31.2 | 291 | 1.20 [−3.94, 6.34] | Bergholdt 2013 | ||
| 79 | 26 | 80 | 81 | 24 | 80 | −2.00 [−9.75, 5.75] | de Leeuw 2013 | ||||
| 79.2 | 24.9 | 108 | 79.7 | 27.6 | 101 | −0.50 [−7.64, 6.64] | Hollingworth 2013 | ||||
| 12 months | 80.26 | 26.65 | 268 | 82.44 | 24.7 | 300 | −2.18 [−6.42, 2.06] | Braeken 2013 | |||
| 81 | 27 | 80 | 85 | 25 | 80 | −4.00 [−12.06, 4.06] | de Leeuw 2013 | ||||
| 80.5 | 26.4 | 106 | 84.1 | 21.9 | 103 | −3.60 [−10.17, 2.97] | Hollingworth 2013 | ||||
| 14 months | 78.8 | 29.57 | 235 | 78 | 28.25 | 239 | 0.80 [−4.41, 6.01] | Bergholdt 2013 | |||
| Role functioning (emotional) | 4th follow‐up visit | SF‐36 | 69 | 44 | 104 | 60 | 44 | 95 | 9.00 [−3.24, 21.24] | Detmar 2002 | |
| 66 | N/A | 108 | 68 | N/A | 110 | N/E | Hilarius 2008 | ||||
| Role functioning (physical) | 36 | 42 | 104 | 31 | 41 | 95 | 5.00 [−6.54, 16.54] | Detmar 2002 | |||
| 30 | N/A | 108 | 33 | N/A | 110 | N/E | Hilarius 2008 | ||||
| Emotional functioning | 1 month | EORTC QLQ‐C30 | 78.28 | 15.93 | 28 | 65.87 | 20.51 | 28 | 12.41 [2.79, 22.03] | Bramsen 2008 | |
| 79.1 | 21.1 | 109 | 77.8 | 21.4 | 103 | 1.30 [−4.42, 7.02] | Hollingworth 2013 | ||||
| FACT‐C | 21.2 | 2 | 20 | 19.4 | 3.7 | 21 | 1.80 [−0.01, 3.61] | Young 2010 | |||
| 6 weeks | EORTC QLQ‐C30 | 74.2 | 22.925 | 42 | 76.4 | 20.825 | 43 | −2.20 [−11.52, 7.12] | Nimako 2015 | ||
| 76.6 | 25 | 43 | 76.4 | 20.825 | 43 | 0.20 [−9.53, 9.93] | |||||
| 2 months | 81.43 | 24.62 | 55 | 73.23 | 24.1 | 53 | 8.20 [−0.99, 17.39] | Schofield 2013 | |||
| 3 months | 78.38 | 22.75 | 268 | 79.46 | 20.68 | 300 | −1.08 [−4.67, 2.51] | Braeken 2013 | |||
| 75.31 | 26.7 | 55 | 75.51 | 25.26 | 53 | −0.20 [−10.00, 9.60] | Schofield 2013 | ||||
| FACT‐C | 21.8 | 1.9 | 20 | 19.2 | 3.2 | 21 | 2.60 [1.00, 4.20] | Young 2010 | |||
| 6 months | EORTC QLQ‐C30 | 81.6 | 21.17 | 278 | 80.5 | 20.87 | 293 | 1.10 [−2.35, 4.55] | Bergholdt 2013 | ||
| 84 | 19 | 80 | 85 | 19 | 80 | −1.00 [−6.89, 4.89] | de Leeuw 2013 | ||||
| 81.2 | 18 | 108 | 80.3 | 20.7 | 101 | 0.90 [−4.37, 6.17] | Hollingworth 2013 | ||||
| 12 months | 83.66 | 20.8 | 268 | 81.23 | 20.6 | 300 | 2.43 [−0.98, 5.84] | Braeken 2013 | |||
| 82 | 23 | 80 | 85 | 18 | 80 | −3.00 [−9.40, 3.40] | de Leeuw 2013 | ||||
| 78.7 | 21.6 | 106 | 80.3 | 21.4 | 103 | −1.60 [−7.43, 4.23] | Hollingworth 2013 | ||||
| 14 months | 80.8 | 21.93 | 238 | 80.7 | 22 | 240 | 0.10 [−3.84, 4.04] | Bergholdt 2013 | |||
| Cognitive functioning | 1 month | 85.12 | 19.43 | 28 | 75 | 25.46 | 28 | 10.12 [−1.74, 21.98] | Bramsen 2008 | ||
| 79.8 | 20.5 | 109 | 78.2 | 21.8 | 103 | 1.60 [−4.10, 7.30] | Hollingworth 2013 | ||||
| 6 weeks | 76.6 | 25 | 42 | 81.4 | 25 | 43 | −4.80 [−15.43, 5.83] | Nimako 2015 | |||
| 83.7 | 25 | 41 | 81.4 | 25 | 43 | 2.30 [−8.40, 13.00] | |||||
| 2 months | 80.45 | 26.25 | 55 | 75.34 | 25.7 | 53 | 5.11 [−4.69, 14.91] | Schofield 2013 | |||
| 3 months | 83.92 | 19.73 | 268 | 84.27 | 19.49 | 300 | −0.35 [−3.58, 2.88] | Braeken 2013 | |||
| 80.4 | 27.51 | 55 | 77.73 | 26.21 | 53 | 2.67 [−7.46, 12.80] | Schofield 2013 | ||||
| 6 months | 83.9 | 22.02 | 278 | 83 | 21.63 | 290 | 0.90 [−2.69, 4.49] | Bergholdt 2013 | |||
| 88 | 17 | 80 | 87 | 17 | 80 | 1.00 [−4.27, 6.27] | de Leeuw 2013 | ||||
| 81 | 20.3 | 108 | 80.7 | 19.7 | 101 | 0.30 [−5.12, 5.72] | Hollingworth 2013 | ||||
| 12 months | 82.46 | 22.11 | 268 | 82.82 | 19.98 | 300 | −0.36 [−3.84, 3.12] | Braeken 2013 | |||
| 87 | 20 | 80 | 86 | 21 | 80 | 1.00 [−5.35, 7.35] | de Leeuw 2013 | ||||
| 82.9 | 18.6 | 106 | 79.8 | 22.5 | 103 | 3.10 [−2.51, 8.71] | Hollingworth 2013 | ||||
| 14 months | 85.1 | 23.49 | 238 | 82.6 | 23.04 | 245 | 2.50 [−1.65, 6.65] | Bergholdt 2013 | |||
| Social functioning | 1 month | 66.07 | 26.64 | 28 | 61.63 | 29.06 | 28 | 4.44 [−10.16, 19.04] | Bramsen 2008 | ||
| 69 | 31.7 | 109 | 67.3 | 29.7 | 103 | 1.70 [−6.57, 9.97] | Hollingworth 2013 | ||||
| FACT‐C | 23.4 | 3.8 | 20 | 22.6 | 5.4 | 21 | 0.80 [−2.05, 3.65] | Young 2010 | |||
| 6 weeks | EORTC QLQ‐C30 | 73.8 | 25 | 42 | 75.8 | 25 | 42 | −2.00 [−12.69, 8.69] | Nimako 2015 | ||
| 75.8 | 25 | 44 | 75.8 | 25 | 42 | 0.00 [−10.57, 10.57] | |||||
| 2 months | EORTC QLQ‐C30 | 68.28 | 31.96 | 55 | 70.58 | 31.16 | 53 | −2.30 [−14.20, 9.60] | Schofield 2013 | ||
| 3 months | 83.46 | 23.57 | 268 | 81.81 | 22.37 | 300 | 1.65 [−2.14, 5.44] | Braeken 2013 | |||
| 65.03 | 34.11 | 55 | 71.29 | 32.61 | 53 | −6.26 [−18.84, 6.32] | Schofield 2013 | ||||
| FLIC | 11.6 | 2.4 | 60 | 11.4 | 2.3 | 60 | 0.20 [−0.64, 1.04] | Rosenbloom 2007 | |||
| 11.2 | 2.4 | 60 | 11.4 | 2.3 | 60 | −0.20 [−1.04, 0.64] | |||||
| FACT‐C | 24.1 | 3.7 | 20 | 22.8 | 4.4 | 21 | 1.30 [−1.18, 3.78] | Young 2010 | |||
| 6 months | EORTC QLQ‐C30 | 86 | 23.8 | 280 | 85.7 | 23.56 | 295 | 0.30 [−3.57, 4.17] | Bergholdt 2013 | ||
| 91 | 15 | 80 | 90 | 16 | 80 | 1.00 [−3.81, 5.81] | de Leeuw 2013 | ||||
| 78.3 | 26.8 | 108 | 78.2 | 28.2 | 101 | 0.10 [−7.37, 7.57] | Hollingworth 2013 | ||||
| FLIC | 11.4 | 2.3 | 51 | 11.5 | 1.8 | 52 | −0.10 [−0.90, 0.70] | Rosenbloom 2007 | |||
| 11.1 | 2.3 | 51 | 11.5 | 1.8 | 52 | −0.40 [−1.20, 0.40] | |||||
| 12 months | EORTC QLQ‐C30 | 86.99 | 20.73 | 268 | 87.55 | 19.1 | 300 | −0.56 [−3.85, 2.73] | Braeken 2013 | ||
| 90 | 19 | 80 | 91 | 21 | 80 | −1.00 [−7.21, 5.21] | de Leeuw 2013 | ||||
| 81.3 | 27.5 | 106 | 84 | 23.4 | 103 | −2.70 [−9.62, 4.22] | Hollingworth 2013 | ||||
| 14 months | 87.4 | 21.93 | 238 | 88.2 | 22.11 | 242 | −0.80 [−4.74, 3.14] | Bergholdt 2013 | |||
| 4th follow‐up visit | SF‐36 | 65 | 30 | 104 | 63 | 29 | 95 | 2.00 [−6.20, 10.20] | Detmar 2002 | ||
| 69 | N/A | 108 | 65 | N/A | 110 | N/E | Hilarius 2008 | ||||
| Fatigue | 1 month | EORTC QLQ‐C30 | 58.33 | 23.55 | 28 | 43.25 | 28.74 | 28 | 15.08 [1.32, 28.84] | Bramsen 2008 | |
| 3 months | 66.93 | 26.64 | 268 | 67.06 | 25.18 | 300 | −0.13 [−4.41, 4.15] | Braeken 2013 | |||
| 6 months | 65.8 | 28 | 279 | 62.6 | 26.92 | 292 | 3.20 [−1.31, 7.71] | Bergholdt 2013 | |||
| 76 | 21 | 80 | 75 | 23 | 80 | 1.00 [−5.82, 7.82] | de Leeuw 2013 | ||||
| 12 months | 74.07 | 24.15 | 268 | 76.29 | 22.63 | 300 | −2.22 [−6.08, 1.64] | Braeken 2013 | |||
| 81 | 25 | 80 | 78 | 24 | 80 | 3.00 [−4.59, 10.59] | de Leeuw 2013 | ||||
| 14 months | 67.7 | 26.4 | 234 | 67.9 | 26.17 | 244 | −0.20 [−4.91, 4.51] | Bergholdt 2013 | |||
| Nausea/vomiting | 1 month | 82.14 | 22.19 | 28 | 74.4 | 27.02 | 28 | 7.74 [−5.21, 20.69] | Bramsen 2008 | ||
| 3 months | 92.85 | 18.51 | 268 | 95.77 | 12.2 | 300 | −2.92 [−5.53, −0.31] | Braeken 2013 | |||
| FLIC | 11.6 | 2.7 | 60 | 11.4 | 2.7 | 60 | 0.20 [−0.77, 1.17] | Rosenbloom 2007 | |||
| 11.5 | 2.7 | 60 | 11.4 | 2.7 | 60 | 0.10 [−0.87, 1.07] | |||||
| 6 months | EORTC QLQ‐C30 | 92 | 17.12 | 284 | 91.9 | 17.6 | 300 | 0.10 [−2.72, 2.92] | Bergholdt 2013 | ||
| 97 | 13 | 80 | 96 | 13 | 80 | 1.00 [−3.03, 5.03] | de Leeuw 2013 | ||||
| FLIC | 11.8 | 2.7 | 51 | 11.1 | 2.9 | 52 | 0.70 [−0.38, 1.78] | Rosenbloom 2007 | |||
| 11.6 | 3 | 51 | 11.1 | 2.9 | 52 | 0.50 [−0.64, 1.64] | |||||
| 12 months | EORTC QLQ‐C30 | 96.86 | 10.43 | 268 | 96.23 | 12.9 | 300 | 0.63 [−1.29, 2.55] | Braeken 2013 | ||
| 97 | 13 | 80 | 96 | 10 | 80 | 1.00 [−2.59, 4.59] | de Leeuw 2013 | ||||
| 14 months | 94.4 | 13.26 | 236 | 94.5 | 12.69 | 244 | −0.10 [−2.42, 2.22] | Bergholdt 2013 | |||
| Pain | 1 month | 84.52 | 21.24 | 28 | 63.69 | 33.96 | 28 | 20.83 [5.99, 35.67] | Bramsen 2008 | ||
| 3 months | 82.09 | 25.44 | 268 | 81.34 | 23.49 | 300 | 0.75 [−3.29, 4.79] | Braeken 2013 | |||
| 6 months | 78 | 26.91 | 274 | 77 | 27.35 | 283 | 1.00 [−3.51, 5.51] | Bergholdt 2013 | |||
| 85 | 22 | 80 | 86 | 23 | 80 | −1.00 [−7.97, 5.97] | de Leeuw 2013 | ||||
| EORTC QLQ‐H&N35 | 85 | 16 | 80 | 85 | 14 | 80 | 0.00 [−4.66, 4.66] | ||||
| 12 months | EORTC QLQ‐C30 | 85.16 | 22.02 | 268 | 86.33 | 23.22 | 300 | −1.17 [−4.89, 2.55] | Braeken 2013 | ||
| 88 | 22 | 80 | 85 | 22 | 80 | 3.00 [−3.82, 9.82] | de Leeuw 2013 | ||||
| EORTC QLQ‐H&N35 | 86 | 17 | 80 | 86 | 18 | 80 | 0.00 [−5.43, 5.43] | ||||
| 14 months | EORTC QLQ‐C30 | 78.6 | 27.95 | 234 | 78.1 | 26.79 | 241 | 0.50 [−4.43, 5.43] | Bergholdt 2013 | ||
| 4th follow‐up visit | SF‐36 | 68 | 28 | 104 | 66 | 28 | 95 | 2.00 [−5.79, 9.79] | Detmar 2002 | ||
| 74 | N/A | 108 | 75 | N/A | 110 | N/E | Hilarius 2008 | ||||
| Dyspnoea | 1 month | EORTC QLQ‐C30 | 82.14 | 21.24 | 28 | 77.78 | 22.65 | 28 | 4.36 [−7.14, 15.86] | Bramsen 2008 | |
| 3 months | 82.41 | 25.44 | 268 | 81.44 | 27.52 | 300 | 0.97 [−3.39, 5.33] | Braeken 2013 | |||
| 6 months | 82.1 | 27.49 | 286 | 83 | 27.15 | 297 | −0.90 [−5.34, 3.54] | Bergholdt 2013 | |||
| 90 | 20 | 80 | 86 | 23 | 80 | 4.00 [−2.68, 10.68] | de Leeuw 2013 | ||||
| 12 months | 85.9 | 20.73 | 268 | 84.95 | 24.97 | 300 | 0.95 [−2.81, 4.71] | Braeken 2013 | |||
| 88 | 21 | 80 | 88 | 19 | 80 | 0.00 [−6.21, 6.21] | de Leeuw 2013 | ||||
| 14 months | 84.6 | 27.12 | 233 | 86.8 | 25.43 | 245 | −2.20 [−6.92, 2.52] | Bergholdt 2013 | |||
| Insomnia | 1 month | 88.1 | 18.62 | 28 | 69.05 | 29.99 | 28 | 19.05 [5.97, 32.13] | Bramsen 2008 | ||
| 3 months | 73.39 | 30.19 | 268 | 71.83 | 30.13 | 300 | 1.56 [−3.41, 6.53] | Braeken 2013 | |||
| 6 months | 72.7 | 31.73 | 285 | 72.5 | 30.91 | 302 | 0.20 [−4.87, 5.27] | Bergholdt 2013 | |||
| 80 | 28 | 80 | 82 | 25 | 80 | −2.00 [−10.23, 6.23] | de Leeuw 2013 | ||||
| 12 months | 75.73 | 30.27 | 268 | 77.95 | 28.42 | 300 | −2.22 [−7.07, 2.63] | Braeken 2013 | |||
| 81 | 30 | 80 | 82 | 25 | 80 | −1.00 [−9.56, 7.56] | de Leeuw 2013 | ||||
| 14 months | 71.5 | 32.24 | 240 | 70.4 | 31.98 | 248 | 1.10 [−4.60, 6.80] | Bergholdt 2013 | |||
| Appetite loss | 1 month | 75 | 30.93 | 28 | 61.9 | 33.6 | 28 | 13.10 [−3.82, 30.02] | Bramsen 2008 | ||
| 3 months | 85.96 | 26 | 268 | 91.43 | 21.36 | 300 | −5.47 [−9.41, −1.53] | Braeken 2013 | |||
| 6 months | 84.1 | 27.59 | 288 | 85.9 | 27.33 | 301 | −1.80 [−6.24, 2.64] | Bergholdt 2013 | |||
| 87 | 23 | 80 | 91 | 19 | 80 | −4.00 [−10.54, 2.54] | de Leeuw 2013 | ||||
| 12 months | 94.01 | 17.37 | 268 | 93.36 | 18.22 | 300 | 0.65 [−2.28, 3.58] | Braeken 2013 | |||
| 93 | 17 | 80 | 92 | 21 | 80 | 1.00 [−4.92, 6.92] | de Leeuw 2013 | ||||
| 14 months | 92.1 | 19.62 | 239 | 90.4 | 19.91 | 246 | 1.70 [−1.82, 5.22] | Bergholdt 2013 | |||
| Constipation | 1 month | 83.33 | 26.45 | 28 | 79.76 | 24.58 | 28 | 3.57 [−9.80, 16.94] | Bramsen 2008 | ||
| 3 months | 91.6 | 18.73 | 268 | 89.63 | 21.98 | 300 | 1.97 [−1.38, 5.32] | Braeken 2013 | |||
| 6 months | 88.7 | 25.68 | 284 | 87.4 | 25.48 | 299 | 1.30 [−2.85, 5.45] | Bergholdt 2013 | |||
| 92 | 21 | 80 | 94 | 14 | 80 | −2.00 [−7.53, 3.53] | de Leeuw 2013 | ||||
| 12 months | 95.03 | 14.5 | 268 | 92.54 | 17.73 | 300 | 2.49 [−0.16, 5.14] | Braeken 2013 | |||
| 93 | 18 | 80 | 94 | 15 | 80 | −1.00 [−6.13, 4.13] | de Leeuw 2013 | ||||
| 14 months | 91.1 | 23.39 | 236 | 88.1 | 23.19 | 248 | 3.00 [−1.15, 7.15] | Bergholdt 2013 | |||
| Diarrhoea | 1 month | 90.48 | 19.99 | 28 | 83.33 | 23.13 | 28 | 7.15 [−4.17, 18.47] | Bramsen 2008 | ||
| 3 months | 90.29 | 19.71 | 268 | 88.53 | 22.29 | 300 | 1.76 [−1.69, 5.21] | Braeken 2013 | |||
| 6 months | 88.6 | 22.26 | 284 | 88.7 | 21.97 | 299 | −0.10 [−3.69, 3.49] | Bergholdt 2013 | |||
| 95 | 15 | 80 | 94 | 16 | 80 | 1.00 [−3.81, 5.81] | de Leeuw 2013 | ||||
| 12 months | 92.22 | 17.55 | 268 | 92.41 | 17.8 | 300 | −0.19 [−3.10, 2.72] | Braeken 2013 | |||
| 96 | 11 | 80 | 92 | 18 | 80 | 4.00 [−0.62, 8.62] | de Leeuw 2013 | ||||
| 14 months | 90 | 23.49 | 238 | 88.6 | 23.28 | 250 | 1.40 [−2.75, 5.55] | Bergholdt 2013 | |||
| Financial difficulties | 1 month | 90.48 | 21.96 | 28 | 88.1 | 22.62 | 28 | 2.38 [−9.30, 14.06] | Bramsen 2008 | ||
| 3 months | 94.88 | 15.54 | 268 | 93.14 | 17.34 | 300 | 1.74 [−0.96, 4.44] | Braeken 2013 | |||
| 6 months | 92 | 19.69 | 284 | 92.4 | 19.27 | 297 | −0.40 [−3.57, 2.77] | Bergholdt 2013 | |||
| 93 | 17 | 80 | 92 | 20 | 80 | 1.00 [−4.75, 6.75] | de Leeuw 2013 | ||||
| 12 months | 92.98 | 17.96 | 268 | 93.23 | 19.42 | 300 | −0.25 [−3.32, 2.82] | Braeken 2013 | |||
| 92 | 22 | 80 | 93 | 15 | 80 | −1.00 [−6.83, 4.83] | de Leeuw 2013 | ||||
| 14 months | 93.3 | 20.27 | 236 | 93.5 | 20.53 | 242 | −0.20 [−3.86, 3.46] | Bergholdt 2013 | |||
| Swallowing | 6 months | EORTC QLQ‐H&N35 | 96 | 18 | 80 | 89 | 16 | 80 | 7.00 [1.72, 12.28] | de Leeuw 2013 | |
| 12 months | 91 | 19 | 80 | 90 | 15 | 80 | 1.00 [−4.30, 6.30] | ||||
| Senses | 6 months | 83 | 24 | 80 | 86 | 21 | 80 | −3.00 [−9.99, 3.99] | |||
| 12 months | 82 | 26 | 80 | 85 | 23 | 80 | −3.00 [−10.61, 4.61] | ||||
| Speech | 6 months | 88 | 21 | 80 | 92 | 15 | 80 | −4.00 [−9.66, 1.66] | |||
| 12 months | 89 | 19 | 80 | 90 | 19 | 80 | −1.00 [−6.89, 4.89] | ||||
| Social eating | 6 months | 85 | 18 | 80 | 91 | 19 | 80 | −6.00 [−11.74, −0.26] | |||
| 12 months | 90 | 19 | 80 | 91 | 17 | 80 | −1.00 [−6.59, 4.59] | ||||
| Social contact | 6 months | 94 | 10 | 80 | 96 | 9 | 80 | −2.00 [−4.95, 0.95] | |||
| 12 months | 95 | 12 | 80 | 97 | 8 | 80 | −2.00 [−5.16, 1.16] | ||||
| Less sexuality | 6 months | 81 | 26 | 80 | 80 | 29 | 80 | 1.00 [−7.53, 9.53] | |||
| 12 months | 81 | 27 | 80 | 85 | 23 | 80 | −4.00 [−11.77, 3.77] | ||||
| Teeth problems | 6 months | 85 | 28 | 80 | 83 | 27 | 80 | 2.00 [−6.52, 10.52] | |||
| 12 months | 89 | 24 | 80 | 88 | 24 | 80 | 1.00 [−6.44, 8.44] | ||||
| Opening mouth | 6 months | 83 | 29 | 80 | 86 | 23 | 80 | −3.00 [−11.11, 5.11] | |||
| 12 months | 89 | 21 | 80 | 90 | 21 | 80 | −1.00 [−7.51, 5.51] | ||||
| Dry mouth | 6 months | 59 | 33 | 80 | 62 | 35 | 80 | −3.00 [−13.54, 7.54] | |||
| 12 months | 62 | 34 | 80 | 67 | 33 | 80 | −5.00 [−15.38, 5.38] | ||||
| Sticky saliva | 6 months | 66 | 32 | 80 | 77 | 32 | 80 | −11.00 [−20.92, −1.08] | |||
| 12 months | 75 | 32 | 80 | 78 | 29 | 80 | −3.00 [−12.46, 6.46] | ||||
| Coughing | 6 months | 84 | 23 | 80 | 80 | 30 | 80 | 4.00 [−4.28, 12.28] | |||
| 12 months | 80 | 26 | 80 | 85 | 25 | 80 | −5.00 [−12.90, 2.90] | ||||
| Feeling ill | 6 months | 94 | 17 | 80 | 88 | 24 | 80 | 6.00 [−0.44, 12.44] | |||
| 12 months | 93 | 22 | 80 | 91 | 18 | 80 | 2.00 [−4.23, 8.23] | ||||
| Use of painkillers | 6 months | 71 | 46 | 80 | 76 | 43 | 80 | −5.00 [−18.80, 8.80] | |||
| 12 months | 78 | 42 | 80 | 78 | 42 | 80 | 0.00 [−13.02, 13.02] | ||||
| Use of nutritional supplements | 6 months | 78 | 42 | 80 | 87 | 34 | 80 | −9.00 [−20.84, 2.84] | |||
| 12 months | 91 | 28 | 80 | 92 | 27 | 80 | −1.00 [−9.52, 7.52] | ||||
| Use of feeding tube | 6 months | 97 | 18 | 80 | 100 | 0 | 80 | N/E | |||
| 12 months | 97 | 18 | 80 | 97 | 12 | 80 | 0.00 [−4.74, 4.74] | ||||
| Weight loss | 6 months | 84 | 37 | 80 | 83 | 38 | 80 | 1.00 [−10.62, 12.62] | |||
| 12 months | 85 | 36 | 80 | 87 | 33 | 80 | −2.00 [−12.70, 8.70] | ||||
| Weight gain | 6 months | 74 | 44 | 80 | 65 | 48 | 80 | 9.00 [−5.27, 23.27] | |||
| 12 months | 73 | 45 | 80 | 66 | 48 | 80 | 7.00 [−7.42, 21.42] | ||||
| Vitality | 4th follow‐up visit | SF‐36 | 51 | 25 | 104 | 49 | 25 | 95 | 2.00 [−4.95, 8.95] | Detmar 2002 | |
| 56 | N/A | 108 | 51 | N/A | 110 | N/E | Hilarius 2008 | ||||
| Mental health | 70 | 19 | 104 | 68 | 21 | 95 | 2.00 [−3.58, 7.58] | Detmar 2002 | |||
| 72 | N/A | 108 | 72 | N/A | 110 | N/E | Hilarius 2008 | ||||
| Hardship | 3 months | FLIC | 15.3 | 3.5 | 60 | 15.1 | 4.2 | 60 | 0.20 [−1.18, 1.58] | Rosenbloom 2007 | |
| 14.2 | 3.9 | 60 | 15.1 | 4.2 | 60 | −0.90 [−2.35, 0.55] | |||||
| 6 months | 15.2 | 4.1 | 51 | 14.6 | 3.8 | 52 | 0.60 [−0.93, 2.13] | ||||
| 15.4 | 3.6 | 51 | 14.6 | 3.8 | 52 | 0.80 [−0.63, 2.23] | |||||
| Psychological well‐being | 3 months | 32.1 | 4.7 | 60 | 30.4 | 6.5 | 60 | 1.70 [−0.33, 3.73] | |||
| 30.3 | 6.3 | 60 | 30.4 | 6.5 | 60 | −0.10 [−2.39, 2.19] | |||||
| 6 months | 30.6 | 5.9 | 60 | 29.7 | 6.1 | 60 | 0.90 [−1.25, 3.05] | ||||
| 30.1 | 6.9 | 60 | 29.7 | 6.1 | 60 | 0.40 [−1.93, 2.73] | |||||
| Colorectal cancer symptom‐related well‐being | 1 month | FACT‐C | 21.4 | 3.3 | 20 | 20.7 | 5.3 | 21 | 0.70 [−1.99, 3.39] | Young 2010 | |
| 3 months | 22.8 | 3.7 | 20 | 19.8 | 4.1 | 21 | 3.00 [0.61, 5.39] | ||||
| Distress (continuous) | Tension/anxiety | 1 month | POMS | 4.4 | 4.5 | 109 | 4.4 | 4.4 | 103 | 0.00 [−1.20, 1.20] | Hollingworth 2013 |
| 3 months | HADS | 4.66 | 3.68 | 268 | 4.86 | 3.81 | 300 | −0.20 [−0.82, 0.42] | Braeken 2013 | ||
| 6 months | POMS | 4.1 | 4.3 | 108 | 4.1 | 4.4 | 101 | 0.00 [−1.18, 1.18] | Hollingworth 2013 | ||
| 12 months | HADS | 4.57 | 3.90 | 268 | 4.98 | 4.24 | 300 | −0.41 [−1.08, 0.26] | Braeken 2013 | ||
| POMS | 4.1 | 4.2 | 106 | 3.7 | 4.4 | 103 | 0.40 [−0.77, 1.57] | Hollingworth 2013 | |||
| 14 months | 3.56 | 4.12 | 226 | 3.82 | 4.12 | 226 | −0.26 [−1.02, 0.50] | Bergholdt 2013 | |||
| Depression/dejection | 1 month | 4.4 | 6.1 | 109 | 4 | 5.3 | 103 | 0.40 [−1.14, 1.94] | Hollingworth 2013 | ||
| 3 months | HADS | 3.68 | 4.11 | 268 | 3.72 | 3.76 | 300 | −0.04 [−0.69, 0.61] | Braeken 2013 | ||
| 6 months | POMS | 3.7 | 5 | 108 | 3.8 | 5.4 | 101 | −0.10 [−1.51, 1.31] | Hollingworth 2013 | ||
| 12 months | HADS | 3.45 | 3.78 | 268 | 3.7 | 4.08 | 300 | −0.25 [−0.90, 0.40] | Braeken 2013 | ||
| POMS | 3.9 | 5.5 | 106 | 2.9 | 4.5 | 103 | 1.00 [−0.36, 2.36] | Hollingworth 2013 | |||
| 14 months | 3.26 | 4.99 | 229 | 3.85 | 4.93 | 223 | −0.59 [−1.50, 0.32] | Bergholdt 2013 | |||
| Psychological distress (+ subscale) | 1 month | GHQ‐12 | 1.54 | 1.57 | 28 | 3.31 | 1.7 | 28 | −1.77 [−2.63, −0.91] | Bramsen 2008 | |
| Psychological distress (‐ subscale) | 1.55 | 1.43 | 28 | 2.5 | 1.86 | 28 | −0.95 [−1.82, −0.08] | ||||
| Psychological distress (total score) | At discharge from hospital | HADS | N/A | N/A | N/A | N/A | N/A | N/A | 0.9 [−0.1, 1.9] | Singer 2017 | |
| 1 month | DT | 3.09 | 2.8 | 28 | 5.81 | 3.29 | 28 | −2.72 [−4.32, −1.12] | Bramsen 2008 | ||
| 1.9 | 2.1 | 20 | 2.8 | 3 | 21 | −0.90 [−2.48, 0.68] | Young 2010 | ||||
| 2.3 | 1.89 | 346 | 2.4 | 2.91 | 363 | −0.10 [−0.46, 0.26] | Young 2013 | ||||
| 2 months | 2.46 | 2.7 | 55 | 2.91 | 2.62 | 53 | −0.45 [−1.45, 0.55] | Schofield 2013 | |||
| HADS | 10.77 | 8.4 | 55 | 11.15 | 8.23 | 53 | −0.38 [−3.52, 2.76] | ||||
| 3 months | GHQ‐12 | 2.74 | 3.26 | 268 | 2.85 | 3.38 | 300 | −0.11 [−0.66, 0.44] | Braeken 2013 | ||
| PSI | 15 | 12.7 | 123 | 15.5 | 13.1 | 127 | −0.50 [−3.70, 2.70] | Maunsell 1996 | |||
| DT | 2.85 | 2.9 | 55 | 2.99 | 2.77 | 53 | −0.14 [−1.21, 0.93] | Schofield 2013 | |||
| 1.3 | N/A | 20 | 2.1 | N/A | 21 | N/E | Young 2010 | ||||
| 2 | 1.86 | 336 | 2 | 2.86 | 351 | 0.00 [−0.36, 0.36] | Young 2013 | ||||
| HADS | 11.52 | 8.8 | 55 | 10.34 | 8.52 | 53 | 1.18 [−2.09, 4.45] | Schofield 2013 | |||
| N/A | N/A | N/A | N/A | N/A | N/A | 0.3 [−1.0, 1.6] | Singer 2017 | ||||
| POMS (negative affect items) | 6.6 | 6 | 60 | 8.5 | 9.3 | 60 | −1.90 [−4.70, 0.90] | Rosenbloom 2007 | |||
| 7.2 | 7.7 | 60 | 8.5 | 9.3 | 60 | −1.30 [−4.36, 1.76] | |||||
| 6 months | 8.1 | 8.5 | 51 | 8.3 | 8.2 | 52 | −0.20 [−3.43, 3.03] | ||||
| 8.1 | 9.5 | 51 | 8.3 | 8.2 | 52 | −0.20 [−3.63, 3.23] | |||||
| DT | 1.8 | 2.74 | 322 | 1.8 | 2.85 | 350 | 0.00 [−0.42, 0.42] | Young 2013 | |||
| HADS | 9.5 | 8.2 | 341 | 9.4 | 7.2 | 234 | 0.10 [−1.17, 1.37] | Singer 2017 | |||
| 12 months | GHQ‐12 | 1.96 | 3.14 | 268 | 2.14 | 3.22 | 300 | −0.18 [−0.70, 0.34] | Braeken 2013 | ||
| PSI | 13.5 | 12.1 | 123 | 14.6 | 12.3 | 127 | −1.10 [−4.12, 1.92] | Maunsell 1996 | |||
| Anger/hostility | 1 month | POMS | 3 | 4.4 | 109 | 2.9 | 4 | 103 | 0.10 [−1.03, 1.23] | Hollingworth 2013 | |
| 6 months | 2.8 | 3.7 | 108 | 2.6 | 3.3 | 101 | 0.20 [−0.75, 1.15] | ||||
| 12 months | 3.5 | 5 | 106 | 2.5 | 3.7 | 103 | 1.00 [−0.19, 2.19] | ||||
| 14 months | 1.88 | 3.46 | 230 | 2.03 | 3.33 | 223 | −0.15 [−0.78, 0.48] | Bergholdt 2013 | |||
| Confusion/bewilderment | 1 month | 3.2 | 3.4 | 109 | 3.6 | 3.6 | 103 | −0.40 [−1.34, 0.54] | Hollingworth 2013 | ||
| 6 months | 3.1 | 3.3 | 108 | 3.6 | 3.6 | 101 | −0.50 [−1.44, 0.44] | ||||
| 12 months | 3.1 | 3.3 | 106 | 3 | 3.2 | 103 | 0.10 [−0.78, 0.98] | ||||
| 14 months | 2.11 | 3.24 | 231 | 2.45 | 3.15 | 229 | −0.34 [−0.92, 0.24] | Bergholdt 2013 | |||
| Fatigue/inertia | 1 month | 7.2 | 6.4 | 109 | 7.8 | 6 | 103 | −0.60 [−2.27, 1.07] | Hollingworth 2013 | ||
| 6 months | 6.6 | 5.4 | 108 | 6.7 | 5.8 | 101 | −0.10 [−1.62, 1.42] | ||||
| 12 months | 6.1 | 5.4 | 106 | 5.1 | 4.7 | 103 | 1.00 [−0.37, 2.37] | ||||
| 14 months | 4.14 | 8.7 | 234 | 4.56 | 4.35 | 226 | −0.42 [−1.67, 0.83] | Bergholdt 2013 | |||
| Vigour/activity | 1 month | 8.2 | 5.6 | 109 | 8.1 | 5.5 | 103 | 0.10 [−1.39, 1.59] | Hollingworth 2013 | ||
| 6 months | 3.1 | 3.3 | 108 | 3.6 | 3.6 | 101 | −0.50 [−1.44, 0.44] | ||||
| 12 months | 3.1 | 3.3 | 106 | 3 | 3.2 | 103 | 0.10 [−0.78, 0.98] | ||||
| 14 months | 10.09 | 5.98 | 228 | 10.28 | 5.77 | 218 | −0.19 [−1.28, 0.90] | Bergholdt 2013 | |||
| Total mood disturbance | 1 month | 38.09 | 23.5 | 109 | 38.6 | 21.99 | 103 | −0.51 [−6.63, 5.61] | Hollingworth 2013 | ||
| 6 months | 34.46 | 20.87 | 108 | 34.87 | 22 | 101 | −0.41 [−6.23, 5.41] | ||||
| 12 months | 35.1 | 22.85 | 106 | 31.13 | 20.52 | 103 | 3.97 [−1.91, 9.85] | ||||
| 14 months | 4.19 | 18.89 | 210 | 4.87 | 18.5 | 200 | −0.68 [−4.30, 2.94] | Bergholdt 2013 | |||
| Psychosocial well‐being | Emotional impact of the intervention (total) | 1 month | IES | 15.77 | 14.53 | 28 | 25.91 | 12.49 | 28 | −10.14 [−17.24, −3.04] | Bramsen 2008 |
| Emotional impact of the intervention (re‐experiencing) | 8 | 8.04 | 28 | 12.5 | 6.61 | 28 | −4.50 [−8.36, −0.64] | ||||
| Emotional impact of the intervention (avoidance) | 6.19 | 6.64 | 28 | 11.09 | 7.7 | 28 | −4.90 [−8.67, −1.13] | ||||
| Psychosocial adjustment (healthcare orientation) | 6 months | PAIS‐SR | 51 | 8 | 80 | 49 | 9 | 80 | 2.00 [−0.64, 4.64] | de Leeuw 2013 | |
| 12 months | 52 | 9 | 80 | 48 | 8 | 80 | 4.00 [1.36, 6.64] | ||||
| Psychosocial adjustment (vocational environment) | 6 months | 57 | 7 | 80 | 56 | 7 | 80 | 1.00 [−1.17, 3.17] | |||
| 12 months | 54 | 7 | 80 | 54 | 7 | 80 | 0.00 [−2.17, 2.17] | ||||
| Psychosocial adjustment (domestic environment) | 6 months | 43 | 9 | 80 | 42 | 9 | 80 | 1.00 [−1.79, 3.79] | |||
| 12 months | 42 | 9 | 80 | 41 | 9 | 80 | 1.00 [−1.79, 3.79] | ||||
| Psychosocial adjustment (sexual relations) | 6 months | 46 | 8 | 80 | 47 | 9 | 80 | −1.00 [−3.64, 1.64] | |||
| 12 months | 46 | 8 | 80 | 47 | 9 | 80 | −1.00 [−3.64, 1.64] | ||||
| Psychosocial adjustment (extended family relations) | 6 months | 49 | 7 | 80 | 52 | 8 | 80 | −3.00 [−5.33, −0.67] | |||
| 12 months | 49 | 7 | 80 | 49 | 7 | 80 | 0.00 [−2.17, 2.17] | ||||
| Psychosocial adjustment (social environment) | 6 months | 43 | 15 | 80 | 43 | 13 | 80 | 0.00 [−4.35, 4.35] | |||
| 12 months | 42 | 14 | 80 | 42 | 13 | 80 | 0.00 [−4.19, 4.19] | ||||
| Psychosocial adjustment (psychological distress) | 6 months | 45 | 10 | 80 | 45 | 10 | 80 | 0.00 [−3.10, 3.10] | |||
| 12 months | 45 | 11 | 80 | 43 | 10 | 80 | 2.00 [−1.26, 5.26] | ||||
| Psychosocial adjustment (total adjustment) | 6 months | 44 | 12 | 80 | 44 | 13 | 80 | 0.00 [−3.88, 3.88] | |||
| 12 months | 43 | 13 | 80 | 42 | 12 | 80 | 1.00 [−2.88, 4.88] | ||||
| Physical functioning (number of arm problems reported) | 3 months | LES | 1.6 | 1.3 | 122 | 1.6 | 1.4 | 126 | 0.00 [−0.34, 0.34] | Maunsell 1996 | |
| 12 months | 1.3 | 1.4 | 122 | 1.1 | 1.4 | 126 | 0.20 [−0.15, 0.55] | ||||
| Role functioning (household activities performed without help) | 3 months | 1.5 | 1.1 | 123 | 1.6 | 1.2 | 127 | −0.10 [−0.39, 0.19] | |||
| 12 months | 2.1 | 1.2 | 123 | 2 | 1.3 | 127 | 0.10 [−0.21, 0.41] | ||||
| Role functioning (hours worked per week) | 3 months | 22.1 | 15.1 | 123 | 22.4 | 14 | 127 | −0.30 [−3.91, 3.31] | |||
| 12 months | 32.1 | 12.3 | 123 | 31.4 | 16.1 | 127 | 0.70 [−2.84, 4.24] | ||||
| Social functioning (times per week engaged in social activities) | 3 months | 7.4 | 5.8 | 123 | 6.1 | 4.7 | 127 | 1.30 [−0.01, 2.61] | |||
| 12 months | 7.5 | 5.3 | 123 | 6.3 | 5 | 127 | 1.20 [−0.08, 2.48] | ||||
| Social functioning (hours per day devoted to leisure activities) | 3 months | 4.5 | 3 | 123 | 4.3 | 2.4 | 127 | 0.20 [−0.47, 0.87] | |||
| 12 months | 4.1 | 2.6 | 123 | 4.5 | 3.1 | 127 | −0.40 [−1.11, 0.31] | ||||
| Social functioning (times per week engaged in physical activities/sports) | 3 months | 3 | 4.4 | 123 | 4.3 | 5.3 | 127 | −1.30 [−2.51, −0.09] | |||
| 12 months | 3.7 | 4.6 | 123 | 3.6 | 4.2 | 127 | 0.10 [−0.99, 1.19] | ||||
| Marital satisfaction | 3 months | LWMAT | 46.6 | 21 | 76 | 50.5 | 25.3 | 82 | −3.90 [−11.13, 3.33] | ||
| 12 months | 48.5 | 25.1 | 74 | 48.5 | 24.4 | 78 | 0.00 [−7.88, 7.88] | ||||
| Supportive care needs | General unmet need | 1 month | SCNS‐SF34 | 128.7 | 75.4 | 35 | 140.3 | 96.6 | 32 | −11.60 [−53.36, 30.16] | Harrison 2011 |
| 3 months | 98.1 | 84.7 | 32 | 110 | 86.7 | 29 | −11.90 [−54.99, 31.19] | ||||
| 59.9 | 57.85 | 336 | 56.8 | 76.07 | 351 | 3.10 [−6.98, 13.18] | Young 2013 | ||||
| 6 months | 50 | 66.96 | 322 | 46.6 | 67.19 | 350 | 3.40 [−6.75, 13.55] | ||||
| N/A | N/A | N/A | N/A | N/A | N/A | N/E | Thewes 2009 | ||||
| CaSUN | 10 | 13.1 | 30 | 14 | 18 | 30 | −4.00 [−11.97, 3.97] | Harrison 2011 | |||
| Medical communication | 2 months | NA‐ALCP | 2.37 | 1.3 | 55 | 2.21 | 1.24 | 53 | 0.16 [−0.32, 0.64] | Schofield 2013 | |
| 3 months | 2.14 | 1.2 | 55 | 2.03 | 1.16 | 53 | 0.11 [−0.34, 0.56] | ||||
| Psychological/emotional | 1 month | SCNS | 16.6 | 11.2 | 20 | 19.6 | 20.4 | 21 | −3.00 [−13.01, 7.01] | Young 2010 | |
| 2 months | NA‐ALCP | 2.04 | 0.9 | 55 | 1.94 | 0.87 | 53 | 0.10 [−0.23, 0.43] | Schofield 2013 | ||
| 3 months | 2.03 | 0.9 | 55 | 1.84 | 0.80 | 53 | 0.19 [−0.13, 0.51] | ||||
| SCNS | 8.2 | 8.1 | 20 | 17.7 | 18.7 | 21 | −9.50 [−18.25, −0.75] | Young 2010 | |||
| 6 months | SCNS‐SF34 | N/A | N/A | N/A | N/A | N/A | N/A | N/E | Thewes 2009 | ||
| Daily living | 1 month | SCNS | 22.8 | 16.3 | 20 | 25.8 | 19 | 21 | −3.00 [−13.82, 7.82] | Young 2010 | |
| 2 months | NA‐ALCP | 1.69 | 0.8 | 55 | 1.56 | 0.80 | 53 | 0.13 [−0.17, 0.43] | Schofield 2013 | ||
| 3 months | 1.75 | 0.9 | 55 | 1.57 | 0.87 | 53 | 0.18 [−0.15, 0.51] | ||||
| SCNS | 11.8 | 15.6 | 20 | 24.4 | 20.3 | 21 | −12.60 [−23.65, −1.55] | Young 2010 | |||
| 6 months | SCNS‐SF34 | N/A | N/A | N/A | N/A | N/A | N/A | N/E | Thewes 2009 | ||
| Financial | 2 months | NA‐ALCP | 1.76 | 1.1 | 55 | 1.78 | 1.09 | 53 | −0.02 [−0.43, 0.39] | Schofield 2013 | |
| 3 months | 1.7 | 1.1 | 55 | 1.64 | 1.02 | 53 | 0.06 [−0.34, 0.46] | ||||
| Symptoms | 2 months | 1.65 | 0.7 | 55 | 1.9 | 0.73 | 53 | −0.25 [−0.52, 0.02] | |||
| 3 months | 1.67 | 0.8 | 55 | 1.86 | 0.73 | 53 | −0.19 [−0.48, 0.10] | ||||
| Social | 2 months | 1.49 | 0.7 | 55 | 1.43 | 0.66 | 53 | 0.06 [−0.20, 0.32] | |||
| 3 months | 1.54 | 0.7 | 55 | 1.38 | 0.73 | 53 | 0.16 [−0.11, 0.43] | ||||
| Health system and information | 1 month | SCNS | 22.6 | 10.3 | 20 | 23.1 | 18 | 21 | −0.50 [−9.42, 8.42] | Young 2010 | |
| 3 months | 19.4 | 10 | 20 | 4.8 | 7.7 | 21 | 14.60 [9.12, 20.08] | ||||
| 6 months | SCNS‐SF34 | N/A | N/A | N/A | N/A | N/A | N/A | N/E | Thewes 2009 | ||
| Patient care and support | 1 month | SCNS | 18.5 | 7.4 | 20 | 14.4 | 14.9 | 21 | 4.10 [−3.05, 11.25] | Young 2010 | |
| 3 months | 10.8 | 9.5 | 20 | 1.8 | 6.1 | 21 | 9.00 [4.09, 13.91] | ||||
| 6 months | SCNS‐SF34 | N/A | N/A | N/A | N/A | N/A | N/A | N/E | Thewes 2009 | ||
| Sexuality | 1 month | SCNS | 7.9 | 11.9 | 20 | 8.8 | 22.9 | 21 | −0.90 [−12.00, 10.20] | Young 2010 | |
| 3 months | 5.7 | 17.8 | 20 | 3.9 | 9.4 | 21 | 1.80 [−6.98, 10.58] | ||||
| 6 months | SCNS‐SF34 | N/A | N/A | N/A | N/A | N/A | N/A | N/E | Thewes 2009 | ||
| Patient satisfaction | Needs addressed | After clinical visit | PDIS | 4.4 | 0.4 | 27 | 4.2 | 0.7 | 26 | 0.20 [−0.11, 0.51] | Taenzer 2000 |
| 4th follow‐up visit | PSQ‐C | N/A | N/A | 104 | N/A | N/A | 95 | N/E | Detmar 2002 | ||
| N/A | N/A | 108 | N/A | N/A | 110 | N/E | Hilarius 2008 | ||||
| Active involvement | N/A | N/A | 104 | N/A | N/A | 95 | N/E | Detmar 2002 | |||
| N/A | N/A | 108 | N/A | N/A | 110 | N/E | Hilarius 2008 | ||||
| Patient‐physician interaction | After clinical visit | PDIS | 4.5 | 0.4 | 27 | 4.5 | 0.5 | 26 | 0.00 [−0.24, 0.24] | Taenzer 2000 | |
| 4th follow‐up visit | PSQ‐C | N/A | N/A | 104 | N/A | N/A | 95 | N/E | Detmar 2002 | ||
| N/A | N/A | 108 | N/A | N/A | 110 | N/E | Hilarius 2008 | ||||
| Information received | After clinical visit | PDIS | 4.4 | 0.5 | 27 | 4.5 | 0.6 | 26 | −0.10 [−0.40, 0.20] | Taenzer 2000 | |
| MOSPVRQ | 3.65 | 0.67 | 147 | 3.81 | 0.45 | 130 | −0.16 [−0.29, −0.03] | Kutner 1999 | |||
| 4th follow‐up visit | PSQ‐C | N/A | N/A | 104 | N/A | N/A | 95 | N/E | Detmar 2002 | ||
| N/A | N/A | 108 | N/A | N/A | 110 | N/E | Hilarius 2008 | ||||
| Support received | 4.3 | 0.72 | 104 | 4 | 0.89 | 95 | 0.30 [0.07, 0.53] | Detmar 2002 | |||
| N/A | N/A | 108 | N/A | N/A | 110 | N/E | Hilarius 2008 | ||||
| General satisfaction | After clinical visit | MOSPVRQ | 3.63 | 0.68 | 149 | 3.76 | 0.52 | 133 | −0.13 [−0.27, 0.01] | Kutner 1999 | |
| 3 months | PSQ‐III general satisfacion | 23.2 | 3.7 | 60 | 24.6 | 4.2 | 60 | −1.40 [−2.82, 0.02] | Rosenbloom 2007 | ||
| 23 | 4.1 | 60 | 24.6 | 4.2 | 60 | −1.60 [−3.09, −0.11] | |||||
| 6 months | 22.4 | 4.2 | 51 | 24.4 | 4.1 | 52 | −2.00 [−3.60, −0.40] | ||||
| 23.1 | 4.2 | 51 | 24.4 | 4.1 | 52 | −1.30 [−2.90, 0.30] | |||||
| TPVCSQ | 70.7 | 17.1 | 108 | 71.2 | 16.1 | 101 | −0.50 [−5.00, 4.00] | Hollingworth 2013 | |||
| Communication satisfaction | After clinical visit | MOSPVRQ | 3.82 | 0.53 | 149 | 3.87 | 0.36 | 133 | −0.05 [−0.15, 0.05] | Kutner 1999 | |
| 3 months | PSQ‐III communication satisfaction | 21.2 | 2.8 | 60 | 21.4 | 2.3 | 60 | −0.20 [−1.12, 0.72] | Rosenbloom 2007 | ||
| 21.1 | 3 | 60 | 21.4 | 2.3 | 60 | −0.30 [−1.26, 0.66] | |||||
| 6 months | 21.2 | 2.8 | 51 | 20.8 | 3.2 | 52 | 0.40 [−0.76, 1.56] | ||||
| 21.2 | 3 | 51 | 20.8 | 3.2 | 52 | 0.40 [−0.80, 1.60] | |||||
| Time spent with medical doctor satisfaction | After clinical visit | MOSPVRQ | 3.53 | 0.78 | 149 | 3.7 | 0.59 | 133 | −0.17 [−0.33, −0.01] | Kutner 1999 | |
| Skills of the medical doctor satisfaction | 3.78 | 0.54 | 149 | 3.85 | 0.40 | 133 | −0.07 [−0.18, 0.04] | ||||
| Abbreviations: CaSUN (Cancer Survivors’ Unmet Needs Measure); CI (confidence interval); DT (Distress Thermometer); EORTC QLQ‐C30 (European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Core 30); EORTC QLQ‐H&N35 (European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Head and Neck Cancer 35 items); EQ‐5D (EuroQol 5D); FACT‐C (Functional Assessment of Cancer Therapy‐Colorectal); FLIC (Functional Living Index‐Cancer); GHQ‐12 (General Health Questionnaire 12‐item version); HADS (Hospital Anxiety and Depression Scale); HRQoL (health‐related quality of life); IES (Impact of Events Scale); LES (Life Experiences Survey); LWMAT (Locke‐Wallace Marital Adjustment Scale); MD (mean difference); MOSPVRQ (Medical Outcomes Study Patient Visit Rating Questionnaire); NA‐ALCP (Needs Assessment for Advanced Lung Cancer Patients); POMS (Profile of Mood States); PAIS‐SR (Psychosocial Adjustment to Illness Scale – Self Reported); PDIS (Patient‐Doctor Interaction Scale); PSI (Psychiatric Symptom Index); PSQ‐III (Patient Satisfaction Questionnaire III); PSQ‐C (Patient Satisfaction Questionnaire Core); SCNS (Supportive Care Needs Survey); SCNS‐SF34 (Supportive Care Needs Survey‐Short Form); SD (standard deviation); SF‐36 (36‐item Short Form Health Survey); TPVCSQ (Trent Patient Views of Cancer Services Questionnaire); N/A: Not available; N/E: Not estimable. | |||||||||||
4. Evidence summary ‐ continuous outcomes, change from baseline.
| Raw data: continuous outcomes (change from baseline) | |||||||||||
| Main outcome | Suboutcome | Time postintervention | Scale used | Intervention (screening) | Control (usual care) | MD [95% CI] | Study ID | ||||
| Mean | SD | N | Mean | SD | N | ||||||
| HRQoL (continuous) | Global health status | +/− 6 months | EQ‐5D | −0.78 | 23.61 | 59 | −1.2 | 17.91 | 47 | −2.50 [−12.22, 7.22] | Geerse 2017 |
| EORTC QLQ‐C30 | 3.3 | 25.56 | 58 | 5.8 | 25.2 | 44 | 0.42 [−7.64, 8.48] | ||||
| 6 months | 2.6 | 16.51 | 38 | 4 | 16.92 | 52 | −1.40 [−8.38, 5.58] | van der Meulen 2018 | |||
| 12 months | 4.5 | 16.12 | 33 | 3.3 | 16.77 | 45 | 1.20 [−6.17, 8.57] | ||||
| Physical functioning | 4 months | SF‐36 | 18.24 | 20.12 | 48 | 21.14 | 22.31 | 51 | −2.90 [−11.26, 5.46] | Giesler 2005 | |
| +/− 6 months | EORTC QLQ‐C30 | −3.5 | 22.46 | 60 | −1.2 | 21.21 | 50 | −2.30 [−10.48, 5.88] | Geerse 2017 | ||
| 6 months | 1.3 | 13.84 | 38 | 1.4 | 13.80 | 52 | −0.10 [−5.88, 5.68] | van der Meulen 2018 | |||
| 7 months | SF‐36 | 17.43 | 23.5 | 41 | 17.47 | 24.64 | 44 | −0.04 [−10.27, 10.19] | Giesler 2005 | ||
| 12 months | 18.39 | 21.69 | 41 | 19.47 | 23.97 | 44 | −1.08 [−10.79, 8.63] | ||||
| EORTC QLQ‐C30 | 0.9 | 13.19 | 33 | 1 | 13.52 | 45 | −0.10 [−6.09, 5.89] | van der Meulen 2018 | |||
| Role functioning | +/− 6 months | 6.9 | 37.18 | 60 | 0.7 | 33.6 | 49 | 6.20 [−7.10, 19.50] | Geerse 2017 | ||
| Role functioning (emotional) | 4 months | SF‐36 | 13.44 | 34.33 | 48 | 5.7 | 34.87 | 51 | 7.74 [−5.89, 21.37] | Giesler 2005 | |
| 7 months | 11.41 | 27.74 | 41 | 12.33 | 35.1 | 44 | −0.92 [−14.32, 12.48] | ||||
| 12 months | 12.09 | 30.61 | 41 | 1.34 | 42.05 | 44 | 10.75 [−4.81, 26.31] | ||||
| Role functioning (physical) | 4 months | 55.44 | 43.63 | 48 | 40.38 | 49.84 | 51 | 15.06 [−3.36, 33.48] | |||
| 7 months | 50.33 | 51.87 | 41 | 33.61 | 54.24 | 44 | 16.72 [−5.84, 39.28] | ||||
| 12 months | 51.6 | 47.56 | 41 | 35.4 | 52.08 | 44 | 16.20 [−4.98, 37.38] | ||||
| Emotional functioning | +/− 6 months | EORTC QLQ‐C30 | 6.4 | 20.14 | 60 | 1.7 | 24.5 | 49 | 4.70 [−3.85, 13.25] | Geerse 2017 | |
| 6 months | 3.1 | 19.81 | 38 | 2.9 | 20.42 | 52 | 0.20 [−8.19, 8.59] | van der Meulen 2018 | |||
| 12 months | 2 | 19.49 | 33 | 2.6 | 20.02 | 45 | −0.60 [−9.46, 8.26] | van der Meulen 2018 | |||
| Cognitive functioning | +/− 6 months | 2.5 | 20.14 | 60 | 2 | 23.1 | 49 | 0.50 [−7.73, 8.73] | Geerse 2017 | ||
| 6 months | 2.3 | 19.66 | 38 | −1.6 | 20.42 | 52 | 3.90 [−4.46, 12.26] | van der Meulen 2018 | |||
| 12 months | 3.6 | 19.34 | 33 | −0.6 | 19.85 | 45 | 4.20 [−4.59, 12.99] | van der Meulen 2018 | |||
| Social functioning | 4 months | SF‐36 | 18.84 | 30.06 | 48 | 15.95 | 24.35 | 51 | 2.89 [−7.93, 13.71] | Giesler 2005 | |
| +/− 6 months | EORTC QLQ‐C30 | 6.1 | 28.66 | 60 | 4.4 | 23.8 | 49 | 1.70 [−8.15, 11.55] | Geerse 2017 | ||
| 6 months | 2.7 | 23.12 | 38 | 7.8 | 23.73 | 52 | −5.10 [−14.88, 4.68] | van der Meulen 2018 | |||
| 12 months | 2.6 | 22.71 | 33 | 7.9 | 23.44 | 45 | −5.30 [−15.64, 5.04] | van der Meulen 2018 | |||
| 7 months | SF‐36 | 15.07 | 36.02 | 41 | 14.99 | 25.65 | 44 | 0.08 [−13.30, 13.46] | Giesler 2005 | ||
| 12 months | 18.51 | 27.56 | 41 | 12.45 | 30.33 | 44 | 6.06 [−6.25, 18.37] | ||||
| Pain | 4 months | 22.9 | 26.08 | 48 | 16.37 | 27.91 | 51 | 6.53 [−4.11, 17.17] | |||
| 6 months | EORTC QLQ‐C30 | −0.7 | 24.06 | 38 | −9.7 | 24.83 | 52 | 9.00 [−1.20, 19.20] | van der Meulen 2018 | ||
| EORTC QLQ‐H&N35 | −6.3 | 21.07 | 38 | −11.3 | 21.16 | 52 | 5.00 [−3.83, 13.83] | ||||
| 7 months | SF‐36 | 23.96 | 34.75 | 41 | 16.32 | 27.63 | 44 | 7.64 [−5.77, 21.05] | Giesler 2005 | ||
| 12 months | SF‐36 | 24.49 | 29.16 | 41 | 17.48 | 31.62 | 44 | 7.01 [−5.91, 19.93] | |||
| EORTC QLQ‐H&N35 | −7.5 | 21.84 | 33 | −14.6 | 20.88 | 45 | 7.10 [−2.53, 16.73] | van der Meulen 2018 | |||
| EORTC QLQ‐C30 | −1.3 | 23.74 | 33 | −10.8 | 24.47 | 45 | 9.50 [−1.30, 20.30] | ||||
| Fatigue | 6 months | −11.6 | 21.86 | 38 | −10.7 | 22.44 | 52 | −0.90 [−10.15, 8.35] | |||
| 12 months | −13.6 | 39.86 | 33 | −11.8 | 22.08 | 45 | −1.80 [−16.85, 13.25] | ||||
| Nausea/vomiting | 6 months | −0.8 | 12.11 | 38 | −2.6 | 12.33 | 52 | 1.80 [−3.30, 6.90] | |||
| 12 months | 0.6 | 11.87 | 33 | −1.9 | 11.98 | 45 | 2.50 [−2.85, 7.85] | ||||
| Dyspnoea | 6 months | 3.3 | 20.91 | 38 | 2.2 | 21.71 | 52 | 1.10 [−7.79, 9.99] | |||
| 12 months | 1.2 | 20.66 | 33 | 3.7 | 21.39 | 45 | −2.50 [−11.92, 6.92] | ||||
| Insomnia | 6 months | −0.9 | 24.85 | 38 | −4.4 | 25.94 | 52 | 3.50 [−7.09, 14.09] | |||
| 12 months | −3.2 | 24.47 | 33 | −4.1 | 25.33 | 45 | 0.90 [−10.26, 12.06] | ||||
| Appetite loss | 6 months | −8 | 27.68 | 38 | −13.2 | 28.15 | 52 | 5.20 [−6.46, 16.86] | |||
| 12 months | −5.6 | 26.82 | 33 | −12.9 | 27.72 | 45 | 7.30 [−4.92, 19.52] | ||||
| Constipation | 6 months | −6.7 | 21.39 | 38 | −4.6 | 22.07 | 52 | −2.10 [−11.17, 6.97] | |||
| 12 months | −8.1 | 21.10 | 33 | −4.9 | 21.73 | 45 | −3.20 [−12.80, 6.40] | ||||
| Diarrhoea | 6 months | 0.2 | 16.04 | 38 | −2.7 | 16.74 | 52 | 2.90 [−3.93, 9.73] | |||
| 12 months | −1.3 | 15.68 | 33 | −1 | 16.26 | 45 | −0.30 [−7.45, 6.85] | ||||
| Financial difficulties | 6 months | −4.5 | 19.50 | 38 | −0.9 | 20.24 | 52 | −3.60 [−11.89, 4.69] | |||
| 12 months | −3.2 | 19.20 | 33 | −4.5 | 20.02 | 45 | 1.30 [−7.48, 10.08] | ||||
| Swallowing | 6 months | EORTC QLQ‐H&N35 | −13.3 | 23.90 | 38 | −9 | 25.02 | 52 | −4.30 [−14.50, 5.90] | ||
| 12 months | −8.7 | 23.74 | 33 | −11.5 | 23.96 | 45 | 2.80 [−7.91, 13.51] | ||||
| Senses | 6 months | −5.2 | 20.76 | 38 | −4.8 | 20.97 | 52 | −0.40 [−9.12, 8.32] | |||
| 12 months | −3.8 | 20.22 | 33 | −5.1 | 20.54 | 45 | 1.30 [−7.84, 10.44] | ||||
| Speech | 6 months | −8.2 | 19.81 | 38 | −9.7 | 20.42 | 52 | 1.50 [−6.89, 9.89] | |||
| 12 months | −0.9 | 19.20 | 33 | −11.8 | 19.85 | 45 | 10.90 [2.15, 19.65] | ||||
| Social eating | 6 months | −4.5 | 23.75 | 38 | −7.7 | 24.65 | 52 | 3.20 [−6.90, 13.30] | |||
| 12 months | −3.2 | 23.15 | 33 | −10.6 | 23.79 | 45 | 7.40 [−3.12, 17.92] | ||||
| Social contact | 6 months | 0.1 | 13.37 | 38 | 0 | 13.43 | 52 | 0.10 [−5.50, 5.70] | |||
| 12 months | −0.7 | 12.90 | 33 | −1 | 13.18 | 45 | 0.30 [−5.55, 6.15] | ||||
| Sexuality | 6 months | 4.4 | 30.82 | 38 | −6.3 | 31.09 | 52 | 10.70 [−2.24, 23.64] | |||
| 12 months | −4.6 | 30.63 | 33 | −4.9 | 32.00 | 45 | 0.30 [−13.72, 14.32] | ||||
| Teeth | 6 months | −0.2 | 29.56 | 38 | −6.5 | 30.72 | 52 | 6.30 [−6.27, 18.87] | |||
| 12 months | −0.3 | 0.73 | 33 | −1.4 | 30.63 | 45 | 1.10 [−7.85, 10.05] | ||||
| Opening mouth | 6 months | −3.7 | 25.00 | 38 | −8.6 | 25.20 | 52 | 4.90 [−5.59, 15.39] | |||
| 12 months | −3.2 | 24.33 | 33 | −4.8 | 24.47 | 45 | 1.60 [−9.36, 12.56] | ||||
| Dry mouth | 6 months | −6.8 | 32.87 | 38 | −6.1 | 30.17 | 52 | −0.70 [−13.98, 12.58] | |||
| 12 months | −9.2 | 31.95 | 33 | −8.1 | 32.51 | 45 | −1.10 [−15.56, 13.36] | ||||
| Sticky saliva | 6 months | −8.6 | 29.88 | 38 | −5.5 | 30.72 | 52 | −3.10 [−15.75, 9.55] | |||
| 12 months | −8.5 | 29.16 | 33 | −8.3 | 29.78 | 45 | −0.20 [−13.42, 13.02] | ||||
| Coughing | 6 months | −3.3 | 22.64 | 38 | −4.3 | 22.99 | 52 | 1.00 [−8.53, 10.53] | |||
| 12 months | −3.5 | 21.98 | 33 | −9.4 | 22.42 | 45 | 5.90 [−4.06, 15.86] | ||||
| Felt ill | 6 months | −15.3 | 26.73 | 38 | −6.9 | 27.78 | 52 | −8.40 [−19.77, 2.97] | |||
| 12 months | −9.7 | 28.28 | 33 | −11.4 | 26.52 | 45 | 1.70 [−10.67, 14.07] | ||||
| Global quality of life | +/− 6 months | EQ‐5D | −0.01 | 0.31 | 59 | −0.0004 | 0.21 | 47 | −0.01 [−0.11, 0.09] | Geerse 2017 | |
| Sexual function | 4 months | PC‐QOL | 14.63 | 21.29 | 48 | 5.23 | 20.16 | 51 | 9.40 [1.22, 17.58] | Giesler 2005 | |
| 7 months | 21.9 | 22.72 | 41 | 12.6 | 26.33 | 44 | 9.30 [−1.14, 19.74] | ||||
| 12 months | 25.26 | 26.6 | 41 | 15.32 | 27.77 | 44 | 9.94 [−1.62, 21.50] | ||||
| Sexual limitation | 4 months | 7.75 | 16.81 | 48 | 0.41 | 20.56 | 51 | 7.34 [−0.04, 14.72] | |||
| 7 months | 10.68 | 15.93 | 41 | 3.8 | 15.05 | 44 | 6.88 [0.28, 13.48] | ||||
| 12 months | 12.35 | 17.28 | 41 | 3.11 | 19.61 | 44 | 9.24 [1.39, 17.09] | ||||
| Sexual bother | 4 months | −0.95 | 22.12 | 48 | −3.55 | 24.23 | 51 | 2.60 [−6.53, 11.73] | |||
| 7 months | 5.54 | 23.74 | 41 | −0.2 | 19.67 | 44 | 5.74 [−3.57, 15.05] | ||||
| 12 months | 9.21 | 29.63 | 41 | 3.3 | 25.35 | 44 | 5.91 [−5.85, 17.67] | ||||
| Urinary function | 4 months | 13.68 | 16.89 | 48 | 19.51 | 17.56 | 51 | −5.83 [−12.62, 0.96] | |||
| 7 months | 18.86 | 19.71 | 41 | 22.35 | 19.32 | 44 | −3.49 [−11.80, 4.82] | ||||
| 12 months | 19.55 | 23.57 | 41 | 23.09 | 22.34 | 44 | −3.54 [−13.32, 6.24] | ||||
| Urinary limitation | 4 months | 24.17 | 26.48 | 48 | 20.26 | 25.75 | 51 | 3.91 [−6.39, 14.21] | |||
| 7 months | 23.05 | 23.26 | 41 | 17.58 | 24.17 | 44 | 5.47 [−4.61, 15.55] | ||||
| 12 months | 23.4 | 24.14 | 41 | 17.19 | 26.72 | 44 | 6.21 [−4.60, 17.02] | ||||
| Urinary bother | 4 months | 21.16 | 29.16 | 48 | 19.15 | 22.66 | 51 | 2.01 [−8.32, 12.34] | |||
| 7 months | 27.55 | 21.91 | 41 | 20.51 | 21.72 | 44 | 7.04 [−2.24, 16.32] | ||||
| 12 months | 21.76 | 30.93 | 41 | 25.84 | 24.48 | 44 | −4.08 [−15.99, 7.83] | ||||
| Bowel function | 4 months | 4.81 | 15.56 | 48 | 9.19 | 17.58 | 51 | −4.38 [−10.91, 2.15] | |||
| 7 months | 6.79 | 13.97 | 41 | 11.42 | 19.26 | 44 | −4.63 [−11.75, 2.49] | ||||
| 12 months | 4.8 | 16.91 | 41 | 8.35 | 15.71 | 44 | −3.55 [−10.50, 3.40] | ||||
| Bowel limitation | 4 months | 4 | 13.36 | 48 | 3.25 | 10.66 | 51 | 0.75 [−4.03, 5.53] | |||
| 7 months | 6.01 | 11.62 | 41 | 5.04 | 13.88 | 44 | 0.97 [−4.46, 6.40] | ||||
| 12 months | 2.8 | 10.99 | 41 | 3.27 | 10.6 | 44 | −0.47 [−5.07, 4.13] | ||||
| Bowel bother | 4 months | 15.84 | 27.81 | 48 | 7.21 | 24.15 | 51 | 8.63 [−1.66, 18.92] | |||
| 7 months | 15.56 | 24.51 | 41 | 12.18 | 23.96 | 44 | 3.38 [−6.94, 13.70] | ||||
| 12 months | 14 | 23.67 | 41 | 10.22 | 25.49 | 44 | 3.78 [−6.67, 14.23] | ||||
| Cancer worry | 4 months | 12.64 | 23.52 | 48 | 6.34 | 17.65 | 51 | 6.30 [−1.93, 14.53] | |||
| 7 months | 13.9 | 26.12 | 41 | 8.97 | 21.46 | 44 | 4.93 [−5.27, 15.13] | ||||
| 12 months | 14.15 | 25.12 | 41 | 3.07 | 17.68 | 44 | 11.08 [1.78, 20.38] | ||||
| Vitality | 4 months | SF‐36 | 17.7 | 18.65 | 48 | 18.71 | 23.86 | 51 | −1.01 [−9.42, 7.40] | ||
| 7 months | 16.04 | 22.48 | 41 | 11.88 | 24.16 | 44 | 4.16 [−5.76, 14.08] | ||||
| 12 months | 17.02 | 22.37 | 41 | 13.53 | 21.33 | 44 | 3.49 [−5.82, 12.80] | ||||
| Mental health | 4 months | 0.45 | 14.19 | 48 | 1.98 | 13.74 | 51 | −1.53 [−7.04, 3.98] | |||
| 7 months | 4.56 | 12.6 | 41 | 2.34 | 13.48 | 44 | 2.22 [−3.32, 7.76] | ||||
| 12 months | 1.62 | 11.31 | 41 | 2.43 | 14.57 | 44 | −0.81 [−6.33, 4.71] | ||||
| Health perception | 4 months | 7.15 | 17.47 | 48 | 6.69 | 18.44 | 51 | 0.46 [−6.61, 7.53] | |||
| 7 months | 7.88 | 16.88 | 41 | 7.08 | 18.76 | 44 | 0.80 [−6.78, 8.38] | ||||
| 12 months | 3.21 | 19.41 | 41 | 4.82 | 17.59 | 44 | −1.61 [−9.50, 6.28] | ||||
| Health transition | 4 months | −0.4 | 1.13 | 48 | −0.63 | 1 | 51 | 0.23 [−0.19, 0.65] | |||
| 7 months | −0.76 | 1.3 | 41 | −0.61 | 1.03 | 44 | −0.15 [−0.65, 0.35] | ||||
| 12 months | −1.3 | 1.19 | 41 | −1.35 | 1.28 | 44 | 0.05 [−0.48, 0.58] | ||||
| Distress | Breast cancer‐specific distress | 4 months | Adaptation of breast cancer distress tool | −2.16 | 4.4 | 196 | −1.7 | 4.38 | 157 | −0.46 [−1.38, 0.46] | Livingston 2010 |
| 12 months | −2.74 | 3.46 | 194 | −2.96 | 3.5 | 147 | 0.22 [−0.53, 0.97] | ||||
| Overall distress | +/− 6 months | HADS | −2.1 | 7.68 | 59 | −2.4 | 8.91 | 47 | 0.30 [−2.91, 3.51] | Geerse 2017 | |
| Tension/anxiety | 4 months | −2.33 | 3.05 | 196 | −2.34 | 3.04 | 157 | 0.01 [−0.63, 0.65] | Livingston 2010 | ||
| +/− 6 months | −1.3 | 3.87 | 60 | −1.3 | 4.80 | 47 | 0.00 [−1.69, 1.69] | Geerse 2017 | |||
| 12 months | −2.91 | 3.74 | 194 | −3.1 | 3.8 | 147 | 0.19 [−0.62, 1.00] | Livingston 2010 | |||
| Depression/dejection | 4 months | −0.29 | 2.84 | 196 | −0.18 | 2.85 | 157 | −0.11 [−0.71, 0.49] | |||
| +/− 6 months | −0.6 | 4.61 | 59 | −0.9 | 4.9 | 49 | 0.30 [−1.51, 2.11] | Geerse 2017 | |||
| 12 months | −0.92 | 2.47 | 194 | −0.76 | 2.45 | 147 | −0.16 [−0.69, 0.37] | Livingston 2010 | |||
| Psychosocial well‐being | Dyadic cohesion | 4 months | DAS | −0.35 | 4.29 | 48 | −0.27 | 3.42 | 51 | −0.08 [−1.61, 1.45] | Giesler 2005 |
| 7 months | −0.75 | 4.52 | 41 | 0.07 | 4.12 | 44 | −0.82 [−2.66, 1.02] | ||||
| 12 months | −0.41 | 3.62 | 41 | −0.12 | 4.26 | 44 | −0.29 [−1.97, 1.39] | ||||
| Dyadic satisfaction | 4 months | −0.45 | 2.72 | 48 | 0.51 | 4.13 | 51 | −0.96 [−2.33, 0.41] | |||
| 7 months | −0.55 | 3.75 | 41 | 0.36 | 3.72 | 44 | −0.91 [−2.50, 0.68] | ||||
| 12 months | −0.36 | 3.54 | 41 | 1.01 | 3.87 | 44 | −1.37 [−2.95, 0.21] | ||||
| Depression | 2.5 months | CES‐D | N/A | N/A | 97 | N/A | N/A | 94 | N/E | Given 2004 | |
| 4 months | −2.16 | 6.86 | 48 | −1.89 | 7.08 | 51 | −0.27 [−3.02, 2.48] | Giesler 2005 | |||
| 5 months | N/A | N/A | 80 | N/A | N/A | 87 | N/E | Given 2004 | |||
| 6 months | −0.3 | 6.92 | 38 | −1.6 | 1.10 | 52 | 1.30 [−0.92, 3.52] | van der Meulen 2018 | |||
| 7 months | −2.99 | 4.69 | 41 | −0.69 | 7.57 | 44 | −2.30 [−4.96, 0.36] | Giesler 2005 | |||
| 12 months | −3 | 5.58 | 41 | −1.51 | 6.76 | 44 | −1.49 [−4.12, 1.14] | ||||
| 0.4 | 6.59 | 33 | −1.5 | 6.85 | 45 | 1.90 [−1.11, 4.91] | van der Meulen 2018 | ||||
| Worry of cancer | 6 months | Worry of cancer scale | 0.4 | 1.57 | 38 | 0.1 | 1.66 | 52 | 0.30 [−0.37, 0.97] | ||
| 12 months | 0.2 | 1.47 | 33 | 0.2 | 1.71 | 45 | 0.00 [−0.71, 0.71] | ||||
| Patient satisfaction | Total satisfaction | +/− 6 months | PSQ‐III | −0.3 | 12.61 | 55 | 3.4 | 11.65 | 47 | −3.70 [−8.41, 1.01] | Geerse 2017 |
| Overall satisfation | +/− 6 months | −1.4 | 22.99 | 55 | 4.6 | 18.01 | 48 | −6.00 [−13.93, 1.93] | |||
| Accessibility | +/− 6 months | 1.2 | 13.96 | 54 | 5.4 | 13.71 | 47 | −4.20 [−9.61, 1.21] | |||
| Interpersonal manner | +/− 6 months | −1.2 | 14.97 | 56 | 3.1 | 14.55 | 48 | −4.30 [−9.98, 1.38] | |||
| Technical quality | +/− 6 months | −0.9 | 17.06 | 55 | 1.2 | 13.71 | 47 | −2.10 [−8.07, 3.87] | |||
| Abbreviations: CES‐D (Center for Epidemiological Studies Depression Scale); CI (confidence interval); DAS (Dyadic Adjustment Scale); (European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Head and Neck Cancer 35 items); EORTC QLQ‐C30 (European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Core 30); EQ‐5D (EuroQol 5D); HADS (Hospital Anxiety and Depression Scale); HRQoL (health‐related quality of life); MD (mean difference); PC‐QOL (Prostate Cancer‐Related Quality of Life Scales); PSQ‐III (Patient Satisfaction Questionnaire 3rd update); SD (standard deviation); SF‐36 (36‐Item Short Form Health Survey); N/A: Not available; N/E: Not estimable. | |||||||||||
5. Evidence summary ‐ binary outcomes.
| Raw data: binary outcomes | |||||||||
| Main outcome | Suboutcome | Time postintervention | Scale used | Intervention (screening) | Control (usual care) | RR [95% CI] | Study ID | ||
| Events | Total | Events | Total | ||||||
| Distress (proportion yes) | Anxiety | 2 months | HADS | 9 | 103 | 18 | 192 | 0.93 [0.43;2] | Waller 2012 |
| 3 months | 57 | 268 | 64 | 300 | 1 [0.73;1.37] | Braeken 2013 | |||
| 4 months | 11 | 85 | 18 | 192 | 1.38 [0.68;2.79] | Waller 2012 | |||
| 6 months | 5 | 67 | 18 | 192 | 0.8 [0.31;2.06] | ||||
| 12 months | 42 | 268 | 61 | 300 | 0.77 [0.54;1.1] | Braeken 2013 | |||
| DIS/DSM | 0 | 123 | 0 | 127 | N/E | Maunsell 1996 | |||
| Depression | 2 months | HADS | 10 | 103 | 26 | 192 | 0.72 [0.36;1.43] | Waller 2012 | |
| 3 months | 17 | 268 | 23 | 300 | 0.83 [0.45;1.51] | Braeken 2013 | |||
| 4 months | 9 | 85 | 26 | 192 | 0.78 [0.38;1.6] | Waller 2012 | |||
| 6 months | 9 | 67 | 26 | 192 | 0.99 [0.49;2.01] | ||||
| 12 months | 46 | 268 | 46 | 300 | 1.12 [0.77;1.63] | Braeken 2013 | |||
| DIS/DSM | 22 | 123 | 15 | 127 | 1.51 [0.82;2.78] | Maunsell 1996 | |||
| Psychological distress | 3 months | GHQ‐12 | 103 | 268 | 117 | 300 | 0.99 [0.8;1.21] | Braeken 2013 | |
| 12 months | 65 | 268 | 74 | 300 | 0.98 [0.74;1.31] | ||||
| Psychosocial well‐being | Physical health (rated good or excellent) | 12 months | LES | 98 | 123 | 101 | 127 | 1 [0.88;1.14] | Maunsell 1996 |
| Physical health (do not worry moderately or a lot) | 87 | 123 | 85 | 127 | 1.06 [0.89;1.25] | ||||
| Physical health (no arm problems) | 3 months | 27 | 122 | 31 | 126 | 0.9 [0.57;1.41] | |||
| 12 months | 49 | 122 | 63 | 126 | 0.8 [0.61;1.06] | ||||
| Role functioning (working at interview) | 3 months | 11 | 55 | 7 | 56 | 1.6 [0.67;3.82] | |||
| 12 months | 41 | 55 | 43 | 56 | 0.97 [0.79;1.2] | ||||
| Marital relation (still with spouse) | 3 months | LWMAT | 76 | 78 | 82 | 83 | 0.99 [0.94;1.03] | ||
| 12 months | 74 | 78 | 78 | 83 | 1.01 [0.94;1.09] | ||||
| Marital relation (marriage not rated as unhappy) | 3 months | 69 | 78 | 71 | 83 | 1.03 [0.92;1.17] | |||
| 12 months | 70 | 78 | 75 | 83 | 0.99 [0.9;1.1] | ||||
| Marital relation (had sexual relationship with spouse) | 3 months | 59 | 78 | 61 | 83 | 1.03 [0.86;1.23] | |||
| 12 months | 55 | 78 | 55 | 83 | 1.06 [0.86;1.31] | ||||
| Supportive care needs (proportion yes) | Physical symptom and daily living | 2 months | SCNS | 47 | 103 | 98 | 192 | 0.89 [0.69;1.15] | Waller 2012 |
| 4 months | 40 | 85 | 98 | 192 | 0.92 [0.71;1.2] | ||||
| 6 months | 33 | 67 | 98 | 192 | 0.96 [0.73;1.28] | ||||
| Psychological | 2 months | 39 | 103 | 74 | 192 | 0.98 [0.72;1.33] | |||
| 4 months | 30 | 85 | 74 | 192 | 0.92 [0.65;1.28] | ||||
| 6 months | 22 | 67 | 74 | 192 | 0.85 [0.58;1.25] | ||||
| Health system and information | 2 months | 19 | 103 | 54 | 192 | 0.66 [0.41;1.1] | |||
| 4 months | 16 | 85 | 54 | 192 | 0.67 [0.41;1.1] | ||||
| 6 months | 11 | 67 | 54 | 192 | 0.58 [0.32;1.05] | ||||
| Patient care and support | 2 months | 13 | 103 | 26 | 192 | 0.93 [0.50, 1.73] | |||
| 4 months | 9 | 85 | 26 | 192 | 0.78 [0.38, 1.60] | ||||
| 6 months | 3 | 67 | 26 | 192 | 0.33 [0.10, 1.06] | ||||
| Sexuality | 2 months | 9 | 103 | 12 | 192 | 1.40 [0.61, 3.21] | |||
| 4 months | 6 | 85 | 12 | 192 | 1.13 [0.44, 2.91] | ||||
| 6 months | 4 | 67 | 12 | 192 | 0.96 [0.32, 2.86] | ||||
| Spirituality | 2 months | NA‐ACP | 9 | 103 | 17 | 192 | 0.99 [0.46, 2.13] | ||
| 4 months | 7 | 85 | 17 | 192 | 0.93 [0.40, 2.16] | ||||
| 6 months | 6 | 67 | 17 | 192 | 1.01 [0.42, 2.46] | ||||
| Patient satisfaction (proportion yes) | Doctor‐patient relationship | 6 months | DanPEP (top‐evaluation) | N/A | N/A | N/A | N/A | N/E | Bergholdt 2013 |
| Medical care | N/A | N/A | N/A | N/A | N/E | ||||
| Information and support | N/A | N/A | N/A | N/A | N/E | ||||
| Organisation of care | N/A | N/A | N/A | N/A | N/E | ||||
| General practitioner's accessibility | N/A | N/A | N/A | N/A | N/E | ||||
| Patient satisfaction with general practitioner’s contribution to the rehabilitation course | 14 months | Ad hoc question | 109 | 159 | 105 | 159 | 1.04 [0.89, 1.21] | ||
| Abbreviations: CI (confidence interval); DanPEP (Danish Patients Evaluate General Practice); DIS/DSM (Diagnostic Interview Schedule according to Diagnostic and Statistical Manual of Mental Disorders criteria); GHQ‐12 (General Health Questionnaire 12‐item version); HADS (Hospital Anxiety and Depression Scale); LES (Life Experiences Survey); LWMAT (Locke‐Wallace Martial Adjustment Test); NA‐ACP (Needs Assessment for Advanced Cancer Patients); RR (risk ratio); SCNS (Supportive Care Needs Survey); N/A: Not available; N/E: Not estimable. | |||||||||
The subgroup of studies that did find evidence for the effectiveness of screening for the psychosocial well‐being and care needs of people with cancer, as well as the subgroup of studies that did not find any evidence for positive effects, were studied for their intervention characteristics. We could observe no consistency for specific intervention characteristics and effectiveness of the interventions.
Subgroup analysis and investigation of heterogeneity
We expected that potential heterogeneity in outcomes could be induced by clinical and methodological characteristics:
people with cancer characteristics (e.g. age group, gender);
medical characteristics (e.g. cancer type, disease prognosis or stage, type and degree of (pre‐) treatment);
characteristics of the intervention of interest (e.g. simple or complex screening of psychosocial well‐being and care needs; studies that address more than one relevant intervention condition; content of assessment; timing and frequency of assessment).
If we found several studies that focused on these specific characteristics, we performed subgroup analyses. Subgroup analyses based on intervention characteristics could reveal potential consistency of intervention characteristics and intervention results.
Sensitivity analysis
We conducted a sensitivity analysis to assess the robustness of our findings, as intervention effects could be larger in NRCTs and RCTs of lower quality by overestimation. However, this could equally be the other way around, and effects could be underestimated in RCTs (Sidani 2015). We conducted a meta‐analysis including the eligible RCTs and NRCTs. We subsequently repeated meta‐analysis for both separately to explore the impact on the final results. In so doing, we were able to explore the effects of including NRCTs in the review.
Results
Description of studies
Results of the search
The process of record screening and study selection for the review is shown in Figure 1.
1.
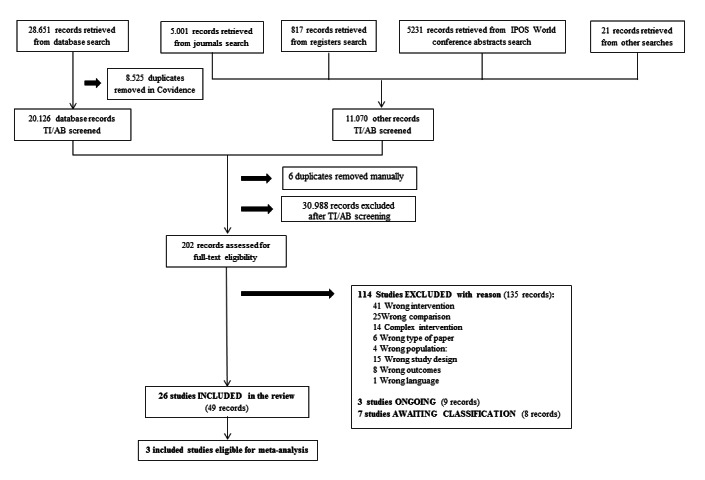
The electronic search of the databases identified 6703 records for MEDLINE, 9739 records for CENTRAL, 1367 records for PsycINFO, 2773 records for CINAHL, and 8069 records for Embase. After de‐duplication in Covidence, 20,126 database records were left for screening. The search of the trial registers identified 44 records for ClinicalTrials.gov, 350 records for the ISRCTN registry, 233 records for the NRT, 103 for RePORTER, and 87 for the UK National Research Register.
Six duplicates that were incorrectly retained after de‐duplication in Covidence were deleted. Screening of the electronic records and records found through screening of the two selected journals, conference abstracts, and reference lists resulted in 202 potentially relevant records. We assessed the full texts of these remaining records for eligibility. We excluded another 114 studies due to the reasons specified in the Excluded studies section. We classified three studies as 'ongoing' (see Characteristics of ongoing studies), and seven as 'awaiting classification' for which there was insufficient information to judge eligibility (see Characteristics of studies awaiting classification). We deemed 26 studies suitable for inclusion in the review.
Included studies
Details of the included studies are presented in the Characteristics of included studies tables. Seven studies were conducted in the Netherlands (Braeken 2013; Bramsen 2008; de Leeuw 2013; Detmar 2002; Geerse 2017; Hilarius 2008; van der Meulen 2018), seven in Australia (Harrison 2011; Livingston 2010; Schofield 2013; Thewes 2009; Waller 2012; Young 2010; Young 2013), five in the USA (Giesler 2005; Given 2004; Kutner 1999; Rosenbloom 2007; Williams 2013), three in the UK (Hollingworth 2013; Nimako 2015; Velikova 2004), two in Canada (Maunsell 1996; Taenzer 2000), one in Denmark (Bergholdt 2013), and one in Germany (Singer 2017). All studies took place between 1990 and 2017 and were written in English.
We contacted the study authors of 14 studies multiple times to request more information or data. Three did not respond. Of the 11 others, only six provided us with extra data.
Design
We included 18 studies with an RCT design. Of these, Detmar 2002 used a longitudinal randomised cross‐over design, and Giesler 2005 used a prospective multisite RCT. Seven studies used an RCT design with two groups, Geerse 2017; Given 2004; Harrison 2011; Hollingworth 2013; Maunsell 1996; Rosenbloom 2007; van der Meulen 2018, and three groups (Nimako 2015; Rosenbloom 2007; Velikova 2004). Five studies used a cluster RCT design with two groups, Bergholdt 2013; Braeken 2013; Kutner 1999; Singer 2017; Young 2013, or three groups (Livingston 2010).
We included eight studies with an NRCT design. Waller 2012 was based on an interrupted time series design, and Williams 2013 conducted a historically controlled study. Two other studies performed a prospective non‐randomised controlled study (de Leeuw 2013; Young 2010). Four studies used a sequential cohort design with repeated measures (Bramsen 2008; Hilarius 2008; Taenzer 2000; Thewes 2009).
Settings
Participants were recruited in general hospitals (Bergholdt 2013; Giesler 2005; Harrison 2011; Hilarius 2008; Hollingworth 2013; Young 2010; Young 2013), university medical centres (Bramsen 2008; Geerse 2017; Giesler 2005; Singer 2017), radiotherapy and oncology departments of academic medical centres (Braeken 2013; Rosenbloom 2007), specialised cancer clinics (de Leeuw 2013; Hollingworth 2013; Maunsell 1996; Schofield 2013; Velikova 2004; Waller 2012), tertiary medicine and care clinics (Livingston 2010; Nimako 2015; Williams 2013), and outpatient clinics of cancer hospitals (Detmar 2002; Kutner 1999; Taenzer 2000; Thewes 2009; van der Meulen 2018). Given 2004 described the settings in which people with cancer were recruited as "institutions", the nature of which remained unclear.
Participants
For all studies, people with cancer were recruited, mostly of both sexes. Maunsell 1996 recruited only women, Giesler 2005 and Livingston 2010 recruited only men. Thirteen studies focused on one or only some specific cancer types, namely: breast cancer (Maunsell 1996); lung cancer (Geerse 2017; Nimako 2015; Schofield 2013; Taenzer 2000); head and neck cancer (de Leeuw 2013; van der Meulen 2018); colorectal cancer (Harrison 2011; Young 2010; Young 2013); prostate cancer (Giesler 2005); prostate and lung cancer (Livingston 2010); and breast, lung, and colorectal cancer (Rosenbloom 2007). The remaining 12 studies defined a broader range of pathologies or made no specifications on type of cancer. In seven studies, people with cancer with metastases or palliative treatment were excluded (Braeken 2013; de Leeuw 2013; Geerse 2017; Giesler 2005; Given 2004; Hollingworth 2013; Maunsell 1996). However, in four other studies advanced diagnosis or palliative treatment was explicitly part of the inclusion criteria (Detmar 2002; Rosenbloom 2007; Schofield 2013; Waller 2012). Nimako 2015 recruited participants after treatment completion. In all other studies people with cancer were recruited at the start of or during active treatment. In 12 studies, the eligible types of treatment were specified (Braeken 2013; Detmar 2002; Geerse 2017; Giesler 2005; Given 2004; Hilarius 2008; Hollingworth 2013; Rosenbloom 2007; Schofield 2013; Williams 2013; Young 2010; Young 2013); chemo‐ and radiotherapy were the most prevalent. Research samples counted between 41, Young 2010, and 1012, Singer 2017, participants, and a total of 7654 participants were included in this review. A detailed description of participants in each study can be found in the Characteristics of included studies table.
Interventions
The characteristics of the studied interventions in the included studies are displayed concisely in Table 8 and in detail below.
6. Characteristics of screening interventions of included studies.
| Study | Theoretical basis | Content of screening (tool) | Interventionist | Screening procedure (frequency) | Conditions needed for implementation |
| Bergholdt 2013 | ‐ | Care needs | Yes nurse |
SI with co‐intervention (1x): results sent to GP, GP encouraged to contact participant | · Interview manual · Interviewers |
| Braeken 2013 | ‐ | Psychosocial problems (SIPP) |
No | Solitary SI (2x): results given to radiotherapist | · Person/system questionnaire management · Training on the SIPP |
| Bramsen 2008 | ‐ | Overall well‐being | Yes psychologist or social worker |
SI with co‐intervention (1x): screening results discussed, summary placed in patient record | · Interviewers |
| de Leeuw 2013 | ‐ | Care needs Psychosocial problems |
Yes nurse |
SI with co‐intervenion (4x): SI part of follow‐up consultations with nurse | · Training for the follow‐up consultations · Training on simple medical checks · Supervision meetings with psychologist |
| Detmar 2002 | ‐ | HRQoL (EORTC) |
No | Solitary SI (3x): physicians and participants received a summary | · Educational sessions on QoL scores · Information pamphlet · Person/system questionnaire management · Assistant available for more information |
| Geerse 2017 | ‐ | Distress (DT&PL) |
No | SI with co‐intervention (min. 4x): discussion of response pattern + referral if necessary | · Person/system questionnaire management · Person for results discussion |
| Giesler 2005 | The proximal‐distal framework | HRQoL | Yes nurse |
SI with co‐intervention (6x): discussion of issues and help/treatment strategies | · Interviewer (monthly) · Computer assessment program · Laptop · Training |
| Given 2004 | Cognitive behavioural model and Bandura’s theory of self‐efficacy | HRQoL | Yes nurse |
SI with co‐intervention (10x): discussion and further direction of care according to computer documentation system | · Computer system with predefined roster of interventions · Training on screening intervention · Interviewer |
| Harrison 2011 | Self‐regulation model of adjustment to illness | Care needs | Yes nurse |
SI with co‐intervention (5x): needs discussion, information and support provision, referral to clinical team members | · Interviewers · Training of interviewers |
| Hilarius 2008 | ‐ | HRQoL (EORTC) |
No | Solitary SI (4x): summary given to participant and nurse | · Person/system for questionnaire management · Education on HRQoL scores · Written materials for participants |
| Hollingworth 2013 | ‐ | Distress (DT&PL) |
Yes nurse or radiographer |
SI with co‐intervention (1x, 2nd possible): results discussion, staff actions/patient actions/referral taken | · Training on DT&PL, role playing, dealing with strong emotions · Source directory with information and guidance for staff |
| Kutner 1999 | ‐ | Care needs | No | Solitary SI (1x): completed forms added to patient's chart | Person/system for questionnaire management |
| Livingston 2010 | ‐ | Biopsychosocial well‐being | Yes nurse |
SI with co‐intervention (4x or 1x): Helpline calls with assessment and support | · A cancer helpline · Interviewers · Training on discussion cancer helpline |
| Maunsell 1996 | ‐ | Distress (GHQ‐20) |
Yes research assistant |
SI with co‐intervention (12x): discussion with social worker and tailored approach | · Interviewer · Social worker that works with patients with high GHQ scores |
| Nimako 2015 | ‐ | HRQoL (EORTC) |
No | Solitary SI (1x): completed questionnaire given to the doctor | · Person/system for questionnaire management · Training on use and interpretation of PROM |
| Rosenbloom 2007 | ‐ | HRQoL (FACT‐G) |
Yes nurse |
SI with co‐intervention (5x): structured interview in case symptoms worse than expected, information shared with treating nurse | · Interviewer |
| Schofield 2013 | ‐ | Care needs (38‐item NAALCP) |
No | SI with co‐intervention (2x): active listening, self‐care education, and communication on unmet needs to the team for management and referral | · Person/system for questionnaire management · Training of health professionals · Consultation materials |
| Taenzer 2000 | ‐ | HRQoL (EORTC) |
No | Solitary SI (1x): report was given to the nurse and physician | · Person/system for questionnaire management · Demonstration on computer program and reading of the report for clinic staff · Volunteer to support completion of the computer EORTC |
| Thewes 2009 | ‐ | Distress (DT) |
No | Solitary SI (1x): information available for staff, staff encouraged to discuss problems and concerns for scores above cut‐off | · Person/system for questionnaire management · Training of staff on screening, instrument, and study |
| van der Meulen 2018 | ‐ | Distress (DT&PL) |
No | SI with co‐intervention (3x or 4x/year): discussion of screening results with nurse, if indicated, basic psychosocial care, minor nursing interventions, or referral to other health care | · Training for nurses |
| Velikova 2004 | ‐ | HRQoL (EORTC) Distress (HADS) |
No | Solitary SI (1x): results given to physicians who were asked to review and use the results | · Person/system for questionnaire management · Training of staff in interpretation of screening scores |
| Waller 2012 | ‐ | Care needs (NAT:PD‐C) |
Yes healthcare professional |
Solitary SI (1x): healthcare professionals use the NAT:PD‐C during consultation and employ resulting insights for discussion and referral | · Palliative care needs assessment guidelines · Training of staff in use of tool · Interviewers for 2‐monthly computer‐assisted telephone interviews |
| Williams 2013 | ‐ | Physical and psychological symptioms (TRSC) |
No | Solitary SI (multiple): clinicians received results prior to consultation | · Person/system for questionnaire management · Training of clinic staff in interpretation of tool results |
| Young 2010 | Self‐regulation model of adjustment to illness | Care needs | Yes nurse |
SI with co‐intervention (5x): nurse provides information, checks understanding, and provides emotional support and advice | · Interviewers · Training for interviewers |
| Young 2013 | Self‐regulation model of adjustment to illness | Care needs | Yes nurse |
SI with co‐intervention (5x): needs discussion, information and support provision, referral to clinical team members | · Training for interviewers · Interviewer for screening calls · Development of detailed, standardised clinical protocols to respond to detected needs |
Abbreviations:
DT: Distress Thermometer DT&PL: Distress Thermometer & Problem List EORTC: European Organisation for Research and Treatment of Cancer FACT‐G: Functional Assessment of Cancer Therapy‐General GHQ‐20: General Health Questionnaire 20‐item version GP: general practitioner HADS: Hospital Anxiety and Depression Scale HRQoL: health‐related quality of life
NAALCP: Needs Assessment for Advanced Lung Cancer Patients NAT:PD‐C: Needs Assessment Tool: Progressive Disease‐Cancer PROM: patient reported outcome measure SI: screening intervention SIPP: Screening Inventory of Psychosocial Problems TRSC: Therapy‐Related Symptom Checklist
Five studies reported a theoretical basis for the studied intervention. Giesler 2005 based their assessment of well‐being on the proximal‐distal framework (Brenner 1995). Given 2004 used the cognitive behavioural model and Bandura’s theory of self‐efficacy to develop their screening intervention (Bandura 1977). The self‐regulation model of adjustment to illness, Leventhal 2016, was the theoretical starting point that Young and colleagues used for the development of their telephone screening intervention (Harrison 2011; Young 2010; Young 2013). The basis for the intervention studied for remaining two studies was findings of a systematic literature review, written recommendations, and guidelines on screening (Giesler 2005; Thewes 2009).
Intervention content and tools
The content of the screening intervention was HRQoL in eight included studies. In five of these studies, the EORTC QLQ‐C30 was used as intervention tool, sometimes with the addition of a cancer type‐specific module (Detmar 2002; Hilarius 2008; Nimako 2015; Taenzer 2000; Velikova 2004). Rosenbloom 2007 used the Functional Assessment of Cancer Therapy‐General (FACT‐G), and in the studies of Giesler 2005 and Given 2004 the content was described without a specific quality of life tool name.
Five studies described their intervention as distress screening. In four of these, the Distress Thermometer (DT) was deployed for this purpose, solely (Thewes 2009); together with the problem list (PL) (Hollingworth 2013); or with the PL and the "referral wish question" (Geerse 2017; van der Meulen 2018). Maunsell 1996 conducted the distress screening with the General Health Questionnaire 20‐item version (GHQ‐20).
Eight studies applied needs assessment. In six of these, no specific instrument was used for this purpose, but the content was described (Bergholdt 2013; de Leeuw 2013; Harrison 2011; Kutner 1999; Young 2010; Young 2013). Schofield 2013 used the 38‐item Needs Assessment for Advanced Lung Cancer Patients, and Waller 2012 used the Needs Assessment Tool: Progressive Disease‐Cancer (NAT:PD‐C).
In six studies, (bio‐)psychosocial symptoms or overall well‐being formed the content for the screening intervention. Braeken 2013 used the Dutch Screening Inventory of Psychosocial Problems (SIPP); Williams 2013 used the Therapy‐Related Symptom Checklist (TRSC); Singer 2017 used the Patient Health Questionnaire Short‐Form, the Generalized Anxiety Screener, and items on fatigue, pain, and financial difficulties from the EORTC QLQ‐C30; and in the remaining three studies no specific instrument was used, but the content of the screen was described (Bramsen 2008; de Leeuw 2013; Livingston 2010).
Intervention mode, frequency, and follow‐up
In 13 studies, the screening intervention took place in the form of self‐completion of a screening tool (Braeken 2013; Detmar 2002; Geerse 2017; Hilarius 2008; Kutner 1999; Nimako 2015; Schofield 2013; Singer 2017; Taenzer 2000; Thewes 2009; van der Meulen 2018; Velikova 2004; Williams 2013), whereas in the other 13 studies an interventionist conducted the screening or assessment. Nurses fulfilled this role in nine studies (Bergholdt 2013; de Leeuw 2013; Giesler 2005; Given 2004; Harrison 2011; Hollingworth 2013; Livingston 2010; Rosenbloom 2007; Young 2010; Young 2013). Other interventionists mentioned were psychologists or social workers (Bramsen 2008), radiographers (Hollingworth 2013), and research assistants (Maunsell 1996). In Waller 2012, healthcare professionals of several disciplines used the NAT:PD‐C during consultation.
Ten studies explored the effect of a ‘solitary screening intervention’, of which the insights on well‐being in people with cancer were communicated to a treating healthcare professional to use in further follow‐up of people with cancer (Braeken 2013; Detmar 2002; Hilarius 2008; Kutner 1999; Nimako 2015; Thewes 2009; Velikova 2004; Waller 2012; Williams 2013). In the remaining studies (Bergholdt 2013; Bramsen 2008; de Leeuw 2013; Geerse 2017; Giesler 2005; Given 2004; Harrison 2011; Hollingworth 2013; Livingston 2010; Maunsell 1996; Rosenbloom 2007; Schofield 2013; Singer 2017; van der Meulen 2018; Young 2010; Young 2013), the screening intervention was combined with guided actions: active results discussion with people with cancer, further assessment of certain problem areas, generation of respond formats, or specified intervention and referral strategies.
There was considerable heterogeneity regarding the number of times that the screening intervention was applied, ranging from one to 12 times. In the intervention of Taenzer 2000 and Kutner 1999, there was no further follow‐up after screening of people with cancer. In all other studies follow‐up varied between four weeks and 18 months.
Further details on intervention procedures are described for each study separately in the Characteristics of included studies table.
Conditions for implementation
A wide variation in conditions was set to implement the screening and assessment interventions studied in the included studies.
In 20 studies, training or educational sessions for the involved care professionals were provided to become familiar with the screening instrument or the intervention procedures, or both (Braeken 2013; de Leeuw 2013; Detmar 2002; Giesler 2005; Given 2004; Harrison 2011; Hilarius 2008; Hollingworth 2013; Livingston 2010; Nimako 2015; Schofield 2013; Singer 2017; Taenzer 2000; Thewes 2009; van der Meulen 2018; Velikova 2004; Waller 2012; Williams 2013; Young 2010; Young 2013). Staffing was required to be able to implement the face‐to‐face and telephone screenings in 11 studies (Bergholdt 2013; Bramsen 2008; Giesler 2005; Given 2004; Harrison 2011; Livingston 2010; Maunsell 1996; Rosenbloom 2007; Waller 2012; Young 2010; Young 2013). In the 13 studies that used a PROM completion for their screening intervention (Braeken 2013; Detmar 2002; Geerse 2017; Hilarius 2008; Kutner 1999; Nimako 2015; Schofield 2013; Singer 2017; Taenzer 2000; Thewes 2009; van der Meulen 2018; Velikova 2004; Williams 2013), a person or system for questionnaire management (giving to people with cancer, collecting, data analysis, giving result reports to people with cancer and/or healthcare professionals) was needed. The authors of seven studies stated that special documents were developed, such as interview manuals, a source directory, standardised clinical protocols, or written material for people with cancer (Bergholdt 2013; Detmar 2002; Hilarius 2008; Hollingworth 2013; Schofield 2013; Waller 2012; Young 2013). Detmar 2002 and Taenzer 2000 made a person (assistant, volunteer) available for people with cancer in case there was a need for extra information.
For detailed information on all of the conditions for implementation in each included study, see the Characteristics of included studies table.
Comparative conditions
In 22 studies, the intervention of interest was only compared to a usual care control group (Bergholdt 2013; Braeken 2013; Bramsen 2008; de Leeuw 2013; Detmar 2002; Geerse 2017; Giesler 2005; Given 2004; Harrison 2011; Hilarius 2008; Hollingworth 2013; Kutner 1999; Maunsell 1996; Schofield 2013; Singer 2017; Taenzer 2000; Thewes 2009; van der Meulen 2018; Waller 2012; Williams 2013; Young 2010; Young 2013). In two studies, a third condition, or "attention control group", was created, with participants completing screening questionnaires, that differed from the intervention condition because the screening results were not shared with the treating physicians (Nimako 2015; Velikova 2004). Rosenbloom 2007 used a third condition described as an "assessment control group" with screening and sharing of screening results, but without a structured interview following the HRQoL assessment in the intervention condition. Livingston 2010 also introduced an extra condition in addition to the intervention and control condition, with a less intensive version of the intervention of interest (one versus four outcalls from the Cancer Helpline).
Outcomes
Most of the included studies measured several of our primary and secondary outcomes of interest.
Outcomes of primary interest
HRQoL
Twenty studies focused on our primary outcome, HRQoL, using a wide variety of measurement tools such as the EORTC QLQ‐C30, its subscales or individual items and its cancer type‐specific modules (Bergholdt 2013; Braeken 2013; Bramsen 2008; de Leeuw 2013; Geerse 2017; Hollingworth 2013; Nimako 2015; Schofield 2013; Singer 2017; van der Meulen 2018; Waller 2012), the 36‐Item Short Form Health Survey (SF‐36) (Detmar 2002; Giesler 2005; Hilarius 2008), the EuroQol 5D (EQ‐5D) (Geerse 2017; Hollingworth 2013), the FLIC (Functional Living Index‐Cancer) (Rosenbloom 2007), the Functional Assessment of Cancer Therapy‐General (FACT‐G) or its disease‐specific versions (Harrison 2011; Hilarius 2008; Velikova 2004; Young 2010; Young 2013), and two lesser‐known tools: the HRQoL Linear Analogue Self‐Assessment (HRQoL‐LASA), Williams 2013, and the Prostate Cancer Quality of Life Instrument (PCQoL) (Giesler 2005).
Distress
Sixteen studies measured the effect of the screening intervention on the distress of people with cancer. The following instruments were used for this purpose: the Profile of Mood States (POMS) (Bergholdt 2013; Hollingworth 2013; Rosenbloom 2007), the Hospital Anxiety and Depression Scale (HADS) (Braeken 2013; Geerse 2017; Livingston 2010; Schofield 2013; Singer 2017; Waller 2012), the General Health Questionnaire 12‐item version (GHQ‐12) (Braeken 2013; Bramsen 2008), the Center for Epidemiological Studies Depression Scale (CES‐D) (Giesler 2005; Given 2004; van der Meulen 2018), the Psychiatric Symptom Index (PSI) (Maunsell 1996), the DT (Schofield 2013; Young 2010; Young 2013), the psychological subscale of the Somatic and Psychological Health Report (PSYCH‐6) (Thewes 2009), and a modified version of an existing distress tool for people with breast cancer (Livingston 2010).
Care needs
Seven studies reported on care needs as outcomes. Five studies used the Supportive Care Needs Survey (SCNS) (Harrison 2011; Thewes 2009; Waller 2012; Young 2010; Young 2013). In addition, Waller 2012 also used the questions on spiritual needs from the Needs Assessment for Advanced Cancer Patients (NA‐ACP). Care needs were further assessed with the Cancer Survivors’ Unmet Needs Measure (CaSUN) (Harrison 2011), the Needs Assessment for Advanced Lung Cancer Patients (NA‐ALCP) (Schofield 2013), or study authors did not specify a tool for needs assessment, but described the content (Bergholdt 2013).
Adverse events
None of the included studies specified adverse events as an outcome.
Outcomes of secondary interest
Satisfaction
Twelve studies measured satisfaction. This outcome concerned satisfaction with the quality of care in general or care from a specific healthcare professional, satisfaction with professional‐patient communication, their active involvement, addressing of needs, information and emotional support received. Satisfaction of people with cancer was surveyed with self‐constructed questions, Bergholdt 2013; Braeken 2013; van der Meulen 2018; Velikova 2004, or with existing tools. Tools used for this purpose were the Danish Patients Evaluate General Practice (Dan‐PEP) (Bergholdt 2013), the Patient Satisfaction Questionnaire (PSQ) (Detmar 2002; Geerse 2017; Hilarius 2008; Rosenbloom 2007), the Trent Patient Views of Cancer Services Questionnaire (TPVCSQ) (Hollingworth 2013), the five‐item Medical Outcomes Study Patient Visit Rating Questionnaire (Kutner 1999), the Quality of Care from Patients Perspective questionnaire (QPP) (Singer 2017), and the Patient‐Doctor Interaction Scale (PDIS) (Taenzer 2000).
Psychosocial well‐being
Other elements of psychosocial well‐being addressed as outcomes in the included studies were marital well‐being (Giesler 2005; Maunsell 1996), health and activity limitation (Maunsell 1996), impact of stressful life events (Bramsen 2008), psychosocial adjustment (de Leeuw 2013), fear of recurrence (van der Meulen 2018), and psychiatric comorbidity (Singer 2017).
Time points
The frequency of outcome measurement in the included studies varied from one to four times for each condition. The studies used the following time points for outcome assessment: baseline, one week, one month, six weeks, seven weeks, eight weeks, 10 weeks, 12 weeks, three months, four months, 20 weeks, 25 weeks, and six, seven, 12, and 14 months. In the included interrupted time series study, outcomes were measured seven times: three times before intervention implementation, at baseline, and four times after implementation (Waller 2012). The timing of outcome assessment in the study of Williams 2013 was variable, as it was linked to the treatment cycles of radio‐ and chemotherapy received by people with cancer.
Excluded studies
We excluded 114 studies for the following reasons.
intervention does not meet protocol: no psychosocial screening intervention effect studied (41 records).
comparison does not meet protocol: no usual care condition without screening to use as comparison (25 records).
complex intervention: screening part of complex intervention, not possible to distinguish effect of screening intervention (14 records).
type of paper does not meet protocol: no original research paper, rather review, recommendations, or letter to the editor (6 records).
population does not meet protocol: no adults with cancer (4 records).
study design does not meet protocol: other than RCT and NRCT (15 records).
outcomes do not meet protocol, but are interesting and related: care outcomes (8 records).
language does not meet protocol: record not in English, French, or Dutch (1 record).
We included studies irrespective of their publication status, reporting, or availability of outcome data (as per MECIR standards).
See Characteristics of excluded studies table.
Risk of bias in included studies
We have presented our 'Risk of bias' judgements separately for RCTs and NRCTs in the 'Risk of bias' tables (see Characteristics of included studies table for RCTs and Table 9 for NRCTs) and visualised for each study in the 'Risk of bias' summaries (see Figure 2; Figure 3). Our judgements about each 'Risk of bias' domain presented as percentages across all included studies are displayed in the 'Risk of bias' graphs (see Figure 4).
7. Risk of bias tables for NRCTs (judged with ROBINS‐I).
| Bramsen 2008 | ||
| Bias | Authors’ judgement | Support for judgement |
| Bias due to confounding | MODERATE RISK | Confounding possible, but not more than we would expect in an RCT on this topic. |
| Bias in selection of participants into the study | LOW RISK | A sequential cohort design was used with first a usual care group (n = 50, during 15 weeks), followed by a screening group (n = 79, 24 weeks). |
| Bias in classification of interventions | NO INFORMATION | Not everyone in the experimental group received the screening interview, only the participants that wanted to talk to a psychosocial worker. When experimental and control groups are compared in Tables 2 and 3, no numbers of participants in each condition are specified, which makes it difficult to know which intervention(s) played a role in potential group differences. |
| Bias due to deviations from intended intervention | NO INFORMATION | There were no deviations in the screening interview intervention mentioned. |
| Bias due to missing data | SERIOUS RISK | Dropout from baseline to 4 weeks' postdischarge: 44% in control phase, 65% in intervention phase; reasons for dropout: death, worsened health condition. It is unclear on what number of participants the analysis for each outcome is based. |
| Bias in measurement of outcomes | MODERATE RISK | Validated PRO's are used to measure the subjective outcomes in both conditions. No extra person for outcome assessment aware of condition allocation. |
| Bias in selection of the reported result | LOW RISK | The outcome measurements and analyses are clearly defined, and there is no indication of selection of the reported analysis from among multiple analyses, and no indication of selection of the cohort or subgroups for analysis and reporting on the basis of the results. |
| OVERALL RISK OF BIAS | SERIOUS RISK study | |
| de Leeuw 2013 | ||
| Bias | Authors’ judgement | Support for judgement |
| Bias due to confounding | SERIOUS RISK | Confounding possible, QoL scores at baseline differ substantially between conditions. |
| Bias in selection of participants into the study | LOW RISK | The study employed a sequential cohort design with an initial cohort of consecutive patients that formed a usual care control group, and after a "wash out" period of 5 months the cohort of the experimental arm was recruited. |
| Bias in classification of interventions | LOW RISK | The classification of interventions is clear. |
| Bias due to deviations from intended intervention | NO INFORMATION | There were no deviations in the screening interview intervention mentioned. |
| Bias due to missing data | SERIOUS RISK | Dropout of participants from baseline to 12 months' assessment +/‐ 22%; non‐responses evenly distributed between both conditions. It is unclear on what number of participants the analysis for each outcome is based. |
| Bias in measurement of outcomes | MODERATE RISK | Validated PRO's are used to measure the subjective outcomes in both conditions. No extra person for outcome assessment aware of condition allocation. |
| Bias in selection of the reported result | LOW RISK | The outcome measurements and analyses are clearly defined, and there is no indication of selection of the reported analysis from among multiple analyses, and no indication of selection of the cohort or subgroups for analysis and reporting on the basis of the results. |
| OVERALL RISK OF BIAS | NO INFORMATION enough to estimate the risk of the study (highest is 'low risk'). | |
| Hilarius 2008 | ||
| Bias | Authors’ judgement | Support for judgement |
| Bias due to confounding | MODERATE RISK | Confounding possible, but not more than we would expect in an RCT on this topic. |
| Bias in selection of participants into the study | LOW RISK | The study employed a sequential cohort design with an initial cohort of 100 consecutive patients that formed the usual care control group, and after a "wash out" period of 2 months the cohort of the experimental arm was recruited. |
| Bias in classification of interventions | LOW RISK | The classification of interventions is clear. |
| Bias due to deviations from intended intervention | NO INFORMATION | There were no deviations in the screening interview intervention mentioned. |
| Bias due to missing data | SERIOUS RISK | Dropout of participants from baseline to 13 and 14 months' assessment +/‐ 27%; non‐responses evenly distributed between both conditions; the 2 most common reasons for dropout were death and cessation of treatment. |
| Bias in measurement of outcomes | MODERATE RISK | For both conditions subjective outcomes were measured with validated and self‐adjusted PRO's. No extra person for outcome assessment aware of condition allocation. |
| Bias in selection of the reported result | LOW RISK | The outcome measurements and analyses are clearly defined, and there is no indication of selection of the reported analysis from among multiple analyses, and no indication of selection of the cohort or subgroups for analysis and reporting on the basis of the results. |
| OVERALL RISK OF BIAS | NO INFORMATION enough to estimate the risk of the study (highest is 'low risk'). | |
| Taenzer 2000 | ||
| Bias | Authors’ judgement | Support for judgement |
| Bias due to confounding | MODERATE RISK | Confounding possible, but not more than we would expect in an RCT on this topic. |
| Bias in selection of participants into the study | LOW RISK | A sequential recruitment design was used to recruit participants during the study period, first for the control group (approximately 25), then for the experimental group (approximately 25). |
| Bias in classification of interventions | LOW RISK | The classification of interventions is clear. |
| Bias due to deviations from intended intervention | NO INFORMATION | No information is reported on whether there is a departure from the intended intervention. |
| Bias due to missing data | LOW RISK | Only 1 outcome time point, so no potential missing data due to loss in follow‐up. "Complete data for 26 participants in the control group and 27 in the experimental group, which were used for all analyses." |
| Bias in measurement of outcomes | MODERATE RISK | The outcomes were measured by an independent research assistant for both conditions, not by clinic staff. |
| Bias in selection of the reported result | LOW RISK | The outcome measurements and analyses are clearly defined, and there is no indication of selection of the reported analysis from among multiple analyses, and no indication of selection of the cohort or subgroups for analysis and reporting on the basis of the results. |
| OVERALL RISK OF BIAS | NO INFORMATION enough to estimate the risk of the study (highest is 'low risk'). | |
| Thewes 2009 | ||
| Bias | Authors’ judgement | Support for judgement |
| Bias due to confounding | SERIOUS RISK | All nursing and psychosocial staff participated in a 2‐hour training session on the rationale for screening, the screening instrument, and the study procedure before the study started. This potentially influenced the alertness to and management of psychosocial concerns in both conditions, with the the potential to influence outcomes for both conditions. |
| Bias in selection of participants into the study | LOW RISK | Study authors mention 2 waves of data collection from consecutive patients: an unscreened cohort and a screened cohort. |
| Bias in classification of interventions | LOW RISK | The classification of interventions is clear. |
| Bias due to deviations from intended intervention | MODERATE RISK | 7 out of 19 participants that reported scores on the DT above the cut‐off did not receive referral because of vacancies of social workers or psychologists (n = 4), clinic staff not being able to contact the participant (n = 1), or unstated reason (n = 2). It is possible that these people were left with unmet care needs despite the use of screening because there was no action in response to the screening results, while this was the case for 10 of the 19 participants in the experimental condition. |
| Bias due to missing data | SERIOUS RISK | Dropout of participants from baseline to 6 months' assessment +/‐ 22%; reasons for dropout: withdrawal and death. Participant characteristics are based on n = 83, however 16 participants died and 2 withdrew during the study period/follow‐up. Since the sample size on which the analysis is based on it is not clear , we assume that they included the records from participants with missing data. |
| Bias in measurement of outcomes | MODERATE RISK | Validated PRO's are used to measure the subjective outcomes in both conditions. No extra person for outcome assessment aware of condition allocation. |
| Bias in selection of the reported result | LOW RISK | The outcome measurements and analyses are clearly defined, and there is no indication of selection of the reported analysis from among multiple analyses, and no indication of selection of the cohort or subgroups for analysis and reporting on the basis of the results. |
| OVERALL RISK OF BIAS | SERIOUS RISK study | |
| Waller 2012 | ||
| Bias | Authors’ judgement | Support for judgement |
| Bias due to confounding | CRITICAL RISK | Substantial deviations from the intended intervention are present and are not adjusted for in the analysis: "control group" (baseline) is much unhealthier (QoL, depression) than "intervention groups" (2 months', 4 months', 6 months' follow‐up). |
| Bias in selection of participants into the study | LOW RISK | A sequential recruitment design was used to include eligible participants. The same approach for inclusion was used in the 2 study phases (first 3 months as intervention group phase, and second 3 months as control group phase). |
| Bias in classification of interventions | LOW RISK | The classification of interventions is clear. |
| Bias due to deviations from intended intervention | MODERATE RISK | A separate publication reports a fidelity (NAT:PD‐C due that were actually completed) of 83%. |
| Bias due to missing data | SERIOUS RISK | A strong variation in sample size across all time points: T‐3 (n = 70); T‐2 (n = 122); T‐1 (n = 160); T0 (n = 192); T1 (n = 103); T2 (n = 85); T3 (n = 67), so the results are not always based on the same sample (dropout of +/‐ 30%). Proportions of missing data differ substantially between "control" and "intervention" AND the nature of the missing data means that risk of bias cannot be removed. |
| Bias in measurement of outcomes | MODERATE RISK | Trained interviewers (not part of the clinical team) telephoned participants every 2 months during the study period to undertake a computer‐assisted interview on the subjective outcome variables. |
| Bias in selection of the reported result | LOW RISK | There is clear evidence that all reported results correspond to all intended outcomes, analyses, and subcohorts (Waller 2010). |
| OVERALL RISK OF BIAS | CRITICAL RISK study | |
| Williams 2013 | ||
| Bias | Authors’ judgement | Support for judgement |
| Bias due to confounding | LOW RISK | Non‐randomised design, but no real confounding expected + thoroughly controlled for potentially confounding factors. |
| Bias in selection of participants into the study | LOW RISK | A sequential recruitment design was used to include eligible participants. The same approach for inclusion was used in the 2 study phases. |
| Bias in classification of interventions | LOW RISK | The classification of interventions is clear. |
| Bias due to deviations from intended intervention | SERIOUS RISK | Problems with implementation fidelity are apparent (amount of screening interventions/outcome measurements ranged from 2 to 11). |
| Bias due to missing data | SERIOUS RISK | Dropout of +/‐ 12%. |
| Bias in measurement of outcomes | NO INFORMATION | Unclear information on outcome assessment (even the 6 items of the HRQoL tool are never mentioned), only tools and timing are mentioned, not who performs assessment, paper/digital. |
| Bias in selection of the reported result | SERIOUS RISK | Results of generalised estimating equations analysis of HRQoL‐LASA on covariates is mentioned, no information about the scores of the items on the HRQoL‐LASA itself. |
| OVERALL RISK OF BIAS | SERIOUS RISK study | |
| Young 2010 | ||
| Bias | Authors’ judgement | Support for judgement |
| Bias due to confounding | NO INFORMATION | No information on missing data, but also no smaller numbers of participants mentioned in the outcome tables than the 20 intervention and 21 control participants mentioned in the section on participants. |
| Bias in selection of participants into the study | LOW RISK | A sequential recruitment design was used to include eligible participants. The same approach for inclusion was used in the 2 study phases (first 3 months as intervention group phase, and second 3 months as control group phase). |
| Bias in classification of interventions | LOW RISK | The classification of interventions is clear. |
| Bias due to deviations from intended intervention | SERIOUS RISK | 1) The control condition followed in time after the intervention condition. The routine of screening and discussion of participant needs during the 'intervention phase' can influence the behaviour and way of working of the interventionist in the 'control phase'. Consequently, the 'usual care' in the control phase is possibly influenced by this and is no longer usual care; 2) not all follow‐up calls of the CONNECT intervention could be done successfully for all participants. |
| Bias due to missing data | NO INFORMATION | No information on missing data, but also no smaller numbers of participants mentioned in the outcome tables than the 20 intervention and 21 control participants mentioned in the section on participant characteristics, so outcomes are probably based on all participants. |
| Bias in measurement of outcomes | NO INFORMATION | Risk of bias seems to be low, the outcomes were measured by an independent researcher that differs from the intervention nurse, but it is not clear if the independent researcher was aware of participants' allocation to intervention or control condition. |
| Bias in selection of the reported result | LOW RISK | Reporting of the results is rather complete, only P values were missing in the results section on psychological distress. The outcome measurements and analyses are clearly defined, and there is no indication of selection of the reported analysis from among multiple analyses, and no indication of selection of the cohort or subgroups for analysis and reporting on the basis of the results. |
| OVERALL RISK OF BIAS | SERIOUS RISK study | |
DT: Distress Thermometer
HRQOL: health related quality of life
HRQOL‐LASA: Health‐Related Quality of Life Linear Analogue Self‐assessment
NAT:PD‐C: Needs Assessment Tool Progressive Diseased Cancer
PRO: patient reported outcome
RCT: randomized clinical trial
QOL: quality of life
2.
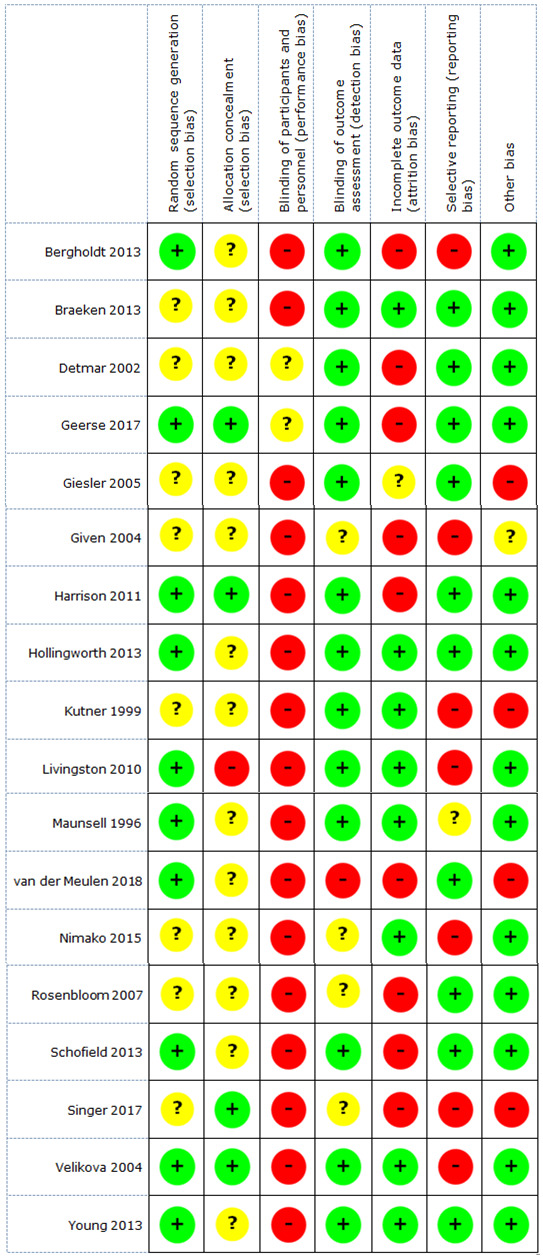
'Risk of bias' summary RCT: review authors' judgements about each risk of bias item for each included RCT
3.
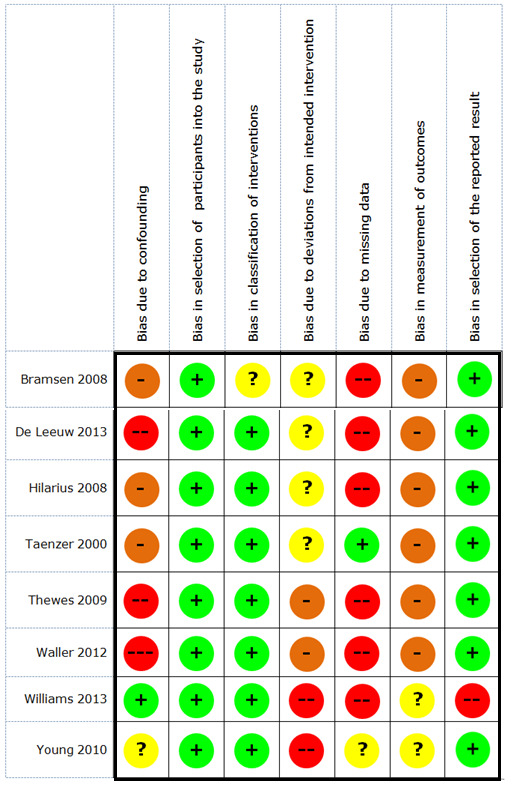
'Risk of bias' summary NRCT: review authors' judgements about each risk of bias item for each included NRCT
4.
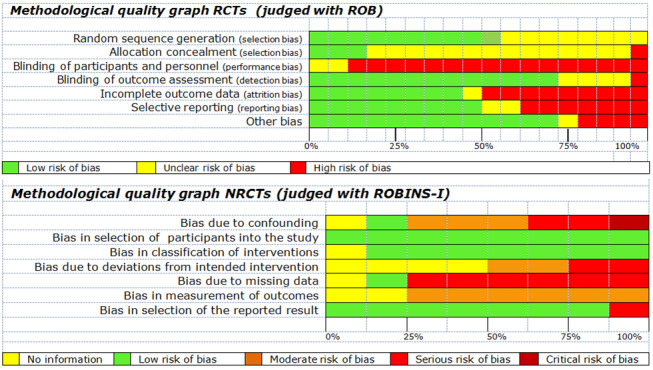
'Risk of bias' graphs: review authors' judgements about each risk of bias item presented as percentages across all included studies
Randomised controlled trials
Our 'Risk of bias' judgements using the Cochrane tool for assessing risk of bias for the 18 included RCTs are shown in Figure 2. Based on sequence generation, blinding of outcome assessors, and reporting on outcomes, we labelled eight studies as 'high risk of bias' studies, Bergholdt 2013; Given 2004; Kutner 1999; Livingston 2010; Nimako 2015; Singer 2017; van der Meulen 2018; Velikova 2004, and five studies as ‘low risk of bias' studies (Geerse 2017; Harrison 2011; Hollingworth 2013; Schofield 2013; Young 2013). We could make no general 'Risk of bias' judgement for five studies as they were assessed as at unclear risk of bias for several domains (Braeken 2013; Detmar 2002; Giesler 2005; Maunsell 1996; Rosenbloom 2007).
Allocation
Sequence generation
Ten RCTs specified the generation of random sequences. Maunsell 1996 and Velikova 2004 generated the sequence with a random number table, and the other eight studies used computer‐generated randomisation schedules (Bergholdt 2013; Geerse 2017; Harrison 2011; Hollingworth 2013; Livingston 2010; Schofield 2013; van der Meulen 2018; Young 2013). The method for sequence generation was unclear for the other eight studies.
Allocation to conditions
In five studies, condition allocation was done by an independent researcher or administrative worker not involved in the clinical care (Geerse 2017; Harrison 2011; Maunsell 1996; Singer 2017; Velikova 2004). In Livingston 2010 there was a risk of bias related to allocation, since the study co‐ordinator and referring specialist were aware of group allocation. Allocation concealment was not specified for the other 12 studies.
Blinding
Blinding of participants and personnel
Due to the nature of the intervention, blinding of participants and personnel was difficult. Consequently, there was a high risk of performance bias in all studies. In three studies participants were blinded and personnel were not (Braeken 2013; Detmar 2002; Velikova 2004). In the other studies neither participants nor healthcare professionals were blinded.
Blinding of outcome assessors
Thirteen studies clearly stated the blinding of outcome assessors, or used self‐report measures to collect outcome data from people with cancer directly. We labelled these studies as at low risk of detection bias, since there was no extra person aware of the condition that could bias the outcome assessment. Four studies did not provide clear information on blinding of outcome assessors (Given 2004; Nimako 2015; Rosenbloom 2007; Singer 2017). In van der Meulen 2018, the person analysing the outcomes was aware of the condition the participants were in.
Incomplete outcome data
Kutner 1999 provided insufficient information to judge attrition bias. In six other studies, dropout rates were low (≤ 15%) and comparable in both conditions, with reasons for dropout reported (Braeken 2013; Hollingworth 2013; Livingston 2010; Maunsell 1996; Nimako 2015; Young 2013). We considered 11 studies to be at high risk of attrition bias due to high dropout (> 15%) (Bergholdt 2013; Detmar 2002; Geerse 2017; Giesler 2005; Given 2004; Harrison 2011; Rosenbloom 2007; Schofield 2013; Singer 2017; van der Meulen 2018; Velikova 2004).
Selective reporting
We judged 10 studies to have a low risk of reporting bias as all prespecified outcomes were reported in the results of the paper or in supplementary files. For several outcomes, Kutner 1999 and Velikova 2004 only reported on significant subscales with P values without further information on group outcomes or spread of data, and were therefore judged as being at high risk of bias for this domain. We found indications of selective reporting due to a lack of reporting on certain outcomes for one or more conditions in Bergholdt 2013, Livingston 2010, Maunsell 1996, Nimako 2015, and Singer 2017, which we therefore considered as at high risk of reporting bias. In Given 2004, the data on patient characteristics were limited, and there was no clear presentation of the concrete data on depression or severity of problems in people with cancer, therefore we judged this study to be at high risk of reporting bias.
Other potential sources of bias
We identified three studies as having other potential sources of bias. In Giesler 2005, no adjustments for multiple comparisons were made, implying that the few positive results have a high risk of type I errors. It is not clear whether the different models composed in Given 2004 are post hoc analyses, or whether they were planned in advance. In the former case, this would have induced high risk of bias for this study. Kutner 1999 reported that adjusted P values were reported to adjust for clustering. However, no information was provided on how this adjustment was done. There is a huge difference in baseline characteristics, which according to the study authors is the result of clustering at the physician level. These differences lose statistical significance when clustering is taken into account. In our opinion, this is problematic and potentially induces bias. In Singer 2017, there is a clear imbalance present between the intervention and the control group with respect to gender, type of cancer, stage of cancer, and likely other unknown factors as well, including the conclusions made. The study of van der Meulen 2018 was underpowered. Additionally, the objective to screen people with cancer three to four times a year (intervention protocol) was not met, potentially biasing the results.
Non‐randomised controlled trials
Our assessment of risk of bias using the ROBINS‐I tool in the eight included NRCTs is shown in Table 9 and Figure 3. Based on the criteria formulated in the Methods section, we labelled one study as a 'critical risk' study (Waller 2012), and six as ‘serious risk' studies (Bramsen 2008; de Leeuw 2013; Hilarius 2008; Thewes 2009; Williams 2013; Young 2010). There was not enough information available to make an overall judgement regarding risk of bias for the remaining NRCT (Taenzer 2000).
Bias due to confounding
Only Williams 2013 was labelled as having a low risk of bias due to confounding, since no real bias in this domain was expected, and the study design thoroughly controlled for potentially confounding factors (baseline HRQoL, education level, age, gender, disease stage, treatment type, days postbaseline). We judged four studies as at moderate risk of bias due to confounding since confounding was possible, but not more than we would expect in an RCT on this topic (Bramsen 2008; Hilarius 2008; Taenzer 2000; Young 2013). In de Leeuw 2013, quality of life scores at baseline differed significantly between conditions, making the chance for confounding likely. All nursing and psychosocial staff in Thewes 2009 participated in training sessions before the study started, which potentially influenced the alertness to and management of psychosocial concerns in both conditions. We found evidence for critical risk of bias due to confounding in one study (Waller 2012): substantial deviations from the intended intervention were present and not adjusted for in the analysis, and baseline characteristics between the control group and intervention groups differed significantly.
Bias in selection of participants into the study
We judged all included NRCTs as having a low risk of bias in selection of participants. Sequential recruitment designs were used to include eligible participants. Each time, the same approach for inclusion was used in the intervention and control phase of the studies.
Bias in classification of interventions
For seven studies the intervention of interest was clear and distinct from the control condition, therefore we assessed these studies as at low risk of bias for classification of interventions. There was no clear information to judge risk of bias for this domain only for Bramsen 2008. Not all participants in the experimental group received the screening interview, that is only the people with cancer who wanted to talk to a psychosocial worker. When experimental and control groups were compared, it was difficult to know which intervention(s) played a role in potential group differences. Consequently, we judged this study to have an unclear risk of bias for classification of interventions.
Bias due to deviations from intended intervention
Four studies reported no information on whether there was a deviation from the intended intervention (Bramsen 2008; de Leeuw 2013; Hilarius 2008; Taenzer 2000), and were therefore classified as having an unclear risk of bias due to deviations from intended intervention. In two studies a certain percentage of participants did not receive the screening intervention as planned (Thewes 2009; Waller 2012), therefore we judged these studies as having a moderate risk of bias due to deviations from the intended intervention. We found evidence for serious risk of bias in Williams 2013, since problems with implementation fidelity were apparent (amount of screening interventions/outcome measurements ranged from 2 to 11).
Bias due to missing data
Young 2010 provided insufficient information to assess bias due to missing data and was therefore classified as having an unclear risk of bias for this domain. In two other studies, dropout rates were low (≤ 15%) and comparable in both conditions, with reasons for dropout reported (Taenzer 2000; Williams 2013); we judged these studies to have a low risk of bias due to missing data. We considered five NRCTs to be at high risk of attrition bias due to high dropout (> 15%) (Bramsen 2008; de Leeuw 2013; Hilarius 2008; Thewes 2009; Waller 2012).
Bias in measurement of outcomes
Williams 2013 and Young 2013 provided insufficient information on outcome assessment to judge bias in measurement of outcomes. Six studies used validated PROMs or trained interviewers (not part of the clinical team) to measure outcomes, resulting in an assessment of low risk of bias for this domain (Bramsen 2008; de Leeuw 2013; Hilarius 2008; Taenzer 2000; Thewes 2009; Waller 2012).
Bias in selection of the reported results
In seven studies the outcome measurements and analyses were clearly defined: there were no indications of selective reporting, data dredging, or biased selection of the study cohort or subgroups (Bramsen 2008; de Leeuw 2013; Hilarius 2008; Taenzer 2000; Thewes 2009; Young 2010). For Waller 2012, there was concrete evidence that the reporting of results was complete. We therefore considered all these NRCTs as having a low risk of bias in selection of the reported results. Results of generalised estimating equations analysis of HRQoL‐LASA on covariates were mentioned in Williams 2013, however no information about the scores of the HRQoL‐LASA items itself was presented, leading to a serious risk of bias in selection of the reported results.
Effects of interventions
See: Table 1
In this section we have described the evidence resulting from the quantitative and qualitative analysis of the included studies.
A meta‐analysis with all included studies was not possible due to considerable heterogeneity in intervention characteristics, outcome measures, outcome time points, and variation in methodological certainty. We have narratively described the effects of interventions for 23 studies. We combined the other three included studies in a meta‐analysis.
We have provided an Evidence Summary with all the results of these studies for the outcomes that fell within the scope of this review (Table 5; Table 6; Table 7).
Narrative description of evidence
The evidence for the studied screening and assessment interventions of 23 studies not included in meta‐analysis is narratively described for all the outcomes of primary and secondary interest for this review (qualitative analysis).
Evidence for outcomes of primary interest
HRQoL
Of the 17 studies (4882 people) that focused on HRQoL, one did not report outcomes for HRQoL (Singer 2017), and eight did not detect an effect of the screening intervention on the HRQoL of people with cancer (Bergholdt 2013; Detmar 2002; Geerse 2017; Hilarius 2008; Nimako 2015; Rosenbloom 2007; van der Meulen 2018; Waller 2012). Eight other studies did find evidence for negative and/or positive effects on one or more domains of HRQoL.
In the study of Braeken 2013, a negative effect was found for ‘role functioning’ (one of the five EORTC QLQ‐C30 functional scales) after three months, in a three‐way interaction model including intervention, time of referral, and time of measurement. However, the raw data did not show large absolute differences between intervention and control groups, both after 3 months (mean difference (MD) −0.10, 95% confidence interval (CI) −4.83 to 4.63; 568 participants) and after 12 months (MD −2.18, 95% CI −6.42 to 2.06; 568 participants).
de Leeuw 2013 found no difference between groups in the EORTC QLQ‐C30 and EORTC QLQ‐H&N35 data at six and 12 months. Nevertheless, the study authors claimed to have found a beneficial effect, since changes in time compared to baseline were significantly larger in the intervention group for three out of five functional scales (physical functioning, role functioning, social functioning); for global health status/quality of life; for six out of nine symptom scales (fatigue, nausea/vomiting, pain, dyspnoea, appetite loss, constipation); and for nine out of 18 head and neck scales (pain, swallowing, senses, speech, social eating, opening mouth, coughing, use of nutritional supplements, weight loss) at six and 12 months. However, the intervention condition had significantly worse scores than the control condition at baseline, compromising the comparability between groups.
Giesler 2005 found no evidence for effects on the SF‐36. However, in the same study positive effects were detected with the PCQoL in one out of the 10 domains at each time point: ‘sexual functioning’ at four months (MD 9.40, 95% CI 1.22 to 17.58; 98 participants); ‘sexual limitations’ at seven months (MD 6.88, 95% CI 0.28 to 13.48; 85 participants); and ‘sexual limitations’ (MD 9.24, 95% CI 1.39 to 17.09; 85 participants) and ‘cancer worry’ (MD 11.08, 95% CI 1.78 to 20.38; 85 participants) at 12 months.
A positive effect on ‘physical functioning’ (1 of the 5 EORTC functioning scales) was measured in Hollingworth 2013 when combining measurements at 1, 6, and 12 months. The mean differences at the individual time points did not differ significantly: 1 month: 1.20 (95% CI −4.32 to 6.72; 209 participants); 6 months: 0.40 (95% CI −4.70 to 5.50; 209 participants); and 12 months: −1.70, 95% CI −6.73 to 3.33; 209 participants). Also, no evidence for effects was found with the EQ‐5D‐3L after 12 months: −0.17, 95% CI −0.45 to 0.10; 209 participants.
The tests of group differences on the five EORTC functional scales at follow‐up assessments were not significant in Schofield 2013. Yet, analysis of change scores indicated between‐group differences on the ‘physical functioning scale’ at 8 weeks (MD 5.38, 95% CI −5.37 to 16.50; effect size 0.23); and on ‘social functioning’ (MD 6.42, 95% CI −5.54 to 18.37; ES 0.24) and ‘role functioning’ at 12 weeks (MD 10.61, 95% CI −4.61 to 25.83; ES 0.32), in favour of the intervention condition. The authors state that these differences indicated greater declines in functioning in the intervention group.
Velikova 2004 observed positive effects of their screening intervention on the FACT‐G total, and on three of four subscales: physical, emotional, and functional well‐being scales (no raw data available).
The EORTC QLQ‐C30 assessment in Bramsen 2008 revealed positive effects for the domain of 'role functioning' (1 out of 5 functioning scales; MD 17.26, 95% CI 0.61 to 33.91; 56 participants) and 'pain' (1 out of 9 symptom scales; MD 20.83, 95% CI 5.99 to 35.67; 56 participants) at four weeks' postbaseline.
Williams 2013 detected a positive effect on HRQoL measured with the HRQoL‐LASA after four months (no raw data available).
For detailed information we refer to the Evidence Summary (Table 5; Table 6; Table 7).
Distress
In 12 of the 14 studies (4780 people) that included distress as an outcome measure, no effect of the intervention on this outcome was detected (Bergholdt 2013; Braeken 2013; Geerse 2017; Giesler 2005; Given 2004; Hollingworth 2013; Livingston 2010; Maunsell 1996; Rosenbloom 2007; Singer 2017; van der Meulen 2018; Waller 2012).
At four weeks' postbaseline, Bramsen 2008 found a significantly higher score in the intervention condition on the ‘positive sub scale’ of the GHQ‐12 (MD −1.77, 95% CI −2.63 to −0.91; 56 participants). No difference in score was found for the 'negative sub scale' or the GHQ Total score (MD −0.95, 95% CI −1.82 to −0.08; 56 participants).
There were no significant group differences on the HADS‐Total (8 weeks: MD −0.38, 95% CI −3.52 to 2.76; 108 participants and 12 weeks: MD 1.18, 95% CI −2.09 to 4.45; 108 participants) and DT (8 weeks: MD −0.45, 95% CI −1.45 to 0.55; 108 participants and 12 weeks: MD −0.14, 95% CI from −1.21 to 0.93; 108 participants) at follow‐up assessments in Schofield 2013. However, change score analysis indicated better scores for the intervention condition at 8 weeks in both measures.
For detailed information we refer to the Evidence Summary (Table 5; Table 6; Table 7).
Care needs
Of the 4 studies (1461 people) that assessed care needs as an outcome, one study did not present any data on this outcome (Bergholdt 2013), and the remaining three studies detected positive or negative effects of the screening intervention they studied.
The study of Thewes 2009 revealed negative effects, since the intervention group reported higher levels of ‘overall unmet needs’, ‘psychological needs’, ‘information needs’, and ‘physical and daily living needs’ (three out of five Supportive Care Needs Survey short‐form (SCNS‐SF) scales) compared to the control group after six months (no raw data available).
Using the same outcome measure, Waller 2012 found a positive effect of their intervention in terms of a lower proportion of people with cancer scoring at least one moderate or high need on two out of five subscales: ‘health system and information needs’ (risk ratio (RR) 0.58, 95% CI 0.32 to 1.05; 259 participants) and ‘care and support needs of people with cancer’ (RR 0.33, 95% CI 0.10 to 1.06; 259 participants) at follow‐up of approximately six months.
In Schofield 2013, change score analysis of the NA‐ALCP data indicated a relative benefit from the intervention for unmet ‘symptom needs’ at 8 weeks (MD −0.25, 95% CI −0.52 to 0.02; 108 participants) and 12 weeks (MD −0.19, 95% CI −0.48 to 0.10; 108 participants) postassessment (1 out of 6 subscales).
For detailed information we refer to the Evidence Summary (Table 5; Table 6; Table 7).
Adverse events
None of the included studies specified adverse events as an outcome, and no spontaneous findings on this were reported. However, three studies reported unfavourable effects of the intervention on certain components of HRQoL (Braeken 2013), care needs of people with cancer (Thewes 2009), and cancer patient satisfaction (Kutner 1999).
Evidence for outcomes of secondary interest
Satisfaction
No evidence for an effect of the screening interventions on participants’ satisfaction was found in eight of the 12 studies that focused on this outcome (Bergholdt 2013; Braeken 2013; Geerse 2017; Hilarius 2008; Hollingworth 2013; Rosenbloom 2007; Taenzer 2000; Velikova 2004). In one study, satisfaction was only an outcome for the intervention group (van der Meulen 2018), while for another study, data for this outcome were not reported (Singer 2017). The two remaining studies showed positive or negative effects as detailed below.
With the PSQ, Detmar 2002 detected more 'satisfaction with emotional support received' in the intervention group at the fourth follow‐up visit (1 out of 5 domains) (no raw data available).
On the other hand, Kutner 1999 found that compared to the control group, the intervention group experienced significantly lower levels of ‘satisfaction on time spent with the physician’ (MD −0.17, 95% CI −0.33 to −0.01; 282 participants) and ‘satisfaction on how physicians’ explanation what was done for the patient’ (MD −0.16, 95% CI −0.29 to −0.03; 282 participants) (the five‐item Medical Outcomes Study Patient Visit Rating Questionnaire).
For detailed information we refer to the Evidence Summary (Table 5; Table 6; Table 7).
Psychosocial well‐being
For other concepts of psychosocial well‐being addressed in the included studies, namely marital well‐being (Giesler 2005; Maunsell 1996), health and activity limitation (Maunsell 1996), impact of stressful life events (Bramsen 2008), psychosocial adjustment (de Leeuw 2013), psychiatric comorbidity (Singer 2017), and fear of cancer recurrence (van der Meulen 2018), no effects of the screening interventions were found.
For detailed information we refer to the Evidence Summary (Table 5; Table 6; Table 7).
Results of meta‐analysis
Of the 26 included studies, we considered two RCTs and one NRCT to be sufficiently homogeneous to pool for meta‐analysis (Harrison 2011; Young 2010; Young 2013).
These studies comprised a nurse‐led intervention where people with cancer who had undergone surgery for colorectal cancer were contacted on a regular basis by phone to discuss their supportive care needs, known as the CONNECT intervention. The first paper by this research group reports a non‐randomised feasibility study (Young 2010), followed by a single‐centre pilot RCT and a large‐scale, multicentre RCT (Harrison 2011; Young 2013). The outcomes investigated in these studies were HRQoL (measured with the FACT‐C scale in all three studies), distress (measured with the DT in Young 2010 and Young 2013), and supportive care needs (measured with the SCNS in all three studies, and at six months with the CaSUN in Harrison 2011).
The NRCT, Young 2010, had follow‐up measurements at one and three months after surgery, while the RCTs, Harrison 2011; Young 2013, measured their outcomes at one, three, and six months after surgery.
Unfortunately, due to differences between studies in reporting (e.g. only subscale scores for SCNS reported in the NRCT versus only total scores in the RCT) and timing of outcome measurements (e.g. supportive care needs measured at one, three, and six months in Harrison 2011 versus only at three and six months in Young 2013), we were not able to combine all reported outcomes. We therefore refer to the Evidence Summary (Table 5; Table 6; Table 7) for full details of all results.
HRQoL
At one‐month postsurgery, none of the studies found a significant effect of the intervention on global health status, leading to an overall MD for the RCTs of 0.02 (95% CI −2.55 to 2.60; P = 0.99; 2 trials; 775 participants; Analysis 1.1) (Figure 5). No heterogeneity was detected (I2 = 0%). The MD for the NRCT was 6.60 (95% CI −4.27 to 17.47; P = 0.23; 1 trial; 41 participants).
1.1. Analysis.
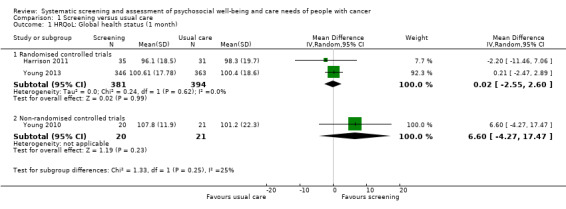
Comparison 1 Screening versus usual care, Outcome 1 HRQoL: Global health status (1 month).
5.
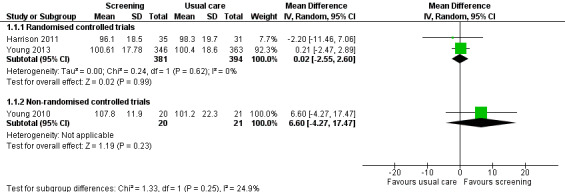
Forest plot of comparison: 1 Screening versus usual care, outcome: 1.1 HRQoL: Global health status (1 month)
We also did not find a significant difference in global health status between the screening intervention and usual care at three months after surgery, with an MD for the RCTs of 0.29 (95% CI −2.38 to 2.95; P = 0.02; 2 trials; 750 participants; Analysis 1.2) (Figure 6). No heterogeneity was detected (I2 = 0%). The NRCT did show a beneficial effect of the screening intervention compared to usual care for HRQoL at three months after surgery, with an MD of 12.70 (95% CI 2.61 to 22.79; P = 0.02; 1 trial; 41 participants).
1.2. Analysis.
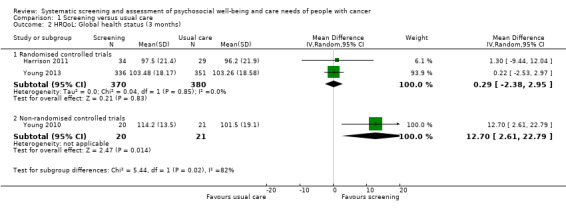
Comparison 1 Screening versus usual care, Outcome 2 HRQoL: Global health status (3 months).
6.
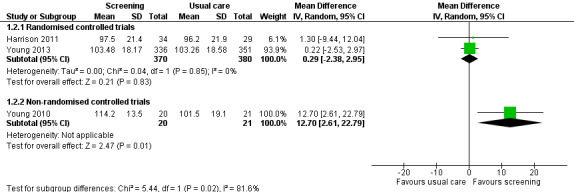
Forest plot of comparison: 1 Screening versus usual care, outcome: 1.2 HRQoL: Global health status (3 months)
The RCTs assessed HRQoL at six months after surgery only (Harrison 2011; Young 2013), and no significant effect of the screening intervention was found, with an MD of 1.65 (95% CI −4.83 to 8.12; P = 0.62; 2 trials; 730 participants; Analysis 1.3) (Figure 7). Heterogeneity between the two RCTs was moderate (I2 = 43%).
1.3. Analysis.
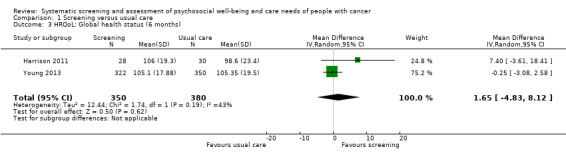
Comparison 1 Screening versus usual care, Outcome 3 HRQoL: Global health status (6 months).
7.
Forest plot of comparison: 1 Screening versus usual care, outcome: 1.3 HRQoL: Global health status (6 months).
Distress
Psychological distress was assessed by the NRCT, Young 2010, and the multicentre RCT, Young 2013, and did not differ at one month after surgery between the screening intervention and usual care condition, with an MD in DT score of −0.10 (95% CI −0.46 to 0.26; P = 0.33; 1 trial; 709 participants; Analysis 1.4) in the RCT and of −0.90 (95% CI −2.48 to 0.68; P = 0.26; 1 trial; 41 participants) in the NRCT (Figure 8). Distress also did not differ at three months, with an MD of 0.0 (95% CI −0.36 to 0.36; P = 1; 1 trial; 687 participants; Analysis 1.5) (Figure 9), or at six months, with an MD of 0.0 (95% CI −0.42 to 0.42; P = 1; 1 trial; 672 participants) (Table 9).
1.4. Analysis.
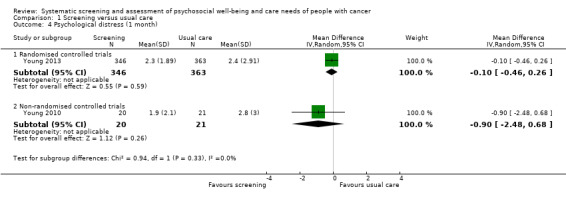
Comparison 1 Screening versus usual care, Outcome 4 Psychological distress (1 month).
8.
Forest plot of comparison: 1 Screening versus usual care, outcome: 1.4 Psychological distress (1 month)
1.5. Analysis.
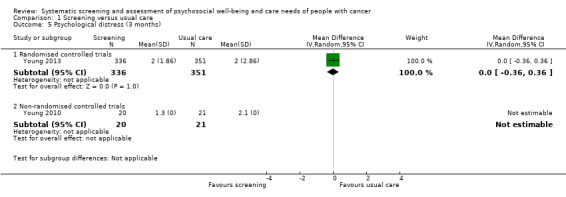
Comparison 1 Screening versus usual care, Outcome 5 Psychological distress (3 months).
9.
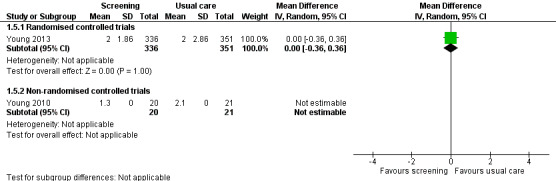
Forest plot of comparison: 1 Screening versus usual care, outcome: 1.5 Psychological distress (3 months)
Supportive care needs
The NRCT included in the meta‐analysis only reported SCNS subscores, and could not be pooled with the other two studies (Young 2010). Supportive care needs, assessed three months after surgery with the SCNS, were reported as a global level of unmet needs in the two RCTs (Harrison 2011; Young 2013), and this level did not differ between screening and usual care, with an MD of 2.32 (95% CI −7.49 to 12.14; P = 0.64; 2 trials; 748 participants; Analysis 1.6) (Figure 10). There was no important heterogeneity (I2 = 0%). This global level of unmet needs was measured at six months with the CaSUN in the pilot RCT (Harrison 2011), and with the SCNS in the multicentre RCT (Young 2013), leading to a standardised mean difference of 0.00 (95% CI −0.22 to 0.22; P = 0.99; 2 trials; 732 participants; Analysis 1.7) (Figure 11). There was no important heterogeneity between studies (I2 = 19%).
1.6. Analysis.
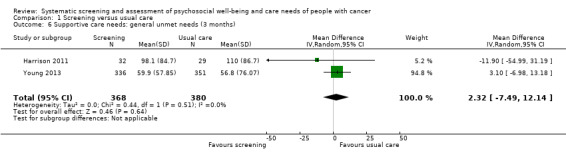
Comparison 1 Screening versus usual care, Outcome 6 Supportive care needs: general unmet needs (3 months).
10.
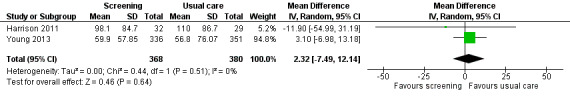
Forest plot of comparison: 1 Screening versus usual care, outcome: 1.6 Supportive care needs: general unmet needs (3 months)
1.7. Analysis.
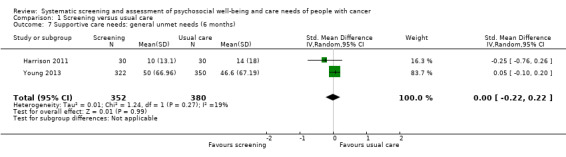
Comparison 1 Screening versus usual care, Outcome 7 Supportive care needs: general unmet needs (6 months).
11.
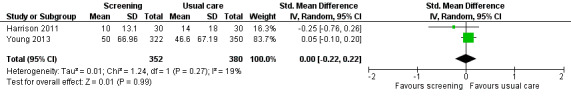
Forest plot of comparison: 1 Screening versus usual care, outcome: 1.7 Supportive care needs: general unmet needs (6 months)
Adverse events
None of the included studies specified adverse events as an outcome, and no spontaneous findings on this were reported. However, evidence from the included NRCT showed an unfavourable effect of the intervention on care needs for people with cancer (Young 2010). At three months after surgery, the intervention group had more unmet care needs in the domains of health and system information (MD 14.60, 95% CI 9.12 to 20.08; P < 0.001) and care and support for people with cancer (MD 9.00, 95% CI 4.09 to 13.91; P = 0.002)
Intervention effects and intervention characteristics
Due to variations in the interventions and the very low certainty of the evidence, we were unable to identify any consistency between intervention characteristics and the effectiveness of these screening interventions.
Within the included studies that displayed a positive effect of the screening intervention on one or more of the outcomes of interest in this review (n = 9), the following characteristics were present:
HRQoL, distress, care needs, psychosocial problems, and overall well‐being were the focus of the screening intervention for three, two, three, one, and two of these studies, respectively;
five had an interventionist for the screening intervention, and four involved self‐reporting of people with a PROM;
four studied a solitary screening intervention, and five studied the effect of a screening intervention with guided actions;
seven provided training for screening interventionists and/or clinic staff to interpret the screening results, or to work with the screening results, and two did not;
four were RCTs and five were NRCTs.
Within the included studies that displayed a negative effect of the screening intervention on one or more of the outcomes of interest in this review (n = 5), the following characteristics were present:
distress, care needs, and psychosocial problems were the focus of the screening intervention for two, two, and one of these studies, respectively;
one had an interventionist for the screening intervention, and four involved self‐reporting of people with a PROM;
three studied a solitary screening intervention, and two studied the effect of a screening intervention with guided actions;
four provided training for screening interventionists and/or clinic staff to interpret the screening results, or to work with the screening results, and one did not;
three were RCTs and two were NRCTs.
Discussion
Summary of main results
The objective of this review was to assess the effect of screening of psychosocial well‐being and care needs of people with cancer. We found 26 eligible studies, of which 18 were RCTs and eight were NRCTs. Five outcomes falling within the scope of this review (HRQoL, distress, needs, satisfaction, and psychosocial well‐being) were addressed in several studies. However, there was considerable heterogeneity in intervention characteristics, measures, and time points for outcome assessment. We could include only three studies in a meta‐analysis, and their pooled estimates did not find evidence for the effectiveness of screening of psychosocial well‐being and care needs in people with cancer. The results of the 23 individual studies not suitable for meta‐analysis varied (Evidence Summary in Table 5; Table 6; Table 7). Some study authors spoke of clinical significance, however no statistically significant effects of the screening interventions were found in 12 studies. The results of 10 studies suggest beneficial effects of the intervention on the well‐being of people with cancer. Importantly, although none of the studies reported adverse events, five studies reported negative effects of screening such as decreased HRQoL, more unmet care needs, and lower patient satisfaction. We judged five RCTs as at low risk of bias. For the remaining studies we judged the risk of bias to be 'high', ‘serious’, or 'critical', or there was insufficient information to judge the methodological certainty of the study. This generally high risk of bias undermines the reliability of the results.
Consequently, the evidence reported in this review does not support the effectiveness of the studied screening interventions.
In addition to reviewing the effectiveness of psychosocial screening interventions, we reviewed different intervention characteristics of included studies (keeping the heterogeneity and low certainty of the evidence in mind) to identify the circumstances in which these interventions were effective. However, we could not detect systematic consistency of intervention characteristics and intervention results. Additionally, the study designs did not seem to coincide with the evidence found.
Overall completeness and applicability of evidence
Our broad search led to the inclusion of a wide variety of screening interventions, populations, and outcomes. We believe that the 26 studies and corresponding evidence that we have found with this systematic literature review are relevant for the review questions. All screening interventions studied are in line with the systematic screening of psychosocial well‐being (quality of life, distress) and care needs that is recommended and described in guidelines by several leading institutions (Accreditation Canada 2008; Holland 2011; IOM 2008; NCCN 2007). Efforts are made to implement these types of interventions in clinical practice spread around the world. We believe that the heterogeneity in intervention characteristics found in this review reflects the variety of psychosocial screening interventions that are used in clinical practice. Consequently, we presume that this systematic review of the available evidence has the potential to have external validity. However, in the light of research it would be better if there was less heterogeneity in the characteristics of the studied interventions, study designs, and characteristics of outcome measurement.
Given that systematic screening of psychosocial well‐being and care needs is widely recommended, we expected to find evidence for positive effects. However, we found limited indications for positive effects. Three possible explanations can be interpreted from this finding.
Firstly, the lack of positive effects could mean that the interventions studied were not effective. Several reasons could explain the lack of effectiveness observed. In several studies, the adherence to the intervention protocol was verified; in other studies this did not seem to be the case. This potential lack of verification of the intervention protocol raises the possibility that not all interventions were performed as intended. Additionally, when intervention conditions were compared to a usual care condition without any form of psychosocial screening, it was often unclear what 'usual care' implied. ‘Usual care’ can vary widely in clinical practice, which raises questions about the extent to which the intervention increased the psychosocial focus and care actions beyond normal routine care of the study settings.
Secondly, the lack of positive effects could also mean that, to be effective, the interventions should be targeted to populations at risk of experiencing elevated levels of distress or needs. We believe that the results of this review might accurately reflect the effectiveness of psychosocial screening interventions in the general population of people with cancer, but might not capture their effectiveness in populations at risk of experiencing elevated levels of needs or distress. The included studies recruited the newly diagnosed and people with advanced cancer, people undergoing surgery, chemotherapy, and radiation, and one study recruited patients with a specific type of cancer. However, earlier studies demonstrated that people that were younger, single, female, and had a worse clinical status, lower quality of life, and socioeconomic status experienced higher levels of distress and care needs compared to the general population of people with cancer (Hulbert‐Williams 2012; Lang 2017; McIllmurray 2001; Pauwels 2013; van Scheppingen 2011). Applying the studied screening interventions in these high‐risk populations may provide more insight into the targeted effectiveness of the intervention. This could clarify why de Leeuw 2013 did not find significant differences between the intervention and the control conditions in the mean outcome scores at six and 12 months, but found that the change score from baseline was significantly higher for the intervention group, that is people in the intervention group initially had a worse clinical status at baseline. We see that only Kutner 1999 recruited a relative young sample of participants (mean age 42 years). However, no positive effect of the intervention was found in this study. The mean age in the other included studies was above 50 years for the majority of participants, even above 60 years in some studies. Several of the other 'risk characteristics' appear in the description of the study samples, however no subgroup analyses were conducted to study the effect of the intervention in the subgroups of younger females, singles, and people with lower socioeconomic status.
Thirdly, the lack of positive effects could also mean that the appropriateness of outcome measures (subjective) should be reconsidered. As stated earlier, using subjective outcomes can introduce bias. It could therefore be valuable to include objective outcomes in future studies and reviews, in addition to subjective outcomes. In 12 included studies, objective measures were added with the number and/or types of referral (Braeken 2013; Bramsen 2008; Singer 2017), patient‐professional communication content and/or length (Detmar 2002; Taenzer 2000; Velikova 2004), information on psychosocial well‐being in patient file (Detmar 2002; Hilarius 2008; Taenzer 2000), healthcare professionals’ awareness of the patient’s well‐being (Detmar 2002; Hilarius 2008), and health service utilisation (Harrison 2011; Hollingworth 2013; Maunsell 1996; Nimako 2015; Singer 2017; Young 2013). In several studies evidence was found for a beneficial effect of psychosocial screening on one or more of these aspects (Bramsen 2008; Detmar 2002; Hilarius 2008; Maunsell 1996; Taenzer 2000).
Quality of the evidence
We identified 26 studies with a total of 7654 participants. Due to the nature of the studied intervention, important methodological limitations may have introduced different types of bias in the findings. For example, response bias may have been fuelled by the difficulty of blinding participants, and by the subjective nature of the outcomes used in the assessment of the intervention. Another key domain of quality weakness in RCTs as well as NRCTs was ‘bias due to missing data’ (> 40% of the studies) (Figure 4). In RCTs there was a lack of clarity on selection bias (> 40% of the studies), and for 50% of the NRCTs the ‘bias due to deviations from intended intervention’ was unclear.
Based on the set criteria, we labelled only five included studies (RCTs) as 'low risk of bias' studies (Geerse 2017; Harrison 2011; Hollingworth 2013; Schofield 2013; Young 2013). We labelled eight RCTs as 'high risk of bias' studies (Bergholdt 2013; Given 2004; Kutner 1999; Livingston 2010; Nimako 2015; Velikova 2004); six NRCTs as ‘serious risk' studies (Bramsen 2008; de Leeuw 2013; Hilarius 2008; Thewes 2009; Williams 2013; Young 2010); and one NRCT as a 'critical risk' study (Waller 2012). For five RCTs, Braeken 2013; Detmar 2002; Giesler 2005; Maunsell 1996; Nimako 2015; Rosenbloom 2007, and one NRCT, Taenzer 2000, there was not enough information to judge overall risk of bias. Consequently, we can say that a majority of the studies included in this review (15/26) were of low methodological quality. The results from the five studies at low risk of bias do not provide convincing evidence to support the beneficial value of screening and assessment of cancer patients’ psychosocial well‐being and care needs on their well‐being.
In addition to judging risk of bias for each study, we used the GRADE system to grade the certainty of the evidence for each primary outcome, which applies a rating of very low to high (Ryan 2016). We started with a baseline rating of high that was downgraded after considering certainty based on risk of bias, inconsistency, indirectness, imprecision, or publication bias. For HRQoL we downgraded for inconsistency due to the large variability in study findings (positive, negative, or absence of effects). For distress and care needs we downgraded for imprecision due to low sample sizes, and lack of data in some studies.
Findings from the included studies on each primary outcome and the corresponding certainty of evidence gradings are shown in Table 1.
Potential biases in the review process
We conducted a thorough search for this review in sources of published and unpublished studies, thereby reducing the chance of publication bias. We also used a wide range of terms to define psychosocial well‐being or distress in the search to avoid missing relevant studies.
For the screening of database records, one review author (BS) screened all records, and was doubled by five other screeners (AVH, BA, GB, JM, PV) for different numbers of records. That not all records were screened by the same two review authors could have caused bias, yet this separation was necessary due to the large number of database records that needed to be screened. To limit the risk of bias, there were frequent exchanges between the review authors involved in the screening of records to ensure that they were mutually well‐tuned with regard to the inclusion and exclusion criteria.
During the title and abstract screening phase, we contacted study authors multiple times to ask for extra information or full texts to determine the eligibility of studies. During data collection, we approached study authors multiple times to request additional data where necessary. As a result, we obtained all the information needed to evaluate most of the studies included in this review.
We assessed the certainty of the included studies with the 'Risk of bias' assessment tool for RCTs and the ROBINS‐I tool for NRCTs (Higgins 2011; Sterne 2016), both of which are Cochrane tools. The latter was recommended by the Cochrane review group, and allowed us to assess the certainty of NRCTs in a nuanced way, with 'low risk', 'moderate risk', 'serious risk', 'critical risk', and 'no information', in comparison with the 'low', 'high', and 'unclear' ratings of the 'Risk of bias' assessment tool for RCTs. However, we noticed that by strictly following the ROBINS‐I tool, NRCTs were assessed more stringently than RCTs were with Cochrane’s standard 'Risk of bias' tool (e.g. an RCT did not fulfil the 'high risk' criteria of the tool, and so received a 'low risk', or positive rating, while an NRCT with similar characteristics fulfilled the 'moderate risk' criteria of the ROBINS‐I, and so received a more negative rating) (Higgins 2011). This may have potentially led to bias in the quality judgements of the included studies. We received permission to combine studies with RCT and NRCT design in this review. However, to make general conclusions it should be possible to generate comparable certainty evaluations with both Cochrane tools.
We originally wanted to study the effect of screening of psychosocial well‐being and care needs on the well‐being of cancer patients and on quality of care (measures with care aspects such as referral, consultation length, and discussion of problems). As this would have resulted in a complex variation of outcomes, we decided to narrow the scope to patients' psychosocial well‐being. On second thought, it may have been more interesting to work with the double scope, since this would have entailed subjective as well as objective outcomes, the latter of which are not prone to reporting bias.
Next to the outcomes, the process of screening is also important, because it can shape the screening results. However, in this review there was no data collection focused on the question ‘Did the consequent intervention specifically address the results of the screening needs identified?’. We assumed that the guided actions matched the needs detected with the screening, but have not verified this.
Agreements and disagreements with other studies or reviews
Prior to our work, several other reviews studied the effectiveness of screening for distress and care needs on cancer patient outcomes (Bidstrup 2011; Carlson 2012; Howell 2012; Luckett 2009; Meijer 2013). In contrast to these reviews, our search was more thorough, consulting more sources to find eligible studies in the published as well as the grey literature (Bidstrup 2011; Carlson 2012). While three previous reviews focused exclusively on RCTs (Bidstrup 2011;Luckett 2009;Meijer 2013), we included RCTs as well as NRCTs based on the belief that RCTs are often not available to address questions about the effects of health system interventions and implementation strategies, due to the nature of the field (Sidani 2015). We believe that these differences, and the fact that the previous reviews were undertaken three to seven years ago, resulted in our identifying more studies eligible for inclusion in the review. We have included 26 studies in the current review, while Bidstrup 2011, Carlson 2012, Howell 2012, Luckett 2009, and Meijer 2013 included six, seven, 14, 14, and one studies, respectively. However, the latter was criticised for its rationale and methodology (Bultz 2013; Price 2013), resulting in very narrow inclusion criteria for studies.
To confine the heterogeneity in included studies, we only included studies with a real usual care condition without screening. This was in contrast to four other reviews (Bidstrup 2011; Carlson 2012; Howell 2012;Luckett 2009), which included studies with a control group that underwent screening without the screening results being shared with physicians or nurses (e.g. Boyes 2006; Carlson 2010; Grassi 2011; McLachlan 2001; Sarna 1998). In our opinion, these studies explored evidence on the effect of making screening results available to care professionals, and not on the effect of the screening on its own.
Previous reviews have concluded that, due to the lack of unambiguous evidence, it is impossible to draw conclusions on the effect of systematic screening and of psychosocial well‐being and care needs in cancer care. Bidstrup 2011 stated “We find it too early to conclude whether psychological screening improves the psychological well‐being of cancer patients”. However, now, six years later, the evidence is moving into the direction of ‘no effect’.
Authors' conclusions
Implications for practice.
During the last decade several calls have been launched to stimulate the design of psychosocial screening programs in clinical practice, and, to support this, consensus‐based guidelines have been written (Carlson 2012;Howell 2012; Lowery 2012; Luckett 2009). With these guidelines, one aimed at answering several questions from clinical practice: ‘What should be the exact content of the screening?’ ‘Which tools should be used?’ ‘What are the appropriate timing and frequency of assessments?' ‘Who should conduct these interventions?’. With this review, we not only attempted to explore the effect of the interventions, but additionally set the objective to add evidence‐based input to the earlier formulated consensus‐based recommendations on intervention characteristics which showed consistency with the effectiveness of the screening interventions.
Some of the included studies suggested some benefits of systematic screening (for health‐related quality of life, quality of life, distress, care needs, patients’ satisfaction, and/or psychosocial well‐being). However, based on the results of this review, screening of the psychosocial well‐being and care needs of people with cancer in general does not seem to be meaningful for the well‐being of these individuals. Attention should possibly be paid to more specific forms of screening in high‐risk populations, or in specific healthcare disciplines. Likewise, we did not find any systematic patterns of cohesion between individual study effects and intervention characteristics. On the basis of the evidence found, it is therefore difficult to say which intervention elements and characteristics should be used in the development of these interventions. Further research is needed to support the guidelines and recommendations for clinical practice with evidence‐based data.
Implications for research.
The results of this review plea for more uniformity in outcomes and reporting; the use of intervention description guidelines; further improvement of methodological certainty in studies; and combining subjective patient‐reported outcomes with objective outcomes.
We advise researchers to use validated, internationally recognised tools such as the European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Core 30 (EORTC QLQ‐C30), 36‐item Short Form Health Survey (SF‐36), Functional Assessment of Cancer Therapy‐General (FACT‐G), General Health Questionnaire 12‐item version (GHQ‐12), Hospital Anxiety and Depression Scale (HADS), Distress Thermometer (DT), Supportive Care Needs Survey (SCNS), and Patient Satisfaction Questionnaire (PSQ) to measure patients’ psychosocial well‐being, care needs, and satisfaction. There has been pleading in recent years for the development of core outcome sets. Core outcome sets are agreed standardised collections of outcomes that should be measured and reported in all trials within a specific field of research (Williamson 2012). The development and use of core outcome sets could reduce heterogeneity in outcomes.
In addition, it is important that study authors clearly describe the intervention content, tools, procedure, and conditions for implementation, so that other researchers can construct and study comparable interventions in other patient populations. This way homogeneity can be pursued, and meta‐analyses could be possible in the future. For example, the Template for Intervention Description and Replication (TIDieR) and the Criteria for Reporting the Development and Evaluation of Complex Interventions in healthcare: revised guideline (CReDECI 2) guidelines were developed to support this purpose (Hoffmann 2014; Mohler 2015).
Although improvement is already being seen, we believe that further efforts should be made to improve the methodological quality of studies, to reduce the risk of bias, and to obtain more reliable and less ambiguous evidence. This review provides several points of attention for this purpose. We believe that well‐developed RCTs as well as NRCTs can have a valuable role in future research on the effectiveness of screening and assessment of psychosocial well‐being and care needs in people with cancer.
When more homogeneity in intervention characteristics can be achieved in combination with an improvement of methodological quality, it will be possible to explore the intervention characteristics that contribute to the effectiveness of these screening interventions.
Finally, we recommend that future studies include the subjective patient‐reported outcome measures together with more objective outcomes, such as biomedical indicators of distress, or care outcomes to detect possible effects in care processes. The latter are less prone to response bias, and care outcomes have shown promising results in several studies (Bramsen 2008; Detmar 2002; Hilarius 2008; Maunsell 1996; Taenzer 2000). At the same time, the use of patient‐reported outcome measures ensures that insights from the patient perspective are obtained, which is of great importance to support the patient‐centred approach in care and research (Boyce 2014).
The evidence from studies conducted so far is not conclusive on the effect of the studied intervention, but suggests the absence of a general intervention effect. We think that future studies in this field should focus on patients in populations at high risk of experiencing increased levels of distress and care needs (e.g. younger, single, female, worse clinical status, lower quality of life, lower socioeconomic status). Focusing on high‐risk patients could permit a determination as to whether screening interventions may have an effect in vulnerable subgroups.
Furthermore, we wonder why some of the interventions in the included studies resulted in negative effects on individuals' health‐related quality of life, care needs, and satisfaction. No explanation for these effects could be found in the intervention characteristics. We may hypothesise that the intervention makes some people more dependent, resulting in an increased expression of problems and care needs. In future studies, more attention needs to be paid to the 'how' and 'why' of negative intervention effects should they occur.
What's new
| Date | Event | Description |
|---|---|---|
| 25 September 2019 | Amended | Updated contact email address for B Schouten. |
Acknowledgements
This review is part of a PhD funded by Limburg Sterk Merk (LSM). We are grateful to Noémie Aubert Bonn for her linguistic advice. We would like to thank Elke van Hoof for her contribution to the review protocol, Nick Van Campenhout for his practical support in the screening of IPOS conference abstracts, and Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers for their support and guidance in conducting this review. This project was supported by the National Institute for Health Research (NIHR), via infrastructure funding to Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Search strategy MEDLINE (Ovid)
1. exp Neoplasms/ 2. (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan* or oncolog* or psycho‐oncolog*).mp. 3. 1 or 2 4. "Quality of Life"/ 5. exp Health Status/ 6. Stress, Psychological/ 7. exp Adaptation, Psychological/ 8. exp Anxiety/ 9. Depression/ 10. Social Support/ 11. (quality of life or QOL or HQOL or HRQOL).mp. 12. (cope or coping).mp. 13. (social support or care need*).mp. 14. ((psychosocial or psycho‐social or psychological or social or emotion* or cogniti* or marital or relational or sexual or financial or spiritual or famil*) adj5 (wellbeing or well‐being or difficult* or function* or dysfunction*)).mp.˜ 15. (health adj status).mp. 16. (distress* or stress* or anxiety or anxious* or depress*).mp. 17. ((psychiat* or adjustment) adj5 disorder).mp. 18. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19. "Outcome Assessment (Health Care)"/ 20. patient outcome assessment/ 21. (PROM or PRO).mp. 22. patient reported outcome*.mp. 23. interview/ 24. Interview, Psychological/ 25. exp Questionnaires/ 26. (questionnaire* or interview*).mp. 27. ((psychosocial or psycho‐social or psychological) adj5 (screen* or assess* or report* or survey* or scale* or instrument*)).mp. 28. exp Psychiatric status rating scales/ 29. (systemat* adj5 assess*).mp. 30. Screen*.mp. 31. (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30).mp. 32. EORTC‐QLQ‐C30.mp. 33. (Short Form Health Survey or SF36).mp. 34. ((Hospital Anxiety and Depression Scale) or HADS).mp. 35. (Distress Thermometer ).mp. 36. (Beck Depression Inventory or BDI).mp. 37. (Supportive Care Needs Survey or Cancer Survivors Unmet Needs or CaSUN).mp. 38. EORTC IN‐PATSAT32.mp. 39. ((Patient Satisfaction and Quality in Oncological Care) or PASQOC).mp. 40. 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 41. 3 and 18 and 40 42. randomized controlled trial.pt. 43. controlled clinical trial.pt. 44. randomized.ab. 45. placebo.ab. 46. clinical trials as topic.sh. 47. randomly.ab. 48. trial.ti. 49. ((before‐after or (before and after)) adj (study or studies)).mp. 50. (CBA adj (study or studies)).mp. 51. interrupted time series.mp. 52. (ITS adj (study or studies)).mp. 53. (repeated measure adj (study or studies)).mp. 54. ((RMS or rms) adj (study or studies)).mp. 55. (historical* control* adj5 (study or studies)).mp. 56. ((HCT or hct) adj (study or studies)).mp. 57. 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 58. 41 and 57 59. exp animals/ not humans.sh. 60. 58 not 59
key: mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier b=abstract ti=title sh=subject heading pt=publication type
Appendix 2. Search strategy CENTRAL
#1. MeSH descriptor: [Neoplasms] explode all trees #2. cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan* or oncolog* or psycho‐oncolog* #3. #1 or #2 #4. MeSH descriptor: [Quality of Life] this term only #5. MeSH descriptor: [Health Status] explode all trees #6.MeSH descriptor: [Stress, Psychological] this term only #7. MeSH descriptor: [Adaptation, Psychological] explode all trees #8. MeSH descriptor: [Anxiety] explode all trees #9. MeSH descriptor: [Depression] this term only #10. MeSH descriptor: [Social Support] this term only #11. quality of life or QOL or HQOL or HRQOL #12. cope or coping #13. social support or care need* #14. ((psychosocial or psycho‐social or psychological or social or emotion* or cogniti* or marital or relational or sexual or financial or spiritual or famil*) near/5 (wellbeing or well‐being or difficult* or function* or dysfunction*)) #15. health near/2 status #16. distress* or stress* or anxiety or anxious* or depress* #17. ((psychiat* or adjustment) near/5 disorder) #18. #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 #19. MeSH descriptor: [Outcome Assessment (Health Care)] this term only #20. MeSH descriptor: [Patient Outcome Assessment] this term only #21. PROM or PRO #22. patient reported outcome* #23. MeSH descriptor: [Interview] this term only #24. MeSH descriptor: [Interview, Psychological] this term only #25. MeSH descriptor: [Surveys and Questionnaires] explode all trees #26. questionnaire* or interview* #27. ((psychosocial or psycho‐social or psychological) near/5 (screen* or assess* or report* or survey* or scale* or instrument*)) #28. MeSH descriptor: [Psychiatric Status Rating Scales] explode all trees #29. systemat* near/5 assess* #30. Screen* #31. European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 #32. EORTC‐QLQ‐C30 #33. Short Form Health Survey or SF36 #34. ((Hospital Anxiety and Depression Scale) or HADS) #35. (Distress Thermometer) #36. (Beck Depression Inventory or BDI) #37. (Supportive Care Needs Survey or Cancer Survivors Unmet Needs or CaSUN) #38. EORTC IN‐PATSAT32 #39. ((Patient Satisfaction and Quality in Oncological Care) or PASQOC) #40. #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 #41. #3 and #18 and #40
Appendix 3. Search strategy Embase (Ovid)
1. exp neoplasm/ 2. (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan* or oncolog* or psycho‐oncolog*).mp. 3. 1 or 2 4. "quality of life"/ 5. exp health status/ 6. mental stress/ 7. exp adaptive behavior/ 8. exp anxiety/ 9. depression/ 10. social support/ 11. (quality of life or QOL or HQOL or HRQOL).mp. 12. (cope or coping).mp. 13. (social support or care need*).mp. 14. ((psychosocial or psycho‐social or psychological or social or emotion* or cogniti* or marital or relational or sexual or financial or spiritual or famil*) adj5 (wellbeing or well‐being or difficult* or function* or dysfunction*)).mp. 15. (health adj status).mp. 16. (distress* or stress* or anxiety or anxious* or depress*).mp. 17. ((psychiat* or adjustment) adj5 disorder).mp. 18. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19. outcome assessment/ 20. patient outcome assessment.mp. 21. (PROM or PRO).mp. 22. patient reported outcome*.mp. 23. interview/ 24. psychologic test/ 25. exp questionnaire/ 26. (questionnaire* or interview*).mp. 27. ((psychosocial or psycho‐social or psychological) adj5 (screen* or assess* or report* or survey* or scale* or instrument*)).mp. 28. exp psychological rating scale/ 29. (systemat* adj5 assess*).mp. 30. Screen*.mp. 31. (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30).mp. 32. EORTC‐QLQ‐C30.mp. 33. (Short Form Health Survey or SF36).mp. 34. ((Hospital Anxiety and Depression Scale) or HADS).mp. 35. Distress Thermometer.mp. 36. (Beck Depression Inventory or BDI).mp. 37. (Supportive Care Needs Survey or Cancer Survivors Unmet Needs or CaSUN).mp. 38. EORTC IN‐PATSAT32.mp. 39. ((Patient Satisfaction and Quality in Oncological Care) or PASQOC).mp. 40. 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 41. 3 and 18 and 40 42. crossover procedure/ 43. double‐blind procedure/ 44. randomized controlled trial/ 45. single‐blind procedure/ 46. random*.mp. 47. factorial*.mp. 48. (crossover* or cross over* or cross‐over*).mp. 49. placebo*.mp. 50. (double* adj blind*).mp. 51. (singl* adj blind*).mp. 52. assign*.mp. 53. allocat*.mp. 54. volunteer*.mp. 55. ((before‐after or (before and after)) adj (study or studies)).mp. 56. (CBA adj (study or studies)).mp. 57. interrupted time series.mp. 58. (ITS adj (study or studies)).mp. 59. (repeated measure adj (study or studies)).mp. 60. ((RMS or rms) adj (study or studies)).mp. 61. (historical* control* adj5 (study or studies)).mp. 62. ((HCT or hct) adj (study or studies)).mp. 63. 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 64. 41 and 63 65. (exp Animal/ or Nonhuman/ or exp Animal Experiment/) not Human/ 66. 64 not 65
Appendix 4. Search strategy PsycINFO (Ovid)
| 1 | exp Neoplasms/ |
| 2 | (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan* or oncolog* or psycho‐oncolog*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] |
| 3 | 1 or 2 |
| 4 | "Quality of Life"/ |
| 5 | Well Being/ |
| 6 | Psychological Stress/ |
| 7 | Psychosocial Rehabilitation/ |
| 8 | Psychosocial Readjustment/ |
| 9 | exp Anxiety/ |
| 10 | "Depression (Emotion)"/ |
| 11 | Distress/ |
| 12 | Stress/ |
| 13 | Social Stress/ |
| 14 | Social Support/ |
| 15 | Needs/ |
| 16 | Health Service Needs/ |
| 17 | Psychological Needs/ |
| 18 | (quality of life or QOL or HQOL).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] |
| 19 | (cope or coping).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] |
| 20 | (social support or care need*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] |
| 21 | ((psychosocial or psycho‐social or psychological or social or emotion* or cogniti* or marital or relational or sexual or financial or spiritual or famil*) adj5 (wellbeing or well‐being or difficult* or function* or dysfunction*)).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] |
| 22 | (distress* or stress* or anxiety or anxious* or depress*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] |
| 23 | 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 |
| 24 | exp Measurement/ |
| 25 | exp Screening/ |
| 26 | (PROM or PRO).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] |
| 27 | patient reported outcome*.mp. |
| 28 | Interviews/ |
| 29 | exp Questionnaires/ |
| 30 | (questionnaire* or interview*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] |
| 31 | ((psychosocial or psycho‐social or psychological) adj5 (screen* or assess* or report* or survey* or scale* or instrument*)).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] |
| 32 | ((European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30) or EORTC‐QLQ‐C30 ).mp. |
| 33 | ((Short Form Health Survey) or SF36).mp. |
| 34 | ((Hospital Anxiety and Depression Scale) or HADS).mp. |
| 35 | (Distress Thermometer).mp. |
| 36 | ((Beck Depression Inventory) or BDI).mp. |
| 37 | ((Supportive Care Needs Survey or SCNS or Cancer Survivors Unmet Needs) or CaSUN).mp. |
| 38 | EORTC IN‐PATSAT32.mp. |
| 39 | ((Patient Satisfaction and Quality in Oncological Care) or PASQOC).mp. |
| 40 | 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 r 35 or 36 or 37 or 38 or 39 |
| 41 | 3 and 23 and 40 |
| 42 | randomized controlled trial.mp. |
| 43 | controlled clinical trial.mp. |
| 44 | randomized.ab. |
| 45 | randomized.ab. |
| 46 | placebo.ab. |
| 47 | exp Clinical Trials/ |
| 48 | randomly.ab. |
| 49 | trial.ti. |
| 50 | ((before‐after or (before and after)) adj (study or studies)).mp. |
| 51 | (CBA adj (study or studies)).mp. |
| 52 | interrupted time series.mp. |
| 53 | (ITS adj (study or studies)).mp. |
| 54 | (repeated measure adj (study or studies)).mp. |
| 55 | 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 |
| 56 | 41 and 54 |
| 57 | limit 56 to human |
Appendix 5. Search strategy CINAHL (EBSCO)
| S56 | S54 not S55 |
| S55 | (MH "Animals") not (MH "Human") |
| S54 | S39 and S53 |
| S53 | S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 |
| S52 | (repeated measure N (study or studies)) |
| S51 | (ITS N (study or studies)) |
| S50 | "interrupted time series" |
| S49 | (CBA N (study or studies)) |
| S48 | ((before‐after or (before and after)) N (study or studies)) |
| S47 | TI trial |
| S46 | AB randomly |
| S45 | AB placebo |
| S44 | AB randomized |
| S43 | "controlled clinical trial" |
| S42 | "randomized controlled trial" |
| S41 | (MH "Clinical Trials+") |
| S40 | (MH "Randomized Controlled Trials") |
| S39 | S7 and S21 and S38 |
| S38 | S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 |
| S37 | ((European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30) or EORTC‐QLQ‐C30) |
| S36 | ((Short Form Health Survey) or SF36) |
| S35 | ((Hospital Anxiety and Depression Scale) or HADS) |
| S34 | (Distress Thermometer) |
| S33 | ((Beck Depression Inventory) or BDI) |
| S32 | ((Supportive Care Needs Survey) or SCNS) or ((Cancer Survivors Unmet Needs) or CaSUN) |
| S31 | EORTC IN‐PATSAT32 |
| S30 | ((Patient Satisfaction and Quality in Oncological Care) or PASQOC) |
| S29 | ((psychosocial or psycho‐social or psychological) N5 (screen* or assess* or report* or survey* or scale* or instrument*)) |
| S28 | (questionnaire* or interview*) |
| S27 | (MH "Questionnaires+") |
| S26 | (MH "Interviews") |
| S25 | "patient reported outcome*" |
| S24 | (PROM or PRO) |
| S23 | (MH "Outcome Assessment") |
| S22 | (MH "Needs Assessment") |
| S21 | S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 |
| S20 | (distress* or stress* or anxiety or anxious* or depress*) |
| S19 | ((psychosocial or psycho‐social or psychological or social or emotion* or cogniti* or marital or relational or sexual or financial or spiritual or famil*) N5 (wellbeing or well‐being or difficult* or function* or dysfunction*)) |
| S18 | (social support or care need*) |
| S17 | (cope or coping) |
| S16 | (quality of life or QOL or HQOL) |
| S15 | (MH "Information Needs") |
| S14 | (MH "Health Services Needs and Demand") |
| S13 | (MH "Social Support (Iowa NOC)") |
| S12 | (MH "Support, Psychosocial") |
| S11 | (MH "Depression") |
| S10 | (MH "Anxiety+") |
| S9 | (MH "Adaptation, Psychological") OR (MH "Psychosocial Adaptation (Iowa NOC)") |
| S8 | (MH "Stress") or (MH "Stress, Psychological") |
| S7 | S1 or S2 or S3 or S4 or S5 or S6 |
| S6 | (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan* or oncolog* or psycho‐oncolog*) |
| S5 | (MH "Carcinoma") |
| S4 | (MH "Oncologic Care") |
| S3 | (MH "Cancer Patients") |
| S2 | (MH "Oncology") |
| S1 | (MH "Neoplasms+") |
Appendix 6. Search strategy ClinicalTrials.gov
Accessed true:clinicaltrials.gov/
Search with indication of conditions:
Study type: Interventional Studies
Condition: cancer
Search terms: ‘psychosocial’, ‘screening’
Appendix 7. Search strategy ISRCTN registry
Accessed true:www.isrctn.com/
Search with advanced search‐option:
Within text search: (‘distress’ OR ‘quality of life’) AND (‘screening’ OR ‘assessment’)
Condition: ‘cancer’
Appendix 8. Search strategy Nederlands Trial Register (NRT)
Accessed true:www.trialregister.nl/trialreg/index.asp
Several searches with individual terms (no combination possible in this register): ‘Psychosocial’, ‘Distress’, ‘Quality of life’, ‘Screening’
Appendix 9. Search strategy RePORTER query tool
Accessed true:projectreporter.nih.gov/reporter.cfm
Search with advanced search‐option: ‘cancer’ AND ‘psychosocial’ AND ‘screening’
Appendix 10. Search strategy UK National Research Register (NRR)
Accessed true:www.journalslibrary.nihr.ac.uk/news/the‐nihr‐journals‐library‐one‐year‐on
Search terms: ‘cancer’ AND ‘psychosocial’ AND ‘screening’
Appendix 11. Data collection and quality assessment file
We used a the following subdivisions to collect data and assess methodological quality.
|
1. Study ID 1st Author Year 2. METHODS Study design Duration study Source 3. PARTICIPANTS Country Participants Setting Inclusion criteria Exclusion criteria 4. INTERVENTION Type Randomisation Aim study Content Of Screen Interventionist Intervention Conditions Intervention Implementation Theoretical basis Comparative condition Protocol adherence Length follow‐up 5. OUTCOMES Primary outcome Secondary outcome Outcome time points 6. STUDY RESULTS Sample size Number analysed Age Gender Results Primary Outcome Results Secondary Outcome |
7. REVIEWERS CONCLUSION Our Primary Outcomes Our Secondary Outcomes 8a. QUALITY ASSESSMENT ‐ RCT Funding info Conflicts of interest Sample size calculation Sequence generation Allocation concealment Blinding patients & staff Blinding outcome assessors Completeness outcome data Reporting on outcome data Other sources of bias Overall RISK OF BIAS in study Notes 8b. QUALITY ASSESSMENT ‐ NRCT Bias due to confounding Bias in selection of participants into the study Bias in classification of interventions Bias due to deviations from intended intervention Bias due to missing data Bias in measurement of outcomes Bias in selection of the reported result Overall RISK OF BIAS in study Notes |
Data and analyses
Comparison 1. Screening versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HRQoL: Global health status (1 month) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Randomised controlled trials | 2 | 775 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐2.55, 2.60] |
| 1.2 Non‐randomised controlled trials | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 6.60 [‐4.27, 17.47] |
| 2 HRQoL: Global health status (3 months) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Randomised controlled trials | 2 | 750 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐2.38, 2.95] |
| 2.2 Non‐randomised controlled trials | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 12.70 [2.61, 22.79] |
| 3 HRQoL: Global health status (6 months) | 2 | 730 | Mean Difference (IV, Random, 95% CI) | 1.65 [‐4.83, 8.12] |
| 4 Psychological distress (1 month) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Randomised controlled trials | 1 | 709 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 4.2 Non‐randomised controlled trials | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.48, 0.68] |
| 5 Psychological distress (3 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Randomised controlled trials | 1 | 687 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.36, 0.36] |
| 5.2 Non‐randomised controlled trials | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Supportive care needs: general unmet needs (3 months) | 2 | 748 | Mean Difference (IV, Random, 95% CI) | 2.32 [‐7.49, 12.14] |
| 7 Supportive care needs: general unmet needs (6 months) | 2 | 732 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.22, 0.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bergholdt 2013.
| Methods | Cluster RCT ‐ with intervention group (IG) and control group (CG) | |
| Participants | Adults with cancer treated for incident cancer at the public regional hospital Country: Denmark Age: CG: mean 63.6 years (62.5 to 64.6); IG: mean 63.2 years (62.2 to 64.3) Sex: CG: 71.4% female; IG: 72.6% female Inclusion criteria
Exclusion criteria
N randomised People: N = 955, IG: n = 486, CG: n = 469 GPs: N = 775; IG: n = 377; CG: n = 398 N in analysis Participants baseline: N = 955; IG: n = 486; CG: n = 469; GPs baseline Participants 6 months: N = 565; IG: n = 273; CG: n =292; GPs 6 months:NA Participants 14 months: N = 318; IG: n = 159; CG: n = 159; GPs 14 months: N = 775; IG: n = 377; CG: n = 398 |
|
| Interventions |
Content of screen:CARE NEEDS: Individual needs for physical, psychological, sexual, social, work‐ and finance‐related rehabilitation (interview guide based on information from studies on needs) Interventionist: 2 RCs to conduct the needs assessment (both nurses with oncological experience, assigned exclusively to the project, and not taking part in the daily routines at the hospital ward) Intervention procedure:SI with co‐intervention to use screening results: RC interviews people about rehabilitation needs, then information about the individual rehabilitation needs is send to the GP + the rehabilitation needs of people with cancer in general, and GP is encouraged to proactively contact the people to facilitate the rehabilitation process Conditions for implementation
Comparative condition: Usual care group Length of follow‐up: 14 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome time points: 6 and 14 months after inclusion (= admission to the hospital after a new cancer diagnosis). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | Danish GP practices allocated to conditions, people automatically allocated to condition of the GP. Unclearif the participant is aware of the randomisation condition of the GP/patient |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of RC that managed the intervention; the staff in the involved departments of the hospital were informed about the study; GPs allocated to the control condition were not informed about the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Validated self‐report questionnaires were used for data collection, "data ware collected in the same way, irrespective of the allocation status". No extra person for outcome assessment aware of condition allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout from baseline to 12 months assessment at both participant (+/− 30%) and GP level (+/− 20%), dropout in both conditions equally distributed |
| Selective reporting (reporting bias) | Unclear risk | There seems to be adequate reporting on every outcome except on "patients rehabilitation needs" (outcome mentioned in protocol, not mentioned elsewhere) |
| Other bias | Low risk | / |
Braeken 2013.
| Methods | Stratified cluster RCT ‐ with intervention group (IG) and control group (CG) | |
| Participants | Adult cancer patients receiving radiotherapy Country: the Netherlands Age: participants: CG: mean 63.6 years (62.5 to 64.6); IG: mean 63.2 years (62.2 to 64.3); GPs: CG: mean 53.3 years (52.5 to 54.1); IG: mean 53.3 years (52.4 to 54.1) Sex: participants: CG: 71.4% female; IG: 72.6% female; GPs: CG: 30.2% female; IG: 36.1% female Inclusion criteria
Exclusion criteria
N randomised: N = 568; IG: n = 268 (n = 136 with baseline assessment, n = 132 without baseline assessment) CG: N = 300 (n = 144 with baseline assessment, n = 156 without baseline assessment) N in analysis: 3 months: n = 640 (IG: n = 356, CG: n = 284); 12 months: n = 491 (IG: n = 230, CG: n = 261) |
|
| Interventions |
Content of screen:PSYCHOSOCIAL PROBLEMS: tool = the Dutch SIPP: 24 items: physical complaints (n = 7), psychological complaints (n = 10), social/financial problems (n = 4), and sexual problems (n = 3) Interventionist: No interventionist for screening act (self‐reported measure) Intervention procedure:solitary SI; participants received SIPP twice: before the first consultation with the radiotherapist and before the consultation at the end of the RT; radiotherapists checked the scores (manual provided with cut‐off scores SIPP); SIPP + judgement radiotherapist ‐> referral for psychosocial support Conditions for implementation:
Comparative condition: Usual care group Length of follow‐up: 12 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome time points: Baseline: participants on odd weeks received a pre‐measurement, participants on even weeks received no pre‐measurement; 3 months; 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear what method was used to generate the sequence |
| Allocation concealment (selection bias) | Unclear risk | Radiotherapists allocated to conditions, participants automatically allocated to condition of the radiotherapist. Unclear if the participant is aware of the randomisation condition of the radiotherapist/patient |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blinding: participants; radiotherapist not blinded (note: asked not to discuss the study with their colleagues of the control group) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were collected with mailed questionnaires No extra person for outcome assessment aware of condition allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout of participants from baseline to 12‐month assessment +/− 14%; equally distributed between both conditions |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | / |
Bramsen 2008.
| Methods | Sequential cohort study with repeated measures ‐ first a control cohort (UCG), sequentially an experimental cohort (IG), a medical records control group from people admitted to the department in the 4 months preceding the control cohort (MRCG) | |
| Participants | Inpatients in department medical oncology Country: the Netherlands Age: IG: mean 53 years (13.1 SD); RealCG: mean 55 years (9.9 SD); MedicalRecordCG: mean 56 years (14.1 SD) Sex: IG: 54% female; RealCG: 43% female; MedicalRecordCG: 47% female Inclusion criteria
Exclusion criteria
N recruited: N = 262; IG: n = 109; RealCG: n = 64; MedicalRecordCG: n = 89 N in analysis: N = 218; IG: n = 79; RealCG: n = 50; MedicalRecordCG: n = 89 |
|
| Interventions |
Content of screen:OVERALL WELL‐BEING: Current overall situation of the participant, physical condition and treatment, emotional condition, social network and living circumstances, religion or philosophy of life, medical history, life events, personality and coping, history of psychosocial care, future perspective, any other issues. Screenings interventionist: A psychologist or social worker (intaker) conducted the semi‐structured screening interview with the participant Intervention procedure: Face‐to‐face psychosocial SI with co‐intervention to use screening results: a semi‐structured interview with a psychologist or social worker guided by a checklist, afterwards rating the presence of problems and needs on 4‐point Likert scale (no special attention needed, mild problems, problems that require attention, serious problems), need for follow‐up contact discussed with participant, if follow‐up requested conclusion summary placed in the participant's medical record Conditions for implementation
Comparative condition:
Length of follow‐up: 4 weeks |
|
| Outcomes |
Primary outcomes
Secondary outcomes: / Outcome time points: baseline; 4 weeks after discharge from hospital |
|
| Notes | For bias judgement on NRCTs, see Table 3; Table 4; Table 9 | |
de Leeuw 2013.
| Methods | Quasi‐experimental prospective single‐centre study ‐ with intervention group (IG) and control group (CG) | |
| Participants | People with head and neck cancer Country: the Netherlands Age: IG: mean 58.4 years (22 to 86); CG: mean 59.2 years (30 to 83) Sex: IG: 32.5% female; CG: 25% female Inclusion criteria
Exclusion criteria
N recruited: N = 160; IG: n = 80; CG: n = 80 N in analysis: N = 160; IG: n = 80; CG: n = 80 |
|
| Interventions |
Content of screen:CARE NEEDS and PSYCHOSOCIAL PROBLEMS
Screenings interventionist: A nurse to conduct the needs assessment Intervention procedure:SI with co‐intervention to use screening results: 6, 30‐minute nursing follow‐up consultations in the first year post‐treatment, conducted in parallel with and preceding the medical routine control visits and included a biopsychosocial needs assessment (13‐item checklist) prior to each consultation Every 3 months, participants were screened for psychosocial problem areas using a specific questionnaire Conditions for implementation
Comparative condition: UCG: Usual care: 5‐year routine control with 6 bimonthly 10‐minute visits to a head and neck surgeon in the first year post‐treatment + nursing follow‐up care (ad hoc problem‐based contacts) Exceptions
Length of follow‐up: 12 months |
|
| Outcomes |
Primary outcomes:
Secondary outcomes: / Outcome time points: 1 month post‐treatment (baseline), 6 and 12 post‐treatment |
|
| Notes | For bias judgement on NRCTs, see Table 3; Table 4; Table 9 | |
Detmar 2002.
| Methods | Longitutinal randomised cross‐over design ‐ with intervention group (IG) and control group (CG) | |
| Participants | Cancer patients undergoing outpatient palliative chemotherapy, and oncologists working in the department of medical oncology Country: the Netherlands Age: Participants: IG: mean 58 years (25 to 84); CG: mean 55 years (24 to 81), P = 0.24; Oncologists: mean 44 years (35 to 53) Sex: Participants: IG: 73% female; CG: 81% female, P = 0.15; Oncologists: 40% female Inclusion criteria
Exclusion criteria
N randomised: Participants: N = 273; IG: n = 131; CG: n = 172; Oncologists: n = 10 N in analysis: Participants: N = 214; IG: n = 100; CG: n = 144; Oncologists: n = 10 |
|
| Interventions |
Content of screen: HRQoL: tool = EORTC QLQ‐C30: 5 functional scales, 9 symptom scales, and 2 General Health and QoL items, no total score can be computed Interventionist: No interventionist for screening act, self‐completion of screening tool Intervention procedure:solitary SI: Participants in IG had a first standard follow‐up visit with oncologist. At 3 following outpatient visits, participants completed HRQoL questionnaire on paper in waiting room immediately before their visit, and the responses were optically scanned, scored, and transformed into a graphic summary Physicians and participants received a copy of the summary before the consultation Conditions for implementation
Comparative condition: Usual care group Length of follow‐up: from the first to the fourth visit for follow‐up of palliative chemotherapy (first study medical visit: baseline assessment for both groups; intervention introduced at second study visit and continued through the fourth study visit) |
|
| Outcomes |
Primary outcomes
Secondary outcomes: / Outcome time points: At the end of the first and fourth follow‐up visit |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear what method was used to generate a sequence |
| Allocation concealment (selection bias) | Unclear risk | Unclear which method was used to conceal the allocation of physicians to conditions |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants: blinded; oncologists: not blinded, act as their own control in other period of the study. Potentially a bias for the oncologists who first had to undertake the intervention period and afterwards the control period (were they providing 'usual care'?) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes of interest were collected with self‐report questionnaires, no extra person for outcome assessment aware of condition allocation. Raters for content coding of audiotaped medical consultations were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout of participants from baseline to fourth study visit +/− 22%; equally distributed between both conditions, explained by death, change of physician, change of hospital |
| Selective reporting (reporting bias) | Low risk | Adequate: conclusions made on the basis of their outcomes of interest, but other outcome results also available in supplementary |
| Other bias | Low risk | / |
Geerse 2017.
| Methods | RCT ‐ with intervention group (IG) and control group (CG) | |
| Participants | Newly diagnosed or recurrent lung cancer patients starting systemic therapy Country: the Netherlands Age: IG: mean 60.6 years (10.5 SD); CG: mean 62.3 years (9.7 SD) Sex: IG: 46% female; CG: 39% female Inclusion criteria
Exclusion criteria
N randomised: N = 223; IG: n = 110; CG: n = 113 N in analysis: N = 111; IG: n = 61; CG: n = 50 completed all 4 assessments |
|
| Interventions |
Content of screen:DISTRESS: tool = DT, PL, and the referral wish question (yes, maybe, no): PL 47 items covering 5 life domains: practical (7 items), social (3 items), emotional (10 items), spiritual (2 items), and physical (25 items) Interventionist: Self‐completion of screening tool, but psychosocial nurse needed for discussion response patterns Intervention procedure:SI with co‐intervention to use screening results: Participants completed DT/PL before their outpatient clinic appointment at baseline ‐ T4 (minimum 4 times). After completion, face‐to‐face with psychosocial nurse to discuss response pattern. Referral DT score was ≥ 4 or if referral wish. Conditions for implementation
Comparative condition: Usual care group Length of follow‐up: From start of treatment (randomisation) until 25 weeks after start of treatment (+/− 6.5 months) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes
Outcome time points: 1, 7, 13, 25 weeks after randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule generated by a validated system (PMX CTM, release 3.3.0 HP2, Propack Data) with use of pseudo‐random number generator and supplied seed number |
| Allocation concealment (selection bias) | Low risk | Randomisation, questionnaire distribution, and data management performed by the independent Netherlands Comprehensive Cancer Organisation (IKNL) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of participants or psychosocial nurses |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes collected with self‐report questionnaires, no extra person for outcome assessment aware of condition allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout from baseline to 24 weeks' postbaseline: 56% in control group, 45% in intervention group; reasons for dropout: death, discontinued participation |
| Selective reporting (reporting bias) | Low risk | All outcome data available in paper or in supplementary file |
| Other bias | Low risk | / |
Giesler 2005.
| Methods | Prospective multisite RCT ‐ with intervention group (IG) and control group (CG) | |
| Participants | Adult patients with prostate carcinoma and their partners Country: USA Age: IG: mean 66.7 years; CG: mean 61.1 years Sex: All male Inclusion criteria
Exclusion criteria: / N randomised: N = 99; IG: n = 48; CG: n = 52 N in analysis: N = 99; IG: n = 48; CG: n = 51 (sample sizes at baseline, 4, 7, 12 months fluctuated slightly due to missing answers), n = 85 at 12 months; dropout equal in IG and CG |
|
| Interventions |
Content of screen:HRQoL: Quality of life problems (sexual functioning, cancer worry, dyadic adjustment, depression, and other cancer‐related problems) Interventionist: A nurse to conduct the screening/assessment Intervention procedure:SI with co‐intervention to use screening results: 6 intervention visits in first 6 months after end treatment, first visit (end therapy): assessment of bowel and urinary function problems; second visit (1 month later): assessment guided by computer assessment program; contacts 3 to 6 (each month on phone): asks to discuss issues and concerns. Menu‐driven computer program provided standardised questions and response formats that the nurse used to elicit and document information concerning QoL problems. If score exceeded threshold for a problem, program was prompted to assess the problem in greater detail and helped identify strategies Conditions for implementation
Comparative condition: Usual care group Length of follow‐up: 12 months (from end of treatment until 6 months later) |
|
| Outcomes |
Primary outcomes
Secondary outcomes: / Outcome time points: Baseline; 4 months, 7 months, and 12 months post‐treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear what method was used to generate the sequence |
| Allocation concealment (selection bias) | Unclear risk | Unclear which method was used to conceal the allocation to conditions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, partners, and nurses not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data collected with computer‐assisted telephone interviews, interviewers were blinded to the group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout of participants from baseline to 12 months' assessment +/− 15%; distributed equally between both conditions (“attriters did not differ from those who completed the study”); reason for dropout: inconvenience. "Because some respondents occasionally failed to answer all items during the interviews, the sample sizes fluctuated slightly"; nowhere stated how much this is |
| Selective reporting (reporting bias) | Low risk | All prespecified data reported |
| Other bias | High risk | No adjustments for multiple testing imply that the few positive results are at high risk of type II errors |
Given 2004.
| Methods | RCT – with an intervention group (IG) and control group (CG) | |
| Participants | Patients diagnosed with a solid tumour and within 56 days of undergoing a first cycle of chemotherapy Country: USA Age: unclear Sex: Almost 80% of the total sample female Inclusion criteria:
Exclusion criteria
N randomised: n = 237; IG: n = 118; CG: n = 119 N in analysis: baseline: n = 237, IG: n = 118, CG: n = 199; 10 weeks: n = 191, IG: n = 97, CG: n = 94; 20 weeks: n = 167, IG: n = 80, CG: n = 87 |
|
| Interventions |
Content of screen:HRQoL: Assessment of severity of problems and extent to which each of these problems impacted QoL ‐ dimensions. Problems assessed: alopecia, anxiety, constipation, depression, diarrhoea, nausea, dyspnoea, fatigue, fever, anorexia, insomnia, mucositis, pain, skin problems, lack of concentration, and physical and work role functioning; QoL dimensions assessed: emotions, relationships with others, sleep, appetite, daily activity, and concentration Interventionist: A nurse to conduct the screening/assessment and broader intervention Intervention procedure:SI with co‐intervention to use screening results: A 10‐contact, 20‐week intervention with symptom assessment. The computer documentation system provided up to 4 intervention strategies for each detected problem selected from the categories: information, counselling and support, co‐ordination of care, and prescribing therapeutic activities. Nurse discussed and entered participants’ choice into computer‐guided protocol. At all subsequent contacts, participants rated the severity and impact on symptoms for each specific intervention. Evaluation of each problem classified as: resolved, improving, no change, or deteriorating. Each of the in‐person sessions took approximately 1 hour Conditions for implementation
Comparative condition: Usual care group Length of follow‐up: 20 weeks |
|
| Outcomes |
Primary outcomes: Depression (CES‐D) Secondary outcomes: / Outcome time points: baseline; 10 and 20 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Unclear which method was used to conceal the allocation to conditions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, partners, and nurses not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of telephone (outcome) interviewers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout from baseline to 20 weeks' postbaseline: 32% in control group, 27% in intervention group |
| Selective reporting (reporting bias) | High risk | Data on participant characteristics are very limited, no clear presentation on the concrete depression data (CES‐D scores) or severity of problems data, only a lot of visuals and text on the assessed interactions. There is also no referral to a supplementary file for the concrete data |
| Other bias | Unclear risk | Not clear whether the differently composed models are post hoc analyses or were planned in advance |
Harrison 2011.
| Methods | RCT ‐ with an intervention group (IG) and a control group (CG) | |
| Participants | Adult colorectal cancer patients that underwent surgery Country: Australia Age: IG: mean 67.2 years; CG: mean 61.8 years Sex: CG: IG: 42% female; CG: 36% female Inclusion criteria
Exclusion criteria: / N randomised: n = 75, IG: n = 38, CG: n = 36 N in analysis: baseline: n = 73, IG: n = 37, CG: n = 36; 1 month: n = 70, IG: n = 36, CG: n = 34; 3 months: n = 65, IG: n = 34, CG: n = 31; 6 months: n = 60, IG: n = 30, CG: n = 30 |
|
| Interventions |
Content of screen:CARE NEEDS: A set of questions acting as a screening tool, designed to address common problems experienced by patients throughout this period. Physical, psychosocial, information, supportive care, and rehabilitation needs are assessed and addressed during each call Interventionist: Colorectal cancer nurse who conducts the telephone screenings Intervention procedure:SI part of a more complex intervention: The CONNECT intervention comprises 5 calls of a nurse following the participants' initial discharge from hospital after surgery (days 3 and 10 and then at 1, 3, and 6 months). Needs are assessed and addressed during each call. Participants also have the opportunity to raise any additional concerns. If the nurse identifies a need, relevant information is provided. Emotional support is given when necessary. Where further clinical advice, or referral, is required, the nurse directs participants back to the appropriate clinical team member to make the relevant appointments and referrals. Conditions for implementation
Comparative condition: Usual care: included a recommended follow‐up appointment with a general practitioner and surgeon Length of follow‐up: 6 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome time points: 1, 3, and 6 months after discharge |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequences were created using a computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Independent researcher randomly allocated participants to intervention or control groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors stated that "blinding of either patients or researchers was not possible" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Care needs and QoL data were collected with self‐report tools for participants, no extra person for outcome assessment aware of condition allocation. Health service utilisation data were collected blind to participants' group status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout of participants from baseline to 6 months' assessment +/− 20%; equally distributed between both conditions for care needs and QoL measurement. Completeness of data on health service utilisation seems to be adequate |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but the published reports included all expected outcomes |
| Other bias | Low risk | / |
Hilarius 2008.
| Methods | Sequential cohort design with repeated measures ‐ with intervention group (IG) and control group (CG) | |
| Participants | Cancer patients who were to begin adjuvant or palliative chemotherapy treatment Country: the Netherlands Age: Participants: IG: mean 57 years; CG: mean 55 years, P = 0.17; Nurses: mean 36 years (26 to 48) Sex: Participants: IG: 61% female; CG: 67% female, P = 0.54; Nurses: 100% female Inclusion criteria
Exclusion criteria: Patients
N recruited: Participants: N = 298; IG: n = 148; CG: n = 150; Nurses: N = 10 N in analysis: Participants: N = 219; IG: n = 111; CG: n = 108; Nurses: N = 10 |
|
| Interventions |
Content of screen:HRQoL: tool = EORTC QLQ‐C30: validated HRQoL measure with 5 functional scales, 9 symptom scales, and 2 General Health and QoL items, no total score can be computed. If applicable, a specific module for breast cancer (QLQ‐BR23), colorectal cancer (QLQ‐CR38), or lung cancer (QLQ‐LC13) was added. Screenings interventionist: No interventionist for screening act, self‐completion of screening tool. Intervention procedure:Solitary SI: Participants completed the EORTC questionnaire on touch screen computer in outpatient clinic. A graphic results summary was generated and given to participant and nurse before consultation (outpatient visit 2, 3, 4, 5 = study visit 1, 2, 3, 4). No specific guidelines were provided on how the HRQoL summary data could/should be used during consultations Conditions for implementation
Comparative condition: CG: usual care Length of follow‐up: 4 consecutive outpatient visits |
|
| Outcomes |
Primary outcomes
Secondary outcomes: / Outcome time points: Second outpatient visit (first study visit = baseline); fifth outpatient visit (fourth study visit) |
|
| Notes | For bias judgement on NRCTs, see Table 3; Table 4; Table 9 | |
Hollingworth 2013.
| Methods | RCT ‐ with intervention group (IG) and usual care control group (UCG) | |
| Participants | Patients undergoing outpatient chemo‐ or radiotherapy Country: UK Age: IG: mean 61 years (12.2 SD); CG: mean 62 years (11.5 SD) Sex: IG: 67.9% female; CG: 59.3% female Inclusion criteria
Exclusion criteria
N randomised: n = 220; IG: n = 112; CG: n = 108 N in analysis: n = 220; IG: n = 112; CG: n = 108 |
|
| Interventions |
Content of screen:DISTRESS: tool = DT, distress by self‐report of participants on an 11‐point scale ranging from 0 ('none') to 10 ('extreme'). PL of physical, practical, family, emotional, and spiritual concerns ('yes' ‐ 'no') refined in this study to a 42‐item list Interventionist: A radiographer or nurse to conduct the screening conversation Intervention procedure:SI with co‐intervention to use screening results: During second week of radiotherapy or second cycle of chemo, participants completed the DT&PL as basis of a therapeutic conversation with the radiographer/nurse: concerns identified, potential solutions discussed, staff actions/patient actions/referral taken. At the discretion of the participant, a second DT&PL meeting could be arranged toward the end of therapy Conditions for implementation
Comparative condition: Usual care group Length of follow‐up: 12 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome time points: Baseline, 1 month, 6 months, 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based 1:1 allocation, stratified by site |
| Allocation concealment (selection bias) | Unclear risk | Unclear which method was used to conceal the allocation to conditions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and therapists were aware of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researcher/outcome assessor was blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout from baseline to 12 months' postbaseline +/− 5%; distributed equally between both conditions; reasons for dropout: death, withdrawal, lost contact |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but the published reports include all expected outcomes |
| Other bias | Low risk | / |
Kutner 1999.
| Methods | Cluster RCT ‐ with an intervention group (IG) and a control group (CG) | |
| Participants | Cancer patients scheduled for a follow‐up visit in an ambulatory cancer clinic Country: USA Age: Physicians: IG: 41.7 ± 6.9, CG: 42.2 ± 6.3; Participants: IG: 51.5 ± 16.4, CG: 55.6 ± 13.3 Sex: Physicians: IG: 33% female, CG: 20% female; Participants: IG: 44% female, CG: 66% female Inclusion criteria
Exclusion criteria N randomised: Physicians: n = 11, IG: n = 6, CG: n = 5; Participants: n = 282, IG: n = 149, CG: n = 133 N in analysis: baseline: Physicians: n = 11, IG: n = 6, CG: n = 5; Participants: n = 282, IG: n = 149, CG: n = 133 |
|
| Interventions |
Content of screen:CARE NEEDS: Needs assessment questionnaire adapted from published instruments, exploring needs in 13 domains: intensive care, financial, self‐care, future, symptom relief, treatment, emotional, spiritual, test, prevention, diagnosis, referral, and advance directives Interventionist: No interventionist for screening act, self‐completion of screening tool Intervention procedure:Solitary SI: Participants completed a pre‐visit needs assessment questionnaire; completed forms were attached to the patient charts prior to the clinic visit. Physicians were aware of this information, but were not instructed in use of the information provided Conditions for implementation: A person or system that gives/sends the pre‐visit questionnaire to participants and attaches it to patient files Comparative condition: Usual care: not further specified Length of follow‐up: No follow‐up |
|
| Outcomes |
Primary outcomes
Secondary outcomes: / Outcome time points: only 1, postvisit |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear what method was used to randomise the physicians |
| Allocation concealment (selection bias) | Unclear risk | Unclear which method was used to conceal the allocation to conditions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of physicians or participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were collected with postvisit questionnaires in both conditions No extra person for outcome assessment aware of condition allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 outcome time point, so no potential for missing data due to loss in follow‐up. No indication for other missing data |
| Selective reporting (reporting bias) | Low risk | Incomplete reporting of outcomes (only significant subscales reported for patient satisfaction, without a measure of the spread of the data) |
| Other bias | High risk | Adjusted P values reported everywhere to adjust for clustering, but no information on how this adjustment was done It is clear that there is a huge difference in baseline characteristics, which is the result of clustering at the physician level, but this becomes non‐significant when "clustering is taken into account". Nevertheless, this remains problematic |
Livingston 2010.
| Methods | Cluster RCT ‐ with 2 intervention groups (IG‐4 and IG‐1) and 1 control group (CG) | |
| Participants | Newly diagnosed prostate cancer and male colorectal cancer patients Country: Australia Age: IG‐4 Outcalls: mean 65.3 years (8.9 SD); IG‐1 Outcall: mean 64.2 years (8.8 SD); CG‐passive referral: mean 63.9 years (9.0 SD) Sex: All male Inclusion criteria
Exclusion criteria
N randomised: N = 571; IG‐4: n = 209; IG‐1: n = 197; CG: n = 165 N in analysis: Variety in sample size according to timing of outcome measurement IG‐4 Outcalls: baseline n = 209, 4 months n = 136, 7 months n = 194, 12 months n = 194 IG‐1 Outcall: baseline n = 225, 4 months n = 183, 7 months n = 174, 12 months n = 166 CG‐Passive Referral: baseline n = 165, 4 months n = 157, 7 months n = 153, 12 months n = 147 |
|
| Interventions |
Content of screen:BIOPSYCHOSOCIAL WELL‐BEING: Discussion of 10 topics during outcall: the cancer diagnosis; treatment/management issues; what to expect from surgery; management of side effects; communication with the specialist; partner/family issues; psychological/emotional and communication concerns; understanding cancer language; diet and nutrition; other support services and availability of written resources. If the participants did not mention a topic, the cancer nurse raised the topic. Interventionist: A Cancer Helpline nurse to conduct the screening/assessment. Intervention procedure:SI with co‐intervention to use screening results: IG‐Active Referral‐4 outcalls (IG‐4): a specialist referral to the Helpline with 4 outcalls to the participant (telephone assessment) within 1 week of diagnosis, 6 weeks, 3 and 6 months postdiagnosis IG‐Active Referral‐1 outcall (IG‐1): a specialist referral to the Helpline and 1 outcall (telephone assessment) within 1 week of diagnosis. If topic (of content of screen) not mentioned by participant, it was raised by the nurse. Conditions for implementation:
Comparative condition: Usual care group Length of follow‐up: 12 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes: / Outcome time points: study entry; 4, 7, and 12 months' postdiagnosis |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers produced by the project co‐ordinator |
| Allocation concealment (selection bias) | High risk | Both study co‐ordinator and referring specialist were aware of intervention group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of intervention group. Blinding of personnel not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not aware of intervention/control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout from baseline to 12 months' postbaseline +/− 12%; equally distributed between both conditions; reasons for dropout: death, withdrawal, refused |
| Selective reporting (reporting bias) | High risk | Incomplete reporting of the data of intervention group 1 outcall |
| Other bias | Low risk | / |
Maunsell 1996.
| Methods | RCT ‐ with intervention group (IG) and control group (CG) | |
| Participants | Women with newly diagnosed localised or regional stage breast cancer Country: Canada Age: IG: mean 54.6 years (12.4 SD); CG: mean 56.3 years (13.2 SD) Sex: All female Inclusion criteria
Exclusion criteria
N randomised: n = 261; IG: n = 131; CG: n = 130 N in analysis: n = 250; IG: n = 123; CG: n = 127 |
|
| Interventions |
Content of screen:DISTRESS: tool = GHQ‐20 measuring increases in psychologic symptoms (somatic items not used for this purpose). GHQ ≥ 5 considered to be symptomatic Interventionist: Telephone screener (research assistant) and social worker to discuss results and give support. Intervention procedure:SI with co‐intervention to use screening results: Systematic telephone screening of psychologic distress, starting at 21 days after randomisation, repeated at 28‐day intervals, for 12 times Participants with high scores were called by social worker to discuss reasons for increased distress, desire for further contact with social worker, and tailored approach Conditions for implementation
Comparative condition: Usual care group Length of follow‐up: 12 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome time points: Baseline, 3 and 12 months after randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Randomisation communication by the clinic secretary with sealed envelopes prepared by the principal investigator, but not clearly stated if the envelopes were opaque and opened sequentially |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The research nurse who carried out the baseline interview was blinded to participants' treatment assignment, no further blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The research nurse who conducted the baseline and all following interviews was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate, dropout from baseline to 12 months' postbaseline +/− 5% |
| Selective reporting (reporting bias) | Unclear risk | It seems that data for some outcomes are not given (e.g. LES) |
| Other bias | Low risk | / |
Nimako 2015.
| Methods | RCT ‐ with intervention group (IG), usual care control group (UCG), and attention control group (ACG) | |
| Participants | Patients of all ages with a diagnosis of a thoracic cancer who had recently completed treatment. Country: UK Age: IG: mean 64.6 years; ACG: mean 64.7 years; UCG: 62.9 years Sex: IG: 44% female; ACG: 45% female; UCG: 46% female Inclusion criteria
Exclusion criteria
N randomised: N = 138; IG: n = 45; ACG: n = 47; UCG: n = 46 N in analysis Baseline measures: N = 138; IG: n = 45; ACG: n = 47; UCG: n = 46 6 weeks' measures: N = 131; IG: n = 42; ACG: n = 45; UCG: n = 44 |
|
| Interventions |
Content of screen:HRQoL: tool = EORTC QLQ‐C30: 5 functional scales, 9 symptom scales, and 2 General Health and QoL items, no total score can be computed Interventionist: No interventionist for screening act, self‐completion of screening tool Intervention procedure:Solitary SI
Conditions for implementation:
Comparative condition: Usual care group Length of follow‐up: 6 weeks |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome time points: baseline and 6 weeks after baseline |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Electronic randomisation is mentioned, however exact method of sequence generation is unclear |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned who allocated the participants to the 3 conditions and how this was done, unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and doctors were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QoL assessments: completed on paper and over the phone, unclear if the telephone assessor was blinded QoL issues identification and management: outcome assessment by the principal investigator based on the record chart completed by the unblinded doctor and the GP letter |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data appear to be complete, dropout from baseline to 6 weeks' postbaseline +/− 7% |
| Selective reporting (reporting bias) | High risk | Only data on the Global Health question of the EORTC QLQ‐C30 was used/reported for control group while whole questionnaire was administered by participants in the control group |
| Other bias | Low risk | / |
Rosenbloom 2007.
| Methods | Stratified 3‐arm RCT ‐ with intervention group (IG), usual care control group (UCG), and assessment control group (ACG) | |
| Participants | Adult patients with advanced cancer Country: USA Age: IG: mean 57.3 years (11.8 SD); ACG: mean 60.2 years (11.0 SD); UCG: mean 60.6 years (9.3 SD) Sex: IG: 67% female; ACG: 70% female; UCG: 64% female Inclusion criteria
Exclusion criteria
N randomised: Unclear: there was dropout due to worsening illness (n = 10) and death (n = 46); analysis techniques were chosen with non‐random missing data in mind N in analysis: N = 213; IG: n = 69; ACG: n = 73; UCG: n = 71 |
|
| Interventions |
Content of screen:HRQoL: tool = FACT‐G: 5 subscales measuring physical, functional, social‐familial, and emotional well‐being, and relation with the physician. Scores on the subscales can be summed to produce a total QoL scale; 9 breast/lung/colon cancer specific items; question if experience of particular symptom was better than/worse than expected Interventionist: An interviewer to conduct the screening Intervention procedure:SI with co‐intervention to use screening results
Conditions for implementation
Comparative condition: UCG: usual care Length of follow‐up: 6 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes: / Outcome time points: baseline; 3 months; 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear what method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Unclear which method was used to conceal the allocation to conditions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and treatment staff were not blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout from baseline to 6 months' assessment +/− 28% |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | / |
Schofield 2013.
| Methods | RCT ‐ with intervention group (IG) and control group (CG) | |
| Participants | Adult patients with inoperable lung cancer Country: Australia Age: IG: mean 62.3 years (9.2 SD); CG: mean 63.8 years (11.4 SD) Sex: IG: 43.6% female; CG: 35.8% female Inclusion criteria
Exclusion criteria
N randomised: N = 108; IG: n = 55; CG: n = 53 N in analysis: N = 108; IG: n = 55; CG: n = 53 |
|
| Interventions |
Content of screen:CARE NEEDS: The 38‐item Needs Assessment for Advanced Lung Cancer Patients with subscales: medical communication/information, psychological/emotional, daily living, financial, symptoms, and social Interventionist: Self‐completion of the needs assessment, but a trained cancer health professional needed for the results discussion Intervention procedure:SI with co‐intervention to use screening results: 2 sessions (treatment commencement and completion): self‐completed needs assessment + intervention with active listening, self‐care education and communication of unmet psychosocial and symptom needs to the multidisciplinary team for management and referral Conditions for implementation
Comparative condition: Usual care group Length of follow‐up: From treatment commencement to 12 weeks' post‐treatment completion: length depends on length of treatment |
|
| Outcomes |
Primary outcomes:
Secondary outcomes: / Outcome time points: baseline; 8‐week post‐treatment completion; 12‐week post‐treatment completion |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, weighted‐biased coin method, including stratification according to scheduled treatment (palliative chemotherapy, radical radiotherapy, and palliative radiotherapy) |
| Allocation concealment (selection bias) | Unclear risk | Unclear which method was used to conceal the allocation of physicians to conditions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding: very involved multidisciplinary team, IG and CG may not have been sufficiently different. Tape‐recorded consultations run by 2 individuals not involved in providing usual care to ensure that there was no contamination between conditions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes collected with self‐report questionnaires, no extra person for outcome assessment aware of condition allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout from baseline to 12 weeks' post‐treatment completion +/− 27%; missing intervention consultations and/or outcome assessment due to scheduling issues, withdrawal, worsened health, death |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | / |
Singer 2017.
| Methods | Cluster RCT ‐ with intervention group (IG) and control group (CG) | |
| Participants | Adult cancer patients receiving oncological treatment in several wards of a university hospital Country: Germany Age: CG: mean 64 years (range 24 to 89); IG: mean 63 years (range 19 to 91) Sex: CG: 29.9% female; IG: 44.4% female Inclusion criteria
Exclusion criteria
N randomised: 13 wards (7 intervention, 6 control), patient sample: n = 1012, IG: n = 570, CG: n = 442 N in analysis Baseline at start of treatment n = 1012, IG: n = 570, CG: n = 442 Analysed for referral at end of treatment n = 1012, IG: n = 570, CG: n = 442 Analysed for well‐being at 0.5 years n = 575, IG: n = 341, CG: n = 234 |
|
| Interventions |
Content of screen:WELL‐BEING: depression (PHQ); anxiety (Generalized Anxiety Screener); fatigue, pain, and financial difficulties (EORTC QLQ‐C30 items) Interventionist: No interventionist for screening act, self‐completion of screening tool on tablet computer. In case help of a research nurse was needed for tool completion, this nurse did not fulfil a role in the clinical team. Intervention procedure:SI with co‐intervention to use screening results
Conditions for implementation
Comparative condition: Usual care, with referral to psychosocial services in case the doctor or nurse felt this was needed for a participant Length of follow‐up: Throughout the whole care trajectory. Screening was applied once and discussed with the participant by the doctor. If the participant and doctor decide that psychological support is needed, or the participant has financial, vocational, or other social problems, the hospital’s psycho‐oncological consultation liaison (CL) service or social service is informed and provides care or support. If necessary, further support in the outpatient setting when the participant is discharged from the hospital is organised by these 2 teams |
|
| Outcomes |
Primary outcomes
Secondary outcomes:
Outcome time points: at the beginning and the end of their hospital stay (= baseline), 3 months and 6 months after baseline |
|
| Notes | This trial was funded by the German Federal Ministry of Health within the framework 'Research Within the German National Cancer Plan' (#NKP‐332‐026) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Allocation was stratified according to the average frequency of CL service in the past 2 years per ward”. Unclear how the sequence was generated |
| Allocation concealment (selection bias) | Low risk | “The Interdisziplinäres Zentrum Klinische Studien performed the randomization independently, and the project manager then unsealed the ward numbers for each arm. Only he knew which number belonged to which ward.” Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “The patients were not told to which group they had been randomized. However, the intervention itself obviously could not be blinded. The doctors could not be blinded because they had to change their consultation behavior in the intervention arm" Participants and doctors are not blinded. Not unlikely that this affects actual outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study nurses collected data either always on intervention wards or always on control wards. They did not change trial arms. Unclear if they were aware of the trial arm they were in. Unclear who collected data after 3 and 6 months |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large (29% IC and 36% CG) dropout. Factors considered to be potentially related with non‐participation and dropout were as follows: age, sex, marital status, education, income, employment status, tumour site, and stage of disease. However, only reasons for non‐participation at baseline were queried in participants |
| Selective reporting (reporting bias) | High risk | The outcomes of primary interest (primary and secondary outcomes) are reported, however not all outcomes specified in the protocol paper are discussed in the published results papers. Authors state that papers on the other outcomes are written but not yet accepted for publication (publication bias) |
| Other bias | High risk | There is a clear imbalance between intervention and control group with respect to gender, type of cancer, stage of cancer, and likely other, unknown factors as well. This is a consequence of a low number clusters, which differ from one another to a great extent. This compromises the conclusions made |
Taenzer 2000.
| Methods | Sequential cohort study ‐ first a control cohort (CG), sequentially an experimental cohort (IG) | |
| Participants | Outpatient lung clinic of specialised cancer centre Country: Canada Age: IG: mean 65.6 years (10.5 SD); CG: mean 64.4 (9.7 SD) Sex: IG: 37% female; CG: 35% Inclusion criteria
Exclusion criteria: Unclear N recruited: N = 57; IG: n = 29; CG: n = 28 N in analysis: N = 53; IG: n = 27; CG: n = 26 |
|
| Interventions |
Content of screen:HRQoL: tool = EORTC QLQ‐C30: validated HRQoL measure with 5 functional scales, 9 symptom scales, and 2 General Health and QoL items, no total score can be computed Screenings interventionist: No interventionist for screening act, self‐completion of screening tool Intervention procedure:Solitary SI: Participants completed the computerised EORTC QLQ‐C30 before their clinic appointment (with help of a trained volunteer if needed); a report was generated and given to the nurse and physician Conditions for implementation
Comparative condition: CG: Usual care: after completion of the clinic appointment participants completed a paper‐and‐pencil version of the EORTC QLQ‐C30. No EORTC report was generated for the clinical staff Length of follow‐up: No follow‐up |
|
| Outcomes |
Primary outcomes
Secondary outcomes: / Outcome time points: 1 single outcome measurement, after the clinical appointment |
|
| Notes | For bias judgement on NRCTs, see Table 3; Table 4; Table 9 | |
Thewes 2009.
| Methods | Sequential cohort study ‐ first a control cohort (CG), sequentially an experimental cohort (IG) | |
| Participants | Rural oncology patients Country: Australia Age: mean age (SD) total sample: 60 years (10.5) Sex: 45.7% woman in the total sample Inclusion criteria:
Exclusion criteria: / N recruited: n = 83; IG: n = 43; CG: n = 40 N in analysis: baseline: N = 83; after 6‐month follow‐up: n = 65 (of 83, 2 withdrew and 16 died); follow‐up questionnaires fully completed: n = 52 |
|
| Interventions |
Content of screen:DISTRESS: Distress Thermometer (DT), a single‐item screening measure that identifies level of distress by self‐report of patients on an 11‐point scale ranging from 0 ('none') to 10 ('extreme') Screenings interventionist: No interventionist for screening act, self‐completion of screening tool Intervention procedure:Solitary SI: Completion of DT at baseline before an initial oncologist rural clinical appointment or chemotherapy education session Comparative condition: CG: usual care without DT screening Length of follow‐up: 6 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes: / Outcome time points: Baseline (before an initial oncologist rural clinical appointment or chemotherapy education session); 6 months after baseline |
|
| Notes | For bias judgement on NRCTs, see Table 3; Table 4; Table 9 | |
van der Meulen 2018.
| Methods | RCT with intervention group (IG) and control group (CG) | |
| Participants | Adult patients with head and neck cancer visiting a university outpatient clinic of oral maxillofacial and otorhinolaryngology Country: the Netherlands Age: CG: mean 64.5 years (11.3 SD); IG: mean 62.4 years (11.5 SD) Sex: CG: 26.39% female; IG: 24.5% female Inclusion criteria
Exclusion criteria: / N randomised: N = 110, CG: n = 57, IG: n = 53 N in analysis: At baseline N = 110, CG: n = 57, IG: n = 53; at 6 months N = 90, CG: n = 52, IG: n = 38; at 12 months N = 78, CG: n = 45, IG: n = 33 |
|
| Interventions |
Content of screen:DISTRESS: Distress Thermometer (DT) + Problem List (PL) Interventionist: No interventionist for screening act, self‐completion of screening tool on paper Intervention procedure:SI with co‐intervention to use screening results
Conditions for implementation: Training for the nurses to increase the skills needed for delivering the intervention in a uniform manner: theoretical background of the DT & PL, practical steps of the procedure, role playing Comparative condition: Usual care, participants received care provided by their head and neck cancer specialist or physician at 2‐month intervals in the first year after cancer treatment and at 2‐month intervals in the second year. No formal time reserved to discuss the participants’ psychosocial concerns. However, opportunity for referral if considered appropriate by treating physician Length of follow‐up: 1 year |
|
| Outcomes |
Primary outcomes
Secondary outcomes: / Outcome time points: 3 times: baseline (i.e. 0 to 6 months after cancer treatment) (M1), at 6 months (M2), and 12 months (M3) after baseline |
|
| Notes | This research was funded by grants from the Dutch Cancer Society and the oral maxillofacial clinic at the University Medical Center Utrecht. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation with a block procedure, stratified by gender, cancer site (oral/oropharyngeal cancer versus hypopharyngeal/laryngeal cancer), and treatment status (new patients, 0 to 3 months after cancer treatment, and 0 to 6 months after cancer treatment). Likely to be adequate |
| Allocation concealment (selection bias) | Unclear risk | Unclear how participants were allocated to the conditions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, participants and nurses conducting the screening discussions could not be blinded. Unclear if the treating physicians were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants completed the outcome questionnaires at home and returned them with the return envelope that was provided. These completed questionnaires were analysed by the researcher who was not blinded to the conditions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large (38% IC and 22% CG) dropout (no collection of reasons for dropout) with significant differences shown between those lost to follow‐up and those who completed the study |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
| Other bias | High risk | Study is underpowered: “a final sample size of 103 patients per group ([1 – 0.542] x 144 + 1), resulting in a total of 206 patients. On the basis of previous studies (de Graeff et al, 1999; van der Meulen et al, 2013), the authors expected that 70% of eligible patients would be included. Therefore, at least 288 patients had to be approached.” While “In six months, 213 patients were invited to participate in the study, of whom 110 (52%) were enrolled.” Study has problems with fidelity of the intervention: the objective to screen patients 3 to 4 times a year was not met, which could have biased the results: “Of the 53 participants allocated to the intervention group, 26 received 1 to 2 sessions, 12 received 3–4 sessions, and 5 received 5 sessions.” |
Velikova 2004.
| Methods | Stratified 3‐arm RCT ‐ with intervention group (IG), usual care control group (UCG), and attention control group (ACG) | |
| Participants | Cancer patients with different tumour types and treatments, and oncology consultants and physicians in training Country: UK Age: IG: mean 55.1 years (13.02 SD); ACG: mean 54.8 years (12.49 SD); UCG: mean 54.7 years (11.67 SD). Sex: IG: 75% female; ACG: 70% female; UCC: 74% female Inclusion criteria: Patients
Exclusion criteria: Patients
N randomised: N = 286; IG: n = 144; ACG: n = 70; UCG: n = 72 (article 2010: n = 258, IG: n = 129, ACG: n = 62, UCG: n = 67) N in analysis: Sample size for analysis (baseline to 6 months): total (286 to 164); IG (144 to 84 or 85?); ACG (70 to 35); UCG (72 to 45) |
|
| Interventions |
Content of screen:HRQoL: tool = EORTC QLQ‐C30: measure with 5 functional scales, 9 symptom scales, and 2 General Health and QoL items, no total score can be computed. Distress: tool = HADS: 14 items, Anxiety (n = 7), Depression (n = 7), total score can be computed Interventionist: No interventionist for screening act, self‐completion of screening tool. Intervention procedure:Solitary SI
Conditions for implementation:
Comparative condition: UCG: usual care Length of follow‐up: 6 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes: / Outcome time points: baseline; after 3 on‐study encounters (approximately 2 to 3 months); 4 months; 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate: randomisation at the level of the participants following an allocation ratio of 2:1:1 in favour of IC and stratified by cancer site |
| Allocation concealment (selection bias) | Low risk | Adequate: the random assignment was carried out by telephone, by the Administrative Office at Cancer Research UK |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blinded, physicians were not. It is possible that the experience with the HRQoL profiles given in the IC influenced physicians’ practice when seeing patients in the control arms. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout of participants from baseline to 6 months' assessment +/− 43% and not equally distributed between conditions (42%, 50%, and 38% for the IG, ACG, and UCG, respectively) |
| Selective reporting (reporting bias) | High risk | Not adequate: means (SD) over time for the FACT‐G scores are given visually, only the exact P values are given |
| Other bias | Low risk | / |
Waller 2012.
| Methods | Quasi‐experimental interrupted time series design ‐ first a control group (CG), sequentially intervention group (IG) | |
| Participants | Patients with advanced cancer Country: Australia Age: At T0: mean 66.1 years (SD 10.7; range 31 to 89) Sex: At T0: 47% female Inclusion criteria
Exclusion criteria: / N recruited: N = 219 consented, n = 195 completed baseline measurement N in analysis: Variable according to time point: T‐3 (n = 70); T‐2 (n = 122); T‐1 (n = 160); T0 (n = 192); T1 (n = 103); T2 (n = 85); T3 (n = 67) |
|
| Interventions |
Content of screen:CARE NEEDS: tool = NAT:PD‐C
Screenings interventionist: Healthcare professionals (several disciplines) use the tool to assess the issues in the consult with the participant Intervention procedure:Solitary SI: healthcare professionals complete the NAT:PD‐C during consultation and use the resulting insights in their discussion of and referral for participants' specific care needs or issues. Conditions for implementation
Comparative condition: CG: usual care without use of the NAT:PD‐C or training of the professionals on the Palliative Care Needs Assessment Guidelines Length of follow‐up: 18 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome time points: 7 times: 6, 4, and 2 months before intervention implementation (T‐3, T‐2, T‐1); at start, 2, 4, and 6 months past intervention implementation (T0, T1, T2, T3) |
|
| Notes | For bias judgement on NRCTs, see Table 3; Table 4; Table 9 | |
Williams 2013.
| Methods | Quasi‐experimental historically controlled study ‐ with control group (CG) and intervention group (IG) | |
| Participants | Adult cancer patients that had started chemo‐ and/or radiotherapy Country: USA Age: IG: mean 58.24 years (9.14 SD), CG: mean 62.33 years (10.49 SD) Sex: IG: 55.2% female; CG: 63.6% female Inclusion criteria
Exclusion criteria: / N recruited: N = 128, IG: n = 64, CG: n = 64 N in analysis: N = 113; IG: n = 58; CG: n = 55 |
|
| Interventions |
Content of screen:PHYSICAL AND PSYCHOLOGICAL SYMPTOMS: tool= TRSC: PROM; 25 symptoms (taste change, loss of appetite, nausea, vomiting, weight loss, sore mouth, cough, sore throat, difficulty swallowing, jaw pain, shortness of breath, numbness of fingers/toes, feeling sluggish, depression, difficulty concentrating, fever, bruising, bleeding, hair loss, skin changes, soreness in vein where chemotherapy was given, difficulty sleeping, pain, decreased interest in sexual activity, constipation) rated using a 5‐point scale; 0 (not present) to 4 (very severe); scores indicate occurrence and severity Screenings interventionist: No interventionist for screening act, self‐completion of screening tool. Intervention procedure:Solitary SI: participants completed TRSC prior to clinical consultation. Clinicians received results of the completed screening intervention form prior to consultation, however they received no training on how to use the form Conditions for implementation
Comparative condition: CG: usual care: chemo‐ and radiotherapy, with a wide range of supportive therapies available. Documentation and management of symptoms is done by clinicians and nurses using the standard clinic interview and medical record Length of follow‐up: 4 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome time points: variable: RT patients completed instruments once weekly on the same day each week. CT patients completed instruments on the day of provider evaluation prior to receiving CT on day 1 of each cycle. The number of RT and CT cycles varied, depending on treatment protocol |
|
| Notes | For bias judgement on NRCTs, see Table 3; Table 4; Table 9 | |
Young 2010.
| Methods | Prospective non‐randomised controlled study – first an intervention group (IG), sequentially a usual care control group (UCG) | |
| Participants | Adult colorectal cancer patients that had undergone surgery Country: Australia Age: IG: mean 66.9 years; CG: mean 64.5 years Sex: IG: 40% female; CG: 50% female. P = 0.4 Inclusion criteria:
Exclusion criteria
N recruited: n = 41; IG: n = 20; CG: n = 21 N in analysis: n = 41; IG: n = 20; CG: n = 21 |
|
| Interventions |
Content of screen:CARE NEEDS: Checklist with 6 areas of potential need (general health, wound, bowel function, investigations/appointments, psychosocial and information needs) Screenings interventionist: Intervention nurse to conduct the telephone screening Intervention procedure:SI with co‐intervention to use screening results: 5 calls in 6 months following participant’s discharge, on days 3 and 10 and at 1, 3, and 6 months. At each time point the nurse makes inquiries regarding each aspect of need on checklist. If a need is identified, nurse provides information, checks understanding, and provides emotional support and advice. The participant is directed back to the clinical team if further clinical advice or referral is warranted Conditions for implementation
Comparative condition: CG: usual care: CG recruited in month 4 to 6 of the study, receiving usual care following discharge from hospital Length of follow‐up: 6 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes: / Outcome time points: 1 month; 3 months |
|
| Notes | For bias judgement on NRCTs, see Table 3; Table 4; Table 9. | |
Young 2013.
| Methods | Cluster RCT ‐ with an intervention group (IG) and a control group (CG) | |
| Participants | Adult patients undergoing surgery for primary colorectal cancer Country: Australia Age: IG: mean 68.6 years; CG: mean 67.0 years Sex: IG: 43.2% female; CG: 45.8% female Inclusion criteria
Exclusion criteria
N randomised: N = 775; IG: n = 398; CG: n = 377 N in analysis Baseline: N = 756, IG: n = 387, CG: n = 369 1 month: N = 709, IG: n = 363, CG: n = 346 3 months: N = 687, IG: n = 336, CG: n = 351 6 months: N = 672, IG: n = 350, CG: n = 322 |
|
| Interventions |
Content of screen:CARE NEEDS: Each call includes 22 standardised screening questions about common physical, psychosocial, information, supportive care, and rehabilitation/follow‐up needs. At 1 month, for cancer patients with type C colon cancer, topic of adjuvant chemotherapy was raised Interventionist: Colorectal cancer nurse who conducts the telephone screenings, employed especially for this study Intervention procedure:SI with co‐intervention to use screening results: 5 scheduled, structured telephone calls on days 3 and 10 and at 1, 3, and 6 months after hospital discharge to screen for needs. Identified needs were addressed by the intervention nurse using detailed, standardised clinical protocols according to the nature and severity of the need and level of clinical risk posed. For low‐risk needs, the nurse provided relevant information and advice so that the participant could seek appropriate assistance from their local care providers. For a serious or potentially high‐risk problem (e.g. suicidal ideation), the intervention nurse contacted a member of the participant’s local healthcare team directly. No independent referrals to other health professionals were made Conditions for implementation
Comparative condition: Usual care group Length of follow‐up: 6 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome time points: 1, 3, and 6 months after discharge |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer‐generated random‐number list |
| Allocation concealment (selection bias) | Unclear risk | Unclear which method was used to conceal the allocation to conditions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and staff were not blinded, intervention group participants received the telephone calls, and hospital staff were contacted by the intervention nurse (not part of clinical team) in case participants had problems or needs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were measured with self‐report questionnaires, no extra person for outcome assessment aware of condition allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors state "Follow‐up participation rates at 1, 3, and 6 months were 91.5%, 88.6%, and 86.7%, respectively". Dropout of participants from baseline to 6 months' assessment +/− 13%; equally distributed between both conditions |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but the published reports include all expected outcomes |
| Other bias | Low risk | / |
Note: In the 'Characteristics of studies' tables, all outcomes mentioned by the study authors are given. However, only the outcomes relevant for this systematic review are further discussed and included in the Evidence Summary (Table 5; Table 6; Table 7)
Abbreviations:
CaSUN: Cancer Survivors’ Unmet Needs Measure CES‐D: Center for Epidemiological Studies Depression Scale CG: control group COOP: the Dartmouth Primary Care Cooperative Information Functional Health Assessment CT: chemotherapy DanPEP: Danish Patients Evaluate General Practice DAS: Dyadic Adjustment Scale DIS/DSM: Diagnostic Interview Schedule according to Diagnostic and Statistical Manual of Mental Disorders criteria DT: Distress Thermometer ECOG: Eastern Cooperative Oncology Group EORTC IN‐PATSAT32: European Organisation for Research and Treatment of Cancer cancer in‐patient satisfaction with care questionnaire EORTC QLQ‐BR23: European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Breast Cancer 23 items EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Core 30 items EORTC QLQ‐CR38: European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Colorectal Cancer 38 items EORTC QLQ‐H&N35: European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Head and Neck Cancer 35 items EORTC QLQ‐LC13: European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Lung Cancer 13 items EQ‐5D: EuroQol 5D EQ‐5D‐3L: EurQol 5D‐lung cancer FACIT‐Sp: Functional Assessment of Chronic Illness Therapy‐Spiritual Well‐Being FACT‐B: Functional Assessment of Cancer Therapy‐Breast FACT‐C: Functional Assessment of Cancer Therapy‐Colorectal FACT‐G: Functional Assessment of Cancer Therapy‐General FACT‐L: Functional Assessment of Cancer Therapy‐Lung FLIC: Functional Living Index‐Cancer GHQ‐12: General Health Questionnaire 12‐item version GHQ‐20: General Health Questionnaire 20‐item version GP: general practitioner HADS: Hospital Anxiety and Depression Scale HRQoL: health‐related quality of life HRQoL‐LASA: Health‐Related Quality of Life Linear Analogue Self‐Assessment IES: Impact of Events Scale IG: intervention group IKNL: Integraal Kankercentrum Nederland LES: Life Experiences Survey LWMAT: Locke‐Wallace martial adjustment test MCQ: Medical Care Questionnaire MHLC: Multidimensional Health Locus of Control questionnaire MRCG: medical records control group
NA: not applicable NA‐ACP: Needs Assessment for Advanced Cancer Patients NAT:PD‐C: Needs Assessment Tool: Progressive Disease‐Cancer NRCT: non‐randomised controlled trial NSCLC: non‐small cell lung cancer PAIS‐SR: Psychosocial Adjustment to Illness‐Self Report PCQoL: Prostate Cancer Quality of Life questionnaire PC‐QOL: Prostate Cancer‐Related Quality of Life Scales PDIS: Patient‐Doctor Interaction Scale PHQ: Patient Health Questionnaire short form PL: Problem List POMS: Profile of Mood States PSI: Psychiatric Symptom Index PSQ: Patient Satisfaction Questionnaire PSQ‐III: Patient Satisfaction Questionnaire 3rd update PSYCH‐6: psychological subscale of the Somatic and Psychological Health Report QoL: quality of life QPP: Quality of Care from the Patient's Perspective questionnaire RC: rehabilitation co‐ordinator RCT: randomised controlled trial ROBINS‐I: Risk Of Bias In Non‐randomized Studies ‐ of Interventions RT: radiotherapy SCID: Structured Clinical Interview SCLC: small cell lung cancer SCNS: Supportive Care Needs Survey SCNS‐SF34: Supportive Care Needs Survey‐Short Form 34 SD: standard deviation SF‐36: 36‐item Short Form Health Survey SI: screening intervention SIPP: Screening Inventory of Psychosocial Problems TPVCSQ: Trent Patient Views of Cancer Services Questionnaire TRSC: Therapy‐Related Symptom Checklist UCG: usual care group WONCA: World Organisation Project of National Colleges and Academics
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bauwens 2014 | Outcomes do not meet protocol, care outcomes |
| Boyes 2006 | Comparison does not meet protocol, no usual care condition without screening |
| Carlson 2010 | Comparison does not meet protocol, no usual care condition without screening |
| Carter 2012 | Study design does not protocol, longitudinal study without control condition |
| Girgis 2014 | Outcomes do not meet protocol, care outcomes |
| Hoekstra‐Weebers 2012 | Comparison does not meet protocol, no usual care condition without screening |
| McLachlan 2001 | Comparison does not meet protocol, no usual care condition without screening |
| Sarna 1998 | Comparison does not meet protocol, no usual care condition without screening. |
| Stanciu 2015 | Comparison does not meet protocol, no usual care condition without screening. |
| Waller 2012a | Outcomes do not meet protocol, care outcomes |
Characteristics of studies awaiting assessment [ordered by study ID]
Amstel 2017.
| Methods | RCT ‐ with intervention group (IG) and control group (CG) |
| Participants | Patients treated with curative intent for breast cancer Country: the Netherlands Age: results not yet available, ≥ 18 years (inclusion criterion) Sex: 100% female (inclusion criterion) Inclusion criteria
Exclusion criteria
N randomised: based on power calculations, a total of 193 patients need to be included to have sufficient power for the primary and secondary outcomes N in analysis: results not yet available |
| Interventions |
Content of screen:DISTRESS: tool = Distress Thermometer (DT): consists of a thermometer ranging from 0 (no distress) to 10 (extreme distress). In addition, the tool contains 47 questions (yes/no answers) related to different issues known as the Problem List (PL). The issues have been categorised into: practical issues, family/social issues, emotional issues, religious/spiritual issues, and physical issues. The DT concludes with the question: 'Would you like to talk with a professional about your problems?' (yes/no/maybe). Interventionist: No interventionist for screening act (self‐reported measure). Intervention procedure:SI with co‐intervention to use screening results: The participant will fill out the DT in the outpatient clinic a few minutes before the appointment, and a trained oncology nurse will discuss the DT results with the participant and ask if she desires a referral. The time allocated to these meetings will be between 5 and 30 minutes, depending on the severity of the distress and the nature of the problems. If the participant reports a DT score of < 5, the nurse will inquire as to whether the participant is sufficiently in control of her situation. The low distress score and the issues marked on the PL are discussed briefly. At a score ≥ 5 on the DT, an extensive exploratory conversation between the nurse and the participant will take place. The outcome of this conversation will be discussed in a psychosocial MDT Conditions for implementation
Comparative condition: Usual care, without using the DT Length of follow‐up: 2 years |
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome time points: Questionnaires are obtained in both arms at baseline, after completion of each type of cancer treatment modality, and during follow‐up, with a 3 and 6 month interval during the first and second year, respectively |
| Notes | Information from conference abstracts and protocol paper available, no further data received from study authors since their results paper has not yet been accepted for publication Registered as ‘Nurse Intervention Project (VIP)’ in ClinicalTrials.gov (NCT01091584) |
Frennet 2011.
| Methods | Multicentre phase II RCT |
| Participants | Frail elderly patients with newly diagnosed cancer Country: not reported Age: mean 79.3 years (SD 5.8) Sex: 57.7% women Inclusion criteria
Exclusion criteria: VES‐13, score < 3/10 N randomised: (ongoing): IG: 53; CG: 58 N in analysis: not applicable (ongoing) |
| Interventions |
Content of screen:Comprehensive geriatric assessment. Exact content of screen unclear Interventionist: not reported Intervention procedure: not reported Conditions for implementation: not reported Comparative condition: conventional oncological management Length of follow‐up: unclear, 6 months? |
| Outcomes |
Primary outcomes: functional decline at 6 months (change in ADL score) Secondary outcomes: unclear Outcome time points: once at 6 months |
| Notes | Information from conference abstract available, no further information received from study authors |
Li 2017.
| Methods | Pre‐post quasi‐experimental design |
| Participants | Oncology patients in general Country: Canada Age: unclear Sex: unclear Inclusion criteria: unclear Exclusion criteria: unclear N randomised: unclear N in analysis: unclear |
| Interventions |
Content of screen:WELL‐BEING: ESAS‐r cut‐offs are used to trigger further ePROM assessment of pain (BPI), fatigue (CFS), anxiety (GAD‐7), and depression (PHQ‐9) Interventionist: unclear Intervention procedure: unclear Conditions for implementation: unclear Comparative condition: unclear Length of follow‐up: unclear |
| Outcomes |
Primary outcomes/Secondary outcomes
Outcome time points: 6 months' pre‐ and post‐iPEHOC intervention implementation |
| Notes | Information from conference abstract available ('Improving patient experience and health outcomes using electronic patient reported outcome measures: effects on distress and health outcomes'), no further information received from study authors |
Mehanna.
| Methods | RCT |
| Participants | Adult patients with head and neck cancer in follow‐up clinic Country: UK Age: adult Sex: men and women Inclusion criteria
Exclusion criteria
N randomised: targeted n = 44 N in analysis: unclear |
| Interventions |
Content of screen:HRQoL: tool = the FACT‐HN Interventionist: no interventionist for the screening act, self‐completion on a touch screen computer. Intervention procedure:Solitary SI: Participants complete the screening on a tablet touch screen computer before their clinic visit, and then take a printout of the results in when seeing the doctor or nurse (doctor‐ and nurse‐led clinics) Conditions for implementation: not reported Comparative condition: not reported Length of follow‐up: unclear, probably no follow‐up |
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome time points: baseline and 4 to 6 weeks following the intervention |
| Notes | Information from ISRCTN registry available, no further information received from study authors |
Munro 1994.
| Methods | RCT – with intervention group (IG) and control group (CG) |
| Participants | Outpatients attending for radiotherapy Country: UK Age: CG: median 65 years (37 to 88); IG: median 63 years (30 to 87) Sex: CG: 58.8% female, 41.2% male; IG: 57.1% female, 42.9% male Inclusion criteria
Exclusion criteria
N randomised: n = 100; IG: n = 49; CG: n = 51 N in analysis: IG: n = 44; CG: n = 51 |
| Interventions |
Content of screen:OVERALL WELL‐BEING: Questions to be asked: 'How are you feeling?' 'Are you having any problems?' 'Have you any further side effects from treatment?' 'Do you need to make an appointment to be seen in the Radiotherapy department before your outpatient appointment?' Participants were asked if they had any additional worries or concerns. Wherever possible, the appropriate action was taken. Interventionist: The telephone calls were made by a member of staff, radiographer, nurse, or doctor who was known to the participant. Intervention procedure:Solitary SI: Semi‐structured telephone calls to the participant on days 4, 8, 14, and 18 after completing radiotherapy. Conditions for implementation: A simple log form needed to record the responses to the set questions and any other relevant information for each telephone call. Comparative condition: Usual care group (i.e. having a once a week consultation in the clinic by a doctor during treatment + no contact during the period between completion of treatment and the first follow‐up visit). Length of follow‐up: Last phone call 18 days after completing radiotherapy, probably no further follow‐up provided as part of the study intervention |
| Outcomes |
Primary outcomes: Adequacy of support (CG + IG): ‘How adequate do you describe the support after treatment?’ Secondary outcomes: Helpfulness of telephone calls (IG): ‘How helpful do you find the telephone calls?’ Outcome time points: 4 weeks after completing radiotherapy treatment (i.e. at the first follow‐up visit) |
| Notes | Information from a journal article (Clinical Oncology, 1994) available, no further information received from study authors |
Powell 2008.
| Methods | RCT – with intervention group with completion (IGC), intervention without completion (IGWC), and control group (CG) |
| Participants | Gynaecologic cancer patients Country: USA Age: IGC: mean 52.2 years (30 to 78); IGWC: mean 47.2 years (27 to 76); CG: mean 49.8 years (24 to 79) Sex: 100% women Inclusion criteria
Exclusion criteria
N randomised: n = 100; IG (IGC + IGWC): n = 49; CG: n = 50 N in analysis: IGC: n = 21; IGWC: n = 28 (however, for IG only n = 45 completed baseline); CG: n = 51 |
| Interventions |
Content of screen:OVERALL WELL‐BEING: Issues and concerns that the woman may have about her symptoms and potential cancer diagnosis Interventionist: psychologist Intervention procedure:Solitary SI: Participants received a single, 1‐hour counselling session with a psychologist that focused on discussing issues and concerns that the woman may have about her symptoms and potential cancer diagnosis Conditions for implementation: having a psychologist in the setting who is available to conduct the counselling sessions with every patient Comparative condition: control group (usual care) Length of follow‐up: 3 months |
| Outcomes |
Primary outcomes/Secondary outcomes
Outcome time points: baseline (at the time of the counselling session); 2 weeks and 3 months after baseline |
| Notes | Information from a journal article (Gynecologic Oncology, 2008) available, no further information received from study authors |
Skorstengaard 2014.
| Methods | Patients from oncology, cardiology, and respiratory departments Country: Denmark Age: not reported Sex: not reported Inclusion criteria: patients from oncology, cardiology, and respiratory departments Exclusion criteria: not reported N randomised: not reported N in analysis: not reported |
| Participants |
Content of screen: well‐being and preferences for end‐of‐life care Interventionist: a healthcare professional conducts the discussion with the participant and if possible a relative Intervention procedure: unclear Conditions for implementation: not reported Comparative condition: usual care Length of follow‐up: unclear |
| Interventions |
Primary outcomes/Secondary outcomes
Outcome time points: unclear, relatives are questioned after participant death |
| Outcomes | Information from conference abstract available, no further information received from study authors |
| Notes |
Abbreviations:
ADL: Activities of Daily Living BPI: Brief Pain Inventory CFS: Chronic Fatique Symptoms CG: control group DT: Distress Thermometer EORTC QLQ‐BR23: European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Breast Cancer 23 items EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Core 30 items EORTC QLQ‐HN35: European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Head and Neck Cancer 35 items ePROM: electronic patient reported outcome measures ESAS‐r: Edmonton Symptom Assessment System‐revised FACIT‐II: Functional Assessment of Chronic Illness Therapy version 2 FACT‐HN: Functional Assessment of Cancer Therapy‐Head and Neck Cancer GAD‐7: Generalized Anxiety Disorder 7‐item scale HADS: Hospital Anxiety and Depression Scale HRQoL: health‐related quality of life IG: intervention group iPEHOC: Improving Patient Experience and Health Outcomes Collaborative MDT: multidisciplinary team PHQ‐9: Patient Health Questionnaire 9‐item depression module POMS: Profile of Mood States RCT: randomised controlled trial VES‐13: Vulnerable Elders Survey.
Characteristics of ongoing studies [ordered by study ID]
Bernacki 2015.
| Trial name or title | Registered as 'Serious Illness Communication Project' in ClinicalTrials.gov (NCT01786811) |
| Methods | Cluster RCT ‐ with intervention group (IG) and control group (CG) |
| Participants | Patients with advanced, incurable cancer and life expectancy of < 12 months and their surrogate Country: USA Age: results not yet available, ≥ 18 years (inclusion criterion) Sex: results not yet available Inclusion criteria
Exclusion criteria: / N randomised: based on power calculations a total of 426 participants (213 per group) will be accrued at an estimated accrual rate of 200 participants per year N in analysis: results not yet available |
| Interventions |
Content of screen:Overall well‐being, information and care preferences: tool = Serious Illness Conversation Guide (SICG): addresses eliciting illness understanding, eliciting decision‐making preferences, sharing prognostic information according to preferences, understanding goals and fears, exploring views on trade‐offs and impaired function, and wishes for family involvement Interventionist: the treating clinician (oncologists) uses the SICG in the outpatient encounter with the participant. Intervention procedure:SI with co‐intervention to use screening results: Participants are sent a letter encouraging them to think about some of the topics raised in the SICG to prepare them for the conversation with their doctor. During the clinical encounter, clinicians use the SICG to conduct participants’ values and goals, document outcomes of the discussion in a structured format in the EMR, and provide participants with a Family Communication Guide to help them continue the discussion at home with their loved ones. Conditions for implementation
Comparative condition: usual care control group Length of follow‐up: at least 1 year or until death |
| Outcomes |
Primary outcomes
Secondary outcomes: anxiety (GAD‐7), depression (PHQ‐9), quality of life (SF‐12v2), therapeutic alliance (Human Connection Scale), quality of communication (QOC), and quality of dying (Brief RCOPE) and death. Outcome time points: baseline, and a following survey every 2 months |
| Starting date | June 2012 |
| Contact information |
Principal Investigator: Rachelle Bernacki, MD, MS Dana‐Farber Cancer Institute Principal Investigator: Atul Gawande, MD, MPH Harvard TH Chan School of Public Health Principal Investigator: Susan Block, MD Dana‐Farber Cancer Institute |
| Notes | Information from conference abstract and protocol paper available, no further data received from study authors. Results paper in progress |
Cooley 2014.
| Trial name or title | Title conference abstract: 'Point‐of‐care clinical decision support for cancer symptom management: results of a group randomized trial' |
| Methods | RCT ‐ with intervention group (IG) and control group (CG) |
| Participants | Cancer patients (no further specification in conference abstract) Country: USA Age: mean age of 63 years Sex: 58% female Inclusion criteria: unclear Exclusion criteria: unclear N randomised: n = 179, number of participants in each condition unclear. N in analysis: unclear |
| Interventions |
Content of screen:BIO‐PSYCHOSOCIAL WELL‐BEING: The symptom assessment resulted in insight on participants’ pain, fatigue, depression, anxiety, and/or dyspnoea Interventionist: Presumably use of a self‐completion tool, no interventionist for the screening act (“patients completed the web based symptom assessment”) Intervention procedure:SI with co‐intervention to use screening results: Participants completed the symptom assessment prior to each visit for 6 months. A tailored report provided a longitudinal symptom report, and suggestions for management were provided to clinicians in the SAMI arm prior to the visit. Conditions for implementation
Comparative condition: usual care condition Length of follow‐up: 6 months |
| Outcomes |
Primary outcomes
Secondary outcomes: management of the target symptoms (chart review) Outcome time points: baseline; 2, 4, and 6 months |
| Starting date | Unclear |
| Contact information | First author conference abstract: Prof Dr Mary E Cooley, Dana‐Farber/Harvard Cancer Institute, Boston |
| Notes | Information from conference abstract available, no further information received from study authors. Results paper in preparation |
Sussman 2012.
| Trial name or title | Title conference abstract: 'Results of a cluster randomized trial to evaluate a nursing lead supportive care intervention in newly diagnosed breast and colorectal cancer patients' |
| Methods | Cluster RCT ‐ with intervention group (IG) and control group (CG) |
| Participants | Newly diagnosed breast and colorectal cancer patients. Country: Canada Age: Results not yet available for the large group, unclear for the subgroup presented with the preliminary results Sex: Results not yet available for the large group, unclear for the subgroup presented in the preliminary results record Inclusion criteria
Exclusion criteria: unclear. N randomised: Results not yet available for the large group, 193 enrolled when preliminary results were presented at the conference N in analysis: Results not yet available for the large group, unclear for the subgroup presented in the preliminary results record |
| Interventions |
Content of screen:CARE NEEDS: No further information on assessment tool and exact content. Interventionist: A person that conducts the in‐person supportive care assessment. Intervention procedure:SI with co‐intervention to use screening results: An in‐person supportive care assessment is conducted followed by ongoing supportive care by telephone or in person including linkage to community services using protocol‐specified guidelines according to identified needs. Conditions for implementation:
Comparative condition: a control group involving usual care practices. Length of follow‐up: 8 weeks |
| Outcomes |
Primary outcomes:
Secondary outcomes:
Outcome time points: at 8 weeks |
| Starting date | Unclear |
| Contact information | First author conference abstract: Dr Jonathan Sussman, Juravinski Cancer Centre, Hamilton, Ontario |
| Notes | Information from conference abstract available, no further data received from study authors. Only preliminiary results were presented at conference, full results paper in progress. |
Abbreviations:
ADL: Activities of Daily Living CG: control group EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Core 30 items EORTC QLQ‐HN35: European Organisation for Research and Treatment of Cancer‐Quality of Life Questionnaire‐Head and Neck Cancer 35 items FACIT‐II: Functional Assessment of Chronic Illness Therapy version 2 FACT‐HN: Functional Assessment of Cancer Therapy‐Head and Neck Cancer GAD‐7: Generalized Anxiety Disorder 7‐item scale HRQoL: health‐related quality of life IG: intervention group PHQ‐9: Patient Health Questionnaire‐9 POMS: Profile of Mood States QOC: Quality of Communication Questionnaire RCT: randomised controlled trial SCNS: Supportive Care Needs Survey SF‐12v2: Short Form‐12 Health Survey version 2 VES‐13: Vulnerable Elders Survey
Differences between protocol and review
'DT' out of search strategy
Protocol: The abbreviation for the distress tool Distress Thermometer (DT) was included in the MEDLINE search strategy published in the Cochrane Review protocol.
Review: The abbreviation 'DT' was not used as a search term in the search strategy for the databases.
Explanation: When we conducted the search in Embase, we noticed that in a large number of records DT was not used as an abbreviation of Distress Thermometer, but of something not related to our review. Following the advice of the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group, we did not include DT in our search strategy in the conduct of the review.
Outcome health‐related quality of life (HRQoL)
Protocol: We specified QoL and HRQoL as separate primary outcomes.
Review: We addressed both in one outcome, namely HRQoL.
Explanation: In the included studies, the terms were used interchangeably, even when the same outcome instruments were used. We chose to combine them into one outcome.
Time span studied
Protocol: We specified that we would include records up to the end of 2015.
Review: We included records up to January 2018.
Explanation: Due to the length of time passed since the submission, review, revision, and acceptance of our Cochrane Review protocol, it was possible to add two additional years to the search.
Management of database records
Protocol: We planned to import and screen all database records in Endnote X6.
Review: We imported and screened all database records in Covidence.
Explanation: Covidence was introduced to Cochrane members as a new and promising tool that would facilitate screening and data extraction. We chose to use Covidence, considering that multiple review authors would be screening at the same time, and that with Covidence a good overview could be maintained.
More than two screeners
Protocol: We specified that all the screening work would be done by the two same screeners (BS and AVH).
Review: Six review authors were involved in the title and abstract screening of database records. BS screened all records, and was doubled for different numbers of records by a second independent screener (AVH, BA, GB, JM, or PV).
Explanation: Due to the large number of database records, it was not possible for AVH to screen all records, so more review authors were involved in this phase of screening.
More than two data extractors
Protocol: We specified that all the data extraction would be done by the two same review authors (BS and AVH).
Review: Three review authors were involved in data extraction and management (BS, AVH, and BA).
Explanation: Compared to the Cochrane Review protocol, an extra review author (BA) participated in data extraction and management.
Dropout rates calculation
Protocol: We had no specific plan to compute the dropout rates for all included studies.
Review: We computed the dropout rates for all included studies.
Explanation: The dropout rates were important in estimating the extent of missing data in the studies, and so these were computed. Based on the literature, a dropout of 15% was set as cut‐off to distinguish between low (≤ 15%) and high dropout (> 15%).
Contributions of authors
Conceptualising the topic for the review: BS, AVH, JH, and PV.
Co‐ordinating the review: BS.
Development of search strategies: BS and AVH.
Undertaking searches: BS.
Screening search results: BS paired with AVH, BA, GB, JM or PV.
Contacting study authors to retrieve papers or for additional information: BS and BA.
Screening retrieved papers and data against eligibility criteria: BS, AVH, and BA.
Data collection of included studies: BS, AVH, and BA.
Certainty assessments of included studies: BS, AVH, and BA.
Entering data into Review Manager 5: BS and BA.
Evidence collection and meta‐analysis: BA.
Narrative analysis: BS.
Results discussion: BS, BA, AVH, GB, and PV.
Providing a methodological perspective: BA, GB, and AVH.
Providing a clinical perspective: PV and JM.
Providing a policy perspective: JH.
Drafting the review text: BS.
Editing the review text: BS, BA, and AVH.
Reviewing the review text: GB, PV, JM, and JH.
Revising the review text: BS, BA.
Sources of support
Internal sources
No sources of support supplied
External sources
There are no external sources of support in terms of funding for the review, Other.
Declarations of interest
Bojoura Schouten: None known. Bert Avau: None known. Geertruida E Bekkering: None known. Patrick Vankrunkelsven: None known. Jeroen Mebis: None known. Johan Hellings: None known. Ann Van Hecke: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bergholdt 2013 {published data only (unpublished sought but not used)}
- Bergholdt SH, Hansen DG, Larsen PV, Kragstrup J, Sondergaard J. A randomised controlled trial to improve the role of the general practitioner in cancer rehabilitation: effect on patients' satisfaction with their general practitioners. BMJ Open 2013;3(7):e002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergholdt SH, Larsen PV, Kragstrup J, Sondergaard J, Hansen DG. Enhanced involvement of general practitioners in cancer rehabilitation: a randomised controlled trial. BMJ Open 2012;2(2):e000764. [DOI: 10.1136/bmjopen-2011-000764] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DG, Bergholdt SH, Holm L, Kragstrup J, Bladt T, Sondergaard J. A complex intervention to enhance the involvement of general practitioners in cancer rehabilitation. Protocol for a randomised controlled trial and feasibility study of a multimodal intervention. Acta Oncologica 2011;50(2):299‐306. [DOI: 10.3109/0284186X.2010.533193] [DOI] [PubMed] [Google Scholar]
Braeken 2013 {published data only (unpublished sought but not used)}
- Braeken AP, Kempen GI, Eekers D, Gils FC, Houben RM, Lechner L. The usefulness and feasibility of a screening instrument to identify psychosocial problems in patients receiving curative radiotherapy: a process evaluation. BMC Cancer 2011;11:479. [DOI: 10.1186/1471-2407-11-479] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braeken AP, Kempen GI, Eekers DB, Houben RM, Gils FC, Ambergen T, et al. Psychosocial screening effects on health‐related outcomes in patients receiving radiotherapy. A cluster randomised controlled trial. Psycho‐Oncology 2013;22(12):2736‐46. [DOI] [PubMed] [Google Scholar]
- Braeken AP, Lechner L, Eekers DB, Houben RM, Gils FC, Ambergen T, et al. Does routine psychosocial screening improve referral to psychosocial care providers and patient‐radiotherapist communication? A cluster randomized controlled trial. Patient Education and Counseling 2013;93(2):289‐97. [DOI: 10.1016/j.pec.2013.06.015] [DOI] [PubMed] [Google Scholar]
- Braeken AP, Lechner L, Gils FC, Houben RM, Eekers D, Ambergen T, et al. The effectiveness of the Screening Inventory of Psychosocial Problems (SIPP) in cancer patients treated with radiotherapy: design of a cluster randomised controlled trial. BMC Cancer 2009;9:177. [DOI: 10.1186/1471-2407-9-177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bramsen 2008 {published data only (unpublished sought but not used)}
- Bramsen I, Linden MH, Eskens FJ, Bijvank EM, Groeningen CJ, Kaufman HJ, et al. Evaluation of a face‐to‐face psychosocial screening intervention for cancer patients: acceptance and effects on quality of life. Patient Education and Counseling 2008;70(1):61‐8. [DOI] [PubMed] [Google Scholar]
de Leeuw 2013 {published data only (unpublished sought but not used)}
- Leeuw J, Prins JB, Teerenstra S, Merkx MA, Marres HA, Achterberg T. Nurse‐led follow‐up care for head and neck cancer patients: a quasi‐experimental prospective trial. Support Care Cancer 2013;21(2):537‐47. [DOI] [PubMed] [Google Scholar]
Detmar 2002 {published data only}
- Detmar SB, Muller MJ, Schornagel JH, Wever LDV, Aaronson NK, Detmar Symone B, et al. Health‐related quality‐of‐life assessments and patient‐physician communication: a randomized controlled trial. JAMA 2002;288(23):3027‐34. [DOI] [PubMed] [Google Scholar]
Geerse 2017 {published data only}
- Geerse OP, Hoekstra‐Weebers JE, Stokroos MH, Burgerhof JG, Groen HJ, Kerstjens HA, et al. Structural distress screening and supportive care for patients with lung cancer on systemic therapy: a randomised controlled trial. European Journal of Cancer 2017;72:37‐45. [DOI] [PubMed] [Google Scholar]
Giesler 2005 {published data only (unpublished sought but not used)}
- Giesler RB, Given B, Given CW, Rawl S, Monahan P, Burns D, et al. Improving the quality of life of patients with prostate carcinoma: a randomized trial testing the efficacy of a nurse‐driven intervention. Cancer 2005;104(4):752‐62. [DOI] [PubMed] [Google Scholar]
Given 2004 {published data only (unpublished sought but not used)}
- Given C, Given B, Rahbar M, Jeon S, McCorkle R, Cimprich B, et al. Does a symptom management intervention affect depression among cancer patients: results from a clinical trial. Psycho‐Oncology 2004;13(11):818‐30. [DOI] [PubMed] [Google Scholar]
Harrison 2011 {published data only (unpublished sought but not used)}
Hilarius 2008 {published data only}
- Hilarius DL, Kloeg PH, Gundy CM, Aaronson NK. Use of health‐related quality‐of‐life assessments in daily clinical oncology nursing practice: a community hospital‐based intervention study. Cancer 2008;113(3):628‐37. [DOI] [PubMed] [Google Scholar]
Hollingworth 2013 {published data only (unpublished sought but not used)}
- Hollingworth W, Metcalfe C, Mancero S, Harris S, Campbell R, Biddle L, et al. Are needs assessments cost effective in reducing distress among patients with cancer? A randomized controlled trial using the distress thermometer and problem list. Journal of Clinical Oncology 2013;31(29):3631‐8. [DOI] [PubMed] [Google Scholar]
Kutner 1999 {published data only (unpublished sought but not used)}
- Kutner JS, Foehner K, Steiner JF. Evaluation of the impact of a pre‐visit questionnaire for addressing cancer patients' information needs. Journal of Cancer Education 1999; Vol. 14, issue 4:248‐53.
Livingston 2010 {published data only (unpublished sought but not used)}
- Livingston PM, White VM, Hayman J, Maunsell E, Dunn SM, Hill D. The psychological impact of a specialist referral and telephone intervention on male cancer patients: a randomised controlled trial. Psycho‐Oncology 2010;19(6):617‐25. [DOI] [PubMed] [Google Scholar]
Maunsell 1996 {published data only}
- Maunsell E, Brisson J, Deschenes L, Frasure‐Smith N. Randomized trial of a psychologic distress screening program after breast cancer: effects on quality of life. Journal of Clinical Oncology 1996;14(10):2747‐55. [DOI] [PubMed] [Google Scholar]
Nimako 2015 {published data only (unpublished sought but not used)}
- Nimako K, Ayite B, Priest K, Severn J, Fries HM, Gunapala R, et al. A randomised assessment of the use of a quality of life questionnaire with or without intervention in patients attending a thoracic cancer clinic. European Journal of Cancer 2015; Vol. 26, issue 4:epub. [DOI] [PubMed]
- Nimako, K, Ayite B, Priest K, Severn J, Fries HM, Gunapala R, et al. A randomised assessment of the use of a quality of life questionnaire with or without intervention in patients attending a thoracic cancer clinic. European Journal of Cancer 2017;26(4):e12402. [DOI] [PubMed] [Google Scholar]
Rosenbloom 2007 {published data only}
- Rosenbloom SK, Victorson DE, Hahn EA, Peterman AH, Cella D. Assessment is not enough: a randomized controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psycho‐Oncology 2007;16(12):1069‐79. [DOI] [PubMed] [Google Scholar]
Schofield 2013 {published data only (unpublished sought but not used)}
- Schofield P, Ugalde A, Gough K, Reece J, Krishnasamy M, Carey M, et al. A tailored, supportive care intervention using systematic assessment designed for people with inoperable lung cancer: a randomised controlled trial. Psycho‐Oncology 2013;22(11):2445‐53. [DOI] [PubMed] [Google Scholar]
Singer 2017 {published and unpublished data}
- Roick J, Danker H, Kersting A, Briest S, Dietrich A, Dietz A, et al. Factors associated with non‐participation and dropout among cancer patients in a cluster‐randomised controlled trial. European Journal of Cancer 2018;27(1):e12645. [DOI] [PubMed] [Google Scholar]
- Singer S, Danker H, Briest S, Dietrich A, Dietz A, Einenkel J, et al. Effect of a structured psycho‐oncological screening and treatment model on mental health in cancer patients (STEPPED CARE): study protocol for a cluster randomized controlled trial. Trials 2014; Vol. 15, issue 1:482. [DOI] [PMC free article] [PubMed]
- Singer S, Danker H, Roick J, Einenkel J, Briest S, Spieker H, et al. Effects of stepped psychooncological care on referral to psychosocial services and emotional well‐being in cancer patients: a cluster‐randomized phase III trial. Psycho‐Oncology 2017;26(10):1675‐83. [DOI] [PubMed] [Google Scholar]
Taenzer 2000 {published data only (unpublished sought but not used)}
- Taenzer P, Bultz BD, Carlson LE, Speca M, DeGagne T, Olson K, et al. Impact of computerized quality of life screening on physician behaviour and patient satisfaction in lung cancer outpatients. Psycho‐Oncology 2000;9(3):203‐13. [DOI] [PubMed] [Google Scholar]
Thewes 2009 {published data only (unpublished sought but not used)}
- Thewes B, Butow P, Stuart‐Harris R, Greater Southern Area Health Service Screening Collaborative Group. Does routine psychological screening of newly diagnosed rural cancer patients lead to better patient outcomes? Results of a pilot study. Australian Journal of Rural Health 2009;17(6):298‐304. [DOI] [PubMed] [Google Scholar]
van der Meulen 2018 {published data only}
- Meulen IC, May AM, Koole R, Ros WJG. A Distress Thermometer intervention for patients with head and neck cancer. Oncology Nursing Forum 2018;45(1):E14‐32. [DOI] [PubMed] [Google Scholar]
Velikova 2004 {published data only (unpublished sought but not used)}
- Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient well‐being: a randomized controlled trial. Journal of Clinical Oncology 2004;22(4):714‐24. [DOI] [PubMed] [Google Scholar]
Waller 2012 {published data only (unpublished sought but not used)}
- Waller A, Girgis A, Johnson C, Lecathelinais C, Sibbritt D, Forstner D, et al. Improving outcomes for people with progressive cancer: interrupted time series trial of a needs assessment intervention. Journal of Pain and Symptom Management 2012;43(3):569‐89. [DOI] [PubMed] [Google Scholar]
Williams 2013 {published data only (unpublished sought but not used)}
- Williams PD, Graham KM, Storlie DL, Pedace TM, Haeflinger KV, Williams DD, et al. Therapy‐related symptom checklist use during treatments at a cancer center. Cancer Nursing 2013;36(3):245‐54. [DOI] [PubMed] [Google Scholar]
Young 2010 {published data only (unpublished sought but not used)}
- Young J, Harrison J, Solomon M, Butow P, Dennis R, Robson D, et al. Development and feasibility assessment of telephone‐delivered supportive care to improve outcomes for patients with colorectal cancer: pilot study of the CONNECT intervention. Supportive Care in Cancer 2010;18(4):461‐70. [DOI] [PubMed] [Google Scholar]
Young 2013 {published data only (unpublished sought but not used)}
- Young JM, Butow PN, Walsh J, Durcinoska I, Dobbins TA, Rodwell L, et al. Multicenter randomized trial of centralized nurse‐led telephone‐based care coordination to improve outcomes after surgical resection for colorectal cancer: the CONNECT intervention. Journal of Clinical Oncology 2013; Vol. 31, issue 28:3585‐91. [DOI] [PubMed]
References to studies excluded from this review
Bauwens 2014 {published data only}
- Bauwens S, Baillon C, Distelmans W, Theuns P. Systematic screening for distress in oncology practice using the Distress Barometer: the impact on referrals to psychosocial care. Psycho‐Oncology 2014;23(7):804‐11. [DOI] [PubMed] [Google Scholar]
Boyes 2006 {published data only}
- Boyes A, Newell S, Girgis A, McElduff P, Sanson‐Fisher R. Does routine assessment and real‐time feedback improve cancer patients' psychosocial well‐being?. European Journal of Cancer Care 2006;15:163‐71. [DOI] [PubMed] [Google Scholar]
Carlson 2010 {published data only}
- Carlson LE, Groff SL, Maciejewski O, Bultz BD. Screening for distress in lung and breast cancer outpatients: a randomized controlled trial. Journal of Clinical Oncology 2010;28(33):4884‐91. [DOI] [PubMed] [Google Scholar]
Carter 2012 {published data only}
- Carter G, Britton B, Clover K, Rogers K, Adams C, McElduff P. Effectiveness of QUICATOUCH: a computerised touch screen evaluation for pain and distress in ambulatory oncology patients in Newcastle, Australia. Psycho‐Oncology 2012;21(11):1149‐57. [DOI] [PubMed] [Google Scholar]
Girgis 2014 {published data only}
- Girgis A, Kelly B, Haas M, Viney R, Descallar J, Candler H, et al. Translating Evidence into Practice ‐ the PACT Study: A Time Series Study Investigating the Impact, Acceptability and Cost of an Integrated Model for Psychosocial Screening, Care and Treatment of Patients With Urological and Head and Neck Cancer. BMJ Open 2014;23(e004147):1‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoekstra‐Weebers 2012 {published data only}
- Hoekstra‐Weebers J, Coyne J, Wiel H. Effects of psychosocial screening on cancer patients' reported quality of life and satisfaction with care. Psycho‐Oncology 2012;8(S2):1‐68. [Google Scholar]
McLachlan 2001 {published data only}
- McLachlan S, Allenby A, Matthews J, Kissane D, Wirth A, Bishop M, et al. A randomised trial of the collection of quality of life (QoL) and cancer needs information via a computer touch screen by ambulatory patients with cancer [abstract]. Proceedings of the American Society of Clinical Oncology. 2001; Vol. 20 (Pt 1):247a, Abstract 987.
Sarna 1998 {published data only}
- Sarna L. Effectiveness of structured nursing assessment of symptom distress in advanced lung cancer. Oncology Nursing Forum 1998;25(6):1041‐8. [PubMed] [Google Scholar]
Stanciu 2015 {published data only}
- Stanciu MA, Morris C, Makin M, Watson E, Bulger J, Evans R, et al. A pilot randomised controlled trial of personalised care after treatment for prostate cancer (TOPCAT‐P): nurse‐led holistic‐needs assessment and individualised psychoeducational intervention: study protocol. BMJ Open 2015;5:e008470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waller 2012a {published data only}
- Waller A, Girgis A, Johnson C, Lecathelinais C, Sibbritt D, Seldon M, et al. Implications of a needs assessment intervention for people with progressive cancer: impact on clinical assessment, response and service utilisation. Psycho‐Oncology 2012;21(5):550‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Amstel 2017 {published data only (unpublished sought but not used)}
- Amstel FKP, Peters MEWJ, Graaf WTA, Prins JB, Ottevanger PB. The distress thermometer in a nurse‐led intervention. Does it improve quality of life? Results of a randomized controlled trial. Psycho‐Oncology. Berlin, 2017; Vol. 26:Wiley.
- Amstel FKP, Prins JB, Graaf WTA, Peters MEWJ, Ottevanger PB. The effectiveness of a nurse‐led intervention with the distress thermometer for patients treated with curative intent for breast cancer: design of a randomized controlled trial. BMC Cancer 2016; Vol. 16:520. [DOI] [PMC free article] [PubMed]
Frennet 2011 {published data only}
- Frennet M, Kerger J, Cornette P, Humblet Y, Lesage V, Saint‐Hubert M. Impact of comprehensive geriatric assessment (CGA) during oncologic treatment in frail elderly patients. European Geriatric Medicine 2011;Conference: 7th Congress of the EUGMS. Malaga Spain. Conference Start: 20110928. Conference End: 20110930:S35. [Google Scholar]
Li 2017 {published data only}
- Li M, Howell DM, Rosberger Z, Prashad A, Moody L, Aslam U, et al. Improving patient experience and health outcomes using electronic patient reported outcome measures: effects on distress and health outcomes. World Congress of Psycho‐Oncology 2017;Berlin:Wiley. [Google Scholar]
Mehanna {published data only}
- ISRCTN12861667. Quality of life in head and neck cancer follow‐up clinics. https://onlinelibrary.wiley.com/doi/abs/10.1111/ecc.12645 2010.
Munro 1994 {published data only}
- Munro AJ, Shaw T, Clarke L, Becker L, Greenwood S. A randomized study of telephone contact following completion of radiotherapy. Clinical Oncology (Royal College of Radiologists (Great Britain)) 1994;6:242‐4. [DOI] [PubMed] [Google Scholar]
Powell 2008 {published data only}
- Powell CB, Kneier A, Chen LM, Rubin M, Kronewetter C, Levine E. A randomized study of the effectiveness of a brief psychosocial intervention for women attending a gynecologic cancer clinic. Gynecologic Oncology 2008;111:137‐43. [DOI] [PubMed] [Google Scholar]
Skorstengaard 2014 {published data only}
- Skorstengaard MH, Brogaard T, Neergaard MA, Jensen AB. Advance care planning ‐ a way to improve end‐of‐life care. Conference: 8th World Research Congress of the European Association for Palliative Care, EAPC 2014. Lleida Spain. Conference Start: 20140605. Conference End: 20140607. Palliative Medicine, 2014:896.
References to ongoing studies
Bernacki 2015 {published data only (unpublished sought but not used)}
- Bernacki R, Hutchings M, Vick J, Smith G, Paladino J, Lipsitz S, et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open 2015; Vol. 5:e009032. [DOI] [PMC free article] [PubMed]
Cooley 2014 {unpublished data only}
- Cooley ME, Blonquist T, Catalano P, Lobach D, Braun I, Halpenny B, et al. Point‐of‐care clinical decision support for cancer symptom management: results of a group randomized trial. Journal of Clinical Oncology 2014; Vol. 32, issue S1:1.
Sussman 2012 {unpublished data only}
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organisation for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85:365‐76. [DOI] [PubMed] [Google Scholar]
Aaronson 1998
- Aaronson NK, Muller M, Cohen PD, Essink‐Bot ML, Fekkes M, Sanderman R, et al. Translation, validation, and norming of the Dutch language version of the SF‐36 Health Survey in community and chronic disease populations. Journal of Clinical Epidemiology 1998;51:1055‐68. [DOI] [PubMed] [Google Scholar]
Accreditation Canada 2008
- Accreditation Canada. Qmentum Program 2009 Standards: Cancer Care and Oncology Services. Ottawa, ON: Accreditation Canada. Accreditation Canada Ver 2, 2008.
Armes 2009
- Armes J, Crowe M, Colbourne L, Morgan H, Murrells T, Oakley C, et al. Patients' supportive care needs beyond the end of cancer treatment: a prospective, longitudinal survey. Journal of Clinical Oncology 2009;27(36):6172‐9. [DOI] [PubMed] [Google Scholar]
Bandura 1977
- Bandura A. Self‐efficacy: toward a unifying theory of behavioral change. Psychological Review 1977;84(2):191‐215. [DOI] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. BDI®‐II Manual. San Antonio, Texas, USA: The Psychological Corporation, 1996. [Google Scholar]
Bidstrup 2011
- Bidstrup PE, Johansen C, Mitchell AJ. Screening for cancer‐related distress: summary of evidence from tools to programmes. Acta Oncologica 2011;50:194‐204. [DOI] [PubMed] [Google Scholar]
Boyce 2014
- Boyce MB, Browne JP, Greenhalgh J. The experiences of professionals with using information from patient‐reported outcome measures to improve the quality of healthcare: a systematic review of qualitative research. BMJ Quality & Safety 2014;23(6):508‐18. [DOI] [PubMed] [Google Scholar]
Boyes 2012
- Boyes AW, Girgis A, D'Este C, Zucca AC. Prevalence and correlates of cancer survivors' supportive care needs 6 months after diagnosis: a population‐based cross‐sectional study. BMC Cancer 2012;12:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bredart 2004
- Bredart A, Mignot V, Rousseau A, Dolbeault S, Beauloye N, Adam V, et al. Validation of the EORTC QLQ‐SAT32 cancer inpatient satisfaction questionnaire by self‐ versus interview‐assessment comparison. Patient Education and Counseling 2004;54(2):207‐12. [DOI] [PubMed] [Google Scholar]
Bredart 2005
- Bredart A, Bottomley A, Blazeby JM, Conroy T, Coens C, D'Haese S, et al. An international prospective study of the EORTC cancer in‐patient satisfaction with care measure (EORTC IN‐PATSAT32). European Journal of Cancer 2005;41(14):2120‐31. [DOI] [PubMed] [Google Scholar]
Breitbart 2015
- Breitbart W, Rosenfeld B, Pessin H, Applebaum A, Kulikowski J, Lichtenthal WG. Meaning‐centered group psychotherapy: an effective intervention for improving psychological well‐being in patients with advanced cancer. Journal of Clinical Oncology 2015;33(7):749‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brenner 1995
- Brenner MH, Curbow B, Legro MW. The proximal‐distal continuum of multiple health outcome measures: the case of cataract surgery. Medical Care 1995;33(4 Suppl):AS236‐44. [PubMed] [Google Scholar]
Browne 2011
- Browne S, Dowie A, Mitchell L, Wyke S, Ziebland S, Campbell N, et al. Patients' needs following colorectal cancer diagnosis: where does primary care fit in?. British Journal of General Practice 2011;61(592):e692‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bultz 2013
- Bultz BD, Carlson LE. A commentary on 'Effects of screening for psychological distress on patient outcomes in cancer: a systematic review'. Journal of Psychosomatic Research 2013;75(1):18‐9. [DOI] [PubMed] [Google Scholar]
Carlson 2003
- Carlson LE, Bultz BD. Cancer distress screening. Needs, models, and methods. Journal of Psychosomatic Research 2003;55(5):403‐9. [DOI] [PubMed] [Google Scholar]
Carlson 2012
- Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. Journal of Clinical Oncology 2012;30(11):1160‐77. [DOI] [PubMed] [Google Scholar]
Carlson 2013
- Carlson LE, Waller A, Groff SL, Giese‐Davis J, Bultz BD. What goes up does not always come down: patterns of distress, physical and psychosocial morbidity in people with cancer over a one year period. Psycho‐Oncology 2013;22(1):168‐76. [DOI] [PubMed] [Google Scholar]
Choi 2012
- Choi KH, Park JH, Park JH, Park JS. Psychosocial needs of cancer patients and related factors: a multi‐center, cross‐sectional study in Korea. Psycho‐Oncology 2012;22(5):1073‐80. [DOI] [PubMed] [Google Scholar]
Cox 2006
- Cox A, Jenkins V, Catt S, Langridge C, Fallowfield L. Information needs and experiences: an audit of UK cancer patients. European Journal of Oncology Nursing 2006;10:263‐72. [DOI] [PubMed] [Google Scholar]
Dettori 2011
- Dettori JR. Loss to follow‐up. Evidence‐Based Spine‐Care Journal 2011;2(1):7‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136:e359‐86. [DOI] [PubMed] [Google Scholar]
Feyer 2008
- Feyer PK, Kleeberg UR, Steingraber M, Gunther W, Behrens M. Frequency of side effects in outpatient cancer care and their influence on patient satisfaction ‐ a prospective survey using the PASQOC questionnaire. Supportive Care in Cancer 2008;16(6):567‐75. [DOI: 10.1007/s00520-008-0422-4] [DOI] [PubMed] [Google Scholar]
Galway 2012
- Galway K, Black A, Cantwell M, Cardwell CR, Mills M, Donnelly M. Psychosocial interventions to improve quality of life and emotional wellbeing for recently diagnosed cancer patients. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007064.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganz 1992
- Ganz PA, Schag CA, Lee JJ, Sim MS. The CARES: a generic measure of health‐related quality of life for patients with cancer. Quality of Life Research 1992;1(1):19‐29. [DOI] [PubMed] [Google Scholar]
Goedendorp 2009
- Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006953.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grassi 2011
- Grassi L, Rossi E, Caruso R, Nanni MG, Pedrazzi S, Sofritti S, et al. Educational intervention in cancer outpatient clinics on routine screening for emotional distress: an observational study. Psycho‐Oncology 2011;20(6):669‐74. [DOI] [PubMed] [Google Scholar]
Grassi 2012
- Grassi L, Watson M. Psychosocial care in cancer: an overview of psychosocial programmes and national cancer plans of countries within the International Federation of Psycho‐Oncology Societies. Psycho‐Oncology 2012;21(10):1027‐33. [DOI] [PubMed] [Google Scholar]
Hack 2010
- Hack TF, Pickles T, Ruether JD, Weir L, Bultz BD, Mackey J, et al. Predictors of distress and quality of life in patients undergoing cancer therapy: impact of treatment type and decisional role. Psycho‐Oncology 2010;19(6):606‐16. [DOI] [PubMed] [Google Scholar]
Harrison 2009
- Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Supportive Care in Cancer 2009;17(8):1117‐28. [DOI] [PubMed] [Google Scholar]
Heins 2013
- Heins MJ, Korevaar JC, Rijken PM, Schellevis FG. For which health problems do cancer survivors visit their General Practitioner?. European Journal of Cancer 2013;49(1):211‐8. [DOI: 10.1016/j.ejca.2012.07.011] [DOI] [PubMed] [Google Scholar]
Hess 2013
- Hess LM, Pohl G. Perspectives of quality care in cancer treatment: a review of the literature. American Health and Drug Benefits 2013;6(6):321‐9. [PMC free article] [PubMed] [Google Scholar]
Heyn 2013
- Heyn L, Finset A, Eide H, Ruland CM. Effects of an interactive tailored patient assessment on patient–clinician communication in cancer care. Psycho‐Oncology 2013;22(1):89‐96. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hodgkinson 2007
- Hodgkinson K, Butow P, Hunt GE, Pendlebury S, Hobbs KM, Lo SK, et al. The development and evaluation of a measure to assess cancer survivors' unmet supportive care needs: the CaSUN (Cancer Survivors' Unmet Needs measure). Psycho‐Oncology 2007;16(9):796‐804. [DOI] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Holland 2011
- Holland J, Watson M, Dunn J. The IPOS new International Standard of Quality Cancer Care: integrating the psychosocial domain into routine care. Psycho‐Oncology 2011;20(7):677‐80. [DOI] [PubMed] [Google Scholar]
Howell 2012
- Howell D, Mayo S, Currie S, Jones G, Boyle M, Hack T, et al. Psychosocial health care needs assessment of adult cancer patients: a consensus‐based guideline. Supportive Care in Cancer 2012;20(12):3343‐54. [DOI] [PubMed] [Google Scholar]
Hulbert‐Williams 2012
- Hulbert‐Williams N, Neal R, Morrison V, Hood K, Wilkinson C. Anxiety, depression and quality of life after cancer diagnosis: what psychosocial variables best predict how patients adjust?. Psycho‐Oncology 2012;21(8):857‐67. [DOI] [PubMed] [Google Scholar]
IOM 2001
- Institute of Medicine, Committee on Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press, 2001. [PubMed] [Google Scholar]
IOM 2008
- Institute of Medicine. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: The National Academies Press, 2008. [PubMed] [Google Scholar]
Kleeberg 2005
- Kleeberg UR, Tews JT, Ruprecht T, Hoing M, Kuhlmann A, Runge C. Patient satisfaction and quality of life in cancer outpatients: results of the PASQOC study. Supportive Care in Cancer 2005;13(5):303‐10. [DOI] [PubMed] [Google Scholar]
Kleeberg 2008
- Kleeberg UR, Feyer P, Gunther W, Behrens M. Patient satisfaction in outpatient cancer care: a prospective survey using the PASQOC questionnaire. Supportive Care in Cancer 2008;16(8):947‐54. [DOI] [PubMed] [Google Scholar]
Knobf 2012
- Knobf MT, Ferrucci LM, Cartmel B, Jones BA, Stevens D, Smith M, et al. Needs assessment of cancer survivors in Connecticut. Journal of Cancer Survivorship ‐ Research and Practice 2012;6(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kristman 2004
- Kristman V, Manno M, Cote P. Loss to follow‐up in cohort studies: how much is too much?. European Journal of Epidemiology 2004;19(8):751‐60. [DOI] [PubMed] [Google Scholar]
Lang 2017
- Lang M, Giese‐Davis J, Patten S, Campbell DJT. Does age matter? Comparing post‐treatment psychosocial outcomes in young adult and older adult cancer survivors with their cancer‐free peers. Psycho‐Oncology 2018;27(5):1404‐11. [DOI] [PubMed] [Google Scholar]
Leventhal 2016
- Leventhal H, Phillips LA, Burns E. The Common‐Sense Model of Self‐Regulation (CSM): a dynamic framework for understanding illness self‐management. Journal of Behavioral Medicine 2016;39(6):935‐46. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowery 2012
- Lowery AE, Greenberg MA, Foster SL, Clark K, Casden DR, Loscalzo M, et al. Validation of a needs‐based biopsychosocial distress instrument for cancer patients. Psycho‐Oncology 2012;21(10):1099‐106. [DOI] [PubMed] [Google Scholar]
Luckett 2009
- Luckett T, Butow PN, King MT. Improving patient outcomes through the routine use of patient‐reported data in cancer clinics: future directions. Psycho‐Oncology 2009;18(11):1129‐38. [DOI] [PubMed] [Google Scholar]
McIllmurray 2001
- McIllmurray MB, Thomas C, Francis B, Morris S, Soothill K, Al‐Hamad A. The psychosocial needs of cancer patients: findings from an observational study. European Journal of Cancer Care 2001;10(4):261‐9. [DOI] [PubMed] [Google Scholar]
Meijer 2013
- Meijer A, Roseman M, Delisle VC, Milette K, Levis B, Syamchandra A, et al. Effects of screening for psychological distress on patient outcomes in cancer: a systematic review. Journal for Psychosomatic Research 2013;75(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyer 2015
- Meyer LA, Nick AM, Shi Q, Wang XS, Williams L, Brock T, et al. Perioperative trajectory of patient reported symptoms: a pilot study in gynecologic oncology patients. Gynecologic Oncology 2015;136(3):440‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mikkelsen 2008
- Mikkelsen T, Sondergaard J, Jensen AB, Olesen F. Cancer rehabilitation: psychosocial rehabilitation needs after discharge from hospital?. Scandinavian Journal of Primary Health Care 2008;26(4):216‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohler 2015
- Mohler R, Kopke S, Meyer G. Criteria for Reporting the Development and Evaluation of Complex Interventions in healthcare: revised guideline (CReDECI 2). Trials 2015;16(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moons 2006
- Moons P, Budts W, Geest S. Critique on the conceptualisation of quality of life: a review and evaluation of different conceptual approaches. International Journal of Nursing Studies 2006;43(7):891‐901. [DOI] [PubMed] [Google Scholar]
NBCC 2003
- National Breast Cancer Centre and National Cancer Control Initiative. Clinical Practice Guidelines for the Psychosocial Care of Adults With Cancer. Camperdown: National Breast Cancer Centre, 2003. [Google Scholar]
NCCN 1999
- National Comprehensive Cancer Network. NCCN practice guidelines for the management of psychosocial distress. Oncology 1999;13(5A):113‐47. [PubMed] [Google Scholar]
NCCN 2007
- National Comprenhensive Cancer Network. Distress Management (v.1.2008). 1st Edition. Vol. 5, National Comprehensive Care Network, Inc, 2007. [Google Scholar]
Parahoo 2013
- Parahoo K, McDonough S, McCaughan E, Noyes J, Semple C, Halstead EJ, et al. Psychosocial interventions for men with prostate cancer. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD008529.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parry 2012
- Parry C, Lomax JB, Morningstar EA, Fairclough DL. Identification and correlates of unmet service needs in adult leukemia and lymphoma survivors after treatment. Journal of Oncology Practice 2012;8(5):e135‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pauwels 2013
- Pauwels EE, Charlier C, Bourdeaudhuij I, Lechner L, Hoof E. Care needs after primary breast cancer treatment. Survivors' associated sociodemographic and medical characteristics. Psycho‐Oncology 2013;22(1):125‐32. [DOI] [PubMed] [Google Scholar]
Price 2013
- Price MA, Dhillon H. Review: insufficient evidence to recommend routine screening for psychological distress among people with cancer. Evidence‐Based Mental Health 2013;16(4):108. [DOI] [PubMed] [Google Scholar]
Ranchor 2012
- Ranchor AV, Fleer J, Sanderman R, Ploeg KM, Coyne JC, Schroevers M. Psychological interventions for cancer survivors and cancer patients in the palliative phase. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009511] [DOI] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ristevski 2015
- Ristevski E, Regan M, Jones R, Breen S, Batson A, McGrail MR. Cancer patient and clinician acceptability and feasibility of a supportive care screening and referral process. Health Expectations 2015;18(3):406‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2016
- Ryan R, Hill S. How to GRADE. cccrg.cochrane.org/author‐resources (accessed prior to 28 February 2019).
Sacket 1997
- Sacket LD, Richardson SW, Rosenberg W. Evidence‐Based Medicine: How to Practice and Teach EBM. New York: Churchill Livingstone, 1997. [Google Scholar]
Sanson‐Fisher 2000
- Sanson‐Fisher R, Girgis A, Boyes A, Bonevski B, Burton L, Cook P. The unmet supportive care needs of patients with cancer. Supportive Care Review Group. Cancer 2000;88(1):226‐37. [DOI] [PubMed] [Google Scholar]
Semple 2013
- Semple C, Parahoo K, Norman A, McCaughan E, Humphris G, Mills M. Psychosocial interventions for patients with head and neck cancer. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009441.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sidani 2015
- Sidani S. Health Intervention Research: Understanding Research Design and Methods. London: Sage Publications Ltd, 2015. [ISBN 978‐1‐4462‐5616‐9] [Google Scholar]
Sterne 2016
- Sterne JAC, Higgins JPT, Reeves BC on behalf of the development group for ROBINS‐I. ROBINS‐I: a tool for assessing Risk Of Bias In Non‐randomized Studies of Interventions, Version 7. www.riskofbias.info (accessed 23 May 2016).
Tuinman 2008
- Tuinman MA, Gazendam‐Donofrio SM, Hoekstra‐Weebers JE. Screening and referral for psychosocial distress in oncologic practice: use of the Distress Thermometer. Cancer 2008;113(4):870‐8. [DOI] [PubMed] [Google Scholar]
van Scheppingen 2011
- Scheppingen C, Schroevers MJ, Smink A, Linden YM, Mul VE, Langendijk JA, et al. Does screening for distress efficiently uncover meetable unmet needs in cancer patients?. Psycho‐Oncology 2011;20(6):655‐63. [DOI] [PubMed] [Google Scholar]
Waller 2010
- Waller A, Girgis A, Johnson C, et al. Facilitating needs based cancder care for people with a chronic disease: evaluation of an intervention using a multi centre interrupted time series design.. BMC Palliative Care 2010;9(2):published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitney 2014
- Whitney RL, Bell JF, Bold RJ, Joseph JG. Mental health needs and service use in a national sample of adult cancer survivors in the USA: has psychosocial care improved?. Psycho‐Oncology 2014;24(1):80‐8. [DOI: 10.1002/pon.3569] [DOI] [PubMed] [Google Scholar]
Williamson 2012
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolff 2015
- Wolff HA, Ihler F, Zeller N, Welz C, Jung K, Canis M, et al. Chemo/radiotherapy after laser microsurgery and selective neck dissection for pN2 head and neck cancer. European Archives of Oto‐rhino‐laryngology 2016;273(6):1533‐41. [DOI] [PubMed] [Google Scholar]
Zabora 2012
- Zabora JR, Macmurray L. The history of psychosocial screening among cancer patients. Journal of Psychosocial Oncology 2012;30(6):625‐35. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]
Zucca 2014
- Zucca A, Sanson‐Fisher R, Waller A, Carey M. Patient‐centred care: making cancer treatment centres accountable. Supportive Care in Cancer 2014;22(7):1989‐97. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Schouten 2016
- Schouten B, Bekkering GE, Vankrunkelsven P, Mebis J, Hoof E, Hellings J, et al. Systematic screening and assessment of psychosocial well‐being and care needs of people with cancer. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012387] [DOI] [PMC free article] [PubMed] [Google Scholar]


