Abstract
Objective
Conversion surgery is a surgery with a purpose of R0 resection in primary advanced gastric cancer (GC) that responded well to systemic chemotherapy. This study aimed to explore the efficacy of conversion surgery for advanced GC.
Methods
A total of 618 advanced GC patients receiving systemic chemotherapy were stratified into low-, moderate- and high-risk groups based on a nomogram-predicted probability of overall survival. The survival of conversion surgery and chemotherapy alone groups was compared using the log-rank test and Cox regression analysis after propensity score matching (PSM).
Results
A nomogram with good discrimination (concordance index: 0.65) and accurate calibration was constructed. After PSM, the median survival time (MST) of conversion surgery was 26.80 months, compared with 16.60 months of chemotherapy alone (P<0.001). Conversion surgery was associated with a longer MST for patients in the low-risk group (30.40 monthsvs. 20.90 months, P=0.013), whereas it was not associated with prolonged survival in the moderate- and high-risk groups (P=0.221 and P=0.131, respectively).
Conclusions
Conversion surgery was associated with longer survival, especially for low-risk population.
Keywords: Gastric cancer, conversion surgery, chemotherapy, risk model
Introduction
Although the incidence of gastric cancer (GC) is decreasing steadily, GC remains the third leading cause of cancer-related death worldwide (1,2). In China, the majority of GC patients have locally advanced or advanced stage disease at the time of diagnosis, and these patients account for more than half of the GC deaths in the world (3,4). Systemic chemotherapy remains the standard treatment strategy for advanced GC. However, despite the development of chemotherapy (5,6), the median survival time (MST) of GC patients is still unsatisfactory (7-10), ranging from 8.6 months to 16.0 months. Advanced GC patients often suffer from progression within 5 to 7 months after first-line chemotherapy, mainly because of acquired chemoresistance.
Theoretically, surgery performed before chemoresistance develops may help to improve the prognosis of patients with advanced GC. For metastatic colorectal cancer, conversion surgery or an oncosurgical approach has been proven to be a critical part of a multimodal treatment strategy after effective chemotherapy (11,12). Unlike metastatic colorectal cancer, the biological behavior of advanced GC is different and consists of varying degrees of tumor burden, including distant lymph node metastasis, hematologic metastasis and peritoneal seeding. Several studies have reported the potential survival benefits of conversion surgery for advanced GC (13-25). Kanda et al. (14) reported the MST of 29 months for stage IV GC patients treated with secondary gastrectomy after S-1-based chemotherapy. Fukuchi et al. (18) reported that the MST of patients treated with chemotherapy plus gastrectomy was 53 months, compared with 14 months in patients treated with chemotherapy alone (P<0.01). As described by Yoshidaet al. (26), advanced GC can be classified into 4 categories according to its different biological behaviors. Yamaguchi et al. (25) demonstrated that advanced GC patients can benefit from conversion surgery, irrespective of these categories. However, substantial selection biases existed in the previous studies. For instance, patients with better performance status (PS), fewer metastatic lesions and better chemotherapy responses had a higher chance of undergoing conversion surgery, thereby accounting for their positive outcomes.
Using propensity score matching (PSM) to minimize selection bias, the present study aimed to evaluate the survival outcomes of patients undergoing conversion surgery and to establish a risk-stratification model to predict the benefits of conversion surgery.
Materials and methods
Patients
Between January 2000 and December 2015, a total of 618 primary advanced gastric adenocarcinoma patients who underwent systemic chemotherapy were identified at Sun Yat-sen University Cancer Center. The clinical and pathological characteristics of the patients were retrospectively reviewed according to the Japanese Classification of Gastric Carcinoma (27). The inclusion criteria were as follows: 1) patients who were pathologically confirmed gastric adenocarcinoma; 2) patients who were clinically diagnosed with distant metastatic lesions including peritoneal seeding, liver metastasis, lung metastasis, para-aortic lymph node (PAN) metastasis, and/or other distant metastasis; and 3) patients who underwent at least one cycle of systemic chemotherapy. Patients were excluded if 1) they had recurrent GC (patients who underwent curative surgery and then the tumor recurred) or 2) the response to chemotherapy could not be evaluated. The Institutional Review Board of Sun Yat-sen University Cancer Center approved the present study.
Before systemic chemotherapy, chest/abdominal/pelvic computed tomography (CT), endoscopic ultrasonography (EUS) or ultrasonography was performed to identify distant metastases. A single non-curative factor included in the following: 1) peritoneal seeding (P1) above the transverse colon (including the greater omentum) without massive ascites; 2) H1 hepatic metastasis (1−4 lesions 1−5 cm in diameter); or 3) 16a1/b2 PAN metastasis.
Chemotherapy, response evaluation and conversion surgery
The chemotherapeutic regimens were inconsistent and included cisplatin, oxaliplatin, fluorouracil, S-1, Taxol, and irinotecan. For patients with human epidermal growth factor receptor-2 (HER-2) cancer [immunohistochemistry (IHC) 3+ or IHC 2+ with fluorescence in situ hybridization (FISH) positive], trastuzumab plus chemotherapy was recommended. After each set of 2−4 cycles, the response to chemotherapy was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) (28) guidelines. The decision to perform conversion surgery was mainly based on the response to chemotherapy and the possibility of R0 resection. Therefore, conversion surgery could be defined as surgery with a purpose of R0 resection for primary advanced GC that responded well to systemic chemotherapy. In our institution, multidisciplinary team (MDT), which consists of surgeons, medical oncologists, radiologists, pathologists and radiotherapists, would be responsible to decide whether the patients should receive conversion surgery or not.
Follow-up
Patients were followed up in the clinic or through telephone calls every 3 months for the first 2 years and every 6 months for 3−5 years, and annually thereafter. In the present study, the median follow-up time was 17.80 (range, 3.00−131.00) months.
Statistical analysis
Categorical variables were presented as numbers with percentages and compared using the χ2 test. The primary outcome was overall survival (OS), which was calculated from the date of the initial chemotherapy to death from any cause or the date of the last follow-up. The OS was estimated with the Kaplan-Meier method, and OS differences were compared using the log-rank test. Cox regression was used in the multivariate analysis of OS. Hazard ratios (HRs) are presented with 95% confidence interval (95% CI). Two-tailed tests were used, and P values less than 0.05 were considered statistically significant.
Variables with P<0.05 in the Cox regression analysis were included in a nomogram. The nomogram was subjected to 1,000 bootstrap samples for internal validation, as quantified using the concordance index (C-index) (29). The C-index values range from 0.5 to 1.0, with 0.5 indicating random chance and 1.0 indicating a perfect ability to discriminate the outcome correctly using the model. Calibration was performed by comparing the nomogram-predicted survival with the observed survival. Patients were grouped into different risk groups (low-, moderate-, and high-risk groups) according to the total points in the nomogram, and the cut-off values were determined as described by Camp et al. (30) using X-tile software (version 3.6.1; Yale University School of Medicine, New Haven, CT, USA).
PSM (31) was used to balance the characteristics between conversion surgery and chemotherapy alone groups. Covariates [peritoneal seeding, ascites, noncurative factors and the objective response rate (ORR) to chemotherapy] that were significantly different between two groups were applied to calculate propensity scores. Then, we chose the 1:1 nearest neighbor matching for the propensity score, with the calipers set at 0.05. After matching, the OS was compared between conversion surgery and chemotherapy alone groups using log-rank tests. Adjusted HRs were calculated using Cox regression after adjustment for peritoneal seeding, ascites, noncurative factors and ORR.
All the statistical analyses were performed using R software (Version 3.4.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient demographics and clinicopathological characteristics
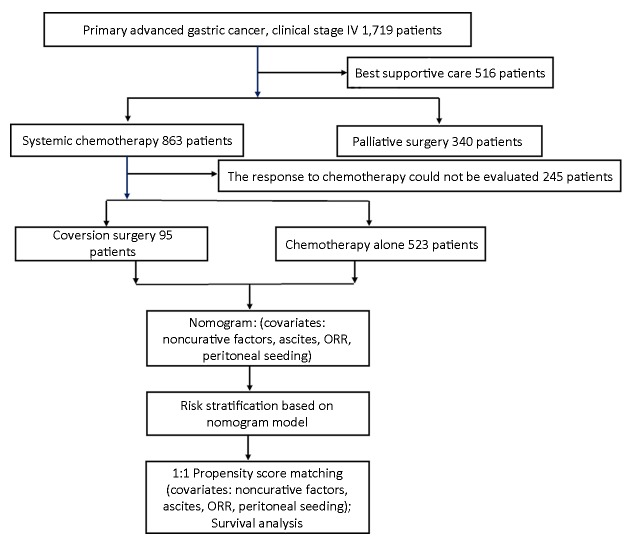
The consort diagram of the patients enrolled in the present study is shown in Figure 1 . The general characteristics of the 618 advanced GC patients who received systemic chemotherapy are summarized in Table 1 . A total of 95 patients underwent conversion surgery. These patients tended to have less peritoneal seeding (P=0.004), less ascites (P=0.009), less noncurative factors (P<0.001) and better responses to chemotherapy (P<0.001), indicating selection biases for conversion surgery. With a median of 17.80 (range, 3.00−131.00) months of follow-up, the MST was 14.50 (95% CI, 13.40−15.70) months for all patients.
1.
Consort diagram of 618 patients enrolled in the present cohort study. Of the 618 patients, 95 patients received conversion surgery. ORR, objective response rate.
1.
Baseline characteristics of metastatic gastric cancer patients who underwent systemic chemotherapy
| Variables | All (N=618)
[n (%)] |
n (%) | ||||||
| Before PSM | After PSM | |||||||
| Conversion surgery (N=95) | Chemotherapy alone (N=523) | P | Conversion surgery (N=95) | Chemotherapy alone (N=95) | P | |||
| Age (year) | 0.200 | |||||||
| ≥65 | 99 (16.0) | 15 (15.8) | 84 (16.1) | 15 (15.8) | 22 (23.2) | |||
| <65 | 519 (84.0) | 80 (84.2) | 439 (83.9) | 80 (84.2) | 73 (76.8) | |||
| Sex ratio (M:F) | 398:220 | 67:28 | 331:192 | 0.175 | 67:28 | 68:27 | 0.873 | |
| PS | 0.726 | 0.388 | ||||||
| ≥2 | 58 (9.4) | 8 (8.4) | 50 (9.6) | 8 (8.4) | 5 (5.3) | |||
| <2 | 560 (90.6) | 87 (91.6) | 473 (90.4) | 87 (91.6) | 90 (94.7) | |||
| Tumor size (cm) | 0.356 | 0.194 | ||||||
| ≥7 | 197 (37.5) | 36 (41.9) | 161 (36.6) | 36 (41.9) | 25 (32.5) | |||
| <7 | 329 (62.5) | 50 (58.1) | 279 (63.4) | 50 (58.1) | 52 (67.5) | |||
| Tumor location | 0.561 | 0.240 | ||||||
| Cardia | 218 (35.3) | 36 (37.9) | 182 (34.8) | 36 (37.9) | 44 (46.3) | |||
| Noncardia | 400 (64.7) | 59 (62.1) | 341 (65.2) | 59 (62.1) | 51 (53.7) | |||
| Tumor differentiation | 0.083 | 0.644 | ||||||
| Poorly differentiated | 500 (83.9) | 74 (77.9) | 426 (85.0) | 74 (77.9) | 66 (75.0) | |||
| Table 1 (continued) | ||||||||
Nomogram for OS and risk-stratification of patients
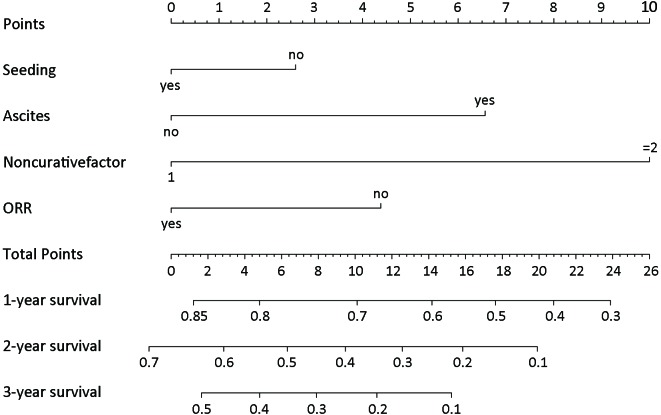
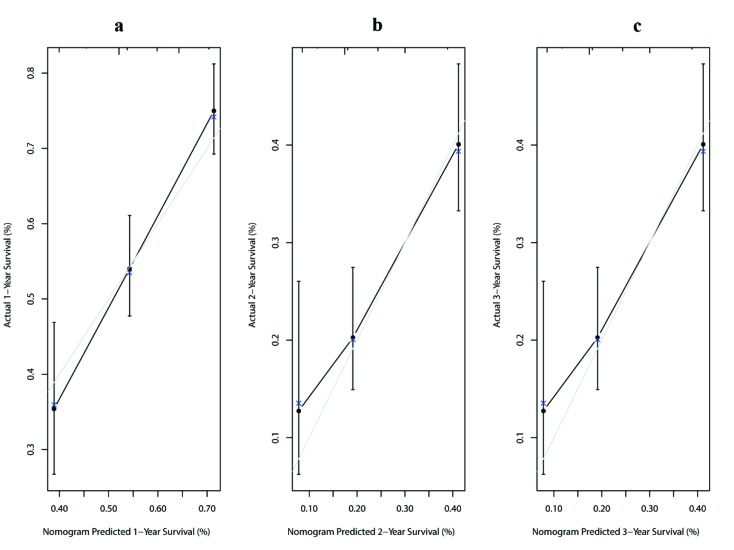
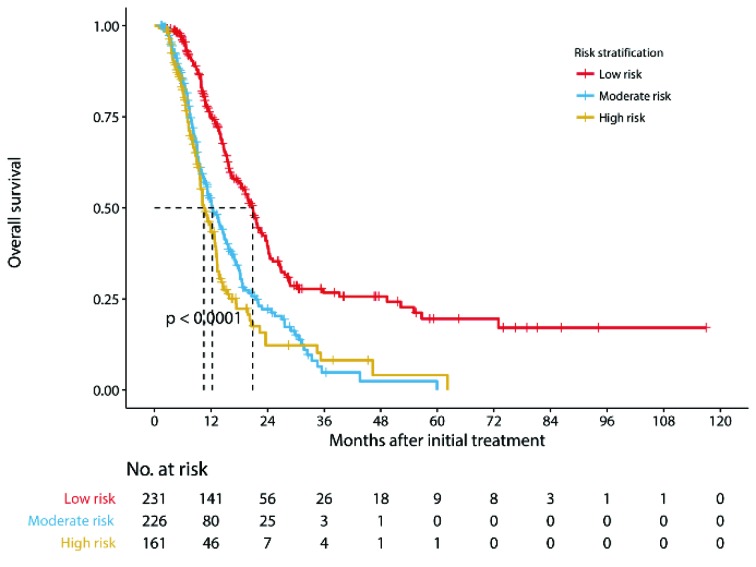
Multivariate analysis for all patients showed that four covariates were prognostic factors for OS: peritoneal seeding, presence of ascites, presence of a single noncurative factor and ORR to chemotherapy (Table 2 ). A nomogram was established with the four covariates to predict the probability of 1-year, 2-year and 3-year OS (Figure 2 ). The number of noncurative factors (10 points) showed the largest contribution to OS, followed by ascites (7 points), ORR (4 points) and peritoneal seeding (3 points) (Supplementary Table S1 ). The C-index for the nomogram was 0.65 (95% CI, 0.58−0.72). Calibration plots of the prognostic nomogram showed accurate agreement between the nomogram-predicted 1-year, 2-year and 3-year OS and the actual 1-year, 2-year and 3-year OS (Supplementary Figure S1 ). After sorting by the total scores (score: 0−13; 14−17; 20−24) (30), patients were grouped into low-risk, moderate-risk and high-risk groups with estimated 2-year OS rates of 39.6% (95% CI, 32.8%−47.7%), 22.2% (95% CI, 16.3%−30.3%), and 12.3% (95% CI, 6.7%−22.6%), respectively (P<0.001, log-rank test) (Supplementary Table S1 , Figure S2 ).
2.
Univariable analysis and Cox proportional hazards regression analysis for all patients
| Variables | Number | Univariable analysis | Multivariable analysis | |||
| P | HR (95% CI) | P | β | |||
| M, male; F, female; PS, performance status; CEA, baseline carcinoembryonic antigen; CA19-9, baseline carbohydrate antigen 19-9; T4b, tumor invading the organs nearby; ORR, objective response rate; HR, hazard ratio; 95% CI, 95% confidence interval; β, β regression coefficients. | ||||||
| Age (years) | 0.168 | |||||
| ≥65 | 15 | |||||
| <65 | 80 | |||||
| Sex ratio (M:F) | 67:28 | 0.016 | ||||
| PS | 0.018 | |||||
| ≥2 | 8 | |||||
| <2 | 87 | |||||
| Tumor size (cm) | 0.050 | |||||
| ≥7 | 36 | |||||
| <7 | 50 | |||||
| Tumor location | 0.017 | |||||
| Cardia | 36 | |||||
| Noncardia | 59 | |||||
| Tumor differentiation | 0.001 | |||||
| Poorly differentiated | 74 | |||||
| Moderately/well-differentiated | 21 | |||||
| CEA (ng/mL) | 0.741 | |||||
| ≥5 | 39 | |||||
| <5 | 46 | |||||
| CA19-9 (U/mL) | 0.018 | |||||
| ≥35 | 38 | |||||
| <35 | 45 | |||||
| Peritoneal seeding | 0.013 | 0.050 | 0.297 | |||
| Yes | 27 | 1.35 (1.00−1.82) | ||||
| No | 68 | Reference | ||||
| T4b | 0.280 | |||||
| Yes | 17 | |||||
| No | 78 | |||||
| Ascites | <0.001 | <0.001 | 0.505 | |||
| Yes | 17 | 1.66 (1.25−2.21) | ||||
| No | 78 | Reference | ||||
| No. of noncurative factors | <0.001 | <0.001 | 0.952 | |||
| 1 | 48 | Reference | ||||
| ≥2 | 47 | 2.59 (1.77−3.80) | ||||
| Chemotherapy response | <0.001 | 0.421 | ||||
| ORR | 61 | <0.001 | Reference | |||
| Without ORR | 34 | 1.52 (1.19−1.95) | ||||
2.
Prognostic nomogram for advanced gastric cancer patients receiving systematic chemotherapy. The total points are generated by summing the points projected on the scale for each variable. Overall survival predictions are indicated on the total points scale. ORR, objective response rate.
S1.
Point assignment and prognostic score
| Variables | Score | Estimated
2-year OS (%) |
| ORR, objective response rate; OS, overall survival; *, total prognostic score was estimated by the nomogram, as seen in Figure S1 . | ||
| Peritoneal seeding | ||
| Yes | 3 | |
| No | 0 | |
| Ascites | ||
| Yes | 7 | |
| No | 0 | |
| No. of noncurative factors | ||
| 1 | 0 | |
| ≥2 | 10 | |
| ORR | ||
| Yes | 0 | |
| No | 4 | |
| Total prognostic score* | ||
| 0−13 | 39.6 | |
| 14−17 | 22.2 | |
| 20−24 | 12.3 | |
S1.
Calibration plots of prognostic nomogram for predicting overall survival (OS) at 1-year (A), 2-year (B), and 3-year (C). The light grey line represents the reference line.
S2.
Kaplan-Meier curves demonstrating overall survival of patients stratified by nomogram-based risk group (P<0.001) (log-rank test).
Survival of conversion surgery and chemotherapy alone groups
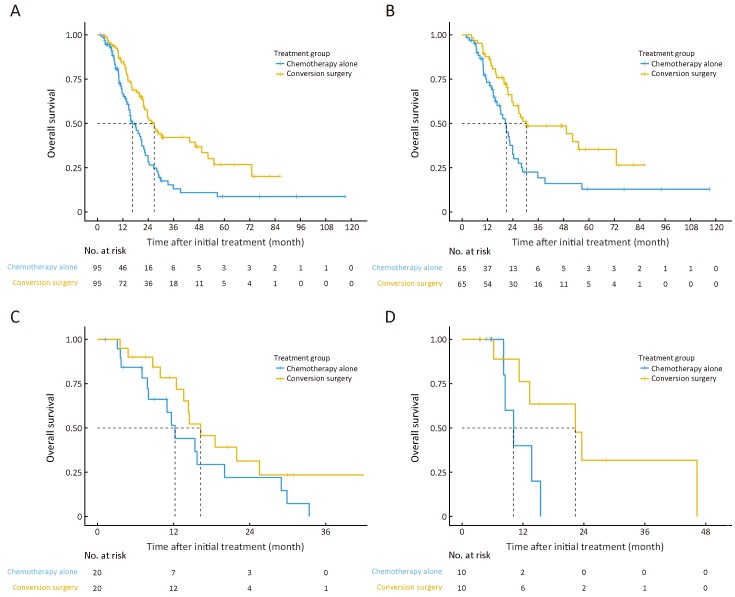
After 1:1 PSM, a total of 190 patients (95 for each group) were finally analyzed. All the characteristics of patients in the conversion surgery and chemotherapy alone groups were balanced after matching (Table 1 ). The MST for patients in the conversion surgery group was 26.80 (95% CI, 21.90−49.30) months compared with patients in the chemotherapy alone group at 16.60 (95% CI, 14.70−21.90) months (P<0.001, log-rank test; adjusted HR, 0.51; 95% CI, 0.36−0.75) (Figure 3A ). Among patients in the low-risk group, the MST of patients treated with conversion surgery was 30.40 [95% CI, 24.20−not reached (NR)] months, while that of patients treated with chemotherapy alone was 20.90 (95% CI, 16.60−24.40) months (P=0.013, log-rank test; adjusted HR, 0.54; 95% CI, 0.33−0.88) (Figure 3B ). No survival differences were observed between patients who underwent conversion surgery and those who were treated with chemotherapy alone in the moderate-risk (MST, 16.30 months vs. 12.20 months; P=0.221, log-rank test; adjusted HR, 0.61; 95% CI, 0.28−1.35) (Figure 3C ) and high-risk groups (MST, 22.30 months vs. 10.20 months; P=0.131, log-rank test; adjusted HR, 0.32; 95% CI, 0.07−1.41) (Figure 3D ).
3.
Kaplan-Meier estimates of overall survival according to treatment between the conversion surgery group and the chemotherapy alone group after propensity score matching (log-rank test). (A) All patients (P<0.001); (B) Low-risk group (P=0.013); (C) Moderate-risk group (P=0.221); and (D) High-risk group (P=0.131).
Discussion
In the present study, a total of 95 advanced GC patients were treated with conversion surgery. After PSM, the survival time of patients in the conversion surgery group was longer than that of patients in the chemotherapy alone group, indicating a potential survival benefit of conversion surgery. Moreover, we found that patients in the low-risk group, but not those in the moderate- or high-risk groups, could benefit from conversion surgery.
Even though systemic chemotherapy has been demonstrated to improve the survival of patients with advanced GC, the prognosis of these patients remains dismal, with a MST of approximately 1 year (7-10). Clinicians have used multimodal treatment strategies to prolong the survival of advanced GC (32-34). Among them, palliative gastrectomy should theoretically reduce tumor burden and facilitate systemic chemotherapy. However, the results of REGATTA trial demonstrated that gastrectomy plus chemotherapy did not prolong the survival of patients with advanced GC compared with chemotherapy alone (32). If advanced GC exhibits a remarkable response to chemotherapy and R0 resection is possible, the usefulness of conversion surgery for improving survival remains unclear. Several previous studies have reported the outcomes of conversion surgery (13-25). Okabe et al. reported that 22 of 41 advanced GC patients underwent R0 resection after induction chemotherapy with S-1 plus cisplatin. The MST of patients treated with R0 resection reached 43.2 months, which was significantly longer than that of patients who did not undergo resection (13). Fukuchi et al. described excellent survival outcomes of patients with unresectable GC after conversion surgery. The 5-year OS of 40 patients after conversion surgery was 43.0%, compared with that of patients who underwent chemotherapy alone at only 1% (18). According to the new classification of advanced GC proposed by Yoshida et al. (26), Yamaguchi et al. reported the long-term survival of patients with advanced GC treated with conversion surgery, with MSTs of 28.3, 30.5, 31.0 and 24.7 months for category 1, 2, 3 and 4, respectively (25). In clinical practice, however, surgical oncologists tend to use surgery for patients with better PS, less tumor burden and better responses to chemotherapy. Substantial selection biases were present in the previous studies, resulting in better outcomes after conversion surgery. Therefore, whether systemic chemotherapy plus conversion surgery had any survival benefit remained uncertain. The present study applied PSM to minimize these selection biases. Our results demonstrated that the MST of patients treated with conversion surgery was 26.80 months, whereas the MST of patients treated with chemotherapy alone was 16.60 months, suggesting favorable survival after conversion surgery. Moreover, we constructed a useful risk-stratification model based on a nomogram. We found that only patients in the low-risk group benefited from conversion surgery. These results help clinicians make decision to perform conversion surgery. For example, for the advanced GC patients with massive ascites [risk factors: ascites (7 point) and noncurative factors ≥2 (10 point)], these patients were grouped into moderate- even high-risk groups, and the conversion surgery should not be recommended for these patients.
It should be discussed with regard to the type of surgical procedure, the timing of the surgery, and the continuation of postoperative chemotherapy. Most previous studies showed that the survival outcomes of patients treated with R0 resection were superior to those of patients treated with R1/2 resection (13-15,18,19,22,25). In our study, R0 resection was performed in 48 (50.5%) patients who underwent conversion surgery. The MST of patients treated with R0 resection exceeded 4 years (49.30 months), whereas the MST of those treated with R1/2 resection was 21.90 months (data not given). The chemotherapeutic regimens and the number of cycles of induction chemotherapy for conversion surgery are debatable. A phase II study reported favorable survival outcomes of the DCS regimen (docetaxel, cisplatin and S-1), with an ORR of 87.1% and a MST of 687 days (35). Several studies (19,22,23) also demonstrated the efficacy of the DCS regimen for patients who have planned conversion surgery. However, the occurrence of grade 3/4 hematologic toxicities with the DCS regimen were high, including 64.5% of patients experiencing leukopenia, 77.4% of patients experiencing neutropenia and 16.1% of patients experiencing febrile neutropenia (35). Therefore, for patients receiving the DCS regimen for conversion surgery, the adverse effects should be carefully monitored. If patients without metastatic disease have planned neoadjuvant chemotherapy, the number of cycles of preoperative chemotherapy range from 2 to 4 (36). However, conversion surgery plays an adjuvant role in improving the survival of patients with advanced GC, on the condition that the tumor burden is minimized by chemotherapy. There is no uniform consensus regarding the number of cycles of induction chemotherapy that should be used. Theoretically, surgery should be performed before chemoresistance develops. Our study has some limitations. First, this was a substantial retrospective study. Second, the time period of patient accrual was up to 15 years, and there were inconsistencies in the treatment strategies used, including the chemotherapeutic regimens, the timing of conversion surgery, and the type of procedure for conversion surgery. Third, the C-index for the nomogram is not very high (0.65) in the present study. Lastly, even though we used PSM to offset selection bias to maximal extent, there may be several unobserved unbalanced covariates because of the retrospective nature of this study. Therefore, a well-designed random controlled trial is still needed to verify the conclusions of this study.
Conclusions
Conversion surgery was associated with prolonged survival, especially for low-risk population. To date, the results of our study promote an understanding of conversion surgery and inform future reasonable prospective randomized controlled trials to further clarify the role of this surgical strategy in the treatment of GC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Yingbo Chen, Email: chenyb@sysucc.org.cn.
Zhiwei Zhou, Email: zhouzhw@sysucc.org.cn.
References
- 1.Torre LA, Bray F, Siegel RL, et al Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Gu X, Zheng R, Xia C, et al Interactions between life expectancy and the incidence and mortality rates of cancer in China: a population-based cluster analysis. Cancer Commun (Lond) 2018;38:44. doi: 10.1186/s40880-018-0308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, et al Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Zheng R, Wang N, et al Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res. 2018;30:291–8. doi: 10.21147/j.issn.1000-9604.2018.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izuishi K, Mori H Recent strategies for treating stage IV gastric cancer: Roles of palliative gastrectomy, chemotherapy, and radiotherapy. J Gastrointestin Liver Dis. 2016;25:87–94. doi: 10.15403/jgld.2014.1121.251.rv2. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsu A Chemotherapy for metastatic gastric cancer: past, present, and future. J Gastroenterol. 2008;43:256–64. doi: 10.1007/s00535-008-2177-6. [DOI] [PubMed] [Google Scholar]
- 7.Koizumi W, Narahara H, Hara T, et al S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21. doi: 10.1016/s1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 8.Bang YJ, Van Cutsem E, Feyereislova A, et al Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 10.Okines AF, Ashley SE, Cunningham D, et al Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for advanced esophagogastric cancer: dose-finding study for the prospective multicenter, randomized, phase II/III REAL-3 trial. J Clin Oncol. 2010;28:3945–50. doi: 10.1200/JCO.2010.29.2847. [DOI] [PubMed] [Google Scholar]
- 11.Poston GJ, Adam R, Alberts S, et al OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005;23:7125–34. doi: 10.1200/JCO.2005.08.722. [DOI] [PubMed] [Google Scholar]
- 12.Adam R, Wicherts DA, de Haas RJ, et al Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009;27:1829–35. doi: 10.1200/JCO.2008.19.9273. [DOI] [PubMed] [Google Scholar]
- 13.Okabe H, Ueda S, Obama K, et al Induction chemotherapy with S-1 plus cisplatin followed by surgery for treatment of gastric cancer with peritoneal dissemination. Ann Surg Oncol. 2009;16:3227–36. doi: 10.1245/s10434-009-0706-z. [DOI] [PubMed] [Google Scholar]
- 14.Kanda T, Yajima K, Kosugi S, et al Gastrectomy as a secondary surgery for stage IV gastric cancer patients who underwent S-1-based chemotherapy: a multi-institute retrospective study. Gastric Cancer. 2012;15:235–44. doi: 10.1007/s10120-011-0100-y. [DOI] [PubMed] [Google Scholar]
- 15.Kim SW The result of conversion surgery in gastric cancer patients with peritoneal seeding. J Gastric Cancer. 2014;14:266–70. doi: 10.5230/jgc.2014.14.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Yu YY, Li W, et al A phase II trial of Xeloda and oxaliplatin (XELOX) neo-adjuvant chemotherapy followed by surgery for advanced gastric cancer patients with para-aortic lymph node metastasis. Cancer Chemother Pharmacol. 2014;73:1155–61. doi: 10.1007/s00280-014-2449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuchi M, Ishiguro T, Ogata K, et al Risk factors for recurrence after curative conversion surgery for unresectable gastric cancer. Anticancer Res. 2015;35:6183–7. [PubMed] [Google Scholar]
- 18.Fukuchi M, Ishiguro T, Ogata K, et al Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. 2015;22:3618–24. doi: 10.1245/s10434-015-4422-6. [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita J, Fushida S, Tsukada T, et al Efficacy of conversion gastrectomy following docetaxel, cisplatin, and S-1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol. 2015;41:1354–60. doi: 10.1016/j.ejso.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Chan DY, Syn NL, Yap R, et al Conversion surgery post-intraperitoneal paclitaxel and systemic chemotherapy for gastric cancer carcinomatosis peritonei. Are we ready? J Gastrointest Surg. 2017;21:425–33. doi: 10.1007/s11605-016-3336-3. [DOI] [PubMed] [Google Scholar]
- 21.Fukuchi M, Mochiki E, Ishiguro T, et al Efficacy of conversion surgery following S-1 plus cisplatin or oxaliplatin chemotherapy for unresectable gastric cancer. Anticancer Res. 2017;37:1343–7. doi: 10.21873/anticanres.11453. [DOI] [PubMed] [Google Scholar]
- 22.Sato Y, Ohnuma H, Nobuoka T, et al Conversion therapy for inoperable advanced gastric cancer patients by docetaxel, cisplatin, and S-1(DCS) chemotherapy: a multi-institutional retrospective study. Gastric Cancer. 2017;20:517–26. doi: 10.1007/s10120-016-0633-1. [DOI] [PubMed] [Google Scholar]
- 23.Uemura N, Kikuchi S, Sato Y, et al A phase II study of modified docetaxel, cisplatin, and S-1(mDCS) chemotherapy for unresectable advanced gastric cancer. Cancer Chemother Pharmacol. 2017;80:707–13. doi: 10.1007/s00280-017-3404-8. [DOI] [PubMed] [Google Scholar]
- 24.Yuan SQ, Nie RC, Chen S, et al Selective gastric cancer patients with peritoneal seeding benefit from gastrectomy after palliative chemotherapy: a propensity score matching analysis. J Cancer. 2017;8:2231–7. doi: 10.7150/jca.18932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi K, Yoshida K, Tanahashi T, et al The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2018;21:315–23. doi: 10.1007/s10120-017-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida K, Yamaguchi K, Okumura N, et al Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19:329–38. doi: 10.1007/s10120-015-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014(ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J, et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Harrell FE Jr., Lee KL, Mark DB Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Camp RL, Dolled-Filhart M, Rimm DL X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 31.Garrido MM, Kelley AS, Paris J, et al Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49:1701–20. doi: 10.1111/1475-6773.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujitani K, Yang HK, Mizusawa J, et al Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–18. doi: 10.1016/s1470-2045(15)00553-7. [DOI] [PubMed] [Google Scholar]
- 33.Nie RC, Chen S, Yuan SQ, et al Significant role of palliative gastrectomy in selective gastric cancer patients with peritoneal dissemination: A propensity score matching analysis. Ann Surg Oncol. 2016;23:3956–63. doi: 10.1007/s10120-016-0633-110.1245/s10434-016-5223-2. [DOI] [PubMed] [Google Scholar]
- 34.Yang XJ, Huang CQ, Suo T, et al Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–81. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato Y, Takayama T, Sagawa T, et al Phase II study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemother Pharmacol. 2010;66:721–8. doi: 10.1007/s00280-009-1215-2. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham D, Allum WH, Stenning SP, et al Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]