1. Overview
Prostate cancer is the second most common cause of cancer in males worldwide, and it is the second leading cause of cancer death in American males behind lung cancer. According to data from China National Cancer Registration Institute, prostate cancer has become the most common tumor in male urinary malignancies since 2008. The incidence rate is about 9.80/100,000 in 2014 and ranks the sixth common malignancy in male malignant tumors. The mortality of prostate cancer is 4.22/100,000, and it is the 9th common cause of death in all male malignancies. It is important to note that the incidence of prostate cancer in China varies widely between urban and rural areas, with especially high incidence in large cities. The prevalence in urban and rural areas is 13.57/100,000 and 5.35/100,000, respectively in 2014. With population over 65 years old accounting for more than 10% of the total population in Shanghai, it could be expected that the incidence of prostate cancer would increase dramatically. In addition, the staging varies widely between China and Western developed countries. In China, only 30% of newly diagnosed patients are clinically localized, and the rest are locally advanced or extensively metastatic disease, who have lost the chance of radical treatment with poor prognosis.
2. Risk factors of prostate cancer
The etiology and pathogenesis of prostate cancer are very complicated, and the exact cause is still unclear. Etiology studies show that prostate cancer is closely related to age, genetic factors and exogenous factors including environmental factors and diet habits.
2.1 Age and genetic factors
The incidence of prostate cancer varies greatly among different races with the highest in African-American, intermediate in whites, and low in Asians, which suggests that genetic factors are one of the most important factors in the pathogenesis of prostate cancer. Epidemiological studies have shown that if an immediate family member (brother or father) developed prostate cancer, the risk of prostate cancer for him would be more than double. With two or more affected close family member, the risk of prostate cancer could increase by 5−11 times. Compared with population without family history of prostate cancer, the disease onset would be 6−7 years earlier for those with family history. Only 9% of patients with prostate cancer are true hereditary disease. Hereditary prostate cancer is defined as three or more affected relatives, or at least two relatives with early-onset, and 43% of patients with hereditary prostate cancer are under 55 years old. The incidence rate increases with aging, with the highest incidence at the age of 65−80 years old.
2.2 Exogenous factors
Epidemiological data show that the incidence of prostate cancer in Asian-American populations will increase significantly after moving to the United States, implying an exogenous factors associated with geographical environment and dietary habits. Currently, exogenous risk factors of prostate cancer are still under study, and some are subject to debate. High alcohol intake has been associated with higher risk of prostate cancer and prostate cancer-specific mortality. Both over-low and over-high vitamin D concentrations are associated with pathogenesis of prostate cancer, especially for high-grade disease. Exposure to sun will increase the level of vitamin D and reduce the risk of prostate cancer. The intake of fried foods is associated with the pathogenesis of prostate cancer. In Asia, the low incidence may associate with high green tea consumption, suggesting the preventive role for prostate cancer. A meta-analysis of carotene showed a reduced tendency for prostate cancer, but the randomized controlled trial did not reach the conclusion. However selenium and Vitamin E supplementation were found not to affect prostate cancer incidence. In hypogonadism patients, supplementation of androgens does not increase the risk of prostate cancer.
3. Pathological classification and grading system
Pathological types of prostate cancer include adenocarcinoma (alveolar adenocarcinoma), intraductal carcinoma, ductal adenocarcinoma, urothelial carcinoma, squamous cell carcinoma, basal cell carcinoma, neuroendocrine tumors, etc. Usually prostate cancer refers to adenocarcinoma since it accounts for the majority of prostate cancer. Gleason score is recommended for pathological grading of prostate adenocarcinoma.
The Gleason score is recommended for the pathological grading of prostate adenocarcinoma. The score system comprises primary and secondary scores, each of which is graded by 5. The degree of differentiation is measured by total score which is the sum of two scores.
The Gleason score is currently the most widely used method for grading prostate adenocarcinoma. After several revisions since its release in 2004, the new WHO classification is described in detail as follows : 1) Gleason grade 1: densely arranged but isolated glands form a well-defined tumor nodule; 2) Gleason grade 2: tumor nodules have microinvasion to surrounding normal tissues, the glands are loosely arranged, and the atypia is greater than grade 1; 3) Gleason grade 3: tumor glands vary in size with irregular shape and invasive growth pattern. Each gland is independent and has clear lumen; 4) Gleason grade 4: tumor glands fuse each other to form a mesh-like shape, or no glandular cavity in the middle of ring-like tissue; and 5) Gleason grade 5: poorly differentiated carcinoma with no obvious ducts, arranged as solid cell nests or single and double cell cords.
The Gleason score is also subject to the following principles: 1) Gleason score 2−5 is not suitable for evaluation of trucut biopsy, and should be used in caution for evaluation of sample obtained in other ways; 2) sieve glands are classified as Gleason 4; 3) if gland is glomerular structure, the grading should be 4; 4) the grading of mucinous adenocarcinoma should be judged according to its growth pattern, and it should not simply graded as Gleason 4; 5) beside sieve glands and glomerular structure, some poorly differentiated and fused glands should be graded as Gleason 4; 6) acne-like necrosis is graded as Gleason 5; 7) ductal adenocarcinoma in the form of sieve and papillary should be Gleason 4, prostatic intraepithelial neoplasia (PIN)-like ductal adenocarcinoma is graded as Gleason grade 3, and graded as Gleason 5 if there is necrosis; 8) in high-grade adenocarcinoma, the low-grade component can be ignored if it is <5%. In contrast, if high-level component exists in biopsy specimen, it should be included in the score regardless of its proportion; 9) in the radical specimen, if >5% of Gleason 5 components were found in the component of Gleason score of 7 (4+3) sample based on the previous biopsy sample, the final score should be Gleason 9 (4+5); if only a small portion of grade 5 components exist, the grade 5 should be reported as the third score; and 10) scoring is not necessary if the change of tumor morphology is obvious after treatment.
The new published WHO grade group system for prostate cancer is based on the new grading system proposed at the 2014 International Society of Urological Pathology (ISUP) Consensus Conference, named the Prostate Cancer Grade Group system (also called ISUP grade). The ISUP system divides prostate cancer into five different groups according to combination of Gleason score since these five different combinations indicate different aggressiveness of the disease: 1) ISUP grade 1 is equivalent to Gleason score ≤6; 2) ISUP grade 2 is equivalent to Gleason score 3+4=7; 3) ISUP grade 3 is equivalent to Gleason score 4+3=7; 4) ISUP grade 4 is equivalent to Gleason score 4+4=8, 3+5=8 and 5+3=8; and 5) ISUP grade 5 is equivalent to Gleason score 9−10.
The most common adapted staging system for prostate cancer is TNM stage system, the 8th edition of which was published on 2017 by the American Joint Committee on Cancer Staging (AJCC) (Table 1 ).
1.
Clinical tumor node metastasis (TNM) classification of prostate cancer
| Category | Definition |
| *, invasion into the prostatic apex or into (but not beyond) the prostatic capsule is not classified as T3, but as T2; 1, metastasis no larger than 0.2 cm can be designated pNmi; 2, when more than one site of metastasis is present, the most advanced category is used. (p)M1c is the most advanced category. | |
| T — Primary | |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Clinically inapparent tumor that is not palpable |
| T1a | Tumor incidental histological finding in 5% or less of tissue resected |
| T1b | Tumor incidental histological finding in more than 5% of tissue resected |
| T1c | Tumor identified by needle biopsy [e.g. because of elevated prostate-specific antigen (PSA)] |
| T2 | Tumor that is palpable and confined within the prostate |
| T2a | Tumor involves one half of one lobe or less |
| T2b | Tumor involves more than half of one lobe, but not both lobes |
| T2c | Tumor involves both lobes |
| T3 | Tumor extends through the prostatic capsule* |
| T3a | Extracapsular extension (unilateral or bilateral) including microscopic bladder neck involvement |
| T3b | Tumor invades seminal vesicle(s) |
| T4 | Tumor is fixed or invades adjacent structures other than seminal vesicles: external sphincter, rectum, levator muscles, and/or pelvic wall |
| N — Regional lymph nodes1 | |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| M — Distant metastasis2 | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Non-regional lymph node(s) |
| M1b | Bone(s) |
| M1c | Other site(s) |
Based on its aggressiveness and metastatic risk, prostate cancer is stratified in six different risk groups: 1) very low risk: T1c, Gleason score ≤6/ISUP grade 1, prostate specific antigen (PSA) <10 ng/mL, positive biopsy fragments ≤3, cancer in each core ≤50%, and PSA density <0.15 ng/(mL·g); 2) low risk: T1−T2a, Gleason score ≤6/ISUP grade 1, PSA<10 ng/mL; 3) favorable intermediate risk: T2b−T2c, or Gleason score 3+4=7/ISUP grade 2, or PSA 10−20 ng/mL, and cancer in each core <50%; 4) unfavorable intermediate risk: T2b−T2c, or Gleason score 3+4=7/ISUP grade 2, or Gleason score 4+3=7/ISUP grade 3, or PSA 10−20 ng/mL; 5) high risk: T3a or Gleason score 8/ISUP grade 4, or Gleason score 9−10/ISUP grade 5, or PSA>20 ng/mL; and 6) very high risk: T3b−T4, or ISUP grade 5, or >4 cores with Gleason score 8−10/ISUP grade 4 or 5.
4. Diagnostic evaluation
4.1 Monitoring and screening for population with high-risk prostate cancer
Screening for prostate cancer has been widely carried out in Western nations. The reduced mortality rate recently in the USA is partly attributed to the widely adopted aggressive prostate cancer screening policy. As more prostate cancers are discovered and treated, the proportion of early prostate cancer is increasing; it might be associated with minor over-diagnosis and over-treatment. Therefore, screening based on the entire population remains a controversy in Western nations. However, since there has been no large-scale screening in China, a considerable number of high-invasive or advanced prostate cancers may exist in the population. Therefore, prostate cancer screening is necessary currently in China. PSA-based prostate cancer screening is recommended for well-informed males over 50, or older than 45 years of age with a family history of prostate cancer.
PSA is a single-chain glycoprotein with serine protease activity secreted by prostate acinar and ductal epithelial cells. It is mostly found in semen and participates in the liquefaction process of semen. PSA is mainly confined to prostate tissue and maintains a low serum level under normal condition. Two forms of PSA exist in serum, 10%−40% of which are free PSA (f-PSA); 60%−90% are complexed PSA containing PSA-ACT, PSA-API and PSA_A2M. The sum of f-PSA and complexed PSA is usually referred to as total serum PSA (t-PSA). Serum PSA will increase when cancerous cells destroy normal tissues, causing large amount of PSA entering the blood. The half-life of PSA is 2−3 d.
PSA-based screening is recommended for males >50 years, or >45 years with a family history of prostate cancer. People should be fully informed about the risk and benefit of PSA-based screening in advance.
Assessment of PSA results: t-PSA>4.0 ng/mL is considered abnormal. Re-testing is needed if initial PSA level is abnormal. Level of serum PSA is affected by age and size of prostate.
F-PSA has certain aided diagnostic value when the total serum PSA is between 4 ng/mL and 10 ng/mL. The recommended normal reference value for f-PSA/t-PSA is >0.16 in China. Prostate biopsy should be considered if the patient’s t-PSA level is 4−10 ng/mL and f-PSA/t-PSA <0.16.
PSA density (PSAD), PSA velocity (PSAV) and PSA doubling time (PSADT) have certain clinical value for diagnosis and prognosis of patients with prostate cancer.
Since PSA shows a poor specificity, researchers have long been searching for new prostate cancer-specific markers. In recent years, PSA isomer 2 (p2PSA) and its derivatives, as well as prostate health index (PHI) and other evaluation indicators have gradually attracted attention. The results suggest that p2PSA is associated with prostate cancer and high-grade disease. PHI has outperformed t-PSA in the diagnosis of prostate cancer, especially with t-PSA between 4−10 ng/mL, which can reduce the number of unnecessary prostate biopsy. PHI is calculated by t-PSA, f-PSA and p2PSA in the following formula: PHI=p2PSA/f-PSA×
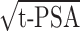 .
.
Prostrate specific membrane antigen (PSMA): PSMA is a membrane-bound glycoprotein with high specificity for benign and malignant epithelial cells of the prostate. PSMA can be detected in normal serum while it is higher in prostate cancer patients. The PSMA values are associated with high-stage disease or androgen-independent status.
PCA3 is a factor expressed in prostate cancer. Among patients with elevated PSA level, PCA3 serves as a diagnostic marker and has a better diagnostic accuracy than t-PSA, f-PSA, etc.
4.2 Genetic testing
The overall incidence of DNA-repair gene mutations in metastatic, localized high-risk and low- to moderate-risk prostate cancer was 11.8%, 6% and 2%, respectively. The understanding on frequency of DNA-repair gene mutations is important for family genetic counseling and better assessment on risk of individual’s cancer pathogenesis. In patients with metastatic castration-resistant prostate cancer (CRPC), the frequency of DNA-repair gene mutations may be higher (up to 25%). Early study in Poly (ADP-ribose) polymerase (PARP) inhibitors has shown clinical benefit in initial studies. Particularly, preliminary data indicate that the PARP inhibitor olaparib is effective in these patients. According to report, defect in DNA repair is indication of tumor sensitivity to platinum agent.
The expert panel suggests that physician should obtain details of family and individual’s cancer history. A genetic counseling is recommended for a family suspected of having genetic cancer syndrome. In addition, due to the high incidence of germline mutations, a germline test for patients with metastatic and high-risk/extremely high-risk clinically localized prostate cancer is recommended and genetic counseling before and after the test is critical.
The data also showed that germline mutation in gene such as BRCA1 has been associated with increased risk of progression for prostate cancer patients with local treatment and with decreased overall survival (OS). If you are considering actively monitoring these patients, you should discuss this information with them.
4.3 Digital rectal examination (DRE)
DRE plays an important role on early diagnosis and staging of prostate cancer. The typical manifestation of prostate cancer consists of palpable hard nodules without tenderness and unclear border. However, no palpable nodules will not exclude the disease. Combined PSA and imaging examination are necessary for comprehensive diagnosis. DRE should be performed after the PSA test since it may squeeze PSA into the blood and affect the accuracy of serum PSA values.
4.4 Magnetic resonance examination
Magnetic resonance imaging (MRI) is one of the most important methods for diagnosing and staging of prostate cancer. Relying on T2-weighted imaging and contrast-enhanced images, prostate cancer usually manifests a weak signal lesions in T2-weighted imaging at periphery zone of prostate, which are significantly different from the normal high signal in the same zoon. It can also accurately detect pelvic lymph node and bone metastasis. multi-parameter MRI (mpMRI) is useful for detecting large, poorly differentiated cancers (ie, Gleason score ≥7/ISUP grade >2), and it plays an important role in the MRI-transrectal ultrasound (MRI-TRUS) fusion targeted biopsy for any suspected lesion of prostate; mpMRI is also helpful in detecting whether extra-capsular is intact or not (T-stage), and it has higher negative predictive value among low-risk patients, and thus can help guidance decision-making in nerve-sparing radical prostatectomy.
Magnetic resonance spectroscopy (MRS) detects different spectral lines in prostate cancer tissues caused by metabolism of citrate, choline and creatinine with normal and proliferative prostate tissues. It reflects changes in cell metabolism with added value to conventional MRI, and thus it will serve as reference value for early diagnosis of prostate cancer.
4.5 Bone scan examination
Currently, bone scan has been the most widely used method for evaluating bone metastases on patients with prostate cancer, a meta-analysis showed that the sensitivity and specificity of bone scans were 79% and 82%, respectively. The positive rate of bone scan was mostly affected by PSA, clinical staging and Gleason score. The positive rate was 2.3% when PSA <10 ng/mL, 5.3% when PSA 10−20 ng/mL, and 16.2% when PSA 20−50 ng/mL. The positive rate of localized prostate cancer was 6.4%, and 49.5% for locally advanced prostate. The positive rate was 5.6% for Gleason score 7, while 29.9% for Gleason score 8. At present, bone scan is recommended when there is bone pain, regardless of PSA, Gleason score and clinical stage.
4.6 Positron emission tomography-computed tomography (PET-CT)
11C-Choline PET/CT has been used to detect and distinguish prostate cancer from benign disease in recent years. The sensitivity and specificity of this technique were 85% and 88%, respectively in biochemical reoccurrence and re-staged patients. 11C-Choline PET/CT may be helpful in detecting distant metastases of prostate cancer.
The sensitivity and specificity of 68Ga-PSMA PET/CT are 86% and 86% for patients with prostate cancer, and 80% and 97% at cancer lesion of the disease, respectively. The diagnostic accuracy is much higher than that of the traditional imaging examination for patients with prostate cancer.
4.7 Prostate biopsy
4.7.1 Indication and contraindication for systemic prostate biopsy
Indications for systemic prostate trucut biopsy include: 1) suspicious nodules found with digital rectal examination at any serum PSA value; 2) suspicious lesions found in trans-rectal ultrasound or MRI at any serum PSA value; 3) PSA> 10 ng/mL; and 4) PSA 4−10 ng/mL, with abnormal f/t-PSA or PSAD value. Contraindications for prostate trucut biopsy include: 1) acute infection or fever; 2) hypertensive crisis; 3) decompensated cardiac failure; 4) diseases with severe bleeding tendency; 5) diabetes mellitus with unstable blood glucose; and 6) serious internal and external hemorrhoids, and perianal or rectal lesions.
4.7.2 Implementation of prostate biopsy
Routine examination before prostate biopsy: MRI should be performed before prostate biopsy in order to assess the stage of prostate cancer since prostate biopsy could interfere the imaging of MRI. Antibiotic prophylaxis is necessary before transrectal prostate biopsy and oral or intravenous antibiotics are recommended. Quinolone is the drug of choice and ciprofloxacin is usually superior to ofloxacin. Increased quinolone resistance is associated with severe post-biopsy infection.
Antibiotic prophylaxis: oral or intravenous prophylactic antibiotics are recommended before TRUS-guided biopsy. Quinolone is the drug of choice. However prophylactic antibiotics are not necessary for transperineal biopsy of prostate.
Intestinal preparation: coloclysis is routine procedure before rectal prostatebiopsy. Kaisell and iodophor instead of enema are recommended before procedure.
Anticoagulation and antiplatelet agents in perioperative period: for patients with risk of cardiovascular, cerebrovascular disease and history of stenting who have long-term use of oral anticoagulation or antiplatelet agents, the risk of bleeding and cardiovascular and cerebrovascular diseases should be assessed during the perioperative period. Related medicines should be used with caution.
Number and location of prostate biopsies: eight or more cores are necessary for patients when the volume of prostate is 30−40 mL. The initial baseline core of 10−12 is recommended. The incidence of complications did not correlate significantly with the number of cores, and thus saturated biopsy could be an option.
4.7.3 Repeated systemic prostate biopsy
Repeated prostate biopsy should be considered if first prostate biopsy is negative, but DRE, follow-up PSA or other bio-derivative levels suggest suspicious prostate cancer. Repeated prostate biopsy should be considered under the following conditions: 1) atypical hyperplasia or high-grade PIN is found in the initial pathological biopsy, especially in multi-cores results; 2) repeated serum PSA>10 ng/mL; 3) repeated serum PSA 4−10 ng/mL, but %f-PSA, PSAD value, DRE or image result is abnormal such as suspicious cancer lesion demonstrated by TRUS or MRI. Imaging-targeted biopsies of suspicious lesions can be obtained through image fusion technology; and 4) PSA control is required every 3 months if the value of PSA 4−10 ng/mL,%f-PSA, PSAD value, DRE and imaging are all normal, however if PSA>10 ng/mL happened again, or PSAV>0.75 ng/mL, repeated prostate biopsy should be necessary.
In addition to routine examination, mpMRI is recommended before repeated prostate biopsy. Targeted mpMRI can significantly improve the positive rate of repeated prostate biopsy and avoid missing the diagnosis of high-risk prostate cancer. However, the schedule and interval between prostate biopsies are still controversial. The interval of three month or longer is recommended till the tissue structure is fully recovered from previous biopsy.
Before repeating prostate biopsy, if suspicious lesion found by MRI, imaging targeted biopsy should be performed again.
4.7.4 Limitations and new strategies for prostate systemic biopsies
The major limitation of systemic transrectal or perineal biopsy is false negativity. It could miss diagnosis of high-risk prostate cancer, or lead to over-diagnosis. Improving the positive rate while avoiding over-diagnosis is a huge challenge in the early diagnosis of prostate cancer. In recent years, prostate biopsy with contrast-enhanced ultrasound, sonoelastography, and mpMRI has shown significant advantages in finding clinically significant prostate cancer and avoiding over-diagnosis.
MRI-guided targeted biopsy can directly extract samples from suspicious lesion and has the highest accuracy. A number of studies have shown that MRI-guided targeted biopsy can improve the detection rate of high-grade prostate cancer during repeated biopsy. However, the procedure is relatively complicated and expensive to promote.
MRI-TRUS fusion technique combines the position accuracy of MRI with the convenience of TRUS-guided biopsy. It significantly increases the positive rate of needle biopsy and the chance of discovering clinically significant prostate cancer while avoiding misdiagnosis on clinically unsignificant lesions. Comparing with MRI, the fusion technique is more convenient and lays the foundation for precise focal therapy.
5. Treatment of prostate cancer
5.1 Treatment for localized prostate cancer
5.1.1 Watchful waiting and active surveillance
5.1.1.1 Watchful waiting
Watchful waiting monitors the progression of prostate cancer to provide palliative treatment when disease-related complaints occur, or examination results changes, or PSA results indicate impending symptoms. The main advantage of watchful waiting is to avoid possible side effects caused by unnecessary treatments such as androgen depletion treatment (ADT). It is generally applicable to patients with life expectancy less than 10 years.
5.1.1.2 Active surveillance
Active surveillance actively and dynamically monitors the progression of prostate cancer. It aims to achieve correct timing for curative treatment in patients with clinically localized prostate cancer. It is generally applicable to low-risk prostate cancer patients with life expectancy more than 10 years. The objective is to delay the possible curative treatment to reduce the side effects caused by treatment without compromising survival.
The inclusion criteria of active surveillance includes life expectancy of more than 10 years, tumor stage cT1 or cT2, PSA ≤10 ng/mL, biopsy Gleason score ≤6, positive biopsy fragments ≤2, and cancerous tissue occupy ≤50% of each biopsy core. Before conducting active monitoring, patients should be informed about the possibilities that they may undergo radical surgery and radiotherapy at some stage in the future. During the follow-up, DRE (at least once a year), PSA (at least once every six months), mpMRI and repeated systemic prostate trucut biopsy (at least once every 3−5 years) should be performed. Active surveillance should be adjusted to active treatment (such as focal or radical treatment) if the pathology has changed after repeated biopsy, such as increased Gleason score, the number of positive needles or the volume of cancer in each core, and/or the progression of the T stage.
5.1.2 Radical prostatectomy
Radical prostatectomy aims to completely remove the tumor while retaining the urinary continence and erectile function as much as possible. Prostatectomy can be performed by open, lapraroscopic or robot-assisted approaches. Therapeutic decisions should be made after all treatments have been discussed by multidisciplinary teams (including urologists, radiation oncologists, medical oncologists and radiologists), and after balancing the benefits and side effects. Appropriate therapeutic modalities should also be discussed together with patients.
5.1.3 Radical treatment for low-risk prostate cancer
Radical prostatectomy is recommended for low-risk patients either by laparoscopic, robot-assisted laparoscopic or open surgery. The mortality rate is only 5%. Intraoperative pelvic lymph node dissection (PLND) is generally not recommended because the percentage of metastatic pelvic lymph nodes is less than 5%.
A randomized clinical trial conducted in 685 patients with early prostate cancer (most with cT2) compared radical prostatectomy with watchful waiting. After a median follow-up period of 12.8 years, there was a significant improvement in tumor-specific survival, OS, risk of metastasis and local progression in the radical prostatectomy group. Mortality was significantly reduced during the 23-year follow-up, with an absolute difference of 11%. In general, the number needed to treat (NNT) to prevent one death was eight. For patients under 65 years old, the NNT is 4. This finding supports the treatment option of radical prostatectomy for clinically localized prostate cancer, even in patients with low-risk prostate cancer.
5.1.4 Radical treatment for intermediate-risk prostate cancer
Radical prostatectomy is still recommended for intermediate-risk patients either by laparoscopic, robotic-assisted laparoscopic or open surgery. The percentage of metastatic pelvic lymph nodes is between 3.7% and 20.1%. Extended lymph node dissection should be performed in intermediate-risk disease if the risk for positive lymph node exceeds 5% with the nomogram (developed at the Memorial Sloan-Kettering Cancer Center) predicting the risk of lymph node metastasis.
For intermediate-risk of prostate cancer, a SPCG-4 study showed that radical prostatectomy can reduce overall mortality, tumor-specific mortality, and distant metastasis of prostate cancer in 18 years after operation. Another PIVOT study showed that radical prostatectomy can reduce overall mortality at 10 years after surgery, but not tumor-specific mortality.
5.1.5 Radical treatment for high-risk prostate cancer
Patients with high-risk prostate cancer are at an increased risk of PSA failure, adjuvant therapy, metastatic progression and death from the disease. Nevertheless, there is no consensus treatment for those patients. Radical prostatectomy is still a reasonable option for patients with tumor which is not fixed to the pelvic wall, or that there is no invasion of the urethral sphincter. Extended pelvic lymph node dissection (ePLND) should be performed in all high-risk patients when undergoing radical prostatectomy since the estimated risk for metastatic pelvic lymph nodes is 15%−40%.
5.1.6 PLND
Although there is insufficient evidence to demonstrate that pelvic lymphadenectomy can improve oncological outcomes, it is generally believed that pelvic lymphadenectomy can provide important information for staging and prognosis, with which nothing else could be matched by any other currently available procedures. The expert panel recommended the use of a nomogram developed at the Memorial Sloan-Kettering Cancer Center to predict the risk of lymph node metastasis, including pre-treatment PSA, clinical stage and Gleason score. Pelvic lymphadenectomy is performed according to the probability of its metastasis. A risk of 2% or 5% is a critical indication to perform ePLND.
Lymph node dissection should be performed with extended pelvic lymphadenectomy. The scope of the dissection includes: the upper boundary is external iliac vessel, the lateral is pelvic wall, the medial is bladder wall, the lower is the bottom of pelvic, the distal end is the Cooper’s ligament, and the proximal end is the internal iliac artery. Several studies have supported the survival advantage of ePLND, which may be due to the removal of micro-metastases lesion. Lymph node dissection can be performed by laparoscopy, robot-assisted laparoscopic or open surgery. The rate of complication for these surgical procedures is similar.
Studies have shown that for cN0 patients, if lymph node dissection in radical prostatectomy confirmed the tumor is pN1, 15-year tumor-specific survival and OS rate would be 45% and 42%, respectively.
The number of removed lymph node and positive lymph nodes, tumor volume within the lymph nodes, and capsular perforation of the nodal metastases are predictors of early recurrence after radical prostatectomy for pN1 patients. A lymph node density greater than 20% was associated with poor prognosis.
5.1.7 Indications for nerve-sparring radical prostatectomy
Nerve-sparing prostatectomy can be performed safely in most males with localized prostate cancer. The absolute contraindications are patients with high-risk of extracapsular disease, such as any cT2c or cT3 P, any Gleason score >7 on biopsy. Preoperative mpMRI may be helpful in patient’s selection.
5.1.8 Adjuvant treatment after radical prostatectomy
For patients with pT3pN0, although PSA<0.1 ng/mL, adjuvant auxiliary or salvage radiotherapy to prostatic fossa should be considered due to increased risk of local relapse factors such as positive margins (highest impact), perforation of prostate capsule, or invasion of seminal vesicles. Adjuvant endocrine therapy may have possible benefit for progression-free survival (PFS) but not OS.
In patients with pN1, the tumor-specific survival rate may reach 80% when conducting early combined adjuvant ADT and adjuvant radiotherapy (if there are poor pathological figures, such as positive margins, perforation of prostate capsule, or invasion of seminal vesicles) after radical prostatectomy.
In a Sureillance, Epidemiology, and End Results (SEER) retrospective study, no significant improvement in OS is observed when adjuvant radiotherapy is combined with radical surgery. And tumor-specific survival was not significantly prolonged either. There is no consensus on the extent of adjuvant radiotherapy although radiotherapy was given to most patients. No conclusion is available on adjuvant chemotherapy after radical surgery, and it is still in the stage of clinical trial.
5.1.9 Surgical treatment for biochemical recurrence after radiotherapy
Radical prostatectomy is a salvage treatment for patients with biochemical recurrence after external beam radiotherapy. But the incidence of complications (including urinary incontinence, erectile dysfunction and bladder neck contracture) is still high compared with radical prostatectomy which serves as an initial therapy. The 10-year overall and tumor-specific survival rates were 54%−89% and 70%−83%, respectively. It is very important to select patients, and salvage prostatectomy should be performed by an experienced surgeon.
6. Radiotherapy for prostate cancer
6.1 External beam radiotherapy (EBRT)
Similar to radical prostatectomy, EBRT is one of the most important radical treatments for prostate cancer patients. The technique includes three-dimensional conformal radiotherapy (3D-CRT), intensity modulated radiotherapy (IMRT), image guided radiation therapy (IGRT), etc. and these are the main technologies for radiation therapy on prostate cancer. The advantage of EBRT is effective, wide range of indication and few complications. It may achieve the similar result as in radical prostatectomy in low-risk patients. EBRT is divided into three categories according to the purpose of radiotherapy on prostate cancer: 1) radical radiotherapy for patients with localized and locally advanced prostate cancer; 2) adjuvant and salvage radiotherapy; and 3) palliative radiotherapy to relieve symptoms and improve quality of life for metastatic cancer patients.
6.1.1 Indications of EBRT for prostate cancer
Localized prostate cancer: for low-risk patients (T1−2a, Gleason ≤6, PSA<10 ng/mL), radical external radiotherapy and radical prostatectomy are first-line treatment, however radical external radiotherapy is preferred for elderly patients. For intermediate-risk patients (T2b or Gleason 7 or PSA 10−20 ng/mL), radical external radiotherapy and surgery are first-line treatment and elderly patients may choose radical external radiotherapy combined with neoadjuvant/synchronous/co-endocrine therapy for 4−6 months. For high-risk patients (≥T2c or Gleason ≥8 or PSA>20 ng/mL), the first-line treatment is external radiotherapy, however long-term combination of neoadjuvant/contemporary/assisted endocrine (2−3 years) treatment is necessary, and surgery is an option.
Locally advanced prostate cancer (T3−4N0M0): The first-line treatment is radical external radiotherapy, and combination with long-term (2−3 years) neoadjuvant/concurrent/adjuvant endocrine therapy is required.
With the development of radiotherapy technology in the past decades, relatively higher radiation doses have been possible for treatment. 3D-CRT using computer software in combination with anatomical CT images may apply a higher cumulative dose with a lower risk of delayed side effect. Currently, the second-generation 3D-technology (IMRT) has been used more in clinical practice. The advantage of IMRT over 3D-CRT is that it reduces the risk of gastrointestinal toxicity in some but not all studies. Daily IGRT must be used to localize prostate in order to achieve the goal of reduction of target boundary and accuracy for either 3D-CRT or IMRT. The conventional dose of 70 Gy is considered insufficient. A total of 75.6−79.2 Gy is appropriate for conventional prostate irradiation (with or without seminal vesicles) in low-risk patients. Intermediate- and high-risk patients can receive up to 81.0 Gy of radiotherapy.
The hyperfractionation with IMRT protocol (2.4−4.0 Gy each, 4−6 weeks) has similar efficacy and toxicity compare with conventional fraction with IMRT. These radiotherapy techniques are considered to replace the conventional fractionated protocols, and the results of clinical randomized trials have shown that dose escalation is associated with improved biochemical outcomes.
Stereotactic body radiotherapy (SBRT) is an emerging therapeutic technology that provides high-dose conformal radiation therapy in equal or less than 5 fractions of treatment. The procedure is only safe under the precise image guidance. SBRT has better biochemical PFS and similar early toxicity (bladder, rectum and quality of life) when compared with standard radiotherapy techniques. However, SBRT may have more serious adverse events compared with IMRT.
6.1.2 Complications of EBRT for prostate cancer
Side effects caused by radiotherapy are associated with single dose, total dose, radiotherapy protocol and irradiated volume. Common acute side effects, including frequent urination, hematuria, diarrhea, hemafecia, etc. would disappear several weeks after radiotherapy. The side effects in late stage include rectal bleeding, radiation cystitis, and so on. The incidence of these complications is significantly reduced after conformal and intensity-modulated radiotherapy, but pelvic radiotherapy may increase the risk of a second primary tumor such as rectal or bladder cancer.
6.1.3 Adjuvant radiotherapy after radical prostatectomy
The probability of 5-year local recurrence was as high as 50% for pT3 patient with extracapsular extension, Gleason score >7 points, and positive margin R1. Three major RCT studies were conducted worldwide that address the issue of postoperative adjuvant radiotherapy. For pT3pN0 patients with postoperative PSA level <0.1 ng/mL and increased risk of local relapse due to positive margin (the highest impact) and/or invasion of the seminal vesicle, there are two options: 1) immediate adjuvant radiotherapy in the surgical area after recovery of urinary function; and 2) close follow-up, starting salvage radiotherapy if PSA>0.5 ng/mL
6.1.4 Radiotherapy for distant metastasis
Radiotherapy is an effective palliative treatment for patients with bone metastases. Isolated symptomatic bone metastases can be treated with EBRT. Short-course irradiation is usually used to treat patients with non-vertebral bone metastases. According to American College of Radiology guidelines, a single dose of 8 Gy is recommended for the treatment.
6.2 Brachytherapy for prostate cancer
Brachytherapy is a technique used for treating localized prostate cancer. By accurately positioning through 3D-treatment planning system, radioactive seeds are implanted into the prostate. It allows the radioactive seeds to be delivered in the treatment area while sparing the rectum and bladder with increasing the local dose and reducing the radiation impact on these organs. It has positive effects and small trauma, and is especially suitable for elderly patients who cannot tolerate radical prostatectomy. Traditionally, brachytherapy has been used in low-risk patients. Early studies have shown that brachytherapy is less effective than EBRT in high-risk patients. With the advance in technology, more evidence suggests that brachytherapy can also play a role in high-risk localized and locally advanced prostate cancer. Two methods of brachytherapy are currently available: low-dose (LDR) and high-dose (HDR) brachytherapy. LDR brachytherapy uses radioactive seeds permanently implanted into the prostate. It allows sufficient radiation dose to prostate lesion while avoiding excessive irradiation to bladder and rectum.
HDR brachytherapy inserts a radioactive source temporarily into the prostate to deliver radiation. It is often combined with EBRT at 40−50 Gy and is a new method to enhance radioactivity in treating high-risk local or locally advanced prostate cancer patients, while minimizing acute or late toxicity.
Brachytherapy combined with EBRT and ADT (2 or 3 years) are common protocol for high-risk patients. The combination is effective, and studies have shown that the 9-year disease-free survival and disease-specific survival rates are 87% and 91%, respectively. HDR brachytherapy patients have a lower risk of urinary frequency, urgency, and rectal pain compared with LDR brachytherapy. The risk of erectile dysfunction after HDR brachytherapy is also lower than that of LDR brachytherapy.
6.3 Proton therapy
Proton beam radiotherapy has been used for cancer therapy since 1950s. Supporters believe that this form of radiotherapy is superior to X-ray (photon)-based radiation in some clinical situations. Proton beams deliver almost all their radiation dose to the end of the particle’s path in prostate. The normal tissues around prostate could be effectively spared. However, side effects caused by these tissues are not common in prostate radiotherapy, and thus the benefits of reducing the dose to these non-critical tissues are not clinically significant. The American Society of Radiation Oncology (ASTRO) believes that there is no clear conclusion on the efficacy of proton beam radiotherapy versus other prostate cancer treatments. Therefore, the role of proton beam radiotherapy in the treatment of localized prostate cancer is still unclear. Although proton beam radiotherapy is not a new technology, its use in the treatment of prostate cancer continues to evolve (still promising, but experimental). ASTRO supports the development of patient data from clinical trials to achieve a consensus on proton beam radiotherapy for prostate cancer, especially comparing proton beam radiotherapy with other radiotherapy methods such as IMRT and brachytherapy.
7. Other treatments for localized prostate cancer
In addition to above therapeutic methods, a variety of other methods have emerged for localized prostate cancer. Cryosurgical ablation of prostate (CSAP) and high-intensity focused ultrasound (HIFU) are relatively mature and supported by some data.
CSAP destroys tumor tissue through localized freezing technique. Studies have shown that 5-year biochemical recurrence-free rate was between 65% and 92% in low-risk patients. Cryotherapy has similar outcomes to radical prostatectomy for unilateral prostate cancer. In a study comparing the effects of cryotherapy and EBRT in T2 or T3 prostate cancer, all patients received neoadjuvant ADT treatment, and results showed no significant difference in 3-year OS and disease-free survival, whereas patients receiving cryotherapy had poor sexual function. However, some studies have suggested that CSAP has a lower biochemical PFS than EBRT, although tumor-specific survival and OS are similar.
Potential indication for CSAP therapy is localized prostate cancer, PSA<20 ng/mL, Gleason score <7 points, low-risk or intermediate-risk prostate cancer but physical status not suitable for radiotherapy or surgery, and prostate volume <40 mL. There is still lack of long-term data on the therapeutic effect for patients over 10 years. Therefore, patients with a life expectancy for more than 10 years should be well-informed.
HIFU achieves therapeutic effect via ultrasonic wave, causing tumor tissue damage through mechanical and thermal effects. HIFU has been used for initial treatment and recurrence after radiotherapy. In a prospective study on 111 patients with localized prostate cancer, the result showed the 2-year survival without other radical treatment was 89%, and the percentage of patients who retained urinary and erectile function at 12 months was 97% and 78%, respectively. After a median follow-up period of 64 months, 48% of patients may avoid the use of ADT.
HIFU is also used in patient with relapse after radiotherapy. The study indicated that after HIFU treatment, the median biochemical recurrence-free survival was 63 months, the 5-year OS was 88%, and the tumor-specific survival was 94%. After a median follow-up period of 64 months, 48% of patients avoided the use of ADT. Other emerging therapies, such as vascular-targeted photodynamic (VTP) therapy, is worth further investigation. In a multicenter, open, phase III randomized controlled trial, 413 low-risk patients received randomized VTP (intravenous parofoline, insert optical fiber into the prostate, followed by laser activation) or dynamic monitoring. After a median follow-up period of 24 months, 28% of patients had disease progression in VTP group, compared with 58% in the active monitoring group. In VTP group, negative prostate biopsy results were more common, and the most common serious adverse event was urinary retention, which was alleviated within 2 months.
8. ADT
ADT is the primary systemic treatment for patients with advanced prostate cancer, or as a neoadjuvant/adjuvant therapy combined with radiotherapy for localized or locally advanced prostate cancer. The accepted castration level is defined as testosterone <50 ng/dL (1.7 nmol/L). Currently, many studies have confirmed that the lower the testosterone level, the better the treatment effect.
8.1 Regimens of ADT
ADT can be achieved with surgical (bilateral orchiectomy) or drug castration, including luteinizing hormone releasing hormone (LHRH, also known as gonadotropin releasing hormone, or GnRH) agonists or antagonists.
The efficacy of bilateral orchiectomy may be similar to LHRH agonist with better safety. Drug or surgical castration combined with an antiandrogen formulation is known as combined androgen blockade. There are no prospective randomized studies confirming that combined androgen blockade has a survival advantage over sequential use of LHRH agonists and antiandrogens currently, but some meta-analysis data suggest that bicalutamide may improve OS rate by 5% to 20% compared with LHRH agonist monotherapy.
8.1.1 Intermittent and continuous ADT
Intermittent ADT treatment resulted in improved quality of life compared with routine continuous regimen as shown in most studies. But some other studies indicated that intermittent ADT has no survival advantage. Therefore, for patients with metastatic prostate cancer, intermittent ADT treatment is only considered in patients with severe adverse events, and patients should be fully informed about the benefits and risks of therapy.
8.1.2 Combination of ADT and chemotherapy
Three large randomized controlled trials have been completed currently in comparing the efficacy of ADT alone and ADT combined with docetaxel on metastatic prostate cancer, including GETUG 15, CHAARTED and STAMPEDE studies. Based on data from those studies, ADT combined with docetaxel should be the standard treatment for patients with newly diagnosed metastatic prostate cancer, as long as the patients can tolerate chemotherapy.
8.2 ADT regimen strategy based on risk group of prostate cancer
8.2.1 ADT for low-risk prostate cancer patients
ADT is often used to treat early-stage low-risk prostate cancer, especially for elderly patients in community hospitals. However, this practice has been questioned. A study of 66,717 elderly patients with T1−T2 prostate cancer have found no survival benefit when compared ADT therapy with observation alone after 15 years. Similar studies have also found that the survival of patients with localized prostate cancer will not benefit from ADT alone, and thus ADT treatment should not be a routine treatment for patients with low-risk early prostate cancer.
8.2.2 ADT for intermediate-risk prostate cancer
The study [of Dana Farber Cancer Institute (DFCI) 95096, Tumor Radiation Therapy Collaboration (RTOG) 9408] demonstrated that 4-month ADT combined with EBRT treatment could improve OS for patients with intermediate-risk prostate cancer, but OS will not be improved further with additional chemotherapy of combination of paclitaxel + estrametine + etoposide.
8.2.3 ADT for high-risk prostate cancer
ADT combined with EBRT is an effective primary treatment for high-risk or very high-risk patients. In a multi-randomized, phase III study, the combination therapy was superior to the single-therapy in tumor-specific survival and OS. More evidence suggests that long-term radiotherapy with neoadjuvant/adjuvant ADT is superior to the corresponding short-term treatment, and ADT treatment should last for 2−3 years. Addition 6 cycles of docetaxel chemotherapy along with ADT combined with EBRT may be used in selected patients. In addition, EBRT combined with brachytherapy may be considered for high-risk patients, with or without neoadjuvant/adjuvant ADT. In addition, radical prostatectomy plus pelvic lymphadenectomy is also a treatment option for young patients with high-risk prostate cancer. Young patients and patients with body in good condition may benefit from it.
8.2.4 ADT for very high-risk prostate cancer
Treatment options for this type of patients include EBRT combined with long-term ADT, EBRT combined with brachytherapy with or without long-term ADT; radical prostatectomy + pelvic lymphadenectomy (tumor is not fixed to pelvic wall) in young patients and patients with body in good condition; ADT or watchful waiting for patients who are not suitable for radical treatment. For some patients, six times of docetaxel chemotherapy should be added for possible benefits after EBRT treatment while continuing ADT therapy.
8.3 ADT for pelvic lymph node metastasis and metastatic prostate cancer
ADT alone or EBRT combined with 2−3 years of neoadjuvant/adjuvant ADT is a treatment option for pelvic lymph node metastatic prostate cancer. ADT is a treatment option for metastatic prostate cancer.
8.4 Treatment of primary lesions under ADT treatment
A retrospective study of the US SEER database and the Munich Cancer Registry database shows that in newly diagnosed metastatic prostate cancer patients, a small number of patients reported improvements in OS and tumor-specific survival from radical resection or brachytherapy. A small sample of prospective studies found that, for some patients with metastatic prostate cancer, radical prostatectomy might improve prognosis with better tumor-specific survival if bone metastase lesion is <3 and patient has received 6-month ADT treatment. Of course, these conclusions are still experimental and needed to be further confirmed by large-scale prospective studies.
8.5 ADT for biochemical recurrence of patients treated with radical prostatectomy
There is no consensus on whether ADT should be used in patients with elevated PSA levels while no clinical evidence of recurrence after radical treatment. Some of these patients eventually die from the cancer. For patients with elevated PSA, the timing of ADT is affected by factors such as PSA growth velocity, anxiety from patient and physician, side effects of ADT, and potential complications of patients. Although early ADT is acceptable, some patients choose close observation until cancer progression, and then choose proper treatments. Although the definition for early and late stages (i.e., determined by PSA levels) remains controversial, early ADT may be better than delayed treatment. Patients are recommended to receive early ADT if PSA increased, and/or PSA doubling time is short, and if they have longer life expectancy.
8.6 Complications of ADT
8.6.1 Adverse effects of conventional ADT
There are various adverse effects for ADT, including hot flash, unstable vasomotor, osteoporosis, high incidence of clinical fractures, obesity, insulin resistance, blood lipid changes, diabetes, kidney damage and risk of cardiovascular disease. Recent evidence suggests a possible link between ADT and cognitive decline or Alzheimer’s disease in future. Overall, the increase of side effects is associated with prolonged ADT therapy.
8.6.2 Bone health in ADT
ADT is associated with an increased fracture risk. In large population studies, ADT increases fracture risk by 21%−54%, and prolonged treatment time makes the risk of fracture even greater. ADT accelerates bone metabolism and reduces bone mineral density. Therefore, treatment for osteoporosis for patients treated with long-term ADT is recommended as following: 1) all males over age of 50 years are supplemented with calcium (1,200 mg daily) and vitamin D3 (800−1,000 IU per day); and 2) when the 10-year probability of male hip fracture is ≥3% or severe osteoporosis-related fracture is ≥20%, additional treatment should be considered.
Denosumab (60 mg every 6 months), zoledronic acid (5 mg intravenously per year) or alendronate (70 mg per week orally) are recommended if ADT treatment will cause absolute fracture risk.
8.6.3 ADT and diabetes and cardiovascular disease
Studies have shown that ADT is associated with increased risk of diabetes and cardiovascular disease. Several mechanisms are associated with the increased incidence: ADT increases fat mass and fasting plasma insulin level, and reduces insulin sensitivity. ADT also increases serum cholesterol and triglyceride levels. Cardiovascular disease and diabetes are the main causes of morbidity and mortality in general population. Due to correlation between ADT and adverse metabolic effects, the association with increased incidence of diabetes and cardiovascular disease suggests that screening and intervention for male patients undergoing ADT are recommended to prevent/treat diabetes and cardiovascular disease.
9. Treatment of castration-resistant prostate cancer (CRPC)
9.1 Definition of CRPC
CRPC refers to one of the following conditions after testosterone reaches the castration level (<50 ng/dL or 1.7 nmol/L): 1) biochemical recurrence, which denotes three consecutive elevation of PSA over one week interval; 2) twice of elevations are above PSA nadir by 50%, and PSA >2 ng/mL; and 3) imaging progression, which denotes the emergence of new lesions, including two or more new bone metastases in bone scan, or new soft tissue lesions evaluated by RECIST standard. Symptomatic progression alone is not enough to diagnose as CRPC and must be subject to further investigation.
9.2 Asymptomatic non-metastatic CPPC (M0CRPC)
Apalutamide and enzalutamide are recommended for M0CRPC since evidences showed that these two medicines could prolong CSS and OS of M0CRPC patients. Abiraterone combined with prednisone should be recommended if patients failed with both apalutamide and enzalutamide. For various reasons (these medicines are neither available in local market nor affordable), patients could choose to be on observation with continuous ADT, and chemotherapy or immunotherapy is not recommended outside the clinical trials for these patients.
9.3 Metastatic CRPC (mCRPC)
9.3.1 Role of castration therapy in patients with mCRPC
Metastatic prostate cancer will eventually become mCRPC, and it is recommended that patients at this stage should maintain ADT treatment.
9.3.2 First-line medical treatment for mCRPC
9.3.2.1 Abiraterone
In April 2011, Food and Drug Administration (FDA) approved the androgen synthesis inhibitor abiraterone acetate (abiraterone) in combination with low-dose prednisone for the treatment of patients with mCRPC who had previously received chemotherapy (docetaxel). The FDA approved abiraterone for patients after docetaxel treatment. The approval is based on the results of a phase III randomized, placebo-controlled clinical trial (COU-AA-301) which conducted in patients with mCRPC who had previously received chemotherapy (docetaxel regimen). Patients were randomized and received abiraterone 1,000 mg per day (n=797) or placebo once-a-day (n=398), both groups received prednisone daily and ADT. In the final analysis, the median survival was 15.8 months for abiraterone vs. 11.2 months for placebo [HR, 0.74; 95% confidence intervival (95% CI), 0.64−0.86; P<0.0001]. The abiraterone group also had improved radiological progression time, decreased PSA and relieved pain.
On December 10, 2012, FDA approved the application of abiraterone prior to docetaxel chemotherapy. The result is based on a phase III randomized COU-AA-302 trial, in which patients with asymptomatic or mild symptomatic mCRPC without visceral metastasis received abiraterone + prednisone + ADT (n=546) and prednisone alone + ADT (n=542). Most patients in the trial did not use anesthetics to relieve pain and never received ketoconazole treatment. After treatment, the common primary endpoint radialogical PFS increased from 8.3 months to 16.5 months (HR, 0.53; P<0.001). In a median follow-up period of 49.2 months, OS was improved in the final analysis (34.7 monthsvs. 30.3 months; HR, 0.81; 95% CI, 0.70−0.93; P=0.003). Key secondary endpoints: time of symptomatic deterioration, start of chemotherapy, pain progression, and PFS of PSA were significantly improved after abiraterone treatment. Reduction of PSA (62%vs. 24%, >50% reduction) and radiological remission (36% vs. 16% RECIST remission) are more common in abiraterone + prednisone + ADT group.
The most common adverse events of abiraterone/prednisone/ADT treatment are fatigue (39%); back or joint discomfort (28%−32%); peripheral edema (28%); diarrhea, nausea or constipation (22%); low blood potassium (17%); hypophosphatemia (24%); atrial fibrillation (4%); muscle discomfort (14%); hot flashes (22%); urinary tract infections; cough; hypertension (22%, 4% had severe high blood pressure); frequent urination and nocturia; indigestion; and upper respiratory tract infection. The most common adverse events which lead to discontinue treatment include elevated aspartate aminotransferase and/or alanine aminotransferase (11%−12%) or heart disease (19%, 6% is severe). Therefore, liver function, potassium and phosphorus levels, and blood pressure should be monitored initially in the period of abiraterone/prednisone/ADT treatment. Symptomatic assessment of heart disease is also necessary, especially for patients with previous history of cardiovascular disease.
9.3.2.2 Enzalutamide
On August 31, 2012, the FDA approved enzalutamide for the treatment of patients with mCRPC who had previously received a chemotherapy regimen containing docetaxel. The approval was based on the results of a phase III randomized placebo-controlled trial (AFFIRM). A total of 1,199 patients were randomized into enzalutamide + ADT and placebo + ADT groups at a ratio of 2:1, and OS was the primary endpoint. After enzalutamide + ADT treatment, the median survival increased from 13.6 months to 18.4 months (HR, 0.63; P<0.001). Survival was improved in all subgroups. There is a significant improvement in secondary endpoints, including the proportion of patients with PSA reduction >50% (54%vs. 2%), radiological remission (29% vs. 4%), radiological PFS (8.3 vs. 2.9 months), and the time to first skeleton-related event (SRE) (16.7 months vs. 13.3 months). Quality of life was assessed with a validated survey, and there was improvement in enzalutamide + ADT group as compared with placebo + ADT group. Adverse events were mild, including fatigue (34% vs. 29%), diarrhea (21% vs. 18%), hot flashes (20% vs. 10%), headache (12% vs. 6%), and epilepsy (0.6% vs. 0%). There was no difference in the incidence of heart disease between the two groups.
The dose of enzalutamide is 160 mg daily. Patients maintain GnRH agonist/antagonist therapy and receive skeletal support medications in the AFFIRM study.
Another phase III trial (PREVAIL) investigated the effects of enzalutamide prior to chemotherapy. A total of 1,717 patients with mCRPC who were initially treated with chemotherapy were randomized to enzalutamide + ADT or placebo + ADT daily. The study was terminated in advance due to the benefits shown in treatment group. Compared with placebo, enzalutamide showed an improvement in median PFS (20.0 months vs. 5.4 months), and median OS (35.3 months vs. 31.3 months), and improvements in secondary endpoints (e.g., the time of start of chemotherapy or the first SRE time).
Two other randomized clinical trials also reported that the efficacy of enzalutamide + ADT was superior to ADT + bicalutamide in treatment of mCRPC in both trials.
In the TERRAIN trial, 375 patients with newly diagnosed mCRPC were randomized to ADT + enzalutamide 160 mg/d or ADT + bicalutamide 50 mg/d at a ratio of 1:1. Compared with ADT + bicalutamide group, PFS (defined as PSA progression, soft tissue progression or other new bone metastases) was significantly better in ADT + enzalutamide group (median time of 15.7 months in ADT + enzalutamide group vs. 5.8 months in ADT + bicalutamide group, HR is 0.44; 95% CI, 0.34−0.57). In the STRIVE trial, 396 patients with newly diagnosed M0CPPC or M1CRPC were randomized to ADT + enzalutamide 160 mg/d or ADT + bicalutamide 50 mg/d at 1:1 ratio. The primary endpoint was PFS, and ADT + enzalutamide reduced the risk of progression or death by 76% (HR, 0.24; 95% CI, 0.18−0.32) compared with ADT + bicalutamide. In choosing antiandrogen as a second-line hormone, the study has shown that enzalutamide is superior to bicalutamide in prolonging PFS in patients with CRPC. Given the side effects’ characteristics of different agents and the high cost of enzalutamide, some patients may still consider bicalutamide. Therefore, enzalutamide is a treatment option for patients with CRPC before and after docetaxel treatment and is a reasonable choice for patients who are not suitable for chemotherapy.
9.3.2.3 Chemotherapy with docetaxel
There are two phase III randomized clinical trials to evaluate the therapeutic effects of docetaxel-based chemotherapy regimens (TAX327 and SWOG9916) in CRPC patients with symptoms or rapid progression. A total of 1,006 patients were recruited in TAX327 study, which tried to compare the efficacy difference between docetaxel (weekly or every 3 weeks) + prednisone and mitoxantrone + prednisone. Docetaxel once every 3 weeks achieved a higher median OS than mitoxantrone + prednisone (18.9 vs. 16.5 months, P=0.009). The survival benefit was maintained during the extended follow-up period. In SWOG9916 trial, docetaxel combined with estramustine showed better survival than mitoxantrone + prednisone.
Docetaxel is approved by the FDA for treatment of mCRPC. The standard protocol is once every three weeks and the alternative protocol is 50 mg/m2 once every two weeks. The trial was based on a large phase II randomized study involving 346 patients with mCRPC. Patients were randomized to docetaxel once every two weeks or docetaxel once every three weeks, with ADT and prednisone maintained therapy in each group. The average survival for patients in two-week group was 19.5 months vs. 17.0 months in three-week group (P=0.015). The two-week group offers better efficacy in disease progression time and PSA decline rate. In addition, docetaxel once every two weeks seems to be well tolerated.
In addition, based on the results of the latest two phase III trials (ECOG 3805/CHAARTED and STAMPEDE), docetaxel is a first-line chemotherapy drug for patients with advanced hormone-sensitive prostate cancer.
In CHAARTED trial, 790 patients with metastatic hormone-sensitive prostate cancer were randomized to receive combination of docetaxel + ADT or ADT alone. The OS of combination group was longer than that of the ADT alone group (57.6 months vs. 44.0 months; HR, 0.61; 95% CI, 0.47−0.80; P<0.001). Subgroup analysis showed that survival benefit appears more pronounced in 65% of subjects with high-volume disease (HR, 0.60; 95% CI, 0.45−0.81; P<0.001). Although the median OS was not achieved in both groups because of small number of patients, it was concluded that patients with low-volume disease could also benefit from docetaxel chemotherapy (HR, 0.60; 95% CI, 0.32−1.13; P=0.11).
The STAMPEDE study is a multi-arm, multi-stage, phase III clinical trial involving M0 and M1 hormone-sensitive prostate cancer. The survival advantage of ADT + docetaxel in M1 patients confirmed in the CHAARTED trial was verified again in this trial. In STAMPEDE trial, 1,087 patients with metastatic hormone-sensitive prostate cancer were not stratified by tumor burden, but the median OS of all M1 patients in the ADT + docetaxel group was 5.4 years compared with 3.6 years in ADT alone group (the difference between the groups is 1.8 years, but 1.1 year in CHAARTED trial). The result of the STAMPEDE trial confirms the results from CHAARTED trial for patients in same condition. Based on GETUG12 trial, docetaxel combined with ADT and EBRT should be considered. In GETUG 12 trial, 413 patients with high-risk or very high-risk localized prostate cancer were randomized to IMRT + ADT or IMRT + ADT + docetaxel + estramustine. After a median follow-up period of 8.8 years, the 8-year recurrence-free survival was 62% in combination group and 50% in ADT alone group (adjusted HR, 0.71; 95% CI, 0.54−0.94; P=0.017). Estramustine was confirmed to increase side effects whereas no enhancement effect was observed in combined with docetaxel, and thus it is not recommended due to results in GETUG 12 trial.
9.3.2.4 Chemotherapy with cabazitaxel
In June 2010, the FDA approved cabazitaxel, a semi-synthetic taxane derivative, for patients with mCRPC who failed docetaxel chemotherapy. In an international phase III trial (TROPIC trial), 755 patients with progressive mCRPC were randomized to cabazitaxel 25 mg/m2 group and mitoxantrone 12 mg/m2 group. The OS for cabazitaxel group was extended by 2.4 months (HR, 0.72; P<0.0001) compared with mitoxantrone group. The improvement in survival in cabazitaxel arm was offset by higher toxic mortality (4.9%vs. 1.9%), which was largely due to sepsis and renal failure. There were 7.5% of patients receiving cabazitaxel had febrile neutropenia compared with 1.3% of mitoxantrone. The incidence of severe diarrhea (6%), fatigue (5%), nausea/vomiting (2%), anemia (11%) and thrombocytopenia (4%) was also higher in patients with cabazitaxel. Thus it is necessary to be vigilant for febrile neutropenia. In an international open phase III non-inferior PROSELICA trial, 1,200 patients with mCRPC who failed docetaxel treatment were randomized to cabazitaxel 20 mg/m2 arm and cabazitaxel 25 mg/m2 arm. The median OS of the low-dose (cabazitaxel 20 mg/m2 arm) was not inferior to the higher-dose [13.4 months (95% CI, 12.19−14.88)vs. 14.5 months (95% CI, 13.47−15.28)], and grade 3−4 adverse events were lower (39.7%vs. 54.5%) in cabazitaxel with 20 mg/m2 arm. In particular, the grade 4 neutropenia rates in the low-dose and high-dose groups were 21.3% and 48.6%, respectively. Currently, cabazitaxel 25 mg/m2 every 3 weeks is still the standard treatment. Cabazitaxel 20 mg/m2 once every 3 weeks was considered for weak patients.
The latest results of phase III FIRSTANA trial indicates that cabazitaxel has clinical implications for mCRPC patients who have not received chemotherapy. The mean OS (primary endpoint) of 20 mg/m2 cabazitaxel, 25 mg/m2 cabazitaxel and 75 mg/m2 docetaxel regimens was 24.5 months, 25.2 months and 24.3 months, respectively. Compared with docetaxel, cabazitaxel has a lower peripheral neuropathy rate, especially in 20 mg/m2 group (12% vs. 25%). Therefore, patients who are not suitable for docetaxel chemotherapy or who have mild peripheral neuropathy may consider cabazitaxel.
9.3.2.5 Sipuleucel-T
In April 2010, Sipuleucel-T was approved by the FDA as the first immunotherapy drug for prostate cancer. The autologous tumor “vaccine” includes collecting presenting leukocytes from the patient, exposing these cells to prostatic acid phosphatase granulocyte macrophage colony stimulating factor (PAP-GM-CSF recombinant chimeric protein), and then reinfuse back to the body. The drug is based on a phase III multicenter randomized double-blind clinical trial (D9902B). A total of 512 patients with mild or asymptomatic mCRPC were randomized to receive either Shipuleucel-T or placebo in a ratio of 2:1. The median survivals was 25.8 months in vaccine group and 21.7 months in control group, respectively. Sipuleucel-T treatment reduced the risk of death by 22% (HR=0.78; 95% CI, 0.61−0.98; P=0.03). Common complications include mild to moderate chills (54.1%), fever (29.3%) and headaches (16.0%). These complications are usually temporary.
9.3.2.6 Radium-223
In May 2013, the US FDA approved radium dichloride (Radium-223) for patients with CRPC, which is a radiopharmaceutical agent that emits alpha particles. This first radiopharmaceutical agent was approved for the treatment of symptomatic bone metastases in CRPC patients without visceral metastasis. Radium-223 can significantly improve OS and extend the time of first SRE. Radium-223 may combine with abiraterone or enzalutamide for asymptomatic patients.
Radiopharmaceutical agents that emit beta radiation are effective and appropriate treatment for patients with extensive metastases, especially when patients are not suitable for effective chemotherapy. Radiopharmaceutical agents, 89Sr and 153Sm, are the most commonly used drugs to treat patients with bone metastases. These patients usually complained multifocal bone pain and system targeted radioactive therapy can relieve the symptom. The side effects are usually mild. Unlike Radium-223, which emits alpha particles, beta particle therapy has no survival advantage and could only be used as palliative treatment.
9.4 Other new medicines for mCRPC
Recent evidence shows that PARP inhibitors can effectively treat patients with mCRPC, especially in the presence of HRD pathway-related gene mutations (such as BRCA1 and BRCA2 gene mutations). PARP inhibitors achieve treating purpose through inhibiting DNA damage repairing and promoting apoptosis of cancerous cells.
Olaparib is a PARP inhibitor and has a promising therapeutic effect on CRPC patients with BRCA1 and BRCA2 mutations. The response rate is as high as 88%, which offer an exciting new opportunity for mCRPC patients.
9.5 Treatment of SREs for patients with mCRPC
In a multicenter study, 643 patients who had mCRPC with asymptomatic or mild symptomatic bone metastases were randomized to intravenous zoledronic acid or placebo every 3 weeks. At the fifteen month, Patients treated with 4 mg zoledronic acid had fewer SRE compared with placebo group (33% vs. 44%, P=0.02). At the 24th month, the median time to first SRE was longer in zoledronic acid group (488 d vs. 321 d; P=0.01). No significant difference was found in OS. Other bisphosphonates did not show any effective prevention on disease-related bone complications. A randomized, double-blind, placebo-controlled study compared the efficacy of denosumab and zoledronic acid in CRPC patients. The absolute incidence of SRE was similar in both groups; however, the median time to first SRE was delayed by 3.6 months in denosumab group compared with the zoledronic acid group (20.7 vs. 17.1 months, non-inferiority P=0.0002, superiority P=0.008). Both groups have similar incidence for severe SREs, which include spinal cord compression (3% vs. 4%), radiation therapy (19% vs. 21%) and pathological fractures (14% vs. 15%).
The incidences of therapy-associated toxicity in both patients treated with zoledronic acid and denosumab were similar, including hypocalcemia (more common in denosumab, 13% vs. 6%), joint pain and osteonecrosis of the jaw (ONJ, the incidence rate is 1%−2%). Although not all, most ONJ patients had history of dental problems.


