Abstract
Objective
To examine the role of store-operated calcium entry (SOCE) and stromal interaction molecule 1 (STIM1) in survival and migration of osteosarcoma cells and investigate what blockade of store-operated Ca2+ contributes to the regulation of osteosarcoma cells.
Methods
First, we examined the expression levels of STIM1 in osteosarcoma cell lines by Western analysis and in tissue specimens by immunohistochemistry. Second, we investigated the effect of SOCE and STIM1 on osteosarcoma cell viability using MTS assays and on cell proliferation using colony formation. Third, we investigated the role of SOCE and STIM1 in cell migration using wound healing assays and Boyden chamber assays. Finally, we studied the effect of SOCE on the nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) activity by luciferase assays.
Results
STIM1 was overexpressed in osteosarcoma cell lines and tissue specimens and was associated with poor survival of osteosarcoma patients. Also, inhibition of SOCE and STIM1 decreased the cell viability and migration of osteosarcoma cells. Furthermore, our results showed that blockade of store-operated Ca2+ channels involved down-regulation of NFATc1 in osteosarcoma cells.
Conclusions
STIM1 is essential for osteosarcoma cell functions, and STIM1 and Ca2+ entry pathway could be further explored as molecular targets in the treatment of osteosarcoma.
Keywords: Calcium, NFATc1, osteosarcoma, SOCE, STIM1
Introduction
Osteosarcoma is the most common malignant bone tumor in children and adolescents (1). With the introduction of chemotherapy in the 1970s, the 5-year survival rate for osteosarcoma has improved up to 65%−70% (2). Nevertheless, no further improvements have been achieved in the last two decades and the survival rate remains at 20%−30% for patients with clinically detectable metastatic disease at initial presentation (3). The lack of improvement in outcomes demands a continuous search for new therapeutic agents.
Ca2+ is a ubiquitous second messenger that regulates a wide range of biologic processes including cell proliferation and migration (4,5). In nonexcitable cells, store-operated calcium entry (SOCE) is a major Ca2+ entry mechanism. Previous studies show that stromal interaction molecule 1 (STIM1) and Orai1 are responsible for SOCE (6). Upon depletion of Ca2+ within the endoplasmic reticulum, STIM1 protein, which functions as a calcium sensor, aggregates into multiple puncta and migrates to the plasma membrane of the endoplasmic reticulum. Orai1 protein, an essential pore-forming component of SOCE channel, also redistributes within the plasma membrane to accumulate at sites opposite to STIM1 and open to mediate Ca2+ entry (7). Dysregulation of Ca2+ signaling has been implicated in tumorigenesis and tumor progression (8). SOCE has been implicated in the proliferation and metastasis of other cancers (9,10), but it has not been investigated in osteosarcoma. In this report, we examined the role of SOCE and STIM1 in survival and migration of osteosarcoma cells and investigated what blockade of store-operated Ca2+ contributed to the regulation of osteosarcoma cells.
Materials and methods
Cell culture
143B, U2OS, MG63, Saos-2, KHOS, and human osteoblast (HOB 1 and HOB 2) cells were incubated in dulbecco’s modified eagle medium (DMEM)-F12 medium containing 10% fetal bovine serum (FBS) and maintained at 37 °C under 5% CO2. HOB 1 and HOB 2 cells were derived from cancellous bone obtained as surgical waste material from orthopedic procedures in accordance with a Mayo Clinic Institutional Review Board (IRB) approved protocol and maintained as explants to generate the osteoblast-like monolayers, as previously described (11).
Immunohistochemistry
Osteosarcoma tissue microarrays (TMA) were purchased from Novus Biologicals (Novus Biologicals, Littleton, USA). The clinical information of TMA samples included follow-up duration and survival status. The immunohistochemistry was carried out using monoclonal anti-STIM1 antibody (Thermo Fisher Scientific, Waltham, USA) and IMMPress (Peroxidase) kit from Vector laboratories (Burlingame, USA). The immunohistochemistry scoring was carried out as described earlier (12). Briefly, the staining intensity of STIM1 protein in tissues was categorized on a scale from 0 to 3 (0 for no staining, 1 for weak staining, 2 for moderate staining and 3 for strong staining). The percentage of immunoreactivity was scored on a scale from 0 to 3 (0 for no positive cells, 1 for <25% of cells positive, 2 for 25%−50% of cells positive and 3 for >50% of cells positive). The staining intensity score and the percentage of immunoreactivity score were then multiplied to attain a composite score (CS). Based on the CS, STIM1 expression in tissues was ranked as high expression (CS≥6) and low expression (CS<6).
MTS assay
Human osteosarcoma cells and HOB cells were plated at a density of 5×104 cells per well into 24-well plates containing 1 mL/well medium. After allowing the cells to attach overnight, the cells were treated with drugs or transfected with siRNAs for 24 h, 48 h and 72 h. The cell survival was measured by MTS-based cell viability assay as per the manufacturer’s protocol (Promega, Madison, USA).
Western analysis
Western analysis was carried out as described earlier (13). Cytoplasmic extracts were prepared from osteosarcoma and HOB cells and extracts containing equal amounts of protein (40 μg) were analyzed by Western blot using anti-STIM1, anti-glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH) (Cell Signaling Technology, Beverly, USA), and anti-ATX (LifeSpan Biosciences, Inc., Seattle, USA) antibodies. Quantitation was performed by densitometry and analyzed using Quantity One software (Bio-Rad, Hercules, USA).
Wound healing assay
Briefly, osteosarcoma cells (2×105) were seeded onto 12-well plates. When at 100% confluence, the cells were scratched with a 200 μL pipette tip across the center of the wells, and washed 2 times with phosphate-buffered saline. The cells were then incubated in the presence and absence of SKF96365. The wounds were observed at 0 h and 12 h after wounding, and photographed using a microscope. Three images were taken per well at each time point using a 10× objective, and the distance between the two edges of the scratch (wound width) was evaluated at 5 sites in each image, using NIH ImageJ software. The cell migration distance was measured by subtracting the wound width at 12 h time point from the wound width at the 0 h time point and then dividing by 2.
Transwell assay
Transwell assay was performed using 6.5 mm Millicell chambers with 8 μm pores (Millipore). Osteosarcoma cells (100,000) in 300 μL of serum free media were added to the upper chamber of the transwell, whereas 500 μL of DMEM supplemented with 10% FBS (used as a chemoattractant) was added to the lower chamber. After 16 h, the nonmigrating cells were removed from the upper surface of each chamber by a cotton swab. Transwell membranes were then stained with crystal violet. Cells that migrated through the membrane to the lower surface were counted in 3 random fields per membrane by microscopy using a 10× objective. The migration for each experiment was expressed as the average number of migrated cells in a field. All experiments were performed in triplicate.
SiRNA transfection
Osteosarcoma cells (5×104) in 24-well plates were transfected with a pool of STIM1 siRNAs (GGUGGUGUCUAUCGUUAUU; UACAGUGGCUGAUCACAUA; CAAUUACCAUGACCCAACA; UCUCUUGACUCGCCAUAAU) and control non-targeting siRNAs (UGGUUUACAUGUCGACUAA) as per the manufacturer’s protocol (Dharmacon, Lafayette, USA). Following 48 h of transfection, the cells were processed for transwell assay, Western blot hybridization, or colony formation assays.
Colony formation assay
Twenty-four hours after control and STIM1 siRNA transfections, the cells were trypsinized and subcultured in 6-well plates at a destiny of 1,000 cells per well in the presence and absence of SKF96365. After 7 d, the cells were fixed and stained with crystal violet. A group of >10 cells was counted as a colony.
Intracellular Ca2+ ([Ca2+]i) imaging
The [Ca2+]i measurements using fluorescent indicators were carried out previously described (14). Briefly, cells were plated on 8-well Labtek glass-bottomed chambers, and incubated in 5 μmol/L Fura-2AM (Invitrogen, Eugene, USA). The cells were then washed and perfused with Hank’s balanced salt solution. Fluorescence was recorded using a real-time imaging system (Metafluor; Molecular Devices, Sunnyvale, CA; Roper Scientific 12-bit camera system) on an inverted Nikon Diaphot microscope (40×/1.3NA objective lens), with [Ca2+]i calculated from the 340/380 ratios based on an in vitro calibration (15).
NFATc1 luciferase assay
Cells were plated at a density of 8×104 cells per well in 12-well plates and transfected with 4 μg of NFATc1/AP-1 reporter plasmid DNA (a kind gift from Dr. Martin Fernandez-Zapico, Mayo Clinic, Rochester, USA) using FuGene as described in the manufacturer’s protocol (Promega, Madison, USA). After 24 h of transfection, the cells were treated with vehicle or treatment groups (CsA, VIVIT, or SKF96365) for 24 h. The cells were harvested and suspended in 300 μL of passive lysis buffer provided in a luciferase assay kit (Promega) and the relative luciferase activity were measured using a luminometer (GloMax 96 microplate luminometer, Promega, Madison, USA). The data were normalized to protein concentration in the lysate.
Statistical analysis
JMP 10.0 Pro software for Windows (SAS Institute Inc., Cary, USA) was used for the statistical analysis. Data were presented as
 from three independent experiments. P<0.05 was considered statistically significant. Statistical comparisons between two groups of data were made using a two-tailed unpaired Student’st-test. Multiple comparisons were determined using one-way analysis of variance followed by the Tukey test for multiple comparisons. Kaplan-Meier survival curve was created to represent disease-specific mortality for patients with low or high STIM1 expression levels. The log-rank test was used to compare differences between two groups.
from three independent experiments. P<0.05 was considered statistically significant. Statistical comparisons between two groups of data were made using a two-tailed unpaired Student’st-test. Multiple comparisons were determined using one-way analysis of variance followed by the Tukey test for multiple comparisons. Kaplan-Meier survival curve was created to represent disease-specific mortality for patients with low or high STIM1 expression levels. The log-rank test was used to compare differences between two groups.
Results
Tumor expression level of STIM1 was associated with clinical outcome
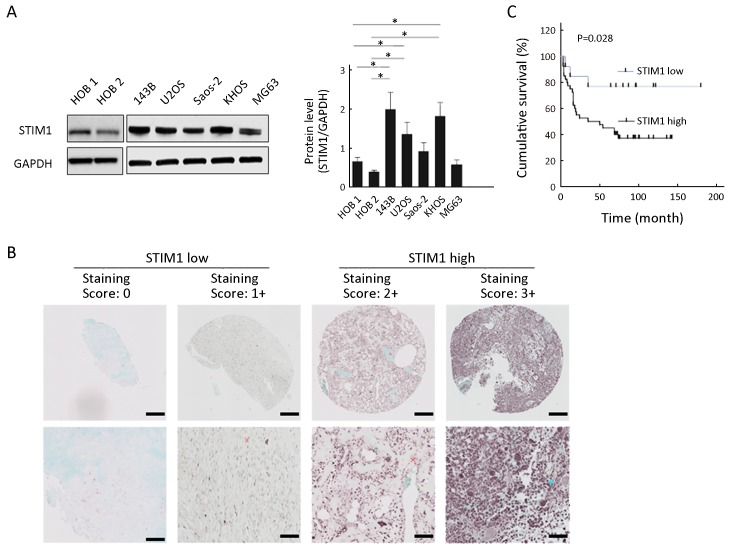
Previous results showed that STIM1 could affect the biologic features of tumor cells. To determine if STIM1 is associated with osteosarcoma, we examined the expression levels of STIM1 in osteosarcoma cell lines. Our results showed that 3 out of 5 cell lines (143B, U2OS and KHOS) demonstrated higher expression of STIM1 protein compared with normal human osteoblast cell lines (HOB 1 and HOB 2) (Figure 1A ). These findings lead us to hypothesize that STIM1 expression correlates with osteosarcoma tumor progression. To test this, we analyzed STIM1 expression in osteosarcoma using TMAs consisting of 53 cases, which included 22 osteoblastic, 20 classical, 9 chondroblastic, 1 telangiectatic, and 1 fibroblastic osteosarcoma samples. The results showed that STIM1 expression in tissues was ranked as high expression in 40 samples and low expression in 13 samples, as displayed by the STIM1 immunoreactivity in these samples (Figure 1B ). Next, tumors were stratified into the osteosarcoma tissues into 2 groups based on STIM1 staining intensity, labelled as STIM1 high and STIM1 low. Our analysis further indicated that compared with the STIM1 low group, the STIM1 high group exhibited poor survival (P=0.028) (Figure 1C ).
1.
Stromal interaction molecule 1 (STIM1) expression level in osteosarcoma cells and tissue specimens. (A) Western analysis. Cytoplasmic extracts from osteosarcoma cells (143B, U2OS, Saos-2, KHOS and MG63) and human osteoblast (HOB 1 and HOB 2) cells were analyzed by Western blot using anti-STIM1 and anti-glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH) antibodies as described in methods. Quantitation was performed by densitometry and normalized to GAPDH expression. Data are presented as
 from three independent experiments. *, P<0.05, compared with HOB 1 and HOB 2; (B) Representative immunohistochemical staining of STIM1 in osteosarcoma using tissue microarrays. The lower panel is a magnified view of a region in the respective image in the upper panel. Scale bars are 200 μm in the upper panel and 50 μm in the lower panel; (C) Overall survival of patients in STIM1 high and STIM1 low groups (P=0.028).
from three independent experiments. *, P<0.05, compared with HOB 1 and HOB 2; (B) Representative immunohistochemical staining of STIM1 in osteosarcoma using tissue microarrays. The lower panel is a magnified view of a region in the respective image in the upper panel. Scale bars are 200 μm in the upper panel and 50 μm in the lower panel; (C) Overall survival of patients in STIM1 high and STIM1 low groups (P=0.028).
Store-operated Ca2+ channels and STIM1 regulated survival of osteosarcoma cells
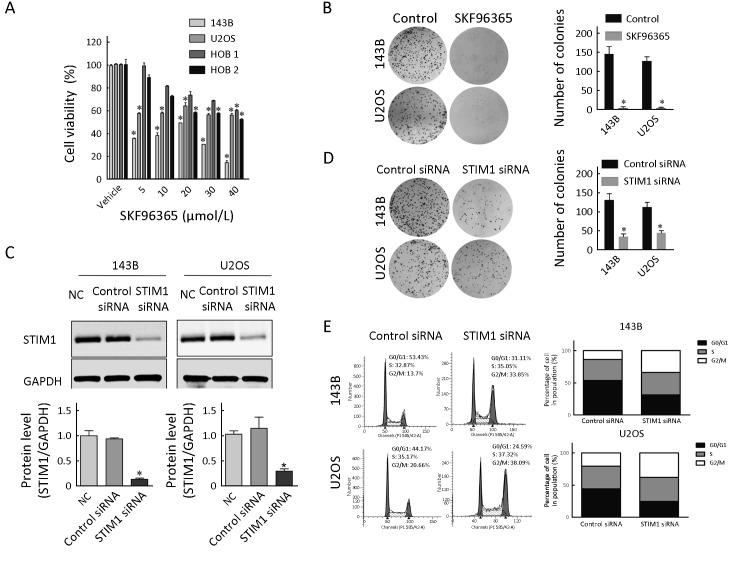
To study the effect of SOCE on osteosarcoma cell survival, we investigated the effect of an inhibitor of SOCE, SKF96365, on osteosarcoma cell viability using MTS assays. The results showed that SKF96365 at the concentrations of 5, 10, 20, 30 and 40 μmol/L decreased the cell survival in 143B and U2OS cells, respectively (Figure 2A ). In addition, the SOCE inhibitor SKF96365 decreased the cell survival in HOB 1 and HOB 2 cells, respectively (Figure 2A ). Also, SKF96365 treatment decreased the colony numbers by 97.2% and 96.7% in 143B and U2OS osteosarcoma cells, respectively (Figure 2B ).
2.
Effect of store-operated calcium entry (SOCE) inhibitor and stromal interaction molecule 1 (STIM1) siRNA on osteosarcoma cell growth. (A) Cell viability. Osteosarcoma cells (143B and U2OS) and normal human osteoblast cells (HOB 1 and HOB 2) treated with SKF96365 for 72 h at the indicated concentrations were analyzed by MTS assays; (B) Colony formation assays and quantitation. 143B and U2OS cells were treated with SKF96365 (5 μmol/L); (C) STIM1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression were analyzed by Western blot hybridization using cytoplasmic extracts from 143B and U2OS cells treated with NC, control siRNA, or STIM1 siRNA for 48 h. The relative protein levels were determined by densitometry; (D) Colony formation assay and quantitation of colonies in 143B and U2OS cells treated with control siRNA or STIM1 siRNAs. Growth inhibition is presented relative to that of control (absence of SKF96365) or control siRNAs. Data are presented as
 from three independent experiments. *, P<0.05, compared with control (absence of SKF96365) or control siRNA; (E) Fluorescence-activated cell sorting (FACS) measurements showed that down-regulated STIM1 expression arrested more cells in G2M stage for 143B and U2OS cells. NC, negative control without siRNA.
from three independent experiments. *, P<0.05, compared with control (absence of SKF96365) or control siRNA; (E) Fluorescence-activated cell sorting (FACS) measurements showed that down-regulated STIM1 expression arrested more cells in G2M stage for 143B and U2OS cells. NC, negative control without siRNA.
To determine the role of STIM1 protein on osteosarcoma cell proliferation, we have performed STIM1 knockdown by siRNA transfections. Our results showed that STIM1 siRNA transfection reduced the protein levels of STIM1 to 30.4% and 33.6%, in 143B and U2OS osteosarcoma cells, respectively (Figure 2C ). The results confirmed that the control siRNA did not have any effect on STIM1 levels. Also, we observed that following siRNA-mediated knockdown of STIM1, the number of colonies decreased by 65.5% and 45.6% in 143B and U2OS cells, respectively (Figure 2D ). Cell cycle distribution analysis was detected by flow cytometry at 48 h after transfection. Down-regulation STIM1 expression arrested more cells in G2M stage for 143B and U2OS cells (Figure 2E ).
Store-operated Ca2+ channels and STIM1 were critical for osteosarcoma cell migration
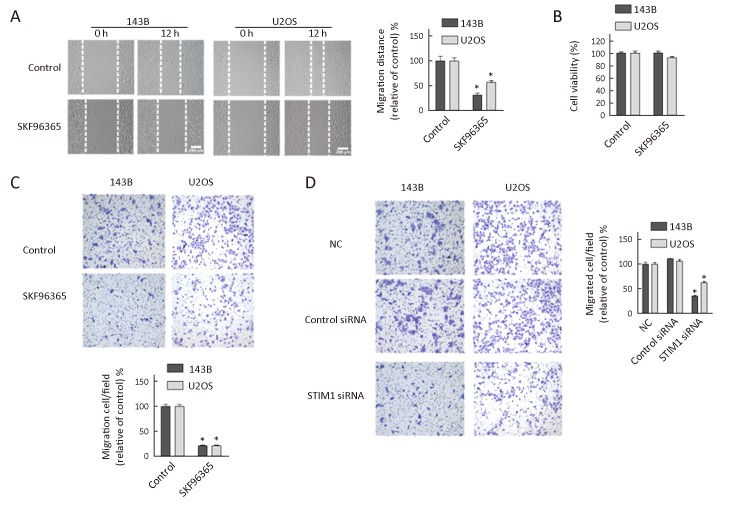
Cell migration is a crucial event during tumor progression and metastasis. To assess the effect of SOCE on osteosarcoma cell migration, we performed a wound healing assay in the presence of SOCE inhibitor SKF96365. Briefly, 143B and U2OS cells were grown to confluence, and a “wound” was made in the middle of the tissue culture plate with a pipette tip and further maintained for 12 h in the presence and absence of SFK96365. The results showed that after 12 h of incubation with 20 μmol/L SKF96365, the relative migration distances in 143B and U2OS cells decreased by 67% and 45%, respectively (Figure 3A ). Also, no detectable level of cell migration was noticed when HOB 1 and HOB 2 cells were cultured in the presence and absence of SKF96365 (data not shown). Our results showed that the inhibition of cell migration by SKF96365 was not due to decreases in cell viability, as SKF96365 did not have any effect on cell viability at 12 h (Figure 3B ). The inhibitory effect of SKF96365 on cell migration was confirmed by another method using Boyden chamber assays which confirmed that SKF96365 blocked the migration of 143B and U2OS cells by 79% and 78.5%, respectively (Figure 3C ).
3.
Effect of store-operated calcium entry (SOCE) inhibitor and stromal interaction molecule 1 (STIM1) siRNA on osteosarcoma cell migration. (A) Wound healing assays. Micrographs of 143B and U2OS cells treated with SKF96365 (20 μmol/L) for 12 h. The migration distance is presented relative to that of control (absence of SKF96365) in each cell line; (B) Cell viability. 143B and U2OS osteosarcoma cells treated with SKF96365 (20 μmol/L) for 12 h and analyzed by MTS assays; (C) Transwell assays and quantitation of migrated cells in 143B and U2OS cultures treated with SKF96365 (20 μmol/L) for 16 h; (D) Transwell assays and quantitation of migrated cells in 143B and U2OS cells treated with control or STIM1 siRNAs for 16 h. The number of migrated cells is presented relative to that of control siRNA. Data are presented as
 from three independent experiments. *, P<0.05; NC, negative control.
from three independent experiments. *, P<0.05; NC, negative control.
To further investigate the role of STIM1 on cell migration, we performed the migration studies using Boyden chamber assays following STIM1 knockdown. The results showed that the migrations were inhibited in 143B and U2OS cells transiently transfected with STIM1 siRNAs by 66% and 40%, respectively (Figure 3D ). Thus, these studies indicated that both pharmacologic and siRNA inhibitors of STIM1 could block cell migration.
Blockade of store-operated Ca2+ channels inhibit osteosarcoma cell functions was associated with down-regulation of NFATc1
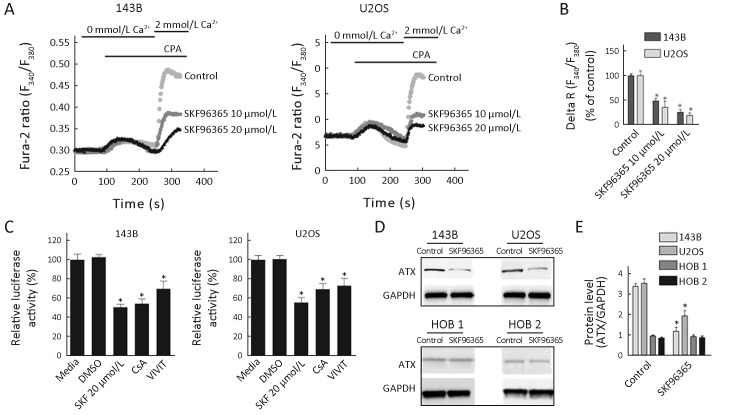
NFATs have been implicated in cancer cell proliferation. Increased Ca2+ can activate calcineurin, which dephosphorylates cytoplasmic NFAT, resulting in its translocation to the nucleus where it forms complexes with other transcription factors and regulates gene transcription (16). In this study, we examined whether blockade of store-operated Ca2+ channels exert a negative effect on osteosarcoma cell survival is associated with down-regulation of NFATc1. Our results showed that the reduction of SOCE was confirmed by Fluo-2-based measurements (Figure 4A ). Store-operated channels are activated when internal Ca2+ stores are empty. The intracellular Ca2+ stores were emptied by cyclopiazonic acid (CPA) in the absence of extracellular Ca2+. Ca2+ influx was then measured by adding 2 mmol/L extracellular Ca2+. Compared with the control group, SKF96365 at the concentrations of 10 and 20 μmol/L decreased the level of Ca2+ influx by 52.5% and 76%, respectively, in 143B cells, and by 65% and 81.4%, respectively, in U2OS cells (Figure 4B ).
4.
Blockade of store-operated Ca2+ channels inhibiting osteosarcoma cell growth is associated with down-regulation of nuclear factor of activated T-cells cytoplasmic 1 (NFATc1). (A) Representative time course recording of store-operated calcium entry (SOCE) in the presence of SKF96365 in osteosarcoma cells. Cells were treated with cyclopiazonic acid (CPA) to passively deplete the intracellular Ca2+ stores followed by application of 2 mmol/L Ca2+; (B) Quantification of CPA-induced SOCE peak value (delta R: Ratiomax−Ratiobase; N=45 cells for each group); (C) NFATc1 luciferase activity. 143B and U2OS cells transfected with reporter plasmids were treated with SKF96365 20 μmol/L, cyclosporine A (CsA) 10 μmol/L or VIVIT 5 μmol/L; (D) Autotaxin (ATX) protein levels in osteosarcoma cells. Cytoplasmic extracts from cells treated with SKF96365 (20 μmol/L) for 24 h were analyzed by Western blot hybridization using anti-ATX and anti-GAPDH antibodies; (E) Quantitation of protein levels by densitometry. Data are presented as
 from three independent experiments. *, P<0.05 (vs. Control).
from three independent experiments. *, P<0.05 (vs. Control).
We also determined the effect of SOCE inhibitor SKF96365 on NFATc1-dependent transcriptional activity by transient transfection assays. The results showed that treatment with SKF96365 markedly decreased the NFATc1-dependent transcription in osteosarcoma cells. The luciferase activities were down in 143B and U2OS cells by 50% and 45%, respectively (Figure 4C ). Also, treatment with cyclosporin A (CsA), an indirect inhibitor of NFAT, and VIVIT, a specific inhibitor of NFAT, demonstrated significant decreases in NFATc1 activities in 143B and U2OS cells (Figure 4C ). The results indicate that CsA decreased NFATc1-dependent luciferase activity by 46% in 143B cells and 31% in U2OS cells. Similarly, VIVIT decreased NFATc1-dependent luciferase activity by 30% and 27% in 143B and U2OS cells, respectively. To confirm the mechanism of inhibition of NFAT by SKF96365, we evaluated the expression of autotaxin (ATX), a product of NFATc1-dependent transcriptional activity (17). Our results showed that ATX protein expression was decreased by 65% in 143B cells and 45% in U2OS cells following treatment with SKF96365 (Figure 4D , E ). And ATX protein expression was not affected by the treatment of SKF96365 in HOB 1 and HOB 2 cell lines (Figure 4D , E ).
Discussion
The present study demonstrates that the expression of STIM1 protein in osteosarcoma specimens positively correlate with poor prognosis. Also, we have found that 3 out of 5 osteosarcoma cell lines examined showed higher levels of STIM1 protein compared with the normal osteoblast cells. The SOCE inhibitor and STIM1 siRNAs inhibited the survival and migration of osteosarcoma cells. Furthermore, it was observed that blockade of store-operated Ca2+ channels involves NFATc1-dependent pathway in osteosarcoma cells. Taken together, our results indicate that STIM1 and SOCE contribute to tumorigenesis and could serve as therapeutic targets in the control of osteosarcoma.
Recent reports indicate that SOCE is essential for the progression of several cancers (9,10,18). Our study reveals that osteosarcoma cells have higher STIM1 protein levels compared with normal osteoblast cells. SiRNA-mediated down-regulation of STIM1 indicates that STIM1 is essential for osteosarcoma cell viability and motility. Also, TMA results show that the expression levels of STIM1 positively correlate with the disease in a wide array of osteosarcoma tissues examined. Thus, these studies suggest that STIM1 expression is associated with the progression of osteosarcoma.
Several studies reveal the involvement of STIM1 and SOCE in regulating cancer cell proliferation. STIM1 and SOCE were critical for cell proliferation in clear cell renal carcinoma cells (19), and colorectal cancer cells (20). Although one study contradicts that STIM1 down-regulation did not affect the proliferation of human breast tumor cells (21), others have reported the tumorigenic role of STIM1 in breast cancer cells (20). In this report, it has been shown that down-regulation of STIM1 by transforming growth factor-β led to suppression of cell proliferation. Current results show that STIM1 siRNA and SKF96365 treatment decrease cell survival in human osteosarcoma cells. The inhibitory effect of STIM1 siRNA was correlated with the treatment time and it did not decrease the tumor growth within 24 h in both 143B and U2OS cells. The effects of STIM1 siRNA became evident at 48 and 72 h.
Other studies show that STIM1 and SOCE could mediate cell migration. Blockade of SOCE and the siRNA-mediated silencing of STIM1 inhibited both migration and invasion in hepatocarcinoma cells (10), cervical cancer cells (9) and colorectal cancer cells (12). Our results corroborate these findings and indicate that cell migration was significantly decreased following the inhibition of SOCE and STIM1 in osteosarcoma cells.
The results show that SOCE inhibitor, SKF96365, decreases osteosarcoma cell survival. Our studies reveal that SOCE and NFATc1 play important roles in the inhibition of osteosarcoma cells by SKF96365 based on the following results: First, we demonstrated the role of store-operated calcium channels on Ca2+ influx in osteosarcoma cells. In Fluo-2-based measurements, SKF96365 decreased the level of Ca2+ influx. Second, we established that the transcriptional activity of NFATc1 was decreased in the presence of SKF96365. This was further supported by decreased expression of autotaxin. Although further studies are needed to understand the molecular mechanisms, to our knowledge, this is the first report that demonstrates the involvement of NFATc1 on osteosarcoma cell regulation.
Conclusions
Our study demonstrates that STIM1 is associated with osteosarcoma cell survival, and SOCE and STIM1 could be further explored as therapeutic targets in the treatment of osteosarcoma.
Acknowledgements
The authors thank Mayo Clinic Scientific Publications for proofreading of this manuscript. This study was supported by Mayo Clinic, USA and Peking University People’s Hospital, Beijing, China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Wei Guo, Email: bonetumor@163.com.
Avudaiappan Maran, Email: maran.avudai@mayo.edu.
References
- 1.Wang S, Ren T, Huang Y, et al. BMPR2 and HIF1-α overexpression in resected osteosarcoma correlates with distant metastasis and patient survival. Chin J Cancer Res. 2017;29:447–54. doi: 10.21147/j.issn.1000-9604.2017.05.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison DC, Carney SC, Ahlmann ER, et al A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. 2012;2012:704872. doi: 10.1155/2012/704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller BJ, Cram P, Lynch CF, Buckwalter JA Risk factors for metastatic disease at presentation with osteosarcoma: an analysis of the SEER database. J Bone Joint Surg Am. 2013;95:e89. doi: 10.2106/JBJS.L.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto MC, Kihara AH, Goulart VA, et al Calcium signaling and cell proliferation. Cell Signal. 2015;27:2139–49. doi: 10.1016/j.cellsig.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Paupe V, Prudent J New insights into the role of mitochondrial calcium homeostasis in cell migration. Biochem Biophys Res Commun. 2018;500:75–86. doi: 10.1016/j.bbrc.2017.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scrimgeour NR, Wilson DP, Barritt GJ, et al Structural and stoichiometric determinants of Ca2+ release-activated Ca2+ (CRAC) channel Ca2+-dependent inactivation . Biochim Biophys Acta. 2014;1838:1281–7. doi: 10.1016/j.bbamem.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Park CY, Hoover PJ, Mullins FM, et al STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–90. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roderick HL, Cook SJ Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival . Nat Rev Cancer. 2008;8:361–75. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 9.Chen YF, Chiu WT, Chen YT, et al Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci U S A. 2011;108:15225–30. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang N, Tang Y, Wang F, et al Blockade of store-operated Ca2+ entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover . Cancer Lett. 2013;330:163–9. doi: 10.1016/j.canlet.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 11.Robey PG, Termine JD Human bone cells in vitro . Calcif Tissue Int. 1985;37:453–60. doi: 10.1007/BF02557826. [DOI] [PubMed] [Google Scholar]
- 12.Wang JY, Sun J, Huang MY, et al STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene. 2015;34:4358–67. doi: 10.1038/onc.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bravo D, Shogren KL, Zuo D, et al 2-Methoxyestradiol-mediated induction of frzb contributes to cell death and autophagy in MG63 osteosarcoma cells. J Cell Biochem. 2017;118:1497–504. doi: 10.1002/jcb.25809. [DOI] [PubMed] [Google Scholar]
- 14.Wylam ME, Sathish V, VanOosten SK, et al Mechanisms of cigarette smoke effects on human airway smooth muscle. PLoS One. 2015;10:e0128778. doi: 10.1371/journal.pone.0128778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White TA, Kannan MS, Walseth TF Intracellular calcium signaling through the cADPR pathway is agonist specific in porcine airway smooth muscle. FASEB J. 2003;17:482–4. doi: 10.1096/fj.02-0622fje. [DOI] [PubMed] [Google Scholar]
- 16.Bai H, Zhu H, Yan Q, et al TRPV2-induced Ca2+-calcineurin-NFAT signaling regulates differentiation of osteoclast in multiple myeloma . Cell Commun Signal. 2018;16:68. doi: 10.1186/s12964-018-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tania M, Khan MA, Zhang H, et al Autotaxin: a protein with two faces. Biochem Biophys Res Commun. 2010;401:493–7. doi: 10.1016/j.bbrc.2010.09.114. [DOI] [PubMed] [Google Scholar]
- 18.Fedida-Metula S, Feldman B, Koshelev V, et al Lipid rafts couple store-operated Ca2+ entry to constitutive activation of PKB/Akt in a Ca2+/calmodulin-, Src- and PP2A-mediated pathway and promote melanoma tumor growth . Carcinogenesis. 2012;33:740–50. doi: 10.1093/carcin/bgs021. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Lkhagvadorj S, Lee MR, et al Orai1 and STIM1 are critical for cell migration and proliferation of clear cell renal cell carcinoma. Biochem Biophys Res Commun. 2014;448:76–82. doi: 10.1016/j.bbrc.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 20.Cheng H, Wang S, Feng R STIM1 plays an important role in TGF-β-induced suppression of breast cancer cell proliferation. Oncotarget. 2016;7:16866–78. doi: 10.18632/oncotarget.7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vashisht A, Trebak M, Motiani RK STIM and Orai proteins as novel targets for cancer therapy. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol. 2015;309:C457-69. doi: 10.1152/ajpcell.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]