ABSTRACT
Glioblastoma multiforme (GBM) is the most common malignant brain tumor in adults. Despite multiple treatment strategies, the prognosis is still poor. This study aimed to evaluate the efficacy of combination treatment of GBM with the histone deacetylase (HDAC) inhibitor panobinostat and dual phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitor BEZ235. GBM cells were exposed to panobinostat and BEZ235 treatment alone or in combination, after which cell viability, proliferation and apoptosis were detected. Furthermore, the inhibitory mechanisms were investigated by Caspase-Glo assay, Western blot and qPCR analysis. We found that combination treatment with panobinostat and BEZ235 synergistically inhibited cell viability, markedly inhibited cell proliferation and induced apoptosis in GBM cells. Mechanistically, cotreatment with panobinostat and BEZ235 increased caspase 3/7 activity, suppressed proliferation- and antiapoptosis-related markers and AKT signaling in GBM cells. Cotreatment with panobinostat and BEZ235 warrants further evaluation in GBM therapy.
Key Words: glioblastoma, panobinostat, BEZ235
INTRODUCTION
Glioblastoma multiforme (GBM) is the most common malignant brain tumor in adults.1 Despite multiple treatment strategies consisting of surgery, radiation and chemotherapy, the median survival is still 12–15 months.2 Therefore, novel therapeutic strategies against GBM are urgently needed.
Currently, epigenetic mechanisms are recognized to play an important role in the pathogenesis of various cancers including GBM.3 Histone deacetylase (HDAC) inhibitors, which can alter histone and non-histone protein functions, are a group of epigenetic therapies investigated for multiple cancers.4 Various HDAC inhibitors, such as panobinostat, vorinostat and sodium valproate, have shown potent efficacy against GBM in preclinical studies either as a single treatment or combined with other therapies.3,5,6 Multiple anti-GBM mechanisms of HDAC inhibitors have been suggested, including the induction of cell cycle arrest, apoptosis, inhibition of angiogenesis and DNA damage repair (DDR).3 Despite encouraging results from preclinical studies, early clinical trials have shown only modest benefit.7-10 Therefore, it is important to explore novel drug combination strategies of HDAC inhibitors to enhance their therapeutic efficacy against GBM.
The PI3K-Akt-mTOR signaling pathway is constitutively activated in approximately 70% of GBM.11 BEZ235 is a dual inhibitor that could inhibit both PI3K and mTOR kinase activity by binding to the ATP-binding cleft of these enzymes.12 BEZ235 has been shown to have antiglioma activity and enhance the radiosensitivity and chemosensitivity of glioma cells in vitro and in vivo.12-14 Recently, BEZ235 has been shown to have synergistic effects with panobinostat in various cancers through multiple mechanisms.15-18 However, whether BEZ235 can also improve the efficacy of panobinostat against GBM remains elusive.
In this study, we explored the efficacy and mechanisms of action of cotreatment with HDAC inhibitor panobinostat and dual PI3K/mTOR inhibitor BEZ235 in GBM cells. We found that combination treatment with panobinostat and BEZ235 synergistically inhibited cell viability, markedly inhibited cell proliferation and induced apoptosis in GBM cells. Mechanistically, cotreatment with panobinostat and BEZ235 increased caspase 3/7 activity, suppressed proliferation- and antiapoptosis-related markers and AKT signaling in GBM cells. Cotreatment with panobinostat and BEZ235 warrants further evaluation in GBM therapy.
MATERIALS AND METHODS
Compounds and cell lines
Panobinostat (S1030) and BEZ235 (S1009) were purchased from Selleck Chem (Houston, TX, USA). U87, U251 and 293T cell lines were obtained from Cell Bank of Chinese Academy of Science (Shanghai, China). U87, U251 and 293T were cultured in Dulbecco’s modified Eagle medium/high glucose (HyClone, Logan, Utah, USA) with 10% fetal bovine serum. U87_Serum_Free were cultured using NeuroCult Neural Stem and Progenitor Cells (NS-A) Proliferation Kit (Human) (05751, Stem Cell Technology, Vancouver, Canada) supplemented with human epidermal growth factor (EGF) basic (20 ng/ml) (AF-100-15-100, PeproTech, New Jersey, USA), human fibroblast growth factor (FGF) basic (20 ng/ml) (100-18B-100, PeproTech), and 0.2% heparin solution (10 ng/ml) (07980, Stem Cell Technology) and passaged for 1.5 months. Mouse neural stem cells (mNSCs) were established from fresh mouse brain tissues. Briefly, neonatal mouse brains were dissected, and the cerebral cortex was dissociated into single cells with Gibco TrypLE Express Enzyme (12604021, Thermo Fisher Scientific, Waltham, USA) for 10 min at 37°C. Excess debris were removed with 22% Percoll solution and mNSCs were cultured in NeuroCult NS-A Proliferation Kit (Mouse) (05702, Stem Cell Technology) supplemented with murine EGF (20 ng/ml) (315-09-100, PeproTech), murine-FGF (20 ng/ml) (450-33-50, PeproTech) and heparin (10 ng/ml).
Cell Viability assays
Celltiter-Glo assay (G7571, Promega) was used for cell viability measurement. Briefly, GBM cells were plated into 96-well plates in at least triplicate and subjected to drug treatment as indicated. Cell viability was detected according to the manufacturer’s protocol. Data were all collected on Synergy H4 Hybrid Reader (BioTek, Winooski, VT, USA).
Western Blot
Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer with 1X protease inhibitor cocktail (0469313200, Roche, Indianapolis, USA) and 1% phosphatase inhibitor cocktail (P0044, Sigma, Woodstock, USA). Pierce™ Bicinchoninic Acid (BCA) Protein Assay Kit (23225, Thermo Fisher Scientific) was used to measure protein concentration according to the manufacturer’s protocol. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed and proteins were transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were incubated for 16–20 hours at 4°C with the following primary antibodies: Akt (pan) (1:1,000, Cell Signaling Technology #2920), Phospho-Akt (Ser473) (1:1,000, Cell Signaling Technology #4060), Beta-Tubulin (1:5000, Abcam #ab6046). The ratio of p-Akt/Akt was calculated by measuring the band intensities using imaging software (ImageJ_v1.8.0).
RNA extraction and RT-qPCR
RNA was isolated from cell lines with TRIzol reagent (15-596-018, Thermo Fisher Scientific) and measured using a Nanodrop 2000 spectrophotometer. Equal amounts of RNA were converted to cDNA using a High Capacity cDNA Reverse Transcription Kit (4368813, Thermo Fisher Scientific). Real-time polymerase chain reaction (RT-qPCR) was performed in 384-well plates using a 7900 HT fast real-time PCR system (Thermo Fisher Scientific). Fold change in gene expression was calculated using the Delta-Delta-Ct (ddCt) method. The qPCR primer sequences and product length were listed as below:
DNA topoisomerase 2-alpha (TOP2A):
Product length: 243
Forward: ACTGAAGGAAGCCCTCAAGA
Reverse: TGTTTTTGTTGCTGCTCTCC
Cyclin D1 (CCND1)
Forward: TTCAAATGTGTGCAGAAGGA
Reverse: GGGATGGTCTCCTTCATCTT
Product length: 221
Antigen KI-67 (MKI67)
Product length: 218
Forward: TTGGAGAATGACTCGTGAGC
Reverse: CGAAGCTTTCAATGACAGGA
Baculoviral inhibitor of apoptosis repeat-containing 5 (BIRC5)
Product length: 161
Forward: AGCCCTTTCTCAAGGACCAC
Reverse: CAGCTCCTTGAAGCAGAAGAA
B-cell lymphoma-extra large (BCL-XL)
Product length: 211
Forward: CTGAATCGGAGATGGAGACC
Reverse: TGGGATGTCAGGTCACTGAA
Cell Proliferation and Apoptosis Assays
The Click-iT 5-ethynyl-2’-deoxyuridine (EdU) Alexa Fluor 647 Flow Cytometry Assay Kit (C10640, Invitrogen, CA, USA) was used for cell proliferation measurement, and EdU+ population represents the proliferating cell population. The Annexin V Fluorescein isothiocyanate (FITC) Apoptosis Detection Kit I (556547, BD Biosciences, CA, USA) was used for cell apoptosis measurement with some minor modifications: DAPI was used instead of PI. Fluorescence-activated cell sorting (FACS) analysis was performed using BD Fortessa FACS machine (BD Biosciences). Flowjo software (FlowJo, LLC, OR, USA) was used for data analysis.
Caspase assays
The Caspase 3/7 Glo Assay (G8092, Promega) was used for caspase activity measurement. Briefly, GBM cells were plated into 96-well plates in at least triplicates and subjected to drug treatment as indicated. Cell caspase activity was measured according to the manufacturer’s protocol, and data were collected on Synergy H4 Hybrid Reader (BioTek, Winooski, VT, USA).
Statistical analyses
Two tailed Student’s t-test was used for comparing two groups in most statistical analyses. *p<0.05, ** p<0.01, *** p<0.001.
RESULTS
Cotreatment with panobinostat and BEZ235 synergistically inhibits GBM cell viability
To determine whether the combined treatment of panobinostat and BEZ235 is synergistic, additive or antagonistic in GBM cells, the combination index (CI) by the Chou and Talalay method was used.19 GBM cell lines, including U87, U251 and serum-free cultured U87 (to mimic stem cell culture condition) were exposed for 72 hours to increasing concentrations of panobinostat or BEZ235 ranging from 12.5 to 400 nM. As shown in Fig. 1A, combined treatment with panobinostat and BEZ235 resulted in a sharp decline in cell viability dose-dependently in each cell line tested. In contrast, agents administered individually had only minimal effects. Fig. 1A demonstrates that the combined treatment of panobinostat and BEZ235 exerted a highly synergistic inhibitory effect against GBM cells with a CI value well below 1.0. Then, we treated U87, U251 and serum-free cultured U87 cell lines with DMSO, panobinostat, BEZ235 or panobinostat/BEZ235 for 24 h, 48 h or 72 h, and the results showed that panobinostat and BEZ235 treatment caused a time-dependent growth disruption of GBM cells (Fig. 1B). Compared with each agent alone, combined treatment of GBM cells with panobinostat and BEZ235 induced a greater inhibition of cell growth in a time-dependent manner (Fig. 1B). Additionally, compared to nontumor cells (mNSC and 293T), panobinostat and BEZ235 induced a greater inhibition of cell growth in GBM cells at the same dosage, suggesting the existence of a therapeutic window (Fig. 1C). The morphological differences of dying GBM cells between the single treatment and combined treatment are shown in Fig. 1D. All these results indicate that cotreatment with panobinostat and BEZ235 inhibits cell viability synergistically in a dose- and time-dependent manner in GBM cells.
Fig. 1. Fig. 1 Cotreatment with panobinostat and BEZ235 synergistically inhibits GBM cell viability.
Fig. 1A: GBM cell lines, including U87, U251 and serum-free cultured U87, were exposed for 72 hours to increasing concentrations of panobinostat, BEZ235 or combined panobinostat/BEZ235 ranging from 12.5 to 400 nM, and cell viability was detected by Celltiter-Glo assay. The combination index (CI) is shown below.
Fig. 1B: Time course tracking of viability of U87, U251 and serum-free cultured U87 cells after treatment with panobinostat, BEZ235 or combined panobinostat/BEZ235 at the indicated drug dosages.
Fig. 1C: Compared to nontumor cells (mNSC and 293T), panobinostat and BEZ235 induced greater inhibition of GBM cell growth at the same dosage.
Fig. 1D: The morphological imaging of GBM cells after DMSO, single drug treatment or combined treatment for 3 days.
Cotreatment with panobinostat and BEZ235 markedly inhibits GBM cell proliferation and induces apoptosis
To examine the inhibitory effect of panobinostat and BEZ235 on the proliferation of GBM cells, U87, U251 and serum-free cultured U87 cells were treated with control vehicle, panobinostat, BEZ235 or the combination of panobinostat and BEZ235 for 24 hours at the indicated concentrations, followed by staining with EdU and FACS analysis. As shown in Fig. 2A, while treatment with panobinostat or BEZ235 alone reduced the percentage of cells positively stained with EDU, combination therapy with panobinostat and BEZ235 reduced the percentage of cells positively stained with EDU to a greater extent in U251 cells and nearly shutdown the proliferation of U87 and serum-free culture U87 cell lines. To determine whether panobinostat and BEZ235 commonly induce apoptosis on GBM cells, U87, U251 and serum-free cultured U87 cells were treated with control vehicle, panobinostat, BEZ235 or the combination of panobinostat and BEZ235 for 48 hours, followed by staining with Annexin V and FACS analysis. As shown in Fig. 2B, while treatment with panobinostat or BEZ235 alone increased the percentage of cells positively stained with Annexin V, combination therapy with panobinostat and BEZ235 increased the percentage of cells positively stained with Annexin V to 80% for U87 cells, 93.65% for U251 cells and 89.72% for serum-free cultured U87 cells. All these results suggest that cotreatment with panobinostat and BEZ235 markedly inhibits cell proliferation and induces apoptosis in GBM cells.
Fig. 2. Cotreatment with panobinostat and BEZ235 markedly inhibits GBM cell proliferation and induces apoptosis.
Fig. 2A: Cell proliferation analyses of GBM cells treated with DMSO, panobinostat, BEZ235 or combined panobinostat/BEZ235 for 24 hours by EdU incorporation FACS assay. Percentages of EdU+ cells are presented on the right of the bar chart.
Fig. 2B: Apoptosis analyses of GBM cells treated with DMSO, panobinostat, BEZ235 or combined panobinostat/BEZ235 for 48 hours by Annexin-V staining FACS assay. Percentages of Annexin-V+ cells are presented on the right of the bar chart.
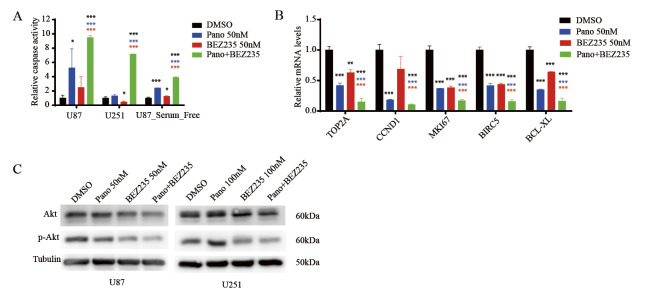
Cotreatment with panobinostat and BEZ235 increases caspase activity and suppresses proliferation- and antiapoptosis-related markers and AKT signaling in GBM cells
To further explore the anti-GBM mechanisms of cotreatment with panobinostat and BEZ235, we first detected caspase activity in U87, U251 and serum-free cultured U87 cells treated with control vehicle, panobinostat, BEZ235 or the combination of panobinostat and BEZ235 for 24 hours. As shown in Fig. 3A, compared with panobinostat or BEZ235 treatment alone, cotreatment with panobinostat and BEZ235 significantly increased caspase activity in all GBM cell lines tested, suggesting cotreatment with panobinostat and BEZ235 induced caspase-dependent apoptosis. Next, we detected mRNA levels of proliferation- and antiapoptosis-related markers, such as TOP2A, CCND1, MKI67, BIRC5 and BCL-XL by qPCR analysis. As shown in Fig. 3B, compared with panobinostat and BEZ235 treatment alone, markers of cell proliferation and antiapoptosis were significantly downregulated by cotreatment with panobinostat and BEZ235, suggesting that panobinostat and BEZ235 commonly regulate oncogene expression in GBM cells. To elucidate the signaling pathway inhibition of the combination of panobinostat and BEZ235 in GBM cells, AKT and p-AKT molecules were measured after 48 h of drug treatment in U87 and U251 cells. As shown in Fig. 3C, there was a pronounced dephosphorylation of AKT by BEZ235 in both cell lines, particularly when combined with panobinostat. The ratio of p-Akt/Akt decreased from 0.60 (panobinostat treatment) and 0.55 (BEZ235 treatment) to 0.52 (combined treatment) for U87 cells and from 0.74 (panobinostat treatment) and 0.60 (BEZ235 treatment) to 0.57 for U251 cells.
Fig. 3. Cotreatment with panobinostat and BEZ235 increases caspase activity and suppresses proliferation- and antiapoptosis-related markers and AKT signaling in GBM cells.
Fig. 3A: Caspase 3/7 activity was detected with Caspase-Glo assay in GBM cells treated with DMSO, panobinostat, BEZ235 or combined panobinostat/BEZ235 at the indicated concentrations for 48 hours.
Fig. 3B: The mRNA expression levels of cell proliferation- and apoptosis-related markers were detected in GBM cells treated with DMSO, panobinostat, BEZ235 or combined panobinostat/BEZ235 at the indicated concentrations for 16 h.
Fig. 3C: Immunoblotting analyses of AKT and p-AKT in GBM cells treated with DMSO, panobinostat, BEZ235 or combined panobinostat/BEZ235 for 16 h. (Blue or red asterisks indicate P values of each single drug treatment compared with combined treatment. Black asterisk indicate P values of each treatment group compared with DMSO group. *p<0.05, ** p<0.01, *** p<0.001, Student’s two-tailed t-test.)
DISCUSSION
GBM remains one of the most fatal cancer types in adults to date. Despite multiple treatment strategies consisting of surgery, radiation and chemotherapy, the median survival is still 12–15 months.20 Therefore, novel therapeutic strategies for GBM treatment are urgently needed.
The enhanced efficacy of a HDAC inhibitor combined with a PI3K/mTOR inhibitor has been observed in several cancer types.15-18 Ellis et al reported that cotreatment with the HDAC inhibitor panobinostat and PI3K/mTOR inhibitor BEZ235 inhibits the viability of gene of phosphate and tension homology deleted on chromosome ten (PTEN)-deficient prostate cancer cells better than each single drug treatment.15 Chen et al found that the HDAC inhibitor trichostatin A and PI3K/mTOR inhibitor BEZ235 synergistically inhibited the growth of breast cancer cells.16 Our study also showed that the HDAC inhibitor panobinostat and PI3K/mTOR inhibitor BEZ235 exerted synergistic anti-GBM activity in vitro. Cotreatment with panobinostat and BEZ235 inhibited cell viability synergistically and markedly inhibited cell proliferation and induced apoptosis with elevated caspase activity in GBM cells. Cotreatment with panobinostat and BEZ235 also suppressed proliferation and antiapoptosis markers and AKT signaling in GBM cells.
Inhibition of cell proliferation and induction of cell death are common synergistic mechanisms of treatment with panobinostat and BEZ235 in various cancers.15-18 Chen et al found that the HDAC inhibitor trichostatin A and PI3K/mTOR inhibitor BEZ235 synergistically inhibited the proliferation of breast cancer cells.16 Venkannagari et al reported that BEZ235 and panobinostat could synergistically suppress pancreatic cancers through the TORC1/4EBP1 signaling pathway and induce apoptosis.18 Here, using a FACS assay with EdU or Annexin-V staining, we also observed that cotreatment with panobinostat and BEZ235 could inhibit cell proliferation or induce apoptosis more potently than each single drug treatment. Similarly, our qPCR results showed that cotreatment with panobinostat and BEZ235 suppressed proliferation-related markers, such as TOP2A, CCND1 and MKI67, and antiapoptosis-related markers, such as BIRC5 and BCL-XL, more significantly than each single drug treatment in GBM cells. B-cell lymphoma-extra large (BCL-XL) is a member of the Bcl-2 family proteins and acts as an antiapoptotic protein by preventing the release of cytochrome c, which leads to caspase activation and ultimately programmed cell death.21 BIRC5 (Survivin) also functions to inhibit caspase activation, thereby leading to negative regulation of apoptosis.22 In agreement with these results, we also observed that caspase 3/7 activity was significantly increased after cotreatment with panobinostat and BEZ235 compared to each single drug treatment, suggesting caspase-dependent apoptosis is one of synergistic mechanisms of cotreatment with panobinostat and BEZ235.
The PI3K-Akt-mTOR signaling pathway is important for the growth of various cancers including GBM.12-14 Although HDAC inhibitors possessed the ability to attenuate activated levels of Akt in tumors, the effect was mostly moderate; therefore, this signaling could be potentially targeted to enhance the antitumor efficacy of HDAC inhibitors.15,18 Indeed, several studies have shown that the synergistic mechanism of combined treatment with panobinostat and BEZ235 may be related to the further depression of the PI3K/Akt/mTOR signaling pathway.15-18 Our Western blot results showed that panobinostat treatment attenuated p-Akt moderately and that its cotreatment with BEZ235 resulted in a profound loss of p-Akt in GBM cells.
In summary, our study has demonstrated that coadministration of panobinostat and BEZ235 synergistically inhibited cell viability, markedly inhibited cell proliferation and induced apoptosis in GBM cells. Mechanistically, cotreatment with panobinostat and BEZ235 increased caspase 3/7 activity, suppressed proliferation- and antiapoptosis-related markers and AKT signaling in GBM cells. Cotreatment with panobinostat and BEZ235 warrants further evaluation in GBM therapy.
ACKNOWLEDGMENTS
This work was supported by Shanghai Jiao Tong University Yi Gong Jiao Cha Fund (YG2016MS74 to Q.L.), Shanghai Shen Kang Hospital Development Center Fund (16CR2031B to J.M.), Shanghai Science and Technology Committee Fund (17411951800 to J.M.) and National Natural Science Foundation of China (81500601 to C.Z., 81702453 to Y.Z.). We also thank all members of Tang lab and Ma lab for their helpful discussions and advice regarding this work.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. [DOI] [PMC free article] [PubMed]
- 2.Anjum K, Shagufta BI, Abbas SQ, et al. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: a review. Biomed Pharmacother. 2017;92:681–689. [DOI] [PubMed]
- 3.Lee DH, Ryu HW, Won HR, Kwon SH. Advances in epigenetic glioblastoma therapy. Oncotarget. 2017;8(11):18577–18589. [DOI] [PMC free article] [PubMed]
- 4.Bezecny P. Histone deacetylase inhibitors in glioblastoma: pre-clinical and clinical experience. Med Oncol. 2014;31(6):985. [DOI] [PubMed]
- 5.Berghauser Pont LM, Kleijn A, Kloezeman JJ, et al. The HDAC inhibitors scriptaid and LBH589 combined with the oncolytic virus Delta24-RGD exert enhanced anti-tumor efficacy in patient-derived glioblastoma cells. PLoS One. 2015;10(5):e0127058. [DOI] [PMC free article] [PubMed]
- 6.Rasmussen RD, Gajjar MK, Jensen KE, Hamerlik P. Enhanced efficacy of combined HDAC and PARP targeting in glioblastoma. Mol Oncol. 2016;10(5):751–763. [DOI] [PMC free article] [PubMed]
- 7.Drappatz J, Lee EQ, Hammond S, et al. Phase I study of panobinostat in combination with bevacizumab for recurrent high-grade glioma. J Neurooncol. 2012;107(1):133–138. [DOI] [PubMed]
- 8.Friday BB, Anderson SK, Buckner J, et al. Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: a north central cancer treatment group study. Neuro Oncol. 2012;14(2):215–221. [DOI] [PMC free article] [PubMed]
- 9.Galanis E, Jaeckle KA, Maurer MJ, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27(12):2052–2058. [DOI] [PMC free article] [PubMed]
- 10.Lee EQ, Reardon DA, Schiff D, et al. Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro Oncol. 2015;17(6):862–867. [DOI] [PMC free article] [PubMed]
- 11.Li QJ, Cai JQ, Liu CY. Evolving molecular genetics of glioblastoma. Chin Med J (Engl). 2016;129(4):464–471. [DOI] [PMC free article] [PubMed]
- 12.Wang WJ, Long LM, Yang N, et al. NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro. Acta Pharmacol Sin. 2013;34(5):681–690. [DOI] [PMC free article] [PubMed]
- 13.Gil del Alcazar CR, Hardebeck MC, Mukherjee B, et al. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin Cancer Res. 2014;20(5):1235–1248. [DOI] [PMC free article] [PubMed]
- 14.Yu Z, Xie G, Zhou G, et al. NVP-BEZ235, a novel dual PI3K-mTOR inhibitor displays anti-glioma activity and reduces chemoresistance to temozolomide in human glioma cells. Cancer Lett. 2015;367(1):58–68. [DOI] [PubMed]
- 15.Ellis L, Ku SY, Ramakrishnan S, et al. Combination antitumor effect of HDACs and the PI3K-Akt-mTOR pathway inhibition in a Pten deficient model of prostate cancer. Oncotarget. 2013;4(12):2225–2236. [DOI] [PMC free article] [PubMed]
- 16.Piao J, Chen L, Quan T, et al. Superior efficacy of co-treatment with the dual PI3K/mTOR inhibitor BEZ235 and histone deacetylase inhibitor Trichostatin A against NSCLC. Oncotarget. 2016;7(37):60169–60180. [DOI] [PMC free article] [PubMed]
- 17.Rahmani M, Aust MM, Benson EC, Wallace L, Friedberg J, Grant S. PI3K/mTOR inhibition markedly potentiates HDAC inhibitor activity in NHL cells through BIM- and MCL-1-dependent mechanisms in vitro and in vivo. Clin Cancer Res. 2014;20(18):4849–4860. [DOI] [PMC free article] [PubMed]
- 18.Venkannagari S, Fiskus W, Peth K, et al. Superior efficacy of co-treatment with dual PI3K/mTOR inhibitor NVP-BEZ235 and pan-histone deacetylase inhibitor against human pancreatic cancer. Oncotarget. 2012;3(11):1416–1427. [DOI] [PMC free article] [PubMed]
- 19.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. [DOI] [PubMed]
- 20.Batash R, Asna N, Schaffer P, Francis N, Schaffer M. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr Med Chem. 2017;24(27):3002–3009. [DOI] [PubMed]
- 21.Karpel-Massler G, Siegelin MD. Bcl-xL inhibition - a novel strategy for glioma therapy. Aging (Albany NY). 2016;8(9):1830–1831. [DOI] [PMC free article] [PubMed]
- 22.Jane EP, Premkumar DR, Sutera PA, Cavaleri JM, Pollack IF. Survivin inhibitor YM155 induces mitochondrial dysfunction, autophagy, DNA damage and apoptosis in Bcl-xL silenced glioma cell lines. Mol Carcinog. 2017;56(4):1251–1265. [DOI] [PMC free article] [PubMed]