Abstract
Background and Purpose
Clozapine is an atypical antipsychotic drug that is very efficacious in treating psychosis, but the risk of severe cardiotoxicity limits its clinical use. The present study investigated the harmful effects of clozapine on myocardium and assessed the involvement of cannabinoid receptors in its cardiotoxicity.
Experimental Approach
Clozapine alone or in combination with selective cannabinoid receptor antagonists or agonists were used to treat mice and cardiomyocytes.
Key Results
Clozapine induced myocardial inflammation and infiltration 7 days after i.p. injection. Mice survival rate and myocardial infiltration, and fibrotic lesions were dose‐dependently worsened by clozapine. Clozapine decreased major endocannabinoid levels in sera and cultured cardiomyocytes. Cannabinoid CB1 receptors decreased in clozapine‐treated hearts and were translocated from cytomembranes to cytoplasm and nuclei, whereas CB2 receptors increased in clozapine‐treated hearts and inversely translocated from nuclei to the cytomembrane. Selective antagonists of CB1 receptors, rimonabant and AM281, but not its selective agonist arachidonyl‐2′‐chloroethylamide, ameliorated clozapine‐induced myocardial inflammatory infiltration and fibrotic lesions. In contrast, selective agonists of CB2 receptors, AM1241 and JWH‐133, but not its selective antagonist AM630, blunted clozapine‐mediated cardiotoxicity in mice. In cultured cardiomyocytes, clozapine increased the pro‐inflammatory factor IL‐1β and the concentrations of myocardial injury markers (LDH and aspartate aminotransferase); these effects were reversed by either a CB1 antagonist or CB2 agonist and further prevented by combined pretreatments.
Conclusions and Implications
Our data provide evidence that cannabinoid CB1 and CB2 receptors have opposite effects and selective antagonists of CB1 or agonists of CB2 receptors might confer protective effects against clozapine in myocardium.
Abbreviations
- 2‐AG
2‐arachidonoylglycerol
- ACEA
arachidonyl‐2′‐chloroethylamide
- AEA
anandamide
- AST
aspartate aminotransferase
- CB receptor
cannabinoid receptor
- cTnI
cardiac troponin I
- EF
ejection fraction
- FAAH
fatty acid amide hydrolase
- IHC
immunohistochemistry
- LVEDd
left ventricular end‐diastolic diameter
- LVESd
left ventricular end‐systolic diameter
- MAGL
monoacylglycerol lipase
What is already known
Clozapine is an efficacious antipsychotic for treating psychosis.
Clozapine has severe cardiotoxicity.
What this study adds
Clozapine imbalanced the endocannabinoid system when causing cardiotoxicity.
CB1 receptor antagonists or CB2 receptor agonists protected against clozapine cardiotoxicity.
What is the clinical significance
Cannabinoid CB1 and CB2 receptors had opposite effects, and selective antagonists of CB1 receptors or agonists of CB2 receptors might confer protective effects against clozapine.
1. INTRODUCTION
Clozapine piperazinyl‐dibenzo‐[1, 4]‐diazepine, a tricyclic dibenzodiazepine, is an atypical antipsychotic drug that is very efficacious in treating psychosis, particularly in patients refractory to other agents (Green et al., 2000). Clozapine appears to be particularly beneficial in patients with positive symptoms (Kelly et al., 2009) and substance use disorder (Kilian, Kerr, Lawrence, & Celermajer, 1999). The use of clozapine reduces the risk of suicidal behaviour in schizophrenic patients (Aguilar & Siris, 2007; Hennen & Baldessarini, 2005) and has also been shown to improve cognitive deficits (Li, Wu, & Li, 2007). However, some adverse effects of clozapine have limited its clinical use (Curto et al., 2016), which is due in large proportion to its cardiotoxicity (Merrill, Dec, & Goff, 2005).
Clozapine has been associated with tachycardia (in at least 10% of clozapine treated patients), hypertension or hypotension, syncope, and electrocardiographical abnormalities (at an incidence of at least 1%; Curto et al., 2016). Other severe or life‐threatening adverse cardiac effects stem from reports of myocarditis in patients treated with this drug (Ronaldson, Fitzgerald, & McNeil, 2015). We have also demonstrated that clozapine is the most common drug associated with antipsychotic‐induced sudden unexpected death (Li, Ye, Zhao, Gao, & Jiang, 2018). Health professionals have warned of the risk of potentially fatal myocarditis, cardiomyopathy, and heart failure (Kilian et al., 1999; La Grenade, Graham, & Trontell, 2001) in association with clozapine therapy. The severe cardiotoxicity of clozapine has even been drawn to the attention of the Food and Drug Administration of the United States (La Grenade et al., 2001), Switzerland (Hagg, Spigset, Bate, & Soderstrom, 2001), and Australia (Haas et al., 2007; Kilian et al., 1999).
The onset of severe cardiotoxicity may develop as early as 8 days after clozapine starting, and most of the patients were less than 50 years of age (Kilian et al., 1999). The mechanism of clozapine‐induced cardiotoxicity is not yet clearly understood. Previous studies showed the presence of cardiac and peripheral blood eosinophilia associated with clozapine cardiotoxicity, indicating a possible IgE‐mediated hypersensitivity reaction (Patel, Lisi, Lathara, & Lipchik, 2012). In addition, numerous reports have shown an increase in the level of ROS and pro‐inflammatory factors following clozapine cardiotoxicity (Abdel‐Wahab & Metwally, 2015; Abdel‐Wahab, Metwally, El‐khawanki, & Hashim, 2014). A recent study also found hypercatecholaminergic states associated with clozapine use, and a β‐adrenoceptor blocking agent may be effective in reducing the incidence and severity of clozapine‐induced myocarditis (Wang et al., 2008). However, blockade of β‐adrenoceptors (one type of GPCR) only caused a partial reduction in TNF‐α levels, postulating that there might be other receptor‐dependent mechanisms driving clozapine‐induced cardiotoxicity (Wang et al., 2008).
The endocannabinoid system is a ubiquitous lipid signalling system involving various physiological processes and comprises cannabinoid receptors (CB receptors), their endogenous ligands (endocannabinoids) and enzymes serving to biosynthesize and degrade endocannabinoids. Major endocannabinoids, such as anandamide (AEA) and 2‐arachidonoylglycerol (2‐AG) elicit tetrahydrocannabinol‐like subjective effects by binding to CB receptors (CB1 or CB2 receptors), and this effect could be reversed after catalysing AEA and 2‐AG by their respective enzyme fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL; O'Sullivan, 2015). The CB2 receptor, expressed peripherally, has been suggested to be cardioprotective after myocardial infarction (Montecucco et al., 2009; Wang et al., 2012); blockade of CB2 receptors using pharmacological antagonists exacerbated ischaemic injury in the rat isolated heart (Lepicier, Bouchard, Lagneux, & Lamontagne, 2003). Cannabinoid CB2 receptors also seem to modulate immunoreactions (Turcotte, Blanchet, Laviolette, & Flamand, 2016) and have an antifibrotic effect in liver fibrosis (Julien et al., 2005) and during skin wound‐healing processes (Wang et al., 2016). The CB1 receptor, in contrast, is considered the most abundant receptor in the mammalian brain since its identification; whereas there have been few relevant studies on its effects in peripheral tissues. Interestingly, it has recently been reported that pharmacological inhibition of the CB1 receptor protects against doxorubicin‐induced cardiotoxicity (Mukhopadhyay et al., 2007) and the cannabinoid CB1 receptor antagonist, rimonabant, protects against acute myocardial infarction (Lim, Davidson, Yellon, & Smith, 2009). Cannabinoid CB1 receptor antagonists seemed to be antifibrotic and this has provided a new strategy for the treatment of liver fibrosis (Teixeira‐Clerc et al., 2006); this suggests that CB1 and CB2 receptors may have opposing effects in peripheral tissues. However, the detailed function of CB receptors in clozapine‐induced cardiotoxicity awaits to be documented.
In the present study we aimed to assess the cardiotoxicity of clozapine in a murine model and investigate the functional role of CB receptors in clozapine‐induced myocardial injury in vivo and in vitro. To determine whether the CB receptors have distinct effects on clozapine cardiotoxicity, dual CB receptor antagonists or agonists were not adopted. Instead, we used a selective antagonist/agonist of each receptor alone or in combination with clozapine treatment. Our data suggest that the CB receptors have opposing effects on the cardiotoxicity induced by the antipsychotic clozapine and indicate that pharmacological therapeutics against drug‐induced cardiotoxicity might need to be receptor specific with regarding to CB receptors.
2. METHODS
2.1. Animal experiments
The experiments were performed using male BALB/cByJSlac mice (~6 weeks old, MGI: 6272006), which were introduced from the Jackson Laboratory to Shanghai Laboratory Animal Centre (Shanghai, China). Animals were housed in cages in a climate‐controlled environment consisting of a 12‐hr light and dark cycle and had continuous access to standardized chow and tap water. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology. All experimental procedures were conducted with the protocols for animal experiments approved by the Institutional Animal Care Committee at the School of Basic Medical Sciences, Fudan University. In particular, humane endpoints were implemented in this study involving animals based on the National Guideline for Replacement, Refinement and Reduction of Animals in Research in China (GB/T 27416‐2014). Generally, for the detail of animal experiments in this study, humane endpoints were based on different categorizing indicators including (a) clinical and behavioural signs (i.e., dullness or no movement in response to handling) and (b) pathophysiological changes (i.e., dramatic drop in body temperature and weak respiration rate and heart beat).
For evaluation of mice survival under clozapine treatment, animals were grouped as vehicle‐treated, clozapine (25 mg·kg−1)‐treated, and clozapine (35 mg·kg−1)‐treated (n = 10 per group). For each group, an aliquot of 0.1 ml clozapine solution was i.p. injected at 1:00 p.m. each day. The number of dropout mice was recorded on a daily basis.
To evaluate the effects of CB receptor activation/inactivation on the histopathological changes and heart function of mice, treatments with the CB1 agonist arachidonyl‐2‐chloroethylamide (ACEA; 5 mg·kg−1), CB1 antagonists rimonabant (4 mg·kg−1) and AM281 (2.5 mg·kg−1), CB2 agonists AM1241 (50 mg·kg−1) and JWH‐133 (2 mg·kg−1), or CB2 antagonist AM630 (5 mg·kg−1) were started 1.5 hr (0.1 ml per mouse) before the clozapine injection and continued i.p. each day until the haemodynamic measurements. Mice were killed after 7 or 14 consecutive days of treatments as indicated. After excision of hearts, heart weight was measured to calculate the ratio of heart weight to body weight on freshly excised hearts. Hearts were dried on a paper towel for 1 min before they were weighed. All efforts were made to minimize suffering of the mice. The doses for CB receptor antagonists/agonists were basically similar to or less than those used previously, as reported in the literature (Mukhopadhyay et al., 2007). For rimonabant, a dose of 10 mg·kg−1 i.p. was reported to cause significant weight loss of obese mice at (Bennetzen, Nielsen, Richelsen, & Pedersen, 2008). We tested the lower dose of 4 mg·kg−1 and found this lower dose caused no significant weight change but tended to improve mice survival (Figure S1a). For AM1241, a previous study used the dose of 20 mg·kg−1 in an infarcted mouse model (Wang et al., 2014). We initially tested this dose and found that the beneficial effects of AM1241 at this dose were not significant. Therefore, we used the dose of 50 mg·kg−1 in concurrent with the clozapine treatments in vivo.
2.2. Echocardiography measurements
Echocardiography was performed on the 10th day after daily injection. Prior to the measurements, mice were anaesthetized with 2% isoflurane. The left ventricle was imaged in the short‐axis view at the midpapillary muscle level, and M‐mode measurements of left ventricular end‐diastolic diameter (LVEDd) and left ventricular end‐systolic diameter (LVESd) were recorded and transferred online to a computer for analysis. Ejection fraction (EF) was measured offline by the Teichholz method (Andren et al., 1995).
2.3. Histopathological examination
Hearts were dissected and cut into two halves in a coronal plane, with one half immediately snap‐frozen for subsequent assays. Ventricles of the second half were used for histological and immunohistochemistry (IHC) studies. The Immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology.
Heart tissues were initially fixed in a 10% neutral formalin solution, then embedded in paraffin, sectioned at a thickness of 4 μm, and stained with haematoxylin and eosin (HE) to examine inflammatory infiltrates. For the purpose of this study, myocarditis was defined as ≥1 collection of inflammatory cells with each collection a minimum of 10 cells. The inflammatory infiltrates were classified in terms of the degree of cellular infiltration and graded on a 5‐point scale ranging from 0 to 4+ (Matsumori, Wang, Abelmann, & Crumpacker, 1985) with minor modifications. A zero score indicated no or the questionable presence of lesions in each category. A 1+ score described a limited focal distribution of myocardial lesions. A 2+ to 3+ score described intermediate severity with multifocal lesions, whereas a 4+ score described the presence of coalescent and extensive lesions over the entire heart tissue examined. In circumstances of sparse infiltration, the lesion was scored as 4+. Inflammation was scored in a blinded manner by two independent pathologists and measured as the numbers of lesions tabulated. The intensity of the inflammatory infiltration was quantitatively assessed using an infiltration index, which was calculated as the infiltration score without controversy.
Fibrotic lesions were examined by Sirius Red staining. After Sirius Red staining, the fibrosis area was automatically calculated in proportion to the area of the slice by ImageJ software (National Institutes of Health, Bethesda, MD, USA). Five fields were randomly selected for each slide, and the fibrotic lesions in each group were then averaged.
IHC analysis was performed using formalin‐fixed and paraffin‐embedded mice hearts. Briefly, slides were initially subjected to antigen retrieval by heating them in a microwave at 100°C for 10 min in 0.1 M citric acid buffer (pH = 6.0) and they were then incubated with corresponding antibodies at 4°C overnight. After secondary antibody incubation at room temperature for 1 hr, the slides were developed in 0.05% diaminobenzidine containing 0.01% hydrogen peroxidase.
2.4. Enzyme‐linked immunosorbent assay
Plasma samples from animal experiments were subject to detection of IL‐1β and cardiac troponin I (cTnI) using elisa. The concentrations of IL‐1β were measured using the mouse IL‐1β elisa kit (Catalogue No. MEC1010, Anogen Biotech., Mississauga, Ontario, Canada), and cTnI was determined using mouse troponin I elisa kit (Catalogue No. CTNI‐1‐HSP, Life Diagnostics Inc., West Chester, PA, USA). The protocol for each elisa test was in accordance with the manufacturers' instructions.
2.5. Determination of activities of myocardial injury markers
The activities of major myocardial injury markers, creatine kinase (CK), LDH, and aspartic transaminase (AST), were estimated in supernatants of cultured cardiomyocytes using a diagnostic kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Supernatants were harvested 24 hr after drug treatments. The absorbance at 660 nm was measured spectrophotometrically to calculate CK levels (Catalogue No. A032). The absorbance was detected for LDH (Catalogue No. A020) and AST (Catalogue No. C010), respectively, on the basis of microplate tests.
2.6. Immunofluorescence analysis
Mouse HL‐1 cardiomyocytes were cultured in 24‐well plates and received clozapine treatments at a final concentration of 40 μM. After a 24‐hr incubation, cells were fixed with acetone for 10 min and washed with cold PBS three times. After being blocked with goat serum for 1 hr, cells were co‐incubated with primary antibodies at 4°C overnight. An Alexa Fluor goat anti‐rabbit IgG was included as the secondary antibody. DAPI was used as a counterstain at a dilution of 1:1,000 for 10 min at room temperature. As negative controls, the primary antibodies were changed into the nonimmune serum from the same species.
2.7. Western blot analysis
Total proteins were extracted from cells or tissue homogenates using RIPA containing protease inhibitor and phosphatase inhibitor cocktail (Calbiochem, EMD Biosciences, San Diego, CA, USA). The protocol used for Western blot analysis was in accordance with our previous study (Li et al., 2016). Briefly, protein was measured by a BCA kit (Pierce, San Francisco, CA, USA), and an equal amount of protein (40 ng) was loaded on 10% SDS‐PAGE gel and transferred onto a PVDF membrane (pore size 0.45 μm, Bio‐Rad, Hercules, CA, USA) using a semidry transfer apparatus (BioRad). The blots were blocked with freshly prepared 5% skimmed milk for 1 hr and incubated with primary antibodies overnight at 4°C. For each primary antibody, this study used tris‐buffered saline containing 0.1% Tween 20 as the diluting solution. The final dilution was 1:1,000 for the primary CB1 antibody and was 1:500 for the CB2 antibody. Membranes were then incubated with corresponding secondary antibodies (1:2,000 dilution in tris‐buffered saline containing 0.1% Tween 20), and the immunoreactivity was detected using enhanced chemiluminescent autoradiography (ECL kit, Amersham, Pittsburgh, PA, USA). GAPDH was synchronously developed as a loading control.
2.8. Quantitative real‐time PCR
Total RNAs from cells or heart tissue homogenates were extracted using TRIzol solution (Invitrogen). After assessment of RNA quality and concentration, an equal amount of RNA (500 ng) was reverse transcribed into cDNA using the HiScript II Q RT SuperMix (Vazyme, Nanjing, China). Quantitative real‐time PCR was performed in an ABI PRISM 7500 Real‐Time System using the AceQ qPCR SYBR Green Master Mix (Vazyme, Nanjing, China). Primers used are listed in Table 1.
Table 1.
Primers used in the qRT‐PCR analysis
| Gene name | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Faah | GAGGCTCCCCTCTGGGTTTA | GCCAGGCTATCCACATCCC |
| Magl | CTTGCCCGTAACCCATCAA | CAACGGGTGGGATTACCTTAC |
| Il‐1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
| Tnf‐α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| Il‐6 | CCAAGAGGTGAGTGCTTCCC | CTGTTGTTCAGACTCTCTCCCT |
| Il‐10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| Gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Note. qRT‐PCR: quantitative real‐time PCR.
2.9. Measurement of endocannabinoid levels by LC/MS/MS
HL‐1 cells were incubated in six‐well plates in CO2 incubator and treated with clozapine at the doses indicated for 12 hr when cells were ~80% confluence per well. Following lipid extraction of cultured cells or whole‐blood samples, the levels of AEA and 2‐AG were quantified by LC/MS/MS as described previously (Dong, Li, Ye, Zheng, & Jiang, 2016).
2.10. Statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. Data are expressed as mean ± SEM. Student's t‐test or one‐way ANOVA was used to compare means of continuous variables where appropriate by SPSS version 16.0 (RRID:SCR_002865; Chicago, IL, USA). If any statistically significant difference was detected, post hoc comparisons were performed using the least significant difference test. The survival analysis was performed by the Kaplan–Meier method and evaluated by means of log‐rank (Mantel–Cox) test. Results yielding two‐tailed values of P < 0.05 were considered statistically significant.
2.11. Materials
Primary rabbit monoclonal antibody against CB1 receptor (RRID:AB_2756361) was purchased from Cell Signaling Technology (Catalogue No. 93815, Boston, MA, USA). Primary rabbit polyclonal anti‐CB2 receptor antibody (RRID:AB_374359) was purchased from Gene Tex Inc. (Catalogue No. GTX23561, Irvine, CA, USA). An HRP‐linked secondary antibody (RRID:AB_628497) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Goat anti‐rabbit secondary antibody Alexa Fluor 555 (Catalogue No. A‐21428, RRID:AB_2535849) and Alexa Fluor 488 (Catalogue No. A‐11008, RRID:AB_143165) were purchased from Invitrogen Inc. (Carlsbad, CA, USA).
Mouse cardiac muscle HL‐1 cells (RRID:CVCL_0303) that derived from mouse atria and contract and retain phenotypic characteristics of the adult cardiomyocyte were obtained from Millipore (Burlington, MA, USA). Cells were cultured in DMEM (Gibco, Invitrogen) containing 10% FBS (Invitrogen), 100 U·ml−1 penicillin, and 100 μg·ml−1 streptomycin at 37°C in a humidified atmosphere of 5% CO2 as we previously described (Li et al., 2014).
2.12. Solutions and chemicals
Antipsychotic clozapine (PubChem CID: 2818) was purchased from Sigma‐Aldrich (St. Louis, MO, USA). Clozapine was dissolved in 0.1 M HCl and pH balanced in PBS to make a stock solution of clozapine (80 mM). The stock solution was then diluted on the basis of use dosage. A selective CB1 antagonist rimonabant (PubChem CID: 104850) and selective CB2 antagonist AM630 (PubChem CID: 4302963) were commercially obtained from Sigma‐Aldrich. A selective CB1 antagonist AM281 (PubChem CID: 4302962) and selective agonist arachidonyl‐2′‐chloroethylamide (ACEA; PubChem CID: 5311007) were purchased from APExBio Technology (Boston, MA, USA) and Tocris Bioscience (Abingdon, Oxfordshire, UK) respectively. Another CB2 agonist JWH‐133 (PubChem CID: 6918505) was purchased from MedChemExpress (Monmouth Junction, NJ, USA). ACEA, rimonabant, and AM630 were prepared as 1, 0.6, and 1 mg·ml−1 working solutions, respectively, in solvents consisting of DMSO, Tween 20, and PBS. A selective CB2 agonist AM1241 (PubChem CID: 10141893) was purchased from Selleck Chemicals (Houston, TX, USA) and prepared as 10 mg·ml−1 working solution in solvents consisting of DMSO and PBS. The highest final concentration of DMSO in external solution was ≤1%, a concentration that had no effect on the survival of mice.
2.13. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
3. RESULTS
3.1. Clozapine induced myocardial injury in a dose‐dependent manner
The maintenance dose of clozapine is approximately 200 mg·day−1 for adults with a clinical prescription. It has also been reported that the dose of clozapine in clinical cases with cardiovascular complications ranges from 100 to 725 mg daily (Kilian et al., 1999). The present study, therefore, adopted two doses of clozapine (25 and 35 mg·kg−1), which were comparable with clinical dosage after conversion from human beings to mice. Initially, we also used another dose of 15 mg·kg−1 together with 25 and 35 mg·kg−1 for respective i.p. injection and found that the dose of 15 mg·kg−1 did not evoke any marked histopathological and functional changes as compared with vehicle‐treated mice (data not shown). Hence, only 25 and 35 mg·kg−1 of clozapine were used in the present study.
We initially assessed the effects of clozapine dosage on mice survival over a 28‐day period. It was found that mice death occurred only in the initial 14 days after starting clozapine. Mice died as early as 3 days after clozapine initiation (35 mg·kg−1), reflecting an acute drug reaction. Compared with vehicle‐injected mice, the loweer dose of clozapine (25 mg·kg−1) caused one dropout on Day 10 (10% dropout rate). The high dose of clozapine (35 mg·kg−1) caused remarkable mortality, four deaths on Day 4 and one each on Days 6, 8, and 11, respectively (Figure 1a). The ratio of heart weight to body weight (HW/BW) was dose‐dependently increased regardless of dosing duration (Figure 1b). Determination of cTnI, a sensitive serum biomarker of myocyte injury, showed that clozapine injection significantly elevated the levels of cTnI, particularly after 14 days of dosing of 35 mg·kg−1, which made up a 45.4% increase as compared with control mice (Figure 1c). Detection of serum pro‐inflammatory factor IL‐1β showed that the treatment with clozapine resulted in an initial decrease in IL‐1β levels, which mirrored the endogenous anti‐inflammatory reaction seen early on after exposure to clozapine. However, the higher dose of clozapine (35 mg·kg−1) significantly increased the serum level of IL‐1β as compared with 25 mg·kg−1 after 14 days (Figure 1d). Moreover, total RNAs were extracted from heart tissues, and qRT‐PCR analysis showed that the transcriptional levels of pro‐inflammatory factors (TNF‐α, IL‐6, and IL‐1β) were enhanced by the clozapine treatments (Figure 1e–g), whereas that of the anti‐inflammatory factor (IL‐10) was decreased significantly by clozapine regardless of low or high dose (Figure 1h). These data suggest that clozapine caused the mice to die and heart dysfunction in a dose‐dependent manner.
Figure 1.
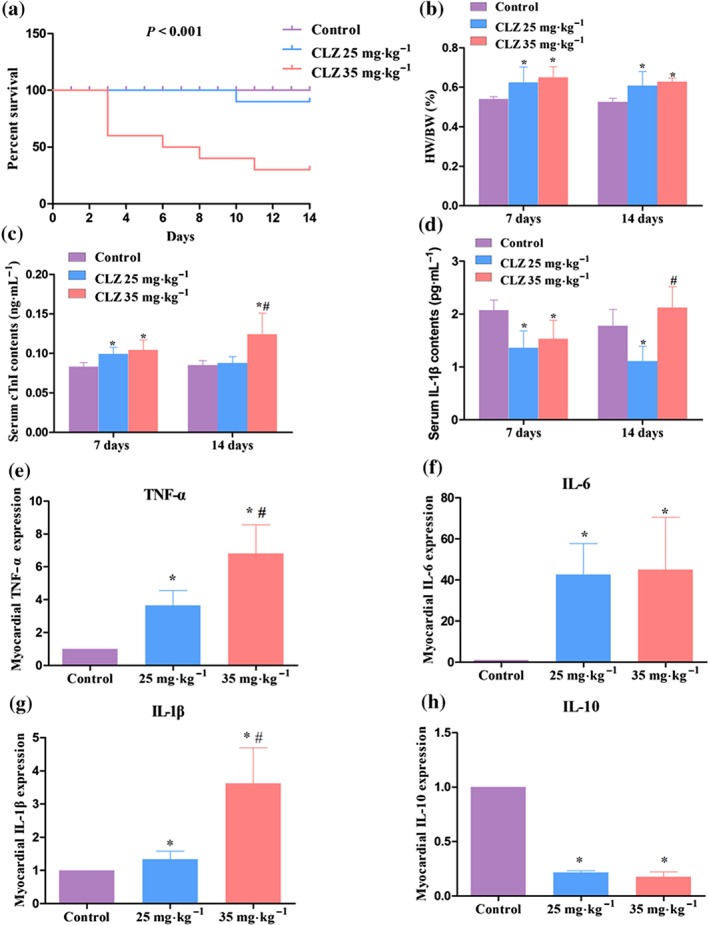
Clozapine (CLZ) induced myocardial injury in a dose‐dependent manner. (a) Mice survival curve was established after 14 days of injection of vehicle (PBS, for control), 25 mg·kg−1 CLZ, and 35 mg·kg−1 CLZ (n = 10 per group). The P value after Kaplan–Meier analysis of survival proportion is indicated. (b) Effects of CLZ treatment on the ratio of heart weight to body weight (HW/BW). (c, d) The serum levels of myocyte injury marker cardiac troponin I (cTnI) and pro‐inflammatory factor IL‐1β were determined using elisa kits. (e–h) Mice hearts were dissected, and total RNAs were extracted for qPCR analysis. The transcriptional activities of pro‐inflammatory factors TNF‐α, IL‐6, and IL‐1β and anti‐inflammatory factor IL‐10 were examined in mice hearts with distinct treatments. n = 5 per group unless otherwise stated. *P < 0.05 versus control; # P < 0.05, 35 versus 25 mg·kg−1
3.2. Effects of clozapine treatment on myocardial histopathology
It was observed that mouse hearts presented abnormal histopathological features after daily treatment with clozapine for 7 days, when inflammation was focally distributed (HE staining) and fibrotic proliferation was observed in the perivascular area or in the myocardial interstitium (Sirius Red staining; Figure 2a). By Day 14, inflammatory infiltrates were intensive and distributed to both subepicardial and endocardial areas. Fibrotic lesions surrounding the inflammation areas were also apparent (Figure 2b). The infiltration index of clozapine‐treated hearts was significantly higher than that of the control hearts on Day 7 and Day 14. Higher dose of clozapine tended to induce severer infiltration on both Day 7 and Day 14 (Figure 2c). Although the fibrotic lesion area was minimal, it was significantly increased in clozapine‐treated hearts regardless of duration (Figure 2d). These observations suggest that clozapine treatment induces changes in myocardial histopathology.
Figure 2.
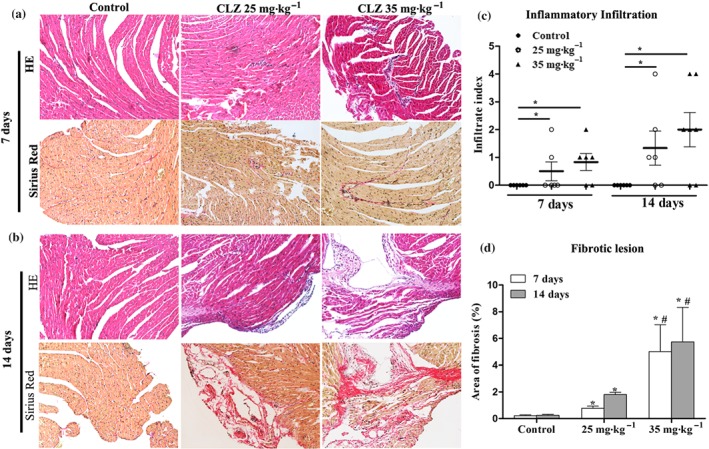
Clozapine (CLZ) treatment induced myocardial histopathological changes. (a, b) Hearts with distinct doses of CLZ were harvested on Day 7 and Day 14, respectively, and subject to haematoxylin and eosin (HE) staining (inflammation infiltration) and Sirius Red staining (fibrotic lesion). Magnification: 200×. (c) Infiltration index of inflammation in each group of mice is shown (n = 6–7 per group). (d) The fraction of fibrotic lesion (%) was calculated using ImageJ software (n = 5 per group). *P < 0.05 versus control; # P < 0.05, 35 versus 25 mg·kg−1
3.3. Effects of clozapine on the endocannabinoid system
LC/MS/MS detection showed that the mouse serum levels of AEA and 2‐AG, two major components of endocannabinoids, were dose‐dependently decreased on both Day 7 and Day 14 (Figure 3a,b). In the cultured cardiomyocytes, it was also confirmed that the myocardial level of 2‐AG decreased in parallel with the increased dose of clozapine (Figure 3c). Interestingly, cannabinoid CB1 receptors were mainly distributed in the cytomembrane before the initiation of the clozapine treatment, whereas CB2 receptors were largely distributed in the nuclei and cytoplasm of intact cardiomyocytes. After 24‐hr exposure to clozapine, CB1 receptors translocated to nuclei and cytoplasm, and CB2 receptors, intriguingly, translocated to the cytomembrane (Figure 3d). It was also noted that the cardiomyocytes seemed to be undergoing cell apoptosis after 24‐hr of clozapine exposure, as demonstrated by chromatin consolidation in DAPI counterstaining (Figure 3d, upper panel at 24 hr). To confirm the inverse translocation of CB receptors, we performed IHC analysis of CB1 and CB2 receptors in the mouse heart tissues. It was observed that the CB1 receptor was present at the myocardial membrane (blue arrow) in control heart and was located mainly in nuclei and cytoplasm in clozapine‐treated heart (red arrow, Figure 3e, 400×). In contrast, the CB2 receptor was mostly distributed in the cytoplasm and nuclei in control heart (red arrow), whereas it was intensively stained in the cytomembrane in clozapine‐treated hearts (blue arrow, Figure 3e, 400×). Of note, the overall staining intensity of CB1 receptors was markedly decreased, whereas that of CB2 receptors increased after continuous clozapine treatments (Figure 3e, 20×). Immunoblotting also showed that the protein level of myocardial CB1 receptors was gradually decreased with extended clozapine treatment, whereas that of CB2 receptors was time‐dependently increased (Figure 3f, upper panel). The protein level of CB1 receptors was further decreased by the higher dose of clozapine, which contrasted with that of CB2 receptors that was increased in response to the higher dose of clozapine (Figure 3f, lower panel). qPCR analysis of myocardial FAAH and MAGL, two genes that encode enzymes that catalyse the hydrolysis of AEA and 2‐AG, respectively, showed that the transcriptional levels of FAAH and MAGL were consistently enhanced by the clozapine treatments (Figure 3g). These data suggest that clozapine imbalances the endocannabinoid system and causes opposite effects on myocardial CB receptors.
Figure 3.
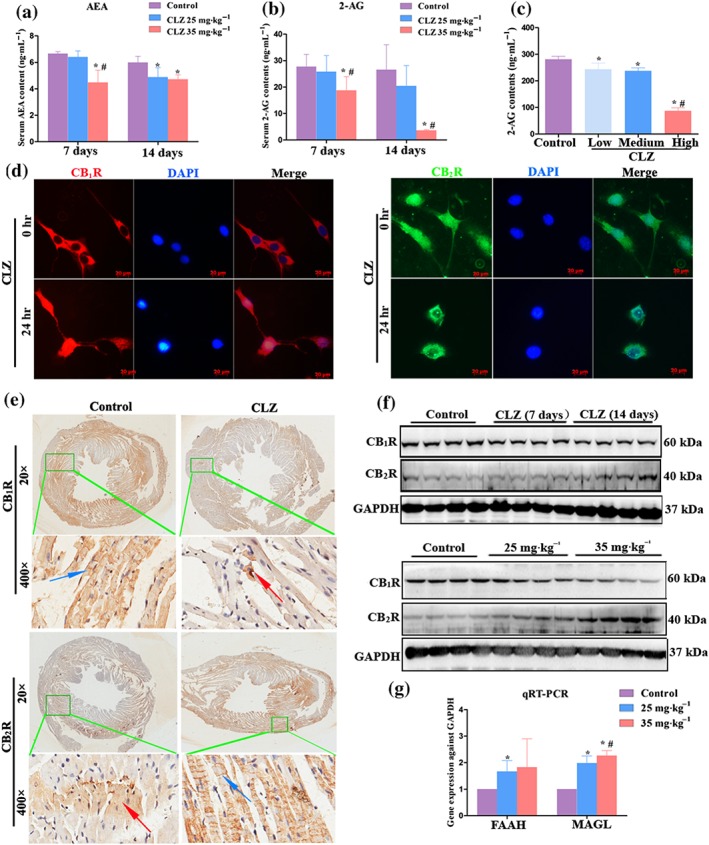
Clozapine (CLZ) imbalanced the endocannabinoid system and caused opposite effects on myocardial cannabinoid receptors. (a, b) The serum levels of anandamide (AEA) and 2‐arachidonoylglycerol (2‐AG), the two main components of endocannabinoids, were determined for each group of mice (n = 5 per group) by LC/MS/MS analysis. (c) Mouse cardiomyocyte HL‐1 cells were cultured with (CLZ group) or without CLZ treatment (control group), and the contents of 2‐AG in each group of cells were determined by LC/MS/MS analysis. The low, medium, and high doses of CLZ were 10, 40, and 160 μM respectively. (d) Immunofluorescence assay was performed to detect the location of CB1 receptors (red) and CB2 receptors (green) in both intact (0 hr) and CLZ‐exposed cardiomyocytes (24 hr). DAPI (blue) was used as a counterstain to show cell nuclei. Scale bar = 20 μm. (e) Immunohistochemistry analysis of cannabinoid receptors in mice hearts with or without CLZ treatments. Low magnification images (20×) showed the overall staining intensity of each marker (CB1 or CB2 receptor), and the green box‐marked regions were further magnified (400×) to show the subcellular location of each marker under control or CLZ treatments. Blue and red arrows indicated membrane and cytoplasm location respectively. (f) Western blot analysis of CB1R and CB2R in the myocardial tissues. Upper panels demonstrated the protein levels of CB1R and CB2R in control hearts, 7‐day CLZ‐treated hearts, and 14‐day CLZ‐treated hearts. The lower panels demonstrate the protein levels of CB1 and CB2 receptors in control heats, low dose (25 mg·kg−1) CLZ‐treated heats, and high dose (35 mg·kg−1) CLZ‐treated hearts. (g) qPCR analysis of myocardial FAAH and MAGL in distinct group of mice (n = 6 per group). *P < 0.05 versus control; # P < 0.05, 35 versus 25 mg·kg−1
3.4. CB1 receptor antagonists or CB2 receptor agonists protected against clozapine‐induced myocardial injury
In the clozapine‐treated mice (35 mg·kg−1), it was observed that pretreatment with rimonabant, a selective antagonist of CB1 receptors, attenuated the histopathological changes on both Day 7 (Figure 4a) and Day 14 (Figure 4b). In particular, the myocardial infiltration index was decreased after 7 days of rimonabant pretreatment, and this protective effect continued on Day 14 (Figure 4c). The area of fibrotic lesion was also decreased significantly on each monitored time point (Figure 4d). Accordingly, the serum levels of cTnI (Figure 4e) and IL‐1β (Figure 4f) were decreased by rimonabant pretreatments, consistent with an ameliorative effect on myocardial impairment. The protective effects of rimonabant were comparable between Day 14 and Day 7, implicating no time‐dependence of this effect. Likewise, AM1241, a selective agonist of CB2 receptors, markedly attenuated the clozapine‐induced histopathological changes on both Day 7 (Figure 4g) and Day 14 (Figure 4h). The infiltration index was significantly decreased after activation of CB2 receptors by AM1241 on Day 14 (Figure 4i). Fibrotic lesions were significantly attenuated by AM1241 pretreatments on both Day 7 and Day 14 (Figure 4j). Consistent with the histopathological examination, serum levels of cTnI (Figure 4k) and IL‐1β (Figure 4l) were accordingly decreased to different extents, mirroring the milder heart dysfunction after activation of CB2 receptors. Of note, pretreatments with either rimonabant or AM1241 tended to decrease the clozapine‐induced mortality (Figure S1a), although these differences did not reach statistical significance. AM1241, but not rimonabant pretreatment, significantly lowered the HW/BW values as compared with clozapine‐treated mice (Figure S1b).
Figure 4.
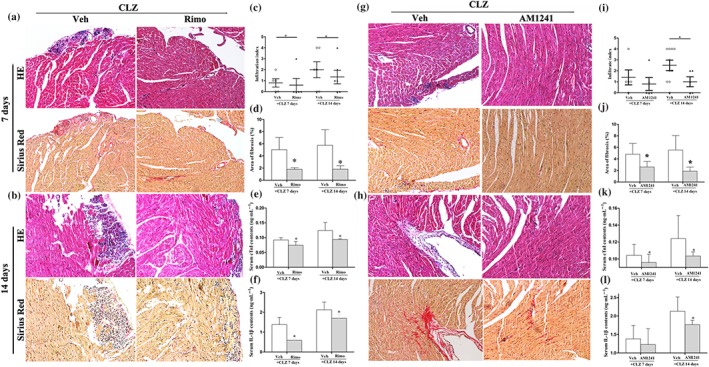
Selective CB1 antagonist (rimonabant) or CB2 agonist (AM1241) protected against clozapine (CLZ)‐induced myocardial injury. (a, b) Mice were pretreated with rimonabant (Rimo, 4 mg·kg−1) prior to daily injection of CLZ (35 mg·kg−1). Hearts were harvested on Day 7 and Day 14 for histopathological examination. Magnification: 200×. (c, d) Infiltration index and area fraction of fibrotic lesions are quantitatively shown in mice with or without rimonabant (Rimo) pretreatment (n = 5–6 per group). (e, f) The serum levels of cTnI and IL‐1β were detected in Rimo‐pretreated mice using elisa kits (n = 5 per group). (g, h) Mice were pretreated with AM1241 (50 mg·kg−1) prior to daily injection of CLZ (35 mg·kg−1). Hearts were harvested on Day 7 and Day 14 for histopathological examination. Magnification: 200×. (i, j) Infiltration index and area fraction of fibrotic lesions were quantitatively shown in mice with or without AM1241 pretreatment (n = 5–10 per group). (k, l) The serum levels of cTnI and IL‐1β were detected in AM1241‐pretreated mice using elisa kits (n = 5 per group). Representative images for haematoxylin and eosin (HE) staining and Sirius Red staining were from serial slices. Mice with vehicle (Veh) PBS pretreatments served as control in each experiment. *P < 0.05 versus Veh group in each category
To determine whether the above beneficial effects were ligand‐specific, another selective CB1 receptor antagonist (AM281) and CB2 receptor agonist (JWH‐133) was additionally used. It was observed that pretreatment with either AM281 or JWH‐133 markedly attenuated the clozapine‐mediated pathological lesions as evidenced by the HE staining and Sirius staining (Figure 5a). The infiltration index was also significantly decreased by either additional ligand (Figure 5b). Furthermore, pretreatment with AM281 or JWH‐133 attenuated the clozapine‐mediated fibrogenesis (Figure 5c). As a reflection of myocardial injury, serum cTnI levels were significantly decreased by both AM281 and JWH‐133 (Figure 5d). Together with the histology and biochemical results, all these observations suggest that selective CB1 antagonists or CB2 receptor agonists protect against clozapine‐induced myocardial injury.
Figure 5.
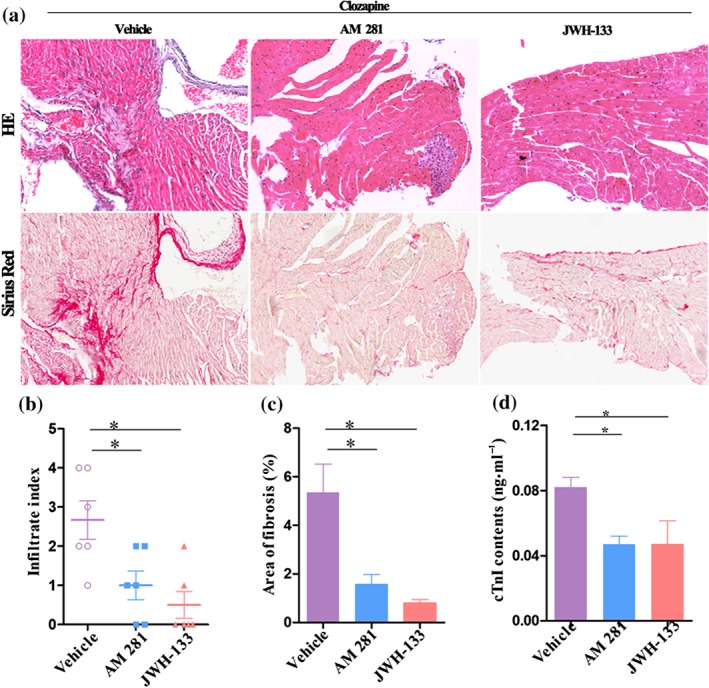
The addition of a CB1 antagonist (AM281) or CB2 agonist (JWH‐133) exerted beneficial effects on clozapine‐medaited cardiotoxicity. (a) Mice were pretreated with AM281 (2.5 mg·kg−1) or JWH‐133 (2 mg·kg−1) prior to daily injection of clozapine (35 mg·kg−1). Hearts were harvested on Day 14 for histopathological examination. Magnification: 200×. (b, c) Infiltration index and fraction of fibrotic lesions were quantitatively calculated in each group (n = 6 per group). (d) The serum levels of cTnI were detected in each group of mice using elisa kits (n = 5 per group). Mice with vehicle (PBS) pretreatments served as control in each experiment. Representative images for haematoxylin and eosin (HE) staining and Sirius Red staining were from serial slices. *P < 0.05 versus vehicle
3.5. CB1 receptor agonist or CB2 receptor antagonist worsened clozapine‐induced myocardial injury
In a second experiment, clozapine‐treated mice were pretreated with a selective CB1 receptor agonist (ACEA) or CB2 receptor antagonist (AM630). We pre‐injected ACEA (a selective agonist of CB1 receptor) into mice, which subsequently received clozapine (25 mg·kg−1) and found that the histopathological changes were exacerbated after 14 days (Figure 6a). The infiltration index and fibrotic lesions were progressively increased by the ACEA pretreatment (Figure 6b,c). Also the ACEA pretreatment caused significantly increased mortality (Figure S1c), although it did not affect the HW/BW ratios (Figure S1d). As biomarkers reflecting myocardial injury, the serum levels of cTnI and IL‐1β were accordingly enhanced in ACEA‐pretreated mice (Figure 6d,e). Likewise, AM630, a selective CB2 receptor antagonist, markedly exacerbated the clozapine‐induced myocardial pathology on Day 14 (Figure 6f). The histopathological results were further confirmed by quantitative analysis of infiltration index (Figure 6g) and fibrotic lesions (Figure 6h) in each group of mice. In particular, co‐treatments of AM630 and clozapine even caused a dramatic increase in fibrotic lesion area to approximate 15%, which represented a seven to eightfold change as compared with single clozapine treatment on Day 14 (Figure 6h). As a consequence, the serum levels of cTnI (Figure 6i) and IL‐1β (Figure 6j) were dramatically elevated by AM630 pretreatments. The effect of AM630 pretreatments on mortality of the mice seemed to be similar to that of clozapine (Figure S1c), and the HW/BW ratio was not significantly affected by AM630 either (Figure S1d). All these findings suggest that activation of CB1 receptors exacerbate, whereas activation of CB2 receptors attenuate myocardial injury.
Figure 6.
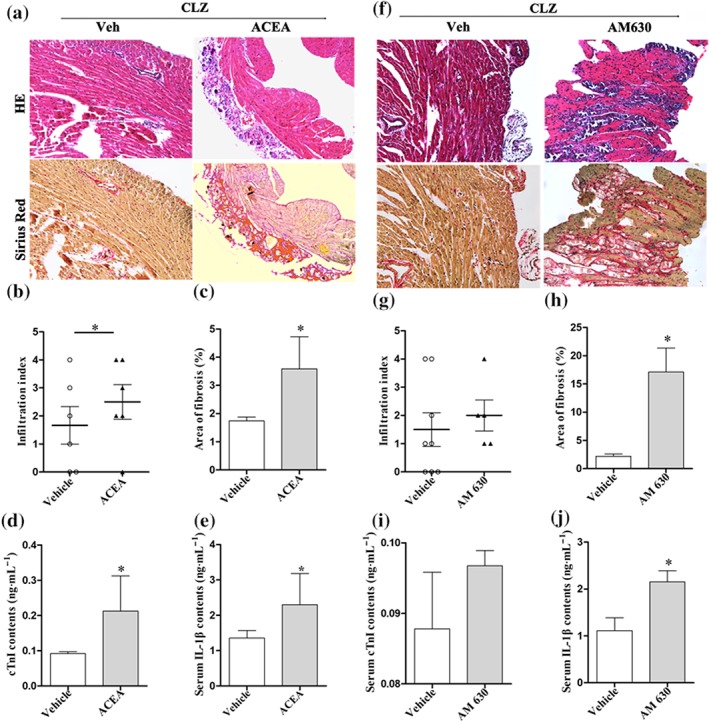
A selective CB1 agonist (ACEA) or CB2 antagonist (AM630) exacerbated clozapine‐induced myocardial injury. (a) Mice were pretreated with ACEA (5 mg·kg−1) prior to daily injection of clozapine (25 mg·kg−1). Hearts were harvested on Day 14 for histopathological examination. Magnification: 200×. (b, c) Infiltration index and fraction of fibrotic lesions were quantitatively compared in mice with or without ACEA pretreatments (n = 6 per group). (d, e) The serum levels of cTnI and IL‐1β were detected in ACEA‐pretreated mice using elisa kits (n = 5 per group). (f) Mice were pretreated with AM630 (5 mg·kg−1) prior to daily injection of clozapine (25 mg·kg−1). Hearts were harvested on Day 14 for histopathological examination. Magnification: 200×. (g, h) Infiltration index and fraction of fibrotic lesions were quantitatively analysed in mice with or without AM630 pretreatments (n = 5–8 per group). (i, j) The serum levels of cTnI and IL‐1β were detected in AM630‐pretreated mice using elisa kits (n = 5 per group). Mice with vehicle (Veh, PBS) pretreatments served as control in each experiment. Representative images for HE staining and Sirius Red staining were from serial slices. *P < 0.05 versus Veh group in each category
3.6. CB1 receptor antagonists or CB2 receptor agonists attenuated clozapine‐induced cardiac dysfunction in vivo
We also analysed left ventricle function on Day 10 after drug initiation. Clozapine treatments were observed to induce significant changes in left ventricle function with higher doses leading to severe heart dysfunction. More importantly, rimonabant and AM281 could significantly attenuate clozapine‐induced changes in LVEDd, LVESd, and EF. The selective CB1 agonist, ACEA, in contrast, aggravated clozapine‐induced left ventricle dysfunction (Figure 7a–c). It was further observed that the CB2 receptor agonists AM1241 and JWH‐133 also blunted clozapine‐induced left ventricle dysfunction as measured by LVEDd, LVESd, and EF parameters (Figure 7d–f). Whereas the CB2 receptor antagonist AM630 significantly worsened the measurements of LVESd (Figure 7e) and EF (Figure 7f). All these in vivo results suggest that CB1 receptor antagonists and CB2 receptor agonists might be protective against clozapine‐induced cardiac dysfunction.
Figure 7.
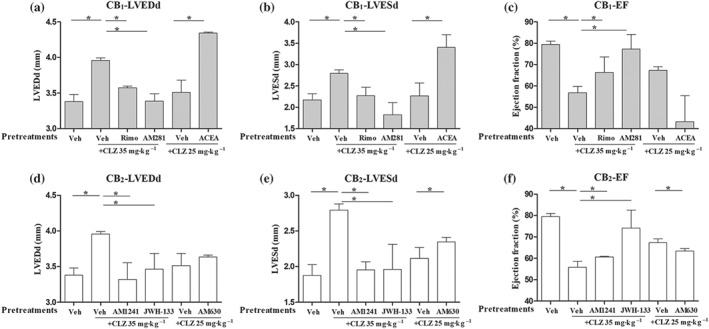
CB1 antagonists or CB2 agonists improved clozapine (CLZ)‐induced cardiac dysfunction in vivo. (a–c) Echocardiography measurements of left ventricle end‐diastolic diameter (LVEDd), left ventricle end‐systolic diameter (LVESd), and ejection fraction (EF) in mice pretreated with CB1 antagonists (rimonabant [Rimo] and AM281) or agonist (ACEA). (d–f) Echocardiography measurements of LVEDd, LVESd, and EF in mice pretreated with CB2 antagonists (AM630) or agonists (AM1241 and JWH‐133). Mice were grouped as indicated. n = 5 per group unless otherwise stated. Veh, vehicle (PBS). *P < 0.05 versus Veh group as indicated
3.7. Pharmacological modulation of cannabinoid receptors protected against clozapine‐induced cardiomyocyte injury in vitro
As shown in Figure S2, single treatments with clozapine significantly decreased the level of 2‐AG, consistent with the observation in Figure 3c. More importantly, pretreatments with either selective CB1 receptor antagonists or CB2 receptor agonists significantly blunted clozapine‐mediated decrease in 2‐AG. Combined pretreatments with a CB1 receptor antagonist and CB2 receptor agonist further increased the 2‐AG contents in HL‐1 cells, confirming the beneficial effects of CB1 receptor antagonists or CB2 receptor agonists on cardiomyocytes. In addition, HL‐1 cardiomyocytes were pretreated with rimonabant and/or AM1241 for 30 min prior to clozapine exposure for 24 hr. Cell supernatants were harvested for biochemical detection. It was found that clozapine exposure caused significant increases in IL‐1β levels. Pretreatment with either rimonabant or AM1241 blocked partially the clozapine‐mediated effects on IL‐1β levels, and a combination of rimonabant and AM1241 further decreased the IL‐1β levels (Figure 8a). In addition, determination of cardiomyocyte injury markers also suggested that single clozapine treatment increased significantly the levels of LDH and AST, two biomarkers of cardiomyocyte injury, but this effect was blocked by either rimonabant or AM1241 pretreatment and further blunted by combined pretreatments (Figure 8b,c). Combined pretreatments of rimonabant and AM1241 even decreased the LDH levels to the basal level (Figure 8b). Similarly, the levels of CK in rimonabant pretreatment alone or together with AM1241 were comparable with the basal level (Figure 8d). These data suggest that pharmacological inhibition of CB1 receptors and/or activation of CB2 receptors ameliorate the inflammatory injury of cardiomyocytes mediated by clozapine.
Figure 8.
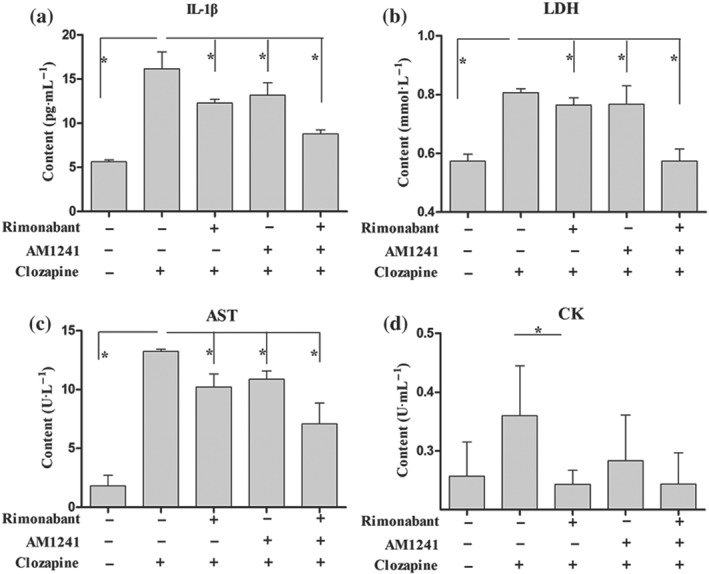
Pharmacological modulation of CB1/CB2 receptors protected against clozapine‐induced cardiomyocyte injury in vitro. Cardiomyocytes were pretreated with rimonabant (2 μM) and/or AM1241 (5 μM) for 30 min before being treated with clozapine for 24 hr and the supernatants were collected. (a) elisa detection of IL‐1β content in supernatants of each group. (b–d) Activities of cardiomyocyte injury markers LDH, aspartate aminotransferase (AST), and creatine kinase (CK) were estimated in supernatants using diagnostic kits. *P < 0.05 as indicated
4. DISCUSSION
The present study investigated clozapine cardiotoxicity and the CB receptor‐dependent mechanisms. Clozapine, the earliest atypical antipsychotic, is very efficacious in treating psychosis, particularly in patients refractory to other agents (Green et al., 2000). However, one serious health safety concern regarding clozapine stems from its severe cardiotoxicity, which limits its clinical use (Kilian et al., 1999; La Grenade et al., 2001). The present study used doses that were comparable with those prescribed clinically and found that myocarditis could be present as early as 7 days after clozapine administration, which was consistent with a clinical observation reporting clozapine‐induced myocarditis 8 days after starting clozapine (Kilian et al., 1999). The higher dose of clozapine reduced the survival rate of mice and was associated with a worse heart function. Histopathological examination also confirmed that clozapine induced dose‐dependent myocardial inflammation and perivascular or interstitial fibrosis, as evidenced by HE staining and Sirius Red staining. Our observations confirm that clozapine induces cardiotoxicity in a dose‐dependent manner.
Clozapine has unique effects on a variety of receptors in the CNS (Schwieler, Linderholm, Nilsson‐Todd, Erhardt, & Engberg, 2008). It has a strong affinity for D4‐dopamine receptors and is a potent inhibitor of 5‐HT, adrenoceptors, histamine, and choline M2 receptors but with weak D2 receptor activity. Although multiple pathophysiological processes such as oxidative stress (Abdel‐Wahab & Metwally, 2015), pro‐inflammatory cytokines, and DNA damage (Abdel‐Wahab et al., 2014) are implicated in clozapine toxicity, no receptor‐dependent mechanisms that transduce extracellular stress into cellular signalling have been revealed. A recent report has found that blockade of β‐adrenoceptors (one type of GPCR) in myocardium could attenuate clozapine‐induced inflammation in mice (Wang et al., 2008), indicating that a GPCR may underlie the cellular receptor mechanisms of clozapine cardiotoxicity. Interestingly, the authors also found that blockade of β‐adrenoceptors produced only a partial reduction in TNF‐α levels, postulating that there might be other GPCRs that drive the clozapine‐induced pro‐inflammatory state in treated subjects (Wang et al., 2008). Hence, one great novelty of this study is the identification of cannabinoid CB1 and CB2 receptors, a subfamily of GPCRs, as critical mediators of clozapine side cardiac effects both in vivo and in vitro.
Receptors that mediate clozapine toxicity in areas outside of the CNS are rarely reported. The present study observed that clozapine treatment decreased the serum levels of major endocannabinoids in mice and in cultured cardiomyocytes. The majority of evidence suggests that the increases in endocannabinoid levels in cardiac disorders are protective (O'Sullivan, 2015). Therefore, the decreases in endocannabinoid levels by clozapine treatment in the present study confirmed the compromised heart function after clozapine treatment. In addition, the protein levels of CB1 receptor were decreased, whereas that of CB2 receptor increased in response to clozapine treatment in mice myocardium. In the cultured cardiomyocytes, the CB1 receptor was observed to translocate from the cytomembrane in intact cells to cytoplasm and nuclei in clozapine‐treated cells, whereas CB2 receptors translocated from the cytoplasm and nuclei in intact cells to the cytomembrane in clozapine‐treated cells. IHC analysis of heart tissues also confirmed the inverse translocation of CB receptors after clozapine treatment. These observations suggest that clozapine imbalanced the endocannabinoid system.
The majority of evidence indicates that endocannabinoids exert cardioprotective effects through CB2 activation but with a role also for CB1 activation. CB2 receptor activation‐mediated cytoprotective effects were consistent across studies. However, the role of CB1 receptors is controversial because in some situations, CB1 receptor activation may be detrimental in the heart (O'Sullivan, 2015). CB1 receptor knockout mice are more susceptible to a chronic heart failure (Liao et al., 2013). A genetic deficiency of CB1 receptors worsened acute heart failure induced by pressure overload in mice (Liao et al., 2012). Blockade of CB1 receptor by its selective antagonist blocked partially the cardioprotective effect of 2‐AG (Lepicier et al., 2003). The above cardioprotective effects of CB1 receptors were challenged by other findings. For examples, pharmacological inhibition or genetic deletion of CB1 receptors attenuated the diabetes‐induced cardiac dysfunction, oxidative stress, inflammation, and fibrosis (receptorajesh et al., 2012). Inhibition of CB1 receptors improved cardiac function and remodelling after myocardial infarction in experimental model of metabolic syndrome (Slavic et al., 2013). In murine models of doxorubicin‐induced cardiotoxicity, activation of CB1 receptors promoted oxidative stress and cardiomyocyte death (Mukhopadhyay et al., 2010), and pharmacological inhibition of CB1 receptors using its selective antagonists protected against doxorubicin‐induced cardiotoxicity (Mukhopadhyay et al., 2007). These conflicting findings suggest that the role of CB1 receptor activation differs across studies and depends on heart disease models.
The present study found that activation of CB2 receptors by its selective agonists (AM1241 or JWH‐133) significantly improved heart function and attenuated myocardial inflammatory infiltrates and fibrotic lesions, whereas inactivation of CB2 receptors by its selective antagonist AM630 worsened clozapine‐mediated inflammation infiltrates and fibrotic processes. The cardioprotective effects of CB1 antagonists/CB2 agonists seemed to be not ligand‐specific, because both CB1 antagonists (rimonabant and AM281) and CB2 agonists (AM1241 and JWH‐133) induced comparable effects in the in vivo functional assays. In addition, activation of CB2 receptors tended to attenuate clozapine‐mediated deaths and significantly decreased the HW/BW ratios. These observations confirmed the cardioprotective effect of CB2 receptors in heart diseases. The CB1 receptor, however, had opposite effects mediated by CB2 receptor. After activation of CB1 receptors by its selective agonist ACEA, clozapine‐mediated mouse mortality was significantly aggravated. Activation of CB1 receptors caused severer myocardial inflammation and fibrotic lesions, whereas inhibition of CB1 receptors protected cardiomyocytes from clozapine‐induced injury. The cardiodepressive effects of CB1 receptors in clozapine‐induced cardiotoxicity were basically similar to those found in two relevant studies, which investigated the role of CB1 receptors in doxotubicin‐induced cardiotoxicity (Mukhopadhyay et al., 2007; Mukhopadhyay et al., 2010). Moreover, pharmacological inhibition of CB1 receptors combined with activation of CB2 receptors exerted stronger protective effects than a single treatment in cultured cardiomyocytes, as evidenced by the 2‐AG contents and also the biomarkers reflecting myocardial injury. These in vitro data suggest the functional contribution of each CB receptor. Taken together, it is concluded that activation of CB2 receptors and inhibition of CB1 receptors protect against the cardiac side effects of clozapine. CB receptors exert opposite effects in clozapine‐induced cardiotoxicity.
Identification of CB receptors and their opposite effects in drug‐induced cardiotoxicity is of great importance. On the one hand, it confirms a previous speculation that other GPCRs are likely to be associated with clozapine‐induced cardiotoxicity (Wang et al., 2008) and might implicate a wider involvement of CB receptors in drug‐induced cardiotoxicity. On the other hand, the opposite effects of CB receptors in drug‐induced cardiotoxicity might suggest that CB receptor dual agonists or antagonists are not necessarily efficacious, and the treatment of drug‐induced cardiotoxicity can be only beneficial when based on specific CB receptor activation or inhibition. It is noteworthy that the use of CB1 antagonists seems to be double edged, on the one hand being marketed for weight loss but on the other hand being tested for improving cardiovascular outcomes (Mukhopadhyay et al., 2007; Topol et al., 2010). The use of rimonabant was also reported to cause serious psychiatric disorders (Christensen, Kristensen, Bartels, Bliddal, & Astrup, 2007). It is therefore mandated to carefully monitor patients' side effects or use a relatively lower but effective dose for clinical intervention of drug cardiotoxicity. In this setting, the use of CB2 agonists might be both beneficial and safer than CB1 antagonists for improving clozapine cardiotoxicity.
Taken together, the results of the present study established a murine model of clozapine‐induced cardiotoxicity and investigated the possible roles of CB receptors in this process. Our data suggest that CB receptors exerted opposite effects on clozapine‐induced myocardial injury. Pharmacological activation of CB2 receptors and inhibition of CB1 receptors were beneficial to clozapine‐induced cardiotoxicity. CB receptor dual agonists or antagonists may not be effective as a treatment for the cardiac side effects of clozapine. Instead, clozapine cardiotoxicity may only be rescued when using specific CB receptor activators or inhibitors.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
L.L., X.D., X.L., Z.P., and Y.Z. performed the experiments, acquired and analysed the data, and prepared the figures. L.L. drafted the manuscript. C.T. analysed the qRT‐PCR data. X.D. and D.Z. performed the LC/MS/MS analysis of endocannabinoids. J.J. provided technical assistance in histopathological staining. A.B. and Z.Z. are senior pathologists who provided professional consultants in determining the infiltration index. L.J. provided critical comments on the study design and data presentation. Y.J. conceived the experiments and proofread the final version of the manuscript.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
Supporting information
Figure S1. Effects of CBR antagonist/agonist on clozapine‐mediated mice survival and heart weight/body weight (HW/BW) ratios. (A, B) Effects of Rimonabant (Rimo, selective CB1 antagonist, 4 mg kg−1) or AM1241 (selective CB2 agonist, 50 mg kg−1) pretreatments on mice survival (n = 10–15 per group) or HW/BW ratio (n = 5–6 per group) after 14‐day exposure to clozapine (CLZ). (C, D) Effects of ACEA (selective CB1 agonist, 5 mg kg−1) or AM630 (selective CB2 antagonist, 5 mg kg−1) pretreatments on mice survival (n = 10–13 per group) or HW/BW ratio (n = 5–6 per group) after 14‐day exposure to CLZ. Mice with vehicle (Veh, PBS) injection served as control for each assay. n.s., no significance. *, P < 0.05 as indicated.
Figure S2. LC–MS/MS analysis of the 2‐AG contents in control cardiac HL‐1 cells and in HL‐1 cells with indicated pretreatments. The final concentrations of JWH‐133 and AM281 were both 1 μM. Doses for other drugs were as reported in Figure 8. *, p < 0.05 as indicated.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support from the University of Maryland School of Medicine (USA) and the China Scholarship Council, Ministry of Education (China) in the form of a PhD Studentship awarded to L. L. This work was financially supported by the National Natural Science Foundation of China (Grants 81571849, 81701861, and 81871525) and the China Postdoctoral Science Foundation (Grants 2016M601507 and 2018T110348).
Li L, Dong X, Tu C, et al. Opposite effects of cannabinoid CB1 and CB2 receptors on antipsychotic clozapine‐induced cardiotoxicity. Br J Pharmacol. 2019;176:890–905. 10.1111/bph.14591
REFERENCES
- Abdel‐Wahab, B. A. , & Metwally, M. E. (2015). Clozapine‐induced cardiotoxicity: Role of oxidative stress, tumour necrosis factor alpha and NF‐κβ. Cardiovascular Toxicology, 15(4), 355–365. 10.1007/s12012-014-9304-9 [DOI] [PubMed] [Google Scholar]
- Abdel‐Wahab, B. A. , Metwally, M. E. , El‐khawanki, M. M. , & Hashim, A. M. (2014). Protective effect of captopril against clozapine‐induced myocarditis in rats: Role of oxidative stress, proinflammatory cytokines and DNA damage. Chemico‐Biological Interactions, 216, 43–52. 10.1016/j.cbi.2014.03.012 [DOI] [PubMed] [Google Scholar]
- Aguilar, E. J. , & Siris, S. G. (2007). Do antipsychotic drugs influence suicidal behavior in schizophrenia? Psychopharmacology Bulletin, 40(3), 128–142. [PubMed] [Google Scholar]
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … Southan, C. (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174(Suppl 1), S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andren, B. , Lind, L. , Larsson, K. , Hedenstierna, G. , Ljunghall, S. , & Lithell, H. (1995). The influence of body composition on left ventricular mass and other echocardiographic and Doppler measurements in 70‐year‐old males. Clinical Physiology, 15(5), 425–433. 10.1111/j.1475-097X.1995.tb00532.x [DOI] [PubMed] [Google Scholar]
- Bennetzen, M. F. , Nielsen, M. P. , Richelsen, B. , & Pedersen, S. B. (2008). Effects on food intake and blood lipids of cannabinoid receptor 1 antagonist treatment in lean rats. Obesity (Silver Spring), 16(11), 2451–2455. 10.1038/oby.2008.390 [DOI] [PubMed] [Google Scholar]
- Christensen, R. , Kristensen, P. K. , Bartels, E. M. , Bliddal, H. , & Astrup, A. (2007). Efficacy and safety of the weight‐loss drug rimonabant: A meta‐analysis of randomised trials. Lancet, 370(9600), 1706–1713. 10.1016/S0140-6736(07)61721-8 [DOI] [PubMed] [Google Scholar]
- Curto, M. , Girardi, N. , Lionetto, L. , Ciavarella, G. M. , Ferracuti, S. , & Baldessarini, R. J. (2016). Systematic review of clozapine cardiotoxicity. Current Psychiatry Reports, 18(7), 68 10.1007/s11920-016-0704-3 [DOI] [PubMed] [Google Scholar]
- Dong, X. , Li, L. , Ye, Y. , Zheng, L. , & Jiang, Y. (2016). Simultaneous determination of major phytocannabinoids, their main metabolites, and common synthetic cannabinoids in urine samples by LC‐MS/MS. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 1033‐1034, 55–64. 10.1016/j.jchromb.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Green, A. I. , Tohen, M. , Patel, J. K. , Banov, M. , DuRand, C. , Berman, I. , … Schatzberg, A. F. (2000). Clozapine in the treatment of refractory psychotic mania. The American Journal of Psychiatry, 157(6), 982–986. 10.1176/appi.ajp.157.6.982 [DOI] [PubMed] [Google Scholar]
- Haas, S. J. , Hill, R. , Krum, H. , Liew, D. , Tonkin, A. , Demos, L. , … McNeil, J. (2007). Clozapine‐associated myocarditis: A review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993‐2003. Drug Safety, 30(1), 47–57. 10.2165/00002018-200730010-00005 [DOI] [PubMed] [Google Scholar]
- Hagg, S. , Spigset, O. , Bate, A. , & Soderstrom, T. G. (2001). Myocarditis related to clozapine treatment. Journal of Clinical Psychopharmacology, 21(4), 382–388. 10.1097/00004714-200108000-00005 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennen, J. , & Baldessarini, R. J. (2005). Suicidal risk during treatment with clozapine: A meta‐analysis. Schizophrenia Research, 73(2–3), 139–145. 10.1016/j.schres.2004.05.015 [DOI] [PubMed] [Google Scholar]
- Julien, B. , Grenard, P. , Teixeira‐Clerc, F. , Van Nhieu, J. T. , Li, L. , Karsak, M. , … Lotersztajn, S. (2005). Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology, 128(3), 742–755. 10.1053/j.gastro.2004.12.050 [DOI] [PubMed] [Google Scholar]
- Kelly, D. L. , Weiner, E. , Ball, M. P. , McMahon, R. P. , Carpenter, W. T. , & Buchanan, R. W. (2009). Remission in schizophrenia: The relationship to baseline symptoms and changes in symptom domains during a one‐year study. Journal of Psychopharmacology, 23(4), 436–441. 10.1177/0269881108093883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian, J. G. , Kerr, K. , Lawrence, C. , & Celermajer, D. S. (1999). Myocarditis and cardiomyopathy associated with clozapine. Lancet, 354(9193), 1841–1845. 10.1016/S0140-6736(99)10385-4 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br J Pharmacol, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Grenade, L. , Graham, D. , & Trontell, A. (2001). Myocarditis and cardiomyopathy associated with clozapine use in the United States. The New England Journal of Medicine, 345(3), 224–225. 10.1056/NEJM200107193450317 [DOI] [PubMed] [Google Scholar]
- Lepicier, P. , Bouchard, J. F. , Lagneux, C. , & Lamontagne, D. (2003). Endocannabinoids protect the rat isolated heart against ischaemia. British Journal of Pharmacology, 139(4), 805–815. 10.1038/sj.bjp.0705313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Li, Y. , Lin, J. , Jiang, J. , He, M. , Sun, D. , … Xue, A. (2016). Phosphorylated myosin light chain 2 (p‐MLC2) as a molecular marker of antemortem coronary artery spasm. Medical Science Monitor, 22, 3316–3327. 10.12659/MSM.900152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Ye, X. , Zhao, Z. , Gao, P. , & Jiang, Y. (2018). Overlooked fatal infectious diseases after long‐term antipsychotic use in patients with psychiatric illness. Schizophrenia Research, 195, 258–259. 10.1016/j.schres.2017.09.033 [DOI] [PubMed] [Google Scholar]
- Li, L. L. , Xue, A. M. , Li, B. X. , Shen, Y. W. , Li, Y. H. , Luo, C. L. , … Zhao, Z. Q. (2014). JMJD2A contributes to breast cancer progression through transcriptional repression of the tumor suppressor ARHI. Breast Cancer Research, 16(3), R56 10.1186/bcr3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. , Wu, X. , & Li, L. (2007). Chronic administration of clozapine alleviates reversal‐learning impairment in isolation‐reared rats. Behavioural Pharmacology, 18(2), 135–145. 10.1097/FBP.0b013e3280d3ee83 [DOI] [PubMed] [Google Scholar]
- Liao, Y. , Bin, J. , Asakura, M. , Xuan, W. , Chen, B. , Huang, Q. , … Kitakaze, M. (2012). Deficiency of type 1 cannabinoid receptors worsens acute heart failure induced by pressure overload in mice. European Heart Journal, 33(24), 3124–3133. 10.1093/eurheartj/ehr246 [DOI] [PubMed] [Google Scholar]
- Liao, Y. , Bin, J. , Luo, T. , Zhao, H. , Ledent, C. , Asakura, M. , … Kitakaze, M. (2013). CB1 cannabinoid receptor deficiency promotes cardiac remodeling induced by pressure overload in mice. International Journal of Cardiology, 167(5), 1936–1944. 10.1016/j.ijcard.2012.05.033 [DOI] [PubMed] [Google Scholar]
- Lim, S. Y. , Davidson, S. M. , Yellon, D. M. , & Smith, C. C. (2009). The cannabinoid CB1 receptor antagonist, rimonabant, protects against acute myocardial infarction. Basic Research in Cardiology, 104(6), 781–792. 10.1007/s00395-009-0034-2 [DOI] [PubMed] [Google Scholar]
- Matsumori, A. , Wang, H. , Abelmann, W. H. , & Crumpacker, C. S. (1985). Treatment of viral myocarditis with ribavirin in an animal preparation. Circulation, 71(4), 834–839. 10.1161/01.CIR.71.4.834 [DOI] [PubMed] [Google Scholar]
- Merrill, D. B. , Dec, G. W. , & Goff, D. C. (2005). Adverse cardiac effects associated with clozapine. Journal of Clinical Psychopharmacology, 25(1), 32–41. 10.1097/01.jcp.0000150217.51433.9f [DOI] [PubMed] [Google Scholar]
- Montecucco, F. , Lenglet, S. , Braunersreuther, V. , Burger, F. , Pelli, G. , Bertolotto, M. , … Steffens, S. (2009). CB2 cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. Journal of Molecular and Cellular Cardiology, 46(5), 612–620. 10.1016/j.yjmcc.2008.12.014 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, P. , Batkai, S. , Rajesh, M. , Czifra, N. , Harvey‐White, J. , Hasko, G. , … Pacher, P. (2007). Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin‐induced cardiotoxicity. Journal of the American College of Cardiology, 50(6), 528–536. 10.1016/j.jacc.2007.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay, P. , Rajesh, M. , Batkai, S. , Patel, V. , Kashiwaya, Y. , Liaudet, L. , … Pacher, P. (2010). CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin‐induced cardiomyopathy and in human cardiomyocytes. Cardiovascular Research, 85(4), 773–784. 10.1093/cvr/cvp369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan, S. E. (2015). Endocannabinoids and the cardiovascular system in health and disease. Handbook of Experimental Pharmacology, 231, 393–422. 10.1007/978-3-319-20825-1_14 [DOI] [PubMed] [Google Scholar]
- Patel, J. J. , Lisi, P. A. , Lathara, Z. , & Lipchik, R. J. (2012). Clozapine‐induced peripheral and pleural fluid eosinophilia. The Annals of Pharmacotherapy, 46(2), e4 10.1345/aph.1Q642 [DOI] [PubMed] [Google Scholar]
- Rajesh, M. , Batkai, S. , Kechrid, M. , Mukhopadhyay, P. , Lee, W. S. , Horvath, B. , … Pacher, P. (2012). Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes, 61(3), 716–727. 10.2337/db11-0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson, K. J. , Fitzgerald, P. B. , & McNeil, J. J. (2015). Clozapine‐induced myocarditis, a widely overlooked adverse reaction. Acta Psychiatrica Scandinavica, 132(4), 231–240. 10.1111/acps.12416 [DOI] [PubMed] [Google Scholar]
- Schwieler, L. , Linderholm, K. R. , Nilsson‐Todd, L. K. , Erhardt, S. , & Engberg, G. (2008). Clozapine interacts with the glycine site of the NMDA receptor: Electrophysiological studies of dopamine neurons in the rat ventral tegmental area. Life Sciences, 83(5–6), 170–175. 10.1016/j.lfs.2008.05.014 [DOI] [PubMed] [Google Scholar]
- Slavic, S. , Lauer, D. , Sommerfeld, M. , Kemnitz, U. R. , Grzesiak, A. , Trappiel, M. , … Kaschina, E. (2013). Cannabinoid receptor 1 inhibition improves cardiac function and remodelling after myocardial infarction and in experimental metabolic syndrome. Journal of Molecular Medicine (Berl), 91(7), 811–823. 10.1007/s00109-013-1034-0 [DOI] [PubMed] [Google Scholar]
- Teixeira‐Clerc, F. , Julien, B. , Grenard, P. , Tran, V. N. J. , Deveaux, V. , Li, L. , … Lotersztajn, S. (2006). CB1 cannabinoid receptor antagonism: A new strategy for the treatment of liver fibrosis. Nature Medicine, 12(6), 671–676. 10.1038/nm1421 [DOI] [PubMed] [Google Scholar]
- Topol, E. J. , Bousser, M. G. , Fox, K. A. , Creager, M. A. , Despres, J. P. , Easton, J. D. , … CRESCENDO Investigators (2010). Rimonabant for prevention of cardiovascular events (CRESCENDO): A randomised, multicentre, placebo‐controlled trial. Lancet, 376(9740), 517–523. 10.1016/S0140-6736(10)60935-X [DOI] [PubMed] [Google Scholar]
- Turcotte, C. , Blanchet, M. R. , Laviolette, M. , & Flamand, N. (2016). The CB2 receptor and its role as a regulator of inflammation. Cellular and Molecular Life Sciences, 73(23), 4449–4470. 10.1007/s00018-016-2300-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. F. , Min, J. Y. , Hampton, T. G. , Amende, I. , Yan, X. , Malek, S. , … Morgan, J. P. (2008). Clozapine‐induced myocarditis: Role of catecholamines in a murine model. European Journal of Pharmacology, 592(1–3), 123–127. 10.1016/j.ejphar.2008.06.088 [DOI] [PubMed] [Google Scholar]
- Wang, L. L. , Zhao, R. , Li, J. Y. , Li, S. S. , Liu, M. , Wang, M. , … Guan, D. W. (2016). Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re‐epithelialization during skin wound healing. European Journal of Pharmacology, 786, 128–136. 10.1016/j.ejphar.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Wang, P. F. , Jiang, L. S. , Bu, J. , Huang, X. J. , Song, W. , Du, Y. P. , & He, B. (2012). Cannabinoid‐2 receptor activation protects against infarct and ischemia–reperfusion heart injury. Journal of Cardiovascular Pharmacology, 59(4), 301–307. 10.1097/FJC.0b013e3182418997 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Ma, S. , Wang, Q. , Hu, W. , Wang, D. , Li, X. , … Cao, F. (2014). Effects of cannabinoid receptor type 2 on endogenous myocardial regeneration by activating cardiac progenitor cells in mouse infarcted heart. Science China. Life Sciences, 57(2), 201–208. 10.1007/s11427-013-4604-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effects of CBR antagonist/agonist on clozapine‐mediated mice survival and heart weight/body weight (HW/BW) ratios. (A, B) Effects of Rimonabant (Rimo, selective CB1 antagonist, 4 mg kg−1) or AM1241 (selective CB2 agonist, 50 mg kg−1) pretreatments on mice survival (n = 10–15 per group) or HW/BW ratio (n = 5–6 per group) after 14‐day exposure to clozapine (CLZ). (C, D) Effects of ACEA (selective CB1 agonist, 5 mg kg−1) or AM630 (selective CB2 antagonist, 5 mg kg−1) pretreatments on mice survival (n = 10–13 per group) or HW/BW ratio (n = 5–6 per group) after 14‐day exposure to CLZ. Mice with vehicle (Veh, PBS) injection served as control for each assay. n.s., no significance. *, P < 0.05 as indicated.
Figure S2. LC–MS/MS analysis of the 2‐AG contents in control cardiac HL‐1 cells and in HL‐1 cells with indicated pretreatments. The final concentrations of JWH‐133 and AM281 were both 1 μM. Doses for other drugs were as reported in Figure 8. *, p < 0.05 as indicated.


