Abstract
Background and Purpose
Extravillous trophoblast (EVT) cells are responsible for decidual stromal invasion, vascular transformation, and the recruitment and functional modulation of maternal leukocytes in the first‐trimester pregnant uterus. An early disruption of EVT function leads to placental insufficiency underlying pregnancy complications such as preeclampsia and fetal growth restriction. Vasoactive intestinal peptide (VIP) is a vasodilating and immune modulatory factor synthesized by trophoblast cells. However, its role in first‐trimester placenta has not been explored. Here, we tested the hypothesis that VIP is involved in first‐trimester EVT outgrowth, spiral artery remodelling, balancing angiogenesis, and maintenance of immune homeostasis.
Experimental Approach
First‐trimester placental tissue (five to nine weeks of gestation) was collected, and was used for EVT outgrowth experiments, immunofluorescence, isolation of decidual natural killer (dNK) cells and decidual macrophages (dMA), and functional assays. Peripheral blood monocytes were differentiated with GM‐CSF and used for angiogenesis assays.
Key Results
In decidua basalis, VIP+ EVT were observed sprouting from cell columns and lining spiral arterioles. EVT migrating from placental explants were also VIP+. VIP increased EVT outgrowth and IL‐10 release, whereas it decreased pro‐inflammatory cytokine production in EVT, dNK cells, and dMA. VIP disrupted endothelial cell networks, both directly and indirectly via an effect on macrophages.
Conclusion and Implications
The results suggest that VIP assists the progress of EVT invasion and vessel remodelling in first‐trimester placental bed in an immunologically “silent” milieu. The effects of VIP in the present ex vivo human placental model endorse its potential as a therapeutic candidate for deep placentation disorders.
Abbreviations
- Ab
antibody
- CD
cluster differentiation
- CM
conditioned medium
- dMA
decidual macrophages
- dNK
decidual natural killer
- EC
endothelial cells
- ECM
extracellular matrix
- EVT
extravillous trophoblast
- FCS
fetal calf serum
- G‐CSF
granulocyte CSF
- HLA‐G
human leukocyte antigen G
- IP‐10/CXCL10
IFN γ‐induced protein 10
- MMP
metalloproteinase
- Scrl
scramble
- siVIP
small interfering VIP
- SMA
smooth muscle actin
- SpA
spiral arterioles
- TIMP
tissue inhibitors of MMP
- TSP‐1
thrombospondin‐1
- VIP
vasoactive intestinal peptide
What is already known
Factors derived from trophoblast cells regulate invasion, remodelling, and immune homeostasis during early placentation.
What this study adds
VIP is one of those factors; it acts through targeting immune, vascular, and trophoblast cells.
What is the clinical significance
The effects of VIP endorse its potential as a therapeutic candidate for deep placentation disorders.
1. INTRODUCTION
Trophoblast invasion of the first‐trimester decidua is a critical step in normal placentation. Extravillous trophoblast (EVT) cells sprout from the cell columns at the anchoring villi (Pijnenborg, Bland, Robertson, & Brosens, 1983) and migrate through the decidual stroma where they interact with maternal leukocytes, vascular, and endothelial cells (EC; Fisher, 2015). They acquire an invasive phenotype with enhanced expression of human leukocyte antigen G (HLA‐G; Davies et al., 2016) and adhesion molecule receptors similar to vascular cells including integrins α1, α5, and αVβ3 (Harris & Aplin, 2007; Spessotto et al., 2006; Sueoka et al., 1997). Decidual invasion by trophoblast cells also entails extracellular matrix (ECM) breakdown associated with an increased expression and activity of matrix metalloproteinases (MMPs), mainly MMP‐2, 9, 12, 14, and 15 as well as the tissue inhibitors of MMP, TIMP1 and TIMP2 (Anacker et al., 2011; Bischof, Meisser, & Campana, 2002; Cohen, Meisser, & Bischof, 2006). EVT cells largely contribute to vascular remodelling through the transformation of spiral arterioles (SpA) into larger diameter vessels with low resistance and high blood flow (Bulmer, Innes, Levey, Robson, & Lash, 2012; Harris et al., 2006; Harris & Aplin, 2007; Pijnenborg et al., 1983).
Cytotrophoblast cells also regulate the recruitment and function of maternal leukocytes in the pregnant uterus (Mor, Aldo, & Alvero, 2017). A trophoblast controlled cytokine micro‐environment conditions maternal leukocyte activation to maintain immune homeostasis (Mor et al., 2017; Mor, Cardenas, Abrahams, & Guller, 2011). Likewise, certain innate response immune cell populations have been implicated in vascular remodelling by trophoblast‐dependent and ‐independent mechanisms (Robson et al., 2012; Smith, Dunk, Aplin, Harris, & Jones, 2009). In particular, decidual macrophages (dMA) and decidual natural killer (dNK) cells are involved in balancing angiogenesis and SpA remodelling through the synthesis of VEGF, CXCL8, and angiopoietins among other factors (Lash et al., 2016; PrabhuDas et al., 2015; Smith et al., 2009). Recently, a cooperative interaction of trophoblast and EC with dMA and dNK was described in vascular transformation in the placental bed during early gestation (Choudhury et al., 2017).
Defective trophoblast invasion and incomplete SpA transformation leading to placental insufficiency are recognized as pathogenic mechanisms of deep placentation disorders (Boeldt & Bird, 2017; Brosens, Pijnenborg, Vercruysse, & Romero, 2011; Fisher, 2015; Huppertz, 2008; Pijnenborg et al., 1991). Among them, preeclampsia, intrauterine growth restriction, and late miscarriage involve exacerbated oxidative stress and inflammation with high rates of maternal and neonatal morbidity and mortality (Khong, De Wolf, Robertson, & Brosens, 1986; Romero, Dey, & Fisher, 2014). So far, early biomarkers of trophoblast dysfunction and defective vascular transformation lack the specificity required as predictive markers; thus, these diseases are diagnosed in the second or third trimester with mild to severe consequences for the mother and neonate (Boeldt & Bird, 2017; Brosens et al., 2011; Romero et al., 2014).
A variety of contact and soluble factors expressed by cytotrophoblast cells mediate local effects on hormone, cytokine, angiogenic, and growth factor synthesis during placental development (Aplin, 2010; Mor et al., 2017). The vasoactive intestinal peptide (VIP, P01282) has vasodilating, pro‐secretory and immunomodulatory effects (Ganea, Hooper, & Kong, 2015; Gomariz et al., 2010; Said, 2007; Waschek, 2013) upon binding high‐affinity receptors VPAC1 and VPAC2 (nomenclature as agreed by the NC‐IUPHAR Subcommittee on Vasoactive Intestinal Peptide Receptors; Harmar et al., 1998; Harmar et al., 2012). VIP is expressed in syncytium and cytrophoblast cells of first‐trimester placenta (Marzioni et al., 2005). VIP promotes progesterone and human chorionic gonadotropin release as well as TGF‐β and chemokine synthesis by human trophoblast‐derived cell lines (Deutsch, Sun, & Kroog, 1990; Fraccaroli et al., 2015; Fraccaroli et al., 2009; Marzioni et al., 2005; Paparini et al., 2015). VPAC1 and VPAC2 receptors were previously identified in human placental trophoblast cells and trophoblast cell lines (Deutsch et al., 1990; Fraccaroli et al., 2009; Marzioni et al., 2005). Recently, both receptors have been implicated in autocrine and paracrine regulation of trophoblast through PKA‐cAMP response element pathways in HTR‐8/SVNeo and Swan 71 cell lines. VIP production and trophoblast cell line migration and invasion were affected by this pathway (Vota et al., 2016). In addition, it displayed embryotrophic effects in mice in vitro if they were explanted with their yolk sac (Gressens et al., 1997). Regarding trophoblast‐immune cell interactions, VIP favours tolerogenic and anti‐inflammatory responses in human cells in vitro, such as monocytes, macrophages, neutrophils and cluster differentiation (CD) 4+ lymphocytes (Calo et al., 2016; Fraccaroli et al., 2015; Fraccaroli et al., 2009; Paparini et al., 2015; Vota et al., 2016) and mice in vivo (Gallino et al., 2016; Hauk et al., 2014).
Here, we tested the hypothesis that invasiveness of EVT in the first trimester is associated with an enhanced VIP expression and that trophoblast‐derived VIP is involved in spiral artery remodelling, balancing angiogenesis, and maintenance of immune homeostasis. Our results point to VIP as a novel mediator of trophoblast differentiation to an invasive phenotype in human first‐trimester placenta. Moreover, we demonstrate that trophoblast‐VIP associates with vascular transformation and the maintenance of a regulated immune micro‐environment: This effect involves the balance of cytokines and angiogenic factors produced by dMA and dNK cells.
2. METHODS
2.1. Primary tissues
First‐trimester placental tissue (5–9 weeks of gestation; n = 31) was collected from women undergoing elective medical and surgical termination of pregnancy. Decidual tissue (5–9 weeks of gestation; n = 18) was collected following surgical termination of pregnancy only. Placental and decidual tissues were dissected as described previously and processed for cell and tissue culture (Smith et al., 2009). Approval was obtained from North West Research Ethics Committee (08/H1010/28). Written informed consent was obtained from all women undergoing termination of pregnancy.
2.2. EVT outgrowths and production of conditioned medium
EVT‐conditioned medium (EVT‐CM; n = 19) was generated as described previously (Wright et al., 2010). Briefly, the terminal portions of villi from 5‐ to 9‐week placenta were dissected out and laid over a collagen I (Corning, Bedford, UK) surface in a 24‐well plate. The explants were cultured in DMEM:F12 (Sigma, Gillingham, Dorset, UK) with 10% fetal calf serum (FCS) in 5% CO2 and 20% O2 for 96 hr. The EVT outgrowths were evaluated under different treatments: 10, 100, or 1000 nM of VIP (BACHEM Cat#3775.0001), 1/1000 dilution of VIP neutralizing antibody (Ab; Abcam Cat# ab78536, RRID:AB_1604043) ± 100 nM VIP and a pharmacological PKA inhibitor, 5 μM H89 dihydrochloride (H89; Sigma ‐Aldrich, UK) ± 100 nM VIP. Microphotographs of each explant were taken before adding the treatment (t0) and every 24 hr. The EVT outgrowths were studied with the ImageJ software, which measured the area of the EVT outgrowths (t96 − t0).
EVT‐CM were then collected after 96 hr and centrifuged at 3,000× g for 5 min to remove any debris and stored at −80°C for further experiments. Outgrowth at 20% oxygen allowed us to obtain conditioned medium (CM) in a 96‐hr time frame, prior to EVT‐induced matrix digestion and loss of cell viability, as described previously (Choudhury et al., 2017).
2.3. Isolation of primary dNK cells and dMA
Primary dNK cells and dMA were isolated from decidual samples as described previously (Zhang et al., 2017). Briefly, decidual samples were washed, minced, and shaken at 37°C for 30 min before passing through a 200‐mm sieve.
Stromal cells and leukocytes were separated from RBCs using a Lymphoprep density gradient (Cambridge, UK). CD56+ dNK cells and CD14+ macrophages were isolated by positive selection using magnetic microbeads (MACS Miltenyi Biotec Cat# 130‐050‐401 and Cat# 130‐091‐097, respectively) from each decidua.
The purity of the cell isolation was measured using flow cytometry after immunostaining for CD56 and CD14, as previously described (Choudhury et al., 2017).
2.4. Blood samples
Blood samples were taken from healthy volunteers, all women of reproductive age (n = 13), who had received no pharmacological treatment for at least 10 days before the day of sampling. Blood was obtained by puncture of the forearm vein, and it was drawn directly into heparin‐containing plastic tubes. Studies were approved by the Argentine Society of Clinical Investigation Board and Ethical Committee (Ref. SAIC 46/14). All healthy donors provided written informed consent for sample collection and subsequent analysis.
2.5. Monocyte isolation and differentiation to macrophages
Peripheral blood mononuclear cells were isolated from individual subjects by Ficoll–Hypaque (GE Healthcare Cat# GE17‐1440‐03), and CD14+ cells were separated by Percoll gradient (GE Healthcare, Sweden), both according to the manufacturer's protocol. Cell population purity (>80%) was checked by flow cytometry analysis with CD14+ labelling as previously (Hauk et al., 2014). For granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) differentiated macrophages, monocytes (5 × 105 cells) were incubated with 100 ng·ml−1 GM‐CSF (Immunotools, APBiotech, Argentina) in RPMI 1640 medium HyClone Laboratories (Logan, UT, USA) for 5 days, as previously described (Paparini et al., 2015).
2.6. Trophoblast cell lines
Two cytotrophoblast cell lines from human pregnancies were used throughout. Swan 71 cell line obtained by telomerase‐mediated transformation of an isolated 7‐week cytotrophoblast (RRID:CVCL_D855; Aplin et al., 2006; Straszewski‐Chavez et al., 2009). Swan 71 was kindly given by Dr Gil Mor (Yale University, New Haven, USA). BeWo cell line was obtained by clonal selection of a chorioncarcinoma (ATCC Cat# CCL‐98, RRID:CVCL_0044). To obtain the conditioned media (CM), Swan 71 cells were cultured for 20 hr in 24‐well flat‐bottom polystyrene plates with DMEM (DMEM:F12) containing 2% (v:v) FCS (Life Technologies, Buenos Aires, Argentina) in the absence (CM) or presence of 10 or 100 nM VIP (Polypeptide, France; CM VIP 100 nM). VIP did not modify the viability of trophoblast cells as tested by the colorimetric 3‐(4,5‐dimethyltiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay and Trypan blue (Vota et al., 2016). Trophoblast cell CM were collected and stored at −20°C until used. To assess that adherent trophoblast cells were not removed during the CM collection procedure, RNA levels were determined and they were below the detection limit.
2.7. VIP silencing in trophoblast cell lines
To transfect BeWo cells with a VIP siRNA (Santa Cruz Biotechnology, Dallas, TX, USA), cells were grown at 60% of confluence and arrested for 3 hr in Optimem®; 100 nM VIP siRNA: Lipofectamine RNAimax (Life Technologies, Grand Island, NY, USA) complex were made in Optimem and incubated for 15 min before being added to the cells in a drop wise manner; 24‐hr post‐transfection, the media were changed for DMEM:F12 supplemented with 10% FCS for another 48 hr. siRNA with a scramble (Scrl) sequence was used as a negative control.
2.8. CD14+ cell cultures
Macrophages (GM‐CSF) were cultured in 24‐well plates (5 × 105 cells per well) in DMEM:F12 2% FCS in the presence or absence of 100 nM VIP, or with CM, or CM (100 nM VIP) for 20 hr. The supernatants were collected and centrifuged at 3,000× g for 5 min to remove any debris and stored at −80°C for further experiments. The cells were harvested and used to analyse gene expression by use of RT‐qPCR.
2.9. Dual immunofluorescence and immunohistochemistry
Immunofluorescence was carried out on formalin‐fixed, paraffin‐embedded human first‐trimester decidua basalis (5–9 weeks of gestation). Serial tissue sections (5 μm) were immunostained for vascular cells (anti‐smooth muscle actin [α‐SMA (Agilent Cat# M0851, RRID:AB_2223500)‐ 1/200] and anti‐CD31 [EC (Agilent Cat# M0823, RRID:AB_2114471)‐ 1/50], EVTs [anti‐HLA‐G (BD Biosciences Cat# 557577, RRID:AB_396753)‐ 1/100 and anti‐αVβ3 integrin (Millipore Cat# MAB1976, RRID:AB_2757810)‐ 1/100] for simple epithelia and leukocyte common Ag: anti‐CD45 (Agilent Cat# GA75161‐2, RRID:AB_2661839)‐ 1/200, anti‐CD14 [monocytes and macrophages (Agilent Cat# M0825, RRID:AB_2291249)‐1/200] and anti‐CD56 [dNK cells (Aligent Cat# R7251, RRID:AB_2282500)‐1/200] using mouse monoclonal Abs, and α‐VIP using rabbit polyclonal Ab (Abcam Cat# ab78536, RRID:AB_1604043)‐1/200. Donkey anti‐rabbit Alexa Fluor 488 Thermo Fisher Scientific Cat# R37118, RRID:AB_2556546), Goat anti‐mouse Alexa Fluor 488 (Thermo Fisher Scientific Cat# A‐11001, RRID:AB_2534069) and Goat anti‐mouse Alexa Fluor 568 (Thermo Fisher Scientific Cat# A‐21144, RRID:AB_2535780) conjugates, 1/500 were used each. To carry out immunofluorescence in EVT outgrowths, PFA was inactivated by pretreatment with a boron hydride solution (10 mg·ml−1). The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology.
For immunohistochemistry, paraffin‐embedded human first‐trimester decidua basalis (5–9 weeks of gestation) was stained using colorimetric detection as described previously. Serial tissue sections (5 μm) were immunostained with anti‐SMA‐1/100, anti‐HLA‐G‐1/100, anti‐CD45, and anti‐VIP‐1/500 Abs. Goat anti‐mouse HRP‐conjugated (Thermo Fisher Scientific Cat# 62‐6520, RRID:AB_2533947) and Donkey anti‐rabbit HRP‐conjugated (Thermo Fisher Scientific Cat# PA1‐86177, RRID:AB_933717) Abs were obtained from Thermo Fischer (1/200), and immunoreactions were performed as described previously (Choudhury et al., 2017).
2.10. BioPlexPro™ human cytokine 27‐Plex assay
The expressions of 27 cytokines in EVT‐CM and the supernatants of dMA and dNK cells were measured by BioPlexPro (BioRad) following the manufacturer's instructions. Briefly, the BioPlex® 200 System and BioPlex™ Human Cytokine Standard 27‐Plex, Group I (Bio‐Rad Cat #m500kcaf0y), were used to assess cytokine levels. This commercial kit measures the levels of most cytokines and chemokines involved in the maternal‐placental interface. The capture sandwich immunoassay format was designed on magnetic beads. The capture Ab‐coupled beads were first incubated with antigen standards, samples, or controls, followed by incubation with biotinylated detection antibodies and reporter streptavidin‐phycoerythrin conjugate. Then, the beads were passed through the BioPlex 200 suspension array reader, which was equipped with two lasers (532 and 635 nm of excitations) that measure the fluorescence of the beads and the bound streptavidin‐phycoerythrin.
A high‐speed digital processor managed the data output, and the BioPlex Manager™ 6.0 software presented the concentration results in pg·ml−1.
2.11. EC culture
Human uterine microvascular EC (HUtMvEC; Lonza, Cologne, Germany), or EC from now on, were cultured as described previously (Amsalem et al., 2014). Upon reaching confluence (Passages 8–10), 1 × 105 cells were seeded per well in a six‐well plate. After being cultured in serum‐containing EC growth basal medium 2 (EBM‐2; Lonza) for 24 hr, the medium was removed and the cells were washed with PBS. The cells were cultured in a 1:1 mixture of serum‐free DMEM:F12 and EBM‐2 for a further 24 hr, then treated with a 1:1 mixture of serum‐free EBM‐2 and either control medium (DMEM:F12) or EVT‐CM for 24 hr. After being washed in PBS, RNA was extracted using the RNeasy mini kit (Qiagen Cat# 74004), followed by treatment with the Ambion DNase kit (Termo Fischer Cat# AM2222) to remove contaminating genomic DNA, both according to the manufacturer's instructions. The RNA sample was assessed for purity and concentration using spectrophotometry and a Nanodrop (2000c; Thermo Scientific).
2.12. Gene expression analysis
RNA extracted from EC treated with EVT‐CM for 24 hr (EVT‐CM from 9 different placenta) and untreated control cells were separately pooled and gene profiling performed using Affymetrix Human Genome U133 Plus 2.0 microarray (Thermo Fischer Cat# 900466). Quality control, normalization, and expression analysis in control and EVT‐CM‐treated groups were as previously described (Li & Wong, 2001; Liu, Milo, Lawrence, & Rattray, 2005; Pearson et al., 2009). Using positional update and matching algorithms, the probability of positive log ratio was calculated (Pearson et al., 2009). Thresholds for significant changes in expression were predefined as probability of positive log ratio scores of >0.9 (for up‐regulated genes) and <0.1 (for down‐regulated genes) and were used to study the VIP/VPAC system in those conditions.
2.13. Reverse transcription and real‐time PCR
To analyse gene expression, real‐time PCR was carried out on individual RNA samples from dNK cells and dMA treated or not with 100 nM VIP, in macrophages (GM‐CSF) treated or not with 100 nM of VIP or CM or CM (VIP) and in BeWo cell line treated with Scrl sequences or a siRNA of VIP for 24 hr (n = 7) as described previously (Vota et al., 2016). A total of 250 ng of RNA was used for reverse transcription using an Affinity Script Multi Temperature cDNA synthesis kit (Agilent Cat# 200436). RT‐qPCR was performed using specific primers (Table S1) with Brilliant III Ultra‐Fast SYBR Green QPCR Master Mix (Agilent Cat# 600882). The PCR data were normalized using two housekeeping genes: YWHAZ and GAPDH (Supporting Information Table S1).
2.14. VIP determination
VIP secretion was quantified in supernatants obtained from human first‐trimester placental explants (with more than 50% of outgrowth, EVT [n = 8], or in those where we did not observe outgrowth, without EVT [n = 6]) after 96 hr of culture using the VIP EIA Kit (Phoenix Pharmaceutical INC Cat# EK‐064‐16) or BeWo cell line transfected with a small interfering VIP (siVIP) or Scrl sequence after 72 hr, as we have described above (n = 7). In brief, 25 μl of antiserum and 50 μl of the standard or sample were incubated in 96‐well immunoplates overnight at 4°C. Then, 25 μl of biotinylated tracer was added and incubated for 2 hr. After five washings with EIA buffer, 100 ml of streptavidin‐HRP were added and incubated at room temperature for 1 hr. After washing with EIA buffer, tetramethylbenzidine solution and 2 N HCl were sequentially added for colour development. Absorbance was determined using the iMark Absorbance Microplate Reader (Bio‐Rad) at 650 and 450 nm for the blue and yellow products respectively. The specificity of the EIA is 100% for VIP (human, rat, mouse, porcine, ovine, and guinea pig). The specificity for VIP (10–28) and VIP from chicken is 34.6% and 28%, respectively, and for PACAP‐27‐NH2 (Human, Rat, Ovine), it is less than 0.02%. The kit has no cross‐reactivity with substance P, endothelin‐1, secretin, glucagon, galanin, somatostatin, or PACAP‐38. The linear range of the EIA for VIP is 0.12–0.93 ng·ml−1.
Intracellular VIP production was measured in BeWo cell line (n = 5) after 72‐hr post‐transfection with siVIP or Scrl RNA sequences by flow cytometry. Briefly, 4 hr before harvesting the cells, they were cultured with the GolgiStop (BD Bioscience, USA). The cells were harvested and fixed/permeabilized with the same kit and incubated with VIP mAb (Santa Cruz Biotechnology, TX, USA) for an hour and then another hour with the donkey anti‐rabbit Alexa Fluor 488 (A21206) secondary Ab. The autofluoresce and positive control were performed to set the system.
2.15. EC tube formation
The release of angiogenic factors by macrophages (GM‐CSF) treated or not with CM of Swan 71 and VIP or dMA treated with VIP was analysed by the ability of the ECs to form tubes; 60 μl of growth factor‐reduced Geltrex (Invitrogen) was plated in a 96‐well plate and incubated at 37°C for 2 hr; 2 × 104 EC were plated on the Geltrex in the presence of VIP (50 or 100 nM) or a 1:2 dilution with DMEM.2% of the supernatants from macrophages (GM‐CSF) ± 100 nM of VIP; or from macrophages (GM‐CSF) incubated with CM of Swan 71 pretreated or not with VIP; or with dMA ± 100 nM of VIP for 8 hr. Microphotographs were taken with an Olympus BX61 (Olympus, Center Valley, PA, USA). Endothelial tube formation was evaluated after 8 hr. Around 15 fields per well were photographed. Adobe Photoshop‐processed photographs were evaluated using the NIH ImageJ Angiogenesis Analyser Plug‐in (ImageJ RRID:SCR_003070). The parameters analysed were meshes and pieces. Pieces consist in the total amount of branches, elements, and isolated elements (Supporting Information Figure S1).
2.16. Statistical analysis
The significance of the results for gene expression in dNK cells and dMA, BioPlex, VIP secretion in explants and EC tube formation by dMA was analysed by the Mann–Whitney test for nonparametric samples. For the gene expression in trophoblast cell lines transfected with siRNA or Scrl sequences, statistically significant difference was determined by the confidence interval of the samples. If the treatment did not contain the value 1, the difference was statistically significantly different. When multiple comparisons were necessary for EC tube formation induced by VIP, one‐way ANOVA with post hoc Holm Sidak test was used. For non‐parametric multiple comparisons, the Kruskal–Wallis test and Dunn's test, post hoc, were used to determine the significance of explants outgrowths area. For the EVT BioPlex, the MMP‐2 expression by dMA, and the EC tube formation by macrophages (GM‐CSF), the Friedman test and Dunn's test, post hoc, were used. Differences between groups were considered significant at P < 0.05 using the GraphPad Prism7 software (GraphPad Prism, RRID:SCR_002798). All the experiments carried out in this work were performed at least five different times as is shown in the figure legend. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology.
2.17. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro et al., 2017a,b; Alexander, Kelly et al., 2017).
A flow chart of methods is shown in Supporting Information Figure S2.
3. RESULTS
3.1. VIP expression in columnar cells of the villi
The expression of VIP in human first‐ and third‐trimester placenta was reported (Marzioni et al., 2005). The syncytiotrophoblast and villous cytotrophoblast are producers of VIP, but EVT were not characterized. Immunostaining of placenta from 5 to 9 weeks of gestation confirmed VIP expression in syncytiotrophoblast and villous cytotrophoblast, in addition to which we found that it was highly expressed in HLA‐G+ cells in cytotrophoblast columns (Figure 1a).
Figure 1.
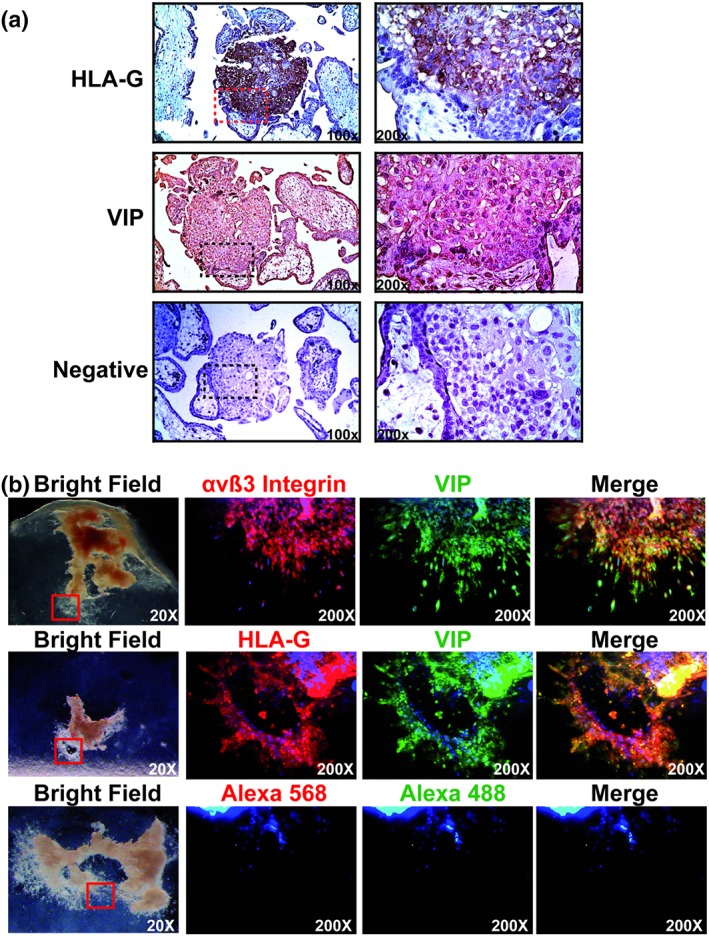
Columnar cells and EVT cells express VIP. (a) Serial placenta sections were stained with anti‐HLA‐G (1/100) or anti‐VIP (1/500) Abs and haematoxylin. The negative control was incubated with the secondary biotinylated Ab. Microphotographs were taken with an Olympus Microscope with 100× and 200× magnification (squared in the left panel). A representative of 10 different placentas at 5–9 weeks of gestation. (b) Five‐ to 9‐week placenta explants (n = 7) were isolated and plated on a collagen I matrix and cultured with DMEM:F12 10% FCS during 96 hr. The explants were immunostained with anti‐αVβ3 integrin (1/100), anti‐HLA‐G (1/100), or anti‐VIP (1/200) Abs, and the bright field microphotographs were taken with an Olympus microscope whereas the immunofluorescence photographs were taken with a Zeiss Clark microscope with Apotometer. The negative controls incubated with the Alexa's Abs (568 and 488 nm) are shown in the panel above. Ab: antibody; EVT: extravillous trophoblast; FCS: fetal calf serum; HLA‐G: human leukocyte antigen G; VIP: vasoactive intestinal peptide
3.2. EVT cells express VIP and it contributes to EVT outgrowth
Since the columnar cells were VIP+, we next explored if EVT were also expressing the polypeptide. Clusters of first‐trimester mesenchymal villi were explanted onto gels of collagen I, and EVT outgrowths measured after 96 hr. HLA‐G co‐localized with VIP in these cells (Figure 1b). αVβ3 integrin is a marker of invasive EVT (Zhou et al., 1997), and we detected some cells co‐expressing it with VIP. A small population of single cytotrophoblasts escapes from the periphery of the EVT outgrowths, and notably, these cells were all strongly VIP+ (Figure 1b). Moreover, those explants that showed more EVT outgrowths presented higher concentrations of VIP secretion compared to explants with low or absent EVT outgrowths (43 ± 5 vs. 9 ± 4 pg·ml−1; P < 0.05).
We next investigated whether exogenous VIP can increase EVT outgrowth from explants. Figure 2a shows that EVT outgrowth was highly increased by 100 nM of VIP, whereas the effect was prevented by an anti‐VIP neutralizing Ab and a PKA inhibitor, H89 (Figure 2b). In some explants, the inhibitory effects of the anti‐VIP Ab and/or H89 were complete, and no EVT outgrowths at all were observed.
Figure 2.
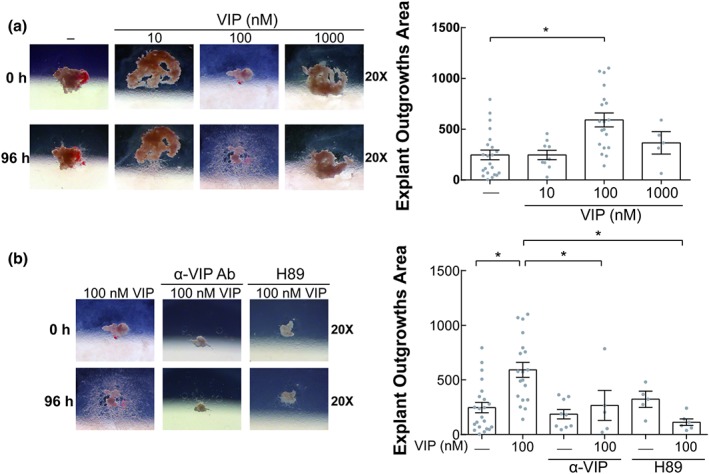
VIP increases EVT outgrowth in placenta explants through a PKA pathway. (a) Placenta explants were cultured in DMEM:F12 10% FCS without or with VIP (10, 100, and 1,000 nM) during 96 hr. In the left panel, representative microphotographs of the EVT outgrowths are shown. In the right panel, results of the EVT outgrowths area of 15 different placenta explants are expressed as mean ± SEM. Each point depicted represents an individual explant. *P < 0.05. Kruskal–Wallis test and post hoc Dunn's test. (b) The explants were cultured in the absence or presence of 100 nM VIP, neutralizing anti‐VIP (α‐VIP) Ab (1/1000), or H89 (5 μM) similar to panel (a). In the left panel, representative microphotographs are shown, and in the right panel, the results are expressed as mean ± SEM of the EVT outgrowth area of 15 different placenta explants. Kruskal–Wallis test and post hoc Dunn's test. EVT: extravillous trophoblast; FCS: fetal calf serum; VIP: vasoactive intestinal peptide
3.3. VIP deficiency in trophoblast cells reduces MMP and TIMP expression
Collagen degradation was often observed around explants suggesting that MMP activity might be a feature of the EVT phenotype that is affected by VIP. It is technically difficult to isolate and transiently transfect first‐trimester primary EVT cells with siRNA, so we next used cytotrophoblast cell lines to explore the expression of genes involved in ECM remodelling and their association with VIP. The cytotrophoblast cell line that expressed the VIP/VPAC system was transfected with siVIP or a Scrl sequences. Reduced levels of VIP expression (Figure 3b) and VIP production and secretion (Supporting information Figure S3a,b, respectively) were observed. Those cells treated with the siVIP revealed decreased expression of MMP‐2, MMP‐9, MMP‐14, MMP‐15, and TIMP‐2 as well as a trend to decrease TIMP‐1 in VIP‐deficient cells (Figure 3c). Moreover, a trend increase in MMP‐2 was also observed when BeWo cell line was cultured with VIP during 24 hr.
Figure 3.
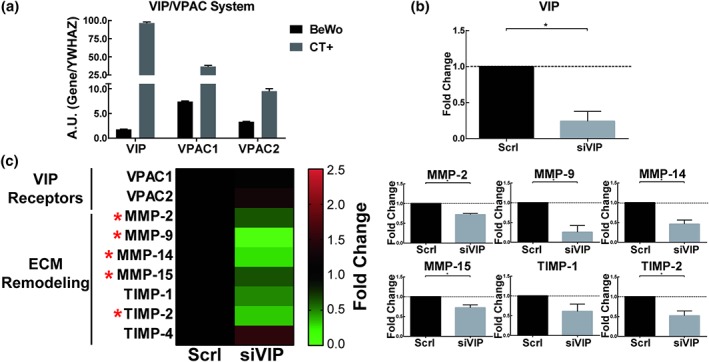
Down‐regulation of ECM remodelling gene expression in VIP‐deficient trophoblast cell lines. BeWo cell line at 60% of confluence was transfected with a VIP‐siRNA (siVIP) or with a Scrl sequence, and after 72 hr, the cells were harvested and used for RT‐qPCR. (a) Basal relative expression of VIP/VPACs is shown in BeWo cell line. Two highly positive controls (CT+) were used: the human epithelial colorectal adenocarcinoma cell line, CACO‐2 for VIP expression, and the human neuroblastoma cell line, SH‐SY5Y for VPAC receptors. Results are expressed as mean ± SEM of arbitrary units (A.U.) gene/housekeeping gene expression (n = 5). (b) The VIP expression in BeWo cell line transfected with VIP siRNA (siVIP) or scramble sequence (Scrl) were studied by qPCR. Results are expressed as mean ± SEM of fold change with respect to siVIP. P < 0.05 of seven independent experiments. (c) In the left panel, a double gradient heat map is shown: green for down‐regulated, black for no‐changes, and red for up‐regulated genes. In the right panel, the results are expressed as mean ± SEM of gene fold change (n = 5). In both, panels (b) and (c) the confidence interval was used to determine if the measurement in siVIP was statistically significant compared to Scrl. ECM, extracellular matrix; MMP, metalloproteinase; TIMP, tissue inhibitor of MMP; VIP, vasoactive intestinal peptide
3.4. EVT cells associated with tissue remodelling are VIP+
To investigate a possible role of VIP in the early stages of arterial remodelling by EVT, we next performed immunostaining assays in sections of decidua basalis at 5–9 weeks. Figure 4 shows a representative SpA in which the remodelling process has not yet started, as deduced from the observed integrity of vascular wall organization. HLA‐G+ EVT cells surrounding remodelled SpA were VIP+, and there were no infiltrating leukocytes. Vessels showing early‐stage remodelling (Figure 5a‐I) contained prominent luminal VIP+ cells associated with the disrupted organization of adjacent mural cells. Notably, HLA‐G+ EVT cells were also positive for VIP. In this arteriole, we also observed a faint expression of CD31, and there was no infiltration of immune cells. Figure 5a‐II shows a more extensively remodelled SpA. In these later stages of physiological changes, diameters were larger, and there were only a few residual vascular cells present and VIP+ cells lined the lumens. We confirmed that some of those VIP+ cells were also HLA‐G+. There were immune cells in the lumens and walls of these vessels and few EC. The difference between early and late SpA remodelling was evidenced by the loss of vascular smooth muscle cells (SMA+), partial loss of ECs (CD31) and the infiltration of immune cells (CD45).
Figure 4.
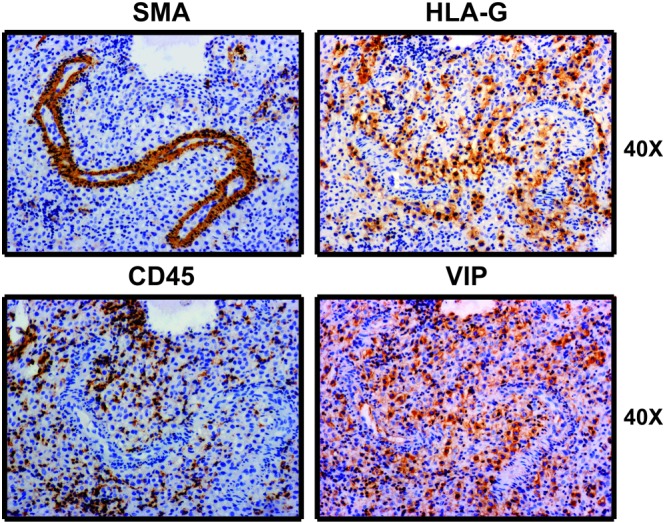
EVT cells surrounding spiral arterioles co‐express VIP and HLA‐G. Serial decidua sections were stained with anti‐HLA‐G, anti‐SMA, anti‐CD45, or anti‐VIP Abs and haematoxylin. Microphotographs were taken with an Olympus Microscope with 40× magnification. Images are representative of at least 15 SpA observed in five decidua basalis (5–9 weeks of pregnancy). Ab: antibody; CD: cluster differentiation; EVT: extravillous trophoblast; HLA‐G: human leukocyte antigen G; SMA: smooth muscle actin; SpA: spiral arterioles; VIP: vasoactive intestinal peptide
Figure 5.
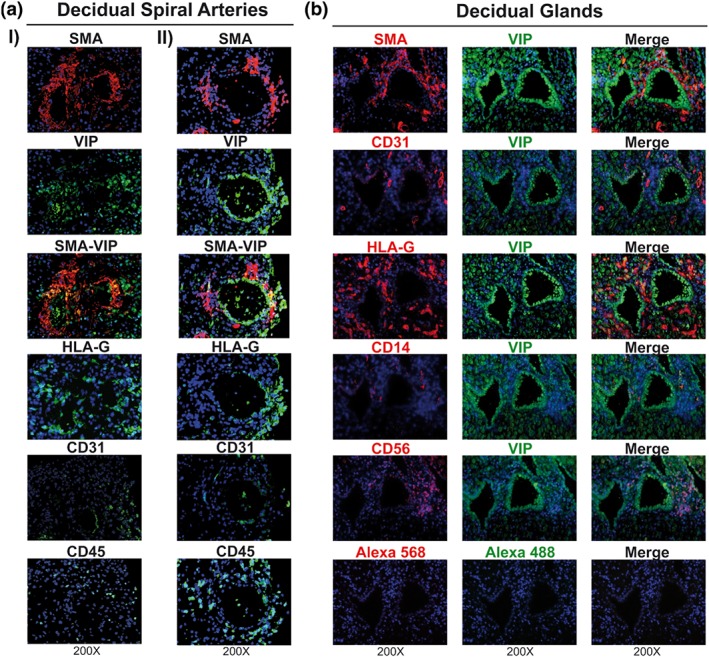
VIP+ EVT cells are associated with SpA remodelling and exocrine glands. (a) Decidua basalis were used to stain with anti‐HLA‐G, anti‐SMA, anti‐CD31, anti‐CD45, or anti‐VIP Abs to explore early (I) or late (II) SpA remodelled. Representative microphotographs of eight decidua basalis are shown. (b) Decidua basalis were stained with anti‐HLA‐G, anti‐SMA, anti‐CD31, anti‐CD14, anti‐CD56, or anti‐VIP Abs to explore maternal glands. The microphotographs were taken with Zeiss Clark microscope with apotometer. The negative controls incubated with the secondary Alexa's Abs (568 and 488 nm) are shown in the panel above. Representative microphotographs of six decidua basalis. Ab: antibody; CD: cluster differentiation; EVT: extravillous trophoblast; HLA‐G: human leukocyte antigen G; SMA: smooth muscle actin; SpA: spiral arterioles; VIP: vasoactive intestinal peptide
We also observed that decidual glandular epithelial cells were highly immunoreactive for VIP and that EVT cells within and adjacent to these glands co‐expressed HLA‐G and VIP. In addition, we observed the presence of abundant dNK cells and a few dMA in the proximity of the glands (Figure 5b).
3.5. VIP‐stimulated EVT cells regulate the inflammatory response
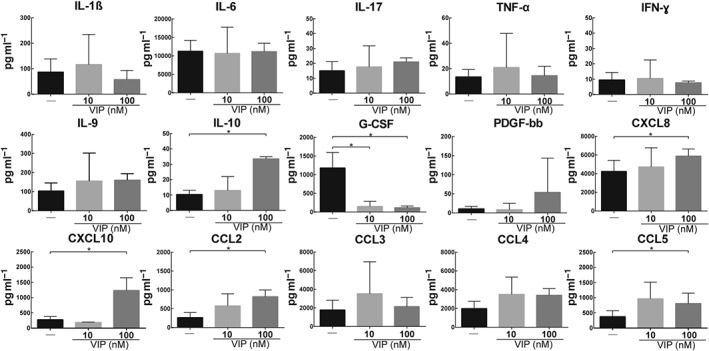
On the basis that EVT cells condition the immune micro‐environment, we wondered if VIP modified their secretion of cytokines. They produced pro‐implantation mediators (IL‐1β and IL‐6), low concentrations of pro‐inflammatory cytokines (IL‐17, TNF‐α, IFN‐γ), IL‐9, an anti‐inflammatory cytokine (IL‐10), PDGF‐BB, and several chemokines (CXCL8. CXCL10, CCL2, CCL3, CCL4, and CCL5). When the EVT cells were stimulated with VIP, there was an increased release of IL‐10 and most of the chemokines (CXCL8, CXCL10, CCL2, and CCL5) whereas granulocyte CSF (G‐CSF) was remarkably decreased (Figure 6).
Figure 6.
VIP modulation of the cytokine secretion by EVT cells. The supernatants obtained from the EVT outgrowths cultured without or with VIP (10 or 100 nM) after 96 hr and were used for a BioPlexPro™ Human Cytokine 27‐Plex Assay. The results are shown as the mean ± SEM. Friedman's test and post hoc Dunn's test (n = 6). EVT: extravillous trophoblast; VIP: vasoactive intestinal peptide
3.6. Functional modulation of dNK cells and dMA by VIP
Taking into account that dNK and dMA are the most abundant immune cells in the decidua during the first trimester and that EVT cells induce immune cell recruitment and condition their activation profile, we next studied the expression and activity of the VIP/VPAC system in these two cell populations. Both dNK and dMA expressed the VPAC1 and VPAC2 receptors, high affinity VIP receptors (Supporting Information Figure S4), but their expressions were not modulated by VIP (Figure 7a‐I,b‐I). We then assessed the immune micro‐environment by BioPlex assay of 27 cytokines in dNK and dMA treated or not with VIP. We observed a decrease in IL‐1β and CXCL8 levels with a trend towards increased IL‐10 in dNK cells (Figure 7a‐II). On the other hand, dMA showed no change in most pro‐inflammatory cytokines, a slight reduction in the secretion of IL‐12 and IL‐2 in parallel with an increment in IL‐10 (Figure 7b‐II). The full mean values of dNK and dMA cytokines expression panel are listed together in the Supporting Information Table S2.
Figure 7.
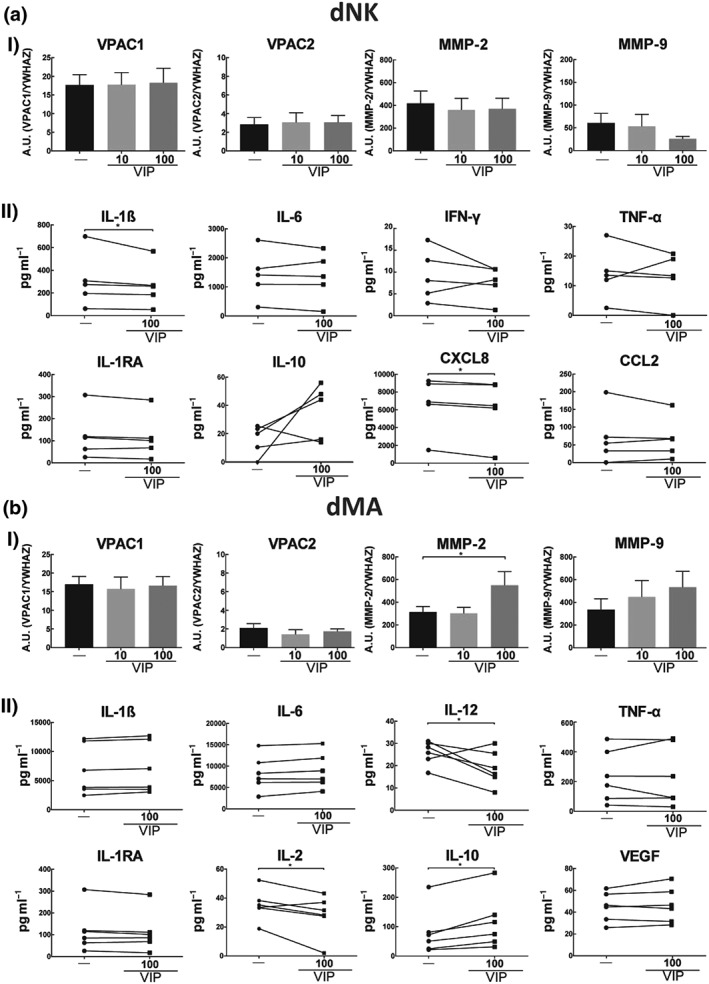
dNK cells and dMA express VIP receptors, and it regulates their functional profile. (a) 5 × 105 dNK cells or (b) 5 × 105 dMA were cultured in RPMI 0% FCS in the absence or presence of VIP (10 or 100 nM). (I) Cells were harvested and used for RT‐qPCR. Each point depicted represents an independent sample. Results are expressed as mean ± SEM of A.U. (gene/YWHAZ)*102. Friedman's test and Dunn's test were used to compare the results (n = 8). (II) The supernatants were used for the BioPlex assay. The results are shown as mean ± SEM. Mann–Whitney test with a post hoc Dunn's test were used to compare the results (n = 9). dMA: decidual macrophages; dNK: decidual natural killer; FCS: fetal calf serum; VIP: vasoactive intestinal peptide
As dNK cells and dMA have an important role in vascular remodelling (Choudhury et al., 2017; Smith et al., 2009), we explored the expression of MMP‐2 and MMP‐9 by RT‐qPCR. dNK cells expressed both MMPs, but they were not modulated by VIP (Figure 7a‐I). dMA also expressed both MMPs, and VIP increased the expression of MMP‐2 (Figure 7b‐I).
3.7. VIP regulates angiogenesis by direct and indirect mechanisms
A pro‐angiogenic effect has been reported for VIP in certain cancer cells (Casibang et al., 2001; Moody, Leyton, Casibang, Pisegna, & Jensen, 2002). A microarray assay showed that EC express both VIP and VIP receptors, and this was not changed by culture with EVT‐CM (Figure 8a). When EC cells in Geltrex® were treated with 100 nM of VIP for 8 hr, a significant reduction was observed in network formation (Figure 8b) as represented by a decrease in meshes and connected elements and an increase in isolated elements. The numbers of branches and pieces remained unchanged (Figure 8c).
Figure 8.
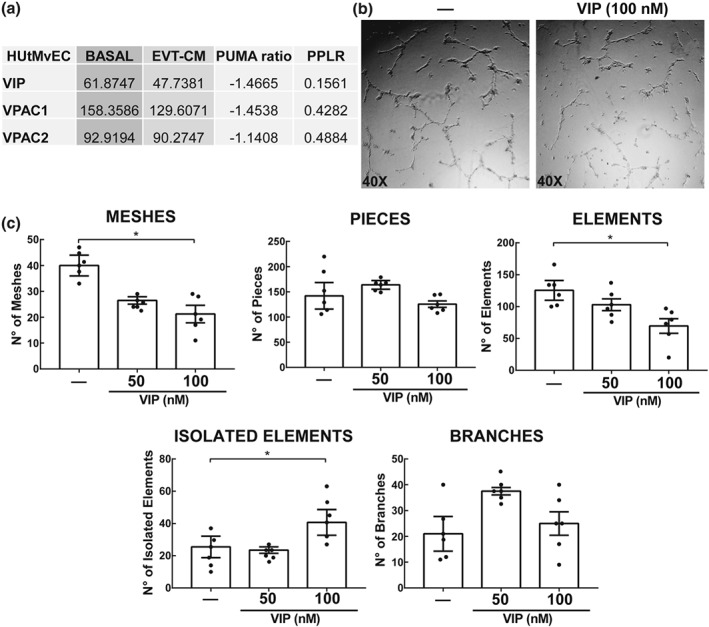
Expression of the VIP/VPAC system in endothelial cells and its effect on tube formation. (a) 1 × 105 HUtMvEC (EC) were cultured in 1:1 mixture of serum‐free EBM‐2 and either DMEM:F12 or EVT‐CM for 24 hr. The RNA was used for Affymetrix Human Genome U133 Plus 2.0 microarray. Expression intensity is measured in arbitrary units. Positional update and matching algorithm (PUMA) ratio (fold change) represents expression from control to treated cells. The PPLR score represents confidence in the observed changes, where PPLR score of 0.9 or 0.1 indicates a significant up‐regulation or down‐regulation respectively (n = 5). (b) 2 × 104 ECs cultured for 8 hr in a Geltrex matrix without or with VIP (50 or 100 nM). Representative microphotographs of EC tube formation ±100 nM VIP (n = 6). (c) Parameters obtained from Angiogenesis Analyser, ImageJ. Each point depicted represents an independent sample. The results are expressed as mean ± SEM of the number of parameter (meshes, pieces, elements, isolated elements, and branches) in macrophages (GM‐CSF). One‐way ANOVA and post hoc Dunn's test were used to compare the results (n = 6). CM: conditioned medium; EBM‐2: EC growth basal medium 2; EC: endothelial cells; EVT: extravillous trophoblast; MA: macrophages; PPLR: probability of positive log ratio; VIP: vasoactive intestinal peptide
Macrophages are maintained at constant levels throughout pregnancy and they are relevant in controlling angiogenesis (Mor et al., 2017). We have previously shown that peripheral blood monocytes differentiated with GM‐CSF (macrophages), when incubated with culture medium conditioned by either of the cytotrophoblast cell lines Swan 71 or HTR‐8/SVNeo pretreated with VIP (CM‐VIP), acquire an activation profile suggestive of an M2 phenotype and reminiscent of dMA (Paparini et al., 2015). We used the 3D gel assay to test the hypothesis that VIP modulates the angiogenic profile of macrophages either directly or indirectly by altering secretions from trophoblast. macrophages (GM‐CSF) treated with CM from either VIP‐stimulated first‐trimester trophoblast cell line or directly VIP‐stimulated dMA both caused a reduction in the number of meshes, pieces, and elements formed by EC, while the number of isolated segments and branches did not change. In contrast, when macrophages (GM‐CSF) were stimulated with VIP, there were increases in meshes, pieces, elements, and branches (Figure 9a–c). To further explore this difference, we performed RT‐qPCR in both types of macrophages. Despite the decrease in tube formation of dMA stimulated with VIP and macrophages (GM‐CSF) treated with CM (VIP), only in the last group was there a significant decrease in the pro‐angiogenic factor VEGF‐A. However, there was an increase in the expression of the anti‐angiogenic factor, thrombospondin‐1 (TSP‐1) in both groups. When macrophages (GM‐CSF) were stimulated with VIP, VEGF‐A expression did not change, whereas no TSP‐1 was detected.
Figure 9.
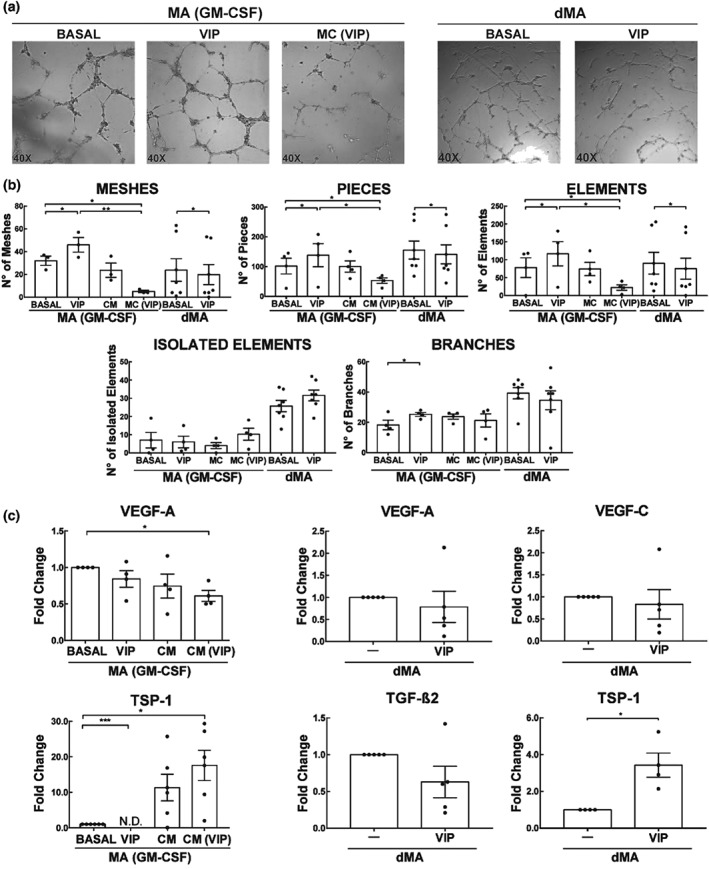
Effect of VIP on EC tube formation by dMA or trophoblast conditioned‐macrophages (GM‐CSF). (a) 2 × 104 EC were cultured for 8 hr in a Geltrex matrix with different treatments. Representative microphotographs of tube formation by macrophages (GM‐CSF) ± 100 nM VIP, CM or CM (VIP), and dMA treated or not with 100 nM of VIP. (b) Parameters obtained from Angiogenesis Analyser, ImageJ. The results are expressed as mean ± SEM of the number of the parameter (meshes, pieces, elements, isolated elements, and branches) in macrophages (GM‐CSF; n = 5) and dMA (n = 8). *P < 0.05. Friedman test and post hoc Dunn's test for macrophages (GM‐CSF) and Mann–Whitney for dMA were used to compare the results. (c) Pro‐ or anti‐angiogenic genes expression analysis in macrophages (G‐CSF) and dMA. The results are expressed as the mean ± SEM of arbitrary units (A.U.) *102, when Ct values were detected, when they were not (N.D.; n = 5). *P < 0.05. Friedman test and post hoc Dunn's test for macrophages (GM‐CSF) and Mann–Whitney for dMA were used to compare the results. CM: conditioned medium; dMA: decidual macrophages; EC: endothelial cells; MA: macrophages; VIP: vasoactive intestinal peptide
4. DISCUSSION
Here, we analysed VIP as a novel regulator of EVT cell function, vascular transformation, and immune modulation in human first‐trimester placenta. We propose that VIP synthesized in first‐trimester placenta assists the progress of EVT invasion and vessel remodelling in an immunologically “silent” milieu. Our results support that VIP expression is associated with an invasive phenotype in trophoblast cells and that it is involved in trophoblast‐mediated vascular transformation and in functional conditioning of dNK and macrophages. These conclusions are based on the following observations: HLA‐G+ columnar cells in anchoring villi and HLA‐G+/αVβ3 integrin+ EVT in placental explants express VIP. EVT outgrowth area increased after treatment with VIP in vitro, and the effect was prevented by either a VIP neutralizing Ab or a PKA pathway inhibitor. Second, EVT localized to the lumen and the adventitia of arteries undergoing active transformation are VIP+. Additionally, in SpA at later stages of remodelling, VIP+ EVT cells were found covering the lumen of the vessel. Likewise, uterine exocrine gland EVT cells also express VIP, appearing as another source of VIP within the decidua. Furthermore, CD45+ immune cells were detected in both SpA and exocrine glands containing VIP+ EVT cells. Third, VIP‐deficient cytotrophoblasts display a lower expression of MMP‐2, 9, 14, 15, and TIMP‐2, confirming a link between VIP endogenous levels and the acquisition of invasive features. Fourth, VIP conditions the cytokine profile of EVT cells, promoting an anti‐inflammatory milieu with higher levels of IL‐10 and chemokines and a pronounced decrease of G‐CSF. Fifth, the most abundant leukocytes present in the first‐trimester decidua, dNK cells and dMA, both express VIP receptors VPAC1 and VPAC2. Similar to the modulation observed in EVT cells, VIP regulated dNK and dMA cytokine secretion with an increase in IL‐10 and decreased or unchanged levels of pro‐inflammatory mediators such as IL‐12. Moreover, dMA treated with VIP showed increased expression of MMP‐2 and the anti‐angiogenic factor TSP‐1 and decreased EC tube formation, pointing to the contribution of VIP to ECM breakdown and angiogenesis balance mediated by dMA. Interestingly, macrophages differentiated in vitro and preconditioned with first‐trimester trophoblast factors and VIP presented a similar profile of dMA, with enhanced TSP‐1 expression and reduced EC tube formation. In the absence of macrophages or dMA‐derived factors, VIP by itself displayed anti‐angiogenic effects on EC as well.
Depending on the apparent routes and decidual structures invaded, distinct functional EVT sub lineages have been identified. Interstitial EVT cells spread within the decidual stroma and contribute to arterial remodelling from the outer side, with concomitant dedifferentiation and apoptosis of vascular smooth muscle cells (Harris & Aplin, 2007; Smith et al., 2009). In contrast, endovascular EVT cells express adhesion molecule receptors similar to vascular cells, aggregate to form plugs that block blood flow in SpA in the first trimester of pregnancy, and are thought to initiate remodelling from the luminal surface (Aplin et al., 2006; Choudhury et al., 2017; Harris et al., 2006). It is interesting to point out that VIP has vasodilating properties that may further contribute to EVT‐mediated transformation. VIP+ EVT cells in the decidual stroma, lining early and late transforming vessels and in uterine glands, may all represent local sources of VIP together with the glandular epithelium. It is noteworthy that explants cultured in the oxygen conditions of our present settings showed an association between amounts of VIP secreted and outgrowth area. Future work will address the interaction of oxygen and VIP actions on the placenta.
Our present results are in line with previous observations that EVT cells and dMA cooperate to effect vascular transformation and immune regulation (Choudhury et al., 2017; Mor et al., 2017). We have reported that trophoblast‐derived signals activate EC to release factors that attract macrophages and dNK into blood vessels (Choudhury et al., 2017). VIP activates trophoblast, EC, and macrophages to produce convergent signals for ECM breakdown and vascular transformation. In line with this, trophoblast VIP deficiency is associated with a reduction in MMP expression, consistent with previously described effects of VIP in trophoblast cell lines (Vota et al., 2016). VIP also has direct disruptive effects on vascular EC networks, as well as indirect effects mediated by macrophages, apparently involving the anti‐angiogenic effect of TSP‐1, and this emerges as a potentially novel mechanism in SpA remodelling. This adds to our previously reported finding that VIP enhances TSP‐1 expression in cytotrophoblast cell lines (Paparini et al., 2015).
Immune modulation is a further and relevant consequence of the effect of VIP on trophoblast and dMA. In particular, the observation that it conditions their cytokine profiles in first‐trimester placenta, with increased IL‐10 and chemokine secretion, extends and validates in an ex vivo human placental model our previous in vitro observations (Paparini et al., 2015). On the other hand, VIP induces a remarkable loss of G‐CSF production by first‐trimester placenta trophoblast. G‐CSF is a potent activator of neutrophils (Gabelloni, Trevani, Sabatté, & Geffner, 2013; Witko‐Sarsat et al., 2011). It is worth noting that the remodelling process entails a pro‐inflammatory response that is regulated, suggesting that neutrophil deactivating signals act at a local level during first trimester. In line with this, we have previously shown that trophoblast cells inhibit neutrophil extracellular trap formation and promote neutrophil apoptosis through VIP‐mediated pathways (Calo et al., 2017). A trophoblast contact‐dependent inhibition of the neutrophil oxidative burst has also been reported (Petty, Kindzelskii, Espinoza, & Romero, 2006). Taking together these observations, it is likely that inhibition of G‐CSF production might be involved in neutrophil deactivation at the maternal‐placental interface in vivo. A hypothetical model of VIP mechanisms in vivo is depicted in Figure 10.
Figure 10.
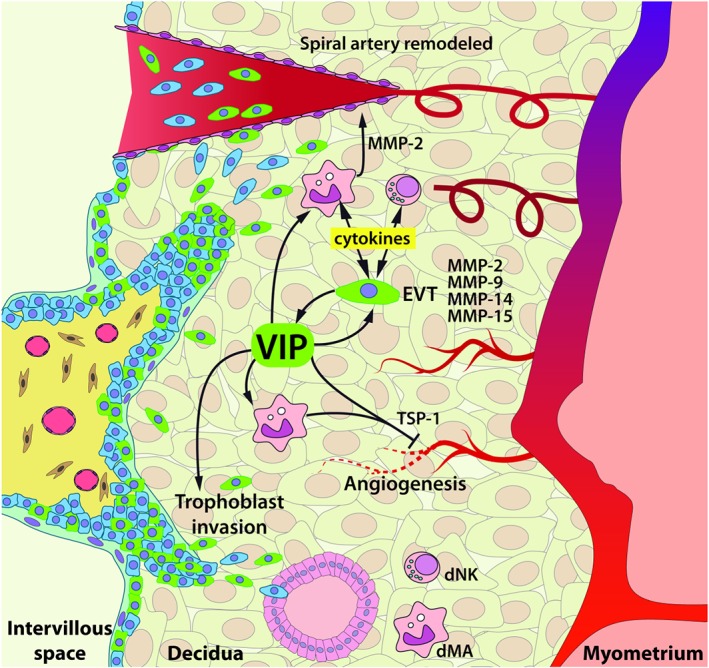
Hypothetical model of VIP effects in first‐trimester placenta. VIP+ EVT cells (green) contribute to ECM remodelling through MMP‐2, 9, 14, and 15 production and MMP‐2 by dMA primed with VIP. Along with this, the immune micro‐environment is shaped to a “silent” milieu with an upregulation of IL‐10 and chemokine production and decrease or no changes in pro‐inflammatory cytokines by EVT, dNK cells, and dMA. VIP+ EVT cells invade the decidual stroma, transform maternal spiral arteries, and are lining the glandular epithelium. VIP regulates EC tube formation directly or indirectly through dMA with high expression of TSP‐1. dMA: decidual macrophages; dNK: decidual natural killer; EC: endothelial cells; ECM: extracellular matrix; EVT: extravillous trophoblast; MMP: metalloproteinase; TSP‐1: thrombospondin‐1; VIP: vasoactive intestinal peptide
Growth factors delivered from maternal circulation, decidual cells, or the syncytium have been proposed to target trophoblast cells and the local vasculature for rescuing undergrown placentas (Aplin, 2010; Cureton et al., 2017; King et al., 2016). Results of the present ex vivo human placental model are in line with the observation that VIP treatment improved pregnancy outcome and restored immune homeostasis in the resorption‐prone CBAxDBA mating and the nonobese diabetic mice (Gallino et al., 2016; Hauk et al., 2014). Also, they are consistent with the results obtained when we treated a VIP gene haploinsufficiency murine pregnancy model with VIP as reported recently (Hauk et al., 2019). Taken together, these results endorse the potential of VIP as a therapeutic candidate for deep placentation disorders.
AUTHOR CONTRIBUTIONS
D.E.P., C.P.L., and J.D.A. designed the whole research; D.E.P. performed all the experiments. R.H.C. collaborated with dMA and dNK isolation and experiments. D.M.V. performed trophoblast cell transfection experiments; M.K‐B. collaborated with the Bioplex assay, and S.F‐S. contributed to placental explant cultures. E.N.G. and V.C.H. collaborated with GM‐CSF‐macrophages experiments. D.E.P., R.R., C.P.L., and J.D.A. analysed data, discussed the results, and wrote the paper.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, and Immunoblotting and Immunochemistry, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1: Primer sequences, product size and annealing temperature used in RT‐qPCR reactions.
Table S2: Cytokines BioPlex assay of dNK cells and dMA treated or not with VIP. 5 × 105 dNK cells or dMA were cultured in RPMI 0% FCS with or without 100 nM VIP. The supernatants were used for the BioPlex assay. The BioPlex Manager™ 6.0 software presented the concentration results in pg/ml. The results are shown as mean ± SEM of pg/ml. * P < 0.05. Mann–Whitney test with a post‐hoc Dunn test.
Figure S1: HUVEC tube formation analysed by angiogenesis analyser plugin. The parameters analysed are: in green (branches); magenta (elements); blue sky (meshes); blue (isolated elements, not shown) and pieces is the sum of elements, branches and isolated elements.
Figure S2: Flow chart of methods. Immunohistochemestry (IHC), Immunofluorescence (IF), Enzyme Immunoassay (EIA), Condition media (CM)
Figure S3: Decrease of VIP production and secretion in BeWo cell line treated with siVIP. BeWo cell line at 60% of confluence were transfected with a VIP‐siRNA (siVIP) or with a scramble sequence (Scrl), after 72 h the cells were harvested and used to explore the VIP production and secretion A) VIP production was studied by flow cytometry. The cells were harvested after culturing 4 h with GolgiStop. A representative dot plot with VIP positive cells of 5 independent experiments is shown. B) The supernatant was used for VIP EIA. Results are expressed as mean ± SEM of pg/ml. * P < 0.05 Student's t‐test of 7 independent experiments. Similar results were obtained with Swan 71 cell line.
Figure S4: Relative expression of VIP receptors in dNK cells and dMA. 5 × 105 dNK cells or dMA were cultured in RPMI 0% FCS with or without 100 nM VIP during 24 h. Basal gene expression of VPACs in dNK cells and dMA was explored by RT‐qPCR. The results are shown as mean ± SEM of A.U. (Gene/YWHAZ) of at least 8 different experiments. The human neuroblastoma cell line, SH‐SY5Y, cDNA was used as positive control (CT+) for VPACs expression.
ACKNOWLEDGEMENTS
We acknowledge the European Molecular Biologist Organization (EMBO) and the Boehringer Ingelheim Fonds for financial support of Daniel Paparini's short‐term stays in the Institute of Human Development, Maternal and Fetal Health Research Centre, University of Manchester. This work was funded by the National Agency of Sciences and Technology from Argentina (PICT 2014‐0657 and PICT 2017‐1536), UBACyT 20020170100317BA, and CONICET fellowship to Daniel Paparini. Ruhul Choudhury was supported in part by funding from the Canadian Institutes for Health Research. The Manchester Maternal and Fetal Health Research Centre is supported by Tommy's Fund, an Action Medical Research Endowment, and the Greater Manchester Clinical Research Network.
Paparini DE, Choudhury RH, Vota DM, et al. Vasoactive intestinal peptide shapes first‐trimester placenta trophoblast, vascular, and immune cell cooperation. Br J Pharmacol. 2019;176:964–980. 10.1111/bph.14609
Contributor Information
Claudia Pérez Leirós, Email: cpleiros@qb.fcen.uba.ar.
John D. Aplin, Email: john.aplin@manchester.ac.uk
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174(Suppl), S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. British Journal of Pharmacology, 174(S1), S225–S271. 10.1111/bph.13876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Overview. British Journal of Pharmacology, 174(S1), S1–S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsalem, H. , Kwan, M. , Hazan, A. , Zhang, J. , Jones, R. L. , Whittle, W. , … Dunk, C. E. (2014). Identification of a novel neutrophil population: Proangiogenic granulocytes in second‐trimester human decidua. Journal of Immunology (Baltimore, Md. : 1950), 193(6), 3070–3079. 10.4049/jimmunol.1303117 [DOI] [PubMed] [Google Scholar]
- Anacker, J. , Segerer, S. E. , Hagemann, C. , Feix, S. , Kapp, M. , Bausch, R. , & Kammerer, U. (2011). Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases. Molecular Human Reproduction, 17(10), 637–652. 10.1093/molehr/gar033 [DOI] [PubMed] [Google Scholar]
- Aplin, J. D. (2010). Developmental cell biology of human villous trophoblast: Current research problems. The International Journal of Developmental Biology, 54(2–3), 323–329. 10.1387/ijdb.082759ja [DOI] [PubMed] [Google Scholar]
- Aplin, J. D. , Straszewski‐Chavez, S. L. , Kalionis, B. , Dunk, C. , Morrish, D. , Forbes, K. , … Knöfler, M. (2006). Trophoblast differentiation: Progenitor cells, fusion and migration—A workshop report. Placenta, 27(Suppl A), S141–S143. 10.1016/j.placenta.2006.01.011 [DOI] [PubMed] [Google Scholar]
- Bischof, P. , Meisser, A. , & Campana, A. (2002). Control of MMP‐9 expression at the maternal‐fetal interface. Journal of Reproductive Immunology, 55(1–2), 3–10. [DOI] [PubMed] [Google Scholar]
- Boeldt, D. S. , & Bird, I. M. (2017). Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. The Journal of Endocrinology, 232(1), R27–R44. 10.1530/JOE-16-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosens, I. , Pijnenborg, R. , Vercruysse, L. , & Romero, R. (2011). The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. American Journal of Obstetrics and Gynecology, 204(3), 193–201. 10.1016/j.ajog.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer, J. N. , Innes, B. A. , Levey, J. , Robson, S. C. , & Lash, G. E. (2012). The role of vascular smooth muscle cell apoptosis and migration during uterine spiral artery remodeling in normal human pregnancy. The FASEB Journal, 26(7), 2975–2985. 10.1096/fj.12-203679 [DOI] [PubMed] [Google Scholar]
- Calo, G. , Sabbione, F. , Vota, D. , Paparini, D. , Ramhorst, R. , Trevani, A. , & Pérez Leirós, C. (2016). Trophoblast cells inhibit neutrophil extracellular trap formation and enhance apoptosis through vasoactive intestinal peptide‐mediated pathways. Human Reproduction, 32(1), 55–64. 10.1093/humrep/dew292 [DOI] [PubMed] [Google Scholar]
- Calo, G. , Sabbione, F. , Vota, D. , Paparini, D. , Ramhorst, R. , Trevani, A. , & Pérez Leirós, C. (2017). Trophoblast cells inhibit neutrophil extracellular trap formation and enhance apoptosis through vasoactive intestinal peptide‐mediated pathways. Human Reproduction, 32(1). 10.1093/humrep/dew292 [DOI] [PubMed] [Google Scholar]
- Casibang, M. , Purdom, S. , Jakowlew, S. , Neckers, L. , Zia, F. , Ben‐Av, P. , … Moody, T. W. (2001). Prostaglandin E2 and vasoactive intestinal peptide increase vascular endothelial cell growth factor mRNAs in lung cancer cells. Lung Cancer (Amsterdam, Netherlands), 31(2–3), 203–212. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11165399, 10.1016/S0169-5002(00)00168-9 [DOI] [PubMed] [Google Scholar]
- Choudhury, R. H. , Dunk, C. E. , Lye, S. J. , Aplin, J. D. , Harris, L. K. , & Jones, R. L. (2017). Extravillous trophoblast and endothelial cell crosstalk mediates leukocyte infiltration to the early remodeling decidual spiral arteriole wall. Journal of Immunology, 198(10), 4115–4128. 10.4049/jimmunol.1601175 [DOI] [PubMed] [Google Scholar]
- Cohen, M. , Meisser, A. , & Bischof, P. (2006). Metalloproteinases and human placental invasiveness. Placenta, 27(8), 783–793. 10.1016/j.placenta.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Cureton, N. , Korotkova, I. , Baker, B. , Greenwood, S. , Wareing, M. , Kotamraju, V. R. , … Harris, L. K. (2017). Selective targeting of a novel vasodilator to the uterine vasculature to treat impaired uteroplacental perfusion in pregnancy. Theranostics, 7(15), 3715–3731. 10.7150/thno.19678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, J. E. , Pollheimer, J. , Yong, H. E. J. , Kokkinos, M. I. , Kalionis, B. , Knöfler, M. , & Murthi, P. (2016). Epithelial‐mesenchymal transition during extravillous trophoblast differentiation. Cell Adhesion & Migration, 10(3), 310–321. 10.1080/19336918.2016.1170258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch, P. J. , Sun, Y. , & Kroog, G. S. (1990). Vasoactive intestinal peptide increases intracellular cAMP and gonadotropin‐alpha gene activity in JEG‐3 syncytial trophoblasts. Constraints posed by desensitization. The Journal of Biological Chemistry, 265(18), 10274–10281. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1693918 [PubMed] [Google Scholar]
- Fisher, S. J. (2015). Why is placentation abnormal in preeclampsia? American Journal of Obstetrics and Gynecology, 213(4 Suppl), S115–S122. 10.1016/j.ajog.2015.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraccaroli, L. , Alfieri, J. , Larocca, L. , Calafat, M. , Roca, V. , Lombardi, E. , … Pérez Leirós, C. (2009). VIP modulates the pro‐inflammatory maternal response, inducing tolerance to trophoblast cells. British Journal of Pharmacology, 156(1), 116–126. 10.1111/j.1476-5381.2008.00055.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraccaroli, L. , Grasso, E. , Hauk, V. , Paparini, D. , Soczewski, E. , Mor, G. , … Ramhorst, R. (2015). VIP boosts regulatory T cell induction by trophoblast cells in an in vitro model of trophoblast?maternal leukocyte interaction. Journal of Leukocyte Biology, 98(1), 49–58. 10.1189/jlb.1A1014-492RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelloni, M. L. , Trevani, A. S. , Sabatté, J. , & Geffner, J. (2013). Mechanisms regulating neutrophil survival and cell death. Seminars in Immunopathology, 35(4), 423–437. 10.1007/s00281-013-0364-x [DOI] [PubMed] [Google Scholar]
- Gallino, L. , Calo, G. , Hauk, V. , Fraccaroli, L. , Grasso, E. , Vermeulen, M. , … Ramhorst, R. (2016). VIP treatment prevents embryo resorption by modulating efferocytosis and activation profile of maternal macrophages in the CBAxDBA resorption prone model. Scientific Reports, 6(September 2015), 18633 10.1038/srep18633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganea, D. , Hooper, K. M. , & Kong, W. (2015). The neuropeptide vasoactive intestinal peptide: Direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiologica (Oxford, England), 213(2), 442–452. 10.1111/apha.12427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomariz, R. P. , Gutiérrez‐Cañas, I. , Arranz, A. , Carrión, M. , Juarranz, Y. , Leceta, J. , & Martínez, C. (2010). Peptides targeting Toll‐like receptor signalling pathways for novel immune therapeutics. Current Pharmaceutical Design, 16(9), 1063–1080. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20030612, 10.2174/138161210790963841 [DOI] [PubMed] [Google Scholar]
- Gressens, P. , Marret, S. , Hill, J. M. , Brenneman, D. E. , Gozes, I. , Fridkin, M. , & Evrard, P. (1997). Vasoactive intestinal peptide prevents excitotoxic cell death in the murine developing brain. The Journal of Clinical Investigation, 100(2), 390–397. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=508202&tool=pmcentrez&rendertype=abstract. 10.1172/JCI119545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar, A. J. , Arimura, A. , Gozes, I. , Journot, L. , Laburthe, M. , Pisegna, J. R. , … Waschek, J. A. (1998). International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase‐activating polypeptide. Pharmacological Reviews, 50(2), 265–270. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9647867 [PMC free article] [PubMed] [Google Scholar]
- Harmar, A. J. , Fahrenkrug, J. , Gozes, I. , Laburthe, M. , May, V. , Pisegna, J. R. , … Said, S. I. (2012). Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase‐activating polypeptide: IUPHAR review 1. British Journal of Pharmacology, 166(1), 4–17. 10.1111/j.1476-5381.2012.01871.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, L. K. , & Aplin, J. D. (2007). Vascular remodeling and extracellular matrix breakdown in the uterine spiral arteries during pregnancy. Reproductive Sciences, 14(8 suppl), 28–34. 10.1177/1933719107309588 [DOI] [PubMed] [Google Scholar]
- Harris, L. K. , Keogh, R. J. , Wareing, M. , Baker, P. N. , Cartwright, J. E. , Aplin, J. D. , & Whitley, G. S. J. (2006). Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a Fas ligand‐dependent mechanism. American Journal of Pathology, 169(5), 1863–1874. 10.2353/ajpath.2006.060265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk, V. , Azzam, S. , Calo, G. , Gallino, L. , Paparini, D. , Franchi, A. , … Pérez Leirós, C. (2014). Vasoactive intestinal peptide induces an immunosuppressant microenvironment in the maternal‐fetal interface of non‐obese diabetic mice and improves early pregnancy outcome. American Journal of Reproductive Immunology (New York, N.Y. : 1989), 71(2), 120–130. 10.1111/aji.12167 [DOI] [PubMed] [Google Scholar]
- Hauk, V. , Vota, D. , Gallino, L. , Calo, G. , Paparini, D. , Merech, F. , … Pérez Leirós, C. (2019). Trophoblast VIP deficiency entails immune homeostasis loss and adverse pregnancy outcome in mice. FASEB Journal, 33, 1801–1810. 10.1096/fj.201800592RR [DOI] [PubMed] [Google Scholar]
- Huppertz, B. (2008). Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension, 51(4), 970–975. 10.1161/HYPERTENSIONAHA.107.107607 [DOI] [PubMed] [Google Scholar]
- Khong, T. Y. , De Wolf, F. , Robertson, W. B. , & Brosens, I. (1986). Inadequate maternal vascular response to placentation in pregnancies complicated by pre‐eclampsia and by small‐for‐gestational age infants. British Journal of Obstetrics and Gynaecology, 93(10), 1049–1059. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3790464. 10.1111/j.1471-0528.1986.tb07830.x [DOI] [PubMed] [Google Scholar]
- King, A. , Ndifon, C. , Lui, S. , Widdows, K. , Kotamraju, V. R. , Agemy, L. , … Harris, L. K. (2016). Tumor‐homing peptides as tools for targeted delivery of payloads to the placenta. Science Advances, 2(5), e1600349 10.1126/sciadv.1600349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash, G. E. , Pitman, H. , Morgan, H. L. , Innes, B. A. , Agwu, C. N. , & Bulmer, J. N. (2016). Decidual macrophages: Key regulators of vascular remodeling in human pregnancy. Journal of Leukocyte Biology, 100(2), 315–325. 10.1189/jlb.1A0815-351R [DOI] [PubMed] [Google Scholar]
- Li, C. , & Wong, W. (2001). Model‐based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proceedings of the National Academy of Sciences, 98(1), 31–36. 10.1073/pnas.011404098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Milo, M. , Lawrence, N. D. , & Rattray, M. (2005). A tractable probabilistic model for Affymetrix probe‐level analysis across multiple chips. Bioinformatics, 21(18), 3637–3644. 10.1093/bioinformatics/bti583 [DOI] [PubMed] [Google Scholar]
- Marzioni, D. , Fiore, G. , Giordano, A. , Nabissi, M. , Florio, P. , Verdenelli, F. , … Castellucci, M. (2005). Placental expression of substance P and vasoactive intestinal peptide: evidence for a local effect on hormone release. The Journal of Clinical Endocrinology & Metabolism, 90(4), 2378–2383. 10.1210/jc.2004-1512 [DOI] [PubMed] [Google Scholar]
- Moody, T. W. , Leyton, J. , Casibang, M. , Pisegna, J. , & Jensen, R. T. (2002). PACAP‐27 tyrosine phosphorylates mitogen activated protein kinase and increases VEGF mRNAs in human lung cancer cells. Regulatory Peptides, 109(1–3), 135–140. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12409225. 10.1016/S0167-0115(02)00196-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor, G. , Aldo, P. , & Alvero, A. B. (2017). The unique immunological and microbial aspects of pregnancy. Nature Reviews Immunology, 17(8), 469–482. 10.1038/nri.2017.64 [DOI] [PubMed] [Google Scholar]
- Mor, G. , Cardenas, I. , Abrahams, V. , & Guller, S. (2011). Inflammation and pregnancy: The role of the immune system at the implantation site. Annals of the New York Academy of Sciences, 1221(1), 80–87. 10.1111/j.1749-6632.2010.05938.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paparini, D. , Grasso, E. , Calo, G. , Vota, D. , Hauk, V. , Ramhorst, R. , & Pérez Leirós, C. (2015). Trophoblast cells primed with vasoactive intestinal peptide enhance monocyte migration and apoptotic cell clearance through αvβ3 integrin portal formation in a model of maternal–placental interaction. Molecular Human Reproduction, 21(12), 930–941. 10.1093/molehr/gav059 [DOI] [PubMed] [Google Scholar]
- Pearson, R. D. , Liu, X. , Sanguinetti, G. , Milo, M. , Lawrence, N. D. , & Rattray, M. (2009). PUMA: A bioconductor package for propagating uncertainty in microarray analysis. BMC Bioinformatics, 10(1), 211 10.1186/1471-2105-10-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty, H. R. , Kindzelskii, A. L. , Espinoza, J. , & Romero, R. (2006). Trophoblast contact deactivates human neutrophils. Journal of Immunology (Baltimore, Md. : 1950), 176(5), 3205–3214. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16493081 [DOI] [PubMed] [Google Scholar]
- Pijnenborg, R. , Anthony, J. , Davey, D. A. , Rees, A. , Tiltman, A. , Vercruysse, L. , & van Assche, A. (1991). Placental bed spiral arteries in the hypertensive disorders of pregnancy. British Journal of Obstetrics and Gynaecology, 98(7), 648–655. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1883787 [DOI] [PubMed] [Google Scholar]
- Pijnenborg, R. , Bland, J. M. , Robertson, W. B. , & Brosens, I. (1983). Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta, 4(4), 397–413. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6634666. 10.1016/S0143-4004(83)80043-5 [DOI] [PubMed] [Google Scholar]
- PrabhuDas, M. , Bonney, E. , Caron, K. , Dey, S. , Erlebacher, A. , Fazleabas, A. , … Yoshinaga, K. (2015). Immune mechanisms at the maternal‐fetal interface: Perspectives and challenges. Nature Immunology, 16(4), 328–334. 10.1038/ni.3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson, A. , Harris, L. K. , Innes, B. A. , Lash, G. E. , Aljunaidy, M. M. , Aplin, J. D. , … Bulmer, J. N. (2012). Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 26(12), 4876–4885. 10.1096/fj.12-210310 [DOI] [PubMed] [Google Scholar]
- Romero, R. , Dey, S. K. , & Fisher, S. J. (2014). Preterm labor: One syndrome, many causes. Science, 345(6198), 760–765. 10.1126/science.1251816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said, S. I. (2007). The discovery of VIP: Initially looked for in the lung, isolated from intestine, and identified as a neuropeptide. Peptides, 28(9), 1620–1621. 10.1016/j.peptides.2007.06.007 [DOI] [PubMed] [Google Scholar]
- Smith, S. D. , Dunk, C. E. , Aplin, J. D. , Harris, L. K. , & Jones, R. L. (2009). Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. The American Journal of Pathology, 174(5), 1959–1971. 10.2353/ajpath.2009.080995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spessotto, P. , Bulla, R. , Danussi, C. , Radillo, O. , Cervi, M. , Monami, G. , … Colombatti, A. (2006). EMILIN1 represents a major stromal element determining human trophoblast invasion of the uterine wall. Journal of Cell Science, 119(Pt 21), 4574–4584. 10.1242/jcs.03232 [DOI] [PubMed] [Google Scholar]
- Straszewski‐Chavez, S. L. , Abrahams, V. M. , Alvero, A. B. , Aldo, P. B. , Ma, Y. , Guller, S. , … Mor, G. (2009). The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta, 30(11), 939–948. 10.1016/j.placenta.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka, K. , Shiokawa, S. , Miyazaki, T. , Kuji, N. , Tanaka, M. , & Yoshimura, Y. (1997). Integrins and reproductive physiology: Expression and modulation in fertilization, embryogenesis, and Implantation. Fertility and Sterility, 67(5), 799–811. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9130881. 10.1016/S0015-0282(97)81388-X [DOI] [PubMed] [Google Scholar]
- Vota, D. , Paparini, D. , Hauk, V. , Toro, A. , Merech, F. , Varone, C. , … Pérez Leirós, C. (2016). Vasoactive intestinal peptide modulates trophoblast‐derived cell line function and interaction with phagocytic cells through autocrine pathways. Scientific Reports, 6 10.1038/srep26364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschek, J. (2013). VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. British Journal of Pharmacology, 169(3), 512–523. 10.1111/bph.12181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witko‐Sarsat, V. , Pederzoli‐Ribeil, M. , Hirsch, E. , Hirsh, E. , Sozzani, S. , & Cassatella, M. A. (2011). Regulating neutrophil apoptosis: New players enter the game. Trends in Immunology, 32(3), 117–124. 10.1016/j.it.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Wright, J. K. , Dunk, C. E. , Amsalem, H. , Maxwell, C. , Keating, S. , & Lye, S. J. (2010). HER1 signaling mediates extravillous trophoblast differentiation in humans. Biology of Reproduction, 83(6), 1036–1045. 10.1095/biolreprod.109.083246 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Dunk, C. E. , Kwan, M. , Jones, R. L. , Harris, L. K. , Keating, S. , & Lye, S. J. (2017). Human dNK cell function is differentially regulated by extrinsic cellular engagement and intrinsic activating receptors in first and second trimester pregnancy. Cellular & Molecular Immunology, 14(2), 203–213. 10.1038/cmi.2015.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Fisher, S. J. , Janatpour, M. , Genbacev, O. , Dejana, E. , Wheelock, M. , & Damsky, C. H. (1997). Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? The Journal of Clinical Investigation, 99(9), 2139–2151. 10.1172/JCI119387 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Primer sequences, product size and annealing temperature used in RT‐qPCR reactions.
Table S2: Cytokines BioPlex assay of dNK cells and dMA treated or not with VIP. 5 × 105 dNK cells or dMA were cultured in RPMI 0% FCS with or without 100 nM VIP. The supernatants were used for the BioPlex assay. The BioPlex Manager™ 6.0 software presented the concentration results in pg/ml. The results are shown as mean ± SEM of pg/ml. * P < 0.05. Mann–Whitney test with a post‐hoc Dunn test.
Figure S1: HUVEC tube formation analysed by angiogenesis analyser plugin. The parameters analysed are: in green (branches); magenta (elements); blue sky (meshes); blue (isolated elements, not shown) and pieces is the sum of elements, branches and isolated elements.
Figure S2: Flow chart of methods. Immunohistochemestry (IHC), Immunofluorescence (IF), Enzyme Immunoassay (EIA), Condition media (CM)
Figure S3: Decrease of VIP production and secretion in BeWo cell line treated with siVIP. BeWo cell line at 60% of confluence were transfected with a VIP‐siRNA (siVIP) or with a scramble sequence (Scrl), after 72 h the cells were harvested and used to explore the VIP production and secretion A) VIP production was studied by flow cytometry. The cells were harvested after culturing 4 h with GolgiStop. A representative dot plot with VIP positive cells of 5 independent experiments is shown. B) The supernatant was used for VIP EIA. Results are expressed as mean ± SEM of pg/ml. * P < 0.05 Student's t‐test of 7 independent experiments. Similar results were obtained with Swan 71 cell line.
Figure S4: Relative expression of VIP receptors in dNK cells and dMA. 5 × 105 dNK cells or dMA were cultured in RPMI 0% FCS with or without 100 nM VIP during 24 h. Basal gene expression of VPACs in dNK cells and dMA was explored by RT‐qPCR. The results are shown as mean ± SEM of A.U. (Gene/YWHAZ) of at least 8 different experiments. The human neuroblastoma cell line, SH‐SY5Y, cDNA was used as positive control (CT+) for VPACs expression.


