Abstract
Gut microbiota (GM) is a collection of bacteria, fungi, archaea, viruses and protozoa, etc. They inhabit human intestines and play an essential role in human health and disease. Close information exchange between the intestinal microbes and the host performs a vital role in digestion, immune defence, nervous system regulation, especially metabolism, maintaining a delicate balance between itself and the human host. Studies have shown that the composition of GM and its metabolites are firmly related to the occurrence of various diseases. More and more researchers have demonstrated that the intestinal microbiota is a virtual ‘organ’ with endocrine function and the bioactive metabolites produced by it can affect the physiological role of the host. With deepening researches in recent years, clinical data indicated that the GM has a significant effect on the occurrence and development of cardiovascular diseases (CVD). This article systematically elaborated the relationship between metabolites of GM and its effects, the relationship between intestinal dysbacteriosis and cardiovascular risk factors, coronary heart disease, myocardial infarction, heart failure and hypertension and the possible pathogenic mechanisms. Regulating the GM is supposed to be a potential new therapeutic target for CVD.
Keywords: cardiovascular diseases, gut microbiota, metabolites, risk factors, treatment
1. THE ROLE OF gut microbiota IN HOST PHYSIOLOGY
Many microbes inhabit the human gastrointestinal tract including viruses and bacteria, fungi and protists, which make up the intestines’ symbiotic microbes.1 The number of bacteria carried by the human body is approximate to about 1014, mostly including anaerobes and the composition of the gut microbiome is similar up to the phylum level (mainly Bacteroidetes and Firmicutes), but the diversity and richness of species are variable between individuals.2, 3, 4, 5 These bacteria can be roughly classified into three categories according to their effects on the human body: (a) Physiological bacteria (symbiotic with the host), such as Bifidobacterium, Lactobacillus. (b) Conditional pathogens, such as Enterobacteriaceae, Enterococcus. (c) Pathogens, such as Proteus, Staphylococcus aureus. The microbiota in the gut has many crucial functions in human health and affects the host via various distinct pathways. Studies have shown that the gut microbiota (GM) is directly involved in the body's nutrient absorption, growth and development, biological barriers, immune regulation, metabolism and many other aspects.6, 7, 8
2. METABOLITES AND EFFECTS OF GM
Metabolites produced by GM include trimethylamine‐N‐oxide (TMAO), bile acids (BAs), short‐chain fatty acids (SCFAs), protocatechuic acid (PCA) and other metabolites, such as phenylacetylglutamine, p‐cresyl sulfate, indoxyl sulfate (IS), enterolactone and H2S.
2.1. Trimethylamine‐N‐oxide
Trimethylamine‐N‐oxide is a biologically active molecule and a putative promoter of chronic diseases including atherosclerosis in humans.9 In the human body, TMAO is established as trimethylamine (TMA). TMA comes directly from foods rich in choline, lecithin and L‐carnitine, such as red meat, eggs, dairy products and saltwater fish.10, 11 Most of TMA is absorbed into the bloodstream and then rapidly oxidized to TMAO by flavin‐containing monooxygenase‐3 (FMO3), a hepatic enzyme.10 TMAO can affect lipid metabolism causing accumulation of cholesterol in cells and it can also have a direct impact on platelet function, promote an inflammatory response and so on. The effect of the TMAO will accelerate the development of atherosclerosis and the level of plasma TMAO and its associated metabolites are directly proportional to the risk of cardiovascular disease (CVD), which will be pointed out in the following sections.12, 13, 14, 15, 16
2.2. Bile acids
Bile acids are amphipathic molecules synthesised from cholesterol in the liver. This process is an important way to eliminate cholesterol from the human body. After eating, BAs stored in the gallbladder are secreted into the intestine to assist the emulsification of dietary fats and assist the intestinal absorption of lipid nutrients and lipid‐soluble vitamins.17, 18 BA itself regulates metabolism, mainly through two receptors, namely the nuclear farnesyl X receptor (FXR) and the G protein‐coupled receptor TGR5.17, 19 BAs can control intestinal bacterial overgrowth, in turn, intestinal bacteria can regulate host metabolism by metabolism BAs.20 Recent studies have shown that BA has a pleiotropic and hormonal activity that regulates lipid and glucose metabolism, controls inflammation and fibrosis, maintains vascular integrity, restores the intestinal barrier and controls ageing and circadian rhythms.19 Further studies have confirmed that FXR activation can reduce intestinal ischemia‐reperfusion injury and preserve internal structure and permeability. Also, FXR agonists decrease the release of pro‐inflammatory cytokines, reduce autophagy inhibition and modulate obesity and related metabolic phenotypes.21, 22
2.3. Short‐chain fatty acids
Short‐chain fatty acids primarily originate from the fermentation of dietary fiber in the gut and they are subsequently absorbed into the bloodstream of the host, in which they interact with host proteins, thereby affecting host physiology.23 Acetate, propionate and butyrate (roughly 60:25:15 in the colon) account for 80% of the SCFA produced by intestinal microflora.24 In addition to their important role as fuel for colonic epithelial cells, SCFAs also modulate different cell signal transduction processes via G protein‐coupled receptors GPR43 and GPR41.23, 25 Recent evidence has suggested that SCFAs also play an important role in glucose and lipid metabolism and butyrate has been demonstrated to decrease LPS translocation, inhibit macrophage activation, thus reducing the production of inflammatory factors and reactive oxygen species (ROS) production.26, 27, 28, 29 SCFAs can also inhibit the inflammatory reaction by reducing the migration and proliferation of immune cells, reducing various cytokines and inducing apoptosis. Therefore, SCFA is considered to have an anti‐inflammatory effect.30
2.4. Protocatechuic acid
Protocatechuic acid is chemically known as 3,4‐dihydroxybenzoic acid and is one of the main metabolites of complex polyphenols such as anthocyanins and procyanidins that are normally found in high concentrations in vegetables and fruit, such as onions, plums, gooseberries and grapes.31, 32 More and more evidence support that PCA can play diverse biological effects by acting on different molecular targets, such as antioxidant, anti‐inflammatory and anti‐hyperglycemic and neuroprotective activity.32 PCA has been implicated in the progression of atherosclerosis. Some scholars concluded that PCA could inhibit OA‐induced vascular smooth muscle cells proliferation, improve the endogenous antioxidant capacity of macrophages and promote cell proliferation and cell survival via IGF‐I signalling.33, 34, 35 Besides, studies have demonstrated that PCA can improve cardiac function and cardiac autonomic balance, prevent cardiac mitochondrial dysfunction and increase anti‐apoptotic protein.36 In a recent experimental study on Type 2 diabetes mouse models it was found that, PCA could significantly stimulate glucose metabolism in skeletal muscle, regulate glycemic and lipid status, reduce the secretion of pro‐inflammatory cytokines.37
2.5. Phenylacetylglutamine, P‐cresyl sulfate, IS, enterolactone and H2S
Proteins in the intestine are metabolized by harmful microorganisms (such as Clostridium perfringens) to produce harmful amines, phenols and ammonia, causing human diseases and discomfort, which have a great impact on the human cardiovascular system.38, 39 Studies have found that phenylacetylglutamine and p‐cresyl sulphateare lower in patients with non‐coronary heart disease and p‐cresyl sulphate is a significant independent predictor of carotid plaque burden.40 Escherichia coli‐based intestinal flora produce a large amount of quinone by metabolizing the tryptophan in food and further oxidize it to indophenol under the action of microbial oxidase. Similar to TMAO, indophenols in the liver are sulphonatedto form IS.41 Excessive accumulation of IS may affect oxidative stress in cardiomyocytes, induce myocardial cell damage and cause vascular endothelial cell damage to inhibit self‐repair and increase vascular access thrombosis.42 Enterolactone is produced primarily by intestinal digestion of fibre‐rich foods. In a meta‐analysis, it was found that the higher risk of acute coronary accidents was lower in patients with higher serum enterolactone and therefore the lower enterolactone may be another risk factor for coronary heart disease (CHD).43 In addition, some sulphate‐reducing bacteria in the human intestinal tract produce a large amount of H2S using sulphate as a substrate. Studies indicate that H2S acts as a gas transmitter in the human body. H2S is an essential mediator of various physiological processes in the human body including cell protection, vasodilation, angiogenesis, blood pressure regulation and heart rate reduction. It plays a major role in CVD (Figure 1).44, 45
Figure 1.
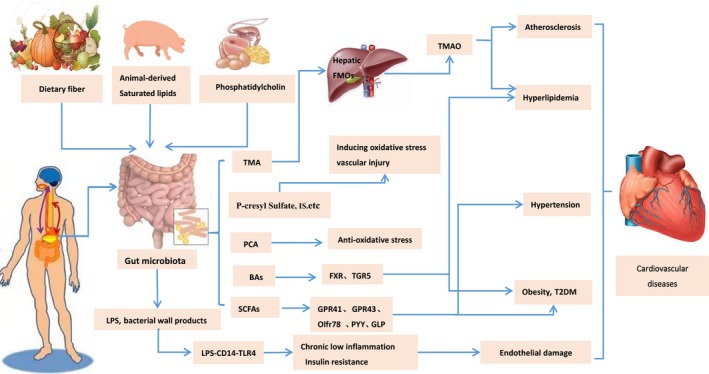
Gut microbiota and its metabolites linked to cardiovascular diseases
3. INTESTINAL DYSBACTERIOSIS AND CARDIOVASCULAR RISK FACTORS
Hyperlipidaemia, obesity, diabetes and atherosclerosis are important risk factors for CVD. Plenty of evidence indicates that the GM is closely related to these four risk factors.
3.1. Hyperlipidaemia
A recent survey found that the GM composition can explain 6.0% of the variation in triglycerides and 4.0% of that in HDL‐C and 4.5% of that in BMI, independent of age, sex and genetics at the human population level.46 Studies have also found that individuals with low microbial richness have increased fasting triglycerides and decreased HDL‐C. Reverse cholesterol transport (RCT) is a key pathway involving the return of excess cholesterol from peripheral tissues to the liver to excretion of bile and eventually to faeces.47 In a recent article, a researcher found that TMAO reduced the expression of cholesterol 7‐alpha‐hydroxylase 1 (CYP7a1), the major BA synthetic enzyme in the catabolism of cholesterol, whose reaction is the rate‐limiting step. This finding may in part explain the impact of TMAO on RCT and upregulation of CYP7a1 has been reported to lead to increasing RCT and reduced atherosclerosis in susceptible mice.48, 49 Recent research shows that BA‐mediated activation of FXR inhibits the activity of CYP7a1 leading to RCT disorders and elevated cholesterol, but reduced triglycerides.17, 50 However, there are also studies showing that FXR knockout mice exhibit high‐density lipoprotein metabolism and RCT leading to elevated cholesterol.51 Recent studies have found that TMAO can cause cholesterol accumulation in cells by increasing the expression of the scavenger receptors, CD36 and SR‐A1 and the formation of foam cells.16, 30 In conclusion, intestinal flora metabolites are closely related to lipid metabolism, but its mechanism of action needs further investigation.
3.2. Obesity and type 2 diabetes mellitus
In 2013, Liou et al transferred caecal contents from RYGB donors to sterile mice and showed that the recipient mice showed significant weight loss indicating a correlation between GM and obesity.52 Study shows that Enterobacter cloacae B29 is part of the direct cause of obesity, which is the first internationally recognized ‘fat bacteria.’53 Recent study indicates the characteristics of the flora associated with obesity, namely the increase in the number of Firmicutes and the reduction of Bacteroides. Similarly, this situation can occur in type 2 diabetes as well.54, 55 GM may cause obesity by reducing fasting‐induced adipokine factor (Fiaf) expression. Mandard et al56 found that Fiaf gene over‐expressing mice showed a 50% reduction in body fat storage compared to wild‐type mice. Intestinal flora can also stimulate the production of various inflammatory factors through the LPS‐CD14‐TLR4 pathway leading to chronic systemic inflammation and further cause obesity and insulin resistance. Animal study showed57 that high‐fat‐fed mice had increased LPS Gram‐negative bacteria in the intestine, elevated plasma LPS levels, causing metabolic endotoxemia and obesity and continued subcutaneous injection of LPS in mice also induced the above reaction. CD14 gene knockdown can reduce the inflammatory state of mice caused by LPS; TLR4 gene knockout can also prevent obesity and insulin resistance caused by high‐fat diet, thereby reducing obesity.58, 59 Obesity is often a leading factor associated with type 2 diabetes mellitus (T2DM), so the above mentioned mechanisms of intestinal flora causing obesity can also increase the risk of T2DM. By combining with GPR41 and GPR43, SCFA can promote intestinal L cells to secrete GLP‐1 and peptide YY (PYY). GLP‐1 can increase glucose‐dependent insulin secretion and promote islet cell proliferation. PYY can improve the survival and function of islet cells. Therefore, intestinal flora disorder can affect the occurrence and development of diabetes by affecting SCFA.60 There is evidence in other studies that activation of FXR and TGR5 mediated by BA can increase insulin sensitivity and regulate GLP‐1 secretion. However, its pathways are complex, many studies use animal models and the formation of a complete BA signalling pathway system needs further study.61 BA can also indirectly activate signal molecules such as inducible nitric oxide synthase, interleukin 18 (IL‐18) and fibroblast growth factor 19 (FGF‐19).62 Related study63 shows that FGF‐19 can improve glucose tolerance, reduce weight gain and increase the metabolic rate. So BA plays a major role in the occurrence and development of diabetes mellitus.
3.3. Atherosclerosis
Hyperlipidaemia is an independent risk factor for atherosclerosis. In the above discussion, we have discussed the effects of GM on lipid metabolism, which we will not state it here. In addition, studies have shown that TMAO can also directly affect platelet function and increase thrombosis risk. When platelets are directly exposed to high levels of TMAO, the intracellular storage of Ca2+ is released, which enhances platelet activation via a variety of agonists (thrombin, ADP, or collagen).15 There are also studies showing that TMAO enhanced the platelet reactivity and thrombosis risk and promoted vascular inflammation by activating the NLRP3 inflammasome.16 Using pyrosequencing to analyse the microbial species of atherosclerotic plaques and intestinal microflora in the same atherosclerotic patients, the results show that several bacteria exist simultaneously, suggesting that bacteria found in the plaque may be derived from intestinal flora.64 In another study using shotgun sequencing method it was found that atherosclerosis patients were rich in Collinsell, and healthy people were rich in Roseburia and Eubacterium.65 The above results suggest that the GM is directly related to the occurrence of atherosclerosis. In the above section, we have already mentioned that BA‐mediated FXR and SCFA, PCA have anti‐inflammatory effects and can regulate glucose metabolism; so the intestinal flora can also inhibit the development of atherosclerosis. However, the core mechanism that causes atherosclerosis remains to be further clarified.
4. GUT MICROBIOTA AND CARDIOVASCULAR DISEASE
4.1. Coronary heart disease
Coronary heart disease is one of the most common CVD in clinical practice, which is extremely harmful to human health. Some scholars have found that plasma TMAO levels are elevated in patients with coronary heart disease and the structure of intestinal flora is also changed and manifested by the decrease of Lactobacillus and Bacillus.66 A research showed that the structural changes in the GM could be used as a diagnostic marker for coronary heart disease.67 Using a high‐choline diet to feed ApoE‐/‐ C57BL mice, results display that higher the serum TMAO level in mice, larger was the area of the sizeable atherosclerotic plaque .68 Tang et al69 were followed up for 3 years in more than 4000 patients with coronary angiography and results showed that the concentration of plasma TMAO was positively correlated with the risk of the cardiovascular endpoint. Among all 2235 patients with coronary heart disease, the all‐cause mortality rate of TMAO (>6.5 mol/L) was 3.9 times higher than that of patients at a low level (<2.5 mol/L). At the same time, independent of the traditional predictors (hypersensitivity C reactive protein, myeloperoxidase, estimated glomerular filtration rate, etc.), TMAO can predict the long‐term risk of death in patients with coronary heart disease. From the perspective of metabonomics, the intestinal microflora metabolite TMAO indirectly proves the clinical prognostic value of intestinal microecology for coronary heart disease. TMAO can also be used to assess the degree of plaque burden of coronary heart disease.70 In short, the current research on TMAO is thorough and the level of TMAO is expected to become the standard for diagnosis and risk assessment of patients with coronary heart disease.
4.2. Myocardial infarction
Different from some other chronic CVD, acute myocardial infarction (AMI) is a critical stress injury to the body. Under this trauma, does the structure of the intestinal microbiota and the metabolites undergo stress changes? In myocardial infarction (MI) patients, typical intestinal flora disorders will occur, probiotics will decrease significantly, pathogenic bacteria will multiply in large numbers and a series of changes such as impaired intestinal barrier function and increased intestinal permeability will occur in the body. Analysing the blood flora of 49 healthy controls, 50 stable coronary heart disease and 100 ST‐segment elevations myocardial infarction (STEMI) patients, the researchers found that after STEMI, the abundance and diversity of blood flora were increased and more than 12% of the blood bacteria were from the intestinal flora. This indicates that a significant increase in intestinal flora in plasma is associated with systemic inflammation and cardiovascular adverse events after STEMI.71 Animal studies showed that AMI rats were parallel to intestinal barrier injury and intestinal flora richness was significantly higher than the sham group. Therefore, the intervention of intestinal flora structure to improve the clinical prognosis of AMI may be a new method of AMI treatment.72 Study has shown that probiotics can reduce cardiac hypertrophy in MI rats.73 Further study also showed that antibiotics could improve the area of MI in AMI mice, which may be related to the regulation of intestinal flora. However, the above study does not show if improvement in the structure of intestinal flora can prevent coronary atherosclerosis and reduce the incidence of AMI.74 In the MI model, left ventricular dysfunction and intestinal perfusion inadequacy, reduce tight junction protein expression, intestinal mucosa damage, increased intestinal permeability, antibiotic treatment to eliminate the intestinal bacterial translocation. It can reduce systemic inflammatory reaction and myocardial injury in model mice.71 Recently, Tang et al75 found that supplementation of SCFA can improve the physiology and survival of ABX (antibiotic‐treated mice) mice after MI and this study also found that MI caused a decrease in Lactobacillus. This indicates that the composition of intestinal flora and SCFA have obvious repair effect on heart tissue after MI. Therefore, the stability of intestinal flora may be of great significance to the rehabilitation of patients with MI.
4.3. Heart failure
As shown in recent studies, changes in intestinal microecology can directly damage cardiac muscle cells and cause cardiac dysfunction. In the mouse model of dietary intervention, it was found that elevated serum TMAO levels in mice resulted in cardiac injury and fibrosis and increased TMAO levels were prone to heart failure (HF).76 There are also studies which found that the levels of TMAO, choline and betaine were involved in left ventricular diastolic dysfunction.77 Also, studies found that, higher the TMAO level in patients with HF, higher the mortality rate in 5 years. Compared with the risk factors for HF, heart and kidney index and systemic inflammatory markers, TMAO can better evaluate the prognostic value.78 After a 1‐year follow‐up of 972 patients with acute HF, investigators found that serum TMAO levels could predict adverse prognostic events, whereas TMAO+NT‐proBNP could predict a higher value.79 A clinically controlled cohort study showed that serum TMAO levels were elevated in patients with chronic HF, and TMAO levels were associated with cardiac function and survival.80 Besides, chronic HF patients had significantly more pathogenic bacteria and Candida than healthy controls, which is a result concluded by multiple researchers. The inflammatory, intestinal permeability and right atrial pressure of these chronic HF patients were significantly increased and these were signals of venous congestion. Also, these correlations were stronger in patients with moderate to severe HF (NYHA heart function III‐IV) than those with mild HF (NYHA cardiac function I‐II).81 Therefore, improving intestinal flora and lowering TMAO level are both expected to improve the prognosis of patients with HF.
4.4. Hypertension
Recent findings show that SCFA bind the G protein‐coupled receptors GPR41 and GPR43 and olfactory receptors olfO78 in the kidney, heart, sympathetic ganglia and blood vessels to modulate blood pressure.82, 83 SCFA can induce the release of renin from the afferent arteriole and increase the blood pressure, which is mediated by Olfo78. This, in turn can be counteracted by the vasodilator action of GPR43.82, 84 GPR41 can increases energy expenditure by stimulating the sympathetic nervous system, but this could also lead to an increase in blood pressure.85 Other researchers have found that TMAO can increase blood pressure and hydrogen sulphate can directly act on blood vessels to modulate blood pressure.86, 87 Studies have shown that high salt may induce hypertension by inducing T helper 17 (TH17) cells, and Lactobacillus can reduce the number of TH17 cells and prevent the deterioration of salt‐sensitive hypertension.88 The Firmicutes and a Bacteroidetes ratio (F/B) was recently reported being increased in spontaneously hypertensive rats and AngII‐induced hypertension rats.89 Some scholars believe that GM facilitates AngII‐induced vascular dysfunction and hypertension, at least in part, by supporting an MCP‐1/IL‐17 driven vascular immune cell infiltration and inflammation.90 In addition, obstructive sleep apnoea (OSA) can increase the risk of systemic hypertension in individuals. Studies have demonstrated that intestinal flora imbalance may be a direct cause of OSA‐induced hypertension.91 There are also studies that confirm probiotics and their fermentation products have been shown in many studies to inhibit the production of nitrogen oxides in macrophages, reduce the species of ROS and increase the absorption of dietary calcium to lower blood pressure (Table 1).92
Table 1.
Association of gut microbiota and related metabolites with cardiovascular disease
| Hyperlipidaemia | Obesity, T2DM | Atherosclerosis | Coronary heart disease | Myocardial infarction | Heart failure | Hypertension | |
|---|---|---|---|---|---|---|---|
| Structural changes in the gut microbiota | Enterobacteria↑ Probiotics↓ | Firmicutes↑Bacteroides↓ | Collinsella↑Roseburiam↓ | Lactobacillus↓Bacillus↓ | Probiotics↓Pathogenic bacteria↑ | CandidaEscherichia coli | Firmicutes/Bacteroidetes ratio↑ |
| Major metabolites | TMAOFXR | SCFABAs | TMAOFXRPCA | TMAO H2SPhenylacetylglutamine,P‐cresyl sulphateIndoxyl sulphate, Enterolactone | SCFA | TMAOIndoxyl sulphate | SCFATMAOH2S |
| Possible mechanism of action | TMAO and FXR significantly inhibits RCT;CYP7a1↓CD36↑SR‐A1↑Foam cells↑Cholesterol↑ | Chronic low‐grade inflammation and insulin resistance;LPS ↑Fiaf↓GLP‐1↓PYY↓FGF‐19↓ | TMAO can directly affect platelet function and increase thrombosis risk;Cholesterol↑NLRP3 ↑FXR↓PCA↓ | TMAO↑Abnormal glucose and lipid metabolism;InflammationPhenylacetylglutamine↑P‐cresyl sulphate↑Indoxyl sulphate↑ Enterolactone ↑ | Gut permeability;Intestinal flora disorder;Lactate ↑LPS↑SCFA↓ | TMAO↑Left ventricle diastolic dysfunction↑Myocardial fibrosis ↑ | SCFA can regulate blood pressure with GPR41, GPR43 and Olfr78;Infusionof Ang II/TMAO associated with blood pressure;H2S can dilate blood vessels and reduce heart rate |
5. THE ROLE OF GUT MICROBIOTA IN CARDIOVASCULAR DISEASE
Gut microbiota is an essential micro‐ecosystem in the human body and even some scholars believe it is the ‘virtual endocrine organ’ of the human body. Summarizing the above and combining current research progress, the mechanism of intestinal flora affecting CVD mainly includes three aspects including: (a) Intestinal flora disorder leads to bacterial endotoxin translocation and promotes the release of inflammatory factors leading to an inflammatory response; (b) intestinal flora disorder leads to abnormal metabolism of substances, which causes CVD such as lipid, glucose and tryptophan metabolism; (c) intestinal flora disorder promotes oxidative stress in the body and aggravates the development of CVD. As the first two mechanisms have already been discussed in the previous discussion. In next section, the discussion would be concentrating on oxidative stress.
Gut microbiota is also involved in the metabolism of purine and uric acid (UA). For example, xanthine dehydrogenase, the key enzyme responsible for the oxidative metabolism of purines is generated by the secretion of Escherichia coli in intestinal bacteria.93 Therefore, the decomposing activity of GM on UA is positively related to the content of Escherichia coli. The study found that the number of E. coli in patients with coronary heart disease increased, blood UA levels increased significantly, whereas high concentrations of UA showed prooxidation. Increased blood UA levels can lead to high level nitrite/nitrate in the blood, decreased bioavailability of NO and oxidative stress.68, 94 There are also studies showing high UA decreased cardiomyocyte viability and increased ROS production in cardiomyocytes.95 In addition, carotenoids, as antioxidants have an anti‐angina effect. The carotenoid genes were increased in healthy patients compared with arteriosclerosis patients.65 The GM disorder causes the decrease of the bacteria containing the synthetic carotenoid gene, the reduction of the level of carotenoid and the weakening of the antioxidant effect, thus promoting the development of AS. There have been studies that rigorously characterize the effect of probiotics on antioxidation regarding the intestinal microbiota composition. Probiotics may modulate the redox status of the host via their metal ion chelating ability, antioxidant systems, regulating signalling pathways, enzyme producing ROS and intestinal microbiota.96 In addition to the above mechanisms, intestinal microflora can also increase the level of nitric oxide through nitrate reduction in the non‐enzymatic pathway.39 At the same time, changes in the structure of GM, also affect the expression and activity of some other molecules acting on the blood such as the AMP‐activated protein kinase, hypoxia‐inducible factor‐1α and peroxisome proliferator‐activated receptor‐γ coactivator‐1α.97 These molecules can promote angiogenesis in skeletal muscle and other parts. Thus, intestinal microflora can not only affect the course of AS but also have potential effects on vascular dilatation, angiogenesis and blood supply.
6. THE TARGET OF FUTURE TREATMENT
At present, the improvement or reversal of intestinal microflora has become a hot spot for CVD, which includes the faecal microbiota transplantation, dietary regulation, probiotics, prebiotics, antibiotic intervention and so on.
6.1. Dietary regulation
Dietary regulation has been proven to be an effective strategy to reduce cardiovascular risk. The Mediterranean diet refers to the eating styles of vegetables, fruits, fish, Cereals, beans and olive oil in the southern European countries on the Mediterranean coast, such as Greece, Spain, France and southern Italy. This diet is especially popular in recent years and is confirmed to prevent CVD and reduce the mortality of CVD in men and women.98 Studies have shown that the level of TMAO in the urine of patients who did not adhere to the Mediterranean diet increased.99 In addition, high fibre diet and acetic acid (fibrous fermentation) can reduce the proportion of F/B, increase the number of bacteriobacterium, reduce myocardial fibrosis in hypertensive mice and prevent the progress of hypertension and HF.100
6.2. Probiotics and prebiotics
Probiotics in animals mainly include lactic acid bacteria, bifidobacteria, actinomycetes, yeasts and so on. Probiotics can inhibit inflammation, protect and repair the intestinal mucosal barrier and improve intestinal function. Probiotics are active microorganisms, which are beneficial to host health. The atherosclerotic plaque of the aorta was less than the control group after feeding the probiotic rhamnosus GG (LGG) to the ApoE‐/‐ mice fed with a high‐fat diet and various biomarkers including endotoxin were improved to some extent.101 Studies showed that after 3 months of treatment with Brucella, patients with chronic HF showed a decrease in creatinine, UA, hsCRP and a decrease in the diameter of the left atrium and a higher left ventricular ejection fraction (LVEF).102 Research has also shown that probiotics can directly absorb cholesterol and reduce the content of cholesterol in the medium and in the process of the growth of probiotics such as lactic acid bacteria, it can promote the metabolism of cholesterol, thus reducing the cholesterol content.103 Prebiotics are a dietary supplement (including isomalt oligosaccharides, oligosaccharides, Bifidus factors, etc.). They has a beneficial effect on the host by selectively stimulating the growth and activity of bacteria. A recent study has shown that probiotics can improve metabolic syndrome and infiltration of small intestinal cells.104 However, probiotics remain at risk of infection and their safety needs further study.
6.3. Fecal microbiota transplantation
Faecal microbiota transplantation (FMT) is a treatment that introduces flora or metabolites in donor faeces into diseased receptors to correct intestinal microecological imbalances and rebuild normal intestinal function. A meta‐analysis speculates that faecal transplantation may be more effective than probiotics in restoring intestinal flora, because faecal flora infusion can overcome the short‐term efficacy of probiotics and make a permanent change in flora.105 At present, FMT has achieved ideal efficacy in the treatment of recurrent or refractory Clostridium difficile infection. Recently, FMT has also been tested as an emerging therapy to manage cardiometabolic disorders. Vrieze et al106 performed FMT treatment in patients with metabolic syndrome and the results showed that insulin sensitivity and intestinal acid production in the faecal donor group were significantly elevated suggesting that FMT could be a potential therapeutic strategy for metabolic‐related diseases. In addition, in a randomized controlled trial, researchers transplanted the faeces of thinner people into fatter ones and 55% of the participants showed improved insulin resistance after 6 weeks.107 However, the use of FMT is currently limited because of its associated risks including the possible transfer of endotoxin or dangerous drugs that may cause new gastrointestinal complications. Further studies are needed to test whether FMT can extend to other aspects of cardiac metabolic disorders.1
6.4. Antibiotics
Antibiotic therapy has greatly disrupted the short‐term and long‐term microbial balance including reducing the richness and diversity of bacterial flora. Murphy et al108 fed mice with vancomycin and the results showed that the number of Firmicutes and Bacteroides decreased significantly, whereas the number of Proteus increased significantly. Tiihonen et al109 reported that the use of non‐steroidal anti‐inflammatory drugs affects the composition of intestinal flora in elderly samples and the use of anti‐inflammatory drugs can reduce the content of isobutyric acid, isovaleric acid and L‐lactic acid. But there are also studies showing that Vancomycin can change the intestinal flora richness, reduce MI area and improve the recovery of mechanical function after ischemia/reperfusion injury in rats.110 Polymyxin B and tobramycin can reduce the endotoxin content of Gram‐negative bacteria in the intestine and faeces and the contents of IL‐1beta, IL‐6 and TNF‐alpha in vivo in patients with HF.111 However, polymyxin B has been clinically restricted because of its toxicity. This situation suggests that we should pay attention to the side effects of antibiotics as well as their clinical effects. The improper use of antibiotics can kill beneficial bacteria in vivo, make pathogenic bacteria resistant and bring various adverse reactions. At present, how to clarify the mechanism of adverse reactions of antibiotics and maximize its clinical curative therapeutic effect is still an problem to be solved.
6.5. TMA inhibitors and other intervention methods
Research shows that choline structural analogues (3,3‐dimethyl‐1‐butanol) can block the choline metabolic pathway, reduce TMA production and play a role in the prevention and treatment of CVD.112 However, studies have shown that drugs that inhibit production of TMA‐FMOs can lead to acute hepatitis and fish odour syndrome.68 Another recent study shows that resveratrol can stimulate the growth of beneficial bacteria in the intestinal tract through the reconstitution of intestinal microflora, thus decreasing the production of TMAO.113 In addition, for colectomy, a study showed that patients with an average age of 45 years of age survived 1000 days after colectomy and compared with other five groups of non‐gastrointestinal surgery patients, colectomy did not reduce the risk of CVD and only reduced the risk of hypertension.114
7. CONCLUSION
Intestinal microecology is the largest microorganism system represented by intestinal microflora and the intestinal microflora together with the host maintains its microecological balance. Once the intestinal microflora is out of balance, it can quickly lead to a series of pathological and physiological changes including CVD. We have a new understanding of the pathogenesis of CVD and the prevention and treatment of CVD, especially for the development of new microecological agents in the clinical trial stage. At present, the research on intestinal microecology and CVD is mostly based on the basis and the internal mechanism is not fully understood. Therefore, the primary and clinical study of intestinal microecology and CVD needs to be further carried out, especially for guiding treatment of coronary heart disease and HF.
CONFLICTS OF INTEREST
The authors confirm that there are no conflicts of interest.
ACKNOWLEDGEMENT
This work was supported by the National Natural Science Foundation of China (no. 81603559).
Jia Q, Xie Y, Lu C, et al. Endocrine organs of cardiovascular diseases: Gut microbiota. J Cell Mol Med. 2019;23:2314‐2323. 10.1111/jcmm.14164
Jia and Xie contributed equally to this work.
Contributor Information
Shichao Lv, Email: 372272027@qq.com.
Junping Zhang, Email: tjzhtcm@163.com.
REFERENCES
- 1. Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242‐249. [DOI] [PubMed] [Google Scholar]
- 3. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cani PD, Delzenne NM. Gut microflora as a target for energy and metabolic homeostasis. Curr Opin Clin Nutr Metab Care. 2007;10:729‐734. [DOI] [PubMed] [Google Scholar]
- 7. Cerf‐Bensussan N, Gaboriau‐Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10:735‐744. [DOI] [PubMed] [Google Scholar]
- 8. Nicholson JK, Holmes E, Kinross J, et al. Host‐gut microbiota metabolic interactions. Science. 2012;336:1262‐1267. [DOI] [PubMed] [Google Scholar]
- 9. Zeisel SH, Warrier M. Trimethylamine N‐oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr. 2017;37:157‐181. [DOI] [PubMed] [Google Scholar]
- 10. Cho CE, Caudill MA. Trimethylamine‐N‐oxide: friend, foe, or simply caught in the cross‐fire? Trends Endocrinol Metab. 2017;28:121‐130. [DOI] [PubMed] [Google Scholar]
- 11. Koeth RA, Levison BS, Culley MK, et al. γ‐Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L‐carnitine to TMAO. Cell Metab. 2014;20:799‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warrier M, Shih DM, Burrows ACM, et al. The TMAO‐generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015;10:326‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spector R. New insight into the cause of atherosclerosis: implications for pharmacology. J Pharmacol Exp Ther. 2016;358:103‐108. [DOI] [PubMed] [Google Scholar]
- 14. Seldin MM, Meng Y, Qi H, et al. Trimethylamine N‐oxide promotes vascular inflammation through signaling of mitogen‐activated protein kinase and nuclear factor‐kB. J Am Heart Assoc. 2016;5:e002767 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen ML, Zhu XH, Ran L, et al. Trimethylamine‐N‐oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3‐SOD2‐mtROS signaling pathway. J Am Heart Assoc. 2017;6:e002238 10.1161/JAHA.117.006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res. 2012;53:1723‐1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Begley M, Sleator RD, Gahan CG, Hill C. Contribution of three bile‐associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes. Infect Immun. 2005;73:894‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adorini L, Schoonjans K, Friedman SL. Megatrends in bile acid receptor research. Hepatol Commun. 2017;1:831‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chiang JYL, Ferrell JM. Bile acid metabolism in liver pathobiology. Gene Expr. 2018;18:71‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang L, Xie C, Nichols RGL, et al. Farnesoid X receptor signaling shapes the gut microbiota and controls hepatic lipid metabolism. mSystems. 2016;1:e00070 10.1128/mSystems.00070-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ceulemans LJ, Verbeke L, Decuypere JP, et al. Farnesoid X receptor activation attenuates intestinal ischemia reperfusion injury in rats. PLoS ONE. 2017;12:e0169331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang W, Zhou L, Guo H, Xu Y. The role of short‐chain fatty acids in kidney injury induced by gut‐derived inflammatory response. Metabolism. 2017;68:20‐30. [DOI] [PubMed] [Google Scholar]
- 24. Marques FZ, Mackay CR, Kaye DM. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol. 2018;15:20‐32. [DOI] [PubMed] [Google Scholar]
- 25. Puddu A, Sanguineti R, Montecucco F, Viviani GL. Evidence for the gut microbiota short‐chain fatty acids as key pathophysiological molecules improving diabetes. Mediators Inflamm. 2014;2014:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Esgalhado M, Kemp JA, Damasceno NR, et al. Short‐chain fatty acids: a link between prebiotics and microbiota in chronic kidney disease. Future Microbiol. 2017;12:1413‐1425. [DOI] [PubMed] [Google Scholar]
- 27. Liu B, Qian J, Wang Q, et al. Butyrate protects rat liver against total hepatic ischemia reperfusion injury with bowel congestion. PLoS ONE. 2014;9:e106184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim CH, Park J, Kim M. Gut microbiota‐derived short‐chain fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woting A, Blaut M. The intestinal microbiota in metabolic disease. Nutrients. 2016;8:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin MC, Ou TT, Chang CH. Protocatechuic acid inhibits oleic acid‐induced vascular smooth muscle cell proliferation through activation of AMP‐activated protein kinase and cell cycle arrest in G0/G1 phase. J Agric Food Chem. 2015;63:235‐241. [DOI] [PubMed] [Google Scholar]
- 32. Masella R, Santangelo C, D'Archivio M, et al. Protocatechuic acid and human disease prevention: biological activities and molecular mechanisms. Curr Med Chem. 2012;19:2901‐2917. [DOI] [PubMed] [Google Scholar]
- 33. Kang Z, Zhu H, Jiang W, Zhang S. Protocatechuic acid induces angiogenesis through PI3K‐Akt‐eNOS‐VEGF signalling pathway. Basic Clin Pharmacol Toxicol. 2013;113:221‐227. [DOI] [PubMed] [Google Scholar]
- 34. Varì R, D'Archivio M, Filesi C, et al. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK‐mediated Nrf2 activation in murine macrophages. J Nutr Biochem. 2011;22:409‐417. [DOI] [PubMed] [Google Scholar]
- 35. Ju DT, Liao HE, Shibu MA, et al. Nerve regeneration potential of protocatechuic acid in RSC96 Schwann cells by induction of cellular proliferation and migration through IGF‐IR‐PI3K‐Akt signaling. Chin J Physiol. 2015;58:412‐419. [DOI] [PubMed] [Google Scholar]
- 36. Semaming Y, Kumfu S, Pannangpetch P, et al. Protocatechuic acid exerts a cardioprotective effect in type 1 diabetic rats. J Endocrinol. 2014;223:13‐23. [DOI] [PubMed] [Google Scholar]
- 37. Bhattacharjee N, Dua TK, Khanra R, et al. Protocatechuic acid, a phenolic from Sansevieria roxburghiana leaves, suppresses diabetic cardiomyopathy via stimulating glucose metabolism, ameliorating oxidative stress, and inhibiting inflammation. Front Pharmacol. 2017;8:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomasova L, Konopelski P, Ufnal M. Gut bacteria and hydrogen sulfide: the new old players in circulatory system homeostasis. Molecules. 2016;21:pii: E1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tiso M, Schechter AN. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE. 2015;10:e0119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bogiatzi C, Gloor G, Allen‐Vercoe E, et al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis. 2018;273:91‐97. [DOI] [PubMed] [Google Scholar]
- 41. Ramezani A, Massy ZA, Meijers B, et al. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. 2016;67:483‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chitalia VC, Shivanna S, Martorell J, et al. Uremic serum and solutes increase post‐vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation. 2013;127:365‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rienks J, Barbaresko J, Nothlings U. Association of polyphenol biomarkers with cardiovascular disease and mortality risk: a systematic review and meta‐analysis of observational studies. Nutrients. 2017;9:415‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dombkowski RA, Russell MJ, Olson KR. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am J Physiol Regul Integr Comp Physiol. 2004;286:678‐685. [DOI] [PubMed] [Google Scholar]
- 45. Nagpure BV, Bian JS. Interaction of hydrogen sulfide with nitric oxide in the cardiovascular system. Oxid Med Cell Longev. 2016;2016:6904327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fu J, Bonder MJ, Cenit MC, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117:817‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakaya K, Ikewaki K. Microbiota and HDL metabolism. Curr Opin Lipidol. 2018;29:18‐23. [DOI] [PubMed] [Google Scholar]
- 48. Miyake JH, Duong‐Polk XT, Taylor JM, et al. Transgenic expression of cholesterol‐7‐alpha‐hydroxylase prevents atherosclerosis in C57BL/6J mice. Arterioscler Thromb Vasc Biol. 2002;22:121‐126. [DOI] [PubMed] [Google Scholar]
- 49. Zong C, Yu Y, Song G, et al. Chitosan oligosaccharides promote reverse cholesterol transport and expression of scavenger receptor BI and CYP7A1 in mice. Exp Biol Med (Maywood). 2012;237:194‐200. [DOI] [PubMed] [Google Scholar]
- 50. Parseus A, Sommer N, Sommer F, et al. Microbiota‐induced obesity requires farnesoid X receptor. Gut. 2017;66:429‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miyazaki‐Anzai S, Masuda M, Levi M, et al. Dual activation of the bile acid nuclear receptor FXR and G‐protein‐coupled receptor TGR5 protects mice against atherosclerosis. PLoS ONE. 2014;9:e108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liou AP, Paziuk M, Luevano JM Jr. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kvit KB, Kharchenko NV. Gut microbiota changes as a risk factor for obesity. Wiad Lek. 2017;70:231‐235. [PubMed] [Google Scholar]
- 55. Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non‐diabetic adults. PLoS ONE. 2010;5:e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mandard S, Zandbergen F, van Straten E, et al. The fasting‐induced adipose factor/angiopoietin‐like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem. 2006;281:934‐944. [DOI] [PubMed] [Google Scholar]
- 57. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761‐1772. [DOI] [PubMed] [Google Scholar]
- 58. Tsukumo DM, Carvalho‐Filho MA, Carvalheira JB, et al. Loss‐of‐function mutation in Toll‐like receptor 4 prevents diet‐induced obesity and insulin resistance. Diabetes. 2007;56:1986‐1998. [DOI] [PubMed] [Google Scholar]
- 59. Davis JE, Gabler NK, Walker‐Daniels J, Spurlock ME. Tlr‐4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring). 2008;16:1248‐1255. [DOI] [PubMed] [Google Scholar]
- 60. Lafferty RA, Flatt PR, Irwin N. Emerging therapeutic potential for peptide YY for obesity‐diabetes. Peptides. 2018;100:269‐274. [DOI] [PubMed] [Google Scholar]
- 61. Brighton CA, Rievaj J, Kuhre RE, et al. Bile acids trigger GLP‐1 release predominantly by accessing basolaterally located G protein‐coupled bile acid receptors. Endocrinology. 2015;156:3961‐3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Inagaki T, Moschetta A, Lee YK, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920‐3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Koren O, Spor A, Felin J, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108:4592‐4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Karlsson FH, Fåk F, Nookaew I, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yamashita T, Emoto T, Sasaki N, Hirata KI. Gut microbiota and coronary artery disease. Int Heart J. 2016;57:663‐671. [DOI] [PubMed] [Google Scholar]
- 67. Emoto T, Yamashita T, Kobayashi T, et al. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels. 2017;32:39‐46. [DOI] [PubMed] [Google Scholar]
- 68. Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Senthong V, Li XS, Hudec T, et al. Plasma trimethylamine N‐oxide, a gut microbe‐generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. 2016;67:2620‐2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou X, Li J, Guo J, et al. Gut‐dependent microbial translocation induces inflammation and cardiovascular events after ST‐elevation myocardial infarction. Microbiome. 2018;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wu ZX, Li SF, Chen H, et al. The changes of gut microbiota after acute myocardial infarction in rats. PLoS ONE. 2017;12:e0180717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gan XT, Ettinger G, Huang CX, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7:491‐499. [DOI] [PubMed] [Google Scholar]
- 74. Lam V, Su J, Hsu A, et al. Intestinal microbial metabolites are linked to severity of myocardial infarction in rats. PLoS ONE. 2016;11:e0160840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tang TW, Chen HC, Chen CY, et al. Loss of gut microbiota alters immune system composition and cripples post‐infarction cardiac repair. Circulation. 2018. 10.1161/CIRCULATIONAHA.118.035235. [DOI] [PubMed] [Google Scholar]
- 76. Organ CL, Otsuka H, Bhushan S, et al. Choline diet and its gut microbe‐derived metabolite, trimethylamine N‐oxide, exacerbate pressure overload‐induced heart failure. Circ Heart Fail. 2016;9:e002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tang WH, Wang Z, Shrestha K, et al. Intestinal microbiota‐dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21:91‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tang WH, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe‐generated metabolite trimethylamine‐N‐oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Suzuki T, Heaney LM, Bhandari SS, et al. Trimethylamine N‐oxide and prognosis in acute heart failure. Heart. 2016;102:841‐848. [DOI] [PubMed] [Google Scholar]
- 80. Trøseid M, Ueland T, Hov JR, et al. Microbiota‐dependent metabolite trimethylamine‐N‐oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717‐726. [DOI] [PubMed] [Google Scholar]
- 81. Pasini E, Aquilani R, Testa C, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4:220‐227. [DOI] [PubMed] [Google Scholar]
- 82. Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota‐derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110:4410‐4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Miyamoto J, Kasubuchi M, Nakajima A, et al. The role of short‐chain fatty acid on blood pressure regulation. Curr Opin Nephrol Hypertens. 2016;25:379‐383. [DOI] [PubMed] [Google Scholar]
- 84. Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5:202‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kimura I, Inoue D, Maeda T, et al. Short‐chain fatty acids and ketones directly regulate sympathetic nervous system via G protein‐coupled receptor 41 (GPR41). Proc Natl Acad Sci USA. 2011;108:8030‐8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota‐dependent trimethylamine N‐oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tomasova L, Dobrowolski L, Jurkowska H, et al. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide. 2016;60:50‐58. [DOI] [PubMed] [Google Scholar]
- 88. Wilck N, Matus MG, Kearney SM, et al. Salt‐responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Karbach SH, Schonfelder T, Brandao I, et al. Gut microbiota promote angiotensin II‐induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016;5:e003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Durgan DJ, Ganesh BP, Cope JL, et al. Role of the gut microbiome in obstructive sleep apnea‐induced hypertension novelty and significance. Hypertension. 2016;67:469‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Daliri EB, Lee BH, Oh DH. Current perspectives on antihypertensive probiotics. Probiotics Antimicrob Proteins. 2017;9:91‐101. [DOI] [PubMed] [Google Scholar]
- 93. Guo Z, Zhang J, Wang Z, et al. Intestinal microbiota distinguish gout patients from healthy humans. Sci Rep. 2016;6:20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Karlsson C, Ahrne S, Molin G, et al. Probiotic therapy to men with incipient arteriosclerosis initiates increased bacterial diversity in colon: a randomized controlled trial. Atherosclerosis. 2010;208:228‐233. [DOI] [PubMed] [Google Scholar]
- 95. Li Z, Shen Y, Chen Y, et al. High uric acid inhibits cardiomyocyte viability through the ERK/P38 pathway via oxidative stress. Cell Physiol Biochem. 2018;45:1156‐1164. [DOI] [PubMed] [Google Scholar]
- 96. Wang Y, Wu Y, Wang Y, et al. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9:e521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kinlay S, Michel T, Leopold JA. The Future of Vascular Biology and Medicine. Circulation. 2016;133:2603‐. 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lopez‐Garcia E, Rodriguez‐Artalejo F, Li TY, et al. The Mediterranean‐style dietary pattern and mortality among men and women with cardiovascular disease. Am J Clin Nutr. 2014;99:172‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. De Filippis F, Pellegrini N, Vannini L, et al. High‐level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812‐1821. [DOI] [PubMed] [Google Scholar]
- 100. Marques FZ, Nelson E, Chu PY, et al. High‐fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964‐977. [DOI] [PubMed] [Google Scholar]
- 101. Chan YK, Brar MS, Kirjavainen PV, et al. High fat diet‐induced atherosclerosis is accompanied with low colonic bacterial diversity and altered abundances that correlates with plaque size, plasma A‐FABP and cholesterol: a pilot study of high‐fat diet and its intervention with Lactobacillus rhamnosus GG (LGG) or telmisartan in ApoE‐/‐ mice. BMC Microbiol. 2016;16:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Costanza AC, Moscavitch SD, Faria Neto HC, Mesquita ET. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double‐blind, placebo‐controlled pilot trial. Int J Cardiol. 2015;179:348‐350. [DOI] [PubMed] [Google Scholar]
- 103. Ebel B, Lemetais G, Beney L, et al. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit Rev Food Sci Nutr. 2014;54:175‐189. [DOI] [PubMed] [Google Scholar]
- 104. Kumar SA, Ward LC, Brown L. Inulin oligofructose attenuates metabolic syndrome in high‐carbohydrate, high‐fat diet‐fed rats. Br J Nutr. 2016;116:1502‐1511. [DOI] [PubMed] [Google Scholar]
- 105. Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693‐702. [DOI] [PubMed] [Google Scholar]
- 106. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913‐916. [DOI] [PubMed] [Google Scholar]
- 107. Kootte RS, Levin E, Salojärvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26:611‐619. [DOI] [PubMed] [Google Scholar]
- 108. Murphy EF, Cotter PD, Hogan A, et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet‐induced obesity. Gut. 2013;62:220‐226. [DOI] [PubMed] [Google Scholar]
- 109. Tiihonen K, Tynkkynen S, Ouwehand A, et al. The effect of ageing with and without non‐steroidal anti‐inflammatory drugs on gastrointestinal microbiology and immunology. Br J Nutr. 2008;100:130‐137. [DOI] [PubMed] [Google Scholar]
- 110. Lam V, Su J, Koprowski S, et al. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26:1727‐1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Conraads VM, Jorens PG, De Clerck LS, et al. Selective intestinal decontamination in advanced chronic heart failure: a pilot trial. Eur J Heart Fail. 2004;6:483‐491. [DOI] [PubMed] [Google Scholar]
- 112. Wang Z, Roberts AB, Buffa JA, et al. Non‐lethalInhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen ML, Yi L, Zhang Y, et al. Resveratrol attenuates trimethylamine‐N‐oxide (TMAO)‐induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio. 2016;7:e02210‐e02215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jensen AB, Ajslev TA, Brunak S, Sorensen TI. Long‐term risk of cardiovascular and cerebrovascular disease after removal of the colonic microbiota by colectomy: a cohort study based on the Danish National Patient Register from 1996 to 2014. BMJ Open. 2015;5:e008702. [DOI] [PMC free article] [PubMed] [Google Scholar]


