Abstract
Chimeric antigen receptor (CAR) T cells have shown great promise in the treatment of hematological and solid malignancies. However, despite the success of this field, there remain some major challenges, including accelerated T cell exhaustion, potential toxicities, and insertional oncogenesis. To overcome these limitations, recent advances in CRISPR technology have enabled targetable interventions of endogenous genes in human CAR T cells. These CRISPR genome editing approaches have unleashed the therapeutic potential of CAR T cell therapy. Here, we summarize the potential benefits, safety concerns, and difficulties in the generation of gene-edited CAR T cells using CRISPR technology.
Keywords: chimeric antigen receptor, CRISPR, gene editing, immunotherapy, cancer, CAR T
Introduction to Chimeric Antigen Receptor T Cell Therapy
Major histocompatibility complex (MHC) molecules play key roles in the surveillance of aberrant proteins of tumor cells. T cell receptors (TCRs) on the surface of T lymphocytes recognize antigenic peptide fragments derived from these aberrant proteins in complex with MHCs (1, 2). The expression of MHC/peptide complexes constitutively occurs on all nucleated cells. Tumor-specific MHC/peptide complexes are considered ideal targets for T cell-based immunotherapies. Diverse strategies have been developed to induce T cell immunity against these tumor epitopes, including cancer vaccination (3), adoptive T cell transfer (4), and TCR engineering (5). In cancer patients, however, tumor cells can effectively escape adoptive immunity via regulatory mechanisms, such as downregulation of MHCs or mutation. Because the presence of relatively fewer MHC molecules on the tumor cell surface limits naive TCR recognition, T cells fail to respond and trigger cascades of immune activation (6).
Recently, the most promising development has been the use of chimeric antigen receptor (CAR) T cell immunotherapy (7). CAR T cell immunotherapy has emerged as a leading curative strategy in the treatment of relapsed hematological malignancies. CAR T cell therapy is based on the immune effect of T cell activation and the principle of transformation through the genetic engineering of T cells. A typical CAR construct comprises a binding domain (single chain antibody fragment, scFv), a transmembrane domain and intracellular signaling domains capable of activating T cells (Figure 1). CARs allow the T cells to be activated independently of MHC. Donor-derived T cells are modified to express multivalent CARs on the cell surface that are responsible for recognizing the tumor-associated antigen (TAA) of tumor cells. Thus, T cells are activated via intracellular signal transduction. CAR designs differ not only in their signaling domains but also in their functional properties. The CAR structures have progressed since the first generation was described in 1989 (8). The first generation of CARs was designed as an scFv linked to the CD3ζ intracellular signaling domain of the TCR through a hinge and a transmembrane domain. Although the CD3ζ signaling domain can trigger activation of T cells, this pattern most likely results in T cell anergy, attenuating T cell activation. Therefore, the first generation of CARs exhibited limited responses in clinical trials (7). To address this limitation, a costimulatory molecule, such as CD28, OX40, or 4-1BB, was incorporated into the intracellular domain for the second generation of CARs. The additional costimulatory domain in the second generation of CARs strikingly improved T-cell proliferation and persistence. To optimize T-cell efficacy, the third generation of CARs has been developed by introducing two costimulatory domains into the CAR structure. Although dual costimulatory domains can enhance the activation and proliferation of T cells, the abundance of cytokines remains to be considered.
Figure 1.
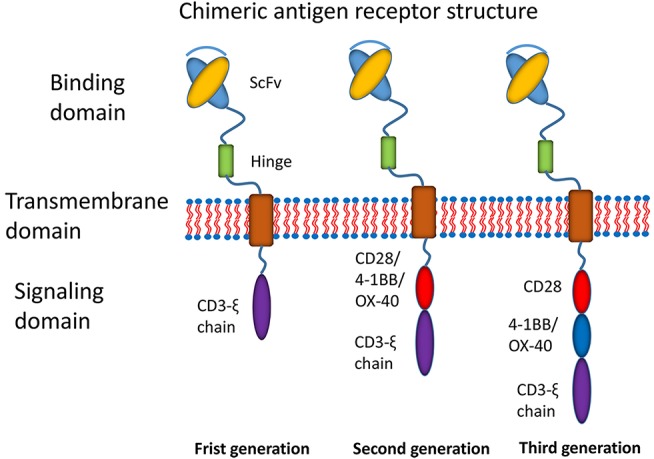
Main structures of chimeric antigen receptors. Three generations of CAR structures. In the first generation of CARs, the binding domain (single chain antibody fragment, scFv) is linked to the signaling domain (CD3ζ) via the transmembrane domain. In the second generation of CARs, the costimulatory molecule (CM1, such as CD28 4-1BB or OX-40) is introduced with the signaling domain (CD3ζ). In the third generation of CARs, the additional costimulatory molecule (CM2) is included.
The CAR T cell approach has provided great advances in the treatment of hematological malignancies. Anti-CD19 CAR T cells have significantly advanced the therapy of human hematological malignancies and were shown to achieve a 90% complete response rate in acute lymphoblastic leukemia (ALL) (9). Tisagenlecleucel, the first anti-CD19 CAR T cell therapy, was approved by the US Food and Drug Administration (US FDA) for the treatment of children and adults with advanced leukemia in 2017 (10, 11). As 2017 ended, there were hundreds of ongoing CAR T cell trials for the treatment of hematologic and solid tumor malignancies (12).
Possible Side Effects of Chimeric Antigen Receptor T Cell Therapy
Although most patients infused with CAR T cells show mild or moderate side effects, potentially severe side effects are still challenging. The prominent toxicities include cytokine release syndrome (CRS), insertional oncogenesis, and neurologic toxicity (13, 14).
Cytokine Release Syndrome
CRS is an unintended side effect due to overactivation of the host immune system. Severe CRS was observed in some patients who received infusion of CAR T cells (15). An abundance of cytokines is released by either the infused CAR T cells or other polarized immune cells. Several clinical studies indicated that 19–43% of patients exhibited CRS when they were treated with anti-CD19 CAR T cells for relapsed/refractory ALL (13, 16). Clinical features of CRS include high fever, muscle pain, malaise, unstable hypotension, fatigue, ang capillary leakage (17). A wide variety of cytokines can be elevated in the serum of patients. Dramatic elevations of inflammatory cytokines, such as INF-γ, IL-2, IL-6, and IL-10, are observed in CRS (18). Occasionally, neurologic toxicity can be associated with anti-CD19 CAR T cell therapy, probably due to the elevated levels of cytokines (16). The use of the anti-IL-6 receptor antibody tocilizumab was demonstrated to exert curative effects for serious cases of CRS in all patients with a high proliferation of CAR T cells (19).
Insertional Oncogenesis
Continuous CAR expression in T cells relies primarily on the delivery of the CAR gene by integrated gamma retroviral (RV) or lentiviral (LV) vectors. The advantages of both systems are high gene-transfer efficiency and stable expression of the CARs. Although both RV and LV vectors have been shown to be safe in intensive biosafety testing, this safety issue remains a concern. LV- or RV-mediated random and uncontrollable integration in the genome are unpredictable (20). Uncontrollable insertions of CAR genes lead to potential oncogenesis, variegated transgene expression, and transcriptional silencing (21). This possibility poses an oncogenic risk for RV/LV-engineered T cells (22). Although RV-driven oncogenesis has not yet been reported in CAR T cell therapy, this phenomenon was observed in clinical trials of hematopoietic stem cell transplantation (23). Additionally, random integration into the genome causes substantial variations in CAR expression levels in a batch of CAR T cells because of the different copy numbers per cell.
Graft-vs.-Host Disease
With the gradual initiation of clinical trials, autologous CAR T cells have shown some disadvantages. In infants or adults who are receiving chemotherapy or radiotherapy, it is difficult to harvest sufficient lymphocytes for CAR T cell manufacture. Thus, the quality of CAR T cells for each patient is uncontrollable and unpredictable. The use of allogeneic CAR T cells has become a solution for these problems. Allogeneic CAR T cells can be expanded ex vivo on a large scale and can be reserved to treat multiple patients (24). The concerned with allogeneic infusion is graft-vs.-host disease (GVHD) between the donor cells and recipients. The repertoire of TCRs and MHCs expressed on allogeneic CAR T cells may potentially induce GVHD in recipients who receive donor CAR T cells (25). A study showed that allogeneic anti-CD19-CAR T cells had clinical benefits for relapsed hematologic malignancies (26). No obvious GVHD was observed in these recipients.
Generation of Potent CAR T Cells With CRISPR Technology
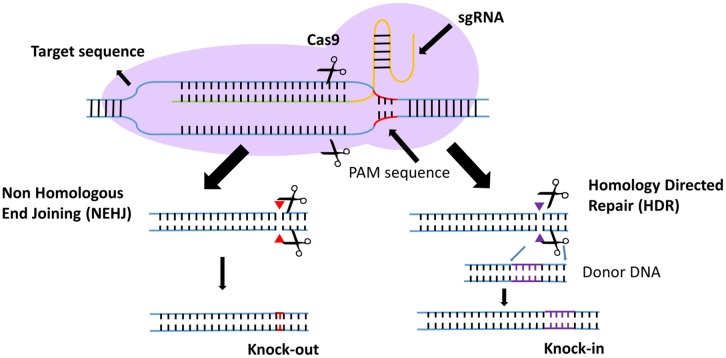
Efforts to enhance the efficacy of CAR T cell therapy have been undertaken, including the selection of extracellular receptors (27), optimization of intracellular costimulatory molecules (28), combination with cytokines(29), and improvement of “on-target/off-tumor” toxicity (30). Effective gene-editing technologies have emerged as tools for cell engineering (31). The properties of three gene-editing tools, including CRISPR, zinc-finger nucleases (ZFNs), and transcription activator-like effector nucleases (TALENs), are summarized in Table 1. The use of CRISPR in genome editing is highly efficient and enables a simple and efficient way to multiplex the processing of T cells (32, 33). Both ZFNs and TALENs have also been adopted to modify T cells for clinical applications (34, 35). However, the recognition of the targetable DNA sequences with ZFNs and TALENs in T cells remains complicated and tedious, resulting in a low gene-editing efficiency. The simultaneous multiplexed genetic manipulations of these techniques are challenging (36). CRISPR/Cas9 systems have been used for the knock-out and knock-in of sequences in mammalian genome editing (Figure 2). In principle, a deletion or insertion at a target gene is introduced by a small RNA (sgRNA)-guided Cas9 nuclease that induces a double-stranded DNA break, which is subsequently repaired by non-homologous end joining (NHEJ) (37). Nucleotide insertions or deletions result in non-sense mutations and loss of gene function. In comparison to NHEJ, a relatively large gene sequence can be delivered to a precise locus in the genome through homology directed repair (HDR) after double-stranded DNA is cleaved by sgRNAs (38–40). The HDR process enables precisely targeted nucleotide replacements at the defined site of interest. Currently, several strategies based on CRISPR are being applied to develop next-generation CAR T cells by multiplexed genome editing (41–43). Such approaches include the knockout of endogenous genes (such as TCRs, MHCs, or self-antigens) to build allogeneic universal CAR T cells (41, 44, 45), the disruption of inhibitory receptors (such as CTLA-4, PD-1, or LAG-3) (44, 46, 47), and the integration of the CAR cassette into the endogenous TCR α constant locus (TRAC) (48, 49) or the C-C chemokine receptor type 5 (CCR5) locus (32) (Table 2).
Table 1.
Comparison of ZFN, TALEN, and CRISPR.
| Property | ZFN | TALEN | CRISPR |
|---|---|---|---|
| Anchor site | 18–36 nt | 30–36 nt | 23 nt |
| Off-target | Low | low | High |
| Complication | High | High | Low |
| Efficiency | Relatively low | Relatively low | High |
| Multiplex | Low | Low | High |
| Methylation sensitivity | High | High | Low |
| Mechanism of action | Zinc finger nuclease for DNA recognition and cleavage | transcription activator-like effector nuclease recognition and DNA cleavage | Guide RNA for DNA recognition and Cas9 endonuclease for cleavage |
Figure 2.
Introduction to the CRISPR gene-editing system. Guided by sgRNAs, the CRISPR-Cas9 nuclease can target short DNA sequences. The PAM specifically creates a sgRNA–target DNA heteroduplex and generates double-strand breaks. Then, the DNA double-strand breaks are repaired by non-homologous end-joining (NHEJ) or homology-directed repair (HDR). In the NHEJ pathway, indels lead to nucleotide deletions or insertions. In the HDR pathway, accessory factors can facilitate genome recombination through the two homology arms, resulting in the knock-in of a gene of interest.
Table 2.
Summary of the CAR-T cells modified with gene editing.
| CAR | Gene-editing method | Targeted gene | Gene editing efficiency (%) | Malignancy | Reference |
|---|---|---|---|---|---|
| KNOCK-OUT | |||||
| CD19 scFv/4-1BB/CD3ζ | Cas9 RNP electroporation | TRAC | 85 | B cell acute lymphoblastic leukemia | (41, 42) |
| β2M | 100 | ||||
| PD-1 | 64.7 | ||||
| CD19 scFv/4-1BB/CD3ζ | Cas9 RNP electroporation | TRAC | 81.7 | B cell acute lymphoblastic leukemia | (45) |
| TRBC | 49.3 | ||||
| β2M | 79.9 | ||||
| CD7 scFv/CD28/4-1BB//CD3ζ | Cas9 RNP electroporation | CD7 | 89.14 | T cell acute lymphoblastic leukemia | (44, 52) |
| EBV-LMP2A CTL | Cas9 plasmid electroporation | PD-1 | 47.4 | Epstein-Barr virus-associated gastric cancer | (46) |
| CD19 scFv/4-1BB/CD3ζ | Cas9 RNP electroporation | LAG-3 | 45–70 | B cell acute lymphoblastic leukemia | (47) |
| KNOCK-IN | |||||
| CD19 scFv/4-1BB/CD3ζ | Cas9 RNP electroporation and transfection with AAV6 encoding CAR | TRAC exon 1 | 50 | B cell lymphoma | (48) |
| CD19 scFv/CD28/CD3ζ | Cas9 RNP electroporation and transfection with AAV encoding CAR | TRAC exon1 | 40 | Adult B acute lymphoblastic leukemia | (49) |
Universal CAR T Cells
Although autologous CAR T cells against B cell malignancies have shown promising results, some clinical studies demonstrated that for some patients, autologous T cells could not be manufactured due to poor lymphocyte counts or low T cell quality and quantity (50). Especially for some patients in infancy, sufficient peripheral blood mononuclear cells (PBMC) cannot be harvested to support T cell manufacture ex vivo. These limitations can be circumvented by utilizing allogeneic T cells. Endogenous TCRs that allogeneic T cells express can recognize the alloantigen of the recipient, resulting in major graft-vs.-host disease (GVHD). Before these allogeneic T cells can be widely used clinically, the issue of GVHD must be resolved (45). Universal allogeneic CAR-T cells are ideal because their manufacture and quality may be more easily controlled and GVHD may be avoided. Several groups have generated allogeneic universal anti-CD19 CAR T cells by deleting multiple genes, such as TRAC, β2M, and MHC, using CRISPR methods (41, 42). Meanwhile, ongoing clinical trials have shown that a suicide gene in the CAR construct can also be used to avoid GVHD after allogeneic CAR T cell injection (25). These results suggest that CAR T cells that utilize multiplexed gene editing generate CAR T cells that are as potent as non-gene-edited T cells.
Until now, most successful CAR T cell therapies have been applied to B cell malignances. For T cell malignances, patients would receive allogeneic T cells rather than autologous CAR T cells. Genomic editing of some antigens, which recognize those “non-self” molecules and are attacked by the host immune system, can broaden the application of CAR T cells. DiPersio et al. reported that fratricide-resistant “off-the-shelf” universal T cells generated with CRISPR gene editing were used for treatment of T-cell malignancies (44). CD7 is a molecule commonly expressed in T lymphocytes. To avoid self-elimination, the CD7 target antigen against malignancies, which is recognized by anti-CD7-CARs, is deleted on CAR T cells (51, 52).
Resistance to PD-1 Inhibition
It is widely accepted that the existence of immune checkpoints (such as PD-1, CTLA-4, and LAG-3) can attenuate the activation of CAR T cells and accelerate T cell exhaustion. PD-1 is a primary inhibitory molecule in T cell transduction (53, 54). The PD-1/PD-L1 pathway plays an important role in the regulation of T cell activation and differentiation (55). High expression of PD-1 accelerates T cell tolerance and exhaustion (56–59). Increasing evidence indicates that blocking the PD-1/PD-L1 axis could partially restore the function of exhausted T cells (54, 60). A recent clinical study demonstrated that treatment with anti-CD19 CAR T cells in combination with an anti-PD-1 antibody was effective in patients with relapsed chronic lymphocytic leukemia (CLL) (61). This anti-PD-1 antibody treatment revives the antitumor response of anti-CD19 CAR T cells in patients who fail to respond to CAR T cell treatment (62). In other cases, unanticipated autoimmune responses are associated with anti-PD-1 checkpoint inhibitors (63). Therefore, ablation of PD-1 with gene editing by CRISPR/Cas9 is an alternative to enhance the antitumor response of CAR T cells in anti-CD19 CAR T cell therapy (41, 42). Ren et al. suggested that depletion of PD-1 genes in anti-prostate stem cell antigen (PSCA) CAR T cells with a Cas9/RNP method significantly enhanced T cell immunity in vivo (42). A significant antitumor response was observed after PD-1 was disrupted by genome editing. Controversially, a study indicated that T-cells without PD-1 were susceptible to exhaustion and lacked long-term durability (64). In regard to other checkpoint targets, no obvious improvement was confirmed when LAG-3 genes were deleted in CAR-T cells using CRISPR/Cas9 (47). Nevertheless, these studies still support the promise of checkpoint inhibition in CAR T cell therapy.
Targeted Integration of CARs
Recently, effective homologous recombination was shown to promote the site-specific integration of large transgenes in the T cell genome (65). In this method, after the DNA of the target gene is cleaved using Cas9 RNPs, a gene of interest is subsequently delivered to the cleavage site using adeno-associated viruses (AAVs). Site-specific transgene integration is achieved by HDR. An anti-CD19 CAR gene has been successfully integrated into the TRAC locus using the combined action of Cas9/RNP and AAV donor vectors (49). Targeting the CAR gene to the TRAC locus not only results in uniform CAR expression but also delays effector T-cell differentiation and exhaustion. Moreover, the insertion of a CAR transgene into a defined location avoids the risk of insertional oncogenesis and places CAR expression under the control of endogenous regulatory elements.
Safety Concerns of CRISPR Gene-Edited CAR-T Cell Therapy
To date, although many limitations of conventional CAR T cells have been addressed with CRISPR gene editing, safety issues must be addressed before these gene-edited cells start to move into clinic. Multiple elements, such as off-target effects, Cas9 activity, target site selection, and sgRNA design, and delivery methods, can determine the efficiency and safety of the CRISPR/Cas9 system.
The first concern of CRISPR gene editing is off-target effects (66). These off-target effects might be beneficial to bacteria and archaea (67). However, several recent studies have reported unintentional CRISPR/Cas9-induced large genomic deletions or gene inversions in various species, including mouse, C. elegans, and rabbit (68–70). For human therapies, clinical safety is particularly important. Several recent studies have reported off-target effects of CRISPR in T-cells. Off-target effects introduce random mutations, thus impacting tumor-suppressor genes or activating oncogenes. Off-target effects were also observed when the TRAC or TRBC locus of CAR-T cells was inserted with CRISPR/Cas9 electroporation (42). A controversial study indicated that CRISPR gene editing could cause hundreds of unintended mutations in the genome when whole-genome sequencing was performed on a CRISPR–Cas9-edited mouse (68). Notably, another study showed that CRISPR/Cas9 genome editing resulted in a p53-mediated DNA damage response in human retinal pigment epithelial cells (71). p53 activation may lead to chromosomal rearrangements and other tumorigenic mutations in cells. Although the outcome of CRISPR-induced p53 activation is unconfirmed, it seems to decrease the gene editing efficiency. Therefore, the off-target issues must be considered in the future development of CRISPR/Cas9-edited CAR T cells. Off-target assays during CRISPR target selection may be performed to manage the safety risk of clinical CAR T trials.
Another safety concern is that unpredicted translocations may occur between double-strand breaks when multiple genes are edited (72). Although such events are rare in T cells, transformation analysis should still be performed to ensure the safety of gene-edited CAR-T therapy. In addition to the safety risk of translocations, altered functions of gene-edited CAR-T cells most likely would cause adverse effects in patients. For example, CRISPR gene disruption in CAR T cells can cause unintended innate immune responses (73).
Perspectives of CRISPR Gene-Edited CAR-T Cell Therapy
In recent, many antitumor approaches have been developed, including target small molecules (74, 75), antibody drugs (76–84), immune cell therapy (85). Among them, CAR T cell therapy aims to treat cancer through the use of the patient's immune system. This type of therapy has many advantages, such as low toxicity and a long duration (86). However, CAR T cell therapy appears to be effective only in a limited portion of patients with hematological malignancies. CRISPR is a cutting-edge technique that can be used to generate CAR T cells with enhanced potency and safety. Although the clinical use of allogeneic donor CAR cells has been recently reported, their use is highly dependent upon either rigorous patient selection or T cell selection (25). Potential GVHD still limits the wide application of allogeneic CAR cells. Taking advantage of CRISPR, the risk of GVHD may be minimized through the deletion of endogenous TCR and MHC molecules. The additional disruption of PD-1 is believed to optimize the antitumor activities of CAR-T cells through the regulation of T-cell functions (32). The safety of gene-edited CAR T cells is the primarily concern because of notorious off-target effects. To minimize the safety risk of off-target effects, careful selection of the target site combined with prior off-target assays will be required during target site selection of CAR T cells. Although skeptics question whether CRISPR gene-edited T cell therapy is safe and ready for the clinical stage, the first CRISPR gene-editing trial using autologous T cells was initiated to treat patients with melanoma, synovial sarcoma, and multiple myeloma in 2016 (87). These potent T cells have shown merits in preclinical studies. The long-term safety profile of gene-edited CAR-T cells should be further examined in the clinic.
Author Contributions
JL, GZ, and LZ wrote part of the manuscript; QZ wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the Science and Technology Development Fund of Macau (FDCT/131/2016/A3, FDCT/0015/2018/A1), the Guangzhou Science and Technology Program (201807010004), and Start-up Research Grand (SRG2016-00082-FHS) and the intramural research program of Faculty of Health Sciences, University of Macau, and National Natural Science Foundation of China (31440041).
References
- 1.Santomasso BD, Roberts WK, Thomas A, Williams T, Blachère NE, Dudley ME, et al. A T cell receptor associated with naturally occurring human tumor immunity. Proc Natl Acad Sci USA. (2007) 104:19073–8. 10.1073/pnas.0704336104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinherz EL. Revisiting the discovery of the αβ TCR complex and its co-receptors. Front Immunol. (2014) 5:583. 10.3389/fimmu.2014.00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grenier JM, Yeung ST, Khanna KM. Combination immunotherapy: taking cancer vaccines to the next level. Front Immunol. (2018) 9:610. 10.3389/fimmu.2018.00610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yee C. Adoptive T cell therapy: addressing challenges in cancer immunotherapy. J Transl Med. (2005) 3:17. 10.1186/1479-5876-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liddy N, Bossi G, Adams KJ, Lissina A, Mahon TM, Hassan NJ, et al. Monoclonal TCR-redirected tumor cell killing. Nat Med. (2012) 18:980–7. 10.1038/nm.2764 [DOI] [PubMed] [Google Scholar]
- 6.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. (2013) 14:1014–22. 10.1038/ni.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med. (2014) 65:333–47. 10.1146/annurev-med-060512-150254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. (1989) 86:10024–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. (2014) 371:1507–17. 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Chen X, Han W, Zhang Y. Tisagenlecleucel, an approved anti-CD19 chimeric antigen receptor T-cell therapy for the treatment of leukemia. Drugs Today. (2017) 53:597–608. 10.1358/dot.2017.53.11.2725754 [DOI] [PubMed] [Google Scholar]
- 11.Prasad V. Immunotherapy: tisagenlecleucel–the first approved CAR-T-cell therapy: implications for payers and policy makers. Nat Rev Clin Oncol. (2018) 15:11–2. 10.1038/nrclinonc.2017.156 [DOI] [PubMed] [Google Scholar]
- 12.Hartmann J, Schussler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. (2017) 9:1183–97. 10.15252/emmm.201607485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncol. (2016) 3:16011. 10.1038/mto.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. (2016) 127:3321–30. 10.1182/blood-2016-04-703751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalaitsidou M, Kueberuwa G, Schutt A, Gilham DE. CAR T-cell therapy: toxicity and the relevance of preclinical models. Immunotherapy. (2015) 7:487–597. 10.2217/imt.14.123 [DOI] [PubMed] [Google Scholar]
- 16.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. (2014) 6:224ra25. 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. (2014) 124:188–95. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith L, Venella K. Cytokine release syndrome: inpatient care for side effects of CAR T-cell therapy. Clin J Oncol Nursing. (2017) 21(2 Suppl.):29–34. 10.1188/17.cjon.s2.29-34 [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Ma N, Okamoto S, Amaishi Y, Sato E, Seo N, et al. Efficient tumor regression by adoptively transferred CEA-specific CAR-T cells associated with symptoms of mild cytokine release syndrome. Oncoimmunology. (2016) 5:e1211218. 10.1080/2162402x.2016.1211218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Riviere I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncol. (2016) 3:16015. 10.1038/mto.2016.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther. (2005) 16:1241–6. 10.1089/hum.2005.16.1241 [DOI] [PubMed] [Google Scholar]
- 22.Jin C, Fotaki G, Ramachandran M, Nilsson B, Essand M, Yu D. Safe engineering of CAR T cells for adoptive cell therapy of cancer using long-term episomal gene transfer. EMBO Mol Med. (2016) 8:702–11. 10.15252/emmm.201505869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Investig. (2008) 118:3132–42. 10.1172/jci35700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh N, Barrett DM. Donor-derived CD19 chimeric antigen receptor T cells. Curr Opin Hematol. (2015) 22:503–8. 10.1097/moh.0000000000000179 [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Jacoby E, Fry TJ. Challenges and opportunities of allogeneic donor-derived CAR T cells. Curr Opin Hematol. (2015) 22:509–15. 10.1097/moh.0000000000000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. (2016) 34:1112–21. 10.1200/jco.2015.64.5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Q, Ahmed M, Tassev DV, Hasan A, Kuo TY, Guo HF, et al. Affinity maturation of T-cell receptor-like antibodies for Wilms tumor 1 peptide greatly enhances therapeutic potential. Leukemia. (2015) 29:2238–47. 10.1038/leu.2015.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai Y, Weng J, Wei X, Qin L, Lai P, Zhao R, et al. Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T Cells. Leukemia. (2018) 32:801–8. 10.1038/leu.2017.249 [DOI] [PubMed] [Google Scholar]
- 29.Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. (2018) 36:346–51. 10.1038/nbt.4086 [DOI] [PubMed] [Google Scholar]
- 30.Xu D, Jin G, Chai D, Zhou X, Gu W, Chong Y, et al. The development of CAR design for tumor CAR-T cell therapy. Oncotarget. (2018) 9:13991–4004. 10.18632/oncotarget.24179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Methods. (2013) 10:957–63. 10.1038/nmeth.2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hale M, Lee B, Honaker Y, Leung WH, Grier AE, Jacobs HM, et al. Homology-directed recombination for enhanced engineering of chimeric antigen receptor T cells. Mol Ther Methods Clin Dev. (2017) 4:192–203. 10.1016/j.omtm.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren J, Zhao Y. Advancing chimeric antigen receptor T cell therapy with CRISPR/Cas9. Protein Cell. (2017) 8:634–43. 10.1007/s13238-017-0410-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. (2014) 370:901–10. 10.1056/NEJMoa1300662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qasim W, Zhan H, Samarasinghe S, Adams S, Amrolia P, Stafford S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. (2017) 9:eaaj2013. 10.1126/scitranslmed.aaj2013 [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Zhao Y. CRISPR/Cas9 genome editing: fueling the revolution in cancer immunotherapy. Curr Res Transl Med. (2018) 66:39–42. 10.1016/j.retram.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 37.Ehrke-Schulz E, Schiwon M, Hagedorn C, Ehrhardt A. Establishment of the CRISPR/Cas9 system for targeted gene disruption and gene tagging. Methods Mol. Biol. (2017) 1654:165–76. 10.1007/978-1-4939-7231-9_11 [DOI] [PubMed] [Google Scholar]
- 38.Zhang JP, Li XL, Li GH, Chen W, Arakaki C, Botimer GD, et al. Efficient precise knockin with a double cut HDR donor after CRISPR/Cas9-mediated double-stranded DNA cleavage. Genome Biol. (2017) 18:35. 10.1186/s13059-017-1164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki K, Tsunekawa Y, Hernandez-Benitez R, Wu J, Zhu J, Kim EJ, et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature. (2016) 540:144–9. 10.1038/nature20565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bak RO, Porteus MH. CRISPR-mediated integration of large gene cassettes using AAV donor vectors. Cell Rep. (2017) 20:750–6. 10.1016/j.celrep.2017.06.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Zhang Y, Cheng C, Cheng AW, Zhang X, Li N, et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. (2017) 27:154–7. 10.1038/cr.2016.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res. (2017) 23:2255–66. 10.1158/1078-0432.CCR-16-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seki A, Rutz S. Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T cells. J Exp Med. (2018) 215:985–97. 10.1084/jem.20171626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper ML, Choi J, Staser K, Ritchey JK, Devenport JM, Eckardt K, et al. An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia. (2018) 32:1970–83. 10.1038/s41375-018-0065-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, et al. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget. (2017) 8:17002–11. 10.18632/oncotarget.15218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su S, Zou Z, Chen F, Ding N, Du J, Shao J, et al. CRISPR-Cas9-mediated disruption of PD-1 on human T cells for adoptive cellular therapies of EBV positive gastric cancer. Oncoimmunology. (2017) 6:e1249558. 10.1080/2162402X.2016.1249558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Zhang X, Cheng C, Mu W, Liu X, Li N, et al. CRISPR-Cas9 mediated LAG-3 disruption in CAR-T cells. Front Med. (2017) 11:554–62. 10.1007/s11684-017-0543-6 [DOI] [PubMed] [Google Scholar]
- 48.MacLeod DT, Antony J, Martin AJ, Moser RJ, Hekele A, Wetzel KJ, et al. Integration of a CD19 CAR into the TCR alpha chain locus streamlines production of allogeneic gene-edited CAR T cells. Mol Ther. (2017) 25:949–61. 10.1016/j.ymthe.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. (2017) 543:113–7. 10.1038/nature21405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh N, Shi J, June CH, Ruella M. Genome-editing technologies in adoptive T cell immunotherapy for cancer. Curr Hematol Malign Rep. (2017) 12:522–9. 10.1007/s11899-017-0417-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Png YT, Vinanica N, Kamiya T, Shimasaki N, Coustan-Smith E, Campana D. Blockade of CD7 expression in T cells for effective chimeric antigen receptor targeting of T-cell malignancies. Blood Adv. (2017) 1:2348–60. 10.1182/bloodadvances.2017009928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomes-Silva D, Srinivasan M, Sharma S, Lee CM, Wagner DL, Davis TH, et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. (2017) 130:285–96. 10.1182/blood-2017-01-761320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. (2010) 207:2187–94. 10.1084/jem.20100643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, et al. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci USA. (2013) 110:E2480–9. 10.1073/pnas.1305394110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. (2015) 33:1974–82. 10.1200/jco.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bai Y, Kan S, Zhou S, Wang Y, Xu J, Cooke JP, et al. Enhancement of the in vivo persistence and antitumor efficacy of CD19 chimeric antigen receptor T cells through the delivery of modified TERT mRNA. Cell Disc. (2015) 1:15040. 10.1038/celldisc.2015.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. (2012) 120:1412–21. 10.1182/blood-2012-02-411678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. (2015) 21:581–90. 10.1038/nm.3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mamonkin M, Heslop HE. Exhausting alloreactivity of donor-derived CAR T cells. Nat Med. (2017) 23:147–8. 10.1038/nm.4276 [DOI] [PubMed] [Google Scholar]
- 60.Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 blockade: have we found the key to unleash the antitumor immune response? Front Immunol. (2017) 8:1597. 10.3389/fimmu.2017.01597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding W, LaPlant BR, Call TG, Parikh SA, Leis JF, He R, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. (2017) 129:3419–27. 10.1182/blood-2017-02-765685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. (2017) 129:1039–41. 10.1182/blood-2016-09-738245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. (2016) 27:1362. 10.1093/annonc/mdw141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med. (2015) 212:1125–37. 10.1084/jem.20142237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schumann K, Lin S, Boyer E, Simeonov DR, Subramaniam M, Gate RE, et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc Natl Acad Sci USA. (2015) 112:10437–42. 10.1073/pnas.1512503112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng R, Lin G, Li J. Potential pitfalls of CRISPR/Cas9-mediated genome editing. FEBS J. (2016) 283:1218–31. 10.1111/febs.13586 [DOI] [PubMed] [Google Scholar]
- 67.Scherer S, Davis RW. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci USA. (1979) 76:4951–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaefer KA, Wu WH, Colgan DF, Tsang SH, Bassuk AG, Mahajan VB. Unexpected mutations after CRISPR-Cas9 editing in vivo. Nat Methods. (2017) 14:547–8. 10.1038/nmeth.4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shin HY, Wang C, Lee HK, Yoo KH, Zeng X, Kuhns T, et al. CRISPR/Cas9 targeting events cause complex deletions and insertions at 17 sites in the mouse genome. Nat Commun. (2017) 8:15464. 10.1038/ncomms15464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song YN, Lai LX, Li ZJ. Large-scale genomic deletions mediated by CRISPR/Cas9 system. Oncotarget. (2017) 8:5647. 10.18632/oncotarget.14543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. (2018) 24:927–30. 10.1038/s41591-018-0049-z [DOI] [PubMed] [Google Scholar]
- 72.Poirot L, Philip B, Schiffer-Mannioui C, Le Clerre D, Chion-Sotinel I, Derniame S, et al. Multiplex genome-edited T-cell manufacturing platform for “Off-the-Shelf” adoptive T-cell immunotherapies. Cancer Res. (2015) 75:3853–64. 10.1158/0008-5472.Can-14-3321 [DOI] [PubMed] [Google Scholar]
- 73.Kim S, Koo T, Jee HG, Cho HY, Lee G, Lim DG, et al. CRISPR RNAs trigger innate immune responses in human cells. Genome Res. (2018) 28:367–73. 10.1101/gr.231936.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Jiang Y, Ding S, Li J, Song N, Ren Y, et al. Small molecule inhibitors reveal allosteric regulation of USP14 via steric blockade. Cell Res. (2018) 28:1186–94. 10.1038/s41422-018-0091-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao Q, Tran H, Dimitrov DS, Cheung NK. A dual-specific anti-IGF-1/IGF-2 human monoclonal antibody alone and in combination with temsirolimus for therapy of neuroblastoma. Int J Cancer. (2015) 137:2243–52. 10.1002/ijc.29588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li DZ, Han BN, Wei R, Yao GY, Chen Z, Liu J, et al. N-terminal alpha-amino group modification of antibodies using a site-selective click chemistry method. mAbs. (2018) 10:712–9. 10.1080/19420862.2018.1463122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Z, Liu J, Chu D, Shan Y, Ma G, Zhang H, et al. A dual-specific IGF-I/II human engineered antibody domain inhibits IGF signaling in breast cancer cells. Int J Biol Sci. (2018) 14:799–806. 10.7150/ijbs.25928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Z, Wang L, Xu T, Wang Q, Kang L, Zhao Q. Generation of bispecific antibodies by Fc heterodimerization and their application. Curr Pharm Biotechnol. (2016) 17:1324–32. 10.2174/1389201017666161018150553 [DOI] [PubMed] [Google Scholar]
- 79.Li D, Gong R, Zheng J, Chen X, Dimitrov DS, Zhao Q. Engineered antibody CH2 domains binding to nucleolin: isolation, characterization and improvement of aggregation. Biochem Biophys Res Commun. (2017) 485:446–53. 10.1016/j.bbrc.2017.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu Y, Chen Z, Zhang P, Zhou L, Jiang T, Chen H, et al. Recombinant-fully-human-antibody decorated highly-stable far-red AIEdots for in vivo HER-2 receptor-targeted imaging. Chem. Commun. (2018) 54:7314–7. 10.1039/c8cc03037e [DOI] [PubMed] [Google Scholar]
- 81.Xu T, Ying T, Wang L, Zhang XD, Wang Y, Kang L, et al. A native-like bispecific antibody suppresses the inflammatory cytokine response by simultaneously neutralizing tumor necrosis factor-alpha and interleukin-17A. Oncotarget. (2017) 8:81860–72. 10.18632/oncotarget.19899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao Q, Ahmed M, Guo HF, Cheung IY, Cheung NK. Alteration of electrostatic surface potential enhances affinity and tumor killing properties of anti-ganglioside GD2 monoclonal antibody hu3F8. J Biol Chem. (2015) 290:13017–27. 10.1074/jbc.M115.650903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao Q, Feng Y, Zhu Z, Dimitrov DS. Human monoclonal antibody fragments binding to insulin-like growth factors I and II with picomolar affinity. Mol Cancer Ther. (2011) 10:1677–85. 10.1158/1535-7163.MCT-11-0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Shan Y, Gao X, Gong R, Zheng J, Zhang XD, et al. Screening and expressing HIV-1 specific antibody fragments in Saccharomyces cerevisiae. Mol Immunol. (2018) 103:279–85. 10.1016/j.molimm.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 85.Chu D, Zhao Q, Yu J, Zhang F, Zhang H, Wang Z. Nanoparticle targeting of neutrophils for improved cancer immunotherapy. Adv Healthc Mater. (2016) 5:1088–93. 10.1002/adhm.201500998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lipowska-Bhalla G, Gilham DE, Hawkins RE, Rothwell DG. Targeted immunotherapy of cancer with CAR T cells: achievements and challenges. Cancer Immunol Immunother. (2012) 61:953–62. 10.1007/s00262-012-1254-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baylis F, McLeod M. First-in-human phase 1 CRISPR gene editing cancer trials: are we ready? Curr Gene Ther. (2017) 17:309–19. 10.2174/1566523217666171121165935 [DOI] [PMC free article] [PubMed] [Google Scholar]