Abstract
Background: Preoperative hydronephrosis (HN) might be associated with adverse outcomes in patients who underwent radical cystectomy (RC). The aim of this study was to evaluate the effect of preoperative HN on the oncological outcomes in patients with bladder cancer who underwent RC by performing a systemic review and meta-analysis.
Methods: A systematic literature review in PubMed, EMBASE, and Scopus was conducted by searching the terms “bladder cancer,” “cystectomy,” and “hydronephrosis” until December 2016, in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines. The calculated end points were advanced disease stage, cancer-specific survival (CSS), and overall survival (OS).
Results: Twenty-four studies involving 10,461 patients who underwent RC were included. Among the patients, 3,121 (29.8%) had preoperative HN. The pooled analysis showed that preoperative HN had a significant association with advanced stage (odds ratio, 2.56, 95% confidence interval [CI], 1.91–3.42, p < 0.00001), lymph node invasion (OR, 2.44, 95% CI, 1.79–3.34, p < 0.00001), CSS (hazard ratio [HR], 1.67, 95% CI, 1.34–2.08, p < 0.00001), and OS (HR, 1.51, 95% CI, 1.30–1.75, p < 0.00001).
Conclusions: Among patients with bladder cancer who underwent RC, preoperative HN could be a significant predictor of bladder cancer survival. However, large well-designed prospective studies are required to confirm the precise prognostic significance of preoperative HN.
Keywords: bladder cancer, hydronephrosis, stage, lymph node invasion, survival
Introduction
Bladder cancer is the ninth most common cancer worldwide, with an estimated 430,000 new cases in 2012. More than 60% of all bladder cancer cases and half of all the 165,000 bladder cancer deaths occur in the less developed regions of the world (1). Radical cystectomy (RC) with pelvic lymph node dissection was regarded as the gold standard surgical method for muscle invasive bladder cancer (MIBC) and high-risk, non-MIBC (NMIBC) (2). Prediction of prognosis after RC was important for patient counseling, application of adjuvant treatment, and/or clinical trial. Several factors have been established to predict the prognosis after RC among patients with bladder cancer, such as pathological stage and lymph node invasion (3). However, another factor is needed to predict prognosis more accurately, therefore, several studies focused on identifying such a factor.
Preoperative HN is a common finding in patients with MIBC, with a reported incidence of up to 57.9% (4–6). Some researchers showed a positive association between prognosis and preoperative HN, and that preoperative HN had a significant association with extravesical disease, positive lymph node status, and cancer-specific survival (7, 8). However, other reports showed controversial results that preoperative HN was associated with higher stage but not with survival (3, 9). With preoperative imaging, prediction of prognosis was considered a relatively important step to decide appropriate treatment.
Therefore, in this study, we conducted a systemic review and meta-analysis of the available literature to obtain more-definitive results regarding the factor of preoperative HN during RC among patients with bladder cancer.
Materials and Methods
A systematic review was performed in accordance with the Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines (10).
Literature Search Strategy
A systematic literature search was performed in PubMed (1950–December 2016), EMBASE (1947–December 2016), and the Scopus Library using “bladder cancer,” “cystectomy,” and “hydronephrosis” as grouped terms.
Overview of the Included Studies
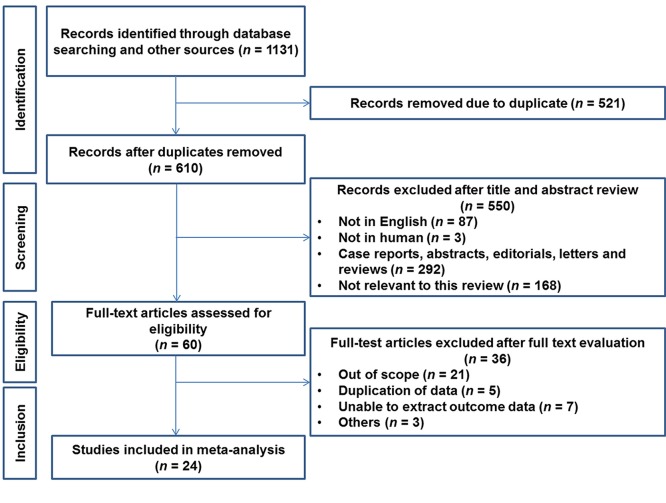
Figure 1 shows the PRISMA flowchart. We collected data from 237 PubMed articles, 380 Scopus articles, and 514 Embase articles, for a total of 1,131 resources. The exclusion criteria were duplicated data, non-English data, and non-human data. Finally, 24 studies we included in the meta-analysis (2–9, 11–26).
Figure 1.
PRISMA statement flow diagram showing the search strategy for meta-analysis.
Meta-Analysis
A meta-analysis was performed to summarize the gross prognostic value of associated factors. The cumulative effects of the factors were calculated using the inverse variance method. As traditional methods (27, 28), we measured the log odds ratio (OR) or hazard ratio (HR) and 95% confidence interval (CI) by using the indirect method. Statistical heterogeneity was investigated using the Cochran Q-test and the I2 statistic, which describes the percentage of total variation among the included studies. Significant heterogeneity was defined as a p-value of < 0.05 for the Cochran Q-test or an I2 statistic of >50% (29, 30). It resulted in the use of the random-effects model based on the Der Simonian method for estimating the tau value (29). To assess the risk of publication bias, we used a funnel plot and the Egger test for outcomes when at least 10 statistically significant studies were included in the meta-analysis (31). The meta-analysis was performed using the RevMan 5.0 statistical software (The Cochrane Collaboration, Copenhagen).
Study Quality Evaluation
The study quality was determined by the Newcastle-Ottawa scale (NOS) for including cohort studies only (32). A total score of five or less was considered low, 6–7 was considered intermediate, and 8–9 high quality.
Results
Study Population
Table 1 shows individual data on the characteristics of the 24 studies and 10,461 patients population included. All the studies contained data from 46 to 1,776 patients selected according to the presence of HN. The proportion of patients without preoperative HN ranged from 45.7 to 83.1% in each cystectomy cohort. Among the 10,461 patients who underwent RC, 7,340 (70.2%) had no HN before RC and 3,121 (29.8%) had a hydronephrotic kidney.
Table 1.
Characteristics of the studies included in the meta-analysis (studies = 24).
| No. | Study | Country | Recruited period | No. of patients | Median age | No HN (%) | Unilateral HN (%) | Bilateral HN (%) | Median fu (m) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Canter | USA | 1988–2003 | 306 | 65.3 | 232 (75.8) | 57 (18.6) | 17 (5.6) | 45.6 |
| 2 | Chapman | USA | 1996–2006 | 308 | 66.4 (mean) | 203 (65.9) | 82 (26.6) | 23 (7.5) | NA |
| 3 | Resorlu | Turkey | 1990–2007 | 241 | 59.8 (mean) | 189 (78.4) | 39 (16.2) | 13 (5.4) | 34 |
| 4 | Asadauskiene | Lithuania | 2000–2008 | 46 | 60.5 | 21 (45.7) | 24 (52.2) | 18 | |
| 5 | Choudhury | UK | 1995–2005 | 88 | 68 | 48 (54.5) | 40 (45.5) | 84 | |
| 6 | Kim | Korea | 1986–2005 | 457 | 60.8 | 321 (79.1) | 74 (18.2) | 11 (2.7) | 66.3 |
| 7 | Stimson | USA | 2001–2007 | 753 | 69 | 509 (67.6) | 183 (24.3) | 61 (8.1) | NA |
| 8 | Hofner | Germany | 1990–2009 | 328 | 64 | 253 (77.2) | 75 (22.8) | 104 | |
| 9 | Lin | China | 2003–2010 | 126 | 60 | 87 (69.0) | 34 (27.0) | 5 (4.0) | 23 |
| 10 | Xie | China | 2003–2011 | 248 | 60 | 198 (79.8) | 50 (20.2) | ||
| 11 | Ahmadi | USA | 1990–2008 | 1,186 | 66.9 (mean) | 970 (81.8) | 216 (18.2) | ||
| 12 | Eisenberg | USA | 1980–2008 | 1,776 | 68 | 1319 (74) | 457 (26) | 10.5 years | |
| 13 | Green | USA | 201 | 72.9 | 112 (72.3) | 43 (27.7) | |||
| 14 | Potretzke | USA | 2002–2012 | 102 | 69.0 | 68 (66.7) | 34 (33.3) | ||
| 15 | Prelević | Serbia | 2002–2012 | 233 | 63.8 (mean) | 109 (46.8) | 78 (33.5) | 46 (19.7) | |
| 16 | Pietzak | USA | 1990–2009 | 275 | 198 (72.0) | 77 (28.0) | 23.2 | ||
| 17 | Racioppi | Italy | 1982–2002 | 1,312 | 64.3 (mean) | 751 (57.2) | 176 (13.4) | 40 (3.0) | 39.0 |
| 18 | Stojadinovic | Serbia | 2002–2012 | 183 | 63.4 (mean) | 77 (42.1) | 106 (57.9) | ||
| 19 | Hirasawa | Japan | 2003–2015 | 136 | 68.6 (mean) | 106 (77.9) | 30 (22.1) | 46.7 | |
| 20 | Martini | Europe | 2011 | 337 | 69 | 258 (76.6) | 79 (23.4) | ||
| 21 | Mitra | USA | 1971–2009 | 828 | 513 (62.0) | 182 (38.0) | |||
| 22 | Aglamis | Turkey | 100 | 56.1 (mean) | 64 (64.0) | 36 (36.0) | 27.7 | ||
| 23 | Fernández | USA | 1989–2012 | 103 | 67 | 79 (76.7) | 22 (21.4) | 60 | |
| 24 | Bartsch | Germany | 1986–2003 | 788 | 65 | 655 (83.1) | 108 (13.7) | 25 (3.2) | 35 |
HN, hydronephrosis.
Locally Advanced Disease Prediction
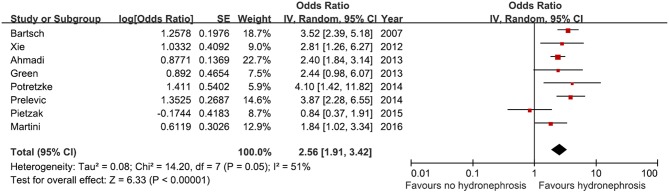
Eight studies involving 3,370 patients were identified for investigation of the prediction of locally advanced disease after RC according to the presence of preoperative HN. All the studies showed an odds ratio (OR) of 1.84 to 4.10 except one study (Figure 2). Among 3,370 patients, 802 (23.8%) had preoperative HN, the pooled analysis showed a significant difference in the prediction of locally advanced disease between the non-HN and HN groups (OR, 2.56, 95% CI, 1.91–3.42, p < 0.00001).
Figure 2.
Forest plot and meta-analysis of advances pathological stage after radical cystectomy according to preoperative hydronephrosis. SE, standard error; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Lymph Node Invasion
Data on the association between lymph node invasion and preoperative HN were available from three studies. The pooled analysis of these studies showed a significant association (OR, 2.44, 95% CI, 1.79–3.34, p < 0.00001, Figure 3).
Figure 3.
Forest plot and meta-analysis of lymph node invasion after radical cystectomy according to preoperative hydronephrosis. SE, standard error; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Cancer-Specific Survival
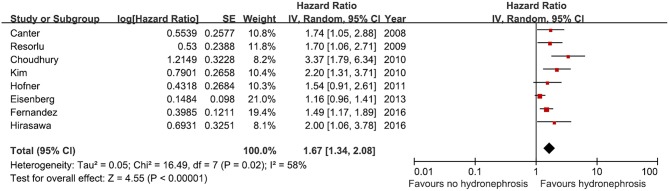
Eight studies were included in the meta-analysis to predict cancer-specific survival after RC. A total 3,435 patients were included, among whom 835 (24.3%) had preoperative HN and 2,600 (75.7%) had no preoperative HN. All the studies showed a positive association between preoperative HN and cancer-specific survival (HR, 1.16–3.37). A significant association was found in five studies but not in three studies (Figure 4). The analysis showed a significant association between bladder cancer-specific survival and preoperative HN among the selected cohorts (HR, 1.67, 95% CI, 1.34–2.08, p < 0.00001).
Figure 4.
Forest plot and meta-analysis of cancer specific survival after radical cystectomy according to preoperative hydronephrosis. SE, standard error; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Overall Survival
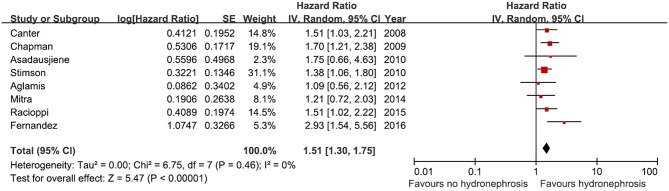
Eight studies were included in a meta-analysis to predict overall survival after RC according to preoperative HN. A total of 3,756 patients were included, among whom 903 (24.0%) had preoperative HN and 2,853 (76.0%) patients had no preoperative HN. From 2008 to 2016, all the included studies showed a positive association between preoperative HN and overall survival (HR, 1.09–2.93). A significant association was observed in five studies but not in three studies (Figure 5). The analysis showed a significant association between overall survival and preoperative HN among the selected cohorts (HR, 1.51, 95% CI, 1.30–1.75, p < 0.00001).
Figure 5.
Forest plot and meta-analysis overall survival after radical cystectomy according to preoperative hydronephrosis. SE, standard error; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Publication Bias
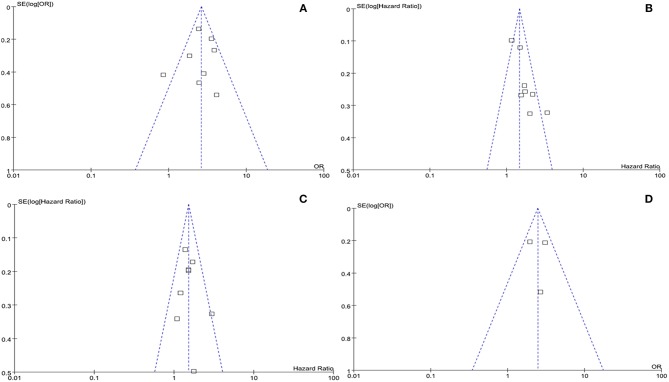
The publication bias of our meta-analysis was assessed using funnel plots. No evidence of significant publication bias was observed (Figure 6). We found no strong evidence for publication bias by graphical inspection. No publication bias was detected in the other meta-analyses (all p > 0.05, Egger's test).
Figure 6.
Funnel plots of (A) advanced pathological stage, (B) cancer specific survival, (C) overall survival. and (D) lymph node invasion. SE, standard error; OR, odds ratio.
Discussion
In this systematic review, we investigated the effect of preoperative HN on bladder cancer prognostic outcomes. A meta-analysis that incorporated 24 studies based on data from 10,461 patients who underwent RC for bladder cancer revealed that preoperative HN was significantly associated with pathologically advanced disease, lymph node invasion, cancer-specific survival, and overall survival.
Leibovitch et al. (33) evaluated 122 patients with invasive bladder cancer and showed for the first time the influence of HN on survival outcomes among patients who underwent RC. They showed ureteral obstruction (54.1%) due to bladder cancer associated with higher stage and grade, and low 5-year survival rate. Similar studies confirmed previous results that HN was an obvious prognostic factor. Thrasher et al. (34) reported their results from 531 patients who underwent RC for bladder cancer and demonstrated that preoperative HN was a significant prognostic factor of cancer-specific survival. Haleblian et al. (35) showed in their results in 1998 that 28% of patients with hydronephrotic bladder cancer had higher tumor stage and poorer survival outcomes.
On the basis of these results, preoperative HN could be a confirmatory factor for the prediction of patient survival after RC. However, some controversial results showed no association between HN and prognosis. Hofner et al. (3) reported their results on relatively long-term outcomes from an analysis of HN as a factor of cancer-specific survival. They showed that HN was positively associated with advanced stage, but not with cancer-specific survival. They emphasized the surgical margin status in a multivariate analysis, after including the aforementioned factors, HN remained a significant prognostic factor. Haleblian et al. (35) from the University of Southern California Group, reported a 5-year cancer-specific survival of 44.7% for patients with HN, which was lower than that of the patients without HN. However, cancer-specific survival was comparable after the follow-up period. More-large-scale data (n = 1,776) from the Mayo Clinic in the United States showed that preoperative HN was associated with a 20% increased risk of bladder cancer-related death. However, HN was not a significant factor in the multivariate analysis with a competing risk analysis (2). In our meta-analysis, eight studies were included in the analysis to examine cancer-specific survival. Six studies showed a positive impact on the increased HR, but two studies showed no significant impact. Finally, merged HR to cancer-specific survival was calculated to be 1.67 (95% CI, 1.34–2.08, p < 0.00001) in 3,435 patients with bladder cancer who underwent RC (2, 3, 7, 11, 13, 14, 22, 26). Overall survival was regarded controversial according to the presence of preoperative HN. In our meta-analysis, three studies showed no significant association between HN and overall survival (5, 24, 25), but five studies showed a significant association (8, 11, 12, 21, 26). The merged HR was 1.51 (95% CI 1.30–1.75, p < 0.00001) in 3,756 patients. As the prognosis of the patients with preoperative HN associated was so poor, more active therapeutic strategies might be beneficial for them (9).
The reason that the presence of preoperative HN had an impact on survival after RC might be the association with preoperative HN and pathological stage. Previous studies showed almost uniform results that HN before RC among patients with bladder cancer was a predictive factor of higher pathological stage. In the study by Leibovitch et al. (33), most cases of hydronephrotic bladder cancer without involvement of the intramucosal ureteral orifices extended to the external layer of the detrusor muscle. In their series, hydronephrotic bladder cancer infiltrating adjacent organs (pT4 tumors) was evident in 58% of patients. Therefore, preoperative HN was an evident factor of advanced disease stage and thus could be an apparent prognostic factor. Another reason should be focused on renal function. A previous study showed a significant cause-specific survival benefit for patients without HN as compared with those with HN (65.9 vs. 32.2% at 5 years) (4). The authors also found a significant difference in serum creatinine levels between patients with and patients without hydronephrotic kidneys (2.4 mg/dl vs. 1.1 mg/dl), and performed a simultaneous nephrectomy in 13.9% during surgical procedures. Therefore, decreased renal function could play an effective role in the prognosis after RC among patients with bladder cancer. Lastly, the appropriate treatment in the presence of HN might be limited to neoadjuvant chemotherapy because of the relative decrease in renal function (11).
This systemic meta-analysis has several limitations. First, most of the included studies had a retrospective design. No prospective study was included for comparison of outcomes according to preoperative HN, although we collected well-designed retrospective studies. Nevertheless, a prospective observational study should be conducted to confirm the significance of HN. Most included studies had an intermediate-to-high NOS score. Second, only few studies had a relatively small number of subjects included, and most of the studies had a relatively large sample size, therefore, publication bias analysis could include most of them. We also examined the differences between unilateral and bilateral HN as prognostic factors. However, only a relatively small number of studies compared unilateral HN to an extension on both kidneys: Nine studies included data for uni- and bilaterality, and only one study presented a statistically significant difference for CSS with a worse outcome among patients with bilateral HN compared to unilateral HN. Due to the lack of evidence no further analyses comparing unilateral and bilateral HN with respect to OS or CSS were possible. Another limitation was heterogeneity. The heterogeneity of studies were found in CSS. And no variables analyzed in the meta-regression contributed to the heterogeneity. In fact, the presence of heterogeneity may result from many other factors. Due to lack of detailed data, we could not use these variables in the meta-regression. Finally, we could not adjust the surgical methods, including urinary reconstruction. The obvious differences among urinary diversion techniques such as ileal conduit, neobladder, or ureterostomy could affect patients' health. However, we could not unify these factors. This could also be elucidated in a future prospective study.
In conclusions, our meta-analysis showed that patients with bladder cancer who had HN before RC had significantly higher pathological stage, higher risk of lymph node invasion, and poorer cancer-specific survival and overall survival than those who did not have HN before RC. Although larger prospective studies should be necessary to confirm our findings, preoperative HN was a confirmatory prognostic factor of survival in patients with bladder cancer.
Author Contributions
JK conceived, designed, supervised experiments. CJ, S-SB, JK, HK, and CK performed the experiments. CJ and JK analyzed the data. JO wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2016R1A2B4011623) and grant of SNUBH-14-2018-011 from SNUBH (Seoul National University Bundang Hospital). The funding source had no role in the study design, in the collection, analysis, and interpretation of data, or in the writing of the report. The corresponding author had full access to all data and had final responsibility to submit the paper for publication.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. (2017) 71:96–108. 10.1016/j.eururo.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg MS, Boorjian SA, Cheville JC, Thompson RH, Thapa P, Kaushik D, et al. The SPARC score: a multifactorial outcome prediction model for patients undergoing radical cystectomy for bladder cancer. J Urol. (2013) 190:2005–10. 10.1016/j.juro.2013.06.022 [DOI] [PubMed] [Google Scholar]
- 3.Hofner T, Haferkamp A, Knapp L, Pahernik S, Hadaschik B, Djakovic N, et al. Preoperative hydronephrosis predicts advanced bladder cancer but is not an independent factor for cancer-specific survival after radical cystectomy. Urol Int. (2011) 86:25–30. 10.1159/000321008 [DOI] [PubMed] [Google Scholar]
- 4.Bartsch GC, Kuefer R, Gschwend JE, de Petriconi R, Hautmann RE, Volkmer BG. Hydronephrosis as a prognostic marker in bladder cancer in a cystectomy-only series. Eur Urol. (2007) 51:690–7; discussion 7–8. 10.1016/j.eururo.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 5.Asadauskiene J, Aleknavicius E, Pipiriene-Zelviene T, Jankevicius F. The value of clinical prognostic factors for survival in patients with invasive urinary bladder cancer. Medicina. (2010) 46:305–14. 10.3390/medicina46050043 [DOI] [PubMed] [Google Scholar]
- 6.Stojadinovic MM, Prelevic RI. External validation of existing nomograms predicting lymph node metastases in cystectomized patients. Int J Clin Oncol. (2015) 20:164–70. 10.1007/s10147-014-0693-3 [DOI] [PubMed] [Google Scholar]
- 7.Kim DS, Cho KS, Lee YH, Cho NH, Oh YT, Hong SJ. High-grade hydronephrosis predicts poor outcomes after radical cystectomy in patients with bladder cancer. J Korean Med Sci. (2010) 25:369–73. 10.3346/jkms.2010.25.3.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stimson CJ, Cookson MS, Barocas DA, Clark PE, Humphrey JE, Patel SG, et al. Preoperative hydronephrosis predicts extravesical and node positive disease in patients undergoing cystectomy for bladder cancer. J Urol. (2010) 183:1732–7. 10.1016/j.juro.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 9.Lin HY, Wang SZ, Chen JX, Chen LW, Xiao J. The prognostic value of hydronephrosis in bladder cancer treated by radical cystectomy. Urologia. (2011) 78:17–21. 10.5301/RU.2011.6444 [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. (2009) 151:W65–94. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 11.Canter D, Guzzo TJ, Resnick MJ, Brucker B, Vira M, Chen Z, et al. Hydronephrosis is an independent predictor of poor clinical outcome in patients treated for muscle-invasive transitional cell carcinoma with radical cystectomy. Urology. (2008) 72:379–83. 10.1016/j.urology.2008.03.053 [DOI] [PubMed] [Google Scholar]
- 12.Chapman DM, Pohar KS, Gong MC, Bahnson RR. Preoperative hydronephrosis as an indicator of survival after radical cystectomy. Urol Oncol. (2009) 27:491–5. 10.1016/j.urolonc.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 13.Resorlu B, Baltaci S, Resorlu M, Ergun G, Abdulmajeed M, Haliloglu AH, et al. Prognostic significance of hydronephrosis in bladder cancer treated by radical cystectomy. Urol Int. (2009) 83:285–8. 10.1159/000241668 [DOI] [PubMed] [Google Scholar]
- 14.Choudhury A, Nelson LD, Teo MT, Chilka S, Bhattarai S, Johnston CF, et al. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. (2010) 70:7017–26. 10.1158/0008-5472.CAN-10-1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie HY, Zhu Y, Yao XD, Zhang SL, Dai B, Zhang HL, et al. Development of a nomogram to predict non-organ-confined bladder urothelial cancer before radical cystectomy. Int Urol Nephrol. (2012) 44:1711–9. 10.1007/s11255-012-0273-2 [DOI] [PubMed] [Google Scholar]
- 16.Ahmadi H, Mitra AP, Abdelsayed GA, Cai J, Djaladat H, Bruins HM, et al. Principal component analysis based pre-cystectomy model to predict pathological stage in patients with clinical organ-confined bladder cancer. BJU Int. (2013) 111:E167–72. 10.1111/j.1464-410X.2012.11502.x [DOI] [PubMed] [Google Scholar]
- 17.Green DA, Rink M, Hansen J, Cha EK, Robinson B, Tian Z, et al. Accurate preoperative prediction of non-organ-confined bladder urothelial carcinoma at cystectomy. BJU Int. (2013) 111:404–11. 10.1111/j.1464-410X.2012.11370.x [DOI] [PubMed] [Google Scholar]
- 18.Potretzke A, Hillman L, Wong K, Shi F, Brower R, Mai S, et al. NLR is predictive of upstaging at the time of radical cystectomy for patients with urothelial carcinoma of the bladder. Urol Oncol. (2014) 32:631–6. 10.1016/j.urolonc.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 19.Prelevic R, Stojadinovic MM, Simic D, Spasic A, Petrovic N. Scoring system development for prediction of extravesical bladder cancer. Vojnosanit Pregl. (2014) 71:851–7. 10.2298/VSP130814040P [DOI] [PubMed] [Google Scholar]
- 20.Pietzak EJ, Sterling ME, Smith ZL, Malkowicz SB, Guzzo TJ. Outcomes of radical cystectomy in potential candidates for bladder preservation therapy. Urology. (2015) 85:869–75. 10.1016/j.urology.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 21.Racioppi M, Salmaso L, Brombin C, Arboretti R, D'Agostino D, Colombo R, et al. The clinical use of statistical permutation test methodology: a tool for identifying predictive variables of outcome. Urol Int. (2015) 94:262–9. 10.1159/000365292 [DOI] [PubMed] [Google Scholar]
- 22.Hirasawa Y, Nakashima J, Yunaiyama D, Sugihara T, Gondo T, Nakagami Y, et al. Sarcopenia as a novel preoperative prognostic predictor for survival in patients with bladder cancer undergoing radical cystectomy. Ann Surg Oncol. (2016) 23:1048–54. 10.1245/s10434-016-5606-4 [DOI] [PubMed] [Google Scholar]
- 23.Martini T, Aziz A, Roghmann F, Rink M, Chun FK, Fisch M, et al. Prediction of locally advanced urothelial carcinoma of the bladder using clinical parameters before radical cystectomy–a prospective multicenter study. Urol Int. (2016) 96:57–64. 10.1159/000433606 [DOI] [PubMed] [Google Scholar]
- 24.Mitra AP, Skinner EC, Schuckman AK, Quinn DI, Dorff TB, Daneshmand S. Effect of gender on outcomes following radical cystectomy for urothelial carcinoma of the bladder: a critical analysis of 1,994 patients. Urol Oncol. (2014) 32:52.e1-9. 10.1016/j.urolonc.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 25.Aglamis E, Toktas G, Unluer E, Tasdemir C, Ceylan C. Prognostic factors in radical cystectomy affecting survival. Arch Med Sci. (2012) 8:650–4. 10.5114/aoms.2012.30288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez MI, Williams SB, Willis DL, Slack RS, Dickstein RJ, Parikh S, et al. Clinical risk stratification in patients with surgically resectable micropapillary bladder cancer. BJU Int. (2017) 119:684–91. 10.1111/bju.13689 [DOI] [PubMed] [Google Scholar]
- 27.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Response to: practical methods for incorporating summary time-to-event data into meta. Authors' reply. Trials. (2013) 14:391. 10.1186/1745-6215-14-391 [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Jeong CW, Kwak C, Kim HH, Ku JH. Association between demographic factors and prognosis in urothelial carcinoma of the upper urinary tract: a systematic review and meta-analysis. Oncotarget. (2017) 8:7464–76. 10.18632/oncotarget.10708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells GA, Shea BJ, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed November 3, 2014).
- 33.Leibovitch I, Ben-Chaim J, Ramon J, Madjar I, Engelberg IS, Goldwasser B. The significance of ureteral obstruction in invasive transitional cell carcinoma of the urinary bladder. J Surg Oncol. (1993) 52:31–5. 10.1002/jso.2930520109 [DOI] [PubMed] [Google Scholar]
- 34.Thrasher JB, Frazier HA, Robertson JE, Dodge RK, Paulson DF. Clinical variables which serve as predictors of cancer-specific survival among patients treated with radical cystectomy for transitional cell carcinoma of the bladder and prostate. Cancer. (1994) 73:1708–15. [DOI] [PubMed] [Google Scholar]
- 35.Haleblian GE, Skinner EC, Dickinson MG, Lieskovsky G, Boyd SD, Skinner DG. Hydronephrosis as a prognostic indicator in bladder cancer patients. J Urol. (1998) 160:2011–4. 10.1016/S0022-5347(01)62228-6 [DOI] [PubMed] [Google Scholar]