Abstract
Novel alternative splicing events were identified from BmNPV-susceptible and -resistant silkworm strains after BmNPV infection using high-throughput RNA-sequencing strategy. In total, 12.82 Gb clean RNA-seq data were generated for the two midgut samples from BmNPV-susceptible and -resistant silkworm strains, and 14.78 Gb clean data for the two fat body samples. The number of alternative splicing events and isoforms in the BmNPV-susceptible silkworm strain was more than that in the BmNPV-resistant silkworm strain. Furthermore, alternative splicing genes uniquely present in BmNPV-resistant silkworm strain were involved in functions about ribosome, whereas, alternative splicing genes uniquely present in BmNPV-susceptible silkworm strain were implicated in functions like DNA helicase activity and signal transduction. Additionally, 33 expressed SR or SR-like proteins were identified, and three genes encoding SR or SR-like proteins (tetratricopeptide repeat protein 14 homolog, ubiquitin carboxyl-terminal hydrolase 32 and zinc finger CCCH domain-containing protein 18) have a higher number of different alternative splicing events between two silkworm strains. The present study suggested BmNPV treatment may have a smaller effect on the mRNA transcription in BmNPV-resistant silkworms than that in BmNPV-susceptible silkworms, and functions of alternative splicing genes are different between the two silkworm strains.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1669-9) contains supplementary material, which is available to authorized users.
Keywords: Bombyx mori nucleopolyhedrovirus, RNA-sequencing, Alternative splicing, SR proteins
Introduction
Bombyx mori nucleopolyhedrovirus (BmNPV) is a common and severe pathogen for silkworm (Bombyx mori), which is of great economic value and a model organism for Lepidoptera insects (Qin et al. 2012). Till now, no therapeutic agents are available to effectively control BmNPV infection. To enhance antiviral capacity of the silkworm, BmNPV-resistant silkworm varieties have been constructed (Xu et al. 2013). However, the viral infection and the defense mechanism in silkworm need to be further investigated. During the invading or spreading process of pathogens, different innate immune responses in silkworm are activated to act against pathogens. The midgut and fat body of silkworm are important immune tissues in the resistance against pathogens (Lekha et al. 2015; Morishima et al. 1997). Previous studies have found a set of genes and proteins (e.g., caspase-1, serine protease and arginine kinase) involved in the resistance against BmNPV in silkworm (Kang et al. 2011; Li et al. 2016; Qin et al. 2012).
Alternative splicing plays an extremely pivotal role in the expansion of protein diversity (Graveley 2001). Due to different splice sites, a single transcript can generate multiple protein isoforms, thus alternative splicing contributes to the apparent discordance between gene number and organismal complexity. A previous study has identified a series of alternative splicing events by transcriptome analysis of silkworm (Shao et al. 2012). Furthermore, some proteins associated with alternative splicing have been found to be correlated with the resistance against BmNPV in silkworm. For instance, a member of the CELF/BRUNOL protein family, CUGBP Elav-like family member 4 (CELF4), which can regulate pre-mRNA alternative splicing, is upregulated in BmNPV-infected midguts of silkworm (Lekha et al. 2015). Besides, an unusual alternative splicing of an antimicrobial peptide enbocin has been discovered in the BmNPV-infected silkworms (Andreeva 2013). However, associations of alternative splicing with silkworm resistance to BmNPV are still elusive. There is no study using a high-throughput technology to analyze the differences of alternative splicing events and related genes between BmNPV-susceptible and BmNPV-resistant silkworm strains after BmNPV infection so far.
In this study, to compare differences of alternative splicing events and related genes between BmNPV-susceptible and BmNPV-resistant silkworms after infection, the RNA-sequencing of midgut and fat body tissues from two silkworm strains Qiufeng (susceptible to BmNPV) and QiufengN (highly resistant to BmNPV) after BmNPV treatment was performed. Alternative splicing events in the midgut and fat body were analyzed, and functions of genes involved in alternative splicing were investigated. Furthermore, number and expression of alternative splicing isoforms were calculated. Additionally, the splicing regulators SR proteins were identified using the transcriptome sequencing data. These results may provide new information for the study of alternative splicing in silkworm resistance to BmNPV.
Materials and methods
Silkworm materials and virus
Two silkworm strains Qiufeng (susceptible to BmNPV) and QiufengN (highly resistant to BmNPV) were maintained at 27 °C and underwent a reversed 12:12 h light/dark cycle. They were fed with mulberry leaves until 5-instar old. BmNPV was obtained from the hemolymph of infected larvae and maintained in our laboratory. After 2 h of hunger, 50 individuals of each strain took 1 × 109 BmNPV/mL virus suspension (7 µL per individual) orally. Midgut tissue was sampled from the two strains respectively after 12 h and fat body after 48 h. The samples were frozen immediately in liquid nitrogen after sampling and stored at − 80 °C.
RNA extraction, cDNA library construction and Illumina deep sequencing
Total RNA in midgut and fat body samples was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Poly (A)—tailed RNA was prepared using magnetic oligo (dT) beads, and was then sheared into fragments and reverse-transcribed to cDNA. Afterwards, the cDNA was blunt-ended, phosphorylated, and added with a single 3′ adenosine moiety and index adapters on the repaired ends. Then, cDNA was amplified using bridge amplification to generate clonal DNA clusters. Insert size and concentration of the cDNA library were detected using Agilent 2100 and Qubit 2.0, respectively. Paired-end sequencing was performed following the Illumina workflow on a HiSeq 2500 (Illumina) sequencer with the use of the Truseq PE Cluster Kit v3-cBot-HS (Illumina).
Data filtering
For the raw data in each sample, the reads were cleaned by removing the adapter sequences, empty reads, reads with N over 5% using Sickle (https://github.com/najoshi/sickle) and SeqPrep program (https://github.com/jstjohn/SeqPrep). In addition, the reads were trimmed by discarding the reads containing more than 20% bases with Q value ≤ 10 in 3′ terminal and reads with adaptor sequences.
Transcriptome alignment
The alignment software TopHat2 (Version 2.1.0, http://ccb.jhu.edu/software/tophat/index.shtml) (Kim et al. 2013) was used for the transcriptome alignment of clean data with the reference genome from Silkworm Genome Database (SilkDB, http://silkworm.genomics.org.cn/). Using the ultra high-throughput short read aligner Bowtie, TopHat2 aligns RNA-Seq reads to mammalian-sized genomes, and then identifies splice junctions between exons by analyzing the mapping results (Kim et al. 2013). The reads that were aligned with the reference genome were defined as mapped reads, and the mapped reads that had a unique location were defined as unique mapped reads.
Analysis of alternative splicing
Alternative splicing patterns were analyzed using Cufflinks (Version 2.2.1, http://cole-trapnell-lab.github.io/cufflinks/) (Trapnell et al. 2012), via comparing with the normal mRNA splicing patterns. Six types of alternative splicing events were distinguished: alternative 5′ splicing sites, alternative 3′ splicing sites, skipped exon, intron retention, alternative last exon, and alternative first exon.
Here, the genes that were involved in alternative splicing events were termed as alternative splicing genes (AS-genes). The AS-genes that were uniquely present in fat body samples were termed as FAS-genes, in midgut samples termed as MAS-genes, in QiufengN race termed as RAS-genes, and in Qiufeng race termed as SAS-genes. The AS-genes with fold change (FC) > 2 between two different samples were identified as differentially expressed AS-genes.
Functional analysis of AS-genes
The Gene Ontology (GO) database (http://geneontology.org/) (Consortium 2015) was used to perform the GO enrichment analysis of FAS-genes, MAS-genes, RAS-genes and SAS-genes, respectively. The p value of each term was calculated by Fisher’s exact test (Preacher and Briggs 2001) and adjusted using the Benjamini–Hochberg method (Benjamini and Hochberg 1995). Only functional terms with adjusted p value < 0.05 were considered significant.
Expression analysis of alternative splicing isoforms
The number of alternative splicing isoforms with different FPKM (Reads per Kilobase of exon model per Million mapped reads) and related genes were calculated. Here, only transcripts and genes with FPKM > 0 were included.
Furthermore, expression similarity analysis of alternative splicing isoforms was performed via constructing a clustering tree using a minimum distance method by R package (Yu et al. 2012).
Identification of SR proteins
Serine/arginine-rich proteins (SR proteins) in the midgut and fat body samples from silkworm were detected as the previous method reported by Shao et al. (Shao et al. 2012). Briefly, a database of all silkworm proteins was generated, and then proteins with more than 10 SR/RS di-peptides plus at least one SR/RS triple di-peptide repeat were identified based on the amino acid composition that is characteristic of SR proteins.
Validation of the accuracy of the AS-genes by real-time quantitative PCR
To validate the results of AS-genes, real-time quantitative PCR experiments were carried out using 7300 Sequence Detection System (ABI) with SYBR Premix Ex TapTM (Takara). The primers of randomly selected ten genes of interest were listed in supplementary table S3, and the Bmactin3 gene was used as the internal reference gene. Amplification conditions were as follows: denaturation at 95 °C for 10 min, followed by 35 cycles of 95 °C for 15 s and 60 °C for 40 s for annealing, and an extension. All of the samples were measured independently three times.
Results
Statistics of sequencing data
In total, 12.82 Gb clean data were generated for the two midgut samples (clean data > 6.40 Gb for each sample), and percentage of base ≥ Q30 for each sample was more than 86.03%. There were a total of 14.78 Gb clean data generated for the two fat body samples (clean data > 7.15 Gb for each sample), and percentage of base ≥ Q30 for each sample was more than 88.00% (Table 1). Based on the alignment, the transcriptome data of the four samples covered 14,612 genes (99.92%) in the silkworm reference genome. The mapped reads accounted for about 70% of reads for each sample (Table 1).
Table 1.
The statistic results of transcriptome data for each sample
| F1 | F2 | M1 | M2 | |
|---|---|---|---|---|
| Clean reads | ||||
| Read number | 28,402,151 | 30,308,707 | 25,420,394 | 25,500,762 |
| Base number | 7,151,259,373 | 7,632,852,036 | 6,402,063,943 | 6,422,482,754 |
| GC content (%) | 45.41 | 46.41 | 49.64 | 49.85 |
| % ≥ Q30 | 88.00 | 88.81 | 86.03 | 87.01 |
| Analysis | ||||
| Mapped reads | 41,047,811 | 47,751,117 | 35,265,473 | 35,824,800 |
| Unique mapped reads | 38,826,519 | 46,243,843 | 29,824,772 | 25,500,762 |
| Map ratio (%) | 72.26 | 78.77 | 69.36 | 70.24 |
| No. of covered genes | 14,612 | 14,612 | 14,314 | 14,314 |
Mapped reads are the reads aligned with the reference genome, and unique mapped reads are the mapped reads having a unique location in the genome. Map ratio means the percentage of mapped reads in the total reads. F1 represents the fat body sample of silkworm strain that is susceptible to BmNPV; F2 represents the fat body sample of silkworm strain that is highly resistant to BmNPV; M1 represents the midgut sample of silkworm strain that is susceptible to BmNPV; M2 represents the midgut sample of silkworm strain that is highly resistant to BmNPV.
Statistics of alternative splicing analysis
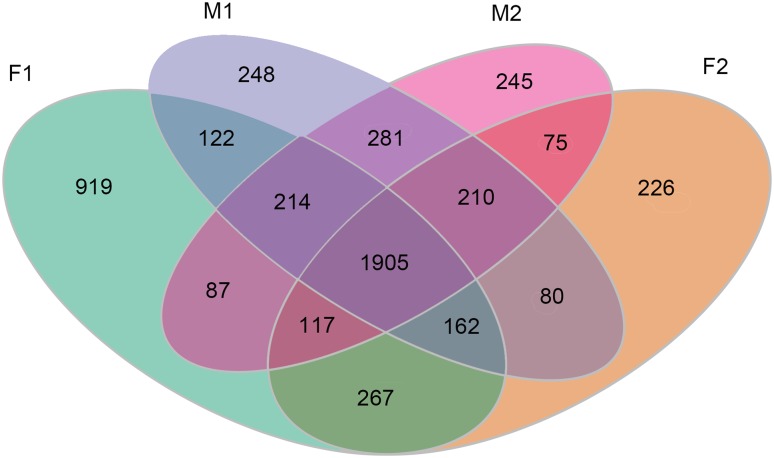
According to the analysis of alternative splicing events for each silkworm sample, the number of skipped exon events was the most (≥ 2257) among the six types of alternative splicing events (detailed in the supplementary table T01-T04). Comparing the two fat body samples of the two silkworm strains, the number of all six types of alternative splicing events in the BmNPV-susceptible silkworms was much more than that in the BmNPV-resistant silkworm. Furthermore, comparing the two midgut samples, the alternative splicing events in the BmNPV-susceptible silkworms were mainly more than that in the BmNPV-resistant silkworm, whereas, the difference between the two strains was small (Table 2). Additionally, the AS-genes in the BmNPV-susceptible silkworms were also more than that in the BmNPV-resistant silkworms (Table 2). The Venn diagram revealed that there were 267 FAS-genes, 281 MAS-genes, 75 RAS-genes, and 122 SAS-genes (Fig. 1, Supplementary table T05).
Table 2.
The number of different alternative splicing events for each silkworm sample
| Sample | Skipped exon | Intron retention | Alternative 5′ splicing sites | Alternative 3′ splicing sites | Alternative last exon | Alternative first exon | Number of AS-genes |
|---|---|---|---|---|---|---|---|
| F1 | 3107 | 481 | 625 | 749 | 860 | 2052 | 3793 |
| F2 | 2257 | 313 | 504 | 602 | 705 | 1556 | 3042 |
| M1 | 2551 | 341 | 530 | 639 | 735 | 1700 | 3222 |
| M2 | 2462 | 343 | 527 | 636 | 739 | 1597 | 3134 |
Fig. 1.
Venn diagram displaying the number of genes involved in alternative splicing in the silkworm midgut and fat body samples. F1 represents the fat body sample of silkworm strain that is susceptible to BmNPV; F2 represents the fat body sample of silkworm strain that is highly resistant to BmNPV; M1 represents the midgut sample of silkworm strain that is susceptible to BmNPV; M2 represents the midgut sample of silkworm strain that is highly resistant to BmNPV
AS-genes represent the genes involved in alternative splicing events. F1 represents the fat body sample of silkworm strain that is susceptible to BmNPV; F2 represents the fat body sample of silkworm strain that is highly resistant to BmNPV; M1 represents the midgut sample of silkworm strain that is susceptible to BmNPV; M2 represents the midgut sample of silkworm strain that is highly resistant to BmNPV.
GO enrichment analysis of AS-genes
To further investigate the difference of biological functions related to alternative splicing between fat body and midgut samples, as well as BmNPV-susceptible and BmNPV-resistant samples, GO enrichment analysis of the FAS-genes, MAS-genes, RAS-genes and SAS-genes was respectively conducted.
The FAS-genes were mainly associated with several GO terms about enzyme activity (e.g., hydrolase activity and carboxypeptidase activity) and metabolic process (e.g., carboxylic acid and one-carbon metabolic processes). Meanwhile, the MAS-genes were significantly related to GO terms about enzyme activity (e.g., phosphodiesterase activity and hydrolase activity) and some biological processes (e.g., signal transduction and translational initiation) (Table S1).
Furthermore, the RAS-genes were significantly involved in several GO terms about ribosome (e.g., structural constituent of ribosome and cellular protein modification process). The SAS-genes were markedly implicated in GO terms like ATP-dependent DNA helicase activity and signal transduction (Table S2).
Real-time quantitative PCR verification of differentially expressed genes
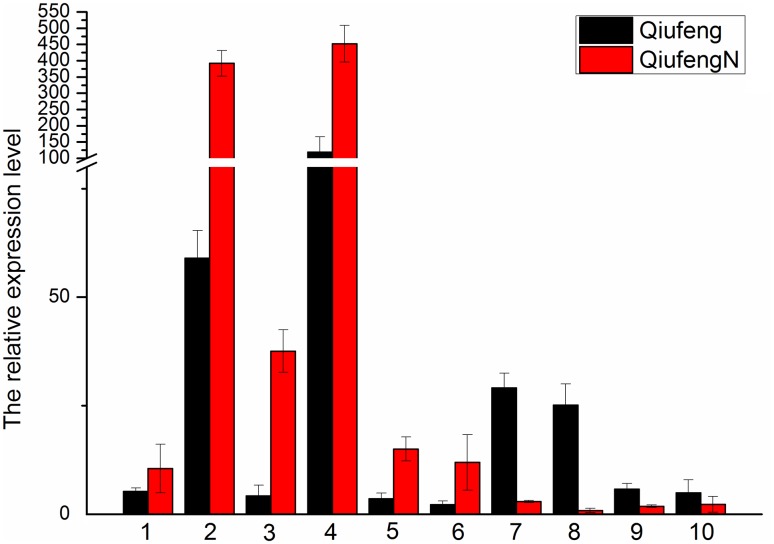
To validate the RNA-seq data, the relative repression of randomly selected ten genes was performed by qRT-PCR (Fig. 2). The qRT-PCR data results showed consistency with the RNA-seq data, suggesting that the RNA-seq data were credible. We assumed that these genes could play an important role in the silkworm BmNPV resistance.
Fig. 2.
qRT-PCR validation of selected AS-genes. The data are the average ± standard error of the three independent replicated qRT-PCR experiments. Indicated is the fold change in expression of selected genes between the Qiufeng and QiufengN silkworm after BmNPV infection as obtained by qRT-PCR on 5th instar samples. (1) Oligoribonuclease (BGIBMGA011592-TA); (2) Eif-4a (BGIBMGA003186-TA); (3) 3-ketoacyl-CoA thiolase (BGIBMGA012661-TA); (4) ribosomal protein S11 (BGIBMGA013792-TA); (5) pseudouridine-5′-phosphate glycosidase (BGIBMGA007781-TA); (6) unknown (BGIBMGA011540-TA); (7) importin subunit alpha-5 (BGIBMGA011351-TA); (8) DNA-damage inducible protein (BGIBMGA012323-TA); (9) serine/arginine repetitive matrix protein 2(BGIBMGA002310-TA); (10) phospholipid-transporting ATPase (BGIBMGA001553-TA)
Expression of alternative splicing isoforms
To reveal the expression of alternative splicing isoforms in the four samples, the isoform count and expression level were calculated. For the fat body samples, there was an obvious difference in the number of expressed isoforms and genes between the BmNPV-susceptible and BmNPV-resistant silkworms (isoforms: 10,420 vs. 9172; genes: 11,756 vs. 10,219), whereas, the number of new genes was the same (1200 vs. 1200). For the midgut samples, the number of expressed isoforms and genes between the two silkworm strains was almost the same (isoforms: 9367 vs. 9326; genes: 10,299 vs. 10,258), but, the new genes in the BmNPV-susceptible silkworms were less than that in the BmNPV-resistant silkworms (788 vs. 942).
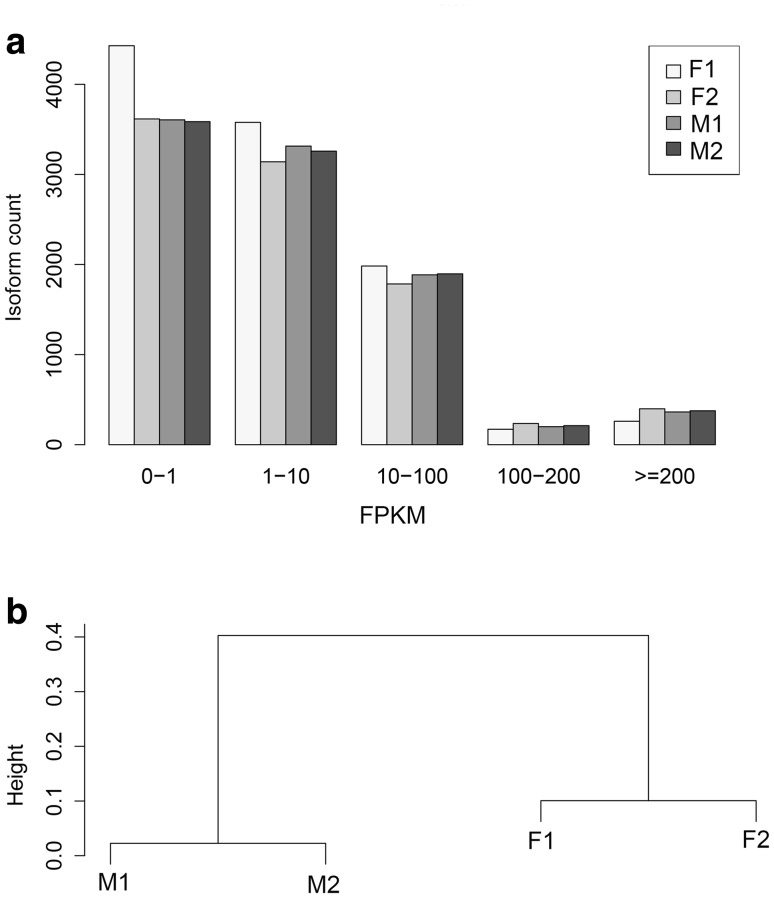
Furthermore, the FPKM of most isoforms in the four samples was distributed at 0–100 (Fig. 3a). According to clustering analysis of the 1905 genes commonly present in the four samples, the cluster dendrogram showed that the gene expression similarity in the same tissue samples (fat body samples or midgut samples) was high, but low in the same strain samples (BmNPV-susceptible or BmNPV-resistant silkworm samples) (Fig. 3b), indicating that gene expression similarity may be almost determined by the tissue type but not the silkworm strain.
Fig. 3.
Expression of alternative splicing isoforms in the silkworm midgut and fat body samples. a The number of alternative splicing isoforms distributing at different FPKM segments. b Cluster dendrogram displaying the expression similarity of alternative splicing isoforms
Analysis of SR proteins
In total, 37 SR or SR-like proteins were identified from the four silkworm samples. Among them, four proteins were not expressed in all of the four samples. The expression of the SR or SR-like protein genes nesprin-1 (BGIBMGA010471) and arginine/serine-rich splicing factor 7 (BGIBMGA010396) in the fat body samples was significantly higher than that in the midgut samples, and other’s gene expression was similar between different tissue samples. Furthermore, the alternative splicing events of 16 SR or SR-like proteins were different between the two fat body samples, and 19 between the two midgut samples (Fig. 4). The top 3 SR or SR-like protein genes with a higher number of different alternative splicing events were tetratricopeptide repeat protein 14 homolog (BGIBMGA005999), ubiquitin carboxyl-terminal hydrolase 32 (BGIBMGA013218) and zinc finger CCCH domain-containing protein 18 (BGIBMGA011662). The alternative splicing types and position information of these three protein genes were listed in Table 3.
Fig. 4.
Heat map of the identified SR or SR-like proteins in the silkworm midgut and fat body samples. Color variation of bars indicates the FPKM change
Table 3.
The different alternative splicing types and position information of the top 3 SR or SR-like protein genes with a higher number of different alternative splicing events
| Sample | Gene | Alternative splicing types | Start site | End site | Start site′ | End site′ | Alternative splicing site |
|---|---|---|---|---|---|---|---|
| Tetratricopeptide repeat protein 14 homolog (BGIBMGA005999) | F1 | Alternative 5′ splicing sites | 4,709,637 | 4,709,786 | 4,709,685 | 4,709,786 | 4,709,637:4,709,684 |
| F1 | Alternative last exon | 4,706,964 | 4,707,132 | 4,704,855 | 4,704,940 | ||
| F1 | Skipped exon | 4,713,653 | 4,714,349 | ||||
| F1 | Skipped exon | 4,714,164 | 4,714,349 | ||||
| F2 | Alternative first exon | 4,713,653 | 4,714,026 | 4,718,133 | 4,718,282 | ||
| F2 | Alternative last exon | 4,706,996 | 4,707,132 | 4,704,855 | 4,704,940 | ||
| M1 | Alternative last exon | 4,706,788 | 4,707,132 | 4,704,855 | 4,704,940 | ||
| M1 | Skipped exon | 4,713,653 | 4,713,845 | ||||
| M1 | Skipped exon | 4,714,164 | 4,714,349 | ||||
| M2 | Alternative last exon | 4,707,027 | 4,707,132 | 4,704,855 | 4,704,940 | ||
| Ubiquitin carboxyl-terminal hydrolase 32 (BGIBMGA013218) | F1 | Alternative first exon | 202,381 | 202,570 | 190,416 | 190,537 | |
| F1 | Alternative last exon | 218,538 | 218,771 | 219,148 | 220,313 | ||
| F1 | Intron retention | 205,981 | 206,060 | ||||
| F1 | Intron retention | 213,780 | 213,851 | ||||
| F1 | Intron retention | 218,907 | 219,148 | ||||
| F2 | Alternative first exon | 202,381 | 202,570 | 190,380 | 190,537 | ||
| F2 | Alternative last exon | 218,538 | 218,770 | 219,237 | 220,314 | ||
| M1 | Alternative first exon | 202,381 | 202,570 | 190,416 | 190,537 | ||
| M1 | Alternative last exon | 218,538 | 218,762 | 219,148 | 220,277 | ||
| M1 | Intron retention | 218,907 | 219,148 | ||||
| M2 | Alternative 3′ splicing sites | 206,480 | 206,620 | 206,496 | 206,620 | 206,480:206,495 | |
| M2 | Alternative first exon | 202,381 | 202,570 | 190,376 | 190,537 | ||
| M2 | Alternative first exon | 202,282 | 202,570 | 190,376 | 190,537 | ||
| M2 | Alternative last exon | 218,538 | 218,772 | 219,237 | 220,277 | ||
| M2 | Intron retention | 205,981 | 206,060 | ||||
| M2 | Skipped exon | 208,413 | 208,486 | ||||
| Zinc finger CCCH domain-containing protein 18 (BGIBMGA011662) | F1 | Skipped exon | 2,314,337 | 2,314,402 | |||
| F2 | Alternative first exon | 2,319,551 | 2,320,337 | 2,322,574 | 2,322,591 | ||
| F2 | Skipped exon | 2,318,994 | 2,319,095 | ||||
| F2 | Skipped exon | 2,319,307 | 2,319,461 | ||||
| F2 | Skipped exon | 2,319,551 | 2,320,339 | ||||
| M1 | Intron retention | 2,316,906 | 2,317,074 | ||||
| M1 | Intron retention | 2,317,074 | 2,317,151 | ||||
| M2 | Alternative first exon | 2,319,551 | 2,320,337 | 2,322,511 | 2,322,593 | ||
| M2 | Skipped exon | 2,318,994 | 2,319,095 | ||||
| M2 | Skipped exon | 2,319,307 | 2,319,461 | ||||
| M2 | Skipped exon | 2,319,551 | 2,320,339 |
“Start site” means the start site of altered exon; “End site” means the end site of altered exon; “Start site′” the start site of retained exon; “End site′” means the end site of retained exon
Discussion
In the present study, a total of 12.82 Gb clean RNA-seq data were generated for the two midgut samples from BmNPV-susceptible and BmNPV-resistant silkworms, and 14.78 Gb clean data for the two fat body samples after infection. Comparing the two fat body samples and two midgut samples from the two silkworm strains, on the whole, the number of alternative splicing events in the BmNPV-susceptible silkworms was more than that in the BmNPV-resistant silkworms. Similar results were observed in the number of alternative splicing isoforms and AS-genes. These results indicated that the BmNPV treatment had a smaller effect on the mRNA transcription in BmNPV-resistant silkworms than that in BmNPV-susceptible silkworms.
Previous studies have found that BmNPV infection results in the changes of mRNA transcription of some protein genes. For example, alternative splicing events were observed in the three subunits of adaptor protein complex-1 (AP-1) post BmNPV infection in B. mori cells (Niu et al. 2012). Besides, BmNPV infection leads to an unusual alternative splicing of an antimicrobial peptide enbocin in silkworm (Andreeva 2013). Moreover, in the BmNPV-infected midguts of silkworms, CELF4 that specifically activates the exonic splicing is upregulated (Lekha et al. 2015), which may induce more alternative splicing events in BmNPV-infected silkworms. These results suggested that after BmNPV treatment, alternative splicing events may occur more frequently in the BmNPV-susceptible silkworms compared with the BmNPV-resistant silkworms.
In this study, according to the functional analysis of AS-genes uniquely present in BmNPV-susceptible and BmNPV-resistant silkworms after infection, we found that RAS-genes were significantly involved in functions about ribosome, such as structural constituent of ribosome and cellular protein modification process. Meanwhile, the SAS-genes were markedly implicated in several functions like ATP-dependent DNA helicase activity and signal transduction. Obviously, the biological functions that RAS-genes and SAS-genes participated in were different. In the BmNPV-susceptible silkworms, after BmNPV infection, NPVs require DNA replication that needs DNA helicase activity to generate more NPVs (Slack and Arif 2007). Besides, after pathogen infection, a series of signal transduction pathways about immunity will be activated during the insect host systemic immune responses (Huang et al. 2009; Hussain and Asgari 2014). Therefore, AS-genes in the BmNPV-susceptible silkworm are predicted to be correlated with DNA replication and signal transduction. By contrast, in the BmNPV-resistant silkworms, we speculated that antiviral proteins were stimulated to be synthetized in ribosome to resist the viral invasion, and special isoforms of some antiviral proteins were generated via alternative splicing. Therefore, in the BmNPV-resistant silkworms, after BmNPV treatment, AS-genes were predicted to be associated with functions about ribosome.
Furthermore, 33 expressed SR or SR-like proteins were identified from the four silkworm samples, and the number of different alternative splicing events of three genes encoding SR or SR-like proteins tetratricopeptide repeat protein 14 homolog (BGIBMGA005999), ubiquitin carboxyl-terminal hydrolase 32 (BGIBMGA013218) and zinc finger CCCH domain-containing protein 18 (BGIBMGA011662) was higher between two silkworm strains, indicating that the three protein genes may generate different kinds of isoforms in silkworm strains with different antiviral ability. SR proteins usually function as activators in pre-mRNA splicing, and alternative splicing type of many SR proteins has a marked impact on alternative splicing and expression of downstream genes (Long and Caceres 2009; Sanford et al. 2005). The evidence of different alternative splicing of the three SR protein genes between BmNPV-susceptible and BmNPV-resistant silkworm varieties still needed further study. As a result, in the resistance to BmNPV of the two silkworm varieties, gene’ alternative splicing and expression are likely much different due to the different alternative splicing of genes encoding SR or SR-like proteins like tetratricopeptide repeat protein 14 homolog, ubiquitin carboxyl-terminal hydrolase 32 and zinc finger CCCH domain-containing protein 18, which is a new discovery of this study.
In conclusion, based on the transcriptome analysis of midgut and fat body from BmNPV-susceptible and BmNPV-resistant silkworms after infection, we found that the number of alternative splicing events and isoforms in the BmNPV-susceptible silkworms was more than that in the BmNPV-resistant silkworms, indicating that the BmNPV treatment had a smaller effect on the mRNA transcription in BmNPV-resistant silkworms than that in BmNPV-susceptible silkworms. Furthermore, functions of AS-genes are different between the two silkworm strains. Different alternative splicing of genes encoding SR or SR-like proteins (tetratricopeptide repeat protein 14 homolog, ubiquitin carboxyl-terminal hydrolase 32 and zinc finger CCCH domain-containing protein 18) between the two silkworm strains may result in different genes’ alternative splicing and expression. These findings have provided new evidence for the investigation on associations of alternative splicing with the silkworm resistance to BmNPV.
Electronic supplementary material
Below is the link to the electronic supplementary material.
T01.AltSplice_info: the alternative splicing information of the fat body of Qiufeng that is susceptible to BmNPV (XLSX 406 KB)
T02.AltSplice_info: the alternative splicing information of the fat body of QiufengN that is resistant to BmNPV (XLSX 314 KB)
T03.AltSplice_info: the alternative splicing information of the midgut of Qiufeng that is susceptible to BmNPV (XLSX 341 KB)
T04.AltSplice_info: the alternative splicing information of the midgut of QiufengN that is resistant to BmNPV (XLSX 331 KB)
T05. Alternative splicing events common between F1, F2, M1, M2 (XLSX 56 KB)
Table S1 The enriched Gene Ontology terms of FAS-genes and MAS-genes. Table S2 The enriched Gene Ontology terms of RAS-genes and SAS-genes. Table S3 Primers of 10 AS-genes of qRT-PCR (DOCX 26 KB)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31802136); the China Agriculture Research System (Sericulture industry, CARS-18); The Natural Science Foundation of the Jiangsu Higher Education Institutions of China (17KJB230001); Key Research & Development program of Zhenjiang (NY2018021, NY2017017).
Compliance with ethical standards
Conflict of interest
The authors declares that they have no conflict interest.
Contributor Information
Heying Qian, Email: qianheying123@163.com.
Anying Xu, Email: srixay@126.com.
References
- Andreeva A. Identification of some proteins of blood and tissue fluid in fish with undeciphered genome. Zh Evol Biokhim Fiziol. 2013;49:394–402. [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- Consortium GO. Gene ontology consortium: going forward. Nucleic Acids Res. 2015;43:D1049–D1056. doi: 10.1093/nar/gku1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley BR. Alternative splicing: increasing diversity in the proteomic world. Trends Genetics. 2001;17:100–107. doi: 10.1016/s0168-9525(00)02176-4. [DOI] [PubMed] [Google Scholar]
- Huang L, Cheng T, Xu P, Cheng D, Fang T, Xia Q. A genome-wide survey for host response of silkworm, Bombyx mori during pathogen Bacillus bombyseptieus infection. PLoS One. 2009;4:e8098. doi: 10.1371/journal.pone.0008098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M, Asgari S. MicroRNAs as mediators of insect host–pathogen interactions and immunity. J Insect Physiol. 2014;70:151–158. doi: 10.1016/j.jinsphys.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Kang L, et al. Arginine kinase is highly expressed in a resistant strain of silkworm (Bombyx mori, Lepidoptera): Implication of its role in resistance to Bombyx mori nucleopolyhedrovirus. Comp Biochem Physiol B Biochem Mol Biol. 2011;158:230–234. doi: 10.1016/j.cbpb.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekha G, Gupta T, Awasthi AK, Murthy GN, Trivedy K, Ponnuvel KM. Genome wide microarray based expression profiles associated with BmNPV resistance and susceptibility in Indian silkworm races of Bombyx mori. Genomics. 2015;106:393–403. doi: 10.1016/j.ygeno.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Li G, Qian H, Luo X, Xu P, Yang J, Liu M, Xu A. Transcriptomic analysis of resistant and susceptible Bombyx mori strains following BmNPV infection provides insights into the antiviral mechanisms. Int J Genomics. 2016;2016:2086346. doi: 10.1155/2016/2086346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JC, Caceres JF. The SR protein family of splicing factors: master regulators of gene expression. Biochem J. 2009;417:15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- Morishima I, Yamano Y, Inoue K, Matsuo N. Eicosanoids mediate induction of immune genes in the fat body of the silkworm, Bombyx mori. FEBS Lett. 1997;419:83–86. doi: 10.1016/s0014-5793(97)01418-x. [DOI] [PubMed] [Google Scholar]
- Niu Y-s, Wang M-x, Liang S, Zhou F, Miao Y-g. Expression and localization of silkworm adaptor protein complex-1 subunits, which were down-regulated post baculovirus infection. Mol Biol Rep. 2012;39:10775–10783. doi: 10.1007/s11033-012-1971-7. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Briggs NE (2001) Calculation for Fisher’s exact test: an interactive calculation tool for Fisher’s exact probability test for 2 x 2 tables [Computer software]. Available from http://quantpsy.org
- Qin L, et al. Comparative proteomic analysis reveals that caspase-1 and serine protease may be involved in silkworm resistance to Bombyx mori nuclear polyhedrosis virus. J Proteom. 2012;75:3630–3638. doi: 10.1016/j.jprot.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Sanford J, Ellis J, Caceres J. Multiple roles of arginine/serine-rich splicing factors in RNA processing. Biochem Soc Trans. 2005;33:443–446. doi: 10.1042/BST0330443. [DOI] [PubMed] [Google Scholar]
- Shao W, et al. Alternative splicing and trans-splicing events revealed by analysis of the Bombyx mori transcriptome. RNA. 2012;18:1395–1407. doi: 10.1261/rna.029751.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J, Arif BM. The baculoviruses occlusion-derived virus: virion structure and function. Adv Virus Res. 2007;69:100. doi: 10.1016/S0065-3527(06)69003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protocols. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Lin C, Qian H, Sun P, Zhang Y, Liu M, Li L. Breeding of a new silkworm variety “Huakang 2” with tolerance to Bombyx mori nucleopolyhedrovirus disease. Sci Seric. 2013;39:275–282. [Google Scholar]
- Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
T01.AltSplice_info: the alternative splicing information of the fat body of Qiufeng that is susceptible to BmNPV (XLSX 406 KB)
T02.AltSplice_info: the alternative splicing information of the fat body of QiufengN that is resistant to BmNPV (XLSX 314 KB)
T03.AltSplice_info: the alternative splicing information of the midgut of Qiufeng that is susceptible to BmNPV (XLSX 341 KB)
T04.AltSplice_info: the alternative splicing information of the midgut of QiufengN that is resistant to BmNPV (XLSX 331 KB)
T05. Alternative splicing events common between F1, F2, M1, M2 (XLSX 56 KB)
Table S1 The enriched Gene Ontology terms of FAS-genes and MAS-genes. Table S2 The enriched Gene Ontology terms of RAS-genes and SAS-genes. Table S3 Primers of 10 AS-genes of qRT-PCR (DOCX 26 KB)