Abstract
Background
Rice blast caused by Magnaporthe oryzae is the most devastating disease in rice production. Compared with seedling blast, panicle blast is considered to be more destructive, which can occur without being preceded by severe seedling blast. However, panicle blast resistance research is rarely reported.
Results
Bodao, a japonica landrace from Taihu Lake region, showed a high level of panicle blast resistance. In this study, a mapping population of 212 recombination inbreeding lines (RILs) was developed from a cross of Bodao and the susceptible cultivar Suyunuo, and the RILs were evaluated for panicle blast resistance in three trials. Two quantitative trait loci (QTLs) qPb11–1 and qPb6–1 for panicle-blast resistance were identified, including a major QTL qPb11–1 (Pb-bd1) on chromosome 11 of Bodao explaining from 55.31% to 71.68% of the phenotype variance, and a minor QTL qPb6–1 on chromosome 6 of Suyunuo explaining from 3.54% to 6.98% of the phenotype variance. With the various segregation populations, Pb-bd1 was fine mapped in a 40.6 Kb region flanked by markers BS83 and BS98, and six candidate genes were identified within this region, including one gene encoding NAC domain-containing protein, one gene encoding unknown expression proteins, two genes encoding nucleotide binding site-leucine rich repeat (NBS-LRR) type disease resistance proteins, and two genes encoding von Willebrand factor type A (VWA) domain containing proteins. For application in rice breeding, three introgression lines of Pb-bd1with significantly enhanced panicle blast resistance were developed by using molecular assisted method (MAS) from the commercial variety Nanjing46 (NJ46).
Conclusion
Two QTLs, qPb11–1(Pb-bd1) and qPb6–1 conferring panicle blast resistance, were identified from japonica landrace Bodao and Suyunuo.qPb11–1(Pb-bd1) was fine mapped in a 40.6 Kb region flanked by marker BS83 and BS98. Three introgression lines of Pb-bd1with significantly enhanced panicle blast resistance were developed by MAS method from the commercial variety NJ46. It indicated that Pb-bd1 would be useful gene source in panicle blast resistance breeding.
Electronic supplementary material
The online version of this article (10.1186/s12284-019-0275-0) contains supplementary material, which is available to authorized users.
Keywords: Rice, Panicle blast resistance, QTL, Fine mapping, MAS, Introgression lines, Resistance breeding
Background
Rice (Oryza sativa L.) is one of the major food crops, and it is the staple food for more than half of the world population (Sharma et al. 2012). Rice blast, caused by the fungal pathogen Magnaporthe oryzae, is one of the most devastating diseases worldwide, which can occur throughout entire rice growth period (Ou 1985). Up to date, more than 100 blast resistance genes were identified in different genotypes of rice and 28 resistance genes among them have been cloned (Ashkani et al. 2016). Pi-b was the first cloned blast resistance gene through a map-based cloning strategy (Wang et al. 1999). Four genes Pi2, Pi9, Pi-gm and Piz-t were located in Pi2 cluster on chromosome 6 (Zhou et al. 2006; Deng et al. 2006; Qu et al. 2006). Six genes Pik, Pik-h, Pik-m, Pik-p, Pi1 and Pi-ke were located in Pik cluster on chromosome 11 (Ashikawa et al. 2008; Chen et al. 2015; Hua et al. 2012; Sharma et al. 2005; Yuan et al. 2011; Zhai et al. 2011). The majority of cloned resistance genes encoded nucleotide binding site-leucine rich repeat (NBS-LRR) proteins (Chen et al. 2010), except for Pi-d2 (encoding a B-lectin receptor kinase) (Kouzai et al. 2013), recessive gene pi21 (encoding a proline-rich protein) (Fukuoka et al. 2009), Bsr-d1 (encoding a C2H2-type transcription factor protein) (Li et al. 2017), and Bsr-k1 (encoding a tetratricopeptide repeats-containing protein) (Zhou et al. 2018). Deng et al. (2017) revealed that epigenetic regulation of Pi-gmR and Pi-gmS can balance the blast resistance and yield in rice. Pi-gmR confered broad-spectrum resistance, and Pi-gmS can increase rice production to counteract the yield lost caused by Pi-gmR. Li et al. (2017) reported Bsr-d1 was an C2H2-type transcription factor conferring broad-spectrum blast resistance, and low expression of this gene could enhance disease resistance by inhibiting degradation of H2O2.
Among the 28 cloned resistance genes, only Pb1 was a panicle blast resistance gene. Pb1 was isolated from the indica cultivar Modan, and encoded a coiled coil-nucleotide binding site-leucine rich repeat (CC-NBS-LRR) protein, conferring durable and broad-spectrum resistance to rice blast (Hayashi et al. 2010; Inoue et al. 2013). Pi25 and Pi64 were associated with both seedling blast and panicle blast resistance and encoded CC-NBS-LRR proteins (Wu et al. 2005; Chen et al. 2011; Ma et al. 2015). In total, eight panicle blast resistant QTLs have been identified. Ishihara et al. (2014) identified two panicle blast resistance QTLs qPbm11 and qPbm9 on chromosome 11 and 9 in japonica cultivar Miyazakimochi. Fang et al. (2016) found one major panicle blast resistance QTL qPbh-11-1 and one minor QTL qPbh-7-1 from japonica landrace Heikezijing. Wang et al. (2016) identified one major panicle blast resistance QTL qPbj-11-1 and three minor QTLs qPbj-7-1, qPbj-6-1 and qPbj-9-1 from japonica landrace Jiangnanwan.
Compared with seedling blast, panicle blast is considered to be more destructive, which can occur without being preceded by severe seedling blast (Katsube and Koshimizu 1970; Hwang et al. 1987; Zhu et al. 2005). Panicle blast can cause direct yield losses up to 70% even 100% in fields by affecting grain sterility, rotting the branch and neck, even losing the entire panicle (Liu et al. 2014; Khan et al. 2014; Roumen 1992; Bonman et al. 1991; Chin 1975; Lu et al. 2015). However, there are few reports about resistance genes or QTLs of panicle blast and the correlation between seedling blast and panicle blast (Koh et al. 1987; Zhuang et al. 2002; Bonman 1992; Fang et al. 2016; Sirithunya et al. 2002). The major difficulty for panicle blast researching is that the inoculation and phenotype identification should be conducted in the fields (Liu et al. 2016; Sirithunya et al. 2002; Zhuang et al. 2002).
So far, the most economical and effective way to control blast disease is introducing resistance genes into susceptible elite cultivars (Hulbert et al. 2001). The resistance genes as Pi1, Pi5, Piz-5, Pita and Pi-gm have been introgressed into various elite cultivars by marker-assisted selection (MAS) method (Sharma et al. 2012; Deng et al. 2017). However, few genes were applied for controlling the panicle blast resistance. Bodao is a japonica landrace from Taihu Lake region and exhibited high leaf blast resistance (Li et al. 2007a, 2007b; Huan et al. 2014). In this study, we fine mapped a panicle blast resistance gene Pb-bd1 in japonica landrace Bodao, and introduced Pb-bd1 into commercial japonica cultivar Nanjing 46 (NJ46) for panicle blast resistance breeding.
Methods
Plant materials and growth
Bodao, a japonica rice (Oryza sativa L.) landrace from Taihu Lake region in China, showed broad-spectrum resistance to rice blast (Li et al. 2007b). Suyunuo, another japonica landrace from the same region, was susceptible to the blast. A RIL population (F2:7) consisting of 212 lines, derived from the cross of Bodao and Suyunuo, was used for QTL mapping of panicle blast resistance in this study.
The RIL population and their parents were grown in the Jiangpu experiment station (Nanjing, Jiangsu Province) in 2014 and 2015 and in the Lingshui experiment station (Lingshui, Hainan Province) in 2014 respectively. Twenty plants of each RIL were grown in two rows per plot. Bodao and Suyunuo were grown adjacent to the plots, as resistant and susceptible controls, respectively. At the booting stage panicles of these plants were inoculated with the blast pathogen.
Inoculation and resistance evaluation
The strain Hoku1 of blast pathogen (Magniporthe oryzae) was used for inoculation in this study, which was provided by Institute of Crop Science, Chinese Academy of Agricultural Sciences. 212 RILs and two parents were inoculated with the pathogen by an injection method as described by Liu et al. (2007). Three panicles of per plant and five plants for each line were inoculated with conidial suspension (3 × 104 conidia/ml). Diseased grain rates were evaluated based on visual assessment of disease severity 3 weeks after inoculation as described by Asaga (1981).
Molecular marker development
According to the International Rice Microsatellite Initiative (IRMI, http://www.gramene.org), 2257 SSR markers were adopted for polymorphism analysis between Bodao and Suyunuo. To find putative InDels, sequence alignments were performed between japonica Nipponbare (http://rgp.dna.affrc.go.jp/) and indica 93–11 (http://www.gramene.org). InDel markers were designed by Primer Premier 5.0, and identified with 8% polyacrylamide gel electrophoresis (PAGE).
Genetic map construction and QTL mapping
With the genotypes and panicle blast resistance phenotypes of all 212 RILs, linkage map construction and QTLs mapping were carried out by the software ICIMapping 4.01 (http://www.isbreeding.net/software). The software parameters were set as follows: a LOD threshold of 2.5, walking speed of 1.0 cM, and calculated from 1000 permutation at a probability of 0.01.
Fine mapping of Pb-bd1 and prediction of candidate genes
BC1F1, BC2F1, BC3F1, BC4F1 and BC5F1 plants were obtained from a cross between Bodao and Suyunuo and backcrossed with recurrent parents Suyunuo. BC4F3 and BC5F3 populations were obtained from selfing of heterozygous BC4F2 and BC5F2, respectively. In this study, 3632 BC3F2, 5240 BC4F3, 1200 BC5F2 and 2928 BC5F3 plants were used for fine mapping the target QTL Pb-bd1. The primers of PCR-based markers used for fine mapping Pb-bd1 are shown in Additional file 1: Table S1.
The genomic sequences in the region of markers BS83 and BS98 on chromosome 11 were downloaded from the RGP (Rice Genome research Program) Web site (https://rgp.dna.affrc.go.jp/index.html.en). Open reading frames in the target region of Pb-bd1 were predicted by GENSCAN (http://genes.mit.edu), FGENSH (http://linux1.softberry.com/), and Rice Genome Automated Annotation System (RiceGAAS) (http://rgp.dna.affrc.go.jp) software (Sakata et al. 2002).
Expression analysis of candidate genes
The GENEVESTIGATOR (http://genevestigator.com/gv/) and Rice Oligo Array Database (http://ricearray.org) were employed to analyze the expression of candidate genes based on 1154 Affymetrix microarray datasets (http://www.ricearray.org/). The expression patterns of five candidate genes P1, P3-P6 were detected in various rice tissues and blast-fungi inoculated seedlings by GENEVESTIGATOR.
The immature panicles at breaking stage of Bodao and Suyunuo inoculated by blast strain Hoku1, were collected at 2 h, 4 h, 8 h, 12 h, 24 h, 48 h and 72 h after inoculation for the expression analysis of candidate genes. The expressions of six candidate genes were detected by real-time PCR methods as described by Huang et al. (2008). The fold changes of target candidate genes relative to the reference gene (18 s-rRNA) was calculated by the 2-△△CT method (Livak and Schmittgen, 2001). All reactions were performed in three replicates. The primers for 6 candidate genes and 18 s-rRNA in quantitative real-time PCR assay are shown in Additional file 2: Table S2.
Development of ingression lines with Pb-bd1
As a blast-resistance donor, Bodao was crossed with a commercial japonica cultivar Nanjing 46 (NJ46) in 2012 at Lingshui. NJ46 was used as a recurrent parent, the BC1F1 and BC2F1 plants with panicle-blast resistance phenotype were selected after inoculated with Hoku1 at Nanjing in 2013. Based on the QTL mapping results at Nanjing in 2014, the panicle blast resistant plants of BC3F1, BC3F2, BC3F3, and BC3F4 were selected with Pb-bd1 linked markers RM7654 and BS79, and the selection was also combined with panicle blast inoculation and agronomy traits identification in the fields.
Three introgression lines (NJ46 + Pb-bd1(a), NJ46 + Pb-bd1(b), NJ46 + Pb-bd1(c)) carrying Pb-bd1 gene, were grown at Nanjing in 2015 for evaluating their panicle blast resistance and agronomic characters, including the diseased grains rates, plant height, grain number/panicle, 1000-grain weight. Diseased grain rates of three introgression lines were evaluated with an injection method as described as above. The introgression lines were also grown in the natural disease-nurseries in Changsha, Hunan province and in Jintan and Ganyu, Jiangsu province for panicle blast evaluation. Ten plants each row and three rows of each introgression line were grown for the evaluation of panicle blast resistance in the natural disease-nurseries. The panicle blast score (1–9) was identified as describe as Ahn (1994).
Results
Characterization of resistance to panicle blast in Bodao
Two parents Bodao and Suyunuo, and 212 RILs derived from these two parents were inoculated with blast strain Hoku1 at the booting stage for panicle blast evaluation at Nanjing and Lingshui in 2014, and Nanjing in 2015. The results from three trials showed that Bodao was high resistant to panicle blast with 9.90–25.92% of diseased grains, while Suyunuo susceptible with 63.15–100.00% of diseased grains (Table 1). The frequency distributions of 212 RILs with various diseased grains rates for three trials were asymmetric and continuous, and the distributions were all predisposed resistance-inclined distribution (Fig. 1). In Pearson’s correlation analysis, the resistance to panicle blast each RIL was a significant correlation in various trails (P ≤ 0.01) (Table 2).
Table 1.
Phenotypic values of panicle blast resistance to isolate Hoku1 in F2:7 RIL population
| Location and year | Isolate | Parents | RIL populationb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bodao | Suyunuo | Mean | Max | Min | SDc | Skewness | Kurtosis | ||
| Lingshui2014 | Hoku1 | 25.92 ± 3.77%a (R) | 100% (S) | 33.85% | 100% | 0 | 0.3357 | 1.0163 | −0.3540 |
| Nanjing 2014 | 10.19 ± 3.41% (R) | 89.08 ± 5.90% (S) | 35.55% | 100% | 0 | 0.2674 | 0.8126 | −0.2598 | |
| Nanjing 2015 | 9.90 ± 1.73% (R) | 63.15 ± 9.36% (S) | 35.80% | 100% | 0 | 0.3236 | 0.6711 | −0.9655 | |
ameans diseased grains (%);
bRIL sample size n = 212, replications r = 3;
cstandard deviation
Fig. 1.
Characterization of panicle blast severity distribution in 212 F2:7 RILs. a-c Distribution of panicle blast diseased grain rate (%) of RILs at Lingshui and Nanjing in 2014, and Nanjing in 2015, respectively. d Panicle blast resistant phenotype of Bodao and Suyunuo after inoculation with strain Hoku1 for 3 weeks
Table 2.
Correlation analysis of panicle blast resistance of F2:7 RILs in three trails
| Lingshui 2014 | Nanjing 2014 | Nanjing 2015 | |
|---|---|---|---|
| Lingshui 2014 | 1 | ||
| Nanjing 2014 | 0.685** | 1 | |
| Nanjing 2015 | 0.803** | 0.792** | 1 |
n = 212, “**” means P ≤ 0.01
QTL identification of panicle blast resistance in Bodao
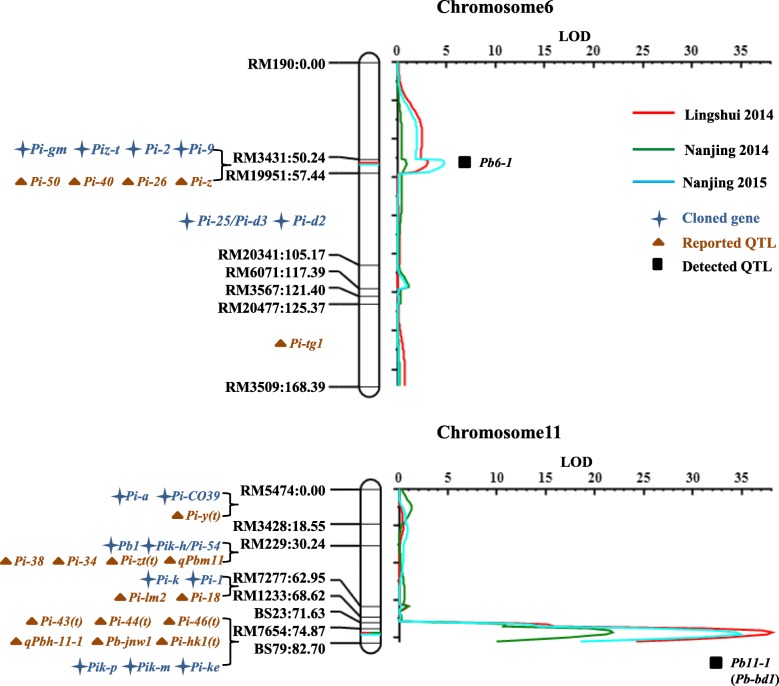
A genetic map with total 1303.34 cM and average 14.98 cM between two adjacent SSR markers was constructed with 87 polymorphic SSR markers selected from 2257 SSR markers. Two panicle blast resistance QTLs qPb11–1 and qPb6–1 were identified by inclusive composite interval mapping (ICIM) method with phenotypic data from three trials at Nanjing and Lingshui in 2014, and Nanjing in 2015 (Table 3, Fig. 2). A major QTL qPb11–1 was detected between marker RM7654 and BS79 on chromosome 11 in all three trials, designated as Pb-bd1 (Fig. 2). It could explain 64.10%, 56.30% and 73.00% of phenotypic variance with LOD scores of 37.63, 21.58 and 35.71, respectively. Another minor QTL qPb6–1 was detected between marker RM3431 and RM19951 on chromosome 6 in two trials at Lingshui in 2014 and Nanjing in 2015, explaining 4.97% and 6.06% of phenotypic variance with LOD scores of 4.24 and 4.28, respectively (Fig. 2).
Table 3.
Identification of panicle blast resistance QTLs in F2:7 RILs population
| QTL | Location and year | Chromosome | Region | LOD | PVE(%) | Add |
|---|---|---|---|---|---|---|
| qPb6–1 | Lingshui 2014 | 6 | RM3431-RM19951 | 4.2383 | 4.9675 | 0.0747 |
| Nanjing 2015 | 6 | RM3431-RM19951 | 4.2843 | 6.0634 | 0.0796 | |
| qPb11–1(Pb-bd1) | Lingshui 2014 | 11 | RM7654-BS79 | 37.6284 | 64.0961 | −0.2872 |
| Nanjing 2014 | 11 | RM7654-BS79 | 21.578 | 56.3005 | −0.2143 | |
| Nanjing 2015 | 11 | RM7654-BS79 | 35.7131 | 73.0038 | −0.2889 |
Fig. 2.
Identification of panicle blast resistance QTLs in Bodao by QTL mapping method. Marker names and their positions are shown on the left linkage group. The color lines indicate logarithm of the odds (LOD) scores
Fine mapping of Pb-bd1
The Pb-bd1 gene was mapped between SSR markers RM1233 and BS79 on chromosome 11 using 11 resistant recombinants screened from 45 BC1F1 plants (Fig. 3a). With segregated population of 3632 BC3F2, 44, 35, 29 and 29 recombinants were identified by the markers RM1233, BS23, BS59, and BS79, respectively. The results showed that Pb-bd1 could be mapped in the region of markers BS23 and BS59 (Fig. 3b). Thirteen recombinants were obtained by further screening segregated populations of 5240 BC4F3, 1200 BC5F2 and 2928 BC5F3, including 8, 6, 6, 5, 4, 4, 4, 5 and 5 recombinants identified by markers BS23, BS86, RM7654, BS84, BS83, BS98, BS97, BS90 and BS59, respectively (Fig. 3c). These 13 recombinants were inoculated with blast strain Hoku1 to evaluate their panicle blast resistance phenotypes. The Pb-bd1 was finally narrowed in the 40.6 kb region between markers BS83 and BS98.
Fig. 3.
Fine mapping of Pb-bd1in Bodao. a Pb-bd1 was located between RM1233-BS79. b Seventy-three recombinants were screened from 3632 BC3F2, and Pb-bd1 was located between markers BS23 and BS59. c 13 recombinants were screened from 5240 BC4F3, 1200 BC5F2 and 2928 BC5F3 population, and Pb-bd1 was finally flanked by markers BS83 and BS98 in the region of 40.6 Kb. d Six Pb-bd1 candidate genes were predicted and the arrows represent the direction of genes.
Candidate genes predicted and their expression
The 40.6 kb of target region conferring Pb-bd1 in the japonica Nipponbare sequence (27,803,976-27,844,340) was covered by two BAC clones OSJNBa0085H07 and OSJNBb0049B20. Six candidate genes were predicted in this region (Fig. 3d), including P1, LOC_Os11g45950; P2, LOC_Os11g45960; P3, LOC_Os11g45970; P4, LOC_Os11g45980; P5, LOC_Os11g45990; P6, LOC_Os11g46000. Among these candidate genes, P1 encodes a NAC domain-containing protein, P2 encodes an unknown expressed protein, P3 and P4 encode NBS-LRR type disease resistance proteins, and P5 and P6 encode von Willebrand factor type A domain (VWA) containing proteins. Compared the genomic sequences of these six genes between two parents Bodao and Suyunuo, P1, P4, P5 and P6 showed differences, and P3 no difference (Table 4), while P2 only existed in resistant parent Bodao.
Table 4.
The predicted candidate genes at the Pb-bd1 region in Bodao
| No. | Annotated genes | position | protein length (AA) | Prediction function | Sequence difference between two parents |
|---|---|---|---|---|---|
| P1 | LOC_Os11g45950 | 27,804,967–27,804,177 | 137 | NAC domain-containing protein 90, putative, expressed | Yes |
| P2 | LOC_Os11g45960 | 27,806,906–27,807,244 | 113 | expressed protein | Yes |
| P3 | LOC_Os11g45970 | 27,818,431–27,812,251 | 1024 | NBS-LRR type disease resistance protein, putative, expressed | No |
| P4 | LOC_Os11g45980 | 27,820,309–27,824,920 | 853 | NBS-LRR type disease resistance protein, putative, expressed | Yes |
| P5 | LOC_Os11g45990 | 27,828,621–27,835,246 | 634 | von Willebrand factor type A domain containing protein, putative, expressed | Yes |
| P6 | LOC_Os11g46000 | 27,841,513–27,845,382 | 599 | von Willebrand factor type A domain containing protein, putative, expressed | Yes |
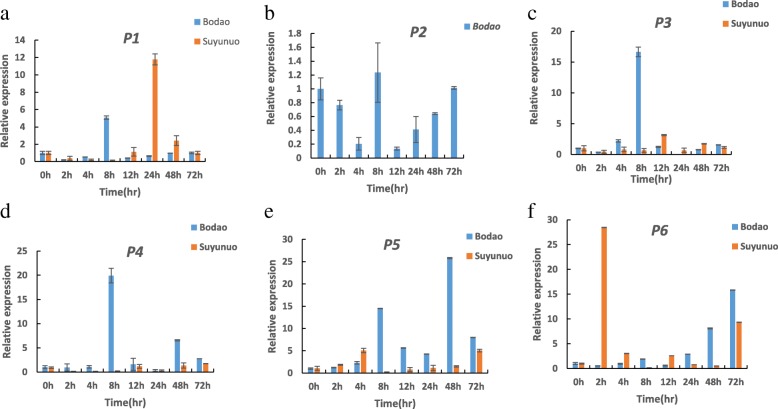
The expression profiles of five candidate genes P1, P3-P6 in various rice tissues and blast fungi inoculated seedlings were investigated based on microarray data deposited in the GENEVESTIGATOR (Fig. 4). The expressions of P1, P4, P5 and P6 could be detected with lower level in panicles, while no expression of P3 in panicle (Fig. 4a). P1, P3 and P4 showed similar expression patterns with obviously higher levels in root and rhizome than other tissues. P5 was expressed in rhizome, shoot, caryopsis, inflorescence, root and seedling with lower level, and P6 was expressed in shoot, caryopsis and leaf with low level (Fig. 4a).
Fig. 4.
Expression patterns of five candidate genes P1, P3-P6 based on microarray data from the GENEVESTIGATOR. a Expression patterns of five candidate genes in various rice tissues; b Expression patterns of five candidate genes in the seedling stage in response to M.oryzae treatments. Heat map showing the levels of gene expression in different rice tissues and blast inoculated seedlings. The P2 gene could not be detected in the microarray data
The expression of P5 was obviously induced at 3 d, 4 d and 6 d after inoculated with blast fungi, and the expression of P6 was induced at 4 d after inoculation. However, the expressions of P1, P3 and P4 were not induced after inoculation of blast fungi (Fig. 4b).
The expression patterns of six candidate genes in Bodao and Suyunuo were detected in immature panicles inoculated by the blast with real-time PCR approach. The expressions of P3, P4 and P5 were obviously induced by blast-inoculation in Bodao, while no significantly induction in Suyunuo (Fig. 5). The expressions of P1 and P6 were induced in both Bodao and Suyunuo, but with different patterns. P1 was induced and reached the peak at 8 h in Bodao, while reached the peak at 24 h in Suyunuo (Fig. 5). The expression of P6 was stably induced and reached the peak at 72 h in Bodao, while it was dramatically induced at 2 h, then decreased to the normal level in Suyunuo (Fig. 5). The expression of P2 was not prominently induced in Bodao.
Fig. 5.
Expression patterns of six candidate genes P1-P6 in Bodao and Suyunuo in immature panicles by real-time PCR methods (a-f). The P2 gene could not be amplified from Suyunuo by PCR. The immature panicles were inoculated by Hoku1 isolate. 18S-rRNA was used as an internal control. Data represent means and standard errors of three replicates.
The introgression lines of Pb-bd1 with enhanced panicle resistance
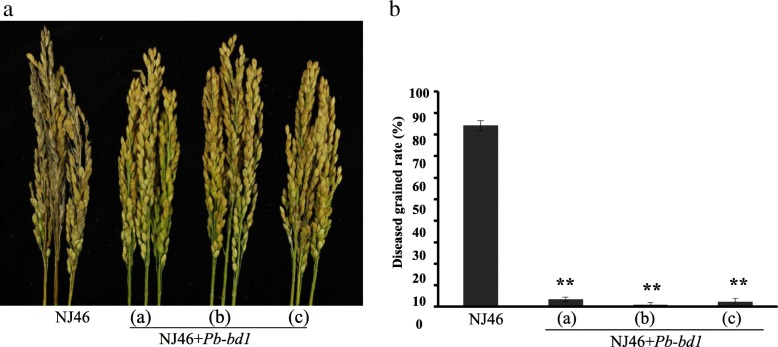
Three Pb-bd1 BC3F4 introgression lines NJ46 + Pb-bd1(a), NJ46 + Pb-bd1(b), NJ46 + Pb-bd1(c) were selected by MAS method with the markers RM7654 and BS79 closely linked to Pb-bd1. After inoculated with blast strain Hoku1, the rates of diseased grains for three introgression lines and susceptible backcross parent NJ46 were 3.56 ± 1.04%, 0.96 ± 0.96%, 2.35 ± 1.68% and 84.31 ± 2.27%, respectively (Fig. 6). It indicates that Pb-bd1 could significantly improve the panicle blast resistance of NJ46. Three introgression lines were also grown in natural disease nurseries in Changsha, Hunan province and in Jintan and Ganyu, Jiangsu province in 2016 and 2017, and they also showed enhanced panicle blast resistance (Table 5).
Fig. 6.
Three introgression lines of NJ46 + Pb-bd1 with enhanced panicle blast resistance. a The resistance phenotypes of NJ46 and the three introgression lines NJ46 + Pb-bd1 (a-c). b The diseased grain rates of NJ46 and the three introgression lines NJ46 + Pb-bd1 (a-c)
Table 5.
Panicle blast resistance score of three introgression lines in the natural disease nurseries
| Plant materials | 2016 | 2017 | |||
|---|---|---|---|---|---|
| Changsha, Hunan | Jintan, Jiangsu | Ganyu, Jiangsu | Changsha, Hunan | Jintan, Jiangsu | |
| NJ46 | 5.0 | 3.5 | 3.0 | 5.0 | 3.0 |
| NJ46 + Pb-bd1(a) | 3.0 | 3.0 | 1.0 | 3.5 | 3.0 |
| NJ46 + Pb-bd1(b) | 2.5 | 1.5 | 1.0 | 3.0 | 2.5 |
| NJ46 + Pb-bd1(c) | 1.0 | 1.0 | 1.0 | 3.0 | 1.5 |
The panicle blast score was 1–9 as describe as Ahn (1994)
The three introgression lines and backcross parent NJ46 were grown in the field in Nanjing to identify their agronomic characters. There were no significant differences in grain number per panicle between introgression lines and NJ46. The height of NJ46 + Pb-bd1(b) and NJ46 + Pb-bd1(c) were 80.33 ± 5.91 cm and 80.33 ± 5.25 cm respectively, significantly shorter than that of NJ46 (96.60 ± 5.28 cm). The 1000-grain weight of NJ46 + Pb-bd1(b) and NJ46 + Pb-bd1(c) were 29.97 ± 1. 31 g and 30.23 ± 0. 82 g respectively, significantly heavier than that of NJ46 (27.76 ± 1. 03 g). There were no significant differences in plant height and 1000-grain weight between NJ46 + Pb-bd1(a) and NJ46 (Table 6, Fig. 6).
Table 6.
Agronomic characters of three introgression lines and their recurrent parent NJ46
| Lines | Plant height (cm) | Grain number/panicle | 1000-grain weight (g) | Diseased grain(%) |
|---|---|---|---|---|
| NJ46 | 96.60 ± 5.28 | 142.92 ± 29.08 | 27.76 ± 1.03 | 84.31 ± 2.27 |
| NJ46 + Pb-bd1(a) | 86.33 ± 1.70 | 138.75 ± 17.98 | 27.41 ± 0.79 | 3.56 ± 1.04** |
| NJ46 + Pb-bd1(b) | 80.33 ± 5.91* | 155.00 ± 14.56 | 29.97 ± 1.31** | 0.96 ± 0.96** |
| NJ46 + Pb-bd1(c) | 80.33 ± 5.25* | 146.50 ± 32.85 | 30.23 ± 0.82** | 2.35 ± 1.68** |
** means P ≤ 0.01, and * means P ≤ 0.05
Discussion
The panicle blast resistant phenotypes in RILs population were relatively stable in various trials
Bodao, one Japonica landrace from Taihu lake region, exhibited high leaf blast resistance (Li et al. 2007a, 2007b; Huan et al. 2014). In this study, Bodao showed high panicle blast resistance with less than 30% the diseased grains, while the diseased grain rate of Suyunuo was over 60–100% in three various trials. The resistance to panicle blast of each RIL was a significant correlation in various trails (P ≤ 0.01). It indicates that the panicle blast resistant phenotypes in RILs population were relatively stable in various trials.
Two panicle blast resistance QTLs qPb11–1(Pb-bd1) and qPb6–1 from Bodao were detected by inoculated with Hoku1
Two panicle blast resistance QTLs qPb11–1(Pb-bd1) and qPb6–1 were detected from Bodao and Suyunuo by inoculated with the strain Hoku1 in this study. The qPb11–1 (Pb-bd1) was the major QTL for panicle blast resistance on chromosome 11 and with 71.68% of contribution to resistance phenotype. Up to date, only one panicle blast resistance gene Pb1 has been cloned and three QTLs conferring panicle blast resistance qPbm11, Pb-jnw1 and qPbh-11–1 were identified on chromosome 11 (Hayashi et al. 2010; Ishihara et al. 2014; Wang et al. 2016; Fang et al. 2016). Pb1, encoding an atypical CC-NBS-LRR protein, was located on the region between M35 and M26 on the short arm of chromosome 11 (Hayashi et al. 2010). The qPbm11 was a major panicle blast resistance QTL flanked by SNP markers aa11000537 and aa11001573 identified in japonica cultivar Miyazakimochi (Ishihara et al. 2014). The Pb-jnw1 flanked by SSR markers RM27273 and RM27381 was a major QTL in Jiangnanwan, conferring resistance to both seedling blast and panicle blast (Wang et al. 2016). The qPbh-11–1 flanked by SSR markers RM27187 and RM27381 was a major QTL for panicle blast resistance in Heikezijing (Fang et al. 2016). The qPb11–1 (Pb-bd1) identified in this study was not located in the same region of Pb1 and qPbm11, but within the region of QTLs Pb-jnw1 and qPbh-11-1 (Additional file 3: Figure S1). qPb11–1 (Pb-bd1), Pb-jnw1 and qPbh-11-1 are identified from landraces Bodao, Jiangnanwan and Heikezijing, respectively, which are all from Taihu lake region. These three QTLs might be the same novel panicle blast resistance gene and it could be confirmed through fine mapping and cloning methods.
One minor panicle blast resistant QTL qPb6–1 was detected between RM3431 and RM19951 on chromosome 6 in Suyunuo, and explained 3.54–6.98% of phenotype variance. In our previous results, a minor panicle blast resistant QTL qPbj-6-1 was also detected on chromosome 6 in Suyunuo with the Jiangnanwan×Suyunuo F2:6 RIL population (Wang et al. 2016). Both of qPb6–1 and qPbj-6-1 were located in the Pi2/Pi9 cluster (Wang et al. 2016). qPb6–1 was detected in two trails in Lingshui in 2014 and in Nanjing in 2015 in this study, while qPbj-6-1 was detected only in one trial (Wang et al. 2016). It indicates that these minor QTLs might be greatly influenced by environmental factors in the fields.
Six candidate genes in Pb-bd1 region were predicted
With the fine mapping populations, Pb-bd1 was finally mapped in a region of 40.6 kb between markers BS83 and BS98, and there were six candidate genes predicted. Among these six candidate genes, P1 encodes a NAC domain-containing protein, and P5 and P6 encode VWA containing proteins. NAC domain containing protein and VWA-containing domain protein have been reported that they can play important roles in rice- blast interactions (Liu and Jambunathan 2005; Lin et al. 2007; Rawat et al. 2012; Sun et al. 2013). P2 encoding unknown expression protein was only existed in resistant cultivar Bodao, and it could be the possible resistance gene Pb-bd1. P3 and P4 encode NBS-LRR type disease resistance proteins. Among the cloned 28 resistant genes, 24 genes encode NBS-LRR-containing proteins, and six resistance genes as Pikm, Pia, Pikp, Pike, Pi-l and Pi-5 were contributed by two adjacent NBS-LRR resistance genes (Zhai et al. 2011; Hua et al. 2012; Chen et al. 2015; Yuan et al. 2011; Ashikawa et al. 2008). These adjacent candidate NBS-LRR genes P3 and P4 could also be the resistance gene Pb-bd1. In our future research, we will further validate the functions of these six candidate genes through gene editing or transgenic complementary methods.
Introgression lines with high panicle blast resistance were good resources for blast resistance breeding
It has been proved that the most effective method to control rice blast disease is using resistance genes (Hulbert et al. 2001). Marker-assisted selection (MAS) is a high effective strategy to introduce the resistance genes into susceptible commercial cultivars. It has been reported that 99.75% of the plants containing the target gene were selected within 5 cM genetic distance between the marker and target gene by the MAS method (Zheng et al. 2009). In this study, three Pb-bd1 introgression lines were developed by MAS method. Compared with NJ46, the Pb-bd1 introgression lines enhanced resistance to panicle blast over 80%, while the QTL qPb11–1 (Pb-bd1) could only explain 55.31–71.68% of resistance phenotypic variance in Bodao. The possible reason could be due to some resistant locus in NJ46 might function with Pb-bd1 to contribute the resistance in the three introgression lines. The agronomic characters of introgression lines showed less significant differences with NJ46. Therefore, Bodao and the introgression lines with high panicle blast resistance were good resources for application in blast resistance breeding.
Additional files
Table S1. Primers of PCR-based markers used for fine mapping Pb-bd1. (DOCX 17 kb)
Table S2. Primers for expression patterns analysis of candidate genes by real-time PCR. (DOCX 16 kb)
Figure S1. The integrated physical map of four panicle blast resistance QTLs. (PPTX 79 kb)
Acknowledgements
We would like to thank Prof. Chen Zhi-Yi and Dr. Liu Yong-Feng for their kindly providing all the blast isolates.
Funding
This research has been supported by grants from the National Key Project for Transgenic Crops (2016ZX08009–003-001), the Fundamental Research Funds for the Central Universities (KYZ201704), the Open Project of State Key Laboratory of Rice Biology (160101), the Natural Science Foundation of China (31871602, 31171516), and Jiangsu Agriculture science and technology innovation fund (CX (15)1054).
Availability of data and materials
The datasets supporting the conclusions of this article are provided within the article and its additional files.
Abbreviations
- MAS
Molecular assisted selection
- NBS-LRR
Nucleotide binding site-leucine rich repeat
- NJ46
Nanjing46
- PAGE
Polyacrylamide gel electrophoresis
- QTLs
Two quantitative trait loci
- RGP
Rice Genome research Program
- VWA
Von Willebrand factor type A domain
Authors’ contributions
YB, NF and HZ raised the project conception and designed the research. NF and XW carried out most experiments and analyzed data. NF and YB wrote the manuscript. LS, YY, ML, CG and HC carried out panicle blast resistance identification in the fields. WH and CY participated in developing the RILs populations. All authors read and approved the final manuscript.
Ethics approval and consent to participate
No applicable.
Consent for publication
No applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hongsheng Zhang, Email: hszhang@njau.edu.cn.
Yongmei Bao, Email: yongmeibao@njau.edu.cn.
References
- Ahn SW. International collaboration on breeding for resistance to rice blast. Rice blast disease. Wallingford: CAB International; 1994. pp. 137–153. [Google Scholar]
- Asaga K. A procedure for evaluating field resistance to blast in rice varieties (in Japanese with English summary) J Cent Agr Exp Sta. 1981;35:51–138. [Google Scholar]
- Ashikawa I, Hayashi N, Yamane H, Kanamori H, Wu J, Matsumoto T, et al. Two adjacent nucleotide-binding site-leucine-rich repeat class genes are required to confer Pikm-specific rice blast resistance. Genetics. 2008;180(4):2267–2276. doi: 10.1534/genetics.108.095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkani S, Rafii MY, Shabanimofrad M, Ghasemzadeh A, Ravanfar SA, Latif MA. Molecular progress on the mapping and cloning of functional genes for blast disease in rice (Oryza sativa L.): current status and future considerations. Crit Rev Biotechnol. 2016;36(2):353–367. doi: 10.3109/07388551.. [DOI] [PubMed] [Google Scholar]
- Bonman JM. Durable resistance to rice blast disease-environmental influences. Euphytica. 1992;63(1):115–123. doi: 10.1007/bf00023917. [DOI] [Google Scholar]
- Bonman JM, Estrada BA, Kim CK, Lee EJ. Assessment of blast disease and yield loss in susceptible and partially resistant rice cultivars in two irrigated lowland environments. Plant Dis. 1991;75(5):462. doi: 10.1094/PD-75-0462. [DOI] [Google Scholar]
- Chen J, Peng P, Tian J, He Y, Zhang L, Liu Z, et al. Pike, a rice blast resistance allele consisting of two adjacent NBS–LRR genes, was identified as a novel allele at the Pik locus. Mol Breeding. 2015;35:1–15. doi: 10.1007/s11032-015-0305-6. [DOI] [Google Scholar]
- Chen J, Shi Y, Liu W, Chai R, Fu Y, Zhuang J, et al. A Pid3 allele from rice cultivar Gumei2 confers resistance to Magnaporthe oryzae. J Genet Genomics. 2011;38(5):209–216. doi: 10.1016/j.jgg.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Chen L, Hamada S, Fujiwara M, Zhu T, Thao NP, Wong HL, et al. The hop/Sti1-Hsp90 chaperone complex facilitates the maturation and transport of a PAMP receptor in rice innate immunity. Cell Host Microbe. 2010;7(3):185–196. doi: 10.1016/j.chom.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Chin KM. Fungicidal control of the rice blast disease. Malays Agric J. 1975;50:221–228. [Google Scholar]
- Deng Y, Zhai K, Xie Z, Yang D, Zhu X, Liu J, et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science. 2017;355:962–965. doi: 10.1126/science.aai8898. [DOI] [PubMed] [Google Scholar]
- Deng Y, Zhu X, Shen Y, He Z. Genetic characterization and fine mapping of the blast resistance locus Pigm(t) tightly linked to Pi2 and Pi9 in a broad-spectrum resistant Chinese variety. Theor Appl Genet. 2006;113(4):705–713. doi: 10.1007/s00122-006-0338-7. [DOI] [PubMed] [Google Scholar]
- Fang N, Wang R, He W, Yin C, Guan C, Chen H, et al. QTL mapping of panicle blast resistance in japonica landrace heikezijing and its application in rice breeding. Mol Breeding. 2016;36(12):171. doi: 10.1007/s11032-016-0603-7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka S, Saka N, Koga H, Ono K, Shimizu T, Ebana K, et al. Loss of function of a proline-containing protein confers durable disease resistance in rice. Science. 2009;325(5943):998–1001. doi: 10.1126/science.1175550. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Inoue H, Kato T, Funao T, Shirota M, Shimizu T, et al. Durable panicle blast-resistance gene Pb1 encodes an atypical CC-NBS-LRR protein and was generated by acquiring a promoter through local genome duplication. Plant J. 2010;64(3):498–510. doi: 10.1111/j.1365-313X.2010.04348.x. [DOI] [PubMed] [Google Scholar]
- Hua L, Wu J, Chen C, Wu W, He X, Lin F, et al. The isolation of Pi1, an allele at the Pik locus which confers broad spectrum resistance to rice blast. Theor Appl Genet. 2012;125(5):1047–1055. doi: 10.1007/s00122-012-1894-7. [DOI] [PubMed] [Google Scholar]
- Huan J, Bao YM, Wu YY, Zeng GY, He WW, Dang LL, et al. (2014) Identification of quantitative trait loci conferring blast resistance in bodao, a japonica rice landrace Genet Mol Res13(4): 9756–9765. doi: 10.4238/2014.November.27.3. [DOI] [PubMed]
- Huang J, Wang MM, Jiang Y, Bao YM, Huang X, Sun H, et al. Expression analysis of rice A20/AN1-type zinc finger genes and characterization of ZFP177 that contributes totemperature stress tolerance. Gene. 2008;420:135–144. doi: 10.1016/j.gene.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Hulbert SH, Webb CA, Smith SM, Sun Q. Resistance gene complexes: evolution and utilization. Annu Rev Phytopathol. 2001;39(1):285–312. doi: 10.1146/annurev.phyto.39.1.285. [DOI] [PubMed] [Google Scholar]
- Hwang BK, Koh YJ, Chung HS. Effects of adult-plant resistance on blast severity and yield of rice. Plant Dis. 1987;71(11):1035–1038. doi: 10.1094/PD-71-1035. [DOI] [Google Scholar]
- Inoue H, Hayashi N, Matsushita A, Liu X, Nakayama A, Sugano S, et al. Blast resistance of CC-NB-LRR protein Pb1 is mediated by WRKY45 through protein-protein interaction. Proc Natl Acad Sci U S A. 2013;110(23):9577–9582. doi: 10.1073/pnas.1222155110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T, Hayano-Saito Y, Oide S, Ebana K, La NT, Hayashi K, et al. Quantitative trait locus analysis of resistance to panicle blast in the rice cultivar Miyazakimochi. Rice. 2014;7(1):2. doi: 10.1186/s12284-014-0002-9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsube T, Koshimizu Y. Influence of blast disease on harvests in rice plant. I Effect of panicle infection on yield components and quality. Bull Tohoku Agric Exp Station. 1970;39:55–96. [Google Scholar]
- Khan MAI, Bhuiyan MR, Hossain MS, Sen PP, Ara A, Siddique MA, et al. Neck blast disease influences grain yield and quality traits of aromatic rice. C R Biol. 2014;337(11):635–641. doi: 10.1016/j.crvi.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Koh YJ, Hwang BK, Chung HS. Adult-plant resistance of rice to leaf blast. Phytopathology. 1987;77(2):232–236. doi: 10.1094/Phyto-77-232. [DOI] [Google Scholar]
- Kouzai Y, Kaku H, Shibuya N, Minami E, Nishizawa Y. Expression of the chimeric receptor between the chitin elicitor receptor CEBiP and the receptor-like protein kinase Pi-d2 leads to enhanced responses to the chitin elicitor and disease resistance against Magnaporthe oryzae in rice. Plant Mol Biol. 2013;81(3):287–295. doi: 10.1007/s11103-012-9998-7. [DOI] [PubMed] [Google Scholar]
- Li P, Shi X, Wang J, Liu C, Zhang H. Molecular mapping of rice blast resistance gene in a japonica landrace Heikezijing from the Taihu Lake area. China Rice Sci. 2007;21:579–584. [Google Scholar]
- Li PF, Shi XL, Wang JF, Zhang HS. Genetic analysis of resistance to rice blast in four japonica landraces from Taihu Lake region. Hereditas. 2007;29(29):1249–1255. doi: 10.1360/yc-007-1249. [DOI] [PubMed] [Google Scholar]
- Li W, Zhu Z, Chern M, Yin J, Yang C, Ran L, et al. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell. 2017;170(1):114–126. doi: 10.1016/j.cell.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Lin R, Zhao W, Meng X, Min W, Peng Y. Rice gene OsNAC19 encodes a novel NAC-domain transcription factor and responds to infection by Magnaporthe grisea. Plant Sci. 2007;172(1):120–130. doi: 10.1016/j.plantsci.2006.07.019. [DOI] [Google Scholar]
- Liu J, Jambunathan NT. Transgenic expression of the von Willebrand a domain of the BONZAI1/COPINE1 protein triggers a lesion-mimic phenotype in Arabidopsis. Planta. 2005;221(1):85–94. doi: 10.1007/s00425-004-1413-4. [DOI] [PubMed] [Google Scholar]
- Liu Q, Yang J, Zhang S, Zhao J, Feng A, Yang T, et al. OsGF14b positively regulates panicle blast resistance but negatively regulates leaf blast resistance in rice. Mol Plant Microbe In. 2016;29(1):46–56. doi: 10.1094/MPMI-03-15-0047-R. [DOI] [PubMed] [Google Scholar]
- Liu S, Yang X, Sun S, Liu C, Wang Y, Zhang C, Gu H. Identification technique of rice resistance to Magnaporthe Grisea. Tianjin Agr Sci. 2007;13:55–58. [Google Scholar]
- Liu W, Liu J, Triplett L, Leach JE, Wang GL. Novel insights into rice innate immunity against bacterial and fungal pathogens. Annu Rev Phytopathol. 2014;52:213–241. doi: 10.1146/annurev-phyto-102313-045926. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expressiondata using real-time quantitative PCR and the 2 -△△CTmethod. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu M, Liu W, Zhu F, Zhang Q, Xia F. The reason analysis of the outbreak of rice blast in 2014 and its the strategy of management. China Plant Prot. 2015;35(6):35–39. [Google Scholar]
- Ma J, Lei C, Xu X, Hao K, Wang J, Cheng Z, et al. Pi64, encoding a novel CC-NBS-LRR protein, confers resistance to leaf and neck blast in rice. Mol Plant Microbe In. 2015;28(5):558–568. doi: 10.1094/mpmi-11-14-0367-r. [DOI] [PubMed] [Google Scholar]
- Ou SH. Rice disease. 2. UK: Commonw Mycol Inst, Kew; 1985. [Google Scholar]
- Qu S, Liu G, Zhou B, Bellizzi M, Zeng L, Dai L, et al. The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics. 2006;172(3):1901–1914. doi: 10.1534/genetics.105.044891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat N, Naga NC, Meenakshi SR, Nair S, Bentur JS. A novel mechanism of gall midge resistance in the rice variety Kavya revealed by microarray analysis. Funct Integr Genomics. 2012;12(2):249–264. doi: 10.1007/s10142-012-0275-2. [DOI] [PubMed] [Google Scholar]
- Roumen EC. Partial resistance to neck blast influenced by stage of panicle development and rice genotype. Euphytica. 1992;64(3):173–182. doi: 10.1007/bf00046046. [DOI] [Google Scholar]
- Sakata K, Nagamura Y, Numa H, Antonio BA, Nagasaki H, Idonuma A, et al. Rice GAAS: an automated annotation system and databasefor rice genome sequence. Nucleic Acids Res. 2002;30:98–102. doi: 10.1093/nar/30.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma TR, Madhav MS, Singh BK, Shanker P, Jana TK, Dalal V, et al. High-resolution mapping, cloning and molecular characterization of the Pi-k(h) gene of rice, which confers resistance to Magnaporthe grisea. Mol Gen Genomic. 2005;274(6):569–578. doi: 10.1007/s00438-005-0035-2. [DOI] [PubMed] [Google Scholar]
- Sharma TR, Rai AK, Gupta SK, Vijayan J, Devanna BN, Ray S. Rice blast management through host-plant resistance: retrospect and rrospects. Agric Res. 2012;1(1):37–52. doi: 10.1007/s40003-011-0003-5. [DOI] [Google Scholar]
- Sirithunya P, Tragoonrung S, Vanavichit A, Pa-In N, Vongsaprom C, Toojinda T. Quantitative trait loci associated with leaf and neck blast resistance in recombinant inbred line population of rice (Oryza sativa) DNA Res. 2002;9(3):79–88. doi: 10.1093/dnares/9.3.79. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhang H, Li D, Lei H, Hong Y, Xin SD, et al. Functions of rice NAC transcriptional factors, ONAC122 and ONAC131, in defense responses against Magnaporthe grisea. Plant Mol Biol. 2013;81(1):41–56. doi: 10.1007/s11103-012-9981-3. [DOI] [PubMed] [Google Scholar]
- Wang R, Fang N, Guan C, He W, Bao Y, Zhang H. Characterization and fine mapping of a blast resistant gene Pi-jnw1 from the japonicarice landrace Jiangnanwan. PLoS One. 2016;11(12):e0169417. doi: 10.1371/journal.pone.0169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Yano M, Yamanouchi U, Iwamoto M, Monna L, Hayasaka H, et al. The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J. 1999;19(1):55–64. doi: 10.1046/j.1365-313X.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- Wu JL, Fan YY, Li DB, Zheng KL, Leung H, Zhuang JY. Genetic control of rice blast resistance in the durably resistant cultivar Gumei 2 against multiple isolates. Theor Appl Genet. 2005;111(1):50–56. doi: 10.1007/s00122-005-1971-2. [DOI] [PubMed] [Google Scholar]
- Yuan B, Zhai C, Wang W, Zeng X, Xu X, Hu H, et al. The Pik-p resistance to Magnaporthe oryzae in rice is mediated by a pair of closely linked CC-NBS-LRR genes. Theor Appl Genet. 2011;122(5):1017–1028. doi: 10.1007/s00122-010-1506-3. [DOI] [PubMed] [Google Scholar]
- Zhai C, Lin F, Dong Z, He X, Yuan B, Zeng X, et al. The isolation and characterization of Pik, a rice blast resistance gene which emerged after rice domestication. New Phytol. 2011;189(1):321–334. doi: 10.1111/j.1469-8137.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Chen C, Zhang J, Xie H. Mapping, cloning of rice blast resistance genes and their application. Mol Plant Breeding. 2009;7(2):385–392. [Google Scholar]
- Zhou B, Qu S, Liu G, Dolan M, Sakai H, Lu G, et al. The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol Plant Microbe In. 2006;19(11):1216. doi: 10.1094/MPMI-19-1216. [DOI] [PubMed] [Google Scholar]
- Zhou X, Liao H, Chern M, Yin J, Chen Y, Wang J, et al. Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance. Proc Natl Acad Sci U S A. 2018;115(12):3174–3179. doi: 10.1073/pnas.1705927115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YY, Fang H, Wang YY, Fan JX, Yang SS, Mew TW, et al. Panicle blast and canopy moisture in rice cultivar mixtures. Phytopathology. 2005;95(4):433–438. doi: 10.1094/Phyto-95-0433. [DOI] [PubMed] [Google Scholar]
- Zhuang JY, Ma WB, Wu JL, Chai RY, Lu J, Fan YY, et al. Mapping of leaf and neck blast resistance genes with resistance gene analog, RAPD and RFLP in rice. Euphytica. 2002;128(3):363–370. doi: 10.1023/a:1021272710294.. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers of PCR-based markers used for fine mapping Pb-bd1. (DOCX 17 kb)
Table S2. Primers for expression patterns analysis of candidate genes by real-time PCR. (DOCX 16 kb)
Figure S1. The integrated physical map of four panicle blast resistance QTLs. (PPTX 79 kb)
Data Availability Statement
The datasets supporting the conclusions of this article are provided within the article and its additional files.