Abstract
Lyme borreliosis is the most common vector-borne zoonosis in the northern hemisphere, and the pathogens causing Lyme borreliosis have distinct, incompletely described transmission cycles involving multiple host groups. The mammal community in Fennoscandia differs from continental Europe, and we have limited data on potential competent and incompetent hosts of the different genospecies of Borrelia burgdorferi sensu lato (sl) at the northern distribution ranges where Lyme borreliosis is emerging. We used qPCR to determine presence of B. burgdorferi sl in tissue samples (ear) from 16 mammalian species and questing ticks from Norway, and we sequenced the 5S–23 S rDNA intergenic spacer region to determine genospecies from 1449 qPCR-positive isolates obtaining 423 sequences. All infections coming from small rodents and shrews were linked to the genospecies B. afzelii, while B. burgdorferi sensu stricto (ss) was only found in red squirrels (Sciurus vulgaris). Red squirrels were also infected with B. afzelii and B. garinii. There was no evidence of B. burgdorferi sl infection in moose (Alces alces), red deer (Cervus elaphus) or roe deer (Capreolus capreolus), confirming the role of cervids as incompetent hosts. In infected questing ticks in the two western counties, B. afzelii (67% and 75%) dominated over B. garinii (27% and 21%) and with only a few recorded B. burgdorferi ss and B. valaisiana. B. burgdorferi ss were more common in adult ticks than in nymphs, consistent with a reservoir in squirrels. Our study identifies potential competent hosts for the different genospecies, which is key to understand transmission cycles at high latitudes of Europe.
Introduction
Understanding the transmission cycles of pathogens circulating in ecosystems is challenging for multi-host systems1. A competent host is defined as a host with the ability to transmit parasites or pathogens such that they effectively infect another host or vector2. The numerical balance between competent (or transmission/reservoir) hosts versus incompetent hosts is key to determine the disease hazard, as formulated in the biodiversity buffers disease or dilution hypothesis3–5. What constitutes an incompetent or competent host differs across pathogens. An important step to understand disease hazard is hence to determine for a given pathogen which hosts are competent and incompetent in different ecosystems. Among the more complicated enzootic transmission cycles are the ones linked to the generalist ticks of the Ixodidae family in the northern hemisphere6–8. These generalist ticks transmit a range of pathogens among which the genospecies forming the Borrelia burgdorferi sensu lato (sl) complex causing Lyme borreliosis are the most common and widespread.
The general pattern of the transmission cycle of B. burgdorferi sl is well-known both in North America, Asia and Europe6–9. The number of nymphs infected with B. burgdorferi sl depends on how many tick larvae get their first blood meal on an infected small vertebrate host10. Co-feeding transmission is less important for this group of pathogens, though considerable more for other pathogens like tick-borne encephalitis virus11. However, beyond this commonality, there is much specificity in terms of dominating hosts both across and within continents12. The B. burgdorferi sensu stricto (ss) pathogen probably migrated from Europe to North America some 60000 years ago13 and this genospecies dominates in North America and evolved into strains infecting birds and small mammals7. In Europe, the main pathogenic genospecies to humans are B. afzelii linked mainly to small rodents, and the B. garinii linked mainly to birds, while B. burgdorferi ss is less common and has been linked to red squirrels (Sciurus vulgaris) in Switzerland14, France15 and gray squirrels (Sciurus carolinensis) in the UK16. However, the competent hosts of the genospecies are partly overlapping and not fully described. Evidence is accumulating that the different genospecies cause different clinical symptoms in humans17–21. It is therefore important to understand the transmission dynamics of each of the different genospecies in different regions.
Ticks are expanding their geographical distribution in northern Europe22,23, and Lyme disease incidence is documented to increase in both Norway24,25 and Finland26. The community of mammals in Fennoscandia is different from continental Europe. This is due to both the colder climate and the post-glacial colonization routes both from south and from northeast via the landbridge towards Russia27. We have limited data on the pattern of genospecies transmission hosts in these northern ecosystems. We here determined presence of different genospecies of B. burgdorferi sl in different vertebrate hosts to determine their role as potential transmission host at the northern distribution range of Ixodes ricinus in Europe. Furthermore, we determined the relative abundance of B. burgdorferi sl genospecies in questing ticks as a basis to understand the hazard of each genospecies known to cause different clinical manifestations.
Results
Infection of B. burgdorferi sl was found in all rodent and shrew species except in the single house mouse (Mus musculus) found (Table 1). With the exception of red squirrels, all sequences of the IGS in rodents and shrews were consistent with infections of B. afzelii. In 17 red squirrels, we found high infection rates of B. burgdorferi sl (88%), of which 9 sequences yielded four B. afzelii, two B. garinii and three B. burgdorferi ss. Infection of B. burgdorferi sl was also found in red fox (Vulpes vulpes) and badger (Meles meles), but not in the single hare (Lepus timidus) included. Attempts to determine the genospecies in red fox and badger were unsuccessful. No infection of B. burgdorferi sl was found in 111 moose, 28 roe deer or 141 red deer (Table 1). In the eastern region, the infection levels were higher in squirrels (Z = 4.060, P < 0.001) and lower in the Eurasian pygmy shrew (Sorex minutus) (Z = −2.922, P = 0.003) compared to the common shrew (S. araneus), the latter had similar infection levels as the bank vole (Myodes glareolus) (Z = 0.206, P = 0.837) and the wood mice (Apodemus sylvaticus) (Z = 0.184, P = 0.854).
Table 1.
The sample sizes (n) and infection prevalence levels in mammals collected from eastern, southern and western Norway.
| Common name | Latin name | Region | n | B. burgdorferi sl | Number of IGS sequences | ||||
|---|---|---|---|---|---|---|---|---|---|
| Neg | Pos | Prev | B. afzelii | B. garinii | B. burgdorferi ss | ||||
| Yellow-necked mouse | Apodemus flavicollis | SF | 19 | 18 | 1 | 0.05 | |||
| Wood mouse | Apodemus sylvaticus | SF | 76 | 68 | 8 | 0.11 | |||
| East | 96 | 71 | 25 | 0.26 | 1 | ||||
| Field vole | Microtus agrestis | SF | 41 | 30 | 11 | 0.27 | 6 | ||
| House mouse | Mus musculus | SF | 1 | 1 | 0 | 0 | |||
| Bank vole | Myodes glareolus | SF | 64 | 56 | 8 | 0.13 | 1 | ||
| East | 333 | 247 | 86 | 0.26 | 23 | ||||
| Water shrew | Neomys fodiens | SF | 15 | 14 | 1 | 0.07 | |||
| East | 1 | 1 | 0 | 0 | |||||
| Common shrew | Sorex araneus | SF | 554 | 519 | 35 | 0.06 | 12 | ||
| East | 271 | 203 | 68 | 0.25 | 17 | ||||
| Taiga shrew | Sorex isodon | SF | 157 | 154 | 3 | 0.02 | 1 | ||
| Eurasian pygmy shrew | Sorex minutus | SF | 55 | 55 | 0 | 0 | |||
| East | 53 | 51 | 2 | 0.04 | |||||
| Red squirrel | Sciurus vulgaris | East | 17 | 2 | 15 | 0.88 | 4 | 2 | 3 |
| Red fox | Vulpes vulpes | East | 6 | 5 | 1 | 0.17 | |||
| Badger | Meles meles | East | 14 | 12 | 2 | 0.14 | |||
| Hare | Lepus timidus | East | 1 | 1 | 0 | 0 | |||
| Roe deer | Capreolus capreolus | East | 28 | 28 | 0 | 0 | |||
| Red deer | Cervus elaphus | SF | 126 | 126 | 0 | 0 | |||
| South | 15 | 15 | 0 | 0 | |||||
| Moose | Alces alces | South | 111 | 111 | 0 | 0 | |||
Neg/Pos: negative/positive for B. burgdorferi sl. Prev: prevalence. SF: Sogn and Fjordane county. East: Østfold and Akershus county. South: Telemark and Vestfold county. Locations are given in Fig. 1.
In questing nymphal ticks with infection of B. burgdorferi sl (Table 2), the genospecies B. afzelii dominated both in the Sogn & Fjordane (66.7%) and Møre & Romsdal (75.2%) county in the western region, with B. garinii being common in both Sogn & Fjordane (26.5%) and Møre & Romsdal (21.0%), while B. valaisiana was less commonly found (Sogn & Fjordane: 6.1%, Møre & Romsdal: 3.8%). Sample sizes for the eastern region was low, and yielded only B. afzelii in nymphs (n = 8). The same main geographic pattern in terms of genospecies distribution was found in adult male and female ticks. The exception was B. burgdorferi ss that were found in 6 adult ticks and one nymphal tick overall, and a logistic regression confirmed a higher frequency of B. burgdorferi ss in adults than nymphs compared to B. afzelii (Z = −2.071, P = 0.044).
Table 2.
An overview of B. burgdorferi sl genospecies in infected questing I. ricinus ticks in % (n) based on the IGS sequences from Sogn & Fjordane (SF), Møre & Romsdal (MR) and Akershus/Østfold (east) counties of Norway. n-tot = total number of questing ticks; n-pos = number of B. burgdorferi sl-positive ticks; n-seq = number of positive samples that was successfully sequenced.
| Life stage | Region | n-tot | n-pos | n-seq | B. afzelii | B. garinii | B. burgdorferi ss | B. valaisiana |
|---|---|---|---|---|---|---|---|---|
| Nymphs | SF | 4857 | 567 | 147 | 66.7% (98) | 26.5% (39) | 0.7% (1) | 6.1% (9) |
| MR | 1771 | 245 | 105 | 75.2% (79) | 21.0% (22) | 0 | 3.8% (4) | |
| East | 872 | 99 | 8 | 100% (8) | 0 | 0 | 0 | |
| Adult males | SF | 626 | 74 | 15 | 66.7% (10) | 20.0% (3) | 6.7% (1) | 6.7% (1) |
| MR | 264 | 35 | 16 | 81.3% (13) | 12.5% (2) | 6.3% (1) | 0 | |
| East | 109 | 16 | 0 | |||||
| Adult females | SF | 624 | 91 | 35 | 65.7% (23) | 20.0% (7) | 5.7% (2) | 8.6% (3) |
| MR | 223 | 34 | 17 | 70.6% (12) | 23.5% (4) | 0 | 5.9% (1) | |
| East | 91 | 22 | 10 | 40.0% (4) | 40.0% (4) | 20.0% (2) | 0 |
To ensure that the B. garinii classification based on sequencing of 5S–23S rDNA intergenic spacer region (IGS) from positive samples was correct and not included B. bavariensis (see Methods), we also ran another genetic marker, namely pepX, on the 83 positive samples for B. garinii, of which 51 yielded successful sequences. We confirmed B. garinii in 49 of the 51 samples, while two cases (one red squirrel in Akershus, one tick nymph in Sogn & Fjordane) came out as B. afzelii, likely due to infection with both B. garinii and B. afzelii (see Discussion).
Discussion
Determining the competence of vertebrate hosts to pathogens is one of several keys necessary to estimation of disease hazard. A competent host is defined by the ability to be a source of infection to ticks14,28. A competent host must be fed on by infected ticks and take up a critical number of the pathogen, and it must allow the pathogen to multiply and to pass on infection to new ticks28. Hence, establishing infection is necessary, but on its own does not prove amplification of a pathogen in a host or transmission to a vector. In some cases, DNA from B. burgdorferi sl can be found without evidence of amplification or transmission to vector ticks29, and PCR cannot separate live and dead pathogen DNA. Several other technical issues, such as the sensitivity and specificity of the PCR, tissue tropisms of the pathogen and timing of host collection can also impede the identification of competent hosts. Although the absence of infection can confirm a vertebrate as an incompetent host, lack of pathogen detection by PCR due to technical limitations, cannot firmly establish this. Ideally, laboratory-based transmission experiments30,31, as well as field studies focusing on vertebrate communities32, are both necessary and complimentary approaches to acquire full understanding of the transmission dynamics of tick-borne pathogens. Our field study hence provides one step towards gaining the required information to identify important potential competent and incompetent hosts at northern latitudes of Europe.
A new potential competent host of B. afzelii
Due to its medical importance and relevance of determining disease hazard, there are a huge number of studies reporting frequency of genospecies of B. burgdorferi sl in Europe. The dominating genospecies in questing ticks is the genospecies B. afzelii almost universally across continental Europe33, while B. garinii dominates in the UK8. Our estimates of 75% and 67% B. afzelii in the two western counties are close to previous estimates from Norway both in south with 62%34, 86% in east and south35, and 68% further north of the western study site reported here36. This main picture was also found in Sweden37. The B. garinii is typically the second most common genospecies mainly linked to birds38,39. Our estimated prevalence in B. burgdorferi sl infected ticks of 21% and 26% B. garinii in the two western counties are also close to previous estimates of 23.4% B. garinii in south34, 12% in east and south35 and 20.8% in west36 of Norway. Further, as reported in these studies, we also found a low prevalence of the bird-borne, but less pathogenic, B. valaisiana.
The commonness of B. afzelii in ticks is linked to the high abundance and spread of small mammals, which is its main reservoir. As commonly reported, the bank vole and the wood mice are dominant hosts of larvae and have often high infection levels with B. afzelii. Wood mice and yellow-necked mice are also considered to be important hosts of B. burgdorferi sl in urban environments40. As we reported earlier41, the common shrew is abundant and important in feeding tick larvae in Norway, as also found in Scotland42. Based on this study, we can add the taiga shrew (S. isodon) to the list of potential competent hosts for B. afzelii in Europe. The taiga shrew has larval tick loads similar to the common shrew (own unpublished data), but prevalence of B. burgdorferi sl was lower (Table 1). This is a rare species in Norway and Red Listed as ‘data deficient’. However, the species is common in Finland43 and further east in Russia. Therefore, the host species involved in transmission may differ in the north relative to continental Europe. There is also a high level of endemism in the Mediterranean small mammals44, suggesting that species involved in circulation of B. afzelii differs with latitude.
Medium-sized hosts infections
Squirrels are sufficiently large in size to be commonly bitten by nymphal ticks14–16, which is required to obtain infections of B. burgdorferi sl8. Their movements on the ground make them regularly exposed to ticks. We indeed found a high infection prevalence in red squirrels. Interestingly, we found infection of three genospecies in red squirrels: B. afzelii, B. burgdorferi ss, and B. garinii. Squirrels seem to regularly be infected by several genospecies of B. burgdorferi sl. In Switzerland, a mixture of B. afzelii and B. burgdorferi ss was found in red squirrels14. In France, prevalence of 18.9% B. burgdorferi s.s., 11.9% B. afzelii, and 3.5% B. garinii was found in red squirrels15. All four genospecies (B. afzelii, B. burgdorferi ss, B. garinii, B. valaisiana) that occur in the United Kingdom were detected in gray squirrels, and the commonly bird-associated B. garinii was most common in the gray squirrels16. Our few reports of B. burgdorferi ss in questing ticks, and mostly in adult ticks, are consistent with a reservoir like squirrels typically having nymphs attached and which are less abundant than small mammals. Another study at the west coast of Norway also found B. burgdorferi ss more commonly in adult than nymphal ticks36.
Infection with several genospecies seem common in medium-sized mammals. More B. burgdorferi sl genospecies were found in Siberian chipmunks (Tamias sibericus) than in the native bank vole45. We only retrieved one hare and it was without infection, but infection of both B. afzelii and B. burgdorferi ss in hare was documented in south Norway46. Hares were also infected with B. garinii in Sweden37. We also documented infection in badger and red fox, but prevalence was low (Table 1). Infection of B. burgdorferi sl has previously been found in red fox47, and badger was reported infected with both B. afzelii and B. valaisiana48. Medium-sized mammals may hence potentially play roles for transmission of multiple genospecies. Hedgehogs are also important for circulation of B. bavariensis49, and that this genospecies have not been found in Norway may be due to the low population numbers of hedgehogs.
Amplification and the challenge of co-infections
A quite high proportion of our positive B. burgdorferi sl samples did not amplify (Tables 1 and 2), which is a requirement in our approach for determining genospecies. The sequencing success decreases with increasing Ct values (i.e., the number of PCR cycles before getting a positive signal). However, only 0.35% (6 of our 865 samples) had Ct values above 40. We cannot identify the cause of lack of amplification with certainty. The most straightforward explanation is that our qPCR-based assay is more sensitive than the conventional PCR50. It is also possible that infections with multiple B. burgdorferi sl genospecies can play a role. Indeed, typing using the pepX marker of our B. garinii-positive samples based on IGS (see Methods) came up with two B. afzelii (one in a red squirrel and one in a tick nymph). As B. garinii and B. afzelii are readily separated by both methods, this is indicative of co-infection. The aim of our study was not to quantify extent of co-infections, but these kind of co-infections may cause detection bias if amplification success by PCR differs between genospecies, or when B. burgdorferi sl genospecies have differences in tissue tropism. That we in the small sample of squirrels identified all 3 genospecies using IGS alone would suggest this was not a strong bias. It is therefore likely a robust result that only B. afzelii was detected in smaller mammals with a much larger sample size, and that this was not due to failure of B. burgdorferi ss to amplify. Multiple infections in medium-sized hosts may provide a platform for genetic re-arrangements between genospecies, becoming a potential ‘melting-pot’51. It can also cause co-infection of genotypes of B. burgdorferi sl in ticks already at the nymphal stage.
Cervids as incompetent hosts
A controversial issue is the extent to which biodiversity dilutes disease in general, and how important incompetent hosts are for Lyme borreliosis hazard52,53. Several lines of evidence have been used to infer that cervids most likely are incompetent hosts in the enzootic cycle of B. burgdorferi sl. Commonly, ticks engorged on cervids are used as evidence54. However, ticks removed from cervids often contain a low level of infection with B. burgdorferi sl55, which may be due to a number of different mechanisms. In our sizeable sample, the complete lack of detection suggest using ear skin infections are likely better in substantiating that cervids are incompetent hosts than using ticks feeding on cervids. The absence of infection are evidence that cervids are incompetent hosts, but not sufficient evidence that they are dilution hosts. The mechanism of the dilution hypothesis is that when competent hosts are outnumbered by incompetent hosts, the probability of vectors feeding on competent hosts is reduced and so the abundance of infected vectors is lowered52. The degree of dilution by cervids depends not only on the relative proportion of the immature tick population fed relative to competent host groups, but also on the absolute number of ticks fed with a change in host population density52. High deer density was linked to increased incidence of Lyme disease in Norway24, which document that deer mainly act as an amplification (or reproduction) host of tick populations rather than as a dilution host that interferes with the Borrelia transmission cycle.
Our study identifies potential competent (or reservoir) and incompetent hosts towards the northern distribution range of ticks in Europe with a different mammal community compared to continental Europe. Future studies are necessary to determine that they are indeed reservoir competent, vector competent and whether the presence of competent and incompetent hosts has a substantial effect on the hazard (density of questing infected nymphs), which will depend on a number of factors including the population densities of the different species involved52.
Material and Methods
Study areas
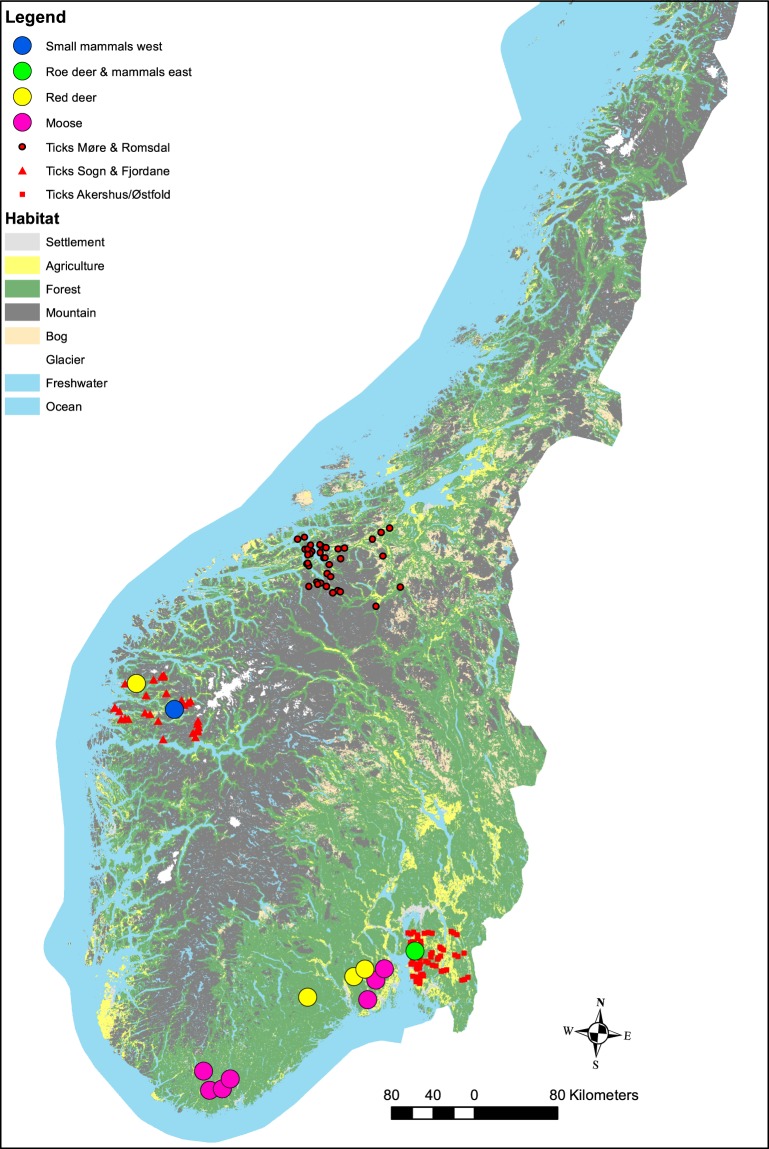
The study areas are spread across the southern part of Norway (Fig. 1). The western coast has a warmer and rainier climate compared to eastern areas of Norway. The location of samples and the main habitat types are given in Fig. 1. A more detailed description of the main habitat types can be found in ref. 24.
Figure 1.
A map of southern Norway with origin of samples of mammal tissue and questing ticks. The ‘roe deer & mammal east’ site include red fox, badger, hare and red squirrel in addition to small rodents and shrews, while ‘small mammals west’ includes small rodents and shrews (Table 1).
Small mammal trapping
Small mammals were captured in spring and fall during 2014–16 in Vestby municipality, Akershus county and 2013–16 in Førde and Askvoll municipalities, Sogn & Fjordane county, Norway. Small mammals captured were sacrificed (cervical dislocation) and transferred to an individual zip-lock plastic bag, marked with station number, trap number and date. All bags were stored in a freezer for later observation. The temporal and regional variation in tick load and infection prevalence of B. burgdorferi sl will be presented elsewhere (own unpublished results).
Medium and large sized mammals
We used different methods to sample as many different species of mammals as possible. In Vestby municipality in Akershus county and surrounding areas, we sampled road-kills of roe deer, badger, fox, red squirrels and hare from spring 2016. Ears from red deer and moose were sampled in connection with ongoing surveillance for Chronic Wasting Disease fall 2016. For these data, hunters provided full heads to the Norwegian Food Safety Authority, which removed an ear that was then frozen and later transported to CEES, University of Oslo. We obtained further red squirrels from hunting (August 2016).
Ethics statement
Permissions to capture rodents and shrews and hunt red squirrels outside of hunting season were given by the Norwegian Environment Agency. Small mammals captured were sacrificed on site (see above). The licence by Norwegian Environment Agency is the only requirement to conform to the Norwegian laws and regulations.
Questing ticks
Questing ticks were sampled regularly along 34 transects in the Sogn & Fjordane county and along 42 transects and 10 other plots in Møre & Romsdal county, western Norway56,57. The temporal and regional variation in prevalence of B. burgdorferi sl in questing nymphs has been presented elsewhere24,58. The questing ticks were all identified to I. rinicus based on morphology.
DNA extraction
Tissue samples from mammals were derived from the ears, sometimes with surrounding skin for the smallest shrew species. The DNA was extracted with Qiagen blood and tissue kit according to the manufactures recommendations. To easier prepare a high number of samples that later could be extracted, we froze the samples after the overnight incubation with ATL buffer and proteinase K. A total of 94 samples were extracted at the time, leaving two spaces empty for controls. The DNA was stored at −80 °C till later use.
qPCR protocol
The extracted DNA were screened for B. burgdorferi sl by realtime PCR (qPCR) in a duplex59 (with A. phagocytophilum, not reported here), at CEES, UiO as in previous work24,60, following Allender et al.61. We used the forward primer CGAGTCTTAAAAGGGCGATTTAGT, the reverse primer GCTTCAGCCTGGCCATAAATAG and the probe [6FAM]AGATGTGGTAGACCCGAAGCCGAGTG[TAMRA] to target the 23 S rRNA gene of B. burgdorferi with the fluorescent colour FAM. qPCR reactions were done with a total volume of 10 µl, using 1 µl of DNA and 9 µl of mastermix. A 96 well plate was filled with 94 samples, one positive control and one negative control. A two step program was used on LightCycler 96. Starting with pre incubation of 600 s of 95 °C followed by 50 cycles of two step amplification with 15 s of 95 °C and 60 s of 60 °C.
Sequencing of 5S–23S rDNA intergenic spacer region (IGS) from positive samples
The DNA from the samples that came up positive from the qPCR, were amplified by conventional PCR, targeting the5S-23S ribosomal RNA intergenic spacer region (IGS) of B. burgdorferi. We used the forward primer B5Sborseq (5′-GAGTTCGCGGGAGAGTAGGTTATTGCC-3′) and the reverse primer 23Sborseq (5′-TCAGGGTACTTAGATGGTTCACTTCC-3′).
To ensure that a product from the PCR was obtained, gel electrophoreses was utilized with 8 µl of DNA on a 1.5% agarose gel. The gel was colored with SYBR™ Gold Nucleic Acid Gel Stain (Invitrogen™). If the PCR was successful showing a clear band on the gel, the DNA was cleaned with ExoSAP-IT™ PCR Product Cleanup Reagent (Applied Biosystems™) and sent to sequencing by the firm BaseClear. The chromatographs of the sequences were visually inspected and the primers sites were trimmed in Bionumerics. Our sequences were used to identify the B. burgdorferi sl genospecies by comparison to sequences of known genospecies from GenBank (See Supplementary Table 1). The cluster analyses were performed in Bionumerics 7.4 (Applied Math, Belgium) as described previously62.
Sequencing of pepX of B. garinii positive samples
As it is not always simple to discriminate between B. bavariensis and B. garinii based on IGS data, the nested PCR pepX was done for confirmation following Margos et al.63.
We used the forward primer pepXF362 (5′-ACAGAGACTTAAGCTTAGCAG-3′) and reverse primer pepXR1172 (5′-GTTCCAATGTCAATAGTTTC-3′) for the initial PCR, and added 1 µl of this product to the nested PCR. The nested primers are the forward primer pepXF449 (5′-TTATTCCAAACCTTGCAATCC-3′) and reverse primer pepXR1115 (5′-TGTGCCTGAAGGAACATTTG-3′).
A total of 10 µl of the nested product was analyzed using the QIAxcel DNA High Resolution Kit on the QIAxcel Advanced System (Qiagen, Hilden, Germany 2018), and samples with a clear band were sent to sequencing by the firm BaseClear. The primer sites of our sequences were manually trimmed and processed. The sequences from this study were used to identify the B. burgdorferi sl genospecies by genetic comparison to sequences of established isolates, as described previously62. PepX sequences of known B. burgdorferi sl genospecies can be retrieved from GenBank or from a Borrelia MLST Database (https://pubmlst.org/borrelia/).
Statistical analysis
We used logistic regression in R vs. 3.4.4 to analyse variation in prevalence of B. burgdorferi sl with mammal species as a factor. However, the presence of species was not balanced across counties, so that we cannot run a full model. For the most common small mammals, we were mainly interested in comparing infection levels relative to medium-sized mammals. Due to the issue of perfect separation for some species (no positives in cervids), we initially applied a one-step-estimator (maxit = 1), but failed to get convergence. We therefore ended up with an analysis restricted to the eastern region and including rodents and shrews, except water shrew due to low sample size. For questing ticks, we ran a logistic regression to test if genospecies B. afzelii and B. burgdorferi ss in questing ticks was found equally often in nymphs or adults (males and females combined) and with county included as a 3-level factor variable.
Supplementary information
Acknowledgements
We are grateful to the Research Council of Norway and the Norwegian Environment Agency funding the #EcoTick project (254469), and to the Faculty of Mathematics and Natural Sciences, UiO, for a scholarship funding a sabbatical of AM at Univ. of Antwerp. We are grateful to Anders Herland for help with the field work, and to Vidar Holthe for the collection of traffic killed mammals.
Author Contributions
A.M. initiated the study and organized collection of samples; V.M.S. sampled parts of the data and did the DNA extraction and qPCR. V.M.S. and R.I.J. prepared data for sequencing under supervision of H.S. A.M. drafted the manuscript with notable input from H.S. and methods parts contributed from V.M.S. and R.I.J. All authors edited and approved the final manuscript.
Data Availability
All data are reported in Tables 1 and 2 within the paper. Sequences obtained in this study are in Genbank with accession numbers MK108437 to MK108914.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41686-0.
References
- 1.Buhnerkempe MG, et al. Eight challenges in modelling disease ecology in multi-host, multi-agent systems. Epidemics. 2015;10:26–30. doi: 10.1016/j.epidem.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin LB, Burgan SC, Gervasi SS. Host competence: An organismal trait to integrate immunology and epidemiology. icb. 2016;56:1225–1237. doi: 10.1093/icb/icw064. [DOI] [PubMed] [Google Scholar]
- 3.Keesing F, et al. Hosts as ecological traps for the vector of Lyme disease. Proc R Soc Lond Ser B. 2009;276:3911–3919. doi: 10.1098/rspb.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LoGiudice K, et al. Impact of host community composition on Lyme disease risk. Ecol. 2008;89:2841–2849. doi: 10.1890/07-1047.1. [DOI] [PubMed] [Google Scholar]
- 5.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nature Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franke J, Hildebrandt A, Dorn W. Exploring gaps in our knowledge on Lyme borreliosis spirochaetes-updates on complex heterogeneity, ecology, and pathogenicity. Ticks Tick Borne Dis. 2013;4:11–25. doi: 10.1016/j.ttbdis.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Coipan, E. C. & Sprong, H. In Ecology and prevention of Lyme disease (eds Braks, M. A. H., Van Wieren, S. E., Takken, W. & Sprong, H.) 41-61 (Wageningen Academic Publishers, Wageningen, 2016).
- 9.Kurtenbach K, et al. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Micro. 2006;4:660–669. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- 10.Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. Plos Biol. 2006;4:1058–1068. doi: 10.1371/journal.pbio.0040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randolph SE, Gern L, Nuttall PA. Co-feeding ticks: epidemiological significance for tick-borne pathogen transmission. Parasitol Today. 1996;12:472–479. doi: 10.1016/S0169-4758(96)10072-7. [DOI] [PubMed] [Google Scholar]
- 12.Kilpatrick AM, et al. Lyme disease ecology in a changing world: consensus, uncertainty and critical gaps for improving control. Phil Trans R Soc London Ser B. 2017;372:20160117. doi: 10.1098/rstb.2016.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter KS, Carpi G, Caccone A, Diuk-Wasser MA. Genomic insights into the ancient spread of Lyme disease across North America. Nature. Ecol Evol. 2017;1:1569–1576. doi: 10.1038/s41559-017-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humair PF, Gern L. Relationship between Borrelia burgdorferi sensu lato species, red squirrels (Sciurus vulgaris) and Ixodes ricinus in enzootic areas in Switzerland. Acta Tropica. 1998;69:213–227. doi: 10.1016/S0001-706X(97)00126-5. [DOI] [PubMed] [Google Scholar]
- 15.Pisanu B, et al. High prevalence of Borrelia burgdorferi s.l. in the European red squirrel Sciurus vulgaris in France. Ticks Tick Borne Dis. 2014;5:1–6. doi: 10.1016/j.ttbdis.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Millins C, et al. An invasive mammal (the gray squirrel, Sciurus carolinensis) commonly hosts diverse and atypical genotypes of the zoonotic pathogen Borrelia burgdorferi sensu lato. Appl Environ Microbiol. 2015;81:4236–4245. doi: 10.1128/AEM.00109-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahfari S, et al. Enzootic origins for clinical manifestations of Lyme borreliosis. Infect Genet Evol. 2017;49:48–54. doi: 10.1016/j.meegid.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Jungnick S, et al. Borrelia burgdorferi sensu stricto and Borrelia afzelii: Population structure and differential pathogenicity. Int J Med Microbiol. 2015;305:673–681. doi: 10.1016/j.ijmm.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Logar M, et al. Comparison of Erythema migrans caused by Borrelia afzelii and Borrelia garinii. Infection. 2004;32:15–19. doi: 10.1007/s15010-004-3042-z. [DOI] [PubMed] [Google Scholar]
- 20.Stanek G, Reiter M. The expanding Lyme Borrelia complex - clinical significance of genomic species? Clin Microbiol Infec. 2011;17:487–493. doi: 10.1111/j.1469-0691.2011.03492.x. [DOI] [PubMed] [Google Scholar]
- 21.Hanincova K, et al. Multilocus sequence typing of Borrelia burgdorferi suggests existence of lineages with differential pathogenic properties in humans. Plos One. 2013;8:e73066. doi: 10.1371/journal.pone.0073066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jore S, et al. Multi-source analysis reveals latitudinal and altitudinal shifts in range of Ixodes ricinus at its northern distribution limit. Parasite Vector. 2011;4:84. doi: 10.1186/1756-3305-4-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaenson TGT, Lindgren E. The range of Ixodes ricinus and the risk of contracting Lyme borreliosis will increase northwards when the vegetation period becomes longer. Ticks Tick Borne Dis. 2011;2:44–49. doi: 10.1016/j.ttbdis.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 24. Mysterud, A. et al. Contrasting emergence of Lyme disease across ecosystems. Nature Comm7, Article 11882 (2016). [DOI] [PMC free article] [PubMed]
- 25. Mysterud, A., Jore, S., Østerås, O. & Viljugrein, H. Emergence of tick-borne diseases at northern latitudes in Europe: a comparative approach. Sci Rep7, article number 16316 (2017). [DOI] [PMC free article] [PubMed]
- 26.Sajanti E, et al. Lyme Borreliosis in Finland, 1995–2014. Emerg Infect Dis. 2017;23:1282. doi: 10.3201/eid2308.161273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taberlet P, Fumagalli L, Wust-Saucy A, Cosson J. Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol. 2002;7:453–464. doi: 10.1046/j.1365-294x.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- 28.Kahl, O., Gern, L., Eisen, L. & Lane, R. S. In Lyme borreliosis: biology, epidemiology and control. (eds Gray, J. S., Kahl, O., Lane, R. S. & Stanek, G.) CABI publishing, Wallingford, Oxfordsire, UK, 2002).
- 29.Kurtenbach K, et al. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl Environ Microbiol. 1998;64:1169–1174. doi: 10.1128/aem.64.4.1169-1174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heylen D, Matthysen E, Fonville M, Sprong H. Songbirds as general transmitters but selective amplifiers of Borrelia burgdorferi sensu lato genotypes in Ixodes rinicus ticks. Environ Microbiol. 2014;16:2859–2868. doi: 10.1111/1462-2920.12304. [DOI] [PubMed] [Google Scholar]
- 31.Heylen D, Fonville M, Leeuwen AD, Sprong H. Co-infections and transmission dynamics in a tick-borne bacterium community exposed to songbirds. Environ Microbiol. 2015;18:988–996. doi: 10.1111/1462-2920.13164. [DOI] [PubMed] [Google Scholar]
- 32. Hofmeester, T. R. et al. Cascading effects of predator activity on tick-borne disease risk. Proc R Soc Lond Ser B284 (2017). [DOI] [PMC free article] [PubMed]
- 33.Hofmeester TR, et al. Few vertebrate species dominate the Borrelia burgdorferi s.l. life cycle. Environ Res Lett. 2016;11:043001. doi: 10.1088/1748-9326/11/4/043001. [DOI] [Google Scholar]
- 34.Kjelland V, Stuen S, Skarpaas T, Slettan A. Prevalence and genotypes of Borrelia burgdorferi sensu lato infection in Ixodes ricinus ticks in southern Norway. Scand J Infect Dis. 2010;42:579–585. doi: 10.3109/00365541003716526. [DOI] [PubMed] [Google Scholar]
- 35.Kjelland V, et al. Tick-borne encephalitis virus, Borrelia burgdorferi sensu lato, Borrelia miyamotoi, Anaplasma phagocytophilum and Candidatus Neoehrlichia mikurensis in Ixodes ricinus ticks collected from recreational islands in southern Norway. Ticks Tick Borne Dis. 2018;9:1098–1102. doi: 10.1016/j.ttbdis.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Tveten AK. Prevalence of Borrelia burgdorferi sensu stricto, Borrelia afzelii, Borrelia garinii, and Borrelia valaisiana in Ixodes ricinus ticks from the northwest of Norway. Scand J Infect Dis. 2013;45:681–687. doi: 10.3109/00365548.2013.799288. [DOI] [PubMed] [Google Scholar]
- 37.Jaenson TGT, et al. Risk indicators for the tick Ixodes ricinus and Borrelia burgdorferi sensu lato in Sweden. Med Vet Entomol. 2009;23:226–237. doi: 10.1111/j.1365-2915.2009.00813.x. [DOI] [PubMed] [Google Scholar]
- 38.Heylen, D. J. A. In Ecology and prevention of Lyme borreliosis. (eds Marieta, A. H. B., Van Wieren, S. E., Takken, W. & Sprong, H.) 91–101 (Wageningen Academic Publishers, Wageningen, 2016).
- 39.Heylen D, et al. Pathogen communities of songbird-derived ticks in Europe’s low countries. Parasite Vector. 2017;10:497. doi: 10.1186/s13071-017-2423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gryczynska A, Gortat T, Kowalec M. Urban rodent reservoirs of Borrelia spp. in Warsaw, Poland. Epidemiol Infect. 2018;146:589–593. doi: 10.1017/S095026881800033X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mysterud A, Byrkjeland R, Qviller L, Viljugrein H. The generalist tick Ixodes ricinus and the specialist tick Ixodes trianguliceps on shrews and rodents in a northern forest ecosystem - a role of body size even among small hosts. Parasite Vector. 2015;8:639. doi: 10.1186/s13071-015-1258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bown KJ, et al. The common shrew (Sorex araneus): a neglected host of tick-borne infections? Vector-Borne Zoonot. 2011;11:947–953. doi: 10.1089/vbz.2010.0185. [DOI] [PubMed] [Google Scholar]
- 43.Hanski I, Kaikusalo A. Distribution and habitat selection of shrews in Finland. Ann Zool Fenn. 1989;26:339–348. [Google Scholar]
- 44.Bilton DT, et al. Mediterranean Europe as an area of endemism for small mammals rather than a source for northwards postglacial colonization. Proc R Soc Lond Ser B. 1998;265:1219. doi: 10.1098/rspb.1998.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marsot M, et al. Introduced Siberian chipmunks (Tamias sibiricus barberi) harbor more-diverse Borrelia burgdorferi sensu lato genospecies than native bank voles (Myodes glareolus) Appl Environ Microbiol. 2011;77:5716–5721. doi: 10.1128/AEM.01846-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kjelland V, et al. Borrelia burgdorferi sensu lato detected in skin of Norwegian mountain hares (Lepus timidus) without signs of dissemination. J Wildl Dis. 2011;47:293–299. doi: 10.7589/0090-3558-47.2.293. [DOI] [PubMed] [Google Scholar]
- 47.Heidrich J, et al. Investigation of skin samples from red foxes (Vulpes vulpes) in Eastern Brandenburg (Germany) for the detection of Borrelia burgdorferi s.l. Zentralblatt für Bakteriologie. 1999;289:666–672. doi: 10.1016/S0934-8840(99)80026-7. [DOI] [PubMed] [Google Scholar]
- 48.Gern L, Sell K. Isolation of Borrelia burgdorferi sensu lato from the skin of the European badger (Meles meles) in Switzerland. Vector-Borne Zoonot. 2008;9:207–208. doi: 10.1089/vbz.2008.0050. [DOI] [PubMed] [Google Scholar]
- 49.Skuballa J, et al. Occurrence of different Borrelia burgdorferi sensu lato genospecies including B. afzelii, B. bavariensis, and B. spielmanii in hedgehogs (Erinaceus spp.) in Europe. Ticks Tick Borne Dis. 2012;3:8–13. doi: 10.1016/j.ttbdis.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Gooskens J, Templeton KE, Claas EC, van Dam AP. Evaluation of an internally controlled real-time PCR targeting the ospA gene for detection of Borrelia burgdorferi sensu lato DNA in cerebrospinal fluid. Clin Microbiol Infec. 2006;12:894–900. doi: 10.1111/j.1469-0691.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 51.Jahfari S, et al. Melting pot of tick-borne zoonoses: the European hedgehog contributes to the maintenance of various tick-borne diseases in natural cycles urban and suburban areas. Parasit Vectors. 2017;10:134. doi: 10.1186/s13071-017-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Randolph SE, Dobson ADM. Pangloss revisisted: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology. 2012;139:847–863. doi: 10.1017/S0031182012000200. [DOI] [PubMed] [Google Scholar]
- 53.Ogden NH, Tsao JI. Biodiversity and Lyme disease: Dilution or amplification? Epidemics. 2009;1:196–206. doi: 10.1016/j.epidem.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Telford SR, Mather TN, Moore SI, Wilson ML, Spielman A. Incompetence of deer as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg. 1988;39:105–109. doi: 10.4269/ajtmh.1988.39.105. [DOI] [PubMed] [Google Scholar]
- 55.Kjelland V, Ytrehus B, Stuen S, Skarpaas T, Slettan A. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks collected from moose (Alces alces) and roe deer (Capreolus capreolus) in southern Norway. Ticks Tick Borne Dis. 2011;2:99–103. doi: 10.1016/j.ttbdis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Qviller L, et al. Landscape level variation in tick abundance relative to seasonal migration pattern of red deer. Plos One. 2013;8:e71299. doi: 10.1371/journal.pone.0071299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qviller, L., Viljugrein, H., Loe, L. E., Meisingset, E. L. & Mysterud, A. The influence of red deer space use on questing tick density and distribution in the landscape. Parasite Vector9, article 545 (2016). [DOI] [PMC free article] [PubMed]
- 58. Mysterud, A. et al. Tick abundance, pathogen prevalence, and disease incidence in two contrasting regions at the northern distribution range of Europe. Parasite Vector11, Article 309 (2018). [DOI] [PMC free article] [PubMed]
- 59.Courtney JW, Kostelnik LM, Zeidner NS, Massung RF. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol. 2004;42:3164–3168. doi: 10.1128/JCM.42.7.3164-3168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mysterud A, Easterday WR, Qviller L, Viljugrein H, Ytrehus B. Spatial and seasonal variation in prevalence of Anaplasma phagocytophilum and Borrelia burgdorferi in Ixodes ricinus ticks in Norway. Parasite Vector. 2013;6:187. doi: 10.1186/1756-3305-6-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allender CJ, Easterday WJ, Van Ert MN, Wagner DM, Keim P. High-throughput extraction of arthropod vector and pathogen DNA using bead milling. Biotechniques. 2004;37:730–734. doi: 10.2144/04375BM03. [DOI] [PubMed] [Google Scholar]
- 62.Coipan EC, et al. Geodemographic analysis of Borrelia burgdorferi sensu lato using the 5S-23S rDNA spacer region. Infect Genet Evol. 2013;17:216–222. doi: 10.1016/j.meegid.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 63.Margos G, et al. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc Natl Acad Sci USA. 2008;105:8730–8735. doi: 10.1073/pnas.0800323105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are reported in Tables 1 and 2 within the paper. Sequences obtained in this study are in Genbank with accession numbers MK108437 to MK108914.