Abstract
The transcription factor Nrf2, which regulates the expression of antioxidant and cytoprotective enzymes, contributes to cell proliferation and resistance to chemotherapy. Nrf2 is also dysregulated in many cancers such as lung, head and neck, and breast cancers, but its role in Epstein-Barr virus (EBV)–transformed B cells is still not understood. Here, we investigated EBV infection-induced Nrf2 activation in B cells by analyzing translocation of Nrf2 from the cytosol to the nucleus. In addition, we confirmed expression of the target genes in response to increased Nrf2 activation in EBV-transformed B cells. We demonstrated that knockdown of LMP1 and 2A blocks the translocation of Nrf2 to the nucleus and reduces ROS production in EBV-transformed B cells. Further, we showed that inhibition of Akt prevents Nrf2 activation. Moreover, knockdown of Nrf2 induces apoptotic cell death in EBV-transformed B cells. In conclusion, our study demonstrates that Nrf2 promotes proliferation of EBV-transformed B cells through the EBV-related proteins LMP1 and 2A and Akt signaling, implicating Nrf2 as a potential molecular target for EBV-associated disease.
Introduction
Epstein-Barr virus (EBV) is a γ-herpes virus that infects more than 90% of individuals worldwide [1]. EBV is the first characterized oncovirus [2], and its function is well known in human cancers, including Burkitt's lymphoma, non-Hodgkin's lymphoma, nasopharyngeal carcinoma), and gastric cancer [3], with up to 400,000 cases each year as estimated by the World Health Organization [4]. EBV encodes a series of functional proteins that support two types of infection: latent and lytic.
Among EBV latent proteins, latent membrane proteins 1 (LMP1) and 2A (LMP2A) are essential for maintenance of the latent infection and EBV-induced B-cell transformation and oncogenesis [5], [6]. The large cytosolic c-terminal activating regions (CTARs) 1 (aa 194-232) and 2 (aa 351-386) of LMP1 provide constitutive activation of cell proliferation, motility, and migration by binding to oncogenic signaling pathways, such as NF-κB, phosphatidylinositol 3-kinase (PI3K)/Akt, ERK, and JAK/STAT [7], [8], [9], [10]. LMP2A also activates PI3K/Akt pathway and contributes to B-cell survival and proliferation [6], [11], [12]. In this way, expression of LMP1 and 2 promoted malignant phenotypes, including suppressed apoptosis, and increased metastasis and invasion in EBV-infected cancer cells [13], [14], [15], [16], [17].
Reactive oxygen species (ROS) play pivotal roles in the body, helpful or harmful depending on the level of ROS. A moderate increase in ROS level activates a signaling pathway that initiates various biological processes, while accumulation of ROS promotes aging and numerous diseases including cancer, cardiovascular, and autoimmune disorder [18], [19], [20].
Mammalian cells have developed an effective system to protect against cellular damage in response to oxidative stress. The activation of nuclear factor erythroid 2-related factor 2 (Nrf2), a redox-sensitive transcription factor, is the first-line defense. Nrf2 has a basic leucine zipper (bZip) motif with a Cap “n” Collar (CNC) structure [21]. Nrf2 binds to the antioxidant response element (ARE, 5′-TGACNNNGC-3) in the promoter region of target genes [22], [23], [24].
Under normal conditions, Nrf2 is isolated from the Kelch-like ECH-associated protein-1 (Keap1) in the cytoplasm. Sequestered Nrf2 is degraded by E3 ubiquitin ligase complex for ubiquitination. However, under oxidative stress, Nrf2 is protected from proteasomal degradation through dissociation from Keap1. Then, Nrf2 is translocated into the nucleus and forms a heterodimer with small Maf proteins. Finally, it binds to ARE sequences and antioxidant genes such as heme oxygenase-1 (HO-1), NAD(P)H-quinone oxidoreductase 1 (NQO-1), glutathione S-transferase (GST), and γ-glutamylcysteine ligase (GCL) [25], [26], [27].
Recently, several studies including our reports have shown that EBV-infected cells have high ROS levels that are modulated by various mechanisms [28], [29]. However, it is still not known whether Nrf2 is activated in EBV-infected cells and how Nrf2 activation is related to expression of EBV-related proteins. Therefore, in this study, we investigated the activation of Nrf2 in EBV-transformed B cells, with a focus on the underlying molecular mechanisms.
Materials and Methods
Cell Culture
Preparation of cell-free EBV virions and generation of EBV-transformed B cells were carried out as described previously [30]. PBMCs were isolated from peripheral blood of human volunteers. These cells were maintained in RPMI 1640 medium (Hyclone, Logan, UT) supplemented with 10% FBS (Hyclone, Logan, UT) and antibiotics under a humidified atmosphere with 5% CO2.
Cell Viability Assay
The cells were seeded at a density of 1-2 × 104 cells/well in a 96-well plate and transfected with siNrf2 for 48 hours. Cell viability was assessed by Cell Tilter 96 Aqueous One Solution Cell Proliferation Assay (Promega Corporation, Madison, WI), according to the manufacturer's instructions. Untreated cells served as a negative control. The experiments were performed in triplicate.
Western blot
Protein concentrations of cell lysates were determined with Bradford reagent (Bio-Rad, Hercules, CA), and 20 μg per lane was separated by 8%-15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis before transferring to Immobilion PVDF membranes (Millipore, Billerica, MA). Membranes were blocked with 5% skim milk in TBS-T buffer (20 mM Tris–HCl pH 7.4, 150 mM NaCl, 0.1% Tween 20) and reacted with primary antibodies at 4°C overnight. We used anti–β-actin, anti–Lamin B and LMP2A (Santa Cruz Biotechnology, Santa Cruz, CA), anti-LMP1 (Abcam, Cambridge, MA), anti-Nrf2, anti-Ho-1, anti–NQO-1, anti-pAkt, anti-Akt, anti–caspase-9, and anti–caspase-3 (Cell Signaling Technology, Beverly, MA) antibodies. Bound primary antibodies were visualized by incubating with horseradish peroxidase–conjugated anti-mouse or anti-rabbit secondary antibodies (Cell Signaling Technology, Beverly, MA) and detected with chemiluminescence detection reagents (Amersham, Buckinghamshire, UK). Densitometry to quantify protein was performed using Image J software.
RNA Interference
EBV-transformed B cells were transfected with scrambled siLMP1 (5′-GGAAUUUGCACGGACAGGUU-3′ and siLMP2A (5’-AACUCCCAAUAUCCAUCUGCUUU-3′, Genolution Pharmaceuticals Inc. Seoul, Republic of Korea) or Nrf2 siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) with Lipofectamine RNAimax (Invitrogen, Carlsbad, CA) for 48 hours according to recommended guidelines.
Nuclear and Cytoplasmic Fractionation
Nuclear and cytoplasmic fractionation of cells was performed using a Nuclear/Cytosol Fractionation kit (BioVision Inc., CA). After treatment with CEB-A and CEB-B, the cytoplasmic extract was harvested by centrifugation at 16,000 g for 5 minutes. The pellet was resuspended in 100 gl of NEB, and the nuclear fraction was harvested by centrifugation at 16,000 g for 10 minutes.
ROS Measurement
Cells were treated or transfected as indicated in the figure legends and stained with 20 μM H2DCF-DA (Sigma-Aldrich, St Louis, MO) for 1 hour. Cells were trypsinized, washed with PBS, and resuspended in 0.5 ml PBS. H2DCF-stained cells were quantitatively analyzed by FACS Calibur flow cytometer (BD Biosciences, San Jose, CA). Flow cytometric data were analyzed using BD Cell Quest software (BD Biosciences, San Jose, CA).
Immunocytochemistry
The cells were plated onto poly-l-lysine–coated slides, fixed in an acetone:methanol solution (1:1) for 10 minutes at −20 °C, and washed twice with PBS for 5 minutes. The cells were then permeabilized in 0.2% Triton X-100 for 5 minutes and washed twice with PBS for 5 minutes. The cells were blocked in 1.5% horse serum in PBS for 30 minutes at room temperature and then incubated overnight in a humidified chamber at 4°C with primary antibodies including rabbit anti-Nrf2 (1:300, Cell Signaling Technology, Danvers, MA). After washing twice with PBS, the cells were incubated with rabbit-FITC secondary antibody (1:500, Sigma-Aldrich, St Louis, MO) for 1 hour at room temperature. They were also stained with 4,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei. Slides were then washed twice with PBS and mounted with DAKO fluorescence mounting medium (DAKO, Santa Clara, CA) before viewing with Olympus BX51 Fluorescence microscope with MetaMorph software (FISH).
TUNEL Staining
EBV-transformed B cells were plated in six-well plates with RPMI medium, transfected with siNrf2 for 48 hours, and plated onto poly-l-lysine–coated slides. They were then fixed in an acetone:methanol solution (1:1) for 10 minutes at −20°C and washed twice with PBS for 5 minutes. The cells were then permeabilized in 0.2% Triton X-100 for 5 minutes and washed with PBS twice for 5 minutes. The slides were stained with terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling (TUNEL) using an in situ cell death detection kit (Millipore, Billerica, MA) in accordance with the manufacturer's instructions.
Statistical Analysis
Data are expressed as the mean ± standard deviation (SD). Statistical analysis was performed using SigmaPlot version 7 and unpaired Student's t tests. Differences were considered statistically significant when P < .001.
Results
Nrf2 activation and ROS up-regulation in EBV-Transformed B cells
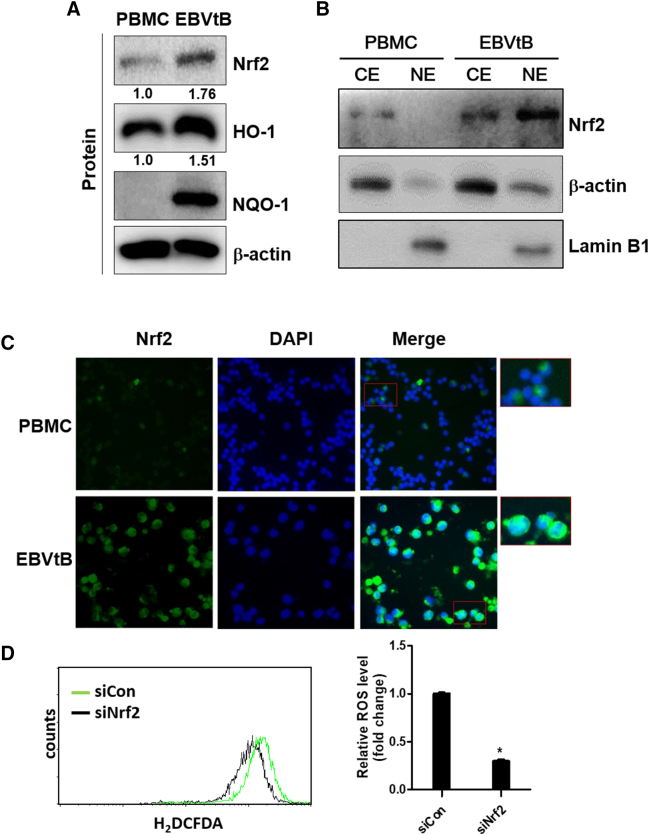
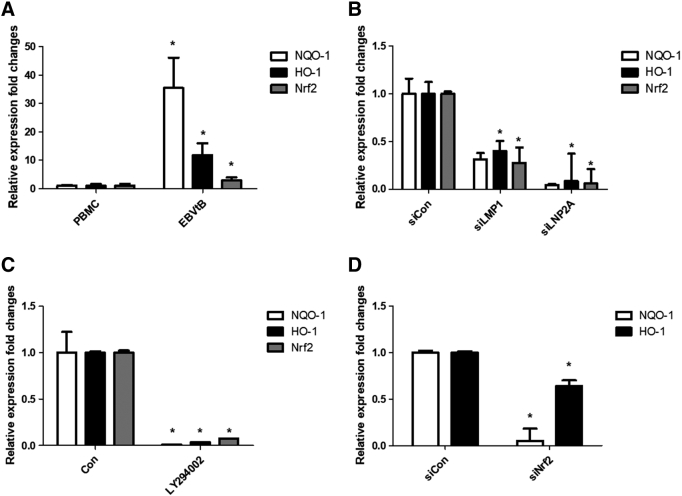
Recently, several studies including our report showed that EBV-infected B cells have high ROS levels [28], [29]. Also, it is well known that the first-line defense system involves activation of Nrf2 transcription factor in response to oxidative stress [22], [23], [24]. Therefore, we determined the levels of Nrf2, HO-1, and NQO-1 in EBV-transformed B cells and PBMCs. As shown in Figure 1A, EBV-transformed B cells expressed high levels of Nrf2, HO-1, and NQO-1 compared to PBMCs. The mRNA level of these proteins is shown in Supplementary Figure 5A. Also, Nrf2 translocated into the nucleus in EBV-transformed B cells but not PBMCs (Figure 1, B and C). Next, we found that the ROS level decreased when Nrf2 expression was inhibited by siRNA in EBV-transformed B cells (Figure 1D). These results suggest that Nrf2 is an important ROS regulator in EBV-transformed B cells.
Figure 1.
Nrf2 activation is upregulated in EBV-infected B cells. (A) Whole cell extract and (B) cytosolic and nuclear extracts were prepared from PBMCs and EBV-transformed B cells. The protein levels of Nrf2, HO-1, and NQO-1 were measured by Western blot analysis. β-Actin and Lamin B1 were used as equal loading control for normalization. CE, cytosol extract; NE, nuclear extract. The fold increase in Nrf2 and HO-1 is indicated numerically, as determined by densitometry.
(C) The expression level of Nrf2 was detected by immunofluorescence. DAPI was used to counter stain the nucleus. Photographs were taken at 200× magnification. (D) EBV-transformed B cells were transfected with siRNA against Nrf2 for 48 hours. ROS were detected with carboxy-H2DCFDA. The data are expressed as the mean ± S.D. *P < .001. The experiments were performed in triplicate.
Supplementary Figure 5.
Level of mRNA expression of Nrf2, HO-1 and NQO-1. Graphs for expression level of Nrf2, NQO-1 and HO-1 were quantified using the ratio against b-actin from (A) PBMCs and EBV-transformed B cells or (B) LMP1 or LMP2A or (D) Nrf2-transfected or (C) LY294002 treated cells. The mRNA levels of Nrf2, HO-1, and NQO-1 were measured by Real-time PCR. The data are expressed as the mean ± S.D. *p < 0.01.
Both LMP1 and 2A Regulate Nrf2 Activation in EBV-Transformed B Cells
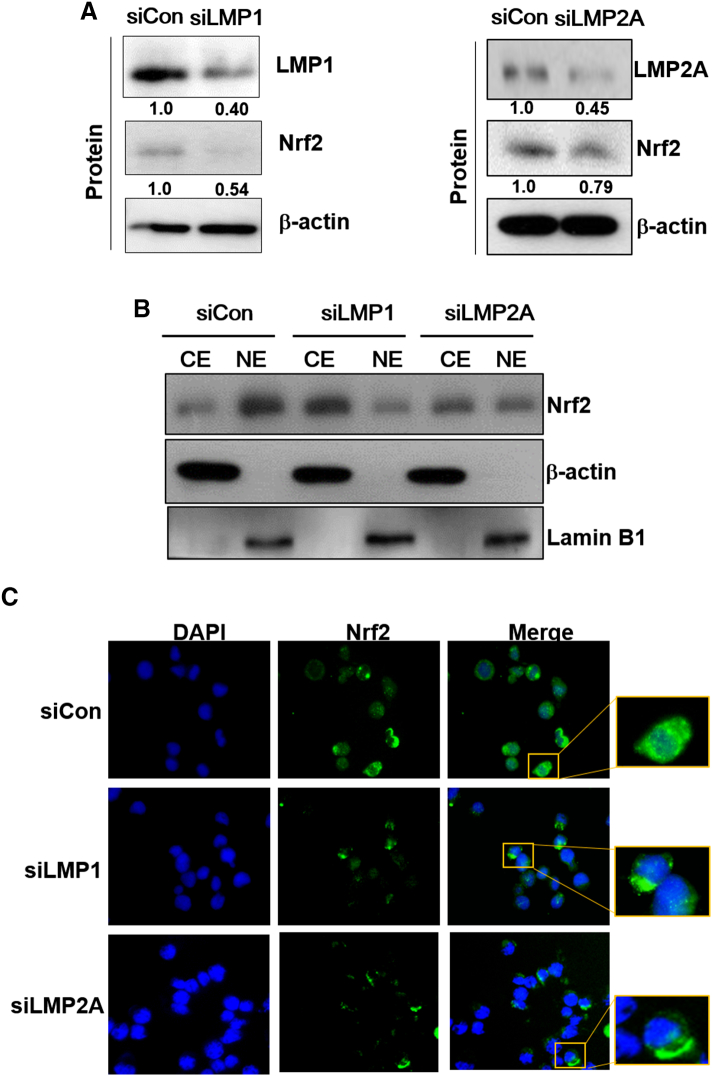
To determine whether the EBV-related key protein LMP1 affects ROS and Nrf2 levels in EBV-transformed B cells, we used siRNA against LMP1 or LMP2A. As shown in Figure 2A, siRNA-mediated inhibition of LMP1or LMP2A resulted in decreased Nrf2 in EBV-transformed B cells. In addition, LMP1 or LMP2A knockdown blocked the translocation of Nrf2 into the nucleus in EBV-transformed B cells but not in control siRNA-transfected cells (Figure 2, B and C). Interestingly, we found that the ROS level was lower in the LMP1 knockdown compared to siRNA-mediated inhibition of Nrf2 (Figure 2D). Because it has been reported that Ctar1 of LMP1 provides constitutive activation of cell proliferation, motility, and migration by binding to oncogenic signaling pathways, such as NF-κB, PI3K/Akt, ERK, and JAK/STAT [7], [8], [9], [10], we next determined the LMP1 required for its activation of Nrf2. We manufactured deletion mutant lacking Ctar1 to test in this study. The results showed that Nrf2 activated with wild-type LMP1 but not its deletion mutants (Supplementary Figure 1, Supplementary Figure 2). Following transfection of siRNA against LMP1 or LMP2A, HO-1 and NQO-1 were downregulated in EBV-transformed B cells (Figure 2E). The mRNA level of these proteins is shown in Supplementary Figure 5B. Also, transfection of wild-type LMP1 increased both HO-1 and NQO-1 expression level. However, transfection of Ctar1-deleted mutants restored the increased NQO-1 level but not HO-1 expression (Supplementary Figure 3). These findings suggest that LMP1 and LMP2A are regulated by the Nrf2/ROS system in EBV-transformed B cells.
Figure 2.
LMP1 and LMP2A activate Nrf2 in EBV-transformed B cells. EBV-transformed B cells were transfected with siRNA against LMP1 or LMP2A for 48 hours. (A) Whole cell extract and (B) cytosolic and nuclear extracts were prepared from siRNA-LMP1 or LMP2A-transfected cells. The protein levels of Nrf2 were measured by Western blot analysis. β-Actin and Lamin B1 were used as equal loading controls for normalization. CE, cytosol extract; NE, nuclear extract. The fold increase in LMP1, LMP2A, and Nrf2 is indicated numerically, as determined by densitometry. (C) The expression level of Nrf2 was detected by immunofluorescence. DAPI was used to counter stain the nucleus. Photographs were taken at 400× magnification. (D) ROS were detected with carboxy-H2DCFDA. The data are expressed as the mean ± S.D. *P < .001. (E) The protein levels of HO-1 and NQO-1 were measured by Western blot analysis. The fold increase in NQO-1 and HO-1 is indicated numerically, as determined by densitometry. The experiments were performed in triplicate.
Supplementary Figure 1.
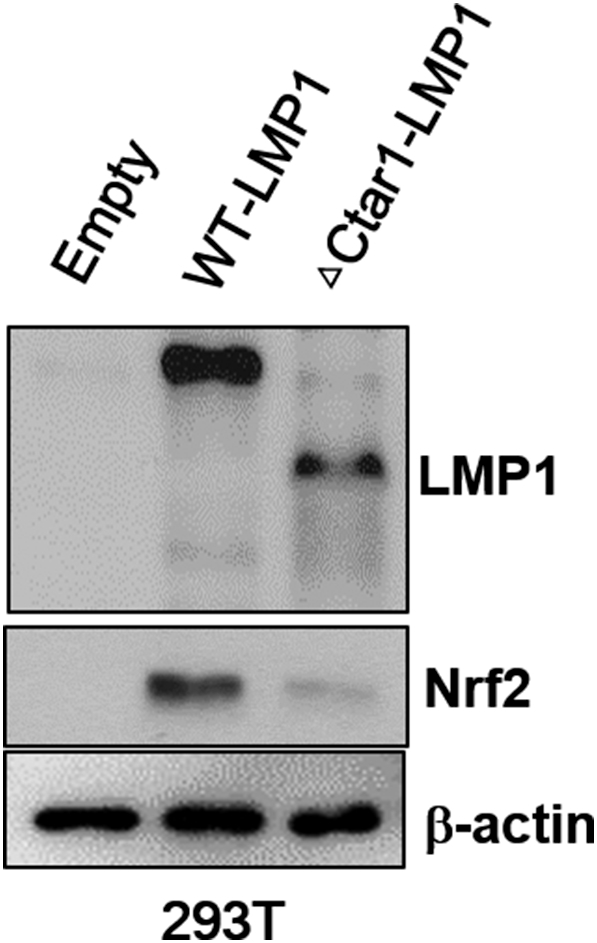
Nrf2 activation is effected by the expression of LMP1 with mutation CTAR1 regions. 293T cells were transfected with wild-type LMP1(WT) or Ctar1 mutant of LMP1 (ΔCtar1) and empty vector. Cell lysates were harvested 28 h after transfection, and western blot were performed with LMP1, Nrf2 and b-actin antibody. b-actin was used as equal loading controls for normalization.
Supplementary Figure 2.
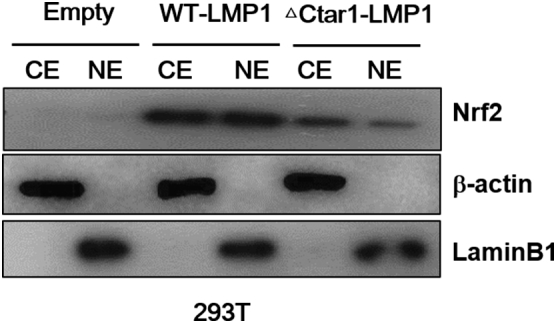
Nrf2 translocation into nucleus is effected by the expression of LMP1 with mutation CTAR1 regions. Cytosolic and nuclear extracts were prepared from 293T cells were transfected with LMP1-△194-232 or wild type (WT) vector plasmid. 48 h later, the expression of the WT and mutant LMP1 proteins were determined by Western blot analysis. b-actin and Lamin B1 were used as equal loading control for normalization. CE: cytosol extract, NE: nuclear extract.
Supplementary Figure 3.
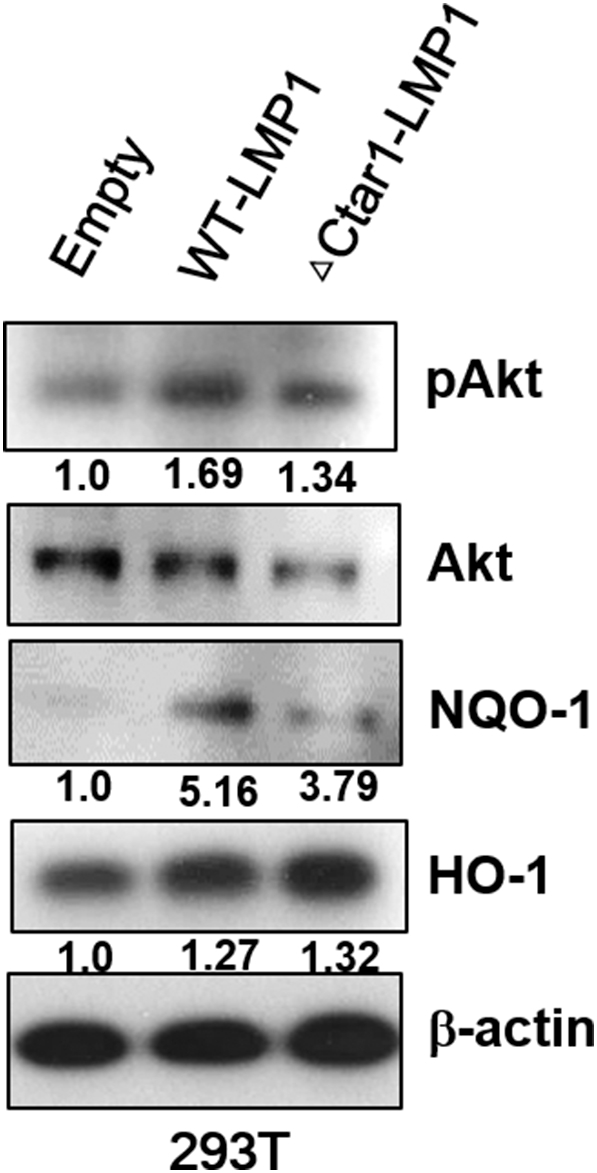
Expression of Nrf2 downstreams and Akt is effected by the expression of LMP1 with mutation CTAR1 regions. 293T cells were transfected with wild-type LMP1(WT) or Ctar1 mutant of LMP1 (ΔCtar1) and empty vector. Cell lysates were harvested 28 h after transfection, and western blot were performed with pAkt, Akt, NQO-1, HO-1 and b-actin antibody. b-actin was used as equal loading controls for normalization. The fold increase in Nrf2 and HO-1 is indicated numerically, as determined by densitometry.
LMP1 and 2A Induce Nrf2 Activation Through Akt Protein in EBV-Transformed B Cells
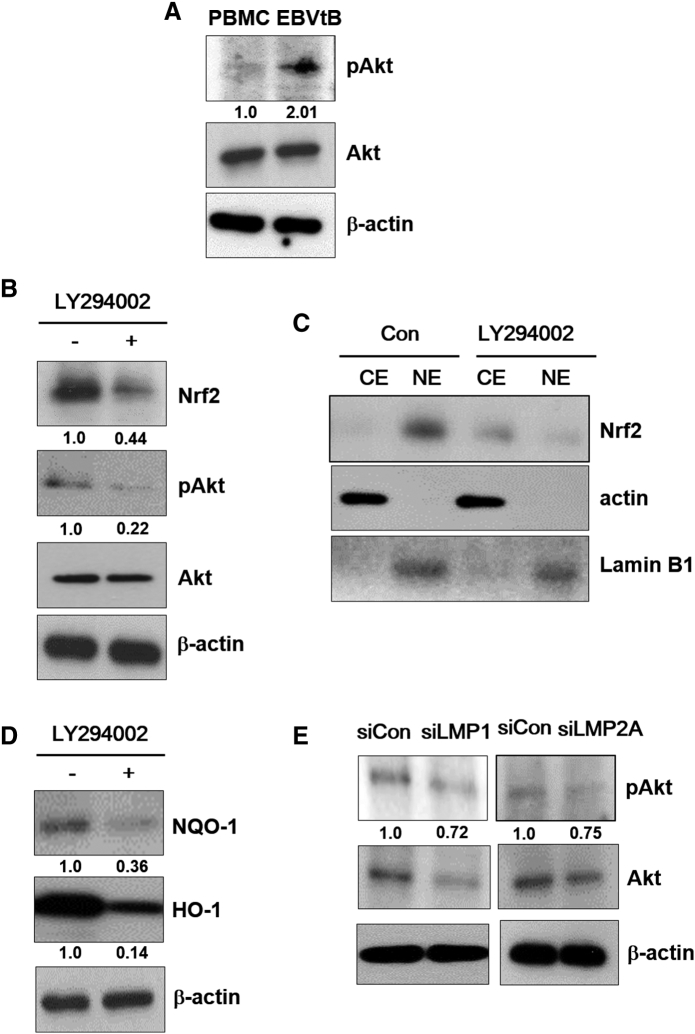
Because both LMP1 protein and ROS can activate Akt [8], we identified the protein levels of both in EBV-transformed B cells. As shown in Figure 3A, activation of Akt was increased in EBV-transformed B cells compared to PBMCs. Next, inhibition of Akt using LY294002 decreased the level of Nrf2 in EBV-transformed B cells (Figure 3B). In addition, LY294002 blocked the translocation of Nrf2 into the nucleus in EBV-transformed B cells but not in control siRNA-transfected cells (Figure 3C). Following treatment with LY294002, HO-1 and NQO-1 were downregulated in EBV-transformed B cells (Figure 3D). The mRNA level of these proteins is shown in Supplementary Figure 5C. Also, transfection of wild-type LMP1 in 293T cells increased in pAkt expression level but not its deletion mutants (Supplementary Figure 3). Moreover, we determined that Akt activation was downregulated by LMP1 or LMP2A knockdown in EBV-transformed B cells (Figure 3E). These data imply that Akt is a key regulator of LMP1- and LMP2A-mediated Nrf2 activation in EBV-transformed B cells.
Figure 3.
Akt activates Nrf2 in EBV-transformed B cells. (A) Cell extracts were prepared from PBMCs and EBV-transformed B cells. The protein levels of pAkt and Akt were measured by Western blot analysis. (B-D) EBV-transformed B cells were treated with LY294002 (10 μM) for 48 hours. (B and D) Whole cell extract and (C) cytosolic and nuclear extracts were prepared from LY294002-treated cells. The protein levels of Nrf2, pAkt, Akt, HO-1, and NQO-1 were measured by Western blot analysis. β-Actin and Lamin B1 were used as equal loading controls for normalization. CE, cytosol extract; NE, nuclear extract. (E) Cell extracts were prepared from siRNA-LMP1 or LMP2A-transfected EBV-transformed B cells. The protein levels of pAkt and Akt were measured by Western blot analysis. The fold increase in pAkt, Nrf2, NQO-1, and HO-1 is indicated numerically, as determined by densitometry.
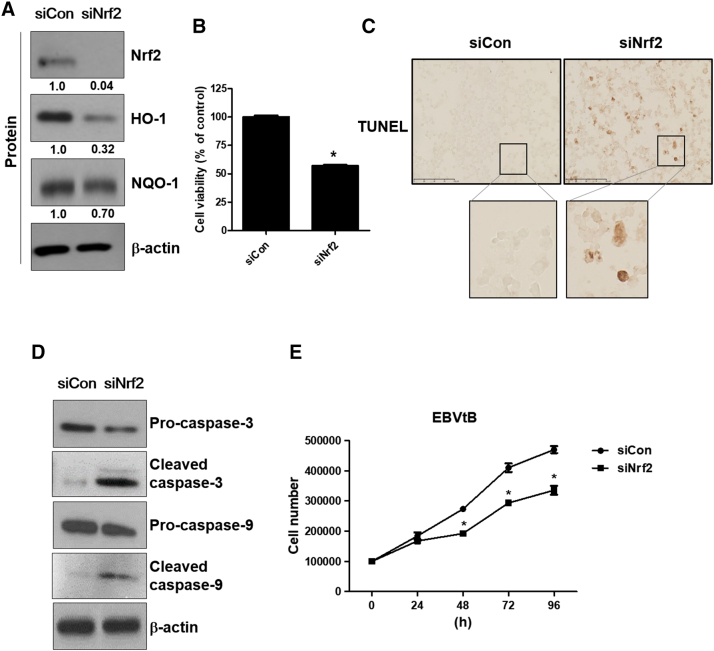
Suppression of Nrf2 Expression Induces Apoptotic Cell Death in EBV-Transformed B Cells
To determine the effects of Nrf2 inhibition on the viability of EBV-transformed B cells, cells were transfected with siRNA against Nrf2 or control for 48 hours. As shown in Figure 4A, knockdown of Nrf2 inhibited expression of Nrf2, HO-1, and NQO-1. The mRNA level of these proteins is shown in Supplementary Figure 5D. These data show that siRNA against Nrf2 is processed in EBV-transformed B cells. Also, knockdown of Nrf2 decreased the cell viability of EBV-transformed B cells compared to control-siRNA–transfected cells (Figure 4B). Next, we evaluated apoptosis in EBV-transformed B cells after knockdown of Nrf2. Through TUNEL staining, we found that siRNA-Nrf2–transfected cells underwent apoptosis, whereas control-siRNA–transfected cells were less sensitive to knockdown of Nrf2-induced apoptosis (Figure 4C). We then examined caspase-3 and -9 cleavage, a hallmark of apoptosis. Following knockdown of Nrf2, cleaved caspase-3 and -9 levels increased in EBV-transformed B cells but not in control-siRNA–transfected cells (Figure 4D). Also, siRNA-mediated knockdown of Nrf2 resulted in a decrease in proliferation activity (Figure 4E). These data clearly show that inhibition of Nrf2 results in selective killing of EBV-infected cells.
Figure 4.
Suppression of Nrf2 induces apoptotic cell death in EBV-transformed B cells. EBV-transformed B cells were transfected with siRNA against Nrf2 for 48 hours. (A) Cell extracts were prepared from siRNA-Nrf2–transfected EBV-transformed B cells. The protein levels of Nrf2, HO-1, and NQO-1 were measured by Western blot analysis. The fold increase in Nrf2, NQO-1, and HO-1 is indicated numerically, as determined by densitometry. (B) Cell viability was evaluated by the MTX assay. The data are expressed as the mean ± S.D. *P < .001. (C) Induction of apoptosis by transfection of siRNA against Nrf2 was identified in EBV-transformed B cells by TUNEL staining. (D) The protein levels of caspase-3 and caspase-9 were measured by Western blot analysis. (E) EBVtB cell was transfected with Nrf2 siRNA or control siRNA. Every 24 hours, cell proliferation was detected using the trypan blue staining assay up to 96 hours. Each line and symbol represent individual siRNA (black circles: control siRNA and black squares: Nrf2 siRNA). The data are expressed as the mean ± S.D. *P < .001 versus each control siRNA.
Discussion
Our study is the first demonstration of the importance of Nrf2 activation induced by the EBV-related protein LMP1 and LMP2A in EBV-transformed B cells. The activation of Nrf2 is due to potentiation of ROS-induced apoptosis via overexpression of Akt.
EBV is the most common virus and infects more than 90% of the world's adult population. EBV can remain asymptomatic for a long time in humans [1], [2] EBV is absent in nontumorous mucosa but present in all cancer cells, in which it has a clonal nature. Therefore, EBV is often considered a dangerous virus [31], [32]. EBV-positive cancer patients often demonstrate a poor response to chemotherapy. Moreover, the presence of EBV is associated with poor prognosis with low survival rate in breast and gastric cancer patients. Also, EBV-positive nasopharyngeal cancer is related to poor radiotherapy response [33], [34], [35]. However, the mechanisms underlying these associations are not very clear. In this study, we demonstrated a new mechanism in EBV-transformed B cells. Therefore, this report is expected to improve the poor prognosis of patients with EBV-associated diseases.
Many studies have shown that Nrf2 helps tumor cell survival and accelerates drug metabolism [36], [37], [38], thus contributing to chemo- or radioresistance [39]. Overexpression of Nrf2 is associated with poor prognosis in cancer [40]. Targeting of Nrf2 signaling impairs tumorigenesis in the lung, pancreas, and colon [41], [42], [43]. However, there is no study on Nrf2 activation in EBV-infected cells. Because several studies including ours have shown that EBV-infected cells have a high ROS level [28], [29], we determined the relationship of Nrf2 signaling with EBV-infected cells. In this study, we observed high expression of Nrf2 (Figure 1A) as well as the activation of Nrf2 through translocation into the nucleus (Figure 1B) in EBV-transformed B cells compared to PBMCs. Inhibition of LMP1 or LMP2A, which is one of several EBV key proteins, was also suppressed by activation of Nrf2 as well as ROS level (Figure 2D). Also, knockdown of LMP1 or LMP2A using siRNA increased caspase-3 and -9 cleavage in EBV-transformed B cells compared to PBMCs (data not shown). These data suggest that targeting LMP1 and LMP2A could suppress ROS levels through Nrf2 inhibition and potentially facilitate the development of agents to treat EBV-infected patients with a poor response to chemotherapy.
We further showed that inhibition of Akt suppressed Nrf2 activation in EBV-transformed B cells (Figure 3B). PI3K/Akt is an important pathway in cell survival and is frequently disordered in several human malignancies. In addition, the PI3K/Akt and RAF/ERK/MAPK pathways are well-known mediators of RAS-mediated transformation and tumorigenesis [44], [45]. Especially, several reports including our previous study have shown that Akt and/or the ERK pathway play an important role in EBV survival [46], [47], [48]. In addition, there is a well-known association of ERK/Akt with Nrf2 pathways [49], [50], [51]. Therefore, we examined that relationship of ERK/Akt with Nrf2 activation in EBV-transformed B cells. As shown in Figure 3A, the Akt is activated in EBV-transformed B cells compared to PBMCs. Inhibition of Akt by LY294002 caused decreased expression and activation of Nrf2 (Figure 3, B-C). Also, inhibition of Akt using LY294002 increased caspase-3 and -9 cleavage in EBV-transformed B cells compared to PBMCs (data not shown). Particularly, knockdown of LMP1 or LMP2A suppressed Akt activation, suggesting that LMP1 and LMP2A are upstream of the Akt pathway (Figure 3E). On the other hand, inhibition of the ERK pathway made little change in Nrf2 activation, although ERK is overexpressed in EBV-transformed B cells compared to PBMCs (data not shown). These results suggest that Nrf2 activation is associated with the Akt pathway in EBV-transformed B cells.
The c-terminal region of LMP1 contains two major c-terminal activating regions (Ctar1 at amino acids 194 to 232 and Ctar2 at amino acids 351 to 386) [52], and we selected deletion mutants lacking Ctar1 (ΔCtar1) to test in this study. As shown in Supplementary Figure 1, Supplementary Figure 2, Supplementary Figure 3, we determined important of LMP1-Ctar1 for Nrf2 activation. The transfection of wild-type LMP1 in 293T cells increased in pAkt and NQO-1 as well as Nrf2 expression level but not Ctar1-deleted mutants. On the other hand, HO-1 level is increased by wild-type LMP1 transfection in 293T cells, but its mutants failed restoration. Because we suspect that HO-1 was affected by other effects, these results are planned for further study. Also, we will study the importance of Ctar2, one of two major c-terminal regions of LMP1.
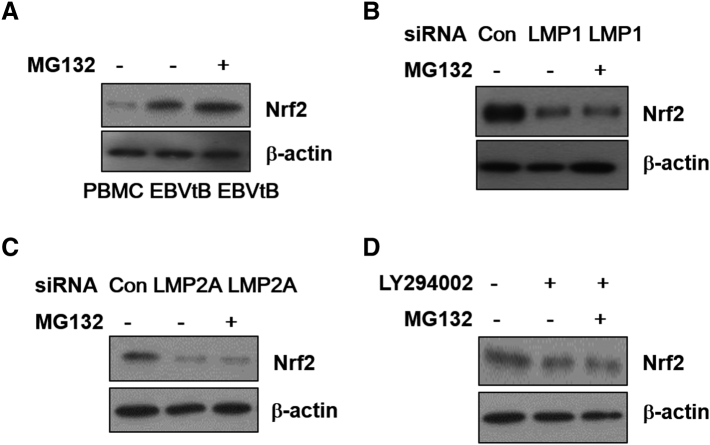
In addition, under normal condition, because Nrf2 is isolated from the Keap1 in the cytoplasm and then sequestered Nrf2 is degraded by E3 ubiquitin ligase complex for ubiquitination, we determined using MG132 as a proteasome inhibitor whether the decrease in Nrf2 expression by each siRNAs or LY294002 was caused by ubiquitination-mediated degradation, but ubiquitination did not affect the decrease (Supplementary Figure 4).
Supplementary Figure 4.
Inhibition of Nrf2 activation by LMP1 or LMP2A or LY294002 was not affect from ubiquitination-mediated degradation. (A) Whole cell extracts were prepared from PBMCs, EBVtB cell and MG132 treated EBVtB cells. EBV-transformed B cells were transfected with siRNA against (B) LMP1 or (C) LMP2A or (D) treated LY294002 and/or with MG132 (5 mM) for 48 h. The protein levels of Nrf2 were measured by Western blot analysis. b-actin were used as equal loading controls for normalization.
In summary, our data demonstrate that LMP1 and LMP2A promote Nrf2 activation through translocation into the nucleus, whereas knockdown of LMP1 or LMP2A blocked these responses in EBV-transformed B cells. Furthermore, we showed that Akt mediated LMP1- or LMP2A-induced Nrf2 activation using the Akt inhibitor LY294002. Also, suppression of Nrf2 using siRNA induced apoptotic cell death in EBV-transformed B cells. Overall, our findings suggest that Nrf2 is a key determinant for the differential response of EBV-infected cells and noninfected cells, and Nrf2 signaling is modulated by LMP1 and LMP2A expression in EBV-transformed B cells. Finally, the Nrf2 pathway is a promising therapeutic strategy for treating EBV-related diseases.
The following are the supplementary data related to this article.
Acknowledgements
This work was supported by 2018 Inje University Busan Paik Hospital research grant.
References
- 1.Khan G., Hashim M.J. Global burden of deaths from Epstein-Barr virus attributable malignancies 1990–2010. Infect Agent Cancer. 2014;9(1):38. doi: 10.1186/1750-9378-9-38. [508 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–768. doi: 10.1038/nrc1452. [nrc1452 pii] [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A., Munger K.L. Epstein-barr virus infection and multiple sclerosis: a review. J NeuroImmune Pharmacol. 2010;5(3):271–277. doi: 10.1007/s11481-010-9201-3. [DOI] [PubMed] [Google Scholar]
- 4.Boshoff C, Weiss R. AIDS-related malignancies. Nat Rev Cancer. 2002;2(5):373–382. doi: 10.1038/nrc797. [DOI] [PubMed] [Google Scholar]
- 5.Dirmeier U, Neuhierl B, Kilger E, Reisbach G, Sandberg ML. Latent membrane protein 1 is critical for efficient growth transformation of human B cells by epstein-barr virus. Cancer Res. 2003;63(11):2982–2989. [PubMed] [Google Scholar]
- 6.Fukuda M, Longnecker R. Epstein-Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/PI3-K/Akt Pathway. J Virol. 2007;81(17):9299–9306. doi: 10.1128/JVI.00537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson C.W., Port R.J., Young L.S. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC) Semin Cancer Biol. 2012;22(2):144–153. doi: 10.1016/j.semcancer.2012.01.004. [S1044-579X(12)00006–5 [pii]] [DOI] [PubMed] [Google Scholar]
- 8.Kung C.P., Meckes D.G., Jr., Raab-Traub N. Epstein-Barr virus LMP1 activates EGFR, STAT3, and ERK through effects on PKCdelta. J Virol. 2011;85(9):4399–4408. doi: 10.1128/JVI.01703-10. [JVI.01703–10 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsao SW, Tramoutanis G, Dawson CW, Lo AK, Huang DP. The significance of LMP1 expression in nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12(6):473–487. doi: 10.1016/s1044579x02000901. [S1044579X02000901 [pii]] [DOI] [PubMed] [Google Scholar]
- 10.Huen DS, Henderson SA, Croom-Carter D, Rowe M. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-kappa B and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995;10(3):549–560. [PubMed] [Google Scholar]
- 11.Scholle F, Bendt KM, Raab-Traub N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol. 2000;74(22):10681–10689. doi: 10.1128/jvi.74.22.10681-10689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swart R, Ruf IK, Sample J, Longnecker R. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-Kinase/Akt pathway. J Virol. 2000;74(22):10838–10845. doi: 10.1128/jvi.74.22.10838-10845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Chen W, Liu P, Zhou J, Liu B. Lentivirus-mediated RNAi knockdown of LMP2A inhibits the growth of the Epstein-Barr–associated gastric carcinoma cell line GT38 in vitro. Exp Ther Med. 2017;13(1):187–193. doi: 10.3892/etm.2016.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ying X, Zhang R, Wang H, Teng Y. Lentivirus-mediated RNAi knockdown of LMP2A inhibits the growth of nasopharyngeal carcinoma cell line C666–1 in vitro. Gene. 2014;542(1):77–82. doi: 10.1016/j.gene.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Lam N, Sandberg ML, Sugden B. High physiological levels of LMP1 result in phosphorylation of eIF2 alpha in Epstein-Barr virus–infected cells. J Virol. 2004;78(4):1657–1664. doi: 10.1128/JVI.78.4.1657-1664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pratt ZL, Zhang J, Sugden B, The latent membrane protein 1 (LMP1) oncogene of Epstein-Barr virus can simultaneously induce and inhibit apoptosis in B cells J Virol. 2012;86(8):4380–4393. doi: 10.1128/JVI.06966-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moody CA, Scott RS, Amirghahari N, Nathan CO, Young LS. Modulation of the cell growth regulator mTOR by Epstein-Barr virus–encoded LMP2A. J Virol. 2005;79(9):5499–5506. doi: 10.1128/JVI.79.9.5499-5506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 20.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91(21):9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chun KS, Kundu J, Kundu JK, Surh YJ. Targeting Nrf2-Keap1 signaling for chemoprevention of skin carcinogenesis with bioactive phytochemicals. Toxicol Lett. 2014;229(1):73–84. doi: 10.1016/j.toxlet.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Prestera T, Talalay P, Alam J, Ahn YI, Lee PJ. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: regulation by upstream antioxidant-responsive elements (ARE) Mol Med. 1995;1(7):827–837. [PMC free article] [PubMed] [Google Scholar]
- 24.Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci U S A. 1997;94(10):5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giudice A, Montella M. Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. BioEssays. 2006;28(2):169–181. doi: 10.1002/bies.20359. [DOI] [PubMed] [Google Scholar]
- 26.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8(1–2):99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- 28.Ding Y, Zhu W, Sun R, Yuan G, Zhang D. Diphenylene iodonium interferes with cell cycle progression and induces apoptosis by modulating NAD(P)H oxidase/ROS/cell cycle regulatory pathways in Burkitt's lymphoma cells. Oncol Rep. 2015;33(3):1434–1442. doi: 10.3892/or.2015.3726. [DOI] [PubMed] [Google Scholar]
- 29.Park GB, Choi Y, Kim YS, Lee HK, Kim D. ROS-mediated JNK/p38-MAPK activation regulates Bax translocation in Sorafenib-induced apoptosis of EBV-transformed B cells. Int J Oncol. 2014;44(3):977–985. doi: 10.3892/ijo.2014.2252. [DOI] [PubMed] [Google Scholar]
- 30.Park GB, Kim YS, Lee HK, Song H, Cho DH. Endoplasmic reticulum stress-mediated apoptosis of EBV-transformed B cells by cross-linking of CD70 is dependent upon generation of reactive oxygen species and activation of p38 MAPK and JNK pathway. J Immunol. 2010;185(12):7274–7284. doi: 10.4049/jimmunol.1001547. [DOI] [PubMed] [Google Scholar]
- 31.Yau TO, Tang CM, Yu J. Epigenetic dysregulation in Epstein-Barr virus–associated gastric carcinoma: disease and treatments. World J Gastroenterol. 2014;20(21):6448–6456. doi: 10.3748/wjg.v20.i21.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marquitz AR, Mathur A, Shair KH, Raab-Traub N. Infection of Epstein-Barr virus in a gastric carcinoma cell line induces anchorage independence and global changes in gene expression. Proc Natl Acad Sci U S A. 2012;109(24):9593–9598. doi: 10.1073/pnas.1202910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou X, Zhao C, Guo Y, Han F, Lu LX. Different clinical significance of pre- and post-treatment plasma Epstein-Barr virus DNA load in nasopharyngeal carcinoma treated with radiotherapy. Clin Oncol (R Coll Radiol) 2011;23(2):128–133. doi: 10.1016/j.clon.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Hu H, Luo ML, Desmedt C, Nabavi S, Yadegarynia S. Epstein-Barr virus infection of mammary epithelial cells promotes malignant transformation. EBioMedicine. 2016;9:148–160. doi: 10.1016/j.ebiom.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoda K, Ichikawa D, Fujita Y, Masuda K, Hiramoto H. Clinical utility of circulating cell-free Epstein-Barr virus DNA in patients with gastric cancer. Oncotarget. 2017;8(17):28796–28804. doi: 10.18632/oncotarget.15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27(20):2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang DD. The Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal. 2010;13(11):1623–1626. doi: 10.1089/ars.2010.3301. [DOI] [PubMed] [Google Scholar]
- 38.Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29(6):1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamadori T, Ishii Y, Homma S, Morishima Y, Kurishima K. Molecular mechanisms for the regulation of Nrf2-mediated cell proliferation in non–small-cell lung cancers. Oncogene. 2012;31(45):4768–4777. doi: 10.1038/onc.2011.628. [DOI] [PubMed] [Google Scholar]
- 40.Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12(8):564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauer AK, Cho HY, Miller-Degraff L, Walker C, Helms K. Targeted deletion of Nrf2 reduces urethane-induced lung tumor development in mice. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim TH, Hur EG, Kang SJ, Kim JA, Thapa D. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. Cancer Res. 2011;71(6):2260–2275. doi: 10.1158/0008-5472.CAN-10-3007. [DOI] [PubMed] [Google Scholar]
- 44.Campbell PM, Groehler AL, Lee KM, Ouellette MM, Khazak V. K-Ras promotes growth transformation and invasion of immortalized human pancreatic cells by Raf and phosphatidylinositol 3-kinase signaling. Cancer Res. 2007;67(5):2098–2106. doi: 10.1158/0008-5472.CAN-06-3752. [DOI] [PubMed] [Google Scholar]
- 45.Zhao JJ, Cheng H, Jia S, Wang L, Gjoerup OV. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc Natl Acad Sci U S A. 2006;103(44):16296–16300. doi: 10.1073/pnas.0607899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson LJ, Longnecker R. EBV LMP2A provides a surrogate pre-B cell receptor signal through constitutive activation of the ERK/MAPK pathway. J Gen Virol. 2008;89(Pt 7):1563–1568. doi: 10.1099/vir.0.2008/001461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan YR, Vatsyayan J, Chang YS, Chang HY. Epstein-Barr virus latent membrane protein 2A upregulates UDP-glucose dehydrogenase gene expression via ERK and PI3K/Akt pathway. Cell Microbiol. 2008;10(12):2447–2460. doi: 10.1111/j.1462-5822.2008.01221.x. [DOI] [PubMed] [Google Scholar]
- 48.Park GB, Kim D, Kim YS, Kim S, Lee HK. The Epstein-Barr virus causes epithelial-mesenchymal transition in human corneal epithelial cells via Syk/src and Akt/Erk signaling pathways. Invest Ophthalmol Vis Sci. 2014;55(3):1770–1779. doi: 10.1167/iovs.13-12988. [DOI] [PubMed] [Google Scholar]
- 49.Jin X, Xu Z, Fan R, Wang C, Ji W. HO1 alleviates cholesterolinduced oxidative stress through activation of Nrf2/ERK and inhibition of PI3K/AKT pathways in endothelial cells. Mol Med Rep. 2017;16(3):3519–3527. doi: 10.3892/mmr.2017.6962. [DOI] [PubMed] [Google Scholar]
- 50.Luo Y, Lu S, Dong X, Xu L, Sun G. Dihydromyricetin protects human umbilical vein endothelial cells from injury through ERK and Akt mediated Nrf2/HO-1 signaling pathway. Apoptosis. 2017;22(8):1013–1024. doi: 10.1007/s10495-017-1381-3. [DOI] [PubMed] [Google Scholar]
- 51.Kim KC, Kang KA, Zhang R, Piao MJ, Kim GY. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int J Biochem Cell Biol. 2010;42(2):297–305. doi: 10.1016/j.biocel.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Wakisaka N, Murono S, Yoshizaki T, Furukawa M, Pagano JS. Epstein-barr virus latent membrane protein 1 induces and causes release of fibroblast growth factor-2. Cancer Res. 2002;62(21):6337–6344. [PubMed] [Google Scholar]