Abstract
Gelatinous foam (GelFoam, Pfizer, Inc, New York, NY) is a low cost, readily available material with a wide range of procedural applications. A novel implementation during computed tomography (CT) guided percutaneous lung biopsy to reduce the rates of pneumothorax leading to further intervention with chest tube placement. We present the imaging and outcome of a patient undergoing this procedure in a community hospital setting.
Keywords: Gelfoam, Surgifoam, Lung biopsy, Pneumothorax, Embolization
Introduction
Percutaneous CT-guided lung core biopsy is a common procedure that is an integral part of the work up of suspicious lung nodules [1]. Although minimally invasive, it is associated with possible complications. Pneumothorax (PTX) is the most common complication of CT-guided percutaneous lung biopsy, with a reported rate ranging from 17% to 40% [1], [2], [3], [4], [5], [6], [7]. Variances in technique and skill are at least partially responsible for the wide range. Most postbiopsy PTX require only observation [1], [3], [7], [8]. The incidence of a PTX requiring chest tube placement is much lower and reportedly ranges from 1% to 14.2% [1], [2], [3], [4], [5], [6], [7].
Lesion size has been hypothesized to affect occurrence of PTX but study findings have been contradictory. Some studies showed lesion size less than 2 cm had a rate of PTX of 33%-60% [2], [6], [9]. Others showed no correlation with size of lesion [10]. The rationale is that smaller lesions potentially require more needle passes and longer procedural time in order to localize the lesion.
Another debatable variable is needle travel distance to the lesion. Some studies correlated longer needle travel distance with increased incidence of PTX and hemorrhage [6]. Others showed that longer needle travel distance was actually protective against PTX in subpleural lesion biopsies when approached at an oblique angle vs perpendicular [11]. Chronic obstructive pulmonary disease (COPD) is associated with a 6-fold increase in biopsy induced PTX vs patients without this risk factor [1], [12].
Graffy et al. studied autologous intraparenchymal blood patching and found a significant decrease in both chest tube placements and hospitalizations post biopsy [13]. Out of 482 patients, there were no adverse effects attributable to the blood patch. Billich et al. showed injection of normal saline solution into the biopsy track was just as effective with no adverse effects [8]. At least 2 clinical trials showed similar reduction in morbidity with gelatinous sponge slurry [7], [14].
Proprietary self-expanding sealant plug devices such as Biosentry (Angiodynamics, Latham, NY) have been shown to be effective in reducing both the rates of PTX and chest tube insertion associated with lung needle biopsy [15], [16]. These devices are not always readily available in small and remote community hospitals due to logistical or price barriers. As a result, alternative techniques have been studied and implemented in attempts to minimize the rates of postbiopsy morbidity and complications in a cost effective manner [7], [13], [14].
GelFoam and Surgifoam (Ethicon, Somerville, NJ) are low cost, readily available products. They are composed of absorbable gelatinous sponge with hemostatic properties.
Chest tube placement and progressive enlargement of postbiopsy PTX have been shown to decrease whenever the needle tract was embolized with gelatinous foam. Tran et al. showed injection of gelatin sponge slurry to have a protective effect against the progression of PTX severity and a decrease in the rate of chest tube placement from 10.7% to 6.9 % [7]. Baadh et al. showed a reduction in the total rate of procedure-related PTX in patients embolized with gelatinous sponge (8.8%) when compared to controls (21%) [14].
When proprietary tract sealant devices are not available, gelatinous foam is a low risk and viable option. Absorbable gelatin foam kits costs under $150 USD [17]. The availability and low cost may also be beneficial in many developing countries that provide lung biopsy services. We seek to illustrate practical means of implementing these new research findings in the community hospital setting, and present our outcomes in this case report. The authors have no conflict of interest. The hospital where this case report took place performs approximately 200 lung biopsies per year and recently started applying gelfoam to the majority of cases.
Materials and methods
Patient
Patient was a 76-year-old male with past medical history of coronary artery disease status postcoronary artery bypass graft. He had a history of hypertension, hyperlipidemia, and chronic alcohol abuse. He also had a history of testicular cancer and was status postorchiectomy. Patient came to the emergency room complaining of generalized weakness. He had no chest pain, no abdominal pain, and no fever or diarrhea recently. Electrocardiogram showed new onset atrial fibrillation.
Chest X-ray showed right lung base consolidation with chronic pleural and parenchymal scarring along with hyperinflation indicative of COPD. Right basilar opacity was present. Subsequent chest CT showed 4.1 × 3.2 × 3.3 cm right middle lobe mass that had markedly increased in size as compared with prior CT (2.2 cm in greatest diameter 1 year prior). There was fluid in the right interlobar fissure. New enlarged paratracheal and right hilar lymph nodes ranging from 2 to 2.5 cm were identified. CT-guided percutaneous lung biopsy was ordered to obtain a tissue sample of the mass.
Procedure
On the day of biopsy, informed consent was obtained. The patient was placed in the supine position. CT scan of the chest demonstrated a 3.3 cm right upper lobe pulmonary mass with an associated small right pleural effusion (see Fig. 1). Moderate sedation was provided with the assistance of trained nursing staff who monitored the patient's level of consciousness and physiologic status throughout the procedure. Subcutaneous 1% lidocaine was administered at the site of puncture for local anesthesia. Utilizing CT guidance, an 18 gauge BioPince (Argon Medical, Frisco, TX) core needle was advanced into the mass. Two core samples were obtained. A Gel-Foam slurry was then injected as the guiding needle was slowly withdrawn. The Gelfoam slurry consisted of pledgets of Gelfoam placed within a 20 mL syringe which was subsequently mixed with 5 mL saline with the use of a 3-way stopcock (see Fig. 2).
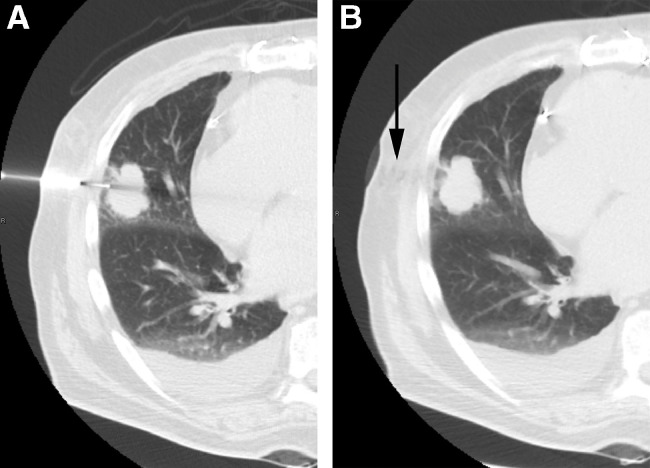
Fig. 1.
Biopsy CT:
(A) Intraprocedure axial CT scan demonstrating trocar needle placement to obtaining 18-guage sample. (B) Postprocedure CT scan demonstrates gelatin sponge slurry within the needle tract (arrow).
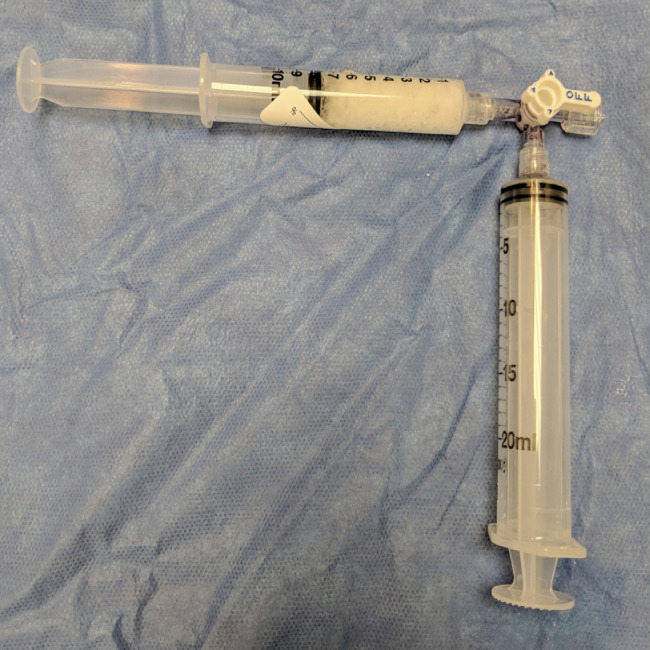
Fig. 2.
Gelfoam slurry preparation:
Gelfoam is torn into small pieces and placed into one syringe. The other syringe is then filled with 5 to 10 mL of normal saline. Both syringes are attached to a three-way stop cock and the slurry is mixed by alternating contents between syringes.
Results
Completion CT scan demonstrated no evidence of hemorrhage or pneumothorax. There was no change in size of a small right pleural effusion. The patient tolerated the procedure well without postprocedure complication. A follow-up chest radiograph obtained 2 hours after biopsy was negative for pneumothorax and the patient was discharged to home. Core needle biopsy tissue was sent to pathology. Histologic findings and immunoprofile were consistent with adenocarcinoma of the lung. The patient later underwent surgical resection of the tumor with postoperative adjuvant chemotherapy. He is currently in remission.
Discussion
The reason we chose GelFoam over other alternatives was availability and low cost. It has very few known contraindications we continue to apply in current lung biopsy cases to mitigate PTX occurrence. This case report is clearly limited in being a single application. It merely shows that it is possible to do so. Our case report focuses on one patient in order to illustrate the application of these findings to clinical practice where necessity spurs us to implement the most readily available and cost effective means while providing the highest level of patient care and safety.
Conclusions
This case report involved a CT-guided lung biopsy in a patient who was at higher risk for post-procedural PTX due to COPD. Recent clinical trials evaluating the efficacy and safety of needle track embolization with gelatinous sludge to reduce the risk of postprocedural morbidities led us to try and implement it. We were able to successfully utilize the gelatinous slurry immediately after taking 2 core samples of a 3.3 cm lung nodule. The patient tolerated the procedure well. There were no complications or pneumothorax. The patient was able to go home after a 2-hour observation.
There is a growing body of research exploring and validating the products available to minimize the risk of a pneumothorax and associated interventions occurring after lung biopsies. Available options include BioSentry, gelatinous foam slurry, autologous blood, and normal saline. All of them have initially shown to have preventative effects and minimal side-effects. Further research directly comparing the available products prospectively to determine which is optimal from a clinical efficacy and cost effectiveness perspective is warranted.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2019.01.009.
Contributor Information
Christopher F. Leopardi, Email: cleopard@student.touro.edu.
Vivek V. Patil, Email: v2patil@gmail.com.
Appendix. Supplementary materials
References
- 1.Winokur R.S., Pua B.B., Sullivan B., Madoff D.C. Percutaneous lung biopsy: technique, efficacy, and complications. Semin Intervent Radiol. 2013;30:121–127. doi: 10.1055/s-0033-1342952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Covey A.M., Gandhi R., Brody L.A., Getrajdman G., Thaler H.T., Brown K.T. Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 2004;15:479–483. doi: 10.1097/01.rvi.0000124951.24134.50. [DOI] [PubMed] [Google Scholar]
- 3.Khan M.F., Straub R., Moghaddam S.R., Maataoui A., Gurung J., Wagner T.O.F. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol. 2008;18:1356–1363. doi: 10.1007/s00330-008-0893-1. [DOI] [PubMed] [Google Scholar]
- 4.Saji H., Nakamura H., Tsuchida T., Tsuboi M., Kawate N., Konaka C. The incidence and the risk of pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy: the angle of the needle trajectory is a novel predictor. Chest. 2002;121:1521–1526. doi: 10.1378/chest.121.5.1521. [DOI] [PubMed] [Google Scholar]
- 5.Wu C.C., Maher M.M., Shepard J-A.O. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. Am J Roentgenol. 2011;196:W678–W682. doi: 10.2214/AJR.10.4659. [DOI] [PubMed] [Google Scholar]
- 6.Yeow K-M., Su I-H., Pan K-T., Tsay P-K., Lui K-W., Cheung Y-C. Risk factors of pneumothorax and bleeding. Chest. 2004;126:748–754. doi: 10.1378/chest.126.3.748. [DOI] [PubMed] [Google Scholar]
- 7.Tran A.A., Brown S.B., Rosenberg J., Hovsepian D.M. Tract embolization with gelatin sponge slurry for prevention of pneumothorax after percutaneous computed tomography-guided lung biopsy. Cardiovasc Intervent Radiol. 2013;37:1546–1553. doi: 10.1007/s00270-013-0823-8. [DOI] [PubMed] [Google Scholar]
- 8.Billich C., Muche R., Brenner G., Schmidt S.A., Krüger S., Brambs H-J. CT-guided lung biopsy: incidence of pneumothorax after instillation of NaCl into the biopsy track. Eur Radiol. 2008;18:1146–1152. doi: 10.1007/s00330-008-0872-6. [DOI] [PubMed] [Google Scholar]
- 9.Cox J.E., Chiles C., McManus C.M., Aquino S.L., Choplin R.H. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology. 1999;212:165–168. doi: 10.1148/radiology.212.1.r99jl33165. [DOI] [PubMed] [Google Scholar]
- 10.Laurent F., Latrabe V., Vergier B., Montaudon M., Vernejoux J-M., Dubrez J. CT-guided transthoracic needle biopsy of pulmonary nodules smaller than 20mm: results with an automated 20-gauge coaxial cutting needle. Clin Radiol. 2000;55:281–287. doi: 10.1053/crad.1999.0368. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka J, Sonomura T, Shioyama Y, Kutsukake Y, Tomita K, Ushimi T, et al. “Oblique path”–the optimal needle path for computed tomography-guided biopsy of small subpleural lesions. Cardiovasc Intervent Radiol n.d.;19:332–4. [PubMed]
- 12.Fish G., Stanley J., Miller K., Schabel S., Sutherland S. Postbiopsy pneumothorax: estimating the risk by chest radiography and pulmonary function tests. Am J Roentgenol. 1988;150:71–74. doi: 10.2214/ajr.150.1.71. [DOI] [PubMed] [Google Scholar]
- 13.Graffy P., Loomis S.B., Pickhardt P.J., Lubner M.G., Kitchin D.R., Lee F.T. Pulmonary intraparenchymal blood patching decreases the rate of pneumothorax-related complications following percutaneous CT-guided needle biopsy. J Vasc Interv Radiol. 2017;28:608–613. doi: 10.1016/j.jvir.2016.12.1217. [DOI] [PubMed] [Google Scholar]
- 14.Baadh A.S., Hoffmann J.C., Fadl A., Danda D., Bhat V.R., Georgiou N. Utilization of the track embolization technique to improve the safety of percutaneous lung biopsy and/or fiducial marker placement. Clin Imaging. 2016;40:1023–1028. doi: 10.1016/j.clinimag.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Ahrar J.U., Gupta S., Ensor J.E., Mahvash A., Sabir S.H., Steele J.R. Efficacy of a self-expanding tract sealant device in the reduction of pneumothorax and chest tube placement rates after percutaneous lung biopsy: a matched controlled study using propensity score analysis. Cardiovasc Intervent Radiol. 2017;40:270–276. doi: 10.1007/s00270-016-1489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaetta J.M., Licht M.O., Fisher J.S., Avelar R.L. A lung biopsy tract plug for reduction of postbiopsy pneumothorax and other complications: results of a prospective, multicenter, randomized, controlled clinical study. J Vasc Interv Radiol. 2010;21:1235–1243. doi: 10.1016/j.jvir.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Jackson C.R., Eavey R.D., Francis D.O. Surgeon Awareness of Operating Room Supply Costs. Ann Otol Rhinol Laryngol. 2016;125:369–377. doi: 10.1177/0003489415614864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.