Abstract
The profile of brain structural dysmorphology of individuals with Alcohol Use Disorders (AUD) involves disruption of the limbic system. In vivo imaging studies report hippocampal volume loss in AUD relative to controls, but only recently has it been possible to articulate different regions of this complex structure. Volumetric analysis of hippocampal regions rather than total hippocampal volume may augment differentiation of disease processes. For example, damage to hippocampal subfield cornu ammonis 1 (CA1) is often reported in Alzheimer's disease (AD), whereas deficits in CA4/dentate gyrus are described in response to stress and trauma. Two previous studies explored the effects of chronic alcohol use on hippocampal subfields: one reported smaller volume of the CA2+3 in alcohol-dependent subjects relative to controls, associated with years of alcohol consumption; the other, smaller volumes of presubiculum, subiculum, and fimbria in alcohol-dependent relative to control men.
The current study, conducted in 24 adults with DSM5-diagnosed AUD (7 women, 53.7 ± 8.8) and 20 controls (7 women, 54.1 ± 9.3), is the first to use FreeSurfer 6.0, which provides state-of-the art hippocampal parcellation, to explore the sensitivity of hippocampal sufields to alcoholism. T1- and T2- images were collected on a GE MR750 system with a 32-channel Nova head coil. FreeSurfer 6.0 hippocampal subfield analysis produced 12 subfields: parasubiculum; presubiculum; subiculum; CA1; CA2+3; CA4; GC-ML-DG (Granule Cell (GC) and Molecular Layer (ML) of the Dentate Gyrus (DG)); molecular layer; hippocampus-amygdala-transition-area (HATA); fimbria; hippocampal tail; hippocampal fissure; and whole volume for left and right hippocampi. A comprehensive battery of neuropsychological tests comprising attention, memory and learning, visuospatial abilities, and executive functions was administered.
Multiple regression analyses of raw volumetric data for each subfields by group, age, sex, hemisphere, and supratentorial volume (svol) showed significant effects of svol (p < .04) on nearly all structures (excluding tail and fissure). Volumes corrected for svol showed effects of age (fimbria, fissure) and group (subiculum, CA1, CA4, GC-ML-DG, HATA, fimbria); CA2+3 showed a diagnosis-by-age interaction indicating older AUD individuals had a smaller volume than would be expected for their age. There were no selective relations between hippocampal subfields and performance on neuropsychological tests, likely due to lack of statistical power.
The current results concur with the previous study identifying CA2+3 as sensitive to alcoholism, extend them by identifying an alcoholism-age interaction, and suggest an imaging phenotype distinguishing AUD from AD and stress/trauma.
Keywords: Alcohol Use Disorder (AUD), Hippocampus, Hippocampal subregions, Hippocampal subfields, Attention, Learning and memory, Visuospatial, Executive functions
Highlights
-
•
Whether alcohol use disorders (AUD) compromise hippocampal volume is disputed.
-
•
A 32-channel head coil acquired high-resolution images.
-
•
The hippocampus was segmented using FreeSurfer 6.0.
-
•
Several subregions showed volume deficits in AUD relative to healthy controls.
-
•
Cornu Ammonis 2+3 showed a alcoholism-by-age interaction.
1. Introduction
A number of in vivo imaging studies have now reported hippocampal volume loss in Alcohol Use Disorders (AUD) relative to controls (Beresford et al., 2006; Cardenas et al., 2007; Kurth et al., 2004; Le Berre et al., 2014; Sullivan et al., 1995; Wilhelm et al., 2008). A recent meta-analysis of 23 studies suggests that problematic alcohol use is associated with significantly smaller hippocampal volume (Wilson et al., 2017); correlations between gray-matter volumes and memory impairments, however, have not always been forthcoming (cf., Pitel et al., 2014). One explanation for the lack of structure/function relations may be that hippocampal subregions have unique cellular and molecular compositions and distinct connectivity profiles that contribute to their differential roles in cognitive and disease processes (Small et al., 2011), often overlooked when examining the structure as a whole (Poppenk and Moscovitch, 2011).
Postmortem histological evaluation of the hippocampus reveals its heterogeneity and complexity. Functionally specialized but tightly interconnected subfields of the hippocampus – the subiculum (subdivided into parasubiculum, presubiculum, and subiculum proper), the Cornu Ammonis (CA) sectors 1–4, and dentate gyrus – play unique roles learning and memory. Thus, improved in vivo MRI-based delineation of hippocampal subregions may permit selective differentiation of hippocampal-based neurocognitive processes in health (Middlebrooks et al., 2017) and disease (Small, 2014). Available evidence suggests that different neurodegenerative disorders affect selective hippocampal subfield volumes: CA1 is frequently reported as atrophied in Mild Cognitive Impairment (MCI) and Alzheimer's disease (AD) (Braak and Braak, 1997; Khan et al., 2015; Saito and Murayama, 2007; Shim et al., 2017; West et al., 2004), but not in other forms of dementia (e.g., Dementia with Lewy Bodies) (Li et al., 2016; Mak et al., 2016). Relative to controls: CA4/dentate gyrus volume is compromised in trauma and post-traumatic stress disorder (Aas et al., 2014; Boen et al., 2014; Hayes et al., 2017; Teicher et al., 2012); significant volume deficits are observed in subfields CA1 and CA4/dentate in patients with hippocampal sclerosis due to temporal lobe epilepsy (Sone et al., 2016); presubiculum is smaller in patients with schizophrenia (Haukvik et al., 2015); and smaller volumes of CA2+3 are reported in Parkinson's disease (PD), prior to treatment (Gyorfi et al., 2017).
In AUD individuals relative to controls, a postmortem subregional analysis suggested lower neuronal counts in CA1–4 regions and dentate gyrus (Bengochea and Gonzalo, 1990). A study using unbiased stereological techniques, however, found that hippocampal volume compromise in AUD was not attributable to neuronal, but white matter loss (Harding et al., 1997). Two previous studies have explored the effects of chronic alcohol use on MRI-based hippocampal subfield delineation. The first included scans at 2 time points (immediately and 2 weeks after withdrawal; T1-weighted magnetization prepared rapid gradient echo (MP-RAGE) collected on a 12-channel head coil) in 42 alcohol-dependent (diagnosed via Structured Clinical Interview for Diagnostic Statistical Manual (DSM)-III-R (SCID)) and 32 healthy control participants. and reported that CA2+3 volume, segmented using FreeSurfer 5.2, was smaller in alcohol-dependent individuals than controls, and was associated with years of alcohol consumption; volume of CA2+3, however, normalized with 2 weeks of abstinence (Kuhn et al., 2014). The more recent report using T1-weighted MP-RAGE images collected on a 32-channel head coil included 26 alcohol-dependent (diagnosed via SCI DSM-IV-TR and abstinent for 3–60 months) and 26 healthy men. Hippocampus segmentation into 7 subfields using FreeSurfer 5.3.0 showed that presubiculum, subiculum, and fimbria volumes were smaller in alcohol-dependent relative to control men (Lee et al., 2016).
It has been asserted that T2-weighted images outperform T1-weighted approaches for successful hippocampal subfield segmentation (Mueller et al., 2018). Furthermore, FreeSurfer 6.0, with automated segmentation of hippocampal subfields based on a statistical atlas of an ultra-high resolution ex vivo MRI data set (Iglesias et al., 2015) solves a number of limitations of the hippocampal atlas distributed with earlier (5.1–5.3) versions of FreeSurfer. Based on intensity models learned directly from the individual scan to be segmented, parcellation is less dependent on magnet type and sequence (Wisse et al., 2014) and more accurately reflects postmortem histological delineation of hippocampal subregions (Harding et al., 1998; Simic et al., 1997).
The current study used a combination of T1 and T2-weighted approaches for hippocampal segmentation using FreeSurfer 6.0 in a 44 adults, 24 with AUD as per DSM5 criteria. It was hypothesized that CA2+3 would be smaller in individuals with AUD relative to controls (Kuhn et al., 2014). Given that alcoholism is conceptualized as a stress surfeit disorder (Koob, 2013; Koob et al., 2014), it was also hypothesized that CA4/dentate gyrus would show shrinkage because of its role in stress (Cole et al., 2010; Gold et al., 2010; Teicher et al., 2012; Wang et al., 2010). Based on a recent report in healthy aging (Zheng et al., 2018), it was additionally hypothesized that in the AUD group only, smaller CA2+3 and CA4 volume would be related to worse memory performance, specifically on tests of delayed recall (i.e., California Verbal Learning Test (CVLT) short and long delay free recall). Because age-related memory decline focused on hippocampal integrity is an active area of investigation (cf., Comper et al., 2017; Mueller et al., 2012), all statistics considered age, in addition to diagnosis, with the hypothesis that a region with a diagnosis-by-age interaction would be likely to correlate with compromised memory performance.
2. Methods
2.1. Participants
The Institutional Review Boards of Stanford University and SRI International approved this study. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki by the signing of consent documents in the presence of staff. Subjects were 24 alcoholics (17 male/7 female) and 20 controls (13 male/7 female) ages 39–74 years (control 54.1 ± 9.3, AUD 53.7 ± 8.8). AUD participants were referred from local outpatient and treatment centers, or recruited during presentations in clinics by project staff and by distribution of flyers at community events. Healthy, control participants were recruited from the local community by referrals and flyers.
All participants were screened with the Structured Clinical Interview for DSM5 (First, 2000), structured health questionnaires, a semi-structured timeline follow-back interview to quantify lifetime alcohol consumption (Skinner, 1982; Skinner and Sheu, 1982), and were given the Clinical Institute Withdrawal Assessment for Alcohol (CIWA). Upon initial assessment, subjects were excluded if they had a significant history of medical (e.g., epilepsy, stroke, multiple sclerosis, uncontrolled diabetes, or loss of consciousness >30 min), psychiatric (i.e., schizophrenia or bipolar I disorder), or neurological disorders (e.g., neurodegenerative disease). Table 1 presents demographic characteristics of the two groups, including, for example, body mass index and scores on the Beck Depression Index (BDI).
Table 1.
Characteristics of the study groups: mean ± SD/frequency count.
| Control (n = 20) | AUD (n = 24) | p-Value⁎ | |
|---|---|---|---|
| N (men/women) | 13/7 | 17/7 | .68 |
| Age (years) | 54.1 ± 9.3 | 53.7 ± 8.8 | .90 |
| Handedness (Right/Left) | 19/1 | 20/4 | .23 |
| Ethnicitya (Caucasian/African American/Asian) | 8/5/7 | 14/9/1 | .03 |
| Body Mass Index | 25.0 ± 3.6 | 28.9 ± 5.7 | .01 |
| Education (years) | 16.2 ± 2.3 | 13.0 ± 1.8 | .0001 |
| Socioeconomic Statusb | 25.4 ± 15.7 | 44.5 ± 13.2 | .0001 |
| Beck Depression Index (BDI) | 5.2 ± 5.2 | 14.7 ± 10.0 | .001 |
| WTAR IQ (predicted full scale) | 102.3 ± 11.2 | 96.3 ± 10.2 | .08 |
| AUD onset age | – | 21.7 ± 6.6 | n.a. |
| Days since last drink | – | 105.7 ± 94.5 | n.a. |
| Lifetime alcohol consumption (kg) | 53.6 ± 81.2 | 1798.3 ± 1638.0 | <.0001 |
| Alcohol consumption years | – | 32.0 ± 9.6 | n.a. |
| CIWA score | – | 23.2 ± 17.7 | n.a. |
| Smoker (never/past/current) | 18/0/2 | 4/6/14 | .0001 |
| Nicotine (daily) | 1.1 ± 3.3 | 4.6 ± 3.9 | .008 |
t-tests used on continuous variables (e.g., age); χ2 used on nominal variables (e.g., handedness).
Self-defined.
Lower score = higher status.
Bold are statistically significant.
Laboratory evaluation identified 2 controls and 4 AUD subjects to be seropositive for hepatitis C virus (HCV) infection. DSM5-diagnosed past substance use disorders (SUD) among the AUD group with severity ranging from mild to severe included 1 women and 10 men for cannabis, 10 men for stimulants, 10 men for cocaine, 1 women and 5 men for opiates, and 2 men for hallucinogens.
Additional data collected for each participant included a blood draw and a comprehensive neuropsychological test battery. Blood samples (~40 cc) were collected and analyzed by Quest Diagnostics for complete blood count with differential, comprehensive metabolic panel, and human immunodeficiency virus (HIV) and hepatitis C (HCV) screening. Participants also completed a comprehensive neuropsychological battery to assess attention, memory and learning, visuospatial abilities, and executive functions. Raw test scores were statistically corrected for age on the control group [mean and standard deviation for control group = 0 ± 1], allowing averaging across tests. Composite scores were then calculated as the mean of all Z-scores of tests comprising each of the functional domains.
Attention:
-
-
Wechsler Memory Scale-Revised (WMS-R) block forward total
-
-
WMS-R block forward span
Memory and learning:
-
-
Wechsler Adult Intelligence Scale (WAIS) digit symbol incidental recall of symbols
-
-
WAIS digit symbol incidental recall of numbers
-
-
California Verbal Learning Test (CVLT) short delay free recall
-
-
CVLT long delay free recall
-
-
Montreal Cognitive Assessment (MOCA) delayed recall
-
-
WMS-R logical memory story A raw score
-
-
WMS-R logical memory story B raw score
Visuospatial abilities:
-
-
Rey-Osterrieth copy raw score
-
-
WMS-R visual reproduction item 1 raw score
-
-
WMS-R visual reproduction item 2 raw score
Executive functions:
-
-
Controlled Oral Word Association Test (F + A + S total)
-
-
Semantic fluency (inanimate objects, animals)
-
-
WAIS digit symbol total time to complete set
-
-
WAIS digit symbol standard score at 90s
-
-
MOCA abstraction score
2.2. MRI acquisition and analysis
Scanning was performed on a GE MR750 system (General Electric Healthcare, Waukesha, WI) with a 32-channel Nova head coil. T1- and T2- weighted scans were collected. T1-weighted scans were cerebral spinal fluid (CSF)-nulled MPRAGE (3D spoiled gradient echo with an inversion pulse) with prospective motion correction (PROMO, repetition time (TR)/echo time (TE)/inversion time (TI)/time between inversions (TS) = 8 ms/3.5 ms/1100 ms/3.0 s, flip angle = 9, filed of view (FOV) = 18 cm, 200 × 200 matrix, 1 mm thickness, 210 slices). T2-weighted scans were 3D fast spin echo with variable refocusing flip angle (T2 Cube) and PROMO (TR/TE: 3500 ms/62 ms, echo train length 84, FOV = 18 cm, 224 × 224 matrix, 1 mm thickness, 210 slices).
T1- and T2- weighted images from each subject were first processed via an established pipeline (Pfefferbaum et al., 2018). The pipeline affinely aligned T2-weighted to the T1-weighted images via FLIRT epi_reg (FSL V5.0.6) (Jenkinson et al., 2002; Jenkinson and Smith, 2001) and then denoised, corrected for image inhomogeneity, performed skull striping, and segmented both scans. Following initial analysis, the processed and aligned T1- and T2-weighted images were given as input, and hippocampal subfield segmentation was generated via the FreeSurfer 6.0 hippocampal subfields module (Iglesias et al., 2015). An estimate of supratentorial brain volume was computed using the SRI24 atlas (Rohlfing et al., 2008) in the same pipeline.
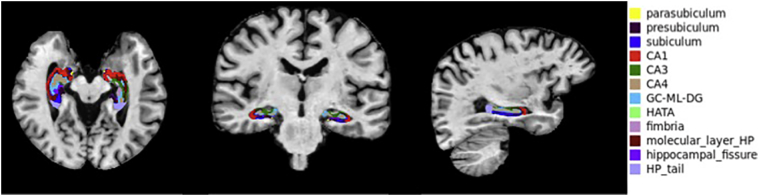
Hippocampal subfield analysis produced 12 subfields: parasubiculum; presubiculum; subiculum; Cornu Ammonis (CA) 1; CA3; CA4; GC-ML-DG (Granule Cell (GC) and Molecular Layer (ML) of the Dentate Gyrus (DG)); molecular layer of hippocampus; hippocampus-amygdala-transition-area (HATA); fimbria; hippocampal tail; and hippocampal fissure, for left and right hippocampi (Fig. 1).
Fig. 1.
Axial, coronal, and sagittal slices through the hippocampus demonstrating parcellation of a fully processed set of images from a 65 year-old control man. Subfields are color-coded.
2.3. Statistical analysis
Demographic analysis used Chi-square tests for categorical variables and t-tests for continuous variables. Subregion analysis initially included a multiple regression for each volume by diagnosis, age, sex, hemisphere, and supratentorial volume (svol). This analysis showed significant effects of svol (p < .04) on nearly all structures (Supplementary Table 1). Thus, data were corrected for svol: each participant's data were entered into a linear regression (lm in R with subfield as a function of supratentorial volume (i.e., svol)); then, the average subfield volume for the whole data set was added to the lm residuals to preserve volume scale. This procedure essentially removed sex effects (women having smaller brains and smaller hippocampi than men). Subfield volumes corrected for svol were then entered into multiple regressions including diagnosis, age, sex, hemisphere, and diagnosis-by-age interaction (Table 2). Two-group t-tests evaluated differences in DSM5 scores, blood analytes, and behavioral performance. In the AUD group only, nonparametric Spearman's ρ evaluated relations between volumes of hippocampal subfields and clinical and cognitive measures.
Table 2.
Multiple regressionsa on svol-corrected data.
| Model r2 | Model p-value | Significant variable(s) | t-Ratio | p-Value | |
|---|---|---|---|---|---|
| Parasubiculum | 0.03 | n.s. | n.a. | ||
| Presubiculum | 0.07 | n.s. | n.a. | ||
| Subiculum | 0.18 | .007 | Diagnosis | −3.8 | .0003 |
| CA1 | 0.13 | .04 | Diagnosis | −3.0 | .004 |
| CA2+3 | 0.12 | .07 | Diagnosis*age | −2.2 | .03 |
| CA4 | 0.15 | .02 | Diagnosis | −2.7 | .008 |
| GC-ML-DG | 0.22 | .001 | Diagnosis | −3.5 | .0007 |
| Age | −2.5 | .02 | |||
| Molecular layer | 0.11 | .08 | Diagnosis | −2.3 | .02 |
| HATA | 0.15 | .02 | Diagnosis | −2.8 | .006 |
| Age | −2.2 | .03 | |||
| Fimbria | 0.32 | <.0001 | Diagnosis | −4.0 | .0001 |
| Sex | 2.1 | .04 | |||
| Age | −4.3 | <.0001 | |||
| Hippocampal tail | 0.04 | n.s. | n.a. | ||
| Hippocampal fissure | 0.25 | .0002 | Age | 4.9 | <.0001 |
| Whole hippocampus | 0.17 | .009 | Diagnosis | −3.4 | .001 |
diagnosis, age, sex, hemisphere, diagnosis*age
3. Results
As expected, the total lifetime alcohol consumption of the AUD group (1798.3 kg) was far greater than that of the controls (53.6 kg). Further, the AUD group had fewer years of education, lower socioeconomic status, and lower scores on the Wechsler Test of Adult Reading (WTAR), which yields a full-scale premorbid IQ estimate, than controls (Table 1).
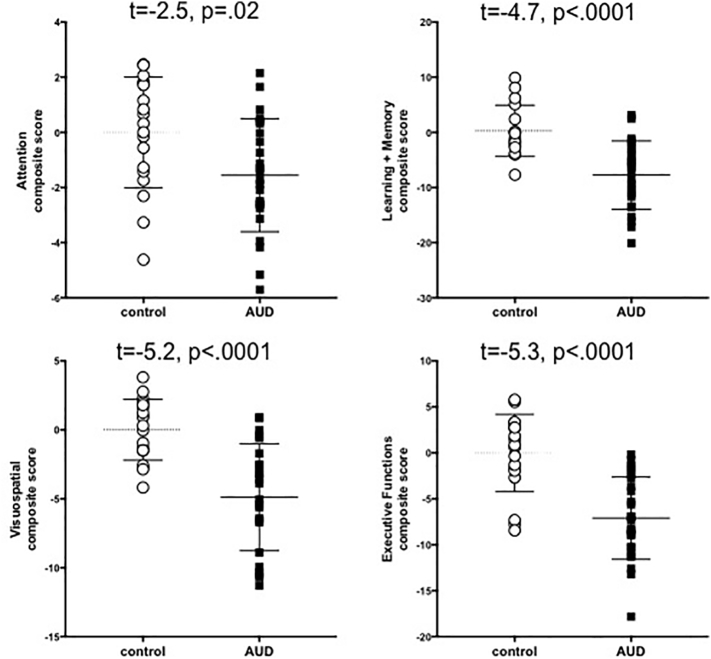
The AUD group performed worse than controls on all 4 neuropsychological composite scores: attention (t = −2.5, p = .02), learning and memory (t = −4.7, p < .0001), visuospatial abilities (t = −5.2, p < .0001), and executive functions (t = −5.3, p < .0001) (Fig. 2).
Fig. 2.
Scatterplots of control (white circles) and AUD (black square) performance on neuropsychological composite scores; mean and SD indicated by lines.
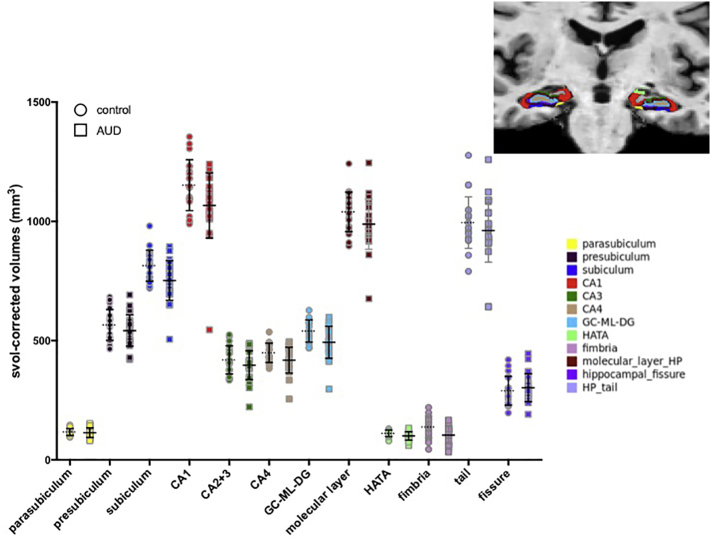
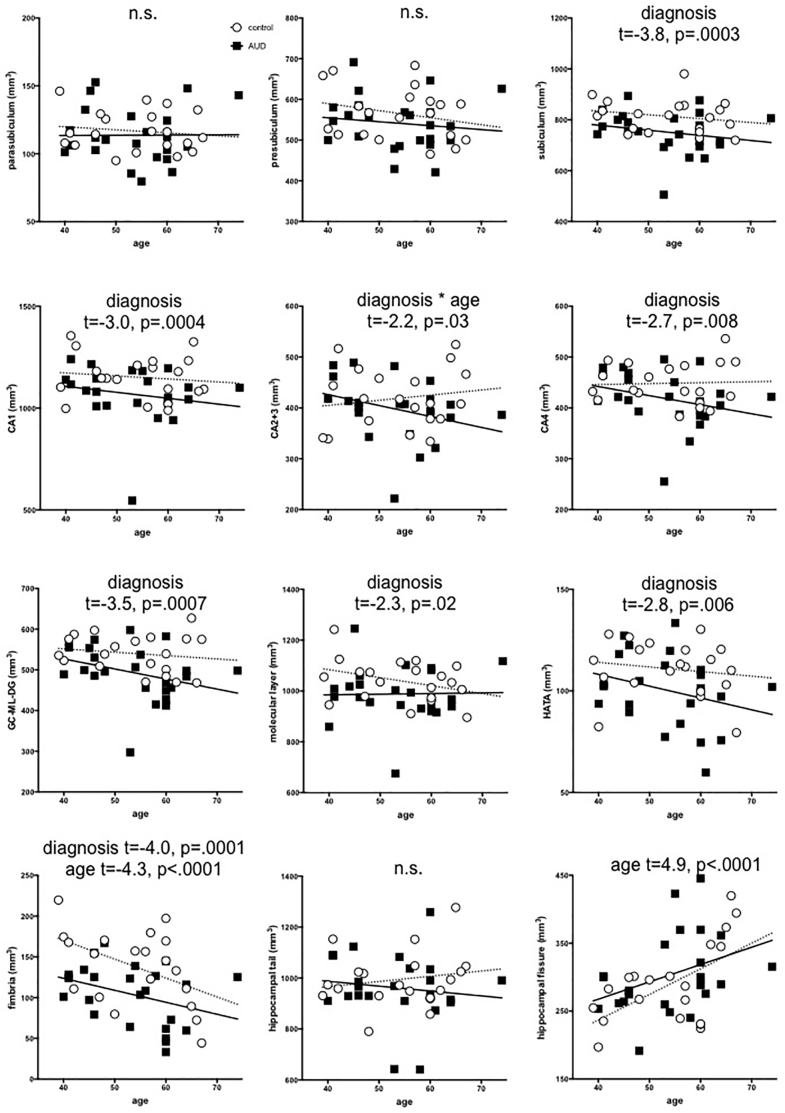
Fig. 3 presents scatter plots of each region by diagnosis to permit estimation of relative size of each left + right subfield. Fig. 4 presents diagnosis-by-age plots for each bilateral hippocampal subfield. Using a directional family-wise Bonferroni correction of p ≤ .008, the multiple regression (i.e., diagnosis, age, sex, hemisphere, and diagnosis-by-age interaction) on svol-corrected hippocampal subfields demonstrated that fimbria was smaller (p < .0001) and the fissure was bigger (p < .0001) with older age; the fimbria showed nominal effects of sex (p = .04, smaller in men); and six subfields were smaller in AUD than healthy controls: subiculum (p = .0003), CA1 (p = .004), CA4 (p = .008), GC-ML-DG (p = .0007), HATA (p = .006), and fimbria (p = .0001). CA2+3 showed diagnosis-by-age interactions (p = .03) such that older AUD participants had a smaller volume than would be expected for their age.
Fig. 3.
Scatterplots of control (circles) and AUD (squares) svol-corrected volumes for each hippocampal subfield; mean and SD indicated by lines. Each region is color-coded according to segmentation shown in inset.
Fig. 4.
Age-by-diagnosis plots of each svol-corrected and left + right summed hippocampal subfield for controls (white circles) and AUD participants (black squares).
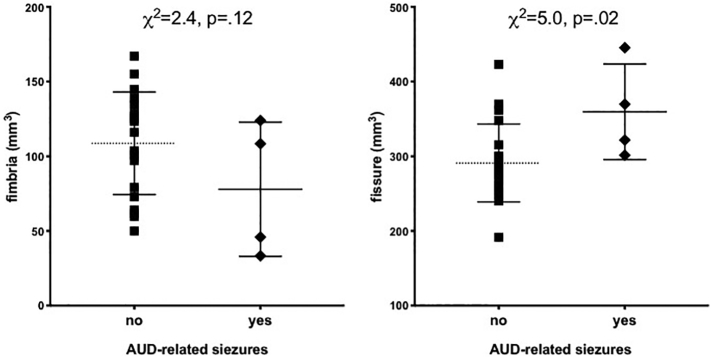
Although evaluated, there were no significant correlations between volumes of hippocampal subregions and any of the demographic, alcohol-related, SUD-related, or behavioral variables distinguishing the two groups (including HCV status, BDI scores, CIWA scores, number of alcohol-related seizures, etc.). Supplementary Fig. 1 shows preliminary evidence that hippocampal subfields (i.e., fimbria and fissure) are selectively compromised by AUD-related seizures. In 4 alcoholics that reported AUD-related seizures, the hippocampal fimbria tended to be smaller (p = .12), and the hippocampal fissure (a CSF-filled space) was larger (p = .02) than in alcoholics reporting to seizures. Supplementary Fig. 2 suggests that in alcoholics, better performance on the WMS-r visual recognition 2 raw score is associated with a larger hippocampal fimbria volume (p = .09).
Supplementary Fig. 1.
Volumes of hippocampal fimbria and fissure (a CSF-filled space) as a function of AUD-related seizures (no – self-report of no seizures; yes – self-report of AUD-related seizures).
Supplementary Fig. 2.
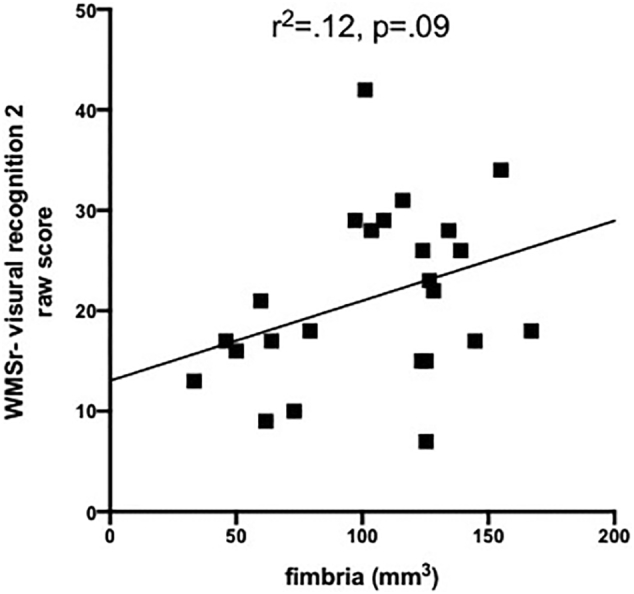
Correlation between fimbria volume and performance on WMSr-visual recognition 2 raw score.
4. Discussion
This study identified hippocampal subfields sensitive to age (fimbria and fissure), AUD (CA1, CA4, GC-ML-DG, HATA, and fimbria), and their interaction (CA2+3). Older age was associated with a smaller fimbria and a larger fissure. Although a number of studies provide evidence for hippocampal subfield volume compromise with older age, results are inconsistent (Daugherty et al., 2016; Kurth et al., 2017; Malykhin et al., 2017; Zheng et al., 2019). Reports include associations between older age and smaller volumes of subiculum and dentate gyrus (Malykhin et al., 2017); cornu ammonis, subiculum, and HATA, but not entorhinal cortex (Kurth et al., 2017); and all subfields, but not parasubiculum (Zheng et al., 2018). Such disparate observations suggest that more work is required in this area to permit differentiation between normal and pathological aging.
The fimbria showed nominal effects of sex (i.e., not statistically significant): despite svol correction, men had smaller volumes than women. Hippocampal subfields are influenced by both age and sex during early childhood (Riggins et al., 2018). In a study including individuals aged 4–22 years, subfield volumes CA2/3, CA4/DG, presubiculum, subiculum, and CA1 (but not fimbria and hippocampal fissure) were bigger in boys than girls, driven by participants under 13 years of age (Krogsrud et al., 2014). In a similar study of individuals aged 8–26 years, no sex differences were found in hippocampal subregion volumes (Tamnes et al., 2018). In older individuals (aged 18–85 years), subiculum had a stronger association with age in men than women (Malykhin et al., 2017). As with the aging findings, research using consistent methods for hippocampal segmentation might help resolve sexually dimorphic differences in hippocampal subfield volumes.
Whole hippocampus volume deficits in AUD men and women have now been reported by a number of neuroimaging groups (e.g., Beresford et al., 2006; De Bellis et al., 2000; Durazzo et al., 2011; Le Berre et al., 2014; Makris et al., 2008). In the current study, CA1, CA4, GC-ML-DG, HATA, and fimbria were smaller in AUD relative to controls, and CA2+3 showed an age-by-alcoholism interaction. Hippocampal subfield vulnerability in AUD has previously been supported by a controlled, longitudinal study of alcoholics soon after withdrawal and then again after 2 weeks of sobriety (Kuhn et al., 2014). Significant volume deficits specific to CA2+3 observed at the initial MRI resolved to control levels 2 weeks later. Greater CA2+3 volume deficits were related to more severe withdrawal symptoms and duration of drinking. Although both the Kuhn study and the current results highlight the relevance of CA2+3 to chronic alcoholism, the time points of evaluation and the results are in fact different: whereas Kuhn et al., 2014 report reversibility of the volume deficit with 2 weeks of abstinence, the alcoholics included in the current study were sober for a minimum of 3 months (i.e., 105.7 ± 94.5 days since last drink). It is important to note that the statistics used in the two studies were different: the Kuhn et al., 2014 study was longitudinal and demonstrated a group-by-time interaction for the CA2+3 subfield, while the present results show a group-by-age interaction. Alternatively, the discrepancy might be due to the limited accuracy of hippocampal segmentation in the Kuhn et al., 2014 study due to low, 1 mm3 spatial resolution of their acquired images and the use of Freesurfer 5.2 for hippocampal segmentation, which had several limitations (e.g., poor resolution of atlas images, poor translation with histological studies Iglesias et al., 2015; Mueller et al., 2018; Schoene-Bake et al., 2014).
The only other in vivo hippocampal subfield analysis, conducted in AUD participants abstinent from alcohol for 3–60 months, was cross-sectional and found significant volume deficits in AUD relative to controls in the left and right subiculum, left presubiculum, and fimbria in addition to the whole hippocampus (Lee et al., 2016). Consistent with the latter study, the current results demonstrate that several hippocampal subregions including subiculum and fimbria were smaller in AUD than controls.
A postmortem study reporting hippocampal volume deficits without neuronal loss implicated hippocampal white matter as the target of alcoholism (Harding et al., 1997). Other factors potentially contributing to alcoholism-related hippocampal volume reductions are glial cell loss (Korbo, 1999), synaptic loss (Freund and Ballinger, 1991), or impeded incorporation of newly formed neurons (He et al., 2005; Le Maitre et al., 2018; Nixon and Crews, 2004). However, considering the report of substantial hippocampal volume recovery within 2 weeks of abstinence (cf., Kuhn et al., 2014) and other support for considerable brain recovery in sobriety (Sullivan and Pfefferbaum, 2005), tissue volume expansion during abstinence from alcoholism is unlikely due to neuronal regeneration (cf., Rakic, 2002).
In contrast to the reported correlation between smaller CA2+3 and duration of regular alcohol consumption (Kuhn et al., 2014), the present study revealed no correlations between hippocampal subregion volumes and demographic, alcohol-related, or neuropsychological performance variables (cf, Lee et al., 2016). Indeed, none of the quantified volumes significantly correlated with relevant measures. This is likely due to the small sample size of the current study. In 5035 dementia- and stroke-free persons from the Rotterdam Study, aged over 45 years, smaller volumes of the fimbria, presubiculum, and subiculum showed strong associations with poor performance on several cognitive domains, including executive but excluding memory functions (Evans et al., 2018). These results are encouraging as preliminary evidence from this study (i.e., Supplementary Figs. 1 and 2) suggests that the fimbria is sensitive to AUD-related seizures and visual recognition (i.e., but not memory).
5. Conclusions
To summarize, the present results provide initial evidence of hippocampal subfields sensitive to age, alcoholism, and their interaction and suggest that CA2+3 can potentially distinguish AUD from AD. A larger sample studied longitudinally may help reveal functions related to hippocampal subfield volumes.
Funding
This work was supported by grants from the National Institute of Alcohol Abuse and Alcoholism R21 AA023582; R01 AA005965; R37 AA010723; U01 AA013521; and National Institute of Mental Health grant R01 MH113406.
Declarations of interest
None.
The following are the supplementary data related to this article.
Multiple regressions* on raw data.
References
- Aas M., Haukvik U.K., Djurovic S., Tesli M., Athanasiu L., Bjella T., Hansson L., Cattaneo A., Agartz I., Andreassen O.A., Melle I. Interplay between childhood trauma and BDNF val66met variants on blood BDNF mRNA levels and on hippocampus subfields volumes in schizophrenia spectrum and bipolar disorders. J. Psychiatr. Res. 2014;59:14–21. doi: 10.1016/j.jpsychires.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Bengochea O., Gonzalo L.M. Effect of chronic alcoholism on the human hippocampus. Histol. Histopathol. 1990;5:349–357. [PubMed] [Google Scholar]
- Beresford T.P., Arciniegas D.B., Alfers J., Clapp L., Martin B., Du Y., Liu D., Shen D., Davatzikos C. Hippocampus volume loss due to chronic heavy drinking. Alcohol. Clin. Exp. Res. 2006;30:1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Boen E., Westlye L.T., Elvsashagen T., Hummelen B., Hol P.K., Boye B., Andersson S., Karterud S., Malt U.F. Smaller stress-sensitive hippocampal subfields in women with borderline personality disorder without posttraumatic stress disorder. J. Psychiatry Neurosci. 2014;39:127–134. doi: 10.1503/jpn.130070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak E., Braak H. Alzheimer's disease: transiently developing dendritic changes in pyramidal cells of sector CA1 of the Ammon's horn. Acta Neuropathol. 1997;93:323–325. doi: 10.1007/s004010050622. [DOI] [PubMed] [Google Scholar]
- Cardenas V.A., Studholme C., Gazdzinski S., Durazzo T.C., Meyerhoff D.J. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J., Toga A.W., Hojatkashani C., Thompson P., Costafreda S.G., Cleare A.J., Williams S.C., Bullmore E.T., Scott J.L., Mitterschiffthaler M.T., Walsh N.D., Donaldson C., Mirza M., Marquand A., Nosarti C., McGuffin P., Fu C.H. Subregional hippocampal deformations in major depressive disorder. J. Affect. Disord. 2010;126:272–277. doi: 10.1016/j.jad.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comper S.M., Jardim A.P., Corso J.T., Gaca L.B., Noffs M.H.S., Lancellotti C.L.P., Cavalheiro E.A., Centeno R.S., Yacubian E.M.T. Impact of hippocampal subfield histopathology in episodic memory impairment in mesial temporal lobe epilepsy and hippocampal sclerosis. Epilepsy Behav. 2017;75:183–189. doi: 10.1016/j.yebeh.2017.08.013. [DOI] [PubMed] [Google Scholar]
- Daugherty A.M., Bender A.R., Raz N., Ofen N. Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus. 2016;26:220–228. doi: 10.1002/hipo.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M.D., Clark D.B., Beers S.R., Soloff P.H., Boring A.M., Hall J., Kersh A., Keshavan M.S. Hippocampal volume in adolescent-onset alcohol use disorders. Am. J. Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Durazzo T.C., Tosun D., Buckley S., Gazdzinski S., Mon A., Fryer S.L., Meyerhoff D.J. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol. Clin. Exp. Res. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T.E., Adams H.H.H., Licher S., Wolters F.J., van der Lugt A., Ikram M.K., O'Sullivan M.J., Vernooij M.W., Ikram M.A. Subregional volumes of the hippocampus in relation to cognitive function and risk of dementia. Neuroimage. 2018;178:129–135. doi: 10.1016/j.neuroimage.2018.05.041. [DOI] [PubMed] [Google Scholar]
- First M. Fourth edition. American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. Text Revision (DSM-IV-TR) [Google Scholar]
- Freund G., Ballinger W.E. Loss of synaptic receptors can precede morphologic changes induced by alcoholism. Alcohol Alcohol. Suppl. 1991;1:385–391. [PubMed] [Google Scholar]
- Gold S.M., Kern K.C., O'Connor M.F., Montag M.J., Kim A., Yoo Y.S., Giesser B.S., Sicotte N.L. Smaller cornu ammonis 2-3/dentate gyrus volumes and elevated cortisol in multiple sclerosis patients with depressive symptoms. Biol. Psychiatry. 2010;68:553–559. doi: 10.1016/j.biopsych.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorfi O., Nagy H., Bokor M., Moustafa A.A., Rosenzweig I., Kelemen O., Keri S. Reduced CA2-CA3 hippocampal subfield volume is related to depression and normalized by l-DOPA in newly diagnosed Parkinson's disease. Front. Neurol. 2017;8:84. doi: 10.3389/fneur.2017.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A.J., Wong A., Svoboda M., Kril J.J., Halliday G.M. Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus. 1997;7:78–87. doi: 10.1002/(SICI)1098-1063(1997)7:1<78::AID-HIPO8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Harding A.J., Halliday G.M., Kril J.J. Variation in hippocampal neuron number with age and brain volume. Cereb. Cortex. 1998;8:710–718. doi: 10.1093/cercor/8.8.710. [DOI] [PubMed] [Google Scholar]
- Haukvik U.K., Westlye L.T., Morch-Johnsen L., Jorgensen K.N., Lange E.H., Dale A.M., Melle I., Andreassen O.A., Agartz I. In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biol. Psychiatry. 2015;77:581–588. doi: 10.1016/j.biopsych.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Hayes J.P., Hayes S., Miller D.R., Lafleche G., Logue M.W., Verfaellie M. Automated measurement of hippocampal subfields in PTSD: evidence for smaller dentate gyrus volume. J. Psychiatr. Res. 2017;95:247–252. doi: 10.1016/j.jpsychires.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Nixon K., Shetty A.K., Crews F.T. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur. J. Neurosci. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Iglesias J.E., Augustinack J.C., Nguyen K., Player C.M., Player A., Wright M., Roy N., Frosch M.P., McKee A.C., Wald L.L., Fischl B., Van Leemput K., Alzheimer's Disease Neuroimaging Initiative A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Khan W., Westman E., Jones N., Wahlund L.O., Mecocci P., Vellas B., Tsolaki M., Kloszewska I., Soininen H., Spenger C., Lovestone S., Muehlboeck J.S., Simmons A., AddNeuroMed consortium and for the Alzheimer’s Disease Neuroimaging Initiative Automated hippocampal subfield measures as predictors of conversion from mild cognitive impairment to alzheimer's disease in two independent cohorts. Brain Topogr. 2015;28:746–759. doi: 10.1007/s10548-014-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Buck C.L., Cohen A., Edwards S., Park P.E., Schlosburg J.E., Schmeichel B., Vendruscolo L.F., Wade C.L., Whitfield T.W., Jr., George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76 Pt B:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbo L. Glial cell loss in the hippocampus of alcoholics. Alcohol. Clin. Exp. Res. 1999;23:164–168. [PubMed] [Google Scholar]
- Krogsrud S.K., Tamnes C.K., Fjell A.M., Amlien I., Grydeland H., Sulutvedt U., Due-Tonnessen P., Bjornerud A., Solsnes A.E., Haberg A.K., Skrane J., Walhovd K.B. Development of hippocampal subfield volumes from 4 to 22 years. Hum. Brain Mapp. 2014;35:5646–5657. doi: 10.1002/hbm.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S., Charlet K., Schubert F., Kiefer F., Zimmermann P., Heinz A., Gallinat J. Plasticity of hippocampal subfield volume cornu ammonis 2+3 over the course of withdrawal in patients with alcohol dependence. JAMA Psychiatry. 2014;71:806–811. doi: 10.1001/jamapsychiatry.2014.352. [DOI] [PubMed] [Google Scholar]
- Kurth C., Wegerer V., Reulbach U., Lewczuk P., Kornhuber J., Steinhoff B.J., Bleich S. Analysis of hippocampal atrophy in alcoholic patients by a Kohonen feature map. Neuroreport. 2004;15:367–371. doi: 10.1097/00001756-200402090-00031. [DOI] [PubMed] [Google Scholar]
- Kurth F., Cherbuin N., Luders E. The impact of aging on subregions of the hippocampal complex in healthy adults. Neuroimage. 2017;163:296–300. doi: 10.1016/j.neuroimage.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Le Berre A.P., Pitel A.L., Chanraud S., Beaunieux H., Eustache F., Martinot J.L., Reynaud M., Martelli C., Rohlfing T., Sullivan E.V., Pfefferbaum A. Chronic alcohol consumption and its effect on nodes of frontocerebellar and limbic circuitry: comparison of effects in France and the United States. Hum. Brain Mapp. 2014;35:4635–4653. doi: 10.1002/hbm.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre T.W., Dhanabalan G., Bogdanovic N., Alkass K., Druid H. Effects of alcohol abuse on proliferating cells, stem/progenitor cells, and immature neurons in the adult human Hippocampus. Neuropsychopharmacology. 2018;43:690–699. doi: 10.1038/npp.2017.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Im S.J., Lee S.G., Stadlin A., Son J.W., Shin C.J., Ju G., Lee S.I., Kim S. Volume of hippocampal subfields in patients with alcohol dependence. Psychiatry Res. Neuroimaging. 2016;258:16–22. doi: 10.1016/j.pscychresns.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Li X., Li D., Li Q., Li Y., Li K., Li S., Han Y. Hippocampal subfield volumetry in patients with subcortical vascular mild cognitive impairment. Sci. Rep. 2016;6 doi: 10.1038/srep20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak E., Su L., Williams G.B., Watson R., Firbank M., Blamire A., O'Brien J. Differential atrophy of hippocampal subfields: a comparative study of dementia with Lewy bodies and Alzheimer Disease. Am. J. Geriatr. Psychiatry. 2016;24:136–143. doi: 10.1016/j.jagp.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Makris N., Oscar-Berman M., Jaffin S.K., Hodge S.M., Kennedy D.N., Caviness V.S., Marinkovic K., Breiter H.C., Gasic G.P., Harris G.J. Decreased volume of the brain reward system in alcoholism. Biol. Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin N.V., Huang Y., Hrybouski S., Olsen F. Differential vulnerability of hippocampal subfields and anteroposterior hippocampal subregions in healthy cognitive aging. Neurobiol. Aging. 2017;59:121–134. doi: 10.1016/j.neurobiolaging.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Middlebrooks E.H., Quisling R.G., King M.A., Carney P.R., Roper S., Colon-Perez L.M., Mareci T.H. The hippocampus: detailed assessment of normative two-dimensional measurements, signal intensity, and subfield conspicuity on routine 3T T2-weighted sequences. Surg. Radiol. Anat. 2017;39:1149–1159. doi: 10.1007/s00276-017-1843-x. [DOI] [PubMed] [Google Scholar]
- Mueller S.G., Laxer K.D., Scanlon C., Garcia P., McMullen W.J., Loring D.W., Meador K.J., Weiner M.W. Different structural correlates for verbal memory impairment in temporal lobe epilepsy with and without mesial temporal lobe sclerosis. Hum. Brain Mapp. 2012;33:489–499. doi: 10.1002/hbm.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.G., Yushkevich P.A., Das S., Wang L., Van Leemput K., Iglesias J.E., Alpert K., Mezher A., Ng P., Paz K., Weiner M.W., Alzheimer's Disease Neuroimaging Initiative Systematic comparison of different techniques to measure hippocampal subfield volumes in ADNI2. Neuroimage Clin. 2018;17:1006–1018. doi: 10.1016/j.nicl.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K., Crews F.T. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J. Neurosci. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A., Kwon D., Brumback T., Thompson W.K., Cummins K., Tapert S.F., Brown S.A., Colrain I.M., Baker F.C., Prouty D., De Bellis M.D., Clark D.B., Nagel B.J., Chu W., Park S.H., Pohl K.M., Sullivan E.V. Altered brain developmental trajectories in adolescents after initiating drinking. Am. J. Psychiatry. 2018;175:370–380. doi: 10.1176/appi.ajp.2017.17040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel A.L., Eustache F., Beaunieux H. Component processes of memory in alcoholism: pattern of compromise and neural substrates. Handb. Clin. Neurol. 2014;125:211–225. doi: 10.1016/B978-0-444-62619-6.00013-6. [DOI] [PubMed] [Google Scholar]
- Poppenk J., Moscovitch M. A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. Neuron. 2011;72:931–937. doi: 10.1016/j.neuron.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurogenesis in adult primates. Prog. Brain Res. 2002;138:3–14. doi: 10.1016/S0079-6123(02)38067-1. [DOI] [PubMed] [Google Scholar]
- Riggins T., Geng F., Botdorf M., Canada K., Cox L., Hancock G.R. Protracted hippocampal development is associated with age-related improvements in memory during early childhood. Neuroimage. 2018;174:127–137. doi: 10.1016/j.neuroimage.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing T., Zahr N.M., Sullivan E.V., Pfefferbaum A. The SRI24 multi-channel brain atlas: construction and applications. Med. Imaging 2008 Image Process. 2008:6914. doi: 10.1117/12.770441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Murayama S. Neuropathology of mild cognitive impairment. Neuropathology. 2007;27:578–584. doi: 10.1111/j.1440-1789.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- Schoene-Bake J.C., Keller S.S., Niehusmann P., Volmering E., Elger C., Deppe M., Weber B. In vivo mapping of hippocampal subfields in mesial temporal lobe epilepsy: relation to histopathology. Hum. Brain Mapp. 2014;35:4718–4728. doi: 10.1002/hbm.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim G., Choi K.Y., Kim D., Suh S.I., Lee S., Jeong H.G., Jeong B. Predicting neurocognitive function with hippocampal volumes and DTI metrics in patients with Alzheimer's dementia and mild cognitive impairment. Brain Behav. 2017;7 doi: 10.1002/brb3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic G., Kostovic I., Winblad B., Bogdanovic N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer's disease. J. Comp. Neurol. 1997;379:482–494. doi: 10.1002/(sici)1096-9861(19970324)379:4<482::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Skinner H.A. Addiction Research Foundation; Toronto, Canada: 1982. Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. [Google Scholar]
- Skinner H.A., Sheu W.J. Reliability of alcohol use indices: the lifetime drinking history and the MAST. J. Stud. Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Small S.A. Isolating pathogenic mechanisms embedded within the hippocampal circuit through regional vulnerability. Neuron. 2014;84:32–39. doi: 10.1016/j.neuron.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S.A., Schobel S.A., Buxton R.B., Witter M.P., Barnes C.A. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone D., Sato N., Maikusa N., Ota M., Sumida K., Yokoyama K., Kimura Y., Imabayashi E., Watanabe Y., Watanabe M., Okazaki M., Onuma T., Matsuda H. Automated subfield volumetric analysis of hippocampus in temporal lobe epilepsy using high-resolution T2-weighed MR imaging. Neuroimage Clin. 2016;12:57–64. doi: 10.1016/j.nicl.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E.V., Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Marsh L., Mathalon D.H., Lim K.O., Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol. Clin. Exp. Res. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Bos M.G.N., van de Kamp F.C., Peters S., Crone E.A. Longitudinal development of hippocampal subregions from childhood to adulthood. Dev. Cogn. Neurosci. 2018;30:212–222. doi: 10.1016/j.dcn.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Anderson C.M., Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Neylan T.C., Mueller S.G., Lenoci M., Truran D., Marmar C.R., Weiner M.W., Schuff N. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch. Gen. Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M.J., Kawas C.H., Stewart W.F., Rudow G.L., Troncoso J.C. Hippocampal neurons in pre-clinical Alzheimer's disease. Neurobiol. Aging. 2004;25:1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Wilhelm J., Frieling H., Hillemacher T., Degner D., Kornhuber J., Bleich S. Hippocampal volume loss in patients with alcoholism is influenced by the consumed type of alcoholic beverage. Alcohol Alcohol. 2008;43:296–299. doi: 10.1093/alcalc/agn002. [DOI] [PubMed] [Google Scholar]
- Wilson S., Bair J.L., Thomas K.M., Iacono W.G. Problematic alcohol use and reduced hippocampal volume: a meta-analytic review. Psychol. Med. 2017;47:2288–2301. doi: 10.1017/S0033291717000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse L.E., Biessels G.J., Geerlings M.I. A critical appraisal of the hippocampal subfield segmentation package in FreeSurfer. Front. Aging Neurosci. 2014;6:261. doi: 10.3389/fnagi.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F., Cui D., Zhang L., Zhang S., Zhao Y., Liu X., Liu C., Li Z., Zhang D., Shi L., Liu Z., Hou K., Lu W., Yin T., Qiu J. The volume of hippocampal subfields in relation to decline of memory recall across the adult lifespan. Front. Aging Neurosci. 2018;10:320. doi: 10.3389/fnagi.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F., Liu Y., Yuan Z., Gao X., He Y., Liu X., Cui D., Qi R., Chen T., Qiu J. Age-related changes in cortical and subcortical structures of healthy adult brains: a surface-based morphometry study. J. Magn. Reson. Imaging. 2019;49:152–163. doi: 10.1002/jmri.26037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple regressions* on raw data.