Highlights
-
•
The tumour microenvironment promotes induction of mesenchymal stromal cells through the production of soluble factors.
-
•
Data from animal models indicates that mesenchymal stromal cells could accelerate pulmonary metastases, promote proliferation of sarcoma cells and increase chemoresistance.
-
•
Pre-activated and use of transduced mesenchymal stromal cells have a positive effect on bone sarcoma.
-
•
There is preliminary clinical evidence for mesenchymal stromal cell in promoting bone regeneration within large bone defects after surgical excision.
Keywords: Bone, Sarcoma, Mesenchymal, Stromal, Cell, Biology, Tissue, Regeneration, Orthopaedic
Abbreviations: AAT, a1-antitrypsin; Abs, antibodies; Ang1, angiopoietin-1; APCs, antigen presenting cells; ASC, adipose-derived stromal/stem cells; BD, bone defect; BMMSCs, bone marrow-derived mesenchymal stromal cells; CAM, cell adhesion molecules; CCL5, chemokine ligand 5; CCR2, chemokine receptor 2; CD, cytosine deaminase; CD, classification determinants; CLUAP1, clusterin associated protein 1; CSPG4, Chondroitin sulfate proteoglycan 4; CXCL12/CXCR4, C-X-C chemokine ligand 12/ C-X-C chemokine receptor 4; CXCL12/CXCR7, C-X-C chemokine ligand 12/ C-X-C chemokine receptor 7; CXCR4, chemokine receptor type 4; CX3CL1, chemokine (C-X3-C motif) ligand 1; DBM, Demineralized Bone Marrow; DKK1, dickkopf-related protein 1; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; FGF-2, fibroblast growth factors-2; FGF-7, fibroblast growth factors-7; GD2, disialoganglioside 2; HER2, human epidermal growth factor receptor 2; HGF, hepatocyte growth factor; HMGB1/RACE, high mobility group box-1 protein/ receptor for advanced glycation end-products; hMSCs, human mesenchymal stromal cells; IDO, indoleamine 2,3-dioxygenase; IFN-α, interferon alpha; IFN-β, interferon beta; IFN-γ, interferon gamma; IGF-1R, insulin-like growth factor 1 receptor; IL-1b, interleukin-1b; IL-2a, interleukin-2a; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; IL11RA, Interleukin 11 Receptor Subunit Alpha; IL-12, interleukin-12; IL-18, interleukin-18; IL-21, interleukin-21; MAGE, melanoma antigen gene; MCP-1, monocyte chemoattractant protein-1; MMP-2, matrix metalloproteinase-2; MMP2/9, matrix metalloproteinase-2/9; MRP, multidrug resistance protein; MSCs, mesenchymal stem/stromal cells; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; OPG, osteoprotegerin; PBS, phosphate-buffered saline; PDGF, platelet-derived growth factor; PDX, patient derived xenograft; PEDF, pigment epithelium-derived factor; PGE2, prostaglandin E2; PI3K/Akt, phosphoinositide 3-kinase/protein kinase B; PTX, paclitaxel; RANK, receptor activator of nuclear factor kappa-B; RANKL, receptor activator of nuclear factor kappa-B ligand; RBCs, red blood cells; RES, reticuloendothelial system; rh-TRAIL, recombinant human tumour necrosis factor related apoptosis-inducing ligand; RNA, ribonucleic acid; SC, stem cells; SCF, stem cells factor; SDF-1, stromal cell-derived factor 1; STAT-3, signal transducer and activator of transcription 3; TAAs, tumour-associated antigens; TCR, T cell receptor; TGF-b1, transforming growth factor beta 1; TGF-b, transforming growth factor beta; TNF-a, tumour necrosis factor alpha; TNF, tumour necrosis factor; TRAIL, tumour necrosis factor related apoptosis-inducing ligand; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; WBCs, white blood cell; 5-FC, 5-fluorocytosine
Abstract
Over the past few decades, there has been growing interest in understanding the molecular mechanisms of cancer pathogenesis and progression, as it is still associated with high morbidity and mortality. Current management of large bone sarcomas typically includes the complex therapeutic approach of limb salvage or sacrifice combined with pre- and postoperative multidrug chemotherapy and/or radiotherapy, and is still associated with high recurrence rates. The development of cellular strategies against specific characteristics of tumour cells appears to be promising, as they can target cancer cells selectively. Recently, Mesenchymal Stromal Cells (MSCs) have been the subject of significant research in orthopaedic clinical practice through their use in regenerative medicine. Further research has been directed at the use of MSCs for more personalized bone sarcoma treatments, taking advantage of their wide range of potential biological functions, which can be augmented by using tissue engineering approaches to promote healing of large defects. In this review, we explore the use of MSCs in bone sarcoma treatment, by analyzing MSCs and tumour cell interactions, transduction of MSCs to target sarcoma, and their clinical applications on humans concerning bone regeneration after bone sarcoma extraction.
1. Introduction
Sarcomas are malignant neoplasms of mesenchymal tissue that are classified as bone or soft tissue in origin [1]. Osteosarcomas and chondrosarcomas are the most common malignant bone tumours, followed by Ewing sarcomas [2]. Osteosarcomas and Ewing sarcomas primarily affect children and adolescents, while chondrosarcoma incidence increases with age [1]. Osteosarcoma and Ewing sarcoma represent 2.4% and 1.4% of childhood cancers, respectively [3]. Ewing sarcoma is a small, round blue cell tumour that in children arises in bone in 80% of the cases, whereas in adults, 75% of primary tumours arise in soft tissue [4]. Osteosarcoma is characterized by the presence of malignant osteoid and occurs primarily in the metaphysis of long tubular bones of young patients with a median age of diagnosis of 15 to 19 years [4], [5]. Following osteosarcoma, chondrosarcoma is the second most frequent primary malignant bone tumour and is characterized by the production of cartilage matrix [6]. The treatment of bone sarcoma is challenging [7]. Intermediate and high-grade sarcomas are more frequently treated with a combination of surgery, chemotherapy and/or radiation therapy. Tumour resection depends on the cancer size and grade, staging and sensitivity to neoadjuvant chemotherapy; it typically requires wide tumour excision along with a margin of healthy tissue around it. Multidrug chemotherapy and radiotherapy are adjuvant treatments which reduce the risk of local and distant relapse, but with major shortcomings including associated morbidity and poor targeting efficiency to a tumour [8].
During the last 30 years, many new approaches to the treatment of bone sarcomas have been developed; however, these have failed to improve 5-year overall survival [8]. Targeting techniques aim to achieve greater accumulation of antineoplastic agents in the tumour whilst maintaining a non-toxic concentration in non-target organs and tissues. Mesenchymal stromal cells (MSCs) may offer an advantage through the delivery of antineoplastic agents or factors such as interferons, interleukins, TNF and antibodies selectively to tumour cells.
The emerging therapeutic role of SCs follows exponential progress in understanding their natural behaviour and characteristics. Stem cells interact with other cell types and are involved in tissue development, regeneration, homeostasis, and evolution by natural selection [9]. Four major categories of stem cells are currently encountered; embryonic, fetal, adult and induced pluripotent SCs [10]. Although all types of SCs can be used for medical research, adult SCs are usually utilized for cellular therapies as they can be easily collected from patients, show high multiplication capability in vitro and can migrate to target tissues of the host [11]. On the other hand, the use of embryonic SCs face legal and ethical restrictions in some countries and could be restricted as they are available in limited quantities [10]. When it comes to SCs isolation from fetus there are practical restrictions as it could harm the fetus and is possible only during the early stages of life [11]. Mesenchymal stromal cells are mainly obtained from adult tissues and are extensively studied for their regenerative capacity through multipotent differentiation [12] and antitumor activity [13]. Although MSCs are usually isolated from the bone marrow, adipose tissue, and cord blood, they can also be obtained from other tissues such as smooth, skeletal and cardiac muscle, spleen, liver, pancreas, lung, testes, menstrual blood, periosteum, dermis, pericytes, trabecular bone, placenta, amniotic fluid, dental pulp, hair follicle and peripheral blood [14]. Moreover, they differentiate into mesodermal, endodermal and ectodermal lineage cells [14]. Currently, two major categories are most widely studied among MSCs; bone marrow MSCs (BMMSCs) and adipose tissue MSCs (ADMSCs) [10]. Mesenchymal stromal cell populations are established in niches; specialized microenvironments that play a central role in the interaction between MSCs and adjacent cells to maintain tissue homeostasis through balancing self-renewal and differentiation [15]. In these microenvironments, MSCs exhibit posterity, produce multiple heterological cell types and, through those multiple cell types, establish an ECM specific for the niche [16]. The niche protects MSCs not only from differentiation or apoptotic stimulus but also from overproduction that may lead to carcinogenesis [17]. Cancer cells exploit similar mechanisms of the MSCs as the tumour sites share common functional features with MSCs niches [18].
The mechanisms of tumour stroma generation and wound healing appear to be contiguous. Mesenchymal stromal cells are attracted to the site of the tumour by tumour-associated inflammatory signals [19], [20]. Several studies have examined cell-cell and paracrine interactions between MSCs and tumour-cells, demonstrating a complicated relationship between them, with MSCs potentially promoting or suspending tumour progression even within the same cancer model [21]. In this review, we explore the feasible therapeutic roles of MSCs in bone sarcoma, analyzing MSCs and tumour interactions, transduction of MSCs to target sarcoma, and clinical applications of MSCs in humans concerning bone regeneration after bone sarcoma extraction.
2. Can we safely place MSCs near tumour cells?
2.1. MSCs and tumour cell interactions
Several factors can influence the interaction between MSCs and cancer cells: the origin and pre-treatment of MSCs, the cancer type and various in vitro and in vivo system-related factors (the ratio of MSCs and cancer cells, simultaneous or sequential injection of MSCs and cancer cells, local versus systemic MSC delivery or kinetics of carcinogenesis) [22].
In vivo and in vitro studies have confirmed that numerous factors such as monocyte chemotactic protein-1 (MCP-1 or CCL2) in breast cancer or stromal cell-derived factor-1 (SDF-1) in prostate, colorectal and breast cancer that are produced by tumour and inflammatory cells promote the homing and migration of MSCs into the tumour microenvironment [23]. The homing and fate of mesenchymal stromal cells have been related to cell to cell interactions that occur between MSCs and tumour cells through cytokine/receptor pairs including stem cell factor (SCF)/c-Kit; monocyte chemoattractant protein-1 (MCP-1)/ chemokine receptor 2 (CCR2); hepatocyte growth factor (HGF)/c-Met; vascular endothelial growth factor/receptor (VEGF/VEGFR); stromal cell-derived factor 1(SDF-1)/CXCR4; and high mobility group box-1 protein/ receptor for advanced glycation end-products (HMGB1/RACE) [24], [25], [26], [27], [28], [29], [30]. Other inflammatory cytokines, growth and angiogenic factors, also enhance migration of MSCs to the tumour site (Table 1).
Table 1.
Inflammatory cytokines, growth and angiogenic factors that enhance MSC migration to the tumour site.
| Tumour released chemotactic factors | Tumour model | Stem Cell type | Mechanism of action |
|---|---|---|---|
| Epidermal growth factor (EGF) | Glioma [194] | hBMMSCs | Migration of SCs into the established human glioma although they were injected at the opposite cerebral hemisphere |
| Pancreatic carcinoma [195] | hBMMSCs | Migration of SCs to tumour blood vessels due to a tumour hypoxia-induced secretion of GFs including EGF | |
| Melanoma, Mouse mammary carcinoma [196] | BMPCs | Migration of SCs to tumour site and recruitment to the growing vasculature | |
| Vascular endothelial growth factor-A (VEGF-A) | Glioma [197] | hMSCs | Enhancement of the migration and invasion of SCs to the tumour |
| Pancreatic carcinoma [195] | hBMMSCs | Migration of SCs from bone marrow to tumour blood vessels mainly due to tumour hypoxia-induced secretion of VEGF | |
| Platelet-derived growth factor (PDGF) | Pancreatic carcinoma [195] | hBMMSCs | Migration of SCs to tumour blood vessels due to tumour hypoxia-induced secretion of PDGF |
| Stromal-derived growth factor-1 (SDF-1) | Osteosarcoma [92] | hMSCs | Enhancement of SCs migration to the tumour site and promotion of growth and metastasis |
| Prostate tumour [198] | ADSCs | Migration of SCs to tumour site is possibly mediated by homing factor SDF-1 (CXCL12) | |
| Interleukin-8 (IL-8) | Hec1a endometrial carcinoma [199] | O-ASC | Recruitment of SCs and possible tumour progression |
| C—C motif chemokine ligand 25 (CCL25) | Multiple Myeloma (MM) [200] | hBMMSCs | Attraction of SCs to MM through the CCL25/CCR9 axis and supportive role in MM cell growth |
| Hematoma-derived growth factor (HDGF) | Breast carcinoma [201] | hBMMSCs | Promotion of SCs chemotaxis to the tumour site |
| Monocyte chemoattractant protein-1 (MCP-1) | Primary & metastatic breast tumours [202] | hMSCs | In vivo and in vitro stimulation of SCs migration to tumour site |
| Urokinase plasminogen activator (uPA)- Urokinase plasminogen activator receptor (uPAR) | Malignant solid tumour (brain, lung, prostate, breast) [203] | NSCs and MSCs | Significantly greater migration of SCs to the tumour expressing high levels of uPA and uPAR |
| Transforming growth factor beta-1 (TGF-β1) | Breast cancer [204] | hBMMSCs | Attraction of SCs in the tumour site |
| C-X-C motif chemokine-1 (CXCL1) | Hec1a endometrial carcinoma [199] | O-ASC | Recruitment of SCs to the tumour and possible tumour progression |
| Neurotrophin-3 | Malignant Glioma [205] | MSC | Combined with IL-8, TGF-beta1 overexpression, mediate tropism of SCs to the tumour site |
| Tissue Inhibitor of Metalloproteinase-1 (TIMP-1) | Glioma [206] | hNSC | Regulation of CD63 and β1 integrin-mediated signalling and enhancement of SCs adhesion and migration |
Factors:
GF: growth factor, EGF: Epidermal growth factor, VEGF-A: Vascular endothelial growth factor-A, PDGF: Platelet-derived growth factor,SDF-1: Stromal-derived growth factor-1, IL-8: Interleukin-8, CCL25: C—C motif chemokine ligand 25, HDGF: Hematoma-derived growth factor, MCP-1: Monocyte chemoattractant protein-1,uPA: Urokinase plasminogen activator,uPAR: Urokinase plasminogen activator receptor,TGF-β1: Transforming growth factor beta-1,CXCL1: C-X-C motif chemokine-1, Neurotrophin-3, TIMP-1: Tissue Inhibitor of Metalloproteinase-1.
Cell types:
hBMMSCs: Human Bone Marrow-derived Mesenchymal Stromal Cells, hMSCs: Human Mesenchymal Stromal Cells, ADSC: Adipose Tissue-derived Stem Cells, O-ASC: Omental Adipose Tissue Stromal Cells, NSCs: Neural Stem Cells, MSCs: Mesenchymal Stromal Cells, MSC: Bone Marrow Stromal Cells, hNSC: Human Neural Stem Cells, MSC: Bone Marrow Stromal Cells, BMPCs: Bone Marrow-derived Perivascular Cells.
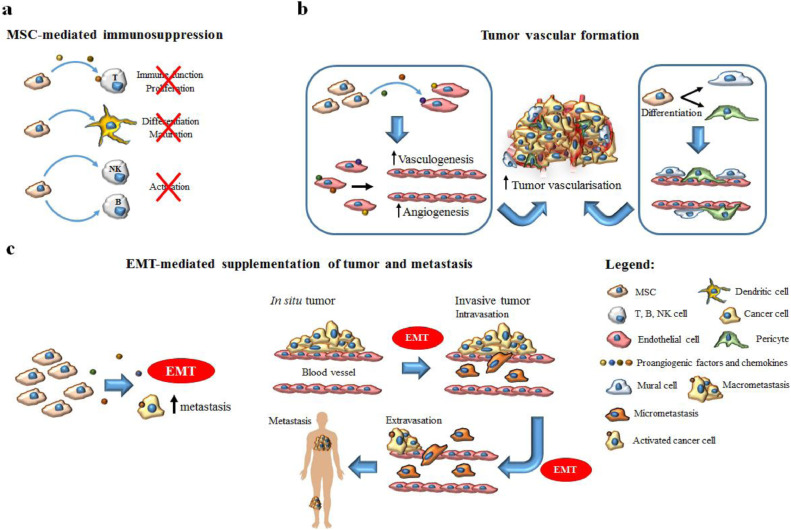
Once MSCs are recruited by cancer cells, they enhance the production of factors like TGF-β, VEGF, SDF-1, and CCL5 or microparticles like exosomes that can either induce or inhibit tumour growth; owing to this bimodal interaction, MSCs have been described as a ”double-edged sword” [23]. The pro- or anti-tumorigenic effect of MSCs on tumour progression depends mainly on the MSC source and the tumour model used [31]. The pro-tumorigenic effect of MSCs includes four main pathways: immunosuppression, tumour angiogenesis and epithelial-mesenchymal transition (EMT)-mediated supplementation of tumour [32](Fig. 1).
Fig. 1.
MSC pro-tumorigenic effect main pathways.
3. Pro-tumorigenic effect
3.1. MSC-mediated immunosuppression
The immunosuppression caused by MSCs promotes immunotolerance and tumour progression [33]. A prerequisite for the immunomodulatory function of MSCs in the tumour microenvironment is their activation by immune cells producing IFN-γ, TNF-a, IL-2a or IL-1b [34], [35], [36]. Once MSCs are activated, they produce a number of molecules (namely TGF-b1, HGF, IDO, PGE2) that inhibit lymphocyte proliferation and suppress the immune function of T lymphocytes, dendritic cell maturation/differentiation, and NK and B-cell activation; simultaneously, MSCs increase the production of regulatory T-cells using a contact-dependent mechanism or by secreting IL-10 and TGF-b, [37], [38], [39], [40], [41]. Regarding specifically T cells, MSCs suppress their activity by inhibiting their proliferation or, by causing apoptosis of already activated T lymphocytes [5].
3.2. Tumour angiogenesis
MSCs promote tumour angiogenesis either by their differentiation into fibroblasts, pericytes, and myofibroblasts or by producing specific growth factors [23]. Proangiogenic factors and chemokines expressed by MSCs, including angiopoietin-1(Ang1), fibroblast growth factors-2 (FGF-2) and −7 (FGF-7), platelet-derived growth factor (PDGF), stromal-derived factor-1 (SDF-1), IL-8 and vascular endothelial growth factor (VEGF) act synergistically on endothelial cells to promote tumour angiogenesis [42], [43], [44]. Other factors with potential pro-angiogenic effect are angiogenin and CCL2 in lymphoma and hepatocyte growth factor, cyclooxygenase, IGF-1 and transforming growth factor-a1 in pancreatic carcinoma [45]. However, in some studies, MSCs suppressed the production of the tumour angiogenic network by inhibiting the growth of endothelial cell-derived capillaries through the production of reactive oxygen species [5].
3.3. EMT-mediated supplementation of a tumour
Epithelial to mesenchymal transition (EMT) has a crucial role in organogenesis, wound healing, tumour progression and metastasis. MSCs facilitate and modulate EMT through the production of regulatory molecules, namely TGF-b, E-cadherin and micro-RNA, resulting in more invasive phenotype [46], [47]. This theory was supported by the upregulation of EMT-specific markers such as N-cadherin, vimentin, Twist and Snail, and the decrease in E-cadherin when breast cancer cells and MSCs were co-cultured [48].
3.4. Metastasis and Anti-apoptotic effects
Based on the type of tumour and MSC status, MSCs and their products can regulate the apoptosis of cancer cells [49], [50], [51], [52]. MSC-induced factors cause vascular formation and secretion of growth factors, promoting invasion of cancer cells and the development of early tumour metastasis [53]. The pro-metastatic effect of MSCs has been proven in breast cancer (CCL5 and IL-17B), hepatocellular carcinoma (MMP2) and osteosarcoma (CXCR4/VEGF axis) [23]. On the other hand, the intravenous injection of UC-MSCs or ADMSCs in mice with mammary tumours reduced the formation of lung metastases [50]. Thus, the role of MSCs in the development of distant metastasis is controversial.
4. Antitumorigenic effect: the role of NF-κB, Akt and Wnt pathways
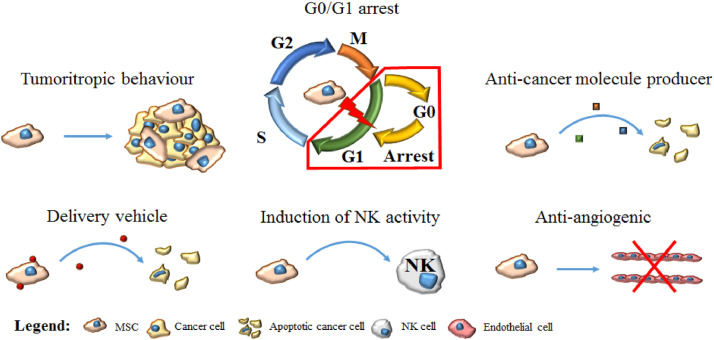
MSCs and cancer cells are thought to use similar signaling pathways regulating self-renewal and differentiation, namely the Wnt/β-catenin, Notch, Shh and BMP pathways [23], [54]. Although the anti-tumorigenic mechanisms of MSCs are not fully understood, it is presumed that they are related to the downregulation of NF-κB, PI3K/Akt, and Wnt pathways [55]. Uneven activation of the Wnt/β-catenin pathway has led to the development of a variety of human tumours [56], [57]. Human umbilical cord-derived MSCs (hUC-MSCs) co-cultured with glioma cells downregulate β-catenin expression in glioma cell lines and increase the levels of secreted DKK1 which is an inhibitor of the Wnt pathway [58]. The anti-tumorigenic and pro-apoptotic effect of MSCs results from the suppression of the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) signaling pathway in some cancer types [59]. However, many factors secreted by MSCs activate the PI3K/Akt pathway in other cancer types and this effect may depend on the metabolic or genetic profile of cancer cells [60]. Existing data from pre-clinical studies regarding the pro-apoptotic effect of MSCs on cancer cells through the downregulation of PI3K/Akt pathway support this antitumor potential in hepatocellular carcinoma, glioma cells, Kaposi's sarcoma, and prostate and breast cancer [61], [62], [63], [64], [65]. Other possible mechanisms that contribute to the anti-tumour potential of MSCs include apoptosis caused by upregulation of TRAIL, G1 arrest, and expression of tumour suppressor genes [23] (Fig. 2).
Fig. 2.
MSC anti-tumorigenic and pro-apoptotic effect.
4.1. Safety of use of MSCs in proximity to a tumour
The unique characteristics of MSCs (self-renewal, differentiation, and tropism to the tumour site) raised expectations of possible therapeutic applications in cancer patients. Major safety issues and knowledge gaps, however, remain to be explored [66].
The role of MSCs in different cancer types is ambiguous. In vivo animal studies of murine melanoma and renal murine adenocarcinoma (Renca kidney cancer cells) showed that co-implantation of MSCs increased tumour size possibly because of MSC-mediated immunosuppression [67], [68]. The production of trophic factors from MSCs including VEGF, chemokine CCL5 and IL6 (activation STAT-3) has been connected with tumour growth and metastasis in Burkitt's lymphoma, breast cancer, colonic cancer and chronic myeloid leukaemia [69], [70], [71], [72], [73]. Rowan et al. investigated the safety of human adipose tissue-derived stromal cells (ADSC) included in fat grafts that were used for reconstruction after surgical resection of a breast tumour. The application of human ADSCs in proximity to breast cancer raised concerns as it was associated with an increase in the migration and metastasis of cancer cells [74]. In an animal study, Zheng et al. scrutinized whether the proximity of breast tumour cells (4T1) and bone marrow-derived mesenchymal stromal cells (BMMSCs) could affect tumour growth. Interestingly, they found that the co-injection of 4T1 cells and BMMSCs accelerated cancer cell proliferation, whereas simultaneous distant injection had the opposite results. When MSCs were injected distantly from cancer cells, they had immunoregulatory action by decreasing the accumulation of myeloid-derived suppressor cells and regulatory T cells at the tumour site [75]. Another mechanism that could increase the aggressiveness of a tumour is the potential differentiation of MSCs to tumour associated fibroblasts that are capable of increasing neovascularization within the tumour stroma and the metastatic rate [76], [77].
4.2. MSCs and bone sarcoma interactions
One human and several animal studies have investigated the safety of using MSCs in proximity to bone sarcomas by exploring MSCs and tumour cell interactions [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93]. Aanstoos et al. evaluated the safety of application of MSCs in mice with osteosarcoma. They used three groups: mice receiving MSCs intravenously or at the surgical site following tumour resection and mice receiving no MSCs; and they evaluated both the development and progression of pulmonary metastasis and local recurrence. Mice that received MSCs intravenously developed the first detectable metastases significantly earlier (2.93 ± 1.90 days) than those that received local injection of MSCs (6.94 ± 6.78 days) and those that received no MSCs (5.93 ± 4.55 days). The recurrence rate and the size of the recurrent tumours, however, were not affected significantly by delivery of the MSCs at the surgical site or intravenously [94].
The co-implantation of rat BMMSCs and COS1NR osteosarcoma cells resulted in the acceleration of non-metastatic cancer settlement and proliferation in a study by Tsukamoto et al. All animals in which MSCs and osteosarcoma cells were co-implanted developed tumour by week 4, compared to those that received only osteosarcoma cells and developed tumour by week 6. However, there was no significant statistical difference in tumour size after six weeks and in the number of metastases between the two groups. On the other hand, intravenous BMMSC injection led to stabilization of the initial tumour size, without altering its histological appearance, and significant enhancement of metastatic lung nodules (17.33 ± 8.39 vs. 2.0 ± 2.0, p = 0.03). The overexpression of the CXCL12/CXCR7 axis was probably involved in osteosarcoma cell-MSCs interaction that led to an increase in pulmonary metastases and tumour progression. Moreover, the elevation of the basement membrane degrading enzymes, MMP-2 and MMP-9 in MSCs, was connected with increased tumour metastasis [95]. The pro-tumorigenic effect of locally administrated MSCs could be correlated with the number of MSCs applied. Lee et al. showed in an animal study that local administration of ADMSCs in low proportions suppress tumour growth, whereas higher proportions show a tumour-promoting effect [88]. In vivo mice studies also indicated that MSCs could promote CXCR-4-mediated osteosarcoma proliferation and pulmonary metastasis through the production of VEGF by MSCs [89]. In vitro co-culture of osteosarcoma cells and BMMSCs showed that SCF-1 secreted from BMMSCs promoted the proliferation and invasion of osteosarcoma cells. In contrast to BMMSCs, osteosarcoma cells expressed higher levels of CXCR4, indicating that the SDF-1/CXCR4 axis plays a pivotal role in osteosarcoma cell proliferation [96]. Another in vivo mice model indicated that SDF-1 could also be secreted from Saos-2 cells in the conditioned medium and enhance the migration of hMSCs to the tumour site, where osteosarcoma cell growth and pulmonary metastasis are promoted by the secretion of CCL5 from exogenous hMSCs [92]. Moreover, pre-clinical data showed that BMMSCs could promote osteosarcoma growth through PI3K/Akt and Ras/Erk intracellular cascades, and enhance metastasis through CXCR4 signaling [91].
Avril et al. investigated the interaction of human osteosarcoma cells with human adipose tissue-derived stromal cells (ADSCs), MSCs obtained from bone marrow, and pre-osteoclasts when co-injected in immunodeficient mice. Adipose tissue-derived stromal cells constitute a population of MSC-like cells that are used in plastic and reconstructive surgery. They found that ADSCs and MSCs increased tumour size while pre-osteoclasts did not; none of them, however, increased metastasis. The cell cycle of proliferating osteosarcoma cells was accelerated by factors secreted by MSCs, in contrast to the quiescent state of dormant tumour cells that remain stable. They concluded that ADSCs/MSCs could be used for reconstructive surgery after osteosarcoma resection; however, they may not be good candidates for targeted cell therapy as they may enhance tumour cell proliferation [97]. Soluble factors secreted from adipose tissue, such as IL-6 and leptin, induced the activation of osteosarcoma cell cycles from G1 to mitosis phases and thus increased the local growth of a tumour. However, they do not promote lung metastasis and osteolysis [80].
Interleukin-6 (IL-6) seems to play a pivotal role in the bidirectional proliferation of human MSCs (hMSCs) with Saos-2 both in vitro and nude mice models. Interestingly, IL-6 produced from the conditioned medium of Saos-2 could inhibit osteogenic differentiation of hMSCs [98]. A probable explanation for IL-6 mediated promotion of osteosarcomas is through the STAT3 signalling pathway. IL-6 secreted from MSCs activates STAT-3 in adjacent osteosarcoma cells in a concentration-dependent manner, promotes their proliferation, protects them from chemotherapeutic drugs such as cisplatin and enhances their migration and invasion properties [82]. Tu et al. also demonstrated that the co-implantation of Saos-2 and MSCs activated STAT-3 by IL-6 and increased the expression of multidrug resistance protein (MRP) and P-glycoprotein that induced chemoresistance in doxorubicin and cisplatin in mice osteosarcoma samples [99]. The ADMSCs-mediated activation of the STAT-3 pathway in osteosarcoma increased MMP2/9 and decreased E-cadherin expression, promoting tumour progression [83].
In assessing the safety of the use of MSCs for bone healing Hernigou et al. retrospectively evaluated 92 patients with chondrosarcoma (31/92), osteosarcoma (35/92), Ewing's sarcoma (28 /92) and other bone tumours (8/92), who received autologous BMMSCs following tumour resection to enhance the healing of the host-to-allograft bone junction. They evaluated the risk of local recurrence after surgical tumour resection at a mean follow-up of 15.4 years. They found no increase in the risk of local recurrence for all cancer types [100]. However, as referred to earlier, there were safety concerns about human adipose-tissue stromal cells (ASC) included in fat grafts that increased migration and metastases of breast tumour and osteosarcoma animal models [74], [101]. Autologous fat grafts used in reconstructive surgery have also been related to late local recurrence, 13 years following the onset of osteosarcoma in a female human patient. Successive pre-clinical models showed that MSCs/stromal cells included in the fatty tissue interact with osteosarcoma cells and induce their proliferation [101].
Studies support the hypotheses that the use of MSCs could accelerate pulmonary metastases after resection of the primary osteosarcoma, promote the proliferation of osteosarcoma cells at the tumour site and increase chemoresistance (Table 2). However, current knowledge refers mainly to in vitro and in vivo animal models and has not yet been translated to the clinical setting. It is not clear whether there is a biological reason suggesting that intravenously administrated MSCs have a predilection for locating within the lungs through their interaction with lung metastases or they are likely to be entrapped in the lungs as they are a first pass tissue. Intracardiac or arterial injection of MSCs in future studies could help us understand the exact mechanism of presumptive acceleration of pulmonary metastases.
Table 2.
In vitro, in vivo and clinical data on safety of stem cells in proximity to bone sarcomas.
| Author/ Year | Model Used | Methods/ Results | Conclusion |
|---|---|---|---|
| Hernigou P/ 2014 [100] | Retrospective cohort study of humans | • 92 humans suffering from chondrosarcoma (31 patients), osteosarcoma (35 patients), Ewing's sarcoma (28 patients) and other bone tumors (8 patients) | • A longer follow-up period is required as some solid malignant tumors are developed after a 20–40- year latent period |
| • Risk of local tumor recurrence after surgical resection and autologous BMMSCs for bone defect reconstruction during 15.4 years mean follow-up is not increased | • Local recurrence when MSCs are administrated beyond the two-year period after bone resection should be investigated | ||
| Aanstoos ME/ 2016 [94] | In vivo animal study | • Adipose tissue-derived MSCs (ADMSCs) were applied in mice with osteosarcoma | • Intravenous delivery of MSCs could promote pulmonary micrometastasis |
| • Study included three groups; mice receiving intravenous MSCs, mice receiving MSCs at the surgical site following resection and mice receiving no MSCs | • Local application at the surgical site appear to be safe in murine model | ||
| • The group of intravenous administrated MSCs presented metastases earlier than the other two groups | • Further investigation is needed before using MSCs in human with osteosarcoma | ||
| Avril P/ 2016 [97] | In vivo animal study | • Human MSCs, ADSCs and pre-osteoclasts were co-injected with human MNNG-HOS osteosarcoma cells in mice | • ADSCs/ MSCs appear not to increase the risk of local recurrence when used for reconstructive surgery after bone tumor resection |
| • MSCs and ADSCs increase MNNG-HOS osteosarcoma growth with-out exacerbation of osteolytic lesions and lung metastases | • ADSCs/ MSCs when used for tumor-targeted cell therapy can enhance tumor progression | ||
| • ADSCs increase tumor growth in a dose-dependent manner | |||
| • ADSCs and MSCs produce factors that may accelerate the cell cycle of proliferating osteosarcoma cells | |||
| Bian Z-Y/ 2010 [98] | In vivo animal study | • Saos-2 with or without co-injection of hMSCs were injected into the proximal tibia of nude mice | • There is a positive feedback loop of IL-6 in interaction between hMSCs and Saos-2 |
| • exogenous hMSCs target the osteosarcoma site and promote its growth and pulmonary metastases | |||
| • ALP levels were increased in response to co-injection of Saos-2 and hMSCs | |||
| • hMSCs and Saos-2 could enhance their own proliferation through IL-6 autocrine | |||
| Perrot P/ 2010 [101] | In vivo animal study | • 21 mice were divided in 3 groups: the control Saos-2 group, Saos-2 + fat group and Saos-2 + cannula group | • Clinicians must be aware of the possible long term local relapse of osteosarcoma after autologous fat graft |
| • Human adipose tissue-delivered stromal cells (ADSC) included in fat grafts can induce osteosarcoma cells proliferation | |||
| Tu B/ 2016 [99] | In vitro and in vivo animal study | • MSCs protect osteosarcoma cells from drug-induced apoptosis | • STAT-3 pathway should be further studied in order to overcome environment-induced chemoresistance |
| • Co-implantation of Saos-2 and MSCs leads to activation of STAT3 by IL-6 and increase not only the expression of multidrug resistance protein (MRP), but also P-glycoprotein |
Factors:
ALP: alkaline phosphatase, MRP: multidrug resistance protein.
Cell types:
BMMSCs: Bone Marrow-derived Mesenchymal Stromal Cells, MSCs: Mesenchymal Stromal Cells, ADMSCs: Adipose tissue-derived MSCs, ADSC: Adipose Tissue-derived Stem Cells, MNNG-HOS: human osteosarcoma cell line, Saos-2: osteosarcoma cell line.
5. Pre-activation and use of transduced MSCs to induce sarcoma cell apoptosis
5.1. Induction of cancer cell death using transduced MSCs
The use of transduced MSCs to express specific anti-cancer proteins selectively to the tumour site offers a promising therapeutic approach. Several proteins, molecules or drug systems have been implicated.
5.1.1. Interferons
Human MSCs genetically engineered to produce interferons have been used to deliver these proteins locally in several cancer models, such gliomas, metastatic breast, melanoma, endometrial, ovarian, hepatocellular, bronchioloalveolar and prostate models. MSCs expressing IFN-β can reduce tumour size, induce apoptosis and increase survival in the aforementioned cancer types [55], [102], [103], [104], [105], [106], [107], [108]. Similarly, IFN-α expressing MSCs were effective in increasing cancer cell apoptosis and suppressing angiogenesis in metastatic melanoma and hepatocellular carcinoma models [109]. IFN-γ-expressing MSCs also inhibited leukemic and lung carcinoma cell proliferation in vitro [35], [110], [111]. However, when IFN-γ-human BMMSCs were tested against H460 cells in an animal model, their pro-tumorigenic effect was superior through the promotion of vascularization [112].
5.1.2. Interleukins
Adenovirus-transduced MSCs that expressed interleukin-12 (IL-12), reduced metastases from subcutaneous tumours [113]. A tumour suppressive effect of IL-12 secreted from genetically modified MSCs was also confirmed in a breast carcinoma model [114], [115]. MSCs developed to deliver IL-2 and IL-21, when injected into murine gliomas and epithelial ovarian cancer respectively, reduced tumour growth and improved survival [116]. Interestingly, transduced BMMSCs expressing IL-18 and IFN-β promoted apoptosis of cancer cells in an intracranial glioma model [117]. Chemokine CX3CL1 intravenously delivered from transduced MSCs to colon cancer, and melanoma reduced the number of lung metastases [118].
5.1.3. Viruses
MSCs have already been used as vectors of oncolytic adenoviruses in several in vitro and in vivo studies and improved survival in murine ovarian, breast and lung tumour models. Viral replication and invasion to the surrounding tumour tissue induced apoptosis of cancer cells [55]. Komarova et al. studied the impact of MSCs infected by genetically modified coxsackie and adenoviruses against animal ovarian cancer. They showed that MSCs mediated infection of cancer cells had better results in decreasing tumour size and increasing of survival rate compared with the directly injected adenovirus [119].
5.1.4. TNF
Cancer cell apoptosis mediated by the tumour necrosis factor (TNF) cytokine superfamily was first proposed almost 30 years ago. TNF-α was the first assessed cytokine that proved to be ineffective in triggering cancer cell apoptosis and also had severe adverse events [120]. Attention has now turned to other members of the TNF superfamily.
5.1.5. TRAIL
Tumour necrosis factor related apoptosis-inducing ligand (TRAIL) [121] is a type 2 transmembrane ligand that causes the death of tumour cells through the extrinsic apoptosis pathway and activation of caspase-8 [122]. TRAIL could be an ideal antitumor candidate for specific tumour types, as in most cases it causes the selective death of malignant cells [123], [124]. However, TRAIL has a short pharmacokinetic half-life when administrated intravenously and this limits its usefulness when given systemically [124]. Genetically modified MSCs expressing TRAIL could offer targeted and prolonged delivery of the ligand [125], [122]. Viral vectors have already been used to develop transduced MSCs that were studied against lung, renal, pancreatic, cervical, breast and colon cancer, malignant pleural mesothelioma, neuroblastoma, rhabdomyosarcoma, squamous cell carcinoma, metastatic malignant fibrous histiocytoma and multiple myeloma [126]. Recently, in vivo and in vitro studies indicated that MSCs expressing TRAIL caused apoptosis more effectively than recombinant human TRAIL (rh-TRAIL), and overcame TRAIL resistance of some cancer types [126]. Moreover, recent data support that the pancreatic cancer sensitivity to MSC-delivered TRAIL could be restored by combined administration of MSCs expressing TRAIL and a paclitaxel (PTX)-based chemotherapy [127].
5.1.6. Antibodies (Abs)
Antibodies that are produced in vitro and then administrated into the systemic circulation have a short-half life and cannot approach the tumour site. To overcome this difficulty, Compte et al. developed genetically engineered hMSCs to secrete a bispecific diabody, resulting in a powerful systemic antitumoral effect even though the diabodies were produced distantly [125]. In a recent animal study, human umbilical cord-derived MSCs were genetically modified to produce a bispecific Ab (Tandab CD3/CD19) against B cell lymphoma and inhibited cancer cell proliferation [128].
5.1.7. Enzyme/prodrug systems
Ghaedi et al. used lentivirus-transduced hMSCs to deliver a1-antitrypsin (AAT) to solid tumours and caused cytotoxicity against umbilical cord vein endothelial cells [129]. The gene-directed enzyme-producing therapy uses MSCs for targeted chemotherapy through a prodrug conversion approach [130]. Uchibori et al. combined MSCs that produced herpes simplex virus-thymidine kinase retroviral vectors with the prodrug ganciclovir leading to glioma cell death both in vitro and in vivo [131].
Similarly, extensively studied enzyme/prodrug systems included cytosine deaminase (CD) combined with 5-fluorocytosine (5-FC) and carboxylesterase with the prodrug CPT-11 (irinotecan) for the treatment of colon cancer, osteosarcoma [132] or glioblastoma and brain stem glioma, respectively [133]. Mesenchymal stem cells engineered to produce the CD enzyme, which converts the substrate 5-FC to the highly toxic 5-fluorouracil, have been used in melanoma and colon cancer. The tumour size was reduced; however, they also caused toxicity to healthy tissues [134], [135].
5.1.8. Other factors
Pigment epithelium-derived factor (PEDF) and NK4 are agents that have been studied against lung carcinoma and gastric cancer, respectively. Transfection of MSCs to secrete these agents was effective in cancer cell apoptosis and suppression of metastases [136], [137], [138], [139].
5.2. Use of transduced MSCs to induce bone sarcoma cell apoptosis
The ability of MSCs to migrate to the primary tumour site raised the possibility of using them like drug or gene carriers. Transduced MSCs producing novel anti-cancer proteins molecules or drug systems such TRAIL, osteoprotegerin, cytosine deaminase/5-fluorocytosine (CD/5-FC) and IL-2 selectively to the tumour site, have also been studied extensively in bone sarcoma models (Table 3).
Table 3.
A list of strategies using transduced MSCs as an anticancer therapy for osteosarcoma.
| Anti-cancer agent | Sarcoma model | Therapeutic strategy | Mechanism of action | Study type |
|---|---|---|---|---|
| TRAIL | Osteosarcoma, Ewing's [140] | Cytotoxic agent | Apoptosis, Anti-angiogenic action | In-vitro and in-vivo animal studies |
| Ewing's [144] | Cytotoxic agent | Apoptosis | In-vitro and in-vivo animal studies | |
| OPG | Osteosarcoma [207] | Cytotoxic agent | Reduction of tumour growth and bone destruction caused by osteosarcoma cells | In-vitro and in-vivo animal studies |
| CD/5-FC | Osteosarcoma [132] | Gene-directed enzyme-producing therapy | Inhibition of DNA and RNA synthesis of tumour cells | In-vitro and in-vivo animal studies |
| IL-12 | Ewing's [156] | Cytotoxic agent | Inhibition of tumour growth | In-vivo animal studies |
Anti-cancer agents:
TRAIL: Tumour necrosis factor related apoptosis-inducing ligand, OPG: Osteoprotegerin CD/5-FC: Cytosine deaminase/ 5-fluorocytosine IL-12: interleukin-12.
5.2.1. TRAIL
Transduced MSCs expressing TRAIL are promising for several cancer types; however, their efficacy against bone sarcomas remains controversial [126]. As noted previously, TRAIL delivered by ADMSCs had a greater anti-tumorigenic effect on osteosarcoma than rh-TRAIL alone. Despite the high levels of OPG, TRAIL cell delivery could be effective against SAOS-2 cells since MSCs offer a longer half-life and more stable TRAIL delivery and secretion of synergizing factors [140]. Transduced MSCs expressing TRAIL have anticancer activity in sarcoma models causing extensive apoptosis of sarcoma and potent antiangiogenic functions both in vitro and in vivo. This pro-apoptotic effect is thought to be controlled by the activation of caspase-8 [140]. Grisendi et al. also demonstrated the effectiveness of the approach in a metastatic lung model of Ewing's sarcoma, as ADMSCs were capable of migrating into the lung and persisting after last inoculation [140].
Several pre-clinical studies have proved the effectiveness of TRAIL against cancer cells, however, some Ewing sarcoma cell lines were TRAIL-resistant in vitro [141], [142], [143]. To overcome this resistance, Guiho et al., developed transduced ADMSCs producing TRAIL that induced cell death even in rh-TRAIL-resistant Ewing sarcoma cell lines. Direct cell-to-cell contact of Ewing sarcoma cells and the transduced ADMSC was necessary to cause cancer cell apoptosis through the clustering of a high level of death receptors. Two different orthotopic in vivo Ewing sarcoma models in mice also confirmed the efficiency of ADMSC expressing TRAIL [144]. In a more recent study, of a para-tibial orthotopic osteosarcoma mouse model using rh-TRAIL resistant K-HOS cells, the delivery of ADMSCs expressing TRAIL into the tumour site did not slow down tumour progression [145]. This was despite promising in vitro data demonstrating that MSC-TRAIL could induce significant K-HOS cell apoptosis in direct co-culture. Possible reasons given for the in vivo findings were the non-optimal target: effector ratio and lack of physical contact between the SCs and cancer cells. Furthermore, two of the MSC inoculated treatment groups demonstrated accelerated tumour progression [145].
5.2.2. Osteoprotegerin (OPG)
Osteoprotegerin is a soluble glycoprotein and a member of the TNF receptor superfamily. It has a crucial role in bone metabolism as a decoy receptor for RANKL in the RANK/RANKL/OPG axis, inhibiting osteoclastogenesis and bone resorption [146]. Osteoprotegerin impedes osteosarcoma progression by inhibiting RANKL whose secretion is enhanced in the bone tumour microenvironment. However, OPG was ineffective outside bone as it could not prevent pulmonary metastasis [147]. Qiao et al. developed MSCs transfected with adenoviruses carrying the OPG gene to prolong OPG's half-life and enhance its delivery directly to a tumour site. When administered to mice with osteosarcoma through the tail vein, the engineered MSCs were capable of migrating to the osteosarcoma site, producing OPG locally, reducing tumour growth and inhibiting bone destruction by osteosarcoma. The tumour volume at day 30 in mice that received MSCs-OPG was reduced by 65.2% compared to those administrated with PBS solution. Additionally, transduced MSCs were detected in treated mice, but with no increase in serum OPG levels was seen even 30 days after systematic administration, demonstrating fewer side effects of systemic administration [148].
5.2.3. CD/5-FC
Cytosine deaminase (CD) is a lethal gene which encodes an enzyme catalyzing the conversion of cytosine into uracil and the non-toxic prodrug 5-fluorocytosine (5-FC) into the cytotoxic agent 5-FU which has anti-tumour activity. The CD/5-FC system is very effective against human cancers by inhibiting DNA synthesis and promoting apoptosis of tumour cells [149].
MSCs expressing the CD/5-FC product (CD/5-FC MSCs) to promote cancer cell apoptosis is a novel but insufficiently studied model. They have been already evaluated in colorectal cancer and lung metastases with promising results [150], [151]. Nguyen Thai et al. assessed the efficacy of CD/5-FC MSCs against human osteosarcoma cells in vitro and in vivo. They administrated MSCs bearing the CD gene combined with the nontoxic 5-FC prodrug. Interestingly, transduced MSCs were more capable of migrating towards osteosarcoma cells than native MSCs. The tumour volume increased at days 2–3 following the administration of CD MSCs, however, when the 5-FC prodrug was administered the tumour volume was gradually decreased from the fifth day. CD MSCs that were taken together with the 5-FC prodrug showed significant cytotoxicity against osteosarcoma cells in an animal model [132].
5.2.4. IL-12
The beneficial activity of IL-12 has been shown in several cancer models; however, initial enthusiasm faded due to severe toxicity with systemic administration. To overcome this, local gene delivery strategies have been used. Aerosol therapy including a polycationic IL-12-gene carrier was effective in reducing both the number of lung metastases and the nodule size in mice with established osteosarcoma lung metastases [152]. In addition, IL-12 increased Fas expression in osteosarcoma and Ewing's sarcoma cells through the enhancement of Fas promoter activity, causing cancer cell apoptosis [153], [154]. The local gene delivery of IL-12 using adenoviral vectors may be effective for Ewing's sarcoma but not in patients with distant tumour metastases [155]. The anti-tumoural effect of IL-12 to osteosarcoma and Ewing's sarcoma and the ability of MSCs to migrate to the tumour site can be combined to deliver IL-12 locally to tumours. Murine MSCs infected with an adenoviral vector carrying the IL-12 gene injected into mice bearing Ewing's sarcoma were localized and produced IL-12 selectively in the tumour. Moreover, MSCs were also found in the lungs, spleen, and liver without harming these organs. p35 and p40 subunits of IL-12 were expressed in the tumour site, inhibiting tumour growth [156].
6. MSCs as delivery vehicles of anti-tumor nanoparticles to trigger bone sarcoma cell death
The tropism of MSCs to cancer tissue suggests their use as vehicles for local delivery of antineoplastic agents. Mesenchymal stem cells can pass through endothelium and following blood flow migrate to the tumour site increasing targeting efficiency and reducing normal tissue toxicity [157]. Moreover, human MSCs can use an active efflux pump system to gain resistance to anticancer drugs they transfer [158], [159]. However, according to a study published by Belmar-Lopez et al., the MSC lineage to be used in a cell therapy should be carefully chosen as its safety and efficacy could vary between tumour types [160]. Moreover, antineoplastic agents that are used in MSC delivery systems would possibly destroy MSCs and lead to failure of this technique. To overcome this obstacle, Duchi et al. developed MSCs loaded with core-shell PMMA nanoparticles (FNPs) and meso‑tetrakis (4-sulfonataphenyl) porphyrin (TPPS) which is a photosensitizer. When these MSCs were co-cultured with human osteosarcoma cell line U2OS-RFP-TUBA1B in vitro and upon light irradiation, they induced controlled osteosarcoma cell death through the generation of reactive oxygen species [161].
7. Clinical evidence of MSC use for bone defect regeneration after osteosarcoma resection
The biological filling of a bone defect [BD] after the surgical resection of a tumour is often difficult. Physiological healing mechanisms cannot cope with defects greater than 2 cm(178). The dominant role of MSCs in bone regeneration and fracture healing [162], [163] combined with their antitumor properties raised the possibility of a role in filling the BD after bone sarcoma therapy [164].
Contemporary engineering and regenerative medicine techniques [165] using molecular [166], [167], [168] and mechanical “guidance” [169], [170], [171] can differentiate ADMSCs into various tissue types. Previous animal studies (157,155) demonstrated the regenerative ability of SCs that were applied either intravenously or topically, loaded on a different type of scaffolds [172], [173], [100]. Although MSCs have been used in humans to promote bone formation, concerning bone sarcoma, there are obvious limitations in their use [101], [174], [175]. Following the first application of MSCs by Herzog in 1951, many steps forward have been accomplished to find better, more effective and less harmful techniques [176] but still, we are far from fully understanding the complex intracellular mechanisms, or safe use in clinical practice and from broad application of MSCs at BDs following osteosarcoma treatment [177]. To the best of our knowledge, three reported techniques use MSCs to fill musculoskeletal BDs after bone tumour extraction.
7.1. Direct local application of MSCs
Hernigou et al. as mentioned earlier, evaluated the local application of autologous BMMSCs to enhance the healing of the host-to-allograft bone junction following bone tumour resection in 92 patients with chondrosarcoma, osteosarcoma, Ewing's sarcoma or other tumours. MSCs were harvested from the patients’ ilium using aspiration, collected in a 30 ml syringe and applied at the host bone-allograft junction. They aimed to retrospectively evaluate the risk of local tumour recurrence after surgical tumour resection at a mean of 15.4 years. Using three control groups, they concluded that the use of MSCs is probably safe, demonstrating no increase in the risk of local recurrence for all cancer types [100]. There were some safety concerns about human ADSC included in fat grafts, however, that were found to increase migration and metastases in a breast tumour and osteosarcoma models [74], [101].
7.2. In vitro differentiation on scaffolds and re-implantation
Morishita et al. used tissue-engineered osteogenic ceramics to fill BDs after bone tumour curettage. They isolated BMMSCs that were osteogenic differentiated to osteoblasts after culture on porous hydroxyapatite ceramics [178], shaped based on specific patient needs and implanted in three patients suffering from a bone tumour. Impressively, the ceramic consolidated with the bone with no relapse nor loosening at the 3-month follow up in all patients. The limb was fully loaded at two and three weeks postoperatively, and no adverse events were reported in the long-term follow-up.
7.3. Formation of 3D like bone grafts
In a recent study, Dufrane et al. presented a novel technique to treat a bone nonunion in extreme clinical and pathophysiological conditions using a human autologous scaffold-free osteogenic 3-dimensional (3D) graft derived from ADSCs [179]. Autologous ADSCs were in vitro differentiated in osteogenic medium, and after 15 to 18 days Demineralized Bone Marrow (DBM) was added to form a 3D scaffold-like graft. Scaffolds were produced and applied to three patients with bone tumours and three patients with non-unions. The 3D grafts were placed at the junction between the native host bone and the bone allograft or the growing prosthesis in cases of tumours. The final osteogenic product was stable and did not induce acute or long-term donor site morbidity up to 4 years after transplantation. This research team reported the use of the same graft in other 11 patients, with bone non-unions [180]. In total, six patients with bone sarcoma were treated (three osteosarcomas, two Ewing sarcomas and one with chondrosarcoma). Except for the DBM, no other donor tissue was used, thus overcoming all the host-to-donor and donor-to-host complications, increasing the safety of the technique and the chances of graft survival significantly. The 3D-scaffold was used as a bridge between the native host bone and the metallic grafts or bone allografts that were used for mechanical stabilization. Abnormal tissue formation was reported in one patient who had osteosarcoma recurrence, likely related to insufficient surgical margins after primary tumour resection. Another patient underwent surgical removal of the graft due to infection. Neither the age of the patients nor the previous chemotherapy affected the bone healing process. The patients had a satisfactory quality of life at three years. A disadvantage of the technique is the time interval between the fat tissue sample extraction and the application of the 3D-like graft that can be as long as four months (107 ± 28 days); however, this is a safe period that minimizes the chance of a subclinical tumour late recurrence.
8. Conclusions and future direction
Novel cellular therapies including Mesenchymal stromal cells, Dendritic cells, Natural Killer cells, Tumour Infiltrating Lymphocytes, Chimeric Antigen Receptor T cells, and γδΤ cells constitute a potential alternative path in bone sarcoma treatment. Using these cell types, we can achieve greater accumulation of antineoplastic agents in the tumour site while reducing adverse effects [181], [182]. Mesenchymal stromal cells therapies have the potential to alter our therapeutic strategies, and improve outcomes for patients.
Mesenchymal stromal cells have promise as they can be used as vectors of antineoplastic agents and for bone defect regeneration after bone sarcoma resection. However, MSCs may not be safe as a sole therapy for bone sarcomas because most existing data suggest that they could accelerate pulmonary metastases after resection of the primary tumour, promote proliferation of cancer cells at the tumour site and increase chemoresistance in doxorubicin and cisplatin when interacting with cancer cells. However, the use of transduced MSCs to express specific anti-cancer proteins selectively to the tumour site is a promising therapeutic approach. MSCs expressing TRAIL, OPG, IL-12 or the CD/5-FC prodrug selectively to tumour site have been studied in-vitro and in-vivo animal models with promising results. Moreover, MSCs could be suitable for delivering drugs in osteosarcoma site using photodynamic techniques.
Bone marrow and adipose tissue were the primary cell source in most studies using MSCs. However, MSCs isolated from neonatal tissues appear to have advantages over adult sources; they are usually discarded without use, can be isolated easily and atraumatically with modern techniques and are associated with fewer adverse effects in clinical trials. Moreover, MSCs isolated from neonatal tissues have a lower risk of mutations and present superior proliferative, regenerative and immunosuppressive potentials over those from adult sources [183]. When it comes to comparison between different neonatal sources, it seems that foetal MSCs, especially those isolated from the chorionic membrane and umbilical cord, show a significantly higher expansion capacity than maternal MSCs [184]. However, the use of foetal MSCs is challenging as their isolation is possible during the early stages of life and can harm fetal development.
Recently, there has been a comparison between MSCs derived from induced pluripotent stem cells and ADMSCs regarding their use for tissue engineering with both presenting vessel [185] and bone formation capabilities [186], [187], topically tumour progression control [188] and ease of use for tissue engineering [189], [190]. In vivo data for the use ADMSCs expressing membrane TRAIL is promising for Ewing's sarcoma. For osteosarcoma, further in vivo study is required. Determining the optimal target: effector ratio and degree of physical contact between the MSCs and cancer cells are areas for future investigation. Also, in vitro findings may not be found in vivo due to the more heterogeneous cell populations. A key need is the ability to study microenvironments using tools which allow for disease modelling and personalized theranostic evaluations. Emerging biofabrication [191] and organ-on-a-chip approaches seem to offer significant promise in this regard, although a critical challenge is the rate and cost of such models [192]. The development of high-throughput techniques to create and assess validated micro-tissue models for specific sarcomas would allow for cellular approaches to be assessed in vitro across heterogeneous populations and on personalized tissue models.
As no defined protocols exist for treating large bone defects after tumour resection, the above studies offer some promising results of different techniques [193]. Technology provides us with the means to “navigate” the MSCs and transform them into other cell types with different capabilities promoting healing and playing an antitumor role. Thus, using tissue engineering technology, we can build a portfolio of smart biomaterials and have a more personalized therapy applied, potentially with antitumor and osteogenic capabilities. Nowadays, we can manipulate the implanted grafts pre- or intra-operatively. Two and three-dimensional co-cultures systems have been widely studied in order to understand the relationship between cancer and surrounding cells, follow cancer progression and study different drugs. 3D co-cultures are gaining importance as they can closely mimic the cancer environment using porous and ECM-like structures that allow a more biological cell response when compared with the 2D cultures. This will be reflected not only on fundamental culture properties, such as proliferation or cell morphology but also in protein and gene expression profiles. These enhanced and more realistic cell properties are appealing for drug screening assays. Concerning bone sarcomas, these strategies can mimic sarcoma biological microenvironment, giving more realistic information about the diseases. Gao et al., thoroughly reviewed this topic.
Although cellular strategies offer promise, cell behaviour is complex and in vitro and animal models are not always predictive of human in vivo response. Thus, better models which allow mechanistic studies using human cells are required. MSC may have value in creating improved patient derived xenograft (PDX) models of osteosarcoma. The addition of MSC to a tumor specimen may enhance implantation into an immunodeficient mouse in a manner superior to that seen with Matrigel alone. We may not be far from the broad application of mesenchymal stromal cells as first-line therapy for bone sarcoma, as there have been made steps to more personalized cell therapy.
Acknowledgments
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there are no conflicts of interest.
Declarations of interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2019.100231.
Appendix. Supplementary materials
References
- 1.Hui J.Y.C. Epidemiology and etiology of sarcomas. Surg. Clin. North Am. 2016 doi: 10.1016/j.suc.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Amankwah E.K., Conley A.P., Reed D.R. Epidemiology and therapies for metastatic sarcoma. Clin. Epidemiol. 2013 doi: 10.2147/CLEP.S28390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ottaviani G., Jaffe N. Pediatric and adolescent osteosarcoma. In: Jaffe N., Bruland O., Bielack S., editors. Cancer Treatment and Research. Springer; Boston, MA: 2009. [DOI] [Google Scholar]
- 4.Grohar P.J., Janeway K.A., Mase L.D., Schiffman J.D. Advances in the treatment of pediatric bone sarcomas. Am. Soc. Clin. Oncol. Educ. B. 2017 doi: 10.14694/EDBK_175378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y.S., Deng Z.H., Zeng C., Lei G.H. JNK pathway in osteosarcoma: pathogenesis and therapeutics, J. Recept. Signal Transduct. 2016;36:465–470. doi: 10.3109/10799893.2015.1122045. [DOI] [PubMed] [Google Scholar]
- 6.Polychronidou G., Karavasilis V., Pollack S.M., Huang P.H., Lee A., Jones R.L. Novel therapeutic approaches in chondrosarcoma. Futur. Oncol. 2017;13:637–648. doi: 10.2217/fon-2016-0226. [DOI] [PubMed] [Google Scholar]
- 7.Bölling T., Hardes J., Dirksen U. Management of bone tumours in paediatric oncology. Clin. Oncol. 2013;25:19–26. doi: 10.1016/j.clon.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Gerrand C., Athanasou N., Brennan B., Grimer R., Judson I., Morland B., Peake D., Seddon B., Whelan J. UK guidelines for the management of bone sarcomas. Clin Sarcoma Res. 2016:6. doi: 10.1186/s13569-016-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissman I.L. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 10.Trávníčková M., Bačáková L. Application of adult mesenchymal stem cells in bone and vascular tissue engineering. Physiol. Res. 2018;67:831–850. doi: 10.33549/physiolres.933820. [DOI] [PubMed] [Google Scholar]
- 11.Shende P., Gupta H., Gaud R.S. Cytotherapy using stromal cells: current and advance multi-treatment approaches. Biomed. Pharmacother. 2018 doi: 10.1016/j.biopha.2017.10.127. [DOI] [PubMed] [Google Scholar]
- 12.Bogdanowicz D.R., Lu H.H. Designing the stem cell microenvironment for guided connective tissue regeneration. Ann. N. Y. Acad. Sci. 2017 doi: 10.1111/nyas.13553. [DOI] [PubMed] [Google Scholar]
- 13.Ramdasi S., Sarang S., Viswanathan C. Potential of mesenchymal stem cell based application in cancer. Int. J. Hematol. Stem Cell Res. 2015;9:41–49. [PMC free article] [PubMed] [Google Scholar]
- 14.Guadix J.A., Zugaza J.L., Gálvez-Martín P. Características, aplicaciones y perspectivas de las células madre mesenquimales en terapia celular. Med. Clin. (Barc) 2017 doi: 10.1016/j.medcli.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 15.Moore K.A., Lemischka I.R. Stem Cells and Their Niches. Science (80-.) 2006;311:1880. doi: 10.1126/science.1110542. http://science.sciencemag.org/content/311/5769/1880.abstract LP-1885. [DOI] [PubMed] [Google Scholar]
- 16.Chacón-Martínez C.A., Koester J., Wickström S.A. Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development. 2018 doi: 10.1242/dev.165399. [DOI] [PubMed] [Google Scholar]
- 17.Mitsiadis T.A., Barrandon O., Rochat A., Barrandon Y., De Bari C. Stem cell niches in mammals. Exp. Cell Res. 2007;313:3377–3385. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Briest F., Berndt A., Clement J., Junker K., Von Eggeling F., Grimm S., Friedrich K. Tumor-stroma interactions in tumorigenesis: lessons from stem cell biology. Front. Biosci. 2012:1871–1887. doi: 10.2741/509. http://www.bioscience.org/2012/v4e/af/509/fulltext.php?bframe=2.htm [DOI] [PubMed] [Google Scholar]
- 19.Dvorak H.F. Tumors: wounds that do not heal. N. Engl. J. Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 20.Spaeth E., Klopp A, Dembinski J., Andreeff M., Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 21.Hong I., Lee H., Kang K. Mesenchymal stem cells and cancer: friends or enemies? Mutat. Res. - Fundam. Mol. Mech. Mutagen. 2014:1–9. doi: 10.1016/j.mrfmmm.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerlin L., Park T.S., Zambidis E.T., Donnenberg V.S., Donnenberg A.D. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie. 2013;95:2235–2245. doi: 10.1016/j.biochi.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.I. Christodoulou, M. Goulielmaki, M. Devetzi, M. Panagiotidis, G. Koliakos, Mesenchymal stem cells in preclinical cancer cytotherapy : a systematic review, 8 (2018) 1–38. [DOI] [PMC free article] [PubMed]
- 24.Magge S.N., Malik S.Z., Royo N.C., Chen H.I., Yu L.Y., Snyder E.Y., O'Rourke D.M., Watson D.J. Role of monocyte chemoattractant protein-1 (MCP-1/CCL2) in migration of neural progenitor cells toward glial tumors. J. Neurosci. Res. 2009 doi: 10.1002/jnr.21983. [DOI] [PubMed] [Google Scholar]
- 25.Sun L., Lee J., Fine H.A. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J. Clin. Invest. 2004;113:1364–1374. doi: 10.1172/JCI20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Son B.-R., Marquez-Curtis L.A., Kucia M., Wysoczynski M., Turner A.R., Ratajczak J., Ratajczak M.Z., Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 27.Imitola J., Raddassi K., Park K.I., Mueller F.-J., Nieto M., Teng Y.D., Frenkel D., Li J., Sidman R.L., Walsh C.A., Snyder E.Y., Khoury S.J. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garzotto D., Giacobini P., Crepaldi T., Fasolo A., De Marchis S. Hepatocyte growth factor regulates migration of olfactory interneuron precursors in the rostral migratory stream through Met-Grb2 coupling. J. Neurosci. 2008;28:5901–5909. doi: 10.1523/JNEUROSCI.1083-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt N.O., Przylecki W., Yang W., Ziu M., Teng Y., Kim S.U., Black P.M., Aboody K.S., Carroll R.S. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;7:623–629. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carbajal K.S., Schaumburg C., Strieter R., Kane J., Lane T.E., James A.A. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc. Natl. Acad. Sci. USA. 2010;107:11068–11073. doi: 10.1073/pnas.1006375107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazennec G., Lam P.Y. Recent discoveries concerning the tumor – mesenchymal stem cell interactions. Biochim. Biophys. Acta. 2016;1866:290–299. doi: 10.1016/j.bbcan.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Dai L., Moniri M.R., Zeng Z., Zhou J.X., Rayat J., Warnock G.L. Potential implications of mesenchymal stem cells in cancer therapy. Cancer Lett. 2011;305:8–20. doi: 10.1016/j.canlet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Sotiropoulou P.A, Papamichail M. Immune properties of mesenchymal stem cells. Methods Mol. Biol. 2007;407:225–243. doi: 10.1007/978-1-59745-536-7_16. [DOI] [PubMed] [Google Scholar]
- 34.Shi Y., Hu G., Su J., Li W., Chen Q., Shou P., Xu C., Chen X., Huang Y., Zhu Z., Huang X., Han X., Xie N., Ren G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 35.Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A.I., Zhao R.C., Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Ghannam S., Bouffi C., Djouad F., Jorgensen C., Noël D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res. Ther. 2010;1:2. doi: 10.1186/scrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corcione A., Benvenuto F., Ferretti E., Giunti D., Cappiello V., Cazzanti F., Risso M., Gualandi F., Mancardi G.L., Pistoia V., Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Mult. Scler. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 38.M. Di Nicola, C. Carlo-stella, M. Magni, M. Milanesi, P.D. Longoni, S. Grisanti, A.M. Gianni, M. Di Nicola, C. Carlo-stella, M. Magni, M. Milanesi, P.D. Longoni, P. Matteucci, S. Grisanti, A.M. Gianni, induced by cellular or nonspecific mitogenic stimuli Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli, 99 (2013) 3838–3843. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed]
- 39.Plumas J., Chaperot L., Richard M.-J., Molens J.-P., Bensa J.-C., Favrot M.-C. Mesenchymal stem cells induce apoptosis of activated T cells. Leuk. Off. J. Leuk. Soc. Am. Leuk. Res. Fund, U.K. 2005;19:1597–1604. doi: 10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]
- 40.Rutella S., Danese S., Leone G. Tolerogenic dendritic cells : cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 41.Prevosto C., Zancolli M., Canevali P., Zocchi M.R., Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 42.Honczarenko M., Le Y., Swierkowski M., Ghiran I., Glodek A.M., Silberstein L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 43.Kinnaird T., Stabile E., Burnett M.S., Lee C.W., Barr S., Fuchs S., Epstein S.E. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 44.Feng B., Chen L. Review of mesenchymal stem cells and tumors: executioner or coconspirator? Cancer Biother. Radiopharm. 2009;24:717–721. doi: 10.1089/cbr.2009.0652. [DOI] [PubMed] [Google Scholar]
- 45.Beckermann B., Kallifatidis G., Groth A., Frommhold D., Apel A., Mattern J., Salnikov A., Moldenhauer G., Wagner W., Diehlmann A., Saffrich R., Schubert M., Ho A., Giese N., Bü Chler M., Friess H., Bü Chler P., Herr I. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br. J. Cancer. 2008;99:622–631. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turley E.A., Veiseh M., Radisky D.C., Bissell M.J. Mechanisms of disease: epithelial-mesenchymal transition - does cellular plasticity fuel neoplastic progression? Nat. Clin. Pract. Oncol. 2008;5:280–290. doi: 10.1038/ncponc1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwatsuki M., Mimori K., Yokobori T., Ishi H., Beppu T., Nakamori S., Baba H., Mori M. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293–299. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin F.T., Dwyer R.M., Kelly J., Khan S., Murphy J.M., Curran C., Miller N., Hennessy E., Dockery P., Barry F.P., Kerin M.J. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment : stimulation of epithelial to mesenchymal transition (EMT) Breast Cancer Res Treat. 2010;124:317–326. doi: 10.1007/s10549-010-0734-1. [DOI] [PubMed] [Google Scholar]
- 49.Tabe Y., Konopleva M., Munsell M.F., Marini F.C., Zompetta C., Tsao T., Zhao S., Pierce S., Igari J., Estey E.H., Andreeff M., Mcqueen T. PML-RAR α is associated with leptin-receptor induction : the role of mesenchymal stem cell – derived adipocytes in APL cell survival. Blood. 2004;103:1815–1822. doi: 10.1182/blood-2003-03-0802. [DOI] [PubMed] [Google Scholar]
- 50.Sun B., Roh K.-H., Park J.-R., Lee S.-R., Park S.-B., Jung J.-W., Kang S.-K., Lee Y.-S., Kang K.-S. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11:289–298. doi: 10.1080/14653240902807026. 1 p following 298. [DOI] [PubMed] [Google Scholar]
- 51.Lanza C., Morando S., Voci A., Canesi L., Principato M.C., Serpero L.D., Mancardi G., Uccelli A., Vergani L. Neuroprotective mesenchymal stem cells are endowed with a potent antioxidant effect in vivo. J. Neurochem. 2009;110:1674–1684. doi: 10.1111/j.1471-4159.2009.06268.x. [DOI] [PubMed] [Google Scholar]
- 52.Lu Y.-R., Yuan Y., Wang X.-J., Wei L.-L., Chen Y.-N., Cong C., Li S.-F., Long D., Tan W.-D., Mao Y.-Q., Zhang J., Li Y.-P., Cheng J.-Q. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol. Ther. 2016;7:245–251. doi: 10.4161/cbt.7.2.5296. [DOI] [PubMed] [Google Scholar]
- 53.Norozi F., Ahmadzadeh A., Shahrabi S., Vosoughi T. Mesenchymal stem cells as a double-edged sword in suppression or progression of solid tumor cells. Tumor Biol. 2016 doi: 10.1007/s13277-016-5187-7. [DOI] [PubMed] [Google Scholar]
- 54.Tannishtha R., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 55.Loebinger M.R., Janes S.M. Stem cells as vectors for antitumour therapy. Thorax. 2010;65:362. doi: 10.1136/thx.2009.128025. http://thorax.bmj.com/content/65/4/362.abstract LP-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moon R.T., Kohn A.D., De Ferrari G.V, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat. Rev. Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 57.Chen G., Shukeir N., Potti A., Sircar K., Aprikian A., Goltzman D., Rabbani S.A. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101:1345–1356. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- 58.Ma S., Liang S., Jiao H. Human umbilical cord mesenchymal stem cells inhibit C6 glioma growth via secretion of dickkopf-1 (DKK1) Mol Cell Biochem. 2014;1:277–286. doi: 10.1007/s11010-013-1836-y. [DOI] [PubMed] [Google Scholar]
- 59.Khakoo A.Y., Pati S., Anderson S.A., Reid W., Elshal M.F., Rovira I.I., Nguyen A.T., Malide D., Combs C.A., Hall G., Zhang J., Raff M., Rogers T.B., Stetler-stevenson W., Frank J.A., Reitz M., Finkel T. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J. Exp. Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torsvik A., Bjerkvig R. Mesenchymal stem cell signaling in cancer progression. Cancer Treat. Rev. 2013;39:180–188. doi: 10.1016/j.ctrv.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Khakoo A.Y., Pati S., Anderson S.A., Reid W., Elshal M.F., Rovira I.I., Nguyen A.T., Malide D., Combs C.A., Hall G., Zhang J., Raff M., Rogers T.B., Stetler-stevenson W., Frank J.A., Reitz M., Finkel T. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi ’ s sarcoma. J. Exp. Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dasari V.R., Kaur K., Velpula K.K., Gujrati M., Fassett D., Klopfenstein J.D., Dinh D.H., Rao J.S. Upregulation of PTEN in glioma cells by cord blood mesenchymal stem cells inhibits migration via downregulation of the PI3K / Akt Pathway. PLoS One. 2010:5. doi: 10.1371/journal.pone.0010350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Sanguinetti A., Bistoni G., Avenia N. Stem cells and breast cancer, where we are? A concise review of literature. G. Chir. 2011;32:438–446. [PubMed] [Google Scholar]
- 64.Han I., Yun M., Kim E., Kim B., Jung M., Kim S. Umbilical cord tissue-derived mesenchymal stem cells induce apoptosis in PC-3 prostate cancer cells through activation of JNK and downregulation of PI3K / AKT signaling. Stem Cell Res. Ther. 2014;5:1–9. doi: 10.1186/scrt443. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Yulyana Y., Ho I.A.W., Sia K.C., Newman J.P., Toh X.Y., Endaya B.B., Chan J.K.Y., Gnecchi M., Huynh H., Chung A.Y.F., Lim K.H., Leong H.S., Iyer N.G., Hui K.M., Lam P.Y.P. Paracrine factors of human fetal mscs inhibit liver cancer growth through reduced activation of IGF-1R / PI3K / Akt Signaling. Am. Soc. Gene Cell Ther. 2014:1–11. doi: 10.1038/mt.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldring C.E.P., Duffy P.A., Benvenisty N., Andrews P.W., Ben-david U., Eakins R., French N., Hanley N.A., Kelly L., Kitteringham N.R., Kurth J., Ladenheim D., Laverty H., Mcblane J., Narayanan G., Patel S., Reinhardt J., Rossi A., Sharpe M., Park B.K., Ami S. Perspective assessing the safety of stem cell therapeutics diagnosis of stem cell. Cell. Stem. Cell. 2011;8:618–628. doi: 10.1016/j.stem.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 67.F. Djouad, P. Plence, C. Bony, P. Tropel, F. Apparailly, J. Sany, Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals, 102 (2003) 3837–3845. doi: 10.1182/blood-2003-04-1193.D.N. [DOI] [PubMed]
- 68.F. Djouad, C. Bony, D. Noel, Earlier onset of syngeneic tumors in the presence of transplantation. (2006) 1060–1066. doi: 10.1097/01.tp.0000236098.13804.0b. [DOI] [PubMed]
- 69.Zhu W., Xu W., Jiang R., Qian H., Chen M., Hu J., Cao W., Han C., Chen Y. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp. Mol. Pathol. 2006;80:267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Sasser A.K., Sullivan N.J., Studebaker A.W., Hendey L.F., Axel A.E., Hall B.M. Interleukin-6 is a potent growth factor for ER-α-positive human breast cancer. FASEB J. 2007;21:3763–3770. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 71.Ramasamy R., Lam E.W.-F., Soeiro I., Tisato V., Bonnet D., Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 72.Karnoub A.E., Dash A.B., Vo A.P., Sullivan A., Brooks M.W., Bell G.W., Richardson A.L., Polyak K., Tubo R., Weinberg R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–665. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 73.Kyriakou C.A., Yong K.L., Benjamin R., Nathwani A.C. Human mesenchymal stem cells (hMSCs) expressing truncated soluble vascular endothelial growth factor receptor (tsFlk-1) following lentiviral-mediated gene transfer inhibit growth of Burkitt ’ s lymphoma in a murine model. J Gene Med. 2006;8:253–264. doi: 10.1002/jgm.840. [DOI] [PubMed] [Google Scholar]
- 74.Rowan B.G., Gimble J.M., Sheng M., Anbalagan M., Jones R.K., Frazier T.P., Asher M., Lacayo E.A., Friedlander P.L., Kutner R., Chiu E.S. Human adipose tissue-derived stromal / stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS One. 2014;9:e89595. doi: 10.1371/journal.pone.0089595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng H., Zou W., Shen J., Xu L., Wang S., Fu Y.-X., Fan W. Opposite effects of coinjection and distant injection of mesenchymal stem cells on breast tumor cell growth. Stem. Cells. Transl. Med. 2016;5:1216–1228. doi: 10.5966/sctm.2015-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hall B., Dembinski J., Sasser A., Studeny M., Andreeff M., Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int. J. Hematol. 2007;86:8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- 77.Spaeth E.L., Dembinski J.L., Sasser A.K., Watson K., Klopp A., Hall B., Andreeff M., Marini F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009:4. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee O., Coathup M., Goodship A., Blunn G. Use of mesenchymal stem cells to facilitate bone regeneration in normal and chemotherapy-treated rats. Tissue Eng. 2005;11:1727–1735. doi: 10.1089/ten.2005.11.1727. [DOI] [PubMed] [Google Scholar]
- 79.Kalia P., Blunn G.W., Miller J., Bhalla A., Wiseman M., Coathup M.J. Do autologous mesenchymal stem cells augment bone growth and contact to massive bone tumor implants? Tissue Eng. 2006;12:1617–1626. doi: 10.1089/ten.2006.12.1617. [DOI] [PubMed] [Google Scholar]
- 80.Avril P., Duteille F., Ridel P., Heymann M.F., De Pinieux G., Rédini F., Blanchard F., Heymann D., Trichet V., Perrot P. Opposite effects of soluble factors secreted by adipose tissue on proliferating and quiescent osteosarcoma cells. Plast. Reconstr. Surg. 2016 doi: 10.1097/01.prs.0000479989.88114.8b. [DOI] [PubMed] [Google Scholar]
- 81.Bian Z.-Y., Fan Q.-M., Li G., Xu W.-T., Tang T.-T. Human mesenchymal stem cells promote growth of osteosarcoma: involvement of interleukin-6 in the interaction between human mesenchymal stem cells and Saos-2. Cancer Sci. 2010 doi: 10.1111/j.1349-7006.2010.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tu B., Du L., Fan Q.-M., Tang Z., Tang T.-T. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y., Chu Y., Yue B., Ma X., Zhang G., Xiang H., Liu Y., Wang T., Wu X., Chen B. Adipose-derived mesenchymal stem cells promote osteosarcoma proliferation and metastasis by activating the STAT3 pathway. Oncotarget. 2017;8:23809–23816. doi: 10.18632/oncotarget.15866. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.impactjournals.com/oncotarget.
- 84.Hernigou P., Flouzat Lachaniette C.H., Delambre J., Chevallier N., Rouard H. Regenerative therapy with mesenchymal stem cells at the site of malignant primary bone tumour resection: what are the risks of early or late local recurrence? Int. Orthop. 2014 doi: 10.1007/s00264-014-2384-0. [DOI] [PubMed] [Google Scholar]
- 85.Perrot P., Rousseau J., Bouffaut A.L., Rédini F., Cassagnau E., Deschaseaux F., Heymann M.F., Heymann D., Duteille F., Trichet V., Gouin F. Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS One. 2010 doi: 10.1371/journal.pone.0010999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aanstoos M.E., Regan D.P., Rose R.J., Chubb L.S., Ehrhart N.P. Do mesenchymal stromal cells influence microscopic residual or metastatic osteosarcoma in a murine model? Clin. Orthop. Relat. Res. 2016 doi: 10.1007/s11999-015-4362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsukamoto S., Honoki K., Fuji H., Tohma Y., Kido A., Mori T., Tsujiuchi T., Tanaka Y. Mesenchymal stem cells promote tumor engraftment and metastatic colonization in rat osteosarcoma model. Int. J. Oncol. 2012 doi: 10.3892/ijo.2011.1220. [DOI] [PubMed] [Google Scholar]
- 88.Lee S.-W., Jeon T.J., Biswal S. Effect of local treatment with adipose tissue-derived mesenchymal stem cells in the early tumorigenesis of osteosarcoma. Oncol. Rep. 2015;33:1381–1387. doi: 10.3892/or.2015.3711. [DOI] [PubMed] [Google Scholar]
- 89.Zhang P., Dong L., Yan K., Long H., Yang T.T., Dong M.Q., Zhou Y., Fan Q.Y., Ma B.A. CXCR4-mediated osteosarcoma growth and pulmonary metastasis is promoted by mesenchymal stem cells through VEGF. Oncol. Rep. 2013 doi: 10.3892/or.2013.2619. [DOI] [PubMed] [Google Scholar]
- 90.Yu F.-X., Hu W.-J., He B., Zheng Y.-H., Zhang Q.-Y., Chen L. Bone marrow mesenchymal stem cells promote osteosarcoma cell proliferation and invasion. World J. Surg. Oncol. 2015 doi: 10.1186/s12957-015-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fontanella R., Pelagalli A., Nardelli A., D'Alterio C., Ieranò C., Cerchia L., Lucarelli E., Scala S., Zannetti A. A novel antagonist of CXCR4 prevents bone marrow-derived mesenchymal stem cell-mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion. Cancer Lett. 2016 doi: 10.1016/j.canlet.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 92.Xu W.T, Bian Z.Y, Fan Q.M, Li G., Tang T.T. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 93.Avril P., Le Nail L.R., Brennan M., Rosset P., De Pinieux G., Layrolle P., Heymann D., Perrot P., Trichet V. Mesenchymal stem cells increase proliferation but do not change quiescent state of osteosarcoma cells: potential implications according to the tumor resection status. J. Bone Oncol. 2016 doi: 10.1016/j.jbo.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aanstoos M., Regan D.P., Ehrhart N.P. Do mesenchymal stromal cells influence microscopic residual or metastatic osteosarcoma in a murine model? Clin. Orthop. Relat. Res. 2016;474:707–715. doi: 10.1007/s11999-015-4362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsukamoto S., Honoki K., Fuji H., Tohma Y., Kido A., Mori T., Tsujiuchi T., Tanaka Y. Mesenchymal stem cells promote tumor engraftment and metastatic colonization in rat osteosarcoma model. Int. J. Oncol. 2012;40:163–169. doi: 10.3892/ijo.2011.1220. [DOI] [PubMed] [Google Scholar]
- 96.Yu F., Hu W., He B., Zheng Y., Zhang Q., Chen L. Bone marrow mesenchymal stem cells promote osteosarcoma cell proliferation and invasion. World J. Surg. Oncol. 2015;13:1–7. doi: 10.1186/s12957-015-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Avril P., Le Nail L.R., Brennan M., Rosset P., De Pinieux G., Layrolle P., Heymann D., Perrot P., Trichet V. Mesenchymal stem cells increase proliferation but do not change quiescent state of osteosarcoma cells: potential implications according to the tumor resection status, J. Bone Oncol. 2016;5:5–14. doi: 10.1016/j.jbo.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bian Z.-Y., Fan Q.-M., Li G., Xu W.-T., Tang T.-T. Human mesenchymal stem cells promote growth of osteosarcoma: involvement of interleukin-6 in the interaction between human mesenchymal stem cells and Saos-2. Cancer Sci. 2010;101:2554–2560. doi: 10.1111/j.1349-7006.2010.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tu B., Zhu J., Liu S., Wang L., Fan Q., Hao Y., Fan C., Tang T.-T. Mesenchymal stem cells promote osteosarcoma cell survival and drug resistance through activation of STAT3. Oncotarget. 2016:7. doi: 10.18632/oncotarget.10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hernigou P., Flouzat Lachaniette C.H., Delambre J., Chevallier N., Rouard H. Regenerative therapy with mesenchymal stem cells at the site of malignant primary bone tumour resection: what are the risks of early or late local recurrence? Int. Orthop. 2014;38:1825–1835. doi: 10.1007/s00264-014-2384-0. [DOI] [PubMed] [Google Scholar]
- 101.Perrot P., Rousseau J., Bouffaut A.L., Rédini F., Cassagnau E., Deschaseaux F., Heymann M.F., Heymann D., Duteille F., Trichet V., Gouin F. Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS One. 2010:5. doi: 10.1371/journal.pone.0010999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Studeny M., Marini F.C., Champlin R.E., Zompetta C., Fidler I.J., Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. http://www.ncbi.nlm.nih.gov/pubmed/12097260 [PubMed] [Google Scholar]
- 103.Horwitz E.M., Andreef M., Frassoni F. Mesenchymal Stromal Cells. Biol. Blood Marrow Transplant. 2007;13:53–57. [Google Scholar]
- 104.Xie C., Xie D.Y., Lin B.L., Zhang G.L., Wang P.P., Peng L., Gao Z.L. Interferon-β gene-modified human bone marrow mesenchymal stem cells attenuate hepatocellular carcinoma through inhibiting AKT/FOXO3a pathway. Br. J. Cancer. 2013 doi: 10.1038/bjc.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yi B.-R., Kang N.-H., Choi K.-C. Antitumor therapeutic effects of cytosine deaminase and interferon-β against endometrial cancer cells using genetically engineered stem cells in vitro. Anticancer Res. 2011;31:2853–2862. [PubMed] [Google Scholar]
- 106.Yi B.-R., Kim S.U., Choi K.-C. Synergistic effect of therapeutic stem cells expressing cytosine deaminase and interferon-beta via apoptotic pathway in the metastatic mouse model of breast cancer. Oncotarget. 2015;7 doi: 10.18632/oncotarget.6719. www.impactjournals.com/oncotarget [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matsuzuka T., Rachakatla R.S., Doi C., Maurya D.K., Ohta N., Kawabata A., Pyle M.M., Pickel L., Reischman J., Marini F., Troyer D., Tamura M. Human umbilical cord matrix–derived stem cells expressing interferon-β gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice. Lung Cancer. 2010;70:28–36. doi: 10.1016/j.lungcan.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dembinski J.L., Wilson S.M., Spaeth E.L., Studeny M., Zompetta C., Samudio I., Roby K., Andreeff M., Marini F.C. Tumor stroma engraftment of gene-modified mesenchymal stem cells as anti-tumor therapy against ovarian cancer. Cytotherapy. 2013 doi: 10.1016/j.jcyt.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Su Y., Cheng R., Zhang J., Qian J., Diao C., Ran J., Zhang H., Li L. Interferon-α2b gene-modified human bone marrow mesenchymal stem cells inhibit hepatocellular carcinoma by reducing the Notch1 levels. Life Sci. 2015 doi: 10.1016/j.lfs.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 110.Li X., Lu Y., Huang W., Xu H., Chen X., Geng Q., Fan H., Tan Y., Xue G., Jiang X. In vitro effect of adenovirus-mediated Human Gamma Interferon Gene Transfer into Human Mesenchymal Stem Cells for Chronic Myelogenous Leukemia. Hematol. Oncol. 2006;24:151–158. doi: 10.1002/hon.779. [DOI] [PubMed] [Google Scholar]
- 111.Yang X., Du J., Xu X., Xu C., Song W. IFN- γ -secreting-mesenchymal stem cells exert an antitumor effect in vivo via the trail pathway. J. Immunol. Res. 2014 doi: 10.1155/2014/318098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Du J., Zhou L., Chen X., Yan S., Ke M., Lu X., Wang Z., Yu W., Xiang A.P. IFN-γ-primed human bone marrow mesenchymal stem cells induce tumor cell apoptosis in vitro via tumor necrosis factor-related apoptosis-inducing ligand. Int. J. Biochem. Cell Biol. 2012 doi: 10.1016/j.biocel.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 113.Chen X., Lin X., Zhao J., Shi W., Zhang H., Wang Y., Kan B., Du L., Wang B., Wei Y., Liu Y., Zhao X. A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Am. Soc. Gene Ther. 2008;16:749–756. doi: 10.1038/mt.2008.3. [DOI] [PubMed] [Google Scholar]
- 114.Seo S.H., Kim K.S., Park S.H., Suh Y.S., Kim S.J., Jeun S.-S., Sung Y.C. The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Ther. 2011;18:488–495. doi: 10.1038/gt.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eliopoulos N., Francois M., Boivin M.-N., Martineau D., Galipeau J. Neo-organoid of marrow mesenchymal stromal cells secreting interleukin-12 for breast cancer therapy. Cancer Res. 2008;68:4810–4818. doi: 10.1158/0008-5472.CAN-08-0160. [DOI] [PubMed] [Google Scholar]
- 116.Nakamura K., Ito Y., Kawano Y., Kurozumi K., Kobune M., Tsuda H., Bizen A., Honmou O., Niitsu Y., Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 117.Xu G., Guo Y., Seng Z., Cui G., Qu J. Bone marrow-derived mesenchymal stem cells co-expressing interleukin-18 and interferon-β exhibit potent antitumor effect against intracranial glioma in rats. Oncol. Rep. 2015 doi: 10.3892/or.2015.4174. [DOI] [PubMed] [Google Scholar]
- 118.Xin H., Kanehira M., Mizugushi H., Hayakawa T., Kikuchi T., Nukiwa T., Saijo Y. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007;25:1618–1626. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- 119.Komarova S., Kawakami Y., Stoff-khalili M.A., Curiel D.T., Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. MolCancer Ther. 2006;5:755–767. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 120.Micheau O., Shirley S., Dufour F. Death receptors as targets. Br. J. Pharmacol. 2013;169:1723–1744. doi: 10.1111/bph.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Evdokiou A., Bouralexis S., Findlay D.M. Chemotherapeutic agents sensitize osteogenic sarcoma cells, but not normal human bone cells, to APO2L / TRAIL-induced apoptosis. Int. J. Cancer. 2002;504:491–504. doi: 10.1002/ijc.10376. [DOI] [PubMed] [Google Scholar]
- 122.Loebinger M.R., Eddaoudi A., Davies D., Janes S.M. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wiley S.R., Schooley K., Smolak P.J., Nicholl J.K., Sutherland F., Smith T.D., Rauch C., Smith C.A., Goodwin R.G. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 124.Ashkenazi A., Pai R.C., Fong S., Leung S., Lawrence D.A., Marsters S.A., Blackie C., Chang L., Mcmurtrey A.E., Hebert A., Deforge L., Koumenis I.L., Lewis D., Harris L., Bussiere J., Koeppen H., Shahrokh Z., Schwall R.H. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Compte M., Cuesta A., Sanchez-Martin D., Alvarez-Vallina L. Tumor immunotherapy using gene-modified human mesenchymal stem cells loaded into synthetic extracellular matrix scaffolds. Stem Cells. 2009;27:753–760. doi: 10.1634/stemcells.2008-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Z. Gamie, K. Kapriniotis, D. Papanikolaou, E. Haagensen, R. Da, C. Ribeiro, K. Dalgarno, A. Krippner-Heidenreich, C. Gerrand, E. Tsiridis, K. Samora Rankin, TNF-related apoptosis-inducing ligand (TRAIL) for bone sarcoma treatment: pre-clinical and clinical data, (2017). doi: 10.1016/j.canlet.2017.08.036. [DOI] [PubMed]
- 127.Rossignoli F., Spano C., Grisendi G., Foppiani E.M., Golinelli G., Mastrolia I., Bestagno M., Candini O., Petrachi T., Recchia A., Miselli F., Rovesti G., Orsi G., Veronesi E., Medici G., Petocchi B., Pinelli M., Horwitz E.M., Conte P., Dominici M. MSC-delivered soluble TRAIL and paclitaxel as novel combinatory treatment for pancreatic adenocarcinoma. Theranostics. 2019:9. doi: 10.7150/thno.27576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang X., Yang Y., Zhang L., Lu Y., Zhang Q., Fan D., Zhang Y. Mesenchymal stromal cells as vehicles of for the treatment of B cell lymphoma combined with IDO pathway inhibitor D -1-methyl-tryptophan. J. Hematol. Oncol. 2017;56:1–14. doi: 10.1186/s13045-017-0397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ghaedi M., Soleimani M., Wu J. Mesenchymal stem cells as vehicles for targeted delivery of anti-angiogenic protein to solid tumors. J. Gene Med. 2011;13:171–180. doi: 10.1002/jgm.1552. [DOI] [PubMed] [Google Scholar]
- 130.Z. Karjoo, X. Chen, A. Hatefi, Progress and problems with the use of suicide genes for targeted cancer therapy,.Progress 2017. doi: 10.1016/j.addr.2015.05.009. [DOI] [PMC free article] [PubMed]
- 131.Uchibori R., Okada T., Ito T. Retroviral vector-producing mesenchymal stem cells for targeted suicide cancer gene therapy. J. Gene Med. 2009;11:373–381. doi: 10.1002/jgm.1313. [DOI] [PubMed] [Google Scholar]
- 132.NguyenThai Q., Sharma N., Luong D.H., Jeong D.K. Targeted inhibition of osteosarcoma tumor growth by bone marrow-derived mesenchymal stem cells expressing cytosine ... Targeted inhibition of osteosarcoma tumor growth by bone marrow-derived mesenchymal stem cells expressing cytosine deaminase / 5- fl uor. J. Gene Med. 2015;17:87–99. doi: 10.1002/jgm.2826. [DOI] [PubMed] [Google Scholar]
- 133.Hagenhoff A., Bruns C.J., Zhao Y., Von Lüttichau I., Spitzweg C., Nelson P.J., Hagenhoff A., Bruns C.J., Zhao Y., Von Lüttichau I., Niess H., Spitzweg C., Nelson P.J. Expert opinion on biological therapy harnessing mesenchymal stem cell homing as an anticancer therapy. Expert Opin. Biol. Ther. 2016;16:1079–1092. doi: 10.1080/14712598.2016.1196179. [DOI] [PubMed] [Google Scholar]
- 134.Kucerova L., Altanerova V., Matuskova M., Kucerova L., Altanerova V., Matuskova M., Tyciakova S., Altaner C. Adipose tissue − derived human mesenchymal stem cells mediated prodrug cancer gene therapy mediated prodrug cancer gene therapy. Cancer Res. 2007;67:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 135.Kucerova L., Matuskova M., Pastorakova A., Tyciakova S., Jakubikova J., Bohovic R., Altanerova V., Altaner C. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J Gene Med. 2008;10:1071–1082. doi: 10.1002/jgm.1239. 2008. [DOI] [PubMed] [Google Scholar]
- 136.Yang L., Zhang Y., Cheng P. Mesenchymal stem cells engineered to secrete pigment epithelium derived factor inhibit tumor metastasis and the formation of malignant ascites in a murine colorectal peritoneal carcinomatosis model Liping. Hum. Gene Ther. 2016;27:1–30. doi: 10.1089/hum.2015.135. [DOI] [PubMed] [Google Scholar]
- 137.C. Zeng, S. Zhou, Mesenchymal stem cell-based NK4 gene therapy in nude mice bearing gastric cancer xenografts, (2014) 2449–2462. [DOI] [PMC free article] [PubMed]
- 138.Q. Chen, P. Cheng, T.A.O. Yin, H. He, L.I. Yang, Y. Wei, X. Chen, Therapeutic potential of bone marrow-derived mesenchymal stem cells producing pigment epithelium-derived factor in lung carcinoma, (2012) 527–534. doi: 10.3892/ijmm.2012.1015. [DOI] [PubMed]
- 139.Zimmerlin L., Donnenberg A.D. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie. 2014;95:1–25. doi: 10.1016/j.biochi.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grisendi G., Spano C., D'Souza N., Dominici M. Mesenchymal progenitors expressing TRAIL induce apoptosis in sarcomas. Stem Cells. 2015:859–869. doi: 10.1002/stem.1903. [DOI] [PubMed] [Google Scholar]
- 141.Mitsiades N., Poulaki V., Mitsiades C., Tsokos M. Ewing's sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5. CANCER Res. 2001;61:2704–2712. [PubMed] [Google Scholar]
- 142.Kontny H.U., Hämmerle K., Klein R., Shayan P., Mackall C.L., Niemeyer C.M. Sensitivity of ewing's sarcoma to TRAIL-induced apoptosis. Cell Death Differ. 2001 doi: 10.1038/sj.cdd.4400836. [DOI] [PubMed] [Google Scholar]
- 143.Van Valen F., Fulda S., Truckenbrod B., Eckervogt V., Sonnemann J., Hillmann A., Rödl R., Hoffmann C., Winkelmann W., Schäfer L., Dockhorn-Dworniczak B., Wessel T., Boos J., Debatin K., Jürgens H. Apoptotic responsiveness of the Ewing’s sarcoma family of tumours to tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) Int. J. Cancer. 2000;88:252–259. doi: 10.1002/1097-0215(20001015)88:2<252::aid-ijc17>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 144.Guiho R., Biteau K., Grisendi G., Taurelle J., Chatelais M., Gantier M. TRAIL delivered by mesenchymal stromal/stem cells counteracts tumor development in orthotopic Ewing sarcoma models. Int. J. Cancer. 2016;139:2802–2811. doi: 10.1002/ijc.30402. [DOI] [PubMed] [Google Scholar]
- 145.Guiho R., Biteau K., Grisendi G., Chatelais M., Brion R., Taurelle J., Renault S., Heymann D., Dominici M., RedinI F. In vitro and in vivo discrepancy in inducing apoptosis by mesenchymal stromal cells delivering membrane-bound tumor necrosis factor–related apoptosis inducing ligand in osteosarcoma pre-clinical models. Cytotherapy. 2018 doi: 10.1016/j.jcyt.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 146.Theoleyre S., Wittrant Y., Tat S.K., Fortun Y., Redini F., Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004 doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 147.Lamoureux F., Richard P., Wittrant Y., Battaglia S., Pilet P., Trichet V., Blanchard F., Gouin F., Pitard B., Heymann D., Redini F. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007 doi: 10.1158/0008-5472.CAN-06-4130. [DOI] [PubMed] [Google Scholar]
- 148.Qiao B., Shui W., Cai L., Guo S., Jiang D. Human mesenchymal stem cells as delivery of osteoprotegerin gene: Homing and therapeutic effect for osteosarcoma. Drug Des. Devel. Ther. 2015 doi: 10.2147/DDDT.S77116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yi B.R., Choi K.J., Kim S.U., Choi K.C. Therapeutic potential of stem cells expressing suicide genes that selectively target human breast cancer cells: evidence that they exert tumoricidal effects via tumor tropism (review) Int. J. Oncol. 2012 doi: 10.3892/ijo.2012.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wei J., Blum S., Unger M., Jarmy G., Lamparter M., Geishauser A., Vlastos G.A., Chan G., Fischer K.D., Rattat D., Debatin K.M., Hatzopoulos A.K., Beltinger C. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell. 2004 doi: 10.1016/s1535-6108(04)00116-3. [DOI] [PubMed] [Google Scholar]
- 151.Kucerova L., Altanerova V., Matuskova M., Tyciakova S., Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007 doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 152.Jia S.-F., Worth L.L., Densmore C.L., Xu B., Duan X., Kleinerman E.S. Aerosol gene therapy with PEI:IL-12 eradicates osteosarcoma lung metastases. Clin. Cancer Res. 2003;9:3462–3468. [PubMed] [Google Scholar]
- 153.Zhou Z. Interleukin-12 Up-regulates fas expression in human osteosarcoma and ewing's sarcoma cells by enhancing its promoter activity. Mol. Cancer Res. 2005 doi: 10.1158/1541-7786.MCR-05-0092. [DOI] [PubMed] [Google Scholar]
- 154.Lafleur E.A., Jia S.-F., Worth L.L., Zhou Z., Owen-Schaub L.B., Kleinerman E.S. Interleukin (IL)-12 and IL-12 Gene Transfer Up-Regulate Fas Expression in Human Osteosarcoma and Breast Cancer Cells. CANCER Res. 2001;61:4066–4071. [PubMed] [Google Scholar]
- 155.Jia S.F., Duan X., Worth L.L., Guan H., Kleinerman E.S. Intratumor murine interleukin-12 gene therapy suppressed the growth of local and distant Ewing's sarcoma. Cancer Gene Ther. 2006 doi: 10.1038/sj.cgt.7700968. [DOI] [PubMed] [Google Scholar]
- 156.Duan X., Guan H., Cao Y., Kleinerman E.S. Murine bone marrow-derived mesenchymal stem cells as vehicles for interleukin-12 gene delivery into ewing sarcoma tumors. Cancer. 2009 doi: 10.1002/cncr.24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Karp J.M., Leng Teo G.S. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 158.Moitra K., Lou H., Dean M. Multidrug efflux pumps and cancer stem cells: insights into multidrug resistance and therapeutic development. Clin. Pharmacol. Ther. 2011;89:491–502. doi: 10.1038/clpt.2011.14. [DOI] [PubMed] [Google Scholar]
- 159.Sadhukha T., O'Brien T.D., Prabha S. Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. J. Control. Release. 2014;196:243–251. doi: 10.1016/j.jconrel.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 160.Belmar-Lopez C., Mendoza G., Oberg D., Burnet J., Simon C., Cervello I., Iglesias M., Ramirez J.C., Lopez-Larrubia P., Quintanilla M., Martin-Duque P. Tissue-derived mesenchymal stromal cells used as vehicles for anti-tumor therapy exert different in vivoeffects on migration capacity and tumor growth. BMC Med. 2013;11:139. doi: 10.1186/1741-7015-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Duchi S., Sotgiu G., Lucarelli E., Ballestri M., Dozza B., Santi S., Guerrini A., Dambruoso P., Giannini S., Donati D., Varchi G. Mesenchymal stem cells as delivery vehicle of porphyrin loaded nanoparticles: effective photoinduced in vitro killing of osteosarcoma. J. Control. Release. 2013:1–13. doi: 10.1016/j.jconrel.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 162.Qin Y., Guan J., Zhang C. Mesenchymal stem cells: mechanisms and role in bone regeneration. Postgrad. Med. J. 2014;90:643–647. doi: 10.1136/postgradmedj-2013-132387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jones E., Yang X. Mesenchymal stem cells and bone regeneration: current status. Injury. 2011;42:562–568. doi: 10.1016/j.injury.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 164.Gamie Z., MacFarlane R.J., Tomkinson A., Moniakis A., Tran G.T., Gamie Y., Mantalaris A., Tsiridis E. Skeletal tissue engineering using mesenchymal or embryonic stem cells: clinical and experimental data. Expert Opin. Biol. Ther. 2014;14:1611–1639. doi: 10.1517/14712598.2014.945414. [DOI] [PubMed] [Google Scholar]
- 165.Toyserkani N.M., Christensen M.L., Sheikh S.P., Sørensen J.A. Adipose-derived stem cells. Ann. Plast. Surg. 2015;75:117–123. doi: 10.1097/SAP.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 166.Xu H.H., Liu S.H., Guo Q.F., Liu Q.H., Li X.Y. Osteogenesis induced in goat bone marrow progenitor cells by recombinant adenovirus coexpressing bone morphogenetic protein 2 and basic fibroblast growth factor. Braz J Med Biol Res. 2013;46:809–814. doi: 10.1590/1414-431X20132929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang X., Guo J., Zhou Y., Wu G. The roles of bone morphogenetic proteins and their signaling in the osteogenesis of adipose-derived stem cells. Tissue Eng. Part B. Rev. 2014;20:84–92. doi: 10.1089/ten.teb.2013.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qin Y., Sun R., Wu C., Wang L., Zhang C. Exosome: A novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int. J. Mol. Sci. 2016:17. doi: 10.3390/ijms17050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wechsler M.E., Hermann B.P., Bizios R. Adult human mesenchymal stem cell differentiation at the cell population and single-cell levels under alternating electric current. Tissue Eng. Part C Methods. 2015;22 doi: 10.1089/ten.tec.2015.0324. ten.TEC.2015.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen Y., Xu J., Huang Z., Yu M., Zhang Y., Chen H., Ma Z., Liao H., Hu J. An innovative approach for enhancing bone defect healing using plga scaffolds seeded with extracorporeal-shock-wave-treated bone marrow mesenchymal stem cells (BMSCs) Sci. Rep. 2017;7:44130. doi: 10.1038/srep44130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Balikov D.A., Fang B., Chun Y.W., Crowder S.W., Prasai D., Lee J.B., Bolotin K.I., Sung H.-J. Directing lineage specification of human mesenchymal stem cells by decoupling electrical stimulation and physical patterning on unmodified graphene. Nanoscale. 2016:8. doi: 10.1039/c6nr04400j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Harada N., Watanabe Y., Sato K., Abe S., Yamanaka K., Sakai Y., Kaneko T., Matsushita T. Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials. 2014;35:7800–7810. doi: 10.1016/j.biomaterials.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 173.Gao X., Zhang X., Song J., Xu X., Xu A., Wang M., Xie B., Huang E., Deng F., Wei S. Osteoinductive peptide-functionalized nanofibers with highly ordered structure as biomimetic scaffolds for bone tissue engineering. Int. J. Nanomedicine. 2015;10:7109–7128. doi: 10.2147/IJN.S94045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang Y., Chu Y., Yue B., Ma X., Zhang G., Xiang H. Adipose-derived mesenchymal stem cells promote osteosarcoma proliferation and metastasis by activating the STAT3 pathway. Oncotarget. 2017;8:23803–23816. doi: 10.18632/oncotarget.15866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cortini M., Massa A., Avnet S., Bonuccelli G. Tumor-activated mesenchymal stromal cells promote osteosarcoma stemness and migratory potential via IL-6 secretion. PLoS One. 2016;11:1–22. doi: 10.1371/journal.pone.0166500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Holzapfel B.M., Wagner F., Martine L.C., Reppenhagen S., Rudert M., Schuetz M., Denham J., Schantz J.-T., Hutmacher D.W. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35:475–487. doi: 10.1007/s10555-016-9635-z. [DOI] [PubMed] [Google Scholar]
- 177.Watanabe Y., Harada N., Sato K., Abe S., Yamanaka K., Matushita T. Stem cell therapy: Is there a future for reconstruction of large bone defects? Injury. 2016;47:S47–S51. doi: 10.1016/S0020-1383(16)30012-2. [DOI] [PubMed] [Google Scholar]
- 178.Morishita T., Honoki K., Ohgushi H., Kotobuki N., Matsushima A., Takakura Y. Tissue engineering approach to the treatment of bone tumors: three cases of cultured bone grafts derived from patients’ mesenchymal stem cells. Artif. Organs. 2006;30:115–118. doi: 10.1111/j.1525-1594.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 179.Dufrane D., Docquier P.-L., Delloye C., Poirel H.A., André W., Aouassar N. Scaffold-free three-dimensional graft from autologous adipose-derived stem cells for large bone defect reconstruction: clinical proof of concept. Medicine (Baltimore) 2015;94:e2220. doi: 10.1097/MD.0000000000002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vériter S., André W., Aouassar N., Poirel H.A., Lafosse A., Docquier P.L., Dufrane D. Human adipose-derived mesenchymal stem cells in cell therapy: safety and feasibility in different “hospital exemption” clinical applications. PLoS One. 2015;10:1–18. doi: 10.1371/journal.pone.0139566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tarek N., Lee D. Natural killer cells for osteosarcoma. In: Kleinerman M.D.E, editor. Advances in Experimental Medicine and Biology. Springer; Cham: 2014. pp. 341–353. [DOI] [PubMed] [Google Scholar]
- 182.Rossig C. Cellular immunotherapy strategies for Ewing sarcoma. Immunotherapy. 2014 doi: 10.2217/imt.14.36. [DOI] [PubMed] [Google Scholar]
- 183.Araújo A.B., Salton G.D., Furlan J.M., Schneider N., Angeli M.H., Laureano Á.M., Silla L., Passos E.P., Paz A.H. Comparison of human mesenchymal stromal cells from four neonatal tissues: amniotic membrane, chorionic membrane, placental decidua and umbilical cord. Cytotherapy. 2017 doi: 10.1016/j.jcyt.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 184.Wu M., Zhang R., Zou Q., Chen Y., Zhou M., Li X., Ran R., Chen Q. Comparison of the biological characteristics of mesenchymal stem cells derived from the human placenta and umbilical cord. Sci. Rep. 2018:8. doi: 10.1038/s41598-018-23396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Freeman F.E., Allen A.B., Stevens H.Y., Guldberg R.E., Mcnamara L.M. Effects of in vitro endochondral priming and pre-vascularisation of human MSC cellular aggregates in vivo. Stem Cell Res. Ther. 2015:1–18. doi: 10.1186/s13287-015-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Klontzas M.E., Kenanidis E.I., Heliotis M., Tsiridis E., Mantalaris A. Bone and cartilage regeneration with the use of umbilical cord mesenchymal stem cells. Expert Opin. Biol. Ther. 2015;15:1541–1552. doi: 10.1517/14712598.2015.1068755. [DOI] [PubMed] [Google Scholar]
- 187.Miao C., Zhou L., Tian L., Zhang Y., Zhang W., Yang F., Liu T., Tang S., Liu F. Osteogenic differentiation capacity of in vitro cultured human skeletal muscle for expedited bone tissue engineering. Biomed Res. Int. 2017 doi: 10.1155/2017/8619385. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhao Q., Gregory C.A, Lee R.H., Reger R.L., Qin L., Hai B., Park M.S., Yoon N., Clough B., McNeill E., Prockop D.J., Liu F. MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs. Proc. Natl. Acad. Sci. U. S. A. 2015;112:530–535. doi: 10.1073/pnas.1423008112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Park B.H., Zhou L., Jang K.Y., Park H.S., Lim J.M., Yoon S.J., Lee S.Y., Kim J.R. Enhancement of tibial regeneration in a rat model by adipose-derived stromal cells in a PLGA scaffold. Bone. 2012;51:313–323. doi: 10.1016/j.bone.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 190.Shao J., Zhang W., Yang T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol. Res. 2015;48:1–7. doi: 10.1186/s40659-015-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jakab K., Norotte C., Marga F., Murphy K., Vunjak-Novakovic G., Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010 doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Caballero D., Kaushik S., Correlo V.M., Oliveira J.M., Reis R.L., Kundu S.C. Organ-on-chip models of cancer metastasis for future personalized medicine: from chip to the patient. Biomaterials. 2017 doi: 10.1016/j.biomaterials.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 193.Dobke M., Mackert G.A. Upper extremity sarcoma: impact of current practice guidelines and controversies on reconstructive approaches. Sicot-J. 2017;3:15. doi: 10.1051/sicotj/2017003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nakamizo A., Marini F., Amano T., Khan A., Studeny M., Gumin J., Chen J., Hentschel S., Vecil G., Dembinski J., Andreeff M., Lang F.F. Human bone marrow–derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 195.Beckermann B.M., Kallifatidis G., Groth A., Frommhold D., Apel A., Mattern J., Salnikov A.V., Moldenhauer G., Wagner W., Diehlmann A., Saffrich R., Schubert M., Ho A.D., Giese N., Büchler M.W., Friess H., Büchler P., Herr I. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br. J. Cancer. 2008 doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Iivanainen E., Lauttia S., Zhang N., Tvorogov D., Kulmala J., Grenman R., Salven P., Elenius K. The EGFR inhibitor gefitinib suppresses recruitment of pericytes and bone marrow-derived perivascular cells into tumor vessels. Microvasc. Res. 2009 doi: 10.1016/j.mvr.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 197.Schichor C., Birnbaum T., Etminan N., Schnell O., Grau S., Miebach S., Aboody K., Padovan C., Straube A., Tonn J.C., Goldbrunner R. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp. Neurol. 2006 doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 198.Lin G., Yang R., Banie L., Wang G., Ning H., Li L.C., Lue T.F., Lin C.S. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate. 2010 doi: 10.1002/pros.21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Klopp A.H., Zhang Y., Solley T., Amaya-Manzanares F., Marini F., Andreeff M., Debeb B., Woodward W., Schmandt R., Broaddus R., Lu K., Kolonin M.G. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin. Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xu S., Menu E., De Becker A., Van Camp B., Vanderkerken K., Van Riet I. Bone marrow-derived mesenchymal stromal cells are attracted by multiple myeloma cell-produced chemokine CCL25 and favor myeloma cell growth in vitro and in vivo. Stem Cells. 2012 doi: 10.1002/stem.787. [DOI] [PubMed] [Google Scholar]
- 201.Lin S.Y., Yang J., Everett A.D., Clevenger C.V., Koneru M., Mishra P.J., Kamen B., Banerjee D., Glod J. The isolation of novel mesenchymal stromal cell chemotactic factors from the conditioned medium of tumor cells. Exp. Cell Res. 2008 doi: 10.1016/j.yexcr.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dwyer R.M., Potter-Beirne S.M., Harrington K.A., Lowery A.J., Hennessy E., Murphy J.M., Barry F.P., O'Brien T., Kerin M.J. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin. Cancer Res. 2007 doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 203.Gutova M., Najbauer J., Frank R.T., Kendall S.E., Gevorgyan A., Metz M.Z., Guevorkian M., Edmiston M., Zhao D., Glackin C.A., Kim S.U., Aboody K.S. Urokinase plasminogen activator and urokinase plasminogen activator receptor mediate human stem cell tropism to malignant solid tumors. Stem Cells. 2008 doi: 10.1634/stemcells.2008-0141. [DOI] [PubMed] [Google Scholar]
- 204.Goldstein R.H., Reagan M.R., Anderson K., Kaplan D.L., Rosenblatt M. Human bone marrow-derived mscs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-10-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Birnbaum T., Roider J., Schankin C.J., Padovan C.S., Schichor C., Goldbrunner R., Straube A. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J. Neurooncol. 2007 doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- 206.Lee S.Y., Kim J.M., Cho S.Y., Kim H.S., Shin H.S., Jeon J.Y., Kausar R., Jeong S.Y., Lee Y.S., Lee M.A. TIMP-1 modulates chemotaxis of human neural stem cells through CD63 and integrin signalling. Biochem. J. 2014 doi: 10.1042/BJ20131119. [DOI] [PubMed] [Google Scholar]
- 207.Qiao B., Shui W., Cai L., Guo S., Jiang D. Human mesenchymal stem cells as delivery of osteoprotegerin gene: homing and therapeutic effect for osteosarcoma. Drug Des. Devel. Ther. 2015 doi: 10.2147/DDDT.S77116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.