Clostridium perfringens enterotoxin (CPE) is a pore-forming toxin that causes the symptoms of common bacterial food poisoning and several non-foodborne human gastrointestinal diseases, including antibiotic-associated diarrhea and sporadic diarrhea. In some cases, CPE-mediated disease can be very severe or fatal due to the involvement of enterotoxemia.
KEYWORDS: enterotoxin, Clostridium perfringens, enterotoxemia, mepacrine
ABSTRACT
Clostridium perfringens enterotoxin (CPE) is a pore-forming toxin that causes the symptoms of common bacterial food poisoning and several non-foodborne human gastrointestinal diseases, including antibiotic-associated diarrhea and sporadic diarrhea. In some cases, CPE-mediated disease can be very severe or fatal due to the involvement of enterotoxemia. Therefore, the development of potential therapeutics against CPE action during enterotoxemia is warranted. Mepacrine, an acridine derivative drug with broad-spectrum effects on pores and channels in mammalian membranes, has been used to treat protozoal intestinal infections in human patients. A previous study showed that the presence of mepacrine inhibits CPE-induced pore formation and activity in enterocyte-like Caco-2 cells, reducing the cytotoxicity caused by this toxin in vitro. Whether mepacrine is similarly protective against CPE action in vivo has not been tested. When the current study evaluated whether mepacrine protects against CPE-induced death and intestinal damage using a murine ligated intestinal loop model, mepacrine protected mice from the enterotoxemic lethality caused by CPE. This protection was accompanied by a reduction in the severity of intestinal lesions induced by the toxin. Mepacrine did not reduce CPE pore formation in the intestine but inhibited absorption of the toxin into the blood of some mice. Protection from enterotoxemic death correlated with the ability of this drug to reduce CPE-induced hyperpotassemia. These in vivo findings, coupled with previous in vitro studies, support mepacrine as a potential therapeutic against CPE-mediated enterotoxemic disease.
INTRODUCTION
By producing Clostridium perfringens enterotoxin (CPE), C. perfringens type F (formerly enterotoxigenic C. perfringens type A [1]) is one of the main causes of bacterial food poisoning (2), with about 1 million cases occurring annually in the United States (3). Additionally, C. perfringens type F strains are implicated in ∼10% of all antibiotic-associated diarrhea (AAD) cases (4). Typically, cases of CPE-associated AAD are more severe and of longer duration than most cases of food poisoning (4, 5), which usually involve diarrhea and abdominal cramps that resolve spontaneously within 24 h (2). However, mortality due to CPE-induced dehydration from diarrhea occasionally occurs, particularly in elderly people affected by this food poisoning (2, 6, 7).
In addition, fatal outbreaks of C. perfringens type F food poisoning have been reported that involved nonelderly people with preexisting drug-induced severe constipation or fecal impaction (6, 8). In those cases, it is presumed that the blockage of diarrhea by those preexisting conditions prolongs contact between CPE and the intestinal mucosa, thereby facilitating absorption of the toxin into the blood for action on distant organs (enterotoxemia). A similar effect is observed in a mouse model of CPE action when the toxin is directly injected into ligated intestinal loops, which are then incubated for up to 4 h (9, 10).
During disease, CPE is produced when C. perfringens sporulates in the intestine and the toxin is later released into the lumen after lysis of the mother cell (2, 5). CPE is a 35-kDa pore-forming protein that contains 319 amino acids with a unique primary sequence (11). Structurally this toxin consists of two domains (12, 13). The C-terminal domain of CPE mediates receptor binding to claudin receptors (14–19), which results in the formation of an ∼90-kDa small complex composed of CPE, receptor, and nonreceptor claudins (5, 17, 20). Using its N-terminal domain, CPE in small complexes oligomerizes, resulting in the formation of a hexameric prepore on the surface of the plasma membrane (5, 17, 21). β-Hairpin loops from the N-terminal domain of CPE extend to form a β-barrel, which inserts into the lipid bilayer to create a pore (22). The resulting CPE oligomeric pore, named the CH-1 large complex, is cation permeable and creates a Ca2+ influx into cells that triggers cell death (10, 23–28).
Practical therapeutic approaches to treat CPE effects in severe clinical scenarios, particularly the often-lethal enterotoxemia, do not currently exist but could be helpful. In the past, CPE binding to rabbit small intestinal mucosal membranes was successfully blocked in a competition assay for claudin binding by using synthetic peptides (16). In the same way, Caco-2 cells and small intestinal loops in rabbits can be protected against CPE using a peptide corresponding to the second extracellular loop sequence of claudin-4 as a receptor decoy (29, 30). However, the potential use of those synthetic peptides as therapeutic tools is limited considering their high cost and likely inactivation in the gastrointestinal tract (31).
Mepacrine is an ∼400-Da acridine derivative (32) that has been used clinically to treat and/or prevent intestinal infections caused by Giardia spp. (33, 34). In an in vitro model using pure synthetic lipid bilayers, the presence of mepacrine was shown to decrease CPE-induced electrophysiologic activity (35). More recently, it was demonstrated that mepacrine also reduces CPE-induced cytotoxicity in enterocyte-like Caco-2 cells in vitro (31). This protection involved at least two mechanisms: (i) the reduction of CPE pore formation by increasing the dissociation of CPE monomers and (ii) the inhibition of CPE pore activity (31). The effective therapeutic use of mepacrine in other intestinal infections, coupled with its ability to protect enterocyte-like cells in vitro from CPE action, provides justification for in vivo investigation of this drug as a potential therapeutic agent against CPE in vivo. Towards that goal, the present study used a ligated mouse intestinal loop model to evaluate the ability of mepacrine to protect against CPE-induced intestinal damage and enterotoxemic death.
RESULTS
Mepacrine protects mice from enterotoxemic death caused by CPE.
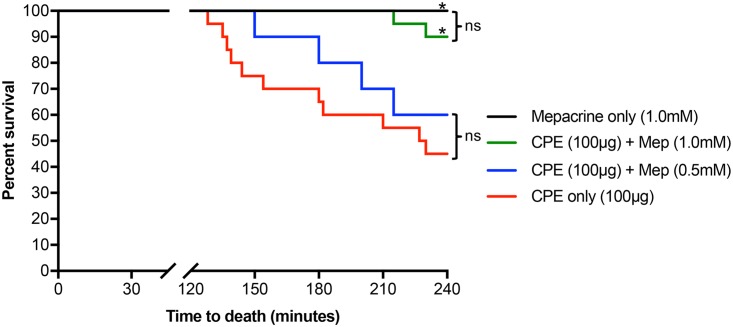
As mentioned in the Introduction, CPE is thought to cause fatal enterotoxemia after being absorbed from the intestine in individuals with severe constipation or fecal impaction, conditions that prolong contact between the toxin and the intestinal mucosa, thereby facilitating CPE absorption into the blood to damage distant organs (6, 8–10). Therefore, we evaluated whether mepacrine can protect mice from enterotoxemic death caused by CPE. In this experiment, mouse intestinal loops were cotreated for a prolonged period (up to 4 h) with 1 ml of Hank’s balanced salt solution (HBSS) containing 100 μg of CPE (a dose known to cause enterotoxemia [9, 10]) and one of two concentrations of mepacrine (0.5 or 1.0 mM). When viability was monitored, all mice (100%) treated with mepacrine alone (no CPE) survived for the full 4-h experimental period. However, mice treated with CPE alone began to die after 2 h. By 4 h, survival was significantly lower (45%) (Fig. 1). The presence of 1.0 mM mepacrine significantly improved survival of mice against CPE-induced enterotoxemia; the first death of mice was observed at 3.5 h, and 90% of mice survived the 4-h experiment. The first death of mice at the lower concentration of mepacrine was not observed until 2.5 h, and 60% of mice survived the entire 4-h experimental period, although this increased survival was not statistically significantly higher than the level for mice treated with CPE in the absence of mepacrine (Fig. 1). Collectively, these results indicated that mepacrine, particularly at high doses, can protect mice from enterotoxemic death induced by CPE.
FIG 1.
Mepacrine protects mice from CPE-induced lethal enterotoxemic death. Mouse intestinal loops received an injection of 1 ml of HBSS containing 100 μg of CPE plus 1.0 mM mepacrine (n = 20), 100 μg of CPE plus 0.5 mM mepacrine (n = 10), 100 μg of CPE alone (n = 20), or 1.0 mM mepacrine alone (n = 10). Incubation of intestinal loops took place for 4 h or until the animals died spontaneously. Mice were observed, and time to death was recorded and plotted. Kaplan-Meier survival curves were compared using log-rank analysis. A P value of <0.05 was regarded as statistically significant. *, P < 0.05 compared with blue and red lines; ns, not significant.
Effects of mepacrine on CPE-induced damage to the mouse small intestine.
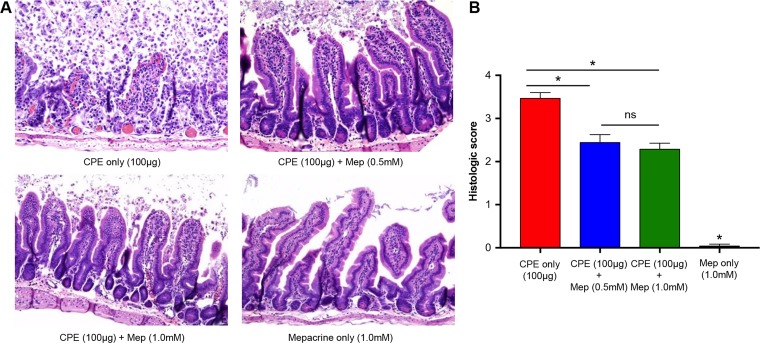
CPE induces intestinal damage in mice that may contribute to toxin absorption and uptake into circulation (36). To assess whether protection from CPE-induced enterotoxemic death correlates with protection from intestinal damage induced by this toxin, sections of challenged intestinal loops were assessed histologically according to the criteria discussed in Materials and Methods. As shown in Fig. 2, CPE induced significant damage to the mouse small intestinal loops. CPE-induced intestinal injury was characterized by villus blunting and fusion, as well as epithelial necrosis and desquamation (Fig. 2A). The overall severity of histopathology scores was significantly reduced when either of the two concentrations of mepacrine (0.5 or 1.0 mM) was coadministered with CPE in small intestinal loops (Fig. 2A and B). Minimal histologic changes developed in control (mepacrine alone) loops over the 4-h experimental duration. Taken together, the results shown in Fig. 2 indicate that treatment with mepacrine reduces CPE-induced intestinal damage in mice.
FIG 2.
Mepacrine reduces CPE-induced intestinal damage in mice. (A) Mouse intestinal loops received an injection of 1 ml of HBSS containing 100 μg of CPE plus 1.0 mM mepacrine (n = 20), 100 μg of CPE plus 0.5 mM mepacrine (n = 10), 100 μg of CPE alone (n = 20), or 1.0 mM mepacrine alone (n = 10). Incubation of intestinal loops took place for 4 h or until the animals died spontaneously. Following cryosectioning and H&E staining, intestinal damage was observed (magnification, ×100). (B) Histological score of intestinal loops treated with the indicated inocula for 4 h. Error bars show standard errors of the means. *, P < 0.05; ns, not significant.
Effects of mepacrine on CH-1 formation in the small intestine.
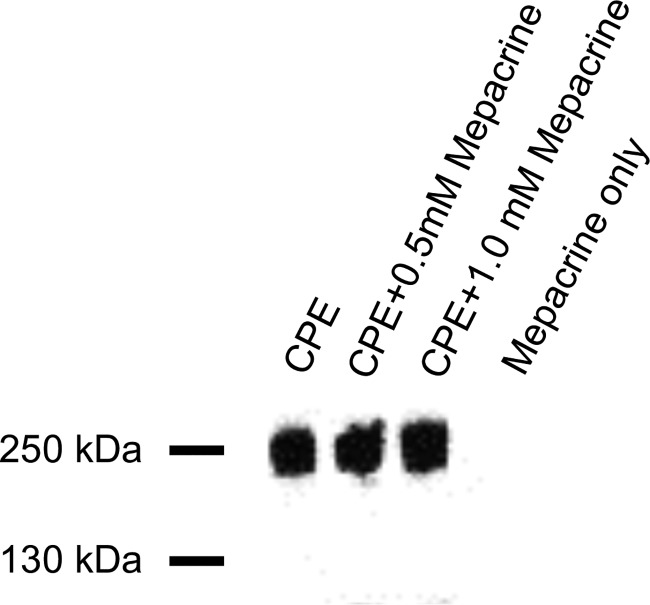
The CH-1 pore complex is required for CPE to cause Caco-2 cell cytotoxicity (29, 31, 37). In previous studies, we showed that the prolonged presence of mepacrine can reduce CH-1 levels present in Caco-2 cells by increasing CPE monomer dissociation from membranes prior to oligomerization (31). Therefore, an experiment was performed to assess whether mepacrine affects CH-1 levels in vivo. When mouse small intestinal loops were challenged with CPE in the presence or absence of either 0.5 mM or 1.0 mM mepacrine concentrations for 4 h, CPE Western blotting detected no significant differences in CH-1 complex levels among these groups of mice (Fig. 3).
FIG 3.
Effects of mepacrine on CH-1 complex formation in the intestine. Mouse small intestinal loops received an injection of 1 ml of HBSS containing 100 μg of CPE plus one of two concentrations of mepacrine (0.5 or 1.0 mM), 100 μg of CPE alone, or 1.0 mM mepacrine alone for 4 h. Intestinal tissue was then homogenized by sonication in RIPA buffer containing protease inhibitor cocktail plus 1 μl Benzonase (Qiagen). The homogenates were then analyzed by SDS-PAGE and Western blotting with a rabbit anti-CPE antibody.
Effects of mepacrine on CPE absorption from the intestine.
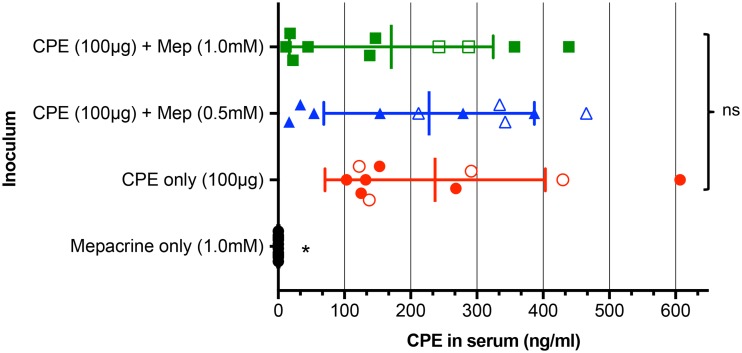
As mentioned previously, CPE gains access to the bloodstream from the intestine to produce enterotoxemia (9). To further evaluate whether mepacrine affects intestinal absorption of CPE, sera were collected from mice whose intestinal loops had been treated for 4 h (or until the animals died spontaneously) with 100 μg of CPE alone, 100 μg of CPE plus one of two concentrations of mepacrine (0.5 or 1.0 mM), or mepacrine alone (1.0 mM). Due to substantial animal-to-animal variation of CPE serum levels in all three groups of CPE-treated mice, the differences in the average CPE concentration in serum samples were not statistically significant between mice that were CPE treated in the presence of mepacrine and those treated in its absence (Fig. 4). However, 7/20 animals treated with both mepacrine and CPE had measurable CPE serum levels of ≤100 ng/ml, while 0/10 mice treated with CPE alone had such low serum CPE concentrations (P = 0.03) (Fig. 4). Notably, none of those animals with <100 ng/ml of serum CPE died. Collectively, these results suggest that mepacrine reduces CPE absorption in some mice and can also inhibit the lethal activity of CPE in serum, particularly when toxin concentrations are low.
FIG 4.
Effects of mepacrine on CPE absorption from the intestine. Mouse small intestinal loops received an injection of 1 ml of HBSS containing 100 μg of CPE plus one of two concentrations of mepacrine (0.5 or 1.0 mM), 100 μg of CPE alone, or 1.0 mM mepacrine alone. Incubation of intestinal loops took place for 4 h or until the animals died spontaneously. Blood samples were then collected by cardiocentesis, and serum samples were evaluated for CPE detection and quantitation by ELISA. Results show the means of samples from 10 mice/group. Closed symbols indicate serum samples obtained from mice that survived the experiment, while open symbols depict serum samples obtained from mice that died during the experiment. Error bars show standard errors of the means. *, P < 0.05; ns, not significant.
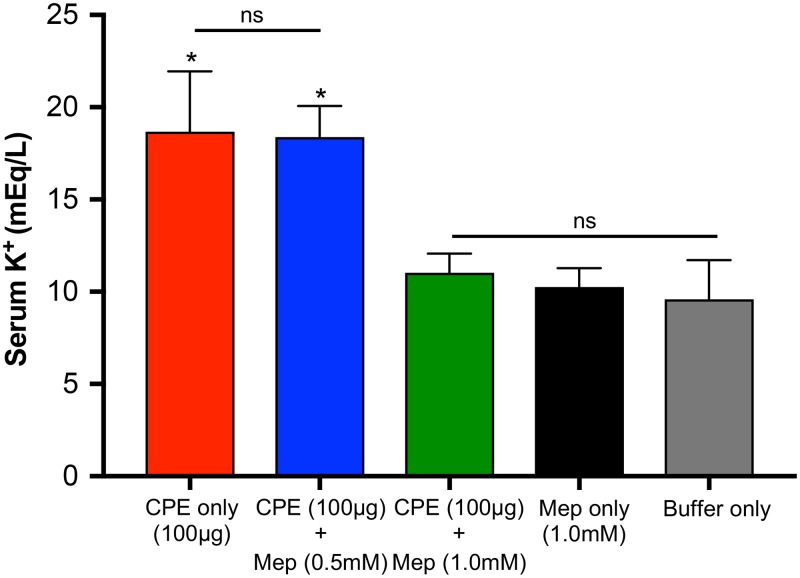
Mepacrine decreases hyperpotassemia caused by CPE.
Previous studies demonstrated that a lethal intravenous (i.v.) CPE injection results in elevated potassium levels in the blood of mice, causing rapid electrocardiogram (ECG) pattern changes and death from cardiac failure (38). Similarly, hyperpotassemia has also been reported in a mouse intestinal loop model of lethal CPE-induced enterotoxemia (9). To evaluate whether mepacrine protection from CPE-induced enterotoxemic death is associated with a reduction of hyperpotassemia, sera were collected from mice challenged with CPE in the absence or presence of 0.5 or 1.0 mM mepacrine and then assessed for potassium levels in the blood by inductively coupled plasma atomic emission spectroscopy (ICP-AES). The basal potassium level measures in sera from control mice receiving buffer only ranged from ∼8 to 12 meq/liter (Fig. 5). Similar values were measured in the mice treated with mepacrine alone. Significantly higher potassium levels were measured in mice receiving an intestinal treatment with CPE alone or CPE with a low concentration of mepacrine. However, cotreatment of CPE and a high concentration of mepacrine significantly reduced serum potassium levels (Fig. 5). Taken together, these results suggest that mepacrine interferes with the rise of potassium in the blood that is associated with CPE-induced lethality.
FIG 5.
Mepacrine reduces CPE-induced hyperpotassemia in mice. Mouse small intestinal loops received an injection of 1 ml of HBSS containing 100 μg of CPE plus one of two concentrations of mepacrine (0.5 or 1.0 mM), 100 μg of CPE alone, 1.0 mM mepacrine alone, or buffer only for 4 h. Incubation of intestinal loops took place for 4 h or until the animals died spontaneously. Blood samples were collected by cardiocentesis. Serum samples were analyzed by utilizing inductively coupled argon plasma emission spectrometry. After the precipitation of proteins, the protein-free supernatant of each sample was analyzed for potassium levels. Results show the means of samples from 8 mice/group. Error bars show standard errors of the means. *, P < 0.05 compared with green, black, and gray bars; ns, not significant.
DISCUSSION
As mentioned in the Introduction, the availability of CPE-targeted therapeutics could be beneficial for treating or preventing severe CPE-associated medical conditions, particularly the often-lethal enterotoxemia due to CPE absorbed from the intestines. An early study suggested that the agent mepacrine is a candidate CPE therapeutic since it interfered with CPE-induced electrophysiologic activity in artificial lipid bilayers (35). A more recent study demonstrated that mepacrine is also able to protect enterocyte-like Caco-2 cells from CPE action in vitro (31). This protection did not involve mepacrine inactivating the CPE protein; instead, this drug interfered with the formation of CPE pores and with the activity of pores already present on CPE-treated Caco-2 cells (31). With this previous information, the present study evaluated the potential therapeutic role of mepacrine against CPE in vivo using a mouse intestinal loop model of severe CPE action, which reproduces CPE-induced intestinal damage and enterotoxemic death in these animals (9, 10).
A first finding of the current study is that mepacrine can provide protection against CPE-induced lethal enterotoxemia. Specifically, this drug provided 4-h protection against CPE concentrations that can be found during food poisoning (i.e., 100 μg/g watery feces), as estimated previously in enzyme-linked immunosorbent assay (ELISA) analyses of stool samples from patients suffering from diarrhea induced by this toxin (39). Protection against enterotoxemic death was efficiently achieved in mice receiving 1.0 mM mepacrine. This concentration lies within the mepacrine dose used clinically to treat intestinal infections caused by Giardia spp. (40).
A second contribution of the present study was the finding that cotreatment of mouse intestinal loops with a high CPE dose and either of two different concentrations of mepacrine reduces the intestinal damage associated with this toxin for at least 4 h. Although intestinal damage was still visible in some areas of the treated loops, the overall improvement in the protection of the intestinal mucosa suggests the possibility of reduced fluid loss during natural CPE-associated intestinal disease in people without fecal impaction, since intestinal damage is needed for CPE to cause luminal fluid accumulation in rabbits (2, 36). Unfortunately, while the static mouse ligated small intestinal loop model is excellent for mimicking the intestinal obstruction thought to cause lethal enterotoxemia in people, this model does not result in intestinal fluid loss despite exhibiting CPE-induced intestinal damage (9). For this reason, we also assessed if mepacrine would inhibit CPE-induced luminal fluid accumulation in a previously described (36) rabbit small intestinal loop model of CPE intestinal fluid accumulation (data not shown). However, we found that mepacrine alone induced increased levels of luminal fluid accumulation similar to those induced by CPE alone in this rabbit model. This finding is not surprising, since a common side effect of using mepacrine to treat Giardia infections in humans is diarrhea (33, 34, 40). This effect was particularly likely given the conditions used in our experiment, i.e., closed rabbit small intestinal loops. In this model, there is no intestinal dilution or excretion of the drug in feces; therefore, any drug side effects would be particularly apparent in this ligated loop model. The question of whether mepacrine can impact the development of CPE-induced intestinal fluid accumulation should be revisited after development of an “open intestine” model of CPE-mediated disease or, better yet, a model of actual type F infection. A type F infection model involving gradual in vivo CPE production would also be more useful than a loop model using a sudden injection of high CPE concentrations to evaluate whether mepacrine can counter diarrhea if administered after CPE exposure. This possibility is suggested by observations that mepacrine offered some protective effects against CPE already bound to Caco-2 cells (31).
The mepacrine-induced reduction in the intestinal damage induced by CPE did not correlate with the reduction of CPE complex formation in the small intestine, as assessed by Western blotting. This is consistent with previous observations in which even lower CPE doses (∼50 μg/ml) were capable of inducing pore formation and causing some histological lesions in challenged small intestinal loops (9). In other words, the concentrations of mepacrine tested in this study against a higher, but still pathophysiologically relevant, CPE dose resemble the effects when lower CPE concentrations act on the intestine. Thus, mepacrine may still be beneficial to reduce mucosal damage in severe clinical situations of CPE-associated intestinal disease, such as enterotoxemia.
Mepacrine-induced protection from CPE enterotoxemic death in the current study was only partially associated with the reduction of CPE absorption from the intestine. Perhaps because of reduced tissue damage, some animals treated with CPE in the presence of mepacrine had very low levels of CPE absorption into the serum. None of those animals with low CPE serum levels died in this study. However, due to substantial animal-to-animal variations, overall toxin levels detected by ELISA in serum samples did not differ significantly in mice treated with mepacrine or left untreated.
Since mepacrine can be absorbed from the intestine (41), it is possible that some protection against CPE-induced enterotoxemia is associated with the action of mepacrine on distant organs targeted by CPE. The i.v. injection of CPE in mice was previously shown to result in binding of this toxin to liver and kidneys (9, 38). The subsequent loss of potassium from these organs to the blood, presumably through the formation of CPE pores, was proposed as a mechanism by which CPE induces cardiac dysfunction and, eventually, death (9, 38). Consistent with that hypothesis, CPE treatment of the liver ex vivo resulted in formation of many CPE pores, identifying this organ as a potential source of the lethal hyperpotassemia observed in mice receiving an i.v. CPE injection (9). Consequently, it is possible that mepacrine can interfere with CPE pore formation and activity on distant organs, such as the liver and kidneys, thereby reducing the associated potassium loss through those pores. In the current study, a high concentration of mepacrine administered in intestinal loops significantly reduced CPE-induced hyperpotassemia in mice. This protection correlated with increased survival of mice for the 4-h experimental period. Potassium levels in these animals were at the level of the control mice receiving either mepacrine or buffer alone (∼8 to 12 meq/liter). These levels lie within reference values for potassium in blood collected via cardiocentesis in albino mice (42).
The exact mechanism by which mepacrine interferes with CPE pore activity is not fully understood (31). However, it seems to act as a generic drug against the activity of many different pores and channels in mammalian membranes (31, 43, 44). Besides CPE, mepacrine has also been shown to inhibit the cytoplasmic entry through membranes of several intracellularly active bacterial toxins, such as Bacillus anthracis lethal toxin and Clostridium botulinum C2 toxin (45). This inhibition involved interfering with the pores formed by these toxins in endosomal membranes. That previous study (45) also showed mepacrine derivatives can be much more active than the native drug against the pore activity of the toxins tested, opening the possibility that more efficient drugs against CPE can be found in the mepacrine family (31, 45).
To our knowledge, this is the first study reporting that mepacrine can protect an animal model from toxin-mediated disease, specifically showing that this drug can reduce CPE-induced lethal enterotoxemia and decrease the severity of intestinal damage caused by this toxin. Future studies are planned to explore the possibility of mepacrine providing protection from other pore-forming toxins in vivo.
MATERIALS AND METHODS
Clostridium perfringens enterotoxin.
CPE was purified to homogeneity from C. perfringens strain NCTC 8238 (ATCC 12916), as described previously (46).
Small intestinal loop challenge.
All procedures involving animals were approved by the University of California, Davis, Committee for Animal Care and Use (permit 18187). Male or female, 20- to 25-g BALB/c mice were anesthetized by intraperitoneal administration of 0.2 ml/10 g of body weight with a mixture of xylazine (0.5 mg/ml) and ketamine (5 mg/ml). Immediately before surgery, the abdomen of each mouse was disinfected with iodine solution (Betadine; Purdue Pharma LP). A midline laparotomy was performed and an ∼10- to 15-cm-long intestinal loop was prepared in the jejunum of each mouse by double ligation of the intestine with careful preservation of the blood supply. For each inoculation (see below), a new sterile 1-ml needle and syringe were used. The incision in the peritoneum, abdominal muscles, and skin were closed in one plane using super glue (Henkel Corporation). Mice were divided into four groups receiving 1 ml of HBSS containing (i) CPE only (100 μg) (n = 20), (ii) mepacrine only (1.0 mM, pH 7.0; Cayman Chemical) (n = 10), (iii) CPE (100 μg) plus mepacrine (0.5 mM, pH 7.0) (n = 10), and (iv) CPE (100 μg) plus mepacrine (1.0 mM, pH 7.0) (n = 20).
Enterotoxemia assay.
The procedure described above was extended to a 4-h incubation period, during which death/survival was recorded. Mice were kept anesthetized until they were euthanized at the end of the 4-h treatment period unless they died spontaneously or developed severe clinical signs necessitating euthanasia.
Histopathology.
Samples of challenged intestinal loops were collected from all animals at the time of death and fixed by immersion in 10% buffered formalin, pH 7.2, for 24 to 72 h. Four-μm-thick sections were then prepared routinely and stained with hematoxylin and eosin (H&E). The sections were examined microscopically by a pathologist in a blinded fashion. A semiquantitative overall severity score of lesions was assigned to each section using an ordinal scale from 0 (no lesions observed) to 4 (most severe). The following criteria were considered in this score: mucus in the lumen, villous blunting, epithelial desquamation, epithelial cell death, cell death in lamina propria, inflammation, dilation of lymphatic vessels, and submucosal edema.
Western blot analysis of CPE complex formation in small intestine.
To assess the effects of mepacrine on the in vivo ability of CPE binding and CH-1 pore complex formation in small intestinal tissues, CPE-treated tissues were collected and frozen. Approximately 50 mg of tissue was homogenized by sonication (QSonica with Amp1 at 40% with three 10-s pulses) in 0.5 ml of radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, protease inhibitor cocktail, pH 7.4 [Products International Corp]) plus 1 μl Benzonase (Qiagen). A 20-μl aliquot of each homogenate was then added to 20 μl of 2× Laemmli buffer. The prepared samples were then loaded onto 4% acrylamide gels, followed by transfer onto nitrocellulose membrane. The blots were blocked with 5% milk in Tris-buffered saline with 0.2% Tween 20 (TBS-T) and then incubated overnight at 4°C with rabbit polyclonal anti-CPE antiserum diluted 1:100 in TBS-T. After three washes with TBS-T, the blot was incubated for 1 h at room temperature with secondary goat polyclonal anti-rabbit Ig horseradish peroxidase-conjugated antibody (Sigma) at a 1:10,000 dilution in TBS-T (Sigma). SuperSignal West Pico substrate (Thermofisher) was used to detect the CPE complex.
Measurement of CPE in serum.
Blood samples were collected, via cardiocentesis, from 10 euthanized or naturally dying mice per group. Sera were then analyzed for the presence of CPE by ELISA using a commercial kit (Techlab) by following the instructions of the manufacturer. Briefly, 50 μl of serum was added to 200 μl of diluent (buffered protein solution plus 0.02% thimerosal). One hundred μl of diluted serum or CPE standards (final CPE range from 0 to 500 ng/ml) then was added to wells of a polystyrene assay U-bottom plate (Falcon) containing 50 μl of conjugate (polyclonal antibody specific for CPE coupled to horseradish peroxidase in a buffered protein solution plus 0.02% thimerosal). Plates were incubated for 2 h at 37°C. After five washes, 100 μl of substrate (buffered solution containing tetramethylbenzidine and peroxide) was added to each well and incubated for 15 min at room temperature. Finally, 50 μl of stop solution (0.6 N sulfuric acid) was added to each well, and after 2 min, the absorbance was read at 450 nm on a microplate reader (Bio-Rad). Most samples were tested in duplicate, and the average reading was used for calculations. The absorbance values of CPE standards were plotted against known concentrations to obtain a standard curve, and the equation of the line of best fit was then used to calculate the concentration of CPE in each serum sample.
Measurement of serum potassium levels.
The trace element screen was performed at the Veterinary Toxicology Laboratory at the California Animal Health and Food Safety Laboratory System, University of California, Davis. Collected sera from mice were analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES). After the precipitation of proteins, the protein-free supernatant of each collected serum sample was analyzed for potassium levels by utilizing an ICP-AES spectrometer (Fisons, Accuris model; Thermo Optek Corporation, Franklin, MA). The accuracy of the ICP-AES results for this element was confirmed by analyzing quality assurance sera obtained from the Veterinary Laboratory Association Quality Assurance Program (Genzyme Diagnostics, Blaine, MD). Data were accepted if the analyzed quality assurance serum values were within 2 standard deviations of the reference values.
Statistical analyses.
Statistical analyses were performed using R for Mac (v 3.3.1). Kaplan-Meier survival curves were compared using log-rank analyses. Histopathological scores were compared by the nonparametric Kruskal-Wallis test followed by the Dunn test as a post hoc analysis. Potassium and CPE levels in serum samples were compared by one-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons as a post hoc analysis. The proportions of CPE-treated animals with serum CPE values of ≤100 ng/ml, with or without mepacrine, were compared by chi-squared analysis. In all cases, a P value of <0.05 was regarded as statistically significant.
ACKNOWLEDGMENTS
We thank the Veterinary Toxicology Laboratory at the California Animal Health and Food Safety Laboratory System for performing the trace element screen.
This work was generously supported by grant R01 AI019844-35 from the National Institute of Allergy and Infectious Diseases. M.A.N. is supported by Becas Chile, CONICYT, Gobierno de Chile.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Rood JI, Adams V, Lacey J, Lyras D, McClane BA, Melville SB, Moore RJ, Popoff MR, Sarker MR, Songer JG, Uzal FA, Van Immerseel F. 2018. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 53:5–10. doi: 10.1016/j.anaerobe.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClane BA, Robertson SL, Li J. 2013. Clostridium perfringens, p 465–489. In Doyle MP, Buchanan RL (ed), Food microbiology: fundamentals and frontiers, 4th ed ASM Press, Washington, DC. [Google Scholar]
- 3.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.091101p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carman RJ. 1997. Clostridium perfringens in spontaneous and antibiotic associated diarrhoea of man and other animals. Rev Med Microbiol 8:S43–S45. [Google Scholar]
- 5.Freedman JC, Shrestha A, McClane BA. 2016. Clostridium perfringens enterotoxin: action, genetics, and translational applications. Toxins 8:73. doi: 10.3390/toxins8030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bos J, Smithee L, McClane B, Distefano RF, Uzal F, Songer JG, Mallonee S, Crutcher JM. 2005. Fatal necrotizing colitis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection. Clin Infect Dis 40:e78–e83. doi: 10.1086/429829. [DOI] [PubMed] [Google Scholar]
- 7.Brynestad S, Granum PE. 2002. Clostridium perfringens and foodborne infections. Int J Food Microbiol 74:195–202. doi: 10.1016/S0168-1605(01)00680-8. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC). 2012. Fatal foodborne Clostridium perfringens illness at a state psychiatric hospital–Louisiana, 2010. MMWR Morb Mortal Wkly Rep 61:605–608. [PubMed] [Google Scholar]
- 9.Caserta JA, Robertson SL, Saputo J, Shrestha A, McClane BA, Uzal FA. 2011. Development and application of a mouse intestinal loop model to study the in vivo action of Clostridium perfringens enterotoxin. Infect Immun 79:3020–3027. doi: 10.1128/IAI.01342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman JC, Navarro MA, Morrell E, Beingesser J, Shrestha A, McClane BA, Uzal FA. 21 June 2018. Evidence that Clostridium perfringens enterotoxin-induced intestinal damage and enterotoxemic death in mice can occur independently of intestinal caspase-3 activation. Infect Immun . doi: 10.1128/IAI.00931-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czeczulin JR, Hanna PC, McClane BA. 1993. Cloning, nucleotide sequencing, and expression of the Clostridium perfringens enterotoxin gene in Escherichia coli. Infect Immun 61:3429–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitadokoro K, Nishimura K, Kamitani S, Fukui-Miyazaki A, Toshima H, Abe H, Kamata Y, Sugita-Konishi Y, Yamamoto S, Karatani H, Horiguchi Y. 2011. Crystal structure of Clostridium perfringens enterotoxin displays features of β-pore-forming toxins. J Biol Chem 286:19549–19555. doi: 10.1074/jbc.M111.228478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briggs DC, Naylor CE, Smedley JG, Lukoyanova N, Robertson S, Moss DS, McClane BA, Basak AK. 2011. Structure of the food-poisoning Clostridium perfringens enterotoxin reveals similarity to the aerolysin-like pore-forming toxins. J Mol Biol 413:138–149. doi: 10.1016/j.jmb.2011.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanna PC, Wnek AP, McClane BA. 1989. Molecular cloning of the 3’ half of the Clostridium perfringens enterotoxin gene and demonstration that this region encodes receptor-binding activity. J Bacteriol 171:6815–6820. doi: 10.1128/jb.171.12.6815-6820.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horiguchi Y, Akai T, Sakaguchi G. 1987. Isolation and function of a Clostridium perfringens enterotoxin fragment. Infect Immun 55:2912–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna PC, Mietzner TA, Schoolnik GK, McClane BA. 1991. Localization of the receptor-binding region of Clostridium perfringens enterotoxin utilizing cloned toxin fragments and synthetic peptides. The 30 C-terminal amino acids define a functional binding region. J Biol Chem 266:11037–11043. [PubMed] [Google Scholar]
- 17.Robertson SL, Smedley JG, Singh U, Chakrabarti G, Van Itallie CM, Anderson JM, McClane BA. 2007. Compositional and stoichiometric analysis of Clostridium perfringens enterotoxin complexes in Caco-2 cells and claudin 4 fibroblast transfectants. Cell Microbiol 9:2734–2755. doi: 10.1111/j.1462-5822.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 18.Harada M, Kondoh M, Ebihara C, Takahashi A, Komiya E, Fujii M, Mizuguchi H, Tsunoda S-I, Horiguchi Y, Yagi K, Watanabe Y. 2007. Role of tyrosine residues in modulation of claudin-4 by the C-terminal fragment of Clostridium perfringens enterotoxin. Biochem Pharmacol 73:206–214. doi: 10.1016/j.bcp.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi A, Komiya E, Kakutani H, Yoshida T, Fujii M, Horiguchi Y, Mizuguchi H, Tsutsumi Y, Tsunoda S, Koizumi N, Isoda K, Yagi K, Watanabe Y, Kondoh M. 2008. Domain mapping of a claudin-4 modulator, the C-terminal region of C-terminal fragment of Clostridium perfringens enterotoxin, by site-directed mutagenesis. Biochem Pharmacol 75:1639–1648. doi: 10.1016/j.bcp.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Wieckowski EU, Wnek AP, McClane BA. 1994. Evidence that an approximately 50-kDa mammalian plasma membrane protein with receptor-like properties mediates the amphiphilicity of specifically bound Clostridium perfringens enterotoxin. J Biol Chem 269:10838–10848. [PubMed] [Google Scholar]
- 21.Smedley JG, McClane BA. 2004. Fine mapping of the N-terminal cytotoxicity region of Clostridium perfringens enterotoxin by site-directed mutagenesis. Infect Immun 72:6914–6923. doi: 10.1128/IAI.72.12.6914-6923.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Theoret JR, Shrestha A, Smedley JG, McClane BA. 2012. Cysteine-scanning mutagenesis supports the importance of Clostridium perfringens enterotoxin amino acids 80 to 106 for membrane insertion and pore formation. Infect Immun 80:4078–4088. doi: 10.1128/IAI.00069-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardy SP, Denmead M, Parekh N, Granum PE. 1999. Cationic currents induced by Clostridium perfringens type A enterotoxin in human intestinal CaCo-2 cells. J Med Microbiol 48:235–243. doi: 10.1099/00222615-48-3-235. [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarti G, Zhou X, McClane BA. 2003. Death pathways activated in CaCo-2 cells by Clostridium perfringens enterotoxin. Infect Immun 71:4260–4270. doi: 10.1128/IAI.71.8.4260-4270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakrabarti G, McClane BA. 2004. The importance of calcium influx, calpain and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin: Ca2+, calpain and calmodulin cause CPE-induced cell death. Cell Microbiol 7:129–146. doi: 10.1111/j.1462-5822.2004.00442.x. [DOI] [PubMed] [Google Scholar]
- 26.McClane BA, Wnek AP, Hulkower KI, Hanna PC. 1988. Divalent cation involvement in the action of Clostridium perfringens type A enterotoxin. Early events in enterotoxin action are divalent cation-independent. J Biol Chem 263:2423–2435. [PubMed] [Google Scholar]
- 27.Matsuda M, Sugimoto N. 1979. Calcium-independent and dependent steps in action of Clostridium perfringens enterotoxin on HeLa and Vero cells. Biochem Biophys Res Commun 91:629–636. doi: 10.1016/0006-291X(79)91568-7. [DOI] [PubMed] [Google Scholar]
- 28.Navarro M, McClane B, Uzal F. 2018. Mechanisms of action and cell death associated with Clostridium perfringens toxins. Toxins 10:212. doi: 10.3390/toxins10050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson SL, Smedley JG, McClane BA. 2010. Identification of a claudin-4 residue important for mediating the host cell binding and action of Clostridium perfringens enterotoxin. Infect Immun 78:505–517. doi: 10.1128/IAI.00778-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrestha A, Robertson SL, Garcia J, Beingasser J, McClane BA, Uzal FA. 2014. A synthetic peptide corresponding to the extracellular loop 2 region of claudin-4 protects against Clostridium perfringens enterotoxin in vitro and in vivo. Infect Immun 82:4778–4788. doi: 10.1128/IAI.02453-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman JC, Hendricks MR, McClane BA. 2017. The potential therapeutic agent mepacrine protects CaCo-2 cells against Clostridium perfringens enterotoxin action. mSphere 2:e00352-17. doi: 10.1128/mSphere.00352-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handfield-Jones RPC. 1949. Chloroquine, proguanil, mepacrine and quinine in the treatment of malaria caused by Plasmodium Falciparum. Ann Trop Med Parasitol 43:345–348. doi: 10.1080/00034983.1949.11685420. [DOI] [PubMed] [Google Scholar]
- 33.Bassily S, Farid Z, Mikhail JW, Kent DC, Lehman JS. 1970. The treatment of Giardia lamblia infection with mepacrine, metronidazole and furazolidone. J Trop Med Hyg 73:15–18. [PubMed] [Google Scholar]
- 34.Meltzer E, Lachish T, Schwartz E. 2014. Treatment of giardiasis after nonresponse to nitroimidazole. Emerg Infect Dis 20:1738–1740. doi: 10.3201/eid2010.140073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardy SP, Ritchie C, Allen MC, Ashley RH, Granum PE. 2001. Clostridium perfringens type A enterotoxin forms mepacrine-sensitive pores in pure phospholipid bilayers in the absence of putative receptor proteins. Biochim Biophys Acta 1515:38–43. doi: 10.1016/S0005-2736(01)00391-1. [DOI] [PubMed] [Google Scholar]
- 36.Smedley JG, Saputo J, Parker JC, Fernandez-Miyakawa ME, Robertson SL, McClane BA, Uzal FA. 2008. Noncytotoxic Clostridium perfringens enterotoxin (CPE) variants localize CPE intestinal binding and demonstrate a relationship between CPE-induced cytotoxicity and enterotoxicity. Infect Immun 76:3793–3800. doi: 10.1128/IAI.00460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kokai-Kun JF, Benton K, Wieckowski EU, McClane BA. 1999. Identification of a Clostridium perfringens enterotoxin region required for large complex formation and cytotoxicity by random mutagenesis. Infect Immun 67:5634–5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugimoto N, Chen YM, Lee SY, Matsuda M, Lee CY. 1991. Pathodynamics of intoxication in rats and mice by enterotoxin of Clostridium perfringens type A. Toxicon 29:751–759. doi: 10.1016/0041-0101(91)90067-2. [DOI] [PubMed] [Google Scholar]
- 39.Berry PR, Rodhouse JC, Hughes S, Bartholomew BA, Gilbert RJ. 1988. Evaluation of ELISA, RPLA, and Vero cell assays for detecting Clostridium perfringens enterotoxin in faecal specimens. J Clin Pathol 41:458–461. doi: 10.1136/jcp.41.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lerman SJ, Walker RA. 1982. Treatment of giardiasis: literature review and recommendations. Clin Pediatr 21:409–414. doi: 10.1177/000992288202100704. [DOI] [PubMed] [Google Scholar]
- 41.Gardner TB, Hill DR. 2001. Treatment of giardiasis. Clin Microbiol Rev 14:114–128. doi: 10.1128/CMR.14.1.114-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serfilippi LM, Pallman DRS, Russell B. 2003. Serum clinical chemistry and hematology reference values in outbred stocks of albino mice from three commonly used vendors and two inbred strains of albino mice. Contemp Top Lab Anim Sci 42:46–52. [PubMed] [Google Scholar]
- 43.Schwartz RD, Mindlin MC. 1988. Inhibition of the GABA receptor-gated chloride ion channel in brain by noncompetitive inhibitors of the nicotinic receptor-gated cation channel. J Pharmacol Exp Ther 244:963–970. [PubMed] [Google Scholar]
- 44.Tamamizu S, Todd AP, McNamee MG. 1995. Mutations in the M1 region of the nicotinic acetylcholine receptor alter the sensitivity to inhibition by quinacrine. Cell Mol Neurobiol 15:427–438. doi: 10.1007/BF02071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kreidler A-M, Benz R, Barth H. 2017. Chloroquine derivatives block the translocation pores and inhibit cellular entry of Clostridium botulinum C2 toxin and Bacillus anthracis lethal toxin. Arch Toxicol 91:1431–1445. doi: 10.1007/s00204-016-1716-9. [DOI] [PubMed] [Google Scholar]
- 46.McDonel JL, McClane BA. 1988. Production, purification, and assay of Clostridium perfringens enterotoxin. Methods Enzymol 165:94–103. doi: 10.1016/S0076-6879(88)65018-X. [DOI] [PubMed] [Google Scholar]