Disseminated infections with the fungal species Cryptococcus neoformans or, less frequently, Cryptococcus gattii are an important cause of mortality in immunocompromised individuals. Central to the virulence of both species is an elaborate polysaccharide capsule that consists predominantly of glucuronoxylomannan (GXM).
KEYWORDS: antibody, capsule, Cryptococcus, fungal, pathogen
ABSTRACT
Disseminated infections with the fungal species Cryptococcus neoformans or, less frequently, Cryptococcus gattii are an important cause of mortality in immunocompromised individuals. Central to the virulence of both species is an elaborate polysaccharide capsule that consists predominantly of glucuronoxylomannan (GXM). Due to its abundance, GXM is an ideal target for host antibodies, and several monoclonal antibodies (mAbs) have previously been derived using purified GXM or whole capsular preparations as antigens. In addition to their application in the diagnosis of cryptococcosis, anti-GXM mAbs are invaluable tools for studying capsule structure. In this study, we report the production and characterization of a novel anti-GXM mAb, Crp127, that unexpectedly reveals a role for GXM remodeling during the process of fungal titanization. We show that Crp127 recognizes a GXM epitope in an O-acetylation-dependent, but xylosylation-independent, manner. The epitope is differentially expressed by the four main serotypes of Cryptococcus neoformans and C. gattii, is heterogeneously expressed within clonal populations of C. gattii serotype B strains, and is typically confined to the central region of the enlarged capsule. Uniquely, however, this epitope redistributes to the capsular surface in titan cells, a recently characterized morphotype where haploid 5-μm cells convert to highly polyploid cells of >10 μm with distinct but poorly understood capsular characteristics. Titan cells are produced in the host lung and critical for successful infection. Crp127 therefore advances our understanding of cryptococcal morphological change and may hold significant potential as a tool to differentially identify cryptococcal strains and subtypes.
INTRODUCTION
As the two main etiological agents of cryptococcosis, Cryptococcus neoformans and Cryptococcus gattii are major contributors to the global health burden imposed by invasive fungal infections (1). While C. neoformans typically manifests as meningitis in immunocompromised individuals, C. gattii infections are not associated with specific immune defects and have been responsible for fatal outbreaks of pneumonia (2–4). Central to the virulence of both species is an elaborate polysaccharide capsule, without which Cryptococcus is rendered avirulent (5, 6). The composition of this capsule is highly variable and differs between yeast cells and titan cells (defined as cells >10 μm in cell body diameter with increased ploidy and altered cell wall and capsule) formed by C. neoformans within the host lung (7–9). Titan cells contribute to pathogenesis by resisting phagocytosis, enhancing dissemination of yeast to the central nervous system, and altering host immune status (7, 9–13).
The cryptococcal capsule consists of ∼90% glucuronoxylomannan (GXM), ∼10% glucuronoxylomannogalactan (GXMGal), and <1% mannoproteins (MPs) (14). GXM is a megadalton polysaccharide containing a backbone of α-(1,3)-mannan that is decorated with β-(1,2)-glucuronic acid, β-(1,2)-xylose, and β-(1,4)-xylose substituents (15). The backbone mannan can also be O-acetylated, although the position at which this modification is added remains unclear for most strains (14–16). Seven repeat motifs, called structure reporter groups (SRGs), contribute to structural variation in GXM (15). All SRGs contain a β-(1,2)-glucuronic acid on their first mannose residue; however, the number of β-(1,2)- and β-(1,4)-xylose substituents varies (15). The extent and position of O-acetyl groups in each SRG remain unclear; however, xylose and O-acetyl groups attached to the same mannose residue appear to be mutually exclusive (17). SRG usage differs between the four main serotypes of Cryptococcus, with each strain designated a serotype based on the reactivity of its capsular material with antibody preparations (18). C. neoformans serotypes A and D tend to biosynthesize GXM containing SRGs with fewer xylose substituents than those from C. gattii serotypes B and C (15, 19).
While the capsule structure differs between serotypes of Cryptococcus, a flexible biosynthetic pathway enables rapid remodeling of the capsule under different environmental conditions (20). In vitro, changes in O-acetylation have been associated with cell aging in C. neoformans (21), reaffirming previous reports that capsules produced within clonal populations are far from homogeneous (19, 22). In vivo, changes in capsule size and structure coincide with infection of different organs and likely enhance fitness through the evasion of host immunity (23–25). In light of these observations, it is perhaps unsurprising that capsules produced by titan cells are structurally distinct from those produced by typical yeast cells (7, 11, 26). As the increased chitin content of cell walls produced by titan cells is associated with the activation of a detrimental TH2 immune response during cryptococcosis (27), it is possible that hitherto-unidentified structural differences in titan cell capsules also contribute to the modulation of host immunity by this C. neoformans morphotype.
Alterations in capsule structure are likely to affect how Cryptococcus is perceived by host immune molecules, with antibodies being particularly sensitive to small changes in molecular structures. Following exposure to cryptococci, immunoglobulin M (IgM) antibodies are the most abundant isotype of antibody produced in response to GXM (28). As a repetitive capsular polysaccharide, GXM is a T-independent type 2 antigen, and antibodies generated against it utilize a restricted set of variable-region gene segments (29). By using monoclonal antibodies (mAbs) in conjunction with mutants harboring specific defects in GXM modification (17, 30, 31), it has been determined that O-acetylation and, to a lesser extent, xylosylation of GXM are important for epitope recognition by anti-GXM antibodies (16, 30). While there is no consensus surrounding the effect of GXM O-acetylation on virulence (17, 32), its influence on antibody binding suggests that changes in GXM O-acetylation could be a strategy deployed by cryptococci to avoid recognition by immune effectors. Additionally, despite the immunomodulatory roles for GXM O-acetylation that have been identified (30, 33), receptors that bind O-acetylated GXM remain elusive (34). Due to the enigmatic nature of this modification within the primary virulence factor of cryptococci, further investigation of GXM O-acetylation will help unravel the complexities of cryptococcal capsule structure with the ultimate aim of understanding the strategies deployed by this fatal fungal pathogen to evade host immunity.
In the present study, we report the generation of Crp127, a murine IgM mAb, using a cocktail of heat-killed C. neoformans H99 (serotype A) cells (35), heat-killed C. gattii R265 (serotype B) cells (36), and their lysates as an immunogen. Characterization of Crp127 demonstrated that it is an O-acetyl-dependent anti-GXM mAb specific for an epitope expressed by the four Cryptococcus serotypes in a serotype-specific manner. Having subsequently found that this epitope is heterogeneously expressed within serotype B populations and is spatially confined to distinct regions of the enlarged capsule across all strains tested, we then turned our attention to its expression by titan cells. Intriguingly, we noticed that the spatial distribution of this epitope differs within the capsules produced by the three C. neoformans morphotypes found within titanizing populations. Further analysis revealed that, under conditions permissive for titanization, cell enlargement coincides with the gradual redistribution of this epitope to the capsule surface.
RESULTS
Crp127 recognizes a capsular epitope located in GXM.
During hybridoma screening, Crp127 was identified as staining the outer zone of live cryptococci. We first assessed whether Crp127 recognizes a capsular component by performing flow cytometric analysis of three GXM-deficient mutants (R265 cap10Δ [37], KN99α cap59Δ [38], and B3501 cap67Δ [39]), a GXMGal-deficient mutant (KN99α uge1Δ [38]), and a mutant lacking both GXM and GXMGal (KN99α cap59Δ uge1Δ [38]), using an Alexa 488-conjugated anti-IgM secondary antibody to label Crp127. Unlike their corresponding wild-type strains, the GXM-deficient mutants were not recognized by Crp127 (cap10Δ, P < 0.05; cap67Δ, P < 0.01; cap59Δ, P < 0.01; cap59Δ uge1Δ, P < 0.01 [by Student’s t test]) (Fig. 1A to C). In contrast, the GXMGal-deficient uge1Δ mutant was bound at levels similar to those for the wild-type strain (P > 0.05) (Fig. 1C). Confocal microscopy corroborated these observations, with no observable binding of Crp127 to GXM-deficient mutants but clear binding of Crp127 to the GXMGal-deficient mutant (Fig. 1D to F). Taken together, these experiments demonstrated that the epitope recognized by Crp127, here referred to as the Crp127 epitope, is a component of GXM.
FIG 1.
Crp127 is an anti-GXM mAb. The ability of Crp127 to bind to GXM- and GXMGal-deficient mutants of C. gattii and C. neoformans was quantified via flow cytometry. (A to C) Scatter plots (top row) and representative histograms (bottom row) for R265 cap10Δ (A); B3501 cap67Δ (B); KN99α cap59Δ, KN99α uge1Δ, and KN99α cap59Δ uge1Δ (C); and their corresponding wild-type (WT) strains. For scatter plots, corrected median fluorescence intensity (MFI) values were calculated by subtracting the MFI value of isotype control cells from the MFI value of the Crp127-treated cells, with data points representing MFI values calculated from three biological replicates performed as independent experiments. Student’s t test was used to test for statistically significant differences between R265 cap10Δ and B3501 cap67Δ and their corresponding wild-type strains, while one-way analysis of variance (ANOVA) followed by Dunnett’s multiple-comparison test was used to test for statistically significant differences between KN99α cap59Δ, KN99α uge1Δ cap59Δ, KN99α uge1Δ, and the wild-type strain KN99α (n = 3) (*, P < 0.05; **, P < 0.01). Histograms show a representative distribution of Crp127 binding for one or all of the strains in the scatter plot above, with the color-coded key provided for reference. Numerical values in the top left and right of each histogram correspond to the MFI value calculated from the strain labeled directly above. (D to F) R265 cap10Δ (D); B3501 cap67Δ (E); KN99α cap59Δ, KN99α uge1Δ, and KN99α uge1Δ cap59Δ (F); and their wild-type strains were labeled for chitin using calcofluor white (CFW) (blue) and Crp127 (goat Alexa 647-conjugated anti-mouse IgM μ-chain) (far red), and maximum-intensity projections were generated from confocal microscopy z-stacks. Presented are representative images merged for transmitted light and Crp127 (left panels) and Crp127 and chitin (right panels). Bars, 5 μm.
GXM O-acetylation is required for Crp127 epitope recognition.
Considering the importance of O-acetylation and xylosylation to the antigenic signature of GXM (30), we proceeded to investigate the effect of these modifications on Crp127 epitope recognition. We first tested the ability of Crp127 to recognize two xylose-deficient mutants (JEC155 uxs1Δ [serotype D] [31] and KN99α uxs1Δ [serotype A] [38]). No significant differences were found between either uxs1Δ mutant and its corresponding wild-type strain (JEC155 uxs1Δ, P > 0.05; KN99α uxs1Δ, P > 0.05) (Fig. 2A), indicating that xylosylation does not impact Crp127 binding. In contrast, however, antibody binding was completely abrogated in the O-acetyl-deficient cas1Δ mutant (P < 0.01) (Fig. 2B), indicating that O-acetylation of GXM is an essential prerequisite for Crp127 epitope recognition.
FIG 2.
Crp127 requires O-acetylation, but not xylosylation, of GXM for epitope recognition. The ability of Crp127 to recognize mutants with specific defects in GXM modification was quantified via flow cytometry. (A to C) Scatter plots (top row) and representative histograms (bottom row) for KN99α uxs1Δ, JEC155 uxs1Δ, and the corresponding wild-type strains (A); JEC156 cas1Δ and wild-type JEC155 (B); and KN99α cas3Δ, KN99α cas31Δ, and wild-type KN99α (C). (D to F) JEC155 uxs1Δ (D), JEC156 cas1Δ (E), KN99α cas31Δ (F), and the corresponding wild-type strains were labeled for chitin, and Crp127 was imaged via confocal microscopy. (G and H) Binding of Crp127 to chemically deacetylated (DeAc) cells of H99 and B3501 was quantified via flow cytometry (G), with a representative histogram presented for H99 (H). (I) Untreated (top) and chemically deacetylated (bottom) H99 cells were labeled for chitin and Crp127 and imaged via confocal microscopy. (J) Binding of 18B7 to chemically deacetylated cells of H99 and B3501 quantified via flow cytometry. (K) Representative cells from the above-described strains labeled for chitin (blue) and O-acetyl-independent mAb F12D2 (far red). For scatter plots, corrected MFI values were calculated by subtracting the MFI value of isotype control cells from the MFI value of the Crp127- or 18B7-treated cells, with data points representing MFI values calculated from three biological replicates performed as independent experiments. Student’s t test was used to test for statistically significant differences between KN99α uxs1Δ, JEC155 uxs1Δ, and JEC156 cas1Δ and their corresponding wild-type strains as well as between untreated and chemically deacetylated cells of the same strain (n = 3). Dunnett’s multiple-comparison test was used to test for statistically significant differences between the KN99α Δcas3 and KN99α Δcas31 mutants and the KN99α wild-type strain (n = 3) (ns, not significant [P > 0.05]; **, P < 0.01). Histograms show a representative distribution of Crp127 or 18B7 binding for one or all of the strains in the scatter plot above, with a color-coded key provided for reference. Numerical values in the top left and right of each histogram correspond to the MFI value calculated from the strain labeled directly above. Representative maximum-intensity projections were merged for transmitted light and Crp127 (far red) (left panels) and Crp127 and chitin (blue) (right panels). Bars, 5 μm.
Having made this observation, we proceeded to test two further mutants in genes implicated in GXM O-acetylation. KN99α cas3Δ exhibits an ∼70% reduction in GXM O-acetylation, whereas KN99α cas31Δ exhibits subtle differences in the sugar composition of GXM but no reduction in GXM O-acetylation (40). Binding of Crp127 to the cas3Δ mutant was slightly reduced compared to binding to the wild-type strain (P > 0.05) (Fig. 2C). This may reflect the reduced density of O-acetylation in GXM produced by this mutant. Surprisingly, however, Crp127 completely failed to recognize the cas31Δ mutant despite this strain retaining an O-acetylation profile similar to that of the wild-type (P < 0.01) (40) (Fig. 2C). To be certain that the O-acetyl-defective mutants tested still produced capsule, we confirmed the binding of O-acetyl-independent anti-GXM mAb F12D2 (41, 42) to each strain (Fig. 2K). Thus, CAS1 and CAS31 contribute to the formation of an O-acetylation-dependent Crp127 epitope.
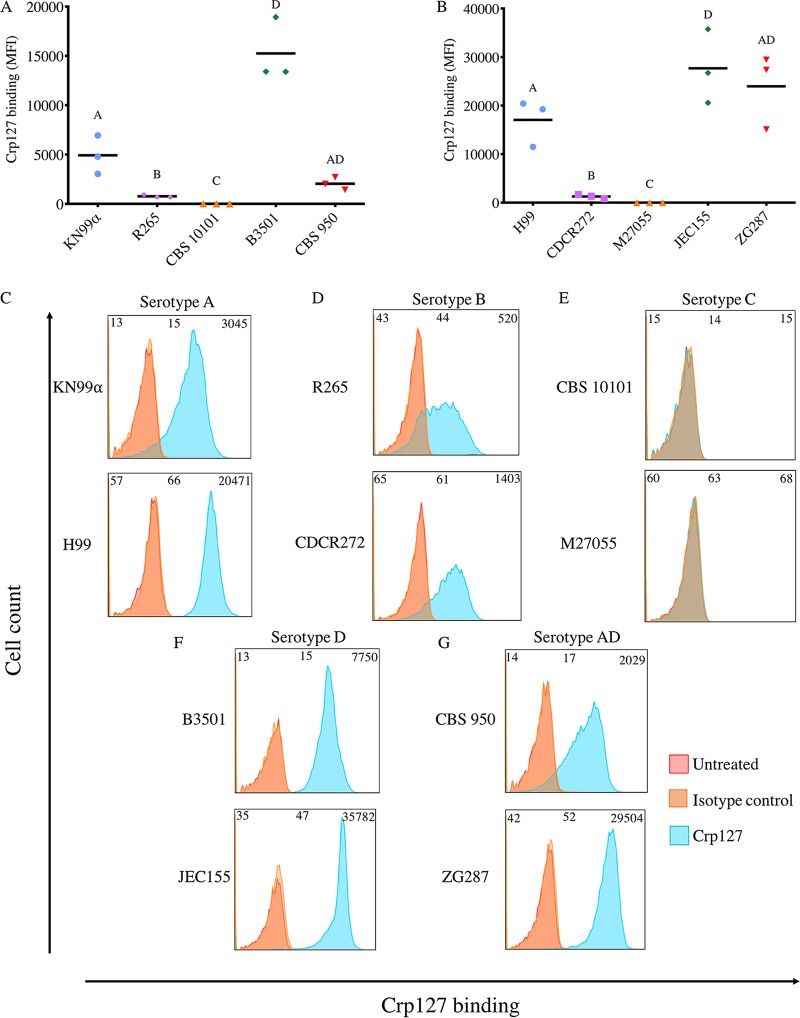
Cryptococcus serotypes differ in their levels of Crp127 epitope recognition.
Differences in the O-acetylation state of GXM contribute to serotype classification and are a source of structural variation within the capsule of cells from a clonal population (21, 30). Therefore, with Crp127 recognizing an O-acetyl-dependent epitope, we next checked for differences in Crp127 staining between the five recognized serotypes of Cryptococcus neoformans and C. gattii, testing two independent strains of each serotype. Flow cytometry analysis demonstrated that Crp127 consistently bound most effectively to serotype D strains (B3501 and JEC155) (Fig. 3A and B), with all cells within these populations exhibiting high-level accessibility of the Crp127 epitope (Fig. 3F). We detected slightly lower binding to serotype A strains (Fig. 3A and B), with high-level homogeneous staining also being seen in the case of H99 but with a proportion of unstained cells from strain KN99α (Fig. 3C). Interestingly, the two serotype AD hybrid strains tested (CBS 950 [43] and ZG287 [44]) were notably different in regard to Crp127 binding (Fig. 3A and B), with CBS 950 exhibiting low-level heterogeneous staining and ZG287 showing high-level homogeneous staining (Fig. 3G).
FIG 3.
Recognition levels of the Crp127 epitope are associated with serotype. The ability of Crp127 to bind to two different strains each of Cryptococcus serotypes A, B, C, D, and AD was quantified using flow cytometry. (A and B) Scatter plots showing corrected MFI values for each strain, which were calculated by subtracting the MFI value of isotype control cells from the MFI value of the corresponding Crp127-treated cells. Data points represent MFI values calculated from three biological replicates performed as independent experiments (n = 3). (C to G) Histograms showing a representative distribution of Crp127 binding for serotype A strains KN99α and H99 (C), serotype B strains R265 and CDCR272 (D), serotype C strains CBS 10101 and M27055 (E), serotype D strains B3501 and JEC155 (F), and serotype AD hybrid strains CBS 950 and ZG287 (G). Fluorescence intensity values for untreated, isotype control, and Crp127-treated cells are presented, with corresponding MFI values displayed at the top left, center, and right of each panel.
The two remaining cryptococcal serotypes, B and C, together represent C. gattii. Serotype B strains R265 and CDCR272 (36) demonstrated significantly lower epitope recognition than C. neoformans serotypes (Fig. 3A and B) and considerable heterogeneity within the population (Fig. 3D). Interestingly, however, serotype C strains were completely unrecognized by Crp127, with neither strain CBS 10101 (45) nor strain M27055 (46) showing detectable staining (Fig. 3A, B, and E). From this, we conclude that there are serotype-specific differences in the availability of the Crp127 epitope, with epitope accessibility being related to serotype in a pattern of D > A ≫ B ⋙ C.
Crp127 exhibits serotype-specific binding patterns that are not associated with opsonic efficacy.
Having identified differential levels of the Crp127 epitope between serotypes using flow cytometry, we next examined their patterns of binding by immunofluorescence microscopy. Indirect immunofluorescence revealed an annular binding pattern for all four strains representing serotypes A and D (Fig. 4A and D). In line with their differences by flow cytometry, the two serotype AD hybrid strains tested showed different patterns of binding, with CBS 950 showing punctate binding and ZG287 showing a mix of annular and punctate staining. Both C. gattii serotype B strains exhibited punctate binding (Fig. 4B and E), while, in agreement with flow cytometry data, no Crp127 binding was detected when imaging serotype C strain CBS 10101 or M27055. However, O-acetyl-independent mAb F12D2 bound well to these strains, suggesting that the lack of Crp127 binding reflects changes in GXM O-acetylation rather than a loss of capsular material (Fig. 4F).
FIG 4.
The immunofluorescence binding pattern of Crp127 correlates with serotype. (A to E) Two Cryptococcus strains each of serotype A (KN99 and H99) (A), serotype B (R265 and CDCR272) (B), serotype C (CBS 10101 and M27055) (C), serotype D (B3501 and JEC155) (D), and serotype AD (CBS 950 and ZG287) (E) were labeled for chitin (CFW) (blue) and Crp127 (goat Alexa 647-conjugated anti-mouse IgM μ-chain) (far red). (F) Representative cells from serotype C strains CBS 10101 and M27055 labeled for chitin (blue) and O-acetyl-independent mAb F12D2 [Alexa 647-conjugated F(ab′)2 goat anti-mouse IgG(H+L)] (far red). Maximum-intensity projections were generated via confocal microscopy. Representative images are shown for each strain. Images are merged for transmitted light and Crp127 (left panels) and Crp127 and chitin (right panels). Bars, 5 μm.
As annular and punctate binding patterns have been associated with opsonic and nonopsonic anti-GXM IgM mAbs, respectively, we tested the ability of Crp127 to opsonize cells from strains KN99α (annular) and R265 (punctate). Unlike positive-control treatments with mAb 18B7 (73) and pooled human serum, Crp127 did not enhance the phagocytosis of either strain by J774 macrophage-like cells in the presence or absence of serum (see Fig. S2 in the supplemental material). In summary, annular binding patterns are associated with the high-level binding of Crp127 to C. neoformans serotype A and D strains. On the other hand, punctate binding is associated with low-level binding of Crp127 to serotype B strains. However, under the conditions tested in this study, neither binding pattern is clearly associated with opsonic efficacy.
Crp127 epitope recognition reflects serotype differences within C. gattii.
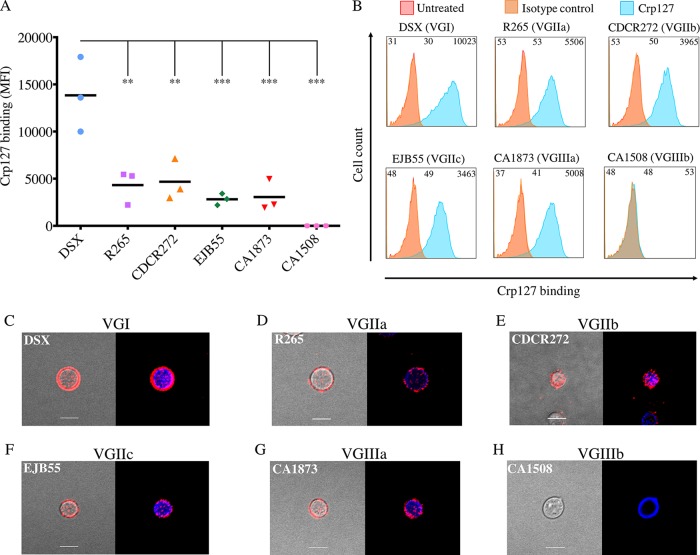
Our data described above indicate that Crp127 binding accurately reflects known serotyping of cryptococcal strains. However, recent genomic data indicate that C. gattii may in fact be composed of several cryptic species (47). We therefore extended our analysis of this species group by investigating a further four C. gattii strains, representing molecular subtypes VGI to VGIII. Similar levels of Crp127 epitope recognition were seen for serotype B strains R265 (subtype VGIIa), CDCR272 (VGIIb), EJB55 (VGIIc) (48), and CA1873 (VGIIIa) (49) (P > 0.05) (Fig. 5A and B); however, a significantly higher level of recognition was seen for the serotype B strain DSX (VGI) (50) (P < 0.01) (Fig. 5A and B). Indirect-immunofluorescence analysis corroborated these findings, with punctate binding being seen for the four strains presenting the epitope at low levels (Fig. 5D to H) and annular binding being seen for strain DSX (Fig. 5C). We also tested strain CA1508 (serotype VGIIIb) (51), a C. gattii strain that, to our knowledge, has not previously been serotyped. Both flow cytometry and indirect-immunofluorescence analyses showed that Crp127 did not recognize this strain (Fig. 5A and H), implying that it is a serotype C strain. In combination with the data presented in Fig. 3, our finding that four out of five serotype B strains were bound similarly by Crp127 suggests that the availability of this epitope is fairly well conserved within this serotype.
FIG 5.
Recognition of the Crp127 epitope is largely consistent within C. gattii serotypes. The ability of Crp127 to bind to six strains of C. gattii that encompass molecular types VGI to VGIIIb was quantified via flow cytometry. (A) Scatter plots showing corrected MFI values for each strain, which were calculated by subtracting the MFI value of isotype control cells from the MFI value of the corresponding Crp127-treated cells. Data points represent MFI values calculated from three biological replicates performed as independent experiments. Tukey’s multiple-comparison test was used to test the statistical significance of differences between the six strains (n = 3) (**, P < 0.01; ***, P < 0.001). (B) Histograms showing a representative distribution of Crp127 binding for strains DSX (type VGI), R265 (VGIIa), CDCR272 (VGIIb), EJB55 (VGIIc), CA1873 (VGIIIa), and CA1508 (VGIIIb). Fluorescence intensity values for untreated, isotype control, and Crp127-treated cells are presented, with the corresponding MFI values displayed at the top left, center, and right of each panel. (C to H) C. gattii strains DSX (C), R265 (D), CDCR272 (E), EJB55 (F), CA1873 (G), and CA1508 (H) were labeled for chitin (CFW) (blue) and Crp127 (goat Alexa 647-conjugated anti-mouse IgM μ-chain) (far red), and maximum-intensity projections were generated via confocal microscopy. Presented are representative images merged for transmitted light and Crp127 (left panels) and Crp127 and chitin (right panels). Bars, 5 μm.
The Crp127 epitope localizes to spatially confined zones of the enlarged capsule, and binding elicits capsular swelling reactions.
Having investigated the binding of Crp127 to cells with a small capsule, we next wished to investigate cells that had been grown under capsule-inducing conditions, given that capsule enlargement occurs shortly after infection of the host. Interestingly, in all of the strains tested, we saw that the Crp127 epitope was spatially confined to distinct capsular regions (Fig. 6). For serotype A strains H99, KN99 (Fig. 6A), and CBS 8336 (52) (Fig. S3F) and serotype D strains JEC21 and B3501 (Fig. 6D), antibody binding was detected in the central zone of the capsule. Serotype B strains differed, with regions adjacent to the cell wall and on the capsule surface being bound by Crp127 in the case of strain R265 but only the single region proximal to the surface being bound in the case of CDCR272 (Fig. 6B). Serotype AD strain ZG287 exhibited a similar pattern of binding to R265, with Crp127 binding to both an inner and an outer region of the capsule; however, strain CBS 950 was bound in a region adjacent to the cell wall (Fig. 6D).
FIG 6.
The Crp127 epitope is spatially confined to distinct capsular regions, and binding elicits capsular swelling reactions distinct from those elicited by 18B7. Cryptococcus cells were grown under capsule-inducing conditions and imaged to determine the location of the Crp127 epitope within the enlarged capsule and characterize the capsular reaction patterns elicited by this antibody. (A to D) Cryptococcus strains of serotype A (KN99α and H99) (A), serotype B (R265 and CDCR272) (B), serotype D (B3501 and JEC155) (C), and serotype AD (CBS 950 and ZG287) (D) were labeled for chitin (CFW) (blue) and Crp127 (goat Alexa 647-conjugated anti-mouse IgM μ-chain) (far red), suspended in India ink to visualize the capsule, and imaged using confocal microscopy. Representative images of a single focal plane are shown for each strain. Presented are images merged for transmitted light and Crp127 (left panels) and Crp127 and chitin (right panels). (E to H) Capsule-induced cells of strains KN99α (E), R265 (F), B3501 (G), and CBS 950 (H) were also left untreated (top right panels) or treated with mAb 18B7 (top right panels) or with mAb Crp127 (bottom panels) and imaged using DIC microscopy to observe capsular reaction patterns. Bars, 5 μm.
The binding of mAbs to capsular GXM alters the refractive index of the enlarged capsule, resulting in capsular swelling reactions that can be visualized using differential interference contrast (DIC) microscopy (53). In testing the ability of Crp127 to produce a capsular swelling reaction with strains KN99α, R265, B3501, and CBS 950, we observed no discernible differences in the reaction patterns produced between strains, with a highly refractive outer rim and a textured inner capsule characteristic of each strain (Fig. 6E to H, bottom panels). Notably, however, Crp127 reaction patterns differed from those elicited by 18B7, which also exhibited a highly refractive outer rim but lacked texture throughout the capsule (Fig. 6E to H, top right panels). Taken together, our studies of Crp127 binding to capsule-induced cells demonstrate that the Crp127 epitope is localized to specific capsular regions and that Crp127 binding produces capsular swelling reactions that are independent of serotype.
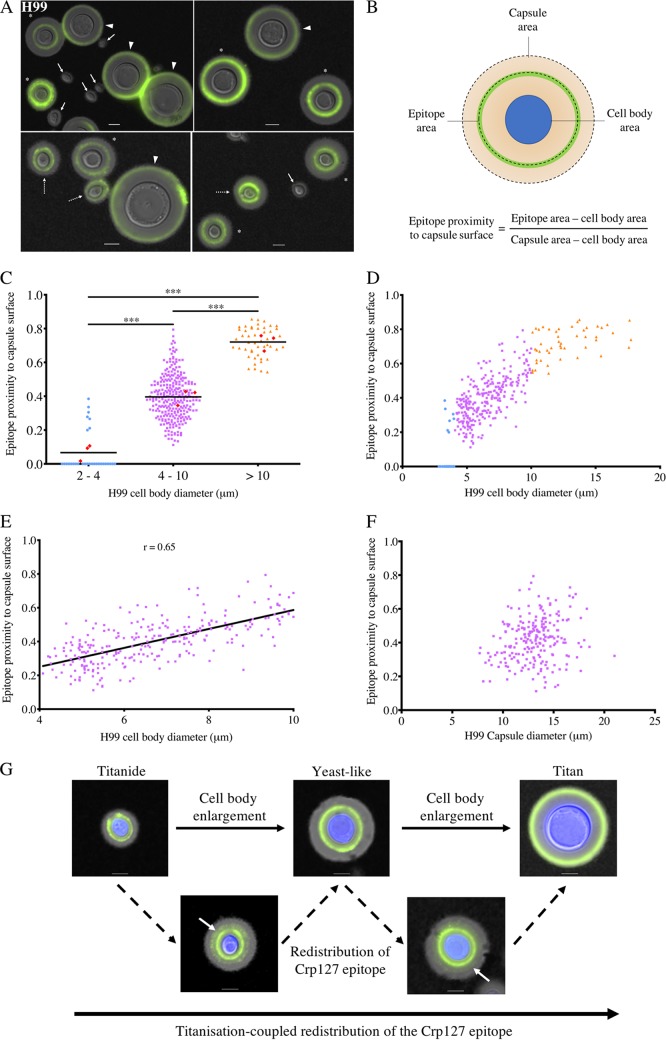
Spatial distributions of the Crp127 epitope differ within the capsules produced by titanide, yeastlike, and titan cells.
Following infection of the host lung, a proportion of C. neoformans cells differentiates into titan cells, a very large morphotype that facilitates pathogenesis and is associated with poor clinical outcomes (8, 12). When grown under titanizing conditions in vitro, C. neoformans forms a heterogeneous population of small, oval-shaped Titanide cells (thin-walled cells 2 to 4 μm in diameter that are distinct from thick-walled 1-μm microcells), yeastlike cells (∼5 μm), and large titan cells (>10 μm) (9, 54). As differences in capsule are known to exist between yeast and titan cells (26, 55), we tested whether Crp127 could distinguish the morphological subtypes found in titanizing populations from strains H99 and KN99α, two closely related strains for which titanization has been extensively studied (7, 9, 11, 26, 56). Indeed, when these strains were grown under titanizing conditions in vitro and imaged, we noticed differences in the spatial distributions of the Crp127 epitope within the capsules produced by cells of different sizes (Fig. 7A and Fig. S3A). Cells with a diameter of 2 to 4 μm were poorly recognized by Crp127 (Fig. 7A), suggesting that these cells did not produce the epitope or that they had budded after the immunostaining procedure. Crp127 bound to a capsular region adjacent to the cell wall in smaller yeast cells, within the central zone of the capsule in larger yeastlike cells, and close to the capsule surface of titan cells (Fig. 7A and Fig. S3A). In order to quantify how cell size affects the capsular distribution of the Crp127 epitope, we determined the ratio between the area of capsule encompassed by the Crp127 epitope and the area of the whole capsule; using this metric, a ratio approaching 1 is indicative of the epitope being found in close proximity to the capsule surface (Fig. 7B). Across three biological repeats (with mean numbers of 111 and 133 cells measured for strains H99 and KN99α, respectively), mean ratios ± standard errors of the means of 0.07 ± 0.02 and 0.05 ± 0.02 were calculated for cells 2 to 4 μm in diameter for strains H99 and KN99α, respectively, consistent with our initial observations that Crp127 bound near the cell wall or not at all in the smallest cells (Fig. 7C and Fig. S3B). For cells with a diameter of 4 to 10 μm, mean ratios were 0.42 ± 0.03 and 0.40 ± 0.01 for strains H99 and KN99α, respectively (Fig. 7C and Fig. S3B), indicating the Crp127 epitope is predominantly located in the central zone of the capsule in 4- to 10-μm cells, as we had previously observed (Fig. 6A). Finally, the mean ratios for cells >10 μm in diameter were 0.72 ± 0.03 and 0.71 ± 0.03 for strains H99 and KN99α, respectively, making them significantly higher than those calculated for both 2- to 4-μm (H99, P < 0.001; KN99α, P < 0.001) (Fig. 7C and Fig. S3B) and 4- to 10-μm (H99, P < 0.001; KN99α, P < 0.001) (Fig. 7C and Fig. S3B) cells. In summary, our results demonstrate that Crp127 binds closer to the capsule surface of titan cells than titanide and yeastlike cells in the widely used serotype A strains H99 and KN99α.
FIG 7.
Spatial distribution of the Crp127 epitope differs within the capsules of the three cell subtypes found in titanizing populations of strain H99, suggesting a model of titanization-coupled epitope redistribution. Cultures of C. neoformans strain H99 that were derived solely from titan cells were investigated for differences in the capsular distribution of the Crp127 epitope. (A) Representative images of cells from strain H99 grown under conditions permissive for titanization, resulting in the formation of titanide cells (block arrows and dashed arrows distinguish no Crp127 binding and binding, respectively), yeastlike cells (asterisks), and titan cells (arrowheads). (B) Schematic representation of how Crp127 epitope proximity to the capsule surface was quantified through analysis of micrographs using ImageJ. Where no antibody binding was detected, the ratio was calculated as zero. (C) Quantification of the proximity of the Crp127 epitope to the capsule surface of 2- to 4-μm, 4- to 10-μm, and >10-μm cells of strain H99. Data points represent results for all individual cells for which the location of the Crp127 epitope was quantified, while the horizontal bars represent the means of data for pooled cells. Red diamonds represent mean values calculated from each of three biological repeats. Tukey’s multiple-comparison test was used to test for statistically significant differences between the three groups (n = 3) (***, P < 0.001). (D and E) Cell body diameter plotted against the epitope proximity to the capsule surface for all cells measured (D) and from cells 4 to 10 μm in cell body diameter (E). (F) Capsule diameter plotted against the epitope proximity to the capsule surface for cells 4 to 10 μm in cell body diameter. (G) Model for titanization-coupled redistribution of the Crp127 epitope. Presented are representative images of titanide, yeastlike, and titan cells (top row) of strain H99 that were recognized by Crp127, in addition to titanide and yeastlike cells exhibiting a second faint ring of antibody binding (white arrows) (bottom row). Bars, 5 μm.
Migration of the Crp127 epitope toward the surface of the capsule coincides with cell enlargement.
To investigate the effect of small changes in cell size on Crp127 epitope distribution, we plotted cell body diameter against epitope proximity to the capsule surface for all H99 and KN99α cells measured (Fig. 7D and Fig. S3C). In doing so, we identified a positive correlation between cell body diameter and epitope proximity to the capsule surface of yeastlike cells. In agreement with this, when plotting only cells with a cell body diameter of 4 to 10 μm, we found a positive correlation between cell body diameter and epitope proximity to the capsule surface in both strains tested (H99, r = 0.65; KN99α, r = 0.66) (Fig. 7E and Fig. S3D). Unlike cell body diameter, capsule diameter did not correlate with epitope proximity to the capsule surface, indicating that changes in capsule size do not explain changes in the proximity of Crp127 to the capsule surface (Fig. 7F and Fig. S3E).
Acknowledging the genetic similarities between strains H99 and KN99α, we also investigated serotype A strain CBS 8336 (52), serotype D strain B3501, and serotype B strain R265. Previously, a C. gattii strain R265 isolate failed to titanize in vitro using the serum induction protocol but was observed to form <10-μm titan-like cells using an alternate protocol (9, 13, 56). Using a different source of R265, we were able to observe limited titan cells in this strain using serum induction (Fig. S3F). In addition, C. neoformans strains CBS 8336 and B3501 both formed Titan cells (Fig. S3F). Although Crp127 binding appeared to be redistributed outward during titanization of CBS 8336 and R265, redistribution was less apparent in the case of B3501 (Fig. S3F). Thus, the extent of epitope redistribution during titanization may vary between strains.
Our results suggest that, in two strains frequently used for the study of titanization, the Crp127 epitope moves gradually to the capsule surface as cells enlarge, raising the question of how this may occur. Throughout our imaging experiments, the binding of Crp127 to the majority of titanide and yeastlike cells (in addition to all titan cells) produced an annular immunofluorescence binding pattern (Fig. 7F, top row). However, we also noticed that some titanide and yeastlike cells produced a second more-faint ring of Crp127 epitope outside this typical annular ring (Fig. 7F, bottom row). This may represent the addition of the Crp127 epitope closer to the capsule surface, partially explaining how the redistribution of this epitope coincides with cell enlargement.
DISCUSSION
In this study, we demonstrate that a capsular epitope recognized by Crp127, an anti-GXM mAb produced in our laboratory, contributes to serotype-specific differences in capsule structure. This epitope traverses the capsule as cells enlarge under conditions permissive for titanization, resulting in its differential distribution throughout the capsule of the three C. neoformans morphotypes found within titanizing populations of two strains used to model cryptococcal titanization. Detailing the accessibility and localization of this epitope adds to the existing body of literature surrounding the variability of the cryptococcal capsule between strains and reveals yet another way in which titan cell capsules are structurally distinct from those produced by yeast cells (21–23, 32, 57).
Based on our examination of a panel of mutants harboring capsule defects, we propose that Crp127 is an anti-GXM mAb whose binding depends on GXM O-acetylation but not xylosylation. When comparing sequences of the complementarity-determining regions (CDRs) from Crp127 with those of four previously characterized anti-GXM IgM mAbs, namely, 2D10, 12A1, 13F1, and 21D2, we found that Crp127 CDRs were significantly different, particularly with regard to the light-chain variable (VL) CDRs. These differences reflect differential gene usage and are likely to manifest as differences in epitope specificity (58, 59). In contrast, when we aligned the heavy-chain variable (VH) and VL sequences from Crp127 with those from anti-GXM IgG1 mAb 302, we noticed that the sequences were extremely similar as a result of identical variable-region gene segment usage by these two mAbs. Identical gene segment usage is not entirely surprising given the restricted set of antibody gene segments utilized by antibodies specific for capsular polysaccharides (29); however, the two mAbs were produced in response to GXM derived from different serotypes of Cryptococcus. Whereas mAb 302 was generated following the immunization of a mouse with serotype D GXM (ATCC 24064) (60), we generated Crp127 through the immunization of a mouse with a cocktail containing both serotype A (H99) and serotype B (R265) GXM. Whichever serotype of GXM activated the B cell from which Crp127 is derived, the sequence similarities between mAbs Crp127 and 302 demonstrate that nearly identical antibodies can be elicited during infection by at least two different serotypes of Cryptococcus.
Crp127 binding shows strong serotype dependence, with serotype D strains being recognized most strongly, followed by serotype A strains. C. gattii serotype B strains show lower, heterogeneous Crp127 epitope recognition and a punctate immunofluorescence binding pattern, while serotype C strains entirely fail to bind the antibody. Interestingly, the predominant SRGs found in GXM produced by serotypes D, A, B, and C contain 1, 2, 3, and 4 xylose substituents, respectively (15, 61). Together with previous observations that β-(1,2)-xylose and O-acetyl groups are not added to the same backbone mannose residue (17, 40), this differential SRG usage may explain the variable Crp127 epitope recognition in one of two ways. For example, the additional xylose substituents present in the predominant SRG found in serotype B and C GXM may prevent the addition of O-acetyl groups in such a way that the Crp127 epitope is not formed. Alternatively, the extra xylose substituents found in these SRGs may sterically hinder binding of Crp127 to its epitope. Studies that further elucidate the roles of specific proteins in GXM biosynthesis, together with advances in techniques that enable chemical synthesis of GXM oligosaccharides, will enhance our understanding of how epitope recognition is achieved by anti-GXM mAbs like Crp127. Intriguingly, a recent transcriptomics study identified CAS31 as being absent from the genome of strain CBS 10101 (62), a serotype C isolate that we subsequently found was not recognized by Crp127. While we cannot rule out the possibility that other factors contribute to the inability of Crp127 to recognize serotype C strains, it is tempting to speculate that the loss of CAS31 function in this lineage may explain its lack of reactivity with Crp127 (32, 34). The molecular basis for CAS31-dependent epitope recognition remains to be determined; however, a cas31Δ strain has been shown to harbor minor alterations in GXM xylose composition (38). Therefore, xylosylation may be in competition with O-acetylation at Crp127 target residues (17). Consistent with this, anti-GXM mAbs CRND-8, 21D2, and 13F1 also fail to recognize cas31Δ mutants (38), suggesting overall changes in capsule organization in this mutant.
Perhaps our most striking observation regarding the Crp127 epitope was its differential distribution throughout the capsules produced by titanide, yeastlike, and titan cells of strains H99 and KN99α. Structural differences in titan capsule compared to yeast capsule have been demonstrated previously by scanning electron microscopy (SEM) and staining with the anticapsule antibody 18B7 (7). Additionally, mAb 18B7 staining of in vivo-derived titan cells was heterogenous across individual titan cells, including annular, exterior, and interior localizations in different cells (7). Using a hypoxic in vitro titan cell induction protocol, Hommel et al. subsequently showed that there were no differences in the localization of the anti-GXM mAb E1, 2D10, or 13F1 in titan cells compared to yeast cells (55). Therefore, the consistent progression of the localization pattern across cell types shown here appears to be a unique feature of the Crp127 epitope (7). The positive correlation between cell size and Crp127 epitope proximity to the capsule surface is suggestive of a scenario whereby the epitope is initially produced in a capsular region adjacent to the cell wall in small titanide cells before redistributing first to the midzone of yeastlike cells and eventually to the capsule surface of titan cells. This finding raises the intriguing question of how the formation and removal of the Crp127 epitope are so tightly spatially controlled within the capsule. One possibility is that the epitope could be formed at the cell surface and then move outward as the capsular material elongates. Therefore, we speculate that since the epitope moves outward at a higher rate than the capsule expands, and since the amount of epitope that initially surrounds a smaller titanide or yeastlike cell would not be sufficient to form the perimeter of capsule encasing a much larger titan cell, we instead favor a model in which the epitope is enzymatically removed and added to different regions of the capsule during growth. For instance, it is possible that GXM decorated with O-acetyl groups is added closer to the capsule surface in larger cells or that such regions are “unmasked” in a different capsular region as the capsule is reshaped during titanization (26).
To summarize, our findings demonstrate that the differential distribution of specific epitopes within the cryptococcal capsule is yet another way in which titan cells can be distinguished from canonical yeast cells. We hope that this will prompt further investigation into how the redistribution of capsular epitopes occurs and what impact this may have on Cryptococcus cell biology. We recently showed that titanization is triggered by exposure to components of the bacterial cell wall (9), while interactions between bacteria and the capsule have previously been described (63, 64). Capsule also contributes to the buoyancy of Cryptococcus cells (65). As such, the importance of redistributing capsular epitopes during titanization should be considered in the context of Cryptococcus cell biology both in the environment and during infection.
MATERIALS AND METHODS
Reagents, strains, and mAbs.
All reagents were purchased from Sigma-Aldrich unless stated otherwise. The Cryptococcus strains used in this study are described in Table S1 in the supplemental material. The anti-GXM mAbs used in this study are described in Table S2.
Growth of cryptococci.
Cryptococcus strains were preserved at −80°C in MicroBank tubes (Thermo Fisher Scientific) prior to being stored on yeast extract-peptone-dextrose (YPD) agar plates at 4°C for a maximum of 30 days. Unless stated otherwise, strains were cultured on a rotary wheel at 20 rpm for 24 h at 25°C in round-bottom culture tubes containing 3 ml YPD broth. To induce capsule growth, Cryptococcus cells were grown in round-bottom culture tubes containing 3 ml Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, and 10% fetal bovine serum (FBS) for 72 h in an incubator at 37°C at 200 rpm.
Hybridoma production and mAb purification.
Cultures of C. neoformans H99 and C. gattii R265 were microcentrifuged (4,000 × g for 5 min) and washed three times in 1 ml Dulbecco’s phosphate-buffered saline (PBS). Washed cultures were then heat killed for 60 min at 65°C. Following heat killing, 20 μl was plated onto YPD agar to confirm that there were no viable cells. Heat-killed H99 and R265 cells were then either lysed (see below) or mixed 1:1 and stored at −20°C prior to inoculation. Fungal cells were lysed using Precellys tubes (catalog number UK05 03961-1-004), using program 6400-2x10-005. Following lysis, lysis beads were microcentrifuged (3,000 × g for 1 min), and the supernatant was collected. H99 and R265 lysates were mixed 1:1 and stored at −20°C.
BALB/c mice were hyperimmunized with heat-killed H99 and R265 cells in addition to their lysates. Hybridomas were generated by a method that has previously been described (66). NS0 immortal fusion partner cells were fused with splenocytes mediated by polyethylene glycol (StemCell Technologies). All animal work was conducted in accordance with Home Office guidelines and following local ethical approval granted under animal license 30/2788. Supernatants from clones were screened for reactivity with H99 and R265 cells using 96-well plates, with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG and anti-mouse IgM antibodies being used to identify positive clones via epifluorescence microscopy. Positive clone 127 was cultured in RPMI 1640 with IgG-depleted FBS, and the supernatant was collected in a MiniPerm bioreactor (Sarstedt). mAb Crp127 was purified from the supernatant using affinity chromatography and ProSep Thiosorb (Millipore).
Hybridoma sequencing and antibody sequence analysis.
Sequencing of hybridomas was carried out by Absolute Antibody Ltd. (UK). Sequencing was performed by whole-transcriptome shotgun sequencing (RNA-Seq). In brief, hybridomas were cultured in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% FBS in an incubator at 37°C and with 5% CO2. Total RNA was extracted from cells, and a barcoded cDNA library was generated through reverse transcription-PCR (RT-PCR) using a random hexamer. Sequencing was performed using an Illumina HiSeq sequencer. Contigs were assembled and annotated for viable antibody sequences (i.e., those not containing stop codons) to confirm the species and isotype of mAb Crp127 as murine and IgM, respectively.
Variable-region gene usage was determined using VBASE2 software (67), and CDRs were predicted using the Kabat numbering system (68). Heavy-chain variable (VH) and light-chain variable (VL) sequences of mAb Crp127 were aligned with antibody sequences that have previously been described (69, 70). Amino acid sequences were aligned using Clustal Omega software (71) and annotated using ESpript software (72).
Immunolabeling.
Cryptococcus cells were immunostained for flow cytometry and microscopy experiments. One milliliter of fungal culture was transferred to a 1.5-ml microcentrifuge tube, microcentrifuged (15,000 × g for 1 min), and washed three times in PBS. Cell density was determined using a hemocytometer and adjusted to 107 cells/ml in a final volume of 200 μl. A total of 20 μg/ml Crp127, F12D2, 18B7, or mouse anti-human IgG (IgM isotype control) was added, and samples were mixed on a rotary wheel at 20 rpm for 1 h at room temperature. Untreated cells for use in flow cytometry experiments were left untreated. After primary antibody treatment, samples were microcentrifuged (15,000 × g for 1 min) and washed three times in PBS to remove unbound primary antibody. A total of 2 μg/ml Alexa 488-conjugated goat anti-mouse IgM (heavy chain) (Thermo Fisher Scientific), Alexa 647-conjugated goat anti-mouse IgM μ-chain (Abcam), or Alexa 647-conjugated F(ab′)2-goat anti-mouse IgG(H+L) (Thermo Fisher Scientific) was added to antibody-treated samples, and samples were mixed on a rotary wheel at 20 rpm for 1 h at room temperature. Secondary antibody was also added to isotype control samples for flow cytometry. For microscopy experiments, 5 μg/ml calcofluor white (CFW) was also added at this stage to label chitin. Following incubation with secondary antibody, samples were again microcentrifuged (15,000 × g for 1 min) and washed three times to remove unbound secondary antibody and CFW.
Flow cytometry.
Flow cytometry experiments were performed with an Attune NxT flow cytometer equipped with an Attune autosampler (Thermo Fisher Scientific). Untreated, isotype control, and either Crp127- or 18B7-treated samples were prepared for each strain or condition tested. Following immunostaining, samples were diluted to 5 × 106 cells/ml, and 200 μl of Cryptococcus cells was put into individual wells of a plastic round-bottom 96-well plate ready for insertion into the Attune autosampler. The sample was collected from each well at a rate of 100 μl/min until 10,000 events were recorded. The 488-nm laser was used to detect primary antibody bound by Alexa 488-conjugated secondary antibodies, with the same voltage being used to power the laser within each experiment. Flow cytometry data were then analyzed using FlowJo (v10) software. Debris was excluded by using the FSC-A-versus-SSC-A gating strategy, followed by exclusion of doublets using the FSC-A-versus-FSC-H gating strategy (Fig. S4). Exclusion of doublets was used to avoid inclusion of cell aggregates that may happen due to incomplete budding, cell-cell adhesion, or antibody-mediated agglutination. Where GXM-deficient mutants were analyzed, samples were gated only to exclude debris due to the inseparable large aggregates formed by these mutants as a result of budding defects. After gating, histograms of fluorescence intensity were plotted, and the median fluorescence intensity (MFI) was determined. Corrected MFI values were calculated by subtracting the MFI value of the mAb-treated sample by that of the corresponding isotype control sample in the case of Crp127 or the untreated sample where 18B7 was used. Across all experiments, MFI values returned from isotype control cells were extremely similar to those returned from untreated cells.
Confocal microscopy.
Following the final wash steps of the immunostaining procedure, 2 μl of stained cryptococcal cells was spotted onto a glass slide and placed under a square glass coverslip. Where visualization of the capsule was necessary, 2 μl India ink was also added to the glass slide. Imaging was performed on a Nikon A1R laser scanning confocal microscope using a 100× lens objective and oil immersion. Alongside transmitted light, 639-nm and 405-nm lasers were used to detect Alexa 647-conjugated secondary antibodies and CFW, respectively. For cells with small capsules, z-stacks spanning 8 μm were generated using steps of 0.27 μm. For capsule-induced cells, z-stacks were taken across 20 μm using steps of 0.66 μm. Generation of maximum-intensity projections (MIPs) and other image processing were performed using NIS-Elements and ImageJ software.
Chemical de-O-acetylation of capsular GXM.
Where chemical de-O-acetylation of the capsule was required, cells were grown in YPD broth that had been adjusted pH 11 with NaOH and sterilized with a 0.22-μm filter. Round-bottom culture tubes containing 3 ml of pH 11 YPD broth were then placed on a rotary wheel turning at 20 rpm for 24 h at 25°C. This method was adapted from that used in a previous study (21).
Phagocytosis assays.
Phagocytosis assays were performed using the murine macrophage-like J774A.1 cell line (mouse BALB/cN; ATCC TIB-67). Cells were cultured in DMEM supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, and 10% FBS, before 1 × 105 cells were seeded onto round glass coverslips that had been placed into wells of a flat-bottom 24-well plate and incubated for 24 h at 37°C and with 5% CO2. Cells of strains R265 and KN99α were opsonized with 18B7 or Crp127 as described above for the first incubation step of the immunostaining procedure. In the same way, cells were opsonized with 10% AB− human serum alone or in combination with Crp127. To achieve a multiplicity of infection (MOI) of 10, 106 R265 or KN99α cells were then resuspended in serum-free DMEM and added to each well of J774A.1 cells. Following infection, each well was gently washed three times with 1 ml of warmed PBS to remove extracellular yeast. The contents of each well were then fixed with 4% paraformaldehyde prior to being washed a further three times. Coverslips were then extracted from their well, any residual PBS was removed by brief submersion in sterile distilled water (dH2O), and the contents were mounted onto glass slides with Prolong Gold antifade mountant (Thermo Fisher Scientific). The total number of internalized yeast cells per 100 J774A.1 cells (phagocytic index) was determined by microscopic examination using a Nikon TE2000-U microscope with a 60× lens objective and oil immersion.
Capsular swelling reactions.
Capsule-induced cells were treated with 50 μg/ml Crp127 or 18B7 as described above for the immunostaining procedure. Two microliters of Cryptococcus cells was then dropped onto a glass slide and placed under a square glass coverslip. Imaging was performed on the differential interference contrast (DIC) channel of a Nikon TE2000-U microscope using a 60× lens objective with oil immersion. Image processing was performed using NIS-Elements and ImageJ software.
Titan cell experiments.
Titan cells that exhibit all the properties of in vivo titan cells were induced in vitro using a previously described protocol (9). C. neoformans H99, KN99α, CBS 8336, and B3501 and C. gattii R265 cells were cultured in glass conical flasks containing 10 ml yeast nitrogen base (YNB) plus 2% glucose at 30°C and at 200 rpm for 24 h. Cells were adjusted to an optical density at 600 nm (OD600) reading of 0.001 before being transferred into 10% heat-inactivated fetal calf serum (HI-FCS) at a final volume of 3 ml in a plastic six-well plate and grown for 72 h at 37°C and with 5% CO2. To begin a culture derived solely from titan cells, cells were passed through an 11-μm filter, trapping only larger cells on the filter paper. This filter paper was then washed in PBS to resuspend titan cells. Between 103 and 104 titan cells were then transferred into 3 ml HI-FCS in a plastic six-well plate and cultured for a further 72 h at 37°C and with 5% CO2. Titanizing populations were prepared for imaging according to the method described above for immunostaining. Imaging was performed on a Nikon TE2000-U microscope using a 60× lens objective with oil immersion.
To quantify the proximity of the Crp127 epitope to the capsule surface, ImageJ software was used to draw regions of interest (ROIs) around the cell body, the immunofluorescence binding pattern of Crp127, and the capsule surface (as determined by India ink staining). For each cell measured, the area of these three ROIs was determined before the area of the cell body was subtracted from the areas calculated for both the Crp127 epitope ROI and the capsule surface ROI. Finally, the area of the Crp127 epitope ROI was divided by the capsule surface ROI as a means of quantifying the proximity of the Crp127 epitope to the capsule surface. For cells where no antibody binding was detected, the ratio was scored as zero. Mean numbers of 111 and 133 cells were measured per biological replicate for strains H99 and KN99α, respectively. Image processing was performed using NIS-Elements software.
Experimental design and statistical analysis.
For each experiment described, three biological repeats were performed as independent experiments that were carried out on different days. All data sets were analyzed using GraphPad Prism 7 or 8 software.
Data availability.
All data needed to evaluate the conclusions drawn in this paper are present in the paper and/or the supplemental material. Additional data related to this paper may be requested from the authors. The Crp127 antibody described here is available via Ximbio.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge our colleagues Tamara Doering (Washington University), Guilhem Janbon (Institut Pasteur), Arturo Casadevall (Johns Hopkins), and Thomas Kozel (University of Nevada) for providing antibodies and strains and for their invaluable advice regarding this project. We are also grateful to Alessandro Di Maio, Leanne Taylor-Smith, and Joao Correia (University of Birmingham) for assistance with confocal microscopy and subsequent image processing.
Experiments were designed and conducted by M.P., X.Z., and E.B. The Crp127 antibody was raised and initially characterized by S.A.J. and M.G. E.R.B. and R.C.M. helped design and oversee this project. Data figures and text were prepared by M.P. and then edited and revised by all the other authors.
We declare no competing interests with this work.
This work was made possible via funding from the Lister Institute for Preventive Medicine and the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013)/ERC grant agreement no. 614562 and from the Biotechnology and Biological Sciences Research Council (BBSRC) via grant BB/R008485/1. R.C.M. is additionally supported by a Wolfson Royal Society research merit award. X.Z. is supported by a studentship from the Darwin Trust. E.R.B. is supported by the UK Biotechnology and Biological Research Council (BB/M014525/1) and the Wellcome Trust (211241/Z/18/Z).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00731-18.
REFERENCES
- 1.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. 2017. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacDougall L, Fyfe M, Romney M, Starr M, Galanis E. 2011. Risk factors for Cryptococcus gattii infection, British Columbia, Canada. Emerg Infect Dis 17:193–199. doi: 10.3201/eid1702.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galanis E, MacDougall L, Kidd S, Morshed M, British Columbia Cryptococcus gattii Working Group. 2010. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999-2007. Emerg Infect Dis 16:251–257. doi: 10.3201/eid1602.090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris JR, Lockhart SR, Debess E, Marsden-Haug N, Goldoft M, Wohrle R, Lee S, Smelser C, Park B, Chiller T. 2011. Cryptococcus gattii in the United States: clinical aspects of infection with an emerging pathogen. Clin Infect Dis 53:1188–1195. doi: 10.1093/cid/cir723. [DOI] [PubMed] [Google Scholar]
- 5.Granger DL, Perfect JR, Durack DT. 1985. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest 76:508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fromtling RA, Shadomy HJ, Jacobson ES. 1982. Decreased virulence in stable, acapsular mutants of Cryptococcus neoformans. Mycopathologia 79:23–29. [DOI] [PubMed] [Google Scholar]
- 7.Zaragoza O, García-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodríguez-Tudela JL, Casadevall A. 2010. Fungal cell gigantism during mammalian infection. PLoS Pathog 6:e1000945. doi: 10.1371/journal.ppat.1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chrétien F, Heitman J, Dromer F, Nielsen K. 2010. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog 6:e1000953. doi: 10.1371/journal.ppat.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dambuza IM, Drake T, Chapuis A, Zhou X, Correia J, Taylor-Smith L, LeGrave N, Rasmussen T, Fisher MC, Bicanic T, Harrison TS, Jaspars M, May RC, Brown GD, Yuecel R, MacCallum DM, Ballou ER. 2018. The Cryptococcus neoformans titan cell is an inducible and regulated morphotype underlying pathogenesis. PLoS Pathog 14:e1006978. doi: 10.1371/journal.ppat.1006978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okagaki LH, Nielsen K. 2012. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot Cell 11:820–826. doi: 10.1128/EC.00121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabtree JN, Okagaki LH, Wiesner DL, Strain AK, Nielsen JN, Nielsen K. 2012. Titan cell production enhances the virulence of Cryptococcus neoformans. Infect Immun 80:3776–3785. doi: 10.1128/IAI.00507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter DA, Fernandes KE, Brockway A, Haverkamp M, Cuomo CA, Van Ogtrop F, Perfect JR. 2018. Phenotypic variability correlates with clinical outcome in Cryptococcus isolates obtained from Botswanan HIV/AIDS patients. bioRxiv doi: 10.1101/418897. [DOI] [PMC free article] [PubMed]
- 13.Zhou X, Ballou ER. 2018. The Cryptococcus neoformans titan cell: from in vivo phenomenon to in vitro model. Curr Clin Microbiol Rep 5:252–260. doi: 10.1007/s40588-018-0107-9. [DOI] [Google Scholar]
- 14.Casadevall A, Coelho C, Cordero RJB, Dragotakes Q, Jung E, Vij R, Wear MP. 1 August 2018. The capsule of Cryptococcus neoformans. Virulence doi: 10.1080/21505594.2018.1431087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherniak R, Valafar H, Morris LC, Valafar F. 1998. Cryptococcus neoformans chemotyping by quantitative analysis of 1H nuclear magnetic resonance spectra of glucuronoxylomannans with a computer-simulated artificial neural network. Clin Diagn Lab Immunol 5:146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherniak R, Reiss E, Slodki ME, Plattner RD, Blumer SO. 1980. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans serotype A. Mol Immunol 17:1025–1032. [DOI] [PubMed] [Google Scholar]
- 17.Janbon G, Himmelreich U, Moyrand F, Improvisi L, Dromer F. 2001. Cas1p is a membrane protein necessary for the O-acetylation of the Cryptococcus neoformans capsular polysaccharide. Mol Microbiol 42:453–467. [DOI] [PubMed] [Google Scholar]
- 18.Dromer F, Gueho E, Ronin O, Dupont B. 1993. Serotyping of Cryptococcus neoformans by using a monoclonal antibody specific for capsular polysaccharide. J Clin Microbiol 31:359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McFadden DC, Fries BC, Wang F, Casadevall A. 2007. Capsule structural heterogeneity and antigenic variation in Cryptococcus neoformans. Eukaryot Cell 6:1464–1473. doi: 10.1128/EC.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFadden D, Zaragoza O, Casadevall A. 2006. The capsular dynamics of Cryptococcus neoformans. Trends Microbiol 14:497–505. doi: 10.1016/j.tim.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Gates-Hollingsworth MA, Kozel TR. 2009. Phenotypic heterogeneity in expression of epitopes in the Cryptococcus neoformans capsule. Mol Microbiol 74:126–138. doi: 10.1111/j.1365-2958.2009.06855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franzot SP, Mukherjee J, Cherniak R, Chen LC, Hamdan JS, Casadevall A. 1998. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect Immun 66:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera J, Feldmesser M, Cammer M, Casadevall A. 1998. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect Immun 66:5027–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlier C, Chrétien F, Baudrimont M, Mordelet E, Lortholary O, Dromer F. 2005. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am J Pathol 166:421–432. doi: 10.1016/S0002-9440(10)62265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Hermoso D, Dromer F, Janbon G. 2004. Cryptococcus neoformans capsule structure evolution in vitro and during murine infection. Infect Immun 72:3359–3365. doi: 10.1128/IAI.72.6.3359-3365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukaremera L, Lee KK, Wagener J, Wiesner DL, Gow NAR, Nielsen K. 2018. Titan cell production in Cryptococcus neoformans reshapes the cell wall and capsule composition during infection. Cell Surf 1:15–24. doi: 10.1016/j.tcsw.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiesner DL, Specht CA, Lee CK, Smith KD, Mukaremera L, Lee ST, Lee CG, Elias JA, Nielsen JN, Boulware DR, Bohjanen PR, Jenkins MK, Levitz SM, Nielsen K. 2015. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog 11:e1004701. doi: 10.1371/journal.ppat.1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houpt DC, Pfrommer GS, Young BJ, Larson TA, Kozel TR. 1994. Occurrences, immunoglobulin classes, and biological activities of antibodies in normal human serum that are reactive with Cryptococcus neoformans glucuronoxylomannan. Infect Immun 62:2857–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohatgi S, Pirofski L-A. 2015. Host immunity to Cryptococcus neoformans. Future Microbiol 10:565–581. doi: 10.2217/fmb.14.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozel TR, Levitz SM, Dromer F, Gates MA, Thorkildson P, Janbon G. 2003. Antigenic and biological characteristics of mutant strains of Cryptococcus neoformans lacking capsular O acetylation or xylosyl side chains. Infect Immun 71:2868–2875. doi: 10.1128/IAI.71.5.2868-2875.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moyrand F, Klaproth B, Himmelreich U, Dromer F, Janbon G. 2002. Isolation and characterization of capsule structure mutant strains of Cryptococcus neoformans. Mol Microbiol 45:837–849. [DOI] [PubMed] [Google Scholar]
- 32.Cleare W, Cherniak R, Casadevall A. 1999. In vitro and in vivo stability a Cryptococcus neoformans glucuronoxylomannan epitope that elicits protective antibodies. Infect Immun 67:3096–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellerbroek PM, Lefeber DJ, van Veghel R, Scharringa J, Brouwer E, Gerwig GJ, Janbon G, Hoepelman AIM, Coenjaerts FEJ. 2004. O-acetylation of cryptococcal capsular glucuronoxylomannan is essential for interference with neutrophil migration. J Immunol 173:7513–7520. doi: 10.4049/jimmunol.173.12.7513. [DOI] [PubMed] [Google Scholar]
- 34.Urai M, Kaneko Y, Ueno K, Okubo Y, Aizawa T, Fukazawa H, Sugita T, Ohno H, Shibuya K, Kinjo Y, Miyazaki Y. 2015. Evasion of innate immune responses by the highly virulent Cryptococcus gattii by altering capsule glucuronoxylomannan structure. Front Cell Infect Microbiol 5:101. doi: 10.3389/fcimb.2015.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perfect JR, Lang SD, Durack DT. 1980. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol 101:177–194. [PMC free article] [PubMed] [Google Scholar]
- 36.Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, MacDougall L, Boekhout T, Kwon-Chung KJ, Meyer W. 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci U S A 101:17258–17263. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu G, Kronstad JW. 2006. Gene disruption in Cryptococcus neoformans and Cryptococcus gattii by in vitro transposition. Curr Genet 49:341–350. doi: 10.1007/s00294-005-0054-x. [DOI] [PubMed] [Google Scholar]
- 38.Moyrand F, Fontaine T, Janbon G. 2007. Systematic capsule gene disruption reveals the central role of galactose metabolism on Cryptococcus neoformans virulence. Mol Microbiol 64:771–781. doi: 10.1111/j.1365-2958.2007.05695.x. [DOI] [PubMed] [Google Scholar]
- 39.Jacobson ES, Ayers DJ. 1979. Auxotrophic mutants of Cryptococcus neoformans. J Bacteriol 139:318–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moyrand F, Chang YC, Himmelreich U, Kwon-Chung KJ, Janbon G. 2004. Cas3p belongs to a seven-member family of capsule structure designer proteins. Eukaryot Cell 3:1513–1524. doi: 10.1128/EC.3.6.1513-1524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Percival A, Thorkildson P, Kozel TR. 2011. Monoclonal antibodies specific for immunorecessive epitopes of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans, reduce serotype bias in an immunoassay for cryptococcal antigen. Clin Vaccine Immunol 18:1292–1296. doi: 10.1128/CVI.05052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandt S, Thorkildson P, Kozel TR. 2003. Monoclonal antibodies reactive with immunorecessive epitopes of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans. Clin Diagn Lab Immunol 10:903–909. doi: 10.1128/CDLI.10.5.903-909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boekhout T, Van Belkum A, Leenders ACAP, Verbrugh HA, Mukamurangwa P, Swinne D, Scheffers WA. 1997. Molecular typing of Cryptococcus neoformans: taxonomic and epidemiological aspects. Int J Syst Bacteriol 47:432–442. doi: 10.1099/00207713-47-2-432. [DOI] [PubMed] [Google Scholar]
- 44.Lengeler KB, Cox GM, Heitman J. 2001. Serotype AD strains of Cryptococcus neoformans are diploid or aneuploid and are heterozygous at the mating-type locus. Infect Immun 69:115–122. doi: 10.1128/IAI.69.1.115-122.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E, IberoAmerican Cryptococcal Study Group. 2003. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis 9:189–195. doi: 10.3201/eid0902.020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Latouche GN, Huynh M, Sorrell TC, Meyer W. 2003. PCR-restriction fragment length polymorphism analysis of the phospholipase B (PLB1) gene for subtyping of Cryptococcus neoformans isolates. Appl Environ Microbiol 69:2080–2086. doi: 10.1128/AEM.69.4.2080-2086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, Falk R, Parnmen S, Lumbsch HT, Boekhout T. 2015. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol 78:16–48. doi: 10.1016/j.fgb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Byrnes EJ, Li W, Lewit Y, Ma H, Voelz K, Ren P, Carter DA, Chaturvedi V, Bildfell RJ, May RC, Heitman J, Heitman J. 2010. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog 6:e1000850. doi: 10.1371/journal.ppat.1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farrer RA, Desjardins CA, Sakthikumar S, Gujja S, Saif S, Zeng Q, Chen Y, Voelz K, Heitman J, May RC, Fisher MC, Cuomo CA. 2015. Genome evolution and innovation across the four major lineages of Cryptococcus gattii. mBio 6:e00868-15. doi: 10.1128/mBio.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Springer DJ, Billmyre RB, Filler EE, Voelz K, Pursall R, Mieczkowski PA, Larsen RA, Dietrich FS, May RC, Filler SG, Heitman J. 2014. Cryptococcus gattii VGIII isolates causing infections in HIV/AIDS patients in southern California: identification of the local environmental source as arboreal. PLoS Pathog 10:e1004285. doi: 10.1371/journal.ppat.1004285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byrnes EJ, Li W, Ren P, Lewit Y, Voelz K, Fraser JA, Dietrich FS, May RC, Chaturvedi S, Chaturvedi V, Heitman J. 2011. A diverse population of Cryptococcus gattii molecular type VGIII in southern Californian HIV/AIDS patients. PLoS Pathog 7:e1002205. doi: 10.1371/journal.ppat.1002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boekhout T, Theelen B, Diaz M, Fell JW, Hop WCJ, Abeln ECA, Dromer F, Meyer W. 2001. Hybrid genotypes in the pathogenic yeast Cryptococcus neoformans. Microbiology 147:891–907. doi: 10.1099/00221287-147-4-891. [DOI] [PubMed] [Google Scholar]
- 53.Mukherjee J, Cleare W, Casadevall A. 1995. Monoclonal antibody mediated capsular reactions (Quellung) in Cryptococcus neoformans. J Immunol Methods 184:139–143. [DOI] [PubMed] [Google Scholar]
- 54.Kress Y, Feldmesser M, Casadevall A. 2001. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 147:2355–2365. doi: 10.1099/00221287-147-8-2355. [DOI] [PubMed] [Google Scholar]
- 55.Hommel B, Mukaremera L, Cordero RJB, Coelho C, Desjardins CA, Sturny-Leclère A, Janbon G, Perfect JR, Fraser JA, Casadevall A, Cuomo CA, Dromer F, Nielsen K, Alanio A. 2018. Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. PLoS Pathog 14:e1006982. doi: 10.1371/journal.ppat.1006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trevijano-Contador N, de Oliveira HC, García-Rodas R, Rossi SA, Llorente I, Zaballos Á, Janbon G, Ariño J, Zaragoza Ó. 2018. Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals. PLoS Pathog 14:e1007007. doi: 10.1371/journal.ppat.1007007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fries BC, Taborda CP, Serfass E, Casadevall A. 2001. Phenotypic switching of Cryptococcus neoformans occurs in vivo and influences the outcome of infection. J Clin Invest 108:1639–1648. doi: 10.1172/JCI13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casadevall A, Mukherjee J, Devi SJ, Schneerson R, Robbins JB, Scharff MD. 1992. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis 165:1086–1093. [DOI] [PubMed] [Google Scholar]
- 59.Mukherjee J, Scharff MD, Casadevall A. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun 60:4534–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eckert TF, Kozel TR. 1987. Production and characterization of monoclonal antibodies specific for Cryptococcus neoformans capsular polysaccharide. Infect Immun 55:1895–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janbon G. 2004. Cryptococcus neoformans capsule biosynthesis and regulation. FEMS Yeast Res 4:765–771. doi: 10.1016/j.femsyr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Farrer RA, Ford CB, Rhodes J, Delorey T, May RC, Fisher M, Cloutman-Green E, Balloux F, Cuomo CA. 2018. Transcriptional heterogeneity of Cryptococcus gattii VGII compared with non-VGII lineages underpins key pathogenicity pathways. bioRxiv doi: 10.1101/396796. [DOI] [PMC free article] [PubMed]
- 63.Abdulkareem AF, Lee HH, Ahmadi M, Martinez LR. 2015. Fungal serotype-specific differences in bacterial-yeast interactions. Virulence 6:652–657. doi: 10.1080/21505594.2015.1066962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saito F, Ikeda R. 2005. Killing of Cryptococcus neoformans by Staphylococcus aureus: the role of cryptococcal capsular polysaccharide in the fungal-bacteria interaction. Med Mycol 43:603–612. [DOI] [PubMed] [Google Scholar]
- 65.Vij R, Cordero RJB, Casadevall A. 2018. The buoyancy of Cryptococcus neoformans is affected by capsule size. mSphere 3:e00534-18. doi: 10.1128/mSphere.00534-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galfrè G, Milstein C. 1981. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol 73:3–46. [DOI] [PubMed] [Google Scholar]
- 67.Retter I, Althaus HH, Münch R, Müller W. 2005. VBASE2, an integrative V gene database. Nucleic Acids Res 33:D671–D674. doi: 10.1093/nar/gki088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu TT, Kabat EA. 1970. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med 132:211–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Casadevall A, DeShaw M, Fan M, Dromer F, Kozel TR, Pirofski LA. 1994. Molecular and idiotypic analysis of antibodies to Cryptococcus neoformans glucuronoxylomannan. Infect Immun 62:3864–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakouzi A, Valadon P, Nosanchuk J, Green N, Casadevall A. 2001. Molecular basis for immunoglobulin M specificity to epitopes in Cryptococcus neoformans polysaccharide that elicit protective and nonprotective antibodies. Infect Immun 69:3398–3409. doi: 10.1128/IAI.69.5.3398-3409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, Cowley AP, Lopez R. 2013. Analysis tool Web services from the EMBL-EBI. Nucleic Acids Res 41:W597–W600. doi: 10.1093/nar/gkt376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman DL, Kozel TR, Lendvai N, Mukherjee J, Pirofski LA, Rivera J, Rosas AL, Scharff MD, Valadon P, Westin K, Zhong Z. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother 42:1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions drawn in this paper are present in the paper and/or the supplemental material. Additional data related to this paper may be requested from the authors. The Crp127 antibody described here is available via Ximbio.