Talaromyces marneffei infection causes talaromycosis (previously known as penicilliosis), a very important opportunistic systematic mycosis in immunocompromised patients. Different virulence mechanisms in T. marneffei have been proposed and investigated.
KEYWORDS: X-ray crystallography, arachidonic acid (AA), lipid-protein interaction, nuclear magnetic resonance, virulence factors
ABSTRACT
Talaromyces marneffei infection causes talaromycosis (previously known as penicilliosis), a very important opportunistic systematic mycosis in immunocompromised patients. Different virulence mechanisms in T. marneffei have been proposed and investigated. In the sera of patients with talaromycosis, Mp1 protein (Mp1p), a secretory galactomannoprotein antigen with two tandem ligand-binding domains (Mp1p-LBD1 and Mp1p-LBD2), was found to be abundant. Mp1p-LBD2 was reported to possess a hydrophobic cavity to bind copurified palmitic acid (PLM). It was hypothesized that capturing of lipids from human hosts by expressing a large quantity of Mp1p is a virulence mechanism of T. marneffei. It was shown that expression of Mp1p enhanced the intracellular survival of T. marneffei by suppressing proinflammatory responses. Mechanistic study of Mp1p-LBD2 suggested that arachidonic acid (AA), a precursor of paracrine signaling molecules for regulation of inflammatory responses, is the major physiological target of Mp1p-LBD2. In this study, we use crystallographic and biochemical techniques to further demonstrate that Mp1p-LBD1, the previously unsolved first lipid binding domain of Mp1p, is also a strong AA-binding domain in Mp1p. These studies on Mp1p-LBD1 support the idea that the highly expressed Mp1p is an effective AA-capturing protein. Each Mp1p can bind up to 4 AA molecules. The crystal structure of Mp1p-LBD1-LBD2 has also been solved, showing that both LBDs are likely to function independently with a flexible linker between them. T. marneffei and potentially other pathogens highly expressing and secreting proteins similar to Mp1p can severely disturb host signaling cascades during proinflammatory responses by reducing the availabilities of important paracrine signaling molecules.
INTRODUCTION
Since the first report in 1956, Talaromyces marneffei (previously known as Penicillium marneffei) is to date the only known pathogenic species in the Talaromyces genus. Talaromycosis caused by T. marneffei was a very important opportunistic systemic mycosis during the AIDS outbreak in the late 1980s, especially in Southeast Asia, including northern Thailand, Vietnam, southern China, Hong Kong, and Taiwan (1–4). Healthy hosts can also be carriers of T. marneffei, but the symptoms of talaromycosis develop only when the immune systems of the hosts are compromised or depressed, for example, in AIDS patients. The first proposed and characterized virulence mechanism of this pathogenic fungus is thermal dimorphism, being able to transit from mycelium form (25°C) to yeast form when its environment reaches the host’s body temperature (37°C) (5, 6).
In the sera of talaromycosis-positive patients, a galactomannoprotein antigen MP1 protein (Mp1p) was highly expressed and secreted by the infecting fungus (7). An enzyme-linked immunosorbent assay (ELISA)-based kit targeting Mp1p was later developed as a diagnostic test (8). However, it remained unknown why a large quantity of Mp1p was produced at the expense of limited resources for T. marneffei residing in human hosts.
Mp1p is a 455-residue protein with two tandem domains, termed lipid binding domains 1 and 2 (Mp1p-LBD1 and Mp1p-LBD2, residues 28 to 180 and residues 188 to 340, respectively), followed by a C-terminal Ser/Thr-rich flexible region, which is thought to be the region of O-glycosylations, and a glycophosphatidylinositol anchor region at the very C terminus. These two domains share 53% sequence identity and 75% sequence similarity. The crystal structure of domain-swapped (open conformation) Mp1p-LBD2 complexed with a copurified palmitic acid (PLM) (16:0), a saturated fatty acid for high-energy storage and other cellular processes, was reported (PDB entry 3L1N), revealing for the first time that Mp1p-LBD2 is a fatty acid binding domain. Mp1p-LBD1 was also described as being unstable in full-length Mp1p; thus, only the structure of Mp1p-LBD2 was reported (9).
Later studies on the infectious and survival behaviors of T. marneffei suggested that Mp1p is indeed a virulence factor in vivo. The life span of the mouse model challenged by an Mp1-knockout strain of T. marneffei was up to 60 days without the development of talaromycosis symptoms, while the same challenge with the wild-type strain could kill the mice within 21 days (10). Subsequent pulldown and lipidomic studies on infected macrophage cell line J774 by T. marneffei showed that arachidonic acid (AA), not PLM, is the dominant fatty acid target of Mp1p in vivo. Furthermore, in the same study, it was reported that the production of both eicosanoids downstream of AA (e.g., prostaglandin E2) and common markers of proinflammatory responses, including tumor necrosis factor alpha and interleukin-6, was significantly reduced in murine macrophage cell line J774 after T. marneffei infection. Detailed molecular interaction between captured AAs and Mp1p-LBD2 was characterized by various biophysical methods, including X-ray crystallography, nuclear magnetic resonance (NMR) titration experiment, and isothermal titration calorimetry (ITC). These results together suggested that Mp1p-LBD2, being a strong AA binder, provides a highly enclosed central hydrophobic cavity (closed conformation) to accommodate up to two AA molecules to suppress inflammation, suggesting a new fungal virulence mechanism (11).
In this study, we focused on the previously uncharacterized LBD1 of Mp1p, Mp1p-LBD1. The crystal structure of Mp1p-LBD1 in complex with copurified PLM was first solved, providing the first structural proof that Mp1p-LBD1 is also a fatty acid-binding domain. Pulldown assay with Mp1p-LBD1 further suggested that, similar to the previously characterized Mp1p-LBD2, AA is the dominant physiological target of Mp1p-LBD1. Liquid chromatography-mass spectrometry (LC-MS) quantification on isolated endogenous full-length Mp1p from an infected murine macrophage cell line showed that the molar ratio of pulled down full-length Mp1p and bound AA is 1 to 4, supporting the idea that both LBDs of Mp1p bind AA molecules in full-length Mp1p in vivo. By X-ray crystallography, NMR titration experiment, and ITC assays, the binding mode of AA molecules in the enclosed hydrophobic cavity of Mp1p-LBD1 was further characterized. A low-resolution crystal structure of Mp1p-LBD1-LBD2 was solved, suggesting independence between the two LBDs in a full-length Mp1p monomer. Thus, the present study enhances our understanding of Mp1p, a novel virulence factor of T. marneffei playing a central role in a novel virulence mechanism to target and suppress inflammation in hosts.
RESULTS
Mp1p-LBD1 is a fatty acid-binding domain.
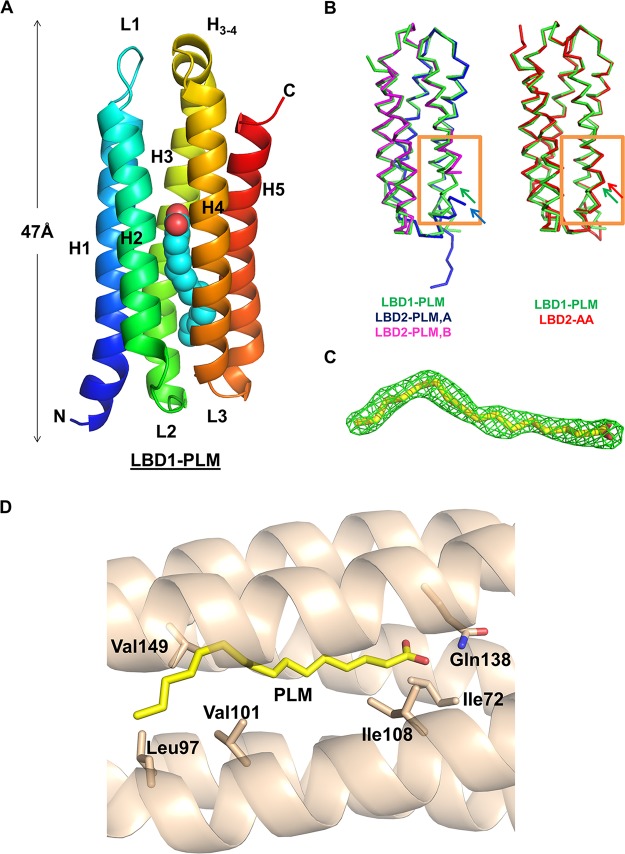
To begin with our analysis on Mp1p-LBD1, we first purified and crystallized recombinant Mp1p-LBD1 from Escherichia coli and solved its crystal structure at 1.80-Å resolution. The yield of recombinant protein purified from E. coli is higher than that from yeast, making it more suitable for both structural and biophysical studies. This domain forms a complex with copurified PLM, demonstrating that Mp1p-LBD1 also serves as a fatty acid binding domain (Fig. 1A), like Mp1p-LBD2. However, with one molecule per asymmetric unit (ASU), the monomeric protein forms a five-helix bundle structure that resembles Mp1p-LBD2 in complex with AAs (PDB entry 5CSD) but not the structure of Mp1p-LBD2 in complex with copurified PLM, which was in an open, domain-swapped form (PDB entry 3L1N) (Fig. 1B). The PLM molecule found in the enclosed central hydrophobic cavity extends from one end to the other, interacting with various hydrophobic amino acid residues along the cavity (Fig. 1D). There is an additional hydrogen bond found between the carboxyl head group of the bound PLM and Gln138 on helix 4. This Gln residue is also involved in hydrogen bonding with singly bound AA in Mp1p-LBD2 (PDB entry 5CSD) but not PLM. Although both Mp1p-LBD1 and Mp1p-LBD2 can bind copurified PLM, the orientation preference of the bound PLM is clearly opposite that in Mp1p-LBD1 when superimposed on the structure of the Mp1p-LBD2-PLM complex (Fig. 2B). This observation of Mp1p-LBD1-PLM complex crystallized in a closed conformation instead of an open conformation, as in Mp1p-LBD2-PLM, despite the high sequence homology between the two domains, supports the hypothesis that the occupation of ligand near the N terminus of helix 3 is correlated with whether Mp1p-LBD prefers an open or closed state in the crystal structure, but the underlying mechanism of this different preference remains to be elucidated.
FIG 1.
Crystallographic structure of Mp1p-LBD1 in complex with copurified PLM at 1.80-Å resolution. (A) Overall monomeric structure of Mp1p-LBD1 complexed with PLM (shown in cyan spheres). The length of this domain was about 47 Å. (B) Alignments of this monomeric Mp1p-LBD1 structure (green) with two domain-swapped, open Mp1p-LBD2 complexed with palmitic acid (PDB entry 3L1N, magenta and blue) (left, 762 main chain atoms with RMSD of 0.668 Å) and monomeric Mp1p-LBD2 complexed with arachidonic acid (PDB entry 5CSD, red) (right, 811 main chain atoms with RMSD of 0.538 Å). Helix 3 of Mp1p-LBD1 resembled the same helix in 5CSD but not 3L1N (regions highlighted in orange boxes), in which helix 3 turns and breaks the continuation at conserved glycine residues (indicated by arrows in corresponding colors). (C) 2mFo-DFc map (green; contour level, 1.0 σ) of PLM in refined model. (D) The amino acid residues involved in interaction between copurified PLM and Mp1p-LBD1.
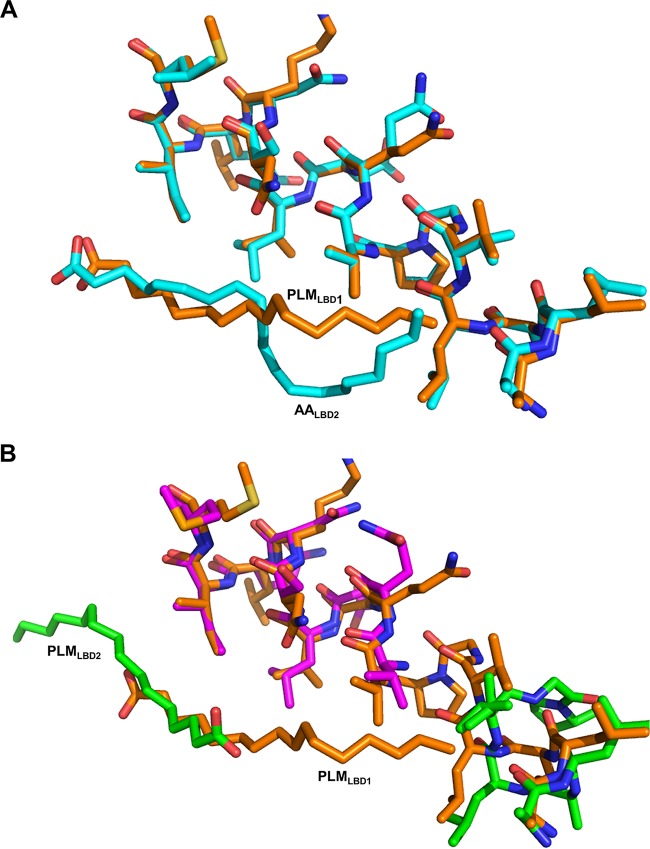
FIG 2.
Superposition of different Mp1p-LBD structures with different ligand positions revealed a possible reason for domain swapping in Mp1p-LBD2 in crystal structures. (A) Alignment of helix 3 from Mp1p-LBD1 complexed with PLM (orange) and Mp1p-LBD2 complexed with AA (5CSD, cyan) near the hinge regions. (B) Alignment of H3 helices from Mp1p-LBD1 complexed with PLM (orange) and LBD2 complexed with PLM (3L1N; green and purple for helix 3 from another Mp1p-LBD2 monomer) near the hinge regions. The PLM in Mp1p-LBD1 and AA in Mp1p-LBD2 locate near the hinge points of both Mp1p-LBDs, providing additional hydrophobic interactions around this region that may stabilize the closed configuration during crystallization. PLM in Mp1p-LBD2, however, locates on the other side of the binding cavity, leading to the absence of the hydrophobic interaction, and may lead to a domain-swapped open structure, as observed in 3L1N.
Mp1p-LBD1 can bind up to 2 AA molecules.
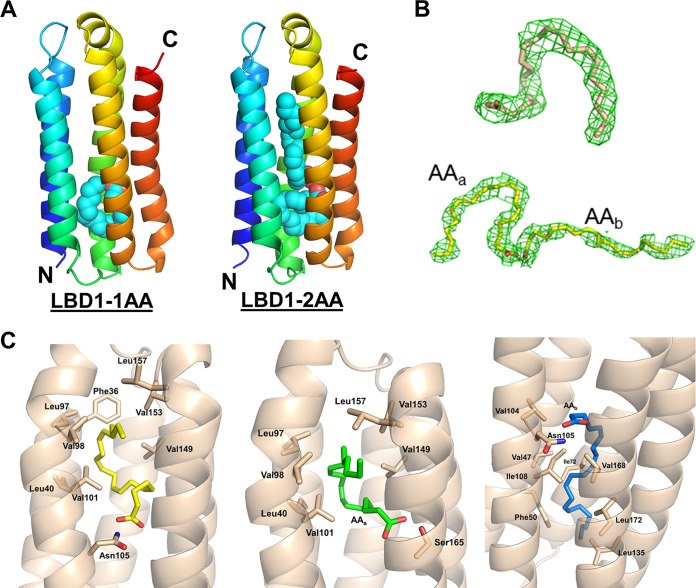
To elucidate the detailed interaction between Mp1p-LBD1 and its physiological target, AA, the complex structure was solved at 2.60-Å resolution. Using purified Mp1p-LBD1 after delipidation, excess AA was added for crystallization. The individual monomeric structure of the Mp1p-LBD1-AA complex does not differ significantly from that of the Mp1p-LBD1-PLM complex or the Mp1p-LBD2-AA complex (Fig. 3A and B). There are ten monomers in an asymmetric unit. In two of ten monomers, single AA molecules with characteristic U-shaped conformation can be successfully modeled close to the N-terminal region of helix 3. In another monomer, two AA molecules are successfully modeled, with one of them showing conserved U-shaped conformation and overlapping the position of the AA in the 1-AA bound form and the other AA showing a more linear conformation and extending its unsaturated alkyl chain to the other side of the cavity (Fig. 4A). In similar positions in other Mp1p-LBD1 monomers, AA models are left unfitted due to the weaker densities observed.
FIG 3.
Mp1p-LBD1 complexed with 1 or 2 AAs. (A) Overall closed monomeric structures of Mp1p-LBD1 complexed with 1 AA molecule (left) and 2 AA molecules (right). Bound AAs are shown in cyan spheres at the middle of the five-helix bundles. (B) 2mFo-DFc maps (green; contour level, 1.0 σ) of AA in refined models. (Top) Singly bound AA. (Bottom) Two AAs in 2-AA bound form. (C) Detailed interaction between AA and Mp1p-LBD1. (Left) Singly bound AA in Mp1p-LBD1-AA form. (Middle) AAa in Mp1p-LBD1-2AA form. Ser165 also provides hydrogen bond. (Right) AAb in Mp1p-LBD1-2AA on the other side of the cavity.
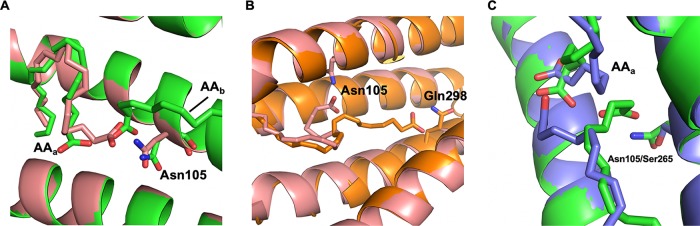
FIG 4.
Asn105 in Mp1p-LBD1 provides additional hydrogen bond to bind AA, leading to shifted AA positions compared to those of AAs in Mp1p-LBD2. (A) Superposition of Mp1p-LBD1-1AA (pink) and Mp1p-LBD1-2AAs (green). AAa in Mp1p-LBD1-2AA superimposes the singly bound AA. Asn105 is involved in forming a hydrogen bond with bound AA in both forms. (B) Superposition of Mp1p-LBD1-1AA (pink) and Mp1p-LBD2-1AAs (orange). It is clear that the interlude of Asn105 preferentially forms a hydrogen bond with singly bound AA in Mp1p-LBD1, while the singly bound AA in Mp1p-LBD2 penetrates deeper into the cavity and forms a hydrogen bond with the conserved glutamine residue (Gln298 in Mp1p-LBD2). (C) Superposition of Mp1p-LBD1-2AA (green) and Mp1p-LBD2-2AAs (blue). The conformation of the 2 bound AAs is largely conserved in both Mp1p-LBDs, but the positions of the head groups differ due to an additionally available hydrogen bond from Asn105 in Mp1p-LBD1.
Superposition of the two AA-bound Mp1p-LBD1 structures to two AA-bound forms of Mp1p-LBD2 (PDB entry 5CSD for 1-AA form and 5FB7 for 2-AA form) shows that there are slight shifts in the positions of the two AAs in Mp1p-LBD1 due to different hydrogen bond networks involved (Fig. 4B and C). In all solved Mp1p-LBD2 structures, the singly bound AA forms a hydrogen bond with conserved Gln298, while both AAs in the 2-AA bound state form a hydrogen bond network with 3 conserved Ser residues. The AAs in both 1-AA and 2-AA bound forms of MP1p-LBD1 make hydrogen bonds with nonconserved Asn105 (Ser265 in Mp1p-LBD2 instead) on helix 3 in Mp1p-LBD1. Thus, the longer polar side chain of Asn105 in the central cavity of Mp1p-LBD1 provides additional hydrogen bond interaction that is absent from Mp1p-LBD2, and this leads to slight shifts of the positions of the AAs in the 2-AA bound forms of Mp1p-LBD1.
Singly bound AA in Mp1p-LBD1 interacts via hydrophobic interactions with Phe36, Leu40, Leu97, Val98, Val101, Val149, Val153, and Leu157 (Fig. 3C, left). The binding of AAa in the 2-AA bound form also involves the same hydrophobic interactions as those in the singly bound AA, except for that of Phe36 (Fig. 3C, middle). In addition, Ser165 provides a hydrogen bond to interact with the carboxylic head group of AAa (Fig. 3C, middle). AAb interacts with the following hydrophobic amino acid residues on the other end of the central cavity: Val47, Phe50, Ile72, Val104, Ile108, Leu135, Val168, and Leu172 (Fig. 3C, right). The hydrophobic residues Ile72, Leu97, Val98, Val101, Ile108, and Val149 are involved in both PLM and AA binding in Mp1p-LBD1.
Crystal structure of Mp1p-LBD1-LBD2.
We successfully crystallized and solved a double-domain structure of Mp1p from LBD1 to LBD2 as a whole at 4.20-Å resolution with 2 molecules per ASU (Fig. 5A). The structure shows that the two Mp1p-LBD1 domains are both in their closed conformation, as in the Mp1p-LBD1-PLM/AA structures reported above, flanking the two Mp1p-LBD2 in their open conformations. This structure is consistent with the individual domain structures of Mp1p-LBD1 and Mp1p-LBD2 complexed with copurified PLM. The densities corresponding to previously missed linkers between Mp1p-LBD1 and Mp1p-LBD2 in the monomers were observed in molecular replacement solution and built in this model (Fig. 5C; see also Fig. S1A in the supplemental material). Moreover, there are minimal contacts observed between Mp1p-LBD1 and Mp1p-LBD2 domains within an Mp1p-LBD1-LBD2 monomer and between two Mp1p-LBD1-LBD2 monomers. These observations suggest that the two Mp1p-LBDs function independently in the full-length Mp1p. Analytical size exclusion chromatography coupled to static light scattering (SLS) experiments, on the other hand, showed that MP1p-LBD1-LBD2 mainly exists as a monomer (expected size, about 35 kDa) when bound to either copurified PLM (37 kDa ± 2.079%) or added AA (34 kDa ± 2.587%).
FIG 5.
Crystal structure of Mp1p-LBD1-LBD2 at 4.20-Å resolution. (A) Pseudodimeric structure of Mp1p-LBD1-LBD2. One monomer is green and the other is yellow. (B) Structure at Mp1p-LBD2 hinge point Gly259 of helix 3 (in full, shown in red) causing the open conformation of Mp1p-LBD2. Thus, this part is the same as the single-domain Mp1p-LBD2-PLM structure (PDB entry 3L1N). (C) The two modelled linkers (shown in red) built between two pairs of Mp1p-LBD1 and Mp1p-LBD2.
Quantification of endogenous full-length Mp1p pulldown shows functional redundancies of LBDs.
Previously, we had shown that endogenous Mp1p could trap AA by coimmunoprecipitation (co-IP) assays, and AA could be identified in the lipid extract of immunoprecipitated products of the T. marneffei-infected cell pellet but not from those of the noninfected cell samples (11). In the present study, we performed quantifications of the pulled down endogenous full-length Mp1p as well as the amount of AA extracted from the Mp1p IP product. The level of Mp1p IP product was found to be 0.864 ± 0.061 pmol per 1 × 106 J774 cell pellet, and the corresponding amount of AA extracted from the Mp1p IP product was 3.10 ± 0.06 pmol (Fig. 6). In comparison, the amount of AA extracted was about 4 molar equivalents of that of full-length Mp1p, which is consistent with the fact that each of the LBDs can trap two AA molecules. The present co-IP experiments indicate that in the endogenous full-length Mp1p, each LBD has trapped two molecules of AA, suggesting that both domains carried equal functionality in terms of their ability to trap cellular AA.
FIG 6.
(A) MS/MS spectra of the precursor ions [M–H]− at m/z 303.2328 on arachidonic acid standard (upper trace) and that of organic extract from the in vitro pulldown experiment (lower trace) at the same collision energy (CE) of 30 to 50 V. (B) MS/MS precursor ion scan on lysophosphatidylcholine (LPC) head group (inset) at m/z 184.0715 at CE of 20 V (upper trace) and 30 to 50 V (lower trace).
Pulldown assay and LC-MS study suggest that AA is a physiological binding target of Mp1p-LBD1 of T. marneffei Mp1p.
Knowing that Mp1p-LBD1 can indeed bind fatty acids leads to speculation that it also serves to bind other important fatty acids when T. marneffei infects a host and, thus, contributes to interrupting host lipid metabolism. We have performed in vitro pulldown experiments on the cell lysate of J774 macrophage cells using recombinant His-tagged Mp1p-LBD1 protein in order to identify its potential cellular target. Lipopolysaccharide (LPS) is the standard chemical for inducing the inflammatory responses with upregulation of cytokines, the lipid mediators, and their metabolites. We have used LPS-activated J774 cells in our pulldown experiment to better simulate the lipid profile of infected macrophages. In order to reveal the dominant higher-affinity binding substrates of Mp1p-LBD1, we used progressively smaller amounts of Mp1p-LBD1 in a series of pulldown experiments. By comparing the results, we could then know the identity of substrates that have higher affinity to Mp1p-LBD1 under the condition of reducing and limited amounts of protein. Table 1 lists the identified substrates from the pulldown experiment of Mp1p-LBD1 against the cell lysate of 3 × 106 LPS-activated J774 cells. Three sets of experiments using 1,000, 250, and 50 μg of bait Mp1p-LBD1 were performed. When 1,000 μg bait protein Mp1p-LBD1 was used, AA, PLM, oleic acid, and 4 lysophosphatidylcholines (LPCs) were found in the pulldown profile list. When the amount of bait was reduced 4 times to 250 μg Mp1p-LBD1, AA, PLM, oleic acid, LPC1, and LPC2 were found. When we further reduced the bait amount to 50 μg Mp1p-LBD1, all LPCs and PLM were no longer detectable in the pulldown list. These results indicate that Mp1p-LBD1 is capable of binding various lipid substrates but AA is the dominant ligand in vivo, even though AA was the least abundant in the pool of cellular lipids (see Table S2 in reference 11). The identities of AA, PLM, and oleic acid in the pulldown lipid extracts were confirmed by using their pure standards. In negative mode, the peak at m/z 303.2323 and elution at room temperature of 16.16 min were found to be significant mass features (MF) in all three pulldown extraction samples (Fig. S2A). Waters MassLynx analysis software (version 4.1; Waters, Milford, MA, USA) suggested a molecular formula of C20H32O2 which nicely matched that of AA. Its identity was subsequently confirmed using a pure AA standard, which showed a well-matched spectrum. The peak at m/z 285 represented a fragment after neutral loss of water from AA, while the loss of the polar ester group exhibited a peak at m/z 259. The cleavage of the remaining aliphatic chain contributed to the other fragments observed in the tandem mass spectrometry (MS/MS) spectrum (Fig. S2A). The identities of the observed LPCs were classified to these lipid classes by their MS/MS fragmentation patterns (Fig. S2B). The characteristic head group of phosphatidylcholine, m/z 184.1, was applied for detection of phosphatidylcholines and LPCs under positive mode. Fatty acid moieties of identified LPCs were determined using the fatty acid scanning (FAS) method described by Ekroos et al. (12) (Table 2). According to their characteristic fragmentation peaks, a series of LPCs were putatively identified in the pulldown extraction samples of Mp1p-LBD1 against the cell lysate of J774 macrophages (Table 1).
TABLE 1.
Profile list of identified lipids from the organic layer extracted under different amounts of Mp1p-LBD1 bait protein
| Low Mp1p-LBD1 (50 μg) | Medium Mp1p-LBD1 (250 μg) | High Mp1p-LBD1 (1,000 μg) |
|---|---|---|
| Arachidonic acid | Arachidonic acid | Arachidonic acid |
| Oleic acid | Oleic acid | Oleic acid |
| Palmitic acid | Palmitic acid | |
| LPC1, 482.3612a | LPC1, 482.3612a | |
| LPC2, 496.3413a | LPC2, 496.3413a | |
| LPC3, 522.3559a | ||
| LPC4, 524.2736a |
Lipids that putatively annotated with their m/z values and matched the fragmentation pattern with lysophosphatidylcholine (LPC).
TABLE 2.
Fatty acid moieties of identified LPCse
| PISa (M + H, 184.1) |
FASc |
Remark | ||
|---|---|---|---|---|
| Precursor ion | Brutto compositionb | Precursor ion | Fatty acid moieties | |
| 496.4 | 16:0 | 554.5 | 16:0 | Palmitoyl-LPC |
| 482.4 | 16:0 | 540.5 | 16:0 | LPC(O-16:0), lyso-PAF C-16d |
| 522.4 | 18:1 | 580.5 | 18:1 | Oleoyl-LPC |
| 524.4 | 18:0 | 582.5 | 18:0 | LPC(18:0) |
PIS represents precursor ion scan on the selected ion fragment of m/z 184.1.
Number of carbon atoms:number of double bonds.
Fatty acid moieties of identified LPCs were determined using FAS method as described by Ekroos et al. (12). FAS [M-H] results were 255.2 (16:0), 281.2 (18:1), and 283.2 (18:0).
PAF, platelet-activating factor.
Identity of each LPC was not characterized. All LPC ions give rise to the same characteristic fragment ion (m/z 184.0735) in high-energy mode (CE, 30 to 50 eV).
NMR titration and ITC of LBD1 with AA molecule show two-step binding process.
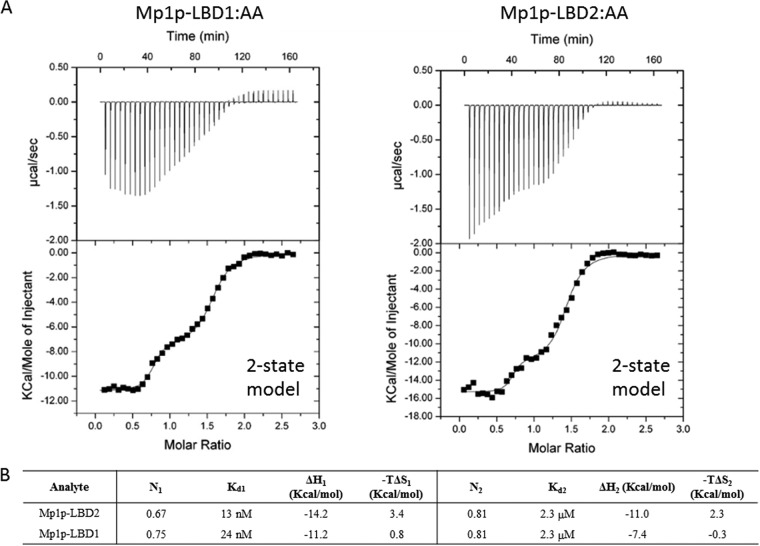
We then verified the AA binding property of Mp1p-LBD1 with NMR and ITC. Figure 7 shows the overlaid two-dimensional 1H-15N heteronuclear single quantum coherence (2D 1H-15N HSQC) NMR spectra of delipidated 15N-labeled Mp1p-LBD1 (blue-colored cross-peaks) and delipidated 15N-labeled Mp1p-LBD1 after the addition of 3.0 molar equivalent of AA (red-colored cross-peaks). Large and specific changes of amide cross-peaks upon the addition of AA indicated that Mp1p-LBD1 could bind to AA, and the binding was site specific. We also carried out ITC to characterize the binding of AA to Mp1p-LBD1. Figure 8 shows the ITC raw heats of binding and isotherms of AA titrated into wild-type Mp1p-LBD1. The isotherm for titration of AA into wild-type Mp1p-LBD1 shows a two-step curve and is best fitted with a two-state model similar to that previously observed in Mp1p-LBD2. Fitting of the ITC isotherm with a two-site model gives the first binding site a high-affinity dissociation constant (Kd) of 24 nM, while the second binding site has a moderate-affinity Kd of 2.3 μM. Compared with Mp1p-LBD2, having 13 nM and 2.3 μM for the first and second AA binding Kds (11), respectively, Mp1p-LBD1’s first AA binding Kd is lower but its second AA binding Kd is similar. Since singly bound AA in Mp1p-LBD1 reported here occupies the same position as the first binding site in Mp1p-LBD2 previously solved (Fig. 4B), we further suggest that the site for the U-shaped AA binding is the high-affinity site of Mp1p-LBD1, while the adjacent site is for the second linear AA.
FIG 7.
NMR titration experiments of 15N-labeled Mp1p-LBD1 with AA. 1H-15N HSQC titration spectra of 15N-Mp1p-LBD1 in the absence (blue) and presence (red) of AA are shown. Mp1p-LBD1 was titrated against AA at pH 8.0. Most peaks displayed slow exchange (peak weakening), while some showed fast exchange (peak shifting).
FIG 8.
ITC of AA binding to Mp1p-LBD1. (Top) Raw heat of binding obtained by ITC when AA was mixed with Mp1p-LBD1. (Bottom) Table of thermodynamic parameters obtained by fitting the ITC data to a two-state binding model (Kd, dissociation constant; ΔH, change in enthalpy; -TΔS, change in entropy; N, number of binding sites; subscripts 1 and 2 refer to the 1st and 2nd binding steps for data fit to a two-state model).
DISCUSSION
Talaromycosis caused by Talaromyces marneffei is a very important opportunistic infection in Southeast Asia, being found mainly in AIDS patients and patients after receiving organ transplantations. One of the key virulence mechanisms known that makes T. marneffei exceptionally pathogenic is its thermal dimorphism to transform to yeast form when in hosts (5, 6). It was later reported that expression of Mp1p also plays an important role in suppressing host proinflammatory responses, because its Mp1p-LBD2 can capture released AA during the onset of proinflammatory responses to reduce the production of downstream eicosanoids (11). In this study, we have further investigated the previously uncharacterized Mp1p-LBD1 of T. marneffei Mp1p and revealed that it structurally resembles Mp1p-LBD2 in terms of folding and strong two-AA binding property. Thus, each Mp1p has two AA-binding domains functioning independently in vivo. Although it is logical from a structural point of view that Mp1p-LBD1 and Mp1p-LBD2 function independently, having two LBDs with similar biological functions may result in avidity for Mp1p. In particular, this might be important for inhibiting and efficient trapping of a ligand. Individually, each binding interaction may be readily broken; however, when many binding interactions are present at the same time, transient unbinding of a single site does not allow the molecule to diffuse away, and binding of that weak interaction is likely to be restored. Antibody is a perfect example for avidity, because each antibody has at least two antigen-binding sites; therefore, antibodies are bivalent to multivalent. For example, IgM is said to have low affinity but high avidity because it has 10 weak binding sites for antigen as opposed to the 2 stronger binding sites of IgG, IgE, and IgD, with higher single binding affinities (13).
Despite both Mp1p-LBD1 and Mp1p-LBD2 being identified first as fatty acid-binding domains by the discovery of singly bound PLM inside their crystal structures, in vivo lipid pulldown experiments suggested that both domains bind to AA most specifically when under an actual pathological environment inside the host, even though other cellular lipids, such as PLM, oleic acid, phosphatidylcholines, and LPC, are more abundant than AA. However, we cannot rule out the possibility that other endogenous hydrophobic ligands also preoccupy the central cavities of the LBDs (for example, PLM). The observation that AA is the only ligand found in Mp1p secreted by T. marneffei in a macrophage cell line (11) supports that both the Mp1p-LBD1 and Mp1p-LBD2 domains will soon be occupied by released AA to displace other occupying lipid molecules during the early steps of proinflammatory responses.
Our structural and biophysical analyses of both Mp1p-LBDs in the full-length Mp1p suggested that they are of equal importance in terms of AA capturing, and each of them can accommodate two AA molecules. It remains unclear why duplication of LBD is present in T. marneffei Mp1p. So far, there are no experimental data on how the full-length Mp1p function would be disrupted in vivo when AA-binding function of either Mp1p-LBD1 or Mp1p-LBD2 is abolished by mutations. We suggest that one advantage of having two functionally duplicated and independent Mp1p-LBDs in a single protein is to double Mp1p-LBD production efficiency. Fast production of a sufficient amount of LBD in the form of full-length Mp1p is critical for the survival of T. marneffei to effectively reduce the availability of AA, because the onset of proinflammatory responses happens after detection of invasion. Concentration of proinflammatory eicosanoids in response to T. marneffei infection might reach a threshold to trigger innate immune responses and eliminate T. marneffei before a sufficient amount of LBD is being secreted and accumulated if the rate of LBD production is low.
Our low-resolution crystal structure of Mp1p-LBD1-LBD2 first demonstrates that the two Mp1p-LBDs are very likely to function independently, since the contact between the two LBDs is minimal. Moreover, a flexible linker was observed connecting these two domains in a monomer. As expected, two Mp1p-LBD1 domains in this Mp1p-LBD1-LBD2 dimeric crystal structure remain as a closed five-helix bundle, exactly the same as the single-domain structure complexed with PLM or AA reported in this study. The Mp1p-LBD2 domains adopt a domain-swapped open conformation exactly the same as that of the Mp1p-LBD2 structure in complex with PLM (PDB entry 3L1N) (9). Despite parallel SLS measurement showing that Mp1p-LBD1-LBD2 remains monomeric in solution regardless of bound ligands, this structure disfavors the explanation that the unusual domain-swapped open conformation of Mp1p-LBD2 is due to crystallization, because the two structures, Mp1p-LBD2-PLM (PDB entry 3L1N) and Mp1p-LBD1-LBD2, both reported here, were crystallized under different conditions and space groups and, hence, different chemical environments for the ASU. Taken together, these observations suggest a model in which Mp1p-LBD2 in full-length Mp1p still adopts an open conformation when not bound to AA in solution. Once bound to the released AA from a host’s proinflammatory responses, Mp1p-LBD2 undergoes conformational changes to adopt a closed conformation. Mp1p-LBD1 possibly goes through a different mechanism from that of Mp1p-LBD2 for AA to enter the central cavity, as the closed conformation is the only conformation observed so far.
We have performed ELISA measurements to estimate the level of expression of Mp1p in infected J774 cell samples. At 48 h postinfection, the levels of free Mp1p were found to be 0.864 ± 0.061 pmol in the cell pellet and 0.116 ± 0.003 pmol in culture supernatant per 1 × 106 J774 cells (see Table S1 in reference 11). The free AA concentration varied by cell types and conditions, e.g., 100 μM (inflamed skin tissue), 13 μM (uninvolved skin) (14), 15 μM (resting islets of Langerhans) (15), and 0.5 to1 μM (resting leukocytes) (16, 17). Our quantitative MS measurements gave total cellular amounts of AA of 33.0 and 28.2 pmol per pellet of 1 × 106 J774 cells infected with Mp1p knockout and wild-type T. marneffei strains, respectively (Table 3). Therefore, the level of free Mp1p is about 3% that of the total cellular AA level. Compared with knockout strain-infected J774 cells, there is a 15% reduction of AA level in J774 cells infected by wild-type T. marneffei. We believe that this observed level of reduction of AA is reasonable given that each full-length Mp1p can trap multiple molecules of AA. AA is a key proinflammatory signal mediator because it generates the downstream eicosanoid family of mediators. These eicosanoids have potent inflammatory actions, some reaching nanomolar scale, and they can also regulate the production of other mediators, including inflammatory cytokines. Since the biosynthesis of eicosanoids depends on the availability of free AA, the base level of free AA in resting cells is under tight control and maintained to be low by locking excess AA into a phospholipid pool in esterified form by the action of coenzyme A synthetase (17). In response to infection by microbes, phospholipase A2 is activated to hydrolyze the ester bonds in membrane phospholipids to yield lysophospholipids and AA to initiate the inflammatory response. This is the first-line host innate immune defense again microbe infection. In our previous study of Mp1p, we showed that Mp1p was able to sequester AA with corresponding downstream effects of reducing AA metabolites, including prostaglandin E2, prostaglandin D2, 15-hydroxyeicosatetraenoic acid, and 11,12-epoxyeicosatrienoic acid, from each of the AA downstream pathways (11). However, no significant changes of AA levels were observed for J774 cells infected with an MP1 knockout strain of T. marneffei, because no Mp1p was expected to be expressed by the MP1 knockout strain (Table 3). Moreover, we also observed a significantly increased production of interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) for the MP1 knockout strain-infected J774 cells (11). Therefore, our present and previous studies on Mp1p have strongly supported that the observed trapping of AA by Mp1p has caused a subtle and significant lowering of cellular AA level with subsequent biological consequences of suppressing downstream AA metabolites and proinflammatory cytokines IL-6 and TNF-α.
TABLE 3.
Cellular level of AA in J774 cells left uninfected or infected with Mp1p knockout and wild-type strains of T. marneffei
| Parameter | Level by infection cell type |
||
|---|---|---|---|
| Noninfected | Mp1p knockout T. marneffei | Wild-type T. marneffei | |
| MS extract samplea [AA] (nM) | 583 ± 36 | 550 ± 30 | 470 ± 20 |
| AAb (pmol/1 × 106 cells) | 35.0 ± 2.2 | 33.0 ± 1.8 | 28.2 ± 0.9 |
| Cellularc [AA] (μM) | 3.50 | 3.30 | 2.82 |
AA concentration in 180 μl extraction buffer from cell lysate containing 3 × 106 cells.
Amount of AA in sample normalized to 1 × 106 cells.
Assuming a cellular volume of ∼10 μl per 1 × 106 J774 cells.
Finally, given that the continuous secretion of Mp1p by T. marneffei is a demanding task for the pathogen under the limiting environment in the host cell, we believe it is reasonable that the amount of Mp1p secreted will be limited to maintain a level near to or slightly lower than the base level of cellular AA to evade host innate immune response by dampening the AA downstream pathway of proinflammatory signals. It is conceivable that Mp1p is designed to trap more than one AA molecule to increase its trapping efficiency in cells. As far as we know, all known AA-binding fatty acid-binding proteins, cyclooxygenases, and lipoxygenases interact with one molecule of AA per binding site. Physiologically, this ability to trap more than one AA molecule per Mp1p will improve the efficiency of anti-inflammatory action by Mp1p through encapsulation of AA, which will be critical at the initial stage of the T. marneffei infection. Furthermore, the plasticity of Mp1p to bind one or more AA molecules with different affinities may reflect the flexibility of this class of AA-binding proteins to sequester AA in response to the abundance of available inflammatory mediators generated by the host.
Following a domain-enhanced lookup time-accelerated BLAST (DELTA-BLAST) search (https://blast.ncbi.nlm.nih.gov/Blast.cgi), many putative Mp1p-LBD1 homologues could be identified in fungal pathogens, such as Aspergillus flavus and Aspergillus fumigatus, with sequence identity ranging from 23% to 44%. The results after filtering the matches for T. marneffei are summarized in Table S1 in the supplemental material. We have characterized a number of these putative Mp1p-like homologues of T. marneffei for serodiagnosis, including Aflmp1 of A. flavus and Afmp1p and Afmp2p of A. fumigatus (18–24). A. flavus and A. fumigatus cause aspergilloma and invasive aspergillosis globally, and they are the most prominent opportunistic fungal pathogens in immunocompromised hosts. In our ongoing investigations of these Mp1p homologues, we have confirmed that Mp1p homologues Afmp1p, Afmp2p, Afmp3p, and Afmp4p are virulence factors of A. fumigatus (25), and they can all bind AA in a five-helix bundle fold similar to that of Mp1p-LBD1 or Mp1p-LBD2, but each carries only a single LBD rather two LBDs. Full structural and functional characterizations of the binding of AA with Afmp1p, Afmp2p, and Afmp4p will be described in a forthcoming manuscript. Here, we showed that these fungi may produce Mp1p or its homologues, which could capture AA to stall the inflammatory process. We are in the process of characterizing other Mp1p homologues identified in our studies. This novel function of trapping key proinflammatory signaling lipid by Mp1p-like virulence factor to evade host innate immunity may be widely present as a virulence mechanism in other fungal pathogens. Thus, Mp1p represents a novel class of fatty acid binding proteins with the function of targeting key proinflammatory signaling lipid to dampen host innate immune response.
MATERIALS AND METHODS
Construction of expression plasmids for E. coli expression system.
Full-length cDNA of Mp1p was obtained from T. marneffei (strain PM1). The pair of primers used for Mp1p-LBD1 (amino acids [aa] 27 to 182 of full-length Mp1) was CAACAAGGATCCACCAAGGACCAGCGTGATG (forward) and CTACTCGAGTTAGCTAATGGAGAAGGCTTCG (reverse). The pair of primers used for the Mp1p-LBD1-LBD2 (aa 27 to 342 of full-length Mp1) construct was CAACAAGGATCCACCAAGGACCAGCGTGATG (forward; same as the forward primer for subcloning of Mp1p-LBD1) and CAACTCGAGTTAAGTGCCGGCGAAG (reverse). PCR experiments were performed on the provided cDNA to subclone the amplified fragments into an ampicillin-resistant expression vector, including an N-terminal hexahistidine tag and SUMO tag (His-SUMO) preceding the target fragments with a Ulp1 cleavage site between them, using restriction enzymes BamHI and XhoI (NEB). Following a standard protocol of 42°C heat shock transformation and selection with 100 μg/ml ampicillin, selected colonies were picked and grown in LB broth for plasmid extraction and sequencing (BGI). Correct plasmids were transformed into E. coli expression strain BL21(DE3) (Invitrogen) to express the recombinant proteins.
Expression and purification of LBD1 and LBD-LBD2 from BL21(DE3).
Four liters of transformed BL21(DE3) was agitated at 37°C and 230 rpm in LB broth with 100 μg/ml ampicillin until the optical density at 600 nm (OD600) was between 0.6 and 0.7. Isopropyl-beta-d-thiogalactopyranoside (IPTG; 0.5 mM) was added for induction at 16°C. After 16 h of induction, cell pellet was harvested by centrifugation and then was lysed by sonication in lysis buffer (25 mM Tris-HCl [pH 7.5], 500 mM NaCl, and 10 mM imidazole). Further centrifugation was performed to obtain the supernatant of soluble total protein. Filtered supernatant was slowly loaded by gravity flow onto 5 ml His60 Superflow Ni-IDA resin (Clontech) equilibrated with lysis buffer. After washing with 25 ml lysis buffer and the same buffer with 50 mM imidazole, remaining bound protein was eluted with a step gradient of 250 mM and 500 mM imidazole. Fractions containing target SUMO-Mp1p-LBD1 were combined, and the His-SUMO tag was released with Ulp1, with simultaneous overnight dialysis against 3 liters of buffer (25 mM Tris-HCl [pH 7.5], 50 mM NaCl) at 4°C. Cleaved protein was slowly reloaded onto 5 ml His60 Superflow Ni-IDA resin equilibrated with buffer (25 mM Tris-HCl [pH 7.5], 50 mM NaCl). Flowthrough containing Mp1p-LBD1 was collected and further purified with a 5-ml HiTrap Q-HP column (GE Healthcare). Target protein was mostly found in the flowthrough (see Fig. S4 in the supplemental material), and it was concentrated at 4°C with an Amicon Ultra unit (3-kDa cutoff) to 40 mg/ml with a calculated extinction coefficient of 2,980 M−1 cm−1.
SUMO-tagged Mp1p-LBD1-LBD2 was overexpressed in the same way as the SUMO-tagged Mp1p-LBD1. The pellet of induced cell was lysed by sonication in lysis buffer (25 mM Tris-HCl [pH 8.0], 500 mM NaCl, and 10 mM imidazole). Further centrifugation was performed to remove cell debris. Filtered supernatant was then slowly loaded by gravity flow onto 5 ml His60 Superflow Ni-IDA resin (Clontech) equilibrated with lysis buffer. After washing with 25 ml lysis buffer and the same buffer with 50 mM imidazole, remaining protein was eluted with a step gradient of 250 mM and 500 mM imidazole. The fractions containing the expressed protein with tag were then further purified with a 5-ml HiTrap Q-HP column (GE Healthcare). Protein was eluted in a single peak with a linear NaCl gradient. The His-SUMO tag was removed with added Ulp1 overnight at 4°C, with slow agitation for thorough mixing. To obtain the target protein without tag, all cleaved sample was loaded onto a 5-ml HisTrap-HP column (GE Healthcare) equilibrated with buffer (25 mM Tris-HCl [pH 8.0], 100 mM NaCl). Target protein was found in the flowthrough with good purity (Fig. S4). It was concentrated at 4°C with an Amicon Ultra unit (10-kDa cutoff) to 40 mg/ml, with a calculated extinction coefficient of 4,470 M−1 cm−1.
Delipidation on purified protein samples and preparation of AA-bound samples.
All recombinant Mp1p constructs from E. coli expression systems are found to contain endogenous fatty acids. The delipidation protocol used to remove the endogenous fatty acids was described before for Mp1p-LBD2 (9). Briefly, 5 ml of the protein sample was mixed with 4 ml of di-isopropyl ether (DIPE) and n-butanol mixture (3:2 [vol/vol]). The total mixture was agitated gently for 30 min at room temperature to perform the removal of bound fatty acid, and the protein-containing aqueous phase and fatty acid-containing organic phase were separated by centrifugation (1,000 × g, 10 min, 4°C). The upper organic phase was removed, and fresh organic solvent mixture was added again to repeat the extraction process at least twice more. Eight ml of DIPE was then added to remove the residual n-butanol in aqueous phase. After another centrifugation to separate the two phases, protein was retrieved by penetrating the bottom of the centrifuge tube with a needle and 5-ml syringe. Dialysis against 1 liter of buffer (25 mM Tris-HCl [pH 7.5], 100 mM NaCl) overnight at 4°C was performed on the retrieved protein to further dilute the remaining organic solvent. Delipidated protein concentrations were determined with the calculated extinction coefficients, and a 6:1 molar excess of freshly prepared 100 mM AA dissolved in 100 mM NaOH on ice was directly added to the delipidated protein. After incubation for 1 h at 4°C, the protein-ligand complexes were concentrated to 40 mg/ml and the buffer was intensively exchanged with the buffers of the untreated proteins with an Amicon Ultra unit (3-kDa cutoff).
Crystallization.
The crystals of Mp1p-LBD1 with copurified PLM (40 mg/ml) were grown by mixing 1 μl of protein sample with 1 μl reservoir solution (0.1 M sodium acetate [pH 4.6], 0.2 M ammonium sulfate, 20% polyethylene glycol [PEG] 12000), using the hanging-drop vapor diffusion method at 298 K. Fully grown and qualified crystals were observed after 1 week (Fig. S4). Cryoprotectant was the same reservoir solution with an additional 16% (vol/vol) glycerol. The crystals of Mp1p-LBD1 complexed with AA (40 mg/ml) were grown by mixing 1 μl of protein sample with 1 μl reservoir solution (0.1 M sodium acetate [pH 4.6], 0.2 M ammonium acetate, 20% PEG 4000), using the hanging-drop vapor diffusion method at 298 K. Fully grown and qualified crystals were observed after 4 days of incubation (Fig. S4). Cryoprotectant was the same reservoir solution with 16% (vol/vol) glycerol. The needle crystals of Mp1p-LBD1-LBD2 were obtained from crystallization conditions of 0.1 M Tris-HCl, pH 8.4, 10 mM NiCl2, 1.1 M Li2SO4, 5% (vol/vol) glycerol after 2 weeks of incubation using the hanging-drop method at 298 K (Fig. S4). The cryoprotectant used was the same as that for the mother liquor with 16% (vol/vol) glycerol.
Crystallographic data collection, phasing, and model refinement.
Diffraction data of Mp1p-LBD1-PLM at 1.80-Å resolution were collected at beamline IO2, DIAMOND, Oxford, UK, at 100 K. Indexing, integrating, and scaling were performed with the XDS package provided on site (26). Data of Mp1p-LBD1-AA at 2.60-Å resolution were collected at beamline PROXIMA-I, SOLEIL, Paris, France, at 100 K. Indexing, integrating, and scaling were performed with the HKL2000 package (27). The Mp1p-LBD1-PLM structure was solved with the apo-Mp1p-LBD2 structure as a search model (PDB entry 5CSD) by the molecular replacement method (MR) using Phaser in the CCP4 package (28). The resultant model of the single solution was then manually mutated to the amino acid sequence of Mp1p-LBD1. Refinement was performed with cycles of manual adjustment of the model using Coot (29) and Refmac5 (30), included in the CCP4 package. Water molecules and PLM molecules were added and checked manually and refined with Refmac5 again to obtain a final model. The Mp1p-LBD1-AA data set was solved with the apo-LBD1 structure as a search model for molecular replacement using Phaser in the CCP4 package. Refinement was performed with cycles of manual adjustment of the model using Coot and Refmac5, included in the CCP4 package. Water molecules and AA molecules were added, checked manually, and refined with Refmac5 again to obtain the final model. The Mp1p-LBD1-LBD2 data set at 4.20-Å resolution was first solved using molecular replacement with closed apo-Mp1p-LBD1 and apo-Mp1p-LBD2 structures as search models. The density map of the single MR solution showed connectivity corresponding to that of previously unobserved linkers and revealed how the two Mp1p-LBD1 and the two Mp1p-LBD2 domains are connected. The cross density at the hinge point (Gly259) in Mp1p-LBD2 showed that both Mp1p-LBD2 domains were in open conformation, as was the case in PDB entry 3L1N (Fig. S1). After manual addition of the missing linkers and removal of unseen side chains due to the relatively low resolution, refinements were performed with cycles of manual adjustment of the model using Coot and Refmac5 in the CCP4 package, including global NCS restraints, jelly body refinement, and Prosmart, to generate fragment constraints. The final models were checked with the online program MolProbity (31). Data collection and refinement statistics are summarized in Table 4. The Ramachandran plots of the three final structures are shown in Fig. S3. All of the graphical presentations of these structures were prepared with graphics software PyMOL (http://www.pymol.org).
TABLE 4.
Data collection and refinement statistics of X-ray structures (molecular replacement)
| Parameter | Value(s) fora: |
||
|---|---|---|---|
| Mp1p-LBD1-PLM | Mp1p-LBD1-AA | Mp1p-LBD1-LBD2 | |
| Data collection | |||
| Space group | P43 | P21 | P41212 |
| Cell dimensions | |||
| a, b, c (Å) | 52.58, 52.58, 57.69 | 65.68, 104.27, 108.10 | 146.36, 146.39, 148.61 |
| α, β, γ (º) | 90.00, 90.00, 90.00 | 90.00, 97.44, 90.00 | 90.00, 90.00, 90.00 |
| Resolution (Å) | 38.86–1.8 (1.85–1.80) | 107.19–2.60 (2.69–2.60) | 50.00–4.20 (4.35–4.20) |
| Rsym or Rmerge | 0.027 (0.583) | 0.074 (0.390) | 0.153 (0.000) |
| I/σI | 22.7 (1.8) | 12.6 (2.16) | 9.520 (1.74) |
| Completeness (%) | 99.1 (95.6) | 94.4 (69.7) | 99.7 (100.0) |
| Redundancy | 3.7 (3.6) | 3.6 (2.8) | 6.2 (6.3) |
| Refinement | |||
| Resolution (Å) | 45.00–1.45 (1.85–1.80) | 107.19–2.60 (2.69–2.60) | 50–4.20 (4.35–4.20) |
| No. of reflections | 14,575 | 44,571 | 12,286 |
| Rwork/Rfree | 0.179/0.221 | 0.256/0.290 | 0.2943/0.3496 |
| No. of atoms | |||
| Protein | 1,151 | 10,929 | 3,217 |
| Ligand | 18 | 88 | NAc |
| Water | 53 | 19 | NA |
| B-factors | |||
| Protein | 36.23 | 38.51 | 135.31 |
| Ligand | 44.55 | 32.20 | NA |
| Water | 40.35 | 36.60 | NA |
| RMSDb | |||
| Bond length (Å) | 0.0071 | 0.0169 | 0.0106 |
| Bond angle (º) | 1.1528 | 1.0730 | 1.2520 |
| MolProbity validation | |||
| MolProbity score (percentile) | 0.84 (100th) | 1.98 (97th) | 1.96 (100th) |
| Clashscore (percentile) | 1.24 (100th) | 12.79 (93th) | 12.87 (97th) |
| Ramachandran favored/outliers (%) | 99.35/0 | 98.48/0.07 | 95.15/0.49 |
Values in parentheses are for the highest-resolution shells.
RMSD, root mean square deviations.
NA, not applicable.
SLS to determine molecular weight of Mp1p-LBD1-LBD2 in solution.
Static light scattering (SLS) experiments were performed with assistance from Y. Zhao’s group (The Hong Kong Polytechnic University, Hong Kong). Purified Mp1p-LBD1-LBD2 samples (with or without AA) were separated with an S75 10/300 column (GE Healthcare) already equilibrated with filtered buffer, i.e., 25 mM Tris-HCl (pH 7.5) and 150 mM NaCl, at 298 K. Protein peaks eluted from columns were immediately measured with a coupled SLS instrument, DynaPro Nanostar (Wyatt), using an estimated extinction coefficient of 4,470 M−1 cm−1. The analysis of protein peaks was performed with the program DYNAMICS.
Pulldown experiment on the cell lysate of J774 macrophage cells with Mp1p-LBD1.
The J774 murine macrophage cell line was grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS) at 37°C. LPS-induced inflammatory response was achieved by stimulating the macrophage with 1 g/ml LPS at 37°C for 24 h. The cells were harvested from confluent cultures and washed three times with 1× phosphate-buffered saline (PBS) buffer at 4°C. Cell suspensions in 1× PBS containing 3 × 106 cells per tube were mixed with 1,000, 250, and 50 μg of soluble delipidated His-tagged Mp1p-LBD1 protein on ice. The mixture was immediately sonicated on ice for 20 s, followed by incubation on ice for 30 min in the presence of 10 μg/ml cOmplete protease inhibitor mixture (Roche Applied Science). The cell pellet was deposited after centrifugation at 10,000 × g for 15 min. The supernatant layer was then incubated with 30 μl of nickel-nitrilotriacetic acid (Ni-NTA) agarose resin (Qiagen) at 4°C for 30 min. The resin was washed with twenty column volumes of 1× PBS buffer supplemented with 10 mM imidazole. Lipid-bound His-tagged Mp1p-LBD1 protein was eluted with 200 μl of PBS buffer containing 200 mM imidazole. Bound lipids in the Mp1p-LBD1 protein were extracted by incubating with organic solvent mixture (DIPE and n-butanol [3:2, vol/vol]) at a 1:1 ratio for 30 min at room temperature. The organic layer was separated by centrifugation (2 min, 13,000 rpm) and subjected to untargeted small-molecule profiling by ultrahigh-performance liquid chromatography-electrospray ionization-quadrupole time of flight mass spectrometry (UHPLC-ESI-QTOFMS), using a lipid profiling method to analyze lipid species, as we have previously described (32, 33). Control pulldown experiments without adding Mp1p-LBD1 to LPS-induced J774 cells were performed with the same procedures. Three biological replicates were performed for each pulldown experiment, and triplicate measurements were made for each pulldown condition.
Untargeted pulldown extract profiling using UHPLC-ESI-QTOFMS.
UHPLC-ESI-QTOFMS analysis was performed using M-class UHPLC (Waters, Milford, MA, USA) coupled with a Synapt G2-Si high-definition mass spectrometer (Waters), accompanied by MassLynx software for QTOF (version 4.1; Waters). A Waters Acquity UPLC BEH C18 column (2.1 by 100 mm, 1.7 μm) was applied for the separation of a wide range of lipids with an injection volume of 8 μl. The column and autosampler temperatures were kept at 45°C and 10°C, respectively. Mobile phase A was LC-MS-grade water containing 0.1% (vol/vol) acetic acid, and mobile phase B was acetonitrile. LC separation was achieved at a flow rate of 0.4 ml/min by applying the following gradient program (where t is time): t = 0 min, 0.5% B; t = 1.5 min, 0.5% B; t = 8 min, 8% B; t = 18 min, 35% B; t = 25 min, 70% B; t = 34 min, 99.5% B; t = 36 min, 99.5% B; and t = 38.1 min, 0.5% B. The total run time was 40 min.
MS conditions.
The following parameters were used for MS: mode of operation, TOF MSE; ionization and capillary voltage, ESI positive (+3,500 V) and negative (−3,500 V); cone voltage, 20.0 V; collision energy, 20 V, with ramp of 30 to 50 V; source temperature, 120°C; desolvation temperature, 350°C; desolvation gas, 500.0 liters/h (N2); acquisition range, 60 to 1,500; calibrant, leucine enkephalin.
Data processing and statistical analysis.
The LC-MS data were processed as described by Lau et al. (34). Multi- and univariate statistical analyses were carried out to identify specific lipids in extraction samples that are present at significantly higher levels in pulldown samples but not in the control samples. Only entries with at least 50% frequency present in either the pulldown sample or control groups were included for further statistical analysis to reduce noise. Volcano plots based on P values of <0.01 combined with fold-change (FC) analysis with an FC of >2 were applied to highlight molecular features (MFs) with high abundance ratios between 2 groups.
Lipid identification.
MFs with significant abundance were selected for MS/MS experiment and analysis. The MS/MS data were processed using Waters MassLynx analysis software (version 4.1) to generate a list of potential molecular formulas. All putative lipids were identified by using exact molecular weights, following the nitrogen rule, using MS2 fragment analysis, and searching the literature and databases (METLIN, Lipidmaps, etc.). Fatty acid moieties of identified LPCs were determined using the FAS method as described by Ekroos et al. (12). Five mM ammonium acetate was spiked into the pulldown lipid extract to yield an anion adduct [M + CH3COO-, M + H + 58]. FAS analysis was achieved by acquiring precursor ion spectra with selected fragment ions containing 16 to 20 carbon atoms and 0 to 4 double bonds under negative ion mode. Collision energy ramped from 25 eV to 35 eV.
NMR and ITC data collection and processing.
AA was titrated into a 15N-labeled delipidated sample of Mp1p-LBD1. Two-dimensional 1H-15N heteronuclear single quantum coherence (2D NHSQC) experiments were acquired at 299.1 K on a Bruker Avance 600-MHz NMR spectrometer (1H and 15N frequencies of 600.133 and 60.818 MHz, respectively) equipped with a 5-mm BBI probe with Z-gradient using standard Bruker pulse sequences. Data were acquired and analyzed using Topspin 3.1 (Bruker). Chemical shifts are given on a parts per million (ppm) scale, and coupling constants are reported in Hz. All 1H chemical shifts were referenced to trimethylsilylpropanoic acid methyl resonance at 0 ppm. Chemical shifts of 15N were referenced indirectly by a gyromagnetic ratio method. Free induction decays were multiplied with an exponential line-broadening function of 0.3 Hz before Fourier transformation. The size of the F2 dimension was 2,048 points, and that of the F1 dimension was 256 points, which were Fourier transformed into 2,048 by 1,024 points. Spectral widths were 9,014.423 by 2,736.807 Hz. Delipidated Mp1p-LBD1 protein samples (100 mM) were loaded into the sample cells and titrated with a 10 mM AA stock solution by an iTC200 microcalorimeter (ultrasensitive calorimetry for life sciences; MicroCal). The starting temperature was 37°C, and the reference in the reference cell was pure autoclaved water. The titrations were performed by injecting 20 consecutive 5-μl aliquots of AA stock solution into the ITC cell containing the Mp1p-LBD1 sample. The titration experiment was performed in triplicate. The binding stoichiometry (n), binding affinity (Kd), enthalpy changes (ΔH), and entropy (ΔS) of the protein-ligand interaction were determined by analyzing the resulting ITC data with the software ORIGIN 7 using appropriate binding models.
Data availability.
Mp1p-LBD1 in complex with copurified PLM was deposited in PDB under accession code 5E7X. Mp1p-LBD1 in complex with AA was deposited in PDB under accession code 5ECF. Mp1p-LBD1-LBD2 was deposited in PDB under accession code 6J6F.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Natural Science Foundation of China (31370739 and 31670753), donation by Michael Tong and the Providence Foundation in memory of L. H. M. Lui, Health and Medical Research Fund (commissioned study) of the Food and Health Bureau of Hong Kong Special Administrative Region (HKM-15-M05 and HKM-15-M07), Research Grant Council Fund of Hong Kong (GRF777512 and GRF17124717, AoE/P-705/16), and research grant from Shenzhen Innovation Committee of Science and Technology (JCYJ20160608140912962). W.-H.L. acknowledges the award of an exchange scholarship that allowed him to spend 12 months with Samar Hasnain at the University of Liverpool, during which time data collection at DIAMOND and SOLEIL was performed. Use of SOLEIL was partly funded by the European Community’s Seventh Framework Program (FP7/2007-2013) under BioStruct-X (grant agreement number 283570 and proposal numbers 2370/5437 to S.V.A. and S. Hasnain).
We thank Y. Zhao’s group (The Hong Kong Polytechnic University, Hong Kong) for help with the SLS measurements. We are thankful for technical support and management from the different synchrotrons used in this work: the data set for Mp1p-LBD1-PLM was collected at beamline IO2, DIAMOND, UK; the data set for Mp1p-LBD1-AA was collected at beamline PROXIMA-1, SOLEIL, France; the data set for Mp1p-LBD2-AA was collected at beamline BL13B1, NSRRC, Taiwan, Republic of China; and the data set for Mp1p-LBD1-LBD2 was collected at beamline BL17U, SSRF, China.
We have no competing financial interests to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00679-18.
REFERENCES
- 1.Cooper CR, Vanittanakom N. 2008. Insights into the pathogenicity of Penicillium marneffei. Future Microbiol 3:43–55. doi: 10.2217/17460913.3.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Ustianowski AP, Sieu TP, Day JN. 2008. Penicillium marneffei infection in HIV. Curr Opin Infect Dis 21:31–36. doi: 10.1097/QCO.0b013e3282f406ae. [DOI] [PubMed] [Google Scholar]
- 3.Wong SS, Siau H, Yuen KY. 1999. Penicilliosis marneffei–West meets East. J Med Microbiol 48:973–975. doi: 10.1099/00222615-48-11-973. [DOI] [PubMed] [Google Scholar]
- 4.Vanittanakom N, Cooper CR Jr, Fisher MC, Sirisanthana T. 2006. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev 19:95–110. doi: 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang E, Wang G, Woo PC, Lau SK, Chow WN, Chong KT, Tse H, Kao RY, Chan CM, Che X, Yuen KY, Cai JJ. 2013. Unraveling the molecular basis of temperature-dependent genetic regulation in Penicillium marneffei. Eukaryot Cell 12:1214–1224. doi: 10.1128/EC.00159-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youngchim S, Vanittanakom N, Hamilton AJ. 1999. Analysis of the enzymatic activity of mycelial and yeast phases of Penicillium marneffei. Med Mycol 37:445–450. doi: 10.1046/j.1365-280X.1999.00235.x. [DOI] [PubMed] [Google Scholar]
- 7.Cao L, Chan CM, Lee C, Wong SS, Yuen KY. 1998. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect Immun 66:966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao L, Chan KM, Chen D, Vanittanakom N, Lee C, Chan CM, Sirisanthana T, Tsang DN, Yuen KY. 1999. Detection of cell wall mannoprotein Mp1p in culture supernatants of Penicillium marneffei and in sera of penicilliosis patients. J Clin Microbiol 37:981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao S, Tung ET, Zheng W, Chong K, Xu Y, Dai P, Guo Y, Bartlam M, Yuen KY, Rao Z. 2010. Crystal structure of the Mp1p ligand binding domain 2 reveals its function as a fatty acid-binding protein. J Biol Chem 285:9211–9220. doi: 10.1074/jbc.M109.057760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo PCY, Lau SKP, Lau CCY, Tung ETK, Chong KTK, Yang FJ, Zhang HM, Lo RKC, Cai JP, Au-Yeung RKH, Ng WF, Tse H, Wong SSY, Xu SM, Lam WH, Tse MK, Sze KH, Kao RY, Reiner NE, Hao Q, Yuen KY. 2016. Mp1p is a virulence factor in Talaromyces (Penicillium) marneffei. PLoS Negl Trop Dis 10:e0004907. doi: 10.1371/journal.pntd.0004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sze KH, Lam WH, Zhang H, Ke YH, Tse MK, Woo PC, Lau SK, Lau CC, Cai JP, Tung ET, Lo RK, Xu S, Kao RY, Hao Q, Yuen KY. 2017. Talaromyces marneffei Mp1p is a virulence factor that binds and sequesters a key proinflammatory lipid to dampen host innate immune response. Cell Chem Biol 24:182–194. doi: 10.1016/j.chembiol.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Ekroos K, Ejsing CS, Bahr U, Karas M, Simons K, Shevchenko A. 2003. Charting molecular composition of phosphatidylcholines by fatty acid scanning and ion trap MS3 fragmentation. J Lipid Res 44:2181–2192. doi: 10.1194/jlr.D300020-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder HW Jr, Cavacini L. 2010. Structure and function of immunoglobulins. J Allergy Clin Immunol 125:S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammarstrom S, Hamberg M, Samuelsson B, Duell EA, Stawiski M, Voorhees JJ. 1975. Increased concentrations of nonesterified arachidonic-acid, 12l-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin-E2, and prostaglandin-F2alpha in epidermis of psoriasis. Proc Natl Acad Sci U S A 72:5130–5134. doi: 10.1073/pnas.72.12.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramanadham S, Gross R, Turk J. 1992. Arachidonic-acid induces an increase in the cytosolic calcium-concentration in single pancreatic-islet beta-cells. Biochem Biophys Res Commun 184:647–653. doi: 10.1016/0006-291X(92)90638-2. [DOI] [PubMed] [Google Scholar]
- 16.Chilton FH, Fonteh AN, Surette ME, Triggiani M, Winkler JD. 1996. Control of arachidonate levels within inflammatory cells. Biochim Biophys Acta 1299:1–15. doi: 10.1016/0005-2760(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 17.Brash AR. 2001. Arachidonic acid as a bioactive molecule. J Clin Investig 107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuen KY, Chan CM, Chan KM, Woo PCY, Che XY, Leung ASP, Cao L. 2001. Characterization of AFMP1: a novel target for serodiagnosis of aspergillosis. J Clin Microbiol 39:3830–3837. doi: 10.1128/JCM.39.11.3830-3837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan CM, Woo PCY, Leung ASP, Lau SKP, Che XY, Cao L, Yuen KY. 2002. Detection of antibodies specific to an antigenic cell wall galactomannoprotein for serodiagnosis of Aspergillus fumigatus aspergillosis. J Clin Microbiol 40:2041–2045. doi: 10.1128/JCM.40.6.2041-2045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo PCY, Chan CM, Leung ASP, Lau SKP, Che XY, Wong SSY, Cao L, Yuen KY. 2002. Detection of cell wall galactomannoprotein Afmp1p in culture supernatants of Aspergillus fumigatus and in sera of aspergillosis patients. J Clin Microbiol 40:4382–4387. doi: 10.1128/JCM.40.11.4382-4387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo PCY, Chong KTK, Leung ASP, Wong SSY, Lau SKP, Yuen KY. 2003. AFLMP1 encodes an antigenic cell wall protein in Aspergillus flavus. J Clin Microbiol 41:845–850. doi: 10.1128/JCM.41.2.845-850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong KTK, Woo PCY, Lau SKP, Huang Y, Yuen KY. 2004. AFMP2 encodes a novel immunogenic protein of the antigenic mannoprotein superfamily in Aspergillus fumigatus. J Clin Microbiol 42:2287–2291. doi: 10.1128/JCM.42.5.2287-2291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo PCY, Chong KTK, Lau CCY, Wong SSY, Lau SKP, Yuen KY. 2006. A novel approach for screening immunogenic proteins in Penicillium marneffei using the delta AFMP1 delta AFMP2 deletion mutant of Aspergillus fumigatus. FEMS Microbiol Lett 262:138–147. doi: 10.1111/j.1574-6968.2006.00376.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang ZY, Cai JP, Qiu LW, Hao W, Pan YX, Tung ETK, Lau CCY, Woo PCY, Lau SKP, Yuen KY, Che XY. 2012. Development of monoclonal antibody-based galactomannoprotein antigen-capture ELISAs to detect Aspergillus fumigatus infection in the invasive aspergillosis rabbit models. Eur J Clin Microbiol Infect Dis 31:2943–2950. doi: 10.1007/s10096-012-1645-3. [DOI] [PubMed] [Google Scholar]
- 25.Woo PCY, Lau SKP, Lau CCY, Tung ETK, Au-Yeung RKH, Cai JP, Chong KTK, Sze KH, Kao RY, Hao Q, Yuen KY. 2018. Mp1p homologues as virulence factors in Aspergillus fumigatus. Med Mycol 56:350–360. doi: 10.1093/mmy/myx052. [DOI] [PubMed] [Google Scholar]
- 26.Kabsch W. 2010. XDS. Acta Crystallogr D Biol Crystallogr 66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otwinowski Z, Minor W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Macromol Crystallogr A 276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 28.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J Appl Crystallogr 40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emsley P, Lohkamp B, Scott WG, Cowtan K. 2010. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murshudov GN, Vagin AA, Dodson EJ. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 31.Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. 2010. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.To KK, Lee K-C, Wong SS, Sze K-H, Ke Y-H, Lui Y-M, Tang BS, Li IW, Lau SK, Hung IF. 2016. Lipid metabolites as potential diagnostic and prognostic biomarkers for acute community acquired pneumonia. Diagn Microbiol Infect Dis 85:249–254. doi: 10.1016/j.diagmicrobio.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.To KKW, Lee KC, Wong SSY, Lo KC, Lui YM, Jahan AS, Wu AL, Ke YH, Law CY, Sze KH, Lau SKP, Woo PCY, Lam CW, Yuen KY. 2015. Lipid mediators of inflammation as novel plasma biomarkers to identify patients with bacteremia. J Infect 70:433–444. doi: 10.1016/j.jinf.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Lau SK, Lam CW, Curreem SO, Lee KC, Lau CC, Chow WN, Ngan AH, To KK, Chan JF, Hung IF, Yam WC, Yuen KY, Woo PC. 2015. Identification of specific metabolites in culture supernatant of Mycobacterium tuberculosis using metabolomics: exploration of potential biomarkers. Emerg Microbes Infect 4:e6. doi: 10.1038/emi.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Mp1p-LBD1 in complex with copurified PLM was deposited in PDB under accession code 5E7X. Mp1p-LBD1 in complex with AA was deposited in PDB under accession code 5ECF. Mp1p-LBD1-LBD2 was deposited in PDB under accession code 6J6F.