Listeria innocua is considered a nonpathogenic Listeria species. Natural atypical hemolytic L. innocua isolates have been reported but have not been characterized in detail.
KEYWORDS: L. innocua, LIPI, inlA, listeriosis, virulence
ABSTRACT
Listeria innocua is considered a nonpathogenic Listeria species. Natural atypical hemolytic L. innocua isolates have been reported but have not been characterized in detail. Here, we report the genomic and functional characterization of representative isolates from the two known natural hemolytic L. innocua clades. Whole-genome sequencing confirmed the presence of Listeria pathogenicity islands (LIPI) characteristic of Listeria monocytogenes species. Functional assays showed that LIPI-1 and inlA genes are transcribed, and the corresponding gene products are expressed and functional. Using in vitro and in vivo assays, we show that atypical hemolytic L. innocua is virulent, can actively cross the intestinal epithelium, and spreads systemically to the liver and spleen, albeit to a lesser degree than the reference L. monocytogenes EGDe strain. Although human exposure to hemolytic L. innocua is likely rare, these findings are important for food safety and public health. The presence of virulence traits in some L. innocua clades supports the existence of a common virulent ancestor of L. monocytogenes and L. innocua.
INTRODUCTION
Listeria innocua is a Gram-positive ubiquitous bacterium, widely distributed in different natural and urban environments and in food (1, 2). L. innocua is a close relative of Listeria monocytogenes species, an important foodborne pathogen and the etiological agent of human listeriosis, a rare but frequently fatal disease (3). In contrast to L. monocytogenes, L. innocua is nonpathogenic to mammals, although excessively rare cases of L. innocua septicemia and meningitis infections have been reported in human (4, 5) and ruminants (6, 7). Typical L. innocua is nonhemolytic, but atypical hemolytic L. innocua isolates have been identified from seafood in Asia (8), pork in North America (9), and poultry in Europe (10), suggesting that atypical hemolytic L. innocua isolates are actually spread worldwide.
The first atypical L. innocua strain (PRL/NW 15B95) was reported in 2004 (8). It was shown to be hemolytic due to the presence of the L. monocytogenes pathogenic island LIPI-1 and considered avirulent based on 50% lethal dose measurement after intravenous infection of carrageenan-treated immunocompromised mice compared to a serotype 4b L. monocytogenes strain (8). Further work also identified the presence of the L. monocytogenes internalin inlA gene, required for mammalian cell invasion (11), in L. innocua LIPI-1-positive isolates (PRL/NW 15B95 and FS J1-023) (12) and showed that these were able to invade human Caco-2 cells at the same levels as the reference L. monocytogenes strain 10403S (13).
Atypical L. innocua isolates carrying the L. monocytogenes pathogenic island LIPI-3 (14) have also been identified and shown to exhibit hemolytic activity on blood agar, when the LIPI-3 llsA gene is under the control of a constitutive promoter (15).
Despite these studies, little is known about the phylogenetic diversity and pathogenicity of hemolytic L. innocua.
In 2016, two unclassified Listeria isolates collected in Finland from apparently healthy bird feces by the Institute for Food Safety and Hygiene (University of Zurich, Switzerland) were sent to the World Health Organization Collaborating Centre for Listeria (Institut Pasteur, France) for identification and characterization. Phenotypic and genomic analyses allowed the classification of these isolates as atypical hemolytic L. innocua. To better understand the genetic organization, virulence potential, and human health risk of these atypical isolates, a detailed genomic and functional characterization was conducted with representatives of all hemolytic L. innocua clades.
RESULTS AND DISCUSSION
Phenotypic analyses and species identification.
Isolates CLIP 2016/00427 and CLIP 2016/00428, obtained from healthy bird feces in Finland, as well as the previously reported atypical FSL J1-023 strain (12) from unknown food origin, exhibited hemolysis, esculinase, and phosphatidylinositol-specific phospholipase C (PI-PLC) activities characteristic of pathogenic L. monocytogenes isolates (see Fig. S1 in the supplemental material). The three isolates belonged to genoserogroup L (characteristic of non-L. monocytogenes [16]). Isolates CLIP 2016/00427 and CLIP 2016/00428 belonged to serotype 6a, whereas FSL J1-023 belonged to serotype 4ab (Table S1). Genome-based species identification of these isolates was consistent with matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) results (17) and confirmed that all isolates belonged to L. innocua species (ANIb, 98.27% ± 1.38% against the Clip11262 genome; accession no. NC_003212).
Phylogenetic analyses and comparative genomics.
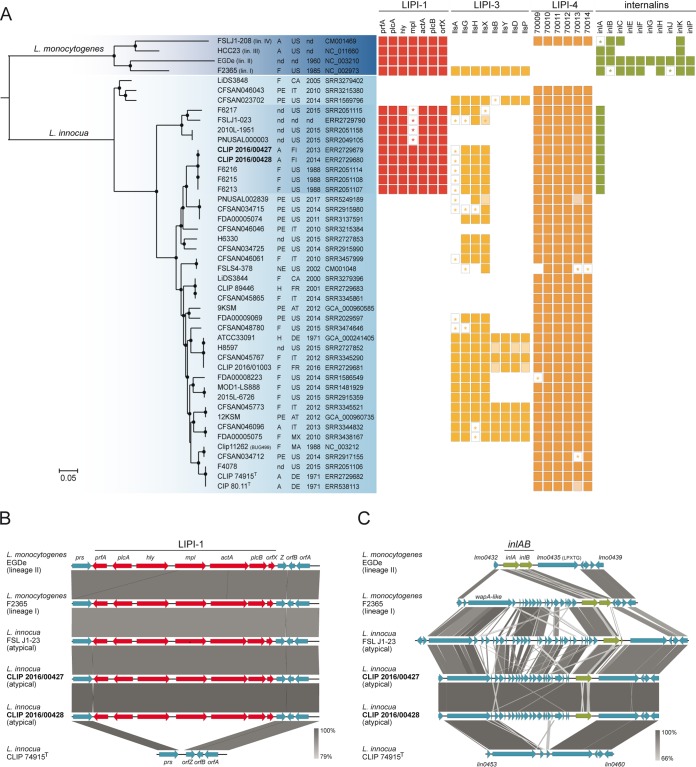
Phylogenetic analysis based on 642,408 core genome single-nucleotide polymorphisms (SNPs) common to L. innocua and L. monocytogenes placed the Finnish atypical L. innocua strains and the previously reported atypical FSL J1-023 strain into two separate genetic clusters (Fig. 1A). The two Finnish isolates formed a monophyletic group within L. innocua species, together with isolates collected from meat samples in the United States in 1988. The same phylogenetic placement was observed using 2,079,497 L. innocua core genome SNPs (Fig. S2). Interestingly, the pathogenic island LIPI-1 was present in all isolates of the two different hemolytic clades (Fig. 1A). The LIPI-1 region consists of 7 genes, including hly, which codes for listeriolysin O (LLO), enabling cytosolic replication, and actA, mediating intra- and intercellular spread (18). While isolates within the Finnish clade carried intact LIPI-1, isolates within the FSL J1-023 clade all harbored a truncated mpl locus. Mpl is required for PlcB activation and its truncation impairs vacuole escape, leading to small infection foci and defective resolution of protrusions into vacuoles (19).
FIG 1.
Phylogenetic and comparative genomic analyses. (A) Rooted maximum likelihood phylogeny of 42 L. innocua genomes based on 642,408 core genome SNPs. Representative genomes of the four L. monocytogenes lineages were used as the outgroup. Circles represent bootstrap branch support values higher than 90% based on 1,000 replicates. Information on the source (A, animal; F, food; H, human; PE, production environment; NE, natural environment; nd, unknown), country, and year of isolation, as well as the NCBI/EMBL/DDBJ accession numbers, is provided in the columns. Colored boxes represent the presence of the different genetic traits. Light-colored boxes represent genes interrupted by end of contigs. Stars represent truncated genes due to the presence of internal stop codons. (B and C) Organization of the LIPI-1 and inlAB loci and their flanking regions. Arrows denote the orientation of genes. Gray blocks denote BLASTN similarities between sequences.
Interestingly, all LIPI-1-positive isolates from the two clades also harbored inlA but not inlB (Fig. 1B and C). In L. monocytogenes, inlA is part of the inlAB operon, which encodes InlA and InlB, involved in invasion and regulated by PrfA in LIPI-1 (11, 20). While InlA mediates invasion of epithelial cells and the crossing of the intestinal barrier, InlB contributes to the crossing of the placental barrier (21–23). The observation that LIPI-1-positive L. innocua isolates harbor InlA but not InlB is consistent with previous findings suggesting that these atypical L. innocua isolates represent an intermediary evolutionary stage between L. monocytogenes and L. innocua species (8, 12, 24). In a scenario where species evolve under different environments from a common pathogenic ancestor possessing LIPI-1 and inlAB loci (25, 26), these atypical isolates may constitute intermediary evolutionary stages with consecutive loss of virulence genes leading to niche restriction, as previously suggested for atypical nonhemolytic L. monocytogenes (27).
Other internalins (inlCEFGHJKP) were absent from all L. innocua genomes, suggesting that, contrary to inlAB, these were absent from the common ancestor and later acquired by L. monocytogenes.
A gene-decaying trend could also be observed within LIPI-3 (14) and, to a lesser degree, in LIPI-4 (28) regions. L. innocua strains carrying the L. monocytogenes-specific LIPI-3 pathogenicity island have also been reported and shown to be hemolytic when the listeriolysin llsA gene, within LIPI-3, is expressed from a constitutive highly active promoter (15). While a few isolates (6/42, 14%) (Fig. 1A) harbored full LIPI-3, the majority of isolates had partial LIPI-3 and/or included multiple point mutations leading to premature stop codons within lls genes. Interestingly, few mutations were present in LIPI-4 (Fig. 1A), which contains genes coding for a putative sugar transport system. LIPI-4 has been shown to be involved in the placental tropism and neurotropism of L. monocytogenes isolates from clonal complex CC4 (28) and is also present in other rare L. monocytogenes lineages and sublineages (29). The presence of LIPI-4 across L. innocua phylogeny (Fig. 1A) suggests that it also has an important role in carbon metabolism in saprophytic environments.
In vitro and in vivo studies.
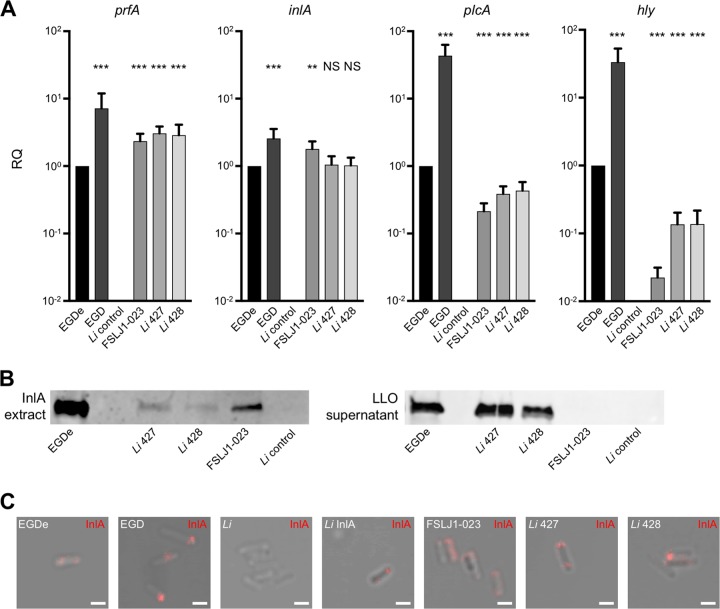
All LIPI-1 tested genes and inlA were transcribed in the atypical L. innocua isolates (Fig. 2A). The EGDe reference strain was used as an L. monocytogenes representative. EGDe is, on average, more virulent than strains from hypovirulent clonal complexes CC9 and CC121 but less virulent than the hypervirulent CC1, CC4, and CC6 strains (28). EGD was used in vitro as a positive control of a PrfA* strain expressing a high level of LIPI-1 genes. Expression of the major regulator prfA was higher in atypical L. innocua than L. monocytogenes EGDe, while expression levels of plcA and hly regulated by prfA were lower, indicating differential regulation of LIPI-1 genes.
FIG 2.
Expression of LIPI-1 and inlA genes in atypical L. innocua. (A) qRT-PCR quantification of prfA, inlA, plcA, and hly transcripts produced in BHI broth at 37°C by L. monocytogenes (EGDe and EGD), L. innocua CLIP 74915T (Li control), and the atypical L. innocua CLIP 2016/00427 (Li 427) and CLIP 2016/00428 (Li 428). Each strain was tested at least three times using independent precultures. gyrB was used as a stable reference gene for normalization. Results are shown as fold change of prfA, inlA, plcA, and hly expression relative to that of EGDe (RQ, relative quantities). A Mann-Whitney test was performed; strains were compared to EGDe (NS, not significant; **, P < 0.01; ***, P < 0.001). (B) InlA and LLO Western blotting of 30 μg proteins of the exponential-phase culture supernatants of each isolate. Immunoblots were performed at least two times. To avoid saturation of signal, strain EGD was not included in this analysis. (C) Cell surface exposure of InlA. Immunofluorescence analysis was performed with mouse monoclonal anti-InlA antibodies revealed with an anti-IgG coupled to Alexa Fluor 555. Scale, 5 μm.
Western blot analysis demonstrated the expression of inlA and hly gene products. Interestingly, protein but not transcript expression levels of InlA were decreased in atypical L. innocua compared to those of EGDe, suggesting a posttranscriptional and/or posttranslational regulation and/or a difference in protein stability (Fig. 2B). While protein quantity differs, InlA had the same tendency to be polar on the surface of both atypical L. innocua and L. monocytogenes at stationary phase, as evidenced by immunofluorescence staining (Fig. 2C) and as previously described for L. monocytogenes (30).
Hemolysis on horse blood agar plates and an opaque halo around colonies on ALOA plates (Fig. S1) reflected LLO and PI-PLC activities, respectively. No difference was detected for hemolysis, while PI-PLC activity was reduced in atypical L. innocua compared to its activity in reference L. monocytogenes strains EGDe and EGD.
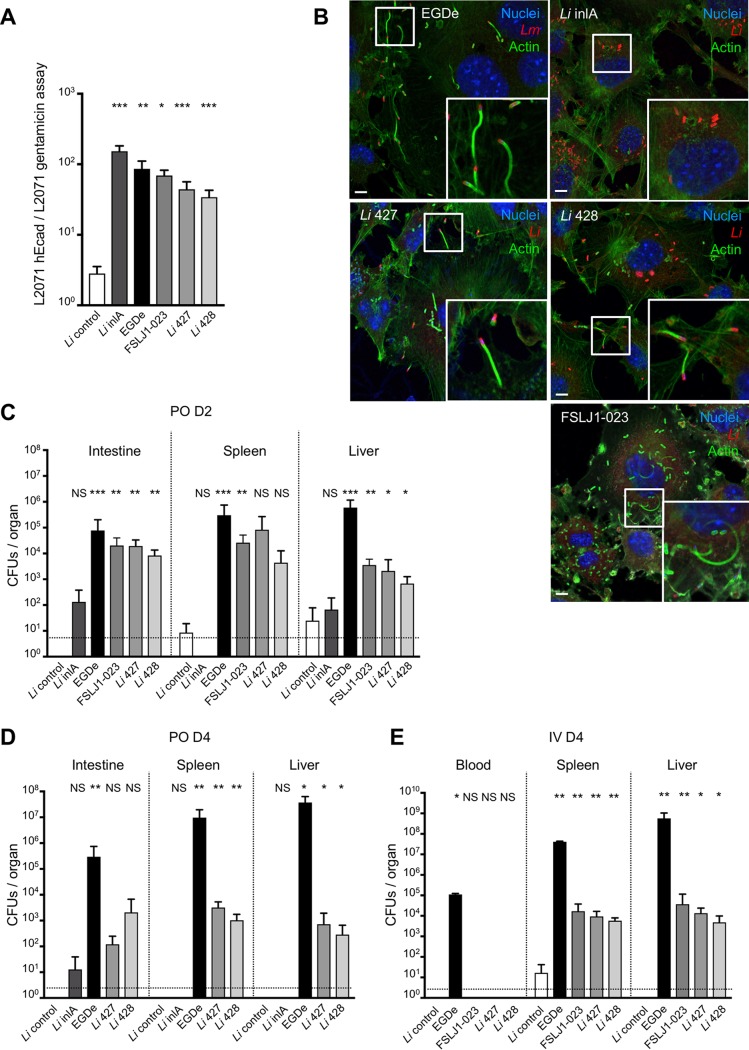
The pathogenicity of L. monocytogenes is linked to its ability to enter nonphagocytic epithelial cells expressing InlA receptor E-cadherin (Ecad), to escape from the phagocytic vacuole in an LLO/PlcA-dependent manner, and to propel itself in the cytoplasm by polymerizing actin in an ActA-dependent manner (11, 31, 32, 33). InlA-dependent entry was specifically tested in L2071 fibroblasts stably transfected for human Ecad (hEcad), in which InlA-Ecad interaction occurs, and compared to entry in control L2071 that does not express Ecad and is therefore not permissive for InlA-dependent invasion, as previously described (31). In contrast to a typical L. innocua reference strain (CLIP 74915) and similar to L. innocua in which L. monocytogenes InlA is stably expressed, all atypical L. innocua strains expressing endogenous InlA entered cells in an hEcad-dependent manner, indicating that InlA from L. innocua interacts with hEcad in a manner similar to that of InlA from L. monocytogenes (Fig. 3A).
FIG 3.
In vitro and in vivo functionality of LIPI-1 and inlA in atypical L. innocua. (A) Gentamicin assay of L. monocytogenes EGDe, L. innocua CLIP 74915T (Li control), L. innocua pAD-inlA mutant (Li inlA), and atypical L. innocua CLIP 2016/00427 (Li 427) and CLIP 2016/00428 (Li 428) in mouse fibroblast L2071 and L2071 hEcad. CFU of gentamicin-resistant bacteria were enumerated. Invasion assays were performed in triplicates and represent at least three independent experiments for each condition tested. Ratio represents CFU in L2071 hEcad divided by CFU in L2071 for each strain. (B) Abilities of L. monocytogenes EGDe, L. innocua pAD-inlA mutant (Li inlA), and atypical L. innocua CLIP 2016/00427 (Li 427) and CLIP 2016/00428 (Li 428) to polymerize host actin were compared after invasion assay on L2071 hEcad cells. Bacteria were detected with anti-L. monocytogenes or anti-L. innocua (red), actin with phalloidin (green), and nuclei with Hoechst (blue). Scale, 5 μm. (C) In vivo assay in KI E16P female mice (n = 6 mice for each condition, n =10 for Li inlA and EGDe) infected orally with 5 × 109 CFU and dissected 48 h after infection. PO, per os. (D) In vivo assay in KI E16P female mice (n = 6 mice for each condition) infected orally with 5 × 109 CFU and dissected 96 h after infection. (E) In vivo assay in KI E16P female mice (n = 6 mice for each condition) infected intravenously (IV) with 5 × 104 CFU and dissected 96 h after infection. For panels A, C, D, and E, a Mann-Whitney test was performed; strains were compared to L. innocua CLIP 74915T (Li control) (NS, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001). Values represent means ± standard deviations.
We next assessed the functionality of LLO/PlcA and ActA by staining intracellular bacteria together with actin. As for the EGDe reference L. monocytogenes strain, we observed actin comet tails in cells infected by atypical L. innocua, in contrast to the control L. innocua expressing InlA but not LIPI-1 genes (Fig. 3B), showing that atypical L. innocua organisms escape from the vacuole and polymerize actin in the cytosol.
InlA, LLO/PlcA, and ActA are major virulence factors of L. monocytogenes in vivo (21, 32, 33). Because mouse Ecad is not a receptor of InlA, we tested the virulence of atypical L. innocua in a mouse model expressing mutated and humanized E-cadherin enabled to interact with InlA (23). Atypical L. innocua was significantly more virulent than control L. innocua and L. innocua expressing InlA alone after oral inoculation (Fig. 3C and D) and intravenous infection (Fig. 3E). Intestinal invasion 2 days postinfection (dpi) likely reflects the functionality of InlA in this model, while invasion of deeper organs after intravenous infection reveals the ability of atypical L. innocua to survive and multiply in the host. At 4 dpi, atypical L. innocua was less virulent than the EGDe control strain after oral and intravenous infection, which could reflect an absence of other unknown virulence factors and/or a defect in regulation of LIPI-1 genes in vivo. As expression of LLO by live L. monocytogenes primes a protective T-cell response against a further L. monocytogenes challenge (34), the ability of atypical L. innocua to induce a protective immune response against L. monocytogenes was investigated. In contrast to control L. innocua, but as for EGDe, albeit to a lesser degree, infection by atypical L. innocua induced a protective immune response to EGDe (Fig. S3), confirming that LIPI-1 genes of atypical L. innocua are expressed and active in an in vivo mouse model.
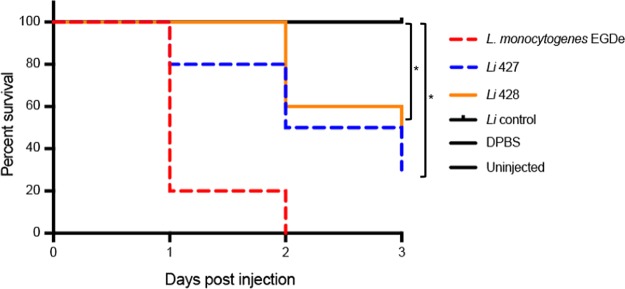
To confirm the virulence phenotype of atypical L. innocua in another in vivo model, we used the previously described zebrafish model (35), which is sensitive to L. monocytogenes infection in an LIPI-1-dependent manner (Fig. 4). At 1 dpi, a survival rate of 20% was observed in wild-type embryos injected with the EGDe positive-control strain, 80% with L. innocua CLIP 2016/00428, and 100% with L. innocua CLIP 2016/00427 and L. innocua negative control. However, at 2 dpi the survival rate dropped to 0% in embryos injected with EGDe and to 50% and 60% with the atypical L. innocua CLIP 2016/00428 and CLIP 2016/00427, respectively. No mortality was observed in embryos injected with typical L. innocua or Dulbecco's phosphate-buffered saline (DPBS) and uninjected controls. Subsequently, the survival percentage dropped to 30% with CLIP 2016/00428 and 50% with CLIP 2016/00427 at 3 dpi. In contrast, survival rates of embryos (100%) in infections using L. innocua remained unaltered over the time course of infection.
FIG 4.
Infection assays in zebrafish. Survival rates of zebrafish embryos after inoculation with L. monocytogenes EGDe, L. innocua (Li control), and atypical L. innocua CLIP 2016/00427 (Li 427) and CLIP 2016/00428 (Li 428) bacterial suspensions in DPBS (n = 30; *, P < 0.05 for comparisons relative to the L. innocua control strain).
Altogether, results show that atypical hemolytic L. innocua strains are virulent and capable of causing infection in mouse and zebrafish models, albeit less than L. monocytogenes. Isolates obtained from healthy bird feces suggest that asymptomatic carriage occurs and that, similar to L. monocytogenes, infection likely depends on host susceptibility factors (3). Moreover, no atypical L. innocua had been previously isolated by the World Health Organization Collaborating Centre for Listeria (Institut Pasteur, France) in the context of food and clinical surveillance (7,236 L. innocua isolates collected between 1987 and 2018). This suggests that hemolytic L. innocua isolates are rare and/or specific to yet-unknown reservoirs and that the absence of reported clinical cases is related to an extremely low degree of exposure (absence of detected exposure in France over the past 30 years).
Although genomic data on L. innocua are still scarce, continuous sequencing efforts will allow us to gain insights into the ecological reservoirs and transmission chains of hemolytic L. innocua.
Conclusions.
In conclusion, we have shown that representatives of the two atypical hemolytic L. innocua clades express functional InlA and LIPI-1 gene products, including LLO, PlcA, and ActA. These strains are therefore virulent, can cross the intestinal epithelial barrier, and can spread systemically to the liver and spleen. Interestingly, atypical L. innocua induces a protective immune response against L. monocytogenes, indicating that atypical L. innocua and L. monocytogenes are closely related, even though they belong to different species.
The results of this study have important implications in risk management and food safety, since L. innocua is often regarded as nonpathogenic. Hemolytic L. innocua, already described in the EN ISO 11290 standard for detection and enumeration of L. monocytogenes in the food chain (36), shall be considered a foodborne pathogen in food regulations, although its low frequency is likely associated with an extremely low risk of infection in human. Whole-genome sequencing has confirmed that hemolytic L. innocua strains contain L. monocytogenes LIPI regions that seem to be progressively lost. These results support previous findings that point toward the existence of a common virulent ancestor of L. monocytogenes and L. innocua, with consecutive loss of virulence traits constraining host adaptation. Interestingly, loss of LIPI-1 activity as well as truncation of InlA has been described in avirulent L. monocytogenes strains (27, 37). This indicates a common path leading from virulence to saprophytism in the Listeria genus.
MATERIALS AND METHODS
Bacterial isolation and phenotypic analyses.
The characteristics of the isolates used in this study are summarized in Table 1. Isolates CLIP 2016/00427 (originally named LM8) and CLIP 2016/0428 (LM31) were collected from healthy bird fecal samples in Finland. The previously reported hemolytic L. innocua FSL J1-023 (kindly provided by Martin Wiedmann, Cornell University) and the L. innocua type strain CLIP 74915T (CIP 80.11T [2]) were used as controls. Pure cultures were obtained by streaking isolated colonies onto Columbia horse blood agar plates (bioMérieux, France) and ALOA agar plates (bioMérieux, France) and incubating overnight at 35°C ± 1°C. Species identification was carried out by MALDI-TOF MS (Biotyper Compass Explorer software v.4.1.60; Bruker Daltonics, Bremen, Germany), as previously described (17).
TABLE 1.
Characteristics of the strains included in this study
| Isolate | Species | Origin | Serotype (genoserogroup) | Hemolysis | LIPI-1 | LIPI-3 | LIPI-4 | inlA | inlBCEFGHJKP | Reference or source |
|---|---|---|---|---|---|---|---|---|---|---|
| CLIP 2016/00427 | L. innocua (atypical) | Animal (healthy duck feces), 2013, Finland | 6a (L) | + | + | + | + | + | − | This study |
| CLIP 2016/00428 | L. innocua (atypical) | Animal (healthy pheasant feces), 2014, Finland | 6a (L) | + | + | + | + | + | − | This study |
| FSL J1-023 | L. innocua (atypical) | Unknown | 4ab (L) | + | + | − | + | + | − | 12 |
| CLIP 74915 (CIP80.11T) | L. innocua | Type strain | 6a (L) | − | − | − | + | − | − | 2 |
| MBHL_0269 Li pAD-inlA | L. innocua | Laboratory construct | 6a (L) | + | − | − | + | + | − | 59 |
| EGDe (BUG1600) | L. monocytogenes | Laboratory strain | 1/2a (IIa) | + | + | − | − | + | + | 60 |
| EGD (BUG600) | L. monocytogenes | Laboratory strain (PrfA*) | 1/2a (IIa) | + | + | − | − | + | + | 61 |
| CLIP 74910 (ATCC 19115) | L. monocytogenes | Laboratory strain | 4b (IVb) | + | + | + | + | + | + (except inlFGJ) | 62 |
Hemolytic activity was tested on Columbia horse blood agar plates (bioMérieux, France) with incubation for 24 and 48 h at 35°C ± 1°C, using L. monocytogenes CLIP 74910 and L. innocua CLIP 74915 as positive and negative controls, respectively. PI-PLC activity was tested on ALOA agar plates (bioMérieux, France) for 24 and 48 h at 35°C ± 1°C, using the same controls.
Serotyping was performed using the slide agglutination method with commercially available Listeria factor sera (Denka Seiken Co., Japan) against somatic (O; I to IX) and flagellar (H; A to D) antigens and the additional X-XV O-factor reference sera available from the World Health Organization Collaborating Centre for Listeria, Institut Pasteur, France (38). Genoserogrouping was performed as described by Doumith et al. (39).
DNA extraction and whole-genome sequencing.
Genomic DNA was extracted using the DNeasy blood and tissue extraction kit (Qiagen, Denmark) as described previously (40). DNA libraries were prepared using the Nextera XT DNA sample kit (Illumina, CA, USA) and sequenced on the Illumina NextSeq 500 platform using paired-end 2 × 150-bp runs.
Raw sequence data were trimmed to eliminate adapters (41), reduce redundant, overrepresented, and overlapping reads (42, 43), correct sequencing errors (44), and discard reads with Phred quality scores below 20, using FqCleaner v.3.0 (Alexis Criscuolo, Institut Pasteur, Paris, France), as previously described (40). Assemblies were obtained by using CLC Assembly Cell v.4.3.0 (Qiagen, Denmark), with estimated library insert sizes ranging from 50 to 850 bp and a minimum contig size of 500 bases. Draft assemblies were annotated with Prokka v.1.12 (45).
Phylogenetic and comparative genomic analyses.
Species identification was confirmed using average nucleotide identity BLAST (ANIb) (46) calculated against reference genomes of Listeria species, as previously described (47).
Isolates were compared to a collection of 40 other L. innocua genomes available from NCBI/EMBL/DDBJ databases, using L. monocytogenes representatives from lineages I to IV as the outgroup. Phylogenies were inferred from the core genome alignments built using Parsnp, implemented in Harvest suite v.1.1.2 (48). Trees were visualized with iTol v.4.2 (49).
Virulence genes were searched using the BLASTN algorithm (50) implemented at the BIGSdb-Lm platform (29, 51, http://bigsdb.pasteur.fr/listeria), with minimum nucleotide identity and alignment length coverage of 70% and word size of 10. Comparative analyses of the LIPI-1, LIPI-3, and inlAB loci were performed using EasyFig v.2.2.2 (52).
LIPI-1 gene expression and protein quantification.
Transcription of prfA, plcA, and hly from LIPI-1 and inlA was assessed by reverse transcription-quantitative PCR (RT-qPCR) in atypical L. innocua and compared to that of the reference strains L. monocytogenes EGDe and EGD (PrfA*), which harbors the Gly145Ser PrfA mutation associated with constitutive PrfA activity. Total RNA was extracted using a phenol-chloroform method (adapted from reference 53). First-strand cDNA was synthesized using Moloney-murine leukemia virus RT enzyme (Invitrogen), and qPCR was performed using the ABI 7500 fast real-time PCR system (Applied Biosystems) and the Power SYBR green PCR master mix (Applied Biosystems) with dedicated primers (Table 2). Data obtained from the RT-qPCR reaction were analyzed using the comparative threshold cycle method with gyrB used as the reference gene for normalization.
TABLE 2.
Primers used in this study
| Gene target | Primer sequence (5′-3′) | Fragment size (bp) | Source |
|---|---|---|---|
| gyrB | Fwd: AGCGATTTTGCCGATTCGTG | 95 | This study |
| Rev: CACCAAAACCAGTACCCATCG | |||
| prfA | Fwd: AACCAATGGGATCCACAAG | 70 | This study |
| Rev: TTCATGATGGTCCCGTTCTC | |||
| hly | Fwd: AAACTTCGGCGCAATCAGTG | 92 | This study |
| Rev: GCCGAAAAATCTGGAAGGTC | |||
| plcA | Fwd: ACAATGGATGTCCGCTCTAC | 68 | This study |
| Rev: GTCTCCGTTATAGCTCATCG | |||
| inlA | Fwd: CAAGGTAAGTGACGTAAGCTC | 118 | This study |
| Rev: CCATGCTTGATCATTCAACCC |
Protein expression was confirmed by immunoblotting. Bacteria were grown in exponential phase (optical density [OD] of 0.8) in brain heart infusion (BHI) medium at 37°C. Cultures were centrifuged, the bacterial pellets were lysed in B-PER II reagent (Thermo Fisher Scientific) to extract membrane proteins, and culture supernatants were incubated with 100% trichloroacetic acid to extract secreted proteins. Protein concentration from total protein extracts was quantified using the bicinchoninic acid protein assay kit (Thermo Fisher Scientific). Thirty μg of protein for each sample was boiled for 10 min in NuPage buffer (Invitrogen) with reducing agent (Invitrogen) and 1× PBS. Samples were then subjected to SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad). Membranes were saturated with PBS supplemented with 0.1% Tween 20 (Sigma-Aldrich) and 5% nonfat milk. Membranes were incubated with primary antibody (InlA, I4.4 [54]; LLO, R176 [55]), washed three times in PBS supplemented with 0.1% Tween 20 (Sigma-Aldrich), incubated with mouse (626520; Invitrogen) or rabbit (NA934V; GE Healthcare) secondary antibody coupled to peroxidase, washed three times in PBS supplemented with 0.1% Tween 20 (Sigma-Aldrich), and revealed by chemiluminescence with an ECL Western blotting detection system (GE Healthcare). Images were acquired with the PXi4 GeneSys software, version 1.5.4.0.
InlA expression was also assessed by immunofluorescence. Bacteria were grown to stationary phase in BHI medium at 37°C. Cultures were harvested by centrifugation and washed with cold PBS. The live bacterial pellets were incubated for 1 h at room temperature with the appropriate primary antibody (I4.4) and then washed with cold PBS and labeled with secondary antibody (Alexa 555 goat anti-mouse) for 1 h at room temperature. All handling of these dyes was performed with minimal exposure to light. A total of 10 μl of live bacteria was spread between a glass slide and an 18-mm square coverslip, gently pressed down, and blotted to remove excess liquid. Images were acquired with an LSM710 confocal Zeiss microscope (40× oil objective).
Cell culture and in vitro intracellular invasion.
Gentamicin survival assays were performed as previously described (56). Briefly, an overnight bacterial culture was diluted 1/20 in BHI liquid medium and incubated at 37°C ± 1°C with shaking until an OD at 600 nm (OD600) of 0.8 was reached. Bacteria were then washed, diluted 100-fold in Dulbecco’s modified Eagle’s medium (DMEM), and added to L2071 or L2071 hEcad cells (31). After 1 h of incubation at 37°C ± 1°C in 5% CO2, cells were rinsed in DMEM plus 10% fetal calf serum and incubated for an additional 1 h with 10 μg·ml−1 gentamicin (Gibco) to kill extracellular bacteria. Cells were then rinsed in DMEM and lysed with 0.1% Triton X-100 in PBS. The number of CFU of intracellular bacteria was determined by plating serial dilutions of the resulting lysates on BHI agar plates. The level of invasion was defined by dividing the number of viable bacteria recovered after gentamicin treatment by the number of viable bacteria that had been inoculated into each well of the 24-well plates. Invasion assays were performed in triplicates and represent at least 3 independent experiments.
Comet tail formation.
L2071 hEcad cells grown on glass coverslips were infected as described above. One h postinfection (hpi), cells were incubated with gentamicin (10 μg·ml−1) for 5 h. Cells were then washed with PBS supplemented with Ca2+ and Mg2+ (Gibco) and then fixed with 4% paraformaldehyde (Electron Microscopy Sciences) in PBS for 15 min at 4°C ± 3°C. Cells were permeabilized for 30 min in PBS supplemented with 0.4% Triton X-100 (Sigma-Aldrich) and 3% bovine serum albumin (BSA) (Sigma-Aldrich) and then labeled with the appropriate primary and secondary antibodies for 1 h at room temperature. Cells on coverslips were mounted in Fluoromount G (Interchim). R6 and R12 rabbit polyclonal antibodies were used for L. innocua and L. monocytogenes staining, respectively (57). Actin was labeled with phalloidin-Alexa 488 and nuclei with Hoechst. Images were acquired with an LSM710 confocal Zeiss microscope (40× oil objective).
Oral and intravenous mouse infections.
All of the procedures used in this study are in agreement with the guidelines of the European Commission for the handling of laboratory animals, directive 86/609/EEC. They were approved by the ethical committee of Institut Pasteur (CETEA-C2EA no. 89) under the number 2015-0015. Infections were performed as previously described (58). L. monocytogenes and L. innocua overnight cultures were diluted in BHI medium to reach mid-log growth phase. For intragastric gavage, 200 μl of bacteria in PBS (5 × 109 CFU/animal) was mixed with 300 μl of CaCO3 (50 mg·ml−1) before injection in animals starved overnight. For intravenous injection, 200 μl of bacteria in PBS (5 × 104 CFU/animal) was injected in the tail vein. For the protective immune assay, 5 × 103 CFU was injected in the tail vein of C57BL/6J mice. Mice were then challenged intravenously 28 dpi with 5 × 105 CFU of EGDe and killed 2 days postchallenge. To monitor bacterial burden, organs were removed from infected killed animals and homogenized. Before homogenization, intestines were rinsed twice with DMEM and incubated for 2 h at room temperature in DMEM supplemented with gentamicin (100 μg·ml−1; Sigma, France). Serial dilutions of cell suspensions in PBS were plated on BHI agar plates. After 24 h of incubation at 37°C ± 1°C, CFU were counted.
Intravenous zebrafish infections.
Two days postfertilization, embryos were manually dechorionated and anesthetized with 200 mg·liter−1 buffered tricaine prior to injection of bacteria. Subsequently, embryos were aligned on an agar plate and injected with 500 CFU of bacteria in 1 to 2 nl of a bacterial suspension in DPBS buffer into the caudal vein. The volume of the injected suspension was adjusted by injection of a droplet into mineral oil and measurement of its approximate diameter over a stage micrometer scale bar. The number of CFU injected at 0 hpi was determined by disintegrating and plating five embryos individually immediately after microinjection of bacteria, which resulted in an accurate determination of the numbers of CFU actually injected. Following injections, infected embryos were allowed to recover in a petri dish with fresh E3 medium for 15 min. To monitor infection kinetics and for survival assays, embryos were transferred into 24‐well plates (one embryo per well) in 1 ml of E3 medium per well, incubated at 28°C, and observed for signs of disease, such as developmental delay (especially swim bladder, decreased speed, acceleration, and locomotory rate), necrosis at the caudal inoculation site, subsequent necrosis in other parts of the body, and eventual death. For survival assays after infection, the number of dead larvae was determined visually on the basis of the absence of heartbeat. The experiments were performed in triplicates. The experiments were conducted in accordance with recommendations of approval no. 216/2012 and by following the guidelines provided by the Veterinary Office of the Public Health Department of the Canton of Zurich (Switzerland). Experiments were carried out until 72 hpi, and at the end of the experiments embryos that were alive were euthanized with an overdose of 4 g·liter−1 buffered tricine. Kaplan-Meier survival analysis and statistics (log‐rank [Mantel-Cox] test) were determined with GraphPad Prism 7 (GraphPad Software, CA, USA). Experiments were performed in triplicate, and P values of <0.05 were considered statistically significant.
Data availability.
All genomes sequenced in this study were deposited in NCBI/EMBL/DDBJ databases under the project accession no. PRJEB27826.
Supplementary Material
ACKNOWLEDGMENTS
We thank Martin Wiedmann (Cornell University) for providing the atypical L. innocua FSL J1-023 strain and Peter Gerner-Smidt and Zuzana Kucerova (CDC/DDID/NCEZID) for proving information on the atypical L. innocua isolates from the United States. We also thank the P2M platform (Institut Pasteur, Paris) for genome sequencing.
M. Lecuit coordinated this study. Bacterial isolation, phenotypic analysis, and DNA extraction were performed by M. Fredriksson-Ahomaa, R. Stephan, and A. Leclercq. P. Thouvenot carried out the serotyping. Genome sequence analyses were performed by A. Moura. M. Lavina, L. Huang, and O. Disson carried out gene expression and protein quantification. Functional studies were performed by M. Lavina, P. Thouvenot, and O. Disson. Zebrafish infections were carried out by A. K. Eshwar. A. Moura and O. Disson wrote the manuscript, with contributions from all coauthors.
This work was supported by Institut Pasteur, Inserm, Santé Publique France, and the University of Zurich.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00758-18.
REFERENCES
- 1.Orsi RH, Wiedmann M. 2016. Characteristics and distribution of Listeria spp., including Listeria species newly described since 2009. Appl Microbiol Biotechnol 100:5273–5287. doi: 10.1007/s00253-016-7552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeliger HP. 1981. Nonpathogenic listeriae: L. innocua sp. n. (Seeliger et Schoofs, 1977) (author’s transl). Zentralbl Bakteriol Mikrobiol Hyg A 249:487–493. doi: 10.1016/S0174-3031(81)80108-4. [DOI] [PubMed] [Google Scholar]
- 3.Charlier C, Perrodeau É, Leclercq A, Cazenave B, Pilmis B, Henry B, Lopes A, Maury MM, Moura A, Goffinet F, Dieye HB, Thouvenot P, Ungeheuer MN, Tourdjman M, Goulet V, de Valk H, Lortholary O, Ravaud P, Lecuit M, MONALISA Study Group. 2017. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect Dis 17:510–519. doi: 10.1016/S1473-3099(16)30521-7. [DOI] [PubMed] [Google Scholar]
- 4.Perrin M, Bemer M, Delamare C. 2003. Fatal case of Listeria innocua bacteremia. J Clin Microbiol 41:5308–5309. doi: 10.1128/JCM.41.11.5308-5309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favaro M, Sarmati L, Sancesario G, Fontana C. 2014. First case of Listeria innocua meningitis in a patient on steroids and eternecept. JMM Case Rep 1:1–5. doi: 10.1099/jmmcr.0.003103. [DOI] [Google Scholar]
- 6.Rocha PRDA, Dalmasso A, Grattarola C, Casalone C, Del Piero F, Bottero MT, Capucchio MT. 2013. Atypical cerebral listeriosis associated with Listeria innocua in a beef bull. Res Vet Sci 94:111–114. doi: 10.1016/j.rvsc.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Walker JK, Morgan JH, McLauchlin J, Grant KA, Shallcross JA. 1994. Listeria innocua isolated from a case of ovine meningoencephalitis. Vet Microbiol 42:245–253. doi: 10.1016/0378-1135(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 8.Johnson J, Jinneman K, Stelma G, Smith BG, Lye D, Ulaszek J, Evsen L, Gendel S, Bennett RW, Pruckler J, Steigerwalt A, Kathariou S, Volokhov D, Rasooly A, Chizhikov V, Fortes E, Duvall RE, Hitchins AD, Messer J, Swaminathan B, Yildirim S, Wiedmann M. 2004. Natural atypical Listeria innocua strains with Listeria monocytogenes pathogenicity island 1 genes. Appl Environ Microbiol 70:4256–4266. doi: 10.1128/AEM.70.7.4256-4266.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno LZ, Paixão R, Gobbi DD, Raimundo DC, Ferreira TP, Hofer E, Matte MH, Moreno AM. 2012. Characterization of atypical Listeria innocua isolated from swine slaughterhouses and meat markets. Res Microbiol 163:268–271. doi: 10.1016/j.resmic.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Milillo SR, Stout JC, Hanning IB, Clement A, Fortes ED, den Bakker HC, Wiedmann M, Ricke SC. 2012. Listeria monocytogenes and hemolytic Listeria innocua in poultry. Poult Sci 91:2158–2163. doi: 10.3382/ps.2012-02292. [DOI] [PubMed] [Google Scholar]
- 11.Gaillard JL, Berche P, Frehel C, Gouin E, Cossart P. 1991. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from Gram-positive cocci. Cell 65:1127–1141. doi: 10.1016/0092-8674(91)90009-N. [DOI] [PubMed] [Google Scholar]
- 12.Volokhov DV, Duperrier S, Neverov AA, George J, Buchrieser C, Hitchins AD. 2007. The presence of the internalin gene in natural atypically hemolytic Listeria innocua strains suggests descent from L. monocytogenes. Appl Environ Microbiol 73:1928–1939. doi: 10.1128/AEM.01796-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.den Bakker HC, Cummings CA, Ferreira V, Vatta P, Orsi RH, Degoricija L, Barker M, Petrauskene O, Furtado MR, Wiedmann M. 2010. Comparative genomics of the bacterial genus Listeria: genome evolution is characterized by limited gene acquisition and limited gene loss. BMC Genomics 11:688. doi: 10.1186/1471-2164-11-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotter PD, Draper LA, Lawton EM, Daly KM, Groeger DS, Casey PG, Ross RP, Hill C. 2008. Listeriolysin S, a novel peptide haemolysin associated with a subset of lineage I Listeria monocytogenes. PLoS Pathog 4:e1000144. doi: 10.1371/journal.ppat.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clayton EM, Daly KM, Guinane CM, Hill C, Cotter PD, Ross PR. 2014. Atypical Listeria innocua strains possess an intact LIPI-3. BMC Microbiol 14:58. doi: 10.1186/1471-2180-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leclercq A, Chenal-Francisque V, Dieye H, Cantinelli T, Drali R, Brisse S, Lecuit M. 2011. Characterization of the novel Listeria monocytogenes PCR serogrouping profile IVb-v1. Int J Food Microbiol 147:74–77. doi: 10.1016/j.ijfoodmicro.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Thouvenot P, Vales G, Bracq-Dieye H, Tessaud-Rita N, Maury MM, Moura A, Lecuit M, Leclercq A. 2018. MALDI-TOF mass spectrometry-based identification of Listeria species in surveillance: a prospective study. J Microbiol Methods 144:29–32. doi: 10.1016/j.mimet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Portnoy DA, Chakraborty T, Goebel W, Cossart P. 1992. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun 60:1263–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez DE, Agaisse H. 2016. The metalloprotease Mpl supports Listeria monocytogenes dissemination through resolution of membrane protrusions into vacuoles. Infect Immun 84:1806–1814. doi: 10.1128/IAI.00130-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lingnau A, Domann E, Hudel M, Bock M, Nichterlein T, Wehland J, Chakraborty T. 1995. Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect Immun 63:3896–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecuit M, Vandormael PS, Lefort J, Huerre M, Gounon P, Dupuy C, Babinet C, Cossart P. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722–1725. doi: 10.1126/science.1059852. [DOI] [PubMed] [Google Scholar]
- 22.Lecuit M, Sonnenburg JL, Cossart P, Gordon JI. 2007. Functional genomic studies of the intestinal response to a foodborne enteropathogen in a humanized gnotobiotic mouse model. J Biol Chem 282:15065–15072. doi: 10.1074/jbc.M610926200. [DOI] [PubMed] [Google Scholar]
- 23.Disson O, Grayo S, Huillet E, Nikitas G, Langa-Vives F, Dussurget O, Ragon M, Le Monnier A, Babinet C, Cossart P, Lecuit M. 2008. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature 455:1114–1118. doi: 10.1038/nature07303. [DOI] [PubMed] [Google Scholar]
- 24.Cai S, Wiedmann M. 2001. Characterization of the prfA virulence gene cluster insertion site in non-hemolytic Listeria spp.: probing the evolution of the Listeria virulence gene island. Curr Microbiol 43:271–277. doi: 10.1007/s002840010300. [DOI] [PubMed] [Google Scholar]
- 25.Schmid MW, Ng EYW, Lampidis R, Emmerth M, Walcher M, Kreft J, Goebel W, Wagner M, Schleifer K-H. 2005. Evolutionary history of the genus Listeria and its virulence genes. Syst Appl Microbiol 28:1–18. doi: 10.1016/j.syapm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 26.den Bakker HC, Bundrant BN, Fortes ED, Orsi RH, Wiedmann M. 2010. A population genetics-based and phylogenetic approach to understanding the evolution of virulence in the genus Listeria. Appl Environ Microbiol 76:6085–6100. doi: 10.1128/AEM.00447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maury MM, Chenal-Francisque V, Bracq-Dieye H, Han L, Leclercq A, Vales G, Moura A, Gouin E, Scortti M, Disson O, Vázquez-Boland JA, Lecuit M. 2017. Spontaneous loss of virulence in natural populations of Listeria monocytogenes. Infect Immun 85:1–13. doi: 10.1128/IAI.00541-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maury MM, Tsai Y-HH, Charlier C, Touchon M, Chenal-Francisque V, Leclercq A, Criscuolo A, Gaultier C, Roussel S, Brisabois A, Disson O, Rocha EPCC, Brisse S, Lecuit M. 2016. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat Genet 48:308–313. doi: 10.1038/ng.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moura A, Criscuolo A, Pouseele H, Maury MM, Leclercq A, Tarr C, Björkman JT, Dallman T, Reimer A, Enouf V, Larsonneur E, Carleton H, Bracq-Dieye H, Katz LS, Jones L, Touchon M, Tourdjman M, Walker M, Stroika S, Cantinelli T, Chenal-Francisque V, Kucerova Z, Rocha EPC, Nadon C, Grant K, Nielsen EM, Pot B, Gerner-Smidt P, Lecuit M, Brisse S. 2016. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat Microbiol 2:16185. doi: 10.1038/nmicrobiol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruck S, Personnic N, Prevost M-C, Cossart P, Bierne H. 2011. Regulated shift from helical to polar localization of Listeria monocytogenes cell wall-anchored proteins. J Bacteriol 193:4425–4437. doi: 10.1128/JB.01154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J 18:3956–3963. doi: 10.1093/emboj/18.14.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaillard J, Berche P, Sansonetti P. 1986. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun 52:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521–531. doi: 10.1016/0092-8674(92)90188-I. [DOI] [PubMed] [Google Scholar]
- 34.Berche P, Gaillard JL, Sansonetti PJ. 1987. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol 138:2266–2271. [PubMed] [Google Scholar]
- 35.Levraud J-P, Disson O, Kissa K, Bonne I, Cossart P, Herbomel P, Lecuit M. 2009. Real-time observation of Listeria monocytogenes-phagocyte interactions in living zebrafish larvae. Infect Immun 77:3651–3660. doi: 10.1128/IAI.00408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.International Organization for Standardization. 2017. EN ISO 11290: microbiology of the food chain–horizontal method for the detection and enumeration of Listeria monocytogenes and of Listeria spp. International Standardization Organization, Geneva, Switzerland. [Google Scholar]
- 37.Jacquet C, Doumith M, Gordon JI, Martin PMV, Cossart P, Lecuit M. 2004. A molecular marker for evaluating the pathogenic potential of foodborne Listeria monocytogenes. J Infect Dis 189:2094–2100. doi: 10.1086/420853. [DOI] [PubMed] [Google Scholar]
- 38.Seeliger HPR, Höhne K. 1979. Serotyping of Listeria monocytogenes and related species. Methods Microbiol 13:31–49. doi: 10.1016/S0580-9517(08)70372-6. [DOI] [Google Scholar]
- 39.Doumith M, Buchrieser C, Glaser P, Jacquet C, Martin P. 2004. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J Clin Microbiol 42:3819–3822. doi: 10.1128/JCM.42.8.3819-3822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moura A, Tourdjman M, Leclercq A, Hamelin E, Laurent E, Fredriksen N, Cauteren D, Van Bracq-Dieye H, Thouvenot P, Vales G, Tessaud-Rita N, Maury MM, Alexandru A, Criscuolo A, Quevillon E, Donguy M, Enouf V, Valk H, De Brisse S, Lecuit M. 2017. Real-time whole-genome sequencing for surveillance of Listeria monocytogenes, France. Emerg Infect Dis 23:1462–1470. doi: 10.3201/eid2309.170336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Criscuolo A, Brisse S. 2013. AlienTrimmer: a tool to quickly and accurately trim off multiple short contaminant sequences from high-throughput sequencing reads. Genomics 102:500–506. doi: 10.1016/j.ygeno.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Magoč T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crusoe MR, Alameldin HF, Awad S, Boucher E, Caldwell A, Cartwright R, Charbonneau A, Constantinides B, Edvenson G, Fay S, Fenton J, Fenzl T, Fish J, Garcia-Gutierrez L, Garland P, Gluck J, González I, Guermond S, Guo J, Gupta A, Herr JR, Howe A, Hyer A, Härpfer A, Irber L, Kidd R, Lin D, Lippi J, Mansour T, McA'Nulty P, McDonald E, Mizzi J, Murray KD, Nahum JR, Nanlohy K, Nederbragt AJ, Ortiz-Zuazaga H, Ory J, Pell J, Pepe-Ranney C, Russ ZN, Schwarz E, Scott C, Seaman J, Sievert S, Simpson J, Skennerton CT, Spencer J, Srinivasan R, Standage D, Stapleton JA, Steinman SR, Stein J, Taylor B, Trimble W, Wiencko HL, Wright M, Wyss B, Zhang Q, Zyme E, Brown CT. 2015. The khmer software package: enabling efficient nucleotide sequence analysis. F1000Res 4:900. doi: 10.12688/f1000research.6924.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Schröder J, Schmidt B. 2013. Musket: a multistage k-mer spectrum-based error corrector for Illumina sequence data. Bioinformatics 29:308–315. doi: 10.1093/bioinformatics/bts690. [DOI] [PubMed] [Google Scholar]
- 45.Seemann T. 2014. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 46.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. 2007. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 47.Leclercq A, Moura A, Vales G, Tessaud-Rita N, Aguilhon C, Lecuit M. 2019. Listeria thailandensis sp. nov. Int J Syst Evol Microbiol 69:74–81. doi: 10.1099/ijsem.0.003097. [DOI] [PubMed] [Google Scholar]
- 48.Treangen TJ, Ondov BD, Koren S, Phillippy AM. 2014. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol 15:524. doi: 10.1186/s13059-014-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Letunic I, Bork P. 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 51.Jolley KA, Maiden MCJ. 2010. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, Balestrino D, Loh E, Gripenland J, Tiensuu T, Vaitkevicius K, Barthelemy M, Vergassola M, Nahori M-A, Soubigou G, Régnault B, Coppée J-Y, Lecuit M, Johansson J, Cossart P. 2009. The Listeria transcriptional landscape from saprophytism to virulence. Nature 459:950–956. doi: 10.1038/nature08080. [DOI] [PubMed] [Google Scholar]
- 54.Mengaud J, Lecuit M, Lebrun M, Nato F, Mazie JC, Cossart P. 1996. Antibodies to the leucine-rich repeat region of internalin block entry of Listeria monocytogenes into cells expressing E-cadherin. Infect Immun 64:5430–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ribet D, Hamon M, Gouin E, Nahori M-A, Impens F, Neyret-Kahn H, Gevaert K, Vandekerckhove J, Dejean A, Cossart P. 2010. Listeria monocytogenes impairs SUMOylation for efficient infection. Nature 464:1192–1195. doi: 10.1038/nature08963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lecuit M, Ohayon H, Braun L, Mengaud J, Cossart P. 1997. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect Immun 65:5309–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dramsi S, Lévi S, Triller A, Cossart P. 1998. Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: an in vitro study. Infect Immun 66:4461–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Disson O, Nikitas G, Grayo S, Dussurget O, Cossart P, Lecuit M. 2009. Modeling human listeriosis in natural and genetically engineered animals. Nat Protoc 4:799–810. doi: 10.1038/nprot.2009.66. [DOI] [PubMed] [Google Scholar]
- 59.Tsai Y-H, Disson O, Bierne H, Lecuit M. 2013. Murinization of internalin extends its receptor repertoire, altering Listeria monocytogenes cell tropism and host responses. PLoS Pathog 9:e1003381. doi: 10.1371/journal.ppat.1003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, Berche P, Bloecker H, Brandt P, Chakraborty T, Charbit A, Chetouani F, Couvé E, De Daruvar A, Dehoux P, Domann E, Domínguez-Bernal G, Duchaud E, Durant L, Dussurget O, Entian KD, Fsihi H, Garcia-Del Portillo F, Garrido P, Gautier L, Goebel W, Gómez-López N, Hain T, Hauf J, Jackson D, Jones LM, Kaerst U, Kreft J, Kuhn M, Kunst F, Kurapkat G, Madueño E, Maitournam A, Mata Vicente J, Ng E, Nedjari H, Nordsiek G, Novella S, De Pablos B, Pérez-Diaz JC, Purcell R, Remmel B, Rose M, Schlueter T, Simoes N, Tierrez A, Vázquez-Boland JA, Voss H, Wehland J, Cossart P. 2001. Comparative genomics of Listeria species. Science 294:849–852. [DOI] [PubMed] [Google Scholar]
- 61.Bécavin C, Bouchier C, Lechat P, Archambaud C, Creno S, Gouin E, Wu Z, Kühbacher A, Brisse S, Pucciarelli MG, García-del Portillo F, Hain T, Portnoy DA, Chakraborty T, Lecuit M, Pizarro-Cerdá J, Moszer I, Bierne H, Cossart P. 2014. Comparison of widely used Listeria monocytogenes strains EGD, 10403S, and EGD-e highlights genomic variations underlying differences in pathogenicity. mBio 5:e00969-14. doi: 10.1128/mBio.00969-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doumith M, Cazalet C, Simoes N, Frangeul L, Jacquet C, Kunst F, Martin P, Cossart P, Glaser P, Buchrieser C. 2004. New aspects regarding evolution and virulence of Listeria monocytogenes revealed by comparative genomics and DNA arrays. Infect Immun 72:1072–1083. doi: 10.1128/IAI.72.2.1072-1083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All genomes sequenced in this study were deposited in NCBI/EMBL/DDBJ databases under the project accession no. PRJEB27826.