Abstract
Aims
To examine the associations of plasma copper concentrations and superoxide dismutase 1 (SOD1) polymorphisms as well as their gene-environment interaction with newly diagnosed impaired glucose regulation (IGR) and type 2 diabetes (T2D).
Methods
We performed a large case-control study in 2520 Chinese Han subjects: 1004 newly diagnosed T2D patients, 512 newly diagnosed IGR patients and 1004 individuals with normal glucose tolerance.
Results
After multivariable adjustment, the ORs (95% CIs) of T2D across tertiles of plasma copper were 1.00 (reference), 1.85 (95% CI: 1.39, 2.45), and 4.21 (95% CI: 3.20, 5.55) (P-trend < 0.001). Each SD increment of ln-transformed plasma copper was associated with 104% higher odds (OR 2.04, 95%CI 1.82–2.28) increment in ORs of T2D. Meanwhile, compared with the GG genotype of rs2070424, the OR of T2D associated with AG and AA genotypes were 1.44 (95% CI 1.15–1.81) and 1.74 (95% CI 1.33–2.28), respectively. In addition, the positive association between plasma copper and T2D was modified by rs2070424 genotypes. The adjusted ORs and 95% CIs of T2D per SD increment of ln-transformed plasma copper were 2.40 (1.93–2.99), 1.85 (1.59–2.16) and 1.76 (1.44–2.15) in rs2070424 GG, AG and GG carriers respectively (P for interaction < 0.05). Similar interactions were also found for IGR and IGR&T2D. When the joint effects were examined, individuals with rs2070424 AA genotype and the highest tertile of plasma copper concentration had a much higher risk of IGR&T2D (OR 5.34, 95% CI 3.48–8.21) than those with rs2070424 GG genotype and the lowest tertile of plasma copper concentrations.
Conclusions
Plasma copper concentrations are positively and significantly associated with IGR as well as T2D, and these associations may be modified by SOD1 polymorphism. Further studies are warranted to elucidate the potential mechanisms.
Keywords: Copper, Gene-environment interaction, Impaired glucose regulation, Superoxide dismutase 1, Type 2 diabetes
Abbreviations: Cu, copper; FFQ, food frequency questionnaire; HDLC, high-density lipoprotein cholesterol; IFG, impaired fasting glucose; IGR, impaired glucose regulation; IGT, impaired glucose tolerance; LDLC, low-density lipoprotein cholesterol; MAF, minor allele frequencies; NGT, normal glucose tolerance; ROS, reactive oxygen species; SNPs, single nucleotide polymorphisms; SOD1, superoxide dismutase 1; TC, total cholesterol; TG, triglyceride; T2D, type 2 diabetes
Graphical abstract
Highlights
-
•
Plasma copper concentrations are positively and significantly associated with IGR as well as T2D.
-
•
Compared with the GG genotype of rs2070424, the risk of T2D associated with AG and AA genotypes were higher.
-
•
The associations between copper and T2D as well as IGR may be modified by SOD1 rs2070424 polymorphism.
-
•
Evaluating the interaction of copper and gene polymorphisms may shed etiologic insight into the copper-diabetes relation.
1. Introduction
The prevalence of type 2 diabetes (T2D) is increasing rapidly and the number of people with diabetes will increase to 629 million by 2045 if these trends continue [1]. Therefore, diabetes is becoming a critical threat to public health and has received considerable attention. Besides the important contribution of environment factors, such as nutrient status [2], genetic variants also play a key role in T2D [3]. Considerable inter-individual variation has been noted in response to the levels of trace elements in patients with impaired glucose regulation (IGR) and T2D, and such variation may be determined by interactions with genetic factors [[4], [5], [6]].
Copper, an essential nutrient ingested from several food sources such as seafood, organ meats, grains and water, is involved in erythropoiesis, immune functions, energy production, glucose metabolism, and neuropeptide synthesis [7]. Numerous copper containing enzymes, such as superoxide dismutase, ceruloplasmin and metallothionein, are known to play important roles in radical scavenging [8]. In animal models, it is well characterized that dietary copper could alter the activities of SOD1 and thus have influences on oxidant defense system [9]. Despite being essential, as a transition metal, copper is an oxidant at high level and its redox properties contribute to the production of excessive damaging reactive oxygen species (ROS) [10], which acts as an important trigger for insulin resistance [11]. In animal models, excess copper has been elucidated to accompany higher ROS level and increased insulin resistance; consequently, treatment with copper chelating agent has been shown to significantly decrease these symptoms in diabetic mice [12]. Consistently, alleviated diabetic organ damages as well as improved antioxidant defences are also observed to be in T2D patients treated with highly-selective copper chelation drugs [13]. A previous meta-analysis involved fifteen eligible studies has demonstrated that diabetes patients carried higher levels of plasma/serum copper than healthy individuals [14]. Nevertheless, none of these studies have considered the potential modification effects of genetic factors.
Superoxide dismutase 1 (SOD1) is an enzyme required copper as a catalytic cofactor for the full enzymatic activity and catalyze the conversion of ROS in intracellular [15]. In animal models, increased expression of SOD1 genes may decrease fasting blood glucose and hemoglobin A1c [16] as well as contribute to the survival of hypertrophied beta cells during chronic hyperglycemia [17]; conversely, genetic disruption of SOD1 gene causes glucose intolerance and impairs beta cell function [18]. Since 1993 the rare mutations in SOD1 were widely studied and identified to be associated with amyotrophic lateral sclerosis [[19], [20], [21]], a degenerative disorder of motor neurons characterized by altered lipid and glucose metabolism, which recently was shown to share several genetic and environmental pathways with diabetes [22]. In general population, several common genetic variations of SOD1 polymorphism has been shown to be associated with diabetes and diabetic complications [[23], [24], [25], [26], [27], [28]]. However, the interaction of copper levels with genetic variation in SOD1 has not been evaluated as a potential determinant of IGR and T2D. Therefore, we hypothesize that the effects of SOD1 variants on diabetes may be modified by plasma copper concentrations and vice versa.
The objective of the present study was thus to examine the associations of plasma copper and SOD1 polymorphism as well as their gene-environment interaction with newly diagnosed IGR and T2D in a large case-control study conducted among a Chinese Han population. Evaluating the interaction of copper exposure and gene polymorphisms may shed etiologic insight into the copper-diabetes relation.
2. Methods
2.1. Study population
The study population consisted of 2520 subjects: 1004 newly diagnosed T2D patients, 512 newly diagnosed IGR patients and 1004 individuals with normal glucose tolerance (NGT). Case subjects were individuals with new-onset IGR and T2D, which were documented in the medical record and consecutively recruited from the outpatient clinics of the Department of Endocrinology at the Tongji Medical College Hospital from January 2013, through December 2016. Concomitantly, healthy control subjects with normal glucose tolerance were selected at random from the population undergoing a routine health checkup in the same hospital, and were matched on age and sex to T2D and IGR subjects by propensity score matching, respectively. The inclusion criteria for subjects recruited were as follows: age ≥30 years, BMI <40 kg/m2, no history of a diagnosis of diabetes, and no history of receiving pharmacological treatment for hyperlipidemia and hypertension. Patients with any clinically systemic disease, acute illness, chronic inflammatory disease or infectious disease were excluded from the study. The study was approved by the Ethics and Human Subject Committee of Tongji Medical College. Written informed consent was obtained from each subject enrolled and all the subjects were of Chinese Han ethnicity.
2.2. Assessment of NGT, IGR and T2D
IGR and T2D were defined by respective diagnostic criteria recommended by the World Health Organization in 1999 [29]. IGR was defined as impaired fasting glucose (IFG) (fasting plasma glucose [FPG] ≥ 6.1 and <7.0 mmol/L and 2-h postglucose load < 7.8 mmol/L) and/or impaired glucose tolerance (IGT) (FPG < 7.0 mmol/L and 2-h postglucose load ≥ 7.8 and <11.1 mmol/L). T2D was diagnosed when FPG ≥7.0 mmol/L and/or 2-h postglucose load ≥11.1 mmol/L. NGT was defined as a FPG <6.1 mmol/L and a 2-h postglucose load of oral glucose tolerance test (OGTT) < 7.8 mmol/L.
2.3. Anthropometric factors and blood parameters
Personal information on sociodemographic data, including sex, age, history of hypertension, family history of diabetes, and lifestyle habits was collected through semi-structured questionnaires by trained interviewers. Smoking status was classified as current, former, and never; similar manner was also utilized to evaluate the alcohol drinking status. Physical activity was classified as regular exercise for at least 60 min per week for more than half a year. Standardized techniques were used for measurements of height (m) and weight (kg). BMI was calculated as weight divided by the square of height (kg/m2). After a 10-h overnight fast, all participants underwent a 75-g OGTT, and venous blood samples were collected at 0 and 2 h for plasma separation. Fasting plasma were used to determination of fasting plasma glucose (FPG), fasting plasma insulin (FPI), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDLC) and plasma copper. 2-h OGTT plasma samples were used only to determine the 2-h postglucose load of OGTT. The HOMA-IR score was computed according to the following formula: [FPI (mU/L) × FPG (mmol/L)] ÷ 22.5. The HOMA index of beta cell function was calculated as [20 × FPI (mU/L)] ÷ [FPG(mmol/L)-3.5].
2.4. Measurement of plasma copper concentrations
Plasma copper concentrations were measured in the Ministry of Education Key Laboratory of Environment and Health at Tongji Medical College of Huazhong University of Science & Technology, using inductively coupled plasma mass spectrometry with an octopole-based collision/reaction cell (Agilent 7700 Series, Tokyo, Japan). We measured case and control specimens randomly in the daily measurement, with laboratory personnel blinded to the case–control status. For quality assurance, we measured metals in standard reference materials once in every 20 samples using certified reference material (ClinChek human plasma controls for trace elements no. 8883 and 8884). The values measured in the reference materials were confirmed to within the recommended range for copper. For no. 8883, we determined a concentration of 917 ± 67 μg/L (certified: 925 ± 185 μg/L), and for no. 8884, we measured 1,314 ± 114 μg/L (certified: 1,363 ± 273 μg/L). Both the intraassay and interassay coefficient of variation of plasma copper were <5%. The limit of detection was 0.009 μg/L, and all study participants had plasma copper levels above the limit of detection.
2.5. Genotyping
Based on identified SOD1 polymorphism loci with diabetes and diabetes or diabetes complications previously [[23], [24], [25], [26], [27], [28]], single nucleotide polymorphisms (SNPs) with minor allele frequencies (MAF) more than 0.05 were selected according to both HapMap HCB and CHB data in dbSNP (http://www.ncbi.nlm.nih.gov/SNP). Two informative SOD1 SNPs (rs2070424 and rs1041704) were selected to test the association between SOD1 and the risk of T2D.
These two common variants were determined by the MassArray system (Agena iPLEXassay, San Diego, United States). Genomic DNA from the peripheral blood sample was isolated by kit (Tiangen biotech, Beijing, China). The sample DNA was amplified by a multiplex Polymerase chain reaction (PCR), which products were then used for locus-specific single-base extension reaction. The alleles were discriminated by mass spectrometry (Agena, San Diego, United States). Overall, the two single nucleotide polymorphisms were successfully genotyped with a call rate of more than 95%. However, only genotype distribution of rs2070424 was in Hardy-Weinberg equilibrium (p > 0.05) for both case and control groups (data not shown). The rs1041702 polymorphism was not involved in further analysis.
2.6. Statistical analysis
General characteristics were summarized according to cases and controls as numbers (percentages) for categorical data, means ± standard deviations (SDs) for parametrically distributed data, and medians (interquartile range) for nonparametrically distributed data. Descriptive statistics were calculated for all demographic and clinical characteristics of the study subjects. Characteristics of NGT, IGR, and T2D were compared using t-test or Mann-Whitney U test for continuous variables, and chi-square tests for categorical variables. Binary logistic regression analysis was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of IGR, T2D and IGR&T2D, in reference to those with normal glucose tolerance. Individual plasma copper concentrations were considered continuous variable and categorized into tertiles according to their distribution among the NGT group (Q1, <794.17 μg/L; Q2, 794.17–937.91 μg/L; Q3, ≥937.91 μg/L). We also calculated the ORs and 95% CIs of IGR, T2D and IGR&T2D per SD increment of the logarithmic transformed copper concentrations. Linear trend P-values were derived by modeling the median value of each quartile as a continuous variable. The ORs and 95% CI were adjusted for potential confounders (see footnotes of Table 2), such as age, sex, BMI, hypertension, family history of diabetes, smoking status, alcohol drinking status, physical activity. We further controlled for multiple T2D risk factors including several oxidative stress related minerals (manganese, magnesium, iron, chromium and selenium) in plasma. Subgroup analyses were performed to evaluate the modification effect in adjusted models for plasma copper concentrations and IGR&T2D group with dichotomous indicator variables, including age (<50, ≥50 years), sex (male, female), BMI (<24.0, ≥24.0), smoking (yes, no), drinking (yes, no), physical activity (yes, no), hypertension (yes, no), as well as family history of diabetes (yes, no). The interactions between these stratification variables and plasma copper were tested by adding multiplicative terms into the multivariate logistic regression models; the likelihood ratio test was used to test the statistical significance of the interaction terms. We further estimated the overall associations of logarithmic transformed plasma copper with IGR, T2D and IGR&T2D using restricted cubic splines with 4 knots at the 20th, 40th, 60th, and 80th percentiles of its distribution via Stata; the reference value was set at the 10th percentile, and the values outside the 5th and 95th percentiles were excluded [30].
Table 2.
Associations of plasma copper concentrations with IGR and T2D.
| Variables | Tertiles of plasma copper concentration (μg/L) |
P value for trend | Per SD of ln-transformed plasma copper | P value | ||
|---|---|---|---|---|---|---|
| Q1 |
Q2 |
Q3 |
||||
| <797.39 (709.25)a | 797.39–956.13 (875.06)a | ≥956.13 (1048.06)a | ||||
| IGR vs. NGT | ||||||
| No. of cases/control subjects | 131/335 | 145/335 | 236/334 | |||
| Crude | 1 | 1.11 (0.84–1.47) | 1.81 (1.39–2.35) | <0.001 | 1.38 (1.25–1.54) | <0.001 |
| Model 1b | 1 | 1.19 (0.89–1.59) | 1.99 (1.50–2.64) | <0.001 | 1.45 (1.29–1.62) | <0.001 |
| Model 2c | 1 | 1.20 (0.89–1.61) | 1.88 (1.41–2.51) | <0.001 | 1.41 (1.26–1.58) | <0.001 |
| Model 3d | 1 | 1.13 (0.84–1.53) | 1.74 (1.28–2.35) | <0.001 | 1.38 (1.22–1.55) | <0.001 |
| T2D vs. NGT | ||||||
| No. of cases/control subjects | 135/335 | 259/335 | 610/334 | |||
| Crude | 1 | 1.92 (1.48–2.48) | 4.53 (3.56–5.76) | <0.001 | 2.00 (1.81–2.20) | <0.001 |
| Model 1b | 1 | 1.91 (1.47–2.50) | 4.43 (3.44–5.70) | <0.001 | 2.00 (1.81–2.22) | <0.001 |
| Model 2c | 1 | 1.89 (1.43–2.50) | 4.25 (3.27–5.53) | <0.001 | 1.97 (1.78–2.20) | <0.001 |
| Model 3d | 1 | 1.85 (1.39–2.45) | 4.21 (3.20–5.55) | <0.001 | 2.04 (1.82–2.28) | <0.001 |
| (IGR & T2D) vs. NGT | ||||||
| No. of cases/control subjects | 266/335 | 404/335 | 846/334 | |||
| Crude | 1 | 1.52 (1.22–1.89) | 3.19 (2.60–3.92) | <0.001 | 1.72 (1.58–1.86) | <0.001 |
| Model 1b | 1 | 1.55 (1.24–1.95) | 3.25 (2.61–4.04) | <0.001 | 1.75 (1.60–1.91) | <0.001 |
| Model 2c | 1 | 1.53 (1.22–1.93) | 3.00 (2.40–3.75) | <0.001 | 1.69 (1.54–1.85) | <0.001 |
| Model 3d | 1 | 1.48 (1.17–1.87) | 2.92 (2.30–3.69) | <0.001 | 1.71 (1.55–1.88) | <0.001 |
Range (median).
Model 1 adjusted for sex, age (≤40, 40–49, 50–59, ≥60) and body mass index (≤18.5, 18.5–23.9, 24–27.9, ≥28).
Model 2 further adjusted for smoking (current, former, and never), drinking (current, former, and never), physical activity (yes or no), hypertension (yes or no), and family history of diabetes (any or none).
Model 3 further adjusted for plasma manganese, plasma magnesium, plasma iron, plasma chromium and plasma selenium.
The distribution of gene genotype was analyzed for deviation from Hardy-Weinberg equilibrium by use of a likelihood ratio test. Binary logistic regression analysis was used to assess the ORs and 95% CI of IGR, T2D and IGR&T2D with rs2070424 polymorphisms (GG, AG, or AA genotypes). The association between plasma copper and IGR, T2D and IGR&T2D stratified by gene polymorphisms were further examined with the same method described in the subgroups analyses. We also estimated the joint association of plasma copper concentrations (tertiles) and rs2070424 polymorphisms with IGR, T2D and IGR&T2D. Statistical power for the rs2070424-copper interaction with IGR&T2D was calculated using QUANTO 1.2.4 (http://biostats.usc.edu/Quanto.html). Assuming an OR of 1.75 per plasma copper tertile and an OR of 1.50 per rs2070424 A allele (allele frequency 48.84%), our study had 80% power to detect an interaction OR of at least 0.80 for IGR&T2D.
All data analyses were carried out with SPSS 20.0 (SPSS Inc., Chicago, IL) and Stata version 12 (StataCorp LP, College Station, TX). P values presented are two tailed with a significance level of 0.05.
3. Results
The characteristics of 2520 participants (1004 T2D, 512 IGR and 1004 NGT) were presented in Table 1. Compared with NGT subjects, the individuals with IGR and T2D had higher BMI, greater prevalence of hypertension and family history of diabetes, higher levels of TG, LDL-C, FPG, FPI and copper as well as lower levels of HDL-C in IGR and T2D, whereas TC was only higher in IGR subjects compared with control subjects. In the insulin sensitivity indexes, we noted lower HOMA-β and higher HOMA-IR in IGR and T2D subjects. Overall, the medians (interquartile range) of the plasma copper concentrations were 874.55 μg/L (760.49–992.97 μg/L) for NGT, 930.18 μg/L (793.01–1085.91 μg/L) for IGR and 1013.19 μg/L (870.35–1184.93 μg/L) for T2D. Allele frequencies of rs2070424 gene polymorphisms were shown in Table 1. The genotype distribution of rs1041740 was departure from Hardy-Weinberg equilibrium (data not shown).
Table 1.
Anthropometric and metabolic characteristics of NGT, IGR and T2D groups.
| Parameters | NGT (n = 1004) | IGR (n = 512) | T2D (n = 1004) |
P value |
|
|---|---|---|---|---|---|
| IGR vs. NGT | T2D vs. NGT | ||||
| Male, n (%) | 627 (62.45) | 332 (64.84) | 588 (58.57) | 0.36 | 0.08 |
| Age (years) | 50.92 ± 11.06 | 50.50 ± 10.99 | 51.21 ± 10.64 | 0.63 | 0.27 |
| BMI (kg/m2) | 23.47 ± 2.94 | 24.98 ± 3.40 | 25.21 ± 3.39 | <0.01 | <0.01 |
| Smoker, n (%) | 401 (39.94) | 201 (40.04) | 347 (34.56) | 0.80 | 0.01 |
| Drinker, n (%) | 337 (33.57) | 189 (37.65) | 351 (34.96) | 0.20 | 0.51 |
| Regular physical activity, n (%) | 379 (37.45) | 176 (34.38) | 378 (37.65) | 0.20 | 0.96 |
| Hypertension, n (%) | 173 (17.23) | 152 (29.69) | 357 (35.56) | <0.01 | <0.01 |
| Family history of diabetes, n (%) | 64 (6.37) | 70 (13.67) | 259 (25.80) | <0.01 | <0.01 |
| Fasting plasma glucose (mmol/L) | 5.55 (5.27–5.82) | 6.30 (6.11–6.57) | 8.03 (7.10–10.49) | <0.01 | <0.01 |
| Fasting plasma insulin (mU/L) | 7.65 (5.23–11.30) | 9.62 (6.77–14.18) | 9.91 (6.49–14.87) | <0.01 | <0.01 |
| HOMA-IR | 1.87 (1.26–2.82) | 2.68 (1.84–3.95) | 3.89 (2.49–6.09) | <0.01 | <0.01 |
| HOMA-β | 76.25 (54.01–109.32) | 70.61 (48.64–103.25) | 42.19 (23.20–70.32) | 0.03 | <0.01 |
| Triglyceride (mmol/L) | 1.32 (0.94–1.72) | 1.45 (0.99–2.26) | 1.75 (1.09–3.50) | <0.01 | <0.01 |
| Total cholesterol (mmol/L) | 4.64 (4.14–5.19) | 4.81 (4.13–5.52) | 4.64 (3.91–5.41) | 0.02 | 0.47 |
| HDL cholesterol (mmol/L) | 1.33 (1.18–1.50) | 1.30 (1.03–1.52) | 1.07 (0.87–1.34) | <0.01 | <0.01 |
| LDL cholesterol (mmol/L) | 2.30 (1.75–2.92) | 2.54 (1.81–3.22) | 2.68 (1.86–3.58) | <0.01 | <0.01 |
| Copper (μg/L) | 874.55 (760.49–992.97) | 930.18 (793.01–1085.91) | 1013.19 (870.35–1184.93) | <0.01 | <0.01 |
| SOD1 rs2070424 | |||||
| Allele G | 1261 (55.43) | 490 (47.85) | 975 (48.56) | <0.01 | <0.01 |
| Allele A | 747 (44.57) | 534 (52.15) | 1033 (51.44) | ||
Note: BMI, body mass index; HDL, high density lipoprotein; LDL, low density lipoprotein; SOD, superoxide dismutase.
Data were presented as n (%) for categorical data, mean (standard deviation) for parametrically distributed data or median (interquartile range) for nonparametrically distributed data.
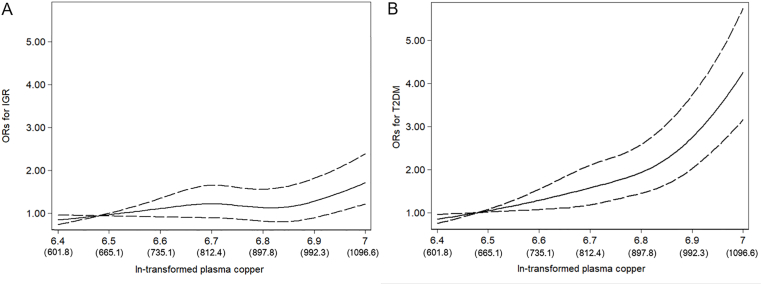
Table 2 presented ORs for IGR and T2D associated with the levels of plasma copper concentrations categorized into tertiles according to their distribution in the NGT subjects. After multivariable adjustment ORs (95% CIs) across tertiles of plasma copper were 1.00 (reference), 1.13 (95% CI: 0.84, 1.53) and 1.74 (95% CI: 1.28, 2.35) (P-trend < 0.001); each SD of ln-transformed plasma copper was associated with a 38% (1.38 [1.22, 1.55]) increment in ORs of IGR. For T2D, the adjusted ORs were 1.00 (reference), 1.85 (95% CI: 1.39, 2.45), and 4.21 (95% CI: 3.20, 5.55) (P-trend < 0.001); each SD of ln-transformed plasma copper was associated with 1.04-fold (2.04 [1.82, 2.28]) increment in ORs of T2D. Similar results were obtained in IGR&T2D groups. In general, the associations between plasma copper and IGR&T2D were consistent across different risk strata subgroups (Table S1), and no significant interaction was found between subgroups. In the spline regression model, the odds of IGR and T2D increase significantly with increasing ln-transformed copper concentrations, especially at levels higher than 900 μg/L (Fig. 1).
Fig. 1.
Representation of restricted cubic spline logistic regression models for ln-transformed copper and risk of IGR (A) and T2D (B). Knots were placed at the 20th, 40th, 60th, and 80th percentiles of ln (plasma copper concentrations). Adjusted ORs (solid line) and 95% CIs (dashed line) for IGR and T2D by ln-transformed plasma copper concentrations. Results were adjusted for sex, age (≤40, 40–49, 50–59, ≥60), BMI (≤18.5, 18.5–23.9, 24–27.9, ≥28), smoking (current, former, and never), drinking (current, former, and never), physical activity (yes or no), hypertension (yes or no), and family history of diabetes (any or none), plasma manganese, plasma magnesium, plasma iron, plasma chromium and plasma selenium.
Minor allele frequencies (A allele) in NGT, IGR, and T2D groups were 0.45, 0.52, and 0.51, respectively. Compared with the GG genotype, after adjustment for age, sex, BMI, smoking, drinking, family history of diabetes, and hypertension, the AG and AA genotypes were associated with increased odds of T2D (OR 1.44 [95% CI 1.15–1.81] and 1.74 [95% CI 1.33–2.28], respectively) (Table 3). Similar associations were found in IGR and IGR&T2D groups. The associations between rs2070424 and IGR&T2D were consistent across different risk strata subgroups, and no significant interaction was found between subgroups (Table S2).
Table 3.
Association of rs2070424 polymorphism with IGR and T2D.
| Controls, n (%) | Cases, n (%) | Crude OR (95% CI) | P Value | Adjusted OR (95% CI)a | P value | |
|---|---|---|---|---|---|---|
| IGR vs. NGT | ||||||
| GG genotype | 320 (31.87) | 125 (24.41) | 1 | 1 | ||
| AG genotype | 473 (47.11) | 240 (46.88) | 1.30 (1.00–1.68) | <0.01 | 1.34 (1.02–1.75) | <0.01 |
| AA genotype | 211 (21.02) | 147 (28.71) | 1.78 (1.33–2.40) | <0.01 | 1.84 (1.35–2.50) | <0.01 |
| AG + AA genotype | 684 (68.13) | 387 (75.59) | 1.45 (1.14–1.84) | <0.01 | 1.49 (1.16–1.92) | <0.01 |
| T2D vs. NGT | ||||||
| GG genotype | 320 (31.87) | 237 (23.61) | 1 | 1 | ||
| AG genotype | 473 (47.11) | 501 (49.90) | 1.43 (1.16–1.76) | <0.01 | 1.44 (1.15–1.81) | <0.01 |
| AA genotype | 211 (21.02) | 266 (26.49) | 1.70 (1.33–2.18) | <0.01 | 1.74 (1.33–2.28) | <0.01 |
| AG + AA genotype | 684 (68.13) | 767 (76.39) | 1.51 (1.24–1.84) | <0.01 | 1.53 (1.24–1.90) | <0.01 |
| (IGR & T2D) vs. NGT | ||||||
| GG genotype | 320 (31.87) | 362 (22.67) | 1 | 1 | ||
| AG genotype | 473 (47.11) | 741 (49.62) | 1.39 (1.15–1.67) | <0.01 | 1.38 (1.13–1.69) | <0.01 |
| AA genotype | 211 (21.02) | 413 (27.71) | 1.73 (1.38–2.16) | <0.01 | 1.77 (1.40–2.25) | <0.01 |
| AG + AA genotype | 684 (68.13) | 1154 (77.33) | 1.49 (1.25–1.78) | <0.01 | 1.50 (1.24–1.81) | <0.01 |
ORs were adjusted for sex, age (≤40, 40–49, 50–59, ≥60), BMI (≤18.5, 18.5–23.9, 24–27.9, ≥28), smoking (current, former, and never), drinking (current, former, and never), physical activity (yes or no), hypertension (yes or no), and family history of diabetes (any or none).
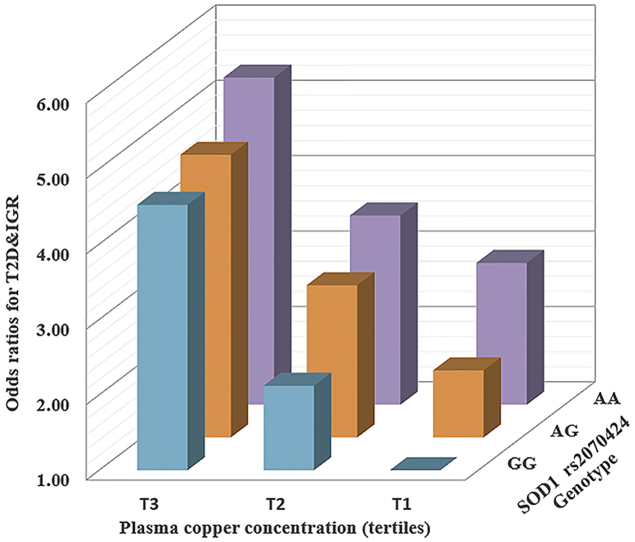
The positive association between plasma copper and T2D was modified by rs2070424 genotypes (Table 4). The effect of plasma copper persisted, whereas appeared to be attenuated in AA and AG genotype carriers than in GG genotype carriers. The adjusted ORs and 95% CIs of T2D per SD increment of ln-transformed plasma copper were 2.40 (1.93–2.99), 1.85 (1.59–2.16) and 1.76 (1.44–2.15) in GG, AG and GG carriers respectively (P for interaction < 0.05). Meanwhile, the observed genetic effects of risk allele A and T2D were likely mitigated by higher plasma copper concentrations (P for interaction < 0.05) (Table S3). Similar interactions were found for IGR and IGR&T2D. When the joint effects were examined, individuals with AA genotype and the highest tertile of plasma copper concentration had a much higher risk of IGR&T2D (OR 5.34 [3.48–8.21]) than those with GG genotype in the lowest copper tertile (reference) (Table 5).
Table 4.
ORs for the association between IGR, T2D and plasma copper concentrations according to gene polymorphisma.
| Variables | Tertiles of plasma copper concentration (mg/L) |
P value for trend | Per SD of ln-transformed plasma copper | P-interaction | ||
|---|---|---|---|---|---|---|
| Q1 |
Q2 |
Q3 |
||||
| <797.39 (709.25)b | 797.39–956.13 (875.06)b | ≥956.13 (1048.06)b | ||||
| IGR vs. NGT | ||||||
| GG | 1 | 1.51 (0.85–2.69) | 3.02 (1.73–5.26) | <0.001 | 1.69 (1.33–2.14) | 0.009 |
| AG | 1 | 1.09 (0.71–1.69) | 1.61 (1.05–2.46) | <0.001 | 1.34 (1.13–1.59) | |
| AA | 1 | 0.94 (0.51–1.72) | 1.24 (0.70–2.22) | 0.380 | 1.24 (0.99–1.55) | |
| T2D vs. NGT | ||||||
| GG | 1 | 2.88 (1.66–4.97) | 6.57 (3.90–11.05) | <0.001 | 2.40 (1.93–2.99) | 0.035 |
| AG | 1 | 1.53 (1.04–2.28) | 3.49 (2.39–5.09) | <0.001 | 1.85 (1.59–2.16) | |
| AA | 1 | 1.57 (0.87–2.83) | 3.24 (1.87–5.62) | <0.001 | 1.76 (1.44–2.15) | |
| (IGR & T2D) vs. NGT | ||||||
| GG | 1 | 2.12 (1.37–3.28) | 4.52 (2.95–6.91) | <0.001 | 1.99 (1.66–2.38) | 0.005 |
| AG | 1 | 1.34 (0.96–1.87) | 2.57 (1.85–3.56) | <0.001 | 1.60 (1.41–1.83) | |
| AA | 1 | 1.20 (0.73–1.97) | 2.12 (1.32–3.44) | <0.001 | 1.51 (1.27–1.79) | |
ORs were adjusted for sex, age (≤40, 40–49, 50–59, ≥60), BMI (≤18.5, 18.5–23.9, 24–27.9, ≥28), smoking (current, former, and never), drinking (current, former, and never), physical activity (yes or no), hypertension (yes or no), and family history of diabetes (any or none).
Range (median).
Table 5.
Joint effects of plasma copper and rs2070424 gene polymorphism for IGR and T2Da.
| Variables | Tertiles of plasma copper concentration (mg/L) |
||
|---|---|---|---|
| Q1 |
Q2 |
Q3 |
|
| <797.39 (709.25)b | 797.39–956.13 (875.06)b | ≥956.13 (1048.06)b | |
| IGR vs. NGT | |||
| GG | 1 | 1.51 (0.85–2.69) | 3.02 (1.73–5.26) |
| AG | 1.77 (1.06–2.95) | 2.36 (1.38–4.06) | 2.64 (1.58–4.40) |
| AA | 3.16 (1.75–5.71) | 3.36 (1.81–6.25) | 3.36 (1.91–5.91) |
| T2D vs. NGT | |||
| GG | 1 | 2.88 (1.66–4.97) | 6.57 (3.90–11.05) |
| AG | 2.15 (1.29–3.58) | 3.98 (2.41–6.56) | 7.29 (4.52–11.76) |
| AA | 2.62 (1.41–4.86) | 4.06 (3.31–7.16) | 7.68 (4.61–12.82) |
| (IGR & T2D) vs. NGT | |||
| GG | 1 | 2.12 (1.37–3.28) | 4.52 (2.95–6.91) |
| AG | 1.89 (1.28–2.82) | 3.02 (2.02–4.52) | 4.72 (3.20–6.96) |
| AA | 2.88 (1.78–4.66) | 3.51 (2.20–6.00) | 5.34 (3.48–8.21) |
ORs were adjusted for sex, age (≤40, 40–49, 50–59, ≥60), BMI (≤18.5, 18.5–23.9, 24–27.9, ≥28), smoking (current, former, and never), drinking (current, former, and never), physical activity (yes or no), hypertension (yes or no), and family history of diabetes (any or none).
Range (median).
4. Discussion
To our knowledge, this was the first study to examine the interaction between plasma copper concentrations and SOD1 genetic variation for IGR and T2D. Elevated plasma copper concentrations were positively associated with higher odds of both newly diagnosed IGR and T2D in a linear dose response manner. As for the SOD1 rs2070424 polymorphism, the risk allele A was confirmed with newly diagnosed IGR and T2D in the Chinese Han population. Moreover, strong interactions were observed for the SOD1 rs2070424 variant and plasma copper concentrations in relation to IGR, T2D and IGR&T2D. Findings from our gene-environment interaction analysis are, thus, consistent with a potential dysfunction of redox mechanisms associated with altered copper levels in T2D.
The observed positive associations of copper with IGR and T2D were largely consistent with findings from most, although not all, population-based studies. A prospective cohort study reports that higher dietary intake of copper, measured via food frequency questionnaire (FFQ), is associated with increased risk of T2D [31]. Consistently, multiple epidemiological studies have elucidated that diabetic patients are characterized by higher level of plasma copper than controls [[32], [33], [34], [35]]. In contrast, a study included twenty young female diabetes patients shows significantly lower mean value of serum copper as compared to the control subjects [36], which may due to rather small sample sizes. Our findings were in accordance with a recent meta-analysis [14], which demonstrate that diabetic patients carry higher levels of plasma/serum copper than healthy individuals. Moreover, population differences are found to affect the distribution of plasma copper concentrations, and the means of plasma copper in Asian populations range from 13.91 to 16.87 μmol/L (890.24–1079.68 μg/L) [14], which is largely comparable to our results. In the current study, the positive association between plasma copper and T2D were examined with adjustment for confounding factors such as lifestyle habits and several oxidative stress related minerals (manganese, magnesium, iron, chromium and selenium) in plasma. In addition, higher level of copper was also observed among newly diagnosed IGR patients, which indicated that the disturbance of copper may have taken place in earlier stage during the occurrence of diabetes.
In the current study, the AG and AA genotype of rs2070424 were associated with a higher risk of IGR and T2D. Several previous studies have highlighted the role of SOD1 in the progression of T2D in animal models [[16], [17], [18]]. Meanwhile, Kase et al. showed that A genotype of rs2070424 associate with a lower expression of SOD1 mRNA in a set of immortalized lymphoblastic cell lines [37]. In epidemiologic studies, researches explored the relation of SOD1 polymorphisms with diabetes and diabetic complications are sparse [[23], [24], [25], [26], [27], [28]], and a common genetic variation of SOD1 rs2070424 polymorphism has been suggested to associate with T2D [23], obesity [38] and Alzheimer's disease [39], where the oxidative disturbance is an important factor involved in the occurrence and development of the diseases. Notably, the MAF of rs2070424 varies widely between ethnicities, which may contribute to genetic heterogeneity in SOD1 gene-T2D associations. In our result, MAF (A allele) of rs2070424 was 0.49 among a Chinese Han population, which is largely comparable to a previous study carried out in China [40].
No previous study has reported the interaction of copper and SOD1 rs2070424 variants with IGR or T2D. Considering copper may contribute to the production of excessive damaging ROS, while SOD1, a copper containing enzyme, also play important roles in radical scavenging, we hypothesized that there might be an interaction between copper and SOD1 polymorphism. When comprehensively analyzing the effects of plasma copper and SOD1 rs2070424 variants on IGR and T2D, we found that the positive association of plasma copper with IGR/T2D was largely attenuated by the rs2070424 A allele and the deleterious effects of copper on IGR even abolished in the AA genotype group. Moreover, the combination of AA genotype and the highest tertile of plasma copper concentration were associated with a much higher ORs of IGR&T2D than the combined GG genotype and the lowest tertile, which confirmed that both the rs2070424 A allele and increased plasma copper could be associated with IGR&T2D. However, as the plasma copper concentration increased, the association between AA genotype and T2D was substantially attenuated. One potential explanation for this interaction is that higher plasma copper might counteract the lower SOD1 activity or expression in AG and AA genotype carriers. Additional studies on these topics are needed to support the biological plausibility of the statistical interaction we report here, such as, investigating the effect of different levels of Cu on the oxidative stress indicators in stably transfected cell line to explain the difference in the fate of SOD1 activity between cells carried the SOD1 rs2070424 mutant type compared to the wild type carriers.
Several mechanisms may explain the important role of plasma copper in the etiology of IGR and T2D. Firstly, because of its redox properties, Cu2+ readily catalyzes both the Fenton [41] and Haber-Weiss [42] reactions, both of which may produce elevated HO• formation. The observed high level of copper in T2D have been positively correlated with ROS generation [43], which may directly cause macromolecular damage or indirectly result in oxidative stress [44], and thus lead to significant deterioration of insulin secretion and beta cell dysfunction [45]. Second, there is also evidence that advanced glycation end products (AGEs), which are positively associated with an increased risk of diabetes complications, could modify susceptible amino acid residues to produce pathological copper-binding sites [46]. The complex of AGEs and Cu2+ catalyze HO• generation and consequently lead to vascular tissue damage in diabetes [47]. Third, Cu2+ ions are found to interact with amylin peptide to produce H2O2 and mediate the aggregation of human amylin into amyloid fibrils [48], which is believed to be of critical importance for the progressive degeneration of islet cells in T2D [49].
Our study needs to be viewed within its strengths and limitations. The strengths of our study included a large sample size necessary to detect gene by environment interaction. Moreover, T2D and IGR subjects were objectively defined based mainly on fasting and postprandial glucose levels from an OGTT. Besides, the subjects with IGR and T2D were confined to the newly diagnosed and drug naive because anti-diabetic drugs may alter the status of copper metabolism and distort the association. Our study also has several limitations. First, the case-control nature of our study does not allow us to infer temporal and causal relationship. Therefore, these findings should be confirmed in further prospective cohort studies. Second, our measurement of copper was confined to plasma compartment, we used plasma copper concentration as a biomarker to measure copper internal exposure to avoid potential bias through dietary assessment, such as systematic measurement error in self-reported dietary exposure and regardless of the digestion, absorption and excretion process in vivo. Third, although we controlled for multiple T2D risk factors including lifestyle habits and several oxidative stress related minerals, we lacked the information on plasma zinc concentrations and inflammatory markers, therefore the possibility of residual confounding could not be ruled out. Forth, it is unclear whether the evaluated polymorphism can induce a change of gene expression or a functional change in SOD1, whereas the main goal of our study was to identify differential associations of copper and diabetes by genetic variants. Additional functional studies of the identified interacting polymorphisms are needed. Finally, all participants in this study were of Chinese Han ethnicity, which minimizes the confounding effects by ethnic background, but may limit the generalizability of the results to other ethnic groups.
In conclusion, the current study showed a positively linear association between plasma copper concentrations and IGR as well as T2D, which may be modified by SOD1 polymorphism. These findings may contribute to the understanding of the etiologic role of copper in T2D development and imply the potential adverse effects of copper may need to be personalized according to genetic background. Further studies are warranted to elucidate the potential mechanisms.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Contribution statement
J.Y. and X.W. contributed to the statistical analysis and interpretation of the results and wrote the manuscript. J.Y., S.L., Y.Z. and S.C. contributed to the acquisition of data and researched the data. Z.S., W.B. and L.L. contributed to the study design. P.L., C.L., Y.H., and W.Y. contributed to the discussion of the project. J.Y., Y.Z., S.C., Z.S., W.B., P.L., W.Y., X.L., X.H. and L.L. reviewed and edited the manuscript. L.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declarations of interest
None.
Acknowledgement
We are thankful to all the study participants for the substantial time and effort they spent in contributing to our study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101172.
Funding
This work was funded by the National Key Research and Development Program of China (2017YFC1600500), the Major International (Regional) Joint Research Project (NSFC 81820108027), the National Institutes of Health (R21 HD091458), the National Natural Science Foundation of China (21537001), the Young Scientists Fund of the National Natural Science Foundation of China (81703214) and the China Postdoctoral Science Foundation (2016M602314).
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.IDF . 2017. IDF Diabetes Atlas 8th. International Diabetes Federation.http://www.diabetesatlas.org/resources/2017-atlas.html Available from: [Google Scholar]
- 2.Bantle J.P., Wylie-Rosett J., Albright A.L. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 3.Cornelis M.C., Hu F.B. Gene-environment interactions in the development of type 2 diabetes: recent progress and continuing challenges. Annu. Rev. Nutr. 2012;32:245–259. doi: 10.1146/annurev-nutr-071811-150648. [DOI] [PubMed] [Google Scholar]
- 4.Shan Z., Bao W., Zhang Y. Interactions between zinc transporter-8 gene (SLC30A8) and plasma zinc concentrations for impaired glucose regulation and type 2 diabetes. Diabetes. 2014;63:1796–1803. doi: 10.2337/db13-0606. [DOI] [PubMed] [Google Scholar]
- 5.Qi L., Meigs J., Manson J.E. HFE genetic variability, body iron stores, and the risk of type 2 diabetes in U.S. women. Diabetes. 2005;54:3567–3572. doi: 10.2337/diabetes.54.12.3567. [DOI] [PubMed] [Google Scholar]
- 6.Galan-Chilet I., Grau-Perez M., De Marco G. A gene-environment interaction analysis of plasma selenium with prevalent and incident diabetes: the Hortega study. Redox Biol. 2017;12:798–805. doi: 10.1016/j.redox.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uriu-Adams J.Y., Keen C.L. Copper, oxidative stress, and human health. Mol. Aspect. Med. 2005;26:268–298. doi: 10.1016/j.mam.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Uauy R., Olivares M., Gonzalez M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998;67:952s–959s. doi: 10.1093/ajcn/67.5.952S. [DOI] [PubMed] [Google Scholar]
- 9.Uriu-Adams J.Y., Rucker R.B., Commisso J.F., Keen C.L. Diabetes and dietary copper alter 67Cu metabolism and oxidant defense in the rat. J. Nutr. Biochem. 2005;16:312–320. doi: 10.1016/j.jnutbio.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Kaler S.G. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat. Rev. Neurol. 2011;7:15–29. doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houstis N., Rosen E.D., Lander E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka A., Kaneto H., Miyatsuka T. Role of copper ion in the pathogenesis of type 2 diabetes. Endocr. J. 2009;56:699–706. doi: 10.1507/endocrj.k09e-051. [DOI] [PubMed] [Google Scholar]
- 13.Cooper G.J., Chan Y.K., Dissanayake A.M. Demonstration of a hyperglycemia-driven pathogenic abnormality of copper homeostasis in diabetes and its reversibility by selective chelation: quantitative comparisons between the biology of copper and eight other nutritionally essential elements in normal and diabetic individuals. Diabetes. 2005;54:1468–1476. doi: 10.2337/diabetes.54.5.1468. [DOI] [PubMed] [Google Scholar]
- 14.Qiu Q., Zhang F., Zhu W., Wu J., Liang M. Copper in diabetes mellitus: a meta-analysis and systematic review of plasma and serum studies. Biol. Trace Elem. Res. 2017;177:53–63. doi: 10.1007/s12011-016-0877-y. [DOI] [PubMed] [Google Scholar]
- 15.Fukai T., Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxidants Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung H., Kim Y.Y., Kim B., Nam H., Suh J.G. Improving glycemic control in model mice with type 2 diabetes by increasing superoxide dismutase (SOD) activity using silk fibroin hydrolysate (SFH) Biochem. Biophys. Res. Commun. 2017;493:115–119. doi: 10.1016/j.bbrc.2017.09.066. [DOI] [PubMed] [Google Scholar]
- 17.Laybutt D.R., Kaneto H., Hasenkamp W. Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to beta-cell survival during chronic hyperglycemia. Diabetes. 2002;51:413–423. doi: 10.2337/diabetes.51.2.413. [DOI] [PubMed] [Google Scholar]
- 18.Muscogiuri G., Salmon A.B., Aguayo-Mazzucato C. Genetic disruption of SOD1 gene causes glucose intolerance and impairs beta-cell function. Diabetes. 2013;62:4201–4207. doi: 10.2337/db13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones C.T., Brock D.J., Chancellor A.M., Warlow C.P., Swingler R.J. Cu/Zn superoxide dismutase (SOD1) mutations and sporadic amyotrophic lateral sclerosis. Lancet (London, England) 1993;342:1050–1051. doi: 10.1016/0140-6736(93)92905-9. [DOI] [PubMed] [Google Scholar]
- 20.Rosen D.R., Siddique T., Patterson D. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 21.Deng H.X., Hentati A., Tainer J.A. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science (New York, NY) 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 22.Logroscino G. Motor neuron disease: are diabetes and amyotrophic lateral sclerosis related? Nat. Rev. Neurol. 2015;11:488–490. doi: 10.1038/nrneurol.2015.145. [DOI] [PubMed] [Google Scholar]
- 23.Haldar S.R., Chakrabarty A., Chowdhury S., Haldar A., Sengupta S., Bhattacharyya M. Oxidative stress-related genes in type 2 diabetes: association analysis and their clinical impact. Biochem. Genet. 2015;53:93–119. doi: 10.1007/s10528-015-9675-z. [DOI] [PubMed] [Google Scholar]
- 24.Neves A.L., Mohammedi K., Emery N. Allelic variations in superoxide dismutase-1 (SOD1) gene and renal and cardiovascular morbidity and mortality in type 2 diabetic subjects. Mol. Genet. Metabol. 2012;106:359–365. doi: 10.1016/j.ymgme.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Mohammedi K., Maimaitiming S., Emery N. Allelic variations in superoxide dismutase-1 (SOD1) gene are associated with increased risk of diabetic nephropathy in type 1 diabetic subjects. Mol. Genet. Metabol. 2011;104:654–660. doi: 10.1016/j.ymgme.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 26.Panduru N.M., Cimponeriu D., Cruce M. Association of +35A/C (intron 3/exon 3) polymorphism in SOD1-gene with diabetic nephropathy in type 1 diabetes. Romanian Journal of morphology and Embryology = Revue Roumaine de Morphologie et Embryologie. 2010;51:37–41. [PubMed] [Google Scholar]
- 27.Flekac M., Skrha J., Hilgertova J., Lacinova Z., Jarolimkova M. Gene polymorphisms of superoxide dismutases and catalase in diabetes mellitus. BMC Med. Genet. 2008;9:30. doi: 10.1186/1471-2350-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Kateb H., Boright A.P., Mirea L. Multiple superoxide dismutase 1/splicing factor serine alanine 15 variants are associated with the development and progression of diabetic nephropathy: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Genetics study. Diabetes. 2008;57:218–228. doi: 10.2337/db07-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alberti K.G., Zimmet P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. J. Br. Diabet. Assoc. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 30.Moon K.A., Guallar E., Umans J.G. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann. Intern. Med. 2013;159:649–659. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eshak E.S., Iso H., Maruyama K., Muraki I., Tamakoshi A. Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: a large population-based prospective cohort study. Clin. Nutr. (Edinburgh, Scotland) 2017 doi: 10.1016/j.clnu.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Viktorinova A., Toserova E., Krizko M., Durackova Z. Altered metabolism of copper, zinc, and magnesium is associated with increased levels of glycated hemoglobin in patients with diabetes mellitus. Metab. Clin. Exp. 2009;58:1477–1482. doi: 10.1016/j.metabol.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 33.Leonhardt W., Hanefeld M., Muller G. Impact of concentrations of glycated hemoglobin, alpha-tocopherol, copper, and manganese on oxidation of low-density lipoproteins in patients with type I diabetes, type II diabetes and control subjects. Clin. Chim. Acta Int. J. Clin. Chem. 1996;254:173–186. doi: 10.1016/0009-8981(96)06384-x. [DOI] [PubMed] [Google Scholar]
- 34.Chen M.D., Lin P.Y., Tsou C.T., Wang J.J., Lin W.H. Selected metals status in patients with noninsulin-dependent diabetes mellitus. Biol. Trace Elem. Res. 1995;50:119–124. doi: 10.1007/BF02789414. [DOI] [PubMed] [Google Scholar]
- 35.Walter R.M., Jr., Uriu-Hare J.Y., Olin K.L. Copper, zinc, manganese, and magnesium status and complications of diabetes mellitus. Diabetes Care. 1991;14:1050–1056. doi: 10.2337/diacare.14.11.1050. [DOI] [PubMed] [Google Scholar]
- 36.Basaki M., Saeb M., Nazifi S., Shamsaei H.A. Zinc, copper, iron, and chromium concentrations in young patients with type 2 diabetes mellitus. Biol. Trace Elem. Res. 2012;148:161–164. doi: 10.1007/s12011-012-9360-6. [DOI] [PubMed] [Google Scholar]
- 37.Kase B.A., Northrup H., Morrison A.C. Association of copper-zinc superoxide dismutase (SOD1) and manganese superoxide dismutase (SOD2) genes with nonsyndromic myelomeningocele. Birth Defects Res. Part A Clin. Mol. Teratol. 2012;94:762–769. doi: 10.1002/bdra.23065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez-Guerrero C., Hernandez-Chavez P., Romo-Palafox I., Blanco-Melo G., Parra-Carriedo A., Perez-Lizaur A. Genetic polymorphisms in SOD (rs2070424, rs7880) and CAT (rs7943316, rs1001179) enzymes are associated with increased body fat percentage and visceral fat in an obese population from Central Mexico. Arch. Med. Res. 2016;47:331–339. doi: 10.1016/j.arcmed.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Spisak K., Klimkowicz-Mrowiec A., Pera J., Dziedzic T., Aleksandra G., Slowik A. rs2070424 of the SOD1 gene is associated with risk of Alzheimer's disease. Neurol. Neurochir. Pol. 2014;48:342–345. doi: 10.1016/j.pjnns.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y.M., Li X.D., Guo X., Liu B., Lin A.H., Rao S.Q. Association between polymorphisms in SOD1 and noise-induced hearing loss in Chinese workers. Acta Otolaryngol. 2010;130:477–486. doi: 10.3109/00016480903253587. [DOI] [PubMed] [Google Scholar]
- 41.Halliwell BG J.M.C. fourth ed. Oxford University Press; Oxford, U K: 2007. Free Radicals in Biology and Medicine. [Google Scholar]
- 42.Kadiiska M.B., Hanna P.M., Hernandez L., Mason R.P. In vivo evidence of hydroxyl radical formation after acute copper and ascorbic acid intake: electron spin resonance spin-trapping investigation. Mol. Pharmacol. 1992;42:723–729. [PubMed] [Google Scholar]
- 43.Hunt J.V., Smith C.C., Wolff S.P. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990;39:1420–1424. doi: 10.2337/diab.39.11.1420. [DOI] [PubMed] [Google Scholar]
- 44.Evans J., Goldfine I., Maddux B., Grodsky G. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 45.El Khattabi I., Sharma A. Preventing p38 MAPK-mediated MafA degradation ameliorates β-cell dysfunction under oxidative stress. Mol. Endocrinol. 2013;27:1078–1090. doi: 10.1210/me.2012-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seifert S., Krause R., Gloe K., Henle T. Metal complexation by the peptide-bound maillard reaction products N(epsilon)-fructoselysine and N(epsilon)-carboxymethyllysine. J. Agric. Food Chem. 2004;52:2347–2350. doi: 10.1021/jf035223y. [DOI] [PubMed] [Google Scholar]
- 47.Chevion M. A site-specific mechanism for free radical induced biological damage: the essential role of redox-active transition metals. Free Radic. Biol. Med. 1988;5:27–37. doi: 10.1016/0891-5849(88)90059-7. [DOI] [PubMed] [Google Scholar]
- 48.Masad A., Hayes L., Tabner B.J. Copper-mediated formation of hydrogen peroxide from the amylin peptide: a novel mechanism for degeneration of islet cells in type-2 diabetes mellitus? FEBS Lett. 2007;581:3489–3493. doi: 10.1016/j.febslet.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 49.Westermark P., Andersson A., Westermark G.T. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol. Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.