Abstract
Electronic cigarette (e-cigarette) use, or vaping, is gaining widespread popularity among adults aged 18–35. Vaping is commercially promoted as a safer alternative to traditional cigarette smoking. Previous studies have reported a close relationship between conventional cigarette smoking and acute eosinophilic pneumonia (AEP), but only one case report to date associates vaping with AEP in a male patient. We present the first case of AEP involving a young female after use of e-cigarettes. Clinicians should consider AEP when evaluating young patients with hypoxic respiratory failure and a recent history of e-cigarette use. This case highlights the need for more research into the relationship between e-cigarettes and AEP.
Keywords: Vaping, Electronic cigarette use, Acute eosinophilic pneumonia
1. Introduction
E-cigarettes are battery-operated devices that heat liquid nicotine, producing an aerosol or vapor, which the user then inhales [1]. E-cigarettes were first developed in 2003, entered the United States in 2006 [2], and have been promoted as a safe and effective alternative to traditional cigarettes [3]. In spite of their rapid rise in popularity and worldwide sales, the effects of e-cigarettes use on short and long-term health are poorly understood. This case describes a young, healthy female who developed hypoxic respiratory failure from acute eosinophilic pneumonia (AEP) after using e-cigarettes.
2. Case report
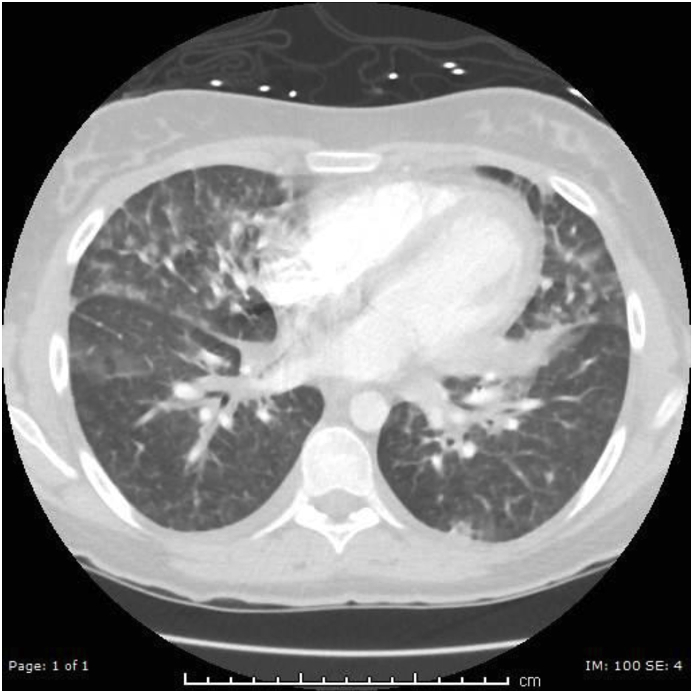
A previously healthy 18-year-old female presented to the Emergency Room after one day of fever, nonproductive cough, difficulty breathing, and pleuritic chest pain. Two months prior to presentation she started vaping using a “Baby Smok Beast Mod” device with 6% nicotine fluid 5 times per day for 30 minutes. She denied traditional cigarette smoking, drug use, exposure to pulmonary irritants, recent respiratory illness or history of deployment to the Middle East. Initial vitals were remarkable for oxygen saturation of 88% on room air, temperature of 102.4 °F, heart rate of 122 beats/min, respiratory rate of 22 breaths/min, and blood pressure of 104/68 mm Hg. On physical exam, she was found to be in mild distress with tachycardia, tachypnea and facial flushing. There was neither accessory muscle use nor chest wall tenderness, and her lungs were clear to auscultation bilaterally. There was no lower extremity edema or calf tenderness. Laboratory tests revealed significant leukocytosis of 19.6 × 109/L with 91.2% granulocytes and 0.5% eosinophils. Initial chest x-ray demonstrated right lower lung airspace consolidation consistent with a pneumonia. Intravenous azithromycin was initiated and she was admitted to the hospital for further monitoring. Overnight, she developed moderate distress with worsening tachycardia, dyspnea, and hypoxemia. Increasing levels of oxygen by nasal cannula were required to maintain her oxygen saturation above 92%, meanwhile her respiratory rate increased to 30 breaths/min. Repeat examination revealed bibasilar inspiratory crackles. D-dimer was elevated to 0.79 mcg/ml. Repeat chest radiograph demonstrated increasing airspace opacities. A computed tomography pulmonary angiogram (CTPA) was completed due to concern for pulmonary embolism (PE). The CTPA excluded PE, but found diffuse ground-glass patchy airspace disease and coalescing nodules (Fig. 1).
Fig. 1.
Computed tomography pulmonary angiogram showing diffuse ground-glass patchy airspace disease.
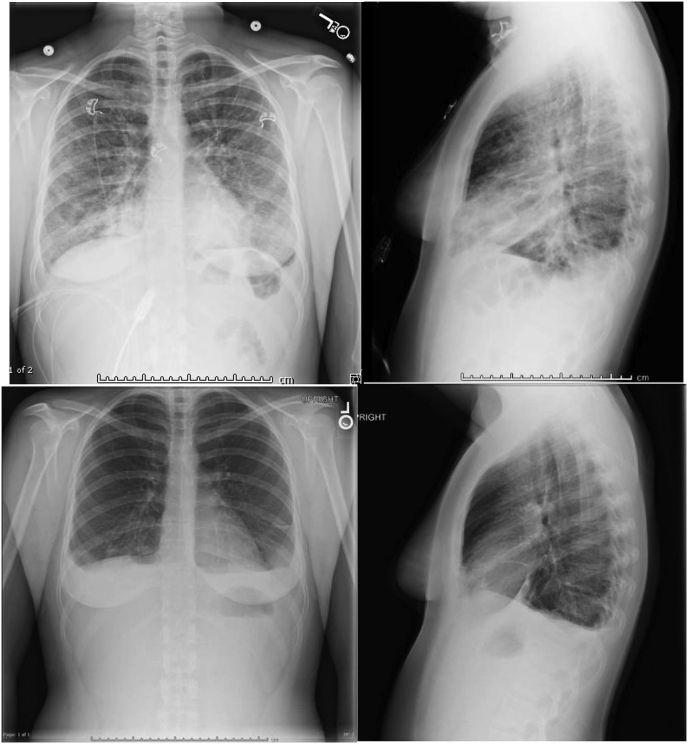
Due to worsening respiratory failure overnight, the patient was transferred to the Intensive Care Unit (ICU) for closer monitoring and respiratory support. Bronchoscopy with bronchial alveolar lavage (BAL) was performed, revealing 26% eosinophils in the lavage fluid. Sputum and BAL cultures were negative for viral, fungal, and bacterial pathogens. No other infectious etiologies to include TB, legionella, strongyloides, coccidioides, or histoplasma were found. Given her acute onset of symptoms, negative alternative workup, and significant BAL eosinophilia the diagnosis of AEP was made. She was started on methylprednisolone 125mg intravenous every 6 hours. After two days she showed significant improvement and was switched to prednisone 60mg oral once daily. She fully recovered 6 days after starting steroids, and was discharged home on oral prednisone with subsequent tapering. On discharge, her vital signs and physical exam were within normal limits. A repeat chest x-ray performed on the day of discharge showed significant improvement of the airspace opacities bilaterally (Fig. 2).
Fig. 2.
Chest radiography before and after steroids treatment. Top left and right: Chest PA and lateral upright series before treatment. Note made of bibasilar airspace opacities. Bottom left and right: Chest PA and lateral upright series after treatment. Significant interval decreased patchy airspace disease within the bilateral lungs.
3. Discussion
Since its original description in 1989 [4], fewer than two hundred AEP cases have been reported; there is a 2:1 male predominance. AEP is a challenging diagnosis to make since patients frequently appear to have a rapidly progressive infectious process with chest radiographs mimicking bacterial pneumonia. A diagnosis of AEP is based upon identification of characteristic symptoms, a detailed patient history, a thorough clinical evaluation, and eosinophilia on BAL [5,6]. Previous studies have reported a close relationship between conventional cigarette smoking and AEP [[7], [8], [9]]. The mechanism by which cigarette smoking induces AEP is suspected to be a strong inflammatory stimulus that recruits macrophages and neutrophils to lung tissue. This induces pro-inflammatory cytokines, such as interleukin (IL)- 5, IL-6, IL-7, and tumor necrosis factor, which may be the inciting event causing eosinophil-rich exudate within the alveoli [10,11].
E-cigarettes are gaining widespread popularity over the past few years with 10.8 million adult users in the United States as of 2016 [12]. Recent studies have shown that the mechanism of inflammation and cytokine stimulation in e-cigarette users is similar to cigarette smokers, including elevation of IL-6 and IL-8 [13,14]. This raises concerns that e-cigarettes may induce AEP and other lung diseases, as their use promotes pulmonary inflammation by a mechanism similar to traditional cigarette smoking. Due to the wide variety of vaping devices and fluid brands (and constituent chemical compounds), more research is required to determine the exact cause of e-cigarette induced AEP.
Physicians should consider AEP in previously healthy patients with hypoxic respiratory failure who have a history of recent e-cigarette use. Previous AEP cases have been associated with traditional cigarettes [7], pulmonary irritants [15], or military service in the Middle East [16]. As E-cigarette use becomes more prevalent, it may become a more common trigger of AEP. In this case of clear association, the patient had no other traditional exposures which could cause AEP, leaving e-cigarettes as the most likely causative irritant. One prior case of e-cigarette-associated AEP was reported in a male patient in 2014 [17]; this is the first case of AEP involving a female after use of e-cigarettes. Although most cases of AEP are diagnosed in the male population, further research may suggest whether AEP is more common in males due to confounding factors such as job preference and smoking prevalence, or organic factors.
References
- 1.Orellana-Barrios M.A., Payne D., Mulkey Z., Nugent K. Electronic cigarettes-A narrative review for clinicians. Am. J. Med. 2015;128(7):674–681. doi: 10.1016/j.amjmed.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Hajek P., Etter J.F., Benowitz N., Eissenberg T., McRobbie H. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction. 2014;109(11):1801–1810. doi: 10.1111/add.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rom O., Pecorelli A., Valacchi G., Reznick A.Z. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann. N. Y. Acad. Sci. 2015;1340:65–74. doi: 10.1111/nyas.12609. [DOI] [PubMed] [Google Scholar]
- 4.Allen J.N., Pacht E.R., Gadek J.E., Davis W.B. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N. Engl. J. Med. 1989;321(9):569–574. doi: 10.1056/NEJM198908313210903. [DOI] [PubMed] [Google Scholar]
- 5.Allen J.N., Davis W.B. Eosinophilic lung diseases. Am. J. Respir. Crit. Care Med. 1994;150(5 Pt 1):1423–1438. doi: 10.1164/ajrccm.150.5.7952571. [DOI] [PubMed] [Google Scholar]
- 6.Philit F., Etienne-Mastroianni B., Parrot A., Guerin C., Robert D., Cordier J.F. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am. J. Respir. Crit. Care Med. 2002;166(9):1235–1239. doi: 10.1164/rccm.2112056. [DOI] [PubMed] [Google Scholar]
- 7.Shintani H., Fujimura M., Yasui M. Acute eosinophilic pneumonia caused by cigarette smoking. Intern. Med. 2000;39(1):66–68. doi: 10.2169/internalmedicine.39.66. [DOI] [PubMed] [Google Scholar]
- 8.Uchiyama H., Suda T., Nakamura Y. Alterations in smoking habits are associated with acute eosinophilic pneumonia. Chest. 2008;133(5):1174–1180. doi: 10.1378/chest.07-2669. [DOI] [PubMed] [Google Scholar]
- 9.Grossi E., Poletti G., Poletti V. Acute eosinophilic pneumonia with respiratory failure: a case likely triggered by cigarette smoking. Monaldi Arch. Chest Dis. 2004;61(1):58–61. [PubMed] [Google Scholar]
- 10.Allen J.N., Liao Z., Wewers M.D., Altenberger E.A., Moore S.A., Allen E.D. Detection of IL-5 and IL-1 receptor antagonist in bronchoalveolar lavage fluid in acute eosinophilic pneumonia. J. Allergy Clin. Immunol. 1996;97(6):1366–1374. doi: 10.1016/s0091-6749(96)70206-3. [DOI] [PubMed] [Google Scholar]
- 11.Kuschner W.G., D'Alessandro A., Wong H., Blanc P.D. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur. Respir. J. 1996;9(10):1989–1994. doi: 10.1183/09031936.96.09101989. [DOI] [PubMed] [Google Scholar]
- 12.Mirbolouk M., Charkhchi P., Kianoush S. Prevalence and distribution of E-cigarette use among U.S. Adults: behavioral risk factor surveillance system, 2016. Ann. Intern. Med. 2018;169(7):429–438. doi: 10.7326/M17-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerner C.A., Sundar I.K., Yao H. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Q., Jiang D., Minor M., Chu H.W. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rom W.N., Weiden M., Garcia R. Acute eosinophilic pneumonia in a New York City firefighter exposed to World Trade Center dust. Am. J. Respir. Crit. Care Med. 2002;166:797. doi: 10.1164/rccm.200206-576OC. [DOI] [PubMed] [Google Scholar]
- 16.Shorr A.F., Scoville S.L., Cersovsky S.B. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. J. Am. Med. Assoc. 2004;292:2997. doi: 10.1001/jama.292.24.2997. [DOI] [PubMed] [Google Scholar]
- 17.Thota D., Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J. Emerg. Med. 2014;47(1):15–17. doi: 10.1016/j.jemermed.2013.09.034. [DOI] [PubMed] [Google Scholar]